JP5560570B2 - 創傷治療用医薬組成物 - Google Patents
創傷治療用医薬組成物 Download PDFInfo
- Publication number
- JP5560570B2 JP5560570B2 JP2009042671A JP2009042671A JP5560570B2 JP 5560570 B2 JP5560570 B2 JP 5560570B2 JP 2009042671 A JP2009042671 A JP 2009042671A JP 2009042671 A JP2009042671 A JP 2009042671A JP 5560570 B2 JP5560570 B2 JP 5560570B2
- Authority
- JP
- Japan
- Prior art keywords
- hyp
- pharmaceutical composition
- gly
- dipeptide
- peptide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 208000027418 Wounds and injury Diseases 0.000 title claims description 35
- 206010052428 Wound Diseases 0.000 title claims description 34
- 239000008194 pharmaceutical composition Substances 0.000 title claims description 22
- 108010050297 hydroxyprolyl-glycine Proteins 0.000 claims description 25
- 239000000126 substance Substances 0.000 claims description 11
- WFDSWNXTPKLLOT-UHNVWZDZSA-N 2-[[(2s,4r)-4-hydroxypyrrolidin-1-ium-2-carbonyl]amino]acetate Chemical compound O[C@H]1CN[C@H](C(=O)NCC(O)=O)C1 WFDSWNXTPKLLOT-UHNVWZDZSA-N 0.000 claims description 9
- 108010016626 Dipeptides Proteins 0.000 claims description 9
- 239000003102 growth factor Substances 0.000 claims description 6
- 239000000843 powder Substances 0.000 claims description 6
- 239000013543 active substance Substances 0.000 claims description 4
- 230000000202 analgesic effect Effects 0.000 claims description 4
- 230000002924 anti-infective effect Effects 0.000 claims description 4
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 4
- 239000002552 dosage form Substances 0.000 claims description 4
- 230000002439 hemostatic effect Effects 0.000 claims description 4
- 239000011248 coating agent Substances 0.000 claims description 3
- 239000006071 cream Substances 0.000 claims description 3
- 239000006196 drop Substances 0.000 claims description 3
- 239000000839 emulsion Substances 0.000 claims description 3
- 239000000499 gel Substances 0.000 claims description 3
- 239000007788 liquid Substances 0.000 claims description 3
- 239000002674 ointment Substances 0.000 claims description 3
- 239000007921 spray Substances 0.000 claims description 3
- 125000003275 alpha amino acid group Chemical group 0.000 claims 2
- 102000008186 Collagen Human genes 0.000 description 16
- 108010035532 Collagen Proteins 0.000 description 16
- 229920001436 collagen Polymers 0.000 description 16
- 108090000765 processed proteins & peptides Proteins 0.000 description 16
- 210000002950 fibroblast Anatomy 0.000 description 13
- 230000001737 promoting effect Effects 0.000 description 12
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 8
- ONPXCLZMBSJLSP-CSMHCCOUSA-N Pro-Hyp Chemical compound C1[C@H](O)C[C@@H](C(O)=O)N1C(=O)[C@H]1NCCC1 ONPXCLZMBSJLSP-CSMHCCOUSA-N 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 238000000034 method Methods 0.000 description 7
- 239000003814 drug Substances 0.000 description 6
- 230000035755 proliferation Effects 0.000 description 6
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- 241000699670 Mus sp. Species 0.000 description 5
- 230000010261 cell growth Effects 0.000 description 5
- 230000012292 cell migration Effects 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 230000005012 migration Effects 0.000 description 5
- 238000013508 migration Methods 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 239000001569 carbon dioxide Substances 0.000 description 4
- 229910002092 carbon dioxide Inorganic materials 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 3
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 3
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 3
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 3
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 3
- 229930182566 Gentamicin Natural products 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 3
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- 229930182816 L-glutamine Natural products 0.000 description 3
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 3
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000000512 collagen gel Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 239000012091 fetal bovine serum Substances 0.000 description 3
- 229940126864 fibroblast growth factor Drugs 0.000 description 3
- 229960002518 gentamicin Drugs 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 238000010647 peptide synthesis reaction Methods 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 230000029663 wound healing Effects 0.000 description 3
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 2
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 2
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 239000004372 Polyvinyl alcohol Substances 0.000 description 2
- 206010072170 Skin wound Diseases 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 108010009583 Transforming Growth Factors Proteins 0.000 description 2
- 102000009618 Transforming Growth Factors Human genes 0.000 description 2
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 230000003187 abdominal effect Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229960002684 aminocaproic acid Drugs 0.000 description 2
- CUFNKYGDVFVPHO-UHFFFAOYSA-N azulene Chemical compound C1=CC=CC2=CC=CC2=C1 CUFNKYGDVFVPHO-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 238000010609 cell counting kit-8 assay Methods 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 230000003301 hydrolyzing effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- WEXRUCMBJFQVBZ-UHFFFAOYSA-N pentobarbital Chemical compound CCCC(C)C1(CC)C(=O)NC(=O)NC1=O WEXRUCMBJFQVBZ-UHFFFAOYSA-N 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 230000037380 skin damage Effects 0.000 description 2
- 210000001626 skin fibroblast Anatomy 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- CIVCELMLGDGMKZ-UHFFFAOYSA-N 2,4-dichloro-6-methylpyridine-3-carboxylic acid Chemical compound CC1=CC(Cl)=C(C(O)=O)C(Cl)=N1 CIVCELMLGDGMKZ-UHFFFAOYSA-N 0.000 description 1
- QEDRTIXTEAXNMY-XVMARJQXSA-N 2-[[(2s,4r)-1-[(2s)-2-aminopropanoyl]-4-hydroxypyrrolidine-2-carbonyl]amino]acetic acid Chemical compound C[C@H](N)C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(O)=O QEDRTIXTEAXNMY-XVMARJQXSA-N 0.000 description 1
- RMMXTBMQSGEXHJ-UHFFFAOYSA-N Aminophenazone Chemical compound O=C1C(N(C)C)=C(C)N(C)N1C1=CC=CC=C1 RMMXTBMQSGEXHJ-UHFFFAOYSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 108010022901 Heparin Lyase Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- WJSNJMXOBDSZDL-WOPDTQHZSA-N L-phenylalanyl-L-hydroxyproline Chemical compound C([C@H](N)C(=O)N1[C@@H](C[C@@H](O)C1)C(O)=O)C1=CC=CC=C1 WJSNJMXOBDSZDL-WOPDTQHZSA-N 0.000 description 1
- VTAJIXDZFCRWBR-UHFFFAOYSA-N Licoricesaponin B2 Natural products C1C(C2C(C3(CCC4(C)CCC(C)(CC4C3=CC2)C(O)=O)C)(C)CC2)(C)C2C(C)(C)CC1OC1OC(C(O)=O)C(O)C(O)C1OC1OC(C(O)=O)C(O)C(O)C1O VTAJIXDZFCRWBR-UHFFFAOYSA-N 0.000 description 1
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 1
- 208000004210 Pressure Ulcer Diseases 0.000 description 1
- 108010094028 Prothrombin Proteins 0.000 description 1
- 102100027378 Prothrombin Human genes 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 102000002262 Thromboplastin Human genes 0.000 description 1
- 235000018936 Vitellaria paradoxa Nutrition 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 150000001413 amino acids Chemical group 0.000 description 1
- 229960000212 aminophenazone Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 229940030225 antihemorrhagics Drugs 0.000 description 1
- 239000002473 artificial blood Substances 0.000 description 1
- 235000013361 beverage Nutrition 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 230000037182 bone density Effects 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 230000035605 chemotaxis Effects 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- -1 coatings Substances 0.000 description 1
- 230000011382 collagen catabolic process Effects 0.000 description 1
- 230000037319 collagen production Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000002316 cosmetic surgery Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000000249 desinfective effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 229960000525 diphenhydramine hydrochloride Drugs 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- SBNKFTQSBPKMBZ-UHFFFAOYSA-N ethenzamide Chemical compound CCOC1=CC=CC=C1C(N)=O SBNKFTQSBPKMBZ-UHFFFAOYSA-N 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 230000019305 fibroblast migration Effects 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- LPLVUJXQOOQHMX-UHFFFAOYSA-N glycyrrhetinic acid glycoside Natural products C1CC(C2C(C3(CCC4(C)CCC(C)(CC4C3=CC2=O)C(O)=O)C)(C)CC2)(C)C2C(C)(C)C1OC1OC(C(O)=O)C(O)C(O)C1OC1OC(C(O)=O)C(O)C(O)C1O LPLVUJXQOOQHMX-UHFFFAOYSA-N 0.000 description 1
- 229960004949 glycyrrhizic acid Drugs 0.000 description 1
- 239000001685 glycyrrhizic acid Substances 0.000 description 1
- UYRUBYNTXSDKQT-UHFFFAOYSA-N glycyrrhizic acid Natural products CC1(C)C(CCC2(C)C1CCC3(C)C2C(=O)C=C4C5CC(C)(CCC5(C)CCC34C)C(=O)O)OC6OC(C(O)C(O)C6OC7OC(O)C(O)C(O)C7C(=O)O)C(=O)O UYRUBYNTXSDKQT-UHFFFAOYSA-N 0.000 description 1
- 235000019410 glycyrrhizin Nutrition 0.000 description 1
- LPLVUJXQOOQHMX-QWBHMCJMSA-N glycyrrhizinic acid Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@H](O[C@@H]1O[C@@H]1C([C@H]2[C@]([C@@H]3[C@@]([C@@]4(CC[C@@]5(C)CC[C@@](C)(C[C@H]5C4=CC3=O)C(O)=O)C)(C)CC2)(C)CC1)(C)C)C(O)=O)[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O LPLVUJXQOOQHMX-QWBHMCJMSA-N 0.000 description 1
- 235000013402 health food Nutrition 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 239000002874 hemostatic agent Substances 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000012567 medical material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- DXGLGDHPHMLXJC-UHFFFAOYSA-N oxybenzone Chemical compound OC1=CC(OC)=CC=C1C(=O)C1=CC=CC=C1 DXGLGDHPHMLXJC-UHFFFAOYSA-N 0.000 description 1
- 229960001173 oxybenzone Drugs 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 229960001412 pentobarbital Drugs 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940039716 prothrombin Drugs 0.000 description 1
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 230000036560 skin regeneration Effects 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000009772 tissue formation Effects 0.000 description 1
- 230000007838 tissue remodeling Effects 0.000 description 1
- GYDJEQRTZSCIOI-LJGSYFOKSA-N tranexamic acid Chemical compound NC[C@H]1CC[C@H](C(O)=O)CC1 GYDJEQRTZSCIOI-LJGSYFOKSA-N 0.000 description 1
- 229960000401 tranexamic acid Drugs 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 150000003952 β-lactams Chemical class 0.000 description 1
Images
Landscapes
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Description
また、コラーゲンは一般にブタ、ウシなどの動物由来であり、感染性物質の混入のリスクが否定できない。
[1]アミノ酸配列がHyp−Glyで表されるジペプチドまたはその薬理学的に許容される誘導体を含有することを特徴とする、創傷治療のための医薬組成物。
[2]経口、皮下または経皮により投与されることを特徴とする、[1]に記載の医薬組成物。
[3]前記ペプチドまたはその誘導体の含有量が0.1〜50重量%である、[1]または[2]に記載の医薬組成物。
[4]前記ペプチドまたはその誘導体の含有量が0.5〜10重量%である、[1]または[2]に記載の医薬組成物。
[5]剤形が、クリーム剤、ゲル剤、軟膏剤、液剤、粉剤、塗布剤、乳剤、噴霧剤、滴剤、散剤、貼付剤またはドレッシング剤である、[1]〜[4]のいずれかに記載の医薬組成物。
[6]さらに、止血活性物質、成長因子、抗感染物質、鎮痛物質および抗炎症物質からなる群より選択される一種類以上を含む、[1]〜[5]のいずれかに記載の医薬組成物。
Hyp−Glyは、固相ペプチド合成法、液相ペプチド合成法などの、一般的なペプチド合成法によって得られる。また、ブタ、ウシ、馬などの動物由来コラーゲンを、希塩酸、希硫酸などの酸性溶液、もしくは水酸化ナトリウム溶液、水酸化カリウム溶液などのアルカリ溶液などで加水分解することによってもHyp−Glyジペプチドを得ることができる。このようにして得られたHyp−Glyジペプチドを含む混合物は、ゲルろ過などの一般的な方法により、Hyp−Glyジペプチドを精製することが望ましい。
ここで、担体としては薬理学的に許容されるものであればよいが、可溶化剤、pH調整剤、賦形剤、保存剤、乳化剤、防腐剤、色素、矯味矯臭剤、増粘剤などが例示される。
用いられるものであれば種類を問わない。例えば、ポリビニルアルコール、グリセロール、キトサン、カルボキシメチルセルロース、コラーゲン、ヒアルロン酸、ポリプロピレングリコールなどが挙げられる。
ここで、止血活性物質としては、プロトロンビン、トロンビン、フィブリノーゲン、フィブリン、フィブロネクチン、ヘパリナーゼが例示される。
また、成長因子としては、上皮細胞増殖因子(EGF)、トランスフォーミング増殖因子(TGF)、線維芽細胞増殖因子(FGF)、血管内皮細胞増殖因子(VEGF)、血小板由来増殖因子(PDGF)が例示される。
また、抗感染物質としては、クロラムフェニコール、マクロライド、キノロン、β−ラクタム、テロラサイクリン、サルファ剤が例示される。
また、鎮痛物質としては、アスピリン、アミノピリン、アセトアミノフェン、エテンザミドが例示される。
また、抗炎症物質としては、オキシベンゾン、トラネキサム酸及びその誘導体、イプシロンアミノカプロン酸、グリチルリチン酸、アズレン、塩酸ジフェンヒドラミン、アミノカプロン酸が例示される。
Hyp−Glyジペプチド又はその誘導体は、医薬組成物中に0.1〜50重量%の割合で含まれることが好ましく、0.5〜10重量%の割合で含まれることがより好ましい。本発明の創傷治療用医薬組成物は、経口、皮下または経皮などの方法により投与することができる。
投与量は創傷の大きさや重傷度などに応じて適宜設定することができる。
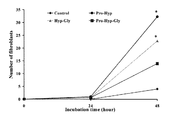
細胞遊走性試験
5週齢のBALB/cマウス(雄)の腹部の皮膚を、3mmのディスクに切り取って、DMEM(Dulbecco's Modified Eagle's Medium)中に採取した。その後、24穴のプラスチックプレートに移し、584μg/mLのL−グルタミン、0.01mg/mLのゲンタマイシン、200nmol/mLの検体(Hyp−Glyジペプチド、Pro−HypジペプチドまたはPro−Hyp−Glyトリペプチド)を含むDMEMを加え、37℃、5容量%炭酸ガス存在下に置いた。その後、マウス皮膚のディスクから1mm以上の距離を遊走した線維芽細胞の数を24時間ごとに顕微鏡により測定した。
結果を図1に示す。図1より、ペプチドを加えないコントロールに比べ、ペプチドを加えたディスクでは細胞遊走促進効果が高いことが分かる。
細胞増殖試験
BALB/cマウスの腹部の皮膚を6−7mm程の幅に培養皿(直径75mm)に切り採り、584μg/mLのL−グルタミン、0.01mg/mLのゲンタマイシン、10容量%のFBS(ウシ胎児血清)を含む8mLのDMEMを加え、37℃、5容量%炭酸ガス存在下で培養した。培養の間、2日ごとに培地を交換し、2週間後に皮膚を取り除き、線維芽細胞を0.25%トリプシン−EDTA溶液で剥離回収した。これを、584μg/mLのL−グルタミン、0.01mg/mLのゲンタマイシン、10容量%のFBS、および、200nmol/mLの検体(Hyp−Glyジペプチド、Pro−HypジペプチドまたはPro−Hyp−Glyトリペプチド)を含むDMEMを加えて5×104
個/mLとなるように調製し、予め作成しておいたコラーゲンゲル(96穴のプラスチックプレートに、0.5%のコラーゲン溶液を含むDMEM100μLを加えて、37℃、5容量%炭酸ガス存在下で静置して調製)に播種し、37℃、5容量%炭酸ガス存在下で培養した。144時間培養した後、Cell Counting Kit−8(同仁化学)を用いて450nmの吸光度を測定することにより細胞数を計測した。
結果を図2に示す。これより、コラーゲンゲル上で、Hyp−Glyジペプチドは、線維芽細胞の増殖を促進することが分かる。
マウスを用いた創傷治療効果の評価
皮膚の全層欠損創傷治癒の実験用としてICRマウス雌(8週齢)を予備飼育した。ペントバルビタール麻酔下でマウスの背部を剪毛し、エタノールで清拭後、背部正中部に生検トレバンφ3mmにて全層欠損創を4箇所作製し、面積を測定した。創面に、最終濃度2%の検体(Hyp−GlyジペプチドまたはPro−Hyp−Glyトリペプチド)と7.5%ポリビニルアルコールを含む溶液10μLを滴下投与した後、防水絆創膏(ニチバン
シアテープ)をはり、周りを瞬間接着剤で密閉した。3日の後、マウスを安楽死させ、創傷部の面積を測定した。各ペプチドを用いたときの、創傷の縮小面積を図3に示した。図3より、コントロール(ペプチドなし)やPro−Hyp−Glyトリペプチドに比べ、Hyp−Glyジペプチドは、創傷の縮小に効果を示すことが分かる。
Claims (6)
- アミノ酸配列がHyp−Glyで表されるジペプチドを含有し、経皮により投与されることを特徴とする、創傷治療のための医薬組成物。
- 剤形が、クリーム剤、ゲル剤、軟膏剤、液剤、粉剤、塗布剤、乳剤、噴霧剤、滴剤、散剤、貼付剤またはドレッシング剤である、請求項1に記載の医薬組成物。
- アミノ酸配列がHyp−Glyで表されるジペプチドを含有し、剤形が、塗布剤、貼付剤またはドレッシング剤である、創傷治療のための医薬組成物。
- 前記ジペプチドの含有量が0.1〜50重量%である、請求項1〜3のいずれか一項に記載の医薬組成物。
- 前記ジペプチドの含有量が0.5〜10重量%である、請求項1〜3のいずれか一項に記載の医薬組成物。
- さらに、止血活性物質、成長因子、抗感染物質、鎮痛物質および抗炎症物質からなる群より選択される一種類以上を含む、請求項1〜5のいずれか一項に記載の医薬組成物。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009042671A JP5560570B2 (ja) | 2009-02-25 | 2009-02-25 | 創傷治療用医薬組成物 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009042671A JP5560570B2 (ja) | 2009-02-25 | 2009-02-25 | 創傷治療用医薬組成物 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010195714A JP2010195714A (ja) | 2010-09-09 |
| JP2010195714A5 JP2010195714A5 (ja) | 2012-01-05 |
| JP5560570B2 true JP5560570B2 (ja) | 2014-07-30 |
Family
ID=42820835
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009042671A Expired - Fee Related JP5560570B2 (ja) | 2009-02-25 | 2009-02-25 | 創傷治療用医薬組成物 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5560570B2 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5459826B2 (ja) * | 2009-03-03 | 2014-04-02 | 林兼産業株式会社 | 皮膚線維芽細胞におけるエラスチン産生促進剤及び血管内皮細胞の増殖促進剤 |
| JP6709440B2 (ja) * | 2018-06-08 | 2020-06-17 | 学校法人福岡大学 | 肥厚性瘢痕の形成抑制用組成物 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1987005219A1 (en) * | 1986-02-28 | 1987-09-11 | Bio-Tech A/S | Pharmaceutical preparation containing a dipeptide with cell growth regultating effect |
| GB2321191B (en) * | 1997-01-17 | 2000-09-27 | Johnson & Johnson Medical | Peptides for use in wound treatment |
| JP2002255847A (ja) * | 2001-02-26 | 2002-09-11 | Miyagi Kagaku Kogyo Kk | コラーゲン産生促進剤、それを含む機能性食品および医薬品 |
| JP2003137807A (ja) * | 2001-11-01 | 2003-05-14 | Miyagi Kagaku Kogyo Kk | コラーゲン産生促進剤、それを含む化粧品、食品および医薬品ならびに皮膚疾患の予防または改善用外用剤 |
| GB0313644D0 (en) * | 2003-06-12 | 2003-07-16 | Univ Bristol | Inhibition of infection |
| JP2005289819A (ja) * | 2004-03-31 | 2005-10-20 | Nippon Meat Packers Inc | 皮膚組織の再生及び肌質改善効果のあるペプチド、並びに該ペプチドを含有する食品、医薬品、又は外用剤 |
| JP4490498B2 (ja) * | 2008-09-30 | 2010-06-23 | 新田ゼラチン株式会社 | 疾病抑制剤 |
-
2009
- 2009-02-25 JP JP2009042671A patent/JP5560570B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2010195714A (ja) | 2010-09-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2424824C2 (ru) | Комплекс амфифильный полимер-pdgf | |
| ES2136618T5 (es) | Curacion de heridas. | |
| EP2938626A1 (en) | Self-assembled ultrashort peptides hydrogels for wound healing, skin care and cosmetics applications | |
| Udhayakumar et al. | Novel fibrous collagen-based cream accelerates fibroblast growth for wound healing applications: in vitro and in vivo evaluation | |
| JP2005290009A (ja) | 線維障害の創傷治療処置 | |
| AU2021202201A1 (en) | Extracellular matrix compositions | |
| CN113769066B (zh) | 一种促进创面愈合的组合物及其制备方法与用途 | |
| JP6348126B2 (ja) | 創傷治癒を促進する生物活性短鎖ペプチド | |
| MX2010007442A (es) | Uso de peptidos para promover la curacion de heridas. | |
| JP7026050B2 (ja) | 慢性創傷を治療するための組成物及び方法 | |
| CN114058663B (zh) | 一种多功能牡蛎活性多肽及其制备方法和应用 | |
| ES2404669T3 (es) | Péptidos bioactivos cortos para modulación celular e inmunológica | |
| JP5560570B2 (ja) | 創傷治療用医薬組成物 | |
| WO2019235292A1 (ja) | 肥厚性瘢痕の形成抑制用組成物 | |
| CN110638999A (zh) | 一种方格星虫胶原肽在创伤修复中的应用 | |
| Novianty et al. | Effect of allicin for re-epithelialization during healing in oral ulcer model | |
| JP2009191015A (ja) | 創傷治癒促進剤 | |
| CN112569346A (zh) | 一种促进伤口愈合的水凝胶及其制备方法 | |
| LV13959B (lv) | Adenilātdezamināzi saturošs hidrogēls un tā izmantošana ādas brūču (čūlu) ārstēšanai | |
| US20240016893A1 (en) | Compositions and methods for treating wounds | |
| CN115721638A (zh) | 月桂酰精氨酸乙酯盐酸盐在制备促伤口愈合药物中的应用 | |
| EP2968402B1 (en) | Medical device having a coating comprising accs | |
| US20240325503A1 (en) | Wound healing and tissue repair composition and method using low molecular weight collagen and bioactive glass | |
| WO2022074523A1 (en) | Primed placental tissue and uses in regenerative medicine | |
| US20230310538A1 (en) | Tissue repair and wound healing composition and method using low molecular weight hydrolyzed collagen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20110331 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111027 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111115 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130625 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130730 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130910 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140513 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140526 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5560570 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |