KR20150126362A - 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 - Google Patents
글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 Download PDFInfo
- Publication number
- KR20150126362A KR20150126362A KR1020157025034A KR20157025034A KR20150126362A KR 20150126362 A KR20150126362 A KR 20150126362A KR 1020157025034 A KR1020157025034 A KR 1020157025034A KR 20157025034 A KR20157025034 A KR 20157025034A KR 20150126362 A KR20150126362 A KR 20150126362A
- Authority
- KR
- South Korea
- Prior art keywords
- solid composition
- cyclohexyl
- pharmaceutically acceptable
- mixture
- rti
- Prior art date
Links
- 239000008247 solid mixture Substances 0.000 title claims abstract description 157
- 238000000034 method Methods 0.000 title claims description 84
- 229940124828 glucokinase activator Drugs 0.000 title 1
- HPGJSAAUJGAMLV-UHFFFAOYSA-N 2-[[2-[[cyclohexyl-(4-propoxycyclohexyl)carbamoyl]amino]-1,3-thiazol-5-yl]sulfanyl]acetic acid Chemical compound C1CC(OCCC)CCC1N(C(=O)NC=1SC(SCC(O)=O)=CN=1)C1CCCCC1 HPGJSAAUJGAMLV-UHFFFAOYSA-N 0.000 claims abstract description 7
- 239000000203 mixture Substances 0.000 claims description 124
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 59
- 239000004094 surface-active agent Substances 0.000 claims description 53
- 239000011230 binding agent Substances 0.000 claims description 52
- 102000030595 Glucokinase Human genes 0.000 claims description 51
- 108010021582 Glucokinase Proteins 0.000 claims description 51
- -1 alkyl sulfate salts Chemical class 0.000 claims description 48
- 239000000843 powder Substances 0.000 claims description 47
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 40
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 30
- 239000008103 glucose Substances 0.000 claims description 30
- 239000000725 suspension Substances 0.000 claims description 29
- 239000008280 blood Substances 0.000 claims description 23
- 210000004369 blood Anatomy 0.000 claims description 23
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 claims description 22
- 229920000053 polysorbate 80 Polymers 0.000 claims description 22
- 239000002904 solvent Substances 0.000 claims description 21
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical group [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 claims description 20
- 238000001704 evaporation Methods 0.000 claims description 20
- 230000008020 evaporation Effects 0.000 claims description 20
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 claims description 20
- 235000019333 sodium laurylsulphate Nutrition 0.000 claims description 20
- 239000007787 solid Substances 0.000 claims description 20
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical group [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 18
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 18
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 18
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 17
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 17
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 16
- 239000004615 ingredient Substances 0.000 claims description 15
- 239000012530 fluid Substances 0.000 claims description 14
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 14
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 14
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 14
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 12
- 239000003937 drug carrier Substances 0.000 claims description 12
- 239000000194 fatty acid Substances 0.000 claims description 12
- 229930195729 fatty acid Natural products 0.000 claims description 12
- 239000007921 spray Substances 0.000 claims description 12
- 238000001694 spray drying Methods 0.000 claims description 12
- 239000002202 Polyethylene glycol Substances 0.000 claims description 11
- 239000002775 capsule Substances 0.000 claims description 11
- 150000001875 compounds Chemical class 0.000 claims description 11
- 238000002156 mixing Methods 0.000 claims description 11
- 229920001223 polyethylene glycol Polymers 0.000 claims description 11
- 229910000027 potassium carbonate Inorganic materials 0.000 claims description 10
- 235000011181 potassium carbonates Nutrition 0.000 claims description 10
- 230000008569 process Effects 0.000 claims description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 9
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 9
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 8
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 8
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 8
- 229920001983 poloxamer Polymers 0.000 claims description 8
- 239000011736 potassium bicarbonate Substances 0.000 claims description 8
- 235000015497 potassium bicarbonate Nutrition 0.000 claims description 8
- 229910000028 potassium bicarbonate Inorganic materials 0.000 claims description 8
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 claims description 8
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 claims description 7
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 claims description 7
- 229920001577 copolymer Polymers 0.000 claims description 7
- 238000001035 drying Methods 0.000 claims description 7
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 7
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 claims description 6
- 235000017550 sodium carbonate Nutrition 0.000 claims description 6
- 229920001214 Polysorbate 60 Polymers 0.000 claims description 5
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims description 5
- 230000003213 activating effect Effects 0.000 claims description 5
- 210000004185 liver Anatomy 0.000 claims description 5
- 229960000502 poloxamer Drugs 0.000 claims description 5
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 claims description 5
- IYKJEILNJZQJPU-UHFFFAOYSA-N acetic acid;butanedioic acid Chemical compound CC(O)=O.OC(=O)CCC(O)=O IYKJEILNJZQJPU-UHFFFAOYSA-N 0.000 claims description 4
- 229940081735 acetylcellulose Drugs 0.000 claims description 4
- 229920002301 cellulose acetate Polymers 0.000 claims description 4
- 239000003085 diluting agent Substances 0.000 claims description 4
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 4
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 claims description 4
- 229940068968 polysorbate 80 Drugs 0.000 claims description 4
- 229920000623 Cellulose acetate phthalate Polymers 0.000 claims description 3
- 239000001856 Ethyl cellulose Substances 0.000 claims description 3
- 229940081734 cellulose acetate phthalate Drugs 0.000 claims description 3
- 235000019325 ethyl cellulose Nutrition 0.000 claims description 3
- 229920001249 ethyl cellulose Polymers 0.000 claims description 3
- 239000004570 mortar (masonry) Substances 0.000 claims description 3
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 claims description 3
- 229920000058 polyacrylate Polymers 0.000 claims description 3
- 238000005507 spraying Methods 0.000 claims description 3
- 125000005591 trimellitate group Chemical group 0.000 claims description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 claims description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 2
- 150000005690 diesters Chemical class 0.000 claims description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 238000004806 packaging method and process Methods 0.000 claims description 2
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 claims description 2
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 claims 3
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 claims 3
- GJJVAFUKOBZPCB-UHFFFAOYSA-N 2-methyl-2-(4,8,12-trimethyltrideca-3,7,11-trienyl)-3,4-dihydrochromen-6-ol Chemical compound OC1=CC=C2OC(CCC=C(C)CCC=C(C)CCC=C(C)C)(C)CCC2=C1 GJJVAFUKOBZPCB-UHFFFAOYSA-N 0.000 claims 2
- 150000003611 tocopherol derivatives Chemical class 0.000 claims 2
- 229930003802 tocotrienol Natural products 0.000 claims 2
- 239000011731 tocotrienol Substances 0.000 claims 2
- 235000019148 tocotrienols Nutrition 0.000 claims 2
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical class C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 claims 1
- 239000004698 Polyethylene Substances 0.000 claims 1
- 238000007605 air drying Methods 0.000 claims 1
- 125000005587 carbonate group Chemical group 0.000 claims 1
- 125000002541 furyl group Chemical group 0.000 claims 1
- 229920000573 polyethylene Polymers 0.000 claims 1
- 239000003826 tablet Substances 0.000 description 74
- 239000002245 particle Substances 0.000 description 42
- 239000008187 granular material Substances 0.000 description 41
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 39
- 239000000243 solution Substances 0.000 description 38
- 235000002639 sodium chloride Nutrition 0.000 description 33
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 33
- 239000012190 activator Substances 0.000 description 31
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 25
- 229960000913 crospovidone Drugs 0.000 description 23
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 23
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 23
- 150000003839 salts Chemical class 0.000 description 23
- 235000019359 magnesium stearate Nutrition 0.000 description 19
- 238000005469 granulation Methods 0.000 description 18
- 230000003179 granulation Effects 0.000 description 18
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 15
- 239000003814 drug Substances 0.000 description 15
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 14
- 229920000881 Modified starch Polymers 0.000 description 14
- 239000008101 lactose Substances 0.000 description 14
- 229960001375 lactose Drugs 0.000 description 14
- 229920002261 Corn starch Polymers 0.000 description 13
- 239000008120 corn starch Substances 0.000 description 13
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 239000002253 acid Substances 0.000 description 11
- 239000002552 dosage form Substances 0.000 description 11
- 239000010410 layer Substances 0.000 description 10
- 238000005550 wet granulation Methods 0.000 description 10
- 239000008279 sol Substances 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- 229910052783 alkali metal Inorganic materials 0.000 description 8
- 201000010099 disease Diseases 0.000 description 8
- 239000006070 nanosuspension Substances 0.000 description 8
- 206010012601 diabetes mellitus Diseases 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- AOBORMOPSGHCAX-UHFFFAOYSA-N Tocophersolan Chemical compound OCCOC(=O)CCC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C AOBORMOPSGHCAX-UHFFFAOYSA-N 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 6
- 239000000314 lubricant Substances 0.000 description 6
- 238000000634 powder X-ray diffraction Methods 0.000 description 6
- 239000007909 solid dosage form Substances 0.000 description 6
- 108010010803 Gelatin Proteins 0.000 description 5
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 5
- 229920002472 Starch Polymers 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 230000026731 phosphorylation Effects 0.000 description 5
- 238000006366 phosphorylation reaction Methods 0.000 description 5
- 235000019698 starch Nutrition 0.000 description 5
- 239000008107 starch Substances 0.000 description 5
- 229940032147 starch Drugs 0.000 description 5
- 201000001320 Atherosclerosis Diseases 0.000 description 4
- 208000002705 Glucose Intolerance Diseases 0.000 description 4
- 206010022489 Insulin Resistance Diseases 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 239000001506 calcium phosphate Substances 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 238000007908 dry granulation Methods 0.000 description 4
- 210000001035 gastrointestinal tract Anatomy 0.000 description 4
- 201000001421 hyperglycemia Diseases 0.000 description 4
- 150000007522 mineralic acids Chemical class 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 229920000136 polysorbate Polymers 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 3
- 208000032928 Dyslipidaemia Diseases 0.000 description 3
- 241001427367 Gardena Species 0.000 description 3
- 208000031226 Hyperlipidaemia Diseases 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 102000004877 Insulin Human genes 0.000 description 3
- 108090001061 Insulin Proteins 0.000 description 3
- 208000017170 Lipid metabolism disease Diseases 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 description 3
- 235000010216 calcium carbonate Nutrition 0.000 description 3
- 229910000389 calcium phosphate Inorganic materials 0.000 description 3
- 235000011010 calcium phosphates Nutrition 0.000 description 3
- 235000010980 cellulose Nutrition 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 239000012458 free base Substances 0.000 description 3
- 230000002440 hepatic effect Effects 0.000 description 3
- 229940125396 insulin Drugs 0.000 description 3
- 208000030159 metabolic disease Diseases 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 210000000496 pancreas Anatomy 0.000 description 3
- 235000021317 phosphate Nutrition 0.000 description 3
- 229920001992 poloxamer 407 Polymers 0.000 description 3
- 201000009104 prediabetes syndrome Diseases 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 210000000813 small intestine Anatomy 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000001488 sodium phosphate Substances 0.000 description 3
- 238000001228 spectrum Methods 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 208000011580 syndromic disease Diseases 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 2
- 239000005995 Aluminium silicate Substances 0.000 description 2
- 229940123208 Biguanide Drugs 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- NBSCHQHZLSJFNQ-GASJEMHNSA-N D-Glucose 6-phosphate Chemical compound OC1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H](O)[C@H]1O NBSCHQHZLSJFNQ-GASJEMHNSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 206010070901 Diabetic dyslipidaemia Diseases 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- VFRROHXSMXFLSN-UHFFFAOYSA-N Glc6P Natural products OP(=O)(O)OCC(O)C(O)C(O)C(O)C=O VFRROHXSMXFLSN-UHFFFAOYSA-N 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 208000001145 Metabolic Syndrome Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 240000007817 Olea europaea Species 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 208000017442 Retinal disease Diseases 0.000 description 2
- 206010038923 Retinopathy Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 2
- 239000002250 absorbent Substances 0.000 description 2
- 230000002745 absorbent Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 235000012216 bentonite Nutrition 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000004958 brain cell Anatomy 0.000 description 2
- GZUXJHMPEANEGY-UHFFFAOYSA-N bromomethane Chemical compound BrC GZUXJHMPEANEGY-UHFFFAOYSA-N 0.000 description 2
- ZCCIPPOKBCJFDN-UHFFFAOYSA-N calcium nitrate Chemical compound [Ca+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ZCCIPPOKBCJFDN-UHFFFAOYSA-N 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000023852 carbohydrate metabolic process Effects 0.000 description 2
- 235000021256 carbohydrate metabolism Nutrition 0.000 description 2
- 239000004359 castor oil Substances 0.000 description 2
- 235000019438 castor oil Nutrition 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 235000011180 diphosphates Nutrition 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 2
- 208000006575 hypertriglyceridemia Diseases 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 2
- 208000017169 kidney disease Diseases 0.000 description 2
- 229960001021 lactose monohydrate Drugs 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- YIXJRHPUWRPCBB-UHFFFAOYSA-N magnesium nitrate Chemical compound [Mg+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O YIXJRHPUWRPCBB-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 238000007909 melt granulation Methods 0.000 description 2
- 230000007102 metabolic function Effects 0.000 description 2
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 description 2
- 229960003105 metformin Drugs 0.000 description 2
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 2
- 229940117841 methacrylic acid copolymer Drugs 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 229960002900 methylcellulose Drugs 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000003094 microcapsule Substances 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 238000009480 moisture-activated dry granulation Methods 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- 201000001119 neuropathy Diseases 0.000 description 2
- 230000007823 neuropathy Effects 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 239000003538 oral antidiabetic agent Substances 0.000 description 2
- 239000006186 oral dosage form Substances 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 208000033808 peripheral neuropathy Diseases 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000002798 polar solvent Substances 0.000 description 2
- 229920001993 poloxamer 188 Polymers 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 230000000291 postprandial effect Effects 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- FGIUAXJPYTZDNR-UHFFFAOYSA-N potassium nitrate Chemical compound [K+].[O-][N+]([O-])=O FGIUAXJPYTZDNR-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K potassium phosphate Substances [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 229940069328 povidone Drugs 0.000 description 2
- 230000006920 protein precipitation Effects 0.000 description 2
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000009491 slugging Methods 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 2
- 235000011069 sorbitan monooleate Nutrition 0.000 description 2
- 239000001593 sorbitan monooleate Substances 0.000 description 2
- 229940035049 sorbitan monooleate Drugs 0.000 description 2
- 210000002784 stomach Anatomy 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 150000003628 tricarboxylic acids Chemical class 0.000 description 2
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical group [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 2
- 229940053446 vitamin E d-alpha Drugs 0.000 description 2
- 230000029663 wound healing Effects 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 1
- MUHFRORXWCGZGE-KTKRTIGZSA-N 2-hydroxyethyl (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCCO MUHFRORXWCGZGE-KTKRTIGZSA-N 0.000 description 1
- RFVNOJDQRGSOEL-UHFFFAOYSA-N 2-hydroxyethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCO RFVNOJDQRGSOEL-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-M 3-carboxy-2,3-dihydroxypropanoate Chemical compound OC(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-M 0.000 description 1
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- XNCOSPRUTUOJCJ-UHFFFAOYSA-N Biguanide Chemical compound NC(N)=NC(N)=N XNCOSPRUTUOJCJ-UHFFFAOYSA-N 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- HZZVJAQRINQKSD-UHFFFAOYSA-N Clavulanic acid Natural products OC(=O)C1C(=CCO)OC2CC(=O)N21 HZZVJAQRINQKSD-UHFFFAOYSA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 206010018429 Glucose tolerance impaired Diseases 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- 206010060378 Hyperinsulinaemia Diseases 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 229920003097 Methocel™ E3 LV Polymers 0.000 description 1
- 229920000715 Mucilage Polymers 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- MHQJUHSHQGQVTM-HNENSFHCSA-N Octadecyl fumarate Chemical compound CCCCCCCCCCCCCCCCCCOC(=O)\C=C/C(O)=O MHQJUHSHQGQVTM-HNENSFHCSA-N 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229920002023 Pluronic® F 87 Polymers 0.000 description 1
- 229930182556 Polyacetal Natural products 0.000 description 1
- 229920002690 Polyoxyl 40 HydrogenatedCastorOil Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- BCKXLBQYZLBQEK-KVVVOXFISA-M Sodium oleate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC([O-])=O BCKXLBQYZLBQEK-KVVVOXFISA-M 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 229940100389 Sulfonylurea Drugs 0.000 description 1
- 229920002253 Tannate Polymers 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000008043 acidic salts Chemical class 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 229940023476 agar Drugs 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229960004977 anhydrous lactose Drugs 0.000 description 1
- 229940125708 antidiabetic agent Drugs 0.000 description 1
- 239000003472 antidiabetic agent Substances 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- 229940092782 bentonite Drugs 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 150000004283 biguanides Chemical class 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 229960003563 calcium carbonate Drugs 0.000 description 1
- JUNWLZAGQLJVLR-UHFFFAOYSA-J calcium diphosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])([O-])=O JUNWLZAGQLJVLR-UHFFFAOYSA-J 0.000 description 1
- 229960001714 calcium phosphate Drugs 0.000 description 1
- 229940043256 calcium pyrophosphate Drugs 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940090805 clavulanate Drugs 0.000 description 1
- HZZVJAQRINQKSD-PBFISZAISA-N clavulanic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 HZZVJAQRINQKSD-PBFISZAISA-N 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 235000019821 dicalcium diphosphate Nutrition 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- ACYGYJFTZSAZKR-UHFFFAOYSA-J dicalcium;2-[2-[bis(carboxylatomethyl)amino]ethyl-(carboxylatomethyl)amino]acetate Chemical compound [Ca+2].[Ca+2].[O-]C(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CC([O-])=O ACYGYJFTZSAZKR-UHFFFAOYSA-J 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000020805 dietary restrictions Nutrition 0.000 description 1
- XZTWHWHGBBCSMX-UHFFFAOYSA-J dimagnesium;phosphonato phosphate Chemical compound [Mg+2].[Mg+2].[O-]P([O-])(=O)OP([O-])([O-])=O XZTWHWHGBBCSMX-UHFFFAOYSA-J 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- SHPKCSFVQGSAJU-UAIGNFCESA-L dipotassium;(z)-but-2-enedioate Chemical compound [K+].[K+].[O-]C(=O)\C=C/C([O-])=O SHPKCSFVQGSAJU-UAIGNFCESA-L 0.000 description 1
- 208000016097 disease of metabolism Diseases 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 229940029980 drug used in diabetes Drugs 0.000 description 1
- 239000002355 dual-layer Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 235000005686 eating Nutrition 0.000 description 1
- 235000006694 eating habits Nutrition 0.000 description 1
- 229940009662 edetate Drugs 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- GDCRSXZBSIRSFR-UHFFFAOYSA-N ethyl prop-2-enoate;2-methylprop-2-enoic acid Chemical compound CC(=C)C(O)=O.CCOC(=O)C=C GDCRSXZBSIRSFR-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 210000001508 eye Anatomy 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000009484 foam granulation Methods 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 229910021485 fumed silica Inorganic materials 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 229960004580 glibenclamide Drugs 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 230000004110 gluconeogenesis Effects 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 230000004153 glucose metabolism Effects 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- ZNNLBTZKUZBEKO-UHFFFAOYSA-N glyburide Chemical compound COC1=CC=C(Cl)C=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZNNLBTZKUZBEKO-UHFFFAOYSA-N 0.000 description 1
- 230000002641 glycemic effect Effects 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000003451 hyperinsulinaemic effect Effects 0.000 description 1
- 201000008980 hyperinsulinism Diseases 0.000 description 1
- TVZISJTYELEYPI-UHFFFAOYSA-N hypodiphosphoric acid Chemical class OP(O)(=O)P(O)(O)=O TVZISJTYELEYPI-UHFFFAOYSA-N 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 239000011872 intimate mixture Substances 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940099584 lactobionate Drugs 0.000 description 1
- JYTUSYBCFIZPBE-AMTLMPIISA-N lactobionic acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O JYTUSYBCFIZPBE-AMTLMPIISA-N 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 239000007942 layered tablet Substances 0.000 description 1
- 230000037356 lipid metabolism Effects 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 150000004668 long chain fatty acids Chemical class 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- GVALZJMUIHGIMD-UHFFFAOYSA-H magnesium phosphate Chemical class [Mg+2].[Mg+2].[Mg+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O GVALZJMUIHGIMD-UHFFFAOYSA-H 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 229940102396 methyl bromide Drugs 0.000 description 1
- LRMHVVPPGGOAJQ-UHFFFAOYSA-N methyl nitrate Chemical compound CO[N+]([O-])=O LRMHVVPPGGOAJQ-UHFFFAOYSA-N 0.000 description 1
- IQSHMXAZFHORGY-UHFFFAOYSA-N methyl prop-2-enoate;2-methylprop-2-enoic acid Chemical compound COC(=O)C=C.CC(=C)C(O)=O IQSHMXAZFHORGY-UHFFFAOYSA-N 0.000 description 1
- 238000009481 moist granulation Methods 0.000 description 1
- YNAVUWVOSKDBBP-UHFFFAOYSA-O morpholinium Chemical compound [H+].C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-O 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 229920001206 natural gum Polymers 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- UWBHMRBRLOJJAA-UHFFFAOYSA-N oxaluric acid Chemical compound NC(=O)NC(=O)C(O)=O UWBHMRBRLOJJAA-UHFFFAOYSA-N 0.000 description 1
- 229940014662 pantothenate Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 230000001991 pathophysiological effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000000578 peripheral nerve Anatomy 0.000 description 1
- 239000008016 pharmaceutical coating Substances 0.000 description 1
- 238000009512 pharmaceutical packaging Methods 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229940075930 picrate Drugs 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229940044519 poloxamer 188 Drugs 0.000 description 1
- 229940044476 poloxamer 407 Drugs 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000008389 polyethoxylated castor oil Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 239000004323 potassium nitrate Substances 0.000 description 1
- 235000010333 potassium nitrate Nutrition 0.000 description 1
- OTYBMLCTZGSZBG-UHFFFAOYSA-L potassium sulfate Chemical compound [K+].[K+].[O-]S([O-])(=O)=O OTYBMLCTZGSZBG-UHFFFAOYSA-L 0.000 description 1
- 229910052939 potassium sulfate Inorganic materials 0.000 description 1
- 235000011151 potassium sulphates Nutrition 0.000 description 1
- VLYFRFHWUBBLRR-UHFFFAOYSA-L potassium;sodium;carbonate Chemical compound [Na+].[K+].[O-]C([O-])=O VLYFRFHWUBBLRR-UHFFFAOYSA-L 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 239000011253 protective coating Substances 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000003340 retarding agent Substances 0.000 description 1
- 238000009490 roller compaction Methods 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 239000011257 shell material Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 229940001593 sodium carbonate Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- OABYVIYXWMZFFJ-ZUHYDKSRSA-M sodium glycocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 OABYVIYXWMZFFJ-ZUHYDKSRSA-M 0.000 description 1
- 239000004317 sodium nitrate Substances 0.000 description 1
- 235000010344 sodium nitrate Nutrition 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- JAJWGJBVLPIOOH-IZYKLYLVSA-M sodium taurocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 JAJWGJBVLPIOOH-IZYKLYLVSA-M 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- ABBQHOQBGMUPJH-UHFFFAOYSA-N sodium;2-hydroxybenzoic acid Chemical compound [Na+].OC(=O)C1=CC=CC=C1O ABBQHOQBGMUPJH-UHFFFAOYSA-N 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 229940035044 sorbitan monolaurate Drugs 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000005563 spheronization Methods 0.000 description 1
- 238000009479 steam granulation Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 229910021653 sulphate ion Inorganic materials 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- RILFGEIJJMTZLZ-UHFFFAOYSA-J tetrapotassium;dioxido-oxo-phosphonato-$l^{5}-phosphane Chemical compound [K+].[K+].[K+].[K+].[O-]P([O-])(=O)P([O-])([O-])=O RILFGEIJJMTZLZ-UHFFFAOYSA-J 0.000 description 1
- RYCLIXPGLDDLTM-UHFFFAOYSA-J tetrapotassium;phosphonato phosphate Chemical compound [K+].[K+].[K+].[K+].[O-]P([O-])(=O)OP([O-])([O-])=O RYCLIXPGLDDLTM-UHFFFAOYSA-J 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000009482 thermal adhesion granulation Methods 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 150000003612 tocotrienol derivatives Chemical class 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 1
- XWKBMOUUGHARTI-UHFFFAOYSA-N tricalcium;diphosphite Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])[O-].[O-]P([O-])[O-] XWKBMOUUGHARTI-UHFFFAOYSA-N 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
- WUUHFRRPHJEEKV-UHFFFAOYSA-N tripotassium borate Chemical compound [K+].[K+].[K+].[O-]B([O-])[O-] WUUHFRRPHJEEKV-UHFFFAOYSA-N 0.000 description 1
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 1
- 229910000406 trisodium phosphate Inorganic materials 0.000 description 1
- 235000019801 trisodium phosphate Nutrition 0.000 description 1
- SOBHUZYZLFQYFK-UHFFFAOYSA-K trisodium;hydroxy-[[phosphonatomethyl(phosphonomethyl)amino]methyl]phosphinate Chemical group [Na+].[Na+].[Na+].OP(O)(=O)CN(CP(O)([O-])=O)CP([O-])([O-])=O SOBHUZYZLFQYFK-UHFFFAOYSA-K 0.000 description 1
- 150000003672 ureas Chemical class 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical class OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/426—1,3-Thiazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/02—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/20—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing sulfur, e.g. dimethyl sulfoxide [DMSO], docusate, sodium lauryl sulfate or aminosulfonic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0007—Effervescent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
- A61K9/1623—Sugars or sugar alcohols, e.g. lactose; Derivatives thereof; Homeopathic globules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1635—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1682—Processes
- A61K9/1694—Processes resulting in granules or microspheres of the matrix type containing more than 5% of excipient
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Obesity (AREA)
- Biochemistry (AREA)
- Physiology (AREA)
- Nutrition Science (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Thiazole And Isothizaole Compounds (AREA)
Abstract
Description
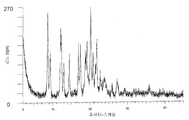
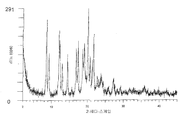
도 2는 Cu-Kα 방사선을 사용하여 수거된, 미분화된 결정형 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산을 함유하는 샘플의 PXRD 분석의 디프렉토그램을 나타낸다.
| 각도(2θ°) | d 값(Å) | 상대적 강도 | 강도 % |
| 2.240 | 39.409 | 10.7 | 3.6 |
| 2.557 | 34.520 | 14.4 | 4.9 |
| 2.812 | 31.396 | 20.4 | 7.0 |
| 3.081 | 28.650 | 9.85 | 3.4 |
| 3.381 | 26.109 | 6.24 | 2.1 |
| 3.869 | 22.818 | 9.47 | 3.2 |
| 4.260 | 20.724 | 3.38 | 1.2 |
| 4.556 | 19.381 | 9.11 | 3.1 |
| 4.924 | 17.932 | 9.33 | 3.2 |
| 6.000 | 14.719 | 13.9 | 4.7 |
| 8.576 | 10.303 | 241 | 81.9 |
| 9.267 | 9.5359 | 147 | 50.1 |
| 11.248 | 7.8604 | 18.2 | 6.2 |
| 12.059 | 7.3334 | 210 | 71.6 |
| 12.283 | 7.1999 | 183 | 62.4 |
| 12.953 | 6.8290 | 106 | 36.2 |
| 14.420 | 6.1377 | 153 | 52.1 |
| 15.704 | 5.6385 | 20.2 | 6.9 |
| 16.827 | 5.2647 | 153 | 52.1 |
| 17.390 | 5.0953 | 165 | 56.3 |
| 18.645 | 4.7551 | 160 | 54.3 |
| 19.117 | 4.6388 | 184 | 62.8 |
| 19.481 | 4.5530 | 60.0 | 20.4 |
| 20.111 | 4.4118 | 293 | 100 |
| 20.754 | 4.2764 | 122 | 41.6 |
| 21.347 | 4.1591 | 73.0 | 24.9 |
| 21.726 | 4.0872 | 174 | 59.4 |
| 22.159 | 4.0085 | 12.0 | 4.1 |
| 22.662 | 3.9206 | 49.8 | 17.0 |
| 22.999 | 3.8639 | 40.9 | 13.9 |
| 23.400 | 3.7985 | 27.3 | 9.3 |
| 23.677 | 3.7547 | 55.3 | 18.8 |
| 23.931 | 3.7154 | 57.4 | 19.5 |
| 24.312 | 3.6581 | 36.8 | 12.5 |
| 24.846 | 3.5806 | 12.8 | 4.4 |
| 25.248 | 3.5245 | 5.44 | 1.9 |
| 25.352 | 3.5103 | 4.47 | 1.5 |
| 25.907 | 3.4364 | 32.2 | 11.0 |
| 27.170 | 3.2794 | 68.6 | 23.4 |
| 27.520 | 3.2385 | 37.9 | 12.9 |
| 28.213 | 3.1606 | 24.4 | 8.3 |
| 29.117 | 3.0644 | 31.8 | 10.8 |
| 34.789 | 2.5767 | 15.8 | 5.4 |
| 38.069 | 2.3619 | 8.85 | 3.0 |
| 40.734 | 2.2133 | 16.7 | 5.7 |
| 44.637 | 2.0284 | 18.9 | 6.4 |
| 각도(2θ°) | d 값(Å) | 상대적 강도 | 강도 % |
| 2.320 | 38.050 | 14.2 | 7.1 |
| 2.400 | 36.782 | 7.47 | 3.7 |
| 2.500 | 35.311 | 17.7 | 8.9 |
| 2.620 | 33.694 | 18.9 | 9.5 |
| 2.908 | 30.361 | 19.6 | 9.8 |
| 3.151 | 28.020 | 7.21 | 3.6 |
| 3.400 | 25.966 | 10.4 | 5.2 |
| 3.900 | 22.638 | 14.3 | 7.2 |
| 4.592 | 19.226 | 13.2 | 6.6 |
| 4.858 | 18.176 | 9.23 | 4.6 |
| 5.340 | 16.536 | 10.7 | 5.4 |
| 7.029 | 12.565 | 13.5 | 6.8 |
| 7.578 | 11.657 | 7.2 | 3.6 |
| 8.581 | 10.296 | 180 | 90.2 |
| 9.255 | 9.5483 | 111 | 55.7 |
| 11.197 | 7.8956 | 12 | 6.0 |
| 12.071 | 7.3259 | 162 | 81.1 |
| 12.296 | 7.1926 | 145 | 73 |
| 12.952 | 6.8299 | 98.9 | 49.6 |
| 14.399 | 6.1463 | 88.7 | 44.5 |
| 14.920 | 6.1463 | 5.22 | 2.6 |
| 16.853 | 5.2564 | 135 | 67.7 |
| 17.382 | 5.0978 | 128 | 64.2 |
| 18.645 | 4.7553 | 95.2 | 47.8 |
| 19.127 | 4.6364 | 134 | 67.5 |
| 19.502 | 4.5482 | 52.5 | 26.4 |
| 20.100 | 4.4142 | 199 | 100 |
| 20.775 | 4.2722 | 78.6 | 39.5 |
| 21.400 | 4.1489 | 56.0 | 28.1 |
| 21.727 | 4.0870 | 137 | 68.6 |
| 22.147 | 4.0106 | 8.78 | 4.4 |
| 22.674 | 3.9185 | 46.7 | 23.4 |
| 23.040 | 3.8571 | 30.2 | 15.1 |
| 23.795 | 3.7364 | 45.1 | 22.7 |
| 24.319 | 3.6570 | 22.1 | 11.1 |
| 24.809 | 3.5859 | 13.8 | 6.9 |
| 25.087 | 3.5468 | 6.74 | 3.4 |
| 25.760 | 3.4557 | 21.4 | 10.8 |
| 25.886 | 3.4392 | 20.8 | 10.4 |
| 26.566 | 3.3526 | 7.4 | 4.0 |
| 27.224 | 3.2731 | 43.8 | 22 |
| 27.520 | 3.2385 | 37.9 | 12.9 |
| 27.577 | 3.2319 | 30.2 | 15.2 |
| 29.342 | 3.0415 | 22.3 | 11.2 |
| 31.328 | 2.8530 | 13.1 | 6.6 |
| 32.860 | 2.7234 | 25.7 | 12.9 |
| 34.695 | 2.5834 | 15.3 | 7.7 |
| 36.845 | 2.4375 | 14.1 | 7.1 |
| 37.869 | 2.3739 | 15.5 | 7.8 |
| 43.839 | 2.0635 | 11.0 | 5.5 |
| 실시예 | 용량 (mg/kg) | C max (ng/mL) | AUC 0 -t (hr*ng/mL) |
| 1 | 10 | 4150 | 40538 |
| 2 | 10 | 6695 | 41256 |
| 3 | 10 | 5875 | 45216 |
| 4 | 10 | 6595 | 52680 |
| 5 | 10 | 4710 | 45986 |
| 6 | 10 | 12500 | 63024 |
| 7 | 10 | 13150 | 58717 |
| 8 | 10 | 16300 | 60884 |
| 9 | 13.1 | 19933 | 140085 |
| 10 | 10 | 21313 | 141501 |
| 11 | 10 | 12713 | 74548 |
| 12 | 10 | 19833 | 150244 |
| 13 | 10 | 13767 | 100724 |
| 실시예 | 용량 (mg/kg) | C max (ng/mL) | AUC 0 -t (hr*ng/mL) |
| 3 | 10.7 | 5303 | 14448 |
| 4 | 10.4 | 4127 | 11724 |
| 6 | 9.8 | 6500 | 14797 |
| 7 | 10.1 | 6113 | 12645 |
| 11 | 9.2 | 7222 | 12697 |
| 14 | 12.6 | 5703 | 15516 |
| 15 | 9.2 | 7787 | 14203 |
| 16 | 9.5 | 2363 | 6558 |
| 17 | 10.0 | 431 | 1985 |
Claims (49)
- {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산 및 수용성 계면활성제를 포함하는 고체 조성물.
- 제1 항에 있어서, 수용성 계면활성제는 황산 알킬 에스테르 염, 담즙산염, 프로필렌 글리콜 지방산 모노- 또는 디에스테르, 폴리에틸렌 글리콜 지방산 에스테르, 폴리옥시에틸렌 소르비탄 지방산 에스테르,폴리옥시에틸렌-폴리옥시프로필렌 코폴리머 또는 차단 코폴리머 계면활성제, 토코페롤 또는 토코트리에놀의 폴리옥시에틸렌 유도체, 천연 오일 및 왁스의 폴리옥시에틸렌 유도체, 소르비탄 지방산 에스테르, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제1 항 또는 제2 항에 있어서, 수용성 계면활성제는 황산 알킬 에스테르 염, 폴리옥시에틸렌 소르비탄 지방산 에스테르, 토코페롤 또는 토코트리에놀의 폴리옥시에틸렌 유도체, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제3 항 중 어느 한 항에 있어서, 수용성 계면활성제는 나트륨 라우릴 술페이트, 폴리소르베이트 80, d-알파-토코페릴 폴리에틸렌 글리콜 숙시네이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제4 항 중 어느 한 항에 있어서, 수용성 계면활성제는 폴리소르베이트 80인 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제4 항 중 어느 한 항에 있어서, 수용성 계면활성제는 나트륨 라우릴 술페이트인 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제4 항 중 어느 한 항에 있어서, 수용성 계면활성제는 d-알파-토코페릴 폴리에틸렌 글리콜 숙시네이트인 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제7 항 중 어느 한 항에 있어서, 고체 조성물에서 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산 대 수용성 계면활성제의 중량/중량 비는 10:1 내지 100:1, 또는 15:1 내지 60:1, 또는 18:1 내지 50:1, 또는 22:1 내지 40:1, 또는 27:1 내지 35:1의 범위에 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제8 항 중 어느 한 항에 있어서, 고체 조성물은 약학적으로 허용 가능한 염기성 부형제를 더 포함하는 것을 특징으로 하는 고체 조성물.
- 제9 항에 있어서, 약학적으로 허용 가능한 염기성 부형제는 카보네이트, 비카보네이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제9 항 또는 제10 항에 있어서, 약학적으로 허용 가능한 염기성 부형제는 나트륨 카보네이트, 나트륨 비카보네이트, 칼륨 카보네이트, 칼륨 비카보네이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제9 항 내지 제11 항 중 어느 한 항에 있어서, 약학적으로 허용 가능한 염기성 부형제는 나트륨 카보네이트, 나트륨 비카보네이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제9 항 내지 제11 항 중 어느 한 항에 있어서, 약학적으로 허용 가능한 염기성 부형제는 칼륨 카보네이트, 칼륨 비카보네이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제9 항 내지 제13 항 중 어느 한 항에 있어서, {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산 대 약학적으로 허용 가능한 염기성 부형제의 중량/중량 비는 1:3 내지 25:1, 또는 1:2 내지 20:1, 또는 1:1 내지 17:1, 또는 2:1 내지 15:1의 범위에 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제14 항 중 어느 한 항에 있어서, 고체 조성물은 바인더를 더 포함하는 것을 특징으로 하는 고체 조성물.
- 제15 항에 있어서, 바인더는 폴리비닐피롤리돈, 히드록실프로필메틸 셀룰로스 아세테이트 숙시네이트, 히드록실프로필메틸 셀룰로스 프탈레이트, 히드록실프로필메틸 셀룰로스, 폴록사머, 히드록실프로필 메틸 셀룰로스 아세테이트, 히드록실프로필 셀룰로스, 히드록시에틸 셀룰로스 아세테이트, 폴리아크릴레이트, 메틸 아크릴레이트메타크릴산 코폴리머, 에틸 아크릴레이트메타크릴산 코폴리머, 셀룰로스 아세테이트 프탈레이트, 셀룰로스 아세테이트 트리멜리테이트, 카르복시메틸 에틸 셀룰로스, 히드록시에틸 셀룰로스, 폴리에틸렌 옥시드, 폴리에틸렌 글리콜, 에틸셀룰로스, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제15 항 또는 제16 항에 있어서, 바인더는 폴리비닐피롤리돈, 히드록실프로필메틸 셀룰로스 아세테이트 숙시네이트, 히드록시프로필메틸 셀룰로스 프탈레이트, 히드록시프로필메틸 셀룰로스, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제17 항 중 어느 한 항에 있어서, 바인더는 폴리비닐피롤리돈, 히드록시프로필메틸 셀룰로스 아세테이트 숙시네이트, 히드록시프로필메틸 셀룰로스 프탈레이트, 히드록시프로필메틸 셀룰로스, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제18 항 중 어느 한 항에 있어서, 바인더는 히드록시프로필메틸 셀룰로스 아세테이트 숙시네이트, 히드록시프로필메틸 셀룰로스 프탈레이트, 또는 이것들의 혼합물인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제19 항 중 어느 한 항에 있어서, 바인더는 히드록시프로필메틸 셀룰로스 아세테이트 숙시네이트인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제18 항 중 어느 한 항에 있어서, 바인더는 폴리비닐피롤리돈인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제18 항 중 어느 한 항에 있어서, 바인더는 히드록시프로필메틸 셀룰로스인 것을 특징으로 하는 고체 조성물.
- 제15 항 내지 제22 항 중 어느 한 항에 있어서, {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산 대 바인더의 중량/중량 비는 25:1 내지 400:1, 또는 35:1 내지 300:1, 또는 50:1 내지 250:1, 또는 65:1 내지 200:1, 또는 75:1 내지 150:1의 범위에 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제23 항 중 어느 한 항에 있어서, 고체 조성물은 약학적으로 허용 가능한 담체 또는 희석제, 또는 이것들의 혼합물을 더 포함하는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제24 항 중 어느 한 항에 있어서, 고체 조성물은 분말의 형태로 되어 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제24 항 중 어느 한 항에 있어서, 고체 조성물은 캡슐의 형태로 되어 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제24 항 중 어느 한 항에 있어서, 고체 조성물은 타블렛의 형태로 되어 있는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제27 항 중 어느 한 항에 있어서, 고체 조성물은 결정형 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산을 포함하는 것을 특징으로 하는 고체 조성물.
- 제1 항 내지 제28 항 중 어느 한 항에 있어서, 고체 조성물은 증발 잔류물을 더 포함하며, 증발 잔류물은 적어도 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산을 포함하는 것을 특징으로 하는 고체 조성물.
- 고체 조성물의 제조 방법으로서,
a) 용액 또는 현탁액을 형성하기 위해 용제의 존재 시 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산 및 수용성 계면활성제를 혼합하는 단계; 및
b) 분말을 형성하기 위해 용액 또는 현탁액으로부터 용제를 제거하는 단계
를 포함하는 방법. - 제30 항에 있어서, 혼합 단계는 용액 또는 현탁액을 형성하기 위해 용제의 존재 시 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산, 약학적으로 허용 가능한 염기성 부형제, 및 수용성 계면활성제를 혼합하는 단계를 포함하는 것을 특징으로 하는 방법.
- 제30 항에 있어서, 혼합 단계는 용액 또는 현탁액을 형성하기 위해 용제의 존재 시 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산, 바인더, 및 수용성 계면활성제를 혼합하는 단계를 포함하는 것을 특징으로 하는 방법.
- 제30 항에 있어서, 혼합 단계는 용액 또는 현탁액을 형성하기 위해 용제의 존재 시 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산, 약학적으로 허용 가능한 염기성 부형제, 바인더, 및 수용성 계면활성제를 혼합하는 단계를 포함하는 것을 특징으로 하는 방법.
- 제30 항 내지 제33 항 중 어느 한 항에 있어서, 제거 단계는 분말을 제거하기 위해 용액 또는 현탁액의 분무 건조를 포함하는 것을 특징으로 하는 방법.
- 제34 항에 있어서, 분무 건조는 분말을 형성하기 위해 고체 약학적으로 허용 가능한 담체에서 용액 또는 현탁액의 분무를 포함하는 것을 특징으로 하는 방법.
- 제34 항 또는 제35 항에 있어서, 분무 건조는 분말을 형성하기 위해 분무 건조기 또는 유동층 건조기/과립화기에서 용액 또는 현탁액의 분무 건조를 포함하는 것을 특징으로 하는 방법.
- 제35 항에 있어서, 고체 약학적으로 허용 가능한 담체는 약학적으로 허용 가능한 염기성 부형제, 약학적으로 허용 가능한 비활성 담체, 또는 이것들의 혼합물를 포함하는 것을 특징으로 하는 방법.
- 제30 항 내지 제33 항 중 어느 한 항에 있어서, 제거 단계는, 선택적으로, 기온보다 더 높은 온도에서 용액의 공기 건조를 포함하는 것을 특징으로 하는 방법.
- 제30 항 내지 제33 항 중 어느 한 항에 있어서, 제거 단계는, 선택적으로, 기온보다 더 높은 온도에서 용액의 유동층 건조를 포함하는 것을 특징으로 하는 방법.
- 제30 항 내지 제39 항 중 어느 한 항에 있어서, 분말은 추가적인 약학적 성분을 포함하는 것을 특징으로 하는 방법.
- 제30 항 내지 제40 항 중 어느 한 항에 있어서,
c) 분말을 타블렛으로 형성하는 단계
를 더 포함하는 것을 특징으로 하는 방법. - 제30 항 내지 제40 항 중 어느 한 항에 있어서,
c) 분말을 캡슐화하는 단계
를 더 포함하는 것을 특징으로 하는 방법. - 제30 항 내지 제40 항 중 어느 한 항에 있어서,
c) 분말을 사세로 포장하는 단계
를 더 포함하는 것을 특징으로 하는 방법. - 제41 항에 있어서, 분말을 타블렛으로의 형성은 다중층 타블렛의 형성을 포함하는 것을 특징으로 하는 방법.
- 타입 2 당뇨병을 치료하는 방법으로서, 인간에게 제1 항 내지 제29 항 중 어느 한 항의 고체 조성물을 투여하는 단계를 포함하며, 고체 조성물은 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산의 치료적 유효량을 포함하는 방법.
- 타입 1 당뇨병을 치료하는 방법으로서, 인간에게 제1 항 내지 제29 항 중 어느 한 항의 고체 조성물을 투여하는 단계를 포함하며, 고체 조성물은 {2-[3-시클로헥실-3-(트랜스-4-프로폭시-시클로헥실)-우레이도]-티아졸-5-일술파닐}-아세트산의 치료적 유효량을 포함하는 방법.
- 인간에서 혈당 농도를 낮추는 방법으로서, 인간에게 제1 항 내지 제29 항 중 어느 한 항의 고체 조성물을 투여하는 단계를 포함하는 방법.
- 인간에서 글루코키나제를 활성화시키는 방법으로서, 인간에게 제1 항 내지 제29 항 중 어느 한 항의 고체 조성물을 투여하는 단계를 포함하는 방법.
- 인간에서 간 글루코스 사용을 증가시키는 방법으로서, 인간에게 제1 항 내지 제29 항 중 어느 한 항의 고체 조성물을 투여하는 단계를 포함하는 방법.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020227000773A KR102694699B1 (ko) | 2013-03-04 | 2014-02-28 | 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361771969P | 2013-03-04 | 2013-03-04 | |
| US61/771,969 | 2013-03-04 | ||
| PCT/US2014/019363 WO2014137799A1 (en) | 2013-03-04 | 2014-02-28 | Solid compositions comprising a glucokinase activator and methods of making and using the same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227000773A Division KR102694699B1 (ko) | 2013-03-04 | 2014-02-28 | 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150126362A true KR20150126362A (ko) | 2015-11-11 |
Family
ID=50288318
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227000773A KR102694699B1 (ko) | 2013-03-04 | 2014-02-28 | 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 |
| KR1020157025034A KR20150126362A (ko) | 2013-03-04 | 2014-02-28 | 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227000773A KR102694699B1 (ko) | 2013-03-04 | 2014-02-28 | 글루코키나제 활성화제를 포함하는 고체 조성물 및 그것의 제조 및 사용 방법 |
Country Status (12)
| Country | Link |
|---|---|
| US (3) | US20160015816A1 (ko) |
| EP (1) | EP2964197B1 (ko) |
| JP (1) | JP6441829B2 (ko) |
| KR (2) | KR102694699B1 (ko) |
| CN (2) | CN105188676A (ko) |
| AU (1) | AU2014226292B2 (ko) |
| CA (1) | CA2903440C (ko) |
| ES (1) | ES2794018T3 (ko) |
| HK (1) | HK1213197A1 (ko) |
| IL (1) | IL240729A0 (ko) |
| MX (1) | MX2015011110A (ko) |
| WO (1) | WO2014137799A1 (ko) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101878252B1 (ko) | 2010-05-26 | 2018-07-13 | 브이티브이 테라퓨틱스 엘엘씨 | 글루코키나아제 활성화제와 병용되는 메트포르민의 용도, 및 메트포르민과 글루코키나아제 활성화제를 포함하는 조성물 |
| KR20150013838A (ko) | 2012-05-17 | 2015-02-05 | 트랜스테크 파르마 엘엘씨 | 당뇨병 치료를 위한 글루코키나아제 활성화제 조성물 |
| CN115715762A (zh) | 2016-12-15 | 2023-02-28 | 华领医药技术(上海)有限公司 | 葡萄糖激酶激活剂的口服制剂及其制备方法 |
| US11541015B2 (en) | 2017-05-17 | 2023-01-03 | Massachusetts Institute Of Technology | Self-righting systems, methods, and related components |
| CN117257753A (zh) * | 2017-05-17 | 2023-12-22 | 麻省理工学院 | 自扶正制品 |
| WO2019222570A1 (en) | 2018-05-17 | 2019-11-21 | Massachusetts Institute Of Technology | Systems for electrical stimulation |
| MX2020008905A (es) | 2018-06-12 | 2020-12-03 | Vtv Therapeutics Llc | Usos terapeuticos de activadores de glucoquinasa en combinacion con insulina o analogos de insulinas. |
| CN113993560B (zh) | 2019-02-01 | 2024-05-07 | 麻省理工学院 | 用于液体注射的系统和方法 |
| WO2021167840A1 (en) | 2020-02-18 | 2021-08-26 | Vtv Therapeutics Llc | Sulfoxide and sulfone glucokinase activators and methods of use thereof |
| CN116194442A (zh) * | 2020-06-08 | 2023-05-30 | 维特卫治疗有限责任公司 | {2-[3-环己基-3-(反式-4-丙氧基-环己基)-脲基]-噻唑-5-基硫烷基}-乙酸的盐或共晶体及其用途 |
| MX2022015524A (es) * | 2020-06-08 | 2023-03-22 | Vtv Therapeutics Llc | Formas cristalinas del acido {2-[3-ciclohexil-3-(trans-4-propoxi-c iclohexil)-ureido]-tiazol-5-ilsulfanil}-acetico y uso de las mismas. |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2551324C (en) * | 2004-01-06 | 2012-11-27 | Novo Nordisk A/S | Heteroaryl-ureas and their use as glucokinase activators |
| CN1910166B (zh) * | 2004-01-06 | 2012-01-04 | 诺和诺德公司 | 杂芳基脲及其作为葡糖激酶活化剂的用途 |
| KR101878252B1 (ko) * | 2010-05-26 | 2018-07-13 | 브이티브이 테라퓨틱스 엘엘씨 | 글루코키나아제 활성화제와 병용되는 메트포르민의 용도, 및 메트포르민과 글루코키나아제 활성화제를 포함하는 조성물 |
| MX2022015524A (es) * | 2020-06-08 | 2023-03-22 | Vtv Therapeutics Llc | Formas cristalinas del acido {2-[3-ciclohexil-3-(trans-4-propoxi-c iclohexil)-ureido]-tiazol-5-ilsulfanil}-acetico y uso de las mismas. |
-
2014
- 2014-02-28 EP EP14710738.7A patent/EP2964197B1/en active Active
- 2014-02-28 WO PCT/US2014/019363 patent/WO2014137799A1/en active Application Filing
- 2014-02-28 CN CN201480011657.6A patent/CN105188676A/zh active Pending
- 2014-02-28 CA CA2903440A patent/CA2903440C/en active Active
- 2014-02-28 KR KR1020227000773A patent/KR102694699B1/ko active IP Right Grant
- 2014-02-28 CN CN202011088593.3A patent/CN112263552A/zh active Pending
- 2014-02-28 JP JP2015561469A patent/JP6441829B2/ja active Active
- 2014-02-28 KR KR1020157025034A patent/KR20150126362A/ko active Application Filing
- 2014-02-28 MX MX2015011110A patent/MX2015011110A/es active IP Right Grant
- 2014-02-28 ES ES14710738T patent/ES2794018T3/es active Active
- 2014-02-28 AU AU2014226292A patent/AU2014226292B2/en active Active
-
2015
- 2015-08-20 IL IL240729A patent/IL240729A0/en unknown
- 2015-08-31 US US14/840,657 patent/US20160015816A1/en not_active Abandoned
-
2016
- 2016-02-03 HK HK16101236.4A patent/HK1213197A1/zh unknown
-
2018
- 2018-10-16 US US16/161,581 patent/US20190046645A1/en not_active Abandoned
-
2021
- 2021-09-21 US US17/480,856 patent/US20220233701A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20190046645A1 (en) | 2019-02-14 |
| ES2794018T3 (es) | 2020-11-17 |
| US20220233701A1 (en) | 2022-07-28 |
| WO2014137799A1 (en) | 2014-09-12 |
| CA2903440A1 (en) | 2014-09-12 |
| AU2014226292B2 (en) | 2018-10-04 |
| IL240729A0 (en) | 2015-10-29 |
| CN112263552A (zh) | 2021-01-26 |
| MX2015011110A (es) | 2015-10-29 |
| EP2964197A1 (en) | 2016-01-13 |
| KR102694699B1 (ko) | 2024-08-12 |
| CN105188676A (zh) | 2015-12-23 |
| KR20220009496A (ko) | 2022-01-24 |
| JP6441829B2 (ja) | 2018-12-19 |
| HK1213197A1 (zh) | 2016-06-30 |
| US20160015816A1 (en) | 2016-01-21 |
| JP2016510741A (ja) | 2016-04-11 |
| CA2903440C (en) | 2021-04-13 |
| EP2964197B1 (en) | 2020-03-25 |
| AU2014226292A1 (en) | 2015-10-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220233701A1 (en) | Solid compositions comprising a glucokinase activator and methods of making and using the same | |
| TWI428333B (zh) | 醫藥組合物 | |
| JP4789806B2 (ja) | パントプラゾール多粒子処方 | |
| JP2009537505A (ja) | インプリタピドを含む薬学的組成物およびこの薬学的組成物の使用方法 | |
| US20150209432A1 (en) | Pharmaceutical compositions of proton pump inhibitor | |
| JP5635491B2 (ja) | 固形医薬組成物 | |
| US20190105397A1 (en) | High-strength oral taxane compositions and methods | |
| US9198901B2 (en) | Tris(hydroxymethyl)aminomethane salts of a small-molecule GLP1R agonist and pharmaceutical compositions and uses thereof | |
| KR102363727B1 (ko) | 생체이용률이 개선된 프란루카스트 함유 고형 제제의 조성물 및 그 제조방법 | |
| US20110064806A1 (en) | Solid compositions comprising an oxadiazoanthracene compound and methods of making and using the same | |
| JP2015514799A (ja) | サルサレートの遅延放出性医薬組成物 | |
| KR101956586B1 (ko) | 약제학적 조성물 및 이의 제조방법 | |
| Azad et al. | Recent Advances in Delivering Strategies of Domperidone: Challenges and Opportunities | |
| WO2024126772A1 (en) | Pharmaceutical composition comprising rupatadine and montelukast |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150911 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20190218 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200804 Patent event code: PE09021S01D |
|
| PN2301 | Change of applicant |
Patent event date: 20201021 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20210611 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20200804 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| E601 | Decision to refuse application | ||
| E801 | Decision on dismissal of amendment | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20211012 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20200804 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| PE0801 | Dismissal of amendment |
Patent event code: PE08012E01D Comment text: Decision on Dismissal of Amendment Patent event date: 20211012 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20210910 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20210115 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20190218 |
|
| A107 | Divisional application of patent | ||
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20220110 |
|
| PJ0201 | Trial against decision of rejection |
Patent event date: 20220110 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20211012 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Patent event date: 20210611 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Appeal identifier: 2022101000047 Request date: 20220110 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2022101000047; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20220110 Effective date: 20230223 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20230223 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20220110 Decision date: 20230223 Appeal identifier: 2022101000047 |