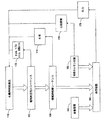
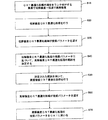
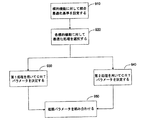
JP5352240B2 - 心臓再同期療法パラメータの最適化 - Google Patents
心臓再同期療法パラメータの最適化 Download PDFInfo
- Publication number
- JP5352240B2 JP5352240B2 JP2008552294A JP2008552294A JP5352240B2 JP 5352240 B2 JP5352240 B2 JP 5352240B2 JP 2008552294 A JP2008552294 A JP 2008552294A JP 2008552294 A JP2008552294 A JP 2008552294A JP 5352240 B2 JP5352240 B2 JP 5352240B2
- Authority
- JP
- Japan
- Prior art keywords
- crt
- parameters
- optimization process
- optimization
- crt optimization
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000005457 optimization Methods 0.000 title claims abstract description 335
- 238000009125 cardiac resynchronization therapy Methods 0.000 title claims description 468
- 238000000034 method Methods 0.000 claims abstract description 356
- 230000008569 process Effects 0.000 claims abstract description 276
- 238000012545 processing Methods 0.000 claims description 46
- 238000002560 therapeutic procedure Methods 0.000 claims description 20
- 238000011282 treatment Methods 0.000 claims description 11
- 230000006870 function Effects 0.000 description 58
- 210000002216 heart Anatomy 0.000 description 45
- 230000000747 cardiac effect Effects 0.000 description 31
- 210000005240 left ventricle Anatomy 0.000 description 21
- 230000002861 ventricular Effects 0.000 description 21
- 230000008602 contraction Effects 0.000 description 16
- 206010019280 Heart failures Diseases 0.000 description 13
- 238000007726 management method Methods 0.000 description 11
- 238000005259 measurement Methods 0.000 description 11
- 210000005242 cardiac chamber Anatomy 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 206010007559 Cardiac failure congestive Diseases 0.000 description 9
- 238000001994 activation Methods 0.000 description 9
- 230000001746 atrial effect Effects 0.000 description 9
- 230000004217 heart function Effects 0.000 description 9
- 230000004913 activation Effects 0.000 description 8
- 238000010586 diagram Methods 0.000 description 8
- 238000004886 process control Methods 0.000 description 8
- 210000005245 right atrium Anatomy 0.000 description 8
- 210000005241 right ventricle Anatomy 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 6
- 210000001992 atrioventricular node Anatomy 0.000 description 5
- 238000007634 remodeling Methods 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 210000002620 vena cava superior Anatomy 0.000 description 5
- 206010003658 Atrial Fibrillation Diseases 0.000 description 4
- 206010003671 Atrioventricular Block Diseases 0.000 description 4
- 230000002169 extracardiac Effects 0.000 description 4
- 230000000004 hemodynamic effect Effects 0.000 description 4
- 230000002441 reversible effect Effects 0.000 description 4
- 230000008901 benefit Effects 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 230000002612 cardiopulmonary effect Effects 0.000 description 3
- 210000003748 coronary sinus Anatomy 0.000 description 3
- 230000010339 dilation Effects 0.000 description 3
- 210000005246 left atrium Anatomy 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 230000002644 neurohormonal effect Effects 0.000 description 3
- 230000033764 rhythmic process Effects 0.000 description 3
- 230000001360 synchronised effect Effects 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 206010006580 Bundle branch block left Diseases 0.000 description 2
- 206010006578 Bundle-Branch Block Diseases 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 208000006029 Cardiomegaly Diseases 0.000 description 2
- 230000004872 arterial blood pressure Effects 0.000 description 2
- 230000036772 blood pressure Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 230000008828 contractile function Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 230000003205 diastolic effect Effects 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 210000005003 heart tissue Anatomy 0.000 description 2
- 201000001715 left bundle branch hemiblock Diseases 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 230000036316 preload Effects 0.000 description 2
- 238000005086 pumping Methods 0.000 description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 230000035939 shock Effects 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 208000004301 Sinus Arrhythmia Diseases 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 238000009530 blood pressure measurement Methods 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 230000011128 cardiac conduction Effects 0.000 description 1
- 229940030602 cardiac therapy drug Drugs 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 238000002565 electrocardiography Methods 0.000 description 1
- 230000005520 electrodynamics Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 210000002837 heart atrium Anatomy 0.000 description 1
- 238000002847 impedance measurement Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 239000000712 neurohormone Substances 0.000 description 1
- 102000008434 neuropeptide hormone activity proteins Human genes 0.000 description 1
- 108040002669 neuropeptide hormone activity proteins Proteins 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000036544 posture Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 210000002321 radial artery Anatomy 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 210000001321 subclavian vein Anatomy 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/3627—Heart stimulators for treating a mechanical deficiency of the heart, e.g. congestive heart failure or cardiomyopathy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/365—Heart stimulators controlled by a physiological parameter, e.g. heart potential
- A61N1/36585—Heart stimulators controlled by a physiological parameter, e.g. heart potential controlled by two or more physical parameters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/365—Heart stimulators controlled by a physiological parameter, e.g. heart potential
- A61N1/368—Heart stimulators controlled by a physiological parameter, e.g. heart potential comprising more than one electrode co-operating with different heart regions
- A61N1/3684—Heart stimulators controlled by a physiological parameter, e.g. heart potential comprising more than one electrode co-operating with different heart regions for stimulating the heart at multiple sites of the ventricle or the atrium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/365—Heart stimulators controlled by a physiological parameter, e.g. heart potential
- A61N1/368—Heart stimulators controlled by a physiological parameter, e.g. heart potential comprising more than one electrode co-operating with different heart regions
- A61N1/3684—Heart stimulators controlled by a physiological parameter, e.g. heart potential comprising more than one electrode co-operating with different heart regions for stimulating the heart at multiple sites of the ventricle or the atrium
- A61N1/36842—Multi-site stimulation in the same chamber
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Radiology & Medical Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Physiology (AREA)
- Biophysics (AREA)
- Hospice & Palliative Care (AREA)
- Electrotherapy Devices (AREA)
- Measuring Pulse, Heart Rate, Blood Pressure Or Blood Flow (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/338,935 US8175703B2 (en) | 2006-01-25 | 2006-01-25 | Cardiac resynchronization therapy parameter optimization |
| US11/338,935 | 2006-01-25 | ||
| PCT/US2006/047216 WO2007087025A1 (en) | 2006-01-25 | 2006-12-11 | Cardiac resynchronization therapy parameter optimization |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009524475A JP2009524475A (ja) | 2009-07-02 |
| JP2009524475A5 JP2009524475A5 (enExample) | 2010-01-28 |
| JP5352240B2 true JP5352240B2 (ja) | 2013-11-27 |
Family
ID=37898508
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008552294A Expired - Fee Related JP5352240B2 (ja) | 2006-01-25 | 2006-12-11 | 心臓再同期療法パラメータの最適化 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8175703B2 (enExample) |
| EP (1) | EP1984074B1 (enExample) |
| JP (1) | JP5352240B2 (enExample) |
| WO (1) | WO2007087025A1 (enExample) |
Families Citing this family (100)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060247693A1 (en) | 2005-04-28 | 2006-11-02 | Yanting Dong | Non-captured intrinsic discrimination in cardiac pacing response classification |
| US7774064B2 (en) | 2003-12-12 | 2010-08-10 | Cardiac Pacemakers, Inc. | Cardiac response classification using retriggerable classification windows |
| US7392086B2 (en) | 2005-04-26 | 2008-06-24 | Cardiac Pacemakers, Inc. | Implantable cardiac device and method for reduced phrenic nerve stimulation |
| US8209013B2 (en) | 2006-09-14 | 2012-06-26 | Cardiac Pacemakers, Inc. | Therapeutic electrical stimulation that avoids undesirable activation |
| US8948867B2 (en) | 2006-09-14 | 2015-02-03 | Cardiac Pacemakers, Inc. | Capture detection with cross chamber backup pacing |
| US8265736B2 (en) | 2007-08-07 | 2012-09-11 | Cardiac Pacemakers, Inc. | Method and apparatus to perform electrode combination selection |
| US9037239B2 (en) | 2007-08-07 | 2015-05-19 | Cardiac Pacemakers, Inc. | Method and apparatus to perform electrode combination selection |
| US8352032B2 (en) * | 2007-11-01 | 2013-01-08 | Cardiac Pacemakers, Inc. | Monitoring right ventricular hemodynamic function during pacing optimization |
| JP5276119B2 (ja) | 2008-02-14 | 2013-08-28 | カーディアック ペースメイカーズ, インコーポレイテッド | 横隔刺激検出のための方法および装置 |
| US9314634B2 (en) * | 2008-11-03 | 2016-04-19 | Pacesetter, Inc. | Initiation tests and guidelines for implementing cardiac therapy |
| US9265951B2 (en) * | 2010-02-12 | 2016-02-23 | The Brigham And Women's Hospital | System and method for automated adjustment of cardiac resynchronization therapy control parameters |
| US20130211472A1 (en) * | 2010-10-27 | 2013-08-15 | St. Jude Medical Ab | Cardiac resynchronization therapy optimization |
| US8639328B2 (en) | 2010-10-29 | 2014-01-28 | Medtronic, Inc. | Cardiac therapy based upon impedance signals |
| JP5805204B2 (ja) | 2010-11-03 | 2015-11-04 | カーディオインサイト テクノロジーズ インコーポレイテッド | 心臓の機能を評価するためのシステム |
| WO2012106297A2 (en) * | 2011-02-01 | 2012-08-09 | Brigham And Women's Hospital, Inc. | System and method for cardiac resychronization therapy control parameter generation using ventricular activation simulation and surface ecg registration |
| US9510763B2 (en) | 2011-05-03 | 2016-12-06 | Medtronic, Inc. | Assessing intra-cardiac activation patterns and electrical dyssynchrony |
| US8617082B2 (en) | 2011-05-19 | 2013-12-31 | Medtronic, Inc. | Heart sounds-based pacing optimization |
| US20120296387A1 (en) | 2011-05-19 | 2012-11-22 | Xusheng Zhang | Phrenic nerve stimulation detection using heart sounds |
| US8777874B2 (en) | 2011-05-24 | 2014-07-15 | Medtronic, Inc. | Acoustic based cough detection |
| US9679869B2 (en) * | 2011-09-02 | 2017-06-13 | Skyworks Solutions, Inc. | Transmission line for high performance radio frequency applications |
| US8886311B2 (en) | 2012-01-27 | 2014-11-11 | Medtronic, Inc. | Techniques for mitigating motion artifacts from implantable physiological sensors |
| US8792998B2 (en) * | 2012-03-28 | 2014-07-29 | Pacesetter, Inc. | Devices, systems and methods for efficient identification of improved CRT parameters |
| US9095718B2 (en) | 2012-04-04 | 2015-08-04 | Medtronic, Inc. | Heart-sounds based adaptive cardiac resynchronization therapy timing parameter optimization system |
| US20130289640A1 (en) * | 2012-04-27 | 2013-10-31 | Medtronic, Inc. | Heart sound-based pacing vector selection system and method |
| US9717915B2 (en) | 2013-03-13 | 2017-08-01 | Cardiac Pacemakers, Inc. | System and method for changing device parameters to control cardiac hemodynamics in a patient |
| US9278219B2 (en) | 2013-03-15 | 2016-03-08 | Medtronic, Inc. | Closed loop optimization of control parameters during cardiac pacing |
| US9931048B2 (en) | 2013-04-30 | 2018-04-03 | Medtronic, Inc. | Systems, methods, and interfaces for identifying effective electrodes |
| US10064567B2 (en) | 2013-04-30 | 2018-09-04 | Medtronic, Inc. | Systems, methods, and interfaces for identifying optimal electrical vectors |
| US9877789B2 (en) | 2013-06-12 | 2018-01-30 | Medtronic, Inc. | Implantable electrode location selection |
| US10251555B2 (en) | 2013-06-12 | 2019-04-09 | Medtronic, Inc. | Implantable electrode location selection |
| US9474457B2 (en) | 2013-06-12 | 2016-10-25 | Medtronic, Inc. | Metrics of electrical dyssynchrony and electrical activation patterns from surface ECG electrodes |
| US9278220B2 (en) | 2013-07-23 | 2016-03-08 | Medtronic, Inc. | Identification of healthy versus unhealthy substrate for pacing from a multipolar lead |
| US9282907B2 (en) | 2013-07-23 | 2016-03-15 | Medtronic, Inc. | Identification of healthy versus unhealthy substrate for pacing from a multipolar lead |
| US9265955B2 (en) | 2013-07-26 | 2016-02-23 | Medtronic, Inc. | Method and system for improved estimation of time of left ventricular pacing with respect to intrinsic right ventricular activation in cardiac resynchronization therapy |
| US9265954B2 (en) | 2013-07-26 | 2016-02-23 | Medtronic, Inc. | Method and system for improved estimation of time of left ventricular pacing with respect to intrinsic right ventricular activation in cardiac resynchronization therapy |
| US9114264B2 (en) | 2013-09-30 | 2015-08-25 | Pacesetter, Inc. | Implantable stimulation devices, and methods and systems for use therewith, that automatically adjust stimulation parameters to improve preload in an HF patient |
| US9789319B2 (en) | 2013-11-21 | 2017-10-17 | Medtronic, Inc. | Systems and methods for leadless cardiac resynchronization therapy |
| US9320446B2 (en) | 2013-12-09 | 2016-04-26 | Medtronic, Inc. | Bioelectric sensor device and methods |
| US9986928B2 (en) | 2013-12-09 | 2018-06-05 | Medtronic, Inc. | Noninvasive cardiac therapy evaluation |
| JP6470291B2 (ja) | 2013-12-18 | 2019-02-13 | カーディアック ペースメイカーズ, インコーポレイテッド | 医療用デバイスにおいて1以上のベクトルの選択を容易にするためのシステム及び方法 |
| US9750942B2 (en) | 2013-12-18 | 2017-09-05 | Cardiac Pacemakers, Inc. | Systems and methods for determining parameters for each of a plurality of vectors |
| EP3082950B1 (en) | 2013-12-18 | 2023-06-21 | Cardiac Pacemakers, Inc. | Method of facilitating selection of a vector for delivering electrical stimulation to a patient |
| US9387330B2 (en) | 2014-01-17 | 2016-07-12 | Medtronic, Inc. | Cardiac resynchronization therapy optimization based on intracardiac impedance and heart sounds |
| US9199086B2 (en) | 2014-01-17 | 2015-12-01 | Medtronic, Inc. | Cardiac resynchronization therapy optimization based on intracardiac impedance |
| US9776009B2 (en) | 2014-03-20 | 2017-10-03 | Medtronic, Inc. | Non-invasive detection of phrenic nerve stimulation |
| US9492671B2 (en) | 2014-05-06 | 2016-11-15 | Medtronic, Inc. | Acoustically triggered therapy delivery |
| US9669224B2 (en) | 2014-05-06 | 2017-06-06 | Medtronic, Inc. | Triggered pacing system |
| US9770584B2 (en) | 2014-05-29 | 2017-09-26 | Cardiac Pacemakers, Inc. | System and methods for treating atrial fibrillation using hemodynamic responses |
| EP3148422B1 (en) | 2014-06-02 | 2023-07-12 | Cardiac Pacemakers, Inc. | Evaluation of hemodynamic response to atrial fibrillation |
| US9591982B2 (en) | 2014-07-31 | 2017-03-14 | Medtronic, Inc. | Systems and methods for evaluating cardiac therapy |
| US9586050B2 (en) | 2014-08-15 | 2017-03-07 | Medtronic, Inc. | Systems and methods for configuration of atrioventricular interval |
| US9586052B2 (en) | 2014-08-15 | 2017-03-07 | Medtronic, Inc. | Systems and methods for evaluating cardiac therapy |
| US9764143B2 (en) | 2014-08-15 | 2017-09-19 | Medtronic, Inc. | Systems and methods for configuration of interventricular interval |
| US11253178B2 (en) | 2015-01-29 | 2022-02-22 | Medtronic, Inc. | Noninvasive assessment of cardiac resynchronization therapy |
| US10004906B2 (en) | 2015-07-16 | 2018-06-26 | Medtronic, Inc. | Confirming sensed atrial events for pacing during resynchronization therapy in a cardiac medical device and medical device system |
| US9731138B1 (en) | 2016-02-17 | 2017-08-15 | Medtronic, Inc. | System and method for cardiac pacing |
| US11219769B2 (en) | 2016-02-26 | 2022-01-11 | Medtronic, Inc. | Noninvasive methods and systems of determining the extent of tissue capture from cardiac pacing |
| US10780279B2 (en) | 2016-02-26 | 2020-09-22 | Medtronic, Inc. | Methods and systems of optimizing right ventricular only pacing for patients with respect to an atrial event and left ventricular event |
| US9802055B2 (en) | 2016-04-04 | 2017-10-31 | Medtronic, Inc. | Ultrasound powered pulse delivery device |
| US10729898B2 (en) | 2016-04-06 | 2020-08-04 | Cardiac Pacemakers, Inc. | Multi-site CRT capture verification |
| US10532213B2 (en) | 2017-03-03 | 2020-01-14 | Medtronic, Inc. | Criteria for determination of local tissue latency near pacing electrode |
| US10987517B2 (en) | 2017-03-15 | 2021-04-27 | Medtronic, Inc. | Detection of noise signals in cardiac signals |
| CN110996784B (zh) | 2017-07-28 | 2023-05-30 | 美敦力公司 | 生成激动时间 |
| CN111050841B (zh) | 2017-07-28 | 2023-09-26 | 美敦力公司 | 心动周期选择 |
| US10694967B2 (en) | 2017-10-18 | 2020-06-30 | Medtronic, Inc. | State-based atrial event detection |
| US10433746B2 (en) | 2017-12-22 | 2019-10-08 | Regents Of The University Of Minnesota | Systems and methods for anterior and posterior electrode signal analysis |
| US10492705B2 (en) | 2017-12-22 | 2019-12-03 | Regents Of The University Of Minnesota | Anterior and posterior electrode signals |
| US10799703B2 (en) | 2017-12-22 | 2020-10-13 | Medtronic, Inc. | Evaluation of his bundle pacing therapy |
| US10786167B2 (en) | 2017-12-22 | 2020-09-29 | Medtronic, Inc. | Ectopic beat-compensated electrical heterogeneity information |
| US11419539B2 (en) | 2017-12-22 | 2022-08-23 | Regents Of The University Of Minnesota | QRS onset and offset times and cycle selection using anterior and posterior electrode signals |
| US10617318B2 (en) | 2018-02-27 | 2020-04-14 | Medtronic, Inc. | Mapping electrical activity on a model heart |
| US10668290B2 (en) | 2018-03-01 | 2020-06-02 | Medtronic, Inc. | Delivery of pacing therapy by a cardiac pacing device |
| US10918870B2 (en) | 2018-03-07 | 2021-02-16 | Medtronic, Inc. | Atrial lead placement for treatment of atrial dyssynchrony |
| WO2019183507A1 (en) | 2018-03-23 | 2019-09-26 | Medtronic, Inc. | Av synchronous vfa cardiac therapy |
| US11058880B2 (en) | 2018-03-23 | 2021-07-13 | Medtronic, Inc. | VFA cardiac therapy for tachycardia |
| US11235159B2 (en) | 2018-03-23 | 2022-02-01 | Medtronic, Inc. | VFA cardiac resynchronization therapy |
| US10780281B2 (en) | 2018-03-23 | 2020-09-22 | Medtronic, Inc. | Evaluation of ventricle from atrium pacing therapy |
| US11285312B2 (en) | 2018-03-29 | 2022-03-29 | Medtronic, Inc. | Left ventricular assist device adjustment and evaluation |
| US10596383B2 (en) | 2018-04-03 | 2020-03-24 | Medtronic, Inc. | Feature based sensing for leadless pacing therapy |
| US10940321B2 (en) | 2018-06-01 | 2021-03-09 | Medtronic, Inc. | Systems, methods, and interfaces for use in cardiac evaluation |
| US11304641B2 (en) | 2018-06-01 | 2022-04-19 | Medtronic, Inc. | Systems, methods, and interfaces for use in cardiac evaluation |
| WO2020065582A1 (en) | 2018-09-26 | 2020-04-02 | Medtronic, Inc. | Capture in ventricle-from-atrium cardiac therapy |
| US11951313B2 (en) | 2018-11-17 | 2024-04-09 | Medtronic, Inc. | VFA delivery systems and methods |
| US12296177B2 (en) | 2018-12-21 | 2025-05-13 | Medtronic, Inc. | Delivery systems and methods for left ventricular pacing |
| US11679265B2 (en) | 2019-02-14 | 2023-06-20 | Medtronic, Inc. | Lead-in-lead systems and methods for cardiac therapy |
| US11547858B2 (en) | 2019-03-29 | 2023-01-10 | Medtronic, Inc. | Systems, methods, and devices for adaptive cardiac therapy |
| US11697025B2 (en) | 2019-03-29 | 2023-07-11 | Medtronic, Inc. | Cardiac conduction system capture |
| US11213676B2 (en) | 2019-04-01 | 2022-01-04 | Medtronic, Inc. | Delivery systems for VfA cardiac therapy |
| US11712188B2 (en) | 2019-05-07 | 2023-08-01 | Medtronic, Inc. | Posterior left bundle branch engagement |
| US11305127B2 (en) | 2019-08-26 | 2022-04-19 | Medtronic Inc. | VfA delivery and implant region detection |
| US11497431B2 (en) | 2019-10-09 | 2022-11-15 | Medtronic, Inc. | Systems and methods for configuring cardiac therapy |
| US12201843B2 (en) | 2019-10-09 | 2025-01-21 | Medtronic, Inc. | Synchronizing external electrical activity |
| US11642533B2 (en) | 2019-11-04 | 2023-05-09 | Medtronic, Inc. | Systems and methods for evaluating cardiac therapy |
| US11813466B2 (en) | 2020-01-27 | 2023-11-14 | Medtronic, Inc. | Atrioventricular nodal stimulation |
| US12383183B2 (en) | 2020-01-30 | 2025-08-12 | Medtronic, Inc. | Disturbance detection and removal in cardiac signals |
| US11911168B2 (en) | 2020-04-03 | 2024-02-27 | Medtronic, Inc. | Cardiac conduction system therapy benefit determination |
| US12023503B2 (en) | 2020-07-30 | 2024-07-02 | Medtronic, Inc. | ECG belt systems to interoperate with IMDs |
| US11813464B2 (en) | 2020-07-31 | 2023-11-14 | Medtronic, Inc. | Cardiac conduction system evaluation |
| US12465770B2 (en) | 2020-07-31 | 2025-11-11 | Medtronic, Inc. | Coronary sinus conduction system pacing and delivery |
| US12280260B2 (en) | 2020-12-02 | 2025-04-22 | Medtronic, Inc. | Evaluation and adjustment of left bundle branch (LBB) pacing therapy |
Family Cites Families (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5101824A (en) * | 1990-04-16 | 1992-04-07 | Siemens-Pacesetter, Inc. | Rate-responsive pacemaker with circuitry for processing multiple sensor inputs |
| US5716382A (en) * | 1995-08-02 | 1998-02-10 | Pacesetter, Inc. | Programmer for an implantable cardiac stimulating device |
| US5800471A (en) * | 1997-10-20 | 1998-09-01 | Cardiac Pacemakers, Inc. | Method for optimizing cardiac performance by determining the optimal pacing mode-AV delay from a transient heart rate signal for use in CHF, brady, and tachy/brady therapy devices |
| JPH11265205A (ja) * | 1998-01-16 | 1999-09-28 | Honda Motor Co Ltd | 同時最適化設計システム |
| US6144880A (en) * | 1998-05-08 | 2000-11-07 | Cardiac Pacemakers, Inc. | Cardiac pacing using adjustable atrio-ventricular delays |
| US7158830B2 (en) * | 1998-05-08 | 2007-01-02 | Cardiac Pacemakers, Inc. | Method and apparatus for optimizing stroke volume during DDD resynchronization therapy using adjustable atrio-ventricular delays |
| FR2779844B1 (fr) * | 1998-06-12 | 2001-07-27 | Ela Medical Sa | Structure d'interconnexion entre organes adressables d'un microcalculateur, notamment pour dispositif medical implantable actif tel que stimulateur ou defibrillateur cardiaque |
| US7181285B2 (en) * | 2000-12-26 | 2007-02-20 | Cardiac Pacemakers, Inc. | Expert system and method |
| US6597951B2 (en) * | 2001-03-16 | 2003-07-22 | Cardiac Pacemakers, Inc. | Automatic selection from multiple cardiac optimization protocols |
| US7113823B2 (en) * | 2001-10-26 | 2006-09-26 | Cardiac Pacemakers, Inc. | Morphology-based optimization of cardiac resynchronization therapy |
| US6832113B2 (en) * | 2001-11-16 | 2004-12-14 | Cardiac Pacemakers, Inc. | Non-invasive method and apparatus for cardiac pacemaker pacing parameter optimization and monitoring of cardiac dysfunction |
| US7392085B2 (en) * | 2001-11-21 | 2008-06-24 | Cameron Health, Inc. | Multiple electrode vectors for implantable cardiac treatment devices |
| US6666826B2 (en) * | 2002-01-04 | 2003-12-23 | Cardiac Pacemakers, Inc. | Method and apparatus for measuring left ventricular pressure |
| DE60215458T2 (de) | 2002-04-03 | 2007-08-23 | Osypka Medical Gmbh | Apparat zur automatischen Bestimmung von hämodynamisch optimalen Herzstimulationsparameterwerten |
| US7228174B2 (en) * | 2002-04-29 | 2007-06-05 | Medtronics, Inc. | Algorithm for the automatic determination of optimal AV an VV intervals |
| US7041061B2 (en) * | 2002-07-19 | 2006-05-09 | Cardiac Pacemakers, Inc. | Method and apparatus for quantification of cardiac wall motion asynchrony |
| US7206634B2 (en) * | 2002-07-26 | 2007-04-17 | Cardiac Pacemakers, Inc. | Method and apparatus for optimizing cardiac pumping performance |
| US6978184B1 (en) | 2002-07-29 | 2005-12-20 | Marcus Frank I | Optimization method for cardiac resynchronization therapy |
| US20050234517A1 (en) * | 2002-08-02 | 2005-10-20 | Frieder Braunschweig | Apparatus and method for hemodynamic-based optimization of cardiac pacing |
| US7343199B2 (en) * | 2002-12-27 | 2008-03-11 | Cardiac Pacemakers, Inc. | Measurement of respiratory sinus arrhythmia using respiratory and electrogram sensors in an implantable device |
| US7013176B2 (en) * | 2003-01-28 | 2006-03-14 | Cardiac Pacemakers, Inc. | Method and apparatus for setting pacing parameters in cardiac resynchronization therapy |
| US7225022B2 (en) * | 2003-03-12 | 2007-05-29 | Cra Associates, Ltd. | Method of optimizing patient outcome from cardiac resynchronization therapy |
| US6871088B2 (en) * | 2003-03-20 | 2005-03-22 | Medtronic, Inc. | Method and apparatus for optimizing cardiac resynchronization therapy |
| US20040220636A1 (en) * | 2003-04-29 | 2004-11-04 | Medtronic, Inc. | Cardiac pacing therapy parameter programming |
| US7130684B2 (en) * | 2003-04-30 | 2006-10-31 | Medtronic, Inc. | Method and apparatus for improving ventricular status using the force interval relationship |
| US7027866B2 (en) | 2003-07-29 | 2006-04-11 | Medtronic, Inc. | Mechanically-based interval optimization for a biventricular pacing engine |
| US7065400B2 (en) * | 2003-08-20 | 2006-06-20 | Pacesetter, Inc. | Method and apparatus for automatically programming CRT devices |
| US7010347B2 (en) * | 2004-02-14 | 2006-03-07 | Pacesetter, Inc. | Optimization of impedance signals for closed loop programming of cardiac resynchronization therapy devices |
| US7617002B2 (en) * | 2003-09-15 | 2009-11-10 | Medtronic, Inc. | Selection of neurostimulator parameter configurations using decision trees |
| CA2548001A1 (en) * | 2003-12-03 | 2005-06-23 | Medtronic, Inc. | Method and apparatus for determining an efficacious atrioventricular delay interval |
| US20050137629A1 (en) * | 2003-12-08 | 2005-06-23 | Dyjach John A. | Trended measurement of cardiac resynchronization therapy |
| US7123960B2 (en) * | 2003-12-22 | 2006-10-17 | Cardiac Pacemakers, Inc. | Method and system for delivering cardiac resynchronization therapy with variable atrio-ventricular delay |
| US7389141B2 (en) * | 2003-12-22 | 2008-06-17 | Cardiac Pacemakers, Inc. | Biatrial pacing optimization for biventricular pacing |
| US7115096B2 (en) * | 2003-12-24 | 2006-10-03 | Cardiac Pacemakers, Inc. | Third heart sound activity index for heart failure monitoring |
| US7203541B2 (en) * | 2004-03-12 | 2007-04-10 | Medtronic, Inc. | Real-time optimization of right to left ventricular timing sequence in bi-ventricular pacing of heart failure patients |
| US7254442B2 (en) * | 2004-03-17 | 2007-08-07 | Medtronic, Inc. | Apparatus and method for “LEPARS” interval-based fusion pacing |
| US20050209649A1 (en) * | 2004-03-17 | 2005-09-22 | Bozidar Ferek-Petric | Mechanical sensing system for cardiac pacing and/or for cardiac resynchronization therapy |
-
2006
- 2006-01-25 US US11/338,935 patent/US8175703B2/en not_active Expired - Fee Related
- 2006-12-11 WO PCT/US2006/047216 patent/WO2007087025A1/en not_active Ceased
- 2006-12-11 EP EP06839302.4A patent/EP1984074B1/en not_active Not-in-force
- 2006-12-11 JP JP2008552294A patent/JP5352240B2/ja not_active Expired - Fee Related
-
2012
- 2012-05-08 US US13/466,431 patent/US20120226328A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009524475A (ja) | 2009-07-02 |
| US8175703B2 (en) | 2012-05-08 |
| US20100023078A1 (en) | 2010-01-28 |
| EP1984074B1 (en) | 2015-04-22 |
| US20120226328A1 (en) | 2012-09-06 |
| EP1984074A1 (en) | 2008-10-29 |
| WO2007087025A1 (en) | 2007-08-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5352240B2 (ja) | 心臓再同期療法パラメータの最適化 | |
| US9066662B2 (en) | System and method for estimating cardiac pressure based on cardiac electrical conduction delays using an implantable medical device | |
| US10016607B2 (en) | Systems and methods for tracking stroke volume using hybrid impedance configurations employing a multi-pole implantable cardiac lead | |
| US8175693B2 (en) | Cardiac resynchronization therapy optimization using electromechanical delay from realtime electrode motion tracking | |
| US9049995B2 (en) | System and method for detecting pulmonary congestion based on stroke volume using an implantable medical device | |
| US8275458B2 (en) | Methods and systems for optimizing cardiac pacing intervals for various physiologic factors | |
| EP2805673B1 (en) | System for evaluating diastolic function based on cardiogenic impedance using an implantable medical device | |
| US7794404B1 (en) | System and method for estimating cardiac pressure using parameters derived from impedance signals detected by an implantable medical device | |
| JP2009521260A (ja) | 非侵襲性血行力学的センサを有する心律動管理システム | |
| US8135468B2 (en) | Systems and methods for estimating left atrial pressure (LAP) in patients with acute mitral valve regurgitation for use by an implantable medical device | |
| CN106456023A (zh) | 用于使用心音来检测房性快速性心律失常的方法和装置 | |
| US20130289641A1 (en) | Method and system for optimizing cardiac pacing settings | |
| US20140039333A1 (en) | Systems and methods for detecting mechanical dyssynchrony and stroke volume for use with an implantable medical device employing a multi-pole left ventricular lead | |
| US8175706B2 (en) | Overlapping pacing and tachyarrhythmia detection zones | |
| US7440804B1 (en) | System and method for measuring ventricular evoked response using an implantable medical device | |
| US9002450B2 (en) | Systems and methods for assessing the sphericity and dimensional extent of heart chambers for use with an implantable medical device | |
| CN111556773A (zh) | 无逐搏通信的双腔起搏 | |
| US9186512B2 (en) | Method and apparatus for organ specific inflammation monitoring | |
| US20100222836A1 (en) | Implantable cardiac stimulator, device and method for monitoring the heart cycle in a human heart | |
| EP2491977B1 (en) | System for adapting pacing settings of a cardiac stimulator | |
| US20240382764A1 (en) | Optimization of atrial ventricular delays with conduction system pacing | |
| US20240382765A1 (en) | Conduction system pacing optimal output setting indicator | |
| WO2024092034A1 (en) | Respiratory sinus arrhythmia (rsa) pacing mode activation | |
| CN117042681A (zh) | 用于心脏监测的间接感测机构 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091207 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091207 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111212 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120312 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120521 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120821 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120828 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20130128 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130528 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130710 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20130716 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130812 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130826 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5352240 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: R3D03 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |