JP6124026B2 - 末梢用途のための改良されたスキャフォールド - Google Patents
末梢用途のための改良されたスキャフォールド Download PDFInfo
- Publication number
- JP6124026B2 JP6124026B2 JP2014533524A JP2014533524A JP6124026B2 JP 6124026 B2 JP6124026 B2 JP 6124026B2 JP 2014533524 A JP2014533524 A JP 2014533524A JP 2014533524 A JP2014533524 A JP 2014533524A JP 6124026 B2 JP6124026 B2 JP 6124026B2
- Authority
- JP
- Japan
- Prior art keywords
- scaffold
- axial
- struts
- segments
- segment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000002093 peripheral effect Effects 0.000 title description 12
- 229920000642 polymer Polymers 0.000 claims description 95
- 210000004204 blood vessel Anatomy 0.000 claims description 23
- 239000000835 fiber Substances 0.000 description 62
- 230000006835 compression Effects 0.000 description 37
- 238000007906 compression Methods 0.000 description 37
- 239000000463 material Substances 0.000 description 21
- 239000010410 layer Substances 0.000 description 19
- 238000005452 bending Methods 0.000 description 17
- 238000002788 crimping Methods 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 14
- 230000033001 locomotion Effects 0.000 description 11
- 229920001432 poly(L-lactide) Polymers 0.000 description 11
- 239000002904 solvent Substances 0.000 description 10
- 230000002792 vascular Effects 0.000 description 10
- 239000012528 membrane Substances 0.000 description 9
- 238000000034 method Methods 0.000 description 9
- 239000012503 blood component Substances 0.000 description 8
- 238000001523 electrospinning Methods 0.000 description 8
- 229920005597 polymer membrane Polymers 0.000 description 8
- 239000011248 coating agent Substances 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 239000003814 drug Substances 0.000 description 7
- 229920001610 polycaprolactone Polymers 0.000 description 7
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 6
- 229920002988 biodegradable polymer Polymers 0.000 description 6
- 239000004621 biodegradable polymer Substances 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 230000008859 change Effects 0.000 description 6
- 230000006378 damage Effects 0.000 description 6
- 239000012530 fluid Substances 0.000 description 6
- 238000002513 implantation Methods 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 206010061218 Inflammation Diseases 0.000 description 5
- 208000031481 Pathologic Constriction Diseases 0.000 description 5
- 208000027418 Wounds and injury Diseases 0.000 description 5
- 239000000853 adhesive Substances 0.000 description 5
- 230000001070 adhesive effect Effects 0.000 description 5
- 210000004351 coronary vessel Anatomy 0.000 description 5
- 230000006837 decompression Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 210000002216 heart Anatomy 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 5
- 238000003825 pressing Methods 0.000 description 5
- 238000007634 remodeling Methods 0.000 description 5
- 208000037803 restenosis Diseases 0.000 description 5
- 208000037804 stenosis Diseases 0.000 description 5
- 230000036262 stenosis Effects 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 210000001367 artery Anatomy 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 229910003460 diamond Inorganic materials 0.000 description 4
- 239000010432 diamond Substances 0.000 description 4
- 210000001105 femoral artery Anatomy 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 4
- 239000004632 polycaprolactone Substances 0.000 description 4
- 239000000622 polydioxanone Substances 0.000 description 4
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 4
- 230000000541 pulsatile effect Effects 0.000 description 4
- 229920006126 semicrystalline polymer Polymers 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 238000002399 angioplasty Methods 0.000 description 3
- 238000010009 beating Methods 0.000 description 3
- 229920001400 block copolymer Polymers 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 230000036760 body temperature Effects 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 230000005684 electric field Effects 0.000 description 3
- 229920005570 flexible polymer Polymers 0.000 description 3
- 238000003698 laser cutting Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 230000000737 periodic effect Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 229920005604 random copolymer Polymers 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- ZRGYUQUHZCEOFH-UHFFFAOYSA-N 1,4-dioxocane-2,3-dione Chemical compound O=C1OCCCCOC1=O ZRGYUQUHZCEOFH-UHFFFAOYSA-N 0.000 description 2
- JJTUDXZGHPGLLC-IMJSIDKUSA-N 4511-42-6 Chemical compound C[C@@H]1OC(=O)[C@H](C)OC1=O JJTUDXZGHPGLLC-IMJSIDKUSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 229920001244 Poly(D,L-lactide) Polymers 0.000 description 2
- 229920000954 Polyglycolide Polymers 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000033115 angiogenesis Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 238000001815 biotherapy Methods 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 208000029078 coronary artery disease Diseases 0.000 description 2
- 238000005336 cracking Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- ZMXDDKWLCZADIW-UHFFFAOYSA-N dimethylformamide Substances CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 210000003090 iliac artery Anatomy 0.000 description 2
- 230000001788 irregular Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- -1 poly (4-hydroxybutylene) Polymers 0.000 description 2
- 229920005594 polymer fiber Polymers 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000013557 residual solvent Substances 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- BYEAHWXPCBROCE-UHFFFAOYSA-N 1,1,1,3,3,3-hexafluoropropan-2-ol Chemical compound FC(F)(F)C(O)C(F)(F)F BYEAHWXPCBROCE-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 1
- 200000000007 Arterial disease Diseases 0.000 description 1
- 206010051113 Arterial restenosis Diseases 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 208000018262 Peripheral vascular disease Diseases 0.000 description 1
- 208000035868 Vascular inflammations Diseases 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229920005603 alternating copolymer Polymers 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- 210000000617 arm Anatomy 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000004323 axial length Effects 0.000 description 1
- 210000000013 bile duct Anatomy 0.000 description 1
- 239000012867 bioactive agent Substances 0.000 description 1
- 229920013641 bioerodible polymer Polymers 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000003486 chemical etching Methods 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 229920006237 degradable polymer Polymers 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000002683 foot Anatomy 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007257 malfunction Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 208000030613 peripheral artery disease Diseases 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 239000006069 physical mixture Substances 0.000 description 1
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 1
- 229920000071 poly(4-hydroxybutyrate) Polymers 0.000 description 1
- 229920001072 poly(l-lactide-co-caprolactone) Polymers 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 239000013047 polymeric layer Substances 0.000 description 1
- 210000003137 popliteal artery Anatomy 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 210000002254 renal artery Anatomy 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 230000002966 stenotic effect Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 210000003270 subclavian artery Anatomy 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/89—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure the wire-like elements comprising two or more adjacent rings flexibly connected by separate members
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2002/825—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents having longitudinal struts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2002/828—Means for connecting a plurality of stents allowing flexibility of the whole structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A61F2002/91558—Adjacent bands being connected to each other connected peak to peak
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A61F2002/91575—Adjacent bands being connected to each other connected peak to trough
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2220/005—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements using adhesives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0013—Horseshoe-shaped, e.g. crescent-shaped, C-shaped, U-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0054—V-shaped
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Veterinary Medicine (AREA)
- Vascular Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Description
この明細書中で言及される全ての刊行物および特許出願は、あたかもそれぞれの個々の刊行物または特許出願が参照によって本明細書に組み入れられるように具体的に個別に示されたかのようにかつあたかも個々の刊行物または特許出願がその中の任意の図を含めて十分に記載されたかのように同じ程度まで参照することにより本明細書に組み入れられる。
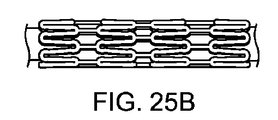
図24Aは、切断されたままの形態の図6Aのパターンと同様のパターンを有する軸方向スキャフォールドセグメントを描く。短いリンクストラットは0.010インチである。図24Bは、クリンプ状態の軸方向スキャフォールドセグメントを描く。セグメントは均一にクリンプすることが分かった。
クリンピングプロセスは以下の3つのステップを含んだ。
1.セグメントを所定長のバルーン上に2mmを超える間隔で配置する。
2.ほぼ完全なクリンプ状態までクリンプする。
3.セグメントを1mmの間隔に設定した後に完全にクリンプする。
Claims (8)
- 端部同士を対向させて配置されて径方向に拡張可能な2つ以上の軸方向スキャフォールドセグメントを備え、
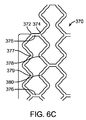
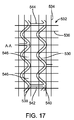
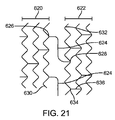
前記軸方向スキャフォールドセグメントのそれぞれが、2つ以上の円筒リングを含み、前記円筒リングが、波状を成す複数のリングストラットから構成されており、前記複数のリングストラットが、複数の山部および複数の谷部を形成しており、
同一の前記軸方向スキャフォールドセグメントにおいて隣り合う円筒リングが、隣り合うリング対を形成する前記隣り合う円筒リングを接続する1つ以上のリンクストラットを備え、
軸方向スキャフォールドセグメントが、高分子チューブから形成され、
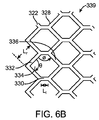
隣り合うリング対のそれぞれについて、前記円筒リングのうちの一方の円筒リングの前記谷部が、他方の円筒リングの前記山部と位置合わせされ、位置合わせされた山部および谷部が2つおきにだけリンクストラットによって接続され、
前記1つ以上のリンクストラットの長さが、1つの前記リングストラットの長さの20%よりも大きく30%以下である、
スキャフォールド。 - 前記軸方向スキャフォールドセグメントが送達バルーン上に配置される、請求項1に記載のスキャフォールド。
- 前記軸方向スキャフォールドセグメントが血管内で展開可能である、請求項1に記載のスキャフォールド。
- 隣り合う軸方向スキャフォールドセグメントの前記端部同士が、前記軸方向スキャフォールドセグメントにおける前記円筒リング間の距離だけ少なくとも離間される、請求項1に記載のスキャフォールド。
- 隣り合う軸方向スキャフォールドセグメントの前記端部同士が、前記リンクストラットの長さの2倍よりも短い距離を隔てて離間される、請求項1に記載のスキャフォールド。
- 軸方向スキャフォールドセグメントのそれぞれが2つまたは3つの円筒リングを含む、請求項1に記載のスキャフォールド。
- 前記軸方向スキャフォールドセグメント同士が前記1つ以上のリンクストラットによって接続されていない、請求項2に記載のスキャフォールド。
- 前記軸方向スキャフォールドセグメントが前記送達バルーンにクリンプされる、請求項7に記載のスキャフォールド。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/252,121 US20130085564A1 (en) | 2011-10-03 | 2011-10-03 | Modified scaffolds for peripheral applications |
| US13/252,121 | 2011-10-03 | ||
| PCT/US2012/043497 WO2013052184A1 (en) | 2011-10-03 | 2012-06-21 | Modified scaffolds for peripheral applications |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2014531275A JP2014531275A (ja) | 2014-11-27 |
| JP2014531275A5 JP2014531275A5 (ja) | 2015-07-23 |
| JP6124026B2 true JP6124026B2 (ja) | 2017-05-10 |
Family
ID=46466893
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014533524A Active JP6124026B2 (ja) | 2011-10-03 | 2012-06-21 | 末梢用途のための改良されたスキャフォールド |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US20130085564A1 (ja) |
| EP (1) | EP2763630B1 (ja) |
| JP (1) | JP6124026B2 (ja) |
| CN (1) | CN103857363B (ja) |
| ES (1) | ES2579609T3 (ja) |
| HK (1) | HK1195239A1 (ja) |
| WO (1) | WO2013052184A1 (ja) |
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8814930B2 (en) | 2007-01-19 | 2014-08-26 | Elixir Medical Corporation | Biodegradable endoprosthesis and methods for their fabrication |
| US8303644B2 (en) * | 2007-05-04 | 2012-11-06 | Abbott Cardiovascular Systems Inc. | Stents with high radial strength and methods of manufacturing same |
| CA2743803C (en) | 2008-11-24 | 2016-12-13 | The Medical Research, Infrastructure, And Health Services Fund Of The Tel Aviv Medical Center | External stent |
| WO2012143925A1 (en) | 2011-04-18 | 2012-10-26 | Vascular Graft Solutions Ltd | Devices and methods for deploying implantable sleeves over blood vessels |
| US9956097B2 (en) | 2012-10-23 | 2018-05-01 | Abbott Cardiovascular Systems Inc. | Methods for vascular restoration therapy |
| EP3060178A4 (en) * | 2013-10-22 | 2017-06-14 | OrbusNeich Medical, Inc. | Medical device for implantation into luminal structures incorporating corrugated structural elements |
| US9480588B2 (en) | 2014-08-15 | 2016-11-01 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9730819B2 (en) | 2014-08-15 | 2017-08-15 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9855156B2 (en) | 2014-08-15 | 2018-01-02 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| WO2016081709A1 (en) * | 2014-11-20 | 2016-05-26 | Boston Scientific Scimed, Inc. | Tracheal implant |
| CN104644295B (zh) * | 2014-12-19 | 2019-07-16 | 上海百心安生物技术有限公司 | 一种可吸收管腔支架及其制备方法 |
| WO2016104128A1 (ja) * | 2014-12-26 | 2016-06-30 | テルモ株式会社 | ステント |
| US9999527B2 (en) * | 2015-02-11 | 2018-06-19 | Abbott Cardiovascular Systems Inc. | Scaffolds having radiopaque markers |
| US11622872B2 (en) | 2016-05-16 | 2023-04-11 | Elixir Medical Corporation | Uncaging stent |
| EP3861961A1 (en) | 2016-05-16 | 2021-08-11 | Elixir Medical Corporation | Uncaging stent |
| JP6960685B2 (ja) * | 2016-10-07 | 2021-11-05 | エフェモラル メディカル インコーポレイテッド | 半径方向に剛性を及び長手方向に可撓性を有する多要素血管内ステント |
| AU2018280236B2 (en) | 2017-06-07 | 2024-06-06 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| EP3710076B1 (en) | 2017-11-13 | 2023-12-27 | Shifamed Holdings, LLC | Intravascular fluid movement devices, systems, and methods of use |
| CN107811734B (zh) * | 2017-11-30 | 2024-03-22 | 苏州恒瑞迪生医疗科技有限公司 | 一种血管内支架及其制备方法 |
| JP7410034B2 (ja) | 2018-02-01 | 2024-01-09 | シファメド・ホールディングス・エルエルシー | 血管内血液ポンプならびに使用および製造の方法 |
| WO2019165311A1 (en) * | 2018-02-23 | 2019-08-29 | Efemoral Medical Llc | Absorbable intravascular devices for the treatment of venous occlusive disease |
| CN108852571A (zh) * | 2018-07-13 | 2018-11-23 | 四川兴泰普乐医疗科技有限公司 | 一种自膨胀式生物可降解聚合物支架及其制备方法 |
| CN110151357A (zh) * | 2019-06-27 | 2019-08-23 | 深圳市创心医疗科技有限公司 | 支架系统及血管支架 |
| EP3996797A4 (en) | 2019-07-12 | 2023-08-02 | Shifamed Holdings, LLC | INTRAVASCULAR BLOOD PUMPS AND METHOD OF USE AND METHOD OF MAKING |
| US11654275B2 (en) | 2019-07-22 | 2023-05-23 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| EP4034184A4 (en) | 2019-09-25 | 2023-10-18 | Shifamed Holdings, LLC | CATHETER BLOOD PUMP AND COLLAPSIBLE BLOOD LINES |
| EP4034192A4 (en) | 2019-09-25 | 2023-11-29 | Shifamed Holdings, LLC | INTRAVASCULAR BLOOD PUMP SYSTEMS AND METHODS OF USE AND CONTROL THEREOF |
| EP4034221B1 (en) | 2019-09-25 | 2024-11-13 | Shifamed Holdings, LLC | Catheter blood pumps and collapsible pump housings |
| CN114615956A (zh) * | 2019-10-11 | 2022-06-10 | 埃夫莫拉尔医疗有限公司 | 随时间降低血管的径向刚性的可吸收血管内装置 |
| CN113367866A (zh) * | 2021-06-23 | 2021-09-10 | 北京航空航天大学 | 一种髂静脉支架 |
| CN118615055A (zh) * | 2024-08-12 | 2024-09-10 | 苏州华岐医疗科技有限公司 | 便于回收的前列腺支架、回收组件及其回收方法 |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998020810A1 (en) * | 1996-11-12 | 1998-05-22 | Medtronic, Inc. | Flexible, radially expansible luminal prostheses |
| US6558415B2 (en) * | 1998-03-27 | 2003-05-06 | Intratherapeutics, Inc. | Stent |
| US6264687B1 (en) * | 1998-04-20 | 2001-07-24 | Cordis Corporation | Multi-laminate stent having superelastic articulated sections |
| US20060122691A1 (en) * | 1998-12-03 | 2006-06-08 | Jacob Richter | Hybrid stent |
| US6187034B1 (en) * | 1999-01-13 | 2001-02-13 | John J. Frantzen | Segmented stent for flexible stent delivery system |
| US6325825B1 (en) * | 1999-04-08 | 2001-12-04 | Cordis Corporation | Stent with variable wall thickness |
| CN2453962Y (zh) * | 2000-12-08 | 2001-10-17 | 杨大智 | 一种镂制正弦波管网式冠状动脉支架 |
| KR20030094286A (ko) * | 2001-03-20 | 2003-12-11 | 지엠피 카르디악 케어, 인크. | 레일 스텐트 |
| US20030135266A1 (en) * | 2001-12-03 | 2003-07-17 | Xtent, Inc. | Apparatus and methods for delivery of multiple distributed stents |
| GB0206061D0 (en) * | 2002-03-14 | 2002-04-24 | Angiomed Ag | Metal structure compatible with MRI imaging, and method of manufacturing such a structure |
| EP1567221A1 (en) * | 2002-11-15 | 2005-08-31 | GMP Cardiac Care, Inc. | Rail stent |
| US7316710B1 (en) * | 2002-12-30 | 2008-01-08 | Advanced Cardiovascular Systems, Inc. | Flexible stent |
| US7479158B2 (en) * | 2004-02-20 | 2009-01-20 | Boston Scientific Scimed, Inc. | Stent with nested flexible connectors for flexibility and crimpability |
| CN2768714Y (zh) * | 2004-11-22 | 2006-04-05 | 微创医疗器械(上海)有限公司 | 一种柔软的血管支架 |
| WO2008030488A2 (en) * | 2006-09-06 | 2008-03-13 | Med Institute, Inc. | Stents with connectors and stabilizing biodegradable elements |
| FR2911063B1 (fr) * | 2007-01-09 | 2009-03-20 | Stentys S A S Soc Par Actions | Structure de pont ruptible pour un stent, et stent incluant de telles structures de pont. |
| EP1958597A1 (de) * | 2007-02-16 | 2008-08-20 | Universität Zürich | Rohrförmige Stützprothese mit Herzklappe insbesondere für Aortenklappenersatz |
| US8133268B2 (en) * | 2007-05-30 | 2012-03-13 | Cordis Corporation | Stent/fiber structural combinations |
| US20090076584A1 (en) * | 2007-09-19 | 2009-03-19 | Xtent, Inc. | Apparatus and methods for deployment of multiple custom-length prostheses |
| US8926688B2 (en) * | 2008-01-11 | 2015-01-06 | W. L. Gore & Assoc. Inc. | Stent having adjacent elements connected by flexible webs |
| CA2714775A1 (en) * | 2008-02-15 | 2009-08-20 | Joseph Michael Thielen | Peripheral overlap stent |
| US20100042202A1 (en) * | 2008-08-13 | 2010-02-18 | Kamal Ramzipoor | Composite stent having multi-axial flexibility |
| US8425587B2 (en) | 2009-09-17 | 2013-04-23 | Abbott Cardiovascular Systems Inc. | Method of treatment with a bioabsorbable stent with time dependent structure and properties and regio-selective degradation |
| US8808353B2 (en) | 2010-01-30 | 2014-08-19 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds having a low crossing profile |
-
2011
- 2011-10-03 US US13/252,121 patent/US20130085564A1/en not_active Abandoned
-
2012
- 2012-06-21 JP JP2014533524A patent/JP6124026B2/ja active Active
- 2012-06-21 WO PCT/US2012/043497 patent/WO2013052184A1/en active Application Filing
- 2012-06-21 ES ES12733319.3T patent/ES2579609T3/es active Active
- 2012-06-21 CN CN201280048531.7A patent/CN103857363B/zh active Active
- 2012-06-21 EP EP12733319.3A patent/EP2763630B1/en not_active Not-in-force
-
2014
- 2014-04-16 US US14/254,812 patent/US20140228930A1/en not_active Abandoned
- 2014-09-01 HK HK14108857.9A patent/HK1195239A1/zh not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| ES2579609T3 (es) | 2016-08-12 |
| US20130085564A1 (en) | 2013-04-04 |
| WO2013052184A1 (en) | 2013-04-11 |
| US20140228930A1 (en) | 2014-08-14 |
| JP2014531275A (ja) | 2014-11-27 |
| CN103857363B (zh) | 2017-10-24 |
| CN103857363A (zh) | 2014-06-11 |
| EP2763630B1 (en) | 2016-04-06 |
| HK1195239A1 (zh) | 2014-11-07 |
| EP2763630A1 (en) | 2014-08-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6124026B2 (ja) | 末梢用途のための改良されたスキャフォールド | |
| US20230093376A1 (en) | Stent | |
| JP6064030B2 (ja) | セグメント化スキャフォールドおよび抹消血管への適用のための送達 | |
| JP5933558B2 (ja) | 連結を壊すように設計された生体吸収性の浅大腿動脈用ステントのパターン | |
| US8961585B2 (en) | Controlled fracture connections for stents | |
| JP2023134672A (ja) | 離脱式ステント | |
| JP2015529102A (ja) | セグメント化スキャフォールドの構造 | |
| US10524941B2 (en) | Device with tensioners | |
| US10722389B2 (en) | Endoluminal stent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150603 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150603 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160426 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160726 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160901 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170307 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170321 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6124026 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |