KR20120052968A - 조직 공학용 장치 및 방법 - Google Patents
조직 공학용 장치 및 방법 Download PDFInfo
- Publication number
- KR20120052968A KR20120052968A KR1020127003354A KR20127003354A KR20120052968A KR 20120052968 A KR20120052968 A KR 20120052968A KR 1020127003354 A KR1020127003354 A KR 1020127003354A KR 20127003354 A KR20127003354 A KR 20127003354A KR 20120052968 A KR20120052968 A KR 20120052968A
- Authority
- KR
- South Korea
- Prior art keywords
- bioactive
- fibers
- pore
- temperature
- pore former
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/4455—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages
- A61F2/4465—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages having a circular or kidney shaped cross-section substantially perpendicular to the axis of the spine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30721—Accessories
- A61F2/30734—Modular inserts, sleeves or augments, e.g. placed on proximal part of stem for fixation purposes or wedges for bridging a bone defect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/10—Ceramics or glasses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/56—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/58—Materials at least partially resorbable by the body
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C10/00—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition
- C03C10/0009—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition containing silica as main constituent
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C11/00—Multi-cellular glass ; Porous or hollow glass or glass particles
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C13/00—Fibre or filament compositions
- C03C13/006—Glass-ceramics fibres
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C4/00—Compositions for glass with special properties
- C03C4/0007—Compositions for glass with special properties for biologically-compatible glass
- C03C4/0014—Biodegradable glass
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/3006—Properties of materials and coating materials
- A61F2002/30062—(bio)absorbable, biodegradable, bioerodable, (bio)resorbable, resorptive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2002/3092—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth having an open-celled or open-pored structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2002/30968—Sintering
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2002/4495—Joints for the spine, e.g. vertebrae, spinal discs having a fabric structure, e.g. made from wires or fibres
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0004—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof bioabsorbable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00329—Glasses, e.g. bioglass
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/18—Modification of implant surfaces in order to improve biocompatibility, cell growth, fixation of biomolecules, e.g. plasma treatment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/02—Materials or treatment for tissue regeneration for reconstruction of bones; weight-bearing implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/38—Materials or treatment for tissue regeneration for reconstruction of the spine, vertebrae or intervertebral discs
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2204/00—Glasses, glazes or enamels with special properties
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2213/00—Glass fibres or filaments
- C03C2213/02—Biodegradable glass fibres
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249924—Noninterengaged fiber-containing paper-free web or sheet which is not of specified porosity
- Y10T428/249928—Fiber embedded in a ceramic, glass, or carbon matrix
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Biomedical Technology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Medicinal Chemistry (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Geochemistry & Mineralogy (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurology (AREA)
- Ceramic Engineering (AREA)
- Dispersion Chemistry (AREA)
- Molecular Biology (AREA)
- Inorganic Chemistry (AREA)
- Biodiversity & Conservation Biology (AREA)
- Manufacturing & Machinery (AREA)
- Crystallography & Structural Chemistry (AREA)
- Materials For Medical Uses (AREA)
- Prostheses (AREA)
- Surgical Instruments (AREA)
Abstract
Description
도 1은 본 발명에 따른 생리활성 조직 스캐폴드의 실시예를 보여주는 약 1000배 확대 비율의 광학 현미경 사진이다.
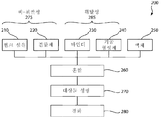
도 2는 도 1의 생리활성 조직 스캐폴드를 형성하기 위한 본 발명에 따른 방법의 실시예의 흐름도이다.
도 3은 도 2 발명의 방법에 따른 경화 단계의 실시예의 흐름도이다.
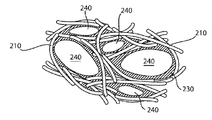
도 4는 본 발명의 방법에 따라 제작된 대상물의 실시예의 개략적인 묘사이다.
도 5는 본 발명에 따른 방법 중 휘발성 성분 제거 단계의 완료에 따른 도 4의 대상물의 개략적인 묘사이다.
도 6은 본 발명에 따른 방법 중 결합 형성 단계의 완료에 따른 도 5의 대상물의 개략적인 묘사이다.
도 7은 본 발명의 재흡수성 조직 스캐폴드의 다양한 실시예를 공지된 표본과 비교한 비교 분석 그래프이다.
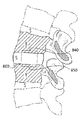
도 8은 척추 이식편으로 제조된 본 발명에 따른 생리활성 조직 스캐폴드의 측면도이다.
도 9는 척추사이 공간 내에 이식된 도 8에 따른 척추 이식편을 갖는 척추 부분의 측면 투시도이다.
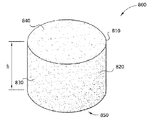
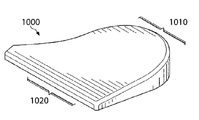
도 10은 골절술 쇄기형으로 제조된 본 발명에 따른 생리활성 조직 스캐폴드의 등각도(isometric view)를 보여주는 개략적인 그림이다.
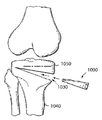
도 11은 뼈 내의 골절술 개방부 안으로 삽입될 수 있도록 구성된 도 10의 골절술 쇄기형의 분해도를 도시하는 개략적인 그림이다.
도 12는 본 발명에 따른 방법의 실시예 중 경화 단계의 대표적인 열 프로파일의 그래프이다.
이미 논의되었듯이, 여기에 나열된 상기 도면이 실시예를 개시하지만, 다른 실시예도 고려된다. 본 공개문헌은 묘사의 방식으로 예시적인 실시예를 나타내며, 이를 제한하지 않는다. 다양한 다른 변형 및 실시예는 여기 개시된 실시예의 원리의 범위 및 사상 내에서 당해 분야의 통상의 기술자에 의해 창안될 수 있다.
| 기공 형성제 | 연소 온도 |
| 활성 탄소 | 621℃ |
| 편상 흑연 | 603℃ |
| HPMC | 375℃ |
| PMMa | 346℃ |
| 목분 | 317℃ |
| 옥수수 전분 | 292℃ |
Claims (18)
- 인공 뼈 보철을 제작하는 방법으로,
기공 형성제 및 소성(plastically) 성형성 뱃지(batch)를 제공하기 위한 액체를 생리활성 섬유 조직과 혼합하는 단계;
생리활성 섬유를 기공 형성제로 분산시켜서 서로 얽혀지고(intertangled) 겹쳐진 생리활성 섬유의 균일한 덩어리(mass)의 성형성 뱃지를 제공하기 위해 상기 성형성 뱃지를 니딩(kneading)하는 단계;
성형된 형태를 제공하기 위하여 성형성 뱃지를 소정의 형상으로 성형하는 단계;
상기 성형된 형태 내의 액체를 제거하기 위해 건조하는 단계;
서로 얽혀지고 겹쳐진 생리활성 유리 섬유 사이에 결합을 형성하기 위하여 기공 형성제의 발열 반응을 이용하는, 결합 형성 온도로 성형된 형태를 가열하는 단계를 포함하는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 생리활성 섬유는 실투 온도를 가지며, 상기 기공 형성제의 발열 반응은 상기 성형된 형태가 실투 온도 미만인 경우에 개시되며, 상기 기공 형성제의 발열 반응은 상기 성형된 형태가 실투 온도에 도달하기 이전에 완료되는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 생리활성 섬유는 실투 온도를 가지면, 상기 기공 형성제의 발열 반응은 상기 성형된 형태가 실투 온도 미만인 경우에 개시되며, 상기 기공 형성제의 발열 반응은 상기 성형된 형태가 실투 온도에 도달한 이후에 완료되는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 성형된 형태를 결합 형서 온도로 가열하는 단계는 공기-제거 가마에서 수행되는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 기공 형성제는 활성 탄소, 편상 흑연, HPMC, PMMA, 목분 및 옥수수 전분으로 구성된 리스트로부터 선택되는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 혼합 단계는 바인더를 더 포함하는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 6 항에 있어서,
상기 혼합 단계는 결합제를 더 포함하는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 혼합 단계는 결합제를 더 포함하는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 제 1 항에 있어서,
상기 가열 단계 이후에 인공 뼈 보철을 어닐링하는 단계를 더 포함하는 것을 특징으로 하는 인공 뼈 보철을 제작하는 방법. - 서로 얽혀진 생리활성 유리 섬유;
강성 3차원 조직 스캐폴드를 형성하도록 서로 얽혀진 생리활성 유리 섬유 사이의 결합;
서로 얽혀진 생리활성 섬유 내에 산재된 휘발성 성분 및 결합의 형성 과정에서 제거됨으로써 기결정된 강성 3차원 조직 스캐폴드 내의 기공 공간; 및
휘발성 성분의 발열 반응을 이용하여 형성된 결합을 포함하는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 휘발성 성분은 기공 형성제를 포함하는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 기공 공간은 인공 뼈 보철 중의 약 40% 내지 약 85%의 다공성을 형성하는 것을 특징으로 인공 뼈 보철. - 제 10 항에 있어서,
상기 기공 공간은 약 10 μm 내지 약 500 μm의 기공 크기를 갖는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 기공 크기는 바이모달(bi-modal) 크기 분포를 갖는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
서로 얽혀진 생리활성 유리 섬유는 탄산나트륨, 탄산칼슘, 오산화인, 약 45 몰% 내지 약 60 몰%의 실리카, 및 약 2 내지 약 10의 인산염에 대한 칼슘의 몰비를 포함하는 조성물을 갖는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 서로 얽혀진 생리활성 유리 섬유는 13-93 유리 섬유를 포함하는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 서로 얽혀진 생리활성 섬유는 다른 조성물을 갖는 생리활성 유리 섬유의 혼합물을 포함하는 것을 특징으로 하는 인공 뼈 보철. - 제 10 항에 있어서,
상기 서로 얽혀진 생리활성 섬유는 약 1 μm 내지 약 200 μm의 직경을 갖는 것을 특징으로 하는 인공 뼈 보철.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US22467509P | 2009-07-10 | 2009-07-10 | |
| US61/224,675 | 2009-07-10 | ||
| US23476809P | 2009-08-18 | 2009-08-18 | |
| US61/234,768 | 2009-08-18 | ||
| PCT/US2010/041333 WO2011005935A2 (en) | 2009-07-10 | 2010-07-08 | Devices and methods for tissue engineering |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20120052968A true KR20120052968A (ko) | 2012-05-24 |
| KR101721276B1 KR101721276B1 (ko) | 2017-03-29 |
Family
ID=43429830
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127003355A Expired - Fee Related KR101683328B1 (ko) | 2009-07-10 | 2010-07-08 | 조직공학용 장치 및 방법 |
| KR1020127003354A Expired - Fee Related KR101721276B1 (ko) | 2009-07-10 | 2010-07-08 | 조직 공학용 장치 및 방법 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127003355A Expired - Fee Related KR101683328B1 (ko) | 2009-07-10 | 2010-07-08 | 조직공학용 장치 및 방법 |
Country Status (10)
| Country | Link |
|---|---|
| US (6) | US8652368B2 (ko) |
| EP (2) | EP2453936B1 (ko) |
| JP (2) | JP5711735B2 (ko) |
| KR (2) | KR101683328B1 (ko) |
| CN (2) | CN102470194B (ko) |
| BR (2) | BR112012000327B8 (ko) |
| CA (2) | CA2767715C (ko) |
| IL (2) | IL217340A (ko) |
| IN (2) | IN2012DN01105A (ko) |
| WO (2) | WO2011005935A2 (ko) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20240029953A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 폭 가변장치 |
| KR20240029947A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 가압유지장치 |
| KR20240029942A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 가압장치 |
Families Citing this family (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3336224B2 (ja) | 1997-05-01 | 2002-10-21 | 新日本製鐵株式会社 | 溶鋼の連続鋳造用鋳型 |
| US10524916B2 (en) | 2006-01-11 | 2020-01-07 | Novabone Products, Llc | Resorbable macroporous bioactive glass scaffold and method of manufacture |
| AU2009335771B2 (en) | 2008-12-18 | 2015-01-29 | 4-Web, Inc. | Truss implant |
| US12279964B2 (en) | 2008-12-18 | 2025-04-22 | 4Web, Llc | Implants having bone growth promoting agents and methods of using such implants to repair bone structures |
| US8287572B2 (en) | 2009-02-11 | 2012-10-16 | Howmedica Osteonics Corp. | Intervertebral implant with integrated fixation |
| US9775721B2 (en) | 2009-07-10 | 2017-10-03 | Bio2 Technologies, Inc. | Resorbable interbody device |
| US20110206828A1 (en) * | 2009-07-10 | 2011-08-25 | Bio2 Technologies, Inc. | Devices and Methods for Tissue Engineering |
| US8652368B2 (en) * | 2009-07-10 | 2014-02-18 | Bio2 Technologies, Inc. | Devices and methods for tissue engineering |
| WO2011059746A1 (en) | 2009-10-29 | 2011-05-19 | Prosidyan, Inc. | Dynamic bioactive bone graft material and methods for handling |
| WO2011053719A1 (en) | 2009-10-29 | 2011-05-05 | Prosidyan, Inc. | Dynamic bioactive bone graft material having an engineered porosity |
| US20120219635A1 (en) * | 2010-05-06 | 2012-08-30 | Bio2 Technologies, Inc. | Devices and Methods for Tissue Engineering |
| US9066998B2 (en) * | 2012-03-02 | 2015-06-30 | Bio2 Technologies, Inc. | Devices and method for tissue engineering |
| US9045362B2 (en) | 2013-03-15 | 2015-06-02 | Mosci Corp. | Bioactive glass scaffolds, and method of making |
| US11225430B2 (en) | 2012-03-26 | 2022-01-18 | Steven Jung | Bioactive glass scaffolds, and method of making |
| KR101941244B1 (ko) * | 2012-03-26 | 2019-01-22 | 모사이 코퍼레이션. | 생활성 유리 지지체 및 이의 제조방법 |
| US8449904B1 (en) | 2012-03-26 | 2013-05-28 | Mosci, Corp. | Bioactive glass scaffolds, and method of making |
| CN102826752B (zh) * | 2012-08-23 | 2015-07-15 | 北京大清生物技术有限公司 | 一种含有准纳米级颗粒的生物活性矿物质粉体、制备方法及其在牙科治疗中的应用 |
| US12115071B2 (en) | 2012-09-25 | 2024-10-15 | 4Web, Llc | Programmable intramedullary implants and methods of using programmable intramedullary implants to repair bone structures |
| US9376540B2 (en) | 2013-01-25 | 2016-06-28 | Eastman Kodak Company | Particles with designed different sized discrete pores |
| US8889178B2 (en) | 2013-03-14 | 2014-11-18 | Prosidyan, Inc | Bioactive porous bone graft compositions in synthetic containment |
| US8883195B2 (en) * | 2013-03-14 | 2014-11-11 | Prosidyan, Inc. | Bioactive porous bone graft implants |
| US9381274B2 (en) | 2013-03-14 | 2016-07-05 | Prosidyan, Inc. | Bone graft implants containing allograft |
| CN103191463B (zh) * | 2013-04-03 | 2014-10-29 | 上海师范大学 | 一种壳聚糖纤维/生物活性玻璃三维有序多孔支架材料的制备方法 |
| EP2984047B1 (en) * | 2013-04-12 | 2022-06-15 | MO-SCI Corporation | Bioactive glass scaffolds, and method of making |
| AU2016200179B2 (en) | 2015-01-14 | 2020-09-17 | Stryker European Operations Holdings Llc | Spinal implant with porous and solid surfaces |
| AU2016200195B2 (en) | 2015-01-14 | 2020-07-02 | Vb Spine Us Opco Llc | Spinal implant with fluid delivery capabilities |
| CA2930123A1 (en) | 2015-05-18 | 2016-11-18 | Stryker European Holdings I, Llc | Partially resorbable implants and methods |
| US20170056190A1 (en) * | 2015-08-27 | 2017-03-02 | Wright Medical Technology, Inc. | Subtalar biofoam wedge |
| US10485897B2 (en) * | 2015-10-12 | 2019-11-26 | Erik Erbe | Osteogenic and angiogenic implant material |
| US11013836B2 (en) * | 2016-06-16 | 2021-05-25 | The Curators Of The University Of Missouri | Inorganic biodegradable substrates for devices and systems |
| EP3459502B1 (en) | 2017-09-20 | 2024-05-22 | Stryker European Operations Holdings LLC | Spinal implants |
| CN109966549B (zh) * | 2017-12-28 | 2021-12-31 | 北京纳通科技集团有限公司 | 一种三维仿生骨修复材料及其制备方法 |
| WO2020008558A1 (ja) * | 2018-07-04 | 2020-01-09 | オリンパス株式会社 | 骨補填材及び骨補填材の製造方法 |
| EP3887331B1 (en) * | 2018-11-26 | 2024-08-14 | Corning Incorporated | Bioactive silicate glasses |
| KR20210149695A (ko) | 2019-02-07 | 2021-12-09 | 바이오레즈, 인크. | 연조직의 수복, 재건, 및 재생을 위한 복합 스캐폴드 |
| EP4057943A4 (en) | 2019-11-15 | 2023-12-13 | 4-web, Inc. | Piezoelectric coated implants and methods of using piezoelectric coated implants to repair bone structures |
| US12246111B2 (en) | 2020-01-30 | 2025-03-11 | SDIP Innovations Pty Ltd | Bioresorbable implant with inside-out resorption for enhanced bone ingrowth and tissue integration and method of manufacturing thereof |
| JP2023517843A (ja) * | 2020-02-24 | 2023-04-27 | プロシディアン・インコーポレイテッド | 生物活性の埋め込み可能な装置、並びに複合生体材料及びその製造方法 |
| US12201531B2 (en) | 2020-07-08 | 2025-01-21 | 4Web, Llc | Implants having bone growth promoting agents contained within biodegradable materials |
| AU2021322190A1 (en) * | 2020-08-03 | 2023-03-02 | Biorez, Inc. | Composite scaffold for the repair, reconstruction, and regeneration of soft tissues |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7241486B2 (en) * | 2001-04-26 | 2007-07-10 | Inion Ltd. | Bone grafting materials |
| US20070162151A1 (en) * | 2006-01-11 | 2007-07-12 | Jiang Chang | Resorbable macroporous bioactive glass scaffold and method of manufacture |
| WO2008141313A1 (en) * | 2007-05-14 | 2008-11-20 | Geo2 Technologies, Inc. | Method and apparatus for an extruded ceramic biosoluble fiber substrate |
| KR20090057392A (ko) * | 2006-08-18 | 2009-06-05 | 지이오2 테크놀로지스 인코포레이티드 | 무기 결합 구조를 갖는 압출 성형 다공성 기판 |
Family Cites Families (84)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0051955B1 (en) * | 1980-11-06 | 1985-04-24 | Charles A. Homsy | Porous body-implantable polytetrafluoroethylene composites |
| US4655777A (en) | 1983-12-19 | 1987-04-07 | Southern Research Institute | Method of producing biodegradable prosthesis and products therefrom |
| SE455103B (sv) | 1985-01-09 | 1988-06-20 | Lars Edgar Martin Ehrnford | Berare for immobilisering av biologiskt aktivt organiskt material, vilken utgores av sammanfogade partiklar av en poros sintrad glasfibermatris |
| US4604097A (en) | 1985-02-19 | 1986-08-05 | University Of Dayton | Bioabsorbable glass fibers for use in the reinforcement of bioabsorbable polymers for bone fixation devices and artificial ligaments |
| US5149857A (en) * | 1989-06-07 | 1992-09-22 | Mitsubishi Gas Chemical Company, Inc. | Process for production of sulfonium compounds |
| US5009822A (en) | 1989-07-17 | 1991-04-23 | University Of Florida | Alumina-or alumina/zirconia-silicon carbide whisker ceramic composites and methods of manufacture |
| US5236458A (en) | 1989-09-06 | 1993-08-17 | S.A. Fbfc International | Bioreactive material for a prosthesis or composite implants |
| DE69115481T2 (de) | 1990-06-01 | 1996-05-02 | Du Pont | Orthopädisches implantat aus verbundwerkstoff mit modulschwankungen |
| EP0672117A4 (en) | 1992-08-13 | 1996-06-12 | Univ Pennsylvania | MATRIX BASED ON A BIOACTIVE MATERIAL FOR THE SYNTHESIS -i (IN VITRO) OF BONE TISSUE. |
| CN1087279A (zh) * | 1992-11-27 | 1994-06-01 | 中国科学院光电技术研究所 | 生物活性玻璃陶瓷人工骨及其制法 |
| US5419857A (en) | 1993-08-17 | 1995-05-30 | Praxair Technology, Inc. | Thermal removal of binders from ceramic-particle bodies |
| US5468544A (en) | 1993-11-15 | 1995-11-21 | The Trustees Of The University Of Pennsylvania | Composite materials using bone bioactive glass and ceramic fibers |
| US5721049A (en) | 1993-11-15 | 1998-02-24 | Trustees Of The University Of Pennsylvania | Composite materials using bone bioactive glass and ceramic fibers |
| US5629186A (en) | 1994-04-28 | 1997-05-13 | Lockheed Martin Corporation | Porous matrix and method of its production |
| FI101129B (sv) * | 1995-01-13 | 1998-04-30 | Vivoxid Oy | Nya bioaktiva glas och deras användning |
| US6162537A (en) | 1996-11-12 | 2000-12-19 | Solutia Inc. | Implantable fibers and medical articles |
| US6471993B1 (en) | 1997-08-01 | 2002-10-29 | Massachusetts Institute Of Technology | Three-dimensional polymer matrices |
| US6296667B1 (en) | 1997-10-01 | 2001-10-02 | Phillips-Origen Ceramic Technology, Llc | Bone substitutes |
| EP1027304B1 (en) | 1997-10-03 | 2005-07-13 | Corning Incorporated | Method for firing ceramic honeycomb bodies |
| BR9814753A (pt) | 1997-12-02 | 2000-10-03 | Corning Inc | Forno em túnel para queima de corpos alveolares cerâmicos |
| US6325963B1 (en) | 1997-12-22 | 2001-12-04 | Corning Incorporated | Method for firing ceramic honeycomb bodies |
| JP4723085B2 (ja) | 1997-12-22 | 2011-07-13 | コーニング インコーポレイテッド | セラミックハニカム体の焼成方法及び焼成に用いられるトンネルキルン |
| US6187329B1 (en) | 1997-12-23 | 2001-02-13 | Board Of Regents Of The University Of Texas System | Variable permeability bone implants, methods for their preparation and use |
| US20030147860A1 (en) | 2002-02-07 | 2003-08-07 | Marchosky J. Alexander | Compositions and methods for forming and strengthening bone |
| US6406498B1 (en) | 1998-09-04 | 2002-06-18 | Bionx Implants Oy | Bioactive, bioabsorbable surgical composite material |
| US6146892A (en) | 1998-09-28 | 2000-11-14 | The Regents Of The University Of Michigan | Fibrillar matrices |
| US6110484A (en) | 1998-11-24 | 2000-08-29 | Cohesion Technologies, Inc. | Collagen-polymer matrices with differential biodegradability |
| FI110063B (fi) | 1998-12-11 | 2002-11-29 | Antti Yli-Urpo | Uusi bioaktiivinen tuote ja sen käyttö |
| US6200347B1 (en) * | 1999-01-05 | 2001-03-13 | Lifenet | Composite bone graft, method of making and using same |
| JP2002539742A (ja) * | 1999-03-17 | 2002-11-19 | シーメンス アクチエンゲゼルシヤフト | 対話のための装置 |
| US6187239B1 (en) * | 1999-03-30 | 2001-02-13 | Tokyo Seihinkaihatsu Kenkyusho | Manufacture method of article similar to unglazed ceramic plate |
| ES2222700T3 (es) * | 1999-04-16 | 2005-02-01 | Rutgers, The State University | Estructuras polimericas porosas para ingenieria tisular. |
| EP1229858B1 (en) | 1999-11-01 | 2007-05-23 | Osteobiologics, Inc. | Biodegradable polymer/ceramic implant material with bimodal degradation profile |
| US6451059B1 (en) | 1999-11-12 | 2002-09-17 | Ethicon, Inc. | Viscous suspension spinning process for producing resorbable ceramic fibers and scaffolds |
| US20040009228A1 (en) * | 1999-11-30 | 2004-01-15 | Pertti Tormala | Bioabsorbable drug delivery system for local treatment and prevention of infections |
| FI20000515A0 (fi) * | 2000-03-07 | 2000-03-07 | Heimo Ylaenen | Menetelmä bioaktiivisen lasin pinnan karhentamiseksi |
| AU2001249437A1 (en) | 2000-03-24 | 2001-10-08 | Lyles, Mark B | Diagnostic devices containing porous material |
| FI20001696L (fi) | 2000-07-21 | 2002-01-22 | Jvs Polymers Oy | Biologisesti aktiivinen materiaali |
| US20020082697A1 (en) | 2000-12-22 | 2002-06-27 | Damien Christopher J. | Implantable osteogenic material |
| US7005135B2 (en) * | 2001-01-30 | 2006-02-28 | Ethicon Inc. | Glass scaffolds with controlled resorption rates and methods for making same |
| WO2002087647A1 (en) * | 2001-04-26 | 2002-11-07 | Eija Pirhonen | Bone grafting materials |
| US6626950B2 (en) | 2001-06-28 | 2003-09-30 | Ethicon, Inc. | Composite scaffold with post anchor for the repair and regeneration of tissue |
| US20040009598A1 (en) | 2001-07-11 | 2004-01-15 | Hench Larry L | Use of bioactive glass compositions to stimulate osteoblast production |
| US20030220692A1 (en) * | 2002-02-09 | 2003-11-27 | Shapiro Irving M. | Preparations of nucleus pulposus cells and methods for their generation, identification, and use |
| US6955716B2 (en) | 2002-03-01 | 2005-10-18 | American Dental Association Foundation | Self-hardening calcium phosphate materials with high resistance to fracture, controlled strength histories and tailored macropore formation rates |
| US8313742B2 (en) | 2002-03-29 | 2012-11-20 | Depuy Acromed, Inc. | Cell-containing bone graft material |
| US7166133B2 (en) | 2002-06-13 | 2007-01-23 | Kensey Nash Corporation | Devices and methods for treating defects in the tissue of a living being |
| WO2004032988A2 (en) | 2002-10-08 | 2004-04-22 | Osteotech, Inc. | Coupling agents for orthopedic biomaterials |
| US20050118236A1 (en) | 2002-12-03 | 2005-06-02 | Gentis Inc. | Bioactive, resorbable scaffolds for tissue engineering |
| US7217293B2 (en) * | 2003-11-21 | 2007-05-15 | Warsaw Orthopedic, Inc. | Expandable spinal implant |
| ITPD20030286A1 (it) | 2003-11-27 | 2005-05-28 | Fidia Advanced Biopolymers Srl | Strutture composite multistrato contenenti acido ialuronico |
| US7625581B2 (en) | 2003-12-19 | 2009-12-01 | Ethicon, Inc. | Tissue scaffolds for use in muscoloskeletal repairs |
| US20070152364A1 (en) | 2005-11-16 | 2007-07-05 | Bilal Zuberi | Process for extruding a porous substrate |
| US20070190108A1 (en) | 2004-05-17 | 2007-08-16 | Arindam Datta | High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair |
| US8597331B2 (en) * | 2004-12-10 | 2013-12-03 | Life Spine, Inc. | Prosthetic spinous process and method |
| US20060216321A1 (en) * | 2005-03-24 | 2006-09-28 | Sdgi Holdings, Inc. | Solvent based processing technologies for making tissue/polymer composites |
| FI20055194A7 (fi) * | 2005-04-27 | 2006-10-28 | Bioretec Oy | Bioabsorboituva ja bioaktiivinen komposiittimateriaali ja menetelmä komposiitin valmistamiseksi |
| WO2006118554A1 (en) | 2005-05-02 | 2006-11-09 | Shanghai Institute Of Ceramics | A degradable, macroporous bioactive glass scaffold as well as its preparation and manufacturing methods |
| US20070055373A1 (en) | 2005-09-08 | 2007-03-08 | Zimmer Spine, Inc. | Facet replacement/spacing and flexible spinal stabilization |
| US20070087059A1 (en) | 2005-10-17 | 2007-04-19 | Frank Everaerts | Bioactive delivery matrix compositions and methods |
| US20070123984A1 (en) | 2005-10-26 | 2007-05-31 | Zimmer Technology, Inc. | Ligament attachment and repair device |
| US20070098799A1 (en) | 2005-10-28 | 2007-05-03 | Zimmer, Inc. | Mineralized Hydrogels and Methods of Making and Using Hydrogels |
| US20090299476A1 (en) * | 2006-05-19 | 2009-12-03 | Ashish Diwan | Tissue prosthesis |
| GB0612028D0 (en) * | 2006-06-16 | 2006-07-26 | Imp Innovations Ltd | Bioactive glass |
| EP1903012B1 (en) * | 2006-09-20 | 2009-12-23 | Inion Oy | Bioactive glass compositions |
| US7923020B2 (en) | 2006-09-29 | 2011-04-12 | Depuy Products, Inc. | Composite for implantation in the body of an animal and method for making the same |
| US7718616B2 (en) | 2006-12-21 | 2010-05-18 | Zimmer Orthobiologics, Inc. | Bone growth particles and osteoinductive composition thereof |
| US7781372B2 (en) | 2007-07-31 | 2010-08-24 | GE02 Technologies, Inc. | Fiber-based ceramic substrate and method of fabricating the same |
| US7855163B2 (en) * | 2007-05-14 | 2010-12-21 | Geo2 Technologies, Inc. | Low coefficient of thermal expansion bonding system for a high porosity ceramic body and methods of manufacture |
| US8641774B2 (en) * | 2007-09-14 | 2014-02-04 | The Curators Of The University Of Missouri | Synthetic osteochondral composite and method of fabrication thereof |
| US20110142902A1 (en) * | 2008-05-27 | 2011-06-16 | Imperial Innovations Limited | Hypoxia Inducing Factor (HIF) Stabilising Glasses |
| US9456890B2 (en) * | 2009-01-15 | 2016-10-04 | The Curators Of The University Of Missouri | Scaffold for bone and tissue repair in mammals |
| US8353966B2 (en) * | 2009-01-15 | 2013-01-15 | The Curators Of The University Of Missouri | Scaffold for bone and tissue repair in mammals |
| EP2243749B1 (en) * | 2009-04-23 | 2015-04-08 | PURAC Biochem BV | Resorbable and biocompatible fibre glass compositions and their uses |
| US20110206828A1 (en) * | 2009-07-10 | 2011-08-25 | Bio2 Technologies, Inc. | Devices and Methods for Tissue Engineering |
| US8652368B2 (en) | 2009-07-10 | 2014-02-18 | Bio2 Technologies, Inc. | Devices and methods for tissue engineering |
| US9775721B2 (en) * | 2009-07-10 | 2017-10-03 | Bio2 Technologies, Inc. | Resorbable interbody device |
| US8287896B2 (en) * | 2010-01-06 | 2012-10-16 | The Curators Of The University Of Missouri | Scaffolds with trace element for tissue regeneration in mammals |
| US8481066B2 (en) * | 2009-07-16 | 2013-07-09 | The Curators Of The University Of Missouri | Scaffold for tissue regeneration in mammals |
| BR112014019797B1 (pt) * | 2012-02-10 | 2019-11-19 | Synthes Gmbh | materiais de implante porosos e métodos relacionados |
| US9066998B2 (en) * | 2012-03-02 | 2015-06-30 | Bio2 Technologies, Inc. | Devices and method for tissue engineering |
| US20140050765A1 (en) * | 2012-08-14 | 2014-02-20 | Bio2 Technologies, Inc. | Devices and Methods for Tissue Engineering |
| US20140212469A1 (en) * | 2013-01-28 | 2014-07-31 | Missouri University Of Science And Technology | Surface functional bioactive glass scaffold for bone regeneration |
| US10238772B2 (en) * | 2013-03-15 | 2019-03-26 | The Curators Of The University Of Missouri | Biodegradable composite scaffold for repairing defects in load-bearing bones |
-
2010
- 2010-07-08 US US12/832,394 patent/US8652368B2/en active Active
- 2010-07-08 WO PCT/US2010/041333 patent/WO2011005935A2/en not_active Ceased
- 2010-07-08 CA CA2767715A patent/CA2767715C/en not_active Expired - Fee Related
- 2010-07-08 EP EP10797831.4A patent/EP2453936B1/en not_active Not-in-force
- 2010-07-08 IN IN1105DEN2012 patent/IN2012DN01105A/en unknown
- 2010-07-08 JP JP2012519718A patent/JP5711735B2/ja not_active Expired - Fee Related
- 2010-07-08 CN CN201080030957.0A patent/CN102470194B/zh not_active Expired - Fee Related
- 2010-07-08 KR KR1020127003355A patent/KR101683328B1/ko not_active Expired - Fee Related
- 2010-07-08 US US12/832,391 patent/US8337876B2/en not_active Expired - Fee Related
- 2010-07-08 EP EP10797833.0A patent/EP2451494B1/en not_active Not-in-force
- 2010-07-08 BR BR112012000327A patent/BR112012000327B8/pt not_active IP Right Cessation
- 2010-07-08 CN CN201080030958.5A patent/CN102470195B/zh not_active Expired - Fee Related
- 2010-07-08 IN IN1107DEN2012 patent/IN2012DN01107A/en unknown
- 2010-07-08 KR KR1020127003354A patent/KR101721276B1/ko not_active Expired - Fee Related
- 2010-07-08 CA CA2767714A patent/CA2767714C/en not_active Expired - Fee Related
- 2010-07-08 JP JP2012519719A patent/JP5711736B2/ja not_active Expired - Fee Related
- 2010-07-08 BR BR112012000326-7A patent/BR112012000326B1/pt not_active IP Right Cessation
- 2010-07-08 WO PCT/US2010/041331 patent/WO2011005933A2/en not_active Ceased
-
2012
- 2012-01-03 IL IL217340A patent/IL217340A/en active IP Right Grant
- 2012-01-03 IL IL217339A patent/IL217339A/en active IP Right Grant
- 2012-04-17 US US13/448,965 patent/US8673016B2/en active Active
- 2012-11-16 US US13/678,644 patent/US8790682B2/en active Active
-
2014
- 2014-02-14 US US14/180,638 patent/US20160158026A9/en not_active Abandoned
-
2016
- 2016-08-03 US US15/227,007 patent/US9968463B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7241486B2 (en) * | 2001-04-26 | 2007-07-10 | Inion Ltd. | Bone grafting materials |
| US20070162151A1 (en) * | 2006-01-11 | 2007-07-12 | Jiang Chang | Resorbable macroporous bioactive glass scaffold and method of manufacture |
| KR20090057392A (ko) * | 2006-08-18 | 2009-06-05 | 지이오2 테크놀로지스 인코포레이티드 | 무기 결합 구조를 갖는 압출 성형 다공성 기판 |
| WO2008141313A1 (en) * | 2007-05-14 | 2008-11-20 | Geo2 Technologies, Inc. | Method and apparatus for an extruded ceramic biosoluble fiber substrate |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20240029953A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 폭 가변장치 |
| KR20240029947A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 가압유지장치 |
| KR20240029942A (ko) | 2022-08-29 | 2024-03-07 | (주)디에이치에스 | 파우치 타입의 이차전지용 가압장치 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9968463B2 (en) | Devices and methods for tissue engineering | |
| US20110206828A1 (en) | Devices and Methods for Tissue Engineering | |
| JP5801382B2 (ja) | 生体組織再生のための医療用デバイスおよび方法 | |
| US20140050765A1 (en) | Devices and Methods for Tissue Engineering | |
| KR20130056874A (ko) | 조직공학용 장치 및 방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U12-oth-PR1002 Fee payment year number: 1 |
|
| PG1601 | Publication of registration |
St.27 status event code: A-4-4-Q10-Q13-nap-PG1601 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: A-4-4-U10-U13-oth-PC1903 Not in force date: 20230324 Payment event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date: 20230324 |