KR20180101452A - 프로리포솜성 테스토스테론 운데카노에이트 제제 - Google Patents
프로리포솜성 테스토스테론 운데카노에이트 제제 Download PDFInfo
- Publication number
- KR20180101452A KR20180101452A KR1020187022494A KR20187022494A KR20180101452A KR 20180101452 A KR20180101452 A KR 20180101452A KR 1020187022494 A KR1020187022494 A KR 1020187022494A KR 20187022494 A KR20187022494 A KR 20187022494A KR 20180101452 A KR20180101452 A KR 20180101452A
- Authority
- KR
- South Korea
- Prior art keywords
- tsx
- dosage form
- pro
- oral dosage
- ratio
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- UDSFVOAUHKGBEK-CNQKSJKFSA-N testosterone undecanoate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](OC(=O)CCCCCCCCCC)[C@@]1(C)CC2 UDSFVOAUHKGBEK-CNQKSJKFSA-N 0.000 title claims abstract description 249
- 229960000746 testosterone undecanoate Drugs 0.000 title claims abstract description 199
- 239000000203 mixture Substances 0.000 title claims abstract description 83
- 238000009472 formulation Methods 0.000 title abstract description 43
- 239000000843 powder Substances 0.000 claims abstract description 70
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 claims abstract description 61
- 239000006185 dispersion Substances 0.000 claims abstract description 56
- 239000000546 pharmaceutical excipient Substances 0.000 claims abstract description 13
- 239000002775 capsule Substances 0.000 claims description 63
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 claims description 62
- 239000006186 oral dosage form Substances 0.000 claims description 43
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 33
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 33
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 33
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 33
- 229960003604 testosterone Drugs 0.000 claims description 30
- 229940079832 sodium starch glycolate Drugs 0.000 claims description 18
- 229920003109 sodium starch glycolate Polymers 0.000 claims description 18
- 239000008109 sodium starch glycolate Substances 0.000 claims description 18
- 239000003814 drug Substances 0.000 claims description 17
- 238000000034 method Methods 0.000 claims description 17
- 229940079593 drug Drugs 0.000 claims description 14
- 239000002552 dosage form Substances 0.000 claims description 10
- 238000011282 treatment Methods 0.000 claims description 9
- 208000011580 syndromic disease Diseases 0.000 claims description 7
- 206010058359 Hypogonadism Diseases 0.000 claims description 4
- 239000008199 coating composition Substances 0.000 claims description 4
- 239000002702 enteric coating Substances 0.000 claims description 4
- 238000009505 enteric coating Methods 0.000 claims description 4
- 230000009246 food effect Effects 0.000 claims description 4
- 235000021471 food effect Nutrition 0.000 claims description 4
- 206010002261 Androgen deficiency Diseases 0.000 claims description 3
- 208000018565 Hemochromatosis Diseases 0.000 claims description 3
- 238000002512 chemotherapy Methods 0.000 claims description 3
- 230000000694 effects Effects 0.000 claims description 3
- 230000006584 pituitary dysfunction Effects 0.000 claims description 3
- 206010016654 Fibrosis Diseases 0.000 claims description 2
- 230000005856 abnormality Effects 0.000 claims description 2
- 230000002411 adverse Effects 0.000 claims description 2
- 208000020832 chronic kidney disease Diseases 0.000 claims description 2
- 230000007882 cirrhosis Effects 0.000 claims description 2
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 claims description 2
- 230000002068 genetic effect Effects 0.000 claims description 2
- 208000015181 infectious disease Diseases 0.000 claims description 2
- 208000027866 inflammatory disease Diseases 0.000 claims description 2
- 229940117841 methacrylic acid copolymer Drugs 0.000 claims description 2
- 229920003145 methacrylic acid copolymer Polymers 0.000 claims description 2
- 238000001959 radiotherapy Methods 0.000 claims description 2
- 231100000398 testicular damage Toxicity 0.000 claims description 2
- 208000022831 chronic renal failure syndrome Diseases 0.000 claims 1
- 238000002203 pretreatment Methods 0.000 claims 1
- 210000002966 serum Anatomy 0.000 claims 1
- 210000001550 testis Anatomy 0.000 claims 1
- 238000002560 therapeutic procedure Methods 0.000 claims 1
- 238000009165 androgen replacement therapy Methods 0.000 abstract description 7
- 239000000243 solution Substances 0.000 description 47
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 44
- 150000002632 lipids Chemical class 0.000 description 36
- AOBORMOPSGHCAX-UHFFFAOYSA-N Tocophersolan Chemical compound OCCOC(=O)CCC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C AOBORMOPSGHCAX-UHFFFAOYSA-N 0.000 description 20
- 235000013305 food Nutrition 0.000 description 20
- 241000282472 Canis lupus familiaris Species 0.000 description 19
- 241001465754 Metazoa Species 0.000 description 18
- 239000003550 marker Substances 0.000 description 18
- 238000000576 coating method Methods 0.000 description 17
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 16
- 238000001035 drying Methods 0.000 description 15
- 238000002360 preparation method Methods 0.000 description 15
- 229920003134 Eudragit® polymer Polymers 0.000 description 14
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 14
- 239000011248 coating agent Substances 0.000 description 14
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- 235000020937 fasting conditions Nutrition 0.000 description 12
- 238000002156 mixing Methods 0.000 description 11
- 238000004090 dissolution Methods 0.000 description 10
- 238000010438 heat treatment Methods 0.000 description 10
- 230000037396 body weight Effects 0.000 description 8
- 235000012000 cholesterol Nutrition 0.000 description 8
- 239000002002 slurry Substances 0.000 description 8
- 239000008280 blood Substances 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 230000003111 delayed effect Effects 0.000 description 7
- 239000002904 solvent Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 6
- 238000011287 therapeutic dose Methods 0.000 description 5
- 210000004731 jugular vein Anatomy 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 210000000813 small intestine Anatomy 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000002596 correlated effect Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000005538 encapsulation Methods 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
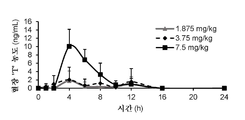
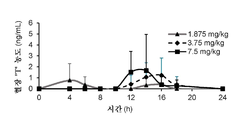
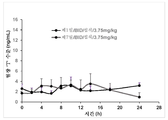
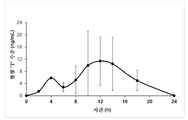
- 230000036470 plasma concentration Effects 0.000 description 3
- 239000004014 plasticizer Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
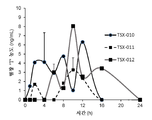
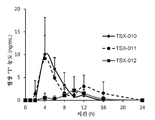
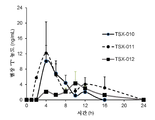
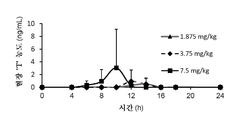
- -1 TSX-010 Chemical compound 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000032683 aging Effects 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 239000003098 androgen Substances 0.000 description 2
- 229920006318 anionic polymer Polymers 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000012738 dissolution medium Substances 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 229940014259 gelatin Drugs 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 230000002267 hypothalamic effect Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 235000012054 meals Nutrition 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 239000005022 packaging material Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000001817 pituitary effect Effects 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 206010000029 5-alpha-reductase deficiency Diseases 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- ORILYTVJVMAKLC-UHFFFAOYSA-N Adamantane Natural products C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 206010056292 Androgen-Insensitivity Syndrome Diseases 0.000 description 1
- 206010006811 Bursitis Diseases 0.000 description 1
- 206010007558 Cardiac failure chronic Diseases 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 208000010228 Erectile Dysfunction Diseases 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 101000945318 Homo sapiens Calponin-1 Proteins 0.000 description 1
- 101000652736 Homo sapiens Transgelin Proteins 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 208000017924 Klinefelter Syndrome Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 108700004960 Pseudovaginal Perineoscrotal Hypospadias Proteins 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010059594 Secondary hypogonadism Diseases 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 208000011622 Testicular disease Diseases 0.000 description 1
- 102100031013 Transgelin Human genes 0.000 description 1
- DOOTYTYQINUNNV-UHFFFAOYSA-N Triethyl citrate Chemical compound CCOC(=O)CC(O)(C(=O)OCC)CC(=O)OCC DOOTYTYQINUNNV-UHFFFAOYSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229940124532 absorption promoter Drugs 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000019771 cognition Effects 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 235000013367 dietary fats Nutrition 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 238000011978 dissolution method Methods 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 201000003585 eunuchism Diseases 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000013022 formulation composition Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- 235000009200 high fat diet Nutrition 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 239000008350 hydrogenated phosphatidyl choline Substances 0.000 description 1
- 201000003368 hypogonadotropic hypogonadism Diseases 0.000 description 1
- 201000001881 impotence Diseases 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 210000004347 intestinal mucosa Anatomy 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960001375 lactose Drugs 0.000 description 1
- 229960001021 lactose monohydrate Drugs 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000004324 lymphatic system Anatomy 0.000 description 1
- 208000037106 male hypogonadism Diseases 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000011129 pharmaceutical packaging material Substances 0.000 description 1
- 150000008105 phosphatidylcholines Chemical class 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000013557 residual solvent Substances 0.000 description 1
- 201000000306 sarcoidosis Diseases 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000036299 sexual function Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- KPTYMLNTUAKIEU-UHFFFAOYSA-N sodium;carbonic acid;dihydrogen borate Chemical compound [Na+].OB(O)[O-].OC(O)=O KPTYMLNTUAKIEU-UHFFFAOYSA-N 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002381 testicular Effects 0.000 description 1
- 229960003410 testosterone decanoate Drugs 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 229940001496 tribasic sodium phosphate Drugs 0.000 description 1
- 239000001069 triethyl citrate Substances 0.000 description 1
- VMYFZRTXGLUXMZ-UHFFFAOYSA-N triethyl citrate Natural products CCOC(=O)C(O)(C(=O)OCC)C(=O)OCC VMYFZRTXGLUXMZ-UHFFFAOYSA-N 0.000 description 1
- 235000013769 triethyl citrate Nutrition 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07J—STEROIDS
- C07J9/00—Normal steroids containing carbon, hydrogen, halogen or oxygen substituted in position 17 beta by a chain of more than two carbon atoms, e.g. cholane, cholestane, coprostane
- C07J9/005—Normal steroids containing carbon, hydrogen, halogen or oxygen substituted in position 17 beta by a chain of more than two carbon atoms, e.g. cholane, cholestane, coprostane containing a carboxylic function directly attached or attached by a chain containing only carbon atoms to the cyclopenta[a]hydrophenanthrene skeleton
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
- A61K9/1277—Preparation processes; Proliposomes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
- A61K31/568—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/683—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols
- A61K31/685—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols one of the hydroxy compounds having nitrogen atoms, e.g. phosphatidylserine, lecithin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4891—Coated capsules; Multilayered drug free capsule shells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/26—Androgens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/06—Drugs for disorders of the endocrine system of the anterior pituitary hormones, e.g. TSH, ACTH, FSH, LH, PRL, GH
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Dispersion Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Endocrinology (AREA)
- Diabetes (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662276452P | 2016-01-08 | 2016-01-08 | |
| US62/276,452 | 2016-01-08 | ||
| US201662394576P | 2016-09-14 | 2016-09-14 | |
| US62/394,576 | 2016-09-14 | ||
| PCT/US2017/012739 WO2017120592A1 (en) | 2016-01-08 | 2017-01-09 | Proliposomal testosterone undecanoate formulations |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180101452A true KR20180101452A (ko) | 2018-09-12 |
Family
ID=59274044
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187022494A Withdrawn KR20180101452A (ko) | 2016-01-08 | 2017-01-09 | 프로리포솜성 테스토스테론 운데카노에이트 제제 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190248830A1 (enExample) |
| EP (1) | EP3399965A4 (enExample) |
| JP (1) | JP2019501199A (enExample) |
| KR (1) | KR20180101452A (enExample) |
| CN (1) | CN108601736A (enExample) |
| WO (1) | WO2017120592A1 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8492369B2 (en) | 2010-04-12 | 2013-07-23 | Clarus Therapeutics Inc | Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same |
| RU2623206C2 (ru) | 2005-04-15 | 2017-06-22 | Кларус Терапьютикс, Инк. | Фармацевтические системы доставки для гидрофобных лекарственных средств и композиций, их содержащих |
| ES3006512T3 (en) | 2017-11-17 | 2025-03-18 | Evonik Operations Gmbh | Process for preparing a coated hard shell capsule |
| US12150945B2 (en) * | 2018-07-20 | 2024-11-26 | Lipocine Inc. | Liver disease |
| US20200197413A1 (en) * | 2018-12-20 | 2020-06-25 | Clarus Therapeutics, Inc. | Methods of treating testosterone deficiency |
| MX2022001417A (es) * | 2019-08-09 | 2022-06-08 | Tesorx Pharma Llc | Formulaciones de undecanoato de testosterona proliposomal. |
| GB202307501D0 (en) * | 2023-05-19 | 2023-07-05 | Lawley Pharmaceuticals Pty Ltd | Process for preparing dispensable testosterone cream |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6759058B1 (en) * | 2001-04-25 | 2004-07-06 | Western Center For Drug Development College Of Pharmacy Western University Of Health Sciences | Enteric-coated proliposomal formulations for poorly water soluble drugs |
| WO2008000534A1 (de) * | 2006-06-30 | 2008-01-03 | Gertrud Langhoff | Solubilisatformulierungen |
| WO2008114274A1 (en) * | 2007-03-19 | 2008-09-25 | Fresenius Kabi Onclology Ltd. | Proliposomal and liposomal compositions |
| EP2229936B1 (en) * | 2009-03-09 | 2015-05-06 | PharmaSol GmbH | Nanonized testosterone formulations for improved bioavailability |
| US20140309202A1 (en) * | 2010-11-30 | 2014-10-16 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| SG11201407349UA (en) * | 2012-05-09 | 2014-12-30 | Univ Western Health Sciences | Proliposomal testosterone formulations |
-
2017
- 2017-01-09 KR KR1020187022494A patent/KR20180101452A/ko not_active Withdrawn
- 2017-01-09 JP JP2018535385A patent/JP2019501199A/ja active Pending
- 2017-01-09 EP EP17736520.2A patent/EP3399965A4/en not_active Withdrawn
- 2017-01-09 US US16/068,190 patent/US20190248830A1/en not_active Abandoned
- 2017-01-09 WO PCT/US2017/012739 patent/WO2017120592A1/en not_active Ceased
- 2017-01-09 CN CN201780010451.5A patent/CN108601736A/zh active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| CN108601736A (zh) | 2018-09-28 |
| WO2017120592A1 (en) | 2017-07-13 |
| EP3399965A4 (en) | 2019-08-21 |
| EP3399965A1 (en) | 2018-11-14 |
| US20190248830A1 (en) | 2019-08-15 |
| JP2019501199A (ja) | 2019-01-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20180101452A (ko) | 프로리포솜성 테스토스테론 운데카노에이트 제제 | |
| JP2023112149A (ja) | パルボシクリブの固形剤形 | |
| JP6356215B2 (ja) | エベロリムスを含む医薬組成物 | |
| EP2763663B1 (en) | Pharmaceutical compositions comprising 40-o-(2-hydroxy)ethyl-rapamycin | |
| US20050249799A1 (en) | Polymeric drug delivery system for hydrophobic drugs | |
| JP2003504331A (ja) | フェノフィブレート含有医薬組成物およびこれを調製するための方法 | |
| JP6468661B2 (ja) | 遅延放出システアミンビーズ処方、ならびにその作製方法および使用方法 | |
| EP3691612B1 (en) | High-strength oral taxane compositions and methods | |
| KR102300335B1 (ko) | 아프레피탄트의 경구용 조성물 | |
| JP2019163327A (ja) | 三種混合製剤 | |
| JPWO2002060448A1 (ja) | 医薬組成物 | |
| WO2011096953A1 (en) | Oral antidepressant formulation with reduced excipient load | |
| CN101647782A (zh) | 长春西汀口服自微乳化微丸及其制备和应用 | |
| EP3582778B1 (en) | Compositions of gallium (iii) complexes for oral administration | |
| US20250325517A1 (en) | Oral dosage forms of elraglusib | |
| TW201811336A (zh) | 前體脂質體十一酸睪固酮調配物 | |
| HK1259799A1 (en) | Proliposomal testosterone undecanoate formulations | |
| TWI867026B (zh) | 前體脂質體十一酸睪固酮調配物 | |
| WO2025122074A1 (en) | Low dose riociguat compositions | |
| HK1212899B (en) | Pharmaceutical compositions comprising everolimus | |
| HK1196567A (en) | Pharmaceutical compositions comprising 40 - o - ( 2 - hydroxy) ethyl - rapamycin | |
| HK1196567B (en) | Pharmaceutical compositions comprising 40 - o - ( 2 - hydroxy) ethyl - rapamycin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180803 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |