KR20110091658A - 기체 액체 접촉기 및 유출물 정화 시스템 및 방법 - Google Patents
기체 액체 접촉기 및 유출물 정화 시스템 및 방법 Download PDFInfo
- Publication number
- KR20110091658A KR20110091658A KR1020117009444A KR20117009444A KR20110091658A KR 20110091658 A KR20110091658 A KR 20110091658A KR 1020117009444 A KR1020117009444 A KR 1020117009444A KR 20117009444 A KR20117009444 A KR 20117009444A KR 20110091658 A KR20110091658 A KR 20110091658A
- Authority
- KR
- South Korea
- Prior art keywords
- gas
- liquid
- gas liquid
- jet
- nozzle
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000007788 liquid Substances 0.000 title claims abstract description 913
- 238000000034 method Methods 0.000 title claims abstract description 177
- 238000000746 purification Methods 0.000 title abstract description 13
- 238000004891 communication Methods 0.000 claims abstract description 38
- 230000003993 interaction Effects 0.000 claims abstract description 16
- 239000007789 gas Substances 0.000 claims description 831
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 138
- MWUXSHHQAYIFBG-UHFFFAOYSA-N nitrogen oxide Inorganic materials O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 claims description 131
- 239000012530 fluid Substances 0.000 claims description 103
- 238000012546 transfer Methods 0.000 claims description 101
- 238000006243 chemical reaction Methods 0.000 claims description 97
- 230000008569 process Effects 0.000 claims description 96
- 229910021529 ammonia Inorganic materials 0.000 claims description 64
- 238000011282 treatment Methods 0.000 claims description 52
- 229910052760 oxygen Inorganic materials 0.000 claims description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 34
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 33
- 239000001301 oxygen Substances 0.000 claims description 33
- 229910001868 water Inorganic materials 0.000 claims description 33
- 239000000126 substance Substances 0.000 claims description 30
- 150000001412 amines Chemical class 0.000 claims description 26
- 229920000642 polymer Polymers 0.000 claims description 23
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 18
- 239000000463 material Substances 0.000 claims description 17
- 238000012545 processing Methods 0.000 claims description 16
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 14
- -1 alkaline earth metal salt Chemical class 0.000 claims description 14
- 239000002253 acid Substances 0.000 claims description 12
- 229910052751 metal Inorganic materials 0.000 claims description 12
- 239000002184 metal Substances 0.000 claims description 12
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 claims description 10
- 239000012267 brine Substances 0.000 claims description 10
- WQYVRQLZKVEZGA-UHFFFAOYSA-N hypochlorite Chemical compound Cl[O-] WQYVRQLZKVEZGA-UHFFFAOYSA-N 0.000 claims description 10
- 239000013535 sea water Substances 0.000 claims description 10
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 claims description 10
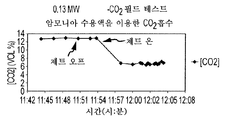
- 239000001569 carbon dioxide Substances 0.000 claims description 9
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 9
- 239000000460 chlorine Substances 0.000 claims description 9
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 claims description 8
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 8
- 150000003863 ammonium salts Chemical class 0.000 claims description 8
- 229910052801 chlorine Inorganic materials 0.000 claims description 6
- 239000002131 composite material Substances 0.000 claims description 6
- 239000012266 salt solution Substances 0.000 claims description 6
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 5
- 229910052815 sulfur oxide Inorganic materials 0.000 claims description 5
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 4
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 4
- 229910052782 aluminium Inorganic materials 0.000 claims description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 4
- 159000000007 calcium salts Chemical class 0.000 claims description 4
- 229910052804 chromium Inorganic materials 0.000 claims description 4
- 239000011651 chromium Substances 0.000 claims description 4
- 229910052802 copper Inorganic materials 0.000 claims description 4
- 239000010949 copper Substances 0.000 claims description 4
- 150000002739 metals Chemical class 0.000 claims description 4
- 229910052759 nickel Inorganic materials 0.000 claims description 4
- XTQHKBHJIVJGKJ-UHFFFAOYSA-N sulfur monoxide Chemical class S=O XTQHKBHJIVJGKJ-UHFFFAOYSA-N 0.000 claims description 4
- 239000004642 Polyimide Substances 0.000 claims description 3
- 229910000831 Steel Inorganic materials 0.000 claims description 3
- 150000002978 peroxides Chemical class 0.000 claims description 3
- 229920003023 plastic Polymers 0.000 claims description 3
- 239000004033 plastic Substances 0.000 claims description 3
- 229920001721 polyimide Polymers 0.000 claims description 3
- 239000010959 steel Substances 0.000 claims description 3
- 239000012855 volatile organic compound Substances 0.000 claims description 3
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 claims description 2
- 150000001447 alkali salts Chemical class 0.000 claims 2
- 159000000003 magnesium salts Chemical class 0.000 claims 2
- 229910002651 NO3 Inorganic materials 0.000 claims 1
- 229910052736 halogen Inorganic materials 0.000 claims 1
- 150000002367 halogens Chemical class 0.000 claims 1
- 238000003672 processing method Methods 0.000 claims 1
- 238000003491 array Methods 0.000 abstract description 18
- 239000002250 absorbent Substances 0.000 description 189
- 230000002745 absorbent Effects 0.000 description 189
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 168
- 239000003546 flue gas Substances 0.000 description 130
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 125
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 87
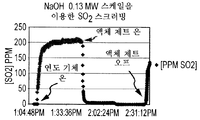
- 229910005965 SO 2 Inorganic materials 0.000 description 86
- 238000010521 absorption reaction Methods 0.000 description 70
- 239000000356 contaminant Substances 0.000 description 59
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 47
- 239000002904 solvent Substances 0.000 description 45
- 239000000654 additive Substances 0.000 description 44
- 239000012071 phase Substances 0.000 description 43
- 235000011114 ammonium hydroxide Nutrition 0.000 description 39
- 238000013461 design Methods 0.000 description 38
- 230000003647 oxidation Effects 0.000 description 38
- 238000007254 oxidation reaction Methods 0.000 description 38
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 36
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 35
- 239000006096 absorbing agent Substances 0.000 description 34
- 239000011575 calcium Substances 0.000 description 29
- 239000000243 solution Substances 0.000 description 29
- 238000012360 testing method Methods 0.000 description 28
- 239000003344 environmental pollutant Substances 0.000 description 27
- 231100000719 pollutant Toxicity 0.000 description 27
- 239000001099 ammonium carbonate Substances 0.000 description 26
- 239000007791 liquid phase Substances 0.000 description 26
- 239000000203 mixture Substances 0.000 description 26
- 229910004013 NO 2 Inorganic materials 0.000 description 25
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical group OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 24
- 239000000376 reactant Substances 0.000 description 24
- 239000011734 sodium Substances 0.000 description 24
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 22
- 230000008901 benefit Effects 0.000 description 22
- 239000000047 product Substances 0.000 description 21
- 239000003153 chemical reaction reagent Substances 0.000 description 20
- 238000010586 diagram Methods 0.000 description 20
- 238000005201 scrubbing Methods 0.000 description 20
- 239000007787 solid Substances 0.000 description 20
- 239000003054 catalyst Substances 0.000 description 19
- 239000010881 fly ash Substances 0.000 description 19
- 239000012528 membrane Substances 0.000 description 19
- 239000002585 base Substances 0.000 description 18
- 230000001965 increasing effect Effects 0.000 description 17
- 230000002829 reductive effect Effects 0.000 description 17
- 150000003839 salts Chemical class 0.000 description 17
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 16
- 235000012501 ammonium carbonate Nutrition 0.000 description 16
- 230000000996 additive effect Effects 0.000 description 15
- 238000002485 combustion reaction Methods 0.000 description 15
- 238000005086 pumping Methods 0.000 description 15
- 239000007921 spray Substances 0.000 description 15
- 229910002091 carbon monoxide Inorganic materials 0.000 description 14
- 238000013459 approach Methods 0.000 description 13
- 239000006227 byproduct Substances 0.000 description 13
- 238000003795 desorption Methods 0.000 description 13
- 238000004519 manufacturing process Methods 0.000 description 13
- 230000001590 oxidative effect Effects 0.000 description 13
- 230000003071 parasitic effect Effects 0.000 description 13
- 239000003381 stabilizer Substances 0.000 description 13
- 239000000725 suspension Substances 0.000 description 13
- 238000001556 precipitation Methods 0.000 description 12
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 11
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 11
- 235000019738 Limestone Nutrition 0.000 description 11
- 239000003245 coal Substances 0.000 description 11
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 11
- 239000006028 limestone Substances 0.000 description 11
- 238000001179 sorption measurement Methods 0.000 description 11
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 10
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 10
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 10
- 239000007800 oxidant agent Substances 0.000 description 10
- 239000002245 particle Substances 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 9
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 9
- 241000196324 Embryophyta Species 0.000 description 9
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 9
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 9
- 235000011941 Tilia x europaea Nutrition 0.000 description 9
- 239000007864 aqueous solution Substances 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 9
- 229910052791 calcium Inorganic materials 0.000 description 9
- 239000003337 fertilizer Substances 0.000 description 9
- 239000000446 fuel Substances 0.000 description 9
- 238000002347 injection Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- 239000004571 lime Substances 0.000 description 9
- 229910052753 mercury Inorganic materials 0.000 description 9
- 239000012621 metal-organic framework Substances 0.000 description 9
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 9
- 239000002244 precipitate Substances 0.000 description 9
- 230000009257 reactivity Effects 0.000 description 9
- 229910052717 sulfur Inorganic materials 0.000 description 9
- VTLYFUHAOXGGBS-UHFFFAOYSA-N Fe3+ Chemical group [Fe+3] VTLYFUHAOXGGBS-UHFFFAOYSA-N 0.000 description 8
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 8
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 8
- 238000002835 absorbance Methods 0.000 description 8
- 239000003513 alkali Substances 0.000 description 8
- 239000000440 bentonite Substances 0.000 description 8
- 229910000278 bentonite Inorganic materials 0.000 description 8
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical group O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 8
- 238000000354 decomposition reaction Methods 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000010440 gypsum Substances 0.000 description 8
- 229910052602 gypsum Inorganic materials 0.000 description 8
- 229910001385 heavy metal Inorganic materials 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 238000011068 loading method Methods 0.000 description 8
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 8
- 239000002243 precursor Substances 0.000 description 8
- 235000017550 sodium carbonate Nutrition 0.000 description 8
- 229910000029 sodium carbonate Inorganic materials 0.000 description 8
- 239000011593 sulfur Substances 0.000 description 8
- FEWFXBUNENSNBQ-UHFFFAOYSA-N 2-hydroxyacrylic acid Chemical compound OC(=C)C(O)=O FEWFXBUNENSNBQ-UHFFFAOYSA-N 0.000 description 7
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 7
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 7
- 239000000908 ammonium hydroxide Substances 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 7
- 238000005516 engineering process Methods 0.000 description 7
- 239000003673 groundwater Substances 0.000 description 7
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 7
- 239000011777 magnesium Substances 0.000 description 7
- 230000007246 mechanism Effects 0.000 description 7
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 7
- 239000003595 mist Substances 0.000 description 7
- 238000012856 packing Methods 0.000 description 7
- 239000002002 slurry Substances 0.000 description 7
- 229910052708 sodium Inorganic materials 0.000 description 7
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 7
- 239000002594 sorbent Substances 0.000 description 7
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 6
- 239000004202 carbamide Substances 0.000 description 6
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 6
- 238000009833 condensation Methods 0.000 description 6
- 230000005494 condensation Effects 0.000 description 6
- 230000003750 conditioning effect Effects 0.000 description 6
- 238000001816 cooling Methods 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- 239000002608 ionic liquid Substances 0.000 description 6
- CRVGTESFCCXCTH-UHFFFAOYSA-N methyl diethanolamine Chemical compound OCCN(C)CCO CRVGTESFCCXCTH-UHFFFAOYSA-N 0.000 description 6
- 238000002156 mixing Methods 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 230000036961 partial effect Effects 0.000 description 6
- 230000008929 regeneration Effects 0.000 description 6
- 238000011069 regeneration method Methods 0.000 description 6
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 6
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 5
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 5
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 5
- 229910000019 calcium carbonate Inorganic materials 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 230000005484 gravity Effects 0.000 description 5
- 239000011630 iodine Substances 0.000 description 5
- 229910052740 iodine Inorganic materials 0.000 description 5
- 238000003754 machining Methods 0.000 description 5
- 229910052749 magnesium Inorganic materials 0.000 description 5
- 239000000395 magnesium oxide Substances 0.000 description 5
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 5
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 238000005457 optimization Methods 0.000 description 5
- 238000004064 recycling Methods 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000006722 reduction reaction Methods 0.000 description 5
- 238000007789 sealing Methods 0.000 description 5
- 230000009919 sequestration Effects 0.000 description 5
- PAWQVTBBRAZDMG-UHFFFAOYSA-N 2-(3-bromo-2-fluorophenyl)acetic acid Chemical compound OC(=O)CC1=CC=CC(Br)=C1F PAWQVTBBRAZDMG-UHFFFAOYSA-N 0.000 description 4
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 4
- 239000004372 Polyvinyl alcohol Substances 0.000 description 4
- LOUPRKONTZGTKE-WZBLMQSHSA-N Quinine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-WZBLMQSHSA-N 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 4
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 4
- 235000011130 ammonium sulphate Nutrition 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 238000005520 cutting process Methods 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 230000004907 flux Effects 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 239000003345 natural gas Substances 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 238000010979 pH adjustment Methods 0.000 description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 description 4
- 229910000027 potassium carbonate Inorganic materials 0.000 description 4
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 4
- 238000004148 unit process Methods 0.000 description 4
- QGJOPFRUJISHPQ-UHFFFAOYSA-N Carbon disulfide Chemical compound S=C=S QGJOPFRUJISHPQ-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 229910019093 NaOCl Inorganic materials 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 239000005708 Sodium hypochlorite Substances 0.000 description 3
- 238000000862 absorption spectrum Methods 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000012298 atmosphere Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 3
- 239000000920 calcium hydroxide Substances 0.000 description 3
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 3
- 235000011116 calcium hydroxide Nutrition 0.000 description 3
- GBAOBIBJACZTNA-UHFFFAOYSA-L calcium sulfite Chemical compound [Ca+2].[O-]S([O-])=O GBAOBIBJACZTNA-UHFFFAOYSA-L 0.000 description 3
- 235000010261 calcium sulphite Nutrition 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 238000010531 catalytic reduction reaction Methods 0.000 description 3
- 230000007797 corrosion Effects 0.000 description 3
- 238000005260 corrosion Methods 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 238000009760 electrical discharge machining Methods 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 230000005284 excitation Effects 0.000 description 3
- 230000004151 fermentation Effects 0.000 description 3
- 239000000835 fiber Substances 0.000 description 3
- 150000004820 halides Chemical class 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 239000013049 sediment Substances 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 235000010265 sodium sulphite Nutrition 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- WJCNZQLZVWNLKY-UHFFFAOYSA-N thiabendazole Chemical compound S1C=NC(C=2NC3=CC=CC=C3N=2)=C1 WJCNZQLZVWNLKY-UHFFFAOYSA-N 0.000 description 3
- 238000003466 welding Methods 0.000 description 3
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 235000001258 Cinchona calisaya Nutrition 0.000 description 2
- 239000004593 Epoxy Substances 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 2
- PRXLCSIMRQFQMX-UHFFFAOYSA-N [O].[I] Chemical compound [O].[I] PRXLCSIMRQFQMX-UHFFFAOYSA-N 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 238000010564 aerobic fermentation Methods 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- JJWKPURADFRFRB-UHFFFAOYSA-N carbonyl sulfide Chemical compound O=C=S JJWKPURADFRFRB-UHFFFAOYSA-N 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 238000001311 chemical methods and process Methods 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 150000001805 chlorine compounds Chemical class 0.000 description 2
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 description 2
- 238000004581 coalescence Methods 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000005202 decontamination Methods 0.000 description 2
- 230000003588 decontaminative effect Effects 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 239000012717 electrostatic precipitator Substances 0.000 description 2
- 238000005265 energy consumption Methods 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 238000000855 fermentation Methods 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000005431 greenhouse gas Substances 0.000 description 2
- 159000000011 group IA salts Chemical class 0.000 description 2
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 2
- 239000003295 industrial effluent Substances 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 238000009434 installation Methods 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 2
- 239000000347 magnesium hydroxide Substances 0.000 description 2
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 2
- 235000012254 magnesium hydroxide Nutrition 0.000 description 2
- 229910017604 nitric acid Inorganic materials 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 239000013618 particulate matter Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000010248 power generation Methods 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 229960000948 quinine Drugs 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000013341 scale-up Methods 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 238000004513 sizing Methods 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 2
- 239000012265 solid product Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-L sulfite Chemical class [O-]S([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-L 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 2
- 239000002918 waste heat Substances 0.000 description 2
- 238000004065 wastewater treatment Methods 0.000 description 2
- PCXJASANFWPUMO-UHFFFAOYSA-N 1-(methylamino)propan-1-ol Chemical compound CCC(O)NC PCXJASANFWPUMO-UHFFFAOYSA-N 0.000 description 1
- MGWGWNFMUOTEHG-UHFFFAOYSA-N 4-(3,5-dimethylphenyl)-1,3-thiazol-2-amine Chemical compound CC1=CC(C)=CC(C=2N=C(N)SC=2)=C1 MGWGWNFMUOTEHG-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 102000003846 Carbonic anhydrases Human genes 0.000 description 1
- 108090000209 Carbonic anhydrases Proteins 0.000 description 1
- 102000016938 Catalase Human genes 0.000 description 1
- 108010053835 Catalase Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- PQUCIEFHOVEZAU-UHFFFAOYSA-N Diammonium sulfite Chemical compound [NH4+].[NH4+].[O-]S([O-])=O PQUCIEFHOVEZAU-UHFFFAOYSA-N 0.000 description 1
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 238000003916 acid precipitation Methods 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 238000003915 air pollution Methods 0.000 description 1
- 229910001854 alkali hydroxide Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 238000000889 atomisation Methods 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000003637 basic solution Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000003660 carbonate based solvent Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- HFNQLYDPNAZRCH-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O.OC(O)=O HFNQLYDPNAZRCH-UHFFFAOYSA-N 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 150000001804 chlorine Chemical class 0.000 description 1
- RCTYPNKXASFOBE-UHFFFAOYSA-M chloromercury Chemical compound [Hg]Cl RCTYPNKXASFOBE-UHFFFAOYSA-M 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 230000003749 cleanliness Effects 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 239000000571 coke Substances 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000000110 cooling liquid Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 238000006477 desulfuration reaction Methods 0.000 description 1
- 230000023556 desulfurization Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 150000002169 ethanolamines Chemical class 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000010304 firing Methods 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 150000002222 fluorine compounds Chemical class 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000003502 gasoline Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 239000000383 hazardous chemical Substances 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000002343 natural gas well Substances 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N nitrogen dioxide Inorganic materials O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000010908 plant waste Substances 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 239000012286 potassium permanganate Substances 0.000 description 1
- 239000012716 precipitator Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000003303 reheating Methods 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000001081 sequentional extraction Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- UKLNMMHNWFDKNT-UHFFFAOYSA-M sodium chlorite Chemical compound [Na+].[O-]Cl=O UKLNMMHNWFDKNT-UHFFFAOYSA-M 0.000 description 1
- 229960002218 sodium chlorite Drugs 0.000 description 1
- 229940079827 sodium hydrogen sulfite Drugs 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000000935 solvent evaporation Methods 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000003476 subbituminous coal Substances 0.000 description 1
- 150000003464 sulfur compounds Chemical class 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000026676 system process Effects 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000006276 transfer reaction Methods 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000002912 waste gas Substances 0.000 description 1
- 239000002351 wastewater Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 238000005200 wet scrubbing Methods 0.000 description 1
- 238000009763 wire-cut EDM Methods 0.000 description 1
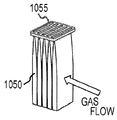
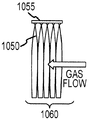
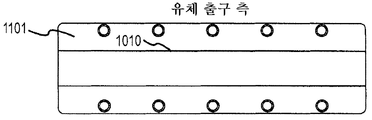
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01S—DEVICES USING THE PROCESS OF LIGHT AMPLIFICATION BY STIMULATED EMISSION OF RADIATION [LASER] TO AMPLIFY OR GENERATE LIGHT; DEVICES USING STIMULATED EMISSION OF ELECTROMAGNETIC RADIATION IN WAVE RANGES OTHER THAN OPTICAL
- H01S3/00—Lasers, i.e. devices using stimulated emission of electromagnetic radiation in the infrared, visible or ultraviolet wave range
- H01S3/09—Processes or apparatus for excitation, e.g. pumping
- H01S3/095—Processes or apparatus for excitation, e.g. pumping using chemical or thermal pumping
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
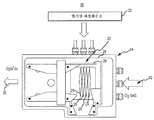
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/14—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by absorption
- B01D53/18—Absorbing units; Liquid distributors therefor
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01S—DEVICES USING THE PROCESS OF LIGHT AMPLIFICATION BY STIMULATED EMISSION OF RADIATION [LASER] TO AMPLIFY OR GENERATE LIGHT; DEVICES USING STIMULATED EMISSION OF ELECTROMAGNETIC RADIATION IN WAVE RANGES OTHER THAN OPTICAL
- H01S3/00—Lasers, i.e. devices using stimulated emission of electromagnetic radiation in the infrared, visible or ultraviolet wave range
- H01S3/02—Constructional details
- H01S3/03—Constructional details of gas laser discharge tubes
- H01S3/036—Means for obtaining or maintaining the desired gas pressure within the tube, e.g. by gettering, replenishing; Means for circulating the gas, e.g. for equalising the pressure within the tube
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/10—Oxidants
- B01D2251/106—Peroxides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/20—Reductants
- B01D2251/206—Ammonium compounds
- B01D2251/2065—Ammonium hydroxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/30—Alkali metal compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/40—Alkaline earth metal or magnesium compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/80—Organic bases or salts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/30—Sulfur compounds
- B01D2257/302—Sulfur oxides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/30—Sulfur compounds
- B01D2257/304—Hydrogen sulfide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/40—Nitrogen compounds
- B01D2257/404—Nitrogen oxides other than dinitrogen oxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/40—Nitrogen compounds
- B01D2257/406—Ammonia
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/60—Heavy metals or heavy metal compounds
- B01D2257/602—Mercury or mercury compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2259/00—Type of treatment
- B01D2259/12—Methods and means for introducing reactants
- B01D2259/124—Liquid reactants
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01S—DEVICES USING THE PROCESS OF LIGHT AMPLIFICATION BY STIMULATED EMISSION OF RADIATION [LASER] TO AMPLIFY OR GENERATE LIGHT; DEVICES USING STIMULATED EMISSION OF ELECTROMAGNETIC RADIATION IN WAVE RANGES OTHER THAN OPTICAL
- H01S3/00—Lasers, i.e. devices using stimulated emission of electromagnetic radiation in the infrared, visible or ultraviolet wave range
- H01S3/14—Lasers, i.e. devices using stimulated emission of electromagnetic radiation in the infrared, visible or ultraviolet wave range characterised by the material used as the active medium
- H01S3/22—Gases
- H01S3/2215—Iodine compounds or atomic iodine
Landscapes
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Engineering & Computer Science (AREA)
- Optics & Photonics (AREA)
- Plasma & Fusion (AREA)
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Gas Separation By Absorption (AREA)
- Treating Waste Gases (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10060608P | 2008-09-26 | 2008-09-26 | |
| US10056408P | 2008-09-26 | 2008-09-26 | |
| US10059108P | 2008-09-26 | 2008-09-26 | |
| US61/100,591 | 2008-09-26 | ||
| US61/100,564 | 2008-09-26 | ||
| US61/100,606 | 2008-09-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110091658A true KR20110091658A (ko) | 2011-08-12 |
Family
ID=42060040
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117009444A Ceased KR20110091658A (ko) | 2008-09-26 | 2009-07-06 | 기체 액체 접촉기 및 유출물 정화 시스템 및 방법 |
Country Status (9)
| Country | Link |
|---|---|
| EP (1) | EP2329567A4 (enExample) |
| JP (1) | JP5777215B2 (enExample) |
| KR (1) | KR20110091658A (enExample) |
| CN (1) | CN102217152B (enExample) |
| AU (1) | AU2009297005B2 (enExample) |
| CA (1) | CA2737637A1 (enExample) |
| MX (1) | MX2011003098A (enExample) |
| NZ (1) | NZ592100A (enExample) |
| WO (1) | WO2010036436A1 (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102012214065A1 (de) * | 2012-08-08 | 2014-02-13 | EnBW Energie Baden-Württemberg AG | Verfahren zum Entfernen von Quecksilber aus Rauchgas in einem Nasswäscher |
| DE102014105030A1 (de) * | 2014-04-09 | 2015-10-15 | Heinz Tischmacher | Vorrichtung sowie Verfahren zum Herstellen von Düngemitteln aus Abgasen einer Produktionsanlage |
| CN103920384B (zh) * | 2014-04-25 | 2016-02-17 | 中国人民解放军63605部队 | 一种常压法吸收高浓度氮氧化物的设备 |
| US9216934B1 (en) * | 2014-09-29 | 2015-12-22 | Cameron Solutions, Inc. | System and method for pH control of lean MEG product from MEG regeneration and reclamation packages |
| CN104359723B (zh) * | 2014-11-21 | 2018-01-23 | 中国环境科学研究院 | 用于采集大气中过氧化物的雾液吸收装置 |
| JP6740036B2 (ja) | 2016-06-30 | 2020-08-12 | 株式会社東芝 | 二酸化炭素回収システムおよび排ガス処理方法 |
| CN107551763A (zh) * | 2016-09-18 | 2018-01-09 | 叶涛 | 一种有机物废气的环保处理方法及其设备 |
| CN106902633B (zh) * | 2017-03-13 | 2020-06-16 | 华北电力大学(保定) | 脱除烟气中元素汞的仿生酶吸收剂及其制备方法和应用 |
| WO2019010344A2 (en) | 2017-07-06 | 2019-01-10 | Qatar Foundation For Education, Science And Community Development | ACID GAS REMOVAL SYSTEM FOR REMOVING ACIDIC GAS FROM GASEOUS HYDROCARBONS |
| CN108585534B (zh) * | 2018-07-25 | 2021-07-13 | 湖北鸿创科技有限公司 | 一种玻璃蚀刻用鼓泡装置 |
| CN111115575A (zh) * | 2020-01-17 | 2020-05-08 | 中国华能集团清洁能源技术研究院有限公司 | 一种基于燃烧前co2捕集系统的等温变换系统 |
| CN112591769A (zh) * | 2020-12-16 | 2021-04-02 | 湖南福千府生物科技有限公司 | 一种配置亚硫酸钠溶液装置 |
| CN112933866B (zh) * | 2021-03-22 | 2022-07-22 | 哈尔滨工程大学 | 一种可用于有害气体净化处理的气液两相引射器 |
| CA3222376A1 (en) * | 2021-04-13 | 2022-10-20 | Enviro Ambient Corporation | System and methods for carbon dioxide capture and recovery |
| US20250041789A1 (en) * | 2021-12-03 | 2025-02-06 | Michigan Technological University | SIMULTANEOUS REMOVAL OF CARBON DIOXIDE, NOx and SOx USING SINGLE STAGE ABSORPTION COLUMN AND RELATED METHODS |
| CN114307693B (zh) * | 2022-01-04 | 2022-11-11 | 大连理工大学 | 一种MOFs与聚合物双连续的混合基质膜的制备方法 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1256808A (en) | 1917-06-05 | 1918-02-19 | Lanston Monotype Machine Co | Mold for casting printers' leads and the like. |
| US3948608A (en) * | 1972-03-24 | 1976-04-06 | Weir Jr Alexander | Apparatus for treating stack gases |
| US3985860A (en) * | 1975-09-22 | 1976-10-12 | Pullman Incorporated | Method for oxidation of SO2 scrubber sludge |
| US4246245A (en) * | 1979-01-02 | 1981-01-20 | Bechtel International Corporation | SO2 Removal |
| US4968328A (en) * | 1986-05-16 | 1990-11-06 | Duke Eddie D | De-mister baffle and assembly |
| US4948402A (en) * | 1988-12-09 | 1990-08-14 | Davis Water & Waste Industries, Inc. | Modular air scrubber system |
| JPH05293332A (ja) * | 1992-04-21 | 1993-11-09 | Showa Shell Sekiyu Kk | 揮発性有機化合物含有ガスの除去方法 |
| US5395482A (en) * | 1992-11-13 | 1995-03-07 | Fuji Photo Film Co., Ltd. | Ultra high purity vapor phase treatment |
| JPH11114354A (ja) * | 1997-10-14 | 1999-04-27 | Japan Energy Corp | 二酸化炭素吸収装置の防食方法 |
| JPH11116223A (ja) * | 1997-10-17 | 1999-04-27 | Mayekawa Mfg Co Ltd | 自家発電設備を備えた炭酸飲料工場におけるco2 の製造方法とその製造装置 |
| JPH11197499A (ja) * | 1998-01-17 | 1999-07-27 | Kawasaki Heavy Ind Ltd | 気液反応装置 |
| US6875247B2 (en) * | 2000-06-06 | 2005-04-05 | Battelle Memorial Institute | Conditions for fluid separations in microchannels, capillary-driven fluid separations, and laminated devices capable of separating fluids |
| JP2002136828A (ja) * | 2000-11-07 | 2002-05-14 | Fuji Photo Film Co Ltd | 気液接触装置及び気液接触方法 |
| US20040131531A1 (en) * | 2001-04-20 | 2004-07-08 | Geerlings Jacobus Johannes Cornelis | Process for mineral carbonation with carbon dioxide |
| WO2003080228A1 (en) * | 2002-03-19 | 2003-10-02 | Mykrolis Corporation | Hollow fiber membrane contact apparatus and process |
| JP2005525924A (ja) * | 2002-05-16 | 2005-09-02 | スチュアート ショーティス、グレアム | 粒子分離 |
| CA2528789C (en) * | 2003-07-09 | 2009-12-22 | Belco Technologies Corporation | Wet scrubbing apparatus and method for controlling nox emissions |
| US7379487B2 (en) * | 2005-02-14 | 2008-05-27 | Neumann Information Systems, Inc. | Two phase reactor |
| US7318855B2 (en) * | 2005-03-30 | 2008-01-15 | The Boeing Company | Gas/liquid separation utilizing structured separator material |
| US20070085227A1 (en) * | 2005-10-13 | 2007-04-19 | Tonkovich Anna L | Multi-phase contacting process using microchannel technology |
-
2009
- 2009-07-06 AU AU2009297005A patent/AU2009297005B2/en not_active Ceased
- 2009-07-06 JP JP2011529041A patent/JP5777215B2/ja not_active Expired - Fee Related
- 2009-07-06 MX MX2011003098A patent/MX2011003098A/es active IP Right Grant
- 2009-07-06 WO PCT/US2009/049707 patent/WO2010036436A1/en not_active Ceased
- 2009-07-06 CN CN2009801459322A patent/CN102217152B/zh not_active Expired - Fee Related
- 2009-07-06 CA CA2737637A patent/CA2737637A1/en not_active Abandoned
- 2009-07-06 KR KR1020117009444A patent/KR20110091658A/ko not_active Ceased
- 2009-07-06 NZ NZ592100A patent/NZ592100A/xx not_active IP Right Cessation
- 2009-07-06 EP EP20090816647 patent/EP2329567A4/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| MX2011003098A (es) | 2011-08-03 |
| JP5777215B2 (ja) | 2015-09-09 |
| CN102217152B (zh) | 2013-06-12 |
| WO2010036436A1 (en) | 2010-04-01 |
| JP2012503541A (ja) | 2012-02-09 |
| CN102217152A (zh) | 2011-10-12 |
| NZ592100A (en) | 2013-11-29 |
| AU2009297005B2 (en) | 2014-05-29 |
| EP2329567A1 (en) | 2011-06-08 |
| AU2009297005A1 (en) | 2010-04-01 |
| CA2737637A1 (en) | 2010-04-01 |
| EP2329567A4 (en) | 2012-03-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8105419B2 (en) | Gas liquid contactor and effluent cleaning system and method | |
| AU2009297005B2 (en) | Gas liquid contactor and effluent cleaning system and method | |
| US8168148B2 (en) | Flue-gas purification and reclamation system and method thereof | |
| US9308494B2 (en) | Flue-gas purification and reclamation system and method thereof | |
| US7052662B2 (en) | NOx, Hg, and SO2 removal using alkali hydroxide | |
| US7964170B2 (en) | Method and apparatus for the removal of carbon dioxide from a gas stream | |
| US8529852B2 (en) | Flue-gas purification and reclamation system and method thereof | |
| US20170029343A1 (en) | Sulfur enhanced nitrogen production from emission scrubbing | |
| US20200368711A1 (en) | Removal of atmospheric pollutants from gas, related apparatuses, processes and uses thereof | |
| CN111514716A (zh) | 烟气脱硫、脱氮、脱汞净化方法和设备 | |
| CN105344214A (zh) | 一种液态催化剂烟气净化一体化系统及工艺 | |
| CN212881806U (zh) | 烟气脱硫、脱氮、脱汞净化设备 | |
| US20250135393A1 (en) | Enhancement of carbon dioxide absorption capture with a frothing agent and related systems and methods | |
| RU2575714C2 (ru) | Система очистки и утилизации дымового газа и способ | |
| HK1078303B (en) | Nox, hg, and so2 removal using ammonia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110425 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20140701 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20151016 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20160226 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20151016 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |