KR20190089851A - 4-메틸-5-(피라진-2-일)-3h-1,2-디티올-3-티온의 제제, 맛-개질 제제, 및 그의 제조 및 사용 방법 - Google Patents
4-메틸-5-(피라진-2-일)-3h-1,2-디티올-3-티온의 제제, 맛-개질 제제, 및 그의 제조 및 사용 방법 Download PDFInfo
- Publication number
- KR20190089851A KR20190089851A KR1020197010518A KR20197010518A KR20190089851A KR 20190089851 A KR20190089851 A KR 20190089851A KR 1020197010518 A KR1020197010518 A KR 1020197010518A KR 20197010518 A KR20197010518 A KR 20197010518A KR 20190089851 A KR20190089851 A KR 20190089851A
- Authority
- KR
- South Korea
- Prior art keywords
- composition
- crystals
- liquid
- weight
- bulking agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- CKNAQFVBEHDJQV-UHFFFAOYSA-N oltipraz Chemical compound S1SC(=S)C(C)=C1C1=CN=CC=N1 CKNAQFVBEHDJQV-UHFFFAOYSA-N 0.000 title claims description 25
- 238000002360 preparation method Methods 0.000 title claims description 13
- 239000001962 taste-modifying agent Substances 0.000 title 1
- 239000000203 mixture Substances 0.000 claims abstract description 860
- 239000013078 crystal Substances 0.000 claims abstract description 382
- 238000000034 method Methods 0.000 claims abstract description 209
- 239000007788 liquid Substances 0.000 claims description 301
- 239000004067 bulking agent Substances 0.000 claims description 178
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 177
- 239000008194 pharmaceutical composition Substances 0.000 claims description 154
- -1 alkenyl ether Chemical compound 0.000 claims description 106
- 239000003381 stabilizer Substances 0.000 claims description 91
- 206010028116 Mucosal inflammation Diseases 0.000 claims description 89
- 201000010927 Mucositis Diseases 0.000 claims description 88
- 239000000796 flavoring agent Substances 0.000 claims description 80
- 239000000725 suspension Substances 0.000 claims description 78
- 239000007787 solid Substances 0.000 claims description 77
- 239000002904 solvent Substances 0.000 claims description 77
- 229920000642 polymer Polymers 0.000 claims description 70
- 235000019634 flavors Nutrition 0.000 claims description 65
- 239000000654 additive Substances 0.000 claims description 63
- 235000013355 food flavoring agent Nutrition 0.000 claims description 58
- 239000004094 surface-active agent Substances 0.000 claims description 55
- 230000000996 additive effect Effects 0.000 claims description 53
- 230000008569 process Effects 0.000 claims description 51
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 claims description 49
- 229920000053 polysorbate 80 Polymers 0.000 claims description 49
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 48
- 238000011282 treatment Methods 0.000 claims description 46
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 44
- 235000021028 berry Nutrition 0.000 claims description 43
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 43
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 42
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 39
- 229920001577 copolymer Polymers 0.000 claims description 39
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 39
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 claims description 36
- 239000003795 chemical substances by application Substances 0.000 claims description 35
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerol Natural products OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 34
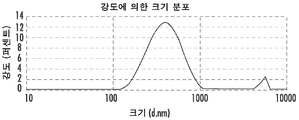
- 238000002296 dynamic light scattering Methods 0.000 claims description 30
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 claims description 28
- 229940068968 polysorbate 80 Drugs 0.000 claims description 28
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 28
- 235000003599 food sweetener Nutrition 0.000 claims description 24
- 235000019333 sodium laurylsulphate Nutrition 0.000 claims description 24
- 239000003765 sweetening agent Substances 0.000 claims description 24
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 23
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 claims description 23
- 239000003125 aqueous solvent Substances 0.000 claims description 23
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 claims description 23
- 238000011068 loading method Methods 0.000 claims description 23
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 claims description 22
- 238000001694 spray drying Methods 0.000 claims description 22
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 21
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 21
- 229920001223 polyethylene glycol Polymers 0.000 claims description 21
- 229920001983 poloxamer Polymers 0.000 claims description 20
- 229920000193 polymethacrylate Polymers 0.000 claims description 20
- 239000008280 blood Substances 0.000 claims description 19
- 210000004369 blood Anatomy 0.000 claims description 19
- 229920001993 poloxamer 188 Polymers 0.000 claims description 19
- 239000003814 drug Substances 0.000 claims description 17
- 238000004519 manufacturing process Methods 0.000 claims description 17
- 239000004376 Sucralose Substances 0.000 claims description 16
- 235000019408 sucralose Nutrition 0.000 claims description 16
- BAQAVOSOZGMPRM-QBMZZYIRSA-N sucralose Chemical compound O[C@@H]1[C@@H](O)[C@@H](Cl)[C@@H](CO)O[C@@H]1O[C@@]1(CCl)[C@@H](O)[C@H](O)[C@@H](CCl)O1 BAQAVOSOZGMPRM-QBMZZYIRSA-N 0.000 claims description 16
- 235000006679 Mentha X verticillata Nutrition 0.000 claims description 15
- 235000002899 Mentha suaveolens Nutrition 0.000 claims description 15
- 235000001636 Mentha x rotundifolia Nutrition 0.000 claims description 15
- 239000002202 Polyethylene glycol Substances 0.000 claims description 15
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 15
- 229960000502 poloxamer Drugs 0.000 claims description 15
- 229920002307 Dextran Polymers 0.000 claims description 14
- 229920003083 Kollidon® VA64 Polymers 0.000 claims description 14
- AMTWCFIAVKBGOD-UHFFFAOYSA-N dioxosilane;methoxy-dimethyl-trimethylsilyloxysilane Chemical compound O=[Si]=O.CO[Si](C)(C)O[Si](C)(C)C AMTWCFIAVKBGOD-UHFFFAOYSA-N 0.000 claims description 14
- 229940083037 simethicone Drugs 0.000 claims description 14
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 13
- 235000011187 glycerol Nutrition 0.000 claims description 13
- 239000000243 solution Substances 0.000 claims description 13
- 229940079593 drug Drugs 0.000 claims description 11
- 235000010980 cellulose Nutrition 0.000 claims description 10
- 229920002678 cellulose Polymers 0.000 claims description 10
- 239000001913 cellulose Substances 0.000 claims description 10
- 229920001531 copovidone Polymers 0.000 claims description 10
- 239000008369 fruit flavor Substances 0.000 claims description 10
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims description 10
- 239000003755 preservative agent Substances 0.000 claims description 10
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims description 9
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 claims description 9
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 claims description 9
- 229920003134 Eudragit® polymer Polymers 0.000 claims description 9
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 9
- 229930006000 Sucrose Natural products 0.000 claims description 9
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims description 9
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims description 9
- 125000002091 cationic group Chemical group 0.000 claims description 9
- 229940119744 dextran 40 Drugs 0.000 claims description 9
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 claims description 9
- 239000000314 lubricant Substances 0.000 claims description 9
- 229940041616 menthol Drugs 0.000 claims description 9
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 9
- 229920001451 polypropylene glycol Polymers 0.000 claims description 9
- 239000005720 sucrose Substances 0.000 claims description 9
- 229910019142 PO4 Inorganic materials 0.000 claims description 8
- 150000001298 alcohols Polymers 0.000 claims description 8
- 210000001072 colon Anatomy 0.000 claims description 8
- 238000001816 cooling Methods 0.000 claims description 8
- 239000010452 phosphate Substances 0.000 claims description 8
- WLAMNBDJUVNPJU-UHFFFAOYSA-N 2-methylbutyric acid Chemical compound CCC(C)C(O)=O WLAMNBDJUVNPJU-UHFFFAOYSA-N 0.000 claims description 7
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 7
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims description 7
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 7
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 7
- 239000000194 fatty acid Substances 0.000 claims description 7
- 229930195729 fatty acid Natural products 0.000 claims description 7
- 239000008101 lactose Substances 0.000 claims description 7
- WBZFUFAFFUEMEI-UHFFFAOYSA-M Acesulfame k Chemical compound [K+].CC1=CC(=O)[N-]S(=O)(=O)O1 WBZFUFAFFUEMEI-UHFFFAOYSA-M 0.000 claims description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 6
- 108010011485 Aspartame Proteins 0.000 claims description 6
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 6
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 claims description 6
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 6
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 6
- 229930195725 Mannitol Natural products 0.000 claims description 6
- 229920003081 Povidone K 30 Polymers 0.000 claims description 6
- 235000010358 acesulfame potassium Nutrition 0.000 claims description 6
- 229960004998 acesulfame potassium Drugs 0.000 claims description 6
- 239000000619 acesulfame-K Substances 0.000 claims description 6
- 239000000605 aspartame Substances 0.000 claims description 6
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 claims description 6
- 235000010357 aspartame Nutrition 0.000 claims description 6
- 229960003438 aspartame Drugs 0.000 claims description 6
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 6
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 6
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 6
- 229940071826 hydroxyethyl cellulose Drugs 0.000 claims description 6
- 239000000594 mannitol Substances 0.000 claims description 6
- 235000010355 mannitol Nutrition 0.000 claims description 6
- 229920001282 polysaccharide Polymers 0.000 claims description 6
- 239000005017 polysaccharide Substances 0.000 claims description 6
- 230000002335 preservative effect Effects 0.000 claims description 6
- LZZYPRNAOMGNLH-UHFFFAOYSA-M Cetrimonium bromide Chemical compound [Br-].CCCCCCCCCCCCCCCC[N+](C)(C)C LZZYPRNAOMGNLH-UHFFFAOYSA-M 0.000 claims description 5
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 5
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 claims description 5
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 5
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 5
- 229960000541 cetyl alcohol Drugs 0.000 claims description 5
- 229940008099 dimethicone Drugs 0.000 claims description 5
- 235000013870 dimethyl polysiloxane Nutrition 0.000 claims description 5
- 239000004205 dimethyl polysiloxane Substances 0.000 claims description 5
- 150000002016 disaccharides Chemical class 0.000 claims description 5
- SYELZBGXAIXKHU-UHFFFAOYSA-N dodecyldimethylamine N-oxide Chemical compound CCCCCCCCCCCC[N+](C)(C)[O-] SYELZBGXAIXKHU-UHFFFAOYSA-N 0.000 claims description 5
- 239000002552 dosage form Substances 0.000 claims description 5
- 239000008103 glucose Substances 0.000 claims description 5
- 239000008123 high-intensity sweetener Substances 0.000 claims description 5
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 claims description 5
- 150000002772 monosaccharides Chemical class 0.000 claims description 5
- 235000013615 non-nutritive sweetener Nutrition 0.000 claims description 5
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 5
- 229920000136 polysorbate Polymers 0.000 claims description 5
- 229940069328 povidone Drugs 0.000 claims description 5
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 claims description 5
- 239000000600 sorbitol Substances 0.000 claims description 5
- 235000010356 sorbitol Nutrition 0.000 claims description 5
- 230000000699 topical effect Effects 0.000 claims description 5
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 claims description 4
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 claims description 4
- CHHHXKFHOYLYRE-UHFFFAOYSA-M 2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)- Chemical compound [K+].CC=CC=CC([O-])=O CHHHXKFHOYLYRE-UHFFFAOYSA-M 0.000 claims description 4
- 229920000858 Cyclodextrin Polymers 0.000 claims description 4
- 229930091371 Fructose Natural products 0.000 claims description 4
- 239000005715 Fructose Substances 0.000 claims description 4
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 claims description 4
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 claims description 4
- 229920001214 Polysorbate 60 Polymers 0.000 claims description 4
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 claims description 4
- 150000005215 alkyl ethers Chemical class 0.000 claims description 4
- 239000007864 aqueous solution Substances 0.000 claims description 4
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 claims description 4
- RNPXCFINMKSQPQ-UHFFFAOYSA-N dicetyl hydrogen phosphate Chemical compound CCCCCCCCCCCCCCCCOP(O)(=O)OCCCCCCCCCCCCCCCC RNPXCFINMKSQPQ-UHFFFAOYSA-N 0.000 claims description 4
- 229940093541 dicetylphosphate Drugs 0.000 claims description 4
- 239000003085 diluting agent Substances 0.000 claims description 4
- 235000010445 lecithin Nutrition 0.000 claims description 4
- 239000000787 lecithin Substances 0.000 claims description 4
- 229940067606 lecithin Drugs 0.000 claims description 4
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 claims description 4
- 235000010241 potassium sorbate Nutrition 0.000 claims description 4
- 239000004302 potassium sorbate Substances 0.000 claims description 4
- 229940069338 potassium sorbate Drugs 0.000 claims description 4
- 239000000811 xylitol Substances 0.000 claims description 4
- 235000010447 xylitol Nutrition 0.000 claims description 4
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 claims description 4
- 229960002675 xylitol Drugs 0.000 claims description 4
- 239000004384 Neotame Substances 0.000 claims description 3
- 229920002125 Sokalan® Polymers 0.000 claims description 3
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 claims description 3
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 claims description 3
- MRUAUOIMASANKQ-UHFFFAOYSA-N cocamidopropyl betaine Chemical compound CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O MRUAUOIMASANKQ-UHFFFAOYSA-N 0.000 claims description 3
- 239000002274 desiccant Substances 0.000 claims description 3
- 150000002334 glycols Chemical class 0.000 claims description 3
- 239000008297 liquid dosage form Substances 0.000 claims description 3
- 235000019412 neotame Nutrition 0.000 claims description 3
- HLIAVLHNDJUHFG-HOTGVXAUSA-N neotame Chemical compound CC(C)(C)CCN[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 HLIAVLHNDJUHFG-HOTGVXAUSA-N 0.000 claims description 3
- 108010070257 neotame Proteins 0.000 claims description 3
- 229940044519 poloxamer 188 Drugs 0.000 claims description 3
- 229950008882 polysorbate Drugs 0.000 claims description 3
- 235000019204 saccharin Nutrition 0.000 claims description 3
- 229940081974 saccharin Drugs 0.000 claims description 3
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 claims description 3
- 239000000375 suspending agent Substances 0.000 claims description 3
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical class C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 claims description 2
- HBXWUCXDUUJDRB-UHFFFAOYSA-N 1-octadecoxyoctadecane Chemical compound CCCCCCCCCCCCCCCCCCOCCCCCCCCCCCCCCCCCC HBXWUCXDUUJDRB-UHFFFAOYSA-N 0.000 claims description 2
- IEORSVTYLWZQJQ-UHFFFAOYSA-N 2-(2-nonylphenoxy)ethanol Chemical compound CCCCCCCCCC1=CC=CC=C1OCCO IEORSVTYLWZQJQ-UHFFFAOYSA-N 0.000 claims description 2
- HNUQMTZUNUBOLQ-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[2-[2-(2-octadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO HNUQMTZUNUBOLQ-UHFFFAOYSA-N 0.000 claims description 2
- AMRBZKOCOOPYNY-QXMHVHEDSA-N 2-[dimethyl-[(z)-octadec-9-enyl]azaniumyl]acetate Chemical compound CCCCCCCC\C=C/CCCCCCCC[N+](C)(C)CC([O-])=O AMRBZKOCOOPYNY-QXMHVHEDSA-N 0.000 claims description 2
- JDRSMPFHFNXQRB-CMTNHCDUSA-N Decyl beta-D-threo-hexopyranoside Chemical compound CCCCCCCCCCO[C@@H]1O[C@H](CO)C(O)[C@H](O)C1O JDRSMPFHFNXQRB-CMTNHCDUSA-N 0.000 claims description 2
- QWZLBLDNRUUYQI-UHFFFAOYSA-M Methylbenzethonium chloride Chemical compound [Cl-].CC1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 QWZLBLDNRUUYQI-UHFFFAOYSA-M 0.000 claims description 2
- 229920001213 Polysorbate 20 Polymers 0.000 claims description 2
- 229920001219 Polysorbate 40 Polymers 0.000 claims description 2
- ZUAAPNNKRHMPKG-UHFFFAOYSA-N acetic acid;butanedioic acid;methanol;propane-1,2-diol Chemical compound OC.CC(O)=O.CC(O)CO.OC(=O)CCC(O)=O ZUAAPNNKRHMPKG-UHFFFAOYSA-N 0.000 claims description 2
- 239000002671 adjuvant Substances 0.000 claims description 2
- 229940073507 cocamidopropyl betaine Drugs 0.000 claims description 2
- 229940073499 decyl glucoside Drugs 0.000 claims description 2
- 229960003964 deoxycholic acid Drugs 0.000 claims description 2
- 229930182478 glucoside Natural products 0.000 claims description 2
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 claims description 2
- LAPRIVJANDLWOK-UHFFFAOYSA-N laureth-5 Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCO LAPRIVJANDLWOK-UHFFFAOYSA-N 0.000 claims description 2
- PYIDGJJWBIBVIA-UYTYNIKBSA-N lauryl glucoside Chemical compound CCCCCCCCCCCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O PYIDGJJWBIBVIA-UYTYNIKBSA-N 0.000 claims description 2
- 229940048848 lauryl glucoside Drugs 0.000 claims description 2
- 229960002285 methylbenzethonium chloride Drugs 0.000 claims description 2
- 229920000847 nonoxynol Polymers 0.000 claims description 2
- YYELLDKEOUKVIQ-UHFFFAOYSA-N octaethyleneglycol monododecyl ether Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCO YYELLDKEOUKVIQ-UHFFFAOYSA-N 0.000 claims description 2
- 229920002113 octoxynol Polymers 0.000 claims description 2
- 229940066429 octoxynol Drugs 0.000 claims description 2
- HEGSGKPQLMEBJL-RKQHYHRCSA-N octyl beta-D-glucopyranoside Chemical compound CCCCCCCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O HEGSGKPQLMEBJL-RKQHYHRCSA-N 0.000 claims description 2
- 229920001992 poloxamer 407 Polymers 0.000 claims description 2
- 229940044476 poloxamer 407 Drugs 0.000 claims description 2
- 229920002401 polyacrylamide Polymers 0.000 claims description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 claims description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 claims description 2
- 235000010483 polyoxyethylene sorbitan monopalmitate Nutrition 0.000 claims description 2
- 239000000249 polyoxyethylene sorbitan monopalmitate Substances 0.000 claims description 2
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 claims description 2
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 claims description 2
- 229940068977 polysorbate 20 Drugs 0.000 claims description 2
- 229940101027 polysorbate 40 Drugs 0.000 claims description 2
- 229940113124 polysorbate 60 Drugs 0.000 claims description 2
- 229940068965 polysorbates Drugs 0.000 claims description 2
- 229920006316 polyvinylpyrrolidine Polymers 0.000 claims description 2
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 claims description 2
- NRHMKIHPTBHXPF-TUJRSCDTSA-M sodium cholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 NRHMKIHPTBHXPF-TUJRSCDTSA-M 0.000 claims description 2
- FRHNXUKHAUWMOQ-UHFFFAOYSA-M sodium;16-methylheptadecanoate Chemical compound [Na+].CC(C)CCCCCCCCCCCCCCC([O-])=O FRHNXUKHAUWMOQ-UHFFFAOYSA-M 0.000 claims description 2
- CMCBDXRRFKYBDG-UHFFFAOYSA-N 1-dodecoxydodecane Chemical compound CCCCCCCCCCCCOCCCCCCCCCCCC CMCBDXRRFKYBDG-UHFFFAOYSA-N 0.000 claims 1
- 229920002675 Polyoxyl Polymers 0.000 claims 1
- 229920004890 Triton X-100 Polymers 0.000 claims 1
- 239000013504 Triton X-100 Substances 0.000 claims 1
- 150000001408 amides Chemical class 0.000 claims 1
- 239000002826 coolant Substances 0.000 claims 1
- DTPCFIHYWYONMD-UHFFFAOYSA-N decaethylene glycol Chemical compound OCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO DTPCFIHYWYONMD-UHFFFAOYSA-N 0.000 claims 1
- 150000002170 ethers Chemical class 0.000 claims 1
- 150000004676 glycans Chemical class 0.000 claims 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims 1
- IEUQRELXPXKBDR-UHFFFAOYSA-M sodium N,N-dimethyldodecan-1-amine oxide dodecyl sulfate Chemical compound [Na+].CCCCCCCCCCCC[N+](C)(C)[O-].CCCCCCCCCCCCOS([O-])(=O)=O IEUQRELXPXKBDR-UHFFFAOYSA-M 0.000 claims 1
- FHHPUSMSKHSNKW-SMOYURAASA-M sodium deoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 FHHPUSMSKHSNKW-SMOYURAASA-M 0.000 claims 1
- AISMNBXOJRHCIA-UHFFFAOYSA-N trimethylazanium;bromide Chemical compound Br.CN(C)C AISMNBXOJRHCIA-UHFFFAOYSA-N 0.000 claims 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 76
- 210000004027 cell Anatomy 0.000 description 47
- 241001465754 Metazoa Species 0.000 description 43
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 42
- 239000000843 powder Substances 0.000 description 39
- 238000003801 milling Methods 0.000 description 38
- 238000009472 formulation Methods 0.000 description 31
- 208000003265 stomatitis Diseases 0.000 description 26
- 239000002245 particle Substances 0.000 description 25
- 229920003151 Eudragit® RL polymer Polymers 0.000 description 24
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 24
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 22
- 208000025865 Ulcer Diseases 0.000 description 22
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 20
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 20
- 150000001875 compounds Chemical class 0.000 description 20
- 238000001959 radiotherapy Methods 0.000 description 20
- 235000019605 sweet taste sensations Nutrition 0.000 description 20
- 238000001238 wet grinding Methods 0.000 description 20
- 235000019640 taste Nutrition 0.000 description 19
- 239000003981 vehicle Substances 0.000 description 19
- 230000000694 effects Effects 0.000 description 18
- 239000004615 ingredient Substances 0.000 description 18
- 239000003642 reactive oxygen metabolite Substances 0.000 description 18
- 239000007900 aqueous suspension Substances 0.000 description 17
- 235000019658 bitter taste Nutrition 0.000 description 17
- 239000002775 capsule Substances 0.000 description 17
- 229920006317 cationic polymer Polymers 0.000 description 17
- 239000008368 mint flavor Substances 0.000 description 17
- 239000000523 sample Substances 0.000 description 17
- 235000019441 ethanol Nutrition 0.000 description 16
- 239000012535 impurity Substances 0.000 description 16
- DNKKLDKIFMDAPT-UHFFFAOYSA-N n,n-dimethylmethanamine;2-methylprop-2-enoic acid Chemical compound CN(C)C.CC(=C)C(O)=O.CC(=C)C(O)=O DNKKLDKIFMDAPT-UHFFFAOYSA-N 0.000 description 16
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 15
- 238000002156 mixing Methods 0.000 description 15
- 229940068196 placebo Drugs 0.000 description 14
- 239000000902 placebo Substances 0.000 description 14
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 13
- 238000004458 analytical method Methods 0.000 description 13
- 230000008859 change Effects 0.000 description 13
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 13
- 238000004108 freeze drying Methods 0.000 description 13
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 13
- 239000007921 spray Substances 0.000 description 13
- 231100000397 ulcer Toxicity 0.000 description 13
- 108010024636 Glutathione Proteins 0.000 description 12
- 239000004480 active ingredient Substances 0.000 description 12
- 239000003963 antioxidant agent Substances 0.000 description 12
- 230000015572 biosynthetic process Effects 0.000 description 12
- 229960003180 glutathione Drugs 0.000 description 12
- 230000036542 oxidative stress Effects 0.000 description 12
- 239000003826 tablet Substances 0.000 description 12
- 235000006708 antioxidants Nutrition 0.000 description 11
- 230000002354 daily effect Effects 0.000 description 11
- 238000005259 measurement Methods 0.000 description 11
- 239000000377 silicon dioxide Substances 0.000 description 11
- 244000078534 Vaccinium myrtillus Species 0.000 description 10
- 239000006185 dispersion Substances 0.000 description 10
- 230000003834 intracellular effect Effects 0.000 description 10
- 238000011200 topical administration Methods 0.000 description 10
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 9
- 239000000872 buffer Substances 0.000 description 9
- 238000009826 distribution Methods 0.000 description 9
- 238000001035 drying Methods 0.000 description 9
- 230000014509 gene expression Effects 0.000 description 9
- 238000000227 grinding Methods 0.000 description 9
- 230000005923 long-lasting effect Effects 0.000 description 9
- 230000007935 neutral effect Effects 0.000 description 9
- 239000006187 pill Substances 0.000 description 9
- 238000001556 precipitation Methods 0.000 description 9
- 230000009467 reduction Effects 0.000 description 9
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 8
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 8
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 8
- 238000013019 agitation Methods 0.000 description 8
- 239000002518 antifoaming agent Substances 0.000 description 8
- 230000003078 antioxidant effect Effects 0.000 description 8
- 229960003237 betaine Drugs 0.000 description 8
- 239000003086 colorant Substances 0.000 description 8
- 239000012530 fluid Substances 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 230000006872 improvement Effects 0.000 description 8
- 239000006194 liquid suspension Substances 0.000 description 8
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000002736 nonionic surfactant Substances 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- 229910052708 sodium Inorganic materials 0.000 description 8
- 229940083542 sodium Drugs 0.000 description 8
- 229960004793 sucrose Drugs 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 7
- 206010028980 Neoplasm Diseases 0.000 description 7
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 7
- 238000002512 chemotherapy Methods 0.000 description 7
- 230000001186 cumulative effect Effects 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 239000003995 emulsifying agent Substances 0.000 description 7
- 210000000214 mouth Anatomy 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 229920000058 polyacrylate Polymers 0.000 description 7
- 229960004063 propylene glycol Drugs 0.000 description 7
- 108090000623 proteins and genes Proteins 0.000 description 7
- 125000004307 pyrazin-2-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 7
- 238000001953 recrystallisation Methods 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 235000015112 vegetable and seed oil Nutrition 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- 208000031648 Body Weight Changes Diseases 0.000 description 6
- 206010015150 Erythema Diseases 0.000 description 6
- 102000003896 Myeloperoxidases Human genes 0.000 description 6
- 108090000235 Myeloperoxidases Proteins 0.000 description 6
- 240000007651 Rubus glaucus Species 0.000 description 6
- 235000017537 Vaccinium myrtillus Nutrition 0.000 description 6
- 230000004579 body weight change Effects 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 6
- 235000020971 citrus fruits Nutrition 0.000 description 6
- VFLDPWHFBUODDF-FCXRPNKRSA-N curcumin Chemical compound C1=C(O)C(OC)=CC(\C=C\C(=O)CC(=O)\C=C\C=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-FCXRPNKRSA-N 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 206010013781 dry mouth Diseases 0.000 description 6
- 235000013399 edible fruits Nutrition 0.000 description 6
- 231100000321 erythema Toxicity 0.000 description 6
- 238000011156 evaluation Methods 0.000 description 6
- 229960001375 lactose Drugs 0.000 description 6
- 239000000178 monomer Substances 0.000 description 6
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 6
- 235000019198 oils Nutrition 0.000 description 6
- 230000005855 radiation Effects 0.000 description 6
- 238000004904 shortening Methods 0.000 description 6
- 235000012239 silicon dioxide Nutrition 0.000 description 6
- 238000013112 stability test Methods 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 5
- 241000207199 Citrus Species 0.000 description 5
- 208000019505 Deglutition disease Diseases 0.000 description 5
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 5
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 5
- 229920003091 Methocel™ Polymers 0.000 description 5
- 102000007456 Peroxiredoxin Human genes 0.000 description 5
- 108010033024 Phospholipid Hydroperoxide Glutathione Peroxidase Proteins 0.000 description 5
- 102100023410 Phospholipid hydroperoxide glutathione peroxidase Human genes 0.000 description 5
- 235000011034 Rubus glaucus Nutrition 0.000 description 5
- 235000009122 Rubus idaeus Nutrition 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 5
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 5
- 102000019197 Superoxide Dismutase Human genes 0.000 description 5
- 108010012715 Superoxide dismutase Proteins 0.000 description 5
- 235000003095 Vaccinium corymbosum Nutrition 0.000 description 5
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 5
- 229960004308 acetylcysteine Drugs 0.000 description 5
- 229920006318 anionic polymer Polymers 0.000 description 5
- 235000021014 blueberries Nutrition 0.000 description 5
- 238000000546 chi-square test Methods 0.000 description 5
- 235000015165 citric acid Nutrition 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 239000013530 defoamer Substances 0.000 description 5
- 238000010790 dilution Methods 0.000 description 5
- 239000012895 dilution Substances 0.000 description 5
- 238000004090 dissolution Methods 0.000 description 5
- SFNALCNOMXIBKG-UHFFFAOYSA-N ethylene glycol monododecyl ether Chemical compound CCCCCCCCCCCCOCCO SFNALCNOMXIBKG-UHFFFAOYSA-N 0.000 description 5
- 235000012907 honey Nutrition 0.000 description 5
- 150000002632 lipids Chemical class 0.000 description 5
- 229960001855 mannitol Drugs 0.000 description 5
- 210000004877 mucosa Anatomy 0.000 description 5
- 239000003960 organic solvent Substances 0.000 description 5
- 108030002458 peroxiredoxin Proteins 0.000 description 5
- 150000004804 polysaccharides Chemical class 0.000 description 5
- 230000002265 prevention Effects 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 238000001878 scanning electron micrograph Methods 0.000 description 5
- 239000001509 sodium citrate Substances 0.000 description 5
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 5
- 229940035049 sorbitan monooleate Drugs 0.000 description 5
- 235000011069 sorbitan monooleate Nutrition 0.000 description 5
- 239000001593 sorbitan monooleate Substances 0.000 description 5
- 230000000087 stabilizing effect Effects 0.000 description 5
- 235000000346 sugar Nutrition 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 230000024883 vasodilation Effects 0.000 description 5
- 235000009492 vitamin B5 Nutrition 0.000 description 5
- 239000011675 vitamin B5 Substances 0.000 description 5
- 239000001993 wax Substances 0.000 description 5
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 4
- 235000013162 Cocos nucifera Nutrition 0.000 description 4
- 244000060011 Cocos nucifera Species 0.000 description 4
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 4
- 108010010803 Gelatin Proteins 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 239000004166 Lanolin Substances 0.000 description 4
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 4
- 240000007472 Leucaena leucocephala Species 0.000 description 4
- 206010028813 Nausea Diseases 0.000 description 4
- 244000179853 Sideroxylon dulcificum Species 0.000 description 4
- 235000011341 Sideroxylon dulcificum Nutrition 0.000 description 4
- 229930003571 Vitamin B5 Natural products 0.000 description 4
- 230000002776 aggregation Effects 0.000 description 4
- POJWUDADGALRAB-UHFFFAOYSA-N allantoin Chemical compound NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 description 4
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 235000021029 blackberry Nutrition 0.000 description 4
- FAPWYRCQGJNNSJ-UBKPKTQASA-L calcium D-pantothenic acid Chemical compound [Ca+2].OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O.OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O FAPWYRCQGJNNSJ-UBKPKTQASA-L 0.000 description 4
- 229960002079 calcium pantothenate Drugs 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 239000001768 carboxy methyl cellulose Substances 0.000 description 4
- 239000004359 castor oil Substances 0.000 description 4
- 235000019438 castor oil Nutrition 0.000 description 4
- 230000003833 cell viability Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 239000001530 fumaric acid Substances 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 210000001035 gastrointestinal tract Anatomy 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 239000007903 gelatin capsule Substances 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011852 gelatine desserts Nutrition 0.000 description 4
- 229960001031 glucose Drugs 0.000 description 4
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 4
- 235000019388 lanolin Nutrition 0.000 description 4
- 229940039717 lanolin Drugs 0.000 description 4
- 235000019359 magnesium stearate Nutrition 0.000 description 4
- 229920000609 methyl cellulose Polymers 0.000 description 4
- 235000010981 methylcellulose Nutrition 0.000 description 4
- 239000001923 methylcellulose Substances 0.000 description 4
- 230000008693 nausea Effects 0.000 description 4
- 150000007524 organic acids Chemical class 0.000 description 4
- 230000004792 oxidative damage Effects 0.000 description 4
- 235000019161 pantothenic acid Nutrition 0.000 description 4
- 239000011713 pantothenic acid Substances 0.000 description 4
- 229940055726 pantothenic acid Drugs 0.000 description 4
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 230000035807 sensation Effects 0.000 description 4
- 235000019615 sensations Nutrition 0.000 description 4
- APSBXTVYXVQYAB-UHFFFAOYSA-M sodium docusate Chemical group [Na+].CCCCC(CC)COC(=O)CC(S([O-])(=O)=O)C(=O)OCC(CC)CCCC APSBXTVYXVQYAB-UHFFFAOYSA-M 0.000 description 4
- 229960002920 sorbitol Drugs 0.000 description 4
- 230000006641 stabilisation Effects 0.000 description 4
- 238000011105 stabilization Methods 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 239000002562 thickening agent Substances 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- 239000008158 vegetable oil Substances 0.000 description 4
- 239000004034 viscosity adjusting agent Substances 0.000 description 4
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 3
- XDFNWJDGWJVGGN-UHFFFAOYSA-N 2-(2,7-dichloro-3,6-dihydroxy-9h-xanthen-9-yl)benzoic acid Chemical compound OC(=O)C1=CC=CC=C1C1C2=CC(Cl)=C(O)C=C2OC2=CC(O)=C(Cl)C=C21 XDFNWJDGWJVGGN-UHFFFAOYSA-N 0.000 description 3
- ROJOGNLUQZQVTP-UHFFFAOYSA-N 2-(4-methyl-3H-dithiol-5-yl)pyrazine Chemical compound N1=C(C=NC=C1)C1=C(CSS1)C ROJOGNLUQZQVTP-UHFFFAOYSA-N 0.000 description 3
- RFVNOJDQRGSOEL-UHFFFAOYSA-N 2-hydroxyethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCO RFVNOJDQRGSOEL-UHFFFAOYSA-N 0.000 description 3
- 240000002234 Allium sativum Species 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 240000009088 Fragaria x ananassa Species 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- 239000004909 Moisturizer Substances 0.000 description 3
- ATGQXSBKTQANOH-UWVGARPKSA-N N-oleoylphytosphingosine Chemical compound CCCCCCCCCCCCCC[C@@H](O)[C@@H](O)[C@H](CO)NC(=O)CCCCCCC\C=C/CCCCCCCC ATGQXSBKTQANOH-UWVGARPKSA-N 0.000 description 3
- 102000003945 NF-kappa B Human genes 0.000 description 3
- 108010057466 NF-kappa B Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 238000009012 ROS assay kit Methods 0.000 description 3
- 235000017848 Rubus fruticosus Nutrition 0.000 description 3
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 235000021307 Triticum Nutrition 0.000 description 3
- 244000098338 Triticum aestivum Species 0.000 description 3
- 240000006365 Vitis vinifera Species 0.000 description 3
- 235000014787 Vitis vinifera Nutrition 0.000 description 3
- 206010052428 Wound Diseases 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 239000003945 anionic surfactant Substances 0.000 description 3
- 229940121363 anti-inflammatory agent Drugs 0.000 description 3
- 239000002260 anti-inflammatory agent Substances 0.000 description 3
- 239000012062 aqueous buffer Substances 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 229960005070 ascorbic acid Drugs 0.000 description 3
- 238000000889 atomisation Methods 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 239000007853 buffer solution Substances 0.000 description 3
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 3
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 3
- 239000003093 cationic surfactant Substances 0.000 description 3
- 229940099417 ceramide 2 Drugs 0.000 description 3
- 229940044176 ceramide 3 Drugs 0.000 description 3
- 229940081733 cetearyl alcohol Drugs 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 235000012000 cholesterol Nutrition 0.000 description 3
- 229940107161 cholesterol Drugs 0.000 description 3
- 230000035597 cooling sensation Effects 0.000 description 3
- 229920006037 cross link polymer Polymers 0.000 description 3
- 229940109262 curcumin Drugs 0.000 description 3
- 235000012754 curcumin Nutrition 0.000 description 3
- 239000004148 curcumin Substances 0.000 description 3
- VFLDPWHFBUODDF-UHFFFAOYSA-N diferuloylmethane Natural products C1=C(O)C(OC)=CC(C=CC(=O)CC(=O)C=CC=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-UHFFFAOYSA-N 0.000 description 3
- PGQAXGHQYGXVDC-UHFFFAOYSA-N dodecyl(dimethyl)azanium;chloride Chemical compound Cl.CCCCCCCCCCCCN(C)C PGQAXGHQYGXVDC-UHFFFAOYSA-N 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 239000011888 foil Substances 0.000 description 3
- 229960002737 fructose Drugs 0.000 description 3
- 235000004611 garlic Nutrition 0.000 description 3
- 238000005469 granulation Methods 0.000 description 3
- 230000003179 granulation Effects 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 239000003701 inert diluent Substances 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 239000012669 liquid formulation Substances 0.000 description 3
- 229960002160 maltose Drugs 0.000 description 3
- 230000035800 maturation Effects 0.000 description 3
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 3
- 229960004963 mesalazine Drugs 0.000 description 3
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 230000001333 moisturizer Effects 0.000 description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 235000008390 olive oil Nutrition 0.000 description 3
- 239000012188 paraffin wax Substances 0.000 description 3
- 230000001681 protective effect Effects 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 238000004062 sedimentation Methods 0.000 description 3
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- RTVVXRKGQRRXFJ-UHFFFAOYSA-N sodium;2-sulfobutanedioic acid Chemical compound [Na].OC(=O)CC(C(O)=O)S(O)(=O)=O RTVVXRKGQRRXFJ-UHFFFAOYSA-N 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 229910021653 sulphate ion Inorganic materials 0.000 description 3
- 230000009967 tasteless effect Effects 0.000 description 3
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 3
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical compound CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 2
- FLPJVCMIKUWSDR-UHFFFAOYSA-N 2-(4-formylphenoxy)acetamide Chemical compound NC(=O)COC1=CC=C(C=O)C=C1 FLPJVCMIKUWSDR-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- CYPKANIKIWLVMF-UHFFFAOYSA-N 2-[(2-oxo-3,4-dihydro-1h-quinolin-5-yl)oxy]acetic acid Chemical compound N1C(=O)CCC2=C1C=CC=C2OCC(=O)O CYPKANIKIWLVMF-UHFFFAOYSA-N 0.000 description 2
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 2
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Natural products NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 description 2
- 241001116389 Aloe Species 0.000 description 2
- 238000012935 Averaging Methods 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- SOGAXMICEFXMKE-UHFFFAOYSA-N Butylmethacrylate Chemical compound CCCCOC(=O)C(C)=C SOGAXMICEFXMKE-UHFFFAOYSA-N 0.000 description 2
- 101100136727 Caenorhabditis elegans psd-1 gene Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 2
- 235000005979 Citrus limon Nutrition 0.000 description 2
- 244000131522 Citrus pyriformis Species 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- AERBNCYCJBRYDG-UHFFFAOYSA-N D-ribo-phytosphingosine Natural products CCCCCCCCCCCCCCC(O)C(O)C(N)CO AERBNCYCJBRYDG-UHFFFAOYSA-N 0.000 description 2
- XMSXQFUHVRWGNA-UHFFFAOYSA-N Decamethylcyclopentasiloxane Chemical compound C[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 XMSXQFUHVRWGNA-UHFFFAOYSA-N 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 2
- 235000016623 Fragaria vesca Nutrition 0.000 description 2
- 235000011363 Fragaria x ananassa Nutrition 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 244000179291 Mahonia aquifolium Species 0.000 description 2
- 244000070406 Malus silvestris Species 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 2
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 2
- 240000005561 Musa balbisiana Species 0.000 description 2
- AOMUHOFOVNGZAN-UHFFFAOYSA-N N,N-bis(2-hydroxyethyl)dodecanamide Chemical compound CCCCCCCCCCCC(=O)N(CCO)CCO AOMUHOFOVNGZAN-UHFFFAOYSA-N 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- 229920002385 Sodium hyaluronate Polymers 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 235000011941 Tilia x europaea Nutrition 0.000 description 2
- 240000006909 Tilia x europaea Species 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 235000009754 Vitis X bourquina Nutrition 0.000 description 2
- 235000012333 Vitis X labruscana Nutrition 0.000 description 2
- 206010047700 Vomiting Diseases 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 238000005054 agglomeration Methods 0.000 description 2
- 230000004931 aggregating effect Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 229940072056 alginate Drugs 0.000 description 2
- 125000005250 alkyl acrylate group Chemical group 0.000 description 2
- 229960000458 allantoin Drugs 0.000 description 2
- 235000011399 aloe vera Nutrition 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 239000000730 antalgic agent Substances 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 229940027983 antiseptic and disinfectant quaternary ammonium compound Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000013011 aqueous formulation Substances 0.000 description 2
- YSJGOMATDFSEED-UHFFFAOYSA-M behentrimonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCCCCCC[N+](C)(C)C YSJGOMATDFSEED-UHFFFAOYSA-M 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 235000012216 bentonite Nutrition 0.000 description 2
- 229960000686 benzalkonium chloride Drugs 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 2
- 229920001400 block copolymer Polymers 0.000 description 2
- 239000002981 blocking agent Substances 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 239000006172 buffering agent Substances 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 description 2
- 229960002882 calcipotriol Drugs 0.000 description 2
- 235000013877 carbamide Nutrition 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical group 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 229940106189 ceramide Drugs 0.000 description 2
- 229940074979 cetyl palmitate Drugs 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- RTIXKCRFFJGDFG-UHFFFAOYSA-N chrysin Chemical compound C=1C(O)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=CC=C1 RTIXKCRFFJGDFG-UHFFFAOYSA-N 0.000 description 2
- 239000007979 citrate buffer Substances 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 230000000112 colonic effect Effects 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 229940097362 cyclodextrins Drugs 0.000 description 2
- 229940086555 cyclomethicone Drugs 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- OSVXSBDYLRYLIG-UHFFFAOYSA-N dioxidochlorine(.) Chemical compound O=Cl=O OSVXSBDYLRYLIG-UHFFFAOYSA-N 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 2
- NOPFSRXAKWQILS-UHFFFAOYSA-N docosan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCCCCCO NOPFSRXAKWQILS-UHFFFAOYSA-N 0.000 description 2
- UKMSUNONTOPOIO-UHFFFAOYSA-N docosanoic acid Chemical compound CCCCCCCCCCCCCCCCCCCCCC(O)=O UKMSUNONTOPOIO-UHFFFAOYSA-N 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- GVGUFUZHNYFZLC-UHFFFAOYSA-N dodecyl benzenesulfonate;sodium Chemical compound [Na].CCCCCCCCCCCCOS(=O)(=O)C1=CC=CC=C1 GVGUFUZHNYFZLC-UHFFFAOYSA-N 0.000 description 2
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- QVBODZPPYSSMEL-UHFFFAOYSA-N dodecyl sulfate;2-hydroxyethylazanium Chemical compound NCCO.CCCCCCCCCCCCOS(O)(=O)=O QVBODZPPYSSMEL-UHFFFAOYSA-N 0.000 description 2
- 239000003974 emollient agent Substances 0.000 description 2
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 2
- 229940093471 ethyl oleate Drugs 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000005454 flavour additive Substances 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 239000003205 fragrance Substances 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 210000001648 gingival epithelial cell Anatomy 0.000 description 2
- 229960005150 glycerol Drugs 0.000 description 2
- 229940075529 glyceryl stearate Drugs 0.000 description 2
- YMAWOPBAYDPSLA-UHFFFAOYSA-N glycylglycine Chemical compound [NH3+]CC(=O)NCC([O-])=O YMAWOPBAYDPSLA-UHFFFAOYSA-N 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- PXDJXZJSCPSGGI-UHFFFAOYSA-N hexadecanoic acid hexadecyl ester Natural products CCCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCCC PXDJXZJSCPSGGI-UHFFFAOYSA-N 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 235000014655 lactic acid Nutrition 0.000 description 2
- 239000004310 lactic acid Substances 0.000 description 2
- 239000004571 lime Substances 0.000 description 2
- 239000006193 liquid solution Substances 0.000 description 2
- 229920001684 low density polyethylene Polymers 0.000 description 2
- 239000004702 low-density polyethylene Substances 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 238000000386 microscopy Methods 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 230000003020 moisturizing effect Effects 0.000 description 2
- 229940069822 monoethanolamine lauryl sulfate Drugs 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 239000002324 mouth wash Substances 0.000 description 2
- 210000004400 mucous membrane Anatomy 0.000 description 2
- XGZOMURMPLSSKQ-UHFFFAOYSA-N n,n-bis(2-hydroxyethyl)octadecanamide Chemical compound CCCCCCCCCCCCCCCCCC(=O)N(CCO)CCO XGZOMURMPLSSKQ-UHFFFAOYSA-N 0.000 description 2
- 239000002159 nanocrystal Substances 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 229940055577 oleyl alcohol Drugs 0.000 description 2
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 2
- 229940113162 oleylamide Drugs 0.000 description 2
- 229950008687 oltipraz Drugs 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 150000002895 organic esters Chemical class 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 2
- 239000003002 pH adjusting agent Substances 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 229940100460 peg-100 stearate Drugs 0.000 description 2
- 239000008363 phosphate buffer Substances 0.000 description 2
- 229940033329 phytosphingosine Drugs 0.000 description 2
- 239000003495 polar organic solvent Substances 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920002689 polyvinyl acetate Polymers 0.000 description 2
- 239000011118 polyvinyl acetate Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 239000008213 purified water Substances 0.000 description 2
- 125000001453 quaternary ammonium group Chemical group 0.000 description 2
- ARIWANIATODDMH-UHFFFAOYSA-N rac-1-monolauroylglycerol Chemical compound CCCCCCCCCCCC(=O)OCC(O)CO ARIWANIATODDMH-UHFFFAOYSA-N 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 229940080264 sodium dodecylbenzenesulfonate Drugs 0.000 description 2
- 229940010747 sodium hyaluronate Drugs 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 235000011008 sodium phosphates Nutrition 0.000 description 2
- 239000008109 sodium starch glycolate Substances 0.000 description 2
- 229920003109 sodium starch glycolate Polymers 0.000 description 2
- 229940079832 sodium starch glycolate Drugs 0.000 description 2
- YWIVKILSMZOHHF-QJZPQSOGSA-N sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2- Chemical compound [Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 YWIVKILSMZOHHF-QJZPQSOGSA-N 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N squalane Chemical compound CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 230000035882 stress Effects 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000003319 supportive effect Effects 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000008719 thickening Effects 0.000 description 2
- 235000010384 tocopherol Nutrition 0.000 description 2
- 239000011732 tocopherol Substances 0.000 description 2
- 229960001295 tocopherol Drugs 0.000 description 2
- 229930003799 tocopherol Natural products 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 235000019166 vitamin D Nutrition 0.000 description 2
- 239000011710 vitamin D Substances 0.000 description 2
- 230000008673 vomiting Effects 0.000 description 2
- 239000007762 w/o emulsion Substances 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- 235000010493 xanthan gum Nutrition 0.000 description 2
- 239000000230 xanthan gum Substances 0.000 description 2
- 229920001285 xanthan gum Polymers 0.000 description 2
- 229940082509 xanthan gum Drugs 0.000 description 2
- 229960003487 xylose Drugs 0.000 description 2
- 229910001928 zirconium oxide Inorganic materials 0.000 description 2
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 2
- WTVHAMTYZJGJLJ-UHFFFAOYSA-N (+)-(4S,8R)-8-epi-beta-bisabolol Natural products CC(C)=CCCC(C)C1(O)CCC(C)=CC1 WTVHAMTYZJGJLJ-UHFFFAOYSA-N 0.000 description 1
- WWUZIQQURGPMPG-UHFFFAOYSA-N (-)-D-erythro-Sphingosine Natural products CCCCCCCCCCCCCC=CC(O)C(N)CO WWUZIQQURGPMPG-UHFFFAOYSA-N 0.000 description 1
- RGZSQWQPBWRIAQ-CABCVRRESA-N (-)-alpha-Bisabolol Chemical compound CC(C)=CCC[C@](C)(O)[C@H]1CCC(C)=CC1 RGZSQWQPBWRIAQ-CABCVRRESA-N 0.000 description 1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- CUNWUEBNSZSNRX-RKGWDQTMSA-N (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;(z)-octadec-9-enoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O CUNWUEBNSZSNRX-RKGWDQTMSA-N 0.000 description 1
- FKCOHLNFINRFFR-RROPMPDHSA-N (2s)-6-amino-2-[[(2s)-2-[[(2s)-6-amino-2-(hexadecanoylamino)hexanoyl]amino]-3-methylbutanoyl]amino]hexanoic acid;2,2,2-trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCCCCCCCCCCCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(O)=O FKCOHLNFINRFFR-RROPMPDHSA-N 0.000 description 1
- 229920002818 (Hydroxyethyl)methacrylate Polymers 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- FFJCNSLCJOQHKM-CLFAGFIQSA-N (z)-1-[(z)-octadec-9-enoxy]octadec-9-ene Chemical compound CCCCCCCC\C=C/CCCCCCCCOCCCCCCCC\C=C/CCCCCCCC FFJCNSLCJOQHKM-CLFAGFIQSA-N 0.000 description 1
- LPMBTLLQQJBUOO-KTKRTIGZSA-N (z)-n,n-bis(2-hydroxyethyl)octadec-9-enamide Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)N(CCO)CCO LPMBTLLQQJBUOO-KTKRTIGZSA-N 0.000 description 1
- QGLWBTPVKHMVHM-KTKRTIGZSA-N (z)-octadec-9-en-1-amine Chemical compound CCCCCCCC\C=C/CCCCCCCCN QGLWBTPVKHMVHM-KTKRTIGZSA-N 0.000 description 1
- JCIIKRHCWVHVFF-UHFFFAOYSA-N 1,2,4-thiadiazol-5-amine;hydrochloride Chemical compound Cl.NC1=NC=NS1 JCIIKRHCWVHVFF-UHFFFAOYSA-N 0.000 description 1
- QLAJNZSPVITUCQ-UHFFFAOYSA-N 1,3,2-dioxathietane 2,2-dioxide Chemical compound O=S1(=O)OCO1 QLAJNZSPVITUCQ-UHFFFAOYSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- HNSDLXPSAYFUHK-UHFFFAOYSA-N 1,4-bis(2-ethylhexyl) sulfosuccinate Chemical compound CCCCC(CC)COC(=O)CC(S(O)(=O)=O)C(=O)OCC(CC)CCCC HNSDLXPSAYFUHK-UHFFFAOYSA-N 0.000 description 1
- 229940043375 1,5-pentanediol Drugs 0.000 description 1
- VUQPJRPDRDVQMN-UHFFFAOYSA-N 1-chlorooctadecane Chemical compound CCCCCCCCCCCCCCCCCCCl VUQPJRPDRDVQMN-UHFFFAOYSA-N 0.000 description 1
- YAOJJEJGPZRYJF-UHFFFAOYSA-N 1-ethenoxyhexane Chemical group CCCCCCOC=C YAOJJEJGPZRYJF-UHFFFAOYSA-N 0.000 description 1
- ZTDMACCDLOOFJP-AFEZEDKISA-N 1-ethyl-2-[(z)-octadec-9-enyl]-4,5-dihydro-1h-imidazol-1-ium;methyl sulfate Chemical compound COS([O-])(=O)=O.CCCCCCCC\C=C/CCCCCCCCC1=NCC[NH+]1CC ZTDMACCDLOOFJP-AFEZEDKISA-N 0.000 description 1
- ZQCIPRGNRQXXSK-UHFFFAOYSA-N 1-octadecoxypropan-2-ol Chemical compound CCCCCCCCCCCCCCCCCCOCC(C)O ZQCIPRGNRQXXSK-UHFFFAOYSA-N 0.000 description 1
- KZVIUXKOLXVBPC-UHFFFAOYSA-N 16-methylheptadecanamide Chemical compound CC(C)CCCCCCCCCCCCCCC(N)=O KZVIUXKOLXVBPC-UHFFFAOYSA-N 0.000 description 1
- JSOVGYMVTPPEND-UHFFFAOYSA-N 16-methylheptadecyl 2,2-dimethylpropanoate Chemical compound CC(C)CCCCCCCCCCCCCCCOC(=O)C(C)(C)C JSOVGYMVTPPEND-UHFFFAOYSA-N 0.000 description 1
- JNAYPSWVMNJOPQ-UHFFFAOYSA-N 2,3-bis(16-methylheptadecanoyloxy)propyl 16-methylheptadecanoate Chemical compound CC(C)CCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCC(C)C)COC(=O)CCCCCCCCCCCCCCC(C)C JNAYPSWVMNJOPQ-UHFFFAOYSA-N 0.000 description 1
- 239000000263 2,3-dihydroxypropyl (Z)-octadec-9-enoate Substances 0.000 description 1
- HVIQCFRUGUHMKW-UHFFFAOYSA-N 2-(2-hydroxyethyl)-n-[2-hydroxy-3-[2-(2-hydroxyethyl)octadecanoylamino]propyl]octadecanamide Chemical compound CCCCCCCCCCCCCCCCC(CCO)C(=O)NCC(O)CNC(=O)C(CCO)CCCCCCCCCCCCCCCC HVIQCFRUGUHMKW-UHFFFAOYSA-N 0.000 description 1
- XKUNZBPXIJSNGV-UHFFFAOYSA-N 2-(4-methyl-1-sulfanylidene-3H-dithiol-5-yl)pyrazine Chemical compound S=S1SCC(C)=C1C1=CN=CC=N1 XKUNZBPXIJSNGV-UHFFFAOYSA-N 0.000 description 1
- FKOKUHFZNIUSLW-UHFFFAOYSA-N 2-Hydroxypropyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(C)O FKOKUHFZNIUSLW-UHFFFAOYSA-N 0.000 description 1
- NLMKTBGFQGKQEV-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-hexadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO NLMKTBGFQGKQEV-UHFFFAOYSA-N 0.000 description 1
- ZKWJQNCOTNUNMF-QXMHVHEDSA-N 2-[dimethyl-[3-[[(z)-octadec-9-enoyl]amino]propyl]azaniumyl]acetate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O ZKWJQNCOTNUNMF-QXMHVHEDSA-N 0.000 description 1
- BMYCCWYAFNPAQC-UHFFFAOYSA-N 2-[dodecyl(methyl)azaniumyl]acetate Chemical compound CCCCCCCCCCCCN(C)CC(O)=O BMYCCWYAFNPAQC-UHFFFAOYSA-N 0.000 description 1
- GGBPWDGQNOAAQC-UHFFFAOYSA-N 2-ethylhexyl hexadecanoate;2-octylhexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(CC)CCCC.CCCCCCCCCCCCCCC(C(O)=O)CCCCCCCC GGBPWDGQNOAAQC-UHFFFAOYSA-N 0.000 description 1
- OPJWPPVYCOPDCM-UHFFFAOYSA-N 2-ethylhexyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(CC)CCCC OPJWPPVYCOPDCM-UHFFFAOYSA-N 0.000 description 1
- OYINQIKIQCNQOX-UHFFFAOYSA-M 2-hydroxybutyl(trimethyl)azanium;chloride Chemical compound [Cl-].CCC(O)C[N+](C)(C)C OYINQIKIQCNQOX-UHFFFAOYSA-M 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- 229940100555 2-methyl-4-isothiazolin-3-one Drugs 0.000 description 1
- LEACJMVNYZDSKR-UHFFFAOYSA-N 2-octyldodecan-1-ol Chemical compound CCCCCCCCCCC(CO)CCCCCCCC LEACJMVNYZDSKR-UHFFFAOYSA-N 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- QJQNWKPSKDLCOX-UHFFFAOYSA-N 3,3-diamino-2-hydroxybutanoic acid Chemical compound NC(C(C(=O)O)O)(C)N QJQNWKPSKDLCOX-UHFFFAOYSA-N 0.000 description 1
- UIVPNOBLHXUKDX-UHFFFAOYSA-N 3,5,5-trimethylhexyl 3,5,5-trimethylhexanoate Chemical compound CC(C)(C)CC(C)CCOC(=O)CC(C)CC(C)(C)C UIVPNOBLHXUKDX-UHFFFAOYSA-N 0.000 description 1
- MIDXCONKKJTLDX-UHFFFAOYSA-N 3,5-dimethylcyclopentane-1,2-dione Chemical compound CC1CC(C)C(=O)C1=O MIDXCONKKJTLDX-UHFFFAOYSA-N 0.000 description 1
- NCZPCONIKBICGS-UHFFFAOYSA-N 3-(2-ethylhexoxy)propane-1,2-diol Chemical compound CCCCC(CC)COCC(O)CO NCZPCONIKBICGS-UHFFFAOYSA-N 0.000 description 1
- MZPBGKHCHOCSOL-UHFFFAOYSA-N 3-(dodecylamino)propanoic acid;sodium Chemical compound [Na].CCCCCCCCCCCCNCCC(O)=O MZPBGKHCHOCSOL-UHFFFAOYSA-N 0.000 description 1
- RMTFNDVZYPHUEF-XZBKPIIZSA-N 3-O-methyl-D-glucose Chemical compound O=C[C@H](O)[C@@H](OC)[C@H](O)[C@H](O)CO RMTFNDVZYPHUEF-XZBKPIIZSA-N 0.000 description 1
- RZRNAYUHWVFMIP-GDCKJWNLSA-N 3-oleoyl-sn-glycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-GDCKJWNLSA-N 0.000 description 1
- TZZGHGKTHXIOMN-UHFFFAOYSA-N 3-trimethoxysilyl-n-(3-trimethoxysilylpropyl)propan-1-amine Chemical compound CO[Si](OC)(OC)CCCNCCC[Si](OC)(OC)OC TZZGHGKTHXIOMN-UHFFFAOYSA-N 0.000 description 1
- SJZRECIVHVDYJC-UHFFFAOYSA-M 4-hydroxybutyrate Chemical compound OCCCC([O-])=O SJZRECIVHVDYJC-UHFFFAOYSA-M 0.000 description 1
- NYCXYKOXLNBYID-UHFFFAOYSA-N 5,7-Dihydroxychromone Natural products O1C=CC(=O)C=2C1=CC(O)=CC=2O NYCXYKOXLNBYID-UHFFFAOYSA-N 0.000 description 1
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 1
- 235000009434 Actinidia chinensis Nutrition 0.000 description 1
- 244000298697 Actinidia deliciosa Species 0.000 description 1
- 235000009436 Actinidia deliciosa Nutrition 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- 241000205585 Aquilegia canadensis Species 0.000 description 1
- 241000219195 Arabidopsis thaliana Species 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 235000003261 Artemisia vulgaris Nutrition 0.000 description 1
- 240000006891 Artemisia vulgaris Species 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 206010003805 Autism Diseases 0.000 description 1
- 208000020706 Autistic disease Diseases 0.000 description 1
- 235000010082 Averrhoa carambola Nutrition 0.000 description 1
- 240000006063 Averrhoa carambola Species 0.000 description 1
- 235000000832 Ayote Nutrition 0.000 description 1
- 208000031729 Bacteremia Diseases 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 235000021357 Behenic acid Nutrition 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 241000167854 Bourreria succulenta Species 0.000 description 1
- 235000014698 Brassica juncea var multisecta Nutrition 0.000 description 1
- 235000006008 Brassica napus var napus Nutrition 0.000 description 1
- 240000000385 Brassica napus var. napus Species 0.000 description 1
- 235000006618 Brassica rapa subsp oleifera Nutrition 0.000 description 1
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 235000004936 Bromus mango Nutrition 0.000 description 1
- 239000004255 Butylated hydroxyanisole Substances 0.000 description 1
- DBFHTTOOJDMDTI-KCMGSBMCSA-N C(CCCCCCCCCCCCCCCCC)(=O)O.CC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.C(CCCCCCCCCCCCCCCCC)(=O)O.C(CCCCCCCCCCCCCCCCC)(=O)O.CC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO Chemical compound C(CCCCCCCCCCCCCCCCC)(=O)O.CC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.C(CCCCCCCCCCCCCCCCC)(=O)O.C(CCCCCCCCCCCCCCCCC)(=O)O.CC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO DBFHTTOOJDMDTI-KCMGSBMCSA-N 0.000 description 1
- YDNKGFDKKRUKPY-JHOUSYSJSA-N C16 ceramide Natural products CCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)C=CCCCCCCCCCCCCC YDNKGFDKKRUKPY-JHOUSYSJSA-N 0.000 description 1
- HNFXBGYAIIDHGB-UHFFFAOYSA-N CC1=C(C=NC(=O)N1)C2=NC=CN=C2 Chemical compound CC1=C(C=NC(=O)N1)C2=NC=CN=C2 HNFXBGYAIIDHGB-UHFFFAOYSA-N 0.000 description 1
- BYUQATUKPXLFLZ-UIOOFZCWSA-N CCCCCCCCCCCCCCCC(=O)NCC(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CN=CN1 Chemical compound CCCCCCCCCCCCCCCC(=O)NCC(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CN=CN1 BYUQATUKPXLFLZ-UIOOFZCWSA-N 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 235000009467 Carica papaya Nutrition 0.000 description 1
- 240000006432 Carica papaya Species 0.000 description 1
- 239000004155 Chlorine dioxide Substances 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 244000241235 Citrullus lanatus Species 0.000 description 1
- 235000012828 Citrullus lanatus var citroides Nutrition 0.000 description 1
- 240000000560 Citrus x paradisi Species 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 240000008067 Cucumis sativus Species 0.000 description 1
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 1
- 240000004244 Cucurbita moschata Species 0.000 description 1
- 235000009854 Cucurbita moschata Nutrition 0.000 description 1
- 235000009804 Cucurbita pepo subsp pepo Nutrition 0.000 description 1
- 244000007835 Cyamopsis tetragonoloba Species 0.000 description 1
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 description 1
- SNPLKNRPJHDVJA-ZETCQYMHSA-N D-panthenol Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCCO SNPLKNRPJHDVJA-ZETCQYMHSA-N 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- 235000011511 Diospyros Nutrition 0.000 description 1
- 244000236655 Diospyros kaki Species 0.000 description 1
- 239000003109 Disodium ethylene diamine tetraacetate Substances 0.000 description 1
- 206010013911 Dysgeusia Diseases 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 235000015489 Emblica officinalis Nutrition 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010053177 Epidermolysis Diseases 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- 229920000896 Ethulose Polymers 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 239000001859 Ethyl hydroxyethyl cellulose Substances 0.000 description 1
- FPVVYTCTZKCSOJ-UHFFFAOYSA-N Ethylene glycol distearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCOC(=O)CCCCCCCCCCCCCCCCC FPVVYTCTZKCSOJ-UHFFFAOYSA-N 0.000 description 1
- 229920003148 Eudragit® E polymer Polymers 0.000 description 1
- 229920003136 Eudragit® L polymer Polymers 0.000 description 1
- 229920003152 Eudragit® RS polymer Polymers 0.000 description 1
- 229920003137 Eudragit® S polymer Polymers 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 235000017048 Garcinia mangostana Nutrition 0.000 description 1
- 240000006053 Garcinia mangostana Species 0.000 description 1
- 235000000885 Garcinia xanthochymus Nutrition 0.000 description 1
- 244000119461 Garcinia xanthochymus Species 0.000 description 1
- 240000001238 Gaultheria procumbens Species 0.000 description 1
- 235000007297 Gaultheria procumbens Nutrition 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 102100038073 General transcription factor II-I Human genes 0.000 description 1
- 102100039696 Glutamate-cysteine ligase catalytic subunit Human genes 0.000 description 1
- 102100033398 Glutamate-cysteine ligase regulatory subunit Human genes 0.000 description 1
- 102000006587 Glutathione peroxidase Human genes 0.000 description 1
- 102100033039 Glutathione peroxidase 1 Human genes 0.000 description 1
- 108700016172 Glutathione peroxidases Proteins 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 108010008488 Glycylglycine Proteins 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101001032427 Homo sapiens General transcription factor II-I Proteins 0.000 description 1
- 101001034527 Homo sapiens Glutamate-cysteine ligase catalytic subunit Proteins 0.000 description 1
- 101000870644 Homo sapiens Glutamate-cysteine ligase regulatory subunit Proteins 0.000 description 1
- 101001014936 Homo sapiens Glutathione peroxidase 1 Proteins 0.000 description 1
- 101000973778 Homo sapiens NAD(P)H dehydrogenase [quinone] 1 Proteins 0.000 description 1
- 101000588302 Homo sapiens Nuclear factor erythroid 2-related factor 2 Proteins 0.000 description 1
- 101001064864 Homo sapiens Polyunsaturated fatty acid lipoxygenase ALOX12 Proteins 0.000 description 1
- 101000605122 Homo sapiens Prostaglandin G/H synthase 1 Proteins 0.000 description 1
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 1
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 description 1
- 235000018481 Hylocereus undatus Nutrition 0.000 description 1
- 244000157072 Hylocereus undatus Species 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 235000015459 Lycium barbarum Nutrition 0.000 description 1
- 244000241838 Lycium barbarum Species 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 235000002823 Mahonia aquifolium Nutrition 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 235000011430 Malus pumila Nutrition 0.000 description 1
- 235000015103 Malus silvestris Nutrition 0.000 description 1
- 235000014826 Mangifera indica Nutrition 0.000 description 1
- 240000007228 Mangifera indica Species 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 244000061354 Manilkara achras Species 0.000 description 1
- 235000011339 Manilkara zapota Nutrition 0.000 description 1
- 235000014435 Mentha Nutrition 0.000 description 1
- 241001072983 Mentha Species 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 241000699673 Mesocricetus auratus Species 0.000 description 1
- 208000003445 Mouth Neoplasms Diseases 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- BBAFBDLICMHBNU-MFZOPHKMSA-N N-(2-hydroxyoctadecanoyl)-4-hydroxysphinganine Chemical compound CCCCCCCCCCCCCCCCC(O)C(=O)N[C@@H](CO)[C@H](O)[C@H](O)CCCCCCCCCCCCCC BBAFBDLICMHBNU-MFZOPHKMSA-N 0.000 description 1
- OTGQIQQTPXJQRG-UHFFFAOYSA-N N-(octadecanoyl)ethanolamine Chemical compound CCCCCCCCCCCCCCCCCC(=O)NCCO OTGQIQQTPXJQRG-UHFFFAOYSA-N 0.000 description 1
- WAYLDHLWVYQNSQ-KEFDUYNTSA-N N-2-hydroxylignoceroylsphingosine Chemical compound CCCCCCCCCCCCCCCCCCCCCCC(O)C(=O)N[C@@H](CO)[C@H](O)\C=C\CCCCCCCCCCCCC WAYLDHLWVYQNSQ-KEFDUYNTSA-N 0.000 description 1
- CRJGESKKUOMBCT-VQTJNVASSA-N N-acetylsphinganine Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@H](CO)NC(C)=O CRJGESKKUOMBCT-VQTJNVASSA-N 0.000 description 1
- OPKOKAMJFNKNAS-UHFFFAOYSA-N N-methylethanolamine Chemical compound CNCCO OPKOKAMJFNKNAS-UHFFFAOYSA-N 0.000 description 1
- 102100022365 NAD(P)H dehydrogenase [quinone] 1 Human genes 0.000 description 1
- 206010029350 Neurotoxicity Diseases 0.000 description 1
- 102100031701 Nuclear factor erythroid 2-related factor 2 Human genes 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 206010031009 Oral pain Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 240000000908 Phyllanthus acidus Species 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 108010020346 Polyglutamic Acid Proteins 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920000289 Polyquaternium Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 102100031949 Polyunsaturated fatty acid lipoxygenase ALOX12 Human genes 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102100038277 Prostaglandin G/H synthase 1 Human genes 0.000 description 1
- 241000508269 Psidium Species 0.000 description 1
- 206010037763 Radiation mucositis Diseases 0.000 description 1
- 231100000991 Reactive Oxygen Species (ROS) Photosafety Assay Toxicity 0.000 description 1
- 235000011552 Rhamnus crocea Nutrition 0.000 description 1
- 235000003942 Rubus occidentalis Nutrition 0.000 description 1
- 244000111388 Rubus occidentalis Species 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 235000018735 Sambucus canadensis Nutrition 0.000 description 1
- 244000151637 Sambucus canadensis Species 0.000 description 1
- 241000220217 Sapotaceae Species 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 235000002597 Solanum melongena Nutrition 0.000 description 1
- 244000061458 Solanum melongena Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- IYFATESGLOUGBX-YVNJGZBMSA-N Sorbitan monopalmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O IYFATESGLOUGBX-YVNJGZBMSA-N 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- 239000004147 Sorbitan trioleate Substances 0.000 description 1
- PRXRUNOAOLTIEF-ADSICKODSA-N Sorbitan trioleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC PRXRUNOAOLTIEF-ADSICKODSA-N 0.000 description 1
- 108010073771 Soybean Proteins Proteins 0.000 description 1
- 235000009184 Spondias indica Nutrition 0.000 description 1
- 240000003186 Stachytarpheta cayennensis Species 0.000 description 1
- 235000009233 Stachytarpheta cayennensis Nutrition 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 108010021188 Superoxide Dismutase-1 Proteins 0.000 description 1
- 102000008221 Superoxide Dismutase-1 Human genes 0.000 description 1
- 235000016639 Syzygium aromaticum Nutrition 0.000 description 1
- 244000223014 Syzygium aromaticum Species 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- PLZVEHJLHYMBBY-UHFFFAOYSA-N Tetradecylamine Chemical compound CCCCCCCCCCCCCCN PLZVEHJLHYMBBY-UHFFFAOYSA-N 0.000 description 1
- 244000141804 Theobroma grandiflorum Species 0.000 description 1
- 235000002424 Theobroma grandiflorum Nutrition 0.000 description 1
- AOBORMOPSGHCAX-UHFFFAOYSA-N Tocophersolan Chemical compound OCCOC(=O)CCC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C AOBORMOPSGHCAX-UHFFFAOYSA-N 0.000 description 1
- 206010044221 Toxic encephalopathy Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- PHYFQTYBJUILEZ-UHFFFAOYSA-N Trioleoylglycerol Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCCCCCCCC)COC(=O)CCCCCCCC=CCCCCCCCC PHYFQTYBJUILEZ-UHFFFAOYSA-N 0.000 description 1
- 108010021111 Uncoupling Protein 2 Proteins 0.000 description 1
- 240000001717 Vaccinium macrocarpon Species 0.000 description 1
- 235000012545 Vaccinium macrocarpon Nutrition 0.000 description 1
- 235000002118 Vaccinium oxycoccus Nutrition 0.000 description 1
- 235000009499 Vanilla fragrans Nutrition 0.000 description 1
- 244000263375 Vanilla tahitensis Species 0.000 description 1
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 1
- 206010047163 Vasospasm Diseases 0.000 description 1
- 241000759263 Ventia crocea Species 0.000 description 1
- 229930003316 Vitamin D Natural products 0.000 description 1
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 1
- 235000018936 Vitellaria paradoxa Nutrition 0.000 description 1
- 241001135917 Vitellaria paradoxa Species 0.000 description 1
- 235000009392 Vitis Nutrition 0.000 description 1
- 241000219095 Vitis Species 0.000 description 1
- LDDUCKDUDZVHLN-UHFFFAOYSA-N [2-hydroxy-3-[2-hydroxy-3-(16-methylheptadecanoyloxy)propoxy]propyl] 16-methylheptadecanoate Chemical compound CC(C)CCCCCCCCCCCCCCC(=O)OCC(O)COCC(O)COC(=O)CCCCCCCCCCCCCCC(C)C LDDUCKDUDZVHLN-UHFFFAOYSA-N 0.000 description 1
- MIUIRGGKIICMBP-NFOZDHADSA-N [27-oxo-27-[[(2s,3s,4r)-1,3,4-trihydroxyoctadecan-2-yl]amino]heptacosyl] octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)[C@H](O)CCCCCCCCCCCCCC MIUIRGGKIICMBP-NFOZDHADSA-N 0.000 description 1
- BKZCZANSHGDPSH-KTKRTIGZSA-N [3-(2,3-dihydroxypropoxy)-2-hydroxypropyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)COCC(O)CO BKZCZANSHGDPSH-KTKRTIGZSA-N 0.000 description 1
- OGELJRHPEZALCC-UHFFFAOYSA-N [3-(2,3-dihydroxypropoxy)-2-hydroxypropyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)OCC(O)COCC(O)CO OGELJRHPEZALCC-UHFFFAOYSA-N 0.000 description 1
- ZGBFGAHZKZQSLG-UMCOJZBLSA-N [30-oxo-30-[[(e,2s,3r,6r)-1,3,6-trihydroxyoctadec-4-en-2-yl]amino]triacontyl] (9z,12z)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCC[C@@H](O)\C=C\[C@@H](O)[C@H](CO)NC(=O)CCCCCCCCCCCCCCCCCCCCCCCCCCCCCOC(=O)CCCCCCC\C=C/C\C=C/CCCCC ZGBFGAHZKZQSLG-UMCOJZBLSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- HSUGRPOJOBRRBK-SXBSVMRRSA-N acetic acid;(2s)-n-[(2s)-4-amino-1-(benzylamino)-1-oxobutan-2-yl]-1-(3-aminopropanoyl)pyrrolidine-2-carboxamide Chemical compound CC(O)=O.CC(O)=O.N([C@@H](CCN)C(=O)NCC=1C=CC=CC=1)C(=O)[C@@H]1CCCN1C(=O)CCN HSUGRPOJOBRRBK-SXBSVMRRSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- RGZSQWQPBWRIAQ-LSDHHAIUSA-N alpha-Bisabolol Natural products CC(C)=CCC[C@@](C)(O)[C@@H]1CCC(C)=CC1 RGZSQWQPBWRIAQ-LSDHHAIUSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- BTBJBAZGXNKLQC-UHFFFAOYSA-N ammonium lauryl sulfate Chemical compound [NH4+].CCCCCCCCCCCCOS([O-])(=O)=O BTBJBAZGXNKLQC-UHFFFAOYSA-N 0.000 description 1
- 229940063953 ammonium lauryl sulfate Drugs 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 239000002280 amphoteric surfactant Substances 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000012296 anti-solvent Substances 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 239000002216 antistatic agent Substances 0.000 description 1
- 235000021016 apples Nutrition 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- BTFJIXJJCSYFAL-UHFFFAOYSA-N arachidyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCO BTFJIXJJCSYFAL-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000005311 autocorrelation function Methods 0.000 description 1
- 235000021302 avocado oil Nutrition 0.000 description 1
- 239000008163 avocado oil Substances 0.000 description 1
- FMBMJZOGMAKBLM-UHFFFAOYSA-N azane;sulfo dodecanoate Chemical compound [NH4+].CCCCCCCCCCCC(=O)OS([O-])(=O)=O FMBMJZOGMAKBLM-UHFFFAOYSA-N 0.000 description 1
- 235000021015 bananas Nutrition 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 229940116226 behenic acid Drugs 0.000 description 1
- 229940070718 behentrimonium Drugs 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- JBIROUFYLSSYDX-UHFFFAOYSA-M benzododecinium chloride Chemical compound [Cl-].CCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 JBIROUFYLSSYDX-UHFFFAOYSA-M 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 229960004217 benzyl alcohol Drugs 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- IEEFCJHQASBKMD-UHFFFAOYSA-N benzylazanium;2-methylpyridine;chloride Chemical compound [Cl-].CC1=CC=CC=N1.[NH3+]CC1=CC=CC=C1 IEEFCJHQASBKMD-UHFFFAOYSA-N 0.000 description 1
- 239000003613 bile acid Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- MKHVZQXYWACUQC-UHFFFAOYSA-N bis(2-hydroxyethyl)azanium;dodecyl sulfate Chemical compound OCCNCCO.CCCCCCCCCCCCOS(O)(=O)=O MKHVZQXYWACUQC-UHFFFAOYSA-N 0.000 description 1
- 229940036350 bisabolol Drugs 0.000 description 1
- HHGZABIIYIWLGA-UHFFFAOYSA-N bisabolol Natural products CC1CCC(C(C)(O)CCC=C(C)C)CC1 HHGZABIIYIWLGA-UHFFFAOYSA-N 0.000 description 1
- 235000019636 bitter flavor Nutrition 0.000 description 1
- 235000007123 blue elder Nutrition 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 1
- 229940043253 butylated hydroxyanisole Drugs 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 229940051246 camellia oleifera leaf extract Drugs 0.000 description 1
- 235000013736 caramel Nutrition 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 229940077731 carbohydrate nutrients Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000008004 cell lysis buffer Substances 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 238000002737 cell proliferation kit Methods 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 238000012200 cell viability kit Methods 0.000 description 1
- ZVEQCJWYRWKARO-UHFFFAOYSA-N ceramide Natural products CCCCCCCCCCCCCCC(O)C(=O)NC(CO)C(O)C=CCCC=C(C)CCCCCCCCC ZVEQCJWYRWKARO-UHFFFAOYSA-N 0.000 description 1
- 229940048864 ceramide 1 Drugs 0.000 description 1
- 150000001783 ceramides Chemical class 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 229940056318 ceteth-20 Drugs 0.000 description 1
- 229960002798 cetrimide Drugs 0.000 description 1
- 229960001927 cetylpyridinium chloride Drugs 0.000 description 1
- YMKDRGPMQRFJGP-UHFFFAOYSA-M cetylpyridinium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCC[N+]1=CC=CC=C1 YMKDRGPMQRFJGP-UHFFFAOYSA-M 0.000 description 1
- WOWHHFRSBJGXCM-UHFFFAOYSA-M cetyltrimethylammonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCC[N+](C)(C)C WOWHHFRSBJGXCM-UHFFFAOYSA-M 0.000 description 1
- 229940119217 chamomile extract Drugs 0.000 description 1
- 235000020221 chamomile extract Nutrition 0.000 description 1
- 150000001793 charged compounds Chemical class 0.000 description 1
- 238000002144 chemical decomposition reaction Methods 0.000 description 1
- 235000019693 cherries Nutrition 0.000 description 1
- 235000019398 chlorine dioxide Nutrition 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- MXOAEAUPQDYUQM-UHFFFAOYSA-N chlorphenesin Chemical compound OCC(O)COC1=CC=C(Cl)C=C1 MXOAEAUPQDYUQM-UHFFFAOYSA-N 0.000 description 1
- 235000019219 chocolate Nutrition 0.000 description 1
- 235000015838 chrysin Nutrition 0.000 description 1
- 229940043370 chrysin Drugs 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 229940031728 cocamidopropylamine oxide Drugs 0.000 description 1
- 229940052366 colloidal oatmeal Drugs 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 235000004634 cranberry Nutrition 0.000 description 1
- 239000010638 cranberry seed oil Substances 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- ZAKOWWREFLAJOT-UHFFFAOYSA-N d-alpha-Tocopheryl acetate Natural products CC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-UHFFFAOYSA-N 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-M decanoate Chemical compound CCCCCCCCCC([O-])=O GHVNFZFCNZKVNT-UHFFFAOYSA-M 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000008260 defense mechanism Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- 231100000020 developmental retardation Toxicity 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- SHLKYEAQGUCTIO-UHFFFAOYSA-N diazanium;4-dodecoxy-4-oxo-3-sulfobutanoate Chemical compound [NH4+].[NH4+].CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O.CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O SHLKYEAQGUCTIO-UHFFFAOYSA-N 0.000 description 1
- SOROIESOUPGGFO-UHFFFAOYSA-N diazolidinylurea Chemical compound OCNC(=O)N(CO)C1N(CO)C(=O)N(CO)C1=O SOROIESOUPGGFO-UHFFFAOYSA-N 0.000 description 1
- 229960001083 diazolidinylurea Drugs 0.000 description 1
- 229960004698 dichlorobenzyl alcohol Drugs 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-M dihydrogenphosphate Chemical compound OP(O)([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-M 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 235000019301 disodium ethylene diamine tetraacetate Nutrition 0.000 description 1
- 229940079886 disodium lauryl sulfosuccinate Drugs 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- KHIQYZGEUSTKSB-UHFFFAOYSA-L disodium;4-dodecoxy-4-oxo-3-sulfobutanoate Chemical compound [Na+].[Na+].CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O.CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O KHIQYZGEUSTKSB-UHFFFAOYSA-L 0.000 description 1
- FXPVUWKFNGVHIZ-UHFFFAOYSA-L disodium;dodecyl sulfate Chemical compound [Na+].[Na+].CCCCCCCCCCCCOS([O-])(=O)=O.CCCCCCCCCCCCOS([O-])(=O)=O FXPVUWKFNGVHIZ-UHFFFAOYSA-L 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 229960000735 docosanol Drugs 0.000 description 1
- 229940018602 docusate Drugs 0.000 description 1
- ILRSCQWREDREME-UHFFFAOYSA-N dodecanamide Chemical compound CCCCCCCCCCCC(N)=O ILRSCQWREDREME-UHFFFAOYSA-N 0.000 description 1
- DLAHAXOYRFRPFQ-UHFFFAOYSA-N dodecyl benzoate Chemical compound CCCCCCCCCCCCOC(=O)C1=CC=CC=C1 DLAHAXOYRFRPFQ-UHFFFAOYSA-N 0.000 description 1
- DDXLVDQZPFLQMZ-UHFFFAOYSA-M dodecyl(trimethyl)azanium;chloride Chemical compound [Cl-].CCCCCCCCCCCC[N+](C)(C)C DDXLVDQZPFLQMZ-UHFFFAOYSA-M 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 235000007124 elderberry Nutrition 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 231100000284 endotoxic Toxicity 0.000 description 1
- 230000002346 endotoxic effect Effects 0.000 description 1
- 229940095399 enema Drugs 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- HQPMKSGTIOYHJT-UHFFFAOYSA-N ethane-1,2-diol;propane-1,2-diol Chemical compound OCCO.CC(O)CO HQPMKSGTIOYHJT-UHFFFAOYSA-N 0.000 description 1
- FYUWIEKAVLOHSE-UHFFFAOYSA-N ethenyl acetate;1-ethenylpyrrolidin-2-one Chemical compound CC(=O)OC=C.C=CN1CCCC1=O FYUWIEKAVLOHSE-UHFFFAOYSA-N 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 1
- 229940093499 ethyl acetate Drugs 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 229960001617 ethyl hydroxybenzoate Drugs 0.000 description 1
- 235000019326 ethyl hydroxyethyl cellulose Nutrition 0.000 description 1
- 235000010944 ethyl methyl cellulose Nutrition 0.000 description 1
- 239000001761 ethyl methyl cellulose Substances 0.000 description 1
- 235000010228 ethyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004403 ethyl p-hydroxybenzoate Substances 0.000 description 1
- 229940100524 ethylhexylglycerin Drugs 0.000 description 1
- NUVBSKCKDOMJSU-UHFFFAOYSA-N ethylparaben Chemical compound CCOC(=O)C1=CC=C(O)C=C1 NUVBSKCKDOMJSU-UHFFFAOYSA-N 0.000 description 1
- 235000008995 european elder Nutrition 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000009093 first-line therapy Methods 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000013022 formulation composition Substances 0.000 description 1
- 235000021588 free fatty acids Nutrition 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- FOYKKGHVWRFIBD-UHFFFAOYSA-N gamma-tocopherol acetate Natural products CC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1 FOYKKGHVWRFIBD-UHFFFAOYSA-N 0.000 description 1
- 235000021472 generally recognized as safe Nutrition 0.000 description 1
- 238000009650 gentamicin protection assay Methods 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 150000002327 glycerophospholipids Chemical class 0.000 description 1
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 1
- 229940068939 glyceryl monolaurate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 229940043257 glycylglycine Drugs 0.000 description 1
- 235000002532 grape seed extract Nutrition 0.000 description 1
- 235000020688 green tea extract Nutrition 0.000 description 1
- 229940094952 green tea extract Drugs 0.000 description 1
- 244000144993 groups of animals Species 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 108091005708 gustatory receptors Proteins 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 238000011134 hematopoietic stem cell transplantation Methods 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- PYGSKMBEVAICCR-UHFFFAOYSA-N hexa-1,5-diene Chemical group C=CCCC=C PYGSKMBEVAICCR-UHFFFAOYSA-N 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- DWMMZQMXUWUJME-UHFFFAOYSA-N hexadecyl octanoate Chemical compound CCCCCCCCCCCCCCCCOC(=O)CCCCCCC DWMMZQMXUWUJME-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 239000007970 homogeneous dispersion Substances 0.000 description 1
- 229940106579 hops extract Drugs 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 239000001906 humulus lupulus l. absolute Substances 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 150000001261 hydroxy acids Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- ZCTXEAQXZGPWFG-UHFFFAOYSA-N imidurea Chemical compound O=C1NC(=O)N(CO)C1NC(=O)NCNC(=O)NC1C(=O)NC(=O)N1CO ZCTXEAQXZGPWFG-UHFFFAOYSA-N 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000013101 initial test Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229940100554 isononyl isononanoate Drugs 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 229940097750 laminaria ochroleuca extract Drugs 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 229940061515 laureth-4 Drugs 0.000 description 1
- 229940031957 lauric acid diethanolamide Drugs 0.000 description 1
- 229940094506 lauryl betaine Drugs 0.000 description 1
- IZWSFJTYBVKZNK-UHFFFAOYSA-N lauryl sulfobetaine Chemical compound CCCCCCCCCCCC[N+](C)(C)CCCS([O-])(=O)=O IZWSFJTYBVKZNK-UHFFFAOYSA-N 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 229960005015 local anesthetics Drugs 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000001525 mentha piperita l. herb oil Substances 0.000 description 1
- 239000001683 mentha spicata herb oil Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 229940012189 methyl orange Drugs 0.000 description 1
- STZCRXQWRGQSJD-GEEYTBSJSA-M methyl orange Chemical compound [Na+].C1=CC(N(C)C)=CC=C1\N=N\C1=CC=C(S([O-])(=O)=O)C=C1 STZCRXQWRGQSJD-GEEYTBSJSA-M 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 229960001047 methyl salicylate Drugs 0.000 description 1
- BEGLCMHJXHIJLR-UHFFFAOYSA-N methylisothiazolinone Chemical compound CN1SC=CC1=O BEGLCMHJXHIJLR-UHFFFAOYSA-N 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000004200 microcrystalline wax Substances 0.000 description 1
- 235000019808 microcrystalline wax Nutrition 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 235000014569 mints Nutrition 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 1
- RZRNAYUHWVFMIP-UHFFFAOYSA-N monoelaidin Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-UHFFFAOYSA-N 0.000 description 1
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 1
- 235000019796 monopotassium phosphate Nutrition 0.000 description 1
- 210000002200 mouth mucosa Anatomy 0.000 description 1
- 229940051866 mouthwash Drugs 0.000 description 1
- 230000004678 mucosal integrity Effects 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- ONHFWHCMZAJCFB-UHFFFAOYSA-N myristamine oxide Chemical compound CCCCCCCCCCCCCC[N+](C)(C)[O-] ONHFWHCMZAJCFB-UHFFFAOYSA-N 0.000 description 1
- 229940078812 myristyl myristate Drugs 0.000 description 1
- SKDZEPBJPGSFHS-UHFFFAOYSA-N n,n-bis(2-hydroxyethyl)tetradecanamide Chemical compound CCCCCCCCCCCCCC(=O)N(CCO)CCO SKDZEPBJPGSFHS-UHFFFAOYSA-N 0.000 description 1
- KKXWPVVBVWBKBL-UHFFFAOYSA-N n,n-diethylethanamine;dodecyl hydrogen sulfate Chemical compound CC[NH+](CC)CC.CCCCCCCCCCCCOS([O-])(=O)=O KKXWPVVBVWBKBL-UHFFFAOYSA-N 0.000 description 1
- JXTPJDDICSTXJX-UHFFFAOYSA-N n-Triacontane Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCC JXTPJDDICSTXJX-UHFFFAOYSA-N 0.000 description 1
- RKISUIUJZGSLEV-UHFFFAOYSA-N n-[2-(octadecanoylamino)ethyl]octadecanamide Chemical compound CCCCCCCCCCCCCCCCCC(=O)NCCNC(=O)CCCCCCCCCCCCCCCCC RKISUIUJZGSLEV-UHFFFAOYSA-N 0.000 description 1
- BOUCRWJEKAGKKG-UHFFFAOYSA-N n-[3-(diethylaminomethyl)-4-hydroxyphenyl]acetamide Chemical compound CCN(CC)CC1=CC(NC(C)=O)=CC=C1O BOUCRWJEKAGKKG-UHFFFAOYSA-N 0.000 description 1
- JRKSTJIPGYBLNV-UHFFFAOYSA-N n-[3-[dodecanoyl(2-hydroxyethyl)amino]-2-hydroxypropyl]-n-(2-hydroxyethyl)dodecanamide Chemical compound CCCCCCCCCCCC(=O)N(CCO)CC(O)CN(CCO)C(=O)CCCCCCCCCCC JRKSTJIPGYBLNV-UHFFFAOYSA-N 0.000 description 1
- PDRAHDYJNDYBNK-UHFFFAOYSA-N n-[3-[hexadecanoyl(2-hydroxyethyl)amino]-2-hydroxypropyl]-n-(2-hydroxyethyl)hexadecanamide Chemical compound CCCCCCCCCCCCCCCC(=O)N(CCO)CC(O)CN(CCO)C(=O)CCCCCCCCCCCCCCC PDRAHDYJNDYBNK-UHFFFAOYSA-N 0.000 description 1
- DVEKCXOJTLDBFE-UHFFFAOYSA-N n-dodecyl-n,n-dimethylglycinate Chemical compound CCCCCCCCCCCC[N+](C)(C)CC([O-])=O DVEKCXOJTLDBFE-UHFFFAOYSA-N 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 230000003533 narcotic effect Effects 0.000 description 1
- 235000021096 natural sweeteners Nutrition 0.000 description 1
- 201000008383 nephritis Diseases 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 230000007135 neurotoxicity Effects 0.000 description 1
- 231100000228 neurotoxicity Toxicity 0.000 description 1
- VVGIYYKRAMHVLU-UHFFFAOYSA-N newbouldiamide Natural products CCCCCCCCCCCCCCCCCCCC(O)C(O)C(O)C(CO)NC(=O)CCCCCCCCCCCCCCCCC VVGIYYKRAMHVLU-UHFFFAOYSA-N 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 231100000065 noncytotoxic Toxicity 0.000 description 1
- 230000002020 noncytotoxic effect Effects 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- WNIFXKPDILJURQ-JKPOUOEOSA-N octadecyl (2s,4as,6ar,6as,6br,8ar,10s,12as,14br)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,14b-dodecahydro-1h-picene-2-carboxylate Chemical compound C1C[C@H](O)C(C)(C)[C@@H]2CC[C@@]3(C)[C@]4(C)CC[C@@]5(C)CC[C@@](C(=O)OCCCCCCCCCCCCCCCCCC)(C)C[C@H]5C4=CC(=O)[C@@H]3[C@]21C WNIFXKPDILJURQ-JKPOUOEOSA-N 0.000 description 1
- KSCKTBJJRVPGKM-UHFFFAOYSA-N octan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCCCCCC[O-].CCCCCCCC[O-].CCCCCCCC[O-].CCCCCCCC[O-] KSCKTBJJRVPGKM-UHFFFAOYSA-N 0.000 description 1
- AEIJTFQOBWATKX-UHFFFAOYSA-N octane-1,2-diol Chemical compound CCCCCCC(O)CO AEIJTFQOBWATKX-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 229920002114 octoxynol-9 Polymers 0.000 description 1
- IIGMITQLXAGZTL-UHFFFAOYSA-N octyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCC IIGMITQLXAGZTL-UHFFFAOYSA-N 0.000 description 1
- 235000014593 oils and fats Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- FATBGEAMYMYZAF-KTKRTIGZSA-N oleamide Chemical compound CCCCCCCC\C=C/CCCCCCCC(N)=O FATBGEAMYMYZAF-KTKRTIGZSA-N 0.000 description 1
- 229960002969 oleic acid Drugs 0.000 description 1
- 235000021313 oleic acid Nutrition 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 229940100690 oral cream Drugs 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 229940042125 oral ointment Drugs 0.000 description 1
- 239000000668 oral spray Substances 0.000 description 1
- 229940041678 oral spray Drugs 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 229940093441 palmitoyl oligopeptide Drugs 0.000 description 1
- 229940094912 palmitoyl tripeptide-5 Drugs 0.000 description 1
- 229940101267 panthenol Drugs 0.000 description 1
- 235000020957 pantothenol Nutrition 0.000 description 1
- 239000011619 pantothenol Substances 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- WCVRQHFDJLLWFE-UHFFFAOYSA-N pentane-1,2-diol Chemical compound CCCC(O)CO WCVRQHFDJLLWFE-UHFFFAOYSA-N 0.000 description 1
- XLMFDCKSFJWJTP-UHFFFAOYSA-N pentane-2,3-diol Chemical compound CCC(O)C(C)O XLMFDCKSFJWJTP-UHFFFAOYSA-N 0.000 description 1
- 235000019477 peppermint oil Nutrition 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229940097156 peroxyl Drugs 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229960005323 phenoxyethanol Drugs 0.000 description 1
- 229940057874 phenyl trimethicone Drugs 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 229940067107 phenylethyl alcohol Drugs 0.000 description 1
- 229940096826 phenylmercuric acetate Drugs 0.000 description 1
- PDTFCHSETJBPTR-UHFFFAOYSA-N phenylmercuric nitrate Chemical compound [O-][N+](=O)O[Hg]C1=CC=CC=C1 PDTFCHSETJBPTR-UHFFFAOYSA-N 0.000 description 1
- AERBNCYCJBRYDG-KSZLIROESA-N phytosphingosine Chemical compound CCCCCCCCCCCCCC[C@@H](O)[C@@H](O)[C@@H](N)CO AERBNCYCJBRYDG-KSZLIROESA-N 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 201000010065 polycystic ovary syndrome Diseases 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 229920000259 polyoxyethylene lauryl ether Polymers 0.000 description 1
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- GNSKLFRGEWLPPA-UHFFFAOYSA-M potassium dihydrogen phosphate Chemical compound [K+].OP(O)([O-])=O GNSKLFRGEWLPPA-UHFFFAOYSA-M 0.000 description 1
- PHZLMBHDXVLRIX-UHFFFAOYSA-M potassium lactate Chemical compound [K+].CC(O)C([O-])=O PHZLMBHDXVLRIX-UHFFFAOYSA-M 0.000 description 1
- 235000011085 potassium lactate Nutrition 0.000 description 1
- 239000001521 potassium lactate Substances 0.000 description 1
- 229960001304 potassium lactate Drugs 0.000 description 1
- 229940078491 ppg-15 stearyl ether Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000011027 product recovery Methods 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229940093625 propylene glycol monostearate Drugs 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- 230000008817 pulmonary damage Effects 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 235000015136 pumpkin Nutrition 0.000 description 1
- 210000001747 pupil Anatomy 0.000 description 1
- DCBSHORRWZKAKO-UHFFFAOYSA-N rac-1-monomyristoylglycerol Chemical compound CCCCCCCCCCCCCC(=O)OCC(O)CO DCBSHORRWZKAKO-UHFFFAOYSA-N 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 235000021013 raspberries Nutrition 0.000 description 1
- 230000006950 reactive oxygen species formation Effects 0.000 description 1
- 229940096976 rectal foam Drugs 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 239000001044 red dye Substances 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 238000002390 rotary evaporation Methods 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000004626 scanning electron microscopy Methods 0.000 description 1
- 108010048734 sclerotin Proteins 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 230000014860 sensory perception of taste Effects 0.000 description 1
- 235000019613 sensory perceptions of taste Nutrition 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 229940057910 shea butter Drugs 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000002210 silicon-based material Substances 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010378 sodium ascorbate Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 description 1
- 229960005055 sodium ascorbate Drugs 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 229940001607 sodium bisulfite Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 1
- 229940083575 sodium dodecyl sulfate Drugs 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 229940057950 sodium laureth sulfate Drugs 0.000 description 1
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 description 1
- 229940045885 sodium lauroyl sarcosinate Drugs 0.000 description 1
- 229940079862 sodium lauryl sarcosinate Drugs 0.000 description 1
- 235000010268 sodium methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 description 1
- NTYZDAJPNNBYED-UHFFFAOYSA-M sodium;2-(2-dodecanoyloxypropanoyloxy)propanoate Chemical compound [Na+].CCCCCCCCCCCC(=O)OC(C)C(=O)OC(C)C([O-])=O NTYZDAJPNNBYED-UHFFFAOYSA-M 0.000 description 1
- SXHLENDCVBIJFO-UHFFFAOYSA-M sodium;2-[2-(2-dodecoxyethoxy)ethoxy]ethyl sulfate Chemical compound [Na+].CCCCCCCCCCCCOCCOCCOCCOS([O-])(=O)=O SXHLENDCVBIJFO-UHFFFAOYSA-M 0.000 description 1
- IWMMSZLFZZPTJY-UHFFFAOYSA-M sodium;3-(dodecylamino)propane-1-sulfonate Chemical compound [Na+].CCCCCCCCCCCCNCCCS([O-])(=O)=O IWMMSZLFZZPTJY-UHFFFAOYSA-M 0.000 description 1
- LLKGTXLYJMUQJX-UHFFFAOYSA-M sodium;3-[2-carboxyethyl(dodecyl)amino]propanoate Chemical compound [Na+].CCCCCCCCCCCCN(CCC(O)=O)CCC([O-])=O LLKGTXLYJMUQJX-UHFFFAOYSA-M 0.000 description 1
- PESXGULMKCKJCC-UHFFFAOYSA-M sodium;4-methoxycarbonylphenolate Chemical compound [Na+].COC(=O)C1=CC=C([O-])C=C1 PESXGULMKCKJCC-UHFFFAOYSA-M 0.000 description 1
- DUXXGJTXFHUORE-UHFFFAOYSA-M sodium;4-tridecylbenzenesulfonate Chemical compound [Na+].CCCCCCCCCCCCCC1=CC=C(S([O-])(=O)=O)C=C1 DUXXGJTXFHUORE-UHFFFAOYSA-M 0.000 description 1
- CRPCXAMJWCDHFM-UHFFFAOYSA-M sodium;5-oxopyrrolidine-2-carboxylate Chemical compound [Na+].[O-]C(=O)C1CCC(=O)N1 CRPCXAMJWCDHFM-UHFFFAOYSA-M 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 229940035044 sorbitan monolaurate Drugs 0.000 description 1
- 235000011071 sorbitan monopalmitate Nutrition 0.000 description 1
- 239000001570 sorbitan monopalmitate Substances 0.000 description 1
- 229940031953 sorbitan monopalmitate Drugs 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 229960005078 sorbitan sesquioleate Drugs 0.000 description 1
- 235000019337 sorbitan trioleate Nutrition 0.000 description 1
- 229960000391 sorbitan trioleate Drugs 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 229940001941 soy protein Drugs 0.000 description 1
- 235000019721 spearmint oil Nutrition 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- WWUZIQQURGPMPG-KRWOKUGFSA-N sphingosine Chemical compound CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)CO WWUZIQQURGPMPG-KRWOKUGFSA-N 0.000 description 1
- 229940032094 squalane Drugs 0.000 description 1
- 238000012430 stability testing Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 229940057981 stearalkonium chloride Drugs 0.000 description 1
- 229960004274 stearic acid Drugs 0.000 description 1
- WNIFXKPDILJURQ-UHFFFAOYSA-N stearyl glycyrrhizinate Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C)CCC(C(=O)OCCCCCCCCCCCCCCCCCC)(C)CC5C4=CC(=O)C3C21C WNIFXKPDILJURQ-UHFFFAOYSA-N 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 235000021012 strawberries Nutrition 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 235000020238 sunflower seed Nutrition 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 239000003760 tallow Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 230000035923 taste sensation Effects 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 125000005207 tetraalkylammonium group Chemical group 0.000 description 1
- DZKXJUASMGQEMA-UHFFFAOYSA-N tetradecyl tetradecanoate Chemical compound CCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCC DZKXJUASMGQEMA-UHFFFAOYSA-N 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 238000005382 thermal cycling Methods 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 229920000428 triblock copolymer Polymers 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 150000003627 tricarboxylic acid derivatives Chemical class 0.000 description 1
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 1
- LINXHFKHZLOLEI-UHFFFAOYSA-N trimethyl-[phenyl-bis(trimethylsilyloxy)silyl]oxysilane Chemical compound C[Si](C)(C)O[Si](O[Si](C)(C)C)(O[Si](C)(C)C)C1=CC=CC=C1 LINXHFKHZLOLEI-UHFFFAOYSA-N 0.000 description 1
- SZYJELPVAFJOGJ-UHFFFAOYSA-N trimethylamine hydrochloride Chemical group Cl.CN(C)C SZYJELPVAFJOGJ-UHFFFAOYSA-N 0.000 description 1
- VLPFTAMPNXLGLX-UHFFFAOYSA-N trioctanoin Chemical compound CCCCCCCC(=O)OCC(OC(=O)CCCCCCC)COC(=O)CCCCCCC VLPFTAMPNXLGLX-UHFFFAOYSA-N 0.000 description 1
- PHYFQTYBJUILEZ-IUPFWZBJSA-N triolein Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(OC(=O)CCCCCCC\C=C/CCCCCCCC)COC(=O)CCCCCCC\C=C/CCCCCCCC PHYFQTYBJUILEZ-IUPFWZBJSA-N 0.000 description 1
- 229940117972 triolein Drugs 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 235000019798 tripotassium phosphate Nutrition 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 229910000406 trisodium phosphate Inorganic materials 0.000 description 1
- 235000019801 trisodium phosphate Nutrition 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- WGIWBXUNRXCYRA-UHFFFAOYSA-H trizinc;2-hydroxypropane-1,2,3-tricarboxylate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O WGIWBXUNRXCYRA-UHFFFAOYSA-H 0.000 description 1
- 229920001664 tyloxapol Polymers 0.000 description 1
- MDYZKJNTKZIUSK-UHFFFAOYSA-N tyloxapol Chemical compound O=C.C1CO1.CC(C)(C)CC(C)(C)C1=CC=C(O)C=C1 MDYZKJNTKZIUSK-UHFFFAOYSA-N 0.000 description 1
- 229960004224 tyloxapol Drugs 0.000 description 1
- 230000036269 ulceration Effects 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
- 150000003672 ureas Chemical class 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 229940099259 vaseline Drugs 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 235000019155 vitamin A Nutrition 0.000 description 1
- 239000011719 vitamin A Substances 0.000 description 1
- 235000019156 vitamin B Nutrition 0.000 description 1
- 239000011720 vitamin B Substances 0.000 description 1
- 150000003710 vitamin D derivatives Chemical class 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 235000019168 vitamin K Nutrition 0.000 description 1
- 239000011712 vitamin K Substances 0.000 description 1
- 229940046008 vitamin d Drugs 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000011746 zinc citrate Substances 0.000 description 1
- 235000006076 zinc citrate Nutrition 0.000 description 1
- 229940068475 zinc citrate Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4965—Non-condensed pyrazines
- A61K31/497—Non-condensed pyrazines containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/32—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. carbomers, poly(meth)acrylates, or polyvinyl pyrrolidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/146—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1635—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Dispersion Chemistry (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Plural Heterocyclic Compounds (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN201611031045 | 2016-09-12 | ||
| IN201611031046 | 2016-09-12 | ||
| IN201611031046 | 2016-09-12 | ||
| IN201611031045 | 2016-09-12 | ||
| US201662412701P | 2016-10-25 | 2016-10-25 | |
| US62/412,701 | 2016-10-25 | ||
| PCT/IB2017/001231 WO2018047002A1 (en) | 2016-09-12 | 2017-09-12 | Formulations of 4-methyl-5-(pyrazin-2-yl)-3h-1.2-dithiole-3-thione, taste-modified formulations, and methods of making and using same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190089851A true KR20190089851A (ko) | 2019-07-31 |
Family
ID=60569946
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197010518A Ceased KR20190089851A (ko) | 2016-09-12 | 2017-09-12 | 4-메틸-5-(피라진-2-일)-3h-1,2-디티올-3-티온의 제제, 맛-개질 제제, 및 그의 제조 및 사용 방법 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US11185544B2 (enExample) |
| EP (1) | EP3509641A1 (enExample) |
| JP (2) | JP2019533008A (enExample) |
| KR (1) | KR20190089851A (enExample) |
| CN (1) | CN109996563A (enExample) |
| AU (1) | AU2017322544B2 (enExample) |
| BR (1) | BR112019004639A2 (enExample) |
| CA (1) | CA3036630A1 (enExample) |
| PH (1) | PH12019500530A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019531344A (ja) * | 2016-09-12 | 2019-10-31 | エスティー アイピー ホールディング エージー | 4−メチル−5−(ピラジン−2−イル)−3h−1,2−ジチオール−3−チオンの処方物、ならびにそれらの製造方法及び使用方法 |
| US11260604B2 (en) * | 2018-03-14 | 2022-03-01 | Andrew Reynolds | Method and system for dispensing molten wax into molds by means of a desktop apparatus |
Family Cites Families (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7067116B1 (en) | 2000-03-23 | 2006-06-27 | Warner-Lambert Company Llc | Fast dissolving orally consumable solid film containing a taste masking agent and pharmaceutically active agent at weight ratio of 1:3 to 3:1 |
| CA2408152A1 (en) | 2000-05-05 | 2001-11-15 | Wisconsin Alumni Research Foundation | Compositions and methods for protecting cells during cancer chemotherapy and radiotherapy |
| US7012148B2 (en) | 2001-09-25 | 2006-03-14 | Trustees Of Dartmouth College | Compositions and methods for thionation during chemical synthesis reactions |
| US7199122B2 (en) | 2001-10-02 | 2007-04-03 | Fox Chase Cancer Center | Methods for inhibiting angiogenesis |
| US20050182128A1 (en) | 2002-02-13 | 2005-08-18 | Stephen Lam | Use of anethole dithiolethione in lung cancer chemoprevention |
| KR100491318B1 (ko) | 2002-11-26 | 2005-05-24 | 씨제이 주식회사 | 올티프라즈(Oltipraz) 제조방법 |
| KR20050014960A (ko) * | 2003-08-01 | 2005-02-21 | 씨제이 주식회사 | B형 간염 치료 및 간 조직 재생용 약제학적 조성물 |
| ZA200604862B (en) | 2003-12-22 | 2007-10-31 | Alcon Inc | Agents for treatment of glaucomatous retinopathy and optic neuropathy |
| KR100629771B1 (ko) * | 2004-01-27 | 2006-09-28 | 씨제이 주식회사 | 결정성이 감소되거나 무정형화된 올티프라즈의 제조방법 |
| PL2486942T3 (pl) | 2004-11-24 | 2019-05-31 | Meda Pharmaceuticals Inc | Kompozycje zawierające azelastynę oraz sposoby ich stosowania |
| FR2879444B1 (fr) | 2004-12-22 | 2007-05-18 | Oreal | Utilisation d'un compose capable d'augmenter le taux de glutathion dans les melanocytes pour le traitement de la canitie |
| US20070036733A1 (en) | 2005-08-12 | 2007-02-15 | Takasago International Corp. (Usa) | Sensation masking composition |
| BRPI0616821B1 (pt) | 2005-08-15 | 2022-06-07 | Givaudan Sa | Método para proporcionar um efeito refrescante em um produto e produto possuindo um efeito refrescante |
| US7803353B2 (en) | 2006-03-29 | 2010-09-28 | The Procter & Gamble Company | Oral care compositions having improved consumer aesthetics and taste |
| GB0704718D0 (en) | 2007-03-12 | 2007-04-18 | Prendergast Patrick T | Compounds and methods for preventing and treating mucositis |
| WO2008151460A2 (en) | 2007-06-13 | 2008-12-18 | Givaudan Sa | Cooling compounds |
| US20110003747A1 (en) | 2007-07-20 | 2011-01-06 | Coloumbe Pierre A | Use of nrf2 inducers to treat epidermolysis bullosa simplex and related diseases |
| EP2244699A2 (en) | 2008-01-31 | 2010-11-03 | Mcneil-PPC, Inc | Edible film-strips for immediate release of active ingredients |
| US8858995B2 (en) | 2008-03-10 | 2014-10-14 | University Of Louisville Research Foundation, Inc. | Methods and compositions for controlled delivery of phytochemical agents |
| US8142806B2 (en) | 2008-03-10 | 2012-03-27 | University Of Louisville Research Foundation, Inc. | Methods and compositions for controlled delivery of phytochemical agents |
| KR101057485B1 (ko) | 2008-08-04 | 2011-08-17 | 서울대학교산학협력단 | 1,2-디티올티온 유도체를 함유하는 엘엑스알-알파 과다발현으로 인한 질병의 예방 및 치료용 약학 조성물 |
| WO2010128026A2 (en) | 2009-05-05 | 2010-11-11 | Givaudan Sa | Organic compounds |
| US10463611B2 (en) * | 2011-06-08 | 2019-11-05 | Sti Pharma, Llc | Controlled absorption water-soluble pharmaceutically active organic compound formulation for once-daily administration |
| ES2632443T3 (es) * | 2011-09-22 | 2017-09-13 | Viiv Healthcare Uk Limited | Compuestos de pirrolopiridinona y procedimientos de tratamiento del VIH |
| WO2014100403A1 (en) | 2012-12-19 | 2014-06-26 | Kashiv Pharma, Llc | Supersaturated stabilized nanoparticles for poorly soluble drugs |
| WO2015023889A1 (en) | 2013-08-16 | 2015-02-19 | Luminus Biosciences Inc. | Rapidly-dissolving thin film formulation of water soluble digitalis glycoside for the treatment of congestive heart disease |
| CA2937766A1 (en) | 2014-02-27 | 2015-09-03 | The Procter & Gamble Company | Oral care compositions with a reduced bitter taste perception |
| WO2016207914A2 (en) | 2015-06-25 | 2016-12-29 | St Ip Holding Ag | Methods for preparing oltipraz |
| US20160376259A1 (en) * | 2015-06-25 | 2016-12-29 | St Ip Holding Ag | Methods for Preparing Oltipraz |
| ITUB20159655A1 (it) | 2015-12-23 | 2017-06-23 | Bormioli Rocco Spa | Capsula di sicurezza di un contenitore. |
| ITUA20162141A1 (it) | 2016-03-31 | 2017-10-01 | Bormioli Pharma Spa | Capsula di chiusura |
| JP2019531344A (ja) * | 2016-09-12 | 2019-10-31 | エスティー アイピー ホールディング エージー | 4−メチル−5−(ピラジン−2−イル)−3h−1,2−ジチオール−3−チオンの処方物、ならびにそれらの製造方法及び使用方法 |
| KR20180123796A (ko) | 2017-05-10 | 2018-11-20 | 서울대학교산학협력단 | Nrf2-NEMO 경로 활성화를 통한 과중력으로 인한 세포괴사의 억제용 조성물 및 방법 |
-
2017
- 2017-09-12 CA CA3036630A patent/CA3036630A1/en active Pending
- 2017-09-12 BR BR112019004639A patent/BR112019004639A2/pt not_active IP Right Cessation
- 2017-09-12 CN CN201780069887.1A patent/CN109996563A/zh active Pending
- 2017-09-12 JP JP2019535991A patent/JP2019533008A/ja active Pending
- 2017-09-12 AU AU2017322544A patent/AU2017322544B2/en not_active Expired - Fee Related
- 2017-09-12 EP EP17808566.8A patent/EP3509641A1/en active Pending
- 2017-09-12 KR KR1020197010518A patent/KR20190089851A/ko not_active Ceased
-
2019
- 2019-03-12 PH PH12019500530A patent/PH12019500530A1/en unknown
- 2019-03-12 US US16/351,122 patent/US11185544B2/en active Active
-
2021
- 2021-10-25 US US17/509,965 patent/US20220296589A1/en not_active Abandoned
-
2023
- 2023-05-09 JP JP2023077049A patent/JP2023113634A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US20220296589A1 (en) | 2022-09-22 |
| BR112019004639A2 (pt) | 2019-07-16 |
| JP2019533008A (ja) | 2019-11-14 |
| US11185544B2 (en) | 2021-11-30 |
| PH12019500530A1 (en) | 2020-02-24 |
| CA3036630A1 (en) | 2018-03-15 |
| US20190209557A1 (en) | 2019-07-11 |
| AU2017322544B2 (en) | 2022-06-02 |
| AU2017322544A1 (en) | 2019-05-02 |
| EP3509641A1 (en) | 2019-07-17 |
| JP2023113634A (ja) | 2023-08-16 |
| CN109996563A (zh) | 2019-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220202735A1 (en) | Methods of making and using compositions comprising flavonoids | |
| US10213443B2 (en) | Tetracycline topical formulations, preparation and uses thereof in treating an ocular condition | |
| RU2589830C2 (ru) | ЛЕКАРСТВЕННАЯ ДОЗИРОВАННАЯ ФОРМА, КОТОРАЯ СОДЕРЖИТ 6'-ФТОР-(N-МЕТИЛ- ИЛИ N, N-ДИМЕТИЛ-)-4-ФЕНИЛ-4', 9'-ДИГИДРО-3'Н-СПИРО[ЦИКЛОГЕКСАН-1, 1'-ПИРАНО[3, 4, b]ИНДОЛ]-4-АМИН | |
| US20210052580A1 (en) | Combination Compositions and Therapies Comprising 4-Methyl-5-(Pyrazin-2-yl)-3H-1,2-Dithiole-3-Thione, and Methods of Making and Using Same | |
| CN115135307A (zh) | 细胞递送用载体 | |
| WO2018047013A1 (en) | Formulations of 4-methyl-5-(pyrazin-2-yl)-3h-1, 2-dithiole-3-thione, and methods of making and using same | |
| EP3328361B1 (en) | Drug loaded nanoresin particles | |
| JP2023113634A (ja) | 4-メチル-5-(ピラジン-2-イル)-3h-1,2-ジチオール-3-チオンの処方物、味覚修飾処方物、ならびにそれらの製造方法及び使用方法 | |
| WO2018047002A1 (en) | Formulations of 4-methyl-5-(pyrazin-2-yl)-3h-1.2-dithiole-3-thione, taste-modified formulations, and methods of making and using same | |
| US11426403B2 (en) | Formulations of 4-methyl-5-(pyrazin-2-yl)-3H-1,2-dithiole-3-thione, and methods of making and using same | |
| KR102247748B1 (ko) | 수분초 겔 및 수분초 추출물을 포함하는 피부보습용 조성물 | |
| TR201911262T4 (tr) | Melatonin veya bunun türevleri ile koenzim q10 içeren bileşim ve bunun cilt yaşlanmasına karşı kullanımı. | |
| US11135220B1 (en) | Methods of treating viral infections with formulated compositions comprising 4-methyl-5-(pyrazin-2-yl)-3H-1,2-dithiole-3-thione | |
| JP4590224B2 (ja) | トラニラストまたは薬理学的に許容される塩を含有する懸濁性医薬組成物 | |
| KR101847720B1 (ko) | 자초추출물을 함유하는 조성물 | |
| RU2469706C2 (ru) | Фармацевтическая композиция для трансдермального применения для увеличения активности лекарственных веществ и снижения их побочных эффектов |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190412 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200911 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20221017 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230707 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240129 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230707 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20221017 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |