KR20160060133A - 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 - Google Patents
다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 Download PDFInfo
- Publication number
- KR20160060133A KR20160060133A KR1020167010607A KR20167010607A KR20160060133A KR 20160060133 A KR20160060133 A KR 20160060133A KR 1020167010607 A KR1020167010607 A KR 1020167010607A KR 20167010607 A KR20167010607 A KR 20167010607A KR 20160060133 A KR20160060133 A KR 20160060133A
- Authority
- KR
- South Korea
- Prior art keywords
- arg arg
- leu
- ala
- val
- gln
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000004048 modification Effects 0.000 title description 6
- 238000012986 modification Methods 0.000 title description 6
- 238000001476 gene delivery Methods 0.000 title description 2
- 239000002105 nanoparticle Substances 0.000 claims abstract description 111
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 106
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 54
- 239000000377 silicon dioxide Substances 0.000 claims abstract description 53
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 50
- 229920001184 polypeptide Polymers 0.000 claims abstract description 48
- 229920006317 cationic polymer Polymers 0.000 claims abstract description 46
- 102000040430 polynucleotide Human genes 0.000 claims abstract description 44
- 108091033319 polynucleotide Proteins 0.000 claims abstract description 44
- 239000002157 polynucleotide Substances 0.000 claims abstract description 44
- 238000000034 method Methods 0.000 claims abstract description 43
- 229920000642 polymer Polymers 0.000 claims abstract description 43
- 238000000576 coating method Methods 0.000 claims abstract description 42
- 125000002091 cationic group Chemical group 0.000 claims abstract description 40
- 239000011248 coating agent Substances 0.000 claims abstract description 40
- 229920006318 anionic polymer Polymers 0.000 claims abstract description 33
- 230000003834 intracellular effect Effects 0.000 claims abstract description 9
- 230000014509 gene expression Effects 0.000 claims description 37
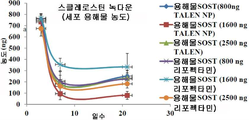
- 102000019307 Sclerostin Human genes 0.000 claims description 35
- 108050006698 Sclerostin Proteins 0.000 claims description 35
- 229920002643 polyglutamic acid Polymers 0.000 claims description 21
- 229920002873 Polyethylenimine Polymers 0.000 claims description 20
- 108010033040 Histones Proteins 0.000 claims description 17
- 108090000623 proteins and genes Proteins 0.000 claims description 15
- 241000282414 Homo sapiens Species 0.000 claims description 13
- 108010011110 polyarginine Proteins 0.000 claims description 13
- 101800000868 Tail peptide Proteins 0.000 claims description 12
- 102400001102 Tail peptide Human genes 0.000 claims description 12
- 101710163270 Nuclease Proteins 0.000 claims description 9
- 239000002773 nucleotide Substances 0.000 claims description 9
- 125000003729 nucleotide group Chemical group 0.000 claims description 9
- 235000018102 proteins Nutrition 0.000 claims description 9
- 102000004169 proteins and genes Human genes 0.000 claims description 9
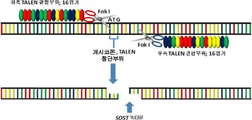
- 238000010459 TALEN Methods 0.000 claims description 5
- 108010043645 Transcription Activator-Like Effector Nucleases Proteins 0.000 claims description 5
- 108010041308 Endothelial Growth Factors Proteins 0.000 claims description 3
- 230000002227 vasoactive effect Effects 0.000 claims description 3
- 230000001939 inductive effect Effects 0.000 claims 2
- 238000001890 transfection Methods 0.000 abstract description 28
- 210000004027 cell Anatomy 0.000 description 61
- BUZMZDDKFCSKOT-CIUDSAMLSA-N Glu-Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O BUZMZDDKFCSKOT-CIUDSAMLSA-N 0.000 description 36
- QVVDVENEPNODSI-BTNSXGMBSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylidene Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O QVVDVENEPNODSI-BTNSXGMBSA-N 0.000 description 31
- 108020004414 DNA Proteins 0.000 description 30
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 26
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 25
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 25
- 230000000694 effects Effects 0.000 description 24
- LTFLDDDGWOVIHY-NAKRPEOUSA-N Val-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N LTFLDDDGWOVIHY-NAKRPEOUSA-N 0.000 description 20
- 239000013612 plasmid Substances 0.000 description 19
- XVZCXCTYGHPNEM-IHRRRGAJSA-N (2s)-1-[(2s)-2-[[(2s)-2-amino-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(O)=O XVZCXCTYGHPNEM-IHRRRGAJSA-N 0.000 description 18
- 108010068380 arginylarginine Proteins 0.000 description 16
- 239000010410 layer Substances 0.000 description 16
- UWZLBXOBVKRUFE-HGNGGELXSA-N Gln-Ala-His Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)N)N UWZLBXOBVKRUFE-HGNGGELXSA-N 0.000 description 15
- KVYVOGYEMPEXBT-GUBZILKMSA-N Gln-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(N)=O KVYVOGYEMPEXBT-GUBZILKMSA-N 0.000 description 15
- BMOFUVHDBROBSE-DCAQKATOSA-N Val-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N BMOFUVHDBROBSE-DCAQKATOSA-N 0.000 description 15
- 238000007792 addition Methods 0.000 description 15
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 13
- KLALXKYLOMZDQT-ZLUOBGJFSA-N Ala-Ser-Asn Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(N)=O KLALXKYLOMZDQT-ZLUOBGJFSA-N 0.000 description 12
- SOEXCCGNHQBFPV-DLOVCJGASA-N Gln-Val-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SOEXCCGNHQBFPV-DLOVCJGASA-N 0.000 description 12
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 12
- KLSUAWUZBMAZCL-RHYQMDGZSA-N Leu-Thr-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(O)=O KLSUAWUZBMAZCL-RHYQMDGZSA-N 0.000 description 12
- QGVBFDIREUUSHX-IFFSRLJSSA-N Thr-Val-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O QGVBFDIREUUSHX-IFFSRLJSSA-N 0.000 description 12
- CCDFBRZVTDDJNM-GUBZILKMSA-N Ala-Leu-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O CCDFBRZVTDDJNM-GUBZILKMSA-N 0.000 description 11
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 11
- QITBQGJOXQYMOA-ZETCQYMHSA-N Gly-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)CN QITBQGJOXQYMOA-ZETCQYMHSA-N 0.000 description 11
- QKIBIXAQKAFZGL-GUBZILKMSA-N Leu-Cys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QKIBIXAQKAFZGL-GUBZILKMSA-N 0.000 description 11
- PRBLYKYHAJEABA-SRVKXCTJSA-N Gln-Arg-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O PRBLYKYHAJEABA-SRVKXCTJSA-N 0.000 description 10
- DLISPGXMKZTWQG-IFFSRLJSSA-N Glu-Thr-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O DLISPGXMKZTWQG-IFFSRLJSSA-N 0.000 description 10
- 210000000963 osteoblast Anatomy 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- PNALXAODQKTNLV-JBDRJPRFSA-N Ala-Ile-Ala Chemical compound C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O PNALXAODQKTNLV-JBDRJPRFSA-N 0.000 description 8
- FHQRLHFYVZAQHU-IUCAKERBSA-N Gly-Lys-Gln Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O FHQRLHFYVZAQHU-IUCAKERBSA-N 0.000 description 8
- RGPWUJOMKFYFSR-QWRGUYRKSA-N His-Gly-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O RGPWUJOMKFYFSR-QWRGUYRKSA-N 0.000 description 8
- ZFNLIDNJUWNIJL-WDCWCFNPSA-N Leu-Glu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZFNLIDNJUWNIJL-WDCWCFNPSA-N 0.000 description 8
- JDBQSGMJBMPNFT-AVGNSLFASA-N Leu-Pro-Val Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O JDBQSGMJBMPNFT-AVGNSLFASA-N 0.000 description 8
- DFXQCCBKGUNYGG-GUBZILKMSA-N Lys-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN DFXQCCBKGUNYGG-GUBZILKMSA-N 0.000 description 8
- FISHYTLIMUYTQY-GUBZILKMSA-N Pro-Gln-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1 FISHYTLIMUYTQY-GUBZILKMSA-N 0.000 description 8
- 230000030648 nucleus localization Effects 0.000 description 8
- OKEWAFFWMHBGPT-XPUUQOCRSA-N Ala-His-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CN=CN1 OKEWAFFWMHBGPT-XPUUQOCRSA-N 0.000 description 7
- 229910019142 PO4 Inorganic materials 0.000 description 7
- 102000014128 RANK Ligand Human genes 0.000 description 7
- 108010025832 RANK Ligand Proteins 0.000 description 7
- XERQKTRGJIKTRB-CIUDSAMLSA-N Ser-His-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CO)N)CC1=CN=CN1 XERQKTRGJIKTRB-CIUDSAMLSA-N 0.000 description 7
- 108010047857 aspartylglycine Proteins 0.000 description 7
- 230000033558 biomineral tissue development Effects 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 230000015556 catabolic process Effects 0.000 description 7
- 230000009918 complex formation Effects 0.000 description 7
- 238000006731 degradation reaction Methods 0.000 description 7
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 7
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 238000003197 gene knockdown Methods 0.000 description 7
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 7
- 108010050848 glycylleucine Proteins 0.000 description 7
- 108010015792 glycyllysine Proteins 0.000 description 7
- 239000010452 phosphate Substances 0.000 description 7
- 108010070643 prolylglutamic acid Proteins 0.000 description 7
- JAMAWBXXKFGFGX-KZVJFYERSA-N Ala-Arg-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JAMAWBXXKFGFGX-KZVJFYERSA-N 0.000 description 6
- 125000000129 anionic group Chemical group 0.000 description 6
- 230000027455 binding Effects 0.000 description 6
- 230000001687 destabilization Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 150000007523 nucleic acids Chemical class 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 5
- LWUWMHIOBPTZBA-DCAQKATOSA-N Ala-Arg-Lys Chemical compound NC(=N)NCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CCCCN)C(O)=O LWUWMHIOBPTZBA-DCAQKATOSA-N 0.000 description 5
- 102000015735 Beta-catenin Human genes 0.000 description 5
- 108060000903 Beta-catenin Proteins 0.000 description 5
- HTTSBEBKVNEDFE-AUTRQRHGSA-N Glu-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N HTTSBEBKVNEDFE-AUTRQRHGSA-N 0.000 description 5
- HEWWNLVEWBJBKA-WDCWCFNPSA-N Lys-Gln-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN HEWWNLVEWBJBKA-WDCWCFNPSA-N 0.000 description 5
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 5
- XTAUQCGQFJQGEJ-NHCYSSNCSA-N Val-Gln-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N XTAUQCGQFJQGEJ-NHCYSSNCSA-N 0.000 description 5
- 108010087924 alanylproline Proteins 0.000 description 5
- 108010093581 aspartyl-proline Proteins 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 108020004999 messenger RNA Proteins 0.000 description 5
- 238000001000 micrograph Methods 0.000 description 5
- 102000039446 nucleic acids Human genes 0.000 description 5
- 108020004707 nucleic acids Proteins 0.000 description 5
- 229920000744 poly(arginines) Polymers 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- NHWYNIZWLJYZAG-XVYDVKMFSA-N Ala-Ser-His Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N NHWYNIZWLJYZAG-XVYDVKMFSA-N 0.000 description 4
- OMMIEVATLAGRCK-BYPYZUCNSA-N Asp-Gly-Gly Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)NCC(O)=O OMMIEVATLAGRCK-BYPYZUCNSA-N 0.000 description 4
- BVFQOPGFOQVZTE-ACZMJKKPSA-N Cys-Gln-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O BVFQOPGFOQVZTE-ACZMJKKPSA-N 0.000 description 4
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 4
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 4
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 4
- HDOYNXLPTRQLAD-JBDRJPRFSA-N Ile-Ala-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)O)N HDOYNXLPTRQLAD-JBDRJPRFSA-N 0.000 description 4
- CDGLBYSAZFIIJO-RCOVLWMOSA-N Ile-Gly-Gly Chemical compound CC[C@H](C)[C@H]([NH3+])C(=O)NCC(=O)NCC([O-])=O CDGLBYSAZFIIJO-RCOVLWMOSA-N 0.000 description 4
- YJRSIJZUIUANHO-NAKRPEOUSA-N Ile-Val-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)O)N YJRSIJZUIUANHO-NAKRPEOUSA-N 0.000 description 4
- 102100022699 Lymphoid enhancer-binding factor 1 Human genes 0.000 description 4
- VDGTVWFMRXVQCT-GUBZILKMSA-N Pro-Glu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 VDGTVWFMRXVQCT-GUBZILKMSA-N 0.000 description 4
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 4
- OOKCGAYXSNJBGQ-ZLUOBGJFSA-N Ser-Asn-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OOKCGAYXSNJBGQ-ZLUOBGJFSA-N 0.000 description 4
- PURRNJBBXDDWLX-ZDLURKLDSA-N Ser-Thr-Gly Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CO)N)O PURRNJBBXDDWLX-ZDLURKLDSA-N 0.000 description 4
- 230000009102 absorption Effects 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 108010070944 alanylhistidine Proteins 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 150000007942 carboxylates Chemical class 0.000 description 4
- -1 cationic amino acid Chemical class 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 108010038320 lysylphenylalanine Proteins 0.000 description 4
- 210000004940 nucleus Anatomy 0.000 description 4
- 108020003175 receptors Proteins 0.000 description 4
- 102000005962 receptors Human genes 0.000 description 4
- 230000011664 signaling Effects 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- RIIVUOJDDQXHRV-SRVKXCTJSA-N Arg-Lys-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O RIIVUOJDDQXHRV-SRVKXCTJSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- UFNSPPFJOHNXRE-AUTRQRHGSA-N Gln-Gln-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UFNSPPFJOHNXRE-AUTRQRHGSA-N 0.000 description 3
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 3
- GLYJPWIRLBAIJH-UHFFFAOYSA-N Ile-Lys-Pro Natural products CCC(C)C(N)C(=O)NC(CCCCN)C(=O)N1CCCC1C(O)=O GLYJPWIRLBAIJH-UHFFFAOYSA-N 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 108010043958 Peptoids Proteins 0.000 description 3
- IFMDQWDAJUMMJC-DCAQKATOSA-N Pro-Ala-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O IFMDQWDAJUMMJC-DCAQKATOSA-N 0.000 description 3
- DWGFLKQSGRUQTI-IHRRRGAJSA-N Pro-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1 DWGFLKQSGRUQTI-IHRRRGAJSA-N 0.000 description 3
- 238000011529 RT qPCR Methods 0.000 description 3
- 108091081024 Start codon Proteins 0.000 description 3
- LKJCABTUFGTPPY-HJGDQZAQSA-N Thr-Pro-Gln Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O LKJCABTUFGTPPY-HJGDQZAQSA-N 0.000 description 3
- 108050003627 Wnt Proteins 0.000 description 3
- 102000013814 Wnt Human genes 0.000 description 3
- 230000021736 acetylation Effects 0.000 description 3
- 238000006640 acetylation reaction Methods 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 108010077245 asparaginyl-proline Proteins 0.000 description 3
- 108010068265 aspartyltyrosine Proteins 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 239000013613 expression plasmid Substances 0.000 description 3
- 108010025306 histidylleucine Proteins 0.000 description 3
- 210000002997 osteoclast Anatomy 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 230000002035 prolonged effect Effects 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 108010073969 valyllysine Proteins 0.000 description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- DVWVZSJAYIJZFI-FXQIFTODSA-N Ala-Arg-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O DVWVZSJAYIJZFI-FXQIFTODSA-N 0.000 description 2
- WDIYWDJLXOCGRW-ACZMJKKPSA-N Ala-Asp-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WDIYWDJLXOCGRW-ACZMJKKPSA-N 0.000 description 2
- OQCPATDFWYYDDX-HGNGGELXSA-N Ala-Gln-His Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O OQCPATDFWYYDDX-HGNGGELXSA-N 0.000 description 2
- BLGHHPHXVJWCNK-GUBZILKMSA-N Ala-Gln-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O BLGHHPHXVJWCNK-GUBZILKMSA-N 0.000 description 2
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 2
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 2
- AETQNIIFKCMVHP-UVBJJODRSA-N Ala-Trp-Arg Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AETQNIIFKCMVHP-UVBJJODRSA-N 0.000 description 2
- 108091023037 Aptamer Proteins 0.000 description 2
- HJVGMOYJDDXLMI-AVGNSLFASA-N Arg-Arg-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N HJVGMOYJDDXLMI-AVGNSLFASA-N 0.000 description 2
- MAISCYVJLBBRNU-DCAQKATOSA-N Arg-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N MAISCYVJLBBRNU-DCAQKATOSA-N 0.000 description 2
- GRRXPUAICOGISM-RWMBFGLXSA-N Arg-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O GRRXPUAICOGISM-RWMBFGLXSA-N 0.000 description 2
- BSYKSCBTTQKOJG-GUBZILKMSA-N Arg-Pro-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O BSYKSCBTTQKOJG-GUBZILKMSA-N 0.000 description 2
- XYOVHPDDWCEUDY-CIUDSAMLSA-N Asn-Ala-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O XYOVHPDDWCEUDY-CIUDSAMLSA-N 0.000 description 2
- PCKRJVZAQZWNKM-WHFBIAKZSA-N Asn-Asn-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O PCKRJVZAQZWNKM-WHFBIAKZSA-N 0.000 description 2
- HUAOKVVEVHACHR-CIUDSAMLSA-N Asn-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N HUAOKVVEVHACHR-CIUDSAMLSA-N 0.000 description 2
- CDGHMJJJHYKMPA-DLOVCJGASA-N Asn-Phe-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CC(=O)N)N CDGHMJJJHYKMPA-DLOVCJGASA-N 0.000 description 2
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 2
- RGKKALNPOYURGE-ZKWXMUAHSA-N Asp-Ala-Val Chemical compound N[C@@H](CC(=O)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O RGKKALNPOYURGE-ZKWXMUAHSA-N 0.000 description 2
- HMQDRBKQMLRCCG-GMOBBJLQSA-N Asp-Arg-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HMQDRBKQMLRCCG-GMOBBJLQSA-N 0.000 description 2
- DTNUIAJCPRMNBT-WHFBIAKZSA-N Asp-Gly-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](C)C(O)=O DTNUIAJCPRMNBT-WHFBIAKZSA-N 0.000 description 2
- KTTCQQNRRLCIBC-GHCJXIJMSA-N Asp-Ile-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O KTTCQQNRRLCIBC-GHCJXIJMSA-N 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 230000004568 DNA-binding Effects 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- XFKUFUJECJUQTQ-CIUDSAMLSA-N Gln-Gln-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O XFKUFUJECJUQTQ-CIUDSAMLSA-N 0.000 description 2
- YPMDZWPZFOZYFG-GUBZILKMSA-N Gln-Leu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YPMDZWPZFOZYFG-GUBZILKMSA-N 0.000 description 2
- CVRUVYDNRPSKBM-QEJZJMRPSA-N Gln-Trp-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N CVRUVYDNRPSKBM-QEJZJMRPSA-N 0.000 description 2
- FYBSCGZLICNOBA-XQXXSGGOSA-N Glu-Ala-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FYBSCGZLICNOBA-XQXXSGGOSA-N 0.000 description 2
- NCWOMXABNYEPLY-NRPADANISA-N Glu-Ala-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O NCWOMXABNYEPLY-NRPADANISA-N 0.000 description 2
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 2
- IRXNJYPKBVERCW-DCAQKATOSA-N Glu-Leu-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O IRXNJYPKBVERCW-DCAQKATOSA-N 0.000 description 2
- ZIYGTCDTJJCDDP-JYJNAYRXSA-N Glu-Phe-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZIYGTCDTJJCDDP-JYJNAYRXSA-N 0.000 description 2
- JRDYDYXZKFNNRQ-XPUUQOCRSA-N Gly-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN JRDYDYXZKFNNRQ-XPUUQOCRSA-N 0.000 description 2
- STVHDEHTKFXBJQ-LAEOZQHASA-N Gly-Glu-Ile Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O STVHDEHTKFXBJQ-LAEOZQHASA-N 0.000 description 2
- GWNIGUKSRJBIHX-STQMWFEESA-N Gly-Tyr-Arg Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)CN)O GWNIGUKSRJBIHX-STQMWFEESA-N 0.000 description 2
- ZVXMEWXHFBYJPI-LSJOCFKGSA-N Gly-Val-Ile Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZVXMEWXHFBYJPI-LSJOCFKGSA-N 0.000 description 2
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 2
- VJJSDSNFXCWCEJ-DJFWLOJKSA-N His-Ile-Asn Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O VJJSDSNFXCWCEJ-DJFWLOJKSA-N 0.000 description 2
- UXSATKFPUVZVDK-KKUMJFAQSA-N His-Lys-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC1=CN=CN1)N UXSATKFPUVZVDK-KKUMJFAQSA-N 0.000 description 2
- ZVKDCQVQTGYBQT-LSJOCFKGSA-N His-Pro-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O ZVKDCQVQTGYBQT-LSJOCFKGSA-N 0.000 description 2
- 102000003893 Histone acetyltransferases Human genes 0.000 description 2
- 108090000246 Histone acetyltransferases Proteins 0.000 description 2
- 101001045751 Homo sapiens Hepatocyte nuclear factor 1-alpha Proteins 0.000 description 2
- 101000972291 Homo sapiens Lymphoid enhancer-binding factor 1 Proteins 0.000 description 2
- RWIKBYVJQAJYDP-BJDJZHNGSA-N Ile-Ala-Lys Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN RWIKBYVJQAJYDP-BJDJZHNGSA-N 0.000 description 2
- IGJWJGIHUFQANP-LAEOZQHASA-N Ile-Gly-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CCC(=O)N)C(=O)O)N IGJWJGIHUFQANP-LAEOZQHASA-N 0.000 description 2
- NGKPIPCGMLWHBX-WZLNRYEVSA-N Ile-Tyr-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N NGKPIPCGMLWHBX-WZLNRYEVSA-N 0.000 description 2
- KXUKTDGKLAOCQK-LSJOCFKGSA-N Ile-Val-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O KXUKTDGKLAOCQK-LSJOCFKGSA-N 0.000 description 2
- XBBKIIGCUMBKCO-JXUBOQSCSA-N Leu-Ala-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XBBKIIGCUMBKCO-JXUBOQSCSA-N 0.000 description 2
- STAVRDQLZOTNKJ-RHYQMDGZSA-N Leu-Arg-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STAVRDQLZOTNKJ-RHYQMDGZSA-N 0.000 description 2
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 2
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 2
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 2
- UCDHVOALNXENLC-KBPBESRZSA-N Leu-Gly-Tyr Chemical compound CC(C)C[C@H]([NH3+])C(=O)NCC(=O)N[C@H](C([O-])=O)CC1=CC=C(O)C=C1 UCDHVOALNXENLC-KBPBESRZSA-N 0.000 description 2
- LXKNSJLSGPNHSK-KKUMJFAQSA-N Leu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N LXKNSJLSGPNHSK-KKUMJFAQSA-N 0.000 description 2
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 2
- SVBJIZVVYJYGLA-DCAQKATOSA-N Leu-Ser-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O SVBJIZVVYJYGLA-DCAQKATOSA-N 0.000 description 2
- AAKRWBIIGKPOKQ-ONGXEEELSA-N Leu-Val-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AAKRWBIIGKPOKQ-ONGXEEELSA-N 0.000 description 2
- 108090001093 Lymphoid enhancer-binding factor 1 Proteins 0.000 description 2
- FZIJIFCXUCZHOL-CIUDSAMLSA-N Lys-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN FZIJIFCXUCZHOL-CIUDSAMLSA-N 0.000 description 2
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 2
- VHXMZJGOKIMETG-CQDKDKBSSA-N Lys-Ala-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCCCN)N VHXMZJGOKIMETG-CQDKDKBSSA-N 0.000 description 2
- SJNZALDHDUYDBU-IHRRRGAJSA-N Lys-Arg-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(O)=O SJNZALDHDUYDBU-IHRRRGAJSA-N 0.000 description 2
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 2
- QOJDBRUCOXQSSK-AJNGGQMLSA-N Lys-Ile-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(O)=O QOJDBRUCOXQSSK-AJNGGQMLSA-N 0.000 description 2
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 2
- VVURYEVJJTXWNE-ULQDDVLXSA-N Lys-Tyr-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O VVURYEVJJTXWNE-ULQDDVLXSA-N 0.000 description 2
- HMZPYMSEAALNAE-ULQDDVLXSA-N Lys-Val-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O HMZPYMSEAALNAE-ULQDDVLXSA-N 0.000 description 2
- OXHSZBRPUGNMKW-DCAQKATOSA-N Met-Gln-Arg Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O OXHSZBRPUGNMKW-DCAQKATOSA-N 0.000 description 2
- DGNZGCQSVGGYJS-BQBZGAKWSA-N Met-Gly-Asp Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O DGNZGCQSVGGYJS-BQBZGAKWSA-N 0.000 description 2
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 108091093037 Peptide nucleic acid Proteins 0.000 description 2
- HTTYNOXBBOWZTB-SRVKXCTJSA-N Phe-Asn-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N HTTYNOXBBOWZTB-SRVKXCTJSA-N 0.000 description 2
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 2
- DEZCWWXTRAKZKJ-UFYCRDLUSA-N Phe-Phe-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O DEZCWWXTRAKZKJ-UFYCRDLUSA-N 0.000 description 2
- 108010020346 Polyglutamic Acid Proteins 0.000 description 2
- OBVCYFIHIIYIQF-CIUDSAMLSA-N Pro-Asn-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O OBVCYFIHIIYIQF-CIUDSAMLSA-N 0.000 description 2
- AJCRQOHDLCBHFA-SRVKXCTJSA-N Pro-His-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O AJCRQOHDLCBHFA-SRVKXCTJSA-N 0.000 description 2
- RUDOLGWDSKQQFF-DCAQKATOSA-N Pro-Leu-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O RUDOLGWDSKQQFF-DCAQKATOSA-N 0.000 description 2
- IXUGADGDCQDLSA-FXQIFTODSA-N Ser-Gln-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N IXUGADGDCQDLSA-FXQIFTODSA-N 0.000 description 2
- OJPHFSOMBZKQKQ-GUBZILKMSA-N Ser-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CO OJPHFSOMBZKQKQ-GUBZILKMSA-N 0.000 description 2
- SZRNDHWMVSFPSP-XKBZYTNZSA-N Ser-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N)O SZRNDHWMVSFPSP-XKBZYTNZSA-N 0.000 description 2
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 2
- 108020004459 Small interfering RNA Proteins 0.000 description 2
- VFEHSAJCWWHDBH-RHYQMDGZSA-N Thr-Arg-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VFEHSAJCWWHDBH-RHYQMDGZSA-N 0.000 description 2
- SLUWOCTZVGMURC-BFHQHQDPSA-N Thr-Gly-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O SLUWOCTZVGMURC-BFHQHQDPSA-N 0.000 description 2
- VYEHBMMAJFVTOI-JHEQGTHGSA-N Thr-Gly-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O VYEHBMMAJFVTOI-JHEQGTHGSA-N 0.000 description 2
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 2
- 102100030627 Transcription factor 7 Human genes 0.000 description 2
- ASQFIHTXXMFENG-XPUUQOCRSA-N Val-Ala-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O ASQFIHTXXMFENG-XPUUQOCRSA-N 0.000 description 2
- OVLIFGQSBSNGHY-KKHAAJSZSA-N Val-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N)O OVLIFGQSBSNGHY-KKHAAJSZSA-N 0.000 description 2
- SYOMXKPPFZRELL-ONGXEEELSA-N Val-Gly-Lys Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)O)N SYOMXKPPFZRELL-ONGXEEELSA-N 0.000 description 2
- LKUDRJSNRWVGMS-QSFUFRPTSA-N Val-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LKUDRJSNRWVGMS-QSFUFRPTSA-N 0.000 description 2
- JAKHAONCJJZVHT-DCAQKATOSA-N Val-Lys-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)O)N JAKHAONCJJZVHT-DCAQKATOSA-N 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 102000044880 Wnt3A Human genes 0.000 description 2
- 108700013515 Wnt3A Proteins 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 2
- 108010038633 aspartylglutamate Proteins 0.000 description 2
- 210000002798 bone marrow cell Anatomy 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 230000004700 cellular uptake Effects 0.000 description 2
- 230000000536 complexating effect Effects 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 230000005782 double-strand break Effects 0.000 description 2
- 238000013265 extended release Methods 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- HPAIKDPJURGQLN-UHFFFAOYSA-N glycyl-L-histidyl-L-phenylalanine Natural products C=1C=CC=CC=1CC(C(O)=O)NC(=O)C(NC(=O)CN)CC1=CN=CN1 HPAIKDPJURGQLN-UHFFFAOYSA-N 0.000 description 2
- 108010027668 glycyl-alanyl-valine Proteins 0.000 description 2
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 2
- 108010078326 glycyl-glycyl-valine Proteins 0.000 description 2
- 108010037850 glycylvaline Proteins 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 108010028295 histidylhistidine Proteins 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- KXCLCNHUUKTANI-RBIYJLQWSA-N keratan Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@H](COS(O)(=O)=O)O[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@H](O[C@@H](O[C@H]3[C@H]([C@@H](COS(O)(=O)=O)O[C@@H](O)[C@@H]3O)O)[C@H](NC(C)=O)[C@H]2O)COS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@H]1O KXCLCNHUUKTANI-RBIYJLQWSA-N 0.000 description 2
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 2
- 108010047926 leucyl-lysyl-tyrosine Proteins 0.000 description 2
- 108010030617 leucyl-phenylalanyl-valine Proteins 0.000 description 2
- 108010025153 lysyl-alanyl-alanine Proteins 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000032965 negative regulation of cell volume Effects 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 239000013047 polymeric layer Substances 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 241000894007 species Species 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- DSLBDPPHINVUID-REOHCLBHSA-N (2s)-2-aminobutanediamide Chemical compound NC(=O)[C@@H](N)CC(N)=O DSLBDPPHINVUID-REOHCLBHSA-N 0.000 description 1
- LCTORNIWLGOBPB-GASJEMHNSA-N (3r,4s,5s,6r)-2-amino-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound NC1(O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O LCTORNIWLGOBPB-GASJEMHNSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- 102100036601 Aggrecan core protein Human genes 0.000 description 1
- 108010067219 Aggrecans Proteins 0.000 description 1
- FJVAQLJNTSUQPY-CIUDSAMLSA-N Ala-Ala-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN FJVAQLJNTSUQPY-CIUDSAMLSA-N 0.000 description 1
- QDRGPQWIVZNJQD-CIUDSAMLSA-N Ala-Arg-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O QDRGPQWIVZNJQD-CIUDSAMLSA-N 0.000 description 1
- CXZFXHGJJPVUJE-CIUDSAMLSA-N Ala-Cys-Leu Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)O)N CXZFXHGJJPVUJE-CIUDSAMLSA-N 0.000 description 1
- PAIHPOGPJVUFJY-WDSKDSINSA-N Ala-Glu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PAIHPOGPJVUFJY-WDSKDSINSA-N 0.000 description 1
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 1
- QPBSRMDNJOTFAL-AICCOOGYSA-N Ala-Leu-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QPBSRMDNJOTFAL-AICCOOGYSA-N 0.000 description 1
- BHTBAVZSZCQZPT-GUBZILKMSA-N Ala-Pro-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N BHTBAVZSZCQZPT-GUBZILKMSA-N 0.000 description 1
- XCIGOVDXZULBBV-DCAQKATOSA-N Ala-Val-Lys Chemical compound CC(C)[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CCCCN)C(O)=O XCIGOVDXZULBBV-DCAQKATOSA-N 0.000 description 1
- SVHRPCMZTWZROG-DCAQKATOSA-N Arg-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N SVHRPCMZTWZROG-DCAQKATOSA-N 0.000 description 1
- CYXCAHZVPFREJD-LURJTMIESA-N Arg-Gly-Gly Chemical compound NC(=N)NCCC[C@H](N)C(=O)NCC(=O)NCC(O)=O CYXCAHZVPFREJD-LURJTMIESA-N 0.000 description 1
- OVQJAKFLFTZDNC-GUBZILKMSA-N Arg-Pro-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O OVQJAKFLFTZDNC-GUBZILKMSA-N 0.000 description 1
- LRPZJPMQGKGHSG-XGEHTFHBSA-N Arg-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)O LRPZJPMQGKGHSG-XGEHTFHBSA-N 0.000 description 1
- BECXEHHOZNFFFX-IHRRRGAJSA-N Arg-Ser-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BECXEHHOZNFFFX-IHRRRGAJSA-N 0.000 description 1
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 1
- 102000004452 Arginase Human genes 0.000 description 1
- 108700024123 Arginases Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- GMRGSBAMMMVDGG-GUBZILKMSA-N Asn-Arg-Arg Chemical compound C(C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N GMRGSBAMMMVDGG-GUBZILKMSA-N 0.000 description 1
- HLTLEIXYIJDFOY-ZLUOBGJFSA-N Asn-Cys-Asn Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O HLTLEIXYIJDFOY-ZLUOBGJFSA-N 0.000 description 1
- OKZOABJQOMAYEC-NUMRIWBASA-N Asn-Gln-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OKZOABJQOMAYEC-NUMRIWBASA-N 0.000 description 1
- PPMTUXJSQDNUDE-CIUDSAMLSA-N Asn-Glu-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PPMTUXJSQDNUDE-CIUDSAMLSA-N 0.000 description 1
- KLKHFFMNGWULBN-VKHMYHEASA-N Asn-Gly Chemical compound NC(=O)C[C@H](N)C(=O)NCC(O)=O KLKHFFMNGWULBN-VKHMYHEASA-N 0.000 description 1
- ZTRJUKDEALVRMW-SRVKXCTJSA-N Asn-His-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CC(=O)N)N ZTRJUKDEALVRMW-SRVKXCTJSA-N 0.000 description 1
- YGHCVNQOZZMHRZ-DJFWLOJKSA-N Asn-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC(=O)N)N YGHCVNQOZZMHRZ-DJFWLOJKSA-N 0.000 description 1
- NVWJMQNYLYWVNQ-BYULHYEWSA-N Asn-Ile-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O NVWJMQNYLYWVNQ-BYULHYEWSA-N 0.000 description 1
- JLNFZLNDHONLND-GARJFASQSA-N Asn-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N JLNFZLNDHONLND-GARJFASQSA-N 0.000 description 1
- FODVBOKTYKYRFJ-CIUDSAMLSA-N Asn-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)N)N FODVBOKTYKYRFJ-CIUDSAMLSA-N 0.000 description 1
- HPASIOLTWSNMFB-OLHMAJIHSA-N Asn-Thr-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O HPASIOLTWSNMFB-OLHMAJIHSA-N 0.000 description 1
- OERMIMJQPQUIPK-FXQIFTODSA-N Asp-Arg-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O OERMIMJQPQUIPK-FXQIFTODSA-N 0.000 description 1
- SOYOSFXLXYZNRG-CIUDSAMLSA-N Asp-Arg-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O SOYOSFXLXYZNRG-CIUDSAMLSA-N 0.000 description 1
- NZWDWXSWUQCNMG-GARJFASQSA-N Asp-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)C(=O)O NZWDWXSWUQCNMG-GARJFASQSA-N 0.000 description 1
- VWWAFGHMPWBKEP-GMOBBJLQSA-N Asp-Met-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(=O)O)N VWWAFGHMPWBKEP-GMOBBJLQSA-N 0.000 description 1
- DRCOAZZDQRCGGP-GHCJXIJMSA-N Asp-Ser-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DRCOAZZDQRCGGP-GHCJXIJMSA-N 0.000 description 1
- 102100021277 Beta-secretase 2 Human genes 0.000 description 1
- 101710150190 Beta-secretase 2 Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 108091033409 CRISPR Proteins 0.000 description 1
- 108091079001 CRISPR RNA Proteins 0.000 description 1
- 238000010354 CRISPR gene editing Methods 0.000 description 1
- 229920002567 Chondroitin Polymers 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- DEVDFMRWZASYOF-ZLUOBGJFSA-N Cys-Asn-Asp Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O DEVDFMRWZASYOF-ZLUOBGJFSA-N 0.000 description 1
- WTNLLMQAFPOCTJ-GARJFASQSA-N Cys-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CS)N)C(=O)O WTNLLMQAFPOCTJ-GARJFASQSA-N 0.000 description 1
- VDUPGIDTWNQAJD-CIUDSAMLSA-N Cys-Lys-Cys Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CS)C(O)=O VDUPGIDTWNQAJD-CIUDSAMLSA-N 0.000 description 1
- QQAYIVHVRFJICE-AEJSXWLSSA-N Cys-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CS)N QQAYIVHVRFJICE-AEJSXWLSSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-VANFPWTGSA-N D-mannopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@@H]1O AEMOLEFTQBMNLQ-VANFPWTGSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- LWDGZZGWDMHBOF-FXQIFTODSA-N Gln-Glu-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O LWDGZZGWDMHBOF-FXQIFTODSA-N 0.000 description 1
- CLPQUWHBWXFJOX-BQBZGAKWSA-N Gln-Gly-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O CLPQUWHBWXFJOX-BQBZGAKWSA-N 0.000 description 1
- GLAPJAHOPFSLKL-SRVKXCTJSA-N Gln-His-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)N)N GLAPJAHOPFSLKL-SRVKXCTJSA-N 0.000 description 1
- QBLMTCRYYTVUQY-GUBZILKMSA-N Gln-Leu-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QBLMTCRYYTVUQY-GUBZILKMSA-N 0.000 description 1
- WOMUDRVDJMHTCV-DCAQKATOSA-N Glu-Arg-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WOMUDRVDJMHTCV-DCAQKATOSA-N 0.000 description 1
- MLCPTRRNICEKIS-FXQIFTODSA-N Glu-Asn-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MLCPTRRNICEKIS-FXQIFTODSA-N 0.000 description 1
- FLQAKQOBSPFGKG-CIUDSAMLSA-N Glu-Cys-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FLQAKQOBSPFGKG-CIUDSAMLSA-N 0.000 description 1
- VGUYMZGLJUJRBV-YVNDNENWSA-N Glu-Ile-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O VGUYMZGLJUJRBV-YVNDNENWSA-N 0.000 description 1
- HRBYTAIBKPNZKQ-AVGNSLFASA-N Glu-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(O)=O HRBYTAIBKPNZKQ-AVGNSLFASA-N 0.000 description 1
- LKOAAMXDJGEYMS-ZPFDUUQYSA-N Glu-Met-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LKOAAMXDJGEYMS-ZPFDUUQYSA-N 0.000 description 1
- SOEPMWQCTJITPZ-SRVKXCTJSA-N Glu-Met-Lys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N SOEPMWQCTJITPZ-SRVKXCTJSA-N 0.000 description 1
- HQOGXFLBAKJUMH-CIUDSAMLSA-N Glu-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)O)N HQOGXFLBAKJUMH-CIUDSAMLSA-N 0.000 description 1
- SOYWRINXUSUWEQ-DLOVCJGASA-N Glu-Val-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCC(O)=O SOYWRINXUSUWEQ-DLOVCJGASA-N 0.000 description 1
- PUUYVMYCMIWHFE-BQBZGAKWSA-N Gly-Ala-Arg Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PUUYVMYCMIWHFE-BQBZGAKWSA-N 0.000 description 1
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 1
- LXXLEUBUOMCAMR-NKWVEPMBSA-N Gly-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)CN)C(=O)O LXXLEUBUOMCAMR-NKWVEPMBSA-N 0.000 description 1
- HQRHFUYMGCHHJS-LURJTMIESA-N Gly-Gly-Arg Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N HQRHFUYMGCHHJS-LURJTMIESA-N 0.000 description 1
- IDOGEHIWMJMAHT-BYPYZUCNSA-N Gly-Gly-Cys Chemical compound NCC(=O)NCC(=O)N[C@@H](CS)C(O)=O IDOGEHIWMJMAHT-BYPYZUCNSA-N 0.000 description 1
- QPTNELDXWKRIFX-YFKPBYRVSA-N Gly-Gly-Gln Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O QPTNELDXWKRIFX-YFKPBYRVSA-N 0.000 description 1
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 1
- INLIXXRWNUKVCF-JTQLQIEISA-N Gly-Gly-Tyr Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 INLIXXRWNUKVCF-JTQLQIEISA-N 0.000 description 1
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 1
- ULZCYBYDTUMHNF-IUCAKERBSA-N Gly-Leu-Glu Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ULZCYBYDTUMHNF-IUCAKERBSA-N 0.000 description 1
- IUKIDFVOUHZRAK-QWRGUYRKSA-N Gly-Lys-His Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IUKIDFVOUHZRAK-QWRGUYRKSA-N 0.000 description 1
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 1
- OOCFXNOVSLSHAB-IUCAKERBSA-N Gly-Pro-Pro Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 OOCFXNOVSLSHAB-IUCAKERBSA-N 0.000 description 1
- ABPRMMYHROQBLY-NKWVEPMBSA-N Gly-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)CN)C(=O)O ABPRMMYHROQBLY-NKWVEPMBSA-N 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229920002971 Heparan sulfate Polymers 0.000 description 1
- IPIVXQQRZXEUGW-UWJYBYFXSA-N His-Ala-His Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IPIVXQQRZXEUGW-UWJYBYFXSA-N 0.000 description 1
- LSQHWKPPOFDHHZ-YUMQZZPRSA-N His-Asp-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N LSQHWKPPOFDHHZ-YUMQZZPRSA-N 0.000 description 1
- RAVLQPXCMRCLKT-KBPBESRZSA-N His-Gly-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RAVLQPXCMRCLKT-KBPBESRZSA-N 0.000 description 1
- NDKSHNQINMRKHT-PEXQALLHSA-N His-Ile-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC1=CN=CN1)N NDKSHNQINMRKHT-PEXQALLHSA-N 0.000 description 1
- ZRSJXIKQXUGKRB-TUBUOCAGSA-N His-Ile-Thr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZRSJXIKQXUGKRB-TUBUOCAGSA-N 0.000 description 1
- ZFDKSLBEWYCOCS-BZSNNMDCSA-N His-Phe-Lys Chemical compound C([C@@H](C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@@H](N)CC=1NC=NC=1)C1=CC=CC=C1 ZFDKSLBEWYCOCS-BZSNNMDCSA-N 0.000 description 1
- 102100039869 Histone H2B type F-S Human genes 0.000 description 1
- 102000006947 Histones Human genes 0.000 description 1
- 101001035372 Homo sapiens Histone H2B type F-S Proteins 0.000 description 1
- 101001043594 Homo sapiens Low-density lipoprotein receptor-related protein 5 Proteins 0.000 description 1
- 101000808011 Homo sapiens Vascular endothelial growth factor A Proteins 0.000 description 1
- AQTWDZDISVGCAC-CFMVVWHZSA-N Ile-Asp-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N AQTWDZDISVGCAC-CFMVVWHZSA-N 0.000 description 1
- UBHUJPVCJHPSEU-GRLWGSQLSA-N Ile-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N UBHUJPVCJHPSEU-GRLWGSQLSA-N 0.000 description 1
- LPXHYGGZJOCAFR-MNXVOIDGSA-N Ile-Glu-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N LPXHYGGZJOCAFR-MNXVOIDGSA-N 0.000 description 1
- PNDMHTTXXPUQJH-RWRJDSDZSA-N Ile-Glu-Thr Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H]([C@H](O)C)C(=O)O PNDMHTTXXPUQJH-RWRJDSDZSA-N 0.000 description 1
- FFAUOCITXBMRBT-YTFOTSKYSA-N Ile-Lys-Ile Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FFAUOCITXBMRBT-YTFOTSKYSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- 101710134930 Import motor subunit, mitochondrial Proteins 0.000 description 1
- 229920000288 Keratan sulfate Polymers 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- KVRKAGGMEWNURO-CIUDSAMLSA-N Leu-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N KVRKAGGMEWNURO-CIUDSAMLSA-N 0.000 description 1
- BOFAFKVZQUMTID-AVGNSLFASA-N Leu-Gln-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N BOFAFKVZQUMTID-AVGNSLFASA-N 0.000 description 1
- LOLUPZNNADDTAA-AVGNSLFASA-N Leu-Gln-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LOLUPZNNADDTAA-AVGNSLFASA-N 0.000 description 1
- QVFGXCVIXXBFHO-AVGNSLFASA-N Leu-Glu-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O QVFGXCVIXXBFHO-AVGNSLFASA-N 0.000 description 1
- PRZVBIAOPFGAQF-SRVKXCTJSA-N Leu-Glu-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O PRZVBIAOPFGAQF-SRVKXCTJSA-N 0.000 description 1
- LAPSXOAUPNOINL-YUMQZZPRSA-N Leu-Gly-Asp Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O LAPSXOAUPNOINL-YUMQZZPRSA-N 0.000 description 1
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 1
- HGFGEMSVBMCFKK-MNXVOIDGSA-N Leu-Ile-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O HGFGEMSVBMCFKK-MNXVOIDGSA-N 0.000 description 1
- QJXHMYMRGDOHRU-NHCYSSNCSA-N Leu-Ile-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O QJXHMYMRGDOHRU-NHCYSSNCSA-N 0.000 description 1
- IEWBEPKLKUXQBU-VOAKCMCISA-N Leu-Leu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IEWBEPKLKUXQBU-VOAKCMCISA-N 0.000 description 1
- 102100021926 Low-density lipoprotein receptor-related protein 5 Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- WGLAORUKDGRINI-WDCWCFNPSA-N Lys-Glu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGLAORUKDGRINI-WDCWCFNPSA-N 0.000 description 1
- YDDDRTIPNTWGIG-SRVKXCTJSA-N Lys-Lys-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O YDDDRTIPNTWGIG-SRVKXCTJSA-N 0.000 description 1
- MSSABBQOBUZFKZ-IHRRRGAJSA-N Lys-Pro-His Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCCCN)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O MSSABBQOBUZFKZ-IHRRRGAJSA-N 0.000 description 1
- XATKLFSXFINPSB-JYJNAYRXSA-N Lys-Tyr-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O XATKLFSXFINPSB-JYJNAYRXSA-N 0.000 description 1
- OHXUUQDOBQKSNB-AVGNSLFASA-N Lys-Val-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O OHXUUQDOBQKSNB-AVGNSLFASA-N 0.000 description 1
- VWJFOUBDZIUXGA-AVGNSLFASA-N Lys-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCCCN)N VWJFOUBDZIUXGA-AVGNSLFASA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 208000003263 MASS syndrome Diseases 0.000 description 1
- QDMUMFDBUVOZOY-GUBZILKMSA-N Met-Arg-Cys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O)N QDMUMFDBUVOZOY-GUBZILKMSA-N 0.000 description 1
- ZEDVFJPQNNBMST-CYDGBPFRSA-N Met-Arg-Ile Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZEDVFJPQNNBMST-CYDGBPFRSA-N 0.000 description 1
- FZUNSVYYPYJYAP-NAKRPEOUSA-N Met-Ile-Ala Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O FZUNSVYYPYJYAP-NAKRPEOUSA-N 0.000 description 1
- DJJBHQHOZLUBCN-WDSOQIARSA-N Met-Lys-Trp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DJJBHQHOZLUBCN-WDSOQIARSA-N 0.000 description 1
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- UNLYPPYNDXHGDG-IHRRRGAJSA-N Phe-Gln-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 UNLYPPYNDXHGDG-IHRRRGAJSA-N 0.000 description 1
- HOYQLNNGMHXZDW-KKUMJFAQSA-N Phe-Glu-Arg Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HOYQLNNGMHXZDW-KKUMJFAQSA-N 0.000 description 1
- AUJWXNGCAQWLEI-KBPBESRZSA-N Phe-Lys-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O AUJWXNGCAQWLEI-KBPBESRZSA-N 0.000 description 1
- OAOLATANIHTNCZ-IHRRRGAJSA-N Phe-Met-Asp Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N OAOLATANIHTNCZ-IHRRRGAJSA-N 0.000 description 1
- 229920000805 Polyaspartic acid Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- YFNOUBWUIIJQHF-LPEHRKFASA-N Pro-Asp-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O YFNOUBWUIIJQHF-LPEHRKFASA-N 0.000 description 1
- SZZBUDVXWZZPDH-BQBZGAKWSA-N Pro-Cys-Gly Chemical compound OC(=O)CNC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1 SZZBUDVXWZZPDH-BQBZGAKWSA-N 0.000 description 1
- HQVPQXMCQKXARZ-FXQIFTODSA-N Pro-Cys-Ser Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O HQVPQXMCQKXARZ-FXQIFTODSA-N 0.000 description 1
- XZONQWUEBAFQPO-HJGDQZAQSA-N Pro-Gln-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XZONQWUEBAFQPO-HJGDQZAQSA-N 0.000 description 1
- AQGUSRZKDZYGGV-GMOBBJLQSA-N Pro-Ile-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O AQGUSRZKDZYGGV-GMOBBJLQSA-N 0.000 description 1
- UREQLMJCKFLLHM-NAKRPEOUSA-N Pro-Ile-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O UREQLMJCKFLLHM-NAKRPEOUSA-N 0.000 description 1
- WOIFYRZPIORBRY-AVGNSLFASA-N Pro-Lys-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O WOIFYRZPIORBRY-AVGNSLFASA-N 0.000 description 1
- CGSOWZUPLOKYOR-AVGNSLFASA-N Pro-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 CGSOWZUPLOKYOR-AVGNSLFASA-N 0.000 description 1
- CZCCVJUUWBMISW-FXQIFTODSA-N Pro-Ser-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O CZCCVJUUWBMISW-FXQIFTODSA-N 0.000 description 1
- 241000242739 Renilla Species 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 101150098533 SOST gene Proteins 0.000 description 1
- YMEXHZTVKDAKIY-GHCJXIJMSA-N Ser-Asn-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(O)=O YMEXHZTVKDAKIY-GHCJXIJMSA-N 0.000 description 1
- KMWFXJCGRXBQAC-CIUDSAMLSA-N Ser-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N KMWFXJCGRXBQAC-CIUDSAMLSA-N 0.000 description 1
- LALNXSXEYFUUDD-GUBZILKMSA-N Ser-Glu-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LALNXSXEYFUUDD-GUBZILKMSA-N 0.000 description 1
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 1
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 1
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 1
- 108091027967 Small hairpin RNA Proteins 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- VASYSJHSMSBTDU-LKXGYXEUSA-N Thr-Asn-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N)O VASYSJHSMSBTDU-LKXGYXEUSA-N 0.000 description 1
- NRUPKQSXTJNQGD-XGEHTFHBSA-N Thr-Cys-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NRUPKQSXTJNQGD-XGEHTFHBSA-N 0.000 description 1
- UDQBCBUXAQIZAK-GLLZPBPUSA-N Thr-Glu-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UDQBCBUXAQIZAK-GLLZPBPUSA-N 0.000 description 1
- SIMKLINEDYOTKL-MBLNEYKQSA-N Thr-His-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](C)C(=O)O)N)O SIMKLINEDYOTKL-MBLNEYKQSA-N 0.000 description 1
- PUEWAXRPXOEQOW-HJGDQZAQSA-N Thr-Met-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(O)=O PUEWAXRPXOEQOW-HJGDQZAQSA-N 0.000 description 1
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 1
- ZHDQRPWESGUDST-JBACZVJFSA-N Trp-Phe-Gln Chemical compound C([C@H](NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(=O)N[C@@H](CCC(N)=O)C(O)=O)C1=CC=CC=C1 ZHDQRPWESGUDST-JBACZVJFSA-N 0.000 description 1
- XXJDYWYVZBHELV-TUSQITKMSA-N Trp-Trp-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CNC4=CC=CC=C43)C(=O)N[C@@H](CCCCN)C(=O)O)N XXJDYWYVZBHELV-TUSQITKMSA-N 0.000 description 1
- NSOMQRHZMJMZIE-GVARAGBVSA-N Tyr-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NSOMQRHZMJMZIE-GVARAGBVSA-N 0.000 description 1
- AYHSJESDFKREAR-KKUMJFAQSA-N Tyr-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 AYHSJESDFKREAR-KKUMJFAQSA-N 0.000 description 1
- NGALWFGCOMHUSN-AVGNSLFASA-N Tyr-Gln-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NGALWFGCOMHUSN-AVGNSLFASA-N 0.000 description 1
- ILTXFANLDMJWPR-SIUGBPQLSA-N Tyr-Ile-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N ILTXFANLDMJWPR-SIUGBPQLSA-N 0.000 description 1
- AZZLDIDWPZLCCW-ZEWNOJEFSA-N Tyr-Ile-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O AZZLDIDWPZLCCW-ZEWNOJEFSA-N 0.000 description 1
- XJPXTYLVMUZGNW-IHRRRGAJSA-N Tyr-Pro-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O XJPXTYLVMUZGNW-IHRRRGAJSA-N 0.000 description 1
- YYLHVUCSTXXKBS-IHRRRGAJSA-N Tyr-Pro-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YYLHVUCSTXXKBS-IHRRRGAJSA-N 0.000 description 1
- VPEFOFYNHBWFNQ-UFYCRDLUSA-N Tyr-Pro-Tyr Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 VPEFOFYNHBWFNQ-UFYCRDLUSA-N 0.000 description 1
- PQPWEALFTLKSEB-DZKIICNBSA-N Tyr-Val-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O PQPWEALFTLKSEB-DZKIICNBSA-N 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- XLDYBRXERHITNH-QSFUFRPTSA-N Val-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)C(C)C XLDYBRXERHITNH-QSFUFRPTSA-N 0.000 description 1
- CFSSLXZJEMERJY-NRPADANISA-N Val-Gln-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O CFSSLXZJEMERJY-NRPADANISA-N 0.000 description 1
- YCMXFKWYJFZFKS-LAEOZQHASA-N Val-Gln-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCMXFKWYJFZFKS-LAEOZQHASA-N 0.000 description 1
- SZTTYWIUCGSURQ-AUTRQRHGSA-N Val-Glu-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SZTTYWIUCGSURQ-AUTRQRHGSA-N 0.000 description 1
- LAYSXAOGWHKNED-XPUUQOCRSA-N Val-Gly-Ser Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LAYSXAOGWHKNED-XPUUQOCRSA-N 0.000 description 1
- VENKIVFKIPGEJN-NHCYSSNCSA-N Val-Met-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N VENKIVFKIPGEJN-NHCYSSNCSA-N 0.000 description 1
- ZXYPHBKIZLAQTL-QXEWZRGKSA-N Val-Pro-Asp Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZXYPHBKIZLAQTL-QXEWZRGKSA-N 0.000 description 1
- NHXZRXLFOBFMDM-AVGNSLFASA-N Val-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C NHXZRXLFOBFMDM-AVGNSLFASA-N 0.000 description 1
- UGFMVXRXULGLNO-XPUUQOCRSA-N Val-Ser-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O UGFMVXRXULGLNO-XPUUQOCRSA-N 0.000 description 1
- CEKSLIVSNNGOKH-KZVJFYERSA-N Val-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](C(C)C)N)O CEKSLIVSNNGOKH-KZVJFYERSA-N 0.000 description 1
- DOBHJKVVACOQTN-DZKIICNBSA-N Val-Tyr-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=C(O)C=C1 DOBHJKVVACOQTN-DZKIICNBSA-N 0.000 description 1
- PMKQKNBISAOSRI-XHSDSOJGSA-N Val-Tyr-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N PMKQKNBISAOSRI-XHSDSOJGSA-N 0.000 description 1
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 101150063416 add gene Proteins 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 235000009697 arginine Nutrition 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010008355 arginyl-glutamine Proteins 0.000 description 1
- 108010091092 arginyl-glycyl-proline Proteins 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000001479 atomic absorption spectroscopy Methods 0.000 description 1
- 230000002902 bimodal effect Effects 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- DLGJWSVWTWEWBJ-HGGSSLSASA-N chondroitin Chemical compound CC(O)=N[C@@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1OC1[C@H](O)[C@H](O)C=C(C(O)=O)O1 DLGJWSVWTWEWBJ-HGGSSLSASA-N 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 108010054813 diprotin B Proteins 0.000 description 1
- 230000002222 downregulating effect Effects 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 230000007783 downstream signaling Effects 0.000 description 1
- 238000002296 dynamic light scattering Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 238000001917 fluorescence detection Methods 0.000 description 1
- 238000002073 fluorescence micrograph Methods 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 108010033719 glycyl-histidyl-glycine Proteins 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 210000002288 golgi apparatus Anatomy 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 102000058223 human VEGFA Human genes 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 108010043322 lysyl-tryptophyl-alpha-lysine Proteins 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 230000034701 macropinocytosis Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 108010056582 methionylglutamic acid Proteins 0.000 description 1
- 108091070501 miRNA Proteins 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 230000025608 mitochondrion localization Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 239000002539 nanocarrier Substances 0.000 description 1
- 239000002121 nanofiber Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000001937 non-anti-biotic effect Effects 0.000 description 1
- 210000000633 nuclear envelope Anatomy 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 108010064470 polyaspartate Proteins 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010090894 prolylleucine Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000004055 small Interfering RNA Substances 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 230000004960 subcellular localization Effects 0.000 description 1
- 239000012134 supernatant fraction Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- ORZHVTYKPFFVMG-UHFFFAOYSA-N xylenol orange Chemical compound OC(=O)CN(CC(O)=O)CC1=C(O)C(C)=CC(C2(C3=CC=CC=C3S(=O)(=O)O2)C=2C=C(CN(CC(O)=O)CC(O)=O)C(O)=C(C)C=2)=C1 ORZHVTYKPFFVMG-UHFFFAOYSA-N 0.000 description 1
- 125000002256 xylenyl group Chemical class C1(C(C=CC=C1)C)(C)* 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/645—Polycationic or polyanionic oligopeptides, polypeptides or polyamino acids, e.g. polylysine, polyarginine, polyglutamic acid or peptide TAT
- A61K47/6455—Polycationic oligopeptides, polypeptides or polyamino acids, e.g. for complexing nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6923—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being an inorganic particle, e.g. ceramic particles, silica particles, ferrite or synsorb
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6927—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores
- A61K47/6929—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L77/00—Compositions of polyamides obtained by reactions forming a carboxylic amide link in the main chain; Compositions of derivatives of such polymers
- C08L77/04—Polyamides derived from alpha-amino carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L79/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen with or without oxygen or carbon only, not provided for in groups C08L61/00 - C08L77/00
- C08L79/04—Polycondensates having nitrogen-containing heterocyclic rings in the main chain; Polyhydrazides; Polyamide acids or similar polyimide precursors
- C08L79/08—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/90—Stable introduction of foreign DNA into chromosome
- C12N15/902—Stable introduction of foreign DNA into chromosome using homologous recombination
- C12N15/907—Stable introduction of foreign DNA into chromosome using homologous recombination in mammalian cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0008—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition
- A61K48/0025—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid
- A61K48/0041—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid the non-active part being polymeric
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/95—Protection of vectors from inactivation by agents such as antibodies or enzymes, e.g. using polymers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases [RNase]; Deoxyribonucleases [DNase]
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Nanotechnology (AREA)
- Ceramic Engineering (AREA)
- Inorganic Chemistry (AREA)
- Immunology (AREA)
- Physical Education & Sports Medicine (AREA)
- Rheumatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361881072P | 2013-09-23 | 2013-09-23 | |
| US61/881,072 | 2013-09-23 | ||
| PCT/US2014/057000 WO2015042585A1 (en) | 2013-09-23 | 2014-09-23 | Nanoparticle-mediated gene delivery, genomic editing and ligand-targeted modification in various cell populations |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160060133A true KR20160060133A (ko) | 2016-05-27 |
Family
ID=52689537
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167010607A Abandoned KR20160060133A (ko) | 2013-09-23 | 2014-09-23 | 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US10526616B2 (OSRAM) |
| EP (2) | EP3520822A1 (OSRAM) |
| JP (1) | JP6595459B2 (OSRAM) |
| KR (1) | KR20160060133A (OSRAM) |
| CN (1) | CN105579068A (OSRAM) |
| AU (1) | AU2014321215B2 (OSRAM) |
| CA (2) | CA3175320A1 (OSRAM) |
| ES (1) | ES2719112T3 (OSRAM) |
| IL (1) | IL244585B (OSRAM) |
| PT (1) | PT3049116T (OSRAM) |
| RU (1) | RU2670512C2 (OSRAM) |
| WO (1) | WO2015042585A1 (OSRAM) |
Families Citing this family (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6261500B2 (ja) | 2011-07-22 | 2018-01-17 | プレジデント アンド フェローズ オブ ハーバード カレッジ | ヌクレアーゼ切断特異性の評価および改善 |
| US20150044192A1 (en) | 2013-08-09 | 2015-02-12 | President And Fellows Of Harvard College | Methods for identifying a target site of a cas9 nuclease |
| US9359599B2 (en) | 2013-08-22 | 2016-06-07 | President And Fellows Of Harvard College | Engineered transcription activator-like effector (TALE) domains and uses thereof |
| US9228207B2 (en) | 2013-09-06 | 2016-01-05 | President And Fellows Of Harvard College | Switchable gRNAs comprising aptamers |
| US9737604B2 (en) | 2013-09-06 | 2017-08-22 | President And Fellows Of Harvard College | Use of cationic lipids to deliver CAS9 |
| US9322037B2 (en) | 2013-09-06 | 2016-04-26 | President And Fellows Of Harvard College | Cas9-FokI fusion proteins and uses thereof |
| US9068179B1 (en) | 2013-12-12 | 2015-06-30 | President And Fellows Of Harvard College | Methods for correcting presenilin point mutations |
| US10077453B2 (en) | 2014-07-30 | 2018-09-18 | President And Fellows Of Harvard College | CAS9 proteins including ligand-dependent inteins |
| EP3365356B1 (en) | 2015-10-23 | 2023-06-28 | President and Fellows of Harvard College | Nucleobase editors and uses thereof |
| US10188749B2 (en) | 2016-04-14 | 2019-01-29 | Fred Hutchinson Cancer Research Center | Compositions and methods to program therapeutic cells using targeted nucleic acid nanocarriers |
| JP7231935B2 (ja) | 2016-08-03 | 2023-03-08 | プレジデント アンド フェローズ オブ ハーバード カレッジ | アデノシン核酸塩基編集因子およびそれらの使用 |
| EP3497214B1 (en) | 2016-08-09 | 2023-06-28 | President and Fellows of Harvard College | Programmable cas9-recombinase fusion proteins and uses thereof |
| WO2018039438A1 (en) | 2016-08-24 | 2018-03-01 | President And Fellows Of Harvard College | Incorporation of unnatural amino acids into proteins using base editing |
| EP3526320A1 (en) | 2016-10-14 | 2019-08-21 | President and Fellows of Harvard College | Aav delivery of nucleobase editors |
| KR20210035022A (ko) * | 2016-12-14 | 2021-03-31 | 리간달 인코포레이티드 | 핵산 및/또는 단백질 적재물 전달을 위한 조성물 및 방법 |
| US10745677B2 (en) | 2016-12-23 | 2020-08-18 | President And Fellows Of Harvard College | Editing of CCR5 receptor gene to protect against HIV infection |
| EP3565535A4 (en) | 2017-01-05 | 2020-12-30 | Fred Hutchinson Cancer Research Center | SYSTEMS AND METHODS FOR IMPROVING THE EFFECTIVENESS OF A VACCINE |
| WO2018165504A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Suppression of pain by gene editing |
| WO2018165631A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Cancer vaccine |
| KR20190127797A (ko) | 2017-03-10 | 2019-11-13 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | 시토신에서 구아닌으로의 염기 편집제 |
| KR20240116572A (ko) | 2017-03-23 | 2024-07-29 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | 핵산 프로그램가능한 dna 결합 단백질을 포함하는 핵염기 편집제 |
| US11560566B2 (en) | 2017-05-12 | 2023-01-24 | President And Fellows Of Harvard College | Aptazyme-embedded guide RNAs for use with CRISPR-Cas9 in genome editing and transcriptional activation |
| WO2019023680A1 (en) | 2017-07-28 | 2019-01-31 | President And Fellows Of Harvard College | METHODS AND COMPOSITIONS FOR EVOLUTION OF BASIC EDITORS USING PHAGE-ASSISTED CONTINUOUS EVOLUTION (PACE) |
| EP3676376B1 (en) | 2017-08-30 | 2025-01-15 | President and Fellows of Harvard College | High efficiency base editors comprising gam |
| CN111757937A (zh) | 2017-10-16 | 2020-10-09 | 布罗德研究所股份有限公司 | 腺苷碱基编辑器的用途 |
| WO2019118949A1 (en) | 2017-12-15 | 2019-06-20 | The Broad Institute, Inc. | Systems and methods for predicting repair outcomes in genetic engineering |
| US20210148918A1 (en) * | 2018-04-17 | 2021-05-20 | Purdue Research Foundation | Modifying surface of a live cell and the uses thereof |
| EP3781683A4 (en) * | 2018-04-18 | 2022-02-16 | Ligandal, Inc. | Methods and compositions for genome editing |
| WO2019210005A1 (en) * | 2018-04-24 | 2019-10-31 | Ligandal, Inc. | Methods and compositions for genome editing |
| IL314291A (en) * | 2018-04-27 | 2024-09-01 | Genedit Inc | Cationic polymer and use for biomolecule delivery |
| US12157760B2 (en) | 2018-05-23 | 2024-12-03 | The Broad Institute, Inc. | Base editors and uses thereof |
| TWI764022B (zh) * | 2018-07-30 | 2022-05-11 | 中央研究院 | 凝聚複合物的治療性奈米粒子及其治療細菌的用途 |
| JP2022502384A (ja) | 2018-09-25 | 2022-01-11 | 国立大学法人 東京大学 | 両親媒性ポリアミノ酸、該両親媒性ポリアミノ酸を用いたブロックコポリマー、および該両親媒性ポリアミノ酸または該ブロックコポリマーと核酸とを含む複合体 |
| US12281338B2 (en) | 2018-10-29 | 2025-04-22 | The Broad Institute, Inc. | Nucleobase editors comprising GeoCas9 and uses thereof |
| US12485136B2 (en) | 2018-12-03 | 2025-12-02 | Takeda Pharmaceuticals U.S.A., Inc. | Methods for the treatment of trinucleotide repeat expansion disorders associated with MLH3 activity |
| CA3123617A1 (en) | 2018-12-20 | 2020-06-25 | Praxis Precision Medicines, Inc. | Compositions and methods for the treatment of kcnt1 related disorders |
| WO2020154500A1 (en) | 2019-01-23 | 2020-07-30 | The Broad Institute, Inc. | Supernegatively charged proteins and uses thereof |
| MX2021011426A (es) | 2019-03-19 | 2022-03-11 | Broad Inst Inc | Metodos y composiciones para editar secuencias de nucleótidos. |
| EP3956349A1 (en) | 2019-04-17 | 2022-02-23 | The Broad Institute, Inc. | Adenine base editors with reduced off-target effects |
| US20220296683A1 (en) * | 2019-09-20 | 2022-09-22 | University College Dublin | Oral delivery system |
| US12435330B2 (en) | 2019-10-10 | 2025-10-07 | The Broad Institute, Inc. | Methods and compositions for prime editing RNA |
| MX2022014008A (es) | 2020-05-08 | 2023-02-09 | Broad Inst Inc | Métodos y composiciones para la edición simultánea de ambas cadenas de una secuencia de nucleótidos de doble cadena objetivo. |
| WO2021231680A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of methyl-cpg binding protein 2 (mecp2) |
| WO2021231673A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of leucine rich repeat kinase 2 (lrrk2) |
| EP4150078A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of argininosuccinate lyase (asl) |
| EP4150077A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of transmembrane channel-like protein 1 (tmc1) |
| WO2021231692A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of otoferlin (otof) |
| WO2021231679A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of gap junction protein beta 2 (gjb2) |
| CA3162416C (en) | 2020-05-15 | 2023-07-04 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of argininosuccinate synthetase (ass1) |
| EP4150089A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of retinoschisin 1 (rs1) |
| AR122534A1 (es) | 2020-06-03 | 2022-09-21 | Triplet Therapeutics Inc | Métodos para el tratamiento de los trastornos de expansión por repetición de nucleótidos asociados con la actividad de msh3 |
| US20240263177A1 (en) | 2021-05-20 | 2024-08-08 | Korro Bio, Inc. | Methods and Compositions for Adar-Mediated Editing |
| WO2022256283A2 (en) | 2021-06-01 | 2022-12-08 | Korro Bio, Inc. | Methods for restoring protein function using adar |
| WO2023278410A1 (en) | 2021-06-29 | 2023-01-05 | Korro Bio, Inc. | Methods and compositions for adar-mediated editing |
| US20230194709A9 (en) | 2021-06-29 | 2023-06-22 | Seagate Technology Llc | Range information detection using coherent pulse sets with selected waveform characteristics |
| US20250352667A1 (en) | 2021-10-22 | 2025-11-20 | Korro Bio, Inc. | Methods and compositions for disrupting nrf2-keap1 protein interaction by adar mediated rna editing |
| IL313660A (en) | 2021-12-22 | 2024-08-01 | Camp4 Therapeutics Corp | Modulation of gene transcription using antisense oligonucleotides targeting regulatory RNAs |
| JP2025522380A (ja) | 2022-06-10 | 2025-07-15 | キャンプ4 セラピューティクス コーポレイション | 調節rnaを標的とするアンチセンスオリゴヌクレオチドを用いたプログラニュリン発現の調節方法 |
| KR20250113490A (ko) | 2022-12-01 | 2025-07-25 | 캠프4 테라퓨틱스 코포레이션 | 조절 rna를 표적화하는 안티센스 올리고뉴클레오타이드를 사용하는 syngap1 유전자 전사의 조절 |
| WO2025015338A1 (en) | 2023-07-13 | 2025-01-16 | Korro Bio, Inc. | Rna-editing oligonucleotides and uses thereof |
| WO2025015335A1 (en) | 2023-07-13 | 2025-01-16 | Korro Bio, Inc. | Rna-editing oligonucleotides and uses thereof |
| WO2025128799A1 (en) | 2023-12-12 | 2025-06-19 | Korro Bio, Inc. | Double-stranded rna-editing oligonucleotides and uses thereof |
| WO2025255388A1 (en) | 2024-06-05 | 2025-12-11 | Camp4 Therapeutics Corporation | Modulation of syngap1 gene transcription using antisense oligonucleotides targeting regulatory rnas |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6051429A (en) | 1995-06-07 | 2000-04-18 | Life Technologies, Inc. | Peptide-enhanced cationic lipid transfections |
| US6379966B2 (en) | 1999-02-26 | 2002-04-30 | Mirus Corporation | Intravascular delivery of non-viral nucleic acid |
| US7098032B2 (en) | 2001-01-02 | 2006-08-29 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| RU2295954C2 (ru) | 2000-09-28 | 2007-03-27 | Чирон Корпорейшн | Микрочастицы для доставки гетерологичных нуклеиновых кислот |
| US20040102606A1 (en) | 2001-04-24 | 2004-05-27 | Danuta Balicki | Histone H2A -derived peptides useful in gene delivery |
| US6805904B2 (en) | 2002-02-20 | 2004-10-19 | International Business Machines Corporation | Process of forming a multilayer nanoparticle-containing thin film self-assembly |
| AU2003234196A1 (en) * | 2002-04-22 | 2003-11-03 | University Of Florida | Functionalized nanoparticles and methods of use |
| US20070026069A1 (en) | 2003-03-28 | 2007-02-01 | Shastri Venkatram P | Biommetic hierarchies using functionalized nanoparticles as building blocks |
| WO2005020965A2 (en) | 2003-08-21 | 2005-03-10 | Southwest Research Institute | Skeletally targeted nanoparticles |
| WO2005084326A2 (en) | 2004-03-05 | 2005-09-15 | Board Of Regents, The University Of Texas System | Multilayered nanomedicine delivery system and method |
| WO2005123142A1 (en) | 2004-06-10 | 2005-12-29 | Abraxis Bioscience, Inc. | A method using inorganic nanoparticles as non-viral vectors for gene therapy |
| US20060088599A1 (en) | 2004-08-02 | 2006-04-27 | Prasad Paras N | Amino functionalized ORMOSIL nanoparticles as delivery vehicles |
| EP2131848A4 (en) | 2007-02-16 | 2012-06-27 | Merck Sharp & Dohme | COMPOSITIONS AND METHODS FOR REINFORCED ACTIVITY OF BIOLOGICAL ACTIVE MOLECULES |
| US20100196492A1 (en) | 2007-03-08 | 2010-08-05 | Green Jordan J | Electrostatic coating of particles for drug delivery |
| WO2009058913A2 (en) | 2007-10-29 | 2009-05-07 | University Of Massachusetts | Encapsulated nanoparticles for nucleic acid delivery |
| US8323618B2 (en) | 2007-11-07 | 2012-12-04 | University Of Houston System | Ultrasmall superparamagnetic iron oxide nanoparticles and uses thereof |
| CA2703852A1 (en) | 2007-11-09 | 2009-05-14 | Northeastern University | Self-assembling micelle-like nanoparticles for systemic gene delivery |
| US7999025B2 (en) | 2008-01-28 | 2011-08-16 | University Of Utah Research Foundation | Asymmetrically-functionalized nanoparticles organized on one-dimensional chains |
| US8324333B2 (en) | 2008-06-05 | 2012-12-04 | Wisconsin Alumni Research Foundation | Anionic charge-dynamic polymers for release of cationic agents |
| DK2417262T3 (da) | 2009-04-07 | 2015-06-22 | Dow Agrosciences Llc | Nanopartikelmedieret tilførsel af sekvensspecifikke nukleaser |
| ES2696825T3 (es) * | 2009-12-10 | 2019-01-18 | Univ Minnesota | Modificación del ADN inducida por el efector TAL |
| JPWO2011096408A1 (ja) | 2010-02-02 | 2013-06-10 | 国立大学法人 東京大学 | 複合体微粒子およびその製造方法、ならびに該複合体微粒子を用いた薬学組成物 |
| US9074187B2 (en) | 2011-03-21 | 2015-07-07 | Board Of Trustees Of The University Of Arkansas | Nanostructural materials that increase mineralization in bone cells and affect gene expression through miRNA regulation and applications of same |
| JP5996630B2 (ja) * | 2011-04-05 | 2016-09-21 | セレクティスCellectis | コンパクトtale−ヌクレアーゼを作製する方法及びその使用 |
| US8450107B1 (en) | 2011-11-30 | 2013-05-28 | The Broad Institute Inc. | Nucleotide-specific recognition sequences for designer TAL effectors |
| CN104507458B (zh) | 2012-06-20 | 2018-05-22 | 滑铁卢大学 | 粘膜粘合剂纳米颗粒递送系统 |
| WO2014093701A1 (en) | 2012-12-12 | 2014-06-19 | The Broad Institute, Inc. | Functional genomics using crispr-cas systems, compositions, methods, knock out libraries and applications thereof |
-
2014
- 2014-09-23 CN CN201480052449.0A patent/CN105579068A/zh active Pending
- 2014-09-23 EP EP18248103.6A patent/EP3520822A1/en active Pending
- 2014-09-23 CA CA3175320A patent/CA3175320A1/en active Pending
- 2014-09-23 CA CA2924535A patent/CA2924535C/en active Active
- 2014-09-23 US US15/024,264 patent/US10526616B2/en active Active
- 2014-09-23 AU AU2014321215A patent/AU2014321215B2/en active Active
- 2014-09-23 WO PCT/US2014/057000 patent/WO2015042585A1/en not_active Ceased
- 2014-09-23 JP JP2016516540A patent/JP6595459B2/ja not_active Expired - Fee Related
- 2014-09-23 KR KR1020167010607A patent/KR20160060133A/ko not_active Abandoned
- 2014-09-23 EP EP14846422.5A patent/EP3049116B1/en active Active
- 2014-09-23 ES ES14846422T patent/ES2719112T3/es active Active
- 2014-09-23 RU RU2016115721A patent/RU2670512C2/ru active
- 2014-09-23 PT PT14846422T patent/PT3049116T/pt unknown
-
2016
- 2016-03-14 IL IL24458516A patent/IL244585B/en active IP Right Grant
-
2019
- 2019-11-27 US US16/697,308 patent/US20200181642A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| RU2670512C2 (ru) | 2018-10-23 |
| CN105579068A (zh) | 2016-05-11 |
| JP2016533322A (ja) | 2016-10-27 |
| US20160230189A1 (en) | 2016-08-11 |
| CA3175320A1 (en) | 2015-03-26 |
| CA2924535A1 (en) | 2015-03-26 |
| RU2016115721A3 (OSRAM) | 2018-03-21 |
| EP3049116A1 (en) | 2016-08-03 |
| AU2014321215A1 (en) | 2016-04-21 |
| IL244585A0 (en) | 2016-04-21 |
| IL244585B (en) | 2019-11-28 |
| WO2015042585A1 (en) | 2015-03-26 |
| EP3049116B1 (en) | 2019-01-02 |
| ES2719112T3 (es) | 2019-07-08 |
| CA2924535C (en) | 2022-12-13 |
| PT3049116T (pt) | 2019-04-03 |
| RU2016115721A (ru) | 2017-10-30 |
| JP6595459B2 (ja) | 2019-10-23 |
| AU2014321215B2 (en) | 2020-07-16 |
| US20200181642A1 (en) | 2020-06-11 |
| US10526616B2 (en) | 2020-01-07 |
| EP3520822A1 (en) | 2019-08-07 |
| EP3049116A4 (en) | 2017-05-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20160060133A (ko) | 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 | |
| Winton et al. | Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates | |
| Suh et al. | Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation | |
| CN104981478B (zh) | 细胞穿透肽、包含该肽的缀合物、及包含该缀合物的组合物 | |
| Kim et al. | RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system | |
| Vaissière et al. | A retro-inverso cell-penetrating peptide for siRNA delivery | |
| EP4556567A2 (en) | Rationally-designed synthetic peptide shuttle agents for delivering polypeptide cargos from an extracellular space to the cytosol and/or nucleus of a target eukaryotic cell, uses thereof, methods and kits relating to same | |
| KR20240172763A (ko) | 핵산 및/또는 단백질 적재물 전달을 위한 조성물 및 방법 | |
| Sadeghian et al. | Design, engineering and preparation of a multi-domain fusion vector for gene delivery | |
| CN104768967A (zh) | 细胞穿透性肽、包含该肽的缀合物、及包含该缀合物的组合物 | |
| KR20220031545A (ko) | 펩타이드 기반 비단백질성 카고 전달 | |
| Cheraghi et al. | Development of a targeted anti-HER2 scFv chimeric peptide for gene delivery into HER2-positive breast cancer cells | |
| Lee et al. | Identification of cell‐penetrating osteogenic peptide from copine‐7 protein and its delivery system for enhanced bone formation | |
| Oh et al. | Multimeric Amphipathic α‐Helical Sequences for Rapid and Efficient Intracellular Protein Transport at Nanomolar Concentrations | |
| Du et al. | ATG101 single-stranded antisense RNA-loaded triangular DNA nanoparticles control human pulmonary endothelial growth via regulation of cell macroautophagy | |
| Nirasawa et al. | Development of A2G80 peptide-gene complex for targeted delivery to muscle cells | |
| HK1225274B (en) | Nanoparticle-mediated gene delivery, genomic editing and ligand-targeted modification in various cell populations | |
| HK1225274A1 (en) | Nanoparticle-mediated gene delivery, genomic editing and ligand-targeted modification in various cell populations | |
| JP7725041B2 (ja) | コラーゲン結合型膜透過性ペプチド、並びに、該ペプチド及びコラーゲン若しくはコラーゲン誘導体を含む運搬体 | |
| KR20220057542A (ko) | 펩타이드 내포 페리틴 | |
| Goyal | Paralog specific role of COPI pathway in P19 neuronal differentiation | |
| KR20170002983A (ko) | 항암 및 항염증 활성을 갖는 약물 복합체 | |
| Shen | Understanding the Biological Barriers to Jurkat Transfection using Non-Viral Peptides | |
| Ping | Exploiting the Protein Corona Around Gold Nanoparticles in Modulation of Protein Expression | |
| 서진숙 | Study for intracellular targeting based on peptides for regulation of stem cell differentiation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160422 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20190724 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200729 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20210125 |
|
| PC1904 | Unpaid initial registration fee |