KR20160002709A - 초고순도 테트라하이드로카나비놀-11-오익산 - Google Patents
초고순도 테트라하이드로카나비놀-11-오익산 Download PDFInfo
- Publication number
- KR20160002709A KR20160002709A KR1020157024773A KR20157024773A KR20160002709A KR 20160002709 A KR20160002709 A KR 20160002709A KR 1020157024773 A KR1020157024773 A KR 1020157024773A KR 20157024773 A KR20157024773 A KR 20157024773A KR 20160002709 A KR20160002709 A KR 20160002709A
- Authority
- KR
- South Korea
- Prior art keywords
- acid
- fold
- receptor
- affinity
- composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/658—Medicinal preparations containing organic active ingredients o-phenolic cannabinoids, e.g. cannabidiol, cannabigerolic acid, cannabichromene or tetrahydrocannabinol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Pain & Pain Management (AREA)
- Ophthalmology & Optometry (AREA)
- Physiology (AREA)
- Nutrition Science (AREA)
- Transplantation (AREA)
- Gastroenterology & Hepatology (AREA)
- Urology & Nephrology (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Rheumatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Pyrane Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361763630P | 2013-02-12 | 2013-02-12 | |
| US61/763,630 | 2013-02-12 | ||
| US201361837743P | 2013-06-21 | 2013-06-21 | |
| US61/837,743 | 2013-06-21 | ||
| PCT/US2014/016050 WO2014127016A2 (en) | 2013-02-12 | 2014-02-12 | Ultrapure tetrahydrocannabinol-11-oic acids |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197036267A Division KR20190139327A (ko) | 2013-02-12 | 2014-02-12 | 초고순도 테트라하이드로카나비놀-11-오익산 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160002709A true KR20160002709A (ko) | 2016-01-08 |
Family
ID=51354671
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157024773A Ceased KR20160002709A (ko) | 2013-02-12 | 2014-02-12 | 초고순도 테트라하이드로카나비놀-11-오익산 |
| KR1020197036267A Ceased KR20190139327A (ko) | 2013-02-12 | 2014-02-12 | 초고순도 테트라하이드로카나비놀-11-오익산 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197036267A Ceased KR20190139327A (ko) | 2013-02-12 | 2014-02-12 | 초고순도 테트라하이드로카나비놀-11-오익산 |
Country Status (9)
| Country | Link |
|---|---|
| US (8) | US20150141501A1 (enExample) |
| EP (2) | EP3851101A1 (enExample) |
| JP (3) | JP6689078B2 (enExample) |
| KR (2) | KR20160002709A (enExample) |
| CN (2) | CN105228613A (enExample) |
| AU (2) | AU2014216440B2 (enExample) |
| BR (1) | BR112015019180A8 (enExample) |
| CA (1) | CA2900982C (enExample) |
| WO (1) | WO2014127016A2 (enExample) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3851101A1 (en) | 2013-02-12 | 2021-07-21 | Corbus Pharmaceuticals, Inc. | Ultrapure tetrahydrocannabinol-11-oic acids |
| JP2019515927A (ja) * | 2016-04-29 | 2019-06-13 | コーバス ファーマシューティカルズ インク. | 感染症の処置のための方法 |
| MX2019000348A (es) * | 2016-07-11 | 2019-03-28 | Intec Pharma Ltd | Formulaciones gastrorretentivas orales y usos de las mismas. |
| US10239808B1 (en) | 2016-12-07 | 2019-03-26 | Canopy Holdings, LLC | Cannabis extracts |
| EP3745884A1 (en) | 2018-01-31 | 2020-12-09 | Canopy Holdings, Llc | Hemp powder |
| CA3101626A1 (en) * | 2018-05-31 | 2019-12-05 | Corbus Pharmaceuticals Inc. | Cannabinoids and uses thereof |
| CA3119729A1 (en) | 2018-10-10 | 2020-04-16 | Treehouse Biotech, Inc. | Synthesis of cannabigerol |
| US20200121614A1 (en) * | 2018-10-18 | 2020-04-23 | Bluegrass Farmacueticals, LLC | Cannabinoid-infused transparent hydrogel skin patch |
| WO2020183456A1 (en) * | 2019-03-10 | 2020-09-17 | Bol Pharma Ltd. | Cannabinoid combinations for treating chronic pain in dialysis patients |
| WO2021072325A1 (en) | 2019-10-11 | 2021-04-15 | Corbus Pharmaceuticals, Inc. | Compositions of ajulemic acid and uses thereof |
| US20220023253A1 (en) * | 2020-07-21 | 2022-01-27 | Np Pharma Holdings, Llc | Cannabinoid Compositions and Methods for Treating Joint Pain and Inflammation |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2186485B (en) | 1986-02-13 | 1988-09-07 | Ethical Pharma Ltd | Slow release formulation |
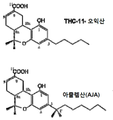
| US5338753A (en) | 1992-07-14 | 1994-08-16 | Sumner H. Burstein | (3R,4R)-Δ6 -tetrahydrocannabinol-7-oic acids useful as antiinflammatory agents and analgesics |
| US8586767B2 (en) | 1999-03-22 | 2013-11-19 | Craig Rick Travis | Method for treatment of HIV and diseases of immune dysregulation |
| US6566560B2 (en) | 1999-03-22 | 2003-05-20 | Immugen Pharmaceuticals, Inc. | Resorcinolic compounds |
| US6974835B2 (en) | 2000-05-17 | 2005-12-13 | Indevus Pharmaceuticals, Inc. | Methods for decreasing cell proliferation based on (3r,4r)-Δ8-tetrahydrocannabinol-11-oic acids |
| US7507767B2 (en) | 2001-02-08 | 2009-03-24 | Schering Corporation | Cannabinoid receptor ligands |
| EP1409473A2 (en) * | 2001-03-07 | 2004-04-21 | Websar Innovations Inc. | CONVERSION OF CBD TO $g(D)?8 -THC AND $g(D)?9 -THC |
| WO2004050011A2 (en) * | 2002-12-04 | 2004-06-17 | Pharmos Corporation | High enantiomeric purity dexanabinol for pharmaceutical copositions |
| IL153277A0 (en) * | 2002-12-04 | 2003-07-06 | Pharmos Corp | High enantiomeric purity dexanabinol for pharmaceutical compositions |
| US7413748B2 (en) | 2002-12-13 | 2008-08-19 | Purdue Pharma L.P. | Transdermal buprenorphine to treat pain in sickle cell crisis |
| WO2004058251A1 (en) | 2002-12-19 | 2004-07-15 | University Of Massachusetts | Cannabinoid analogs as peroxisome proliferator activated nuclear receptor gamma activators |
| US20050070596A1 (en) | 2003-05-12 | 2005-03-31 | David Baker | Methods for treatment of inflammatory diseases using CT-3 or analogs thereof |
| US20050009903A1 (en) * | 2003-06-10 | 2005-01-13 | Martin Billy R. | CB2-selective cannabinoid analogues |
| KR20080000660A (ko) | 2005-04-21 | 2008-01-02 | 오르펀 메디칼, 인크. | 초순수 4-메틸피라졸의 제조 방법 |
| TWI366460B (en) * | 2005-06-16 | 2012-06-21 | Euro Celtique Sa | Cannabinoid active pharmaceutical ingredient for improved dosage forms |
| US20070037873A1 (en) | 2005-08-08 | 2007-02-15 | Zurier Robert B | Airway remodeling treatments |
| US20070060639A1 (en) | 2005-09-09 | 2007-03-15 | University Of Kentucky | Compositions and methods for intranasal delivery of tricyclic cannabinoids |
| WO2007055806A1 (en) * | 2005-10-31 | 2007-05-18 | Indevus Pharmaceuticals, Inc. | Anti-emetic uses of (3r,4r)-δ8-tetrahydrocannabinol-11-oic acids |
| GB2432312A (en) * | 2005-11-01 | 2007-05-23 | Gw Pharma Ltd | Pharmaceutical compositions for the treatment of pain |
| ES2581212T3 (es) | 2005-11-07 | 2016-09-02 | Murty Pharmaceuticals, Inc. | Administración mejorada de tetahidrocannabinol |
| US20080054300A1 (en) * | 2006-06-30 | 2008-03-06 | Philip Gene Nikkel | Body contact structure and method for the reduction of drain lag and gate lag in field effect transistors |
| US8058227B2 (en) | 2006-10-03 | 2011-11-15 | Medical University Of South Carolina | Method of treating fibrosis in a subject in need thereof comprising administering a composition comprising a CSD |
| US8044071B2 (en) | 2007-10-18 | 2011-10-25 | Abbott Laboratories | Method for reducing side effects of CB2 receptor agonist therapy using a combination of a selective CB2 receptor agonist and a selective CB1 receptor antagonist |
| WO2009158499A2 (en) | 2008-06-25 | 2009-12-30 | University Of North Texas Health Science Center At Fort Worth | Prevention of bacterial growth and biofilm formation by ligands that act on cannabinoidergic systems |
| US8877188B2 (en) | 2010-05-04 | 2014-11-04 | The Brigham And Women's Hospital, Inc. | Detection and treatment of non-dermal fibrosis |
| WO2012048045A1 (en) | 2010-10-05 | 2012-04-12 | Jb Therapeutics, Inc. | Compositions, dosages, and methods of using tetrahydrocannabinol derivatives |
| US20120309820A1 (en) * | 2011-06-04 | 2012-12-06 | Jb Therapeutics Inc. | Methods of treating fibrotic diseases using tetrahydrocannabinol-11-oic acids |
| EP3851101A1 (en) | 2013-02-12 | 2021-07-21 | Corbus Pharmaceuticals, Inc. | Ultrapure tetrahydrocannabinol-11-oic acids |
| US20150328198A1 (en) | 2014-05-16 | 2015-11-19 | The University Of North Carolina At Chapel Hill | Methods of treating methicillin-resistant staphylococcus aureus (mrsa) using ppar-gamma agonists |
-
2014
- 2014-02-12 EP EP20208635.1A patent/EP3851101A1/en not_active Withdrawn
- 2014-02-12 CN CN201480020828.1A patent/CN105228613A/zh active Pending
- 2014-02-12 EP EP14752090.2A patent/EP2956133A4/en not_active Withdrawn
- 2014-02-12 AU AU2014216440A patent/AU2014216440B2/en not_active Ceased
- 2014-02-12 CA CA2900982A patent/CA2900982C/en active Active
- 2014-02-12 KR KR1020157024773A patent/KR20160002709A/ko not_active Ceased
- 2014-02-12 JP JP2015557221A patent/JP6689078B2/ja not_active Expired - Fee Related
- 2014-02-12 KR KR1020197036267A patent/KR20190139327A/ko not_active Ceased
- 2014-02-12 CN CN201911271506.5A patent/CN110946854A/zh active Pending
- 2014-02-12 BR BR112015019180A patent/BR112015019180A8/pt not_active Application Discontinuation
- 2014-02-12 WO PCT/US2014/016050 patent/WO2014127016A2/en not_active Ceased
- 2014-11-18 US US14/546,116 patent/US20150141501A1/en not_active Abandoned
-
2016
- 2016-07-26 US US15/220,127 patent/US10085964B2/en active Active
- 2016-11-09 US US15/347,059 patent/US9801849B2/en not_active Expired - Fee Related
- 2016-11-09 US US15/347,104 patent/US9820964B2/en not_active Expired - Fee Related
-
2017
- 2017-09-07 US US15/698,544 patent/US10154986B2/en active Active
-
2018
- 2018-09-22 JP JP2018178162A patent/JP2019031505A/ja active Pending
- 2018-11-05 AU AU2018258159A patent/AU2018258159B2/en not_active Ceased
- 2018-11-21 US US16/198,381 patent/US10369131B2/en active Active
-
2019
- 2019-05-29 US US16/425,196 patent/US11052066B2/en not_active Expired - Fee Related
-
2021
- 2021-06-02 US US17/336,759 patent/US20220117931A1/en not_active Abandoned
- 2021-08-19 JP JP2021133797A patent/JP2021185166A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20160002709A (ko) | 초고순도 테트라하이드로카나비놀-11-오익산 | |
| US20230100789A1 (en) | Method for activating AMPK and the use of Adenine | |
| AU2016207118B2 (en) | Diphenyl derivative and uses thereof | |
| AU2019203668B2 (en) | Compound for activating AMPK and uses thereof | |
| WO2022256540A1 (en) | Methods of use of ppar agonist compounds and pharmaceutical compositions thereof | |
| CN105163732B (zh) | 用于治疗眼科疾病和病症的方法 | |
| HK40026965A (en) | Ultrapure tetrahydrocannabinol-11-oic acids | |
| EP2588462A1 (en) | Metronidazole esters for treating rosacea | |
| CN108546268B (zh) | 用于治疗动脉粥样硬化的化合物及其应用 | |
| CN107823208A (zh) | 吗啉类化合物在制备治疗和预防肾纤维化药物中的应用 | |
| CN107628936A (zh) | 香兰素的提取方法及其应用 | |
| CN119745887A (zh) | 小分子激动剂tepp-46在制备预防和/或治疗主动脉瘤及相关心血管疾病的药物中的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150910 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20160212 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20161221 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20171121 Patent event code: PE09021S02D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| E801 | Decision on dismissal of amendment | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20180713 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20171121 Comment text: Final Notice of Reason for Refusal Patent event code: PE06011S02I Patent event date: 20161221 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| PE0801 | Dismissal of amendment |
Patent event code: PE08012E01D Comment text: Decision on Dismissal of Amendment Patent event date: 20180713 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20180521 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20170721 |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09011S01I Patent event date: 20180713 Comment text: Decision to Refuse Application Patent event code: PX09012R01I Patent event date: 20180521 Comment text: Amendment to Specification, etc. Patent event code: PX09012R01I Patent event date: 20170721 Comment text: Amendment to Specification, etc. |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20181119 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20190906 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20190520 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20181119 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20181012 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20180713 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20180521 Comment text: Final Notice of Reason for Refusal Patent event code: PX06013S02I Patent event date: 20171121 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20170721 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20161221 |
|
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20191206 |
|
| PJ0201 | Trial against decision of rejection |
Patent event date: 20191206 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20190906 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Patent event date: 20180713 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20210316 Appeal identifier: 2019101004044 Request date: 20191206 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2019101004044; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20191206 Effective date: 20210316 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20210316 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20191206 Decision date: 20210316 Appeal identifier: 2019101004044 |