JP5756060B2 - Biogas biological desulfurization apparatus and biological desulfurization method - Google Patents
Biogas biological desulfurization apparatus and biological desulfurization method Download PDFInfo
- Publication number
- JP5756060B2 JP5756060B2 JP2012147623A JP2012147623A JP5756060B2 JP 5756060 B2 JP5756060 B2 JP 5756060B2 JP 2012147623 A JP2012147623 A JP 2012147623A JP 2012147623 A JP2012147623 A JP 2012147623A JP 5756060 B2 JP5756060 B2 JP 5756060B2
- Authority
- JP
- Japan
- Prior art keywords
- gas
- hydrogen sulfide
- biological desulfurization
- biogas
- oxygen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000006477 desulfuration reaction Methods 0.000 title claims description 149
- 230000023556 desulfurization Effects 0.000 title claims description 149
- 238000000034 method Methods 0.000 title claims description 65
- 239000007789 gas Substances 0.000 claims description 341
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 claims description 230
- 229910000037 hydrogen sulfide Inorganic materials 0.000 claims description 230
- 239000001301 oxygen Substances 0.000 claims description 121
- 229910052760 oxygen Inorganic materials 0.000 claims description 121
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 120
- 239000000945 filler Substances 0.000 claims description 97
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 52
- 238000012545 processing Methods 0.000 claims description 35
- 244000005700 microbiome Species 0.000 claims description 28
- 239000007788 liquid Substances 0.000 claims description 18
- 238000004364 calculation method Methods 0.000 claims description 13
- 238000000855 fermentation Methods 0.000 claims description 10
- 230000004151 fermentation Effects 0.000 claims description 10
- 238000011144 upstream manufacturing Methods 0.000 claims description 8
- 239000010815 organic waste Substances 0.000 claims description 7
- 230000008054 signal transmission Effects 0.000 claims description 7
- 238000007599 discharging Methods 0.000 claims description 6
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 103
- 238000006243 chemical reaction Methods 0.000 description 48
- 238000002474 experimental method Methods 0.000 description 25
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 22
- 229910052717 sulfur Inorganic materials 0.000 description 18
- 239000011593 sulfur Substances 0.000 description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 18
- 239000012530 fluid Substances 0.000 description 17
- 238000012360 testing method Methods 0.000 description 13
- 238000011156 evaluation Methods 0.000 description 11
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 8
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 238000003860 storage Methods 0.000 description 8
- 241000894006 Bacteria Species 0.000 description 7
- 238000010790 dilution Methods 0.000 description 7
- 239000012895 dilution Substances 0.000 description 7
- 238000012795 verification Methods 0.000 description 7
- 239000000446 fuel Substances 0.000 description 6
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 5
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 229910002092 carbon dioxide Inorganic materials 0.000 description 4
- 239000001569 carbon dioxide Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 229910001882 dioxygen Inorganic materials 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- -1 polyethylene Polymers 0.000 description 4
- 238000005507 spraying Methods 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- 239000010802 sludge Substances 0.000 description 3
- 230000019635 sulfation Effects 0.000 description 3
- 238000005670 sulfation reaction Methods 0.000 description 3
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- 230000003009 desulfurizing effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 description 2
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- RBTBFTRPCNLSDE-UHFFFAOYSA-N 3,7-bis(dimethylamino)phenothiazin-5-ium Chemical compound C1=CC(N(C)C)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 RBTBFTRPCNLSDE-UHFFFAOYSA-N 0.000 description 1
- 241000257465 Echinoidea Species 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 238000003916 acid precipitation Methods 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 241001148470 aerobic bacillus Species 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000000567 combustion gas Substances 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000002354 daily effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960000907 methylthioninium chloride Drugs 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 238000003918 potentiometric titration Methods 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 229910001961 silver nitrate Inorganic materials 0.000 description 1
- 238000005486 sulfidation Methods 0.000 description 1
- 238000005987 sulfurization reaction Methods 0.000 description 1
- 239000002351 wastewater Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/74—General processes for purification of waste gases; Apparatus or devices specially adapted therefor
- B01D53/84—Biological processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/46—Removing components of defined structure
- B01D53/48—Sulfur compounds
- B01D53/52—Hydrogen sulfide
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F3/00—Biological treatment of water, waste water, or sewage
- C02F3/28—Anaerobic digestion processes
- C02F3/2826—Anaerobic digestion processes using anaerobic filters
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F3/00—Biological treatment of water, waste water, or sewage
- C02F3/28—Anaerobic digestion processes
- C02F3/2866—Particular arrangements for anaerobic reactors
- C02F3/2893—Particular arrangements for anaerobic reactors with biogas recycling
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L3/00—Gaseous fuels; Natural gas; Synthetic natural gas obtained by processes not covered by subclass C10G, C10K; Liquefied petroleum gas
- C10L3/06—Natural gas; Synthetic natural gas obtained by processes not covered by C10G, C10K3/02 or C10K3/04
- C10L3/08—Production of synthetic natural gas
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L3/00—Gaseous fuels; Natural gas; Synthetic natural gas obtained by processes not covered by subclass C10G, C10K; Liquefied petroleum gas
- C10L3/06—Natural gas; Synthetic natural gas obtained by processes not covered by C10G, C10K3/02 or C10K3/04
- C10L3/10—Working-up natural gas or synthetic natural gas
- C10L3/101—Removal of contaminants
- C10L3/102—Removal of contaminants of acid contaminants
- C10L3/103—Sulfur containing contaminants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M47/00—Means for after-treatment of the produced biomass or of the fermentation or metabolic products, e.g. storage of biomass
- C12M47/18—Gas cleaning, e.g. scrubbers; Separation of different gases
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/95—Specific microorganisms
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/05—Biogas
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/10—Inorganic compounds
- C02F2101/101—Sulfur compounds
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2209/00—Controlling or monitoring parameters in water treatment
- C02F2209/26—H2S
- C02F2209/265—H2S in the gas phase
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/20—Air quality improvement or preservation, e.g. vehicle emission control or emission reduction by using catalytic converters
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/30—Fuel from waste, e.g. synthetic alcohol or diesel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W10/00—Technologies for wastewater treatment
- Y02W10/10—Biological treatment of water, waste water, or sewage
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Microbiology (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Sustainable Development (AREA)
- Water Supply & Treatment (AREA)
- Hydrology & Water Resources (AREA)
- Biodiversity & Conservation Biology (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Gas Separation By Absorption (AREA)
- Treatment Of Sludge (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Description
本願発明は、バイオガスの生物学的脱硫装置及び生物学的脱硫方法に係わり、詳しくはメタン発酵処理の工程で発生するバイオガスに含まれる硫化水素を硫酸に転換して効率的に処理する技術に関する。 The present invention relates to a biological desulfurization apparatus and biological desulfurization method for biogas, and more specifically, a technology for efficiently treating hydrogen sulfide contained in biogas generated in the methane fermentation process by converting it to sulfuric acid. About.
有機性廃棄物または有機性廃水は水処理分野においてメタン発酵により処理され、メタンガスを主成分とするバイオガスが発生する。バイオガスはメタン発酵の方法によって濃度は異なるものの、主成分としてメタンを65〜85%、二酸化炭素を15〜35%、硫化水素を1000〜6000ppm程度含んでいる。発生したバイオガス中のメタンをボイラーの燃料として利用が可能であり、ボイラーから発生した蒸気は加温設備にて有効利用できる。また、バイオガスはガスエンジンの燃料となり、発電も可能である。 Organic waste or organic wastewater is treated by methane fermentation in the field of water treatment, and biogas mainly composed of methane gas is generated. Although the concentration of biogas varies depending on the method of methane fermentation, it contains 65 to 85% methane, 15 to 35% carbon dioxide, and 1000 to 6000 ppm hydrogen sulfide as main components. Methane in the generated biogas can be used as fuel for the boiler, and steam generated from the boiler can be used effectively in the heating facility. Biogas also serves as fuel for gas engines and can generate electricity.
バイオガス中に含まれる硫化水素は、燃焼の際に亜硫酸ガス(SO2)に酸化され、発生する亜硫酸ガスは水分に溶解すると硫酸となり、大気中に放出されると酸性雨の原因となるだけでなく、燃焼ガスが施設内で冷却されると凝縮した水分によって硫酸となり、腐食などの問題を生じさせる。
そのため、バイオガスを利用するためには、硫化水素を除去することが重要な課題となっている。
Hydrogen sulfide contained in biogas is oxidized to sulfurous acid gas (SO 2 ) during combustion, and the generated sulfurous acid gas becomes sulfuric acid when dissolved in moisture and causes acid rain when released into the atmosphere. In addition, when the combustion gas is cooled in the facility, the condensed moisture turns into sulfuric acid, which causes problems such as corrosion.
Therefore, in order to use biogas, removal of hydrogen sulfide has become an important issue.
バイオガス中の硫化水素除去方法には、乾式脱硫方法があり、酸化鉄を主成分としたペレット状の脱硫剤を用いて硫化水素を除去する。乾式脱硫方法においては、硫化水素は、酸化鉄と化学的に反応するため、脱硫剤の硫化水素の除去量は、酸化鉄の存在量に概ね比例する。脱硫剤の硫化水素除去反応に関与する酸化鉄がなくなると除去性能は低下し、新規剤に交換する必要がある。 As a method for removing hydrogen sulfide in biogas, there is a dry desulfurization method, in which hydrogen sulfide is removed using a pellet-shaped desulfurization agent mainly composed of iron oxide. In the dry desulfurization method, since hydrogen sulfide chemically reacts with iron oxide, the amount of hydrogen sulfide removed by the desulfurizing agent is approximately proportional to the amount of iron oxide present. When the iron oxide involved in the hydrogen sulfide removal reaction of the desulfurizing agent disappears, the removal performance deteriorates and it is necessary to replace it with a new agent.
他の脱硫方法には、本願発明のように微生物を利用した生物学的脱硫方法がある。生物学的脱硫方法は、バイオガスに微量の空気又は酸素を供給して、硫化水素を微生物により、以下の(式1)(式2)に示す反応経路で硫黄(S)または硫酸(H2SO4)を生成させて除去する方法である。(式1)(式2)に関与する微生物は、充填材表面に付着したり浮遊することが可能であり、硫黄酸化細菌である好気性菌が自然界に多く存在する。微生物が関与するために、温度や水分は微生物の生存環境として必須である。 Other desulfurization methods include biological desulfurization methods using microorganisms as in the present invention. In the biological desulfurization method, a trace amount of air or oxygen is supplied to a biogas, and hydrogen sulfide is converted by microorganisms into sulfur (S) or sulfuric acid (H 2 ) in the reaction pathway shown in the following (formula 1) and (formula 2). This is a method of generating and removing SO 4 ). The microorganisms involved in (Formula 1) and (Formula 2) can adhere to or float on the surface of the filler, and there are many aerobic bacteria that are sulfur-oxidizing bacteria in nature. Since microorganisms are involved, temperature and moisture are essential for the living environment of microorganisms.
H2S + 1/2O2 → S + H2O (式1)
S + 3/2O2 + H2O → H2SO4 (式2)
H 2 S + 1 / 2O 2 → S + H 2 O (Formula 1)
S + 3 / 2O 2 + H 2 O →
(式1)は硫化水素が硫黄酸化細菌により、単体硫黄(S)を生成する反応である。酸素が硫化水素の1/2mol以下の場合の主反応である。酸素が硫化水素の1/2molを超える場合には、硫黄酸化細菌によってさらに(式2)の反応を行い、硫酸(H2SO4)が生成する。硫化水素がすべて硫酸(H2SO4)に転換するには、硫黄酸化細菌の存在下で、理論的には酸素が硫化水素の2mol以上必要となる。 (Formula 1) is a reaction in which hydrogen sulfide generates elemental sulfur (S) by sulfur-oxidizing bacteria. This is the main reaction when oxygen is 1/2 mol or less of hydrogen sulfide. When oxygen exceeds 1/2 mol of hydrogen sulfide, the reaction of (Formula 2) is further performed by sulfur-oxidizing bacteria, and sulfuric acid (H 2 SO 4 ) is generated. In order to convert all the hydrogen sulfide into sulfuric acid (H 2 SO 4 ), 2 mol or more of hydrogen sulfide is theoretically required in the presence of sulfur-oxidizing bacteria.
生物学的脱硫技術の一例として、特許文献1がある。
本方式では、処理が悪くなると、除去した硫化水素の一部は硫黄として析出し充填材に付着し、一部は硫酸に転換されている。析出した硫黄に対し、生物学的脱硫塔に水を張って曝気により剥離して処理性能を回復させる技術が記載されている。
担体に硫黄の析出がある場合、硫黄酸化菌が生成硫黄の付着により、生物反応が阻害されるため、当初の硫化水素除去能が加速度的に低下する欠点がある。
There exists patent document 1 as an example of biological desulfurization technique.
In this method, when the treatment becomes worse, a part of the removed hydrogen sulfide is precipitated as sulfur and adheres to the filler, and a part is converted to sulfuric acid. A technique is described in which the precipitated sulfur is filled with water in a biological desulfurization tower and peeled off by aeration to recover the treatment performance.
In the case where sulfur is deposited on the carrier, the sulfur-oxidizing bacterium has a disadvantage that the initial hydrogen sulfide removing ability is reduced at an accelerated rate because the biological reaction is inhibited by the formation of sulfur.
別な技術である特許文献2には、脱硫塔による処理ガスを循環させており、循環量の制御は脱硫塔後段に設置した圧力調整タンクの圧力値によって制御されており、圧力調整タンク後段でのガス利用設備で処理済のバイオガスの利用がない場合は圧力調整タンクにガスは貯留され、圧力調整タンク内のガスを脱硫塔への循環ガスとしている。
In
本方式において高濃度の硫化水素を含むバイオガスが流入した場合、圧力調整タンクの後段のガス利用設備で処理されたバイオガスが利用されていればバイオガスは循環されず、硫化水素の負荷が高い状態で処理されるため、硫黄が析出して脱硫性能が低下する原因を回避できないという欠点がある。 When biogas containing high-concentration hydrogen sulfide flows in this method, biogas is not circulated and the load of hydrogen sulfide is reduced if the biogas processed in the gas utilization facility at the latter stage of the pressure adjustment tank is used. Since it is processed in a high state, there is a drawback that it is impossible to avoid the cause of the sulfur being precipitated and the desulfurization performance being lowered.
また、酸素含有気体の供給は、脱硫塔からの処理ガス流量に合わせて調整されており、脱硫塔後段の処理ガス流出ラインに設置した酸素濃度計で管理している。
本方式で酸素含有気体の供給量を制御した場合、硫黄が析出すると酸素を消費されなくなり、処理ガス中の酸素濃度が高くなり、酸素含有気体の供給量を低下させるように制御する。このため、本来は硫酸に転換するのに必要な酸素が不足して、硫黄の析出が促進され、処理性能がより低下する欠点がある。
The supply of the oxygen-containing gas is adjusted according to the flow rate of the processing gas from the desulfurization tower, and is managed by an oxygen concentration meter installed in the processing gas outflow line at the latter stage of the desulfurization tower.
When the supply amount of the oxygen-containing gas is controlled by this method, oxygen is not consumed when sulfur is deposited, and the oxygen concentration in the processing gas is increased, and the supply amount of the oxygen-containing gas is controlled to be reduced. For this reason, there is a defect that oxygen necessary for conversion to sulfuric acid is insufficient, sulfur deposition is promoted, and processing performance is further deteriorated.
本願発明が解決しようとする課題は、上述した諸問題に鑑み、高負荷での硫化水素を効率的に処理し、且つ処理する硫化水素を硫酸に転換することで装置内の閉塞をなくし、洗浄などの工程をなくして低コストで処理が可能なバイオガスの生物学的脱硫装置及び生物学的脱硫方法を提供することを目的とする。 The problem to be solved by the present invention is that, in view of the above-mentioned problems, the hydrogen sulfide under high load is efficiently treated, and the hydrogen sulfide to be treated is converted into sulfuric acid, thereby eliminating the blockage in the apparatus and cleaning. It is an object of the present invention to provide a biogas biological desulfurization apparatus and a biological desulfurization method that can be processed at low cost by eliminating the above processes.
上記課題を解決するため、本願発明の生物学的脱硫装置及び生物学的脱硫方法は、以下の技術的特徴を備えている。
(1) 有機性廃棄物をメタン発酵させて発生したバイオガスから生物学的脱硫塔内に循環液を散水して生物学的に硫化水素を除去する生物学的脱硫装置において、
該生物学的脱硫塔の端部よりバイオガスを流入するためのバイオガス流入ラインを設け、
該生物学的脱硫塔内に微生物が付着する充填材からなる充填層を設け、
該生物学的脱硫塔のもう一方の端部であり該充填層の後段に処理ガスを排出するための処理ガス流出ラインを設け、
該処理ガスの一部を生物学的脱硫塔の前記バイオガスが流入する端部に循環するための循環ガスラインを設け、
該バイオガス流入ラインに硫化水素濃度計とガス流量計を設け、
該循環ガスラインに循環ガス量の調節機構を設け、
該硫化水素濃度計によるバイオガスの硫化水素濃度値と、該ガス流量計によるガス流量値から硫化水素負荷量を算出するための演算器を設け、
該演算器の硫化水素負荷量の算出結果により、充填材に接触するガスの硫化水素濃度が所定の範囲内となるように、前記循環ガス量の調節機構を作動させる循環ガスの信号伝達機構を具備することを特徴とする生物学的脱硫装置である。
In order to solve the above problems, the biological desulfurization apparatus and the biological desulfurization method of the present invention have the following technical features.
(1) In a biological desulfurization apparatus for removing hydrogen sulfide biologically by sprinkling a circulating liquid from a biogas generated by methane fermentation of organic waste into a biological desulfurization tower,
A biogas inflow line for introducing biogas from the end of the biological desulfurization tower is provided;
Providing a packed bed made of a filler to which microorganisms adhere in the biological desulfurization tower;
A processing gas outlet line for discharging the processing gas at the other end of the biological desulfurization tower and after the packed bed;
Providing a circulation gas line for circulating a part of the processing gas to an end of the biological desulfurization tower into which the biogas flows;
A hydrogen sulfide concentration meter and a gas flow meter are provided in the biogas inflow line,
Provide a circulating gas amount adjusting mechanism in the circulating gas line,
An arithmetic unit for calculating a hydrogen sulfide load amount from the hydrogen sulfide concentration value of the biogas by the hydrogen sulfide concentration meter and the gas flow value by the gas flow meter is provided,
A circulating gas signal transmission mechanism for operating the circulating gas amount adjusting mechanism so that the hydrogen sulfide concentration of the gas contacting the filler is within a predetermined range based on the calculation result of the hydrogen sulfide load amount of the computing unit. A biological desulfurization apparatus comprising:
(2) 該バイオガス流入ライン及び/又は該生物学的脱硫塔内に酸素含有気体を流入するための酸素含有気体流入ラインを設け、
該酸素含有気体流入ラインに酸素含有気体量の供給調節機構を設け、
該演算器の硫化水素負荷量の算出結果により、前記酸素含有気体量の供給調節機構を作動させる酸素含有気体の信号伝達機構を設けたことを特徴とする上記(1)に記載の生物学的脱硫装置である。
(2) providing an oxygen-containing gas inflow line for flowing an oxygen-containing gas into the biogas inflow line and / or the biological desulfurization tower;
A supply adjustment mechanism for the oxygen-containing gas amount is provided in the oxygen-containing gas inflow line,
The biological transmission apparatus according to (1) above, wherein a signal transmission mechanism for an oxygen-containing gas that operates the supply adjustment mechanism for the oxygen-containing gas amount is provided based on the calculation result of the hydrogen sulfide load amount of the computing unit. Desulfurization equipment.
(3) 有機性廃棄物をメタン発酵させて発生したバイオガスから生物学的脱硫塔内に循環液を散水して生物学的に硫化水素を除去する生物学的脱硫方法において、
該生物学的脱硫塔内に微生物が付着する充填材からなる充填層を設け、
該生物学的脱硫塔内の該充填層の上流側にバイオガスを流入するバイオガス流入工程と、
該生物学的脱硫塔内の該充填層の下流側に処理ガスを排出する処理ガス流出工程と、
該処理ガスの一部を該生物学的脱硫塔内の該充填層の上流側に循環する循環ガス工程とを有し、
該バイオガス流入工程における流入されるバイオガスの硫化水素濃度とガス流量とから、硫化水素負荷量を算出し、該算出結果により、充填材に接触するガスの硫化水素濃度が所定の範囲内となるように、該循環ガス工程の循環ガス量を調節することを特徴とする生物学的脱硫方法である。
(3) In a biological desulfurization method in which hydrogen sulfide is biologically removed by sprinkling a circulating liquid from a biogas generated by methane fermentation of organic waste into a biological desulfurization tower,
Providing a packed bed made of a filler to which microorganisms adhere in the biological desulfurization tower;
A biogas inflow step of flowing biogas upstream of the packed bed in the biological desulfurization tower;
A process gas outflow step for discharging a process gas downstream of the packed bed in the biological desulfurization tower;
A circulating gas step for circulating a part of the processing gas upstream of the packed bed in the biological desulfurization tower,
The hydrogen sulfide load amount is calculated from the hydrogen sulfide concentration and gas flow rate of the biogas flowing in the biogas inflow step, and the calculation result shows that the hydrogen sulfide concentration of the gas contacting the filler is within a predetermined range. Thus, the biological desulfurization method is characterized in that the amount of circulating gas in the circulating gas step is adjusted.
(4) 該生物学的脱硫塔内の該充填層の上流側に酸素含有気体を流入する酸素含有気体流入工程を有し、
前記硫化水素負荷量の算出結果により、該酸素含有気体流入工程における酸素含有気体の供給量を調節することを特徴とする上記(3)に記載の生物学的脱硫方法である。
(4) having an oxygen-containing gas inflow step of flowing an oxygen-containing gas upstream of the packed bed in the biological desulfurization tower;
The biological desulfurization method according to (3), wherein the supply amount of the oxygen-containing gas in the oxygen-containing gas inflow step is adjusted based on the calculation result of the hydrogen sulfide load.
(5) 該生物学的脱硫塔内の該充填層の上流側では、ガス中の硫化水素濃度が50〜1000ppmであることを特徴とする上記(3)又は(4)に記載の生物学的脱硫方法である。 (5) The biological fluid as described in (3) or (4) above, wherein the hydrogen sulfide concentration in the gas is 50 to 1000 ppm upstream of the packed bed in the biological desulfurization tower This is a desulfurization method.
(6) 前記ガス中の硫化水素濃度が、150〜500ppmであることを特徴とする上記(5)に記載の生物学的脱硫方法である。 (6) The biological desulfurization method according to (5) above, wherein the hydrogen sulfide concentration in the gas is 150 to 500 ppm.
本願発明の生物学的脱硫装置及び生物学的脱硫方法を用いてバイオガスを処理することで、除去した硫化水素を硫酸に転換して硫黄の析出による閉塞の問題を解消し、高効率の生物学的な脱硫処理が維持される。
また、バイオガスの硫化水素濃度とガス流量から硫化水素負荷量を算出し、該硫化水素負荷量から循環ガスの量や酸素含有気体の供給量を調節することで、4.0kg/(m3・day)までの硫化水素負荷量に対して、硫酸転換率が100%で処理できることを確認している。
By treating the biogas using the biological desulfurization apparatus and biological desulfurization method of the present invention, the removed hydrogen sulfide is converted to sulfuric acid to eliminate the problem of clogging due to the precipitation of sulfur, and a highly efficient biological The chemical desulfurization process is maintained.
Further, by calculating the hydrogen sulfide load amount from the hydrogen sulfide concentration and gas flow rate of the biogas, and adjusting the amount of circulating gas and the supply amount of oxygen-containing gas from the hydrogen sulfide load amount, 4.0 kg / (m 3 -It has been confirmed that the sulfuric acid conversion rate can be treated at 100% with respect to the hydrogen sulfide load up to day).
従来の生物学的脱硫方法では、本願発明の特徴である硫化水素濃度とガス流量の積で算出される硫化水素負荷量に対する概念がない。このため、低負荷での処理においても一定量の酸素含有気体を供給するため、バイオガス中に酸素含有気体成分が多くなり、バイオガスのメタン濃度が低下し燃料としての価値が低下する。硫化水素濃度が高濃度の場合、硫黄の析出を防止する操作条件がないために、反応等に流入したバイオガス中の硫化水素が硫黄酸化細菌の酸化反応が析出硫黄で阻害され、たとえ酸素含有気体量が十分にあったとしても、酸化細菌量の不足で硫酸までの反応が十分にできずに硫黄が析出する。生物学的脱硫塔の担体が3割程度硫黄に覆われると硫化水素が処理されずに排出されるといった処理不十分が起こる。 In the conventional biological desulfurization method, there is no concept for the hydrogen sulfide load amount calculated by the product of the hydrogen sulfide concentration and the gas flow rate, which is a feature of the present invention. For this reason, since a certain amount of oxygen-containing gas is supplied even in a low-load process, the oxygen-containing gas component increases in the biogas, and the methane concentration of the biogas decreases, reducing the value of the fuel. When the hydrogen sulfide concentration is high, there is no operating condition to prevent the precipitation of sulfur, so the hydrogen sulfide in the biogas flowing into the reaction etc. inhibits the oxidation reaction of sulfur oxidizing bacteria with the precipitated sulfur, even if it contains oxygen Even if there is a sufficient amount of gas, the reaction to sulfuric acid cannot be sufficiently performed due to the insufficient amount of oxidized bacteria, and sulfur is deposited. If the support of the biological desulfurization tower is covered with about 30% of sulfur, hydrogen sulfide will be discharged without being processed, resulting in insufficient processing.
析出した硫黄は疎水性であるため、充填材に付着すると充填材表面に付着した微生物の表面を覆い活性を低下させる。硫黄は充填材の深部方向に向かって析出し続け、最終的には生物学的脱硫塔内の充填材を閉塞させる。硫黄は、一度析出すると充填材からの剥離が困難であり、何らかの手段で剥離処理を施しても当初の処理性能には戻らないため、生物学的脱硫の処理性能を維持するためには硫黄を析出させないで処理する工夫が肝要である。 Since the precipitated sulfur is hydrophobic, when it adheres to the filler, it covers the surface of the microorganisms adhering to the filler surface and reduces the activity. Sulfur continues to precipitate toward the depth of the filler, eventually closing the filler in the biological desulfurization tower. Sulfur is difficult to remove from the filler once deposited, and it does not return to the original treatment performance even if the stripping treatment is performed by some means. Therefore, sulfur should be used to maintain the biological desulfurization treatment performance. It is important to devise treatment without depositing.
本願発明者らは、硫黄を析出させずに生物学的脱硫の処理性能を維持する条件についてバイオガスプラントに装置を設置して検討した。 The inventors of the present application have examined the conditions for maintaining the treatment performance of biological desulfurization without precipitating sulfur by installing an apparatus in a biogas plant.
ここで、後述するガスの名称について、次のように定義する。
・バイオガス:メタン発酵によって発生したガスのことであり、酸素は含有していない。
・酸素含有気体:酸素を含む気体のことである。
・希釈ガス:酸素を含んでいないガスのことであり、バイオガスに混合させて硫化水素濃度を調節するためのガスである。本願発明の検証実験では窒素ガスを用いている。
・循環ガス:処理ガスの一部が循環ガス量供給調節機構によって再び生物学的脱硫塔に流入するガスのことである。
・充填材接触ガス:バイオガスと、希釈ガスと酸素含有気体との混合ガスまたは、バイオガスと循環ガスと酸素含有気体の混合ガスのことであり、生物学的脱硫塔内に流入し充填材に接触するガスのことである。
・処理ガス:生物学的脱硫塔から排出したガスのことである。
Here, the name of the gas to be described later is defined as follows.
・ Biogas: A gas generated by methane fermentation and does not contain oxygen.
-Oxygen-containing gas: A gas containing oxygen.
Diluting gas: A gas that does not contain oxygen, and is a gas that is mixed with biogas to adjust the hydrogen sulfide concentration. In the verification experiment of the present invention, nitrogen gas is used.
Circulating gas: A gas in which a part of the processing gas flows again into the biological desulfurization tower by the circulating gas amount supply control mechanism.
-Filler contact gas: A mixed gas of biogas, dilution gas and oxygen-containing gas, or a mixed gas of biogas, circulating gas and oxygen-containing gas, which flows into the biological desulfurization tower and fills It is the gas that comes into contact with the gas.
・ Processing gas: Gas discharged from a biological desulfurization tower.
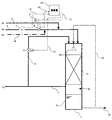
本願発明に係る検証実験は、次のような方法で実施した。検証実験で使用した生物学的脱硫装置を図1に示す。
バイオガス硫化水素濃度を希釈ガスで希釈するため、図1の脱硫装置には、希釈ガスライン14を設けた。
生物学的脱硫塔1に充填材を充填し、生物学的脱硫塔1に流入するバイオガス0aは、生物学的脱硫塔1の頂部から下向流で通気し、生物学的脱硫塔1の底部から処理ガス0cを排出した。
希釈ガス0gは、希釈ガス流入ライン14を通ってガス流量計4後段のバイオガス流入ライン2に供給した。
酸素含有気体0bは、酸素含有気体流入ライン5を通ってガス流量計4後段のバイオガス流入ライン2に供給した。
循環液は、生物学的脱硫塔1の下部の循環液貯留液槽1bから生物学的脱硫塔1の上部へ送られ、充填材に散水した。
酸素含有気体供給量は、除去硫化水素の硫酸への転換と微生物の活性が維持できるように、60L/day供給した。
散水量は、充填材が高湿度環境にあればよく、充填材に付着した微生物が処理の最中に微生物が活動できるのに十分な量とし、200L/dayとした。
処理温度についても同様に、反応に関与する微生物が活動できる環境下となるように、35℃に設定した。
充填材接触ガス濃度ごとに、表1に示すようにRunとして区分けし、本検証実験は図1で示す生物学的脱硫装置を3機用いて3Runずつ並行して行なった。実験の評価期間は30日間とした。
The verification experiment according to the present invention was carried out by the following method. The biological desulfurization apparatus used in the verification experiment is shown in FIG.
In order to dilute the biogas hydrogen sulfide concentration with a diluent gas, the desulfurization apparatus of FIG.
The biodesulfurization tower 1 is filled with a filler, and the biogas 0a flowing into the biological desulfurization tower 1 is vented in a downward flow from the top of the biological desulfurization tower 1. Process gas 0c was discharged from the bottom.
Dilution gas 0 g was supplied to the
The oxygen-containing gas 0b was supplied to the
The circulating liquid was sent from the circulating
The oxygen-containing gas was supplied at 60 L / day so that the conversion of the removed hydrogen sulfide into sulfuric acid and the activity of the microorganisms could be maintained.
The amount of water spraying is sufficient if the filler is in a high-humidity environment. The amount of water sprayed is 200 L / day, which is sufficient for the microorganisms attached to the filler to be active during the treatment.
Similarly, the treatment temperature was set to 35 ° C. so as to be in an environment where microorganisms involved in the reaction could be active.
Each filler contact gas concentration was classified as Run as shown in Table 1, and this verification experiment was performed in parallel by 3 Run using 3 biological desulfurization apparatuses shown in FIG. The evaluation period of the experiment was 30 days.
バイオガス中の硫化水素濃度は、バイオガス流入ライン2に設置した硫化水素濃度計3にて測定した。
硫化水素濃度計で計測できないその他のガスについて、充填材接触ガスは、バイオガス流量計4と生物学的脱硫塔1の頂部の間のバイオガス流入ライン2から吸引ポンプを用いてテドラバッグに採取した。処理ガス0cは、処理ガス流出ライン7から吸引ポンプを用いてテドラバッグに採取した。
テドラバッグに採取したガス中の硫化水素濃度は、硫化水素用検知管(ガステック製ガス検知管;4H)を用いて測定した。硫化水素濃度計3の値と硫化水素用検知管の値については、同一のガスに対し同じ濃度の値を示すことを確認した。
The hydrogen sulfide concentration in the biogas was measured with a hydrogen
For other gases that cannot be measured with a hydrogen sulfide concentration meter, the filler contact gas was collected from a
The concentration of hydrogen sulfide in the gas collected in the Tedra bag was measured using a hydrogen sulfide detector tube (Gastec gas detector tube: 4H). Regarding the value of the hydrogen
処理性能は、硫化水素除去率から単位充填材当たりの硫化水素除去量を計算して評価した。設定硫化水素負荷量2.0kg/(m3・day)に対し、硫化水素除去率が90%以上(単位充填材当たりの硫化水素除去量として1.8kg/(m3・day)以上)で処理が良好であると判断した。
硫化水素除去率の算出方法を以下の(式3)に示し、単位充填材当たりの硫化水素除去量の算出方法を以下の(式4)に示す。
硫化水素除去率[%]=(充填材接触ガス硫化水素濃度−処理ガス硫化水素濃度))[ppm]/充填材接触ガス硫化水素濃度[ppm]×100 (式3)
単位充填材当たりの硫化水素除去量[kg/(m3・day)]=設定硫化水素負荷量[kg/(m3・day)]×除去率[%]/100 (式4)
The treatment performance was evaluated by calculating the amount of hydrogen sulfide removed per unit filler from the hydrogen sulfide removal rate. With a set hydrogen sulfide load of 2.0 kg / (m 3 · day), the hydrogen sulfide removal rate is 90% or more (1.8 kg / (m 3 · day or more as the amount of hydrogen sulfide removed per unit filler)) The treatment was judged to be good.
The calculation method of the hydrogen sulfide removal rate is shown in the following (Formula 3), and the calculation method of the hydrogen sulfide removal amount per unit filler is shown in the following (Formula 4).
Hydrogen sulfide removal rate [%] = (filler contact gas hydrogen sulfide concentration−treatment gas hydrogen sulfide concentration)) [ppm] / filler contact gas hydrogen sulfide concentration [ppm] × 100 (Equation 3)
Hydrogen sulfide removal amount per unit filler [kg / (m 3 · day)] = set hydrogen sulfide load [kg / (m 3 · day)] × removal rate [%] / 100 (Formula 4)
硫酸の転換状況を把握するため、循環液中の硫酸濃度についても調査した。
循環液は、生物学的脱硫塔下部の循環液貯留液槽から1日に1回の頻度でドレンコックにより採取した。
循環液採取量は、循環液量が著しく変動して実験条件に影響を及ぼさない量として、100mLとし、硫酸の測定は、イオンクロマトグラフ法で硫酸イオン濃度を測定した。
循環液は、日毎にブロー水を排水し、ブロー水量と同量の補給水を供給して循環液量を一定に保った。
In order to understand the conversion status of sulfuric acid, the concentration of sulfuric acid in the circulating fluid was also investigated.
The circulating fluid was collected from the circulating fluid reservoir at the bottom of the biological desulfurization tower by a drain cock once a day.
The amount of the circulating fluid collected was set to 100 mL so that the amount of the circulating fluid was not significantly affected and the experimental conditions were affected, and the sulfuric acid concentration was measured by an ion chromatography method.
As for the circulating fluid, the blow water was drained every day, and the same amount of make-up water as the amount of blow water was supplied to keep the circulating fluid amount constant.
硫酸転換率の算出は、1日当たりの硫酸転換量と、1日当たりの除去硫化水素量から求められ、1日当たりの硫酸転換量の算出方法を以下の(式5)に示し、1日当たりの除去硫化水素量を以下の(式6)に示し、硫酸転換率を以下の(式7)に示す。
硫酸転換量[kg−H2SO4/day]=(当日の硫酸濃度−前日の硫酸濃度)[kg−H2SO4/(L・day)]×循環液量[L] (式5)
除去硫化水素量[kg−H2S/day]=単位充填材当たりの硫化水素除去量[kg/(m3・day)]×充填容量[m3] (式6)
硫酸転換率[%]=(硫酸転換量×(32/96)[kg−S/day])/(除去硫化水素量[kg−H2S/day]×(32/34)[kg−S/kg−H2S])×100 (式7)
The sulfuric acid conversion rate is calculated from the sulfuric acid conversion amount per day and the amount of removed hydrogen sulfide per day. The calculation method of the sulfuric acid conversion amount per day is shown in the following (Formula 5), and the removal sulfurization per day. The amount of hydrogen is shown in the following (formula 6), and the sulfuric acid conversion rate is shown in the following (formula 7).
Sulfuric conversion amount [kg-H 2 SO 4 / day] = ( day of sulfuric acid concentration - sulfuric acid concentration of the previous day) [kg-H 2 SO 4 / (L · day)] × circulating fluid quantity [L] (Equation 5)
Removal hydrogen sulfide amount [kg-H 2 S / day] = hydrogen sulfide removal amount per unit filler [kg / (m 3 · day)] × filling capacity [m 3 ] (Formula 6)
Sulfuric acid conversion rate [%] = (sulfuric acid conversion amount × (32/96) [kg-S / day]) / (removed hydrogen sulfide amount [kg-H 2 S / day] × (32/34) [kg-S / Kg-H 2 S]) × 100 (Formula 7)
生物学的脱硫方式で必要な酸素量について説明する。
生物学的脱硫方式で消費される酸素には、微生物による硫酸化での必要酸素量(OO)と、微生物の呼吸に必要な酸素量(OR)がある。本願発明の生物学的脱硫塔に供給する酸素含有気体供給量[kg−O2/day]は、OO+ORとなる。
硫酸化での必要酸素量(OO)は、以下の(式8)で表される。
OO[kg−O2/day]=除去硫化水素量[kg−H2S/day]×32/34[kg−O2/kg/H2S]×2 (式8)
1m3の充填材を用いて2kg/(m3・day)の硫化水素負荷量で硫化水素を硫酸に酸化するときのOOは、(式8)より3.8[kg−O2/day]である。
The amount of oxygen required for biological desulfurization will be described.
Oxygen consumed in the biological desulfurization system includes an oxygen amount necessary for sulfation by microorganisms (O 2 O 3 ) and an oxygen amount necessary for respiration of microorganisms (O R ). Oxygen-containing gas supply amount supplied to the biological desulfurization tower of the
The amount of oxygen required for sulfation (O 2 O 3 ) is expressed by the following (formula 8).
O O [kg-O 2 / day] = removal of hydrogen sulfide amount [kg-H 2 S / day ] × 32/34 [kg-
生物学的脱硫において必要な酸素は、ガス体で供給される。
酸素含有気体として純酸素ガスを25℃で供給する場合には、純酸素ガス量は以下の(式9)で表される。
純酸素ガス量[m3−O2/day]=OO[kg−O2/day]/32×22.4×(273+25)/273/1000 (式9)
The oxygen required for biological desulfurization is supplied in gaseous form.
When pure oxygen gas is supplied as an oxygen-containing gas at 25 ° C., the amount of pure oxygen gas is expressed by the following (formula 9).
Pure oxygen gas amount [m 3 -O 2 / day] = O O [kg-
酸素含有気体として空気(酸素を21v/v%含有;25℃)を用いる場合、OOを含む空気量は以下の(式10)で表される。
空気量[m3−air/day]=純酸素ガス量[m3−O2/day]×(100/21) (式10)
When air (containing 21 v / v% oxygen; 25 ° C.) is used as the oxygen-containing gas, the amount of
Air amount [m 3 −air / day] = pure oxygen gas amount [m 3 −O 2 / day] × (100/21) (Formula 10)
微生物量について、充填材1m3当たりの付着量は 1kg−SS/m3で、呼吸速度は5〜10mg−O2/(g−SS・hr)であることが実験によりわかった。
充填材1m3あたりに付着している微生物は1kg−SSであり、ORは0.12〜0.24kg−O2/dayである。
このように、ORは、OOに比べて十分に小さいものの、微生物の活動を阻害しないようにするためにも、発明者らの実験によりOOの1.5〜3倍量の酸素含有気体供給量が好ましいことがわかった。
供給する酸素量がOOの1.5倍未満の場合は、微生物の硫酸化が遅れ、OOの3倍以上となると、処理ガス中に未反応の酸素含有気体が多く含まれ、処理ガス中のメタン濃度が下がり、燃料の価値が下がる。
Regarding the amount of microorganisms, it was experimentally found that the adhesion amount per 1 m 3 of filler was 1 kg-SS / m 3 and the respiration rate was 5 to 10 mg-O 2 / (g-SS · hr).
Microorganisms adhering per filler 1 m 3 is 1kg-SS, O R is 0.12~0.24kg-O 2 / day.
Thus, O R, while O O sufficiently smaller than the, in order not to inhibit microbial activity, 1.5 to 3 oxygen content of volumes of O O by our experiments It has been found that a gas supply rate is preferred.
When the amount of oxygen to be supplied is less than 1.5 times that of
検証実験でのガス処理条件を表1に示す。
バイオガス中の硫化水素濃度は6000ppm、バイオガス流量は1m3/dayであり、設定硫化水素負荷量を2kg/(m3・day)とした。
酸素含有気体の供給量は、OOの1.5倍量とした。
Table 1 shows the gas treatment conditions in the verification experiment.
The hydrogen sulfide concentration in the biogas was 6000 ppm, the biogas flow rate was 1 m 3 / day, and the set hydrogen sulfide load was 2 kg / (m 3 · day).
The supply amount of the oxygen-containing gas was 1.5 times the amount of
RunK−1−1は、バイオガスのみを処理し、希釈ガスは用いなかった。接触時間は340secだった。
RunK−1−2〜RunK−1−11は、希釈ガスを1〜59m3/dayの範囲で供給し、充填材接触ガス硫化水素濃度を100〜6000ppmの範囲で調節した。
RunK−1−2では、充填材接触ガス流量を2m3/dayとしたとき、接触時間は170secであり、RunK−1−11では、充填材接触ガス流量を60m3/dayとしたとき、接触時間は6secだった。
RunK-1-1 treated only biogas and did not use dilution gas. The contact time was 340 seconds.
In RunK-1-2 to RunK-1-11, a dilution gas was supplied in the range of 1 to 59 m 3 / day, and the filler contact gas hydrogen sulfide concentration was adjusted in the range of 100 to 6000 ppm.
In Run K-1-2, when the filler contact gas flow rate is 2 m 3 / day, the contact time is 170 sec. In Run K-1-1, when the filler contact gas flow rate is 60 m 3 / day, the contact time is 170 m. The time was 6 seconds.
充填材接触ガス硫化水素濃度が生物学的脱硫処理におよぼす影響に関する実験結果を表2に示す。表中の実験結果の値は、評価30日目の値を記載した。
RunK−1−1において、評価開始から30日目には、処理ガス硫化水素濃度は5850ppm検出した。このときの硫化水素除去率3%であり、単位充填材当たりの硫化水素除去量は0.05kg/(m3・day)だった。硫酸転換率は30%となり、硫黄が析出した。
Table 2 shows the experimental results regarding the influence of the filler contact gas hydrogen sulfide concentration on the biological desulfurization treatment. The values of the experimental results in the table are the values on the 30th day of evaluation.
In Run K-1-1, on the 30th day from the start of evaluation, the processing gas hydrogen sulfide concentration was detected at 5850 ppm. The hydrogen sulfide removal rate at this time was 3%, and the amount of hydrogen sulfide removed per unit filler was 0.05 kg / (m 3 · day). The sulfuric acid conversion was 30%, and sulfur was precipitated.
RunK−1−2〜RunK−1−9では、充填材接触ガス流量の増加に伴って硫化水素除去率は増加した。
RunK−1−6〜RunK−1−9では、充填材接触ガス硫化水素濃度を150〜500ppmの範囲で運転すると、硫化水素除去率90%以上であり、単位充填材当たりの硫化水素除去量1.8kg/(m3・day)以上で処理できた。硫酸転換率は100%だった。
In RunK-1-2 to RunK-1-9, the hydrogen sulfide removal rate increased as the filler contact gas flow rate increased.
In Run K-1-6 to Run K-1-9, when the filler contact gas hydrogen sulfide concentration is operated in the range of 150 to 500 ppm, the hydrogen sulfide removal rate is 90% or more, and the amount of hydrogen sulfide removed per unit filler is 1 It was possible to process at 8 kg / (m 3 · day) or more. The sulfuric acid conversion rate was 100%.
RunK−1−10〜RunK−1−11では、希釈ガス流量を増加させると硫化水素除去率は低下し、除去率は75%以下となり、単位充填材当たりの硫化水素除去量は1.5 kg/(m3・day)以下だった。しかし、硫酸転換率は100%を維持した。 In RunK-1-10 to RunK-1-11, when the dilution gas flow rate is increased, the hydrogen sulfide removal rate decreases, the removal rate becomes 75% or less, and the hydrogen sulfide removal amount per unit filler is 1.5 kg. / (M 3 · day) or less. However, the sulfuric acid conversion rate was maintained at 100%.
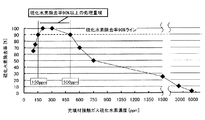
表2の充填材接触ガス硫化水素濃度と硫化水素除去率の関係を図2に示す。
充填材接触ガス硫化水素濃度が120ppmよりも低いとき、硫化水素除去率は75%以下だった。
充填材接触ガス硫化水素濃度が150ppm〜500ppmの濃度範囲で処理した場合、硫化水素除去率は90%以上で処理できた。
充填材接触ガス硫化水素濃度が600ppm以上になると、硫化水素濃度の増加に伴って硫化水素除去率は低下した。
The relationship between the filler contact gas hydrogen sulfide concentration in Table 2 and the hydrogen sulfide removal rate is shown in FIG.
When the filler contact gas hydrogen sulfide concentration was lower than 120 ppm, the hydrogen sulfide removal rate was 75% or less.
When the filler contact gas hydrogen sulfide concentration was processed in the concentration range of 150 ppm to 500 ppm, the hydrogen sulfide removal rate could be processed at 90% or more.
When the hydrogen sulfide concentration of the filler contact gas was 600 ppm or more, the hydrogen sulfide removal rate decreased as the hydrogen sulfide concentration increased.
表2に示すように、RunK−1−1では、充填材接触ガス硫化水素濃度は6000ppmであり、このとき単位充填材当たりの硫化水素除去量は0.05 kg/(m3・day)だった。
RunK−1−7およびRunK−1−8では、充填材接触ガス硫化水素濃度はそれぞれ300ppmと200ppmであり、単位充填材当たりの硫化水素除去量は2.0kg/(m3・day)だった。
As shown in Table 2, in RunK-1-1, the filler contact gas hydrogen sulfide concentration was 6000 ppm, and at this time, the amount of hydrogen sulfide removed per unit filler was 0.05 kg / (m 3 · day). It was.
In RunK-1-7 and RunK-1-8, the hydrogen sulfide concentration of the filler contact gas was 300 ppm and 200 ppm, respectively, and the amount of hydrogen sulfide removed per unit filler was 2.0 kg / (m 3 · day). .
ガスと充填材の接触時間は、充填材接触ガス流量の増加に伴って短くなった。
RunK−1−1〜RunK−1−8では接触時間が短くなると単位充填材当たりの硫化水素除去量は増加し、RunK−1−8では接触時間11secであり、単位充填材当たりの硫化水素除去量は2.0kg/(m3・day)だった。
しかし、RunK−1−9では接触時間が8secであり、単位充填材当たりの硫化水素除去量は1.8kg/(m3・day)だった。
さらに、RunK−1−10において接触時間が6secとなると、単位充填材当たりの硫化水素除去量は1.5kg/(m3・day)に低下した。
The contact time between the gas and the filler became shorter as the filler contact gas flow rate increased.
In RunK-1-1-1 to RunK-1-8, when the contact time is shortened, the amount of hydrogen sulfide removed per unit filler increases, and in RunK-1-8, the contact time is 11 sec. Hydrogen sulfide removed per unit filler The amount was 2.0 kg / (m 3 · day).
However, in Run K-1-9, the contact time was 8 sec, and the amount of hydrogen sulfide removed per unit filler was 1.8 kg / (m 3 · day).
Furthermore, when the contact time was 6 sec in Run K-1-10, the amount of hydrogen sulfide removed per unit filler decreased to 1.5 kg / (m 3 · day).
したがって、接触時間が短くなると単位充填材当たりの硫化水素除去量は増加するものの、更に接触時間が短くなると単位充填材当たりの硫化水素除去量は低下した。
これは、充填材接触ガス流量が増えて接触時間が短くなると、充填材に付着した微生物が十分に硫化水素を処理できず未処理の硫化水素が系外に流出することに起因する。
Therefore, although the removal amount of hydrogen sulfide per unit filler increases as the contact time becomes shorter, the removal amount of hydrogen sulfide per unit filler decreases as the contact time becomes shorter.
This is because when the contact gas flow rate of the filler is increased and the contact time is shortened, microorganisms attached to the filler cannot sufficiently treat hydrogen sulfide, and untreated hydrogen sulfide flows out of the system.
以下、検証実験結果を踏まえ、本願発明の実施の形態を説明する。本願発明の生物学的脱硫装置の一例を図3に示す。
本願発明では、有機性廃棄物をメタン発酵させて発生したバイオガスから生物学的脱硫塔1内に循環液を散水して生物学的に硫化水素を除去する生物学的脱硫装置において、該生物学的脱硫塔の端部よりバイオガスを流入するためのバイオガス流入ライン2を設け、該生物学的脱硫塔内に微生物が付着する充填材からなる充填層1aを設け、該生物学的脱硫塔のもう一方の端部であり該充填層の後段に処理ガスを排出するための処理ガス流出ライン7を設け、該処理ガスの一部を生物学的脱硫塔の前記バイオガスが流入する端部に循環するための循環ガスライン8を設け、該バイオガス流入ライン2に硫化水素濃度計3とガス流量計4を設け、該循環ガスラインに循環ガス量の調節機構9を設け、該硫化水素濃度計によるバイオガス中の硫化水素濃度値と、該ガス流量計によるガス流量値から硫化水素負荷量を算出するための演算器11を設け、該演算器の硫化水素負荷量の算出結果により、前記循環ガス量の調節機構を作動させる循環ガスの信号伝達機構12を具備することを特徴とする。
Hereinafter, an embodiment of the present invention will be described based on the verification experiment result. An example of the biological desulfurization apparatus of the present invention is shown in FIG.
In the present invention, in a biological desulfurization apparatus that biologically removes hydrogen sulfide by sprinkling a circulating liquid into a biological desulfurization tower 1 from biogas generated by methane fermentation of organic waste. A
本願発明者らは、本願発明の生物学的脱硫装置を用いて、長期間の連続実験を行ない、バイオガス中の硫化水素の濃度変動やバイオガスの流量変動のある条件でも効率よく、かつ安定して処理が行なえる方法について検討した。 The inventors of the present invention conducted a long-term continuous experiment using the biological desulfurization apparatus of the present invention, and were efficient and stable even under conditions where the concentration of hydrogen sulfide in biogas and the flow rate of biogas varied. Then, the method that can be processed was examined.
有機性廃棄物をメタン発酵させて発生したバイオガスから生物学的に硫化水素を除去する生物学的脱硫装置の一例を図3に示すが、本願発明は本実施態様に限定されない。 An example of a biological desulfurization apparatus that biologically removes hydrogen sulfide from biogas generated by methane fermentation of organic waste is shown in FIG. 3, but the present invention is not limited to this embodiment.
バイオガス0aを流入するためのバイオガス流入ライン2は、生物学的脱硫塔1の頂部に直結しており、バイオガス流入ライン2には硫化水素濃度計3とガス流量計4を設けている。
流化水素濃度値は、硫化水素濃度信号入力ライン15から演算器に入力し、ガス流量値は、ガス流量信号入力ライン16から演算器に入力する。
酸素含有気体流入ライン5は、バイオガス流入ライン2に直結しており、酸素含有気体0bの供給量は、酸素含有気体供給調節機構6によって調節した。
The
The fluidized hydrogen concentration value is input from the hydrogen sulfide concentration
The oxygen-containing
微生物が付着する充填材はポリエチレン製であり、形状がφ15mm×h15mmの円筒状のもので比表面積が1000m2/m3であり、生物学的脱硫塔1の充填層1aに充填した。
処理ガス流出ライン7は、生物学的脱硫塔1の下部に直結しており、処理ガス0cが系外へ排出する。
The packing material to which microorganisms adhere is made of polyethylene, has a cylindrical shape of φ15 mm × h15 mm, has a specific surface area of 1000 m 2 / m 3 , and is packed in the packed
The processing
循環ガスライン8は処理ガス流出ライン7から分岐し、生物学的脱硫塔1の頂部に直結し、処理ガス0cの一部を循環させた。
循環ガス量は、循環ガス量調節機構9によって調節した。
循環液貯留液槽1bからの循環液0dは、生物学的脱硫塔1の上部から散水した。循環液貯留液槽1bから循環液0d中の硫酸濃度を調整するために間欠的に循環液の一部をブロー水0eとして排出し、補給水0fを補給して循環液貯留液槽1bの水量を一定に保った。
The circulating
The amount of circulating gas was adjusted by the circulating gas
The circulating fluid 0d from the circulating
ここで、バイオガス流入ラインは、生物学的脱硫塔の頂部に直結しているが、生物学的脱硫塔の側面から直結してもよい。この場合、処理ガス流出ラインは、充填層と循環液貯留液槽の間に位置する生物学的脱硫塔の側面に直結している。
また、図3ではバイオガスは下向流で流れる仕組みであるが、バイオガス流入ラインを生物学的脱硫塔の充填層と循環液貯留液槽の間に位置する側面に直結して上向流でガスを流してもよい。この場合、処理ガス流出ラインは、充填層と生物学的脱硫塔の頂部の間の生物学的脱硫塔の側面に直結してもよく、生物学的脱硫塔の頂部に直結してもよい。
Here, the biogas inflow line is directly connected to the top of the biological desulfurization tower, but may be directly connected from the side of the biological desulfurization tower. In this case, the processing gas outflow line is directly connected to the side surface of the biological desulfurization tower located between the packed bed and the circulating liquid storage tank.
Further, in FIG. 3, the biogas flows downward, but the biogas inflow line is directly connected to the side surface located between the packed bed of the biological desulfurization tower and the circulating liquid storage tank, and the upward flow The gas may be flowed with. In this case, the process gas outflow line may be directly connected to the side of the biological desulfurization tower between the packed bed and the top of the biological desulfurization tower, or may be directly connected to the top of the biological desulfurization tower.
酸素含有気体供給調節機構は、ブロワなどの供給手段を用いてガスを供給してもよく、供給量の調節は、ブロワの回転数をインバータ制御してもよく、ブロワの後段にバルブ設置してバルブの開度で制御してもよい。
酸素含有気体は、酸素を含んでいる気体のことであり、空気または、純酸素または、酸素発生器により酸素濃度を調整したガスを用いてもよい。
The oxygen-containing gas supply adjustment mechanism may supply gas using supply means such as a blower, and the supply amount may be adjusted by controlling the number of rotations of the blower with an inverter. You may control by the opening degree of a valve | bulb.
The oxygen-containing gas is a gas containing oxygen, and air, pure oxygen, or a gas whose oxygen concentration is adjusted by an oxygen generator may be used.
酸素含有気体流入ラインは、バイオガス流入ラインに直結してもよく、生物学的脱硫塔の頂部に直結してもよく、生物学的脱硫塔の側面から直結してもよい。
図3では酸素含有気体は下向流で流れる仕組みであるが、バイオガスを上向流で流す場合には、酸素含有気体流入ラインを生物学的脱硫塔の充填層と循環液貯留液槽の間に位置する側面に直結してもよい。
The oxygen-containing gas inflow line may be directly connected to the biogas inflow line, may be directly connected to the top of the biological desulfurization tower, or may be directly connected from the side of the biological desulfurization tower.
In FIG. 3, the oxygen-containing gas flows in a downward flow. However, when the biogas flows in an upward flow, the oxygen-containing gas inflow line is connected to the packed bed of the biological desulfurization tower and the circulating liquid storage tank. You may connect directly to the side surface located in between.
微生物が付着する充填材は、pH1以下の強酸性下で使用できるような素材のものであればよく、例えば材質がポリエチレンやポリプロピレン、塩化ビニル、ポリウレタンなどの有機性物質が好ましい。
充填材の形状は、筒状や、網状骨格パイプやボール状やウニ状が好ましい。比表面積は50〜1000m2/m3の範囲が好ましい。空隙率は、80〜96%の範囲が好ましい。
The filler to which microorganisms adhere can be any material that can be used under strong acidity of pH 1 or lower. For example, organic materials such as polyethylene, polypropylene, vinyl chloride, and polyurethane are preferable.
The shape of the filler is preferably a cylinder, a reticulated skeleton pipe, a ball or a sea urchin. The specific surface area is preferably in the range of 50 to 1000 m 2 / m 3 . The porosity is preferably in the range of 80 to 96%.
ガス流量計は、オリフィス流量計や、容積流量計や、渦流量計や、流速式流量計等を用いることができ、容積式流量計は、実測乾式ガスメーターや、実測湿式を用いることができ、さらに、実測乾式ガスメーターは、膜式あるいは回転子式等を用いてもよい。 As the gas flow meter, an orifice flow meter, a volumetric flow meter, a vortex flow meter, a flow rate type flow meter, etc. can be used, and the positive displacement flow meter can use a measured dry gas meter or a measured wet type, Further, the actual dry gas meter may be a membrane type or a rotor type.
硫化水素濃度計は、定電位電解式による測定方法、硝酸銀電位差滴定法、イオン電極法、メチレンブルー吸光光度法、ガスクロマトグラフ法等を用いてもよい。また、検知管による硫化水素を測定してもよい。 For the hydrogen sulfide concentration meter, a measurement method by a potentiostatic electrolytic method, a silver nitrate potentiometric titration method, an ion electrode method, a methylene blue absorptiometry, a gas chromatograph method, or the like may be used. Moreover, you may measure the hydrogen sulfide by a detection tube.
該酸素含有気体供給調節機構及び/または循環ガス量供給調節機構を制御する方法は、上記の方法によって得られたガス流量及び/または硫化水素濃度の値をもとに、物理的な制御でも電気的な信号による制御でもよい。 The method for controlling the oxygen-containing gas supply adjustment mechanism and / or the circulating gas amount supply adjustment mechanism is based on the gas flow rate and / or the hydrogen sulfide concentration value obtained by the above-described method. It may be controlled by a typical signal.
図3では、処理ガス流出ラインは下向流で処理したガスを排出する仕組みであるが、バイオガスを上向流で流す場合には、生物学的脱硫塔の頂部と充填層の間に位置する生物学的脱硫塔の側面に直結してもよい。 In FIG. 3, the processing gas outflow line is a mechanism for discharging the gas processed in the downward flow, but when the biogas flows in the upward flow, it is positioned between the top of the biological desulfurization tower and the packed bed. It may be directly connected to the side of the biological desulfurization tower.
循環ガスラインは、処理ガス流出ラインから分岐してもよく、生物学的脱硫塔端部に直結してもよい。また、充填塔の頂部と充填層の間に位置する側面に直結してもよい。
図3では循環ガスラインを生物学的脱硫塔の頂部に循環させているが、脱硫塔内を上向流で処理する場合には、循環ラインが充填層と循環液貯留液槽の間の生物学的脱硫塔の側面に直結するよう構成してもよい。
循環ガス量調節機構は、ブロワなどの供給手段を用いてガスを供給してもよく、供給量の調節は、ブロワの回転数をインバータで制御してもよく、ブロワの後段にバルブ設置してバルブの開度で制御してもよい。
The circulating gas line may be branched from the process gas outflow line or directly connected to the end of the biological desulfurization tower. Moreover, you may connect directly to the side surface located between the top part of a packed tower, and a packed bed.
In FIG. 3, the circulation gas line is circulated to the top of the biological desulfurization tower. However, when the inside of the desulfurization tower is treated with an upward flow, the circulation line is disposed between the packed bed and the circulating liquid storage tank. You may comprise so that it may connect directly to the side surface of a chemical desulfurization tower.
The circulating gas amount adjusting mechanism may supply gas using supply means such as a blower. The supply amount may be adjusted by controlling the number of rotations of the blower with an inverter, and installing a valve in the subsequent stage of the blower. You may control by the opening degree of a valve | bulb.
散水ラインの一端は、循環液貯留液槽に貯留した循環液の水位よりも十分に低い位置の生物学的脱硫塔の側面に直結していることが好ましく、もう一端の散水ラインは生物学的脱硫塔の頂部と充填層の間に位置する生物学的脱硫塔の側面に直結してもよく、生物学的脱硫塔の頂部に直結してもよい。循環液は、ポンプなどの送液手段により散水ラインに連結される。 One end of the sprinkling line is preferably directly connected to the side of the biological desulfurization tower at a position sufficiently lower than the level of the circulating liquid stored in the circulating liquid storage tank, and the other end of the sprinkling line is biological It may be directly connected to the side of the biological desulfurization tower located between the top of the desulfurization tower and the packed bed, or may be directly connected to the top of the biological desulfurization tower. The circulating fluid is connected to the watering line by liquid feeding means such as a pump.
演算器は、バイオガス中の硫化水素濃度とバイオガス流量から硫化水素負荷量を演算できることが好ましく、酸素含有気体供給量は、硫化水素負荷量に基づき制御することが好ましく、充填材接触ガス硫化水素濃度範囲が50〜1000ppm、好ましくは150〜500ppmになるように循環ガス量を調節することが好ましい。 The computing unit is preferably capable of calculating the hydrogen sulfide load from the hydrogen sulfide concentration in the biogas and the biogas flow rate, and the oxygen-containing gas supply amount is preferably controlled based on the hydrogen sulfide load, and the filler contact gas sulfide It is preferable to adjust the amount of circulating gas so that the hydrogen concentration range is 50 to 1000 ppm, preferably 150 to 500 ppm.
本願発明者らは、バイオガス中の硫化水素濃度とバイオガス流量の積から求められる硫化水素負荷量を演算し、硫化水素負荷量の値をもとに酸素含有気体の供給量を自動調節して余分な酸素含有気体供給量を含まずに最適な量を供給させた。
具体的には、バイオガス中の硫化水素濃度とバイオガス流量は逐次演算器に入力され、演算器によって逐次硫化水素負荷量が演算され、硫化水素負荷量に基づき、予め記憶された演算式に則って適切な量の酸素含有気体を供給できるように、酸素含有気体供給調節機構をフィードフォワード制御した。
The inventors of the present application calculate the hydrogen sulfide load obtained from the product of the hydrogen sulfide concentration in the biogas and the biogas flow rate, and automatically adjust the supply amount of the oxygen-containing gas based on the value of the hydrogen sulfide load. Thus, an optimum amount was supplied without including an excess oxygen-containing gas supply amount.
Specifically, the hydrogen sulfide concentration and biogas flow rate in the biogas are input to the sequential calculator, the hydrogen sulfide load is calculated sequentially by the calculator, and the calculation formula stored in advance is based on the hydrogen sulfide load. Accordingly, the oxygen-containing gas supply adjusting mechanism was feedforward controlled so that an appropriate amount of oxygen-containing gas could be supplied.
また、本願発明者らは、適切な充填材接触ガス硫化水素濃度で生物学的脱硫処理できるような方法について検討した。具体的には、バイオガス中の硫化水素濃度が設定濃度以上(たとえば濃度として500ppm以上)になった場合、循環ガス量調節機構を稼動させ、充填材接触ガス硫化水素濃度が所定の濃度(例えば300ppm)となるように、循環ガス量を演算し、所定の循環ガス量を供給するように循環ガス量供給調節機構をフィードフォワード制御した。 In addition, the inventors of the present application have studied a method capable of biological desulfurization treatment with an appropriate filler contact gas hydrogen sulfide concentration. Specifically, when the hydrogen sulfide concentration in the biogas is equal to or higher than a set concentration (for example, 500 ppm or more as a concentration), the circulating gas amount adjusting mechanism is operated, and the filler contact gas hydrogen sulfide concentration is set to a predetermined concentration (for example, The circulating gas amount was calculated so as to be 300 ppm), and the circulating gas amount supply adjusting mechanism was feedforward controlled so as to supply a predetermined circulating gas amount.
循環ガス量調節機構の制御フローチャートの一例を図4に示す。
循環ガス量調節機構を硫化水素負荷量で制御する方法のフローチャートを図4−(a)に示す。
バイオガス中の硫化水素濃度とバイオガス流量から硫化水素負荷量が計算され、硫化水素負荷量をもとに、循環ガス量を計算して循環ガス量調節機構を作動させる。
また循環ガス量調節機構の別の制御方法として、硫化水素濃度で制御する方法もあり、図4−(b)に示す。
本方式では、バイオガス中の硫化水素濃度をもとに循環ガス量を計算して循環ガス量調節機構を作動させる。
An example of a control flowchart of the circulating gas amount adjusting mechanism is shown in FIG.
FIG. 4- (a) shows a flowchart of a method for controlling the circulating gas amount adjusting mechanism with the hydrogen sulfide load.
The hydrogen sulfide load is calculated from the hydrogen sulfide concentration in the biogas and the biogas flow rate. Based on the hydrogen sulfide load, the circulating gas amount is calculated and the circulating gas amount adjusting mechanism is operated.
As another control method of the circulating gas amount adjusting mechanism, there is a method of controlling by the hydrogen sulfide concentration, which is shown in FIG.
In this system, the circulating gas amount is calculated based on the hydrogen sulfide concentration in the biogas and the circulating gas amount adjusting mechanism is operated.
次に、酸素含有気体供給調節機構の制御フローチャートの一例を図5に示す。
バイオガス中の硫化水素濃度とバイオガス流量から硫化水素負荷量が計算され、硫化水素負荷量をもとに、酸素含有気体供給量を計算して、酸素含有気体供給調節機構を作動させる。
Next, an example of a control flowchart of the oxygen-containing gas supply adjusting mechanism is shown in FIG.
The hydrogen sulfide load amount is calculated from the hydrogen sulfide concentration in the biogas and the biogas flow rate, the oxygen-containing gas supply amount is calculated based on the hydrogen sulfide load amount, and the oxygen-containing gas supply adjustment mechanism is operated.
次に、他の生物学的脱硫技術で得られた知見に基づき、生物学的脱硫処理したガスを生物学的脱硫塔上部へ循環させて処理したときの性能について検討した。
図3の生物学的脱硫装置において、生物学的脱硫塔1の中にポリエチレン製で、比表面積が1000m2/m3であり、φ15mm×h15mmの円筒状の充填材を2mとなるように充填した。生物学的脱硫塔に流入するガスは、生物学的脱硫塔1の頂部から下向流で流した。循環液として活性汚泥を用い、生物学的脱硫塔下部の循環液貯留液槽に貯留し、ポンプによって生物学的脱硫塔上部へ送られ、ガス方向に並行して散水した。
Next, based on the knowledge obtained by other biological desulfurization techniques, the performance when the biological desulfurized gas was circulated to the upper part of the biological desulfurization tower and processed was examined.
In the biological desulfurization apparatus of FIG. 3, the biological desulfurization tower 1 is made of polyethylene, has a specific surface area of 1000 m 2 / m 3 , and is filled with a cylindrical filler of φ15 mm × h15 mm to 2 m. did. The gas flowing into the biological desulfurization tower was allowed to flow downward from the top of the biological desulfurization tower 1. Activated sludge was used as the circulating liquid, stored in a circulating liquid storage tank at the lower part of the biological desulfurization tower, sent to the upper part of the biological desulfurization tower by a pump, and sprinkled in parallel with the gas direction.
本実験は、硫化水素濃度6000ppmのバイオガスを用いた。
ここで、試験区1ではバイオガス中の硫化水素濃度6000ppmであり、試験区2ではバイオガス中の硫化水素濃度3000ppmであり、試験区3ではバイオガス中の硫化水素濃度1500ppmである。
バイオガス中のメタン濃度は80%、二酸化炭素濃度は20%であり、実施期間を通してほぼ一定だった。
In this experiment, biogas having a hydrogen sulfide concentration of 6000 ppm was used.
Here, in test section 1, the hydrogen sulfide concentration in the biogas is 6000 ppm, in
The methane concentration in the biogas was 80% and the carbon dioxide concentration was 20%, which was almost constant throughout the implementation period.
試験区1におけるガス処理条件を表3に示す。硫化水素濃度;6000ppmのバイオガスを1m3/dayで供給し、循環ガス量は、9〜49 m3/dayの範囲で調節した。
試験区2におけるガス処理条件を表4に示す。硫化水素濃度;3000ppmのバイオガスを2m3/dayで供給し、循環ガス量は、8〜48 m3/dayの範囲で調節した。
試験区3におけるガス処理条件を表5に示す。硫化水素濃度;1500ppmのバイオガスを4m3/dayで供給し、循環ガス量は、6〜46 m3/dayの範囲で調節した。
Table 3 shows the gas treatment conditions in Test Zone 1. Hydrogen sulfide concentration: 6000 ppm of biogas was supplied at 1 m 3 / day, and the amount of circulating gas was adjusted in the range of 9 to 49 m 3 / day.
Table 4 shows the gas treatment conditions in
Table 5 shows the gas treatment conditions in the
本実験では、酸素30v/v%および窒素70v/v%に調整した酸素含有気体を用い、酸素含有気体供給量は、60L/dayとした。
散水量は、充填材が高湿度環境にあればよく、充填材に付着した微生物が処理の最中に微生物が活動できるのに十分な量とし、200L/dayとした。
処理温度についても同様に、反応に関与する微生物が活動できる環境下となるように、35℃に設定した。
充填材接触ガス流量ごとにRunとして区分けし、本実験は図3で示す生物学的脱硫装置を3機用いて並行して行なった。実験の評価期間は30日間とした。
In this experiment, an oxygen-containing gas adjusted to 30 v / v% oxygen and 70 v / v% nitrogen was used, and the oxygen-containing gas supply amount was 60 L / day.
The amount of water spraying is sufficient if the filler is in a high-humidity environment. The amount of water sprayed is 200 L / day, which is sufficient for the microorganisms attached to the filler to be active during the treatment.
Similarly, the treatment temperature was set to 35 ° C. so as to be in an environment where microorganisms involved in the reaction could be active.
Each of the filler contact gas flow rates was classified as Run, and this experiment was performed in parallel using three biological desulfurization apparatuses shown in FIG. The evaluation period of the experiment was 30 days.
バイオガス中の硫化水素濃度は、バイオガス流入ライン2に設置した硫化水素濃度計3にて測定した。
硫化水素濃度計で計測できないその他のガスについて、充填材接触ガスは、バイオガス流量計4と生物学的生物学的脱硫塔1の頂部の間のバイオガス流量ガスライン2から吸引ポンプを用いてテドラバッグに採取した。処理ガス0cは、処理ガス流出ライン7から吸引ポンプを用いてテドラバッグに採取した。
テドラバッグに採取したガス中の硫化水素濃度は、硫化水素用検知管(ガステック製ガス検知管;4H)を用いて測定した。
The hydrogen sulfide concentration in the biogas was measured with a hydrogen
For other gases that cannot be measured with a hydrogen sulfide concentration meter, the filler contact gas is drawn from a biogas
The concentration of hydrogen sulfide in the gas collected in the Tedra bag was measured using a hydrogen sulfide detector tube (Gastec gas detector tube: 4H).
実験結果について説明する。
試験区1における実験結果を表6に、試験区2における実験結果を表7に、試験区3における実験結果を表8に示す。表中の実験結果の値は、評価30日目の値を記載した。
試験区1の結果について、RunJ−1−1は、充填材接触ガス流量は10m3/dayであり、接触時間は34secだった。本期間における処理ガス硫化水素濃度は平均して3000ppmだった。このときの硫化水素除去率50%であり、単位充填材当たりの硫化水素除去量は1.0kg/(m3・day)であり、硫酸転換率は100%だった。
The experimental results will be described.
The experimental results in Test Group 1 are shown in Table 6, the experimental results in
Regarding the results of test section 1, Run J-1-1 had a filler contact gas flow rate of 10 m 3 / day and a contact time of 34 sec. The treatment gas hydrogen sulfide concentration during this period averaged 3000 ppm. The hydrogen sulfide removal rate at this time was 50%, the hydrogen sulfide removal amount per unit filler was 1.0 kg / (m 3 · day), and the sulfuric acid conversion rate was 100%.
RunJ−1−2で充填材接触ガス流量を12m3/dayに増やしたときの接触時間は28secだった。本期間の処理ガス硫化水素濃度は、平均して450ppmだった。このときの硫化水素除去率93%となり、単位充填材当たりの硫化水素除去量1.9kg/(m3・day)であり。硫酸転換率は100%だった。 The contact time when the filler contact gas flow rate was increased to 12 m 3 / day with Run J-1-2 was 28 sec. The treatment gas hydrogen sulfide concentration during this period averaged 450 ppm. The hydrogen sulfide removal rate at this time was 93%, and the amount of hydrogen sulfide removed per unit filler was 1.9 kg / (m 3 · day). The sulfuric acid conversion rate was 100%.
循環ガス量を増やすと(RunJ−1−3〜RunJ−1−7)、充填材接触ガス流量が40m3/day(RunJ−1−7)までは硫化水素除去率90%以上であり、単位充填材当たりの硫化水素除去量は1.8kg/(m3・day)以上だった。この時の接触時間は8〜23secだった。硫酸転換率は100%だった。 When the amount of circulating gas is increased (Run J-1-3 to Run J-1-7), the hydrogen sulfide removal rate is 90% or more until the filler contact gas flow rate is 40 m 3 / day (Run J-1-7). The amount of hydrogen sulfide removed per filler was 1.8 kg / (m 3 · day) or more. The contact time at this time was 8 to 23 sec. The sulfuric acid conversion rate was 100%.
しかし、充填材接触ガス流量を50m3/dayまで増やすと(RunJ−1−8)、接触時間は6secとなり、硫化水素除去率は65%に低下し、単位充填材当たりの硫化水素除去量は1.3kg/(m3・day)に低下した。このときの硫酸転換率は100%だった。 However, when the filler contact gas flow rate is increased to 50 m 3 / day (Run J-1-8), the contact time becomes 6 sec, the hydrogen sulfide removal rate decreases to 65%, and the amount of hydrogen sulfide removed per unit filler is It decreased to 1.3 kg / (m 3 · day). The sulfuric acid conversion rate at this time was 100%.
RunJ−1−9は、従来方式としてバイオガスをそのまま処理させた。評価30日目の処理ガス硫化水素は5850ppmに達し、硫化水素除去率は3%であり、単位充填材当たりの硫化水素除去量は0.05kg/(m3・day)だった。硫酸転換率は30%となり硫黄が析出した。 RunJ-1-9 was treated with biogas as it was as a conventional method. The treatment gas hydrogen sulfide on the 30th day of evaluation reached 5850 ppm, the hydrogen sulfide removal rate was 3%, and the hydrogen sulfide removal amount per unit filler was 0.05 kg / (m 3 · day). The sulfuric acid conversion rate was 30% and sulfur was precipitated.
試験区2および試験区3においても処理性能は試験区1と同じ傾向を示し、充填材接触ガス流量が12〜40m3/dayの範囲では硫化水素除去率90%以上であり、従来方式と比べて本願発明による処理方式は高負荷でも処理性能は良好だった。
In the
循環ガス方式による処理可能な硫化水素負荷量について検証した。実験装置は、実施例1と同じ生物学的脱硫装置を用いた。循環ガス方式による処理可能な硫化水素負荷量に関する実験のガス処理条件および実験結果を表9に示す。
生物学的脱硫塔に流入するガスは、生物学的脱硫塔の頂部から下向流で流した。
循環液は、ポンプによって生物学的脱硫塔の上部へ送られ、ガス方向に並行して散水した。
硫化水素濃度3000ppmのバイオガスを用い、処理ガスを循環させて充填材接触ガス濃度が300ppmとなるように循環ガス量を調整した。
バイオガス中のメタン濃度は80%、二酸化炭素濃度は20%であり、実施期間を通してほぼ一定だった。
本実験の酸素含有気体供給量は、60L/dayとした。
散水量は、充填材が高湿度環境にあればよく、充填材に付着した微生物が処理の最中に微生物が活動できるのに十分な量とし、200L/dayとした。
処理温度についても同様に、反応に関与する微生物が活動できる環境下となるように、35℃に設定した。
充填材接触ガス流量ごとにRunとして区分けし、本実験は図3で示す生物学的脱硫装置を3機用いて並行して行なった。実験の評価期間は30日間とした。表中の実験結果の値は、評価30日目の値を記載した。
The hydrogen sulfide load that can be treated by the circulating gas method was verified. As the experimental apparatus, the same biological desulfurization apparatus as in Example 1 was used. Table 9 shows the gas treatment conditions and the experimental results of the experiment on the hydrogen sulfide load that can be treated by the circulating gas system.
The gas flowing into the biological desulfurization tower flowed downward from the top of the biological desulfurization tower.
The circulating liquid was sent to the upper part of the biological desulfurization tower by a pump and sprinkled in parallel with the gas direction.
Biogas having a hydrogen sulfide concentration of 3000 ppm was used, and the amount of circulating gas was adjusted so that the treatment gas was circulated and the filler contact gas concentration was 300 ppm.
The methane concentration in the biogas was 80% and the carbon dioxide concentration was 20%, which was almost constant throughout the implementation period.
The supply amount of oxygen-containing gas in this experiment was 60 L / day.
The amount of water spraying is sufficient if the filler is in a high-humidity environment. The amount of water sprayed is 200 L / day, which is sufficient for the microorganisms attached to the filler to be active during the treatment.
Similarly, the treatment temperature was set to 35 ° C. so as to be in an environment where microorganisms involved in the reaction could be active.
Each of the filler contact gas flow rates was classified as Run, and this experiment was performed in parallel using three biological desulfurization apparatuses shown in FIG. The evaluation period of the experiment was 30 days. The values of the experimental results in the table are the values on the 30th day of evaluation.
実験結果について説明する。
RunJ−4−1は、バイオガス流量2m3/dayに対して循環ガス量を18m3/day供給し、充填材接触ガス流量を20m3/dayにて処理した。接触時間は17secであり、硫化水素負荷量は2.0kg/(m3・day)だった。
処理ガスから硫化水素は検出されず、単位充填材当たりの硫化水素除去量は2.0kg/(m3・day)であり、硫酸転換率は100%だった。
The experimental results will be described.
RunJ-4-1 is
Hydrogen sulfide was not detected from the treated gas, the amount of hydrogen sulfide removed per unit filler was 2.0 kg / (m 3 · day), and the sulfuric acid conversion rate was 100%.
RunJ−4−2は、バイオガス流量3m3/dayに対して循環ガス量を27m3/day供給し、充填材接触ガス流量を30m3/dayにて処理した。接触時間は11secであり、硫化水素負荷量は3.0kg/(m3・day)だった。
処理ガスからは硫化水素は検出されず、単位充填材当たりの硫化水素除去量は3.0kg/(m3・day)であり、硫酸転換率は100%だった。
RunJ-4-2 is
Hydrogen sulfide was not detected from the treated gas, the amount of hydrogen sulfide removed per unit filler was 3.0 kg / (m 3 · day), and the sulfuric acid conversion rate was 100%.
RunJ−4−3では、バイオガス流量3.5m3/dayに対して循環ガス量31.5m3/day供給し、充填材接触ガス流量を35m3/dayにて処理した。接触時間は10secであり、硫化水素負荷量は3.5kg/(m3・day)だった。
処理ガスの硫化水素は150ppmであり、硫化水素除去率は95%となり、単位充填材当たりの硫化水素除去量は3.3kg/(m3・day)だった。硫酸転換率は100%だった。
In Run J-4-3, a circulating gas amount of 31.5 m 3 / day was supplied to a biogas flow rate of 3.5 m 3 / day, and the filler contact gas flow rate was treated at 35 m 3 / day. The contact time was 10 sec, and the hydrogen sulfide load was 3.5 kg / (m 3 · day).
The processing gas hydrogen sulfide was 150 ppm, the hydrogen sulfide removal rate was 95%, and the amount of hydrogen sulfide removed per unit filler was 3.3 kg / (m 3 · day). The sulfuric acid conversion rate was 100%.
RunJ−4−4では、バイオガス流量が4.0m3/dayに対して循環ガス量を36m3/day供給し、充填材接触ガス流量を40m3/dayにて処理した。接触時間は8secであり、硫化水素負荷量4.0kg/(m3・day)だった。処理ガス中の硫化水素濃度は300ppmであり、硫化水素除去率は90%となり、単位充填材当たりの硫化水素除去量は3.6kg/(m3・day)だった。硫酸転換率は100%だった。 In RunJ-4-4, biogas flow rate of the circulating gas volume and 36m 3 / day fed to 4.0 m 3 / day, a filler-contacting gas flow was treated with 40 m 3 / day. The contact time was 8 sec, and the hydrogen sulfide load was 4.0 kg / (m 3 · day). The concentration of hydrogen sulfide in the process gas was 300 ppm, the hydrogen sulfide removal rate was 90%, and the amount of hydrogen sulfide removed per unit filler was 3.6 kg / (m 3 · day). The sulfuric acid conversion rate was 100%.
RunJ−4−5では、バイオガス流量4.2m3/dayに対して循環ガス量を37.8m3/day供給し、充填材接触ガス流量を42m3/dayにて処理した。接触時間は7secであり、硫化水素負荷量は4.2kg/(m3・day)だった。処理ガス中の硫化水素濃度は2500ppmであり、硫化水素除去率は17%になり、単位充填材当たりの硫化水素除去量は0.7kg/(m3・day)だった。硫酸転換率も20%に著しく低下した。 In RunJ-4-5, the circulating gas amount to the biogas flow rate 4.2m 3 / day 37.8m 3 / to day supply, the filler contact the gas flow rate processed by 42m 3 / day. The contact time was 7 sec, and the hydrogen sulfide load was 4.2 kg / (m 3 · day). The concentration of hydrogen sulfide in the process gas was 2500 ppm, the hydrogen sulfide removal rate was 17%, and the amount of hydrogen sulfide removed per unit filler was 0.7 kg / (m 3 · day). The sulfuric acid conversion rate was also significantly reduced to 20%.
したがって、本願発明である循環ガス方式を適用し、実施例1にて高負荷で処理できた充填材接触ガスの硫化水素濃度を300ppmとなるようにバイオガス流量と循環ガス量を一定に供給して処理したところ、4.0kg/(m3・day)までの硫化水素負荷量であれば硫酸転換率が100%で処理できることがわかった。 Therefore, by applying the circulating gas method according to the present invention, the biogas flow rate and the circulating gas amount are constantly supplied so that the hydrogen sulfide concentration of the filler contact gas that can be processed at a high load in Example 1 is 300 ppm. As a result, it was found that the sulfuric acid conversion rate could be 100% when the hydrogen sulfide loading was 4.0 kg / (m 3 · day).
実験装置は、実施例1と同じ装置を用い、酸素含有気体供給量の制御方法に関する本願発明の効果について検証した。
生物学的脱硫塔に流入するガスは、生物学的脱硫塔の頂部から下向流で流した。
種汚泥として活性汚泥を用い、生物学的脱硫塔の下部の循環液貯留液槽に貯留し、ポンプによって生物学的脱硫塔の上部へ送られ、ガス方向に並行して散水した。
酸素含有気体は、空気(酸素として21v/v%)を用いた。
The experiment apparatus used the same apparatus as Example 1, and verified about the effect of this invention regarding the control method of oxygen-containing gas supply amount.
The gas flowing into the biological desulfurization tower flowed downward from the top of the biological desulfurization tower.
Activated sludge was used as seed sludge, stored in a circulating liquid storage tank at the bottom of the biological desulfurization tower, sent to the upper part of the biological desulfurization tower by a pump, and sprinkled in parallel with the gas direction.
Air (21 v / v% as oxygen) was used as the oxygen-containing gas.
本実験でのバイオガス中の硫化水素濃度は、時間ごとに次のように日変動した。
0:00〜 8:00(期間1): 1000ppm
8:00〜20:00(期間2): 6000ppm
20:00〜 0:00(期間3): 1000ppm
バイオガスの流量は、実験を通して1m3/dayで一定とした。
バイオガス中のメタン濃度は65%、二酸化炭素濃度は35%であり、実施期間を通してほぼ一定だった。
The hydrogen sulfide concentration in the biogas in this experiment fluctuated daily as follows for each hour.
0:00 to 8:00 (period 1): 1000 ppm
8:00 to 20:00 (period 2): 6000 ppm
20: 00 to 0:00 (period 3): 1000 ppm
The flow rate of biogas was fixed at 1 m 3 / day throughout the experiment.
The methane concentration in biogas was 65% and the carbon dioxide concentration was 35%, which were almost constant throughout the implementation period.
酸素含有気体供給量の制御方法に関する実験の条件および結果を表10に示す。
本願発明における酸素含有気体供給量は、硫化水素負荷量で制御する方式とした。
酸素含有気体供給量は、OOの1.5倍量を供給するように設定し、硫化水素負荷量の変動に合わせて酸素含有気体供給量を変更した。つまり、期間1および期間3において硫化水素負荷量が0.3kg/(m3・day)のとき、前述の(式8)〜(式10)に基づき10L/dayの酸素含有気体を供給した。
期間2において硫化水素負荷量が2.0kg/(m3・day)のとき、(式8)〜(式10)に基づき60L/dayの酸素含有気体を供給した。
Table 10 shows the experimental conditions and results regarding the method for controlling the oxygen-containing gas supply rate.
The oxygen-containing gas supply amount in the present invention is controlled by the hydrogen sulfide load amount.
The oxygen-containing gas supply amount was set to supply 1.5 times the amount of
In
一方、対照系列の酸素供給方式は、バイオガス流量に対して一定の割合の酸素含有気体を供給することとした。
つまり、本実験で供給した酸素含有気体供給量は、本願発明で供給した酸素含有気体供給量と同じとするため、実験を通して35L/dayで一定供給した。
On the other hand, the oxygen supply system of the control series decided to supply oxygen-containing gas at a certain ratio with respect to the biogas flow rate.
That is, since the oxygen-containing gas supply amount supplied in this experiment was the same as the oxygen-containing gas supply amount supplied in the present invention, a constant supply was made at 35 L / day throughout the experiment.
循環ガス量は、充填材接触ガス硫化水素濃度が300〜500ppmとなるように制御した。
散水量は、充填材が高湿度環境にあればよく、充填材に付着した微生物が処理の最中に微生物が活動できるのに十分な量とし、200L/dayとした。処理温度についても同様に、反応に関与する微生物が活動できる環境下となるように、35℃に設定した。充填材接触ガス濃度ごとにRunとして区分けし、本実験は図3で示す生物学的脱硫装置を2機用いて並行して行なった。評価期間は30日間とした。表中の実験結果の値は、評価30日目の値を記載した。
The amount of circulating gas was controlled so that the filler contact gas hydrogen sulfide concentration was 300 to 500 ppm.
The amount of water spraying is sufficient if the filler is in a high-humidity environment. The amount of water sprayed is 200 L / day, which is sufficient for the microorganisms attached to the filler to be active during the treatment. Similarly, the treatment temperature was set to 35 ° C. so as to be in an environment where microorganisms involved in the reaction could be active. Each of the filler contact gas concentrations was classified as Run, and this experiment was performed in parallel using two biological desulfurization apparatuses shown in FIG. The evaluation period was 30 days. The values of the experimental results in the table are the values on the 30th day of evaluation.
実験結果について説明する。
本願発明の期間1および期間3において、酸素含有気体供給量を10L/dayとしたとき、硫化水素除去率は100%、硫酸転換率は100%で処理できた。
対照系列の期間1および期間3において、酸素含有気体供給量を35L/dayとしたとき、硫化水素除去率100%であり、硫酸転換率は100%だった。
本願発明の期間2において、酸素含有気体供給量を60L/dayとしたとき、硫化水素除去率は100%、硫酸転換率は100%で処理できた。
対照系列の期間2において、酸素含有気体供給量を35L/dayとしたとき、処理ガス中の硫化水素濃度は1000ppmであり、硫化水素除去率83%で、硫酸転換率は80%だった。
The experimental results will be described.
In
In period 1 and
In
In
本願発明での期間1および期間3では、酸素含有気体供給量がバイオガス流量1m3/dayに対し10L/dayであれば硫酸転換率は100%となり、これ以上の酸素は必要ない。本期間でのバイオガス中のメタン濃度は65%であり、処理ガス中のメタン濃度は64.3%だった。
対照系列での期間1および期間3では、酸素含有気体供給量がバイオガス流量1m3/dayに対し35L/dayと本願発明の3.5倍供給しており、未処理の酸素が処理ガス中に余分に含まれる。本期間でのバイオガス中のメタン濃度は65%であり、処理ガス中のメタン濃度は、62.8%となり、バイオガスの燃料としての価値が低下し、ボイラー等の失火の危険性がある。
In the period 1 and the
In period 1 and
本願発明のように硫化水素負荷量で酸素含有気体供給量を制御する方が負荷に追随して適切な量の酸素含有気体が供給され、硫化水素の除去性能および硫酸転換率も100%で処理でき、安定処理できた。
したがって、バイオガスへの酸素含有気体供給量の制御方式は、硫化水素負荷量の変動に合わせて処理する方が優位性は確認された。
Controlling the oxygen-containing gas supply amount with the hydrogen sulfide load amount as in the present invention allows an appropriate amount of oxygen-containing gas to be supplied following the load, and the hydrogen sulfide removal performance and sulfuric acid conversion rate are also 100%. And stable processing.
Therefore, it was confirmed that the method of controlling the supply amount of oxygen-containing gas to biogas is superior in that it is processed according to the fluctuation of the hydrogen sulfide load.
次に、対象比較で酸素量を増やしたときの除去性能について検討した。
酸素含有気体供給量の制御方法に関する比較実験のガス処理条件および実験結果を表11に示す。
Next, the removal performance when the amount of oxygen was increased in the target comparison was examined.
Table 11 shows gas treatment conditions and experimental results of a comparative experiment regarding the method of controlling the oxygen-containing gas supply rate.
本願発明の実験条件は、実施例2と同じである。
対照系列では、酸素含有気体供給量を35L/dayから60L/dayに変更して一定供給した。表中の実験結果の値は、評価期間30日目の値を記載した。
The experimental conditions of the present invention are the same as in Example 2.
In the control series, the oxygen-containing gas supply amount was changed from 35 L / day to 60 L / day, and a constant supply was made. The value of the experimental result in the table is the value on the 30th day of the evaluation period.
実験結果について説明する。
本願発明の期間1および期間3において、酸素含有気体供給量を10L/dayとしたとき、硫化水素除去率は100%、硫酸転換率は100%で処理できた。期間2において、酸素含有気体供給量を60L/dayとしたとき、硫化水素除去率は100%、硫酸転換率は100%で処理できた。
対照系列の期間1および期間3において、酸素含有気体供給量を60L/dayとしたとき、硫化水素除去率100%であり、硫酸転換率は100%だった。期間2において、酸素含有気体供給量を60L/dayとしたとき、硫化水素除去率100%で処理でき、硫酸転換率も100%で処理できた。
The experimental results will be described.
In
In period 1 and
本願発明の期間1および期間3での酸素含有気体供給量(バイオガス流量1m3/dayに対し10L/day)であれば、硫酸転換率は100%となり、これ以上の酸素は必要ない。本期間でのバイオガス中のメタン濃度は65%であり、処理ガス中のメタン濃度は64.3%だった。
これに対し、対照系列の期間1および期間3での酸素含有気体供給量は、本願発明の6倍(バイオガス流量1m3/dayに対し60L/day)供給しており、対照系列の処理ガス中には未処理の酸素が余分に含まれる。本期間でのバイオガス中のメタン濃度は65%であり、処理ス中のメタン濃度は61.0%となり、バイオガスの燃料としての価値が低下し、ボイラー等の失火の危険性がある。
If the oxygen-containing gas supply amount in the period 1 and
In contrast, the supply amount of oxygen-containing gas in the period 1 and
以上説明したように、本願発明によれば、高負荷での硫化水素を効率的に処理し、且つ処理する硫化水素を硫酸に転換することで装置内の閉塞をなくし、洗浄などの工程をなくして低コストで処理が可能なバイオガスの生物学的脱硫装置及び生物学的脱硫方法を提供することが可能となる。 As described above, according to the present invention, hydrogen sulfide under a high load is efficiently treated, and the hydrogen sulfide to be treated is converted into sulfuric acid, thereby eliminating clogging in the apparatus and eliminating steps such as cleaning. It is possible to provide a biogas biological desulfurization apparatus and a biological desulfurization method that can be processed at low cost.
0a バイオガス
0b 酸素含有気体
0c 処理ガス
0d 循環液
0e ブロー水
0f 補給水
0g 希釈ガス
1 生物学的脱硫塔
1a 充填層
1b 循環液貯留液槽
2 バイオガス流入ライン
3 硫化水素濃度計
4 ガス流量計
5 酸素含有気体流入ライン
6 酸素含有気体供給調節機構
7 処理ガス流出ライン
8 循環ガスライン
9 循環ガス量調節機構
10 散水ライン
11 演算器
12 循環ガスの信号伝達機構
13 酸素含有気体の信号伝達機構
14 希釈ガスライン
15 硫化水素濃度信号入力ライン
16 ガス流量信号入力ライン
0a Biogas 0b Oxygen-containing gas 0c Treatment gas 0d Circulating fluid 0e Blow water 0f Make-up water 0g Diluted gas 1
Claims (6)
該生物学的脱硫塔の端部よりバイオガスを流入するためのバイオガス流入ラインを設け、
該生物学的脱硫塔内に微生物が付着する充填材からなる充填層を設け、
該生物学的脱硫塔のもう一方の端部であり該充填層の後段に処理ガスを排出するための処理ガス流出ラインを設け、
該処理ガスの一部を生物学的脱硫塔の前記バイオガスが流入する端部に循環するための循環ガスラインを設け、
該バイオガス流入ラインに硫化水素濃度計とガス流量計を設け、
該循環ガスラインに循環ガス量の調節機構を設け、
該硫化水素濃度計によるバイオガスの硫化水素濃度値と、該ガス流量計によるガス流量値から硫化水素負荷量を算出するための演算器を設け、
該演算器の硫化水素負荷量の算出結果により、充填材に接触するガスの硫化水素濃度が所定の範囲内となるように、前記循環ガス量の調節機構を作動させる循環ガスの信号伝達機構を具備することを特徴とする生物学的脱硫装置。 In a biological desulfurization apparatus that biologically removes hydrogen sulfide by sprinkling a circulating liquid from biogas generated by methane fermentation of organic waste into a biological desulfurization tower,
A biogas inflow line for introducing biogas from the end of the biological desulfurization tower is provided;
Providing a packed bed made of a filler to which microorganisms adhere in the biological desulfurization tower;
A processing gas outlet line for discharging the processing gas at the other end of the biological desulfurization tower and after the packed bed;
Providing a circulation gas line for circulating a part of the processing gas to an end of the biological desulfurization tower into which the biogas flows;
A hydrogen sulfide concentration meter and a gas flow meter are provided in the biogas inflow line,
Provide a circulating gas amount adjusting mechanism in the circulating gas line,
An arithmetic unit for calculating a hydrogen sulfide load amount from the hydrogen sulfide concentration value of the biogas by the hydrogen sulfide concentration meter and the gas flow value by the gas flow meter is provided,
A circulating gas signal transmission mechanism for operating the circulating gas amount adjusting mechanism so that the hydrogen sulfide concentration of the gas contacting the filler is within a predetermined range based on the calculation result of the hydrogen sulfide load amount of the computing unit. A biological desulfurization apparatus comprising:
該酸素含有気体流入ラインに酸素含有気体量の供給調節機構を設け、
該演算器の硫化水素負荷量の算出結果により、前記酸素含有気体量の供給調節機構を作動させる酸素含有気体の信号伝達機構を設けたことを特徴とする請求項1に記載の生物学的脱硫装置。 Providing an oxygen-containing gas inflow line for flowing an oxygen-containing gas into the biogas inflow line and / or the biological desulfurization tower;
A supply adjustment mechanism for the oxygen-containing gas amount is provided in the oxygen-containing gas inflow line,
2. The biological desulfurization according to claim 1, further comprising an oxygen-containing gas signal transmission mechanism for operating the oxygen-containing gas amount supply adjustment mechanism based on a calculation result of the hydrogen sulfide load amount of the computing unit. apparatus.
該生物学的脱硫塔内に微生物が付着する充填材からなる充填層を設け、
該生物学的脱硫塔内の該充填層の上流側にバイオガスを流入するバイオガス流入工程と、
該生物学的脱硫塔内の該充填層の下流側に処理ガスを排出する処理ガス流出工程と、
該処理ガスの一部を該生物学的脱硫塔内の該充填層の上流側に循環する循環ガス工程とを有し、
該バイオガス流入工程における流入されるバイオガスの硫化水素濃度とガス流量とから、硫化水素負荷量を算出し、該算出結果により、充填材に接触するガスの硫化水素濃度が所定の範囲内となるように、該循環ガス工程の循環ガス量を調節することを特徴とする生物学的脱硫方法。 In the biological desulfurization method of removing hydrogen sulfide biologically by sprinkling the circulating liquid into the biological desulfurization tower from the biogas generated by methane fermentation of organic waste,
Providing a packed bed made of a filler to which microorganisms adhere in the biological desulfurization tower;
A biogas inflow step of flowing biogas upstream of the packed bed in the biological desulfurization tower;
A process gas outflow step for discharging a process gas downstream of the packed bed in the biological desulfurization tower;
A circulating gas step for circulating a part of the processing gas upstream of the packed bed in the biological desulfurization tower,
The hydrogen sulfide load amount is calculated from the hydrogen sulfide concentration and gas flow rate of the biogas flowing in the biogas inflow step, and the calculation result shows that the hydrogen sulfide concentration of the gas contacting the filler is within a predetermined range. Thus, the biological desulfurization method characterized by adjusting the amount of circulating gas in the circulating gas step.
前記硫化水素負荷量の算出結果により、該酸素含有気体流入工程における酸素含有気体の供給量を調節することを特徴とする請求項3に記載の生物学的脱硫方法。 An oxygen-containing gas inflow step for flowing an oxygen-containing gas upstream of the packed bed in the biological desulfurization tower;
The biological desulfurization method according to claim 3, wherein the supply amount of the oxygen-containing gas in the oxygen-containing gas inflow step is adjusted based on the calculation result of the hydrogen sulfide load.
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012147623A JP5756060B2 (en) | 2012-06-29 | 2012-06-29 | Biogas biological desulfurization apparatus and biological desulfurization method |
| PCT/JP2013/067186 WO2014002926A1 (en) | 2012-06-29 | 2013-06-24 | Device and method for biological desulfurization of biogas |
| SG11201408671QA SG11201408671QA (en) | 2012-06-29 | 2013-06-24 | Device and method for biological desulfurization of biogas |
| MYPI2014703904A MY182278A (en) | 2012-06-29 | 2013-06-24 | Device and method for biological desulfurization from biogas |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012147623A JP5756060B2 (en) | 2012-06-29 | 2012-06-29 | Biogas biological desulfurization apparatus and biological desulfurization method |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015108933A Division JP6215257B2 (en) | 2015-05-28 | 2015-05-28 | Biological desulfurization method of biogas |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2014008466A JP2014008466A (en) | 2014-01-20 |
| JP2014008466A5 JP2014008466A5 (en) | 2015-05-28 |
| JP5756060B2 true JP5756060B2 (en) | 2015-07-29 |
Family
ID=49783073
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012147623A Active JP5756060B2 (en) | 2012-06-29 | 2012-06-29 | Biogas biological desulfurization apparatus and biological desulfurization method |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP5756060B2 (en) |
| MY (1) | MY182278A (en) |
| SG (1) | SG11201408671QA (en) |
| WO (1) | WO2014002926A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5767723B2 (en) * | 2014-01-16 | 2015-08-19 | 荏原実業株式会社 | Biological desulfurization apparatus and biological desulfurization method |
| JP5767722B2 (en) * | 2014-01-16 | 2015-08-19 | 荏原実業株式会社 | Biological desulfurization apparatus and desulfurization method |
| JP6170197B1 (en) * | 2016-02-29 | 2017-07-26 | 荏原実業株式会社 | Desulfurization system and desulfurization method |
| CN110157512A (en) * | 2019-06-27 | 2019-08-23 | 山东清沂山石化科技有限公司 | A kind of processing unit and method of the device in Gas that PETROLEUM PROCESSING generates |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3802161B2 (en) * | 1996-10-25 | 2006-07-26 | 株式会社タクマ | Biological treatment of malodorous gases |
| JP2006143780A (en) * | 2004-11-16 | 2006-06-08 | Toshiba Corp | Biogas purification system |
| JP4344773B1 (en) * | 2008-07-22 | 2009-10-14 | 株式会社神鋼環境ソリューション | Digestion gas desulfurization method and apparatus |
-
2012
- 2012-06-29 JP JP2012147623A patent/JP5756060B2/en active Active
-
2013
- 2013-06-24 MY MYPI2014703904A patent/MY182278A/en unknown
- 2013-06-24 SG SG11201408671QA patent/SG11201408671QA/en unknown
- 2013-06-24 WO PCT/JP2013/067186 patent/WO2014002926A1/en active Application Filing
Also Published As
| Publication number | Publication date |
|---|---|
| MY182278A (en) | 2021-01-18 |
| WO2014002926A1 (en) | 2014-01-03 |
| SG11201408671QA (en) | 2015-01-29 |
| JP2014008466A (en) | 2014-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5756060B2 (en) | Biogas biological desulfurization apparatus and biological desulfurization method | |
| JP6170197B1 (en) | Desulfurization system and desulfurization method | |
| JP5756061B2 (en) | Biological desulfurization apparatus and desulfurization method for biogas | |
| EP3333130A1 (en) | Water treatment system, power generation plant, and method for controlling water treatment system | |
| Ramos et al. | Where does the removal of H2S from biogas occur in microaerobic reactors? | |
| JP2006037074A (en) | Method for removing sulfur compound from biogas | |
| JP3750648B2 (en) | Digestion gas desulfurization apparatus and desulfurization method | |
| CN103977703A (en) | Biogas biological desulfurization system | |
| JP6215257B2 (en) | Biological desulfurization method of biogas | |
| JP2014506528A (en) | Apparatus and method for reducing the content of hydrogen peroxide and peracetic acid in a water stream | |
| JP2015214695A (en) | Apparatus and method for biologically desulfurizing biogas | |
| JP2008094985A (en) | Organism desulfurization method and organism desulfurization apparatus | |
| JP6752181B2 (en) | Desulfurization system and desulfurization method | |
| JP6101740B2 (en) | Biological desulfurization method of biogas | |
| JP5925179B2 (en) | Biological desulfurization apparatus and biological desulfurization method | |
| JP5767723B2 (en) | Biological desulfurization apparatus and biological desulfurization method | |
| CN201308814Y (en) | Biological desulphurization treatment apparatus | |
| US11420883B2 (en) | Method and system for controlling odor in water system | |
| JP4199394B2 (en) | Control method of absorbent concentration in thiosulfate denitration method | |
| JP5767722B2 (en) | Biological desulfurization apparatus and desulfurization method | |
| JP6430451B2 (en) | Wet deodorization apparatus and deodorization method | |
| JP2004351262A (en) | Method and apparatus for wet type flue gas desulfurization | |
| JP7030582B2 (en) | Desulfurization equipment and biogas desulfurization method | |
| WO2023243236A1 (en) | Wastewater treatment method and wastewater treatment apparatus | |
| JPS6330080B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20141208 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150410 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20150410 |
|
| TRDD | Decision of grant or rejection written | ||
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20150423 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150428 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150528 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5756060 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |