JP4381663B2 - Weather resistant flame retardant resin composition and electric wire - Google Patents
Weather resistant flame retardant resin composition and electric wire Download PDFInfo
- Publication number
- JP4381663B2 JP4381663B2 JP2002233107A JP2002233107A JP4381663B2 JP 4381663 B2 JP4381663 B2 JP 4381663B2 JP 2002233107 A JP2002233107 A JP 2002233107A JP 2002233107 A JP2002233107 A JP 2002233107A JP 4381663 B2 JP4381663 B2 JP 4381663B2
- Authority
- JP
- Japan
- Prior art keywords
- weight
- resin composition
- parts
- molecular weight
- light stabilizer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000011342 resin composition Substances 0.000 title claims description 27
- 239000003063 flame retardant Substances 0.000 title claims description 11
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 title claims description 10
- 239000004611 light stabiliser Substances 0.000 claims description 35
- 150000001412 amines Chemical class 0.000 claims description 23
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 claims description 18
- 229910052751 metal Inorganic materials 0.000 claims description 17
- 239000002184 metal Substances 0.000 claims description 17
- 239000011248 coating agent Substances 0.000 claims description 15
- 238000000576 coating method Methods 0.000 claims description 15
- 239000006097 ultraviolet radiation absorber Substances 0.000 claims description 7
- 239000003381 stabilizer Substances 0.000 claims description 3
- 239000012190 activator Substances 0.000 claims 1
- 239000000463 material Substances 0.000 description 22
- 229920000098 polyolefin Polymers 0.000 description 18
- 238000002156 mixing Methods 0.000 description 16
- 230000000052 comparative effect Effects 0.000 description 15
- -1 polypropylene, ethylene-ethyl acrylate copolymer Polymers 0.000 description 14
- 239000006078 metal deactivator Substances 0.000 description 12
- 230000000694 effects Effects 0.000 description 11
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 9
- 238000000034 method Methods 0.000 description 8
- 239000006096 absorbing agent Substances 0.000 description 7
- 229910052802 copper Inorganic materials 0.000 description 7
- 239000010949 copper Substances 0.000 description 7
- 230000006866 deterioration Effects 0.000 description 6
- 238000013329 compounding Methods 0.000 description 5
- 229920001577 copolymer Polymers 0.000 description 4
- 238000004132 cross linking Methods 0.000 description 4
- 238000009792 diffusion process Methods 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 238000009413 insulation Methods 0.000 description 4
- 239000000654 additive Substances 0.000 description 3
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 3
- 239000004020 conductor Substances 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 239000005038 ethylene vinyl acetate Substances 0.000 description 3
- 239000012212 insulator Substances 0.000 description 3
- 229920001684 low density polyethylene Polymers 0.000 description 3
- 239000004702 low-density polyethylene Substances 0.000 description 3
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 3
- 239000000347 magnesium hydroxide Substances 0.000 description 3
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 3
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 3
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 2
- 239000012964 benzotriazole Substances 0.000 description 2
- 238000002485 combustion reaction Methods 0.000 description 2
- 239000003431 cross linking reagent Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 238000010894 electron beam technology Methods 0.000 description 2
- 229920006244 ethylene-ethyl acrylate Polymers 0.000 description 2
- 239000005042 ethylene-ethyl acrylate Substances 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 229920000578 graft copolymer Polymers 0.000 description 2
- 150000004677 hydrates Chemical class 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- QUAMTGJKVDWJEQ-UHFFFAOYSA-N octabenzone Chemical compound OC1=CC(OCCCCCCCC)=CC=C1C(=O)C1=CC=CC=C1 QUAMTGJKVDWJEQ-UHFFFAOYSA-N 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- VVUWYXJTOLSMFV-UHFFFAOYSA-N (2-hydroxy-4-octylphenyl)-phenylmethanone Chemical compound OC1=CC(CCCCCCCC)=CC=C1C(=O)C1=CC=CC=C1 VVUWYXJTOLSMFV-UHFFFAOYSA-N 0.000 description 1
- ARVUDIQYNJVQIW-UHFFFAOYSA-N (4-dodecoxy-2-hydroxyphenyl)-phenylmethanone Chemical compound OC1=CC(OCCCCCCCCCCCC)=CC=C1C(=O)C1=CC=CC=C1 ARVUDIQYNJVQIW-UHFFFAOYSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- NALFRYPTRXKZPN-UHFFFAOYSA-N 1,1-bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane Chemical compound CC1CC(C)(C)CC(OOC(C)(C)C)(OOC(C)(C)C)C1 NALFRYPTRXKZPN-UHFFFAOYSA-N 0.000 description 1
- UBRWPVTUQDJKCC-UHFFFAOYSA-N 1,3-bis(2-tert-butylperoxypropan-2-yl)benzene Chemical compound CC(C)(C)OOC(C)(C)C1=CC=CC(C(C)(C)OOC(C)(C)C)=C1 UBRWPVTUQDJKCC-UHFFFAOYSA-N 0.000 description 1
- MEZZCSHVIGVWFI-UHFFFAOYSA-N 2,2'-Dihydroxy-4-methoxybenzophenone Chemical compound OC1=CC(OC)=CC=C1C(=O)C1=CC=CC=C1O MEZZCSHVIGVWFI-UHFFFAOYSA-N 0.000 description 1
- ZXDDPOHVAMWLBH-UHFFFAOYSA-N 2,4-Dihydroxybenzophenone Chemical compound OC1=CC(O)=CC=C1C(=O)C1=CC=CC=C1 ZXDDPOHVAMWLBH-UHFFFAOYSA-N 0.000 description 1
- XMNIXWIUMCBBBL-UHFFFAOYSA-N 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)OOC(C)(C)C1=CC=CC=C1 XMNIXWIUMCBBBL-UHFFFAOYSA-N 0.000 description 1
- ZMWRRFHBXARRRT-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4,6-bis(2-methylbutan-2-yl)phenol Chemical compound CCC(C)(C)C1=CC(C(C)(C)CC)=CC(N2N=C3C=CC=CC3=N2)=C1O ZMWRRFHBXARRRT-UHFFFAOYSA-N 0.000 description 1
- OLFNXLXEGXRUOI-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4,6-bis(2-phenylpropan-2-yl)phenol Chemical compound C=1C(N2N=C3C=CC=CC3=N2)=C(O)C(C(C)(C)C=2C=CC=CC=2)=CC=1C(C)(C)C1=CC=CC=C1 OLFNXLXEGXRUOI-UHFFFAOYSA-N 0.000 description 1
- LHPPDQUVECZQSW-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4,6-ditert-butylphenol Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=CC(N2N=C3C=CC=CC3=N2)=C1O LHPPDQUVECZQSW-UHFFFAOYSA-N 0.000 description 1
- ITLDHFORLZTRJI-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-5-octoxyphenol Chemical compound OC1=CC(OCCCCCCCC)=CC=C1N1N=C2C=CC=CC2=N1 ITLDHFORLZTRJI-UHFFFAOYSA-N 0.000 description 1
- DOTYDHBOKPPXRB-UHFFFAOYSA-N 2-butyl-2-[(3,5-ditert-butyl-4-hydroxyphenyl)methyl]propanedioic acid Chemical compound CCCCC(C(O)=O)(C(O)=O)CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 DOTYDHBOKPPXRB-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- UKTZJXRJZBVHKL-UHFFFAOYSA-N 2h-benzotriazole Chemical class C1=CC=C2NN=NC2=C1.C1=CC=C2NN=NC2=C1 UKTZJXRJZBVHKL-UHFFFAOYSA-N 0.000 description 1
- CBXLKTJBDANMLE-UHFFFAOYSA-N 5-oxo-5-(2,2,6,6-tetramethylpiperidin-4-yl)oxypentane-1,2,3-tricarboxylic acid Chemical compound CC1(C)CC(OC(=O)CC(C(CC(O)=O)C(O)=O)C(O)=O)CC(C)(C)N1 CBXLKTJBDANMLE-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- 229920010126 Linear Low Density Polyethylene (LLDPE) Polymers 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 239000006057 Non-nutritive feed additive Substances 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 229920010346 Very Low Density Polyethylene (VLDPE) Polymers 0.000 description 1
- 239000004708 Very-low-density polyethylene Substances 0.000 description 1
- XTXAZHSPVHPNSL-UHFFFAOYSA-N [4-[4-(4-benzoyl-3-hydroxyphenoxy)butoxy]-2-hydroxyphenyl]-phenylmethanone Chemical compound C=1C=C(C(=O)C=2C=CC=CC=2)C(O)=CC=1OCCCCOC(C=C1O)=CC=C1C(=O)C1=CC=CC=C1 XTXAZHSPVHPNSL-UHFFFAOYSA-N 0.000 description 1
- LGLRENMSBBZYTN-UHFFFAOYSA-N [4-[6-(4-benzoyl-3-hydroxyphenoxy)hexoxy]-2-hydroxyphenyl]-phenylmethanone Chemical compound C=1C=C(C(=O)C=2C=CC=CC=2)C(O)=CC=1OCCCCCCOC(C=C1O)=CC=C1C(=O)C1=CC=CC=C1 LGLRENMSBBZYTN-UHFFFAOYSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000005260 alpha ray Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 150000008366 benzophenones Chemical class 0.000 description 1
- 230000005250 beta ray Effects 0.000 description 1
- FLPKSBDJMLUTEX-UHFFFAOYSA-N bis(1,2,2,6,6-pentamethylpiperidin-4-yl) 2-butyl-2-[(3,5-ditert-butyl-4-hydroxyphenyl)methyl]propanedioate Chemical compound C1C(C)(C)N(C)C(C)(C)CC1OC(=O)C(C(=O)OC1CC(C)(C)N(C)C(C)(C)C1)(CCCC)CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 FLPKSBDJMLUTEX-UHFFFAOYSA-N 0.000 description 1
- WXNRYSGJLQFHBR-UHFFFAOYSA-N bis(2,4-dihydroxyphenyl)methanone Chemical compound OC1=CC(O)=CC=C1C(=O)C1=CC=C(O)C=C1O WXNRYSGJLQFHBR-UHFFFAOYSA-N 0.000 description 1
- SODJJEXAWOSSON-UHFFFAOYSA-N bis(2-hydroxy-4-methoxyphenyl)methanone Chemical compound OC1=CC(OC)=CC=C1C(=O)C1=CC=C(OC)C=C1O SODJJEXAWOSSON-UHFFFAOYSA-N 0.000 description 1
- FQUNFJULCYSSOP-UHFFFAOYSA-N bisoctrizole Chemical compound N1=C2C=CC=CC2=NN1C1=CC(C(C)(C)CC(C)(C)C)=CC(CC=2C(=C(C=C(C=2)C(C)(C)CC(C)(C)C)N2N=C3C=CC=CC3=N2)O)=C1O FQUNFJULCYSSOP-UHFFFAOYSA-N 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- XFWJKVMFIVXPKK-UHFFFAOYSA-N calcium;oxido(oxo)alumane Chemical compound [Ca+2].[O-][Al]=O.[O-][Al]=O XFWJKVMFIVXPKK-UHFFFAOYSA-N 0.000 description 1
- 238000010382 chemical cross-linking Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- NLCKLZIHJQEMCU-UHFFFAOYSA-N cyano prop-2-enoate Chemical class C=CC(=O)OC#N NLCKLZIHJQEMCU-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- PWZFXELTLAQOKC-UHFFFAOYSA-A dialuminum;hexamagnesium;carbonate;hexadecahydroxide;tetrahydrate Chemical compound O.O.O.O.[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Al+3].[Al+3].[O-]C([O-])=O PWZFXELTLAQOKC-UHFFFAOYSA-A 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- TVIDDXQYHWJXFK-UHFFFAOYSA-N dodecanedioic acid Chemical compound OC(=O)CCCCCCCCCCC(O)=O TVIDDXQYHWJXFK-UHFFFAOYSA-N 0.000 description 1
- MCPKSFINULVDNX-UHFFFAOYSA-N drometrizole Chemical compound CC1=CC=C(O)C(N2N=C3C=CC=CC3=N2)=C1 MCPKSFINULVDNX-UHFFFAOYSA-N 0.000 description 1
- IAJNXBNRYMEYAZ-UHFFFAOYSA-N ethyl 2-cyano-3,3-diphenylprop-2-enoate Chemical compound C=1C=CC=CC=1C(=C(C#N)C(=O)OCC)C1=CC=CC=C1 IAJNXBNRYMEYAZ-UHFFFAOYSA-N 0.000 description 1
- TUKWPCXMNZAXLO-UHFFFAOYSA-N ethyl 2-nonylsulfanyl-4-oxo-1h-pyrimidine-6-carboxylate Chemical compound CCCCCCCCCSC1=NC(=O)C=C(C(=O)OCC)N1 TUKWPCXMNZAXLO-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 229920001038 ethylene copolymer Polymers 0.000 description 1
- 229920006225 ethylene-methyl acrylate Polymers 0.000 description 1
- 229920005680 ethylene-methyl methacrylate copolymer Polymers 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 238000007765 extrusion coating Methods 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 230000005251 gamma ray Effects 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229960001545 hydrotalcite Drugs 0.000 description 1
- 229910001701 hydrotalcite Inorganic materials 0.000 description 1
- 230000005865 ionizing radiation Effects 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 229920000092 linear low density polyethylene Polymers 0.000 description 1
- 239000004707 linear low-density polyethylene Substances 0.000 description 1
- 229920005679 linear ultra low density polyethylene Polymers 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229920001179 medium density polyethylene Polymers 0.000 description 1
- 239000004701 medium-density polyethylene Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 150000001451 organic peroxides Chemical class 0.000 description 1
- DXGLGDHPHMLXJC-UHFFFAOYSA-N oxybenzone Chemical compound OC1=CC(OC)=CC=C1C(=O)C1=CC=CC=C1 DXGLGDHPHMLXJC-UHFFFAOYSA-N 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229910000077 silane Inorganic materials 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000012756 surface treatment agent Substances 0.000 description 1
- WUPCFMITFBVJMS-UHFFFAOYSA-N tetrakis(1,2,2,6,6-pentamethylpiperidin-4-yl) butane-1,2,3,4-tetracarboxylate Chemical compound C1C(C)(C)N(C)C(C)(C)CC1OC(=O)CC(C(=O)OC1CC(C)(C)N(C)C(C)(C)C1)C(C(=O)OC1CC(C)(C)N(C)C(C)(C)C1)CC(=O)OC1CC(C)(C)N(C)C(C)(C)C1 WUPCFMITFBVJMS-UHFFFAOYSA-N 0.000 description 1
- NAQGCFBMSYBDFZ-UHFFFAOYSA-N tetrakis(2,2,6,6-tetramethylpiperidin-4-yl) propane-1,1,2,3-tetracarboxylate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)CC(C(=O)OC1CC(C)(C)NC(C)(C)C1)C(C(=O)OC1CC(C)(C)NC(C)(C)C1)C(=O)OC1CC(C)(C)NC(C)(C)C1 NAQGCFBMSYBDFZ-UHFFFAOYSA-N 0.000 description 1
- OIRRCMCATRAYJG-UHFFFAOYSA-N tris(2,2,6,6-tetramethylpiperidin-4-yl) 1H-triazine-2,4,6-tricarboxylate Chemical compound CC1(NC(CC(C1)OC(=O)N1NC(=CC(=N1)C(=O)OC1CC(NC(C1)(C)C)(C)C)C(=O)OC1CC(NC(C1)(C)C)(C)C)(C)C)C OIRRCMCATRAYJG-UHFFFAOYSA-N 0.000 description 1
- HBUNLJQRZABWAM-UHFFFAOYSA-N tris(2,2,6,6-tetramethylpiperidin-4-yl) 2-hydroxypropane-1,2,3-tricarboxylate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)CC(O)(C(=O)OC1CC(C)(C)NC(C)(C)C1)CC(=O)OC1CC(C)(C)NC(C)(C)C1 HBUNLJQRZABWAM-UHFFFAOYSA-N 0.000 description 1
- HAJIOQHUJLPSAL-UHFFFAOYSA-N tris(2,2,6,6-tetramethylpiperidin-4-yl) benzene-1,3,5-tricarboxylate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)C1=CC(C(=O)OC2CC(C)(C)NC(C)(C)C2)=CC(C(=O)OC2CC(C)(C)NC(C)(C)C2)=C1 HAJIOQHUJLPSAL-UHFFFAOYSA-N 0.000 description 1
- FDINTOZPTMFQRL-UHFFFAOYSA-N tris(2,2,6,6-tetramethylpiperidin-4-yl) butane-1,2,3-tricarboxylate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)C(C)C(C(=O)OC1CC(C)(C)NC(C)(C)C1)CC(=O)OC1CC(C)(C)NC(C)(C)C1 FDINTOZPTMFQRL-UHFFFAOYSA-N 0.000 description 1
- 229920001866 very low density polyethylene Polymers 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Landscapes
- Insulated Conductors (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Description
【0001】
【発明の属する技術分野】
本発明は、燃焼時にハロゲン系ガス等の有害ガスの発生がないとともに、機械的強度、耐熱性、電気特性、難燃性及び耐候性をバランス良く兼ね備えた、例えば、屋内や屋外に設置される照明用機器のリード線の絶縁被覆材料又はシース材料などとして好適な耐候性難燃樹脂組成物と、該樹脂組成物からなる被覆を備えた電線に関する。
【0002】
【従来の技術】
オレフィン系ポリマーは、優れた電気特性を有し、安価で加工性も良いことから従来、絶縁電線の絶縁被覆材料又はシース材料などとして広く用いられている。しかし、オレフィン系ポリマーは、それ自体が可燃性物質であるため、安全性、防火上の問題から高度な難燃性を付与する必要があり、その方法としては、オレフィン系ポリマーに、ハロゲン系の難燃剤を配合する方法が広く採用されてきた。しかしながら、これらは燃焼時に多量のハロゲン系ガスを発生し、周囲の機器への腐食性、人体への有害性等が問題となって、近年、ハロゲン系ガスを発生しないことが要望され、難燃剤として水酸化アルミニウムや水酸化マグネシウムのような金属水和物を配合する方法が提案されている。しかしながら、この場合、オレフィン系ポリマーに多量の金属水和物を配合させることにより、ハロゲン系の難燃剤を配合した場合と同程度の高度な難燃性を付与することはできるものの、耐候性、特に耐光性が低下してしまうという欠点があった。耐候性が不充分であると、例えば、屋内や屋外に設置される照明用機器のリード線の絶縁被覆材料又はシース材料などとして使用することが困難である。そこで、従来では、オレフィン系ポリマーに金属水和物を配合した樹脂組成物に、光安定剤や紫外線吸収剤を配合して耐候性を付与するなどの対策が取られている。
【0003】
【発明が解決しようとする課題】
しかしながら、このような方法によって得られた樹脂組成物を屋内や屋外に設置される照明用機器のリード線の絶縁被覆材料などとして使用した場合、樹脂組成物中に配合した光安定剤が次第に表面側に移行してブリードアウトし、光安定剤の効果が損なわれて耐候性が長期間持続しないという問題点があった。そこで、本発明者らは、このような従来技術の問題点を解決するべく種々検討した結果、オレフィン系ポリマーに金属水和物を配合した難燃樹脂組成物に、分子量の異なる二種類の光安定剤と紫外線吸収剤とを併用し、更にそれらを特定量配合することにより、機械的強度、耐熱性、電気特性、難燃性及び耐候性をバランス良く兼ね備えた樹脂組成物が得られることを見い出し、本発明に至った。
【0004】
【課題を解決するための手段】
即ち、本発明の請求項1による耐候性難燃樹脂組成物は、(a)オレフィン系ポリマー100重量部に対し、(b)金属水和物150重量部以上220重量部以下、(c)高分子量ヒンダードアミン系光安定剤0.2重量部以上10重量部以下、(d)分子量600以上900以下の低分子量ヒンダードアミン系光安定剤0.2重量部以上10重量部以下、(e)紫外線吸収剤0.1重量部以上1重量部以下及び(f)ヒドラジン系金属不活性化剤0.2重量部以上1重量部以下を配合することを特徴とするものである。
又、請求項2による電線は、請求項1記載の樹脂組成物からなる被覆を備えたことを特徴とするものである。
又、請求項3による電線は、請求項2記載の電線において、前記樹脂組成物からなる被覆が、架橋されていることを特徴とするものである。
【0005】
【発明の実施の形態】
以下、本発明の樹脂組成物を構成する各成分について説明する。
(a)オレフィン系ポリマー
本発明で使用されるオレフィン系ポリマーとしては、低密度ポリエチレン(LDPE)、中密度ポリエチレン、高密度ポリエチレン、超低密度ポリエチレン(VLDPE)、直鎖状低密度ポリエチレン(LLDPE)、直鎖状超低密度ポリエチレン、ポリプロピレン等のホモポリマー、エチレン−アクリル酸エチル共重合体(EEA)、エチレン−アクリル酸メチル共重合体、エチレン−メタクリル酸エチル共重合体、エチレン−メタクリル酸メチル共重合体、エチレン−酢酸ビニル共重合体(EVA)、エチレン−酢酸ビニル−スチレングラフト共重合体、エチレン−アクリル酸エチル−無水マレイン酸共重合体、エチレン−無水マレイン酸グラフト共重合体、エチレン−プロピレン共重合体、エチレン−プロピレン−ジエン共重合体等のエチレン系共重合体が挙げられる。これらのオレフィン系ポリマーの内、いずれを使用しても良いが、後述する光安定剤や紫外線吸収剤との相溶性の点から、ポリエチレン(LDPE、LLDPE、VLDPE)や、EVA、EEAなどが好ましい。これらのポリオレフィン系ポリマーは、1種のみを単独で使用しても2種以上併用しても良い。
【0006】
(b)金属水和物
本発明で使用される金属水和物としては、水酸化マグネシウム、水酸化アルミニウム、水酸化カルシウム、ハイドロタルサイト、ドーソナイト、アルミン酸カルシウムなどが挙げられる。これらの金属水和物の内、いずれを使用しても良いが、オレフィン系ポリマーの分解温度付近で結晶水を放出し、しかも吸熱量の大きい水酸化マグネシウムや水酸化アルミニウムは、特に難燃効果が高いため好ましい。又、これらの金属水和物は、例えば、ラウリン酸、ステアリン酸、オレイン酸などの高級脂肪酸、又はこれらのアルミニウム、マグネシウム、カルシウム塩などの高級脂肪酸塩、シラン系やチタネート系の表面処理剤によって表面処理されたものが、オレフィン系ポリマーとの親和性を良くし、分散性を良くするために好ましく用いられる。
【0007】
金属水和物は、オレフィン系ポリマー100重量部に対し、150重量部以上220重量部以下配合することが好ましい。金属水和物の配合量が150重量部未満では、目的とする充分な難燃性(例えば、JIS C 3005に規定される60度傾斜難燃試験に合格するレベル)を得ることが困難となってしまい、又、220重量部を超えると、樹脂組成物の機械的強度が低下し、更には、耐候性が低下してしまう。
【0008】
本発明においては、耐候性を付与する目的で光安定剤を配合するのであるが、この際、分子量の異なる二種類の光安定剤(高分子量ヒンダードアミン系光安定剤と、低分子量ヒンダードアミン系光安定剤)、を併用する必要がある。それぞれを単独で使用した場合には、充分な耐候性を付与することができない。これは以下のような理由による。つまり、通常、太陽光や照明器具から発せられる紫外線によって電線の絶縁被覆材料やシース材料が劣化する場合は、その表面から進行する。このため、光安定剤としては、内部から表面に拡散し易いものが有効と言える。低分子量のヒンダードアミン系光安定剤は、分子の大きさが小さく拡散し易いため、初期段階においては有効に劣化を防止することができるのであるが、これらは次第にブリードアウトして外部に逸散してしまうため、光安定剤の効果が損なわれて耐候性が長期間持続しなくなってしまう。一方、高分子量のヒンダードアミン系光安定剤は、拡散速度が遅いため、表面劣化に対して内部からの拡散による劣化防止効果は短期的にはあまり期待できないが、逸散しにくい性質を有していることから劣化防止効果が持続し、耐候性が長期間持続する。従って、これら二種類のヒンダードアミン系光安定剤をそれぞれ一定量以上配合することにより、初期においては低分子量の光安定剤が、長期においては高分子量の光安定剤がそれぞれ劣化防止効果を発揮し、少量の配合量で長期間の耐候性を付与することができる。
【0009】
(c)高分子量ヒンダードアミン系光安定剤
高分子量ヒンダードアミン系光安定剤としては、分子量が1000を超えるものが用いられる。このようなものとしては、例えば、オリゴマー型のHALSであるポリ[6−(1,1,3,3−テトラメチルブチル)イミノ−1,3,5−トリアジン−2,4−ジイル][(2,2,6,6−テトラメチル−4−ピペリジル)イミノ]ヘキサメチレン[(2,2,6,6−テトラメチル−4−ピペリジル)イミノ]や、コハク酸ジメチル−1−(2−ヒドロキシエチル)−4−ヒドロキシ−2,2,6,6−テトラメチルピペリジン重縮合物などが挙げられる。より具体的には、キマソーブ944LD、チヌビン622LDなどの商品名で市販されているものを使用することができる。
【0010】
これらの高分子量ヒンダードアミン系光安定剤は、オレフィン系ポリマー100重量部に対し、0.2重量部以上10重量部以下配合することが好ましい。高分子量ヒンダードアミン系光安定剤の配合量が0.2重量部未満では、目的とする充分な耐候性を得ることができず、又、10重量部を超えると、増量による耐候性向上効果が期待できないだけでなく、分散性の問題により機械的強度が低下し、更には、ブルーミングが生じてしまう。
【0011】
(d)低分子量ヒンダードアミン系光安定剤
低分子量ヒンダードアミン系光安定剤としては、分子量が1000以下、好ましくは900以下、更に好ましくは600以上900以下程度のものが用いられる。分子量が600未満であると、拡散速度が速すぎるため、極短期間で外部に逸散してしまう可能性があり、分子量が900を超えると、拡散速度が遅くなるため、初期段階における劣化防止効果が薄くなってしまう可能性がある。このようなものとしては、例えば、トリス(2,2,6,6−テトラメチル−4−ピペリジル)ベンゼン−1,3,5−トリカルボキシレート、トリス(2,2,6,6−テトラメチル−4−ピペリジル)−2−アセトキシプロパン−1,2,3−トリカルボキシレート、トリス(2,2,6,6−テトラメチル−4−ピペリジル)−2−ヒドロキシプロパン−1,2,3−トリカルボキシレート、トリス(2,2,6,6−テトラメチル−4−ピペリジル)トリアジン−2,4,6−トリカルボキシレート、トリス(2,2,6,6−テトラメチル−4−ピペリジル)ブタン−1,2,3−トリカルボキシレート、テトラキス(2,2,6,6−テトラメチル−4−ピペリジル)プロパン−1,1,2,3−テトラカルボキシレート、テトラキス(2,2,6,6−テトラメチル−4−ピペリジル)1,2,3,4−ブタンテトラカルボキシレート、テトラキス(1,2,2,6,6−ペンタメチル−4−ピペリジル)1,2,3,4−ブタンテトラカルボキシレート、2−(3,5−ジ−t−ブチル−4−ヒドロキシベンジル)−2−n−ブチルマロン酸ビス(1,2,2,6,6−ペンタメチル−4−ピペリジル)などが挙げられる。より具体的には、アデカスタブLA−57、アデカスタブLA−52、チヌビン144などの商品名で市販されているものを使用することができる。
【0012】
これらの低分子量ヒンダードアミン系光安定剤は、オレフィン系ポリマー100重量部に対し、0.2重量部以上10重量部以下配合することが好ましい。低分子量ヒンダードアミン系光安定剤の配合量が0.2重量部未満では、目的とする充分な耐候性を得ることができず、又、10重量部を超えると、増量による耐候性向上効果が期待できないだけでなく、分散性の問題により機械的強度が低下し、更には、ブルーミングが生じてしまう。
【0013】
(e)紫外線吸収剤
紫外線吸収剤としては、例えば、2−(2’−ヒドロキシ−3’,5’−ジ−t−ブチルフェニル)ベンゾトリアゾール、2−(3,5−ジ−t−アミル−2−ヒドロキシフェニル)ベンゾトリアゾール、2−(2’−ヒドロキシ−5’−メチル−フェニル)ベンゾトリアゾール、2−(2’−ヒドロキシ−5’−t−オクチルフェニル)ベンゾトリアゾール、2−(2’−ヒドロキシ−3’,5’−ジ−t−アミルフェニル)ベンゾトリアゾール、2−〔2’−ヒドロキシ−3’−(3’’,4’’,5’’,6’’−テトラヒドロ−フタルイミドメチル)−5’−メチルフェニル〕ベンゾトリアゾール、2,2’−メチレンビス〔4−(1,1,3,3−テトラメチルブチル)−6−(2H−ベンゾトリアゾール−2−イル)フェノール〕、2−〔2−ヒドロキシ−3,5−ビス(α,α−ジメチルベンジル)フェニル〕−2H−ベンゾトリアゾール、2−(2−ヒドロキシ−4−オクチルオキシフェニル)−2H−ベンゾトリアゾール、2−(2H−ベンゾトリアゾール−2−イル)−4−メチル−6−(3,4,5,6−テトラヒドロフタルイミジルメチル)フェノールなどのベンゾトリアゾール系、2−ヒドロキシ−4−メトキシベンゾフェノン、2,4−ジヒドロキシベンゾフェノン、2,2’−ジヒドロキシ−4−メトキシベンゾフェノン、2,2’−ジヒドロキシ−4,4’−ジメトキシベンゾフェノン、2−ヒドロキシ−4−n−オクトキシベンゾフェノン、2,2’,4,4’−テトラヒドロキシベンゾフェノン、4−ドデシロキシ−2−ヒドロキシベンゾフェノン、3,5−ジ−t−ブチル−4−ヒドロキシベンゾイル酸,n−ヘクサデシルエステル、1,4−ビス(4−ベンゾイル−3−ヒドロキシフェノキシ)ブタン、1,6−ビス(4−ベンゾイル−3−ヒドロキシフェノキシ)ヘキサンなどのベンゾフェノン系、エチル−2−シアノ−3,3−ジフェニルアクリレートに代表されるシアノアクリレート系などが挙げられる。より具体的には、チヌビン320、チヌビン328、チヌビン234、アデカスタブLA31、SEESORB102、SEESORB103、SEESORB501などの商品名で市販されているものを使用することができる。
【0014】
これらの紫外線吸収剤は、オレフィン系ポリマー100重量部に対し、0.1重量部以上1重量部以下配合することが好ましい。紫外線吸収剤の配合量が0.1重量部未満では、目的とする充分な耐候性を得ることができず、又、1重量部を超えると、紫外線吸収剤自身の色相により樹脂組成物が黄色く着色され、機械的強度及び耐熱性も低下し、更には、ブルーミングが生じてしまう。
【0015】
(f)ヒドラジン系金属不活性化剤
本発明においては、上記の成分の加えて、(f)ヒドラジン系金属不活性化剤を更に配合しても良い。ヒドラジン系金属不活性化剤を配合することにより、得られる樹脂組成物の耐候性を保持しつつ、銅害を防止することができ、更に耐熱性を向上することができる。ヒドラジン系金属不活性化剤としては、例えば、デカメチレンジカルボン酸ジサリチロイルヒドラジドなどが挙げられる。これらの金属不活性化剤は、オレフィン系ポリマー100重量部に対し、0.2重量部以上1重量部以下配合することが好ましい。金属不活性化剤の配合量が0.2重量部未満では、銅害防止の作用が充分でなく、耐熱性を向上する作用が得られない。又、1重量部を超えると耐候性が低下してしまい、更には高価となってしまう。
【0016】
本発明においては、上記の各構成材料以外にも、本発明の目的を阻害しない範囲内で、従来、電線、ケーブルにおいて一般的に使用されている各種の添加剤を配合しても良い。このような添加剤としては、例えば、難燃助剤、架橋剤、架橋助剤、滑剤、軟化剤、分散剤、酸化防止剤、加工助剤、安定剤、着色剤、顔料などが挙げられる。これらの各種添加剤を上記の各構成材料に必要に応じて配合したものを、インターナルミキサー、一軸混練機、二軸混練機等の公知の混練機を使用して充分に混練りすることによって本発明の樹脂組成物を得ることができる。
【0017】
このようにして得られた本発明の樹脂組成物を公知の方法によって導体周上に押出被覆し、その後、適宜に架橋を施すことにより、本発明の他の態様による電線を得ることができる。この際、被覆の厚さが0.3mm以上である電線などに本発明の樹脂組成物を適用した場合には、特に本発明の樹脂組成物の有する優れた特徴が顕著に発現することになる。被覆の厚さが0.3mm未満の場合、絶縁体内部に存在する光安定剤の量が不足するため、耐候性に問題が生じてしまう可能性がある。
【0018】
架橋方法は特に限定されず、例えば、1,3−ビス(t−ブチルペルオキシイソプロピル)ベンゼン、1,1−ビス(t−ブチルペルオキシ)−3,3,5−トリメチルシクロヘキサン、ジクミルパーオキサイド等の有機過酸化物を架橋剤として使用した化学架橋法、X線、γ線、電子線、陽子線、重陽子線、α線、β線等の電離性放射線を使用した照射架橋法などが挙げられる。
【0019】
【実施例】
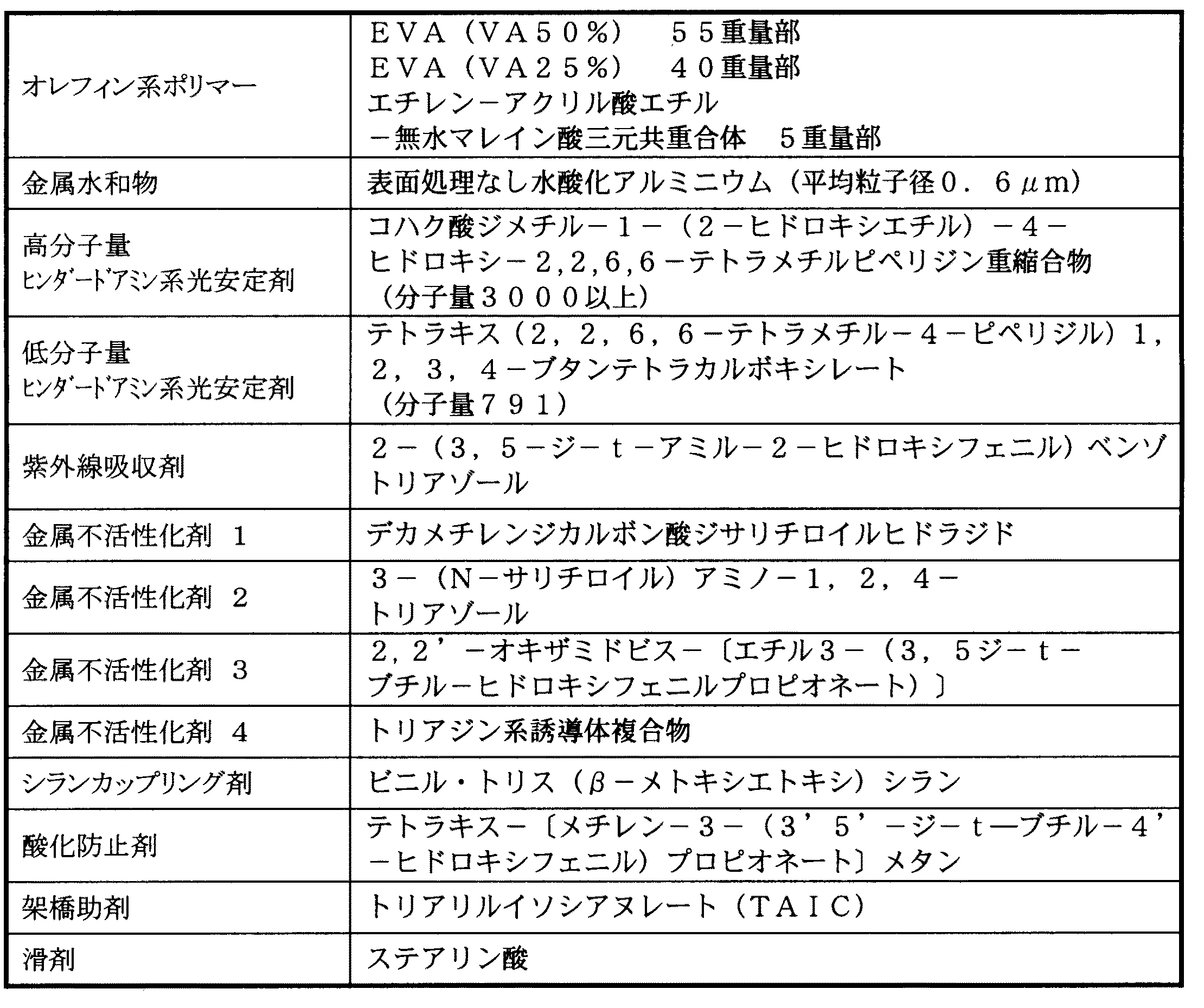
以下に本発明の実施例を比較例と併せて説明する。この実施例で使用した各配合材料の詳細は表4に示す通りである。
【0020】
表4に示した配合材料を二軸混練機で充分に混練りし、得られた樹脂組成物をペレット化した後、L/D=24の25mmφ押出機に供給して、シリンダー150℃、ヘッド160℃の温度条件にて、0.8φの錫めっき軟銅線からなる導体周上に0.8mmの肉厚で押出被覆した。その後、加速電圧650kV、照射線量100kGyの条件で電子線を照射し、仕上外径2.4mmφの架橋電線を製造した。
【0021】
ここで、このようにして得られた合計17種類の架橋電線を試料として、機械的強度(引張強度、伸び)、耐熱性、電気特性、難燃性及び耐候性について、それぞれ評価を行った。結果は表1及び表2に示した。
【0022】
評価方法は以下の通りである。
機械的強度
JIS C 3005(2000)に準拠して、引張強度と伸びを測定した。各々の要求特性は、電気用品安全法の絶縁体要求特性から引用し、引張強度が10MPa以上、伸びが200%以上とした。
【0023】
耐熱性
180℃に保持された恒温槽内に168時間放置した後取り出し、JIS C3005(2000)に準拠して、引張強度残率、伸び残率をそれぞれ測定した。各々の要求特性は、引張強度残率が70%以上、伸び残率が50%以上とした。
【0024】
電気特性
JIS C 3005(2000)に準拠して、水中耐電圧試験を実施した。試験の判断基準は、電気用品安全法の絶縁体要求特性から引用し、AC1.5kVで1分間保持して絶縁破壊が起きなかったものを合格とした。
【0025】
難燃性
JIS C 3005(2000)に準拠して、60度難燃性試験を実施した。試験の判断基準は、60秒以内に自己消火したものを合格とした。
【0026】
耐候性
回転式ギヤオーブンの中央に位置している400W透明型高圧水銀ランプ光源から240mm離れた位置に試料をセットし、120℃、1500時間放置した後取り出し、伸びを測定した。要求特性は、伸びが50%以上とした。
【0027】
【表1】
【0028】
【表2】
【0029】
【表3】
【0030】
【表4】
【0031】
参考例1〜3及び比較例1、2
金属水和物の配合量が本発明の範囲内に入っている参考例1〜3は、諸特性を満足していることが確認された。これに対し、比較例1は、金属水和物の配合量が100重量部と本発明の下限値である150重量部を下回るため、その結果、難燃性が不合格となり、又、比較例2は、金属水和物の配合量が250重量部と本発明の上限値である220重量部を超えているため、その結果、機械的強度(伸び)と耐候性が不合格となることが確認された。
【0032】
参考例4、5及び比較例3、4
高分子量ヒンダードアミン系光安定剤の配合量が本発明の範囲内に入っている参考例4、5は、諸特性を満足していることが確認された。これに対し、比較例3は、高分子量ヒンダードアミン系光安定剤を配合していないため、その結果、耐候性が不合格となり、又、比較例4は、高分子量ヒンダードアミン系光安定剤の配合量が20重量部と本発明の上限値である10重量部を超えているため、その結果、機械的強度(引張強度、伸び)が不合格となることが確認された。
【0033】
参考例6、7及び比較例5、6
低分子量ヒンダードアミン系光安定剤の配合量が本発明の範囲内に入っている参考例6、7は、諸特性を満足していることが確認された。これに対し、比較例5は、高分子量ヒンダードアミン系光安定剤を配合していないため、その結果、耐候性が不合格となり、又、比較例6は、低分子量ヒンダードアミン系光安定剤の配合量が20重量部と本発明の上限値である10重量部を超えているため、その結果、機械的強度(引張強度、伸び)が不合格となることが確認された。
【0034】
参考例8、9及び比較例7、8
紫外線吸収剤の配合量が本発明の範囲内に入っている参考例8、9は諸特性を満足していることが確認された。これに対し、比較例7は、紫外線吸収剤を配合していないため、その結果、耐候性が不合格となり、又、比較例8は、紫外線吸収剤の配合量が3重量部と本発明の上限値である1重量部を超えているため、その結果、機械的強度(引張強度、伸び)及び耐熱性(伸び残率)が不合格となることが確認された。
【0035】
配合材料として、更に金属不活性化剤を加えて得られた合計6種類の架橋電線を試料として、機械的強度(引張強度、伸び)、耐熱性、電気特性、難燃性、耐候性に加え、耐銅害性について、それぞれ評価を行った。結果は表3に示した。
【0036】
耐銅害性の評価方法は以下の通りである。
耐銅害性
導体を取り出したチューブ状サンプルの表面に銅粉末を塗布した状態で、180℃の恒温槽内に168時間放置した後取り出し、伸びを測定した。要求特性は、伸びが50%以上とした。
【0037】
実施例1、2及び比較例9〜12
ヒドラジン系金属不活性化剤の配合量が本発明の範囲内に入っている実施例1、2は、諸特性に加えて耐銅害性も満足し、更に、金属不活性化剤を配合していない参考例2と比べて耐熱性の伸び残率が向上し、70%台となっていることが確認された。これに対し、比較例9は、ヒドラジン系金属不活性化剤の配合量が5重量部と本発明の上限値である1重量部を超えているため、耐熱性は向上しているものの、耐候性が不合格となることが確認された。又、比較例10〜12では、ヒドラジン系以外の金属不活性化剤を使用しているため、耐熱性の向上はなく、耐候性も不合格となるとともに、耐銅害性の試験では激しく劣化してしまい、伸びを測定することができなかった。
【0038】
本発明は上記の実施例に限定されるものではない。上記の実施例では本発明に係る樹脂組成物を電線の絶縁被覆材料として使用したが、複数の電線を組み合わせたケーブルのシース材料、コード状ヒータの絶縁被覆材料、チューブの構成材料などとしても使用可能である。
【0039】
【発明の効果】
以上詳述したように本発明によれば、オレフィン系ポリマーに金属水和物を配合した難燃樹脂組成物に、分子量の異なる二種類の光安定剤と紫外線吸収剤とを併用し、更にそれらを特定量配合することにより、機械的強度、耐熱性、電気特性、難燃性及び耐候性をバランス良く兼ね備えた樹脂組成物を得ることができた。この樹脂組成物は、屋内や屋外に設置される照明用機器のリード線の絶縁被覆材料又はシース材料などとして好適である。[0001]
BACKGROUND OF THE INVENTION
The present invention does not generate harmful gases such as halogen-based gases during combustion, and has a good balance of mechanical strength, heat resistance, electrical characteristics, flame retardancy, and weather resistance, for example, installed indoors or outdoors. The present invention relates to a weather-resistant flame-retardant resin composition suitable as an insulating coating material or sheath material for lead wires of lighting equipment, and an electric wire provided with a coating made of the resin composition.
[0002]
[Prior art]
Olefin polymers have been widely used as insulation coating materials or sheath materials for insulated wires because they have excellent electrical properties, are inexpensive and have good workability. However, since the olefin polymer itself is a flammable substance, it is necessary to impart a high degree of flame retardancy from the viewpoint of safety and fire prevention. Methods of blending flame retardants have been widely adopted. However, they generate a large amount of halogen-based gas at the time of combustion, causing problems such as corrosiveness to surrounding equipment and harmfulness to human bodies. In recent years, it has been demanded not to generate halogen-based gas. A method of blending a metal hydrate such as aluminum hydroxide or magnesium hydroxide has been proposed. However, in this case, by adding a large amount of metal hydrate to the olefin polymer, it is possible to impart the same high level of flame retardancy as when a halogen flame retardant is blended, but the weather resistance, In particular, there was a disadvantage that the light resistance was lowered. If the weather resistance is insufficient, it is difficult to use, for example, as an insulation coating material or a sheath material for lead wires of lighting equipment installed indoors or outdoors. Therefore, conventionally, measures have been taken such as adding a light stabilizer or an ultraviolet absorber to a resin composition in which a metal hydrate is blended with an olefin polymer to impart weather resistance.
[0003]
[Problems to be solved by the invention]
However, when the resin composition obtained by such a method is used as an insulation coating material for lead wires of lighting equipment installed indoors or outdoors, the light stabilizer blended in the resin composition gradually becomes a surface. There was a problem that the effect of the light stabilizer was impaired and the weather resistance did not last for a long time. Therefore, as a result of various studies to solve the problems of the prior art, the present inventors have found that a flame retardant resin composition in which a metal hydrate is blended with an olefin polymer has two types of light having different molecular weights. By using a stabilizer and an ultraviolet absorber in combination, and further blending them in a specific amount, a resin composition having a well-balanced mechanical strength, heat resistance, electrical properties, flame retardancy, and weather resistance can be obtained. As a result, the present invention has been achieved.
[0004]
[Means for Solving the Problems]
That is, the weather resistant flame retardant resin composition according to claim 1 of the present invention comprises (a) 100 parts by weight of an olefin polymer, (b) 150 parts by weight or more and 220 parts by weight or less of metal hydrate, (c) high 0.2 to 10 parts by weight of molecular weight hindered amine light stabilizer, (d) 0.2 to 10 parts by weight of low molecular weight hindered amine light stabilizer having a molecular weight of 600 to 900, (e) UV absorber 0.1 to 1 part by weight and (f) hydrazine-based metal deactivator 0.2 to 1 part by weight are blended.
An electric wire according to claim 2 is provided with a coating made of the resin composition according to claim 1.
An electric wire according to claim 3 is the electric wire according to claim 2, wherein the coating made of the resin composition is crosslinked.
[0005]
DETAILED DESCRIPTION OF THE INVENTION
Hereinafter, each component which comprises the resin composition of this invention is demonstrated.
(A) Olefin Polymer As the olefin polymer used in the present invention, low density polyethylene (LDPE), medium density polyethylene, high density polyethylene, very low density polyethylene (VLDPE), linear low density polyethylene (LLDPE) , Homopolymers such as linear ultra-low density polyethylene and polypropylene, ethylene-ethyl acrylate copolymer (EEA), ethylene-methyl acrylate copolymer, ethylene-ethyl methacrylate copolymer, ethylene-methyl methacrylate Copolymer, ethylene-vinyl acetate copolymer (EVA), ethylene-vinyl acetate-styrene graft copolymer, ethylene-ethyl acrylate-maleic anhydride copolymer, ethylene-maleic anhydride graft copolymer, ethylene -Propylene copolymer, ethylene-pro Len - ethylene copolymers such as diene copolymers. Any of these olefin polymers may be used, but polyethylene (LDPE, LLDPE, VLDPE), EVA, EEA, and the like are preferable from the viewpoint of compatibility with the light stabilizer and ultraviolet absorber described below. . These polyolefin polymers may be used alone or in combination of two or more.
[0006]
(B) Metal Hydrate Examples of the metal hydrate used in the present invention include magnesium hydroxide, aluminum hydroxide, calcium hydroxide, hydrotalcite, dosonite, calcium aluminate and the like. Any of these metal hydrates may be used. Magnesium hydroxide and aluminum hydroxide, which release crystal water near the decomposition temperature of the olefin polymer and have a large endotherm, are particularly flame retardant. Is preferable because of high. In addition, these metal hydrates can be obtained, for example, with higher fatty acids such as lauric acid, stearic acid, and oleic acid, or higher fatty acid salts such as aluminum, magnesium, and calcium salts, and silane-based and titanate-based surface treatment agents. The surface-treated product is preferably used in order to improve the affinity with the olefin polymer and improve the dispersibility.
[0007]
The metal hydrate is preferably blended in an amount of 150 to 220 parts by weight with respect to 100 parts by weight of the olefin polymer. When the amount of the metal hydrate is less than 150 parts by weight, it is difficult to obtain the desired sufficient flame retardancy (for example, a level that passes the 60-degree inclined flame retardant test specified in JIS C 3005). Moreover, when it exceeds 220 weight part, the mechanical strength of a resin composition will fall, and also a weather resistance will fall.
[0008]
In the present invention, a light stabilizer is blended for the purpose of imparting weather resistance. At this time, two kinds of light stabilizers having different molecular weights (a high molecular weight hindered amine light stabilizer and a low molecular weight hindered amine light stabilizer) are used. Agent). When each is used alone, sufficient weather resistance cannot be imparted. This is due to the following reasons. That is, when the insulation coating material or sheath material of the electric wire deteriorates due to sunlight or ultraviolet rays emitted from a lighting fixture, the process proceeds from the surface. For this reason, it can be said that a light stabilizer that is easily diffused from the inside to the surface is effective. Low molecular weight hindered amine light stabilizers are small in size and easy to diffuse, so they can effectively prevent deterioration at the initial stage, but they gradually bleed out and dissipate to the outside. Therefore, the effect of the light stabilizer is impaired and the weather resistance is not sustained for a long time. On the other hand, high-molecular-weight hindered amine light stabilizers have a slow diffusion rate, so the deterioration prevention effect due to diffusion from the inside against surface deterioration cannot be expected in the short term, but has the property of not easily dissipating. Therefore, the effect of preventing deterioration is sustained and the weather resistance is maintained for a long time. Therefore, by blending each of these two types of hindered amine light stabilizers in a certain amount or more, the low molecular weight light stabilizer in the initial stage, and the high molecular weight light stabilizer in the long term each exerts an effect of preventing deterioration. Long-term weather resistance can be imparted with a small amount.
[0009]
(C) High molecular weight hindered amine light stabilizer As the high molecular weight hindered amine light stabilizer, one having a molecular weight exceeding 1000 is used. As such, for example, poly [6- (1,1,3,3-tetramethylbutyl) imino-1,3,5-triazine-2,4-diyl] [( 2,2,6,6-tetramethyl-4-piperidyl) imino] hexamethylene [(2,2,6,6-tetramethyl-4-piperidyl) imino] and dimethyl-1- (2-hydroxysuccinate) And ethyl) -4-hydroxy-2,2,6,6-tetramethylpiperidine polycondensate. More specifically, commercially available products such as Kimasorb 944LD and Tinuvin 622LD can be used.
[0010]
These high molecular weight hindered amine light stabilizers are preferably blended in an amount of 0.2 to 10 parts by weight with respect to 100 parts by weight of the olefin polymer. If the blending amount of the high molecular weight hindered amine light stabilizer is less than 0.2 parts by weight, the desired sufficient weather resistance cannot be obtained, and if it exceeds 10 parts by weight, an effect of improving weather resistance by increasing the amount is expected. Not only is it impossible, but the mechanical strength is lowered due to the problem of dispersibility, and further blooming occurs.
[0011]
(D) Low molecular weight hindered amine light stabilizer As the low molecular weight hindered amine light stabilizer, one having a molecular weight of 1000 or less, preferably 900 or less, more preferably 600 or more and 900 or less is used. If the molecular weight is less than 600, the diffusion rate is too high, so it may be dissipated to the outside in a very short period. If the molecular weight exceeds 900, the diffusion rate will be slowed, preventing deterioration in the initial stage. The effect may be diminished. For example, tris (2,2,6,6-tetramethyl-4-piperidyl) benzene-1,3,5-tricarboxylate, tris (2,2,6,6-tetramethyl) -4-piperidyl) -2-acetoxypropane-1,2,3-tricarboxylate, tris (2,2,6,6-tetramethyl-4-piperidyl) -2-hydroxypropane-1,2,3- Tricarboxylate, tris (2,2,6,6-tetramethyl-4-piperidyl) triazine-2,4,6-tricarboxylate, tris (2,2,6,6-tetramethyl-4-piperidyl) Butane-1,2,3-tricarboxylate, tetrakis (2,2,6,6-tetramethyl-4-piperidyl) propane-1,1,2,3-tetracarboxylate, teto Kiss (2,2,6,6-tetramethyl-4-piperidyl) 1,2,3,4-butanetetracarboxylate, tetrakis (1,2,2,6,6-pentamethyl-4-piperidyl) 1, 2,3,4-butanetetracarboxylate, 2- (3,5-di-tert-butyl-4-hydroxybenzyl) -2-n-butylmalonate bis (1,2,2,6,6-pentamethyl -4-piperidyl) and the like. More specifically, commercially available products such as ADK STAB LA-57, ADK STAB LA-52, and Tinuvin 144 can be used.
[0012]
These low molecular weight hindered amine light stabilizers are preferably blended in an amount of 0.2 to 10 parts by weight with respect to 100 parts by weight of the olefin polymer. If the blending amount of the low molecular weight hindered amine light stabilizer is less than 0.2 parts by weight, the desired sufficient weather resistance cannot be obtained, and if it exceeds 10 parts by weight, an effect of improving weather resistance by increasing the amount is expected. Not only is it impossible, but the mechanical strength is lowered due to the problem of dispersibility, and further blooming occurs.
[0013]
(E) UV absorbers Examples of UV absorbers include 2- (2′-hydroxy-3 ′, 5′-di-t-butylphenyl) benzotriazole and 2- (3,5-di-t-amyl). 2-hydroxyphenyl) benzotriazole, 2- (2′-hydroxy-5′-methyl-phenyl) benzotriazole, 2- (2′-hydroxy-5′-t-octylphenyl) benzotriazole, 2- (2 '-Hydroxy-3', 5'-di-t-amylphenyl) benzotriazole, 2- [2'-hydroxy-3 '-(3 ", 4", 5 ", 6" -tetrahydro- Phthalimidomethyl) -5′-methylphenyl] benzotriazole, 2,2′-methylenebis [4- (1,1,3,3-tetramethylbutyl) -6- (2H-benzotriazol-2-yl) phenol] , 2- [2 Hydroxy-3,5-bis (α, α-dimethylbenzyl) phenyl] -2H-benzotriazole, 2- (2-hydroxy-4-octyloxyphenyl) -2H-benzotriazole, 2- (2H-benzotriazole- Benzotriazoles such as 2-yl) -4-methyl-6- (3,4,5,6-tetrahydrophthalimidylmethyl) phenol, 2-hydroxy-4-methoxybenzophenone, 2,4-dihydroxybenzophenone, 2 , 2'-dihydroxy-4-methoxybenzophenone, 2,2'-dihydroxy-4,4'-dimethoxybenzophenone, 2-hydroxy-4-n-octoxybenzophenone, 2,2 ', 4,4'-tetrahydroxy Benzophenone, 4-dodecyloxy-2-hydroxybenzophenone, 3,5-di-t -Butyl-4-hydroxybenzoyl acid, n-hexadecyl ester, 1,4-bis (4-benzoyl-3-hydroxyphenoxy) butane, 1,6-bis (4-benzoyl-3-hydroxyphenoxy) hexane, etc. Examples thereof include benzophenone series and cyanoacrylate series typified by ethyl-2-cyano-3,3-diphenyl acrylate. More specifically, those commercially available under trade names such as Tinuvin 320, Tinuvin 328, Tinuvin 234, Adeka Stub LA31, SEESORB102, SEESORB103, and SEESORB501 can be used.
[0014]
These ultraviolet absorbers are preferably blended in an amount of 0.1 to 1 part by weight with respect to 100 parts by weight of the olefin polymer. If the blending amount of the UV absorber is less than 0.1 parts by weight, the desired sufficient weather resistance cannot be obtained, and if it exceeds 1 part by weight, the resin composition becomes yellow due to the hue of the UV absorber itself. It will be colored, the mechanical strength and heat resistance will be lowered, and blooming will occur.
[0015]
(F) Hydrazine-based metal deactivator In the present invention, in addition to the above components, (f) hydrazine-based metal deactivator may be further blended. By blending a hydrazine-based metal deactivator, copper damage can be prevented while maintaining the weather resistance of the resulting resin composition, and the heat resistance can be further improved. Examples of the hydrazine-based metal deactivator include decamethylene dicarboxylic acid disalicyloyl hydrazide. These metal deactivators are preferably blended in an amount of 0.2 to 1 part by weight per 100 parts by weight of the olefin polymer. When the compounding amount of the metal deactivator is less than 0.2 parts by weight, the effect of preventing copper damage is not sufficient, and the effect of improving heat resistance cannot be obtained. On the other hand, when the amount exceeds 1 part by weight, the weather resistance is lowered and the cost is further increased.
[0016]
In the present invention, in addition to the above-described constituent materials, various additives that are conventionally used in electric wires and cables may be blended within a range that does not impair the object of the present invention. Examples of such additives include flame retardant aids, crosslinking agents, crosslinking aids, lubricants, softeners, dispersants, antioxidants, processing aids, stabilizers, colorants, pigments, and the like. By kneading these various additives with the above-mentioned constituent materials as necessary using a known kneader such as an internal mixer, a uniaxial kneader, or a biaxial kneader. The resin composition of the present invention can be obtained.
[0017]
The thus obtained resin composition of the present invention is extrusion coated on the circumference of the conductor by a known method, and then subjected to appropriate crosslinking, whereby the electric wire according to another aspect of the present invention can be obtained. At this time, when the resin composition of the present invention is applied to an electric wire having a coating thickness of 0.3 mm or more, the excellent characteristics of the resin composition of the present invention are particularly remarkably exhibited. . When the thickness of the coating is less than 0.3 mm, the amount of the light stabilizer present inside the insulator is insufficient, which may cause a problem in weather resistance.
[0018]
The crosslinking method is not particularly limited. For example, 1,3-bis (t-butylperoxyisopropyl) benzene, 1,1-bis (t-butylperoxy) -3,3,5-trimethylcyclohexane, dicumyl peroxide, etc. Chemical crosslinking method using organic peroxide as a crosslinking agent, irradiation crosslinking method using ionizing radiation such as X-ray, γ-ray, electron beam, proton beam, deuteron beam, α-ray, β-ray, etc. It is done.
[0019]
【Example】
Examples of the present invention will be described below together with comparative examples. The details of each compounding material used in this example are as shown in Table 4.
[0020]
The compounding materials shown in Table 4 were sufficiently kneaded with a twin-screw kneader, and the resulting resin composition was pelletized and then supplied to a 25 mmφ extruder with L / D = 24. Under a temperature condition of 160 ° C., extrusion coating was performed with a thickness of 0.8 mm on a conductor circumference made of 0.8φ tin-plated annealed copper wire. Thereafter, an electron beam was irradiated under the conditions of an acceleration voltage of 650 kV and an irradiation dose of 100 kGy to produce a bridged electric wire having a finished outer diameter of 2.4 mmφ.
[0021]
Here, using a total of 17 types of cross-linked electric wires thus obtained as samples, mechanical strength (tensile strength, elongation), heat resistance, electrical properties, flame retardancy, and weather resistance were evaluated. The results are shown in Tables 1 and 2.
[0022]
The evaluation method is as follows.
Mechanical strength Tensile strength and elongation were measured according to JIS C 3005 (2000). Each required characteristic is cited from the required characteristic of an insulator in the Electrical Appliance and Material Safety Law, and the tensile strength is 10 MPa or more and the elongation is 200% or more.
[0023]
The sample was left for 168 hours in a thermostat maintained at 180 ° C. and then taken out, and the residual tensile strength and the residual elongation were measured in accordance with JIS C3005 (2000). The required properties were a tensile strength residual ratio of 70% or more and an elongation residual ratio of 50% or more.
[0024]
Electrical characteristics An underwater withstand voltage test was performed in accordance with JIS C 3005 (2000). Judgment criteria of the test were quoted from the required characteristics of insulators in the Electrical Appliance and Material Safety Law, and those that were held at AC 1.5 kV for 1 minute and did not break down were regarded as acceptable.
[0025]
Flame retardance A 60 degree flame retardancy test was carried out according to JIS C 3005 (2000). The judgment criteria of the test were determined to be acceptable if they were self-extinguished within 60 seconds.
[0026]
A sample was set at a position 240 mm away from a 400 W transparent high-pressure mercury lamp light source located in the center of the weather-resistant rotating gear oven, taken out at 120 ° C. for 1500 hours, and then measured for elongation. The required characteristics were an elongation of 50% or more.
[0027]
[Table 1]
[0028]
[Table 2]
[0029]
[Table 3]
[0030]
[Table 4]
[0031]
Reference Examples 1 to 3 and Comparative Examples 1 and 2
It was confirmed that Reference Examples 1 to 3 in which the blending amount of the metal hydrate was within the scope of the present invention satisfied various characteristics. On the other hand, in Comparative Example 1, since the blending amount of the metal hydrate is less than 100 parts by weight and 150 parts by weight which is the lower limit of the present invention, as a result, the flame retardancy is rejected. 2, since the compounding amount of the metal hydrate exceeds 250 parts by weight and 220 parts by weight, which is the upper limit of the present invention, mechanical strength (elongation) and weather resistance may be rejected as a result. confirmed.
[0032]
Reference Examples 4 and 5 and Comparative Examples 3 and 4
It was confirmed that Reference Examples 4 and 5 in which the blending amount of the high molecular weight hindered amine light stabilizer was within the scope of the present invention satisfied various characteristics. On the other hand, since Comparative Example 3 does not contain a high molecular weight hindered amine light stabilizer, as a result, the weather resistance is unacceptable. In Comparative Example 4, the high molecular weight hindered amine light stabilizer is added. Exceeds 20 parts by weight and 10 parts by weight which is the upper limit of the present invention. As a result, it was confirmed that the mechanical strength (tensile strength, elongation) was rejected.
[0033]
Reference Examples 6 and 7 and Comparative Examples 5 and 6
It was confirmed that Reference Examples 6 and 7 in which the blending amount of the low molecular weight hindered amine light stabilizer was within the scope of the present invention satisfied various characteristics. On the other hand, since Comparative Example 5 does not contain a high molecular weight hindered amine light stabilizer, as a result, the weather resistance is unacceptable. In Comparative Example 6, the low molecular weight hindered amine light stabilizer is added. Exceeds 20 parts by weight and 10 parts by weight which is the upper limit of the present invention. As a result, it was confirmed that the mechanical strength (tensile strength, elongation) was rejected.
[0034]
Reference Examples 8 and 9 and Comparative Examples 7 and 8
It was confirmed that Reference Examples 8 and 9 in which the blending amount of the ultraviolet absorber was within the range of the present invention satisfied various characteristics. On the other hand, since Comparative Example 7 does not contain an ultraviolet absorber, as a result, the weather resistance is rejected. In Comparative Example 8, the amount of the ultraviolet absorber is 3 parts by weight. Since it exceeded 1 weight part which is an upper limit, as a result, it was confirmed that mechanical strength (tensile strength, elongation) and heat resistance (elongation residual ratio) fail.
[0035]
In addition to mechanical strength (tensile strength, elongation), heat resistance, electrical properties, flame resistance, and weather resistance, a total of six types of cross-linked electric wires obtained by adding a metal deactivator as a compounding material were used as samples. The copper damage resistance was evaluated. The results are shown in Table 3.
[0036]
The copper damage resistance evaluation method is as follows.
With the copper powder applied to the surface of the tubular sample from which the copper-resistant conductor was taken out, it was left in a constant temperature bath at 180 ° C. for 168 hours, taken out, and the elongation was measured. The required characteristics were an elongation of 50% or more.
[0037]
Examples 1 and 2 and Comparative Examples 9-12
Examples 1 and 2 in which the blending amount of the hydrazine-based metal deactivator falls within the scope of the present invention satisfy the copper damage resistance in addition to the various characteristics, and further, a metal deactivator is blended. It was confirmed that the heat-resisting elongation remaining ratio was improved as compared with Reference Example 2 which was not in the range of 70%. On the other hand, in Comparative Example 9, since the blending amount of the hydrazine-based metal deactivator exceeds 5 parts by weight and 1 part by weight which is the upper limit of the present invention, the heat resistance is improved, but the weather resistance It was confirmed that the property was rejected. In Comparative Examples 10 to 12, since a metal deactivator other than hydrazine is used, the heat resistance is not improved, the weather resistance is also rejected, and the copper damage resistance test is severely deteriorated. As a result, the elongation could not be measured.
[0038]
The present invention is not limited to the above embodiments. In the above embodiments, the resin composition according to the present invention is used as an insulating coating material for electric wires. However, it is also used as a sheath material for cables in which a plurality of electric wires are combined, an insulating coating material for cord heaters, a constituent material for tubes, and the like. Is possible.
[0039]
【The invention's effect】
As described above in detail, according to the present invention, two types of light stabilizers and ultraviolet absorbers having different molecular weights are used in combination with a flame retardant resin composition in which a metal hydrate is blended with an olefin polymer, and further, By blending a specific amount, a resin composition having a good balance of mechanical strength, heat resistance, electrical properties, flame retardancy, and weather resistance could be obtained. This resin composition is suitable as an insulating coating material or sheath material for lead wires of lighting equipment installed indoors or outdoors.
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002233107A JP4381663B2 (en) | 2002-08-09 | 2002-08-09 | Weather resistant flame retardant resin composition and electric wire |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002233107A JP4381663B2 (en) | 2002-08-09 | 2002-08-09 | Weather resistant flame retardant resin composition and electric wire |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004067974A JP2004067974A (en) | 2004-03-04 |
| JP4381663B2 true JP4381663B2 (en) | 2009-12-09 |
Family
ID=32018326
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002233107A Expired - Fee Related JP4381663B2 (en) | 2002-08-09 | 2002-08-09 | Weather resistant flame retardant resin composition and electric wire |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4381663B2 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4717347B2 (en) * | 2003-12-25 | 2011-07-06 | 株式会社クラベ | Weather resistant flame retardant resin composition and electric wire |
| JP2012087184A (en) * | 2010-10-18 | 2012-05-10 | Fujikura Ltd | Resin composition, and electric wire and cable |
| WO2019210178A1 (en) | 2018-04-27 | 2019-10-31 | Dow Global Technologies Llc | Polymeric composition containing a light stabilizer |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2844967B2 (en) * | 1991-06-26 | 1999-01-13 | 住友化学工業株式会社 | Polyolefin resin composition |
| JPH07145288A (en) * | 1993-11-22 | 1995-06-06 | Sumitomo Electric Ind Ltd | Resin composition and heat-shrinkable tube made from the same |
| JP3395439B2 (en) * | 1995-03-01 | 2003-04-14 | 豊田合成株式会社 | Resin molding |
| JPH0995566A (en) * | 1995-09-29 | 1997-04-08 | Yazaki Corp | Non-halogen flame retardant heat resistant resin composition |
| JP3696007B2 (en) * | 1999-10-25 | 2005-09-14 | 昭和電線電纜株式会社 | Insulated wire / cable |
| JP3727545B2 (en) * | 2000-02-22 | 2005-12-14 | 協和化学工業株式会社 | Heat degradation, water resistance, insulation, flame retardant insulated wires and cables |
| JP4690517B2 (en) * | 2000-03-22 | 2011-06-01 | 三井・デュポンポリケミカル株式会社 | Ethylene copolymer composition |
| JP4753480B2 (en) * | 2001-03-08 | 2011-08-24 | 株式会社Adeka | Weatherproof / electron beam resistant flame retardant resin composition and wire coating material comprising the resin composition |
-
2002
- 2002-08-09 JP JP2002233107A patent/JP4381663B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2004067974A (en) | 2004-03-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101601286B1 (en) | Highly flame-resistant polymer composition for electrical wire insulation and electrical wire produced therewith | |
| CA2786433C (en) | Composition for low smoke, flame retardant, halogen-free, thermoplastic insulation showing good electrical properties in water | |
| CN102076761A (en) | Vinyl chloride resin composition and insulated wire using same | |
| WO2005056667A1 (en) | Crosslinkable flame-retardant resin composition, and insulated electrical wire and wire harness each obtained with the same | |
| JP2015072743A (en) | Wire and cable | |
| CN110741446A (en) | Cable insulator | |
| JP4717347B2 (en) | Weather resistant flame retardant resin composition and electric wire | |
| KR101837498B1 (en) | Halogen-Free Flame Retarding Resin Composition Having Improved Leading-in and Insulated Electric Wire Using the same | |
| JP2006286529A (en) | Non-halogen flame retardant wire / cable | |
| JP2010095638A (en) | Non-halogen flame retardant resin composition and non-halogen flame retardant electric wire | |
| JP2012097217A (en) | Flame-retardant insulation member | |
| JP4379947B2 (en) | Flame-retardant resin composition and its insulated wires, tubes, heat-shrinkable tubes, flat cables, and high-voltage wires for direct current | |
| JPH0995566A (en) | Non-halogen flame retardant heat resistant resin composition | |
| JP3966632B2 (en) | Wire covering resin composition and insulated wire | |
| JP4381663B2 (en) | Weather resistant flame retardant resin composition and electric wire | |
| JP2020050703A (en) | Non-halogen flame-retardant resin composition, insulation wire, and cable | |
| JP2016095994A (en) | Electric wire and cable | |
| JP2001206993A (en) | Heat-resistant flame-retardant composition | |
| JP2016095993A (en) | Electric wires and cables | |
| JP2016095992A (en) | Electric wires and cables | |
| JP2001166188A (en) | Fire-retardant cable with fewer coated optical fibers | |
| JP2927058B2 (en) | Heat-resistant flame-retardant insulated wire and method of manufacturing the same | |
| JP2018190585A (en) | LAN cable | |
| JP2811970B2 (en) | Flame retardant electrical cable | |
| JP2004346100A (en) | Non-halogen flame-retardant resin composition and electric wire coated with the composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050630 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20070801 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20070814 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20071010 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071225 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080225 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20081202 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090123 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20090316 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090901 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090916 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121002 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4381663 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121002 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151002 Year of fee payment: 6 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |