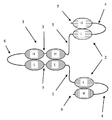
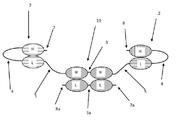
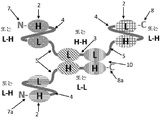
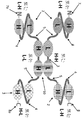
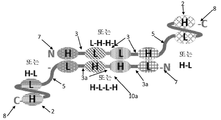
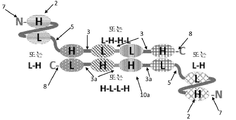
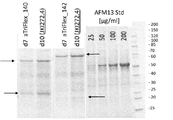
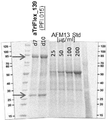
KR20180057720A - 다가 Fv 항체 - Google Patents
다가 Fv 항체 Download PDFInfo
- Publication number
- KR20180057720A KR20180057720A KR1020187013310A KR20187013310A KR20180057720A KR 20180057720 A KR20180057720 A KR 20180057720A KR 1020187013310 A KR1020187013310 A KR 1020187013310A KR 20187013310 A KR20187013310 A KR 20187013310A KR 20180057720 A KR20180057720 A KR 20180057720A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- gly
- thr
- val
- tyr
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 451
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 402
- 229920001184 polypeptide Polymers 0.000 claims abstract description 400
- 239000000427 antigen Substances 0.000 claims abstract description 216
- 102000036639 antigens Human genes 0.000 claims abstract description 216
- 108091007433 antigens Proteins 0.000 claims abstract description 216
- 230000027455 binding Effects 0.000 claims abstract description 167
- 230000009977 dual effect Effects 0.000 claims description 48
- 229940121354 immunomodulator Drugs 0.000 claims description 37
- 239000012642 immune effector Substances 0.000 claims description 36
- 206010028980 Neoplasm Diseases 0.000 claims description 23
- 238000000034 method Methods 0.000 claims description 20
- 125000000539 amino acid group Chemical group 0.000 claims description 16
- 230000003612 virological effect Effects 0.000 claims description 5
- 239000003814 drug Substances 0.000 claims description 4
- 239000012634 fragment Substances 0.000 abstract description 11
- 210000004027 cell Anatomy 0.000 description 160
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 126
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 101
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 92
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 90
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 46
- 108010078144 glutaminyl-glycine Proteins 0.000 description 46
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 45
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 45
- 210000004899 c-terminal region Anatomy 0.000 description 44
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 40
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 40
- 108010089804 glycyl-threonine Proteins 0.000 description 37
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 35
- 206010057190 Respiratory tract infections Diseases 0.000 description 35
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 34
- WLVLIYYBPPONRJ-GCJQMDKQSA-N Asn-Thr-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O WLVLIYYBPPONRJ-GCJQMDKQSA-N 0.000 description 33
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 33
- 108010079364 N-glycylalanine Proteins 0.000 description 33
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 33
- PCDUALPXEOKZPE-DXCABUDRSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoic acid Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O PCDUALPXEOKZPE-DXCABUDRSA-N 0.000 description 31
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 31
- 108010003137 tyrosyltyrosine Proteins 0.000 description 30
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 description 29
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 description 29
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 29
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 28
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 28
- 241000880493 Leptailurus serval Species 0.000 description 28
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 description 28
- 108010060035 arginylproline Proteins 0.000 description 28
- OZHXXYOHPLLLMI-CIUDSAMLSA-N Cys-Lys-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OZHXXYOHPLLLMI-CIUDSAMLSA-N 0.000 description 27
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 27
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 26
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 26
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 26
- 108010069926 arginyl-glycyl-serine Proteins 0.000 description 26
- 108010034529 leucyl-lysine Proteins 0.000 description 25
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 24
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 24
- BGWKULMLUIUPKY-BQBZGAKWSA-N Pro-Ser-Gly Chemical compound OC(=O)CNC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 BGWKULMLUIUPKY-BQBZGAKWSA-N 0.000 description 24
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 24
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 24
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 24
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 24
- 108010070783 alanyltyrosine Proteins 0.000 description 24
- 108010008355 arginyl-glutamine Proteins 0.000 description 24
- 108010010147 glycylglutamine Proteins 0.000 description 24
- KUDREHRZRIVKHS-UWJYBYFXSA-N Ala-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O KUDREHRZRIVKHS-UWJYBYFXSA-N 0.000 description 23
- HGKHPCFTRQDHCU-IUCAKERBSA-N Arg-Pro-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O HGKHPCFTRQDHCU-IUCAKERBSA-N 0.000 description 23
- XOKGKOQWADCLFQ-GARJFASQSA-N Gln-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XOKGKOQWADCLFQ-GARJFASQSA-N 0.000 description 23
- DNAZKGFYFRGZIH-QWRGUYRKSA-N Gly-Tyr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 DNAZKGFYFRGZIH-QWRGUYRKSA-N 0.000 description 23
- 101100334515 Homo sapiens FCGR3A gene Proteins 0.000 description 23
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 23
- 210000000822 natural killer cell Anatomy 0.000 description 23
- 210000004881 tumor cell Anatomy 0.000 description 23
- IDEODOAVGCMUQV-GUBZILKMSA-N Glu-Ser-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IDEODOAVGCMUQV-GUBZILKMSA-N 0.000 description 22
- GBYYQVBXFVDJPJ-WLTAIBSBSA-N Gly-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)CN)O GBYYQVBXFVDJPJ-WLTAIBSBSA-N 0.000 description 21
- LLGTYVHITPVGKR-RYUDHWBXSA-N Phe-Gln-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O LLGTYVHITPVGKR-RYUDHWBXSA-N 0.000 description 21
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 21
- 108010024078 alanyl-glycyl-serine Proteins 0.000 description 21
- 108010038320 lysylphenylalanine Proteins 0.000 description 21
- 108090000623 proteins and genes Proteins 0.000 description 21
- 108010038745 tryptophylglycine Proteins 0.000 description 21
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 20
- BPCLGWHVPVTTFM-QWRGUYRKSA-N Phe-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O BPCLGWHVPVTTFM-QWRGUYRKSA-N 0.000 description 20
- GNRMAQSIROFNMI-IXOXFDKPSA-N Phe-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O GNRMAQSIROFNMI-IXOXFDKPSA-N 0.000 description 20
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 20
- 108010041407 alanylaspartic acid Proteins 0.000 description 20
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 20
- 108010072644 valyl-alanyl-prolyl-glycine Proteins 0.000 description 20
- 108010073969 valyllysine Proteins 0.000 description 20
- HTSSXFASOUSJQG-IHPCNDPISA-N Asp-Tyr-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HTSSXFASOUSJQG-IHPCNDPISA-N 0.000 description 19
- DZLQXIFVQFTFJY-BYPYZUCNSA-N Cys-Gly-Gly Chemical compound SC[C@H](N)C(=O)NCC(=O)NCC(O)=O DZLQXIFVQFTFJY-BYPYZUCNSA-N 0.000 description 19
- OSCLNNWLKKIQJM-WDSKDSINSA-N Gln-Ser-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O OSCLNNWLKKIQJM-WDSKDSINSA-N 0.000 description 19
- FMNHBTKMRFVGRO-FOHZUACHSA-N Gly-Asn-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CN FMNHBTKMRFVGRO-FOHZUACHSA-N 0.000 description 19
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 19
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 19
- OQPNSDWGAMFJNU-QWRGUYRKSA-N Ser-Gly-Tyr Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OQPNSDWGAMFJNU-QWRGUYRKSA-N 0.000 description 19
- CAJFZCICSVBOJK-SHGPDSBTSA-N Thr-Ala-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAJFZCICSVBOJK-SHGPDSBTSA-N 0.000 description 19
- RVMNUBQWPVOUKH-HEIBUPTGSA-N Thr-Ser-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RVMNUBQWPVOUKH-HEIBUPTGSA-N 0.000 description 19
- RMRFSFXLFWWAJZ-HJOGWXRNSA-N Tyr-Tyr-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 RMRFSFXLFWWAJZ-HJOGWXRNSA-N 0.000 description 19
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 19
- 108010092854 aspartyllysine Proteins 0.000 description 19
- OMMDTNGURYRDAC-NRPADANISA-N Ala-Glu-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OMMDTNGURYRDAC-NRPADANISA-N 0.000 description 18
- IOFVWPYSRSCWHI-JXUBOQSCSA-N Ala-Thr-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C)N IOFVWPYSRSCWHI-JXUBOQSCSA-N 0.000 description 18
- ALHMNHZJBYBYHS-DCAQKATOSA-N Asn-Lys-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O ALHMNHZJBYBYHS-DCAQKATOSA-N 0.000 description 18
- SYZZMPFLOLSMHL-XHNCKOQMSA-N Gln-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SYZZMPFLOLSMHL-XHNCKOQMSA-N 0.000 description 18
- PAOHIZNRJNIXQY-XQXXSGGOSA-N Gln-Thr-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O PAOHIZNRJNIXQY-XQXXSGGOSA-N 0.000 description 18
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 18
- INCJJHQRZGQLFC-KBPBESRZSA-N Leu-Phe-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(O)=O INCJJHQRZGQLFC-KBPBESRZSA-N 0.000 description 18
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 18
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 18
- RDFQNDHEHVSONI-ZLUOBGJFSA-N Ser-Asn-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O RDFQNDHEHVSONI-ZLUOBGJFSA-N 0.000 description 18
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 18
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 18
- NGALWFGCOMHUSN-AVGNSLFASA-N Tyr-Gln-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NGALWFGCOMHUSN-AVGNSLFASA-N 0.000 description 18
- MJXNDRCLGDSBBE-FHWLQOOXSA-N Val-His-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N MJXNDRCLGDSBBE-FHWLQOOXSA-N 0.000 description 18
- 239000012636 effector Substances 0.000 description 18
- 108010026333 seryl-proline Proteins 0.000 description 18
- IOFDDSNZJDIGPB-GVXVVHGQSA-N Gln-Leu-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O IOFDDSNZJDIGPB-GVXVVHGQSA-N 0.000 description 17
- PULPZRAHVFBVTO-DCAQKATOSA-N Pro-Glu-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PULPZRAHVFBVTO-DCAQKATOSA-N 0.000 description 17
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 17
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 17
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 17
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 17
- RLMISHABBKUNFO-WHFBIAKZSA-N Ala-Ala-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O RLMISHABBKUNFO-WHFBIAKZSA-N 0.000 description 16
- QLSRIZIDQXDQHK-RCWTZXSCSA-N Arg-Val-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QLSRIZIDQXDQHK-RCWTZXSCSA-N 0.000 description 16
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 16
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 16
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 16
- ULZCYBYDTUMHNF-IUCAKERBSA-N Gly-Leu-Glu Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ULZCYBYDTUMHNF-IUCAKERBSA-N 0.000 description 16
- OHUKZZYSJBKFRR-WHFBIAKZSA-N Gly-Ser-Asp Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O OHUKZZYSJBKFRR-WHFBIAKZSA-N 0.000 description 16
- KAFOIVJDVSZUMD-UHFFFAOYSA-N Leu-Gln-Gln Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-UHFFFAOYSA-N 0.000 description 16
- YXPJCVNIDDKGOE-MELADBBJSA-N Lys-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)N)C(=O)O YXPJCVNIDDKGOE-MELADBBJSA-N 0.000 description 16
- GODBLDDYHFTUAH-CIUDSAMLSA-N Met-Asp-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCC(O)=O GODBLDDYHFTUAH-CIUDSAMLSA-N 0.000 description 16
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 16
- NAXPHWZXEXNDIW-JTQLQIEISA-N Phe-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 NAXPHWZXEXNDIW-JTQLQIEISA-N 0.000 description 16
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 16
- 108010016616 cysteinylglycine Proteins 0.000 description 16
- 108010057083 glutamyl-aspartyl-leucine Proteins 0.000 description 16
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 16
- AXFMEGAFCUULFV-BLFANLJRSA-N (2s)-2-[[(2s)-1-[(2s,3r)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]pentanedioic acid Chemical compound CC[C@@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AXFMEGAFCUULFV-BLFANLJRSA-N 0.000 description 15
- NVWJMQNYLYWVNQ-BYULHYEWSA-N Asn-Ile-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O NVWJMQNYLYWVNQ-BYULHYEWSA-N 0.000 description 15
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 15
- TVTZEOHWHUVYCG-KYNKHSRBSA-N Gly-Thr-Thr Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O TVTZEOHWHUVYCG-KYNKHSRBSA-N 0.000 description 15
- 108010065920 Insulin Lispro Proteins 0.000 description 15
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 15
- FMFNIDICDKEMOE-XUXIUFHCSA-N Leu-Val-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FMFNIDICDKEMOE-XUXIUFHCSA-N 0.000 description 15
- SLQDSYZHHOKQSR-QXEWZRGKSA-N Met-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCSC SLQDSYZHHOKQSR-QXEWZRGKSA-N 0.000 description 15
- UBRMZSHOOIVJPW-SRVKXCTJSA-N Ser-Leu-Lys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O UBRMZSHOOIVJPW-SRVKXCTJSA-N 0.000 description 15
- KZTLZZQTJMCGIP-ZJDVBMNYSA-N Thr-Val-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KZTLZZQTJMCGIP-ZJDVBMNYSA-N 0.000 description 15
- UMPVMAYCLYMYGA-ONGXEEELSA-N Val-Leu-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O UMPVMAYCLYMYGA-ONGXEEELSA-N 0.000 description 15
- 108010081404 acein-2 Proteins 0.000 description 15
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 15
- MCYJBCKCAPERSE-FXQIFTODSA-N Arg-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N MCYJBCKCAPERSE-FXQIFTODSA-N 0.000 description 14
- IIQIOFVDFOLCHP-UHFFFAOYSA-N Asn-Pro-Ser-Ser Chemical compound NC(=O)CC(N)C(=O)N1CCCC1C(=O)NC(CO)C(=O)NC(CO)C(O)=O IIQIOFVDFOLCHP-UHFFFAOYSA-N 0.000 description 14
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 14
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 14
- NJLLRXWFPQQPHV-SRVKXCTJSA-N Asp-Tyr-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O NJLLRXWFPQQPHV-SRVKXCTJSA-N 0.000 description 14
- PONUFVLSGMQFAI-AVGNSLFASA-N Gln-Asn-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PONUFVLSGMQFAI-AVGNSLFASA-N 0.000 description 14
- VNTGPISAOMAXRK-CIUDSAMLSA-N Gln-Pro-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O VNTGPISAOMAXRK-CIUDSAMLSA-N 0.000 description 14
- UZZXGLOJRZKYEL-DJFWLOJKSA-N His-Asn-Ile Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UZZXGLOJRZKYEL-DJFWLOJKSA-N 0.000 description 14
- ZNTSGDNUITWTRA-WDSOQIARSA-N His-Trp-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C(C)C)C(O)=O ZNTSGDNUITWTRA-WDSOQIARSA-N 0.000 description 14
- UKTUOMWSJPXODT-GUDRVLHUSA-N Ile-Asn-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N UKTUOMWSJPXODT-GUDRVLHUSA-N 0.000 description 14
- KAFOIVJDVSZUMD-DCAQKATOSA-N Leu-Gln-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-DCAQKATOSA-N 0.000 description 14
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 14
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 14
- NKKFVJRLCCUJNA-QWRGUYRKSA-N Lys-Gly-Lys Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN NKKFVJRLCCUJNA-QWRGUYRKSA-N 0.000 description 14
- WRXOPYNEKGZWAZ-FXQIFTODSA-N Met-Ser-Cys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(O)=O WRXOPYNEKGZWAZ-FXQIFTODSA-N 0.000 description 14
- YFXXRYFWJFQAFW-JHYOHUSXSA-N Phe-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N)O YFXXRYFWJFQAFW-JHYOHUSXSA-N 0.000 description 14
- HVKMTOIAYDOJPL-NRPADANISA-N Ser-Gln-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O HVKMTOIAYDOJPL-NRPADANISA-N 0.000 description 14
- BBPCSGKKPJUYRB-UVOCVTCTSA-N Thr-Thr-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O BBPCSGKKPJUYRB-UVOCVTCTSA-N 0.000 description 14
- NJLQMKZSXYQRTO-FHWLQOOXSA-N Tyr-Glu-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 NJLQMKZSXYQRTO-FHWLQOOXSA-N 0.000 description 14
- CDHQEOXPWBDFPL-QWRGUYRKSA-N Tyr-Gly-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CDHQEOXPWBDFPL-QWRGUYRKSA-N 0.000 description 14
- 108010087924 alanylproline Proteins 0.000 description 14
- 239000000539 dimer Substances 0.000 description 14
- 108010020688 glycylhistidine Proteins 0.000 description 14
- FEZJJKXNPSEYEV-CIUDSAMLSA-N Arg-Gln-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O FEZJJKXNPSEYEV-CIUDSAMLSA-N 0.000 description 13
- DBWYWXNMZZYIRY-LPEHRKFASA-N Asp-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)O)N)C(=O)O DBWYWXNMZZYIRY-LPEHRKFASA-N 0.000 description 13
- QXHVOUSPVAWEMX-ZLUOBGJFSA-N Asp-Asp-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXHVOUSPVAWEMX-ZLUOBGJFSA-N 0.000 description 13
- GYWQGGUCMDCUJE-DLOVCJGASA-N Asp-Phe-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(O)=O GYWQGGUCMDCUJE-DLOVCJGASA-N 0.000 description 13
- WMLFFCRUSPNENW-ZLUOBGJFSA-N Asp-Ser-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O WMLFFCRUSPNENW-ZLUOBGJFSA-N 0.000 description 13
- GCACQYDBDHRVGE-LKXGYXEUSA-N Asp-Thr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC(O)=O GCACQYDBDHRVGE-LKXGYXEUSA-N 0.000 description 13
- WZZSKAJIHTUUSG-ACZMJKKPSA-N Glu-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O WZZSKAJIHTUUSG-ACZMJKKPSA-N 0.000 description 13
- SBCYJMOOHUDWDA-NUMRIWBASA-N Glu-Asp-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SBCYJMOOHUDWDA-NUMRIWBASA-N 0.000 description 13
- HVYWQYLBVXMXSV-GUBZILKMSA-N Glu-Leu-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O HVYWQYLBVXMXSV-GUBZILKMSA-N 0.000 description 13
- QSVCIFZPGLOZGH-WDSKDSINSA-N Gly-Glu-Ser Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O QSVCIFZPGLOZGH-WDSKDSINSA-N 0.000 description 13
- VAXIVIPMCTYSHI-YUMQZZPRSA-N Gly-His-Asp Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)CN VAXIVIPMCTYSHI-YUMQZZPRSA-N 0.000 description 13
- IRJWAYCXIYUHQE-WHFBIAKZSA-N Gly-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)CN IRJWAYCXIYUHQE-WHFBIAKZSA-N 0.000 description 13
- POJJAZJHBGXEGM-YUMQZZPRSA-N Gly-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN POJJAZJHBGXEGM-YUMQZZPRSA-N 0.000 description 13
- KOYUSMBPJOVSOO-XEGUGMAKSA-N Gly-Tyr-Ile Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KOYUSMBPJOVSOO-XEGUGMAKSA-N 0.000 description 13
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 13
- UCOCBWDBHCUPQP-DCAQKATOSA-N Leu-Arg-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O UCOCBWDBHCUPQP-DCAQKATOSA-N 0.000 description 13
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 13
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 13
- DIBZLYZXTSVGLN-CIUDSAMLSA-N Lys-Ser-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O DIBZLYZXTSVGLN-CIUDSAMLSA-N 0.000 description 13
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 13
- VOOINLQYUZOREH-SRVKXCTJSA-N Met-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCSC)N VOOINLQYUZOREH-SRVKXCTJSA-N 0.000 description 13
- KSIPKXNIQOWMIC-RCWTZXSCSA-N Met-Thr-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KSIPKXNIQOWMIC-RCWTZXSCSA-N 0.000 description 13
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 13
- NMZXJDSKEGFDLJ-DCAQKATOSA-N Ser-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CO)N)C(=O)N[C@@H](CCCCN)C(=O)O NMZXJDSKEGFDLJ-DCAQKATOSA-N 0.000 description 13
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 13
- NJEMRSFGDNECGF-GCJQMDKQSA-N Thr-Ala-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O NJEMRSFGDNECGF-GCJQMDKQSA-N 0.000 description 13
- OYTNZCBFDXGQGE-XQXXSGGOSA-N Thr-Gln-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](C)C(=O)O)N)O OYTNZCBFDXGQGE-XQXXSGGOSA-N 0.000 description 13
- JRAUIKJSEAKTGD-TUBUOCAGSA-N Thr-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N JRAUIKJSEAKTGD-TUBUOCAGSA-N 0.000 description 13
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 13
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 13
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 13
- XHWCDRUPDNSDAZ-XKBZYTNZSA-N Thr-Ser-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O XHWCDRUPDNSDAZ-XKBZYTNZSA-N 0.000 description 13
- UMFLBPIPAJMNIM-LYARXQMPSA-N Thr-Trp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O)N)O UMFLBPIPAJMNIM-LYARXQMPSA-N 0.000 description 13
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 13
- RERRMBXDSFMBQE-ZFWWWQNUSA-N Trp-Met-Gly Chemical compound CSCC[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N RERRMBXDSFMBQE-ZFWWWQNUSA-N 0.000 description 13
- SMLCYZYQFRTLCO-UWJYBYFXSA-N Tyr-Cys-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O SMLCYZYQFRTLCO-UWJYBYFXSA-N 0.000 description 13
- XDGPTBVOSHKDFT-KKUMJFAQSA-N Tyr-Met-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(O)=O XDGPTBVOSHKDFT-KKUMJFAQSA-N 0.000 description 13
- TYFLVOUZHQUBGM-IHRRRGAJSA-N Tyr-Ser-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 TYFLVOUZHQUBGM-IHRRRGAJSA-N 0.000 description 13
- BUPRFDPUIJNOLS-UFYCRDLUSA-N Tyr-Tyr-Met Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCSC)C(O)=O BUPRFDPUIJNOLS-UFYCRDLUSA-N 0.000 description 13
- VFOHXOLPLACADK-GVXVVHGQSA-N Val-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N VFOHXOLPLACADK-GVXVVHGQSA-N 0.000 description 13
- SUGRIIAOLCDLBD-ZOBUZTSGSA-N Val-Trp-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(=O)O)C(=O)O)N SUGRIIAOLCDLBD-ZOBUZTSGSA-N 0.000 description 13
- 108010038633 aspartylglutamate Proteins 0.000 description 13
- 108010027345 wheylin-1 peptide Proteins 0.000 description 13
- ZDILXFDENZVOTL-BPNCWPANSA-N Ala-Val-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDILXFDENZVOTL-BPNCWPANSA-N 0.000 description 12
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 12
- PRLPSDIHSRITSF-UNQGMJICSA-N Arg-Phe-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PRLPSDIHSRITSF-UNQGMJICSA-N 0.000 description 12
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 12
- LTDGPJKGJDIBQD-LAEOZQHASA-N Asn-Val-Gln Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LTDGPJKGJDIBQD-LAEOZQHASA-N 0.000 description 12
- ALMIMUZAWTUNIO-BZSNNMDCSA-N Asp-Tyr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ALMIMUZAWTUNIO-BZSNNMDCSA-N 0.000 description 12
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 12
- MFVQGXGQRIXBPK-WDSKDSINSA-N Gly-Ala-Glu Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFVQGXGQRIXBPK-WDSKDSINSA-N 0.000 description 12
- CQZDZKRHFWJXDF-WDSKDSINSA-N Gly-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CN CQZDZKRHFWJXDF-WDSKDSINSA-N 0.000 description 12
- UGTHTQWIQKEDEH-BQBZGAKWSA-N L-alanyl-L-prolylglycine zwitterion Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O UGTHTQWIQKEDEH-BQBZGAKWSA-N 0.000 description 12
- DRRXXZBXDMLGFC-IHRRRGAJSA-N Lys-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN DRRXXZBXDMLGFC-IHRRRGAJSA-N 0.000 description 12
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 12
- BTPAWKABYQMKKN-LKXGYXEUSA-N Ser-Asp-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BTPAWKABYQMKKN-LKXGYXEUSA-N 0.000 description 12
- KMWFXJCGRXBQAC-CIUDSAMLSA-N Ser-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N KMWFXJCGRXBQAC-CIUDSAMLSA-N 0.000 description 12
- HAYADTTXNZFUDM-IHRRRGAJSA-N Ser-Tyr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O HAYADTTXNZFUDM-IHRRRGAJSA-N 0.000 description 12
- MXNAOGFNFNKUPD-JHYOHUSXSA-N Thr-Phe-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MXNAOGFNFNKUPD-JHYOHUSXSA-N 0.000 description 12
- IEZVHOULSUULHD-XGEHTFHBSA-N Thr-Ser-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O IEZVHOULSUULHD-XGEHTFHBSA-N 0.000 description 12
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 12
- ZPFLBLFITJCBTP-QWRGUYRKSA-N Tyr-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O ZPFLBLFITJCBTP-QWRGUYRKSA-N 0.000 description 12
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 12
- PWRITNSESKQTPW-NRPADANISA-N Val-Gln-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N PWRITNSESKQTPW-NRPADANISA-N 0.000 description 12
- KZKMBGXCNLPYKD-YEPSODPASA-N Val-Gly-Thr Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O KZKMBGXCNLPYKD-YEPSODPASA-N 0.000 description 12
- 108010037850 glycylvaline Proteins 0.000 description 12
- 108010024607 phenylalanylalanine Proteins 0.000 description 12
- 108010070643 prolylglutamic acid Proteins 0.000 description 12
- 108010029020 prolylglycine Proteins 0.000 description 12
- 102000004169 proteins and genes Human genes 0.000 description 12
- 108010061238 threonyl-glycine Proteins 0.000 description 12
- ROLXPVQSRCPVGK-XDTLVQLUSA-N Ala-Glu-Tyr Chemical compound N[C@@H](C)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O ROLXPVQSRCPVGK-XDTLVQLUSA-N 0.000 description 11
- CBWCQCANJSGUOH-ZKWXMUAHSA-N Asn-Val-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O CBWCQCANJSGUOH-ZKWXMUAHSA-N 0.000 description 11
- SFRQEQGPRTVDPO-NRPADANISA-N Cys-Gln-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O SFRQEQGPRTVDPO-NRPADANISA-N 0.000 description 11
- BJVBMSTUUWGZKX-JYJNAYRXSA-N Gln-Tyr-His Chemical compound N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O BJVBMSTUUWGZKX-JYJNAYRXSA-N 0.000 description 11
- IWAXHBCACVWNHT-BQBZGAKWSA-N Gly-Asp-Arg Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IWAXHBCACVWNHT-BQBZGAKWSA-N 0.000 description 11
- GNPVTZJUUBPZKW-WDSKDSINSA-N Gly-Gln-Ser Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GNPVTZJUUBPZKW-WDSKDSINSA-N 0.000 description 11
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 11
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 11
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 11
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 11
- ZGKVPOSSTGHJAF-HJPIBITLSA-N Ile-Tyr-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CO)C(=O)O)N ZGKVPOSSTGHJAF-HJPIBITLSA-N 0.000 description 11
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 11
- SKRGVGLIRUGANF-AVGNSLFASA-N Lys-Leu-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SKRGVGLIRUGANF-AVGNSLFASA-N 0.000 description 11
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 11
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 11
- FSPGBMWPNMRWDB-AVGNSLFASA-N Phe-Cys-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N FSPGBMWPNMRWDB-AVGNSLFASA-N 0.000 description 11
- IILUKIJNFMUBNF-IHRRRGAJSA-N Phe-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O IILUKIJNFMUBNF-IHRRRGAJSA-N 0.000 description 11
- FQUUYTNBMIBOHS-IHRRRGAJSA-N Phe-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N FQUUYTNBMIBOHS-IHRRRGAJSA-N 0.000 description 11
- CDVFZMOFNJPUDD-ACZMJKKPSA-N Ser-Gln-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CDVFZMOFNJPUDD-ACZMJKKPSA-N 0.000 description 11
- CKDXFSPMIDSMGV-GUBZILKMSA-N Ser-Pro-Val Chemical compound [H]N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O CKDXFSPMIDSMGV-GUBZILKMSA-N 0.000 description 11
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 11
- FGBLCMLXHRPVOF-IHRRRGAJSA-N Ser-Tyr-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FGBLCMLXHRPVOF-IHRRRGAJSA-N 0.000 description 11
- MQCPGOZXFSYJPS-KZVJFYERSA-N Thr-Ala-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MQCPGOZXFSYJPS-KZVJFYERSA-N 0.000 description 11
- DIPIPFHFLPTCLK-LOKLDPHHSA-N Thr-Gln-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N)O DIPIPFHFLPTCLK-LOKLDPHHSA-N 0.000 description 11
- DIHPMRTXPYMDJZ-KAOXEZKKSA-N Thr-Tyr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N)O DIHPMRTXPYMDJZ-KAOXEZKKSA-N 0.000 description 11
- XLMDWQNAOKLKCP-XDTLVQLUSA-N Tyr-Ala-Gln Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N XLMDWQNAOKLKCP-XDTLVQLUSA-N 0.000 description 11
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 11
- JIODCDXKCJRMEH-NHCYSSNCSA-N Val-Arg-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N JIODCDXKCJRMEH-NHCYSSNCSA-N 0.000 description 11
- ZXYPHBKIZLAQTL-QXEWZRGKSA-N Val-Pro-Asp Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZXYPHBKIZLAQTL-QXEWZRGKSA-N 0.000 description 11
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 11
- 150000001413 amino acids Chemical class 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 108010064235 lysylglycine Proteins 0.000 description 11
- 108010044292 tryptophyltyrosine Proteins 0.000 description 11
- MREVELMMFOLESM-HOCLYGCPSA-N Gly-Trp-Val Chemical compound [H]NCC(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C(C)C)C(O)=O MREVELMMFOLESM-HOCLYGCPSA-N 0.000 description 10
- UAVQIQOOBXFKRC-BYULHYEWSA-N Ile-Asn-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O UAVQIQOOBXFKRC-BYULHYEWSA-N 0.000 description 10
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 10
- ODRREERHVHMIPT-OEAJRASXSA-N Leu-Thr-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ODRREERHVHMIPT-OEAJRASXSA-N 0.000 description 10
- GHQFLTYXGUETFD-UFYCRDLUSA-N Met-Tyr-Tyr Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N GHQFLTYXGUETFD-UFYCRDLUSA-N 0.000 description 10
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 10
- HUPLKEHTTQBXSC-YJRXYDGGSA-N Thr-Ser-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUPLKEHTTQBXSC-YJRXYDGGSA-N 0.000 description 10
- ARKBYVBCEOWRNR-UBHSHLNASA-N Trp-Ser-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O ARKBYVBCEOWRNR-UBHSHLNASA-N 0.000 description 10
- NKUGCYDFQKFVOJ-JYJNAYRXSA-N Tyr-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NKUGCYDFQKFVOJ-JYJNAYRXSA-N 0.000 description 10
- BZWUSZGQOILYEU-STECZYCISA-N Val-Ile-Tyr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BZWUSZGQOILYEU-STECZYCISA-N 0.000 description 10
- 238000004113 cell culture Methods 0.000 description 10
- AWAXZRDKUHOPBO-GUBZILKMSA-N Ala-Gln-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O AWAXZRDKUHOPBO-GUBZILKMSA-N 0.000 description 9
- BJNUAWGXPSHQMJ-DCAQKATOSA-N Arg-Gln-Met Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O BJNUAWGXPSHQMJ-DCAQKATOSA-N 0.000 description 9
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 9
- LTUVYLVIZHJCOQ-KKUMJFAQSA-N Glu-Arg-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LTUVYLVIZHJCOQ-KKUMJFAQSA-N 0.000 description 9
- SYAYROHMAIHWFB-KBIXCLLPSA-N Glu-Ser-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O SYAYROHMAIHWFB-KBIXCLLPSA-N 0.000 description 9
- ZRHDPZAAWLXXIR-SRVKXCTJSA-N Leu-Lys-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O ZRHDPZAAWLXXIR-SRVKXCTJSA-N 0.000 description 9
- PRSBSVAVOQOAMI-BJDJZHNGSA-N Lys-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCCCN PRSBSVAVOQOAMI-BJDJZHNGSA-N 0.000 description 9
- BPMRXBZYPGYPJN-WHFBIAKZSA-N Ser-Gly-Asn Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O BPMRXBZYPGYPJN-WHFBIAKZSA-N 0.000 description 9
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 9
- IOVBCLGAJJXOHK-SRVKXCTJSA-N Ser-His-His Chemical compound C([C@H](NC(=O)[C@H](CO)N)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IOVBCLGAJJXOHK-SRVKXCTJSA-N 0.000 description 9
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 9
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 9
- BKVICMPZWRNWOC-RHYQMDGZSA-N Thr-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)[C@@H](C)O BKVICMPZWRNWOC-RHYQMDGZSA-N 0.000 description 9
- YKZVPMUGEJXEOR-JYJNAYRXSA-N Val-Val-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N YKZVPMUGEJXEOR-JYJNAYRXSA-N 0.000 description 9
- 108010068265 aspartyltyrosine Proteins 0.000 description 9
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 9
- 108010087823 glycyltyrosine Proteins 0.000 description 9
- 108010028295 histidylhistidine Proteins 0.000 description 9
- 108010051242 phenylalanylserine Proteins 0.000 description 9
- 239000013598 vector Substances 0.000 description 9
- JBGSZRYCXBPWGX-BQBZGAKWSA-N Ala-Arg-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CCCN=C(N)N JBGSZRYCXBPWGX-BQBZGAKWSA-N 0.000 description 8
- HJDNZFIYILEIKR-OSUNSFLBSA-N Arg-Ile-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O HJDNZFIYILEIKR-OSUNSFLBSA-N 0.000 description 8
- UPAGTDJAORYMEC-VHWLVUOQSA-N Asn-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC(=O)N)N UPAGTDJAORYMEC-VHWLVUOQSA-N 0.000 description 8
- 102100021935 C-C motif chemokine 26 Human genes 0.000 description 8
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 8
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 8
- CIVXDCMSSFGWAL-YUMQZZPRSA-N Cys-Lys-Gly Chemical compound C(CCN)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CS)N CIVXDCMSSFGWAL-YUMQZZPRSA-N 0.000 description 8
- HNAUFGBKJLTWQE-IFFSRLJSSA-N Gln-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CCC(=O)N)N)O HNAUFGBKJLTWQE-IFFSRLJSSA-N 0.000 description 8
- LCNXZQROPKFGQK-WHFBIAKZSA-N Gly-Asp-Ser Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O LCNXZQROPKFGQK-WHFBIAKZSA-N 0.000 description 8
- KMSGYZQRXPUKGI-BYPYZUCNSA-N Gly-Gly-Asn Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC(N)=O KMSGYZQRXPUKGI-BYPYZUCNSA-N 0.000 description 8
- PDAWDNVHMUKWJR-ZETCQYMHSA-N Gly-Gly-His Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CNC=N1 PDAWDNVHMUKWJR-ZETCQYMHSA-N 0.000 description 8
- AAHSHTLISQUZJL-QSFUFRPTSA-N Gly-Ile-Ile Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AAHSHTLISQUZJL-QSFUFRPTSA-N 0.000 description 8
- BHPQOIPBLYJNAW-NGZCFLSTSA-N Gly-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN BHPQOIPBLYJNAW-NGZCFLSTSA-N 0.000 description 8
- CSMYMGFCEJWALV-WDSKDSINSA-N Gly-Ser-Gln Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(N)=O CSMYMGFCEJWALV-WDSKDSINSA-N 0.000 description 8
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 8
- UXZMINKIEWBEQU-SZMVWBNQSA-N His-Trp-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC3=CN=CN3)N UXZMINKIEWBEQU-SZMVWBNQSA-N 0.000 description 8
- 101000897493 Homo sapiens C-C motif chemokine 26 Proteins 0.000 description 8
- YKZAMJXNJUWFIK-JBDRJPRFSA-N Ile-Ser-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)O)N YKZAMJXNJUWFIK-JBDRJPRFSA-N 0.000 description 8
- ZLFNNVATRMCAKN-ZKWXMUAHSA-N Ile-Ser-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N ZLFNNVATRMCAKN-ZKWXMUAHSA-N 0.000 description 8
- LSXGADJXBDFXQU-DLOVCJGASA-N Phe-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 LSXGADJXBDFXQU-DLOVCJGASA-N 0.000 description 8
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 8
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 8
- PZZJMBYSYAKYPK-UWJYBYFXSA-N Ser-Ala-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O PZZJMBYSYAKYPK-UWJYBYFXSA-N 0.000 description 8
- IQGJAHMZWBTRIF-UBHSHLNASA-N Trp-Asp-Asn Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N IQGJAHMZWBTRIF-UBHSHLNASA-N 0.000 description 8
- OJCISMMNNUNNJA-BZSNNMDCSA-N Tyr-Tyr-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 OJCISMMNNUNNJA-BZSNNMDCSA-N 0.000 description 8
- YMTOEGGOCHVGEH-IHRRRGAJSA-N Val-Lys-Lys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O YMTOEGGOCHVGEH-IHRRRGAJSA-N 0.000 description 8
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 8
- 238000005734 heterodimerization reaction Methods 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- UXJCMQFPDWCHKX-DCAQKATOSA-N Arg-Arg-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O UXJCMQFPDWCHKX-DCAQKATOSA-N 0.000 description 7
- NXDXECQFKHXHAM-HJGDQZAQSA-N Arg-Glu-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NXDXECQFKHXHAM-HJGDQZAQSA-N 0.000 description 7
- ATABBWFGOHKROJ-GUBZILKMSA-N Arg-Pro-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O ATABBWFGOHKROJ-GUBZILKMSA-N 0.000 description 7
- ISJWBVIYRBAXEB-CIUDSAMLSA-N Arg-Ser-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O ISJWBVIYRBAXEB-CIUDSAMLSA-N 0.000 description 7
- GMUOCGCDOYYWPD-FXQIFTODSA-N Asn-Pro-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O GMUOCGCDOYYWPD-FXQIFTODSA-N 0.000 description 7
- DPWDPEVGACCWTC-SRVKXCTJSA-N Asn-Tyr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O DPWDPEVGACCWTC-SRVKXCTJSA-N 0.000 description 7
- NKCZYEDZTKOFBG-GUBZILKMSA-N Gln-Gln-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NKCZYEDZTKOFBG-GUBZILKMSA-N 0.000 description 7
- MADFVRSKEIEZHZ-DCAQKATOSA-N Gln-Gln-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N MADFVRSKEIEZHZ-DCAQKATOSA-N 0.000 description 7
- IKFZXRLDMYWNBU-YUMQZZPRSA-N Gln-Gly-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N IKFZXRLDMYWNBU-YUMQZZPRSA-N 0.000 description 7
- NPSWCZIRBAYNSB-JHEQGTHGSA-N Gly-Gln-Thr Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NPSWCZIRBAYNSB-JHEQGTHGSA-N 0.000 description 7
- IALQAMYQJBZNSK-WHFBIAKZSA-N Gly-Ser-Asn Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O IALQAMYQJBZNSK-WHFBIAKZSA-N 0.000 description 7
- FGPLUIQCSKGLTI-WDSKDSINSA-N Gly-Ser-Glu Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O FGPLUIQCSKGLTI-WDSKDSINSA-N 0.000 description 7
- CQMFNTVQVLQRLT-JHEQGTHGSA-N Gly-Thr-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O CQMFNTVQVLQRLT-JHEQGTHGSA-N 0.000 description 7
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 7
- BQIIHAGJIYOQBP-YFYLHZKVSA-N Ile-Trp-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N3CCC[C@@H]3C(=O)O)N BQIIHAGJIYOQBP-YFYLHZKVSA-N 0.000 description 7
- QDSKNVXKLPQNOJ-GVXVVHGQSA-N Leu-Gln-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O QDSKNVXKLPQNOJ-GVXVVHGQSA-N 0.000 description 7
- FKQPWMZLIIATBA-AJNGGQMLSA-N Leu-Lys-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FKQPWMZLIIATBA-AJNGGQMLSA-N 0.000 description 7
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 7
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 7
- UZVKFARGHHMQGX-IUCAKERBSA-N Met-Gly-Met Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCSC UZVKFARGHHMQGX-IUCAKERBSA-N 0.000 description 7
- LVVBAKCGXXUHFO-ZLUOBGJFSA-N Ser-Ala-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O LVVBAKCGXXUHFO-ZLUOBGJFSA-N 0.000 description 7
- JGUWRQWULDWNCM-FXQIFTODSA-N Ser-Val-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O JGUWRQWULDWNCM-FXQIFTODSA-N 0.000 description 7
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 7
- 210000001744 T-lymphocyte Anatomy 0.000 description 7
- GLQFKOVWXPPFTP-VEVYYDQMSA-N Thr-Arg-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O GLQFKOVWXPPFTP-VEVYYDQMSA-N 0.000 description 7
- FDQXPJCLVPFKJW-KJEVXHAQSA-N Thr-Met-Tyr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N)O FDQXPJCLVPFKJW-KJEVXHAQSA-N 0.000 description 7
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 7
- CDRYEAWHKJSGAF-BPNCWPANSA-N Tyr-Ala-Met Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O CDRYEAWHKJSGAF-BPNCWPANSA-N 0.000 description 7
- XYNFFTNEQDWZNY-ULQDDVLXSA-N Tyr-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N XYNFFTNEQDWZNY-ULQDDVLXSA-N 0.000 description 7
- HZWPGKAKGYJWCI-ULQDDVLXSA-N Tyr-Val-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(O)=O HZWPGKAKGYJWCI-ULQDDVLXSA-N 0.000 description 7
- JTWIMNMUYLQNPI-WPRPVWTQSA-N Val-Gly-Arg Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N JTWIMNMUYLQNPI-WPRPVWTQSA-N 0.000 description 7
- JQTYTBPCSOAZHI-FXQIFTODSA-N Val-Ser-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N JQTYTBPCSOAZHI-FXQIFTODSA-N 0.000 description 7
- USXYVSTVPHELAF-RCWTZXSCSA-N Val-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N)O USXYVSTVPHELAF-RCWTZXSCSA-N 0.000 description 7
- 241000700605 Viruses Species 0.000 description 7
- 230000000890 antigenic effect Effects 0.000 description 7
- 108010077245 asparaginyl-proline Proteins 0.000 description 7
- 230000001472 cytotoxic effect Effects 0.000 description 7
- 108010050848 glycylleucine Proteins 0.000 description 7
- 108010003700 lysyl aspartic acid Proteins 0.000 description 7
- GJLXVWOMRRWCIB-MERZOTPQSA-N (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanamide Chemical compound C([C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(N)=O)C1=CC=C(O)C=C1 GJLXVWOMRRWCIB-MERZOTPQSA-N 0.000 description 6
- PPINMSZPTPRQQB-NHCYSSNCSA-N 2-[[(2s)-1-[(2s)-2-[[(2s)-2-amino-3-methylbutanoyl]amino]propanoyl]pyrrolidine-2-carbonyl]amino]acetic acid Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PPINMSZPTPRQQB-NHCYSSNCSA-N 0.000 description 6
- MPLOSMWGDNJSEV-WHFBIAKZSA-N Ala-Gly-Asp Chemical compound [H]N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O MPLOSMWGDNJSEV-WHFBIAKZSA-N 0.000 description 6
- RMAWDDRDTRSZIR-ZLUOBGJFSA-N Ala-Ser-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O RMAWDDRDTRSZIR-ZLUOBGJFSA-N 0.000 description 6
- RTZCUEHYUQZIDE-WHFBIAKZSA-N Ala-Ser-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RTZCUEHYUQZIDE-WHFBIAKZSA-N 0.000 description 6
- ZJLORAAXDAJLDC-CQDKDKBSSA-N Ala-Tyr-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O ZJLORAAXDAJLDC-CQDKDKBSSA-N 0.000 description 6
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 6
- CNBIWSCSSCAINS-UFYCRDLUSA-N Arg-Tyr-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CNBIWSCSSCAINS-UFYCRDLUSA-N 0.000 description 6
- IOTKDTZEEBZNCM-UGYAYLCHSA-N Asn-Asn-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOTKDTZEEBZNCM-UGYAYLCHSA-N 0.000 description 6
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- IGNGBUVODQLMRJ-CIUDSAMLSA-N Gln-Ala-Met Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O IGNGBUVODQLMRJ-CIUDSAMLSA-N 0.000 description 6
- QYTKAVBFRUGYAU-ACZMJKKPSA-N Gln-Asp-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O QYTKAVBFRUGYAU-ACZMJKKPSA-N 0.000 description 6
- CVRUVYDNRPSKBM-QEJZJMRPSA-N Gln-Trp-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N CVRUVYDNRPSKBM-QEJZJMRPSA-N 0.000 description 6
- QGWXAMDECCKGRU-XVKPBYJWSA-N Gln-Val-Gly Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(N)=O)C(=O)NCC(O)=O QGWXAMDECCKGRU-XVKPBYJWSA-N 0.000 description 6
- FBEJIDRSQCGFJI-GUBZILKMSA-N Glu-Leu-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O FBEJIDRSQCGFJI-GUBZILKMSA-N 0.000 description 6
- WKJKBELXHCTHIJ-WPRPVWTQSA-N Gly-Arg-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCN=C(N)N WKJKBELXHCTHIJ-WPRPVWTQSA-N 0.000 description 6
- QPDUVFSVVAOUHE-XVKPBYJWSA-N Gly-Gln-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)CN)C(O)=O QPDUVFSVVAOUHE-XVKPBYJWSA-N 0.000 description 6
- RWYCOSAAAJBJQL-KCTSRDHCSA-N Ile-Gly-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N RWYCOSAAAJBJQL-KCTSRDHCSA-N 0.000 description 6
- SVBAHOMTJRFSIC-SXTJYALSSA-N Ile-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(=O)N)C(=O)O)N SVBAHOMTJRFSIC-SXTJYALSSA-N 0.000 description 6
- LZHJZLHSRGWBBE-IHRRRGAJSA-N Leu-Lys-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O LZHJZLHSRGWBBE-IHRRRGAJSA-N 0.000 description 6
- LZWNAOIMTLNMDW-NHCYSSNCSA-N Lys-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N LZWNAOIMTLNMDW-NHCYSSNCSA-N 0.000 description 6
- PIXVFCBYEGPZPA-JYJNAYRXSA-N Lys-Phe-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N PIXVFCBYEGPZPA-JYJNAYRXSA-N 0.000 description 6
- ABHVWYPPHDYFNY-WDSOQIARSA-N Met-His-Trp Chemical compound C([C@H](NC(=O)[C@@H](N)CCSC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C1=CN=CN1 ABHVWYPPHDYFNY-WDSOQIARSA-N 0.000 description 6
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 6
- ULIWFCCJIOEHMU-BQBZGAKWSA-N Pro-Gly-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 ULIWFCCJIOEHMU-BQBZGAKWSA-N 0.000 description 6
- HEQPKICPPDOSIN-SRVKXCTJSA-N Ser-Asp-Tyr Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HEQPKICPPDOSIN-SRVKXCTJSA-N 0.000 description 6
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 6
- LRZLZIUXQBIWTB-KATARQTJSA-N Ser-Lys-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LRZLZIUXQBIWTB-KATARQTJSA-N 0.000 description 6
- FZEUTKVQGMVGHW-AVGNSLFASA-N Ser-Phe-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O FZEUTKVQGMVGHW-AVGNSLFASA-N 0.000 description 6
- OSFZCEQJLWCIBG-BZSNNMDCSA-N Ser-Tyr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OSFZCEQJLWCIBG-BZSNNMDCSA-N 0.000 description 6
- YEDSOSIKVUMIJE-DCAQKATOSA-N Ser-Val-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O YEDSOSIKVUMIJE-DCAQKATOSA-N 0.000 description 6
- KPNSNVTUVKSBFL-ZJDVBMNYSA-N Thr-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KPNSNVTUVKSBFL-ZJDVBMNYSA-N 0.000 description 6
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 6
- CURFABYITJVKEW-QTKMDUPCSA-N Thr-Val-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O CURFABYITJVKEW-QTKMDUPCSA-N 0.000 description 6
- RNDWCRUOGGQDKN-UBHSHLNASA-N Trp-Ser-Asp Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O RNDWCRUOGGQDKN-UBHSHLNASA-N 0.000 description 6
- XGJLNBNZNMVJRS-NRPADANISA-N Val-Glu-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O XGJLNBNZNMVJRS-NRPADANISA-N 0.000 description 6
- DEGUERSKQBRZMZ-FXQIFTODSA-N Val-Ser-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DEGUERSKQBRZMZ-FXQIFTODSA-N 0.000 description 6
- PDASTHRLDFOZMG-JYJNAYRXSA-N Val-Tyr-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=C(O)C=C1 PDASTHRLDFOZMG-JYJNAYRXSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 239000012228 culture supernatant Substances 0.000 description 6
- 108010087914 epidermal growth factor receptor VIII Proteins 0.000 description 6
- 108010056582 methionylglutamic acid Proteins 0.000 description 6
- 108091033319 polynucleotide Proteins 0.000 description 6
- 102000040430 polynucleotide Human genes 0.000 description 6
- 239000002157 polynucleotide Substances 0.000 description 6
- 108010077112 prolyl-proline Proteins 0.000 description 6
- 108010031719 prolyl-serine Proteins 0.000 description 6
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 6
- FVNAUOZKIPAYNA-BPNCWPANSA-N Ala-Met-Tyr Chemical compound CSCC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 FVNAUOZKIPAYNA-BPNCWPANSA-N 0.000 description 5
- MUGAESARFRGOTQ-IGNZVWTISA-N Ala-Tyr-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N MUGAESARFRGOTQ-IGNZVWTISA-N 0.000 description 5
- YSUVMPICYVWRBX-VEVYYDQMSA-N Arg-Asp-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YSUVMPICYVWRBX-VEVYYDQMSA-N 0.000 description 5
- WVNFNPGXYADPPO-BQBZGAKWSA-N Arg-Gly-Ser Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O WVNFNPGXYADPPO-BQBZGAKWSA-N 0.000 description 5
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 5
- WSXDIZFNQYTUJB-SRVKXCTJSA-N Asp-His-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O WSXDIZFNQYTUJB-SRVKXCTJSA-N 0.000 description 5
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 5
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- CLPQUWHBWXFJOX-BQBZGAKWSA-N Gln-Gly-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O CLPQUWHBWXFJOX-BQBZGAKWSA-N 0.000 description 5
- ZMXZGYLINVNTKH-DZKIICNBSA-N Gln-Val-Phe Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ZMXZGYLINVNTKH-DZKIICNBSA-N 0.000 description 5
- FTMLQFPULNGION-ZVZYQTTQSA-N Gln-Val-Trp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O FTMLQFPULNGION-ZVZYQTTQSA-N 0.000 description 5
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 5
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 5
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 5
- LBRCLQMZAHRTLV-ZKWXMUAHSA-N Ile-Gly-Ser Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LBRCLQMZAHRTLV-ZKWXMUAHSA-N 0.000 description 5
- LZDNBBYBDGBADK-UHFFFAOYSA-N L-valyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C(C)C)C(O)=O)=CNC2=C1 LZDNBBYBDGBADK-UHFFFAOYSA-N 0.000 description 5
- QUYCUALODHJQLK-CIUDSAMLSA-N Lys-Asp-Asp Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O QUYCUALODHJQLK-CIUDSAMLSA-N 0.000 description 5
- FHIAJWBDZVHLAH-YUMQZZPRSA-N Lys-Gly-Ser Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FHIAJWBDZVHLAH-YUMQZZPRSA-N 0.000 description 5
- PPHLBTXVBJNKOB-FDARSICLSA-N Met-Ile-Trp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O PPHLBTXVBJNKOB-FDARSICLSA-N 0.000 description 5
- PNHRPOWKRRJATF-IHRRRGAJSA-N Met-Tyr-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 PNHRPOWKRRJATF-IHRRRGAJSA-N 0.000 description 5
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 5
- 108010066427 N-valyltryptophan Proteins 0.000 description 5
- UIMCLYYSUCIUJM-UWVGGRQHSA-N Pro-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 UIMCLYYSUCIUJM-UWVGGRQHSA-N 0.000 description 5
- ZUGXSSFMTXKHJS-ZLUOBGJFSA-N Ser-Ala-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O ZUGXSSFMTXKHJS-ZLUOBGJFSA-N 0.000 description 5
- OHKFXGKHSJKKAL-NRPADANISA-N Ser-Glu-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OHKFXGKHSJKKAL-NRPADANISA-N 0.000 description 5
- LDKDSFQSEUOCOO-RPTUDFQQSA-N Tyr-Thr-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LDKDSFQSEUOCOO-RPTUDFQQSA-N 0.000 description 5
- 102100033178 Vascular endothelial growth factor receptor 1 Human genes 0.000 description 5
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 108010043240 arginyl-leucyl-glycine Proteins 0.000 description 5
- 238000006471 dimerization reaction Methods 0.000 description 5
- 108010049041 glutamylalanine Proteins 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 108010009962 valyltyrosine Proteins 0.000 description 5
- WDIYWDJLXOCGRW-ACZMJKKPSA-N Ala-Asp-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WDIYWDJLXOCGRW-ACZMJKKPSA-N 0.000 description 4
- NBTGEURICRTMGL-WHFBIAKZSA-N Ala-Gly-Ser Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O NBTGEURICRTMGL-WHFBIAKZSA-N 0.000 description 4
- VHEVVUZDDUCAKU-FXQIFTODSA-N Ala-Met-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(O)=O VHEVVUZDDUCAKU-FXQIFTODSA-N 0.000 description 4
- YHBDGLZYNIARKJ-GUBZILKMSA-N Ala-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N YHBDGLZYNIARKJ-GUBZILKMSA-N 0.000 description 4
- VNFSAYFQLXPHPY-CIQUZCHMSA-N Ala-Thr-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VNFSAYFQLXPHPY-CIQUZCHMSA-N 0.000 description 4
- 108010088751 Albumins Proteins 0.000 description 4
- 102000009027 Albumins Human genes 0.000 description 4
- RYEWQKQXRJCHIO-SRVKXCTJSA-N Asp-Asn-Tyr Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 RYEWQKQXRJCHIO-SRVKXCTJSA-N 0.000 description 4
- CELPEWWLSXMVPH-CIUDSAMLSA-N Asp-Asp-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O CELPEWWLSXMVPH-CIUDSAMLSA-N 0.000 description 4
- BOXNGMVEVOGXOJ-UBHSHLNASA-N Asp-Trp-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC(=O)O)N BOXNGMVEVOGXOJ-UBHSHLNASA-N 0.000 description 4
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 4
- LVRKAFPPFJRIOF-GARJFASQSA-N Gln-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N LVRKAFPPFJRIOF-GARJFASQSA-N 0.000 description 4
- AYBKPDHHVADEDA-YUMQZZPRSA-N Gly-His-Asn Chemical compound [H]NCC(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O AYBKPDHHVADEDA-YUMQZZPRSA-N 0.000 description 4
- FOCSWPCHUDVNLP-PMVMPFDFSA-N His-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC4=CN=CN4)N FOCSWPCHUDVNLP-PMVMPFDFSA-N 0.000 description 4
- FUKDBQGFSJUXGX-RWMBFGLXSA-N Lys-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCCN)N)C(=O)O FUKDBQGFSJUXGX-RWMBFGLXSA-N 0.000 description 4
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 4
- BRKHVZNDAOMAHX-BIIVOSGPSA-N Ser-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N BRKHVZNDAOMAHX-BIIVOSGPSA-N 0.000 description 4
- CTLVSHXLRVEILB-UBHSHLNASA-N Ser-Asn-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CO)N CTLVSHXLRVEILB-UBHSHLNASA-N 0.000 description 4
- RNFKSBPHLTZHLU-WHFBIAKZSA-N Ser-Cys-Gly Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)NCC(=O)O)N)O RNFKSBPHLTZHLU-WHFBIAKZSA-N 0.000 description 4
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 4
- FBLNYDYPCLFTSP-IXOXFDKPSA-N Ser-Phe-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FBLNYDYPCLFTSP-IXOXFDKPSA-N 0.000 description 4
- PIQRHJQWEPWFJG-UWJYBYFXSA-N Ser-Tyr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O PIQRHJQWEPWFJG-UWJYBYFXSA-N 0.000 description 4
- GFDUZZACIWNMPE-KZVJFYERSA-N Thr-Ala-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O GFDUZZACIWNMPE-KZVJFYERSA-N 0.000 description 4
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 4
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 4
- KIMOCKLJBXHFIN-YLVFBTJISA-N Trp-Ile-Gly Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O)=CNC2=C1 KIMOCKLJBXHFIN-YLVFBTJISA-N 0.000 description 4
- WPVGRKLNHJJCEN-BZSNNMDCSA-N Tyr-Asp-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 WPVGRKLNHJJCEN-BZSNNMDCSA-N 0.000 description 4
- HRHYJNLMIJWGLF-BZSNNMDCSA-N Tyr-Ser-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 HRHYJNLMIJWGLF-BZSNNMDCSA-N 0.000 description 4
- SYFHQHYTNCQCCN-MELADBBJSA-N Tyr-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O SYFHQHYTNCQCCN-MELADBBJSA-N 0.000 description 4
- GBIUHAYJGWVNLN-UHFFFAOYSA-N Val-Ser-Pro Natural products CC(C)C(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O GBIUHAYJGWVNLN-UHFFFAOYSA-N 0.000 description 4
- WUFHZIRMAZZWRS-OSUNSFLBSA-N Val-Thr-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C(C)C)N WUFHZIRMAZZWRS-OSUNSFLBSA-N 0.000 description 4
- 108010044940 alanylglutamine Proteins 0.000 description 4
- 230000003013 cytotoxicity Effects 0.000 description 4
- 231100000135 cytotoxicity Toxicity 0.000 description 4
- 239000013604 expression vector Substances 0.000 description 4
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 4
- 108010085059 glutamyl-arginyl-proline Proteins 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 230000008685 targeting Effects 0.000 description 4
- 108010084932 tryptophyl-proline Proteins 0.000 description 4
- IGXNPQWXIRIGBF-KEOOTSPTSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoic acid Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IGXNPQWXIRIGBF-KEOOTSPTSA-N 0.000 description 3
- -1 APRIL Proteins 0.000 description 3
- HXNNRBHASOSVPG-GUBZILKMSA-N Ala-Glu-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O HXNNRBHASOSVPG-GUBZILKMSA-N 0.000 description 3
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 3
- VQBULXOHAZSTQY-GKCIPKSASA-N Ala-Trp-Phe Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O VQBULXOHAZSTQY-GKCIPKSASA-N 0.000 description 3
- YBZMTKUDWXZLIX-UWVGGRQHSA-N Arg-Leu-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YBZMTKUDWXZLIX-UWVGGRQHSA-N 0.000 description 3
- KXEGPPNPXOKKHK-ZLUOBGJFSA-N Asn-Asp-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O KXEGPPNPXOKKHK-ZLUOBGJFSA-N 0.000 description 3
- ZAESWDKAMDVHLL-RCOVLWMOSA-N Asn-Val-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O ZAESWDKAMDVHLL-RCOVLWMOSA-N 0.000 description 3
- PBVLJOIPOGUQQP-CIUDSAMLSA-N Asp-Ala-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O PBVLJOIPOGUQQP-CIUDSAMLSA-N 0.000 description 3
- MRQQMVZUHXUPEV-IHRRRGAJSA-N Asp-Arg-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O MRQQMVZUHXUPEV-IHRRRGAJSA-N 0.000 description 3
- JNNVNVRBYUJYGS-CIUDSAMLSA-N Asp-Leu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O JNNVNVRBYUJYGS-CIUDSAMLSA-N 0.000 description 3
- MJJIHRWNWSQTOI-VEVYYDQMSA-N Asp-Thr-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O MJJIHRWNWSQTOI-VEVYYDQMSA-N 0.000 description 3
- NXQCSPVUPLUTJH-WHFBIAKZSA-N Cys-Ser-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O NXQCSPVUPLUTJH-WHFBIAKZSA-N 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108060006698 EGF receptor Proteins 0.000 description 3
- 102000001301 EGF receptor Human genes 0.000 description 3
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 3
- RWQCWSGOOOEGPB-FXQIFTODSA-N Gln-Ser-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O RWQCWSGOOOEGPB-FXQIFTODSA-N 0.000 description 3
- ZSIDREAPEPAPKL-XIRDDKMYSA-N Glu-Trp-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCC(=O)O)N ZSIDREAPEPAPKL-XIRDDKMYSA-N 0.000 description 3
- PMSDOVISAARGAV-FHWLQOOXSA-N Glu-Tyr-Phe Chemical compound C([C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 PMSDOVISAARGAV-FHWLQOOXSA-N 0.000 description 3
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 3
- MYXNLWDWWOTERK-BHNWBGBOSA-N Gly-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN)O MYXNLWDWWOTERK-BHNWBGBOSA-N 0.000 description 3
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 3
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 3
- 108091006905 Human Serum Albumin Proteins 0.000 description 3
- 102000008100 Human Serum Albumin Human genes 0.000 description 3
- RQJUKVXWAKJDBW-SVSWQMSJSA-N Ile-Ser-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N RQJUKVXWAKJDBW-SVSWQMSJSA-N 0.000 description 3
- ZSESFIFAYQEKRD-CYDGBPFRSA-N Ile-Val-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(=O)O)N ZSESFIFAYQEKRD-CYDGBPFRSA-N 0.000 description 3
- WIYDLTIBHZSPKY-HJWJTTGWSA-N Ile-Val-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 WIYDLTIBHZSPKY-HJWJTTGWSA-N 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- 229930182816 L-glutamine Natural products 0.000 description 3
- YSKSXVKQLLBVEX-SZMVWBNQSA-N Leu-Gln-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 YSKSXVKQLLBVEX-SZMVWBNQSA-N 0.000 description 3
- NRFGTHFONZYFNY-MGHWNKPDSA-N Leu-Ile-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NRFGTHFONZYFNY-MGHWNKPDSA-N 0.000 description 3
- VKVDRTGWLVZJOM-DCAQKATOSA-N Leu-Val-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O VKVDRTGWLVZJOM-DCAQKATOSA-N 0.000 description 3
- PNDCUTDWYVKBHX-IHRRRGAJSA-N Met-Asp-Tyr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 PNDCUTDWYVKBHX-IHRRRGAJSA-N 0.000 description 3
- OBPCXINRFKHSRY-SDDRHHMPSA-N Met-Met-Pro Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@@H]1C(=O)O)N OBPCXINRFKHSRY-SDDRHHMPSA-N 0.000 description 3
- VSJAPSMRFYUOKS-IUCAKERBSA-N Met-Pro-Gly Chemical compound CSCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O VSJAPSMRFYUOKS-IUCAKERBSA-N 0.000 description 3
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 3
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- IUVYJBMTHARMIP-PCBIJLKTSA-N Phe-Asp-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O IUVYJBMTHARMIP-PCBIJLKTSA-N 0.000 description 3
- 229920002873 Polyethylenimine Polymers 0.000 description 3
- VYWNORHENYEQDW-YUMQZZPRSA-N Pro-Gly-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 VYWNORHENYEQDW-YUMQZZPRSA-N 0.000 description 3
- INDVYIOKMXFQFM-SRVKXCTJSA-N Pro-Lys-Gln Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O INDVYIOKMXFQFM-SRVKXCTJSA-N 0.000 description 3
- WOIFYRZPIORBRY-AVGNSLFASA-N Pro-Lys-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O WOIFYRZPIORBRY-AVGNSLFASA-N 0.000 description 3
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 3
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 3
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 3
- TYYBJUYSTWJHGO-ZKWXMUAHSA-N Ser-Asn-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O TYYBJUYSTWJHGO-ZKWXMUAHSA-N 0.000 description 3
- SFZKGGOGCNQPJY-CIUDSAMLSA-N Ser-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)N SFZKGGOGCNQPJY-CIUDSAMLSA-N 0.000 description 3
- LQESNKGTTNHZPZ-GHCJXIJMSA-N Ser-Ile-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O LQESNKGTTNHZPZ-GHCJXIJMSA-N 0.000 description 3
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 3
- NERYDXBVARJIQS-JYBASQMISA-N Ser-Trp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CO)N)O NERYDXBVARJIQS-JYBASQMISA-N 0.000 description 3
- 108091008874 T cell receptors Proteins 0.000 description 3
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 3
- PZVGOVRNGKEFCB-KKHAAJSZSA-N Thr-Asn-Val Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)N)O PZVGOVRNGKEFCB-KKHAAJSZSA-N 0.000 description 3
- KRPKYGOFYUNIGM-XVSYOHENSA-N Thr-Asp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O KRPKYGOFYUNIGM-XVSYOHENSA-N 0.000 description 3
- VUKVQVNKIIZBPO-HOUAVDHOSA-N Thr-Asp-Trp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N)O VUKVQVNKIIZBPO-HOUAVDHOSA-N 0.000 description 3
- LIXBDERDAGNVAV-XKBZYTNZSA-N Thr-Gln-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O LIXBDERDAGNVAV-XKBZYTNZSA-N 0.000 description 3
- DJDSEDOKJTZBAR-ZDLURKLDSA-N Thr-Gly-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O DJDSEDOKJTZBAR-ZDLURKLDSA-N 0.000 description 3
- KSVMDJJCYKIXTK-IGNZVWTISA-N Tyr-Ala-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 KSVMDJJCYKIXTK-IGNZVWTISA-N 0.000 description 3
- DYEGCOJHFNJBKB-UFYCRDLUSA-N Tyr-Arg-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 DYEGCOJHFNJBKB-UFYCRDLUSA-N 0.000 description 3
- CVXURBLRELTJKO-BWAGICSOSA-N Tyr-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)O CVXURBLRELTJKO-BWAGICSOSA-N 0.000 description 3
- BIWVVOHTKDLRMP-ULQDDVLXSA-N Tyr-Pro-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O BIWVVOHTKDLRMP-ULQDDVLXSA-N 0.000 description 3
- DJEVQCWNMQOABE-RCOVLWMOSA-N Val-Gly-Asp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)O)C(=O)O)N DJEVQCWNMQOABE-RCOVLWMOSA-N 0.000 description 3
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 3
- 108010005233 alanylglutamic acid Proteins 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- 230000006037 cell lysis Effects 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 231100000050 cytotoxic potential Toxicity 0.000 description 3
- 238000002784 cytotoxicity assay Methods 0.000 description 3
- 231100000263 cytotoxicity test Toxicity 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 108010084389 glycyltryptophan Proteins 0.000 description 3
- 239000000833 heterodimer Substances 0.000 description 3
- 108010025306 histidylleucine Proteins 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 238000009169 immunotherapy Methods 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 108010057821 leucylproline Proteins 0.000 description 3
- 108010005942 methionylglycine Proteins 0.000 description 3
- 238000002823 phage display Methods 0.000 description 3
- 108010090894 prolylleucine Proteins 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 230000002269 spontaneous effect Effects 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- OZRFYUJEXYKQDV-UHFFFAOYSA-N 2-[[2-[[2-[(2-amino-3-carboxypropanoyl)amino]-3-carboxypropanoyl]amino]-3-carboxypropanoyl]amino]butanedioic acid Chemical compound OC(=O)CC(N)C(=O)NC(CC(O)=O)C(=O)NC(CC(O)=O)C(=O)NC(CC(O)=O)C(O)=O OZRFYUJEXYKQDV-UHFFFAOYSA-N 0.000 description 2
- XWTNPSHCJMZAHQ-QMMMGPOBSA-N 2-[[2-[[2-[[(2s)-2-amino-4-methylpentanoyl]amino]acetyl]amino]acetyl]amino]acetic acid Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(=O)NCC(O)=O XWTNPSHCJMZAHQ-QMMMGPOBSA-N 0.000 description 2
- UCIYCBSJBQGDGM-LPEHRKFASA-N Ala-Arg-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N UCIYCBSJBQGDGM-LPEHRKFASA-N 0.000 description 2
- LSLIRHLIUDVNBN-CIUDSAMLSA-N Ala-Asp-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN LSLIRHLIUDVNBN-CIUDSAMLSA-N 0.000 description 2
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 2
- YYAVDNKUWLAFCV-ACZMJKKPSA-N Ala-Ser-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O YYAVDNKUWLAFCV-ACZMJKKPSA-N 0.000 description 2
- SYIFFFHSXBNPMC-UWJYBYFXSA-N Ala-Ser-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N SYIFFFHSXBNPMC-UWJYBYFXSA-N 0.000 description 2
- GIVWETPOBCRTND-DCAQKATOSA-N Arg-Gln-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O GIVWETPOBCRTND-DCAQKATOSA-N 0.000 description 2
- XZFONYMRYTVLPL-NHCYSSNCSA-N Asn-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N XZFONYMRYTVLPL-NHCYSSNCSA-N 0.000 description 2
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 2
- JDHOJQJMWBKHDB-CIUDSAMLSA-N Asp-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC(=O)O)N JDHOJQJMWBKHDB-CIUDSAMLSA-N 0.000 description 2
- KYQNAIMCTRZLNP-QSFUFRPTSA-N Asp-Ile-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O KYQNAIMCTRZLNP-QSFUFRPTSA-N 0.000 description 2
- BRRPVTUFESPTCP-ACZMJKKPSA-N Asp-Ser-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O BRRPVTUFESPTCP-ACZMJKKPSA-N 0.000 description 2
- MGSVBZIBCCKGCY-ZLUOBGJFSA-N Asp-Ser-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MGSVBZIBCCKGCY-ZLUOBGJFSA-N 0.000 description 2
- BJDHEININLSZOT-KKUMJFAQSA-N Asp-Tyr-Lys Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(O)=O BJDHEININLSZOT-KKUMJFAQSA-N 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- BPHKULHWEIUDOB-FXQIFTODSA-N Cys-Gln-Gln Chemical compound SC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O BPHKULHWEIUDOB-FXQIFTODSA-N 0.000 description 2
- 108010046276 FLP recombinase Proteins 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- RZSLYUUFFVHFRQ-FXQIFTODSA-N Gln-Ala-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O RZSLYUUFFVHFRQ-FXQIFTODSA-N 0.000 description 2
- LOJYQMFIIJVETK-WDSKDSINSA-N Gln-Gln Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(O)=O LOJYQMFIIJVETK-WDSKDSINSA-N 0.000 description 2
- ILKYYKRAULNYMS-JYJNAYRXSA-N Gln-Lys-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ILKYYKRAULNYMS-JYJNAYRXSA-N 0.000 description 2
- WLRYGVYQFXRJDA-DCAQKATOSA-N Gln-Pro-Pro Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 WLRYGVYQFXRJDA-DCAQKATOSA-N 0.000 description 2
- KUBFPYIMAGXGBT-ACZMJKKPSA-N Gln-Ser-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KUBFPYIMAGXGBT-ACZMJKKPSA-N 0.000 description 2
- WTJIWXMJESRHMM-XDTLVQLUSA-N Gln-Tyr-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O WTJIWXMJESRHMM-XDTLVQLUSA-N 0.000 description 2
- UTKUTMJSWKKHEM-WDSKDSINSA-N Glu-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O UTKUTMJSWKKHEM-WDSKDSINSA-N 0.000 description 2
- VPKBCVUDBNINAH-GARJFASQSA-N Glu-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)O)N)C(=O)O VPKBCVUDBNINAH-GARJFASQSA-N 0.000 description 2
- JVSBYEDSSRZQGV-GUBZILKMSA-N Glu-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(O)=O JVSBYEDSSRZQGV-GUBZILKMSA-N 0.000 description 2
- JRCUFCXYZLPSDZ-ACZMJKKPSA-N Glu-Asp-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O JRCUFCXYZLPSDZ-ACZMJKKPSA-N 0.000 description 2
- QSTLUOIOYLYLLF-WDSKDSINSA-N Gly-Asp-Glu Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QSTLUOIOYLYLLF-WDSKDSINSA-N 0.000 description 2
- LPHQAFLNEHWKFF-QXEWZRGKSA-N Gly-Met-Ile Chemical compound [H]NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LPHQAFLNEHWKFF-QXEWZRGKSA-N 0.000 description 2
- JQFILXICXLDTRR-FBCQKBJTSA-N Gly-Thr-Gly Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)NCC(O)=O JQFILXICXLDTRR-FBCQKBJTSA-N 0.000 description 2
- BXDLTKLPPKBVEL-FJXKBIBVSA-N Gly-Thr-Met Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(O)=O BXDLTKLPPKBVEL-FJXKBIBVSA-N 0.000 description 2
- NGRPGJGKJMUGDM-XVKPBYJWSA-N Gly-Val-Gln Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O NGRPGJGKJMUGDM-XVKPBYJWSA-N 0.000 description 2
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- UROVZOUMHNXPLZ-AVGNSLFASA-N His-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 UROVZOUMHNXPLZ-AVGNSLFASA-N 0.000 description 2
- DEMIXZCKUXVEBO-BWAGICSOSA-N His-Thr-Tyr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N)O DEMIXZCKUXVEBO-BWAGICSOSA-N 0.000 description 2
- 101000878602 Homo sapiens Immunoglobulin alpha Fc receptor Proteins 0.000 description 2
- 101001034652 Homo sapiens Insulin-like growth factor 1 receptor Proteins 0.000 description 2
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 2
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 2
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 2
- 101000589305 Homo sapiens Natural cytotoxicity triggering receptor 2 Proteins 0.000 description 2
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 2
- NCSIQAFSIPHVAN-IUKAMOBKSA-N Ile-Asn-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N NCSIQAFSIPHVAN-IUKAMOBKSA-N 0.000 description 2
- UAQSZXGJGLHMNV-XEGUGMAKSA-N Ile-Gly-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N UAQSZXGJGLHMNV-XEGUGMAKSA-N 0.000 description 2
- KBAPKNDWAGVGTH-IGISWZIWSA-N Ile-Ile-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 KBAPKNDWAGVGTH-IGISWZIWSA-N 0.000 description 2
- NSPNUMNLZNOPAQ-SJWGOKEGSA-N Ile-Tyr-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N NSPNUMNLZNOPAQ-SJWGOKEGSA-N 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 102100038005 Immunoglobulin alpha Fc receptor Human genes 0.000 description 2
- 102100039688 Insulin-like growth factor 1 receptor Human genes 0.000 description 2
- 102100025390 Integrin beta-2 Human genes 0.000 description 2
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 2
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 2
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 2
- HPBCTWSUJOGJSH-MNXVOIDGSA-N Leu-Glu-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HPBCTWSUJOGJSH-MNXVOIDGSA-N 0.000 description 2
- VGPCJSXPPOQPBK-YUMQZZPRSA-N Leu-Gly-Ser Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O VGPCJSXPPOQPBK-YUMQZZPRSA-N 0.000 description 2
- JKSIBWITFMQTOA-XUXIUFHCSA-N Leu-Ile-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O JKSIBWITFMQTOA-XUXIUFHCSA-N 0.000 description 2
- ZJZNLRVCZWUONM-JXUBOQSCSA-N Leu-Thr-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O ZJZNLRVCZWUONM-JXUBOQSCSA-N 0.000 description 2
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 2
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 2
- NRQRKMYZONPCTM-CIUDSAMLSA-N Lys-Asp-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O NRQRKMYZONPCTM-CIUDSAMLSA-N 0.000 description 2
- CENKQZWVYMLRAX-ULQDDVLXSA-N Lys-Phe-Met Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O CENKQZWVYMLRAX-ULQDDVLXSA-N 0.000 description 2
- VHTOGMKQXXJOHG-RHYQMDGZSA-N Lys-Thr-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O VHTOGMKQXXJOHG-RHYQMDGZSA-N 0.000 description 2
- 108010031099 Mannose Receptor Proteins 0.000 description 2
- 241000204031 Mycoplasma Species 0.000 description 2
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 2
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 2
- 102100032851 Natural cytotoxicity triggering receptor 2 Human genes 0.000 description 2
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 2
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 2
- WSRWHZRUOCACLJ-UWVGGRQHSA-N Pro-Gly-His Chemical compound C([C@@H](C(=O)O)NC(=O)CNC(=O)[C@H]1NCCC1)C1=CN=CN1 WSRWHZRUOCACLJ-UWVGGRQHSA-N 0.000 description 2
- VTFXTWDFPTWNJY-RHYQMDGZSA-N Pro-Leu-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VTFXTWDFPTWNJY-RHYQMDGZSA-N 0.000 description 2
- SMFQZMGHCODUPQ-ULQDDVLXSA-N Pro-Lys-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SMFQZMGHCODUPQ-ULQDDVLXSA-N 0.000 description 2
- HBOABDXGTMMDSE-GUBZILKMSA-N Ser-Arg-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O HBOABDXGTMMDSE-GUBZILKMSA-N 0.000 description 2
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 2
- ILZAUMFXKSIUEF-SRVKXCTJSA-N Ser-Ser-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ILZAUMFXKSIUEF-SRVKXCTJSA-N 0.000 description 2
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 2
- DKGRNFUXVTYRAS-UBHSHLNASA-N Ser-Ser-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DKGRNFUXVTYRAS-UBHSHLNASA-N 0.000 description 2
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 2
- QYBRQMLZDDJBSW-AVGNSLFASA-N Ser-Tyr-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O QYBRQMLZDDJBSW-AVGNSLFASA-N 0.000 description 2
- BIWBTRRBHIEVAH-IHPCNDPISA-N Ser-Tyr-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O BIWBTRRBHIEVAH-IHPCNDPISA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 2
- DGDCHPCRMWEOJR-FQPOAREZSA-N Thr-Ala-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DGDCHPCRMWEOJR-FQPOAREZSA-N 0.000 description 2
- YLXAMFZYJTZXFH-OLHMAJIHSA-N Thr-Asn-Asp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O YLXAMFZYJTZXFH-OLHMAJIHSA-N 0.000 description 2
- GUHLYMZJVXUIPO-RCWTZXSCSA-N Thr-Met-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O GUHLYMZJVXUIPO-RCWTZXSCSA-N 0.000 description 2
- DEGCBBCMYWNJNA-RHYQMDGZSA-N Thr-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)[C@@H](C)O DEGCBBCMYWNJNA-RHYQMDGZSA-N 0.000 description 2
- AKHDFZHUPGVFEJ-YEPSODPASA-N Thr-Val-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AKHDFZHUPGVFEJ-YEPSODPASA-N 0.000 description 2
- BPGDJSUFQKWUBK-KJEVXHAQSA-N Thr-Val-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BPGDJSUFQKWUBK-KJEVXHAQSA-N 0.000 description 2
- BGWSLEYVITZIQP-DCPHZVHLSA-N Trp-Phe-Ala Chemical compound C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(O)=O BGWSLEYVITZIQP-DCPHZVHLSA-N 0.000 description 2
- ZHDQRPWESGUDST-JBACZVJFSA-N Trp-Phe-Gln Chemical compound C([C@H](NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(=O)N[C@@H](CCC(N)=O)C(O)=O)C1=CC=CC=C1 ZHDQRPWESGUDST-JBACZVJFSA-N 0.000 description 2
- WMIUTJPFHMMUGY-ZFWWWQNUSA-N Trp-Pro-Gly Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)N)C(=O)NCC(=O)O WMIUTJPFHMMUGY-ZFWWWQNUSA-N 0.000 description 2
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 2
- NZFCWALTLNFHHC-JYJNAYRXSA-N Tyr-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NZFCWALTLNFHHC-JYJNAYRXSA-N 0.000 description 2
- HSBZWINKRYZCSQ-KKUMJFAQSA-N Tyr-Lys-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O HSBZWINKRYZCSQ-KKUMJFAQSA-N 0.000 description 2
- QPBJXNYYQTUTDD-KKUMJFAQSA-N Tyr-Met-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N QPBJXNYYQTUTDD-KKUMJFAQSA-N 0.000 description 2
- VYQQQIRHIFALGE-UWJYBYFXSA-N Tyr-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VYQQQIRHIFALGE-UWJYBYFXSA-N 0.000 description 2
- GQVZBMROTPEPIF-SRVKXCTJSA-N Tyr-Ser-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GQVZBMROTPEPIF-SRVKXCTJSA-N 0.000 description 2
- NZBSVMQZQMEUHI-WZLNRYEVSA-N Tyr-Thr-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N NZBSVMQZQMEUHI-WZLNRYEVSA-N 0.000 description 2
- KUXCBJFJURINGF-PXDAIIFMSA-N Tyr-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC3=CC=C(C=C3)O)N KUXCBJFJURINGF-PXDAIIFMSA-N 0.000 description 2
- RZAGEHHVNYESNR-RNXOBYDBSA-N Tyr-Trp-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RZAGEHHVNYESNR-RNXOBYDBSA-N 0.000 description 2
- VJOWWOGRNXRQMF-UVBJJODRSA-N Val-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 VJOWWOGRNXRQMF-UVBJJODRSA-N 0.000 description 2
- QPPZEDOTPZOSEC-RCWTZXSCSA-N Val-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](C(C)C)N)O QPPZEDOTPZOSEC-RCWTZXSCSA-N 0.000 description 2
- LCHZBEUVGAVMKS-RHYQMDGZSA-N Val-Thr-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(O)=O LCHZBEUVGAVMKS-RHYQMDGZSA-N 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 108010011559 alanylphenylalanine Proteins 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000001588 bifunctional effect Effects 0.000 description 2
- DEGAKNSWVGKMLS-UHFFFAOYSA-N calcein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(O)=O)CC(O)=O)=C(O)C=C1OC1=C2C=C(CN(CC(O)=O)CC(=O)O)C(O)=C1 DEGAKNSWVGKMLS-UHFFFAOYSA-N 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 230000003412 degenerative effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 206010015037 epilepsy Diseases 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 210000003714 granulocyte Anatomy 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 239000000710 homodimer Substances 0.000 description 2
- 239000012678 infectious agent Substances 0.000 description 2
- 210000004964 innate lymphoid cell Anatomy 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- 108010073093 leucyl-glycyl-glycyl-glycine Proteins 0.000 description 2
- 238000012417 linear regression Methods 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 229960002378 oftasceine Drugs 0.000 description 2
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 2
- 238000005498 polishing Methods 0.000 description 2
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 108010020532 tyrosyl-proline Proteins 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- OVKKNJPJQKTXIT-JLNKQSITSA-N (5Z,8Z,11Z,14Z,17Z)-icosapentaenoylethanolamine Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCO OVKKNJPJQKTXIT-JLNKQSITSA-N 0.000 description 1
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 1
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 description 1
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 1
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 1
- REAQAWSENITKJL-DDWPSWQVSA-N Ala-Met-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O REAQAWSENITKJL-DDWPSWQVSA-N 0.000 description 1
- BFMIRJBURUXDRG-DLOVCJGASA-N Ala-Phe-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 BFMIRJBURUXDRG-DLOVCJGASA-N 0.000 description 1
- XKXAZPSREVUCRT-BPNCWPANSA-N Ala-Tyr-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CC1=CC=C(O)C=C1 XKXAZPSREVUCRT-BPNCWPANSA-N 0.000 description 1
- DEAGTWNKODHUIY-MRFFXTKBSA-N Ala-Tyr-Trp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DEAGTWNKODHUIY-MRFFXTKBSA-N 0.000 description 1
- QHUOOCKNNURZSL-IHRRRGAJSA-N Arg-Tyr-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O QHUOOCKNNURZSL-IHRRRGAJSA-N 0.000 description 1
- YNDLOUMBVDVALC-ZLUOBGJFSA-N Asn-Ala-Ala Chemical compound C[C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CC(=O)N)N YNDLOUMBVDVALC-ZLUOBGJFSA-N 0.000 description 1
- AYKKKGFJXIDYLX-ACZMJKKPSA-N Asn-Gln-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O AYKKKGFJXIDYLX-ACZMJKKPSA-N 0.000 description 1
- RVHGJNGNKGDCPX-KKUMJFAQSA-N Asn-Phe-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N RVHGJNGNKGDCPX-KKUMJFAQSA-N 0.000 description 1
- NSTBNYOKCZKOMI-AVGNSLFASA-N Asn-Tyr-Glu Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O NSTBNYOKCZKOMI-AVGNSLFASA-N 0.000 description 1
- DGKCOYGQLNWNCJ-ACZMJKKPSA-N Asp-Glu-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O DGKCOYGQLNWNCJ-ACZMJKKPSA-N 0.000 description 1
- UZNSWMFLKVKJLI-VHWLVUOQSA-N Asp-Ile-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O UZNSWMFLKVKJLI-VHWLVUOQSA-N 0.000 description 1
- DWOGMPWRQQWPPF-GUBZILKMSA-N Asp-Leu-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O DWOGMPWRQQWPPF-GUBZILKMSA-N 0.000 description 1
- OAMLVOVXNKILLQ-BQBZGAKWSA-N Asp-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](N)CC(O)=O OAMLVOVXNKILLQ-BQBZGAKWSA-N 0.000 description 1
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 1
- UEFODXNXUAVPTC-VEVYYDQMSA-N Asp-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O UEFODXNXUAVPTC-VEVYYDQMSA-N 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 206010008909 Chronic Hepatitis Diseases 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 102100030886 Complement receptor type 1 Human genes 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 1
- 238000012270 DNA recombination Methods 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 241000725619 Dengue virus Species 0.000 description 1
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- 102000009024 Epidermal Growth Factor Human genes 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- XZWYTXMRWQJBGX-VXBMVYAYSA-N FLAG peptide Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CC=C(O)C=C1 XZWYTXMRWQJBGX-VXBMVYAYSA-N 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 1
- VZRAXPGTUNDIDK-GUBZILKMSA-N Gln-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N VZRAXPGTUNDIDK-GUBZILKMSA-N 0.000 description 1
- CXRWMMRLEMVSEH-PEFMBERDSA-N Glu-Ile-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O CXRWMMRLEMVSEH-PEFMBERDSA-N 0.000 description 1
- NJCALAAIGREHDR-WDCWCFNPSA-N Glu-Leu-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NJCALAAIGREHDR-WDCWCFNPSA-N 0.000 description 1
- JVZLZVJTIXVIHK-SXNHZJKMSA-N Glu-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCC(=O)O)N JVZLZVJTIXVIHK-SXNHZJKMSA-N 0.000 description 1
- BKMOHWJHXQLFEX-IRIUXVKKSA-N Glu-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CCC(=O)O)N)O BKMOHWJHXQLFEX-IRIUXVKKSA-N 0.000 description 1
- 102100036263 Glutamyl-tRNA(Gln) amidotransferase subunit C, mitochondrial Human genes 0.000 description 1
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 1
- XUORRGAFUQIMLC-STQMWFEESA-N Gly-Arg-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CN)O XUORRGAFUQIMLC-STQMWFEESA-N 0.000 description 1
- LURCIJSJAKFCRO-QWRGUYRKSA-N Gly-Asn-Tyr Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O LURCIJSJAKFCRO-QWRGUYRKSA-N 0.000 description 1
- KQDMENMTYNBWMR-WHFBIAKZSA-N Gly-Asp-Ala Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O KQDMENMTYNBWMR-WHFBIAKZSA-N 0.000 description 1
- DGKBSGNCMCLDSL-BYULHYEWSA-N Gly-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)CN DGKBSGNCMCLDSL-BYULHYEWSA-N 0.000 description 1
- FXGRXIATVXUAHO-WEDXCCLWSA-N Gly-Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCCN FXGRXIATVXUAHO-WEDXCCLWSA-N 0.000 description 1
- MKIAPEZXQDILRR-YUMQZZPRSA-N Gly-Ser-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN MKIAPEZXQDILRR-YUMQZZPRSA-N 0.000 description 1
- FKESCSGWBPUTPN-FOHZUACHSA-N Gly-Thr-Asn Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O FKESCSGWBPUTPN-FOHZUACHSA-N 0.000 description 1
- 101800001649 Heparin-binding EGF-like growth factor Proteins 0.000 description 1
- 102400001369 Heparin-binding EGF-like growth factor Human genes 0.000 description 1
- 208000009889 Herpes Simplex Diseases 0.000 description 1
- AWHJQEYGWRKPHE-LSJOCFKGSA-N His-Ala-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AWHJQEYGWRKPHE-LSJOCFKGSA-N 0.000 description 1
- UOAVQQRILDGZEN-SRVKXCTJSA-N His-Asp-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O UOAVQQRILDGZEN-SRVKXCTJSA-N 0.000 description 1
- STOOMQFEJUVAKR-KKUMJFAQSA-N His-His-His Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1N=CNC=1)C(=O)N[C@@H](CC=1N=CNC=1)C(O)=O)C1=CNC=N1 STOOMQFEJUVAKR-KKUMJFAQSA-N 0.000 description 1
- 108010093488 His-His-His-His-His-His Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000727061 Homo sapiens Complement receptor type 1 Proteins 0.000 description 1
- 101000908391 Homo sapiens Dipeptidyl peptidase 4 Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101001001786 Homo sapiens Glutamyl-tRNA(Gln) amidotransferase subunit C, mitochondrial Proteins 0.000 description 1
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101000971538 Homo sapiens Killer cell lectin-like receptor subfamily F member 1 Proteins 0.000 description 1
- 101000868279 Homo sapiens Leukocyte surface antigen CD47 Proteins 0.000 description 1
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 1
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 description 1
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 1
- 101001109503 Homo sapiens NKG2-C type II integral membrane protein Proteins 0.000 description 1
- 101000589301 Homo sapiens Natural cytotoxicity triggering receptor 1 Proteins 0.000 description 1
- 101000971513 Homo sapiens Natural killer cells antigen CD94 Proteins 0.000 description 1
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 1
- 101001098352 Homo sapiens OX-2 membrane glycoprotein Proteins 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 101000904787 Homo sapiens Serine/threonine-protein kinase ATR Proteins 0.000 description 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 description 1
- 101000946860 Homo sapiens T-cell surface glycoprotein CD3 epsilon chain Proteins 0.000 description 1
- 101000795167 Homo sapiens Tumor necrosis factor receptor superfamily member 13B Proteins 0.000 description 1
- 101000801255 Homo sapiens Tumor necrosis factor receptor superfamily member 17 Proteins 0.000 description 1
- 241000598171 Human adenovirus sp. Species 0.000 description 1
- GRRNUXAQVGOGFE-UHFFFAOYSA-N Hygromycin-B Natural products OC1C(NC)CC(N)C(O)C1OC1C2OC3(C(C(O)C(O)C(C(N)CO)O3)O)OC2C(O)C(CO)O1 GRRNUXAQVGOGFE-UHFFFAOYSA-N 0.000 description 1
- 102000009438 IgE Receptors Human genes 0.000 description 1
- 108010073816 IgE Receptors Proteins 0.000 description 1
- QADCTXFNLZBZAB-GHCJXIJMSA-N Ile-Asn-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C)C(=O)O)N QADCTXFNLZBZAB-GHCJXIJMSA-N 0.000 description 1
- WTOAPTKSZJJWKK-HTFCKZLJSA-N Ile-Cys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N WTOAPTKSZJJWKK-HTFCKZLJSA-N 0.000 description 1
- GTSAALPQZASLPW-KJYZGMDISA-N Ile-His-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N GTSAALPQZASLPW-KJYZGMDISA-N 0.000 description 1
- JODPUDMBQBIWCK-GHCJXIJMSA-N Ile-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O JODPUDMBQBIWCK-GHCJXIJMSA-N 0.000 description 1
- WKSHBPRUIRGWRZ-KCTSRDHCSA-N Ile-Trp-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)NCC(=O)O)N WKSHBPRUIRGWRZ-KCTSRDHCSA-N 0.000 description 1
- ZUWSVOYKBCHLRR-MGHWNKPDSA-N Ile-Tyr-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCCN)C(=O)O)N ZUWSVOYKBCHLRR-MGHWNKPDSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102100025304 Integrin beta-1 Human genes 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 101150069255 KLRC1 gene Proteins 0.000 description 1
- 102100021458 Killer cell lectin-like receptor subfamily F member 1 Human genes 0.000 description 1
- LJHGALIOHLRRQN-DCAQKATOSA-N Leu-Ala-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N LJHGALIOHLRRQN-DCAQKATOSA-N 0.000 description 1
- OGCQGUIWMSBHRZ-CIUDSAMLSA-N Leu-Asn-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O OGCQGUIWMSBHRZ-CIUDSAMLSA-N 0.000 description 1
- YRRCOJOXAJNSAX-IHRRRGAJSA-N Leu-Pro-Lys Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)O)N YRRCOJOXAJNSAX-IHRRRGAJSA-N 0.000 description 1
- 102100032913 Leukocyte surface antigen CD47 Human genes 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 1
- VMTYLUGCXIEDMV-QWRGUYRKSA-N Lys-Leu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCCCN VMTYLUGCXIEDMV-QWRGUYRKSA-N 0.000 description 1
- JYVCOTWSRGFABJ-DCAQKATOSA-N Lys-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCCN)N JYVCOTWSRGFABJ-DCAQKATOSA-N 0.000 description 1
- JOSAKOKSPXROGQ-BJDJZHNGSA-N Lys-Ser-Ile Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JOSAKOKSPXROGQ-BJDJZHNGSA-N 0.000 description 1
- CAVRAQIDHUPECU-UVOCVTCTSA-N Lys-Thr-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAVRAQIDHUPECU-UVOCVTCTSA-N 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 description 1
- DBMLDOWSVHMQQN-XGEHTFHBSA-N Met-Ser-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DBMLDOWSVHMQQN-XGEHTFHBSA-N 0.000 description 1
- IIHMNTBFPMRJCN-RCWTZXSCSA-N Met-Val-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IIHMNTBFPMRJCN-RCWTZXSCSA-N 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 108010008707 Mucin-1 Proteins 0.000 description 1
- 108010063954 Mucins Proteins 0.000 description 1
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 102100022683 NKG2-C type II integral membrane protein Human genes 0.000 description 1
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 102100021462 Natural killer cells antigen CD94 Human genes 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 1
- 102400000058 Neuregulin-1 Human genes 0.000 description 1
- 108090000556 Neuregulin-1 Proteins 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- 102100037589 OX-2 membrane glycoprotein Human genes 0.000 description 1
- 239000012570 Opti-MEM I medium Substances 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- NOFBJKKOPKJDCO-KKXDTOCCSA-N Phe-Ala-Tyr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O NOFBJKKOPKJDCO-KKXDTOCCSA-N 0.000 description 1
- AUJWXNGCAQWLEI-KBPBESRZSA-N Phe-Lys-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O AUJWXNGCAQWLEI-KBPBESRZSA-N 0.000 description 1
- BSKMOCNNLNDIMU-CDMKHQONSA-N Phe-Thr-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O BSKMOCNNLNDIMU-CDMKHQONSA-N 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- JARJPEMLQAWNBR-GUBZILKMSA-N Pro-Asp-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JARJPEMLQAWNBR-GUBZILKMSA-N 0.000 description 1
- CLNJSLSHKJECME-BQBZGAKWSA-N Pro-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H]1CCCN1 CLNJSLSHKJECME-BQBZGAKWSA-N 0.000 description 1
- QKDIHFHGHBYTKB-IHRRRGAJSA-N Pro-Ser-Phe Chemical compound N([C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 QKDIHFHGHBYTKB-IHRRRGAJSA-N 0.000 description 1
- 101710098940 Pro-epidermal growth factor Proteins 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 241000508269 Psidium Species 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 1
- 102100029986 Receptor tyrosine-protein kinase erbB-3 Human genes 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- HBZBPFLJNDXRAY-FXQIFTODSA-N Ser-Ala-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O HBZBPFLJNDXRAY-FXQIFTODSA-N 0.000 description 1
- UGJRQLURDVGULT-LKXGYXEUSA-N Ser-Asn-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UGJRQLURDVGULT-LKXGYXEUSA-N 0.000 description 1
- PVDTYLHUWAEYGY-CIUDSAMLSA-N Ser-Glu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PVDTYLHUWAEYGY-CIUDSAMLSA-N 0.000 description 1
- MUARUIBTKQJKFY-WHFBIAKZSA-N Ser-Gly-Asp Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O MUARUIBTKQJKFY-WHFBIAKZSA-N 0.000 description 1
- WLJPJRGQRNCIQS-ZLUOBGJFSA-N Ser-Ser-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O WLJPJRGQRNCIQS-ZLUOBGJFSA-N 0.000 description 1
- PMTWIUBUQRGCSB-FXQIFTODSA-N Ser-Val-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O PMTWIUBUQRGCSB-FXQIFTODSA-N 0.000 description 1
- JZRYFUGREMECBH-XPUUQOCRSA-N Ser-Val-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O JZRYFUGREMECBH-XPUUQOCRSA-N 0.000 description 1
- 102100023921 Serine/threonine-protein kinase ATR Human genes 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 206010042971 T-cell lymphoma Diseases 0.000 description 1
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 description 1
- 102100035794 T-cell surface glycoprotein CD3 epsilon chain Human genes 0.000 description 1
- UNURFMVMXLENAZ-KJEVXHAQSA-N Thr-Arg-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O UNURFMVMXLENAZ-KJEVXHAQSA-N 0.000 description 1
- YAAPRMFURSENOZ-KATARQTJSA-N Thr-Cys-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N)O YAAPRMFURSENOZ-KATARQTJSA-N 0.000 description 1
- KBBRNEDOYWMIJP-KYNKHSRBSA-N Thr-Gly-Thr Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KBBRNEDOYWMIJP-KYNKHSRBSA-N 0.000 description 1
- UYTYTDMCDBPDSC-URLPEUOOSA-N Thr-Ile-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N UYTYTDMCDBPDSC-URLPEUOOSA-N 0.000 description 1
- STUAPCLEDMKXKL-LKXGYXEUSA-N Thr-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O STUAPCLEDMKXKL-LKXGYXEUSA-N 0.000 description 1
- GRIUMVXCJDKVPI-IZPVPAKOSA-N Thr-Thr-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O GRIUMVXCJDKVPI-IZPVPAKOSA-N 0.000 description 1
- LVRFMARKDGGZMX-IZPVPAKOSA-N Thr-Tyr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CC1=CC=C(O)C=C1 LVRFMARKDGGZMX-IZPVPAKOSA-N 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 208000037280 Trisomy Diseases 0.000 description 1
- DQDXHYIEITXNJY-BPUTZDHNSA-N Trp-Gln-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N DQDXHYIEITXNJY-BPUTZDHNSA-N 0.000 description 1
- 108010028230 Trp-Ser- His-Pro-Gln-Phe-Glu-Lys Proteins 0.000 description 1
- 102100029675 Tumor necrosis factor receptor superfamily member 13B Human genes 0.000 description 1
- 102100033726 Tumor necrosis factor receptor superfamily member 17 Human genes 0.000 description 1
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 1
- MBFJIHUHHCJBSN-AVGNSLFASA-N Tyr-Asn-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MBFJIHUHHCJBSN-AVGNSLFASA-N 0.000 description 1
- NXRGXTBPMOGFID-CFMVVWHZSA-N Tyr-Ile-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O NXRGXTBPMOGFID-CFMVVWHZSA-N 0.000 description 1
- UPODKYBYUBTWSV-BZSNNMDCSA-N Tyr-Phe-Cys Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CS)C(O)=O)C1=CC=C(O)C=C1 UPODKYBYUBTWSV-BZSNNMDCSA-N 0.000 description 1
- SZEIFUXUTBBQFQ-STQMWFEESA-N Tyr-Pro-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O SZEIFUXUTBBQFQ-STQMWFEESA-N 0.000 description 1
- AKKYBQGHUAWPJR-MNSWYVGCSA-N Tyr-Thr-Trp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N)O AKKYBQGHUAWPJR-MNSWYVGCSA-N 0.000 description 1
- BMPPMAOOKQJYIP-WMZOPIPTSA-N Tyr-Trp Chemical compound C([C@H]([NH3+])C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C([O-])=O)C1=CC=C(O)C=C1 BMPPMAOOKQJYIP-WMZOPIPTSA-N 0.000 description 1
- MWUYSCVVPVITMW-IGNZVWTISA-N Tyr-Tyr-Ala Chemical compound C([C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 MWUYSCVVPVITMW-IGNZVWTISA-N 0.000 description 1
- CFSSLXZJEMERJY-NRPADANISA-N Val-Gln-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O CFSSLXZJEMERJY-NRPADANISA-N 0.000 description 1
- DAVNYIUELQBTAP-XUXIUFHCSA-N Val-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N DAVNYIUELQBTAP-XUXIUFHCSA-N 0.000 description 1
- IEBGHUMBJXIXHM-AVGNSLFASA-N Val-Lys-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)O)N IEBGHUMBJXIXHM-AVGNSLFASA-N 0.000 description 1
- CKTMJBPRVQWPHU-JSGCOSHPSA-N Val-Phe-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)O)N CKTMJBPRVQWPHU-JSGCOSHPSA-N 0.000 description 1
- GBIUHAYJGWVNLN-AEJSXWLSSA-N Val-Ser-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N GBIUHAYJGWVNLN-AEJSXWLSSA-N 0.000 description 1
- OFTXTCGQJXTNQS-XGEHTFHBSA-N Val-Thr-Ser Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N)O OFTXTCGQJXTNQS-XGEHTFHBSA-N 0.000 description 1
- VTIAEOKFUJJBTC-YDHLFZDLSA-N Val-Tyr-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N VTIAEOKFUJJBTC-YDHLFZDLSA-N 0.000 description 1
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 1
- 102000009524 Vascular Endothelial Growth Factor A Human genes 0.000 description 1
- 108010084455 Zeocin Proteins 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000003990 capacitor Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000011210 chromatographic step Methods 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 238000012761 co-transfection Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 108091008034 costimulatory receptors Proteins 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 108010009297 diglycyl-histidine Proteins 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- GRRNUXAQVGOGFE-NZSRVPFOSA-N hygromycin B Chemical compound O[C@@H]1[C@@H](NC)C[C@@H](N)[C@H](O)[C@H]1O[C@H]1[C@H]2O[C@@]3([C@@H]([C@@H](O)[C@@H](O)[C@@H](C(N)CO)O3)O)O[C@H]2[C@@H](O)[C@@H](CO)O1 GRRNUXAQVGOGFE-NZSRVPFOSA-N 0.000 description 1
- 229940097277 hygromycin b Drugs 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 108010053037 kyotorphin Proteins 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 108010017391 lysylvaline Proteins 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 229940056360 penicillin g Drugs 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 239000000779 smoke Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 108010060175 trypsinogen activation peptide Proteins 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/283—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against Fc-receptors, e.g. CD16, CD32, CD64
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3076—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells against structure-related tumour-associated moieties
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/35—Valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/626—Diabody or triabody
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Virology (AREA)
- Cell Biology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15189665.1A EP3156417A1 (en) | 2015-10-13 | 2015-10-13 | Multivalent fv antibodies |
| EP15189665.1 | 2015-10-13 | ||
| PCT/EP2016/074642 WO2017064221A1 (en) | 2015-10-13 | 2016-10-13 | Multivalent fv antibodies |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180057720A true KR20180057720A (ko) | 2018-05-30 |
Family
ID=54325413
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187013310A Ceased KR20180057720A (ko) | 2015-10-13 | 2016-10-13 | 다가 Fv 항체 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US11572415B2 (enExample) |
| EP (2) | EP3156417A1 (enExample) |
| JP (1) | JP6936497B2 (enExample) |
| KR (1) | KR20180057720A (enExample) |
| CN (1) | CN108473577A (enExample) |
| AU (1) | AU2016336866B2 (enExample) |
| BR (1) | BR112018007445A2 (enExample) |
| CA (1) | CA3001765A1 (enExample) |
| IL (1) | IL258638B2 (enExample) |
| MX (1) | MX2018004547A (enExample) |
| SG (2) | SG11201803083XA (enExample) |
| WO (1) | WO2017064221A1 (enExample) |
| ZA (1) | ZA201802543B (enExample) |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MA44381A (fr) | 2015-06-15 | 2019-01-23 | Numab Innovation Ag | Format d'anticorps hétérodimères multispécifiques |
| AU2018295119B2 (en) * | 2017-06-25 | 2024-10-03 | Systimmune, Inc. | Multi-specific antibodies and methods of making and using thereof |
| JP7288913B2 (ja) | 2018-03-14 | 2023-06-08 | アフィメッド ゲゼルシャフト ミット ベシュレンクテル ハフツンク | 二重特異性egfr/cd16抗原結合タンパク質 |
| CN111527108A (zh) * | 2018-03-27 | 2020-08-11 | 西雅图免疫公司 | 制导和导航控制蛋白的制造和使用方法 |
| US20240132626A1 (en) * | 2018-04-13 | 2024-04-25 | Eli Lilly And Company | Fab-Based Trispecific Antibodies |
| BR112020020828A2 (pt) * | 2018-04-13 | 2021-01-19 | Affimed Gmbh | Proteínas de ligação, composições farmacêuticas, método para o tratamento ou melhoria de uma doença, para tratar e/ou prevenir uma doença, para tratar um paciente e para tratar câncer e anticorpos |
| US20210179734A1 (en) * | 2018-04-17 | 2021-06-17 | Invenra Inc. | Trivalent trispecific antibody constructs |
| AU2019327155A1 (en) | 2018-08-27 | 2021-03-18 | Affimed Gmbh | Cryopreserved NK cells preloaded with an antibody construct |
| CN115066274A (zh) * | 2019-09-25 | 2022-09-16 | 斯图加特大学 | 三价结合分子 |
| CN118459602A (zh) * | 2019-11-06 | 2024-08-09 | 西雅图免疫公司 | 制导和导航控制蛋白及其制备和使用方法 |
| AU2020414409A1 (en) | 2019-12-27 | 2022-06-16 | Affimed Gmbh | Method for the production of bispecific FcyRIIl x CD30 antibody construct |
| TWI874613B (zh) * | 2020-03-17 | 2025-03-01 | 美商西雅圖免疫公司 | 微型導引和導航控制(miniGNC)類抗體蛋白及其製造和使用方法 |
| CA3187272A1 (en) | 2020-10-08 | 2022-04-14 | Thorsten Ross | Trispecific binders |
| EP4255931A1 (en) * | 2020-12-03 | 2023-10-11 | Amgen Inc. | Immunoglobuline constructs with multiple binding domains |
| JP2024510098A (ja) * | 2021-02-19 | 2024-03-06 | イノベント バイオロジクス(スーチョウ)カンパニー,リミティド | 抗GPRC5DxBCMAxCD3三重特異性抗体およびその使用 |
| IL308154A (en) | 2021-07-30 | 2023-12-01 | Affimed Gmbh | Double structure antibodies |
| IL311765A (en) * | 2021-09-29 | 2024-05-01 | Modex Therapeutics Inc | Antigen-binding polypeptides, antigen-binding polypeptide complexes and methods of using them |
| CN114316060B (zh) * | 2021-12-15 | 2023-06-13 | 北京市肿瘤防治研究所 | 抗人cd19与cd206双特异性抗体及其制备方法和应用 |
| US20230272069A1 (en) * | 2022-02-28 | 2023-08-31 | Institute Of Hematology And Blood Diseases Hospital, Chinese Academy Of Medical Sciences | Chimeric Antigen Receptor Targeting CD22 and CD19 and Application thereof |
| EP4572772A1 (en) | 2022-08-17 | 2025-06-25 | Capstan Therapeutics, Inc. | Conditioning for in vivo immune cell engineering |
| WO2024249954A1 (en) | 2023-05-31 | 2024-12-05 | Capstan Therapeutics, Inc. | Lipid nanoparticle formulations and compositions |
| WO2025076127A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025076113A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Ionizable cationic lipids with conserved spacing and lipid nanoparticles |
| WO2025179294A2 (en) | 2024-02-22 | 2025-08-28 | Capstan Therapeutics, Inc. | Immune engineering amplification |
| WO2025217454A2 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Ionizable cationic lipids and lipid nanoparticles |
| WO2025217452A1 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025235413A1 (en) * | 2024-05-05 | 2025-11-13 | Duke University | Compositions targeting cancer antigens and methods of use thereof |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69329974T2 (de) | 1992-12-04 | 2001-07-19 | Medical Research Council, London | Multivalente und multispezifische bindungsproteine, deren herstellung und verwendung |
| DE69535719T2 (de) * | 1994-01-07 | 2009-03-19 | Mochida Pharmaceutical Co. Ltd. | Fas-antigenbindender ligand |
| US5989830A (en) * | 1995-10-16 | 1999-11-23 | Unilever Patent Holdings Bv | Bifunctional or bivalent antibody fragment analogue |
| EP1294904A1 (en) * | 2000-06-30 | 2003-03-26 | Vlaams Interuniversitair Instituut voor Biotechnologie vzw. | Heterodimeric fusion proteins |
| ES2276735T3 (es) * | 2001-09-14 | 2007-07-01 | Affimed Therapeutics Ag | Anticuerpos fv multimericos de cadena sencilla en tandem. |
| AU2002364531A1 (en) * | 2001-12-26 | 2003-07-24 | Ibc Pharmaceuticals | Methods of generating multispecific, multivalent agents from vh and vl domains |
| US20120237442A1 (en) | 2005-04-06 | 2012-09-20 | Ibc Pharmaceuticals, Inc. | Design and Construction of Novel Multivalent Antibodies |
| GB0510790D0 (en) * | 2005-05-26 | 2005-06-29 | Syngenta Crop Protection Ag | Anti-CD16 binding molecules |
| CA2657385A1 (en) * | 2006-07-13 | 2008-01-17 | Naoki Kimura | Cell death inducer |
| EP2014680A1 (en) | 2007-07-10 | 2009-01-14 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Recombinant, single-chain, trivalent tri-specific or bi-specific antibody derivatives |
| GB201003701D0 (en) * | 2010-03-05 | 2010-04-21 | Cilian Ag | System for the expression of a protein |
| FR2959416B1 (fr) * | 2010-05-03 | 2012-06-22 | Monoclonal Antibodies Therapeutics Mat Biopharma | Utilisation d'anticorps anti-cd71 pour la preparation d'un medicament |
| EP2655415A4 (en) | 2010-12-22 | 2016-03-09 | Abbvie Inc | THREE VARIABLE DOMAIN LINK PROTEINS AND USES THEREOF |
| TWI671315B (zh) * | 2011-03-28 | 2019-09-11 | 法商賽諾菲公司 | 具有交叉結合區定向之雙重可變區類抗體結合蛋白 |
| ES2784631T3 (es) * | 2012-12-03 | 2020-09-29 | Novimmune Sa | Anticuerpos anti-CD47 y métodos de uso de los mismos |
| WO2014106004A2 (en) | 2012-12-28 | 2014-07-03 | Abbvie, Inc. | High-throughput system and method for identifying antibodies having specific antigen binding activities |
| US20150017120A1 (en) * | 2013-06-13 | 2015-01-15 | Massachusetts Institute Of Technology | Synergistic tumor treatment with extended-pk il-2 and adoptive cell therapy |
| EP2930188A1 (en) * | 2014-04-13 | 2015-10-14 | Affimed Therapeutics AG | Trifunctional antigen-binding molecule |
| MA44381A (fr) * | 2015-06-15 | 2019-01-23 | Numab Innovation Ag | Format d'anticorps hétérodimères multispécifiques |
-
2015
- 2015-10-13 EP EP15189665.1A patent/EP3156417A1/en not_active Withdrawn
-
2016
- 2016-10-13 SG SG11201803083XA patent/SG11201803083XA/en unknown
- 2016-10-13 EP EP16791328.4A patent/EP3362478A1/en not_active Withdrawn
- 2016-10-13 JP JP2018519031A patent/JP6936497B2/ja active Active
- 2016-10-13 SG SG10202004094VA patent/SG10202004094VA/en unknown
- 2016-10-13 CN CN201680073236.5A patent/CN108473577A/zh active Pending
- 2016-10-13 IL IL258638A patent/IL258638B2/en unknown
- 2016-10-13 MX MX2018004547A patent/MX2018004547A/es unknown
- 2016-10-13 AU AU2016336866A patent/AU2016336866B2/en not_active Ceased
- 2016-10-13 BR BR112018007445A patent/BR112018007445A2/pt not_active Application Discontinuation
- 2016-10-13 KR KR1020187013310A patent/KR20180057720A/ko not_active Ceased
- 2016-10-13 WO PCT/EP2016/074642 patent/WO2017064221A1/en not_active Ceased
- 2016-10-13 CA CA3001765A patent/CA3001765A1/en active Pending
-
2018
- 2018-04-11 US US15/950,733 patent/US11572415B2/en active Active
- 2018-04-17 ZA ZA2018/02543A patent/ZA201802543B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| AU2016336866B2 (en) | 2023-05-25 |
| SG11201803083XA (en) | 2018-05-30 |
| ZA201802543B (en) | 2020-01-29 |
| EP3362478A1 (en) | 2018-08-22 |
| IL258638B (en) | 2022-12-01 |
| CN108473577A (zh) | 2018-08-31 |
| IL258638B2 (en) | 2023-04-01 |
| AU2016336866A1 (en) | 2018-05-10 |
| EP3156417A1 (en) | 2017-04-19 |
| WO2017064221A1 (en) | 2017-04-20 |
| CA3001765A1 (en) | 2017-04-20 |
| US20190040155A1 (en) | 2019-02-07 |
| BR112018007445A2 (pt) | 2019-12-10 |
| IL258638A (en) | 2018-06-28 |
| RU2018126966A3 (enExample) | 2020-04-20 |
| RU2018126966A (ru) | 2020-02-10 |
| JP2018537415A (ja) | 2018-12-20 |
| JP6936497B2 (ja) | 2021-09-15 |
| MX2018004547A (es) | 2018-11-09 |
| SG10202004094VA (en) | 2020-05-28 |
| US11572415B2 (en) | 2023-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11572415B2 (en) | Multivalent FV antibodies | |
| US20220048994A1 (en) | Trifunctional antigen-binding molecule | |
| JP7095078B2 (ja) | キメラポリペプチド及びその使用 | |
| CN111527109B (zh) | 以抗体Fc区为骨架的融合蛋白二聚体及其应用 | |
| CA2861816C (en) | Light chain-bridged bispecific antibody | |
| KR102629905B1 (ko) | 항-pd-l1/항-pd-1 천연 항체 구조-유사 헤테로다이머 이중특이성 항체 및 그의 제조 | |
| EP3600407A1 (en) | Improved antigen binding receptors | |
| CA3130508A1 (en) | Antibody molecules that bind to nkp30 and uses thereof | |
| US20240299543A1 (en) | Self-polarizing immune cells | |
| RU2785766C9 (ru) | Мультивалентные fv-антитела | |
| RU2785766C2 (ru) | Мультивалентные fv-антитела | |
| US20250312453A1 (en) | Antibody targeting ccr8 and its applications | |
| HK1260025A1 (en) | Multivalent fv antibodies | |
| WO2025093867A1 (en) | Invariant natural killer t cell | |
| WO2025191154A1 (en) | Therapeutic agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180510 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20211008 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20240613 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240906 Comment text: Decision to Refuse Application Patent event code: PE06012S01D |