JP6853819B2 - Wntシグナル伝達経路阻害剤およびその治療用途 - Google Patents
Wntシグナル伝達経路阻害剤およびその治療用途 Download PDFInfo
- Publication number
- JP6853819B2 JP6853819B2 JP2018518629A JP2018518629A JP6853819B2 JP 6853819 B2 JP6853819 B2 JP 6853819B2 JP 2018518629 A JP2018518629 A JP 2018518629A JP 2018518629 A JP2018518629 A JP 2018518629A JP 6853819 B2 JP6853819 B2 JP 6853819B2
- Authority
- JP
- Japan
- Prior art keywords
- mmol
- alkyl
- substituted
- mixture
- unsubstituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000004156 Wnt signaling pathway Effects 0.000 title description 12
- 239000003112 inhibitor Substances 0.000 title description 4
- 230000001225 therapeutic effect Effects 0.000 title description 4
- 150000001875 compounds Chemical class 0.000 claims description 158
- -1 C 1- C 6 alkyl Chemical group 0.000 claims description 58
- 150000004820 halides Chemical class 0.000 claims description 45
- 125000000217 alkyl group Chemical group 0.000 claims description 30
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 25
- 229910052760 oxygen Inorganic materials 0.000 claims description 24
- 229910052799 carbon Inorganic materials 0.000 claims description 23
- 239000008194 pharmaceutical composition Substances 0.000 claims description 23
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 23
- 229910052717 sulfur Inorganic materials 0.000 claims description 23
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 21
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 19
- 210000004027 cell Anatomy 0.000 claims description 17
- 201000010099 disease Diseases 0.000 claims description 17
- 125000005842 heteroatom Chemical group 0.000 claims description 17
- 125000003545 alkoxy group Chemical group 0.000 claims description 16
- 229910052757 nitrogen Inorganic materials 0.000 claims description 15
- 229910052739 hydrogen Inorganic materials 0.000 claims description 14
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims description 12
- 239000003814 drug Substances 0.000 claims description 12
- 150000003839 salts Chemical class 0.000 claims description 12
- 102000013814 Wnt Human genes 0.000 claims description 10
- 108050003627 Wnt Proteins 0.000 claims description 10
- 241000124008 Mammalia Species 0.000 claims description 8
- 230000001404 mediated effect Effects 0.000 claims description 8
- 229940079593 drug Drugs 0.000 claims description 7
- 125000000623 heterocyclic group Chemical group 0.000 claims description 7
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 6
- 201000009030 Carcinoma Diseases 0.000 claims description 6
- 125000002252 acyl group Chemical group 0.000 claims description 6
- 238000011282 treatment Methods 0.000 claims description 6
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 claims description 5
- 206010016654 Fibrosis Diseases 0.000 claims description 5
- 206010039491 Sarcoma Diseases 0.000 claims description 5
- 230000004761 fibrosis Effects 0.000 claims description 5
- 206010009944 Colon cancer Diseases 0.000 claims description 4
- 125000004442 acylamino group Chemical group 0.000 claims description 4
- 125000004471 alkyl aminosulfonyl group Chemical group 0.000 claims description 4
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 4
- 125000000266 alpha-aminoacyl group Chemical group 0.000 claims description 4
- 208000029742 colonic neoplasm Diseases 0.000 claims description 4
- 208000035475 disorder Diseases 0.000 claims description 4
- 239000012453 solvate Substances 0.000 claims description 4
- 229940124597 therapeutic agent Drugs 0.000 claims description 4
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 claims description 3
- 206010004146 Basal cell carcinoma Diseases 0.000 claims description 3
- 206010061692 Benign muscle neoplasm Diseases 0.000 claims description 3
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 3
- 206010006187 Breast cancer Diseases 0.000 claims description 3
- 208000026310 Breast neoplasm Diseases 0.000 claims description 3
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 claims description 3
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 3
- 208000005243 Chondrosarcoma Diseases 0.000 claims description 3
- 208000006168 Ewing Sarcoma Diseases 0.000 claims description 3
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 claims description 3
- 206010025323 Lymphomas Diseases 0.000 claims description 3
- 201000004458 Myoma Diseases 0.000 claims description 3
- 206010029260 Neuroblastoma Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 3
- 206010060862 Prostate cancer Diseases 0.000 claims description 3
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 3
- 208000032056 Radiation Fibrosis Syndrome Diseases 0.000 claims description 3
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 3
- 201000009594 Systemic Scleroderma Diseases 0.000 claims description 3
- 206010042953 Systemic sclerosis Diseases 0.000 claims description 3
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 3
- 208000008383 Wilms tumor Diseases 0.000 claims description 3
- 125000005422 alkyl sulfonamido group Chemical group 0.000 claims description 3
- 201000010881 cervical cancer Diseases 0.000 claims description 3
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 3
- 206010017758 gastric cancer Diseases 0.000 claims description 3
- 201000010536 head and neck cancer Diseases 0.000 claims description 3
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 3
- 208000036971 interstitial lung disease 2 Diseases 0.000 claims description 3
- 208000032839 leukemia Diseases 0.000 claims description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 3
- 201000008026 nephroblastoma Diseases 0.000 claims description 3
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 3
- 201000008968 osteosarcoma Diseases 0.000 claims description 3
- 201000002528 pancreatic cancer Diseases 0.000 claims description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 3
- 230000002062 proliferating effect Effects 0.000 claims description 3
- 201000002793 renal fibrosis Diseases 0.000 claims description 3
- 201000011549 stomach cancer Diseases 0.000 claims description 3
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 3
- 125000006559 (C1-C3) alkylamino group Chemical group 0.000 claims description 2
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 2
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 claims description 2
- 201000000053 blastoma Diseases 0.000 claims description 2
- 229910052805 deuterium Inorganic materials 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 201000008184 embryoma Diseases 0.000 claims description 2
- 201000001441 melanoma Diseases 0.000 claims description 2
- 230000002207 retinal effect Effects 0.000 claims description 2
- 229910052722 tritium Inorganic materials 0.000 claims description 2
- 125000005115 alkyl carbamoyl group Chemical group 0.000 claims 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 185
- 239000000203 mixture Substances 0.000 description 134
- 235000019439 ethyl acetate Nutrition 0.000 description 82
- 238000000034 method Methods 0.000 description 77
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 69
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 64
- 239000007787 solid Substances 0.000 description 61
- 239000011734 sodium Substances 0.000 description 55
- 239000000243 solution Substances 0.000 description 50
- 238000001816 cooling Methods 0.000 description 47
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 46
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 45
- 239000000460 chlorine Substances 0.000 description 44
- 238000002360 preparation method Methods 0.000 description 44
- 238000010898 silica gel chromatography Methods 0.000 description 44
- 239000012044 organic layer Substances 0.000 description 39
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 33
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 32
- 239000003208 petroleum Substances 0.000 description 31
- 239000012267 brine Substances 0.000 description 24
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 24
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 22
- 239000012299 nitrogen atmosphere Substances 0.000 description 22
- 238000010992 reflux Methods 0.000 description 18
- 125000001072 heteroaryl group Chemical group 0.000 description 16
- 238000005481 NMR spectroscopy Methods 0.000 description 14
- 125000003118 aryl group Chemical group 0.000 description 14
- 238000009472 formulation Methods 0.000 description 14
- 239000000706 filtrate Substances 0.000 description 13
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 12
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 12
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 12
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 12
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 12
- 230000015572 biosynthetic process Effects 0.000 description 11
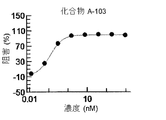
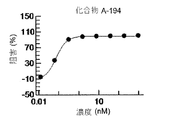
- 238000002810 primary assay Methods 0.000 description 11
- 239000007858 starting material Substances 0.000 description 11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- 125000005549 heteroarylene group Chemical group 0.000 description 10
- 239000010410 layer Substances 0.000 description 10
- 206010028980 Neoplasm Diseases 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 8
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 8
- 238000004440 column chromatography Methods 0.000 description 8
- 125000000753 cycloalkyl group Chemical group 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 7
- 125000004432 carbon atom Chemical group C* 0.000 description 7
- 230000037361 pathway Effects 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- BDTRIDKONHOQQN-UHFFFAOYSA-N 4h-pyrimidin-5-one Chemical compound O=C1CN=CN=C1 BDTRIDKONHOQQN-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 102000015735 Beta-catenin Human genes 0.000 description 6
- 108060000903 Beta-catenin Proteins 0.000 description 6
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 6
- 125000000732 arylene group Chemical group 0.000 description 6
- 125000004429 atom Chemical group 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- ZCSHNCUQKCANBX-UHFFFAOYSA-N lithium diisopropylamide Chemical compound [Li+].CC(C)[N-]C(C)C ZCSHNCUQKCANBX-UHFFFAOYSA-N 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- DGMIGAHDDPJOPN-UHFFFAOYSA-N 4,6-dichloro-5-fluoropyrimidine Chemical compound FC1=C(Cl)N=CN=C1Cl DGMIGAHDDPJOPN-UHFFFAOYSA-N 0.000 description 5
- BDAGIHXWWSANSR-UHFFFAOYSA-N Formic acid Chemical compound OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 5
- 101100030361 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) pph-3 gene Proteins 0.000 description 5
- 125000003277 amino group Chemical group 0.000 description 5
- 230000033228 biological regulation Effects 0.000 description 5
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- DSNCFDPCEPPERK-UHFFFAOYSA-N ClC1=C(C(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C)F Chemical compound ClC1=C(C(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C)F DSNCFDPCEPPERK-UHFFFAOYSA-N 0.000 description 4
- KFSALLZNNHJKRD-UHFFFAOYSA-N FC=1C(=NC=NC=1C1=CC2=CC=CC=C2C=C1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C Chemical compound FC=1C(=NC=NC=1C1=CC2=CC=CC=C2C=C1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C KFSALLZNNHJKRD-UHFFFAOYSA-N 0.000 description 4
- NMDFRINJWNMIRB-UHFFFAOYSA-N FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC1=CC=C(C=C1)C1=CC(=[N+](C=C1)[O-])C Chemical compound FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC1=CC=C(C=C1)C1=CC(=[N+](C=C1)[O-])C NMDFRINJWNMIRB-UHFFFAOYSA-N 0.000 description 4
- ILEIXYBRCAFHCY-UHFFFAOYSA-N FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC=1C=C2C=CC(=CC2=CC=1)C#N Chemical compound FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC=1C=C2C=CC(=CC2=CC=1)C#N ILEIXYBRCAFHCY-UHFFFAOYSA-N 0.000 description 4
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 238000005755 formation reaction Methods 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- OYRRZWATULMEPF-UHFFFAOYSA-N pyrimidin-4-amine Chemical compound NC1=CC=NC=N1 OYRRZWATULMEPF-UHFFFAOYSA-N 0.000 description 4
- 210000000130 stem cell Anatomy 0.000 description 4
- 125000001424 substituent group Chemical group 0.000 description 4
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 4
- CXNIUSPIQKWYAI-UHFFFAOYSA-N xantphos Chemical compound C=12OC3=C(P(C=4C=CC=CC=4)C=4C=CC=CC=4)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=CC=CC=1)C1=CC=CC=C1 CXNIUSPIQKWYAI-UHFFFAOYSA-N 0.000 description 4
- ICSNLGPSRYBMBD-UHFFFAOYSA-N 2-aminopyridine Chemical compound NC1=CC=CC=N1 ICSNLGPSRYBMBD-UHFFFAOYSA-N 0.000 description 3
- FZZMTSNZRBFGGU-UHFFFAOYSA-N 2-chloro-7-fluoroquinazolin-4-amine Chemical compound FC1=CC=C2C(N)=NC(Cl)=NC2=C1 FZZMTSNZRBFGGU-UHFFFAOYSA-N 0.000 description 3
- VMFALDWCEQUFSX-UHFFFAOYSA-N 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(N=CC=C2)C2=C1 VMFALDWCEQUFSX-UHFFFAOYSA-N 0.000 description 3
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 3
- BYSUWZLWJRDTGS-UHFFFAOYSA-N CC1=NC=CC(=C1)C1=CC=C(CNC2=NC=CC(=C2)C2=CC3=CC=CC=C3C=C2)C=C1 Chemical compound CC1=NC=CC(=C1)C1=CC=C(CNC2=NC=CC(=C2)C2=CC3=CC=CC=C3C=C2)C=C1 BYSUWZLWJRDTGS-UHFFFAOYSA-N 0.000 description 3
- BECDVNDOPZYROV-UHFFFAOYSA-N ClC1=C(C(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=[N+](C=C1)[O-])C)F Chemical compound ClC1=C(C(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=[N+](C=C1)[O-])C)F BECDVNDOPZYROV-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- LBPURGIIEPFEDZ-UHFFFAOYSA-N FC=1C(=NC=NC=1C1=CC2=CC=CC=C2C=C1)N(CC1=CC=C(C=C1)C1=CC(=NC=C1)C)C Chemical compound FC=1C(=NC=NC=1C1=CC2=CC=CC=C2C=C1)N(CC1=CC=C(C=C1)C1=CC(=NC=C1)C)C LBPURGIIEPFEDZ-UHFFFAOYSA-N 0.000 description 3
- HOZMPHCGCXTCBK-UHFFFAOYSA-N FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC=1C=C2C=CC(=CC2=CC=1)C(=O)N Chemical compound FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CC=1)NCC=1C=C2C=CC(=CC2=CC=1)C(=O)N HOZMPHCGCXTCBK-UHFFFAOYSA-N 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- KEWQOEQVFSBVLX-UHFFFAOYSA-N N1=CC=CC2=CC(=CC=C12)C1=NC(=NS1)NCC=1C=NC2=CC(=CC=C2C=1)C#N Chemical compound N1=CC=CC2=CC(=CC=C12)C1=NC(=NS1)NCC=1C=NC2=CC(=CC=C2C=1)C#N KEWQOEQVFSBVLX-UHFFFAOYSA-N 0.000 description 3
- VDTBUCSXFLNVSG-UHFFFAOYSA-N NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)C#N Chemical compound NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)C#N VDTBUCSXFLNVSG-UHFFFAOYSA-N 0.000 description 3
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 3
- 102000044880 Wnt3A Human genes 0.000 description 3
- 108700013515 Wnt3A Proteins 0.000 description 3
- LRFASXOTTIOBEW-UHFFFAOYSA-N [5-fluoro-6-(2-methylpyridin-4-yl)pyridin-3-yl]methanamine Chemical compound C1=NC(C)=CC(C=2C(=CC(CN)=CN=2)F)=C1 LRFASXOTTIOBEW-UHFFFAOYSA-N 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000012230 colorless oil Substances 0.000 description 3
- 230000001066 destructive effect Effects 0.000 description 3
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 235000019253 formic acid Nutrition 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 125000001188 haloalkyl group Chemical group 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 239000000543 intermediate Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- RLOWWWKZYUNIDI-UHFFFAOYSA-N phosphinic chloride Chemical compound ClP=O RLOWWWKZYUNIDI-UHFFFAOYSA-N 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 3
- LJEFUOGGCMPGHN-UHFFFAOYSA-N (2,3-difluoropyridin-4-yl)boronic acid Chemical compound OB(O)C1=CC=NC(F)=C1F LJEFUOGGCMPGHN-UHFFFAOYSA-N 0.000 description 2
- WWEHSVLHWVCRCW-UHFFFAOYSA-N (6-chloro-5-fluoropyridin-3-yl)methanamine Chemical compound NCC1=CN=C(Cl)C(F)=C1 WWEHSVLHWVCRCW-UHFFFAOYSA-N 0.000 description 2
- QUIAPFCZANBSTK-UHFFFAOYSA-N (6-iodo-5-methoxypyridin-3-yl)methanol Chemical compound COC1=CC(CO)=CN=C1I QUIAPFCZANBSTK-UHFFFAOYSA-N 0.000 description 2
- AEROXRIZWDKASJ-UHFFFAOYSA-N 2-bromo-5-(bromomethyl)pyridine-3-carbonitrile Chemical compound BrC1=C(C#N)C=C(C=N1)CBr AEROXRIZWDKASJ-UHFFFAOYSA-N 0.000 description 2
- XVPDCSLVBVJOGX-UHFFFAOYSA-N 2h-thiadiazol-3-amine Chemical compound NN1NSC=C1 XVPDCSLVBVJOGX-UHFFFAOYSA-N 0.000 description 2
- CEUHPOVLEQUFCC-UHFFFAOYSA-N 3,5-dichloro-1,2,4-thiadiazole Chemical compound ClC1=NSC(Cl)=N1 CEUHPOVLEQUFCC-UHFFFAOYSA-N 0.000 description 2
- ORTHGCWYABMWNU-UHFFFAOYSA-N 3-chloro-5-quinolin-6-yl-1,2,4-thiadiazole Chemical compound ClC1=NSC(=N1)C=1C=C2C=CC=NC2=CC=1 ORTHGCWYABMWNU-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- PPYZUBLDYPTDSU-UHFFFAOYSA-N 4-naphthalen-2-ylpyridin-2-amine Chemical compound C1=C(C=CC2=CC=CC=C12)C1=CC(=NC=C1)N PPYZUBLDYPTDSU-UHFFFAOYSA-N 0.000 description 2
- FFBVBZPIQJQLEF-UHFFFAOYSA-N 5-fluoro-4-(1,7-naphthyridin-6-yl)-1H-pyrimidin-6-one Chemical compound FC=1C(=NC=NC=1C=1C=C2C=CC=NC2=CN=1)O FFBVBZPIQJQLEF-UHFFFAOYSA-N 0.000 description 2
- IIUTXCOBPSSCDS-UHFFFAOYSA-N 6-(aminomethyl)naphthalene-2-carbonitrile Chemical compound C1=C(C#N)C=CC2=CC(CN)=CC=C21 IIUTXCOBPSSCDS-UHFFFAOYSA-N 0.000 description 2
- VLCWWCSDVFKUBL-UHFFFAOYSA-N BrC1=CC=C(CNC2=NC=CC(=C2)C2=CC3=CC=CC=C3C=C2)C=C1 Chemical compound BrC1=CC=C(CNC2=NC=CC(=C2)C2=CC3=CC=CC=C3C=C2)C=C1 VLCWWCSDVFKUBL-UHFFFAOYSA-N 0.000 description 2
- NSHWFQZFOHJQRU-UHFFFAOYSA-N BrCC1=CC=C2C=C(C=NC2=C1)C#N Chemical compound BrCC1=CC=C2C=C(C=NC2=C1)C#N NSHWFQZFOHJQRU-UHFFFAOYSA-N 0.000 description 2
- DGCFPQYUJXXRTR-UHFFFAOYSA-N BrCC=1C=NC2=CC(=CC=C2C=1)C#N Chemical compound BrCC=1C=NC2=CC(=CC=C2C=1)C#N DGCFPQYUJXXRTR-UHFFFAOYSA-N 0.000 description 2
- OETBVLCSPLXDGL-UHFFFAOYSA-N CC1=NC=CC(=C1)C1=CC=C(CNC2=CC(=NC=N2)N2C(C3=C(N=C(N=C3)SC)CC2)=O)C=C1 Chemical compound CC1=NC=CC(=C1)C1=CC=C(CNC2=CC(=NC=N2)N2C(C3=C(N=C(N=C3)SC)CC2)=O)C=C1 OETBVLCSPLXDGL-UHFFFAOYSA-N 0.000 description 2
- DONHFJKQYCASQY-UHFFFAOYSA-N CC1=NC=CC(=C1)C1=NC=C(C=N1)CN Chemical compound CC1=NC=CC(=C1)C1=NC=C(C=N1)CN DONHFJKQYCASQY-UHFFFAOYSA-N 0.000 description 2
- IIUKHAXOZDXSIT-UHFFFAOYSA-N COC=1C(=NC=C(C=1)CN)C1=CC(=NC=C1)C Chemical compound COC=1C(=NC=C(C=1)CN)C1=CC(=NC=C1)C IIUKHAXOZDXSIT-UHFFFAOYSA-N 0.000 description 2
- ALODPBLTEOGSQL-UHFFFAOYSA-N CS(=O)(=O)C1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1 Chemical compound CS(=O)(=O)C1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1 ALODPBLTEOGSQL-UHFFFAOYSA-N 0.000 description 2
- VGOIUMGBLMUEGU-UHFFFAOYSA-N CSC1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1 Chemical compound CSC1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1 VGOIUMGBLMUEGU-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- NBGFARDUXWHUFY-UHFFFAOYSA-N ClC1=C(C(=NC=N1)C(=O)OCC)F Chemical compound ClC1=C(C(=NC=N1)C(=O)OCC)F NBGFARDUXWHUFY-UHFFFAOYSA-N 0.000 description 2
- FQMCWSXIQFPHJI-UHFFFAOYSA-N ClC1=C(C(=NC=N1)NCC=1C=C2C=CC(=CC2=CC=1)C#N)F Chemical compound ClC1=C(C(=NC=N1)NCC=1C=C2C=CC(=CC2=CC=1)C#N)F FQMCWSXIQFPHJI-UHFFFAOYSA-N 0.000 description 2
- PULCHMADXXGMMI-UHFFFAOYSA-N ClC1=C(C(=NC=N1)NCC=1C=NC(=NC=1)C1=CC(=NC=C1)C)F Chemical compound ClC1=C(C(=NC=N1)NCC=1C=NC(=NC=1)C1=CC(=NC=C1)C)F PULCHMADXXGMMI-UHFFFAOYSA-N 0.000 description 2
- KEVDQJIWMUMWQF-UHFFFAOYSA-N FC=1C(=NC=NC=1C#CC=1C=NC=CC=1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)F Chemical compound FC=1C(=NC=NC=1C#CC=1C=NC=CC=1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)F KEVDQJIWMUMWQF-UHFFFAOYSA-N 0.000 description 2
- RCUJXXGXEXZORM-UHFFFAOYSA-N FC=1C(=NC=NC=1OC)C(=O)OCC Chemical compound FC=1C(=NC=NC=1OC)C(=O)OCC RCUJXXGXEXZORM-UHFFFAOYSA-N 0.000 description 2
- 229910004373 HOAc Inorganic materials 0.000 description 2
- LRWBVEDOWGHEMX-UHFFFAOYSA-N IC1=C(C=C(C=N1)CN)OC Chemical compound IC1=C(C=C(C=N1)CN)OC LRWBVEDOWGHEMX-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 239000005089 Luciferase Substances 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- DUNLXXJEMRYHLG-UHFFFAOYSA-N N(=[N+]=[N-])CC1=CC=C2C=C(C=NC2=C1)C#N Chemical compound N(=[N+]=[N-])CC1=CC=C2C=C(C=NC2=C1)C#N DUNLXXJEMRYHLG-UHFFFAOYSA-N 0.000 description 2
- YMZIZPQFUIWFGJ-UHFFFAOYSA-N N(=[N+]=[N-])CC=1C=C(C(=NC=1)I)OC Chemical compound N(=[N+]=[N-])CC=1C=C(C(=NC=1)I)OC YMZIZPQFUIWFGJ-UHFFFAOYSA-N 0.000 description 2
- LDRLPACNKWPUDK-UHFFFAOYSA-N N(=[N+]=[N-])CC=1C=NC2=CC(=CC=C2C=1)C#N Chemical compound N(=[N+]=[N-])CC=1C=NC2=CC(=CC=C2C=1)C#N LDRLPACNKWPUDK-UHFFFAOYSA-N 0.000 description 2
- TYRHDAZPKQQYLT-UHFFFAOYSA-N N1=CC=CC2=CC(=CC=C12)C1=NC(=NC=C1)NCC1=CC=C2C=C(C=NC2=C1)C#N Chemical compound N1=CC=CC2=CC(=CC=C12)C1=NC(=NC=C1)NCC1=CC=C2C=C(C=NC2=C1)C#N TYRHDAZPKQQYLT-UHFFFAOYSA-N 0.000 description 2
- BSZWLYBWWBPZFX-UHFFFAOYSA-N NCC1=CC=C2C=C(C=NC2=C1)C#N Chemical compound NCC1=CC=C2C=C(C=NC2=C1)C#N BSZWLYBWWBPZFX-UHFFFAOYSA-N 0.000 description 2
- CJZYCGXEGAPXOI-UHFFFAOYSA-N NCC=1C=NC(=C(C#N)C=1)Br Chemical compound NCC=1C=NC(=C(C#N)C=1)Br CJZYCGXEGAPXOI-UHFFFAOYSA-N 0.000 description 2
- SYRRVMTWHDXNJF-UHFFFAOYSA-N NCC=1C=NC2=CC(=CC=C2C=1)C#N Chemical compound NCC=1C=NC2=CC(=CC=C2C=1)C#N SYRRVMTWHDXNJF-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 2
- UCPYNZJOMGURGF-UHFFFAOYSA-N [4-(2-methylpyridin-4-yl)phenyl]methanamine Chemical compound C1=NC(C)=CC(C=2C=CC(CN)=CC=2)=C1 UCPYNZJOMGURGF-UHFFFAOYSA-N 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 150000001345 alkine derivatives Chemical class 0.000 description 2
- 125000005256 alkoxyacyl group Chemical group 0.000 description 2
- 125000003282 alkyl amino group Chemical group 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 150000001642 boronic acid derivatives Chemical group 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 238000006555 catalytic reaction Methods 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- FAOQYBVMAYMMCJ-UHFFFAOYSA-N ethyl 5-fluoro-6-oxo-1H-pyrimidine-4-carboxylate Chemical compound FC=1C(=NC=NC=1O)C(=O)OCC FAOQYBVMAYMMCJ-UHFFFAOYSA-N 0.000 description 2
- 239000012458 free base Substances 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 238000007429 general method Methods 0.000 description 2
- 238000005469 granulation Methods 0.000 description 2
- 230000003179 granulation Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 150000002391 heterocyclic compounds Chemical class 0.000 description 2
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 2
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 description 2
- KPTRDYONBVUWPD-UHFFFAOYSA-N naphthalen-2-ylboronic acid Chemical compound C1=CC=CC2=CC(B(O)O)=CC=C21 KPTRDYONBVUWPD-UHFFFAOYSA-N 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 125000004043 oxo group Chemical group O=* 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 230000008439 repair process Effects 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 238000001694 spray drying Methods 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- AQRLNPVMDITEJU-UHFFFAOYSA-N triethylsilane Chemical compound CC[SiH](CC)CC AQRLNPVMDITEJU-UHFFFAOYSA-N 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- MHCBYPRBJFPLPP-UHFFFAOYSA-N (2-chloropyrimidin-5-yl)methanamine Chemical compound NCC1=CN=C(Cl)N=C1 MHCBYPRBJFPLPP-UHFFFAOYSA-N 0.000 description 1
- HCBCJAFWOBCSRJ-UHFFFAOYSA-N (6-cyanonaphthalen-2-yl) trifluoromethanesulfonate Chemical compound C1=C(C#N)C=CC2=CC(OS(=O)(=O)C(F)(F)F)=CC=C21 HCBCJAFWOBCSRJ-UHFFFAOYSA-N 0.000 description 1
- 125000006619 (C1-C6) dialkylamino group Chemical group 0.000 description 1
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 description 1
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 description 1
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 1
- 125000006708 (C5-C14) heteroaryl group Chemical group 0.000 description 1
- KOFLVDBWRHFSAB-UHFFFAOYSA-N 1,2,4,5-tetrahydro-1-(phenylmethyl)-5,9b(1',2')-benzeno-9bh-benz(g)indol-3(3ah)-one Chemical compound C1C(C=2C3=CC=CC=2)C2=CC=CC=C2C23C1C(=O)CN2CC1=CC=CC=C1 KOFLVDBWRHFSAB-UHFFFAOYSA-N 0.000 description 1
- DNCYBUMDUBHIJZ-UHFFFAOYSA-N 1h-pyrimidin-6-one Chemical compound O=C1C=CN=CN1 DNCYBUMDUBHIJZ-UHFFFAOYSA-N 0.000 description 1
- AIXBVJWFQNYGJA-UHFFFAOYSA-N 2,3-difluoro-4-iodopyridine Chemical compound FC1=NC=CC(I)=C1F AIXBVJWFQNYGJA-UHFFFAOYSA-N 0.000 description 1
- HIOKTELNLIIACQ-UHFFFAOYSA-N 2-(2-methylpyridin-4-yl)pyrimidine Chemical compound C1=NC(C)=CC(C=2N=CC=CN=2)=C1 HIOKTELNLIIACQ-UHFFFAOYSA-N 0.000 description 1
- OXULGZNPTSIVHO-UHFFFAOYSA-N 2-bromo-5-methylpyridine-3-carbonitrile Chemical compound CC1=CN=C(Br)C(C#N)=C1 OXULGZNPTSIVHO-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 1
- CLRPXACRDTXENY-UHFFFAOYSA-N 3-ethynylpyridine Chemical compound C#CC1=CC=CN=C1 CLRPXACRDTXENY-UHFFFAOYSA-N 0.000 description 1
- YVTGWZIQJIIIOE-UHFFFAOYSA-N 3-fluoro-2-(2-methylpyridin-4-yl)pyridine Chemical compound C1=NC(C)=CC(C=2C(=CC=CN=2)F)=C1 YVTGWZIQJIIIOE-UHFFFAOYSA-N 0.000 description 1
- 125000005917 3-methylpentyl group Chemical group 0.000 description 1
- 125000005925 3-methylpentyloxy group Chemical group 0.000 description 1
- GRFNBEZIAWKNCO-UHFFFAOYSA-N 3-pyridinol Chemical compound OC1=CC=CN=C1 GRFNBEZIAWKNCO-UHFFFAOYSA-N 0.000 description 1
- ZRYZBQLXDKPBDU-UHFFFAOYSA-N 4-bromobenzaldehyde Chemical compound BrC1=CC=C(C=O)C=C1 ZRYZBQLXDKPBDU-UHFFFAOYSA-N 0.000 description 1
- DFOHHQRGDOQMKG-UHFFFAOYSA-N 4-chloro-2-methylsulfanylpyrimidine Chemical compound CSC1=NC=CC(Cl)=N1 DFOHHQRGDOQMKG-UHFFFAOYSA-N 0.000 description 1
- RQMWVVBHJMUJNZ-UHFFFAOYSA-N 4-chloropyridin-2-amine Chemical compound NC1=CC(Cl)=CC=N1 RQMWVVBHJMUJNZ-UHFFFAOYSA-N 0.000 description 1
- URZFWMPMGOMSSY-UHFFFAOYSA-N 7-methylquinoline-3-carbonitrile Chemical compound C1=C(C#N)C=NC2=CC(C)=CC=C21 URZFWMPMGOMSSY-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- FGUUSXIOTUKUDN-IBGZPJMESA-N C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 Chemical compound C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 FGUUSXIOTUKUDN-IBGZPJMESA-N 0.000 description 1
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 1
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 description 1
- OWUVURWWJBRNDR-WAVHTBQISA-N CC/N=C(\C(\F)=C(/N)\NCc1cnc(-c2ccnc(C)c2)nc1)/Cl Chemical compound CC/N=C(\C(\F)=C(/N)\NCc1cnc(-c2ccnc(C)c2)nc1)/Cl OWUVURWWJBRNDR-WAVHTBQISA-N 0.000 description 1
- DRFBBHYBUFDNFH-UHFFFAOYSA-N CC1=NC=CC(=C1)C2=C(C=C(C=N2)CN3CN=CC(=C3Cl)F)F Chemical compound CC1=NC=CC(=C1)C2=C(C=C(C=N2)CN3CN=CC(=C3Cl)F)F DRFBBHYBUFDNFH-UHFFFAOYSA-N 0.000 description 1
- RAVXUBDLEUFQMP-UHFFFAOYSA-N CC=1C=NC2=CC(=CC=C2C=1)C#N Chemical compound CC=1C=NC2=CC(=CC=C2C=1)C#N RAVXUBDLEUFQMP-UHFFFAOYSA-N 0.000 description 1
- RGOUMCCQGQALBH-UHFFFAOYSA-N COc1ncnc(-c(nc2)cc3c2nccc3)c1F Chemical compound COc1ncnc(-c(nc2)cc3c2nccc3)c1F RGOUMCCQGQALBH-UHFFFAOYSA-N 0.000 description 1
- 102000005403 Casein Kinases Human genes 0.000 description 1
- 108010031425 Casein Kinases Proteins 0.000 description 1
- 102100034357 Casein kinase I isoform alpha Human genes 0.000 description 1
- PBAOVJPXPLPKKS-UHFFFAOYSA-N Cc1cc(C2N=CC(CN)=CN2)ccn1 Chemical compound Cc1cc(C2N=CC(CN)=CN2)ccn1 PBAOVJPXPLPKKS-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- BRGCCZGIFJELDG-UHFFFAOYSA-N ClC1=C(C(=NC=N1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)F)F Chemical compound ClC1=C(C(=NC=N1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)F)F BRGCCZGIFJELDG-UHFFFAOYSA-N 0.000 description 1
- ZXZLMQIXYSYHOS-UHFFFAOYSA-N ClC1=CC(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C Chemical compound ClC1=CC(=NC=N1)NCC1=CC=C(C=C1)C1=CC(=NC=C1)C ZXZLMQIXYSYHOS-UHFFFAOYSA-N 0.000 description 1
- OCLJVHBXCDEXNR-UHFFFAOYSA-N ClC1=NSC(=N1)C=1C=C2C=CC=NC2=CN=1 Chemical compound ClC1=NSC(=N1)C=1C=C2C=CC=NC2=CN=1 OCLJVHBXCDEXNR-UHFFFAOYSA-N 0.000 description 1
- 102000006311 Cyclin D1 Human genes 0.000 description 1
- 108010058546 Cyclin D1 Proteins 0.000 description 1
- 102000017944 Dishevelled Human genes 0.000 description 1
- 108050007016 Dishevelled Proteins 0.000 description 1
- 241000289669 Erinaceus europaeus Species 0.000 description 1
- RIYMALQOHRXRRB-UHFFFAOYSA-N FC=1C(=NC=C(C=1)CNC1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1)C1=CC(=NC=C1)C Chemical compound FC=1C(=NC=C(C=1)CNC1=NC=CC(=N1)C=1C=C2C=CC=NC2=CC=1)C1=CC(=NC=C1)C RIYMALQOHRXRRB-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 108091007911 GSKs Proteins 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102000004103 Glycogen Synthase Kinases Human genes 0.000 description 1
- 102000003693 Hedgehog Proteins Human genes 0.000 description 1
- 108090000031 Hedgehog Proteins Proteins 0.000 description 1
- 101000994700 Homo sapiens Casein kinase I isoform alpha Proteins 0.000 description 1
- 101001043594 Homo sapiens Low-density lipoprotein receptor-related protein 5 Proteins 0.000 description 1
- LELOWRISYMNNSU-UHFFFAOYSA-N Hydrocyanic acid Natural products N#C LELOWRISYMNNSU-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 108010015167 Low Density Lipoprotein Receptor-Related Protein-5 Proteins 0.000 description 1
- 102000001770 Low Density Lipoprotein Receptor-Related Protein-5 Human genes 0.000 description 1
- 102000001774 Low Density Lipoprotein Receptor-Related Protein-6 Human genes 0.000 description 1
- 108010015179 Low Density Lipoprotein Receptor-Related Protein-6 Proteins 0.000 description 1
- 102100021926 Low-density lipoprotein receptor-related protein 5 Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- PFEVMXYWVNKFOP-UHFFFAOYSA-N N1=CC=CC2=CC(=NC=C12)C1=NC(=NC=C1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)C#N Chemical compound N1=CC=CC2=CC(=NC=C12)C1=NC(=NC=C1)NCC=1C=C(C(=NC=1)C1=CC(=NC=C1)C)C#N PFEVMXYWVNKFOP-UHFFFAOYSA-N 0.000 description 1
- JUIYRKBTIPDEHL-YDEXQGNWSA-N NN/C(/Cl)=C(\C(Cl)=N)/F Chemical compound NN/C(/Cl)=C(\C(Cl)=N)/F JUIYRKBTIPDEHL-YDEXQGNWSA-N 0.000 description 1
- 239000007832 Na2SO4 Substances 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 102100021709 Rho guanine nucleotide exchange factor 4 Human genes 0.000 description 1
- 101710128386 Rho guanine nucleotide exchange factor 4 Proteins 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 102000000479 TCF Transcription Factors Human genes 0.000 description 1
- 108010016283 TCF Transcription Factors Proteins 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- XPOLVIIHTDKJRY-UHFFFAOYSA-N acetic acid;methanimidamide Chemical compound NC=N.CC(O)=O XPOLVIIHTDKJRY-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 125000002009 alkene group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000002355 alkine group Chemical group 0.000 description 1
- 125000004422 alkyl sulphonamide group Chemical group 0.000 description 1
- 230000029936 alkylation Effects 0.000 description 1
- 238000005804 alkylation reaction Methods 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000019552 anatomical structure morphogenesis Effects 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 125000004966 cyanoalkyl group Chemical group 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 238000007907 direct compression Methods 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000007580 dry-mixing Methods 0.000 description 1
- 210000001671 embryonic stem cell Anatomy 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 210000002514 epidermal stem cell Anatomy 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- DOJUQNDTUGUKLL-UHFFFAOYSA-N ethyl 2-methylsulfanylpyrimidine-4-carboxylate Chemical compound CCOC(=O)C1=CC=NC(SC)=N1 DOJUQNDTUGUKLL-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 230000003779 hair growth Effects 0.000 description 1
- 230000009459 hedgehog signaling Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 229940030980 inova Drugs 0.000 description 1
- 210000004966 intestinal stem cell Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000007909 melt granulation Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 1
- IXWUNGYYIXCLLJ-UHFFFAOYSA-N methyl 6-iodo-5-methoxypyridine-3-carboxylate Chemical compound COC(=O)C1=CN=C(I)C(OC)=C1 IXWUNGYYIXCLLJ-UHFFFAOYSA-N 0.000 description 1
- NQMRYBIKMRVZLB-UHFFFAOYSA-N methylamine hydrochloride Chemical compound [Cl-].[NH3+]C NQMRYBIKMRVZLB-UHFFFAOYSA-N 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 210000005155 neural progenitor cell Anatomy 0.000 description 1
- 210000001178 neural stem cell Anatomy 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 1
- AHHWIHXENZJRFG-UHFFFAOYSA-N oxetane Chemical compound C1COC1 AHHWIHXENZJRFG-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 238000000059 patterning Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 208000015768 polyposis Diseases 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000009117 preventive therapy Methods 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- 150000003222 pyridines Chemical class 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 238000006268 reductive amination reaction Methods 0.000 description 1
- 238000003571 reporter gene assay Methods 0.000 description 1
- 238000006798 ring closing metathesis reaction Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 230000008470 skin growth Effects 0.000 description 1
- 210000002460 smooth muscle Anatomy 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 238000007614 solvation Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- YBRBMKDOPFTVDT-UHFFFAOYSA-N tert-butylamine Chemical compound CC(C)(C)N YBRBMKDOPFTVDT-UHFFFAOYSA-N 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 125000006337 tetrafluoro ethyl group Chemical group 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 150000003557 thiazoles Chemical class 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 125000003866 trichloromethyl group Chemical group ClC(Cl)(Cl)* 0.000 description 1
- 125000004205 trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000005550 wet granulation Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/472—Non-condensed isoquinolines, e.g. papaverine
- A61K31/4725—Non-condensed isoquinolines, e.g. papaverine containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/53—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with three nitrogens as the only ring hetero atoms, e.g. chlorazanil, melamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/541—Non-condensed thiazines containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D513/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00
- C07D513/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00 in which the condensed system contains two hetero rings
- C07D513/04—Ortho-condensed systems
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Pyridine Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
Description
本出願は、2015年10月8日に出願された中国特許出願201510645986.2;2016年3月31日に出願された中国特許出願201610195733.4;2016年3月31に出願された中国特許出願201610195731.5;2016年8月16日に出願された中国特許出願201610670828.7;2016年9月26日に出願された中国特許出願201610850360.X;2016年9月26日に出願された中国特許出願201610850358.2;および2016年9月26日に出願された中国特許出願201610850357.8の利益を請求し、これらの全ては本明細書中に参考として援用される。
本発明は、一般的に複素環化合物に関し、およびより特定すると哺乳動物における癌などのWntシグナル伝達経路媒介疾患を標的とする療法に有用である新規複素環化合物に関する。
Wntシグナル伝達経路は、細胞増殖、組織パターン形成、および細胞の運命、移動、形態形成および機能、ならびに細胞の生存および変性における調節の役割を含む、発生経路において重要な役割を有する(Logan and Nusse, Annu. Rev. Cell Dev. Biol. (2004) 20:781-810)。近年、幹細胞に対する研究の間、Wntシグナル伝達経路は表皮幹細胞、腸幹細胞、造血幹細胞、神経幹細胞、胚幹細胞および腫瘍幹細胞の維持を調節することが見い出されている(Reya and Clevers, Nature (2005) 434:843-850)。
1つの局面において、本発明は式Iの化合物あるいはその薬学的に許容可能な塩、溶媒和物、立体異性体または互変異性体を提供する:
AはA1、A2、A3またはA4であり;
UはU1、U2、U3またはU4であり;
LはL1、L2またはL3であり;
QはQ1、Q2、Q3またはQ4であり;
ZはZ1、Z2、Z3またはZ4であり;
A1は
X1〜X7は独立してNおよびC-R13から選ばれ;
A2は
A3は
A4は
X13およびX15は独立してO、N、SまたはC-R4cであり、そしてX14はNまたはCであり;
U1は
X8〜X10は独立してNおよびC-R14から選ばれ;
G1およびG2は独立してNおよびCから選ばれ;
V1およびV2は独立してN、O、SおよびC-R15から選ばれ;
WはV3、V4 - V5またはV4 = V5であり、ここでV3〜V5は独立してN、O、SおよびC-R16から選ばれ、ここでV4はV1と結合しており;そして
V5はV2と結合しており;
U2はC6-C10アリール、5-12員ヘテロアリール、11-13員ヘテロシクロフェニルまたは10-13員ヘテロシクロヘテロアリールであり、ここでヘテロシクロおよびヘテロアリールは独立してN、OおよびSから選ばれる1〜4個のヘテロ原子を含み;そして
C6-C10アリール、5-12員ヘテロアリール、11-13員ヘテロシクロフェニルおよび10-13員ヘテロシクロヘテロアリールは無置換または1〜4個のR6a基で置換されており;
U3は-OR14b、-NR14bR15b、無置換または1〜6個のR13b基で置換されたC6-C12アリール、無置換または1〜6個のR13b基で置換された5-14員ヘテロアリール、無置換または1〜6個のR13b基で置換された11-13員ヘテロシクロフェニル、無置換または1〜6個のR13b基で置換された10-13員ヘテロシクロヘテロアリール、5-6員アリールまたはヘテロアリールで置換されたC2-C8アルケニル、5-6員アリールまたはヘテロアリールで置換されたC2-C8アルキニル、または無置換または1〜2個のR13b基で置換された
X11はNまたはC-R13bであり;
U4は
X8〜X10は独立してNおよびC-R15cから選ばれ;
G1およびG2は独立してNおよびCから選ばれ;
V1およびV2は独立してN、O、SおよびC-R16cから選ばれ;
WはV3、V4 - V5またはV4 = V5であり、ここでV3〜V5は独立してN、O、SおよびC-R17cから選ばれ、ここでV4はV1と結合しており;そして
V5はV2と結合しており;
L1は
L2は
Y2は空白、-O-、-S-、-N(R18b)-または-C(R18b)(R19b)-であり;
L3は-C(R2c)(R3c)-または-NHCH2CH2-であり;
Q1はC6-C10アリーレンまたは5-12員ヘテロアリーレンであり、ここで5-12員ヘテロアリーレンは独立してN、OおよびSから選ばれる1〜4個のヘテロ原子を含み;そして
C6-C10アリールおよび5-12員ヘテロアリーレンは無置換または1〜4個のR17基で置換されており;
Q2はC6-C10アリーレンまたは5-10員ヘテロアリーレンであり、ここで5-10員ヘテロアリーレンは独立してN、OおよびSから選ばれる1〜4個のヘテロ原子を含み;そして
C6-C10アリーレンおよび5-10員ヘテロアリーレンは無置換または1〜4個のR4a基で置換されており;
Q3は無置換または1〜6個のR6b基で置換されたC6-C12アリーレン、無置換または1〜6個のR6b基で置換された5-14員ヘテロアリーレン、無置換または1〜6個のR6b基で置換されたC3-C6シクロアルキレン、無置換または1〜6個のR6b基で置換されたC3-C6ヘテロシクレン、または無置換または1〜2個のR6b基で置換された
X12はNまたはC-R6bであり;
Q4はC6-C10アリーレンまたは5-12員ヘテロアリーレンであり、ここで5-12員ヘテロアリーレンは独立してN、OおよびSから選ばれる1〜4個のヘテロ原子を含み;そして
C6-C10アリーレンおよび5-12員ヘテロアリーレンは無置換または1〜4個のR5c基で置換されており;
Z1は-CN、C6アリール、5-6員ヘテロアリール、または
C6アリールおよび5-6員ヘテロアリールは無置換または1〜3個のR18基で置換されており;
Y1はOまたはNR20であり;そして
qは0、1、2または3であり;
Z2はH、-CN、ハライド、-OH、C1-C8アルキル、C3-C8シクロアルキル、C2-C8アルケニル、C2-C8アルキニル、C1-C8アルキルスルホニル、C1-C8アシル、アミノアシル、C1-C8アシルアミノ、C1-C8アルキルカルバモイルアミノ、C1-C8アルコキシカルバモイル、C1-C8アルキルスルホンアミド、C1-C8アルキルアミノスルホニル、C1-C8アルコキシ、C2-C8アルコキシアシル、フェニル、5-6員ヘテロアリール、または5-7員ヘテロシクロであり、ここで5-6員ヘテロアリールおよび5-7員ヘテロシクロは独立してN、OおよびSから選ばれる1個以上のヘテロ原子を含み;そして
フェニル、5-6員ヘテロアリールおよび5-7員ヘテロ環は無置換または1〜3個のR5a基で置換されており;
Z3はH、-CN、ハライド、-OH、無置換または1〜3個のR12b基で置換されたC1-C8アルキル、C3-C8シクロアルキル、C2-C8アルケニル、C2-C8アルキニル、C1-C8アルキルスルホニル、C1-C8アシル、アミノアシル、C1-C8アシルアミノ、C1-C8アルキルカルバモイルアミノ、C1-C8アルコキシカルバモイル、C1-C8アルキルスルホンアミド、C1-C8アルキルアミノスルホニル、C1-C8アルコキシ、C2-C8アルコキシアシル、無置換または1〜3個のR12b基で置換された-N(R7b)(R8b)、無置換または1〜3個のR9b基で置換されたフェニル、無置換または1〜3個のR10b基で置換された5-6員ヘテロアリール、または無置換または1〜3個のR11b基で置換された5-7員ヘテロシクロであり、ここで5-6員ヘテロアリールおよび5-7員ヘテロシクロは独立してN、OおよびSから選ばれる1〜3個のヘテロ原子を含み;
Z4は-CN、C6アリール、5-6員ヘテロアリール、または
C6アリールおよび5-6員ヘテロアリールは無置換または1〜3個のR6c基で置換されており;
Y3はOまたはNR8cであり;そして
qは0、1、2または3であり;
R1〜R3は独立してHおよびC1-C6アルキルから選ばれ、ここでC1-C6アルキルは無置換または1〜3個のハライド、-CN、-OH、C1-C3アルキル、C3-C5シクロアルキル、またはC1-C3アルコキシ基で置換されており;
R4はH、ハライド、-CN、-OH、-NO2、C1-C6アルキル、C3-C8シクロアルキル、またはC1-C6アルコキシであり、ここでC1-C6アルキル、C3-C8シクロアルキルおよびC1-C6アルコキシは無置換または1〜3個のハライドで置換されており;
R5およびR6は独立してH、ハライド、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキルおよびC1-C6アルコキシから選ばれ、ここでC1-C6アルキル、C3-C6シクロアルキルおよびC1-C6アルコキシは無置換または1〜3個のハライドで置換されており;
R7およびR8は独立してHおよびC1-C6アルキルから選ばれ、またはR7およびR8は一緒にオキソ(=O)であり;
R9〜R12は独立してH、C1-C6アルキルから選ばれ;
R13はH、ハライド、-CN、-OH、アミノ、-NO2、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシ、C1-C6アルキルチオまたは1-ピロリジノであり、ここでアミノ、C1-C6アルキルおよびC1-C6アルコキシは無置換または1〜3個のハライド、C1-C3アルキルまたはC3-C6シクロアルキル基で置換されており;
R14〜R17は独立してH、ハライド、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシおよびC1-C6アルキルチオから選ばれ、ここでアミノ、C1-C6アルキルおよびC1-C6アルコキシは無置換または1〜3個のハライド、C1-C3アルキルまたはC3-C6シクロアルキル基で置換されており;
R18はH、ハライド、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキルまたはC1-C6アルコキシであり、ここでアミノ、C1-C6アルキルおよびC1-C6アルコキシは無置換または1〜3個のハライド、C1-C3アルキルまたはC3-C6シクロアルキル基で置換されており;
R19およびR20は独立してHおよびC1-C3アルキルから選ばれ、またはR19およびR20はそれらが結合する隣接した原子と一緒になって環構造を形成し;
R4a、R5aおよびR6aは独立してハライド、-CN、および-OHから選ばれ、またはR4a、R5aおよびR6aは独立してC1-C8アルキル、C3-C8シクロアルキル、C1-C8アルコキシ、C2-C8アルケニル、C2-C8アルキニル、C1-C8アルキルスルホニル、C1-C8アシル、アミノアシル、C1-C8アシルアミノ、C1-C8アルキルカルバモイルアミノ、C1-C8アルコキシカルバモイル、C1-C8アルキルスルホンアミド、C1-C8アルキルアミノスルホニル、C2-C8アルコキシアシル、および3-8員ヘテロ環から選ばれ、これらの全ては無置換または1〜3個のハライド、-OH、-CN、C1-C3アルキル、C1-C3アルコキシルまたはC3-C8シクロアルキル基で置換されており、ここで3-8員ヘテロ環はN、OまたはSからの1個以上のヘテロ原子を含み;
R2mはH、重水素、三重水素、ハライド、-OH、-CN、C1-C8アルキル、C3-C8シクロアルキルまたはC1-C8アルコキシであり;
R2bおよびR3bは独立してH、無置換またはハライド、-CN、-OH、C1-C6アルキル、C3-C5シクロアルキルおよびC1-C3アルコキシから選ばれる1〜3個の基で置換されたC1-C6アルキルから選ばれ;
R4bおよびR5bは独立してH、ハライド、-CN、アミノ、C1-C8アルキル、C3-C8シクロアルキル、C1-C8アルコキシ、およびC1-C3アルキルアミノから選ばれ;
R6bはH、ハライド、-CNまたは-OHであり、またはR6bはアミノ、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシ、およびC1-C3アルキルアミノから選ばれ、これらの全ては無置換または1〜3個のハライド、C1-C3アルキル、またはC3-C6シクロアルキル基で置換されており;
R7bおよびR8bは独立してH、C1-C8アルキルおよびC3-C8シクロアルキルから選ばれ、これらの後者の2つは無置換または1〜3個のR12bで置換されており、またはR7およびR8はそれらが結合する隣接した原子と一緒になって環構造を形成し;
R9bおよびR10bは独立してH、ハライド、-CNおよび-OHから選ばれ、またはR9bおよびR10bは独立してアミノ、C1-C6アルキル、C3-C6シクロアルキル、およびC1-C6アルコキシから選ばれ、これらの全ては無置換または1〜3個のハライド、C1-C3アルキル、またはC3-C6シクロアルキル基で置換されており;
R11bはH、ハライド、-CN、-OH、アミノ、無置換またはハライド、C3-C6シクロアルキル、オキソ、またはC1-C6アルコキシで置換されたC1-C6アルキルであり;
R12bはH、-CN、-OH、アミノ、無置換またはハライド、C3-C6シクロアルキル、オキソ、またはC1-C6アルコキシで置換されたC1-C6アルキルであり;
R13bはH、ハライド、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシ、C2-C6アルキルカルバモイルアミノ、C2-C6アルコキシカルバモイル、C1-C3アルキルスルホニル、-N(R16b)S(O)2-C1-C3アルキル、または-N(R16b)C(O)-C1-C3アルキルであり;
R14bおよびR15bは独立してH、無置換または1〜3個のR20b基で置換されたC3-C6シクロアルキル、無置換または1〜3個のR20b基で置換されたC1-C6アルキル、無置換または1〜3個のR20b基で置換された5-6員アリール、無置換または1〜3個のR20b基で置換された5-6員ヘテロアリール、および無置換または1〜3個のR20b基で置換された5-6員ヘテロシクロから選ばれ、ここで5-6員ヘテロアリールおよび5-7員ヘテロシクロは独立してN、OおよびSから選ばれる1〜3個のヘテロ原子を含み、またはR14bおよびR15bはそれらが結合する隣接した原子と一緒になって環構造を形成し;
R16b〜R19bは独立してHおよび無置換またはハライドで置換されたC1-C6アルキルから選ばれ;
R20bはH、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシ、5-6員アリール、または5-6員ヘテロアリールであり、ここで5-6員ヘテロアリールは独立してN、OおよびSから選ばれる1〜3個のヘテロ原子を含み;
R21bはHまたはC1-C6アルキルであり;
R2cおよびR3cは独立してHおよび無置換または1〜3個のハライドで置換されたC1-C6アルキルから選ばれ;
R4cはH、ハライド、C1-C3アルキル、C3-C5シクロアルキル、またはC1-C3アルコキシであり;
R5cおよびR6cは独立してH、ハライド、-CN、-OH、アミノ、無置換または1〜3個のハライドで置換されたC1-C6アルキル、C3-C5シクロアルキル、およびC1-C6アルコキシから選ばれ;
R7cおよびR8cは独立してHおよびC1-C6アルキルから選ばれ、またはR7cおよびR8cはそれらが結合する隣接した原子と一緒になって環構造を形成し;
R9c〜R14cは独立してH、C1-C6アルキルおよび-CF3から選ばれ;
R15c〜R17cは独立してH、ハライド、-CN、-OH、アミノ、無置換または1〜3個のハライドで置換されたC1-C6アルキル、C3-C5シクロアルキル、およびC1-C6アルコキシから選ばれる。
別の実施態様において、式Iの化合物は
1つの実施態様において、式Iの化合物は
別の実施態様において、式Iの化合物は、無置換または1〜3個のR15基で置換された
1つの実施態様において、式Iの化合物は、無置換または1〜3個のR4a基で置換された
1つの実施態様において、式Iの化合物は、無置換または1〜3個のR5a基で置換された
別の実施態様において、式Iの化合物は、無置換または1〜6個のR6b基で置換された
1つの実施態様において、式Iの化合物は、無置換または1〜2個のR10b基で置換された
別の実施態様において、式Iの化合物は、無置換または1〜6個のR13c基で置換された
別の実施態様において、式Iの化合物は、
1つの実施態様において、式Iの化合物は、
定義
化合物は、本明細書中、一般的に標準の命名法を用いて記載される。不斉中心を有する化合物について、(特に明記しない限り)全ての光学異性体およびそれらの混合物が包含されることを理解すべきである。さらに、炭素-炭素二重結合を有する化合物はZ-およびE-体で生じ得、該化合物の全ての異性体形態は特に指定がない限り本発明に含まれる。化合物が種々の互変異性体形態で存在する場合、記載した化合物はいずれか1つの特定の互変異性体に限定されず、むしろ全ての互変異性体形態を包含すると意図される。
1つの実施態様は、式Iの化合物、またはその立体異性体、互変異性体、水和物、溶媒和物または薬学的に許容可能な塩、および少なくとも1種の薬学的に許容可能な賦形剤を含有する医薬組成物を提供する。
本発明の方法は、広範囲の細胞、組織および器官の修復および/または機能的性能の調節におけるヘッジホッグシグナル伝達を阻害し、そして神経組織、骨および軟骨の形成および修復の調節、精子形成の調節、平滑筋の調節、肺、肝臓および原腸から発生する他の器官の調節、造血機能の調節、皮膚および毛髪の成長の調節などにわたる治療および美容用途を有する少なくとも1つの式Iの化合物の使用を含む。従って、本発明の方法および組成物は、ヘッジホッグタンパク質の阻害剤が関与し得るので、全てのこのような使用のための主題の阻害剤の使用を含む。さらに、主題の方法は、培養物中に提供される細胞上(インビトロ)、または全動物における細胞上(インビボ)で実施され得る。
全ての試薬および溶媒は、市販で入手した。必要であれば、全ての試薬および溶媒は、標準的な技術によって精製した:テトラヒドロフランはナトリウムからの蒸留によって精製した。全ての薄層クロマトグラフィー(TLC、GF254)分析およびカラム精製(100-200メッシュ)は、シリカゲル(Qingdao Haiyang Chemical Co.Ltd.またはYantai Chemical Co. Ltd.)上で行い、そしてスポットは254 nmでのUV可視化およびI2蒸気またはリンモリブデン酸によって現れた。全ての核磁気共鳴スペクトルは、示した通り、400 MHzでのVarian unity INOVA 400NB分光光度計または300 MHzでのVarian Vnmrs分光光度計を用いて記録した。LC-MSは、Agela Durashell C18 3.5 μm 4.6×50 mmカラムを用いたAgilent 1100システムを用いて行った。勾配は、示した実行時間(例えば、5分)、流速1.8 mL/minで5/95〜95/5の勾配を有する0.1 NH4HCO3水溶液およびアセトニトリルを用いて行った。
実施例1:5-フルオロ-N-(4-(2-メチルピリジン-4-イル)ベンジル)-6-(ナフタレン-2-イル)ピリミジン-4-アミン(A-1)の調製
1次アッセイは、Wnt経路スーパートップフラッシュ(STF)レポーター遺伝子アッセイに基づく:
EK293 STF安定クローン(「スーパートップフラッシュ」TCF-ルシフェラーゼレポータープラスミドで安定にトランスフェクトしたHEK293細胞)を完全培養培地(6 μg/mL ブラストサイジンおよび10% FBSを含む、4 mM L-Gln、1.5 g/L重炭酸ナトリウムおよび4.5 g/Lグルコースを有するDMEM)中に維持した。L Wnt3A細胞(CRL-2647、ATCC)を10% FBS(Hyclone)を含むDMEM(Gibico)中に維持した。HEK293 STFおよびL Wnt3A細胞を90%コンフルエンスで採集し、そして細胞懸濁物を1:1(HEK293 STF:L Wnt3A)の固定比で混合した。混合した細胞懸濁物の100 μL/ウェルを96-ウェル-プレートに12,000細胞/ウェルの最終細胞濃度で加え、次いでさらに24時間培養し、その後化合物を加えた。
Claims (7)
- 式I:
AはA2またはA3であり;
UはU1であり;
LはL1、L2またはL3であり;
A2は
A3は
U1は
X8〜X10は独立してNおよびC-R14から選ばれ;
G1およびG2は独立してNおよびCから選ばれ;
V1およびV2は独立してN、O、SおよびC-R15から選ばれ;
WはV3、V4 - V5またはV4= V5であり、ここでV3〜V5は独立してN、O、SおよびC-R16から選ばれ、ここでV4はV1と結合しており;そして
V5はV2と結合しており;
L1は
L2は
Y2は空白、-O-、-S-、-N(R18b)-または-C(R18b)(R19b)-であり;
L3は-C(R2c)(R3c)-または-NHCH2CH2-であり;
Qは、それぞれ無置換または1〜3個のR4a基で置換された
Zは、それぞれ無置換または1〜3個のR5a基で置換された
R1〜R3は独立してHおよびC1-C6アルキルから選ばれ、ここでC1-C6アルキルは無置換または1〜3個のハライド、-CN、-OH、C1-C3アルキル、C3-C5シクロアルキル、またはC1-C3アルコキシ基で置換されており;
R14〜R16は独立してH、ハライド、-CN、-OH、アミノ、C1-C6アルキル、C3-C6シクロアルキル、C1-C6アルコキシおよびC1-C6アルキルチオから選ばれ、ここでアミノ、C1-C6アルキルおよびC1-C6アルコキシは無置換または1〜3個のハライド、C1-C3アルキルまたはC3-C6シクロアルキル基で置換されており;
R4aおよびR5aは独立してハライド、-CN、および-OHから選ばれ、またはR4aおよびR5aは独立してC1-C8アルキル、C3-C8シクロアルキル、C1-C8アルコキシ、C2-C8アルケニル、C2-C8アルキニル、C1-C8アルキルスルホニル、C1-C8アシル、アミノアシル、C1-C8アシルアミノ、C1-C8アルキルカルバモイルアミノ、C1-C8アルコキシカルバモイル、C1-C8アルキルスルホンアミド、C1-C8アルキルアミノスルホニル、C2-C8アルコキシアシル、および3-8員ヘテロ環から選ばれ、これらの全ては無置換または1〜3個のハライド、-OH、-CN、C1-C3アルキル、C1-C3アルコキシルまたはC3-C8シクロアルキル基で置換されており、ここで3-8員ヘテロ環はN、OまたはSから選ばれる1個以上のヘテロ原子を含み;
R2mはH、重水素、三重水素、ハライド、-OH、-CN、C1-C8アルキル、C3-C8シクロアルキルまたはC1-C8アルコキシであり;
R2bおよびR3bは独立してH、無置換またはハライド、-CN、-OH、C1-C6アルキル、C3-C5シクロアルキルおよびC1-C3アルコキシから選ばれる1〜3個の基で置換されたC1-C6アルキルから選ばれ;
R4bおよびR5bは独立してH、ハライド、-CN、アミノ、C1-C8アルキル、C3-C8シクロアルキル、C1-C8アルコキシ、およびC1-C3アルキルアミノから選ばれ;
R16b〜R19bは独立してHおよび無置換またはハライドで置換されたC1-C6アルキルから選ばれ;
R2cおよびR3cは独立してHおよび無置換または1〜3個のハライドで置換されたC1-C6アルキルから選ばれる
の化合物、あるいはその薬学的に許容可能な塩、溶媒和物、立体異性体または互変異性体。 - 治療有効量の請求項1の化合物および薬学的に許容可能な担体を含有する医薬組成物。
- 請求項1の化合物、またはその医薬組成物を、必要に応じて第2の治療剤と組み合わせて含有する、Wnt-媒介疾患を患う哺乳動物におけるWnt-媒介疾患の治療に使用するための医薬組成物であって、該疾患が、全身性硬化症、皮膚線維症、突発性肺線維症、腎線維症、肝線維症、薬物誘発線維症、放射線誘発線維症、大腸癌、乳癌、頭頸部扁平上皮癌、食道扁平上皮癌、非小細胞肺癌、胃癌、膵臓癌、白血病、リンパ腫、神経芽細胞腫、網膜芽細胞腫、肉腫、骨肉腫、軟骨肉腫、ユーイング肉腫、横紋筋肉腫、脳腫瘍、ウィルムス腫瘍、基底細胞癌、黒色腫、頭頸部癌、子宮頸癌および前立腺癌からなる群から選ばれる細胞増殖性疾患である、医薬組成物。
Applications Claiming Priority (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201510645986.2 | 2015-10-08 | ||
| CN201510645986.2A CN105254613A (zh) | 2015-10-08 | 2015-10-08 | 具有Wnt信号通路抑制活性的杂环化合物及其应用 |
| CN201610195733.4 | 2016-03-31 | ||
| CN201610195731 | 2016-03-31 | ||
| CN201610195731.5 | 2016-03-31 | ||
| CN201610195733 | 2016-03-31 | ||
| CN201610670828.7 | 2016-08-16 | ||
| CN201610670828.7A CN107759584B (zh) | 2016-08-16 | 2016-08-16 | 一种具有Wnt信号通路抑制活性的氨基五元杂环化合物及其应用 |
| CN201610850357.8 | 2016-09-26 | ||
| CN201610850358.2A CN107286136B (zh) | 2016-03-31 | 2016-09-26 | 一种3-氟吡啶杂环化合物及其应用 |
| CN201610850358.2 | 2016-09-26 | ||
| CN201610850360.XA CN106565673B (zh) | 2015-10-08 | 2016-09-26 | 具有Wnt信号通路抑制活性的5-氟嘧啶杂环化合物及其应用 |
| CN201610850360.X | 2016-09-26 | ||
| CN201610850357.8A CN107286135B (zh) | 2016-03-31 | 2016-09-26 | 一种具有Wnt信号通路抑制活性的杂环化合物及其应用 |
| PCT/US2016/055851 WO2017062688A1 (en) | 2015-10-08 | 2016-10-06 | Wnt signaling pathway inhibitors and therapeutic applications thereof |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018535949A JP2018535949A (ja) | 2018-12-06 |
| JP2018535949A5 JP2018535949A5 (ja) | 2019-11-14 |
| JP6853819B2 true JP6853819B2 (ja) | 2021-03-31 |
Family
ID=62621830
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018518629A Active JP6853819B2 (ja) | 2015-10-08 | 2016-10-06 | Wntシグナル伝達経路阻害剤およびその治療用途 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US10450300B2 (ja) |
| EP (1) | EP3359154B1 (ja) |
| JP (1) | JP6853819B2 (ja) |
| KR (1) | KR102698411B1 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3164264A1 (en) * | 2020-01-02 | 2021-07-08 | Helmholtz-Zentrum Fur Infektionsforschung Gmbh | Novel pqsr inverse agonists |
| CN112612873B (zh) * | 2020-12-25 | 2023-07-07 | 上海德拓信息技术股份有限公司 | 一种基于nlp技术的集中性事件挖掘方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61205261A (ja) * | 1985-03-08 | 1986-09-11 | Sagami Chem Res Center | 6―置換―5―フルオロウラシル誘導体 |
| US4617393A (en) * | 1985-07-19 | 1986-10-14 | American Home Products Corporation | 5-substituted-6-aminopyrimidines, composition and uses as cardiotonic agents for increasing cardiac contractility |
| US7135575B2 (en) * | 2003-03-03 | 2006-11-14 | Array Biopharma, Inc. | P38 inhibitors and methods of use thereof |
| UY31048A1 (es) | 2007-04-25 | 2008-11-28 | Astrazeneca Ab | Nuevos compuestos de pirimidina y usos de los mismos |
| US20090054392A1 (en) * | 2007-08-20 | 2009-02-26 | Wyeth | Naphthylpyrimidine, naphthylpyrazine and naphthylpyridazine analogs and their use as agonists of the wnt-beta-catenin cellular messaging system |
| TW201011017A (en) * | 2008-08-19 | 2010-03-16 | Astrazeneca Ab | Chemical compounds 495-1 |
| GB0818241D0 (en) | 2008-10-06 | 2008-11-12 | Cancer Res Technology | Compounds and their use |
| US20110189097A1 (en) | 2009-11-09 | 2011-08-04 | Dritan Agalliu | Use of WNT inhibitor to inhibit angiogenesis in the CNS |
| EP2702043A1 (en) * | 2011-04-29 | 2014-03-05 | Exelixis, Inc. | Inhibitors of inducible form of 6-phosphofructose-2-kinase |
| US9493444B2 (en) | 2012-01-28 | 2016-11-15 | Merck Patent Gmbh | Azaheterocyclic compounds |
| PH12017500997A1 (en) * | 2012-04-04 | 2018-02-19 | Samumed Llc | Indazole inhibitors of the wnt signal pathway and therapeutic uses thereof |
| EP2968348A4 (en) * | 2013-03-12 | 2016-11-09 | Curegenix Inc | COMPOUNDS FOR THE TREATMENT OF CANCER |
| AU2014244506A1 (en) | 2013-03-14 | 2015-09-17 | Curegenix, Inc. | Compounds for treatment of fibrosis diseases |
| RU2719422C2 (ru) | 2014-08-04 | 2020-04-17 | Нуэволюшон А/С | Необязательно конденсированные гетероциклил-замещенные производные пиримидина, пригодные для лечения воспалительных, метаболических, онкологических и аутоиммунных заболеваний |
| US9771349B2 (en) * | 2014-09-19 | 2017-09-26 | Forma Therapeutics, Inc. | Pyridinyl quinolinone derivatives as mutant-isocitrate dehydrogenase inhibitors |
| AU2015340215B2 (en) * | 2014-10-29 | 2018-11-15 | Dong-A St Co., Ltd | Novel pyridopyrimidinone compounds for modulating the catalytic activity of histone lysine demethylases (KDMs) |
-
2016
- 2016-10-06 EP EP16854369.2A patent/EP3359154B1/en active Active
- 2016-10-06 JP JP2018518629A patent/JP6853819B2/ja active Active
- 2016-10-06 KR KR1020187013073A patent/KR102698411B1/ko active IP Right Grant
- 2016-10-06 US US15/766,799 patent/US10450300B2/en active Active
-
2019
- 2019-10-15 US US16/653,217 patent/US20200048223A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20180244651A1 (en) | 2018-08-30 |
| JP2018535949A (ja) | 2018-12-06 |
| EP3359154A4 (en) | 2019-05-15 |
| US10450300B2 (en) | 2019-10-22 |
| US20200048223A1 (en) | 2020-02-13 |
| EP3359154B1 (en) | 2021-05-26 |
| KR20180061363A (ko) | 2018-06-07 |
| KR102698411B1 (ko) | 2024-08-26 |
| EP3359154A1 (en) | 2018-08-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2753552C (en) | N- (hetero)aryl, 2- (hetero)aryl-substituted acetamides for use as wnt signaling modulators | |
| CN104379583B (zh) | 作为wnt信号传导抑制剂的化合物、组合物及其应用 | |
| WO2015057963A1 (en) | Fgfr4 inhibitors | |
| JP7025359B2 (ja) | ネクローシス阻害剤であるヘテロアリール化合物、その組成物及び用途 | |
| CN102558173B (zh) | 抑制wnt信号传导的化合物、组合物及其应用 | |
| WO2018017435A1 (en) | Heteroaryl compounds as inhibitors of necrosis, composition and application thereof | |
| JP7033588B2 (ja) | 癌の治療に有用なparp1、parp2、および/またはチューブリンの阻害剤としてのフタラジン誘導体 | |
| KR20160124083A (ko) | Wnt 경로 조절제 | |
| JP6853819B2 (ja) | Wntシグナル伝達経路阻害剤およびその治療用途 | |
| CA3103879A1 (en) | 1,2,4-triazole derivatives as tankyrase inhibitors | |
| WO2017062688A1 (en) | Wnt signaling pathway inhibitors and therapeutic applications thereof | |
| KR20150105302A (ko) | 헤지 호그 신호 경로의 억제제로서 환상 술폰아미드 함유 유도체 | |
| CN114728998A (zh) | 靶向cd73和腺苷受体的磺酰胺化合物 | |
| AU2012203023C1 (en) | N-(hetero)aryl, 2- (hetero)aryl-substituted acetamides for use as Wnt signaling modulators | |
| CN104530042A (zh) | 抑制wnt信号传导的化合物、组合物及其应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191003 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20191003 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20200910 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200929 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201224 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210216 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210312 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6853819 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |