JP4309136B2 - 制御目的にeegの複雑さを使用する閉ループ薬物投与方法及び装置 - Google Patents
制御目的にeegの複雑さを使用する閉ループ薬物投与方法及び装置 Download PDFInfo
- Publication number
- JP4309136B2 JP4309136B2 JP2002590824A JP2002590824A JP4309136B2 JP 4309136 B2 JP4309136 B2 JP 4309136B2 JP 2002590824 A JP2002590824 A JP 2002590824A JP 2002590824 A JP2002590824 A JP 2002590824A JP 4309136 B2 JP4309136 B2 JP 4309136B2
- Authority
- JP
- Japan
- Prior art keywords
- patient
- signal
- hypnotic
- complexity
- eeg
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims description 32
- 238000012377 drug delivery Methods 0.000 title description 3
- 230000000147 hypnotic effect Effects 0.000 claims description 113
- 206010002091 Anaesthesia Diseases 0.000 claims description 50
- 230000037005 anaesthesia Effects 0.000 claims description 50
- 229940079593 drug Drugs 0.000 claims description 41
- 239000003814 drug Substances 0.000 claims description 41
- 230000003444 anaesthetic effect Effects 0.000 claims description 32
- 239000003326 hypnotic agent Substances 0.000 claims description 29
- 230000000694 effects Effects 0.000 claims description 28
- 238000005259 measurement Methods 0.000 claims description 23
- 230000003595 spectral effect Effects 0.000 claims description 18
- 238000012546 transfer Methods 0.000 claims description 14
- 230000002526 effect on cardiovascular system Effects 0.000 claims description 13
- 210000003205 muscle Anatomy 0.000 claims description 9
- 230000003285 pharmacodynamic effect Effects 0.000 claims description 8
- 238000001228 spectrum Methods 0.000 claims description 7
- 238000004422 calculation algorithm Methods 0.000 claims description 5
- 230000029058 respiratory gaseous exchange Effects 0.000 claims description 3
- 238000010183 spectrum analysis Methods 0.000 claims description 3
- 230000003993 interaction Effects 0.000 claims description 2
- 230000000144 pharmacologic effect Effects 0.000 claims description 2
- 238000000537 electroencephalography Methods 0.000 description 66
- 230000006870 function Effects 0.000 description 13
- 238000001647 drug administration Methods 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 208000003443 Unconsciousness Diseases 0.000 description 9
- 238000013459 approach Methods 0.000 description 9
- 210000004556 brain Anatomy 0.000 description 9
- 239000006200 vaporizer Substances 0.000 description 9
- 230000008859 change Effects 0.000 description 7
- 238000004458 analytical method Methods 0.000 description 6
- 238000004364 calculation method Methods 0.000 description 6
- 239000008280 blood Substances 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 238000001990 intravenous administration Methods 0.000 description 5
- 238000012544 monitoring process Methods 0.000 description 5
- OLBCVFGFOZPWHH-UHFFFAOYSA-N propofol Chemical compound CC(C)C1=CC=CC(C(C)C)=C1O OLBCVFGFOZPWHH-UHFFFAOYSA-N 0.000 description 5
- 229960004134 propofol Drugs 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 230000006399 behavior Effects 0.000 description 4
- 230000007177 brain activity Effects 0.000 description 4
- 230000002490 cerebral effect Effects 0.000 description 4
- 229960003537 desflurane Drugs 0.000 description 4
- DPYMFVXJLLWWEU-UHFFFAOYSA-N desflurane Chemical compound FC(F)OC(F)C(F)(F)F DPYMFVXJLLWWEU-UHFFFAOYSA-N 0.000 description 4
- 239000003193 general anesthetic agent Substances 0.000 description 4
- 238000010606 normalization Methods 0.000 description 4
- 229940035674 anesthetics Drugs 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 230000000241 respiratory effect Effects 0.000 description 3
- 206010039897 Sedation Diseases 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000036772 blood pressure Effects 0.000 description 2
- 238000007405 data analysis Methods 0.000 description 2
- 230000000857 drug effect Effects 0.000 description 2
- 238000002567 electromyography Methods 0.000 description 2
- 230000000763 evoking effect Effects 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 230000036280 sedation Effects 0.000 description 2
- 208000028399 Critical Illness Diseases 0.000 description 1
- 206010013710 Drug interaction Diseases 0.000 description 1
- 206010021118 Hypotonia Diseases 0.000 description 1
- 206010052904 Musculoskeletal stiffness Diseases 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 241000287463 Phalacrocorax Species 0.000 description 1
- 206010040030 Sensory loss Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000002567 autonomic effect Effects 0.000 description 1
- 230000037086 body physiology Effects 0.000 description 1
- 230000003925 brain function Effects 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- 238000005291 chaos (dynamical) Methods 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000010410 dusting Methods 0.000 description 1
- 229960000305 enflurane Drugs 0.000 description 1
- JPGQOUSTVILISH-UHFFFAOYSA-N enflurane Chemical compound FC(F)OC(F)(F)C(F)Cl JPGQOUSTVILISH-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000003983 inhalation anesthetic agent Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000013178 mathematical model Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 230000036640 muscle relaxation Effects 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000002232 neuromuscular Effects 0.000 description 1
- 238000005312 nonlinear dynamic Methods 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 230000000803 paradoxical effect Effects 0.000 description 1
- 230000002974 pharmacogenomic effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 229960002078 sevoflurane Drugs 0.000 description 1
- DFEYYRMXOJXZRJ-UHFFFAOYSA-N sevoflurane Chemical compound FCOC(C(F)(F)F)C(F)(F)F DFEYYRMXOJXZRJ-UHFFFAOYSA-N 0.000 description 1
- 230000007958 sleep Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/01—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes specially adapted for anaesthetising
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/103—Measuring devices for testing the shape, pattern, colour, size or movement of the body or parts thereof, for diagnostic purposes
- A61B5/11—Measuring movement of the entire body or parts thereof, e.g. head or hand tremor or mobility of a limb
- A61B5/1104—Measuring movement of the entire body or parts thereof, e.g. head or hand tremor or mobility of a limb induced by stimuli or drugs
- A61B5/1106—Measuring movement of the entire body or parts thereof, e.g. head or hand tremor or mobility of a limb induced by stimuli or drugs to assess neuromuscular blockade, e.g. to estimate depth of anaesthesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/316—Modalities, i.e. specific diagnostic methods
- A61B5/369—Electroencephalography [EEG]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/316—Modalities, i.e. specific diagnostic methods
- A61B5/369—Electroencephalography [EEG]
- A61B5/372—Analysis of electroencephalograms
- A61B5/374—Detecting the frequency distribution of signals, e.g. detecting delta, theta, alpha, beta or gamma waves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/316—Modalities, i.e. specific diagnostic methods
- A61B5/389—Electromyography [EMG]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/48—Other medical applications
- A61B5/4821—Determining level or depth of anaesthesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/10—Preparation of respiratory gases or vapours
- A61M16/14—Preparation of respiratory gases or vapours by mixing different fluids, one of them being in a liquid phase
- A61M16/18—Vaporising devices for anaesthetic preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/168—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body
- A61M5/172—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body electrical or electronic
- A61M5/1723—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body electrical or electronic using feedback of body parameters, e.g. blood-sugar, pressure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2505/00—Evaluating, monitoring or diagnosing in the context of a particular type of medical care
- A61B2505/05—Surgical care
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6813—Specially adapted to be attached to a specific body part
- A61B5/6814—Head
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/10—Preparation of respiratory gases or vapours
- A61M16/1005—Preparation of respiratory gases or vapours with O2 features or with parameter measurement
- A61M2016/102—Measuring a parameter of the content of the delivered gas
- A61M2016/1035—Measuring a parameter of the content of the delivered gas the anaesthetic agent concentration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/02—Gases
- A61M2202/0241—Anaesthetics; Analgesics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/04—Heartbeat characteristics, e.g. ECG, blood pressure modulation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/08—Other bio-electrical signals
- A61M2230/10—Electroencephalographic signals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/20—Blood composition characteristics
- A61M2230/205—Blood composition characteristics partial oxygen pressure (P-O2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/30—Blood pressure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/40—Respiratory characteristics
- A61M2230/43—Composition of exhalation
- A61M2230/437—Composition of exhalation the anaesthetic agent concentration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/60—Muscle strain, i.e. measured on the user
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Surgery (AREA)
- Physics & Mathematics (AREA)
- Molecular Biology (AREA)
- Medical Informatics (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Hematology (AREA)
- Emergency Medicine (AREA)
- Psychology (AREA)
- Psychiatry (AREA)
- Pulmonology (AREA)
- Vascular Medicine (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Dentistry (AREA)
- Physiology (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Neurology (AREA)
- Diabetes (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Description
本発明は、2001年5月18日に出願された、米国仮出願番号60/291,873の優先権を主張する。
本発明は、”閉ループ”の方法で催眠薬の投与を制御する方法及び装置に向けられている。
index)を使用して記載されている。Mortier E.他の、麻酔、1998年8月;53(8):749−754を参照する。また、この論文の著者への、公開された欧州特許出願EP959,921を参照する。バイスペクトルインデックスは、MAのファーミンガム(Farmingham)のアスペクト医療システムに専用であり、そして、1つ又はそれ以上の以下の米国特許:4,907,597;5,101,891;5,320,109;及び5,458,117に記載されている。バイスペクトルインデックスは、バイスペクトルインデックス(BIS)と呼ばれる、単一の変数を形成する努力であり、幾つかの催眠薬ついての麻酔の範囲をわたり、鎮静作用と催眠状態のふるまいの評価に関連する。
R. Rampilの、”麻酔でのEEG信号処理のためのプライマー(A Primer for EEG
Signal Processing
in Anesthesia)”、麻酔学、89(1998)、980−1003を参照する。このインデックスについての記載を含む、共通の譲渡人に譲渡された、ここに名前のある及び他の発明者の、米国特許6,731,975を参照する。
J.他の、麻酔学2000;92:1485−7を参照する。EMGに結合されていないバイスペクトルインデックス(BIS)のある逆説的な動作も報告されており;Detsch
O.他の、麻酔英国ジャーナル(British Journal of
Anesthesia)84(1):33−7(3000);Hirota K.他のEurJAnaesth(麻酔)1999年、16、779−783を参照する。
M,他のBr J
麻酔(Anaesth)1997年、2月;78(2):180−4を参照する。聴覚応答は、最低の催眠レベルに持続せず、測定の範囲を制限する。これは、閉ループ催眠薬管理で使用するAEPインデックスの有用性を減らしがちである。また、この技術は患者にイアフォンを装着する必要があり、適切な聴覚を有する患者に制限される。
従って、本発明の目的は、正確で且つ高い応答性の指示を使用し、それにより薬物の投与を改善する、閉ループの方法で患者へ催眠薬の投与を制御する改善された方法と装置を提供することである。本発明で使用される指示は、患者の催眠状態の変化に高速に応答するようにすることが可能である。これは、特に、患者が麻酔状態から意識のある状態となりうることを麻酔医に警告するのに有利である。
本発明では、患者から得られたEEG信号の複雑さの定量化が、患者の催眠レベルを決定し、次に、閉ループで患者への催眠薬の投与を制御するのに使用される。このアプローチは、脳のそれらのような、神経単位系は、種々の非線形な動作を示しそれにより非常にランダムなEEG信号の非線形力学に基づく測定値は、基礎をなす脳の活動の状態への直接的な洞察を可能とするという前提に基づいている。EEGバイオポテンシャル信号は、患者の頭部に付けられた電極から得られる。
complexity measures for physiological signal
analysis)”を参照しこれは、25Hzの遮断周波数へのエントロピー測定を記述し、そして、Bruhn J.他の麻酔、92(2000)、ページ715−726の”デスフルラン麻酔中の麻酔薬効果の脳波記録の測定値としての近似エントロピー(Approximate
Entropy as
an Electroencephalographic
Measure of
Anesthetic Drug Effect during Desflurane
Anesthesia)”は、0.5から32Hzの周波数範囲のエントロピー測定を記述する。また、J.Clin.Monitoring
and Comp.
Vol.16(2000)pg.16のViertio−Oja H他の、”EEG測定から麻酔の深さを決定する新しい方法(New method to
determine depth of anesthesia from
EEG measurement)”を参照し、それは、意識のある状態から意識のない状態への変化は、患者に独立のエントロピーのユニバーサルクリティカル値で発生することを報告している。また、ZhangXS他の、Med.Bio.Eng.Comput.1999,37:327−34も参照する。
J他の、麻酔学92(2000)715−26;麻酔学93(2000)981−5及び、Viertio−Oja
H他の、”EEG信号のエントロピーは、催眠の深さのロバストインデックスである。(Entropy of EEG
signal is
a robust
index for
depth of
hypnosis)”、麻酔学93(2000)A、pg.1369を参照する。これは、催眠のレベルを特徴化するために自然な且つ強い選択としてエントロピーの考慮を保証する。また、エントロピーは、麻酔の全てのレベルで麻酔の深さと相関するので、バイスペクトルインデックス(BIS)として種々のサブパラメータを結合する必要を避ける。第2に、意識のある状態から意識のない状態への変化は、患者に独立なエントロピーのクリティカルレベルで発生することが見つかった。Viertio−Oja
H他のJ.Clin.Monitoring and Computing、Vol.16(2000)pg.16を参照する。第3に、そして、特定の実際の重要な、麻酔から意識のある状態への患者の回復は、クリティカルレベルへ向かったエントロピーの上昇によりしばしば予測されることが可能である。
M.Pincus,Igor M.Gladstone、及びRichard A.Ehrenkranzの、”医療データ分析のための規則正しさの統計(A
regularity statistic for medical data
analysis)”、J.Clin.Monitoring7(1991)、pgs.335−345を参照する。近似エントロピーについてのプログラムは、Bruhn他の麻酔学の論文で述べられている。スペクトルエントロピーと近似エントロピー技術は、EEG信号の複雑さを分析するのに使用されることが分かった。
Lemlel及びJacob Zivの”有限シーケンスの複雑さに関して(On
the complexity
of finite
sequence)”、IEEEトランザクション、IT−22(1976)ページ75−81を参照する。
H.他のJClinical Monitaring and Computing(J医療モニタリング及び計算)、Vol.16(2000)、pg.16を参照する。
Claims (10)
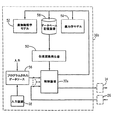
- 患者へ催眠薬を投与する装置であって、
(a)患者についての望ましい催眠レベルに対応する基準信号を入力する手段と、
(b)患者へ催眠薬を投与する麻酔薬送出しユニットと、
(c)患者からEEG信号データを得るセンサと、
(d)EGG信号データの複雑さから患者に存在する催眠レベルを決定し且つ同様なものに対応する信号を供給するために、EEG信号データの複雑さの少なくとも1つの測定値を得るために前記センサに接続された手段と、
(e)前記基準信号を入力する手段及び前記センサに接続された手段に接続された入力と、前記麻酔薬送出しユニットに接続された出力とを有する比較器を有する制御ユニットであって、前記比較器は、患者内に存在する催眠レベルに対応する信号と、前記基準信号とを比較し且つ、比較に従って、麻酔薬送出しユニットと、催眠薬の投与とを制御する出力信号を供給する制御ユニットと、
(f)前記麻酔薬送出しユニットの制御において使用するための、患者内の薬理学的効果と、患者への薬物の投与との間の伝達関数を確立する、前記制御ユニット内の手段と、
(g)前記伝達関数を確立する手段によって前記麻酔薬送出しユニットの動作の制御において使用するための薬物動態学モデル手段及び薬力学モデル手段であって、前記催眠薬と患者との間の相互作用を表すアルゴリズムを有する薬物動態学モデル手段及び薬力学モデル手段と
を有する装置。 - 構成要素(d)は、患者内に存在する催眠レベルを決定するために、EEG信号データのエントロピー、好ましくはスペクトルエントロピー又は近似のエントロピーを測定する手段として更に定義される、請求項1に記載の装置。
- 構成要素(d)は、患者内に存在する催眠レベルを決定するために、Lempel−Ziv複雑さ測定値を使用し及び/又はフラクタルスペクトル分析を実行する手段として更に定義される、請求項1に記載の装置。
- 構成要素(c)は、患者の筋肉の活動からの結果のEMG信号を得るセンサとして更に定義され、構成要素(d)は、EMG信号からのEMG活動の測定値、好ましくは周波数領域パワースペクトルを得て且つ、患者の催眠レベルに対応する信号を供給するために、同じものをEEG信号の複雑さから得られる測定値と共に使用すると更に定義される、請求項1に記載の装置。
- 構成要素(c)は患者の筋肉活動からの結果のEMG信号を得るセンサとして更に定義され、構成要素(d)は、患者の催眠レベルを決定するために、EEG信号の複雑さの得られた測定値と共に使用するための、EEG信号とEMG信号を統合する周波数スペクトルをわたりEEG信号データの複雑さを得る手段として更に定義される、請求項4に記載の装置。
- 患者に対する望ましい心臓血管の特徴に対応する信号を供給する手段;患者から心臓血管の信号データを得る手段と;患者の心臓血管の信号データを望ましい心臓血管の特徴信号と比較する手段と;心臓血管の特徴信号と心臓血管の信号データの比較に従って麻酔薬送出しユニットと催眠薬の投与を制御する手段とを更に有する、請求項1乃至5の何れか一項に記載の装置。
- 患者の呼吸ガス内の揮発性催眠薬の量を測定し且つ麻酔薬送出しユニットを制御するのに使用するために、前記制御ユニットに接続され、及び/又は前記手段は伝達関数を確立するのに使用するために前記伝達関数手段に接続された手段を更に有する、請求項1乃至6の何れか一項に記載の装置。
- 患者から心臓血管のデータを得る手段を更に有し、前記手段は伝達関数を確立するのに使用するために前記伝達関数手段へ接続されている、請求項1乃至7の何れか一項に記載の装置。
- 患者への催眠薬の投与を制御するのに使用するために、患者、催眠薬、医療手順、患者の前の麻酔状態及び内科医の1つ又はそれ以上に関連する情報を提供し及び/又は記憶する手段を更に有する、請求項1乃至8の何れか一項に記載の装置。
- 麻酔の時間の経過につれて情報を発生し、患者への催眠薬の投与を制御するのに発生された情報を使用する手段を有する、請求項1乃至9の何れか一項に記載の装置。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US29187301P | 2001-05-18 | 2001-05-18 | |
| US09/861,878 US6631291B2 (en) | 2001-05-18 | 2001-05-21 | Closed loop drug administration method and apparatus using EEG complexity for control purposes |
| PCT/IB2002/001675 WO2002094099A1 (en) | 2001-05-18 | 2002-05-13 | Closed loop drug administration method and apparatus using eeg complexity for control purposes |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004527328A JP2004527328A (ja) | 2004-09-09 |
| JP2004527328A5 JP2004527328A5 (ja) | 2005-12-22 |
| JP4309136B2 true JP4309136B2 (ja) | 2009-08-05 |
Family
ID=26967021
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002590824A Expired - Lifetime JP4309136B2 (ja) | 2001-05-18 | 2002-05-13 | 制御目的にeegの複雑さを使用する閉ループ薬物投与方法及び装置 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US6631291B2 (ja) |
| EP (1) | EP1389953A1 (ja) |
| JP (1) | JP4309136B2 (ja) |
| WO (1) | WO2002094099A1 (ja) |
Families Citing this family (101)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6738661B1 (en) | 1999-10-22 | 2004-05-18 | Biosynergetics, Inc. | Apparatus and methods for the controllable modification of compound concentration in a tube |
| NZ518740A (en) | 1999-11-08 | 2004-04-30 | Univ Florida | Marker detection method and apparatus to monitor drug compliance |
| US7104963B2 (en) * | 2002-01-22 | 2006-09-12 | University Of Florida Research Foundation, Inc. | Method and apparatus for monitoring intravenous (IV) drug concentration using exhaled breath |
| EP1989998B1 (en) * | 2001-06-13 | 2014-03-12 | Compumedics Medical Innovation Pty Ltd. | Methods and apparatus for monitoring consciousness |
| US20070167853A1 (en) | 2002-01-22 | 2007-07-19 | Melker Richard J | System and method for monitoring health using exhaled breath |
| US7373198B2 (en) * | 2002-07-12 | 2008-05-13 | Bionova Technologies Inc. | Method and apparatus for the estimation of anesthetic depth using wavelet analysis of the electroencephalogram |
| EP2055233B1 (en) * | 2003-01-23 | 2012-10-10 | University of Florida Research Foundation, Incorporated | Method and apparatus for monitoring intravenous (IV) drug concentration using exhaled breath |
| WO2004112603A1 (en) * | 2003-06-19 | 2004-12-29 | Wayne State University | System for identifying patient response to anesthesia infusion |
| US7454393B2 (en) * | 2003-08-06 | 2008-11-18 | Microsoft Corporation | Cost-benefit approach to automatically composing answers to questions by extracting information from large unstructured corpora |
| US7509161B2 (en) * | 2003-10-22 | 2009-03-24 | Instrumentarium Corporation | Method and apparatus for determining the cerebral state of a patient using generalized spectral entropy of the EEG signal |
| DE102004010516A1 (de) * | 2004-03-04 | 2005-09-22 | Bayer Technology Services Gmbh | Verbessertes Verfahren zur zeitlichen Dosierung von Arzneistoffen |
| WO2005116902A2 (en) * | 2004-05-28 | 2005-12-08 | Philips Intellectual Property & Standards Gmbh | System for the noninvasive determination of tracer concentration in blood |
| US7447541B2 (en) * | 2004-06-30 | 2008-11-04 | Instrumentarium Corporation | Monitoring subcortical responsiveness of a patient |
| US20060009733A1 (en) * | 2004-07-07 | 2006-01-12 | Martin James F | Bis closed loop anesthetic delivery |
| DE102004032814B4 (de) * | 2004-07-07 | 2014-07-31 | Dräger Medical GmbH | Vorrichtung zur Steuerung der Wirkstoffzufuhr |
| WO2006008334A1 (en) * | 2004-07-20 | 2006-01-26 | Mega Elektroniikka Oy | Method and device for identifying, measuring and analyzing abnormal neurological responses |
| US20060129324A1 (en) * | 2004-12-15 | 2006-06-15 | Biogenesys, Inc. | Use of quantitative EEG (QEEG) alone and/or other imaging technology and/or in combination with genomics and/or proteomics and/or biochemical analysis and/or other diagnostic modalities, and CART and/or AI and/or statistical and/or other mathematical analysis methods for improved medical and other diagnosis, psychiatric and other disease treatment, and also for veracity verification and/or lie detection applications. |
| US8219187B2 (en) * | 2005-01-27 | 2012-07-10 | Instrumentarium Corporation | Method and apparatus for providing improved assessment of a physiological condition of a patient |
| US7882167B2 (en) * | 2005-02-18 | 2011-02-01 | Beth Israel Deaconess Medical Center | Complexity-based dynamical analysis of a network |
| US7601124B2 (en) * | 2005-02-18 | 2009-10-13 | Beth Israel Deaconess Medical Center | Complexity-based dynamical assay for assessing the toxicity and efficacy of pharmaceutical and other therapeutic interventions |
| US7925338B2 (en) * | 2005-03-24 | 2011-04-12 | General Electric Company | Determination of the anesthetic state of a patient |
| US20070010756A1 (en) * | 2005-07-07 | 2007-01-11 | Viertio-Oja Hanna E | Patient monitoring during drug administration |
| US7850597B2 (en) | 2005-08-26 | 2010-12-14 | Dominick Schillizzi | Interactive hypnotic bio-stabilization system |
| EP1767146A1 (en) * | 2005-09-21 | 2007-03-28 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.V. | Monitoring neuronal signals |
| WO2007041766A1 (en) * | 2005-10-10 | 2007-04-19 | Compumedics Limited | Adaptive real-time line noise suppression for electrical or magnetic physiological signals |
| JP2007111118A (ja) * | 2005-10-18 | 2007-05-10 | Japan Lifeline Co Ltd | 脳病変診断装置及びその使用方法 |
| EP1776977A1 (en) * | 2005-10-21 | 2007-04-25 | General Electric Company | System for delivering anesthesia drugs to a patient |
| DE602006005333D1 (de) | 2006-03-06 | 2009-04-09 | Gen Electric | Automatische Kalibrierung der Sensibilität einer Person gegenüber einem Arzneimittel |
| US7630758B2 (en) * | 2006-06-22 | 2009-12-08 | General Electric Company | Separation of natural and drug-induced sleep of a subject |
| WO2008003049A2 (en) * | 2006-06-28 | 2008-01-03 | The University Of Utah Research Foundation | Distinguishing different drug effects from the electroencephalogram |
| US7914460B2 (en) | 2006-08-15 | 2011-03-29 | University Of Florida Research Foundation, Inc. | Condensate glucose analyzer |
| DE102006045014A1 (de) * | 2006-09-23 | 2008-04-03 | Dräger Medical AG & Co. KG | Verfahren und Vorrichtung zur Überwachung einer Dosierung wenigstens eines Medikaments |
| US20080108970A1 (en) * | 2006-11-08 | 2008-05-08 | Viertio-Oja Hanna E | Control of Drug Administration |
| US20080202523A1 (en) * | 2007-02-23 | 2008-08-28 | General Electric Company | Setting mandatory mechanical ventilation parameters based on patient physiology |
| US20080255469A1 (en) * | 2007-04-12 | 2008-10-16 | Yuan Ze University | Method for Monitoring the Depth of Anesthesia |
| US7920914B2 (en) * | 2007-04-12 | 2011-04-05 | Yuan Ze University | Method for monitoring the depth of anesthesia |
| US9398863B2 (en) * | 2007-06-20 | 2016-07-26 | General Electric Company | Detection of anomalies in measurement of level of hypnosis |
| US8108039B2 (en) * | 2007-07-13 | 2012-01-31 | Neuro Wave Systems Inc. | Method and system for acquiring biosignals in the presence of HF interference |
| WO2009050736A1 (en) * | 2007-10-15 | 2009-04-23 | The Secretary, Department Of Information Technology | An improved automatic anaesthesia delivery system |
| US20100286549A1 (en) * | 2007-12-18 | 2010-11-11 | New York University | System and Method for Assessing Efficacy of Therapeutic Agents |
| WO2009115948A1 (en) * | 2008-03-17 | 2009-09-24 | Philips Intellectual Property & Standards Gmbh | A closed loop system for automatically controlling a physiological variable of a patient |
| US20090247893A1 (en) * | 2008-03-27 | 2009-10-01 | The General Electric Company | Method and apparatus for measuring responsiveness of a subject |
| US20100130811A1 (en) * | 2008-04-24 | 2010-05-27 | Searete Llc | Computational system and method for memory modification |
| US9064036B2 (en) * | 2008-04-24 | 2015-06-23 | The Invention Science Fund I, Llc | Methods and systems for monitoring bioactive agent use |
| US20090312595A1 (en) * | 2008-04-24 | 2009-12-17 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | System and method for memory modification |
| US9026369B2 (en) | 2008-04-24 | 2015-05-05 | The Invention Science Fund I, Llc | Methods and systems for presenting a combination treatment |
| US8930208B2 (en) | 2008-04-24 | 2015-01-06 | The Invention Science Fund I, Llc | Methods and systems for detecting a bioactive agent effect |
| US20100069724A1 (en) * | 2008-04-24 | 2010-03-18 | Searete Llc | Computational system and method for memory modification |
| US20100280332A1 (en) * | 2008-04-24 | 2010-11-04 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Methods and systems for monitoring bioactive agent use |
| US9449150B2 (en) | 2008-04-24 | 2016-09-20 | The Invention Science Fund I, Llc | Combination treatment selection methods and systems |
| US9282927B2 (en) | 2008-04-24 | 2016-03-15 | Invention Science Fund I, Llc | Methods and systems for modifying bioactive agent use |
| US9239906B2 (en) | 2008-04-24 | 2016-01-19 | The Invention Science Fund I, Llc | Combination treatment selection methods and systems |
| US9649469B2 (en) | 2008-04-24 | 2017-05-16 | The Invention Science Fund I Llc | Methods and systems for presenting a combination treatment |
| US9560967B2 (en) | 2008-04-24 | 2017-02-07 | The Invention Science Fund I Llc | Systems and apparatus for measuring a bioactive agent effect |
| US9662391B2 (en) | 2008-04-24 | 2017-05-30 | The Invention Science Fund I Llc | Side effect ameliorating combination therapeutic products and systems |
| US8876688B2 (en) | 2008-04-24 | 2014-11-04 | The Invention Science Fund I, Llc | Combination treatment modification methods and systems |
| US20100063368A1 (en) * | 2008-04-24 | 2010-03-11 | Searete Llc, A Limited Liability Corporation | Computational system and method for memory modification |
| US20090292226A1 (en) * | 2008-05-21 | 2009-11-26 | Ethicon Endo-Surgery, Inc. | Medical system and method employing a drug delivery assembly |
| US11375929B2 (en) | 2008-10-15 | 2022-07-05 | The University Of Tennessee Research Foundation | Method and device for detection of bioavailable drug concentration in a fluid sample |
| WO2010045465A1 (en) | 2008-10-15 | 2010-04-22 | The University Of Tennessee Research Foundation | Method and device for detection of bioavailable drug concentration in a fluid sample |
| US20100121158A1 (en) * | 2008-11-06 | 2010-05-13 | Quevedo Adrian E | Physiologically Modulated Visual Entrainment Device |
| FR2940913B1 (fr) * | 2009-01-15 | 2013-07-19 | Hopital Foch | Systeme de pilotage de moyens d'injection d'agents d'anesthesie ou de sedation |
| FR2940912B1 (fr) | 2009-01-15 | 2013-08-16 | Hopital Foch | Systeme de pilotage de moyens d'injection d'agents d'anesthesie ou de sedation en vue de l'induction de celle-ci |
| US8457731B2 (en) * | 2009-02-16 | 2013-06-04 | Wisconsin Alumni Research Foundation | Method for assessing anesthetization |
| US20100256515A1 (en) * | 2009-04-03 | 2010-10-07 | Egeth Marc J | Communication with and consciousness-assessment of anesthetized surgery patients |
| CN102946797B (zh) * | 2009-08-14 | 2016-12-07 | D·伯顿 | 麻醉和意识深度监测系统 |
| US20110137297A1 (en) * | 2009-09-17 | 2011-06-09 | Kiani Massi Joe E | Pharmacological management system |
| DE102009053256A1 (de) * | 2009-11-06 | 2011-05-19 | Baars, Jan H., Dr. med. | Verfahren zur Bestimmung des Analgesieniveaus eines sedierten oder narkotisierten Individuums |
| US20110108034A1 (en) * | 2009-11-06 | 2011-05-12 | General Electric Company | Method and system for controlling a ventilator |
| US20110209065A1 (en) * | 2010-02-23 | 2011-08-25 | Farmacia Electronica, Inc. | Method and system for consumer-specific communication based on cultural normalization techniques |
| US20130150748A1 (en) * | 2010-07-23 | 2013-06-13 | Quantium Medical S.L. | Apparatus for combining drug effect interaction between anaesthetics and analgesics and electroencephalogram features for precise assessment of the level of consciousness during anaesthesia |
| US9055925B2 (en) | 2010-07-27 | 2015-06-16 | Carefusion 303, Inc. | System and method for reducing false alarms associated with vital-signs monitoring |
| EP2601606A2 (en) * | 2010-08-02 | 2013-06-12 | Koninklijke Philips Electronics N.V. | Method for semantic communication of device data between a source and receiving client |
| US8821397B2 (en) | 2010-09-28 | 2014-09-02 | Masimo Corporation | Depth of consciousness monitor including oximeter |
| US9775545B2 (en) | 2010-09-28 | 2017-10-03 | Masimo Corporation | Magnetic electrical connector for patient monitors |
| US9750430B2 (en) | 2011-04-27 | 2017-09-05 | General Electric Company | Methods of intravenous drug monitoring |
| US11786132B2 (en) | 2011-05-06 | 2023-10-17 | The General Hospital Corporation | Systems and methods for predicting arousal to consciousness during general anesthesia and sedation |
| MX2013012933A (es) | 2011-05-06 | 2014-02-27 | Gen Hospital Corp | Sistema y metodo para rastrear estados cerebrales durante administracion de anestesia. |
| US10556063B2 (en) | 2011-06-20 | 2020-02-11 | Renaudia Medical, Llc | Distributed medication delivery using autonomous delivery device |
| WO2012177798A2 (en) | 2011-06-20 | 2012-12-27 | Renaudia Medical, Llc | Distributed medication delivery system and method having autonomous delivery devices |
| DE102012203897B4 (de) * | 2012-03-13 | 2014-11-27 | Kist Europe Forschungsgesellschaft Mbh | Vorrichtung zur Durchführung einer Anästhesie oder Analgosedierung und Verfahren zum Betreiben einer Vorrichtung zur Durchführung einer Anästhesie oder Analgosedierung |
| US9983162B2 (en) | 2012-06-01 | 2018-05-29 | The University Of Tennessee Research Foundation | Method and device for detection of bioavailable drug concentration |
| US11109789B1 (en) * | 2012-08-08 | 2021-09-07 | Neurowave Systems Inc. | Field deployable brain monitor and method |
| CN104869897B (zh) * | 2012-10-12 | 2018-03-20 | 通用医疗公司 | 用于监测和控制患者的系统 |
| CN103432651B (zh) * | 2012-12-31 | 2016-01-20 | 南京理工大学 | 一种闭环的智能麻醉控制系统 |
| EP2789293A1 (en) * | 2013-04-12 | 2014-10-15 | Commissariat à l'Énergie Atomique et aux Énergies Alternatives | Methods to monitor consciousness |
| US9849241B2 (en) | 2013-04-24 | 2017-12-26 | Fresenius Kabi Deutschland Gmbh | Method of operating a control device for controlling an infusion device |
| US10154815B2 (en) | 2014-10-07 | 2018-12-18 | Masimo Corporation | Modular physiological sensors |
| DE102014015897A1 (de) * | 2014-10-28 | 2016-04-28 | Drägerwerk AG & Co. KGaA | Verfahren und System zum Kontrollieren einer Medikamentendosiereinrichtung |
| US10702208B2 (en) * | 2015-03-31 | 2020-07-07 | Cerenion Oy | Apparatus and method for electroencephalographic examination |
| US10369272B2 (en) * | 2015-06-15 | 2019-08-06 | Enspero Inc. | Multiport delivery device |
| CA2988179A1 (en) * | 2015-06-16 | 2016-12-22 | Quantum Dental Technologies Inc. | System and method of monitoring consumable use based on correlations with diagnostic testing |
| CN105249962B (zh) * | 2015-11-03 | 2019-04-30 | 北京联合大学 | 头皮脑电信号回顾性癫痫发作点检测方法及系统 |
| KR101939574B1 (ko) * | 2015-12-29 | 2019-01-17 | 주식회사 인바디 | 의식 상태 모니터링 방법 및 장치 |
| WO2019127557A1 (zh) * | 2017-12-29 | 2019-07-04 | 深圳迈瑞生物医疗电子股份有限公司 | 麻醉药物识别方法、麻醉脑电信号的处理方法和装置 |
| DE102018110275A1 (de) * | 2018-04-27 | 2019-10-31 | Susanne Koch | Verfahren und Vorrichtung zur Bereitstellung eines Parameters, der auf einen Bewusstseinsverlust eines Patienten unter Narkose hinweist |
| US20210345952A1 (en) * | 2020-05-06 | 2021-11-11 | Janssen Pharmaceuticals, Inc. | Controlling operation of drug administration devices using surgical hubs |
| US11571541B2 (en) * | 2020-10-27 | 2023-02-07 | David Richardson Hubbard, JR. | Apparatus and methods of transcranial stimulation to adjust sensory cortical dendritic spine neck membrane potentials for altering consciousness |
| US20220134000A1 (en) * | 2020-11-05 | 2022-05-05 | Wave Neuroscience, Inc. | Microdosing System, Apparatus, Method |
| CN113230511A (zh) * | 2021-05-25 | 2021-08-10 | 四川大学华西医院 | 一种靶控给药装置 |
| CN113440690A (zh) * | 2021-08-03 | 2021-09-28 | 复旦大学 | 基于肌电信号反馈的智能定量给药电针注射设备 |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2690178A (en) | 1950-11-13 | 1954-09-28 | Research Corp | Automatic apparatus for administering drugs |
| US4417590A (en) * | 1978-06-09 | 1983-11-29 | Beckman Instruments, Inc. | Electroencephalograph |
| US4533346A (en) | 1979-06-26 | 1985-08-06 | Pharmacontrol Corporation | System for automatic feedback-controlled administration of drugs |
| US4421122A (en) * | 1981-05-15 | 1983-12-20 | The Children's Medical Center Corporation | Brain electrical activity mapping |
| US4402222A (en) | 1982-01-26 | 1983-09-06 | Snap-On Tools Corporation | Bolt load determining apparatus |
| FI64281C (fi) * | 1982-01-29 | 1983-11-10 | Instrumentarium Oy | Maetnings- och oevervakningssystem |
| US4753246A (en) | 1986-03-28 | 1988-06-28 | The Regents Of The University Of California | EEG spatial filter and method |
| US4705049A (en) | 1986-08-04 | 1987-11-10 | John Erwin R | Intraoperative monitoring or EP evaluation system utilizing an automatic adaptive self-optimizing digital comb filter |
| US5010891A (en) | 1987-10-09 | 1991-04-30 | Biometrak Corporation | Cerebral biopotential analysis system and method |
| US4907597A (en) | 1987-10-09 | 1990-03-13 | Biometrak Corporation | Cerebral biopotential analysis system and method |
| US5769793A (en) * | 1989-09-08 | 1998-06-23 | Steven M. Pincus | System to determine a relative amount of patternness |
| US5109862A (en) * | 1990-03-19 | 1992-05-05 | Del Mar Avionics | Method and apparatus for spectral analysis of electrocardiographic signals |
| US5101891A (en) | 1991-06-03 | 1992-04-07 | General Motors Corporation | Heat exchanger tubing with improved fluid flow distribution |
| DE69230613T2 (de) * | 1991-07-02 | 2000-12-28 | Inhale Inc | Verfahren und vorrichtung zum abgeben von medikamenten in aerosolform |
| US5458117A (en) | 1991-10-25 | 1995-10-17 | Aspect Medical Systems, Inc. | Cerebral biopotential analysis system and method |
| US5320109A (en) | 1991-10-25 | 1994-06-14 | Aspect Medical Systems, Inc. | Cerebral biopotential analysis system and method |
| US6117066A (en) | 1992-12-04 | 2000-09-12 | Somatics, Inc. | Prevention of seizure arising from medical magnetoictal non-convulsive stimulation therapy |
| US5474082A (en) * | 1993-01-06 | 1995-12-12 | Junker; Andrew | Brain-body actuated system |
| US5566678B1 (en) * | 1993-09-10 | 1999-11-30 | Cadwell Ind Inc | Digital eeg noise synthesizer |
| US6067467A (en) | 1994-02-07 | 2000-05-23 | New York University | EEG operative and post-operative patient monitoring method |
| US5579774A (en) | 1994-03-07 | 1996-12-03 | Camino Neurocare, Inc. | Method and apparatus for monitoring local cerebral physiology |
| GB9511964D0 (en) * | 1995-06-13 | 1995-08-09 | Rdm Consultants Limited | Monitoring an EEG |
| US5995868A (en) | 1996-01-23 | 1999-11-30 | University Of Kansas | System for the prediction, rapid detection, warning, prevention, or control of changes in activity states in the brain of a subject |
| BE1010264A5 (nl) | 1996-03-18 | 1998-04-07 | Struys Michel | Inrichting voor het controleren van een intraveneuze injectie. |
| US5857978A (en) * | 1996-03-20 | 1999-01-12 | Lockheed Martin Energy Systems, Inc. | Epileptic seizure prediction by non-linear methods |
| EP0828225A1 (de) * | 1996-09-04 | 1998-03-11 | Siemens Aktiengesellschaft | Verfahren und Vorrichtung zum Auswerten von EEG-Daten |
| GB9618998D0 (en) | 1996-09-11 | 1996-10-23 | Univ Glasgow | Anaesthesia control |
| US6016449A (en) | 1997-10-27 | 2000-01-18 | Neuropace, Inc. | System for treatment of neurological disorders |
| US6016444A (en) | 1997-12-10 | 2000-01-18 | New York University | Automatic control of anesthesia using quantitative EEG |
| US6128094A (en) * | 1998-07-08 | 2000-10-03 | Hewlett-Packard Company | Printer having processor with instruction cache and compressed program store |
| US20010041964A1 (en) * | 1998-09-14 | 2001-11-15 | George M. Grass | Pharmacokinetic-based drug design tool and method |
| US6731975B1 (en) | 2000-10-16 | 2004-05-04 | Instrumentarium Corp. | Method and apparatus for determining the cerebral state of a patient with fast response |
| US6678548B1 (en) * | 2000-10-20 | 2004-01-13 | The Trustees Of The University Of Pennsylvania | Unified probabilistic framework for predicting and detecting seizure onsets in the brain and multitherapeutic device |
| US6594524B2 (en) * | 2000-12-12 | 2003-07-15 | The Trustees Of The University Of Pennsylvania | Adaptive method and apparatus for forecasting and controlling neurological disturbances under a multi-level control |
-
2001
- 2001-05-21 US US09/861,878 patent/US6631291B2/en not_active Ceased
-
2002
- 2002-05-13 EP EP02727903A patent/EP1389953A1/en not_active Withdrawn
- 2002-05-13 WO PCT/IB2002/001675 patent/WO2002094099A1/en active Application Filing
- 2002-05-13 JP JP2002590824A patent/JP4309136B2/ja not_active Expired - Lifetime
-
2005
- 2005-09-21 US US11/232,411 patent/USRE41291E1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| US20020173729A1 (en) | 2002-11-21 |
| WO2002094099A1 (en) | 2002-11-28 |
| EP1389953A1 (en) | 2004-02-25 |
| USRE41291E1 (en) | 2010-04-27 |
| US6631291B2 (en) | 2003-10-07 |
| JP2004527328A (ja) | 2004-09-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4309136B2 (ja) | 制御目的にeegの複雑さを使用する閉ループ薬物投与方法及び装置 | |
| Leslie et al. | Prediction of movement during propofol/nitrous oxide anesthesia: Performance of concentration, electroencephalographic, pupillary, and hemodynamic indicators | |
| CN107847172B (zh) | 用于评估觉醒、镇静和全身麻醉期间的意识、疼痛和伤害感受的水平的设备和方法 | |
| Funcke et al. | Validation of innovative techniques for monitoring nociception during general anesthesia: a clinical study using tetanic and intracutaneous electrical stimulation | |
| US8512273B2 (en) | Automatic calibration of the sensitivity of a subject to a drug | |
| EP1704819B1 (en) | Determination of the anesthetic state of a patient | |
| EP1495715B1 (en) | A method and apparatus based on combination of three phsysiological parameters for assessment of analgesia during anesthesia or sedation | |
| EP1742155B1 (en) | Determination of the clinical state of a subject | |
| US8715193B2 (en) | Determination of the clinical state of a subject | |
| EP1711104B1 (en) | Method and apparatus for ecg-derived sleep disordered breathing monitoring, detection and classification | |
| EP1757226B1 (en) | Measurement of responsiveness of a patient under anaesthesia | |
| US20020117176A1 (en) | Anaesthesia control system | |
| US20070010756A1 (en) | Patient monitoring during drug administration | |
| JP6626122B2 (ja) | 脳波を検査する装置およびその作動方法 | |
| WO2011054959A1 (en) | Method for determining the level of analgesia of a sedated or narcotized individual | |
| Sinha et al. | Monitoring devices for measuring the depth of anaesthesia–An overview | |
| Beekoo et al. | Analyzing electroencephalography (EEG) waves provides a reliable tool to assess the depth of sevoflurane anesthesia in pediatric patients | |
| Ribeiro et al. | Brain monitoring in dogs using the cerebral state index during the induction of anaesthesia via target-controlled infusion of propofol | |
| Kumar et al. | A depth of anaesthesia index from linear regression of EEG parameters | |
| Wen | Consciousness, EEG and depth of anaesthesia monitoring | |
| Nguyen-Ky et al. | An improved Chaos method for monitoring the depth of anaesthesia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050428 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050428 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080527 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20080819 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20080826 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080926 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090407 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090507 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4309136 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120515 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120515 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130515 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140515 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |