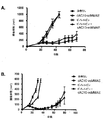
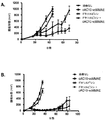
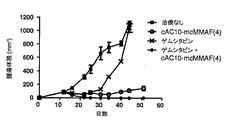
JP5485897B2 - 抗体−薬物複合体の併用療法 - Google Patents
抗体−薬物複合体の併用療法 Download PDFInfo
- Publication number
- JP5485897B2 JP5485897B2 JP2010529007A JP2010529007A JP5485897B2 JP 5485897 B2 JP5485897 B2 JP 5485897B2 JP 2010529007 A JP2010529007 A JP 2010529007A JP 2010529007 A JP2010529007 A JP 2010529007A JP 5485897 B2 JP5485897 B2 JP 5485897B2
- Authority
- JP
- Japan
- Prior art keywords
- antibody
- alkyl
- alkenyl
- alkynyl
- groups
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 0 CC(C(*)NC(C(*)NC(C(*)NC(C(*)NC)=O)=O)=O)=O Chemical compound CC(C(*)NC(C(*)NC(C(*)NC(C(*)NC)=O)=O)=O)=O 0.000 description 2
- DEZOKKOZDXFZIK-UHFFFAOYSA-N CC(CC(N1CCOCCOCC(C)=O)=O)C1=O Chemical compound CC(CC(N1CCOCCOCC(C)=O)=O)C1=O DEZOKKOZDXFZIK-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US97959407P | 2007-10-12 | 2007-10-12 | |
| US60/979,594 | 2007-10-12 | ||
| US2766808P | 2008-02-11 | 2008-02-11 | |
| US61/027,668 | 2008-02-11 | ||
| US4064108P | 2008-03-28 | 2008-03-28 | |
| US61/040,641 | 2008-03-28 | ||
| PCT/US2008/079224 WO2009048967A1 (en) | 2007-10-12 | 2008-10-08 | Combination therapy with antibody-drug conjugates |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011500582A JP2011500582A (ja) | 2011-01-06 |
| JP2011500582A5 JP2011500582A5 (enExample) | 2011-11-10 |
| JP5485897B2 true JP5485897B2 (ja) | 2014-05-07 |
Family
ID=40549535
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010529007A Expired - Fee Related JP5485897B2 (ja) | 2007-10-12 | 2008-10-08 | 抗体−薬物複合体の併用療法 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US8263083B2 (enExample) |
| EP (1) | EP2211885B1 (enExample) |
| JP (1) | JP5485897B2 (enExample) |
| KR (1) | KR101641345B1 (enExample) |
| CN (1) | CN101969970B (enExample) |
| AU (1) | AU2008310908B2 (enExample) |
| CA (1) | CA2699090C (enExample) |
| EA (1) | EA020696B1 (enExample) |
| IL (1) | IL204851A (enExample) |
| MX (1) | MX2010003977A (enExample) |
| NZ (1) | NZ584552A (enExample) |
| WO (1) | WO2009048967A1 (enExample) |
| ZA (1) | ZA201002428B (enExample) |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ583292A (en) | 2003-11-06 | 2012-03-30 | Seattle Genetics Inc | Monomethylvaline compounds capable of conjugation to ligands |
| US7750116B1 (en) * | 2006-02-18 | 2010-07-06 | Seattle Genetics, Inc. | Antibody drug conjugate metabolites |
| RU2557319C2 (ru) | 2007-07-16 | 2015-07-20 | Дженентек, Инк. | ГУМАНИЗИРОВАННЫЕ АНТИТЕЛА ПРОТИВ CD79b И ИММУНОКОНЪЮГАТЫ И СПОСОБЫ ПРИМЕНЕНИЯ |
| MY150531A (en) | 2007-07-16 | 2014-01-30 | Genentech Inc | Anti-cd79b antibodies and immunoconjugates and methods of use |
| AU2008310908B2 (en) | 2007-10-12 | 2014-01-09 | Seagen Inc. | Combination therapy with antibody-drug conjugates |
| DK2657253T3 (en) | 2008-01-31 | 2017-10-09 | Genentech Inc | Anti-CD79b antibodies and immune conjugates and methods of use |
| CA3150199C (en) | 2009-01-09 | 2025-07-22 | Seagen Inc | WEEKLY DOSING REGIMEN FOR ANTI-CD30 ANTIBODY CONJUGATIONS VC-PAB-MMAE - MEDICINE |
| US8758758B1 (en) * | 2010-01-08 | 2014-06-24 | Seattle Genetics, Inc. | Post-relapse treatment of CD30 expressing lymphomas |
| UA115517C2 (uk) * | 2010-02-08 | 2017-11-27 | Ейдженсіс, Інк. | Кон'югат антитіло-лікарський засіб (adc), який зв'язується з білком 161p2f10b |
| ES3004351T3 (en) | 2010-10-22 | 2025-03-12 | Seagen Inc | Synergistic effects between auristatin-based antibody drug conjugates and inhibitors of the pi3k-akt mtor pathway |
| ES2708699T3 (es) * | 2011-09-29 | 2019-04-10 | Seattle Genetics Inc | Determinación de la masa intacta de compuestos de agentes conjugados con proteínas |
| CA2850375C (en) | 2011-10-14 | 2019-07-02 | Seattle Genetics, Inc. | Pyrrolobenzodiazepines and targeted conjugates |
| PT3327027T (pt) | 2011-11-17 | 2021-02-15 | Pfizer | Péptidos citotóxicos e seus conjugados de anticorpos e fármacos |
| EP2812702B1 (en) * | 2012-02-10 | 2019-04-17 | Seattle Genetics, Inc. | Diagnosis and management of CD30-expressing cancers |
| BR112015010436A2 (pt) | 2012-11-07 | 2017-08-22 | Pfizer | Anticorpos anti-notch3 e conjugados anticorpo-fármaco |
| US9353150B2 (en) | 2012-12-04 | 2016-05-31 | Massachusetts Institute Of Technology | Substituted pyrazino[1′,2′:1 ,5]pyrrolo[2,3-b]-indole-1,4-diones for cancer treatment |
| AU2015282627B2 (en) | 2014-06-30 | 2020-04-02 | Glykos Finland Oy | Saccharide derivative of a toxic payload and antibody conjugates thereof |
| DK3262071T3 (da) | 2014-09-23 | 2020-06-15 | Hoffmann La Roche | Fremgangsmåde til anvendelse af anti-CD79b-immunkonjugater |
| EP3223854A1 (en) | 2014-11-25 | 2017-10-04 | ADC Therapeutics SA | Pyrrolobenzodiazepine-antibody conjugates |
| WO2017197045A1 (en) | 2016-05-11 | 2017-11-16 | Movassaghi Mohammad | Convergent and enantioselective total synthesis of communesin analogs |
| WO2018209239A1 (en) | 2017-05-11 | 2018-11-15 | Massachusetts Institute Of Technology | Potent agelastatin derivatives as modulators for cancer invasion and metastasis |
| MX2020003664A (es) * | 2017-10-11 | 2020-10-12 | Seattle Genetics Inc | Metodos de reduccion de efectos secundarios del tratamiento con conjugado anticuerpo anti-cd30-farmaco. |
| CA3077729A1 (en) * | 2017-10-13 | 2019-04-18 | Seattle Genetics, Inc. | Modulating the immune response using antibody-drug conjugates |
| US10640508B2 (en) | 2017-10-13 | 2020-05-05 | Massachusetts Institute Of Technology | Diazene directed modular synthesis of compounds with quaternary carbon centers |
| TWI841554B (zh) | 2018-03-21 | 2024-05-11 | 丹麥商珍美寶股份有限公司 | 以鉑為主之劑與抗組織因子抗體-藥物共軛物的組合治療癌症之方法 |
| KR20210006362A (ko) * | 2018-04-06 | 2021-01-18 | 시애틀 지네틱스, 인크. | 캄토테신 펩타이드 접합체들 |
| UA130009C2 (uk) | 2018-05-07 | 2025-10-15 | Генмаб А/С | Спосіб лікування раку за допомогою комбінації антитіла до pd-1 і кон'югата антитіла до тканинного фактора і лікарського засобу |
| TW202519270A (zh) | 2018-06-07 | 2025-05-16 | 美商思進公司 | 喜樹鹼結合物 |
| TWI844571B (zh) | 2018-10-30 | 2024-06-11 | 丹麥商珍美寶股份有限公司 | 使用抗血管內皮生長因子(vegf)抗體與抗組織因子(tf)抗體-藥物共軛體之組合以治療癌症之方法 |
| WO2020127618A1 (en) * | 2018-12-21 | 2020-06-25 | F. Hoffmann-La Roche Ag | Tumor-targeted agonistic cd28 antigen binding molecules |
| US11535634B2 (en) | 2019-06-05 | 2022-12-27 | Massachusetts Institute Of Technology | Compounds, conjugates, and compositions of epipolythiodiketopiperazines and polythiodiketopiperazines and uses thereof |
| CA3152316A1 (en) * | 2019-10-04 | 2021-04-08 | Scott C. Jeffrey | Camptothecin peptide conjugates |
| JP2023512084A (ja) * | 2020-01-31 | 2023-03-23 | シージェン インコーポレイテッド | 抗cd30抗体薬物コンジュゲート及び非ホジキンリンパ腫の治療のためのその使用 |
| WO2022182415A1 (en) | 2021-02-24 | 2022-09-01 | Massachusetts Institute Of Technology | Himastatin derivatives, and processes of preparation thereof, and uses thereof |
| CN119490595B (zh) * | 2023-08-15 | 2025-11-18 | 东莞市朋志生物科技有限公司 | 一种抗免疫球蛋白e的抗体及其应用 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5165922A (en) * | 1990-05-22 | 1992-11-24 | Bristol-Myers Squibb Company | Synergistic tumor therapy with combinations of biologically active anti-tumor antibodies and chemotherapy |
| US6150508A (en) * | 1996-03-25 | 2000-11-21 | Northwest Biotherapeutics, Inc. | Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen |
| US7090843B1 (en) | 2000-11-28 | 2006-08-15 | Seattle Genetics, Inc. | Recombinant anti-CD30 antibodies and uses thereof |
| US20040018194A1 (en) * | 2000-11-28 | 2004-01-29 | Francisco Joseph A. | Recombinant anti-CD30 antibodies and uses thereof |
| CA2802205C (en) | 2002-07-31 | 2016-01-19 | Seattle Genetics, Inc. | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| US20070134243A1 (en) * | 2004-12-01 | 2007-06-14 | Gazzard Lewis J | Antibody drug conjugates and methods |
| PT3248613T (pt) * | 2005-07-18 | 2022-03-16 | Seagen Inc | Conjugados de ligante de fármaco e beta-glucuronida |
| CA2625998C (en) * | 2005-10-06 | 2015-12-01 | Xencor, Inc. | Optimized anti-cd30 antibodies |
| US7750116B1 (en) * | 2006-02-18 | 2010-07-06 | Seattle Genetics, Inc. | Antibody drug conjugate metabolites |
| WO2008025020A2 (en) * | 2006-08-25 | 2008-02-28 | Seattle Genetics, Inc. | Cd30 binding agents and uses thereof |
| WO2008103916A2 (en) | 2007-02-23 | 2008-08-28 | The Trustees Of Columbia Univeristy In The City Of New York | Compositions and methods for treating cancer or a neurotrophic disorder |
| AU2008310908B2 (en) | 2007-10-12 | 2014-01-09 | Seagen Inc. | Combination therapy with antibody-drug conjugates |
-
2008
- 2008-10-08 AU AU2008310908A patent/AU2008310908B2/en active Active
- 2008-10-08 EP EP08837451.7A patent/EP2211885B1/en active Active
- 2008-10-08 EA EA201070463A patent/EA020696B1/ru unknown
- 2008-10-08 NZ NZ584552A patent/NZ584552A/en unknown
- 2008-10-08 WO PCT/US2008/079224 patent/WO2009048967A1/en not_active Ceased
- 2008-10-08 MX MX2010003977A patent/MX2010003977A/es active IP Right Grant
- 2008-10-08 JP JP2010529007A patent/JP5485897B2/ja not_active Expired - Fee Related
- 2008-10-08 KR KR1020107008660A patent/KR101641345B1/ko active Active
- 2008-10-08 CA CA2699090A patent/CA2699090C/en active Active
- 2008-10-08 CN CN200880111297.1A patent/CN101969970B/zh active Active
- 2008-10-08 US US12/681,599 patent/US8263083B2/en active Active
-
2010
- 2010-04-06 IL IL204851A patent/IL204851A/en active IP Right Grant
- 2010-04-07 ZA ZA2010/02428A patent/ZA201002428B/en unknown
-
2012
- 2012-08-13 US US13/571,313 patent/US8470329B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| IL204851A (en) | 2016-08-31 |
| ZA201002428B (en) | 2011-06-29 |
| AU2008310908A1 (en) | 2009-04-16 |
| HK1151727A1 (zh) | 2012-02-10 |
| CA2699090C (en) | 2016-02-02 |
| US8263083B2 (en) | 2012-09-11 |
| US20130022627A1 (en) | 2013-01-24 |
| US8470329B2 (en) | 2013-06-25 |
| KR20100087288A (ko) | 2010-08-04 |
| IL204851A0 (en) | 2010-11-30 |
| EP2211885A1 (en) | 2010-08-04 |
| US20100215671A1 (en) | 2010-08-26 |
| EA020696B1 (ru) | 2015-01-30 |
| NZ584552A (en) | 2012-07-27 |
| CA2699090A1 (en) | 2009-04-16 |
| CN101969970B (zh) | 2014-10-15 |
| JP2011500582A (ja) | 2011-01-06 |
| KR101641345B1 (ko) | 2016-07-20 |
| EP2211885B1 (en) | 2015-07-29 |
| EP2211885A4 (en) | 2012-09-26 |
| EA201070463A1 (ru) | 2010-10-29 |
| CN101969970A (zh) | 2011-02-09 |
| AU2008310908B2 (en) | 2014-01-09 |
| WO2009048967A1 (en) | 2009-04-16 |
| MX2010003977A (es) | 2010-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5485897B2 (ja) | 抗体−薬物複合体の併用療法 | |
| CN106563128B (zh) | 以奥里斯他汀为主的抗体药物结合物和结合mTOR的抑制剂在制备治疗癌症药物中的用途 | |
| US9919061B2 (en) | CD19 binding agents and uses thereof | |
| US20110070248A1 (en) | Dr5 ligand drug conjugates | |
| US20150079114A1 (en) | Dr5 ligand drug conjugates | |
| JP2007500236A (ja) | 組換え抗cd30抗体とその使用 | |
| TWI873167B (zh) | 以結合191p4d12蛋白質之抗體藥物結合物(adc)治療癌症 | |
| HK1151727B (en) | Combination therapy with antibody-drug conjugates | |
| HK40044344A (en) | Use of antibody drug conjugates comprising tubulin disrupting agents to treat solid tumor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD01 | Notification of change of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7426 Effective date: 20110112 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110112 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110922 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110922 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130625 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130925 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140121 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140220 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5485897 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |