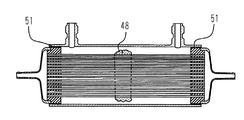
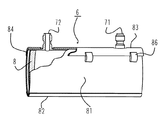
JP4190131B2 - 医療用血液浄化器およびハウジング - Google Patents
医療用血液浄化器およびハウジング Download PDFInfo
- Publication number
- JP4190131B2 JP4190131B2 JP2000133965A JP2000133965A JP4190131B2 JP 4190131 B2 JP4190131 B2 JP 4190131B2 JP 2000133965 A JP2000133965 A JP 2000133965A JP 2000133965 A JP2000133965 A JP 2000133965A JP 4190131 B2 JP4190131 B2 JP 4190131B2
- Authority
- JP
- Japan
- Prior art keywords
- dialysate
- blood
- hollow fiber
- fiber membrane
- housing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000004369 blood Anatomy 0.000 title claims description 222
- 239000008280 blood Substances 0.000 title claims description 221
- 239000012528 membrane Substances 0.000 claims description 163
- 239000012510 hollow fiber Substances 0.000 claims description 157
- 239000000463 material Substances 0.000 claims description 91
- 238000007789 sealing Methods 0.000 claims description 49
- 230000017531 blood circulation Effects 0.000 claims description 26
- 238000005452 bending Methods 0.000 claims description 7
- -1 polyethylene Polymers 0.000 description 29
- 238000004659 sterilization and disinfection Methods 0.000 description 25
- 230000001954 sterilising effect Effects 0.000 description 22
- 239000000835 fiber Substances 0.000 description 19
- 229920000515 polycarbonate Polymers 0.000 description 18
- 239000004417 polycarbonate Substances 0.000 description 18
- 239000002699 waste material Substances 0.000 description 17
- 238000004382 potting Methods 0.000 description 16
- 239000004698 Polyethylene Substances 0.000 description 14
- 229920000573 polyethylene Polymers 0.000 description 14
- 239000004743 Polypropylene Substances 0.000 description 11
- 229920000728 polyester Polymers 0.000 description 11
- 229920001155 polypropylene Polymers 0.000 description 11
- 239000000047 product Substances 0.000 description 11
- 238000000034 method Methods 0.000 description 10
- 239000004793 Polystyrene Substances 0.000 description 9
- 230000000052 comparative effect Effects 0.000 description 9
- 229920001971 elastomer Polymers 0.000 description 9
- 229920000058 polyacrylate Polymers 0.000 description 9
- 229920002647 polyamide Polymers 0.000 description 9
- 229920002223 polystyrene Polymers 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 239000004952 Polyamide Substances 0.000 description 8
- 239000000853 adhesive Substances 0.000 description 8
- 230000001070 adhesive effect Effects 0.000 description 8
- 239000000806 elastomer Substances 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 239000011148 porous material Substances 0.000 description 8
- 238000000746 purification Methods 0.000 description 8
- 238000001631 haemodialysis Methods 0.000 description 7
- 230000000322 hemodialysis Effects 0.000 description 7
- 229920000098 polyolefin Polymers 0.000 description 7
- 229920002635 polyurethane Polymers 0.000 description 7
- 239000004814 polyurethane Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 239000002131 composite material Substances 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- 238000000502 dialysis Methods 0.000 description 6
- 239000004627 regenerated cellulose Substances 0.000 description 6
- 230000008961 swelling Effects 0.000 description 6
- 230000002745 absorbent Effects 0.000 description 5
- 239000002250 absorbent Substances 0.000 description 5
- 239000004745 nonwoven fabric Substances 0.000 description 5
- 229920002492 poly(sulfone) Polymers 0.000 description 5
- 238000011084 recovery Methods 0.000 description 5
- 239000004925 Acrylic resin Substances 0.000 description 4
- 229920000178 Acrylic resin Polymers 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000005020 polyethylene terephthalate Substances 0.000 description 4
- 229920000139 polyethylene terephthalate Polymers 0.000 description 4
- 229920005749 polyurethane resin Polymers 0.000 description 4
- 229920003048 styrene butadiene rubber Polymers 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 229920002803 thermoplastic polyurethane Polymers 0.000 description 4
- 229920000219 Ethylene vinyl alcohol Polymers 0.000 description 3
- 239000004695 Polyether sulfone Substances 0.000 description 3
- 239000004734 Polyphenylene sulfide Substances 0.000 description 3
- 229920006393 polyether sulfone Polymers 0.000 description 3
- 229920000069 polyphenylene sulfide Polymers 0.000 description 3
- 230000008929 regeneration Effects 0.000 description 3
- 238000011069 regeneration method Methods 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 239000004721 Polyphenylene oxide Substances 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 238000004891 communication Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 2
- 239000003822 epoxy resin Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 201000006370 kidney failure Diseases 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000005022 packaging material Substances 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 229920000647 polyepoxide Polymers 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 241000157282 Aesculus Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 239000000385 dialysis solution Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 239000008393 encapsulating agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- CYKDLUMZOVATFT-UHFFFAOYSA-N ethenyl acetate;prop-2-enoic acid Chemical compound OC(=O)C=C.CC(=O)OC=C CYKDLUMZOVATFT-UHFFFAOYSA-N 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 230000005251 gamma ray Effects 0.000 description 1
- 229920000578 graft copolymer Polymers 0.000 description 1
- 235000010181 horse chestnut Nutrition 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 239000012779 reinforcing material Substances 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Chemical class [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 125000001174 sulfone group Chemical group 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
Images
Landscapes
- External Artificial Organs (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000133965A JP4190131B2 (ja) | 2000-05-02 | 2000-05-02 | 医療用血液浄化器およびハウジング |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000133965A JP4190131B2 (ja) | 2000-05-02 | 2000-05-02 | 医療用血液浄化器およびハウジング |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2001314501A JP2001314501A (ja) | 2001-11-13 |
| JP2001314501A5 JP2001314501A5 (enExample) | 2008-09-18 |
| JP4190131B2 true JP4190131B2 (ja) | 2008-12-03 |
Family
ID=18642338
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2000133965A Expired - Fee Related JP4190131B2 (ja) | 2000-05-02 | 2000-05-02 | 医療用血液浄化器およびハウジング |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4190131B2 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5278933B2 (ja) * | 2005-08-16 | 2013-09-04 | 独立行政法人産業技術総合研究所 | ゼオライト膜耐溶媒性封止構造 |
| US8216409B2 (en) * | 2009-12-10 | 2012-07-10 | General Electric Company | Methods for making a housingless hollow fiber filtration apparatus |
-
2000
- 2000-05-02 JP JP2000133965A patent/JP4190131B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2001314501A (ja) | 2001-11-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4623909B2 (ja) | 滅菌液濾過カートリッジ、およびこれを用いる方法 | |
| CA1142705A (en) | Heat sterilization method for artificial organ assemblies | |
| KR101964576B1 (ko) | 살균 용액 제품 백 | |
| US4559034A (en) | Line for use in body fluid treatment | |
| JP4309038B2 (ja) | 医療システム用の滅菌状態保持接続システムならびにそれに用いる雌型接続要素および雄型接続要素 | |
| US4176156A (en) | Method for heat-sterilizing artificial kidneys | |
| EP0090093B1 (en) | A humors processing device | |
| JPS58502036A (ja) | 腹膜透析のための多教室システム | |
| CN204275132U (zh) | 一种可同步实现透析与灌流治疗的血液净化装置 | |
| JP5007997B2 (ja) | 共用封止栓及び血液浄化器 | |
| US20210106744A1 (en) | Medical Device and Method of Manufacturing the Same | |
| US4867739A (en) | Sterilizing method | |
| WO1998022161A1 (en) | Hollow fiber dialyzer | |
| JP4190131B2 (ja) | 医療用血液浄化器およびハウジング | |
| JP2011072814A (ja) | 血液処理フィルター | |
| US4810376A (en) | Medical bag arrangement | |
| JP3821557B2 (ja) | 血液浄化器の製造方法 | |
| JP4144844B2 (ja) | 中空糸膜型透析濾過器 | |
| JPS607496B2 (ja) | 人工腎臓の熱滅菌方法 | |
| JP4245597B2 (ja) | 血液浄化器 | |
| JP2014090889A (ja) | 透析装置 | |
| JPS609818B2 (ja) | 人工臓器 | |
| US20240342351A1 (en) | Multi-part dialyzer | |
| JPS61143072A (ja) | 人工腎臓の熱滅菌方法 | |
| JPS5982868A (ja) | 熱滅菌された中空糸型物質移動装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20070322 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070405 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20070323 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080805 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080912 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080916 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110926 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110926 Year of fee payment: 3 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110926 Year of fee payment: 3 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110926 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120926 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130926 Year of fee payment: 5 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313111 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130926 Year of fee payment: 5 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| LAPS | Cancellation because of no payment of annual fees |