JP5964540B2 - Mvaを使ってがんを処置する方法 - Google Patents
Mvaを使ってがんを処置する方法 Download PDFInfo
- Publication number
- JP5964540B2 JP5964540B2 JP2009531472A JP2009531472A JP5964540B2 JP 5964540 B2 JP5964540 B2 JP 5964540B2 JP 2009531472 A JP2009531472 A JP 2009531472A JP 2009531472 A JP2009531472 A JP 2009531472A JP 5964540 B2 JP5964540 B2 JP 5964540B2
- Authority
- JP
- Japan
- Prior art keywords
- mva
- taxane
- mher2
- antigen
- mice
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 206010028980 Neoplasm Diseases 0.000 title claims description 112
- 201000011510 cancer Diseases 0.000 title claims description 37
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 133
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 123
- 239000000427 antigen Substances 0.000 claims description 96
- 108091007433 antigens Proteins 0.000 claims description 95
- 102000036639 antigens Human genes 0.000 claims description 95
- 238000011282 treatment Methods 0.000 claims description 77
- 229940123237 Taxane Drugs 0.000 claims description 66
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 claims description 61
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 claims description 43
- 229960003668 docetaxel Drugs 0.000 claims description 42
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 40
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 34
- 108090000623 proteins and genes Proteins 0.000 claims description 34
- 229920001184 polypeptide Polymers 0.000 claims description 32
- 230000002476 tumorcidal effect Effects 0.000 claims description 32
- 239000008194 pharmaceutical composition Substances 0.000 claims description 13
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 12
- 238000004113 cell culture Methods 0.000 claims description 12
- 241000700618 Vaccinia virus Species 0.000 claims description 9
- 206010046865 Vaccinia virus infection Diseases 0.000 claims description 5
- 208000007089 vaccinia Diseases 0.000 claims description 5
- 238000002648 combination therapy Methods 0.000 claims description 3
- 230000002147 killing effect Effects 0.000 claims description 3
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 claims 1
- 101710098119 Chaperonin GroEL 2 Proteins 0.000 claims 1
- 206010072219 Mevalonic aciduria Diseases 0.000 description 172
- 241000699670 Mus sp. Species 0.000 description 106
- 210000004027 cell Anatomy 0.000 description 59
- 229960005486 vaccine Drugs 0.000 description 29
- 241000700605 Viruses Species 0.000 description 27
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 26
- 230000004614 tumor growth Effects 0.000 description 26
- 230000028993 immune response Effects 0.000 description 25
- 150000001413 amino acids Chemical class 0.000 description 24
- 239000003656 tris buffered saline Substances 0.000 description 24
- 102000004169 proteins and genes Human genes 0.000 description 22
- 235000001014 amino acid Nutrition 0.000 description 21
- 235000018102 proteins Nutrition 0.000 description 20
- 241001465754 Metazoa Species 0.000 description 19
- 230000000694 effects Effects 0.000 description 17
- 210000004881 tumor cell Anatomy 0.000 description 16
- 210000004072 lung Anatomy 0.000 description 15
- 210000001744 T-lymphocyte Anatomy 0.000 description 14
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 14
- 239000002953 phosphate buffered saline Substances 0.000 description 14
- 230000000259 anti-tumor effect Effects 0.000 description 13
- 230000005875 antibody response Effects 0.000 description 13
- 230000006698 induction Effects 0.000 description 13
- 108060006698 EGF receptor Proteins 0.000 description 12
- 102000005962 receptors Human genes 0.000 description 12
- 108020003175 receptors Proteins 0.000 description 12
- 230000010076 replication Effects 0.000 description 12
- -1 April Proteins 0.000 description 11
- 108010055044 Tetanus Toxin Proteins 0.000 description 11
- 229940127089 cytotoxic agent Drugs 0.000 description 11
- 239000013612 plasmid Substances 0.000 description 11
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 229940118376 tetanus toxin Drugs 0.000 description 10
- 206010006187 Breast cancer Diseases 0.000 description 9
- 208000026310 Breast neoplasm Diseases 0.000 description 9
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 238000002255 vaccination Methods 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 8
- 238000002512 chemotherapy Methods 0.000 description 8
- 108010048367 enhanced green fluorescent protein Proteins 0.000 description 8
- 102000051957 human ERBB2 Human genes 0.000 description 8
- 230000004044 response Effects 0.000 description 8
- 238000002965 ELISA Methods 0.000 description 7
- 108091054438 MHC class II family Proteins 0.000 description 7
- 239000002246 antineoplastic agent Substances 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 230000014509 gene expression Effects 0.000 description 7
- 230000012010 growth Effects 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 238000000034 method Methods 0.000 description 7
- 229930012538 Paclitaxel Natural products 0.000 description 6
- 241000700159 Rattus Species 0.000 description 6
- 230000003321 amplification Effects 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 238000009169 immunotherapy Methods 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 238000003199 nucleic acid amplification method Methods 0.000 description 6
- 229960001592 paclitaxel Drugs 0.000 description 6
- 229920000136 polysorbate Polymers 0.000 description 6
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 6
- 230000005909 tumor killing Effects 0.000 description 6
- 230000003612 virological effect Effects 0.000 description 6
- 102000001301 EGF receptor Human genes 0.000 description 5
- 238000012286 ELISA Assay Methods 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- 102000043131 MHC class II family Human genes 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 238000011284 combination treatment Methods 0.000 description 5
- 210000005260 human cell Anatomy 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 230000006798 recombination Effects 0.000 description 5
- 238000005215 recombination Methods 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 230000009261 transgenic effect Effects 0.000 description 5
- 102100034540 Adenomatous polyposis coli protein Human genes 0.000 description 4
- 101000924577 Homo sapiens Adenomatous polyposis coli protein Proteins 0.000 description 4
- 206010061598 Immunodeficiency Diseases 0.000 description 4
- 108091054437 MHC class I family Proteins 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- 241001183012 Modified Vaccinia Ankara virus Species 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 206010061535 Ovarian neoplasm Diseases 0.000 description 4
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 4
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 230000033289 adaptive immune response Effects 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 230000002238 attenuated effect Effects 0.000 description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 4
- 239000002254 cytotoxic agent Substances 0.000 description 4
- 231100000599 cytotoxic agent Toxicity 0.000 description 4
- 210000002950 fibroblast Anatomy 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 108020001507 fusion proteins Proteins 0.000 description 4
- 102000037865 fusion proteins Human genes 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 230000002163 immunogen Effects 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 230000015788 innate immune response Effects 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 239000006166 lysate Substances 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 230000001681 protective effect Effects 0.000 description 4
- 238000011510 Elispot assay Methods 0.000 description 3
- 241000287828 Gallus gallus Species 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 3
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 3
- 102000043129 MHC class I family Human genes 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 206010033128 Ovarian cancer Diseases 0.000 description 3
- 208000004210 Pressure Ulcer Diseases 0.000 description 3
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 238000002619 cancer immunotherapy Methods 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 3
- 206010017758 gastric cancer Diseases 0.000 description 3
- 102000054766 genetic haplotypes Human genes 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 230000003053 immunization Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000011081 inoculation Methods 0.000 description 3
- 210000003292 kidney cell Anatomy 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 210000001161 mammalian embryo Anatomy 0.000 description 3
- 230000009401 metastasis Effects 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 210000004988 splenocyte Anatomy 0.000 description 3
- 230000002269 spontaneous effect Effects 0.000 description 3
- 201000011549 stomach cancer Diseases 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 238000011830 transgenic mouse model Methods 0.000 description 3
- 239000013603 viral vector Substances 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- 206010001197 Adenocarcinoma of the cervix Diseases 0.000 description 2
- 208000034246 Adenocarcinoma of the cervix uteri Diseases 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 241000271566 Aves Species 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 101710132601 Capsid protein Proteins 0.000 description 2
- 108090000538 Caspase-8 Proteins 0.000 description 2
- 102000056372 ErbB-3 Receptor Human genes 0.000 description 2
- 102400001302 Gasdermin-B, N-terminal Human genes 0.000 description 2
- 102400000921 Gastrin Human genes 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 102000006354 HLA-DR Antigens Human genes 0.000 description 2
- 108010058597 HLA-DR Antigens Proteins 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 108091029795 Intergenic region Proteins 0.000 description 2
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 2
- 102100022430 Melanocyte protein PMEL Human genes 0.000 description 2
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 2
- 230000004988 N-glycosylation Effects 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 101710189818 Non-structural protein 2a Proteins 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 108700026244 Open Reading Frames Proteins 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- 101710151911 Phosphoprotein p30 Proteins 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 2
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 108010008038 Synthetic Vaccines Proteins 0.000 description 2
- 230000029662 T-helper 1 type immune response Effects 0.000 description 2
- 108700019146 Transgenes Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 2
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 239000013592 cell lysate Substances 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 201000006662 cervical adenocarcinoma Diseases 0.000 description 2
- 239000003593 chromogenic compound Substances 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000000185 hemagglutinin Substances 0.000 description 2
- 230000006801 homologous recombination Effects 0.000 description 2
- 238000002744 homologous recombination Methods 0.000 description 2
- 230000002519 immonomodulatory effect Effects 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 230000002998 immunogenetic effect Effects 0.000 description 2
- 230000005847 immunogenicity Effects 0.000 description 2
- 230000006054 immunological memory Effects 0.000 description 2
- 239000002955 immunomodulating agent Substances 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 108020001756 ligand binding domains Proteins 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 230000015654 memory Effects 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 150000007523 nucleic acids Chemical group 0.000 description 2
- 201000008968 osteosarcoma Diseases 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 238000010837 poor prognosis Methods 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 229940124551 recombinant vaccine Drugs 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000011421 subcutaneous treatment Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 239000011534 wash buffer Substances 0.000 description 2
- 238000009736 wetting Methods 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- LKKMLIBUAXYLOY-UHFFFAOYSA-N 3-Amino-1-methyl-5H-pyrido[4,3-b]indole Chemical compound N1C2=CC=CC=C2C2=C1C=C(N)N=C2C LKKMLIBUAXYLOY-UHFFFAOYSA-N 0.000 description 1
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 description 1
- 102100030310 5,6-dihydroxyindole-2-carboxylic acid oxidase Human genes 0.000 description 1
- 101710163881 5,6-dihydroxyindole-2-carboxylic acid oxidase Proteins 0.000 description 1
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 1
- 102100033350 ATP-dependent translocase ABCB1 Human genes 0.000 description 1
- 101100347635 Acanthamoeba castellanii MIC gene Proteins 0.000 description 1
- 102100021305 Acyl-CoA:lysophosphatidylglycerol acyltransferase 1 Human genes 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 102100023635 Alpha-fetoprotein Human genes 0.000 description 1
- 102000052587 Anaphase-Promoting Complex-Cyclosome Apc3 Subunit Human genes 0.000 description 1
- 108700004606 Anaphase-Promoting Complex-Cyclosome Apc3 Subunit Proteins 0.000 description 1
- 102100035526 B melanoma antigen 1 Human genes 0.000 description 1
- 102100038080 B-cell receptor CD22 Human genes 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 101000653197 Beet necrotic yellow vein virus (isolate Japan/S) Movement protein TGB3 Proteins 0.000 description 1
- 102000015735 Beta-catenin Human genes 0.000 description 1
- 108060000903 Beta-catenin Proteins 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 102100022002 CD59 glycoprotein Human genes 0.000 description 1
- 101150108242 CDC27 gene Proteins 0.000 description 1
- MUJJVOYNTCTXIC-UHFFFAOYSA-N CNC(=O)c1ccc2-c3c(C)c(nn3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F Chemical compound CNC(=O)c1ccc2-c3c(C)c(nn3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F MUJJVOYNTCTXIC-UHFFFAOYSA-N 0.000 description 1
- 101150071146 COX2 gene Proteins 0.000 description 1
- 101100114534 Caenorhabditis elegans ctc-2 gene Proteins 0.000 description 1
- 102100025570 Cancer/testis antigen 1 Human genes 0.000 description 1
- 101100507655 Canis lupus familiaris HSPA1 gene Proteins 0.000 description 1
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 description 1
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 1
- 208000009458 Carcinoma in Situ Diseases 0.000 description 1
- 102000004091 Caspase-8 Human genes 0.000 description 1
- 102100026548 Caspase-8 Human genes 0.000 description 1
- 102000005600 Cathepsins Human genes 0.000 description 1
- 108010084457 Cathepsins Proteins 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 241000282552 Chlorocebus aethiops Species 0.000 description 1
- 108010066551 Cholestenone 5 alpha-Reductase Proteins 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102100025680 Complement decay-accelerating factor Human genes 0.000 description 1
- 102100030886 Complement receptor type 1 Human genes 0.000 description 1
- 102100032768 Complement receptor type 2 Human genes 0.000 description 1
- 241000700626 Cowpox virus Species 0.000 description 1
- 102100037364 Craniofacial development protein 1 Human genes 0.000 description 1
- 208000037845 Cutaneous squamous cell carcinoma Diseases 0.000 description 1
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 1
- 102000009508 Cyclin-Dependent Kinase Inhibitor p16 Human genes 0.000 description 1
- 108010009392 Cyclin-Dependent Kinase Inhibitor p16 Proteins 0.000 description 1
- 102100036252 Cyclin-dependent kinase 4 Human genes 0.000 description 1
- 102100024462 Cyclin-dependent kinase 4 inhibitor B Human genes 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 206010011985 Decubitus ulcer Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 101100216227 Dictyostelium discoideum anapc3 gene Proteins 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 101100118548 Drosophila melanogaster Egfr gene Proteins 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 101150049307 EEF1A2 gene Proteins 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 101710147220 Ent-copalyl diphosphate synthase, chloroplastic Proteins 0.000 description 1
- 102400000102 Eosinophil granule major basic protein Human genes 0.000 description 1
- 102100031940 Epithelial cell adhesion molecule Human genes 0.000 description 1
- 102000007317 Farnesyltranstransferase Human genes 0.000 description 1
- 108010007508 Farnesyltranstransferase Proteins 0.000 description 1
- 108091072337 GAGE family Proteins 0.000 description 1
- 102000040452 GAGE family Human genes 0.000 description 1
- 101710113436 GTPase KRas Proteins 0.000 description 1
- 102100039788 GTPase NRas Human genes 0.000 description 1
- 108010052343 Gastrins Proteins 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- OYRVWOGRRQDEQH-MLVLNPCWSA-N Gln-Tyr-Ile-Lys-Ala-Asn-Ser-Lys-Phe-Ile-Gly-Ile-Thr-Glu-Leu Chemical compound C([C@@H](C(=O)N[C@@H](C(C)CC)C(=O)NCC(=O)N[C@@H](C(C)CC)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@@H](N)CCC(N)=O)C(C)CC)C1=CC=CC=C1 OYRVWOGRRQDEQH-MLVLNPCWSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102000005720 Glutathione transferase Human genes 0.000 description 1
- 108010070675 Glutathione transferase Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- NMJREATYWWNIKX-UHFFFAOYSA-N GnRH Chemical compound C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CC(C)C)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 NMJREATYWWNIKX-UHFFFAOYSA-N 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 208000012766 Growth delay Diseases 0.000 description 1
- 108010065192 HER2p63 peptide Proteins 0.000 description 1
- 108010062347 HLA-DQ Antigens Proteins 0.000 description 1
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 1
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 1
- 101001042227 Homo sapiens Acyl-CoA:lysophosphatidylglycerol acyltransferase 1 Proteins 0.000 description 1
- 101000874316 Homo sapiens B melanoma antigen 1 Proteins 0.000 description 1
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101100165850 Homo sapiens CA9 gene Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000897400 Homo sapiens CD59 glycoprotein Proteins 0.000 description 1
- 101000856237 Homo sapiens Cancer/testis antigen 1 Proteins 0.000 description 1
- 101000914324 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 5 Proteins 0.000 description 1
- 101000914321 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 7 Proteins 0.000 description 1
- 101000856022 Homo sapiens Complement decay-accelerating factor Proteins 0.000 description 1
- 101000727061 Homo sapiens Complement receptor type 1 Proteins 0.000 description 1
- 101000941929 Homo sapiens Complement receptor type 2 Proteins 0.000 description 1
- 101000980919 Homo sapiens Cyclin-dependent kinase 4 inhibitor B Proteins 0.000 description 1
- 101100066427 Homo sapiens FCGR1A gene Proteins 0.000 description 1
- 101000744505 Homo sapiens GTPase NRas Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101001014223 Homo sapiens MAPK/MAK/MRK overlapping kinase Proteins 0.000 description 1
- 101000620359 Homo sapiens Melanocyte protein PMEL Proteins 0.000 description 1
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 1
- 101000961414 Homo sapiens Membrane cofactor protein Proteins 0.000 description 1
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 description 1
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 1
- 101000721712 Homo sapiens NTF2-related export protein 1 Proteins 0.000 description 1
- 101000693231 Homo sapiens PDZK1-interacting protein 1 Proteins 0.000 description 1
- 101000617725 Homo sapiens Pregnancy-specific beta-1-glycoprotein 2 Proteins 0.000 description 1
- 101001062222 Homo sapiens Receptor-binding cancer antigen expressed on SiSo cells Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000934341 Homo sapiens T-cell surface glycoprotein CD5 Proteins 0.000 description 1
- 101000626112 Homo sapiens Telomerase protein component 1 Proteins 0.000 description 1
- 101000597785 Homo sapiens Tumor necrosis factor receptor superfamily member 6B Proteins 0.000 description 1
- 101000818517 Homo sapiens Zinc-alpha-2-glycoprotein Proteins 0.000 description 1
- 241000681881 Human mammary tumor virus Species 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 101800000324 Immunoglobulin A1 protease translocator Proteins 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102100020793 Interleukin-13 receptor subunit alpha-2 Human genes 0.000 description 1
- 102100031413 L-dopachrome tautomerase Human genes 0.000 description 1
- 101710093778 L-dopachrome tautomerase Proteins 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 101000758645 Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) UPF0178 protein Lmo1456 Proteins 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 102100031520 MAPK/MAK/MRK overlapping kinase Human genes 0.000 description 1
- 108010010995 MART-1 Antigen Proteins 0.000 description 1
- 239000007993 MOPS buffer Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000051089 Melanotransferrin Human genes 0.000 description 1
- 108700038051 Melanotransferrin Proteins 0.000 description 1
- 108010047230 Member 1 Subfamily B ATP Binding Cassette Transporter Proteins 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- 102100039373 Membrane cofactor protein Human genes 0.000 description 1
- 102000003735 Mesothelin Human genes 0.000 description 1
- 108090000015 Mesothelin Proteins 0.000 description 1
- 102000036436 Metzincins Human genes 0.000 description 1
- 108091007161 Metzincins Proteins 0.000 description 1
- 241000713333 Mouse mammary tumor virus Species 0.000 description 1
- 102100023123 Mucin-16 Human genes 0.000 description 1
- 108010063954 Mucins Proteins 0.000 description 1
- 102000015728 Mucins Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101001062862 Mus musculus Fatty acid-binding protein, adipocyte Proteins 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 241000204031 Mycoplasma Species 0.000 description 1
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 102100029438 Nitric oxide synthase, inducible Human genes 0.000 description 1
- 101710089543 Nitric oxide synthase, inducible Proteins 0.000 description 1
- 241000700629 Orthopoxvirus Species 0.000 description 1
- 108010077077 Osteonectin Proteins 0.000 description 1
- 102000009890 Osteonectin Human genes 0.000 description 1
- 102100025648 PDZK1-interacting protein 1 Human genes 0.000 description 1
- 108060006580 PRAME Proteins 0.000 description 1
- 102000036673 PRAME Human genes 0.000 description 1
- 101150000187 PTGS2 gene Proteins 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 101000653209 Peanut clump virus (isolate 87/TGTA2) Movement protein TGB3 Proteins 0.000 description 1
- 101000621505 Peanut clump virus (isolate 87/TGTA2) Suppressor of RNA silencing Proteins 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 101800004191 Peptide P2 Proteins 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 description 1
- 102100039418 Plasminogen activator inhibitor 1 Human genes 0.000 description 1
- 241000224016 Plasmodium Species 0.000 description 1
- 101000726057 Plasmodium falciparum Circumsporozoite protein Proteins 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 241000700625 Poxviridae Species 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 102100029165 Receptor-binding cancer antigen expressed on SiSo cells Human genes 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 108091077753 SSX family Proteins 0.000 description 1
- 102000042330 SSX family Human genes 0.000 description 1
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 1
- 101710173693 Short transient receptor potential channel 1 Proteins 0.000 description 1
- 101710173694 Short transient receptor potential channel 2 Proteins 0.000 description 1
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 1
- 101000637859 Spinacia oleracea Thylakoid lumenal 17 kDa protein Proteins 0.000 description 1
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 102100025244 T-cell surface glycoprotein CD5 Human genes 0.000 description 1
- 101710109927 Tail assembly protein GT Proteins 0.000 description 1
- 102100024553 Telomerase protein component 1 Human genes 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 1
- LVTKHGUGBGNBPL-UHFFFAOYSA-N Trp-P-1 Chemical compound N1C2=CC=CC=C2C2=C1C(C)=C(N)N=C2C LVTKHGUGBGNBPL-UHFFFAOYSA-N 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102100035284 Tumor necrosis factor receptor superfamily member 6B Human genes 0.000 description 1
- 102000003425 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- 102100027244 U4/U6.U5 tri-snRNP-associated protein 1 Human genes 0.000 description 1
- 101710155955 U4/U6.U5 tri-snRNP-associated protein 1 Proteins 0.000 description 1
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 108010027570 Xanthine phosphoribosyltransferase Proteins 0.000 description 1
- 102100021144 Zinc-alpha-2-glycoprotein Human genes 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 1
- 201000008274 breast adenocarcinoma Diseases 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 238000009566 cancer vaccine Methods 0.000 description 1
- 229940022399 cancer vaccine Drugs 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- AOXOCDRNSPFDPE-UKEONUMOSA-N chembl413654 Chemical compound C([C@H](C(=O)NCC(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)CNC(=O)[C@@H](N)CCC(O)=O)C1=CC=C(O)C=C1 AOXOCDRNSPFDPE-UKEONUMOSA-N 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 210000003837 chick embryo Anatomy 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000009827 complement-dependent cellular cytotoxicity Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011443 conventional therapy Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- 108010048134 estramustine-binding protein Proteins 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 108020005243 folate receptor Proteins 0.000 description 1
- 102000006815 folate receptor Human genes 0.000 description 1
- 239000003517 fume Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 108010066264 gastrin 17 Proteins 0.000 description 1
- GKDWRERMBNGKCZ-RNXBIMIWSA-N gastrin-17 Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)CNC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 GKDWRERMBNGKCZ-RNXBIMIWSA-N 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000005745 host immune response Effects 0.000 description 1
- 229940098197 human immunoglobulin g Drugs 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 230000008348 humoral response Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 230000007365 immunoregulation Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 201000004933 in situ carcinoma Diseases 0.000 description 1
- 210000003000 inclusion body Anatomy 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 108040003607 interleukin-13 receptor activity proteins Proteins 0.000 description 1
- 244000000056 intracellular parasite Species 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 210000005075 mammary gland Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000005621 mannosylation reaction Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000008722 morphological abnormality Effects 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 238000007857 nested PCR Methods 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 230000000242 pagocytic effect Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 230000008884 pinocytosis Effects 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 108010042121 probasin Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 108010014186 ras Proteins Proteins 0.000 description 1
- 102000016914 ras Proteins Human genes 0.000 description 1
- 239000003488 releasing hormone Substances 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 230000001850 reproductive effect Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 201000010106 skin squamous cell carcinoma Diseases 0.000 description 1
- 235000017550 sodium carbonate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000011895 specific detection Methods 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 101150050955 stn gene Proteins 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000000126 substance Chemical class 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 101150047061 tag-72 gene Proteins 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 230000029069 type 2 immune response Effects 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 230000001018 virulence Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/337—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having four-membered rings, e.g. taxol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001103—Receptors for growth factors
- A61K39/001106—Her-2/neu/ErbB2, Her-3/ErbB3 or Her 4/ErbB4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55544—Bacterial toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/24011—Poxviridae
- C12N2710/24111—Orthopoxvirus, e.g. vaccinia virus, variola
- C12N2710/24141—Use of virus, viral particle or viral elements as a vector
- C12N2710/24143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2830/00—Vector systems having a special element relevant for transcription
- C12N2830/60—Vector systems having a special element relevant for transcription from viruses
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
Description
一定の実施形態では、細胞関連ポリペプチド抗原に対する免疫応答を、対象内で生じさせる。そのような実施形態のいくつかでは、細胞関連ポリペプチド抗原が腫瘍関連抗原である。
一定の実施形態において、細胞関連ポリペプチド抗原は、APCの表面にMHCクラスI分子と会合して提示された場合に、ポリペプチド抗原に由来するエピトープをその表面に提示する細胞に対するCTL応答が誘導されるように修飾される。そのような実施形態のいくつかでは、少なくとも一つの第1外来性THエピトープが、提示される際に、APCの表面でMHCクラスII分子と会合する。そのような実施形態のいくつかでは、細胞関連抗原が腫瘍関連抗原である。
さまざまな修飾HER-2ポリペプチド抗原と、それを製造するための方法が、参照により本明細書に組み入れられる米国特許第7,005,498号ならびに米国特許出願公開第2004/0141958号および同第2006/0008465号に記載されている。これらの文書には、HER-2ポリペプチド中の異なる位置にプロミスカスT細胞エピトープを含むさまざまな修飾HER-2ポリペプチド抗原が記載されている。
1.既知および予想CTLエピトープ;
2.関連受容体(特にEGFR)に対する相同性;
3.システイン残基の保存;
4.予想されるループ、α-ヘリックスおよびβ-シート構造;
5.潜在的N-糖鎖付加部位;
6.露出および埋没アミノ酸残基の予想;
7.ドメイン構成。
限定でない一実施形態では、腫瘍関連抗原を含む組換えMVA、例えばMVA-BN-mHER2が、以下のように構築される。複製を許容する細胞タイプ、例えばCEF細胞を使って、細胞培養での組換えによって、最初のウイルスストックを作製する。細胞に、弱毒化ワクシニアウイルス、例えばMVA-BNを接種すると共に、腫瘍関連抗原(例えばmHER2)配列とウイルスゲノムの隣接領域とをコードする組換えプラスミド(例えばpBN146)をトランスフェクトする。限定でない一実施形態では、プラスミドpBN146が、MVA-BN中にも存在する配列(14Lおよび15Lオープンリーディングフレーム)を含有する。mHER2配列は、MVA-BNウイルスゲノムへの組換えが可能になるように、そのMVA-BN配列の間に挿入される。一定の実施形態では、プラスミドが、CEF細胞における組換えコンストラクトの選択が可能なように、1つまたはそれ以上の選択遺伝子を含む選択カセットも含有する。好ましい一実施形態では、組換えMVAが配列番号2を含むポリペプチドをコードする。
細胞毒性剤は、ワクチン効力にとって有益であるだろう免疫調整活性を、サブ殺腫瘍量で示す。しかし、殺腫瘍量(高用量)では、これらの薬剤はワクチン活性にとって有害になりうる。MVA-BN-mHER2処置の過程でマウスに投与されたヒト等価殺腫瘍量のドセタキセルは、ワクチンが誘導する抗HER-2抗体価に影響を及ぼさないことが、ここに証明された。そのうえ、MVA-BN-mHER2によるマウスの処置は、インビボでドセタキセルに対する腫瘍の感受性を増加させた。したがって、MVA-BN-mHER2処置と同時に、またはMVA-BN-mHER2処置に先だって、またはMVA-BN-mHER2処置の後に行われる化学療法は、どちらか一方の処置だけよりも優れている可能性がある。
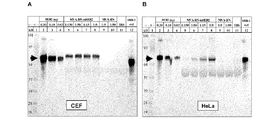
MVA-BN-mHER2の構築および感染細胞におけるタンパク質発現の解析
培養物の同時の感染およびトランスフェクションにより、ウイルスゲノムと組換えプラスミドの間で、相同組換えを起こさせた。インサートを保持するウイルスを単離し、特徴づけ、ウイルスストックを調製した。
ATIプロモーター−mHER2配列−Psプロモーター−gpt−IRES−EGFP。このインサート領域が、細菌組換えプラスミドpBN146中のMVA-BN I4L遺伝子間領域配列(F1およびF2)で挟まれた。このコンストラクトのヌクレオチド配列を以下に示す。
HER2開始および停止コドンを太字で示す。隣接配列を斜体で示す。
p2およびp30配列の破傷風毒素エピトープを太字で示す。
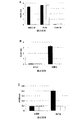
MVA-BN-mHER2で処置されたマウスにおける抗HER-2免疫応答の誘導
MVA-BN-mHER2による処置後に起こる抗HER-2免疫応答の誘導を、免疫学的バックグラウンドまたはハプロタイプが異なる2つのマウス系統BALB/cおよびC57BL/6マウスの両方で評価した。MVA-BN-mHER2による処置後に起こる抗HER-2免疫応答の誘導を、トランスジェニックHER-2マウス系統BALB/c NeuTマウスでも評価した。これらの研究では、2E6〜5E7 TCID50の範囲にわたるさまざまな用量のMVA-BN-mHER2を、以下に詳述するように評価した。以下に述べるように、各処置の前日ならびに処置中および処置後のさまざまな時点で、血液試料を収集した。体液性応答(抗HER-2 IgGの産生)をELISAアッセイによって解析した。最終処置後に脾細胞を集め、細胞性応答をELISpotで解析した。これらの研究を実施例3で説明する。
BALB/c、C57BL/6、およびBALB/c NeuTマウスに、対照溶液(トリス緩衝食塩水(TBS))、または2E6、1E7、もしくは5E7 TCID50のMVA-BN-mHER2を、1日目、15日目および29日目に皮下注射した。これらの試験処置群はそれぞれ5匹とした。0日目、14日目、28日目、42日目および56日目に、血液試料を集めた。各試験群の5匹の動物のそれぞれから得た血清をプールし、ELISAアッセイを使って抗HER-2 IgGの存在について分析した。
MVA-BN-mHER2処置マウスの血清を、上述の手法を使って、同様にELISAで評価した。HER-2、HER-3、およびHER-4 ecd-Fcキメラタンパク質を、微量滴定プレートのウェルにコーティングされる抗原として使用した。モノクローナル抗HER-2抗体(HER-2 Ab;AB-5、Calbiochem)、モノクローナル抗ヒトIg Fcフラグメント抗体(Fc Ab;Southern Biotech)、またはモノクローナルアイソタイプ対照抗体(Contr Ab)を使って抗原を検出した。結果を図3に示す。図3AはELISA対照を示し、モノクローナル抗HER-2抗体がHER-2 ecd-Fc被覆ウェルだけと特異的に反応するのに対して、モノクローナル抗ヒトIg Fcフラグメント抗体は3つのキメラタンパク質の全てと反応する。図3Bおよび3Cは、MVA-BN-mHER2で処置されたC57BL/6マウスとBALB/cマウスのどちらにおいても、血清がHER-2 ecd-Fcキメラだけを検出したことを示している。これらのデータは、どちらのマウス系統でも、MVA-BN-mHER2処置後に誘導される抗体応答は、HER-2に対して高度に特異的であり、上皮成長因子受容体ファミリーの他の一定のメンバー、例えばHER-3およびHER-4とは、交差反応しないことを示している。
抗HER-2 T細胞応答の誘導
BALB/cマウスおよびC57BL/6マウス(各群5匹)に、対照(TBS)または1E7 TCID50のMVA-BN-mHER2を、1日目、15日目、29日目、および43日目に皮下注射した。48日目に動物から脾臓を収集し、各試験群から得た細胞懸濁液を分析用にプールした。インビトロ抗原特異的再刺激後のIFNγ産生を測定するELISpotアッセイによって、T細胞応答の誘導を評価した。再刺激には、HER-2 ecd、MHCクラスI HER-2ペプチド、およびmHER2配列に含まれる破傷風毒素由来の2つのMHCクラスII Tヘルパーペプチドを、個別に使用した。クラスI HER-2ペプチドはアミノ酸配列TYLPTNASL(配列番号3)を持った。MHCクラスII Tヘルパー破傷風毒素ペプチドP2はアミノ酸配列QYIKANSKFIGITEL(配列番号4)(図4ではTTp2と表記)を持ち、MHCクラスII Tヘルパー破傷風毒素ペプチドP30はアミノ酸配列FNNFTVSFWLRVPKVSASHLE(配列番号5)(図4ではTTp30と表記)を持った。
MVA-BN-mHER2処置マウスにおけるTh1免疫調整
上記実施例のデータは、MVA-BNが、強い免疫原性をも示す効率のよい導入遺伝子送達ビヒクルであることを示している。MVAが、痘瘡からの防御を付与するTh1適応免疫応答をトリガーし(Earlら, 2004;Wyattら, 2004)、先天免疫応答も誘導すること(Brutkiewiczら, 1992;Dokunら, 2001)は、以前に報告されている。したがって、MVA-BNの固有の免疫特性は、導入遺伝子に対する免疫応答を調整するのに、潜在的に役立ちうる。
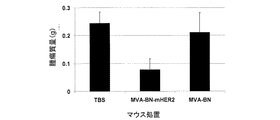
MVA-BN-mHER2で処置されたマウスにおける抗腫瘍活性
予防的処置
予防的状況において腫瘍成長を防止するというMVA-BN-mHER2の能力を、マウスにおける乳がんモデルとして、移植されたTUBO細胞を使って評価した。TUBO細胞は、トランスフォーミングラットHER-2(HER-2/neu)がん遺伝子が導入されたトランスジェニックBALB/cマウスで発生した乳腺癌に由来する(Roveroら, J. Immunol. 165, 5133-5142(2000))。HER-2配列はラットとヒトの間で高度に保存されているので、HER-2のラットまたはヒトホモログのどちらか一方を含むワクチンの効力を評価するために、TUBO細胞は日常的に使用される(Dela Cruzら, Vaccine 23, 4793-4803 (2005))。
定着腫瘍を抑制するMVA-BN-mHER2の能力を、ヒトHER-2を安定して発現させるCT26細胞を用いる実験的肺転移モデルで評価した。CT26は、BALB/cマウスの化学的に誘導された結腸直腸癌である(Brattainら, 1980)。このモデルでは、CT26-HER-2細胞がBALB/cマウスに静脈内注射され、腫瘍小結節が成長する肺における腫瘍量が評価される。
先天免疫をトリガーすることによってMVA-BN-mHER2の抗腫瘍活性に寄与するMVA-BNの能力を、上述のCT26腫瘍モデルで評価した。この実験では、腫瘍チャレンジの日に(この時点で腫瘍量は少ない)、MVA-BN(5E6もしくは5E7 TCID50)またはMVA-BN-mHER2(5E6もしくは5E7 TCID50)のどちらか一方で、マウスを処置した。チャレンジされたマウスの肺において、上述のように腫瘍量を評価した。結果を図7に示す。これらの結果は、MVA-BN(5E7 TCID50)を使った処置による腫瘍成長阻害(TGI)が>70%であったこと(p<0.0001)を示している。MVA-BN(5E6 TCID50)による処置は5E7 TCID50による処置ほど効率がよくなかった(32%TGI;p=0.002)ので、MVA-BNの抗腫瘍活性は用量依存的だった。対照的に、MVA-BN-mHER2(5E6または5E7 TCID50のどちらか一方)で処置したマウスは、類似する防御を示した(>70%TGI;p<0.000001)。
細胞毒性剤との併用療法
C57BL/6マウスに、対照(トリス緩衝食塩水(TBS);5匹のI群)または5E7 TCID50のMVA-BN-mHER2(5匹ずつの9群)による皮下処置を、1日目、22日目および43日目(3週毎×3)に行った。化学療法剤ドセタキセルが抗HER-2抗体誘導に及ぼす影響を、MVA-BN-mHER2処置の1週間前(-7日目)または2日前(-2日目)に殺腫瘍量(33mg/Kg)の薬物で動物を処置することによって評価した。薬物を、1回、2回(3週毎×2)、3回(3週毎×3)または4回(3週毎×4)、iv注射した。動物群の配置、投与レジメンおよびスケジュールを、表2に要約する。
エピトープ/抗原拡大(epitope/antigen spreading)
エピトープ/抗原拡大は、瀕死の腫瘍細胞からのエピトープ/抗原の露出によってトリガーされる免疫応答の誘導によってもたらされる。ワクチンが誘導するエピトープ/抗原拡大は、最大限の抗腫瘍活性にとって著しく有利である。HER-2+腫瘍に対して保護されたマウスは、HER-2を発現させない親腫瘍による2回目のチャレンジに抵抗するので、MVA-BN-mHER2処置はエピトープ/抗原拡大をもたらすことが見出された。したがって、MVA-BN-mHER2は、HER-2以外の腫瘍抗原にも拡大することができる幅広い防御免疫応答のトリガリング(これは、不均一な腫瘍を処置し、腫瘍エスケープを防止するための前提条件である)を可能にする。
NeuTマウスにおける自然発生腫瘍
異種HER-2(例えばヒトHER-2)で処置されたラットHER-2/neuを発現させるトランスジェニックマウス(NeuTマウス)において生じる自然発生腫瘍を遅延させるには、高力価かつ広スペクトルの抗体が要求される。裸のDNAのような異種HER-2のワクチン製剤はこのモデルにおける腫瘍成長を遅延させることができなかったが、ウイルスに基づく製剤は抗腫瘍活性を示した。MVA-BN-mHER2は、処置を腫瘍発生の後期に開始した場合でさえ、NeuTにおける自然発生腫瘍成長を遅延させることが見出された。したがってMVA-BNは抗腫瘍活性を誘導するための優れた抗原製剤を与える。
Claims (19)
- 殺腫瘍量のタキサンとの併用療法のための、HER−2抗原を含むポリペプチドをコードする遺伝子を含む組換え改変ワクシニアアンカラ(MVA)を含有し、タキサンがドセタキセルである、ヒトがん患者を処置するための医薬組成物。
- MVAが、European Collection of Cell Cultures(ECACC)に番号V00083008として寄託された改変ワクシニアアンカラバーバリアンノルディック(MVA−BN)である、請求項1の医薬組成物。
- HER−2抗原を含む前記ポリペプチドが配列番号2を含む、請求項2の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンに先だって投与されるように用いられることを特徴とする、請求項1の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンと同時に投与されるように用いられることを特徴とする、請求項1の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサン後に投与されるように用いられることを特徴とする、請求項1の医薬組成物。
- タキサンが用量75〜100mg/m 2 である、請求項1〜5のいずれか一つの医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンの1〜26週間前に投与されるように用いられることを特徴とする、請求項4の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンの1〜3週間前に投与されるように用いられることを特徴とする、請求項4の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンの2〜60日後に投与されるように用いられることを特徴とする、請求項6の医薬組成物。
- 組換えMVAが殺腫瘍量のタキサンの2〜7日後に投与されるように用いられることを特徴とする、請求項6の医薬組成物。
- HER−2抗原を含むポリペプチドをコードする遺伝子を含む組換えMVAと殺腫瘍量のタキサンとを含有し、タキサンがドセタキセルである、ヒトがん患者を処置するための医薬組成物。
- (a)HER−2抗原を含むポリペプチドをコードする遺伝子を含む組換えMVA;および
(b)組換えMVAを殺腫瘍量のタキサンによる処置に先だって投与するようにという指示書類を含み、
タキサンがドセタキセルである、がん患者を処置するためのキット。 - (a)HER−2抗原を含むポリペプチドをコードする遺伝子を含む組換えMVA;および
(b)組換えMVAを殺腫瘍量のタキサンによる処置と同時に投与するようにという指示書類を含み、
タキサンがドセタキセルである、がん患者を処置するためのキット。 - (a)HER−2抗原を含むポリペプチドをコードする遺伝子を含む組換えMVA;および
(b)組換えMVAを殺腫瘍量のタキサンによる処置後に投与するようにという指示書類を含み、
タキサンがドセタキセルである、がん患者を処置するためのキット。 - HER−2抗原を含む前記ポリペプチドが配列番号2を含む、請求項13のキット。
- HER−2抗原を含む前記ポリペプチドが配列番号2を含む、請求項14のキット。
- HER−2抗原を含む前記ポリペプチドが配列番号2を含む、請求項15のキット。
- HER−2抗原を含むポリペプチドをコードする遺伝子を含み、HER−2抗原を含むポリペプチドが配列番号2を含む、組換えワクシニアウイルス。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US85003106P | 2006-10-06 | 2006-10-06 | |
| US60/850,031 | 2006-10-06 | ||
| PCT/US2007/021436 WO2008045346A2 (en) | 2006-10-06 | 2007-10-05 | Recombinant modified vaccinia ankara encoding a her-2 antigen for use in treating cancer |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014047741A Division JP6124822B2 (ja) | 2006-10-06 | 2014-03-11 | Mvaを使ってがんを処置する方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010505850A JP2010505850A (ja) | 2010-02-25 |
| JP2010505850A5 JP2010505850A5 (ja) | 2010-08-19 |
| JP5964540B2 true JP5964540B2 (ja) | 2016-08-03 |
Family
ID=39283388
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009531472A Active JP5964540B2 (ja) | 2006-10-06 | 2007-10-05 | Mvaを使ってがんを処置する方法 |
| JP2014047741A Active JP6124822B2 (ja) | 2006-10-06 | 2014-03-11 | Mvaを使ってがんを処置する方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014047741A Active JP6124822B2 (ja) | 2006-10-06 | 2014-03-11 | Mvaを使ってがんを処置する方法 |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US7807146B2 (ja) |
| EP (2) | EP2073837B1 (ja) |
| JP (2) | JP5964540B2 (ja) |
| AU (1) | AU2007307080B2 (ja) |
| CA (1) | CA2665068C (ja) |
| DK (2) | DK2596801T3 (ja) |
| ES (1) | ES2500465T3 (ja) |
| IL (1) | IL197633A (ja) |
| NZ (2) | NZ597998A (ja) |
| PT (1) | PT2073837E (ja) |
| WO (1) | WO2008045346A2 (ja) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002042480A2 (en) * | 2000-11-23 | 2002-05-30 | Bavarian Nordic A/S | Modified vaccinia ankara virus variant |
| US7807146B2 (en) * | 2006-10-06 | 2010-10-05 | Bn Immunotherapeutics, Inc. | Methods for treating cancer with a recombinant MVA expressing HER-2 |
| WO2010036652A1 (en) * | 2008-09-23 | 2010-04-01 | Thomas Jefferson University | Cancer vaccines against mucosal antigens and methods of making and using the same |
| MY168733A (en) * | 2010-11-02 | 2018-11-29 | Ericsson Telefon Ab L M | Methods and devices for media description delivery |
| US9659706B2 (en) | 2011-09-22 | 2017-05-23 | The Trustees Of Dartmouth College | Methods for making radially anisotropic thin-film magnetic torroidal cores |
| US9463238B2 (en) | 2011-12-09 | 2016-10-11 | Bavarian Nordic A/S | Recombinant poxvirus vector comprising tetanus toxin fragment C |
| EP2788021B1 (en) | 2011-12-09 | 2017-01-18 | Bavarian Nordic A/S | Poxvirus vector for the expression of bacterial antigens linked to tetanus toxin fragment c |
| US20150283220A1 (en) * | 2012-10-19 | 2015-10-08 | Bavarian Nordic, Inc. | Methods and compositions for the treatment of cancer |
| EP2777711A1 (en) * | 2013-03-11 | 2014-09-17 | Icon Genetics GmbH | Her2/Neu cancer vaccine |
| CN105379295A (zh) | 2013-07-03 | 2016-03-02 | 皇家Kpn公司 | 分段内容的流送 |
| EP3060232B1 (en) | 2013-10-23 | 2018-07-04 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Hla-a24 agonist epitopes of muc1-c oncoprotein and compositions and methods of use |
| EP3261669B1 (en) | 2015-02-25 | 2022-08-03 | Memorial Sloan Kettering Cancer Center | Use of inactivated nonreplicating modified vaccinia virus ankara (mva)as monoimmunotherapy or in combination with immune checkpoint blocking agents for solid tumors |
| CN116173193A (zh) | 2015-04-17 | 2023-05-30 | 纪念斯隆凯特琳癌症中心 | Mva或mvaδe3l作为抗实体瘤的免疫治疗剂的应用 |
| FR3042121A1 (fr) | 2015-10-08 | 2017-04-14 | Jean-Marc Limacher | Composition anti-tumorale |
| BR112018016948A2 (pt) | 2016-02-25 | 2019-01-08 | Memorial Sloan Kettering Cancer Center | mva recombinante ou mva¿e3l que expressa flt3l humano e uso do mesmo como agente imunoterapêutico contra tumores sólidos |
| CA3015650A1 (en) | 2016-02-25 | 2017-08-31 | Memorial Sloan Kettering Cancer Center | Replication competent attenuated vaccinia viruses with deletion of thymidine kinase with and without the expression of human flt3l or gm-csf for cancer immunotherapy |
| CN111107872A (zh) | 2017-05-12 | 2020-05-05 | 纪念斯隆-凯特林癌症中心 | 有用于癌症免疫疗法的牛痘病毒突变体 |
| KR102658198B1 (ko) | 2017-05-15 | 2024-04-16 | 얀센 백신스 앤드 프리벤션 비.브이. | 안정한 바이러스 함유 조성물 |
| CN110603058A (zh) | 2017-05-15 | 2019-12-20 | 扬森疫苗与预防公司 | 含有病毒的稳定组合物 |
| WO2019090343A1 (en) * | 2017-11-06 | 2019-05-09 | Memorial Sloan Kettering Cancer Center | Heat-inactivated vaccinia virus as a vaccine immune adjuvant |
| CN113573729A (zh) | 2019-01-10 | 2021-10-29 | 詹森生物科技公司 | 前列腺新抗原及其用途 |
| IL293051A (en) | 2019-11-18 | 2022-07-01 | Janssen Biotech Inc | calr and jak2 mutant-based vaccines and their uses |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1341245C (en) | 1988-01-12 | 2001-06-05 | F. Hoffmann-La Roche Ag | Recombinant vaccinia virus mva |
| DK96493D0 (da) | 1993-08-26 | 1993-08-26 | Mouritsen Og Elsner Aps | Fremgangsmaade til at inducere antistofresponser mod selvproteiner og autovaccine fremstillet ved fremgangsmaaden |
| JP3926839B2 (ja) | 1993-09-14 | 2007-06-06 | エピミューン,インコーポレイティド | 万能dr−結合性ペプチドを用いる免疫応答の改変 |
| US6326356B1 (en) * | 1996-10-18 | 2001-12-04 | Board Of Regents, The University Of Texas System | Suppression of neu overexpression using a mini-E1A gene |
| UA68327C2 (en) | 1995-07-04 | 2004-08-16 | Gsf Forschungszentrum Fur Unwe | A recombinant mva virus, an isolated eukaryotic cell, infected with recombinant mva virus, a method for production in vitro of polypeptides with use of said cell, a method for production in vitro of virus parts (variants), vaccine containing the recombinant mva virus, a method for immunization of animals |
| AUPO390396A0 (en) | 1996-11-29 | 1996-12-19 | Csl Limited | Novel promiscuous T helper cell epitopes |
| DE19729279A1 (de) | 1997-07-09 | 1999-01-14 | Peter Hildebrandt | Urologisches Implantat, insbesondere Gefäßwandstütze für den Urinaltrakt |
| HUP0103976A3 (en) | 1998-10-05 | 2008-04-28 | Pharmexa As | Novel methods for therapeutic vaccination |
| DE10042598A1 (de) * | 2000-08-30 | 2002-03-28 | Gsf Forschungszentrum Umwelt | Rekombinantes MVA mit der Fähigkeit zur Expression des HER-2/Neu-GENS |
| WO2002042480A2 (en) | 2000-11-23 | 2002-05-30 | Bavarian Nordic A/S | Modified vaccinia ankara virus variant |
| WO2003026581A2 (en) * | 2001-09-26 | 2003-04-03 | Intermune, Inc. | Pharmaceutical compositions and methods for treating cancer |
| DE60325502D1 (de) * | 2002-11-06 | 2009-02-05 | Cyclacel Ltd | Kombination aus docetaxel und einem cdk-hemmer |
| US8663622B2 (en) * | 2002-12-16 | 2014-03-04 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Recombinant vaccine viruses expressing IL-15 and methods using the same |
| CN1511549A (zh) * | 2002-12-27 | 2004-07-14 | 张小丽 | 含有黄芩的抗肿瘤、抗炎症及肿瘤预防药物的组合物 |
| ITMI20030317A1 (it) * | 2003-02-21 | 2004-08-22 | Pharmacia Italia Spa | Terapia combinata comprendente un derivato dell'indolopirrolocarbazolo ed un altro agente antitumorale. |
| US20040197312A1 (en) * | 2003-04-02 | 2004-10-07 | Marina Moskalenko | Cytokine-expressing cellular vaccine combinations |
| CA2526212C (en) * | 2003-05-16 | 2013-08-27 | Hybridon, Inc. | Synergistic treatment of cancer using immunomers in conjunction with chemotherapeutic agents |
| KR20060033870A (ko) | 2003-06-25 | 2006-04-20 | 파멕사 에이/에스 | Her-2 변이체의 정제 |
| DK1641819T3 (da) * | 2003-06-25 | 2009-08-03 | Bn Immunotherapeutics Inc | Oprensning af HER-2-varianter |
| US7951780B2 (en) * | 2004-02-25 | 2011-05-31 | Astellas Pharma Inc. | Antitumor agent |
| NZ556409A (en) * | 2005-02-23 | 2010-09-30 | Bavarian Nordic As | Use of a modified poxvirus for the rapid induction of immunity against a poxvirus or other infectious agents |
| US7807146B2 (en) | 2006-10-06 | 2010-10-05 | Bn Immunotherapeutics, Inc. | Methods for treating cancer with a recombinant MVA expressing HER-2 |
-
2007
- 2007-10-05 US US11/905,876 patent/US7807146B2/en active Active
- 2007-10-05 EP EP07839307.1A patent/EP2073837B1/en active Active
- 2007-10-05 CA CA2665068A patent/CA2665068C/en active Active
- 2007-10-05 DK DK13154196.3T patent/DK2596801T3/en active
- 2007-10-05 WO PCT/US2007/021436 patent/WO2008045346A2/en active Application Filing
- 2007-10-05 ES ES07839307.1T patent/ES2500465T3/es active Active
- 2007-10-05 JP JP2009531472A patent/JP5964540B2/ja active Active
- 2007-10-05 NZ NZ597998A patent/NZ597998A/xx unknown
- 2007-10-05 NZ NZ575388A patent/NZ575388A/en unknown
- 2007-10-05 DK DK07839307.1T patent/DK2073837T3/da active
- 2007-10-05 PT PT78393071T patent/PT2073837E/pt unknown
- 2007-10-05 AU AU2007307080A patent/AU2007307080B2/en active Active
- 2007-10-05 EP EP13154196.3A patent/EP2596801B1/en active Active
-
2009
- 2009-03-17 IL IL197633A patent/IL197633A/en active IP Right Grant
-
2010
- 2010-08-31 US US12/872,156 patent/US8313740B2/en active Active
-
2014
- 2014-03-11 JP JP2014047741A patent/JP6124822B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| EP2596801A1 (en) | 2013-05-29 |
| IL197633A (en) | 2017-03-30 |
| JP2014129416A (ja) | 2014-07-10 |
| PT2073837E (pt) | 2014-09-22 |
| US20110008294A1 (en) | 2011-01-13 |
| US7807146B2 (en) | 2010-10-05 |
| US8313740B2 (en) | 2012-11-20 |
| JP2010505850A (ja) | 2010-02-25 |
| DK2073837T3 (da) | 2014-09-29 |
| NZ597998A (en) | 2013-08-30 |
| AU2007307080A1 (en) | 2008-04-17 |
| AU2007307080B2 (en) | 2014-01-09 |
| EP2073837A2 (en) | 2009-07-01 |
| WO2008045346A3 (en) | 2009-06-04 |
| DK2596801T3 (en) | 2018-08-13 |
| CA2665068A1 (en) | 2008-04-17 |
| ES2500465T3 (es) | 2014-09-30 |
| WO2008045346A2 (en) | 2008-04-17 |
| JP6124822B2 (ja) | 2017-05-10 |
| CA2665068C (en) | 2016-01-05 |
| EP2596801B1 (en) | 2018-05-02 |
| IL197633A0 (en) | 2011-08-01 |
| US20080213302A1 (en) | 2008-09-04 |
| NZ575388A (en) | 2012-03-30 |
| EP2073837B1 (en) | 2014-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6124822B2 (ja) | Mvaを使ってがんを処置する方法 | |
| JP7368305B2 (ja) | 腫瘍抗原を発現するポックスウイルス及びtim-3に対するモノクローナル抗体を用いた癌治療のための併用療法 | |
| CA2702586C (en) | Use of mva to treat prostate cancer | |
| RU2714142C2 (ru) | Комбинированное лекарственное средство для лечения рака с использованием поксвируса, экспрессирующего опухолевый антиген, и антагониста и/или агониста ингибитора имунной контрольной точки | |
| AU2013331328B2 (en) | Methods and compositions for the treatment of cancer | |
| JP2017515837A (ja) | 腫瘍抗原発現組換えポックスウイルス及び免疫チェックポイント分子アンタゴニストまたはアゴニストを用いた癌治療のための併用療法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20100604 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100630 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100630 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121016 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130107 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130115 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130213 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130220 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130415 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20131112 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150904 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20151113 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20151210 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20160318 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160404 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20160318 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160412 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20160413 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20160630 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5964540 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |