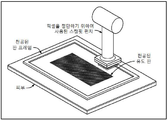
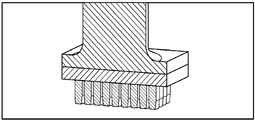
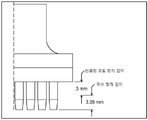
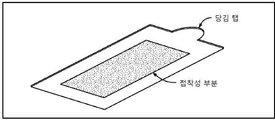
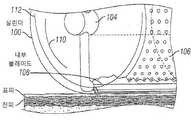
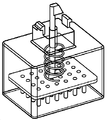
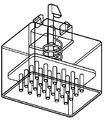
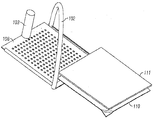
KR20170101184A - 픽셀 어레이 의료용 장치 및 방법 - Google Patents
픽셀 어레이 의료용 장치 및 방법 Download PDFInfo
- Publication number
- KR20170101184A KR20170101184A KR1020177008576A KR20177008576A KR20170101184A KR 20170101184 A KR20170101184 A KR 20170101184A KR 1020177008576 A KR1020177008576 A KR 1020177008576A KR 20177008576 A KR20177008576 A KR 20177008576A KR 20170101184 A KR20170101184 A KR 20170101184A
- Authority
- KR
- South Korea
- Prior art keywords
- scallop
- skin
- array
- skin pixels
- donor site
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 238000000034 method Methods 0.000 title claims abstract description 366
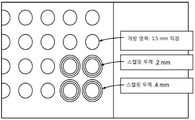
- 235000020637 scallop Nutrition 0.000 claims abstract description 836
- 241000237509 Patinopecten sp. Species 0.000 claims abstract description 714
- 230000007547 defect Effects 0.000 claims abstract description 164
- 241000237503 Pectinidae Species 0.000 claims abstract description 122
- 239000000758 substrate Substances 0.000 claims description 211
- 239000000853 adhesive Substances 0.000 claims description 156
- 230000001070 adhesive effect Effects 0.000 claims description 156
- 230000004044 response Effects 0.000 claims description 85
- 238000005520 cutting process Methods 0.000 claims description 62
- 230000007246 mechanism Effects 0.000 claims description 56
- 210000004761 scalp Anatomy 0.000 claims description 53
- 238000004659 sterilization and disinfection Methods 0.000 claims description 36
- 230000029663 wound healing Effects 0.000 claims description 27
- 239000011148 porous material Substances 0.000 claims description 20
- 230000001954 sterilising effect Effects 0.000 claims description 20
- 238000007920 subcutaneous administration Methods 0.000 claims description 20
- 238000004140 cleaning Methods 0.000 claims description 19
- 230000006698 induction Effects 0.000 claims description 17
- 230000015572 biosynthetic process Effects 0.000 claims description 16
- 230000008878 coupling Effects 0.000 claims description 15
- 238000010168 coupling process Methods 0.000 claims description 15
- 238000005859 coupling reaction Methods 0.000 claims description 15
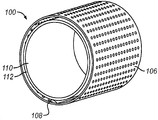
- 238000003491 array Methods 0.000 claims description 10
- 239000000463 material Substances 0.000 claims description 10
- 239000002184 metal Substances 0.000 claims description 9
- 229920000642 polymer Polymers 0.000 claims description 9
- 206010029113 Neovascularisation Diseases 0.000 claims description 4
- 230000002792 vascular Effects 0.000 claims description 3
- 230000002285 radioactive effect Effects 0.000 claims 1
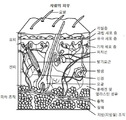
- 210000003491 skin Anatomy 0.000 description 768
- 210000004209 hair Anatomy 0.000 description 59
- 239000002313 adhesive film Substances 0.000 description 53
- 239000010410 layer Substances 0.000 description 40
- 238000002679 ablation Methods 0.000 description 34
- 238000002054 transplantation Methods 0.000 description 30
- 210000001519 tissue Anatomy 0.000 description 26
- 210000004207 dermis Anatomy 0.000 description 23
- 230000036961 partial effect Effects 0.000 description 23
- 231100000241 scar Toxicity 0.000 description 22
- 230000035876 healing Effects 0.000 description 21
- 239000012528 membrane Substances 0.000 description 20
- 208000027418 Wounds and injury Diseases 0.000 description 19
- 238000001356 surgical procedure Methods 0.000 description 19
- 238000012377 drug delivery Methods 0.000 description 18
- 230000037390 scarring Effects 0.000 description 17
- 206010052428 Wound Diseases 0.000 description 16
- 230000008569 process Effects 0.000 description 15
- 208000032544 Cicatrix Diseases 0.000 description 14
- 229920001436 collagen Polymers 0.000 description 14
- 230000037387 scars Effects 0.000 description 14
- 239000003814 drug Substances 0.000 description 13
- 210000002615 epidermis Anatomy 0.000 description 13
- 201000004384 Alopecia Diseases 0.000 description 12
- 239000002537 cosmetic Substances 0.000 description 12
- 230000003111 delayed effect Effects 0.000 description 12
- 230000008021 deposition Effects 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 230000000007 visual effect Effects 0.000 description 12
- 238000002316 cosmetic surgery Methods 0.000 description 11
- 230000002500 effect on skin Effects 0.000 description 11
- 239000000835 fiber Substances 0.000 description 11
- 239000007943 implant Substances 0.000 description 11
- 238000002271 resection Methods 0.000 description 11
- 102000008186 Collagen Human genes 0.000 description 10
- 108010035532 Collagen Proteins 0.000 description 10
- 230000006378 damage Effects 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 230000035755 proliferation Effects 0.000 description 10
- 238000005096 rolling process Methods 0.000 description 9
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 238000000605 extraction Methods 0.000 description 8
- 230000003676 hair loss Effects 0.000 description 8
- 208000014674 injury Diseases 0.000 description 8
- 230000000670 limiting effect Effects 0.000 description 8
- 230000006870 function Effects 0.000 description 7
- 210000003127 knee Anatomy 0.000 description 7
- 210000004003 subcutaneous fat Anatomy 0.000 description 7
- 208000002847 Surgical Wound Diseases 0.000 description 6
- 230000032683 aging Effects 0.000 description 6
- 230000008602 contraction Effects 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 210000003780 hair follicle Anatomy 0.000 description 6
- 238000002513 implantation Methods 0.000 description 6
- 238000002690 local anesthesia Methods 0.000 description 6
- 230000035800 maturation Effects 0.000 description 6
- 230000036573 scar formation Effects 0.000 description 6
- 241000282414 Homo sapiens Species 0.000 description 5
- 206010039580 Scar Diseases 0.000 description 5
- 210000001015 abdomen Anatomy 0.000 description 5
- 210000000038 chest Anatomy 0.000 description 5
- 230000001815 facial effect Effects 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000012546 transfer Methods 0.000 description 5
- 230000008733 trauma Effects 0.000 description 5
- 210000000689 upper leg Anatomy 0.000 description 5
- 206010006803 Burns third degree Diseases 0.000 description 4
- 206010015150 Erythema Diseases 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 210000001217 buttock Anatomy 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 239000002131 composite material Substances 0.000 description 4
- 210000004907 gland Anatomy 0.000 description 4
- 208000024963 hair loss Diseases 0.000 description 4
- 229960001340 histamine Drugs 0.000 description 4
- 238000007726 management method Methods 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 230000003068 static effect Effects 0.000 description 4
- 208000002193 Pain Diseases 0.000 description 3
- 231100000360 alopecia Toxicity 0.000 description 3
- 230000003698 anagen phase Effects 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000004049 embossing Methods 0.000 description 3
- 210000002950 fibroblast Anatomy 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 230000003779 hair growth Effects 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 239000003589 local anesthetic agent Substances 0.000 description 3
- 229960005015 local anesthetics Drugs 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000036407 pain Effects 0.000 description 3
- 230000035515 penetration Effects 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 210000001732 sebaceous gland Anatomy 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 230000002459 sustained effect Effects 0.000 description 3
- 206010002091 Anaesthesia Diseases 0.000 description 2
- 206010010356 Congenital anomaly Diseases 0.000 description 2
- 206010063560 Excessive granulation tissue Diseases 0.000 description 2
- 206010064503 Excessive skin Diseases 0.000 description 2
- 102000018997 Growth Hormone Human genes 0.000 description 2
- 108010051696 Growth Hormone Proteins 0.000 description 2
- 208000034693 Laceration Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 206010041235 Snoring Diseases 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- 210000001789 adipocyte Anatomy 0.000 description 2
- 230000037005 anaesthesia Effects 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- 230000002491 angiogenic effect Effects 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000007012 clinical effect Effects 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 230000001351 cycling effect Effects 0.000 description 2
- 230000006735 deficit Effects 0.000 description 2
- 238000009795 derivation Methods 0.000 description 2
- 238000002224 dissection Methods 0.000 description 2
- 210000001339 epidermal cell Anatomy 0.000 description 2
- 210000001723 extracellular space Anatomy 0.000 description 2
- 210000000416 exudates and transudate Anatomy 0.000 description 2
- 210000001126 granulation tissue Anatomy 0.000 description 2
- 210000004013 groin Anatomy 0.000 description 2
- 239000000122 growth hormone Substances 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 210000003630 histaminocyte Anatomy 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000001000 micrograph Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000000394 mitotic effect Effects 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 230000004118 muscle contraction Effects 0.000 description 2
- 206010033675 panniculitis Diseases 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 239000011241 protective layer Substances 0.000 description 2
- 230000003716 rejuvenation Effects 0.000 description 2
- 238000007634 remodeling Methods 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 230000036548 skin texture Effects 0.000 description 2
- 201000002859 sleep apnea Diseases 0.000 description 2
- 210000004872 soft tissue Anatomy 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 210000000434 stratum corneum Anatomy 0.000 description 2
- 238000005728 strengthening Methods 0.000 description 2
- 210000004304 subcutaneous tissue Anatomy 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000008719 thickening Effects 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 238000013022 venting Methods 0.000 description 2
- 206010003694 Atrophy Diseases 0.000 description 1
- 206010063659 Aversion Diseases 0.000 description 1
- 208000035985 Body Odor Diseases 0.000 description 1
- 108030001720 Bontoxilysin Proteins 0.000 description 1
- 206010006802 Burns second degree Diseases 0.000 description 1
- 102000002585 Contractile Proteins Human genes 0.000 description 1
- 108010068426 Contractile Proteins Proteins 0.000 description 1
- 208000034423 Delivery Diseases 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 208000008454 Hyperhidrosis Diseases 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 206010021639 Incontinence Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- ZFMITUMMTDLWHR-UHFFFAOYSA-N Minoxidil Chemical compound NC1=[N+]([O-])C(N)=CC(N2CCCCC2)=N1 ZFMITUMMTDLWHR-UHFFFAOYSA-N 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 208000004550 Postoperative Pain Diseases 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 206010040799 Skin atrophy Diseases 0.000 description 1
- 208000028990 Skin injury Diseases 0.000 description 1
- 206010067868 Skin mass Diseases 0.000 description 1
- 206010040904 Skin odour abnormal Diseases 0.000 description 1
- 108010057266 Type A Botulinum Toxins Proteins 0.000 description 1
- 210000001766 X chromosome Anatomy 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 206010068168 androgenetic alopecia Diseases 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 230000037444 atrophy Effects 0.000 description 1
- 230000037365 barrier function of the epidermis Effects 0.000 description 1
- 230000003796 beauty Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000036770 blood supply Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 229940089093 botox Drugs 0.000 description 1
- 229940053031 botulinum toxin Drugs 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000001804 debridement Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000004200 deflagration Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000007933 dermal patch Substances 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 231100000321 erythema Toxicity 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000004299 exfoliation Methods 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 201000000475 female stress incontinence Diseases 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 229960004039 finasteride Drugs 0.000 description 1
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 1
- 230000003325 follicular Effects 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 208000002557 hidradenitis Diseases 0.000 description 1
- 230000002962 histologic effect Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000016507 interphase Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 229960003632 minoxidil Drugs 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000006740 morphological transformation Effects 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 210000000651 myofibroblast Anatomy 0.000 description 1
- 230000003227 neuromodulating effect Effects 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 210000004417 patella Anatomy 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000000554 physical therapy Methods 0.000 description 1
- 238000013310 pig model Methods 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 238000004080 punching Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 210000002374 sebum Anatomy 0.000 description 1
- 230000008591 skin barrier function Effects 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 210000001584 soft palate Anatomy 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000005070 sphincter Anatomy 0.000 description 1
- 210000000273 spinal nerve root Anatomy 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 208000013460 sweaty Diseases 0.000 description 1
- 238000010408 sweeping Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 231100000057 systemic toxicity Toxicity 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 230000009772 tissue formation Effects 0.000 description 1
- 229940100611 topical cream Drugs 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000009677 vaginal delivery Effects 0.000 description 1
- 230000008728 vascular permeability Effects 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000009941 weaving Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/322—Skin grafting apparatus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/20—Surgical instruments, devices or methods for vaccinating or cleaning the skin previous to the vaccination
- A61B17/205—Vaccinating by means of needles or other puncturing devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/3205—Excision instruments
- A61B17/32053—Punch like cutting instruments, e.g. using a cylindrical or oval knife
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00743—Type of operation; Specification of treatment sites
- A61B2017/00747—Dermatology
- A61B2017/00752—Hair removal or transplantation
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Transplantation (AREA)
- Surgical Instruments (AREA)
- Radiation-Therapy Devices (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Prostheses (AREA)
- Massaging Devices (AREA)
Applications Claiming Priority (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462044060P | 2014-08-29 | 2014-08-29 | |
| US201462044102P | 2014-08-29 | 2014-08-29 | |
| US201462044078P | 2014-08-29 | 2014-08-29 | |
| US201462044089P | 2014-08-29 | 2014-08-29 | |
| US62/044,089 | 2014-08-29 | ||
| US62/044,060 | 2014-08-29 | ||
| US62/044,078 | 2014-08-29 | ||
| US62/044,102 | 2014-08-29 | ||
| US14/505,090 US10076354B2 (en) | 2010-12-17 | 2014-10-02 | Pixel array medical devices and methods |
| US14/505,090 | 2014-10-02 | ||
| PCT/US2015/047695 WO2016033584A1 (en) | 2014-08-29 | 2015-08-31 | Pixel array medical devices and methods |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170101184A true KR20170101184A (ko) | 2017-09-05 |
Family
ID=55400735
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177008576A Ceased KR20170101184A (ko) | 2014-08-29 | 2015-08-31 | 픽셀 어레이 의료용 장치 및 방법 |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP3185788A4 (enExample) |
| JP (1) | JP2017528216A (enExample) |
| KR (1) | KR20170101184A (enExample) |
| CN (1) | CN107405164B (enExample) |
| AU (2) | AU2015308582A1 (enExample) |
| BR (1) | BR112017004112A2 (enExample) |
| CA (1) | CA2959512A1 (enExample) |
| MX (1) | MX2017002661A (enExample) |
| PH (1) | PH12017500349A1 (enExample) |
| SG (1) | SG11201701521RA (enExample) |
| WO (2) | WO2016033584A1 (enExample) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4578477A3 (en) | 2008-12-02 | 2025-10-08 | Allergan, Inc. | Injection device |
| US10772658B2 (en) * | 2010-12-17 | 2020-09-15 | Srgi Holdings, Llc | Pixel array medical systems, devices and methods |
| CA2900505C (en) | 2013-02-20 | 2023-10-24 | Cytrellis Biosystems, Inc. | Methods and devices for skin tightening |
| US20140350516A1 (en) | 2013-05-23 | 2014-11-27 | Allergan, Inc. | Mechanical syringe accessory |
| US20140350518A1 (en) | 2013-05-23 | 2014-11-27 | Allergan, Inc. | Syringe extrusion accessory |
| WO2015021434A2 (en) | 2013-08-09 | 2015-02-12 | Cytrellis Biosystems, Inc. | Methods and apparatuses for skin treatment using non-thermal tissue ablation |
| WO2015095675A1 (en) | 2013-12-19 | 2015-06-25 | Cytrellis Biosystems, Inc. | Methods and devices for manipulating subdermal fat |
| US10029048B2 (en) | 2014-05-13 | 2018-07-24 | Allergan, Inc. | High force injection devices |
| US10226585B2 (en) | 2014-10-01 | 2019-03-12 | Allergan, Inc. | Devices for injection and dosing |
| CA2967636A1 (en) | 2014-11-14 | 2016-05-19 | Cytrellis Biosystems, Inc. | Devices and methods for ablation of the skin |
| CN107530490B (zh) | 2015-03-10 | 2021-06-25 | 爱力根销售有限责任公司 | 多针注射器 |
| JP6968867B2 (ja) | 2016-03-29 | 2021-11-17 | サイトレリス バイオシステムズ,インコーポレーテッド | 美容用スキンリサーフェシングのためのデバイス及び方法 |
| BR112018070642A2 (pt) | 2016-04-08 | 2019-02-05 | Allergan, Inc. | dispositivo de aspiração e injeção |
| EP3451951A4 (en) * | 2016-05-03 | 2019-12-25 | SRGI Holdings, LLC | MEDICAL SYSTEMS WITH PIXEL ARRANGEMENT, DEVICES AND METHOD |
| CN106166090B (zh) * | 2016-07-30 | 2018-10-02 | 汕头大学医学院 | 毛囊移植中转储存器 |
| US11464954B2 (en) | 2016-09-21 | 2022-10-11 | Cytrellis Biosystems, Inc. | Devices and methods for cosmetic skin resurfacing |
| DE102017106310B4 (de) | 2017-03-23 | 2018-10-11 | Lavenir Bioscience Ag | Vorrichtung und Verfahren zur Erzeugung einer Mikrograftmatrix aus Vollhaut |
| USD867582S1 (en) | 2017-03-24 | 2019-11-19 | Allergan, Inc. | Syringe device |
| DE102020129157A1 (de) | 2020-11-05 | 2022-05-05 | Lavenir Bioscience Ag | Vorrichtung zur Entfernung wenigstens eines Objekts oder einer Agglomeration von Objek-ten aus der Haut |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6669685B1 (en) * | 1997-11-06 | 2003-12-30 | Biolase Technology, Inc. | Tissue remover and method |
| US5922000A (en) * | 1997-11-19 | 1999-07-13 | Redfield Corp. | Linear punch |
| US6589202B1 (en) * | 2000-06-29 | 2003-07-08 | Becton Dickinson And Company | Method and apparatus for transdermally sampling or administering a substance to a patient |
| US6440096B1 (en) * | 2000-07-14 | 2002-08-27 | Becton, Dickinson And Co. | Microdevice and method of manufacturing a microdevice |
| CN1816280B (zh) * | 2003-05-01 | 2012-07-04 | 迈德詹尼克斯公司 | 真皮微器官和产生及应用该真皮微器官的方法和装置 |
| US8535299B2 (en) * | 2004-01-23 | 2013-09-17 | Joseph Giovannoli | Method and apparatus for skin reduction |
| KR101107440B1 (ko) * | 2007-06-26 | 2012-01-19 | 레스토레이션 로보틱스, 인코포레이티드 | 모낭 유닛 발췌 장치 및 결합 조직을 단절하기 위한 그 사용 방법 |
| US20100121307A1 (en) * | 2007-08-24 | 2010-05-13 | Microfabrica Inc. | Microneedles, Microneedle Arrays, Methods for Making, and Transdermal and/or Intradermal Applications |
| EP3892213B1 (en) * | 2010-05-07 | 2023-10-11 | The General Hospital Corporation | Apparatus for tissue grafting and copying |
| US9610093B2 (en) * | 2010-08-06 | 2017-04-04 | Kci Licensing, Inc. | Microblister skin grafting |
| AU2012211122B2 (en) * | 2011-01-28 | 2016-07-07 | The General Hospital Corporation | Method and apparatus for skin resurfacing |
| WO2012145504A1 (en) * | 2011-04-20 | 2012-10-26 | Kci Licensing, Inc. | Skin graft devices and methods |
| US20120323325A1 (en) * | 2011-06-16 | 2012-12-20 | Fulton Judith A | Autologous in situ tissue engineering |
| US20120323139A1 (en) * | 2011-06-17 | 2012-12-20 | Will Richardson | Kit and method for extracting and storing a skin tissue sample |
| EP3563772B1 (en) * | 2012-08-14 | 2022-01-12 | The General Hospital Corporation | Apparatus for tissue harvesting |
| EP2928395B1 (en) * | 2012-12-06 | 2022-02-02 | SRGI Holdings LLC | Pixel array medical devices |
-
2015
- 2015-08-31 MX MX2017002661A patent/MX2017002661A/es unknown
- 2015-08-31 CA CA2959512A patent/CA2959512A1/en active Pending
- 2015-08-31 SG SG11201701521RA patent/SG11201701521RA/en unknown
- 2015-08-31 AU AU2015308582A patent/AU2015308582A1/en not_active Abandoned
- 2015-08-31 KR KR1020177008576A patent/KR20170101184A/ko not_active Ceased
- 2015-08-31 BR BR112017004112A patent/BR112017004112A2/pt not_active IP Right Cessation
- 2015-08-31 WO PCT/US2015/047695 patent/WO2016033584A1/en not_active Ceased
- 2015-08-31 WO PCT/US2015/047721 patent/WO2016033586A1/en not_active Ceased
- 2015-08-31 JP JP2017511818A patent/JP2017528216A/ja active Pending
- 2015-08-31 EP EP15836045.3A patent/EP3185788A4/en not_active Withdrawn
- 2015-08-31 CN CN201580055986.5A patent/CN107405164B/zh not_active Expired - Fee Related
-
2017
- 2017-02-27 PH PH12017500349A patent/PH12017500349A1/en unknown
-
2020
- 2020-04-01 AU AU2020202310A patent/AU2020202310B2/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| AU2015308582A1 (en) | 2017-04-20 |
| EP3185788A1 (en) | 2017-07-05 |
| AU2020202310A1 (en) | 2020-04-23 |
| EP3185788A4 (en) | 2018-02-07 |
| CN107405164A (zh) | 2017-11-28 |
| MX2017002661A (es) | 2018-01-25 |
| WO2016033584A1 (en) | 2016-03-03 |
| CN107405164B (zh) | 2021-02-09 |
| AU2020202310B2 (en) | 2021-01-28 |
| CA2959512A1 (en) | 2016-03-03 |
| SG11201701521RA (en) | 2017-03-30 |
| JP2017528216A (ja) | 2017-09-28 |
| WO2016033586A1 (en) | 2016-03-03 |
| PH12017500349A1 (en) | 2017-07-17 |
| BR112017004112A2 (pt) | 2017-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11844546B2 (en) | Pixel array medical systems, devices and methods | |
| AU2020202310B2 (en) | Pixel array medical devices and methods | |
| EP3579767B1 (en) | Pixel array medical systems and devices | |
| US20200281617A1 (en) | Pixel array medical devices and methods | |
| KR102448313B1 (ko) | 픽셀 어레이 의료 시스템, 장치 및 방법 | |
| KR20150105312A (ko) | 픽셀 어레이 의료용 장치 및 방법 | |
| KR20180110103A (ko) | 픽셀 어레이 의료 시스템, 장치 및 방법 | |
| CA3053243C (en) | Pixel array medical systems, devices and methods | |
| US11490952B2 (en) | Pixel array medical devices and methods | |
| KR102382212B1 (ko) | 픽셀 어레이 의료용 장치 및 방법 | |
| JP2020116398A (ja) | ピクセルアレイ医療デバイスおよび方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170329 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200831 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20221004 Patent event code: PE09021S01D |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20230817 Patent event code: PE09021S02D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240126 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230817 Comment text: Final Notice of Reason for Refusal Patent event code: PE06011S02I Patent event date: 20221004 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |