KR100800984B1 - Dyeing or printing method of manufactured natural polymer and hydrophobic synthetic fiber material - Google Patents
Dyeing or printing method of manufactured natural polymer and hydrophobic synthetic fiber material Download PDFInfo
- Publication number
- KR100800984B1 KR100800984B1 KR1020037008421A KR20037008421A KR100800984B1 KR 100800984 B1 KR100800984 B1 KR 100800984B1 KR 1020037008421 A KR1020037008421 A KR 1020037008421A KR 20037008421 A KR20037008421 A KR 20037008421A KR 100800984 B1 KR100800984 B1 KR 100800984B1
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- dye
- dyeing
- natural polymer
- dyes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- LEUGDXNWCPIUNY-UHFFFAOYSA-N Nc(cccc1C(c(c2ccc34)c3Nc3ccccc3C4=O)=O)c1C2=O Chemical compound Nc(cccc1C(c(c2ccc34)c3Nc3ccccc3C4=O)=O)c1C2=O LEUGDXNWCPIUNY-UHFFFAOYSA-N 0.000 description 1
- XWBLBLRNPLIRFE-UHFFFAOYSA-N O=C(c(cccc1)c1Nc1c2C(c3cccc(Sc4ccccc4)c33)=O)c1ccc2C3=O Chemical compound O=C(c(cccc1)c1Nc1c2C(c3cccc(Sc4ccccc4)c33)=O)c1ccc2C3=O XWBLBLRNPLIRFE-UHFFFAOYSA-N 0.000 description 1
- 0 O=C1c(cc(c(C(c2ccccc22)=O)c3C2=O)N*c2ccccc2)c3Nc2c1cccc2 Chemical compound O=C1c(cc(c(C(c2ccccc22)=O)c3C2=O)N*c2ccccc2)c3Nc2c1cccc2 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B5/00—Dyes with an anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings
- C09B5/24—Dyes with an anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings the heterocyclic rings being only condensed with an anthraquinone nucleus in 1-2 or 2-3 position
- C09B5/34—Anthraquinone acridones or thioxanthrones
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B5/00—Dyes with an anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings
- C09B5/24—Dyes with an anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings the heterocyclic rings being only condensed with an anthraquinone nucleus in 1-2 or 2-3 position
- C09B5/34—Anthraquinone acridones or thioxanthrones
- C09B5/347—Anthraquinone acridones
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3412—Heterocyclic compounds having nitrogen in the ring having one nitrogen atom in the ring
- C08K5/3432—Six-membered rings
- C08K5/3437—Six-membered rings condensed with carbocyclic rings
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/16—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using dispersed, e.g. acetate, dyestuffs
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/16—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using dispersed, e.g. acetate, dyestuffs
- D06P1/20—Anthraquinone dyes
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/34—Material containing ester groups
- D06P3/40—Cellulose acetate
- D06P3/42—Cellulose acetate using dispersed dyestuffs
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/34—Material containing ester groups
- D06P3/52—Polyesters
- D06P3/54—Polyesters using dispersed dyestuffs
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S8/00—Bleaching and dyeing; fluid treatment and chemical modification of textiles and fibers
- Y10S8/92—Synthetic fiber dyeing
- Y10S8/922—Polyester fiber
Landscapes
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Coloring (AREA)
- Other In-Based Heterocyclic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
Abstract
본 발명은 제조된 천연 중합체 또는 소수성 합성 섬유재료를 염색하거나 날염하는 방법으로서, 화학식 1의 염료를 사용함을 특징으로 하는 방법, 제조된 천연 중합체 또는 소수성 합성 섬유재료의 삼색 염색 및 플라스틱 재료의 대량 염색을 위한 이들 염료의 용도 및 이색염색(tone-in-tone dyed) 플라스틱 재료와 제조된 천연 중합체 또는 합성 섬유재료의 조합물을 제조하는 방법에 관한 것이다.The present invention is a method for dyeing or printing a manufactured natural polymer or hydrophobic synthetic fibrous material, characterized by using a dye of formula (1), tricolor dyeing of the produced natural polymer or hydrophobic synthetic fibrous material and mass dyeing of plastic material The use of these dyes and methods for producing combinations of tone-in-tone dyed plastic materials and natural polymers or synthetic fiber materials produced.
화학식 1Formula 1
위의 화학식 1에서,In Formula 1 above,
R1은 수소, 하이드록실 또는 라디칼 -NHCO-R6(여기서, R6은 C1-C6알킬 또는 치환되지 않거나 C1-C4알킬- 또는 할로겐 치환된 페닐이다)이고,R 1 is hydrogen, hydroxyl or radical —NHCO—R 6 , wherein R 6 is C 1 -C 6 alkyl or unsubstituted or C 1 -C 4 alkyl- or halogen substituted phenyl,
R2는 수소, 하이드록실 또는 라디칼 W-R7(여기서, W는 -NHCO- 또는 -S-이고 R7은 C1-C6알킬 또는 치환되지 않거나 C1-C4알킬- 또는 할로겐- 치환된 페닐이다)이고,R 2 is hydrogen, hydroxyl or radical WR 7 where W is -NHCO- or -S- and R 7 is C 1 -C 6 alkyl or unsubstituted or C 1 -C 4 alkyl- or halogen-substituted phenyl )
R3은 수소이고,R 3 is hydrogen,
R4는 수소 또는 하이드록실이고,R 4 is hydrogen or hydroxyl,
R5는 수소, 할로겐, 메톡시, 페녹시 또는 페닐티오이거나,R 5 is hydrogen, halogen, methoxy, phenoxy or phenylthio, or
R3 및 R4는 결합하여 라디칼 (여기서, 환 A 및 B는 독립적으로 추가의 치환체를 가질 수 있다)을 형성한다.R 3 and R 4 combine to form a radical Wherein Rings A and B may independently have additional substituents.
염색, 날염, 소수성 합성 섬유재료, 천연 중합체Dyeing, Printing, Hydrophobic Synthetic Fiber Materials, Natural Polymers
Description
본 발명은 제조된 천연 중합체 또는 소수성 합성 섬유재료를 염색하거나 날염하는 방법에 관한 것이다.The present invention relates to a method for dyeing or printing a produced natural polymer or hydrophobic synthetic fibrous material.
제조된 천연 중합체 또는 소수성 합성 섬유재료를 염색하거나 날염하는 데 유용한 염료, 특히 분산 염료, 즉 수용해 그룹이 없는 염료가 널리 공지되어 있다.Dyes useful for dyeing or printing natural polymers or hydrophobic synthetic fibrous materials produced, in particular disperse dyes, ie dyes without water-soluble groups, are well known.
그러나, 이들 염료로 수득된 염색물 또는 날염물이 특히, 열광견뢰도(hot light fastness)와 관련하여 최고의 요구조건을 항상 완전하게 충족시키는 것은 아님이 밝혀졌다. 따라서, 일반적인 견뢰도가 우수한 열광견뢰도를 갖는 염색물 또는 날염물을 제조하기 위한 신규 방법이 필요하다.However, it has been found that the dyeings or printings obtained with these dyes do not always fully meet the highest requirements, especially with regard to hot light fastness. Therefore, there is a need for a new method for producing dyeings or printings having thermoluminescent fastnesses with good general fastnesses.
본 발명에 이르러, 놀랍게도, 본 발명의 방법으로 수득한 염색물 또는 날염물이 하기에 나타낸 표준 조건을 실질적으로 충족시키는 것으로 밝혀졌다.It has now been surprisingly found that the dyeings or printings obtained by the process of the present invention substantially meet the standard conditions indicated below.
따라서, 본 발명은 제조된 천연 중합체 또는 소수성 합성 섬유재료를 염색하거나 날염시키는 방법으로서, 하기 화학식 1의 염료를 사용함을 특징으로 하는 방법을 제공한다.Accordingly, the present invention provides a method for dyeing or printing a manufactured natural polymer or hydrophobic synthetic fibrous material, using a dye of formula (1).
위의 화학식 4에서,In Formula 4 above,
R1은 수소, 하이드록실 또는 라디칼 -NHCO-R6(여기서, R6은 C1-C 6알킬 또는 치환되지 않거나 C1-C4알킬- 또는 할로겐 치환된 페닐이다)이고,R 1 is hydrogen, hydroxyl or radical —NHCO—R 6 , wherein R 6 is C 1 -C 6 alkyl or unsubstituted or C 1 -C 4 alkyl- or halogen substituted phenyl,
R2는 수소, 하이드록실 또는 라디칼 W-R7(여기서, W는 -NHCO- 또는 -S-이고 R7은 C1-C6알킬 또는 치환되지 않거나 C1-C4알킬- 또는 할로겐-치환된 페닐이다)이고,R 2 is hydrogen, hydroxyl or radical WR 7 where W is -NHCO- or -S- and R 7 is C 1 -C 6 alkyl or unsubstituted or C 1 -C 4 alkyl- or halogen-substituted phenyl )
R3은 수소이고,R 3 is hydrogen,
R4는 수소 또는 하이드록실이고,R 4 is hydrogen or hydroxyl,
R5는 수소, 할로겐, 메톡시, 페녹시 또는 페닐티오이거나,R 5 is hydrogen, halogen, methoxy, phenoxy or phenylthio, or
R3 및 R4는 결합하여 화학식 의 라디칼(여기서, 환 A 및 B는 독립적으로 추가의 치환체를 가질 수 있다)을 형성한다.R 3 and R 4 in combination To form radicals wherein rings A and B may independently have additional substituents.
환 A 및 B에 대해 유용한 임의의 치환체는 특히, 할로겐, C1-C4알킬 및 C1-C 4알콕시를 포함한다. 이들은 1회 이상 존재할 수 있다.Any substituent useful for rings A and B includes, in particular, halogen, C 1 -C 4 alkyl and C 1 -C 4 alkoxy. They may be present one or more times.
C1-C6 알킬 R6 및 R7은 예를 들어, 메틸, 에틸, 프로필, 이소프로필, n-부틸, 이소부틸, 2급-부틸, 3급-부틸, 아밀, 3급-아밀(1,1-디메틸프로필), 1,1,3,3-테트라메틸부틸, 네오펜틸, 헥실, 1-메틸펜틸, 사이클로펜틸, 사이클로헥실 및 이와 관련된 이성체들을 포함한다.C 1 -C 6 alkyl R 6 and R 7 are for example methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, secondary-butyl, tert-butyl, amyl, tert-amyl (1 , 1-dimethylpropyl), 1,1,3,3-tetramethylbutyl, neopentyl, hexyl, 1-methylpentyl, cyclopentyl, cyclohexyl and isomers associated therewith.
페닐상의 치환체 또는 환 A 및 B상의 치환체로서 C1-C4알킬은 메틸, 에틸, 프로필, 이소프로필, n-부틸, 이소부틸, 2급-부틸 및 3급-부틸이다.Substituents on phenyl or C 1 -C 4 alkyl as substituents on rings A and B are methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, secondary-butyl and tert-butyl.
환 A 및 B상의 치환체로서 C1-C4알콕시는 예를 들어, 메톡시, 에톡시, 프로폭시 및 부톡시이다.C 1 -C 4 alkoxy as substituents on rings A and B are, for example, methoxy, ethoxy, propoxy and butoxy.
할로겐은 브롬, 요오드, 특히 염소이다.Halogen is bromine, iodine, especially chlorine.
C1-C6티오알킬 W-R7은 예를 들어, 메틸티오, 에틸티오, 프로필티오 또는 부틸티오이다.C 1 -C 6 thioalkyl WR 7 is, for example, methylthio, ethylthio, propylthio or butylthio.
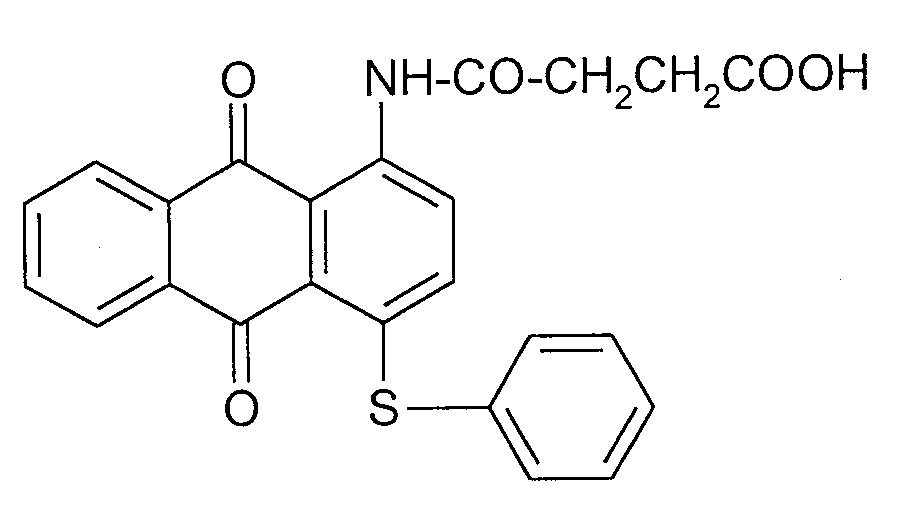
화학식 1의 염료중에서, 화학식 2 내지 6의 청색 염료가 바람직하다.Of the dyes of formula 1, the blue dyes of formulas 2 to 6 are preferred.
화학식 1의 염료중에서, 화학식 7 내지 10의 적색 염료도 바람직하다.Among the dyes of the formula (1), red dyes of the formulas (7) to (10) are also preferred.
화학식 2 및 7의 염료가 특히 바람직하다.Particular preference is given to dyes of formulas (2) and (7).
본 발명에 따라 사용되는 염료는 공지되어 있거나 공지된 방법에 의해 제조될 수 있다.The dyes used according to the invention are known or can be prepared by known methods.
화학식 7의 염료는 신규한 것이고 본 발명의 주요 요지의 일부를 구성한다. The dyes of formula 7 are novel and form part of the main subject of the invention.
화학식 7의 염료는 공지된 방법에 의해 공지된 화합물과 유사하게 제조될 수 있다.Dyes of formula (7) can be prepared analogously to known compounds by known methods.
화학식 7의 염료는 예를 들어, 올레움/클로로설폰산 중의 화학식 50의 화합물을 폐환시키고 수득한 화학식 51의 중간체를 화학식 (CH3)2-CH-COCl의 화합물과 반응시켜 수득한다.The dye of formula (7) is obtained by, for example, ring closing the compound of formula (50) in oleum / chlorosulfonic acid and reacting the intermediate of formula (51) with the compound of formula (CH 3 ) 2- CH-COCl.
화학식 50의 화합물은 공지되어 있고, 공지된 방법에 의해 제조될 수 있다.Compounds of formula 50 are known and can be prepared by known methods.
화학식 1의 염료는 제조된 천연 중합체 및 특히 소수성 합성 섬유재료, 특히, 직물을 염색시키고 날염시키는데 유용하다.The dyes of formula 1 are useful for dyeing and printing natural polymers produced and in particular hydrophobic synthetic fibrous materials, in particular textiles.
이러한 제조된 천연 중합체 또는 소수성 합성 섬유재료를 포함하는, 혼방 직물을 구성하는 직물은 본 발명의 염료로 염색 또는 날염도 가능하다.Fabrics constituting the blended fabric, including such manufactured natural polymer or hydrophobic synthetic fibrous material, can also be dyed or printed with the dye of the present invention.
유용한 제조된 천연 중합체 직물은, 특히 셀룰로스 아세테이트 및 셀룰로스 트리아세테이트이다.Useful manufactured natural polymer fabrics are, in particular, cellulose acetate and cellulose triacetate.
소수성 합성 직물은 특히, 선형 방향족 폴리에스테르, 예를 들어, 테레프탈산 및 글리콜(특히, 에틸렌 글리콜)로부터 형성된 폴리에스테르, 또는 테레프탈산과 1,4-비스(하이드록시메틸)-사이클로헥산과의 축합 산물; 폴리카보네이트, 예를 들어, α,α-디메틸-4,4'-디하이드록시디페닐메탄과 포스겐으로부터 형성된 폴리카보네이트; 또는 폴리비닐 클로라이드 또는 폴리아미드계 섬유이다.Hydrophobic synthetic fabrics are, in particular, polyesters formed from linear aromatic polyesters such as terephthalic acid and glycols (especially ethylene glycol), or condensation products of terephthalic acid with 1,4-bis (hydroxymethyl) -cyclohexane; Polycarbonates such as polycarbonates formed from α, α-dimethyl-4,4'-dihydroxydiphenylmethane and phosgene; Or polyvinyl chloride or polyamide based fibers.
본 발명에 따라 사용한 화학식 1의 염료는 공지된 염색 공정에 따라 직물에 적용된다. 예를 들어, 폴리에스테르 섬유는 80 내지 140℃, 특히, 120 내지 135℃의 온도에서 통상적인 캐리어의 존재 또는 부재하에 통상적인 음이온성 또는 비이온성 분산제의 존재하에 수성 분산액으로부터 흡착 염색된다. 셀룰로스 아세테이트는 바람직하게 60 내지 85℃에서 염색되고 셀룰로스 트리아세테이트는 115℃ 이하의 온도에서 염색된다.The dye of formula 1 used according to the invention is applied to the fabric according to known dyeing processes. For example, polyester fibers are adsorbed and dyed from aqueous dispersions in the presence of conventional anionic or nonionic dispersants in the presence or absence of conventional carriers at temperatures of 80-140 ° C, in particular 120-135 ° C. Cellulose acetate is preferably dyed at 60 to 85 ° C. and cellulose triacetate is dyed at temperatures up to 115 ° C.
본 발명에 따라 사용한 염료는 단지 최소로 인접 모직물 및 면직물을 염색시키는데, 즉, 매우 우수한 모직물 및 면직물 보유성을 나타내어 이들은 폴리에스테르 모직물 및 폴리에스테르-셀룰로스 혼방 직물을 우수하게 염색하기 위해 사용될 수 있다.The dyes used in accordance with the invention only dye minimally adjacent wool and cotton fabrics, ie exhibit very good wool and cotton retaining properties so that they can be used to excellently dye polyester wool and polyester-cellulose blend fabrics.
본 발명에 따라 사용되는 염료는 서머졸 염색법(thermosol), 흡착 염색법 및 연속 염색법에 의해 염색시키고 날염 공정을 위해 유용하다. 흡착 염색법이 바람직하다. 액비는 장치, 기질 및 구성 형태(make-up form)에 의존한다. 그러나, 액비는 광범위한 범위, 예를 들어, 4:1 내지 100:1의 범위내에서 선택될 수 있지만, 바람직하게는 6:1 내지 25:1이다.The dyes used according to the invention are useful for dyeing and printing processes by thermosol, adsorption and continuous dyeing. Adsorption dyeing is preferred. The liquid ratio depends on the device, the substrate and the make-up form. However, the liquid ratio may be selected within a wide range, for example, in the range of 4: 1 to 100: 1, but is preferably 6: 1 to 25: 1.
각각의 염료가 염욕(dyebath) 또는 날염 페이스트에서 사용될 수 있는 양은 광범위한 제한 범위내에서 변하고 이것은 목적하는 색조에 의존한다. 유리한 양은 일반적으로 섬유 중량 또는 날염 페이스트를 기준으로 하여, 0.01중량% 내지 15중량%, 특히 0.1중량% 내지 10중량%이다.The amount that each dye can be used in a dyebath or printing paste varies within wide limits and this depends on the desired color tone. Advantageous amounts are generally from 0.01% to 15% by weight, in particular from 0.1% to 10% by weight, based on fiber weight or printing paste.
언급된 직물은 다양한 가공 형태, 예를 들어, 섬유, 사 또는 웹으로서 또는 제직물 또는 루프 형성 편직물로서 존재할 수 있다.The fabrics mentioned can be present in various processing forms, for example as fibers, yarns or webs or as woven or loop forming knits.
본 발명에 따라 사용되는 염료를, 사용 전에 염료 제제로 전환시키는 것이 유리하다. 이를 위해, 염료를 분쇄하여 이의 입자 크기가 0.01 내지 10㎛가 되게하고, 바람직하게 입자 크기는 평균 0.1 내지 1㎛이다. 분산제의 존재하에 분쇄하는 것이 효과적일 수 있다. 예를 들어, 무수 염료는 분산제와 함께 분쇄되거나 분산제와 함께 페이스트 형태로서 혼련한 다음, 감압하에 또는 분무 건조에 의해 건조된다. 이와 같이 수득한 제제를 사용하여 물을 첨가함에 의해 날염 페이스트 및 염욕을 제조할 수 있다.It is advantageous to convert the dyes used according to the invention into dye preparations before use. To this end, the dye is pulverized so that its particle size is from 0.01 to 10 μm, preferably the particle size is on average 0.1 to 1 μm. Grinding in the presence of a dispersant may be effective. For example, the anhydrous dye is ground with the dispersant or kneaded with the dispersant in paste form and then dried under reduced pressure or by spray drying. Printing pastes and salt baths can be prepared by adding water using the formulation thus obtained.
날염은 통상적인 증점제, 예를 들어, 개질되거나 개질되지 않은 천연 생성물, 예를 들어, 알기네이트, 브리티쉬 검, 아라비아 고무, 결정 검, 구주콩나무 콩가루, 트라가칸트, 카복시메틸셀룰로스, 하이드록시에틸셀룰로스, 전분 또는 합성 생성물, 예를 들어, 폴리아크릴아미드, 폴리아크릴산 또는 이의 공중합체 또는 폴리비닐 알콜을 사용한다.Prints are conventional thickeners, for example modified or unmodified natural products, such as alginates, British gums, gum arabic, crystalline gums, citrus soybean flour, tragacanth, carboxymethylcellulose, hydroxyethylcellulose , Starch or synthetic products, for example polyacrylamide, polyacrylic acid or copolymers thereof or polyvinyl alcohol.
본 발명에 따라 사용되는 염료는 매우 우수한 사용자 견뢰도, 특히, 우수한 승화 견뢰도 및 매우 우수한 광견뢰도를 갖는 균일한 청색조 또는 적색조를 상기된 재료(특히, 폴리에스테르 재료)에 부여한다. 명백한 특징은 매우 우수한 세탁견뢰도이다. 본 발명에 따른 염료의 탁월한 특징은 추가로 흡착성 및 빌드-업(build-up)이 우수하다는 것이다.The dyes used according to the invention impart uniform blue or red shades to the aforementioned materials (particularly polyester materials) having very good user fastnesses, in particular good sublimation fastness and very good light fastness. The obvious feature is very good wash fastness. An excellent feature of the dye according to the invention is that it is further superior in adsorption and build-up.
본 발명에 따라 사용되는 염료는 또한 또 다른 염료와 함께 또는 삼색 염색(trichromatic dyeing)을 위해 적합한 황색 염료와 함께 조합 색조(combination shade)를 생성하기 위해 유용하다.The dyes used according to the invention are also useful for producing combination shades with another dye or with a yellow dye suitable for trichromatic dyeing.
본 발명은 추가로 상기한 방법에 의해 염색되거나 날염된, 제조된 천연 중합체 또는 소수성 합성 섬유재료, 바람직하게는 폴리에스테르 직물을 제공하는 것이다. The present invention further provides a prepared natural polymer or hydrophobic synthetic fibrous material, preferably a polyester fabric, which is dyed or printed by the above-described method.
본 발명은 추가로, 화학식 2 내지 6 중의 하나 이상의 청색 염색 염료를 화학식 7 내지 25 중의 하나 이상의 적색 염색 염료 및 화학식 26의 황색 염색 염료와 함께 사용함을 특징으로 하는, 제조된 천연 중합체, 특히 소수성 합성 섬유를 삼색 염색 또는 날염시키기 방법을 제공한다.The present invention further provides natural polymers prepared, in particular hydrophobic synthesis, characterized by the use of at least one blue dye of formulas 2-6 with at least one red dye of formulas 7-25 and a yellow dye of formula 26 Provided are methods for tricolor dyeing or printing fibers.
화학식 11 내지 26의 염료들은 공지되어 있거나 공지된 방법 대로 공지된 염료와 유사하게 제조될 수 있다.The dyes of formulas 11-26 can be prepared analogously to known dyes by known or known methods.
삼색은 적당히 선택된 황색 또는 오렌지, 적색 및 청색 염색 염료를 색 혼합물로서 부가하여 이에 의해 임의의 목적하는 가시광 스펙트럼(visible spectrum)의 색조가 염료 성분의 양을 적합한 비율로 선택함으로써 조화될 수 있다.The three colors can be matched by adding appropriately selected yellow or orange, red and blue dye dyes as color mixtures whereby the desired hue of the visible spectrum is selected in an appropriate proportion by amount of the dye component.
본 발명에 따른 삼색 과정의 바람직한 양태는, 화학식 2의 청색 염색 염료를 화학식 7의 적색 염색 염료 및 화학식 26의 황색 염색 염료와 함께 사용함을 특징으로 한다.A preferred embodiment of the tricolor process according to the invention is characterized by the use of a blue dye of formula (2) in combination with a red dye of formula (7) and a yellow dye of formula (26).
본 발명에 따른 삼색 과정의 추가의 바람직한 양태는, 화학식 2의 청색 염색 염료를 화학식 14의 적색 염색 염료와 화학식 26의 황색 염색 염료와 함께 사용함을 특징으로 한다.A further preferred embodiment of the tricolor process according to the invention is characterized in that the blue dye of formula (2) is used in combination with the red dye of formula (14) and the yellow dye of formula (26).
본 발명은 추가로, 화학식 2 내지 6 중의 하나 이상의 청색 염색 염료를 화학식 7 내지 25 중의 하나 이상의 적색 염색 염료 및 화학식 26의 황색 염색 염료와 함께 포함하는 삼색 염료 혼합물을 제공한다.The present invention further provides a tricolor dye mixture comprising at least one blue dye of formula 2 to 6 together with at least one red dye of formula 7 to 25 and a yellow dye of formula 26.
삼색 염색 또는 날염을 위한 본 발명의 방법은 각각 통상적인 염색 및 날염 과정에 적용될 수 있다. 물 및 염료 이외에, 염색 용액 또는 날염 페이스트는 추가로 첨가제, 예를 들어, 습윤제, 소포제, 균염제 또는 직물 성질에 영향을 주는 제제, 예를 들어, 연화제, 난연제 또는 방오제, 발수제 및 발유제(oil repellent)및 또한 물 연화제 및 천연 또는 합성 증점제, 예를 들어, 알기네이트 및 셀룰로스 에테르를 함유할 수 있다.The method of the present invention for tricolor dyeing or printing can be applied to conventional dyeing and printing processes, respectively. In addition to water and dyes, dyeing solutions or printing pastes may further contain additives, for example wetting agents, antifoams, leveling agents or agents which affect the fabric properties, for example softeners, flame retardants or antifouling agents, water repellents and oil repellents. and also water softeners and natural or synthetic thickeners such as alginates and cellulose ethers.
삼색 염색 또는 날염을 위한 본 발명의 방법은 또한 예를 들어, 연속 염색 공정 또는 뱃치 및 연속 발포 염색 공정에서 불충분한 용액으로부터 염색시키는데 유용하다.The process of the invention for tricolor dyeing or printing is also useful for dyeing from insufficient solutions, for example in a continuous dyeing process or in batch and continuous foaming dyeing processes.
염색, 특히 흡착 공정에 의한 염색이 바람직하다.Dyeing, in particular dyeing by an adsorption process, is preferred.
염색은 바람직하게 pH 3 내지 7, 특히 3 내지 5에서 수행한다. 액비는 광범위한 제한된 범위 내에서 다양할 수 있고, 예를 들어, 5:1 내지 50:1, 바람직하게 5:1 내지 30:1이다. 염색은 바람직하게 100 내지 140℃, 특히 120 내지 135℃에서 수행한다.Staining is preferably carried out at pH 3 to 7, especially 3 to 5. The liquor ratio can vary within a wide limited range and is for example 5: 1 to 50: 1, preferably 5: 1 to 30: 1. Dyeing is preferably carried out at 100 to 140 ° C, in particular at 120 to 135 ° C.
본 발명의 삼색 혼합물은 또한 대량 착색 플라스틱에 유용하다.The tricolor mixture of the present invention is also useful for bulk colored plastics.
본 발명의 삼색 공정에 사용되는 염료는 삼색 염색 또는 날염에서 다양한 농도에서의 우수한 색조 항구성, 우수한 견뢰도 및 분산도, 특히 매우 우수한 양립성 및 균일한 색상 빌드-업에 있어서 주목할만 하다.The dyes used in the tricolor process of the present invention are notable for their excellent color tone persistence, good fastness and dispersion at various concentrations in tricolor dyeing or printing, in particular very good compatibility and uniform color build-up.
본 발명의 염료 및 본 발명에 따라 사용되는 염료는, 추가로 또한 대량 염색된 플라스틱이, 욕조로부터 날염되거나 염색된 제조된 천연 중합체 또는 소수성 합성 섬유재료와 동일한 색조 및 조건등색(metamerism) 및 동일한 견뢰도를 갖는다는 점에서 플라스틱의 대량 착색을 위해 적합한 것으로 주목할만하며 이로써 염료는 개별적으로 사용될 수 있을 뿐만 아니라 삼색 혼합물 형태로 사용될 수도 있다.The dyes of the invention and the dyes used according to the invention furthermore have the same color tone and metamerism and the same fastness as the produced natural polymer or hydrophobic synthetic fibrous material in which the mass dyed plastic is printed or dyed from the bath. It is noteworthy that they are suitable for mass coloring of plastics in that they can be used individually as well as in the form of a tricolor mixture.
따라서, 본 발명은 추가로, 화학식 1의 동일한 염료를 사용하여 플라스틱 재료를 대량 착색시키고 제조된 천연 중합체 또는 합성 섬유재료를 날염하거나 염색함을 특징으로 하는, 이색염색된(tone-in-tone dyed) 플라스틱 재료와 제조된 천연 중합체 또는 합성 섬유재료, 특히 직물 재료의 조합물을 제조하는 방법을 제공한다.Thus, the present invention further provides tone-in-tone dyed, which is characterized by mass coloring plastic materials using the same dye of formula 1 and printing or dyeing the produced natural polymer or synthetic fibrous material. A method of producing a combination of a plastic material and a natural polymer or synthetic fibrous material, in particular a textile material, produced.
이러한 조합은 예를 들어, 안락 의자 또는 3개의 조립 가구와 같은 가구의 플라스틱 부분 및 씌우개(upholstery) 또는 자동차의 플라스틱 판넬 또는 조립품 및 시트 커버 형성 부분으로 이루어질 수 있다.This combination may consist of plastic parts and upholstery of furniture, for example armchairs or three assembled furniture, or plastic panels or assemblies and seat cover forming parts of an automobile.
본 발명의 목적을 위한 이색염색물은 CIELAB 배위체(△E ≤2.0)(독일 산업 표준 DIN 6174에 따름)를 갖는다.Dichroic dyes for the purposes of the present invention have a CIELAB configuration (ΔE ≦ 2.0) (according to the German industry standard DIN 6174).
흡착 염색에 의해 이색염색물을 수득하기 위해, 염료 양은 염료의 흡착 정도를 고려하여 결정되어야만 하고 이는 폴리에스테르 재료에 대해 통상적으로 80 내지 99.9%, 일반적으로 90 내지 99.9%이다.In order to obtain a dichroic dye by adsorption dyeing, the dye amount must be determined in consideration of the degree of adsorption of the dye, which is usually 80 to 99.9%, generally 90 to 99.9% with respect to the polyester material.
제조된 천연 중합체 또는 합성 섬유재료에 관하여, 당해 기재 및 바람직한 양태가 또한 유효하다.With regard to the natural polymer or synthetic fibrous material produced, the above description and preferred embodiments are also effective.
대량 염색에 유용한 플라스틱은 예를 들어, 염색 가능한 고분자량의 유기 재료(중합체)(유전 상수 2.5 이상), 특히, 폴리에스테르, 폴리카보네이트(PC), 폴리스티렌(PS), 폴리메틸 메타크릴레이트(PMMA), 폴리아미드, 폴리에틸렌, 폴리프로필렌, 스티렌/아크릴로니트릴(SAN) 또는 아크릴로니트릴/부타디엔/스티렌(ABS)를 포함한다. 폴리에스테르 및 폴리아미드가 바람직하다. 특히, 테레프탈산 및 글리콜(특히, 에틸렌 글리콜)의 다중축합에 의해 수득가능한 선형 방향족 폴리에스테르 또는 테레프탈산과 1,4-비스(하이드록시메틸)사이클로헥산과의 축합 생성물, 예를 들어, 폴리에틸렌 테레프탈레이트(PET) 또는 폴리부틸렌 테레프탈레이트(PBTP); 폴리카보네이트, 예를 들어, α,α-디메틸-4,4'-디하이드록시디페닐메탄 및 포스겐으로부터 형성된 폴리카보네이트; 또는 폴리비닐 클로라이드 또는 폴리아미드계 중합체, 예를 들어, 나일론 6 또는 나일론 6.6이 특히 바람직하다. 선형 방향족 폴리에스테르, 예를 들어, 테레프탈산과 글리콜(특히, 에틸렌 글리콜)로부터 형성된 폴리에스테르계 또는 테레프탈산과 1,4-비스(하이드록시메틸)-사이클로헥산과의 축합 생성물계 플라스틱이 매우 특히 바람직하다.Plastics useful for bulk dyeing are, for example, dyeable high molecular weight organic materials (polymers) (dielectric constants 2.5 or higher), in particular polyesters, polycarbonates (PCs), polystyrenes (PS), polymethyl methacrylates (PMMA). ), Polyamide, polyethylene, polypropylene, styrene / acrylonitrile (SAN) or acrylonitrile / butadiene / styrene (ABS). Polyester and polyamides are preferred. In particular, condensation products of linear aromatic polyesters or terephthalic acids with 1,4-bis (hydroxymethyl) cyclohexane obtainable by multicondensation of terephthalic acid and glycols (especially ethylene glycol), for example polyethylene terephthalate ( PET) or polybutylene terephthalate (PBTP); Polycarbonates such as polycarbonates formed from α, α-dimethyl-4,4'-dihydroxydiphenylmethane and phosgene; Or polyvinyl chloride or polyamide-based polymers, for example nylon 6 or nylon 6.6. Very particular preference is given to polyaromatic polyesters, for example polyesters formed from terephthalic acid and glycols (especially ethylene glycol) or condensation product plastics of terephthalic acid with 1,4-bis (hydroxymethyl) -cyclohexane .
플라스틱은 예를 들어, 삼색 혼합물의 염료 성분을 롤 밀 또는 혼합 또는 분쇄 장치를 사용하여 이들 기질에 혼합하여 염료가 플라스틱에 용해되거나 미세하게 분산되도록 하여 염색된다. 이후, 혼합 염료를 갖는 플라스틱은 통상적인 방식, 예를 들어, 캘린더링, 프레싱, 압출, 스프레드 피복, 스피닝, 캐스팅 또는 사출 성형에 의해 가공되어 염색재는 이의 궁극적인 형태를 획득한다. 염료는 또한 실질적인 가공 단계 전에 직접적으로 예를 들어, 연속적인 계량 고체 염료, 예를 들어, 분말 염료 및 과립화되거나 분말인 플라스틱 및 또한 임의의 추가의 물질(예: 첨가제)를 동시에 직접 가공 직전에 혼합 주입 영역인 압출기의 주입구로 첨가되어 혼합될 수 있다. 그러나, 일반적으로 염료를 사전에 플라스틱에 혼합시키는 것이 바람직한데 그 이유는 보다 균일한 염색 기질이 수득될 수 있기 때문이다.Plastics are dyed, for example, by mixing the dye components of the tricolor mixture into these substrates using a roll mill or mixing or grinding apparatus to allow the dye to dissolve or finely disperse in the plastic. The plastic with the mixed dye is then processed by conventional methods, such as calendering, pressing, extrusion, spread coating, spinning, casting or injection molding so that the dyeing material obtains its ultimate form. The dye is also directly before the actual processing step, for example immediately before the simultaneous direct processing of, for example, continuous metered solid dyes, for example powder dyes and granulated or powdered plastics and also any further substances (eg additives) simultaneously. It may be added to the inlet of the extruder which is the mixing injection zone and mixed. In general, however, it is preferable to mix the dyes in advance in the plastics, since a more uniform dyeing substrate can be obtained.
본 발명은 추가로 염색 또는 날염 소수성 섬유재료, 바람직하게, 폴리에스테르 직물 및 또한 상기한 공정에 의해 제공되는 대량 염색 플라스틱을 제공한다.The present invention further provides dyed or printed hydrophobic fibrous materials, preferably polyester fabrics and also bulk dyed plastics provided by the processes described above.
하기의 실시예가 본 발명을 설명한다. 달리 언급되지 않는 한, 부 및 %는 중량 기준이다. 온도는 섭씨 온도이다. 중량부는 그람이 세제곱 센터미터에 관련된 것 처럼 용량부와 관련되어 있다.The following examples illustrate the invention. Unless stated otherwise, parts and percentages are by weight. The temperature is in degrees Celsius. Parts by weight are related to parts of capacity as grams are in cubic centimeters.
실시예 1:Example 1:
반응 용기에 진한 황산 145.0중량부와 클로로설폰산 70.0중량부를 첨가한다. 화학식 50의 화합물 36.0중량부를 25℃에서 첨가하고 이어서 혼합물을 2시간 동안 25 내지 30℃에서 교반한다.145.0 parts by weight of concentrated sulfuric acid and 70.0 parts by weight of chlorosulfonic acid are added to the reaction vessel. 36.0 parts by weight of the compound of formula 50 is added at 25 ° C. and the mixture is then stirred at 25-30 ° C. for 2 hours.
화학식 50Formula 50
이어서 1500중량부의 빙수상에 놓고 침전된 생성물을 흡입 여과하고 중성 세척하고 건조시킨다.It is then placed on 1500 parts by weight of ice water and the precipitated product is suction filtered, neutral washed and dried.
이로써 화학식 51의 화합물 32.0 중량부를 수득한다.This gives 32.0 parts by weight of the compound of formula 51.
화학식 51Formula 51
화학식 53의 화합물 17.0중량부를 피리딘 20중량부 및 니트로벤젠 150중량부에 도입하고 혼합물을 110℃로 가열한다. 니트로벤젠 15중량부중에 이소부티릴 클로라이드 7.2중량부 용액을 약 30분에 걸쳐 110℃에서 적가한다. 이어서, 혼합물을 2시간 동안 교반한다. 이어서 반응 혼합물을 20 내지 40℃로 냉각시키고 직접 여과하거나 메탄올로 희석시킨 후 여과 잔사를 메탄올 및 물로 세척하고 건조시킨다. 이로써 내광 적색 색조로 폴리에스테르를 염색시키는 화학식 7의 염료 18.0중량부를 수득한다.17.0 parts by weight of the compound of formula 53 is introduced into 20 parts by weight of pyridine and 150 parts by weight of nitrobenzene, and the mixture is heated to 110 ° C. To 15 parts by weight of nitrobenzene, a 7.2 parts by weight solution of isobutyryl chloride is added dropwise at 110 ° C. over about 30 minutes. The mixture is then stirred for 2 hours. The reaction mixture is then cooled to 20-40 [deg.] C. and filtered directly or diluted with methanol and then the filter residue is washed with methanol and water and dried. This yields 18.0 parts by weight of a dye of formula (7) which dyes the polyester in a light red hue.
화학식 7Formula 7
실시예 2:Example 2:
폴리에스테르 직물 100g을, 실온에서 용액비를 20:1로 하여 화학식 7의 염료 0.07g, 화학식 2의 염료 0.058g, 화학식 26의 염료 0.57g, 황산암모늄 1g/ℓ 및 시판되는 균염제 0.5g/ℓ를 함유하고 80%의 포름산으로 pH가 4.5 내지 5로 조정된 용액에 침지시킨다. 이어서 용액을 분당 3℃의 속도로 60℃까지 가열하고, 이어서 분당 2℃의 속도로 130℃까지 가열한다. 용액을 140℃에서 60분 동안 유지한다. 이어서 용액을 40℃로 냉각시키고 염색된 폴리에스테르 직물을 물로 세척하고, 70 내지 80℃에서 30% 수산화나트륨 용액 5ml/l, 85% 나트륨 디티오니트 용액 2g/ℓ 및 시판되는 세제 1g/ℓ를 함유하는 욕에서 20분 동안 환원 세정한다. 이어서, 완성된 염색물을 물로 세척하고 건조시킨다. 수득된 염색물은 베이지색이고 우수한 만능의 견뢰도, 특히, 우수한 광견뢰도를 갖는다.100 g of polyester fabric, at room temperature, with a solution ratio of 20: 1, 0.07 g of dye of formula 7, 0.058 g of dye of formula 2, 0.57 g of dye of formula 26, 1 g / l of ammonium sulfate and 0.5 g / l of commercially available leveling agent It was immersed in a solution containing and adjusted to a pH of 4.5 to 5 with 80% formic acid. The solution is then heated to 60 ° C. at a rate of 3 ° C. per minute and then to 130 ° C. at a rate of 2 ° C. per minute. The solution is kept at 140 ° C. for 60 minutes. The solution is then cooled to 40 ° C. and the dyed polyester fabric washed with water, and 5 g / l of 30% sodium hydroxide solution, 2 g / l of 85% sodium dithionite solution and 1 g / l of commercial detergent at 70-80 ° C. Reduction rinse for 20 minutes in the bath containing. The finished dyeings are then washed with water and dried. The dyeings obtained are beige and have good all-round fastness, in particular good light fastness.
실시예 3:Example 3:
폴리에스테르 직물 100g을, 실온에서 용액비를 20:1로 하여 화학식 14의 염료 0.086g, 화학식 2의 염료 0.181g, 화학식 26의 염료 0.41g, 황산암모늄 1g/ℓ 및 시판되는 균염제 0.5g/ℓ를 함유하고 80%의 포름산으로 pH가 4.5 내지 5로 조정된 용액에 침지시킨다. 이어서, 용액을 분당 3℃의 속도로 60℃까지 가열하고, 이어서 분당 2℃의 속도로 130℃까지 가열한다. 용액을 60분동안 140℃에서 유지한다. 이어서 용액을 40℃로 냉각시키고 염색된 폴리에스테르 직물을 물로 세척하고 70 내지 80℃에서 30% 수산화나트륨 용액 5ml/l, 85% 나트륨 디티오니트 용액 2g/ℓ 및 시판되는 세제 1g/ℓ를 포함하는 욕조에서 20분동안 환원 세정한다. 이어서, 완성된 염색물을 물로 세척하고 건조시킨다. 수득된 염색물은 암회색이고 우수한 만능의 견뢰도, 특히, 우수한 광견뢰도를 갖는다.100 g of polyester fabric, at room temperature with a solution ratio of 20: 1, 0.086 g of dye of formula 14, 0.181 g of dye of formula 2, 0.41 g of dye of formula 26, 1 g / l of ammonium sulfate and 0.5 g / l of commercially available leveling agent It was immersed in a solution containing and adjusted to a pH of 4.5 to 5 with 80% formic acid. The solution is then heated to 60 ° C. at a rate of 3 ° C. per minute and then to 130 ° C. at a rate of 2 ° C. per minute. The solution is kept at 140 ° C. for 60 minutes. The solution is then cooled to 40 ° C. and the dyed polyester fabric is washed with water and contains 5 ml / l 30% sodium hydroxide solution, 2 g / l 85% sodium dithionite solution and 1 g / l commercial detergent at 70-80 ° C. 20 minutes reduction cleaning in a bath. The finished dyeings are then washed with water and dried. The dyeings obtained are dark gray and have good all-round fastness, in particular good light fastness.
실시예 4:Example 4:
폴리에스테르 칩(PET Arnite D04-300, DSM) 1200.00g을 130℃에서 4시간 동안예비 건조시키고, 이어서 롤러 랙(roller rack)에서 균질해질때까지 15분동안 분당 60회전 속도로 화학식 7의 염료 0.24g과 혼합한다. 균질의 혼합물을, 최대 온도 275℃에서 6개의 가열 영역을 포함하는 이축 스크류 25mm 압출기(Collin, D 85560 Ebersberg)로 압출시키고 물로 켄칭시키고 터브 에투브(Turb Etuve) TE 25 과립기(MAPAG AG, CH 3001 Bern)에서 과립화시키고, 이어서 130℃에서 4시간 동안 건조시킨다. 우수한 만능의 견뢰도, 특히, 매우 우수한 광견뢰도 및 열광견뢰도를 갖는 적색 폴리에스테르 칩을 수득한다.1200.00 g of polyester chips (PET Arnite D04-300, DSM) were pre-dried at 130 ° C. for 4 hours and then at a rate of 60 revolutions per minute for 15 minutes until homogeneous in a roller rack. Mix with g The homogeneous mixture is extruded with a twin screw 25 mm extruder (Collin, D 85560 Ebersberg) containing six heating zones at a maximum temperature of 275 ° C., quenched with water and a Turb Etuve TE 25 granulator (MAPAG AG, CH 3001 Bern) and then dried at 130 ° C. for 4 hours. A red polyester chip is obtained which has good universal fastness, in particular very good light fastness and hot light fastness.
실시예 5:Example 5:
염료 혼합물 대신에, 화학식 2의 염료 0.7g을 사용하여 실시예 2를 반복한다. 수득한 염색물은 청색이고 우수한 만능의 견뢰도, 특히 우수한 광견뢰도를 갖는다.Instead of the dye mixture, Example 2 is repeated using 0.7 g of the dye of formula (2). The dyeings obtained are blue and have good all-round fastness, especially good light fastness.
Claims (9)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH2510/00 | 2000-12-22 | ||
| CH25102000 | 2000-12-22 | ||
| PCT/EP2001/014690 WO2002051942A1 (en) | 2000-12-22 | 2001-12-13 | Dyeing or printing of manufactured natural polymer and synthetic hydrophobic fibre materials |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20030064858A KR20030064858A (en) | 2003-08-02 |
| KR100800984B1 true KR100800984B1 (en) | 2008-02-05 |
Family
ID=4569746
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020037008421A Expired - Fee Related KR100800984B1 (en) | 2000-12-22 | 2001-12-13 | Dyeing or printing method of manufactured natural polymer and hydrophobic synthetic fiber material |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US6893472B2 (en) |
| EP (1) | EP1343846A1 (en) |
| JP (1) | JP3929401B2 (en) |
| KR (1) | KR100800984B1 (en) |
| CN (1) | CN1247704C (en) |
| AU (1) | AU2002217107A1 (en) |
| BR (1) | BR0116401B1 (en) |
| CA (1) | CA2430606C (en) |
| MX (1) | MXPA03005532A (en) |
| WO (1) | WO2002051942A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003080734A1 (en) * | 2002-03-22 | 2003-10-02 | Ciba Specialty Chemicals Holding Inc. | Anthraquinone dyes |
| BR0308599A (en) * | 2002-03-22 | 2005-02-09 | Ciba Sc Holding Ag | Anthraquinone Dyes |
| DE602004016644D1 (en) * | 2003-04-22 | 2008-10-30 | Huntsman Adv Mat Switzerland | PIGMENT / DYE MIXTURES |
| US20050155163A1 (en) * | 2004-01-21 | 2005-07-21 | Griffin Bruce O. | Dye mixtures |
| DE102005025270A1 (en) * | 2005-06-02 | 2006-12-07 | Dystar Textilfarben Gmbh & Co. Deutschland Kg | Very light fast blue disperse dyes |
| KR101523756B1 (en) * | 2013-05-31 | 2015-06-04 | 한국생산기술연구원 | Organic dye for LCD color filter and composition thereof |
| CN111304934A (en) * | 2020-04-03 | 2020-06-19 | 晋江市祺烽线带有限公司 | Dyeing method of polyester yarn |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1301068A (en) * | 1961-08-04 | 1962-08-10 | Cassella Farbwerke Mainkur Ag | New anthraquinone dyes and their preparation |
| GB963519A (en) * | 1961-08-02 | 1964-07-08 | Cassella Farbwerke Mainkur Ag | Process for printing and dyeing polyester materials |
| DE1278391B (en) * | 1961-07-14 | 1968-09-26 | Cassella Farbwerke Mainkur Ag | Dyeing and printing of fiber material made from high molecular weight polyesters |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB713512A (en) * | 1951-07-30 | 1954-08-11 | Cassella Farbwerke Mainkur Ag | Vat dyestuffs of the anthraquinone series |
| BE595479A (en) * | 1959-09-29 | |||
| BE627049A (en) * | 1962-01-12 | |||
| CH570436A5 (en) * | 1972-07-07 | 1975-12-15 | Ciba Geigy Ag | |
| EP0823505B1 (en) * | 1996-07-12 | 2000-03-08 | Ciba SC Holding AG | Process for trichromic dyeing or printing |
-
2001
- 2001-12-13 AU AU2002217107A patent/AU2002217107A1/en not_active Abandoned
- 2001-12-13 EP EP01272008A patent/EP1343846A1/en not_active Withdrawn
- 2001-12-13 JP JP2002553424A patent/JP3929401B2/en not_active Expired - Fee Related
- 2001-12-13 CA CA2430606A patent/CA2430606C/en not_active Expired - Fee Related
- 2001-12-13 BR BRPI0116401-5B1A patent/BR0116401B1/en not_active IP Right Cessation
- 2001-12-13 CN CNB018209475A patent/CN1247704C/en not_active Expired - Fee Related
- 2001-12-13 US US10/451,170 patent/US6893472B2/en not_active Expired - Lifetime
- 2001-12-13 WO PCT/EP2001/014690 patent/WO2002051942A1/en not_active Ceased
- 2001-12-13 KR KR1020037008421A patent/KR100800984B1/en not_active Expired - Fee Related
- 2001-12-13 MX MXPA03005532A patent/MXPA03005532A/en active IP Right Grant
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1278391B (en) * | 1961-07-14 | 1968-09-26 | Cassella Farbwerke Mainkur Ag | Dyeing and printing of fiber material made from high molecular weight polyesters |
| GB963519A (en) * | 1961-08-02 | 1964-07-08 | Cassella Farbwerke Mainkur Ag | Process for printing and dyeing polyester materials |
| FR1301068A (en) * | 1961-08-04 | 1962-08-10 | Cassella Farbwerke Mainkur Ag | New anthraquinone dyes and their preparation |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1481423A (en) | 2004-03-10 |
| JP3929401B2 (en) | 2007-06-13 |
| WO2002051942A1 (en) | 2002-07-04 |
| CA2430606C (en) | 2011-01-11 |
| US20040068808A1 (en) | 2004-04-15 |
| BR0116401B1 (en) | 2014-09-16 |
| BR0116401A (en) | 2003-11-11 |
| JP2004526874A (en) | 2004-09-02 |
| CN1247704C (en) | 2006-03-29 |
| AU2002217107A1 (en) | 2002-07-08 |
| EP1343846A1 (en) | 2003-09-17 |
| US6893472B2 (en) | 2005-05-17 |
| MX233178B (en) | 2005-12-20 |
| CA2430606A1 (en) | 2002-07-04 |
| MXPA03005532A (en) | 2003-10-24 |
| KR20030064858A (en) | 2003-08-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100847236B1 (en) | Azo dyes, dye mixtures, methods for their preparation and dyeing or printing of hydrophobic fiber materials using them | |
| KR100800984B1 (en) | Dyeing or printing method of manufactured natural polymer and hydrophobic synthetic fiber material | |
| EP1366122B1 (en) | Azo dyes, a process for their preparation and their use in the production of coloured plastics or polymeric colour particles, and in the dyeing or printing of hydrophobic fibre materials | |
| US7435270B2 (en) | Pigment/dye mixtures | |
| KR920000745B1 (en) | Composition of monoazo dyestaffs | |
| US4331584A (en) | Styryl compounds and coloring synthetic resins therewith | |
| TWI428399B (en) | Blue anthraquinone dyes, their preparation and use | |
| US6964689B2 (en) | Anthraquinone dyes, preparation thereof and use thereof | |
| US4527994A (en) | Process for mass-coloring nylon with 1:2 chromium complex azo dye | |
| KR910004554B1 (en) | Dicyanobenzantron compound | |
| US4826505A (en) | Monoazo pyridone compounds and application thereof for dyeing of hydrophobic fiber materials | |
| US5318601A (en) | Dye mixtures containing azo and quinophthalone dyes | |
| US4699982A (en) | Perinone compound | |
| KR840001841B1 (en) | Method for preparing styryl compound | |
| JPH0232162A (en) | Dicyanobenzanthrone compound | |
| KR20080010430A (en) | High Heat-Resistant Blue Disperse Dyes | |
| AU2002229658A1 (en) | Anthraquinone dyes, preparation thereof and use thereof | |
| ZA200304294B (en) | Anthraquinone dyes, preparation thereof and use thereof. | |
| JPH03258859A (en) | Water-insoluble naphthalimide dye | |
| HK1005548A1 (en) | Mixtures of pyridone monoazo dyestuffs | |
| JPH02185569A (en) | Disperse dye composition and method of dyeing or printing hydrophobic fiber material therewith |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| A201 | Request for examination | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U12-oth-PR1002 Fee payment year number: 1 |
|
| PG1601 | Publication of registration |
St.27 status event code: A-4-4-Q10-Q13-nap-PG1601 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-5-5-R10-R18-oth-X000 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| FPAY | Annual fee payment |
Payment date: 20130326 Year of fee payment: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| FPAY | Annual fee payment |
Payment date: 20140128 Year of fee payment: 7 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 7 |
|
| LAPS | Lapse due to unpaid annual fee | ||
| PC1903 | Unpaid annual fee |
St.27 status event code: A-4-4-U10-U13-oth-PC1903 Not in force date: 20150130 Payment event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date: 20150130 |