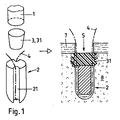
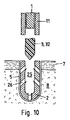
JP6456589B2 - 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 - Google Patents
硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 Download PDFInfo
- Publication number
- JP6456589B2 JP6456589B2 JP2013550721A JP2013550721A JP6456589B2 JP 6456589 B2 JP6456589 B2 JP 6456589B2 JP 2013550721 A JP2013550721 A JP 2013550721A JP 2013550721 A JP2013550721 A JP 2013550721A JP 6456589 B2 JP6456589 B2 JP 6456589B2
- Authority
- JP
- Japan
- Prior art keywords
- suture
- tool
- anchor
- suture anchor
- anchoring
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title claims description 83
- 238000004873 anchoring Methods 0.000 claims description 184
- 229920001169 thermoplastic Polymers 0.000 claims description 81
- 239000004416 thermosoftening plastic Substances 0.000 claims description 77
- 239000012815 thermoplastic material Substances 0.000 claims description 35
- 238000011065 in-situ storage Methods 0.000 claims description 11
- 238000004804 winding Methods 0.000 claims description 6
- 230000014759 maintenance of location Effects 0.000 claims 1
- 210000001519 tissue Anatomy 0.000 description 131
- 239000000463 material Substances 0.000 description 69
- 210000000988 bone and bone Anatomy 0.000 description 49
- 230000008439 repair process Effects 0.000 description 24
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 11
- 230000001054 cortical effect Effects 0.000 description 10
- 230000013011 mating Effects 0.000 description 9
- 229920000642 polymer Polymers 0.000 description 9
- 239000001506 calcium phosphate Substances 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 239000002131 composite material Substances 0.000 description 7
- 210000003041 ligament Anatomy 0.000 description 7
- 230000002093 peripheral effect Effects 0.000 description 7
- 210000002435 tendon Anatomy 0.000 description 7
- 229910000389 calcium phosphate Inorganic materials 0.000 description 6
- 235000011010 calcium phosphates Nutrition 0.000 description 6
- 210000004439 collateral ligament Anatomy 0.000 description 6
- 239000000945 filler Substances 0.000 description 6
- 239000004696 Poly ether ether ketone Substances 0.000 description 5
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 210000002683 foot Anatomy 0.000 description 5
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 5
- 239000007943 implant Substances 0.000 description 5
- 238000007373 indentation Methods 0.000 description 5
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 5
- 229920002530 polyetherether ketone Polymers 0.000 description 5
- 230000000717 retained effect Effects 0.000 description 5
- 230000002739 subcortical effect Effects 0.000 description 5
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- JVTAAEKCZFNVCJ-REOHCLBHSA-N L-lactic acid Chemical compound C[C@H](O)C(O)=O JVTAAEKCZFNVCJ-REOHCLBHSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 208000027418 Wounds and injury Diseases 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 229920006237 degradable polymer Polymers 0.000 description 4
- 229920001432 poly(L-lactide) Polymers 0.000 description 4
- 229920000747 poly(lactic acid) Polymers 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 235000019731 tricalcium phosphate Nutrition 0.000 description 4
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 150000004676 glycans Chemical class 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000010883 osseointegration Methods 0.000 description 3
- 229920002492 poly(sulfone) Polymers 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 239000004632 polycaprolactone Substances 0.000 description 3
- -1 polydioxane (PD) Polymers 0.000 description 3
- 229920006324 polyoxymethylene Polymers 0.000 description 3
- 229920001282 polysaccharide Polymers 0.000 description 3
- 239000005017 polysaccharide Substances 0.000 description 3
- 239000001488 sodium phosphate Substances 0.000 description 3
- 229910000162 sodium phosphate Inorganic materials 0.000 description 3
- 210000004872 soft tissue Anatomy 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 3
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 229920002292 Nylon 6 Polymers 0.000 description 2
- 229920002302 Nylon 6,6 Polymers 0.000 description 2
- 229920001244 Poly(D,L-lactide) Polymers 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 229920000954 Polyglycolide Polymers 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 230000003190 augmentative effect Effects 0.000 description 2
- 230000004323 axial length Effects 0.000 description 2
- 239000011173 biocomposite Substances 0.000 description 2
- 239000012620 biological material Substances 0.000 description 2
- 230000002051 biphasic effect Effects 0.000 description 2
- 239000000316 bone substitute Substances 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 210000004394 hip joint Anatomy 0.000 description 2
- 210000003127 knee Anatomy 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 238000002324 minimally invasive surgery Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- JTIGKVIOEQASGT-UHFFFAOYSA-N proquazone Chemical compound N=1C(=O)N(C(C)C)C2=CC(C)=CC=C2C=1C1=CC=CC=C1 JTIGKVIOEQASGT-UHFFFAOYSA-N 0.000 description 2
- 238000004080 punching Methods 0.000 description 2
- 238000007711 solidification Methods 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 2
- 229940078499 tricalcium phosphate Drugs 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- NSMXQKNUPPXBRG-SECBINFHSA-N (R)-lisofylline Chemical compound O=C1N(CCCC[C@H](O)C)C(=O)N(C)C2=C1N(C)C=N2 NSMXQKNUPPXBRG-SECBINFHSA-N 0.000 description 1
- 229920004943 Delrin® Polymers 0.000 description 1
- 206010061159 Foot deformity Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 208000001963 Hallux Valgus Diseases 0.000 description 1
- 229920000571 Nylon 11 Polymers 0.000 description 1
- 229920000299 Nylon 12 Polymers 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 239000004697 Polyetherimide Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004734 Polyphenylene sulfide Substances 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 208000028484 Urethral disease Diseases 0.000 description 1
- 206010046543 Urinary incontinence Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 210000001361 achilles tendon Anatomy 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 210000001264 anterior cruciate ligament Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000004068 calcium phosphate ceramic Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 210000003109 clavicle Anatomy 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- OEBRKCOSUFCWJD-UHFFFAOYSA-N dichlorvos Chemical compound COP(=O)(OC)OC=C(Cl)Cl OEBRKCOSUFCWJD-UHFFFAOYSA-N 0.000 description 1
- 210000001513 elbow Anatomy 0.000 description 1
- 230000005670 electromagnetic radiation Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 210000001145 finger joint Anatomy 0.000 description 1
- 210000004744 fore-foot Anatomy 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 210000004247 hand Anatomy 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 210000001624 hip Anatomy 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 210000000281 joint capsule Anatomy 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 210000000811 metacarpophalangeal joint Anatomy 0.000 description 1
- 210000001872 metatarsal bone Anatomy 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 210000000452 mid-foot Anatomy 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 210000000426 patellar ligament Anatomy 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 229920001643 poly(ether ketone) Polymers 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001692 polycarbonate urethane Polymers 0.000 description 1
- 239000000622 polydioxanone Substances 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001601 polyetherimide Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920006389 polyphenyl polymer Polymers 0.000 description 1
- 229920000069 polyphenylene sulfide Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 210000000513 rotator cuff Anatomy 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 210000002832 shoulder Anatomy 0.000 description 1
- 210000000323 shoulder joint Anatomy 0.000 description 1
- 210000005070 sphincter Anatomy 0.000 description 1
- 238000009718 spray deposition Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000003356 suture material Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/11—Surgical instruments, devices or methods for performing anastomosis; Buttons for anastomosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0401—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00955—Material properties thermoplastic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0401—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors
- A61B2017/0409—Instruments for applying suture anchors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0401—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors
- A61B2017/0414—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors having a suture-receiving opening, e.g. lateral opening
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0401—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors
- A61B2017/042—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors plastically deformed during insertion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0401—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors
- A61B2017/0438—Suture anchors, buttons or pledgets, i.e. means for attaching sutures to bone, cartilage or soft tissue; Instruments for applying or removing suture anchors slotted, i.e. having a longitudinal slot for enhancing their elasticity
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F04—POSITIVE - DISPLACEMENT MACHINES FOR LIQUIDS; PUMPS FOR LIQUIDS OR ELASTIC FLUIDS
- F04C—ROTARY-PISTON, OR OSCILLATING-PISTON, POSITIVE-DISPLACEMENT MACHINES FOR LIQUIDS; ROTARY-PISTON, OR OSCILLATING-PISTON, POSITIVE-DISPLACEMENT PUMPS
- F04C2270/00—Control; Monitoring or safety arrangements
- F04C2270/04—Force
- F04C2270/042—Force radial
- F04C2270/0421—Controlled or regulated
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Rheumatology (AREA)
- Surgical Instruments (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161437227P | 2011-01-28 | 2011-01-28 | |
| US61/437,227 | 2011-01-28 | ||
| PCT/CH2012/000018 WO2012100359A1 (en) | 2011-01-28 | 2012-01-26 | Method and device for floating a suture anchor with a suture in hard tissue |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016118871A Division JP6370837B2 (ja) | 2011-01-28 | 2016-06-15 | 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 |
| JP2018165963A Division JP2019010531A (ja) | 2011-01-28 | 2018-09-05 | 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2014510555A JP2014510555A (ja) | 2014-05-01 |
| JP2014510555A5 JP2014510555A5 (enExample) | 2015-03-12 |
| JP6456589B2 true JP6456589B2 (ja) | 2019-01-23 |
Family
ID=46584465
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013550721A Expired - Fee Related JP6456589B2 (ja) | 2011-01-28 | 2012-01-26 | 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 |
Country Status (9)
| Country | Link |
|---|---|
| EP (2) | EP2667790B1 (enExample) |
| JP (1) | JP6456589B2 (enExample) |
| KR (1) | KR102022733B1 (enExample) |
| CN (1) | CN103370013B (enExample) |
| BR (1) | BR112013018116B1 (enExample) |
| CA (1) | CA2821362C (enExample) |
| IL (1) | IL227073B (enExample) |
| RU (1) | RU2600664C2 (enExample) |
| WO (1) | WO2012100359A1 (enExample) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9386976B2 (en) * | 2011-01-28 | 2016-07-12 | Sportwelding Gmbh | Method and device for fixating a suture anchor with a suture in hard tissue |
| US8698163B2 (en) * | 2011-09-29 | 2014-04-15 | Toshiba Techno Center Inc. | P-type doping layers for use with light emitting devices |
| US9913637B2 (en) | 2013-03-13 | 2018-03-13 | DePuy Synthes Products, Inc. | Soft tissue fixation system |
| EP2967544B1 (en) * | 2013-03-13 | 2019-04-24 | DePuy Synthes Products, Inc. | Soft tissue fixation system |
| US9901333B2 (en) | 2013-03-13 | 2018-02-27 | DePuy Synthes Products, Inc. | Soft tissue fixation system |
| US10722270B2 (en) * | 2015-09-30 | 2020-07-28 | Spinewelding Ag | System comprising a medical apparatus and a medical device, medical apparatus and surgical method |
| EP3235471A1 (en) * | 2016-04-22 | 2017-10-25 | Kyon AG | An implant system for repair or replacement of tension-carrying connective tissue |
| CN108261228B (zh) * | 2016-12-30 | 2020-02-04 | 江苏风和医疗器材股份有限公司 | 一种穿刺器 |
| US10939937B2 (en) * | 2017-06-29 | 2021-03-09 | Ethicon Llc | Trocar with oblique needle insertion port and perpendicular seal latch |
| US11224465B2 (en) | 2018-12-04 | 2022-01-18 | Spinewelding Ag | Surgical methods for the treatment of spinal stenosis |
| US11311284B2 (en) | 2019-03-06 | 2022-04-26 | Speed Clip Solutions, LLC | Suture tensioning and securement device, system, and methods |
| CN109893189A (zh) * | 2019-04-16 | 2019-06-18 | 江苏尚美医疗器械有限公司 | 一种用于相对硬组织固定缝线的线锚钉固定装置 |
| KR102322486B1 (ko) | 2020-01-28 | 2021-11-05 | 주식회사 바이원 | 올인원 봉합 앵커. |
| DE102020107245A1 (de) * | 2020-03-17 | 2021-09-23 | Karl Storz Se & Co. Kg | Knochenankerelement zum Einbringen in einen Knochen und/oder Fixieren von Gewebe an dem Knochen, Einbringer, Knochenankersystem und Verfahren zum Konfektionieren eines Knochenankersystems |
| CN113208675B (zh) * | 2021-05-24 | 2022-05-10 | 杭州锐健马斯汀医疗器材有限公司 | 一种复合缝线锚植入装置 |
| CN113812992B (zh) * | 2021-10-08 | 2025-09-19 | 北京德益达美医疗科技有限公司 | 一种全缝线锚钉 |
| CN113907818A (zh) * | 2021-11-15 | 2022-01-11 | 北京天助畅运医疗技术股份有限公司 | 一种用于腔镜穿刺缝合器的推钉缝合结构 |
| EP4480456A1 (en) * | 2023-06-20 | 2024-12-25 | Surgical Fusion Technologies GmbH | Implant, instrument and kit for implanting an implant |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5733307A (en) | 1996-09-17 | 1998-03-31 | Amei Technologies, Inc. | Bone anchor having a suture trough |
| US20060161159A1 (en) * | 1999-02-02 | 2006-07-20 | Dreyfuss Peter J | PEEK ribbed suture anchor |
| US6267766B1 (en) * | 1999-05-28 | 2001-07-31 | Stephen S. Burkhart | Suture anchor reel device kit and method |
| US7144414B2 (en) * | 2000-06-27 | 2006-12-05 | Smith & Nephew, Inc. | Surgical procedures and instruments |
| US7335205B2 (en) | 2001-03-02 | 2008-02-26 | Woodwelding Ag | Implants, device and method for joining tissue parts |
| US6508830B2 (en) | 2001-04-30 | 2003-01-21 | Musculoskeletal Transplant Foundation | Suture anchor |
| EP1262152A1 (fr) | 2001-05-25 | 2002-12-04 | Ecole d'ingénieurs | Instruments de façonnage par ultrasons |
| US7008226B2 (en) * | 2002-08-23 | 2006-03-07 | Woodwelding Ag | Implant, in particular a dental implant |
| US7678134B2 (en) | 2003-10-10 | 2010-03-16 | Arthrex, Inc. | Knotless anchor for tissue repair |
| CA2556963A1 (en) | 2004-02-20 | 2005-09-01 | Woodwelding Ag | Implant that can be implanted in osseous tissue, method for producing said implant and corresponding implant |
| AU2005290341B2 (en) * | 2004-09-28 | 2012-01-19 | Surgical Solutions Llc | Suture anchor |
| US20090187216A1 (en) * | 2006-05-18 | 2009-07-23 | Arthrex, Inc. | Fenestrated swivel anchor for knotless fixation of tissue |
| US20090192546A1 (en) | 2005-03-30 | 2009-07-30 | Reinhold Schmieding | Fenestrated suture anchor and method for knotless fixation of tissue |
| GB0524360D0 (en) * | 2005-11-30 | 2006-01-04 | Biocomposites Ltd | Suture anchor |
| US7695495B2 (en) | 2005-12-13 | 2010-04-13 | Arthrex, Inc. | Peek threaded suture anchor |
| US20070260250A1 (en) * | 2006-05-05 | 2007-11-08 | Sdgi Holdings, Inc. | Reinforcement of boney material surrounding a bone implant |
| ES2607224T3 (es) | 2006-09-20 | 2017-03-29 | Woodwelding Ag | Dispositivo para implante en tejido humano o animal y método para implantar y ensamblar el dispositivo |
| WO2008034277A2 (en) | 2006-09-20 | 2008-03-27 | Woodwelding Ag | Implant, implantation device, implantation method |
| EP2465456B1 (en) | 2007-04-20 | 2013-06-19 | Stryker Trauma GmbH | Implantation pin |
| ES2377887T3 (es) | 2007-04-30 | 2012-04-02 | Stryker Trauma Gmbh | Dispositivo para preparar una cavidad simétrica no rotacional en un hueso |
| ES2337510T3 (es) * | 2007-07-13 | 2010-04-26 | Stryker Trauma Gmbh | Pieza manual ultrasonica. |
| EP2205185B1 (en) * | 2007-10-30 | 2014-06-18 | Woodwelding AG | Device for producing an anchorage in human or animal tissue |
| EP2249710B1 (de) * | 2008-03-03 | 2016-04-20 | Woodwelding AG | Vorrichtung zur verankerung eines fadens in gewebe |
| US9226784B2 (en) | 2008-05-01 | 2016-01-05 | Woodwelding Ag | Device and method for establishing an anchorage in tissue |
| ATE531335T1 (de) | 2008-05-21 | 2011-11-15 | Nexilis Ag | Vorrichtung und verfahren zur amelioration von ausnehmungen |
| ES2563286T3 (es) * | 2008-10-23 | 2016-03-14 | Spinewelding Ag | Dispositivo de aumento para el anclaje de objetos en tejido duro |
| EP2221014B1 (en) * | 2009-02-23 | 2015-05-20 | Inion Oy | Implant, implantation tool and kit |
| WO2010127462A1 (en) | 2009-05-06 | 2010-11-11 | Woodwelding Ag | Device for dispensing a material with thermoplastic properties in a flowable state at an operation site in a human or animal patient |
| US9155563B2 (en) * | 2009-11-09 | 2015-10-13 | Spinewelding Ag | Medical device, apparatus, and surgical method |
| ES2853202T3 (es) | 2010-01-27 | 2021-09-15 | Sportwelding Gmbh | Elemento de sujeción adecuado para sujetar un tejido o un elemento protésico correspondiente en una abertura proporcionada en un hueso humano o animal |
-
2012
- 2012-01-26 BR BR112013018116-8A patent/BR112013018116B1/pt not_active IP Right Cessation
- 2012-01-26 JP JP2013550721A patent/JP6456589B2/ja not_active Expired - Fee Related
- 2012-01-26 WO PCT/CH2012/000018 patent/WO2012100359A1/en not_active Ceased
- 2012-01-26 EP EP12705224.9A patent/EP2667790B1/en active Active
- 2012-01-26 KR KR1020137021486A patent/KR102022733B1/ko active Active
- 2012-01-26 CN CN201280006423.3A patent/CN103370013B/zh active Active
- 2012-01-26 CA CA2821362A patent/CA2821362C/en not_active Expired - Fee Related
- 2012-01-26 RU RU2013138864/14A patent/RU2600664C2/ru active
- 2012-01-26 EP EP22153859.8A patent/EP4011302B1/en active Active
-
2013
- 2013-06-20 IL IL227073A patent/IL227073B/en active IP Right Grant
Also Published As
| Publication number | Publication date |
|---|---|
| IL227073B (en) | 2018-04-30 |
| KR102022733B1 (ko) | 2019-09-18 |
| CA2821362C (en) | 2020-06-23 |
| CA2821362A1 (en) | 2012-08-02 |
| JP2014510555A (ja) | 2014-05-01 |
| EP2667790B1 (en) | 2022-03-09 |
| KR20140005968A (ko) | 2014-01-15 |
| WO2012100359A1 (en) | 2012-08-02 |
| EP4011302A1 (en) | 2022-06-15 |
| RU2013138864A (ru) | 2015-03-10 |
| CN103370013A (zh) | 2013-10-23 |
| CN103370013B (zh) | 2016-05-25 |
| BR112013018116A2 (pt) | 2016-11-08 |
| EP2667790A1 (en) | 2013-12-04 |
| BR112013018116B1 (pt) | 2020-12-08 |
| RU2600664C2 (ru) | 2016-10-27 |
| EP4011302B1 (en) | 2024-09-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6370837B2 (ja) | 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 | |
| JP6456589B2 (ja) | 硬組織中に縫合糸付き縫合糸アンカーを固定するための方法および装置 | |
| JP6120776B2 (ja) | 硬組織中に縫合糸付きの縫合糸アンカーまたは頭部付きアンカーを固定するための装置および方法 | |
| KR102058812B1 (ko) | 봉합 앵커를 경조직 내에 고정시키기 위한 장치 및 방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150123 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150123 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20151125 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20151215 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160314 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160415 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160615 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160927 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20170125 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20180502 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180529 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20181003 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20181219 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6456589 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |