JP3695366B2 - Positive electrode active material for lithium ion secondary battery and method for producing the same - Google Patents
Positive electrode active material for lithium ion secondary battery and method for producing the same Download PDFInfo
- Publication number
- JP3695366B2 JP3695366B2 JP2001218250A JP2001218250A JP3695366B2 JP 3695366 B2 JP3695366 B2 JP 3695366B2 JP 2001218250 A JP2001218250 A JP 2001218250A JP 2001218250 A JP2001218250 A JP 2001218250A JP 3695366 B2 JP3695366 B2 JP 3695366B2
- Authority
- JP
- Japan
- Prior art keywords
- positive electrode
- active material
- electrode active
- battery
- ion secondary
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Description
【0001】
【発明の属する技術分野】
本発明は、リチウムイオン二次電池に使用される正極活物質に係り、特に、ガス発生が少なく、電池特性(サイクル特性、高負荷特性)及び熱安定性に優れた正極活物質及びその製造方法に関する。
【0002】
【従来の技術】
近年、携帯用のパソコン、ビデオカメラ等の電子機器に内蔵される電池として、高エネルギー密度を有するリチウムイオン二次電池が採用されている。このリチウムイオン二次電池は、リチウムコバルト複合酸化物等の正極活物質をその支持体である正極集電体に保持してなる正極板、リチウム金属等の負極活物質をその支持体である負極集電体に保持してなる負極板、LiPF6等のリチウム塩を溶解した有機溶媒からなる非水電解液、及び正極板と負極板の間に介在して両極の短絡を防止するセパレータからなっている。このうち、正極板、負極板及びセパレータの薄いシート状に成形されたものを巻回し、金属ラミネート樹脂フィルムの電池ケースに収納したラミネート電池、或いは薄型の金属ケースに収納した電池は、従来の厚型の金属ケースに収納した電池に比べ、電池内のガス発生、発熱又は外部からの加熱により容易に膨張し、電池を格納した電池パックケースまでも膨張変形するという問題があった。
【0003】
従来、リチウムイオン二次電池の正極活物質としてLiCoO2を用いた場合、放電容量を向上する目的で充電電圧を上昇させると、正極活物質の結晶の転移、或いは正極活物質の分解が起こり、コバルト酸からの酸素が放出され、この酸素は非水系電解液を酸化分解し、その結果電池内でガスが発生し、ラミネート電池等において上記問題が起きるため対策を必要とした。
【0004】
同様に、放電容量を向上する目的で充電電圧を上昇させると、正極活物質の結晶転移或いは分解に伴い、電池特性(サイクル特性、高負荷特性)、熱安定性も低下した。また、正極活物質のLiCoO2は導電性が低く、そのため導電性のあるカーボンを被覆することで導電性を改善しているが、カーボンとの接触が悪い場合、サイクル劣化を引き起こす原因となっていた。
【0005】
【発明が解決しようとする課題】
本発明は、上述した事情に鑑みなされたもので、リチウムイオン二次電池のガス発生を低減し、電池特性(サイクル特性、高負荷特性)及び熱安定性を向上できる正極活物質及びその製造方法を提供することを目的とする。
【0006】
【課題を解決するための手段】
本発明者は上述した問題を解決するために鋭意検討した結果、リチウムイオン二次電池の正極活物質として一般式がLivCo1−w−xTiwMxOyXz(Mは少なくとも一種以上のアルカリ土類金属元素、Xは少なくとも一種以上のハロゲン元素を示す。vは0.95≦v≦1.05、wは0<w≦0.05、xは0<x≦0.10、yは1≦y≦2.5、zは0<z≦0.10である。)で表される正極活物質を用いることで、上記課題を解決することができることを見いだし本発明を完成させるに至った。
【0007】
すなわち、本発明のリチウムイオン二次電池用正極活物質は、一般式がLivCo1−w−xTiwMxOyXz(Mは少なくとも一種以上のアルカリ土類金属元素、Xは少なくとも一種以上のハロゲン元素を示す。vは0.95≦v≦1.05、wは0<w≦0.05、xは0<x≦0.10、yは1≦y≦2.5、zは0<z≦0.10である。)で表される正極活物質であって、アルカリ土類金属元素Mとしては、Be、Mg、Ca、Sr、Baが好ましく、特にMg、Ca、Baが好ましい。また、ハロゲン元素Xとしては、F、Cl、Br、Iが好ましく、特にF、Clが好ましい。組成中のLi量(v値)はリチウムイオン二次電池の放電容量及び高負荷容量に影響し、0.95≦v≦1.05の範囲が好ましい。また、組成中のTi量(w値)、M量(x値)及びX量(z値)はリチウムイオン二次電池のガス発生、電池特性(サイクル特性、高負荷特性)及び熱安定性に非常に影響し、0<w≦0.05、0<x≦0.10、0<z≦0.10の範囲が好ましく、さらに0.0001≦w≦0.05、0<x≦0.01、0.001≦z≦0.07の範囲がより好ましい。組成中のO量(y値)については、ハロゲン元素を正極活物質中に導入する方法等により異なり、1≦y≦2.5の範囲である。
【0008】
本発明のリチウムイオン二次電池用正極活物質は、その比表面積が0.2〜2.0m2/gの範囲であることを特徴とする。正極活物質の比表面積はリチウムイオン二次電池のガス発生に非常に影響し、特に上記一般式で表される本発明の正極活物質の場合、比表面積が0.2〜2.0m2/gの範囲でガス発生を大幅に低減することができる。より好ましくは0.4〜0.8m2/gの範囲である。
【0009】
本発明のリチウムイオン二次電池用正極活物質の製造方法は、リチウム化合物、コバルト化合物、チタン化合物、アルカリ土類金属元素を含む化合物、及びハロゲン元素を含む化合物を混合した原料混合物を焼成した後、粉砕することを特徴とする。
【0010】
【発明の実施の形態】
本発明のリチウムイオン二次電池用正極活物質の合成は、下記に示すように、リチウム化合物、コバルト化合物、チタン化合物、アルカリ土類金属元素を含む化合物、及びハロゲン元素を含む化合物を混合した原料混合物を焼成した後、粉砕することによって行われる。
【0011】
リチウム化合物、コバルト化合物、チタン化合物、及びアルカリ土類金属元素を含む化合物としては、酸化物、水酸化物、炭酸塩、硝酸塩、硫酸塩、酢酸塩、ハロゲン化物等が用いられ、ハロゲン元素を含む化合物としては、アルカリ金属のハロゲン化物、アンモニウム塩等が用いられる。例えば、リチウム化合物としては、Li2O、LiOH、Li2CO3、LiHCO3、LiNO3、Li2SO4、Li(CH3COO)、LiCl等が用いられ、コバルト化合物としては、Co3O4、Co2O3、CoCO3、Co(NO3)2、CoCl2等が好ましく用いられる。また、チタン化合物としては、TiO2、Ti(NO3)4、Ti(SO4)2等が用いられ、、アルカリ土類金属元素を含む化合物としては、MgCO3、Mg(NO3)2、MgCl2、CaCO3、Ca(NO3)2、CaCl2、BaCO3、Ba(NO3)2、BaSO4等が用いられ、ハロゲン元素を含む化合物としては、LiF、LiCl、LiBr、LiI、NH4F、NH4Cl、NH4Br、NH4I等が好ましく用いられる。これらの原料の混合は、粉末状の原料をそのまま混合しても良く、水又は有機溶媒を用いてスラリー状として混合しても良い。スラリー状の混合物は乾燥して原料混合物とする。
【0012】
このようにして得られる原料混合物を空気中或いは弱酸化雰囲気で、500〜1000℃の温度範囲で1〜24時間焼成する。好ましくは800〜1000℃の温度範囲で6〜12時間焼成する。焼成温度が500℃未満の場合、未反応の原料が正極活物質に残留し正極活物質の本来の特徴を生かせない。逆に、1000℃を越えると、正極活物質の粒径が大きくなり過ぎて電池特性が低下する。焼成時間は、1時間未満では原料粒子間の拡散反応が進行せず、24時間経過すると拡散反応はほとんど完了しているため、それ以上焼成する必要がない。
【0013】
上記焼成により得られる焼成品をらいかい機を用いて粉砕して、比表面積が0.2〜2.0m2/g、平均粒径が1.0〜12.0μmの範囲の本発明の正極活物質を得る。
【0014】
本発明の正極活物質を用いたリチウムイオン二次電池は、電解液の酸化分解反応が抑制され、電池内で発生するガス量が低減されるため、膨張変形が非常に少なく、電池特性(サイクル特性、高負荷特性)及び熱安定性も向上する。
【0015】
次に、本発明の正極活物質を用いてリチウムイオン二次電池を作製し、ガス発生、電池特性(サイクル特性、高負荷特性)及び熱安定性について測定した結果を説明する。
【0016】
(リチウムイオン二次電池の作製)
正極活物質粉末90重量部と、導電剤としてのアセチレンブラック5重量部と、ポリフッ化ビニリデン5重量部とを混練してペーストを調製し、これを正極集電体に塗布、乾燥して正極板とする。また、負極に天然黒鉛、セパレータに多孔性プロピレンフィルムを用い、電解液としてエチレンカーボネート:ジエチルカーボネイト=1:1(体積比)の混合溶媒にLiPF6を1mol/lの濃度で溶解した溶液を用いてリチウムイオン二次電池を作製する。ここでは、正極板、負極板及びセパレータの薄いシート状に成形されたものを巻回し、金属ラミネート樹脂フィルムの電池ケースに収納したラミネート電池を作製する。
【0017】
(ガス発生の評価)
一般式がLiCo0.998Ti0.001Mg0.001O2Fz、LiCo0.999Ti0.001O2Fz、LiCo0.999Mg0.001O2Fz及びLiCoO2Fzで表される種々の正極活物質を用いてラミネート電池を作製し、充電負荷0.5Cで4.2Vまで定電流充電後、80℃で2日間保存し、ガス発生による電池の膨張率(%)を下記の式から求める(ここで1Cは、1時間で充電又は放電が終了する電流負荷である)。
電池の膨張率={(80℃保存後の電池の厚み−測定前の電池の厚み)/測定前の電池の厚み}×100
【0018】
図1に、上記正極活物質中のF量(z値)と電池の膨張率の関係を示す。この図から明らかなように、本発明の正極活物質LiCo0.998Ti0.001Mg0.001O2Fz(1a)を用いた電池の膨張率は、z値が0<z≦0.10の範囲で低く、特に0.001≦z≦0.07の範囲で非常に低くなっており、電池内で発生するガス量が低減されることがわかる。また、Mg元素を含有しない正極活物質LiCo0.999Ti0.001O2Fz(1b)、Ti元素を含有しない正極活物質LiCo0.999Mg0.001O2Fz(1c)及びMg元素とTi元素を共に含有しない正極活物質LiCoO2Fz(1d)を用いた電池に比べて、膨張率が非常に低いことがわかる。このように、正極活物質中にTi元素、Mg元素、F元素の3種類の元素を全て含むことによって、電池の膨張率は非常に低減される。また、Mg以外のアルカリ土類金属元素、F以外のハロゲン元素を含む場合も同様な特性を示す。
【0019】
上記と同様にして、一般式がLiCo0.999−xTi0.001MgxO2F0.002、LiCo0.999−xTi0.001MgxO2、LiCo1−xMgxO2F0.002及びLiCo1−xMgxO2で表される種々の正極活物質を用いてラミネート電池を作製し、電池の膨張率を求める。図2に、上記正極活物質中のMg量(x値)と電池の膨張率の関係を示す。この図から明らかなように、本発明の正極活物質LiCo0.999−xTi0.001MgxO2F0.002(2a)を用いた電池の膨張率は、x値が0<x≦0.10の範囲で少なく、特に0<x≦0.01の範囲で非常に少なくなっており、電池内で発生するガス量が低減されることがわかる。また、F元素を含有しない正極活物質LiCo0.999−xTi0.001MgxO2(2b)、Ti元素を含有しない正極活物質LiCo1−xMgxO2F0.002(2c)及びF元素とTi元素を共に含有しない正極活物質LiCo1−xMgxO2(2d)を用いた電池に比べて、膨張率が非常に低いことがわかる。このように、正極活物質中にTi元素、Mg元素、F元素の3種類の元素を全て含むことによって、電池の膨張率は非常に低減される。また、Mg以外のアルカリ土類金属元素、F以外のハロゲン元素を含む場合も同様な特性を示す。
【0020】
これは、正極活物質中にアルカリ土類金属元素を固溶させることによって格子定数が小さくなり、充放電に伴う体積変化が減少して、粒子に与える応力が緩和されることにより、正極活物質の分解等が抑制され、電解液の酸化分解反応が抑制される結果、電池内で発生するガス量が低減されるものと考えられる。さらに、固溶していないアルカリ土類金属元素とハロゲン元素とが反応し、正極活物質を被覆することにより、負極への影響が軽減されることからも、ガス量が低減されるものと考えられる。また、正極活物質中のMg量(x値)については、x値が0.10を越えると、電池の膨張率が増加するだけでなく、放電容量も低下するため、0<x≦0.10の範囲が好ましい。
【0021】
次に、比表面積が異なる種々の正極活物質LiCo0.998Ti0.001Mg0.001O2F0.002を用いてラミネート電池を作製し、同様に電池の膨張率(%)を求める。図3に、上記正極活物質の比表面積と電池の膨張率の関係を示す。この図から明らかなように、電池の膨張率は比表面積が2.0m2/g以下で少なく、特に0.8m2/g以下で非常に少なくなっており、電池内で発生するガス量が低減されることがわかる。比表面積が2.0m2/gより大きくなると、正極活物質表面或いはその近傍で起こる電解液の酸化分解反応の反応性が増し、その結果電池内で発生するガス量が増えるものと考えられる。また、比表面積が0.4m2/gより小さいと、正極活物質の粒径が大きくなり過ぎて電池特性が低下する。
【0022】
(サイクル特性の評価)
一般式がLiCo0.999−wTiwMg0.001O2F0.002で表される種々の正極活物質を用いてラミネート電池を作製し、常温(25℃)で、充電負荷0.5Cで4.2Vまで定電流充電後、1.0Cで2.75Vまで放電する充放電を500サイクル行い、500サイクル目の容量維持率(%)を下記の式から求める。
容量維持率=(500サイクル目の放電容量/1サイクル目の放電容量)×100
【0023】
図4に、上記正極活物質中のTi量(w値)と容量維持率の関係を示す。この図から明らかなように、容量維持率はw値が0<w≦0.05の範囲で高く、特に0.0001≦w≦0.05の範囲で非常に高くなっており、サイクル特性が優れていることがわかる。
【0024】
(高負荷特性の評価)
一般式がLiCo0.999−wTiwMg0.001O2F0.002で表される種々の正極活物質を用いてラミネート電池を作製し、充電負荷2.0Cで4.2Vまで定電流充電後、2.0Cで2.75Vまで放電したときの放電容量を高負荷容量(mAh/g)として求める。
【0025】
図5に、上記正極活物質中のTi量(w値)と高負荷容量の関係を示す。この図から明らかなように、高負荷容量はw値が0<w≦0.05の範囲で高く、特に0.0001≦w≦0.05の範囲で非常に高くなっており、高負荷特性が優れていることがわかる。
【0026】
このように正極活物質中にTi元素を含むことによって、正極活物質の結晶転移或いは分解が抑制される結果、電池特性(サイクル特性、高負荷特性)が向上するものと考えられる。
【0027】
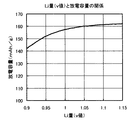
同様に、一般式がLivCo0.998Ti0.001Mg0.001O2F0.002で表される正極活物質を用いてラミネート電池を作製し、高負荷容量(mAh/g)を求める。図6に、正極活物質中のLi量(v値)と高負荷容量の関係を示す。この図から、高負荷容量はv値が1.05より大きくなると低下していることがわかる。
【0028】
また、通常の電流密度で放電させた場合(0.25C)について、図7に正極活物質中のLi量(v値)と放電容量の関係を示す。この図から、放電容量はv値が0.95より小さくなると低下していることがわかる。
【0029】
従って、高負荷容量と通常時の放電容量のいずれも考慮すると、v値は0.95≦v≦1.05の範囲に設定する必要がある。
【0030】
以下、本発明の実施例について説明するが、本発明は具体的実施例のみに限定されるものではないことは言うまでもない。
【0031】
【実施例】
[実施例1]
炭酸リチウム(Li2CO3)、四三酸化コバルト(Co3O4)、二酸化チタン(TiO2)、炭酸マグネシウム(MgCO3)及びフッ化アンモニウム(NH4F)を、v=1.0、w=0.001、x=0.001、z=0.002になるように計量し、乾式混合する。得られた原料混合物を空気中、900℃で10時間焼成した後、らいかい機を用いて粉砕して、比表面積が0.63m2/g、平均粒径が3.5μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2F0.002を得る。
【0032】
なお、比表面積は、窒素ガス吸着による定圧式BET一点法にて測定した値であり、平均粒径は、空気透過法により比表面積を測定し、一次粒子の粒径の平均値を求めたものであり、フィッシャーサブシーブサイザー(F.S.S.S.)を用いて測定した値である。
【0033】
[実施例2]
w=0.005にする以外は実施例1と同様にして、比表面積が0.65m2/g、平均粒径が3.4μmの正極活物質粉末LiCo0.994Ti0.005Mg0.001O2F0.002を得る。
【0034】
[実施例3]
x=0.002にする以外は実施例1と同様にして、比表面積が0.63m2/g、平均粒径が3.3μmの正極活物質粉末LiCo0.997Ti0.001Mg0.002O2F0.002を得る。
【0035】
[実施例4]
x=0.005にする以外は実施例1と同様にして、比表面積が0.63m2/g、平均粒径が3.3μmの正極活物質粉末LiCo0.994Ti0.001Mg0.005O2F0.002を得る。
【0036】
[実施例5]
x=0.01にする以外は実施例1と同様にして、比表面積が0.63m2/g、平均粒径が3.3μmの正極活物質粉末LiCo0.989Ti0.001Mg0.010O2F0.002を得る。
【0037】
[実施例6]
z=0.006にする以外は実施例1と同様にして、比表面積が0.62m2/g、平均粒径が3.5μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2F0.006を得る。
【0038】
[実施例7]
z=0.01にする以外は実施例1と同様にして、比表面積が0.62m2/g、平均粒径が3.5μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2F0.01を得る。
【0039】
[実施例8]
炭酸マグネシウム(MgCO3)の代わりに炭酸カルシウム(CaCO3)を使用する以外は実施例1と同様にして、比表面積が0.59m2/g、平均粒径が3.7μmの正極活物質粉末LiCo0.998Ti0.001Ca0.001O2F0.002を得る。
【0040】
[実施例9]
炭酸マグネシウム(MgCO3)の代わりに炭酸バリウム(BaCO3)を使用する以外は実施例1と同様にして、比表面積が0.62m2/g、平均粒径が3.5μmの正極活物質粉末LiCo0.998Ti0.001Ba0.001O2F0.002を得る。
【0041】
[実施例10]
フッ化アンモニウム(NH4F)の代わりに塩化アンモニウム(NH4Cl)を使用する以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2Cl0.002を得る。
【0042】
[実施例11]
フッ化アンモニウム(NH4F)の代わりに臭化アンモニウム(NH4Br)を使用する以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2Br0.002を得る。
【0043】
[実施例12]
フッ化アンモニウム(NH4F)の代わりに沃化アンモニウム(NH4I)を使用する以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2I0.002を得る。
【0044】
[実施例13]
炭酸マグネシウム(MgCO3)の代わりに炭酸ストロンチウム(SrCO3)を使用する以外は実施例1と同様にして、比表面積が0.62m2/g、平均粒径が3.5μmの正極活物質粉末LiCo0.998Ti0.001Sr0.001O2F0.002を得る。
【0045】
[比較例1]
二酸化チタン(TiO2)、炭酸マグネシウム(MgCO3)及びフッ化アンモニウム(NH4F)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCoO2を得る。
【0046】
[比較例2]
炭酸マグネシウム(MgCO3)、フッ化アンモニウム(NH4F)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.999Ti0.001O2を得る。
【0047】
[比較例3]
二酸化チタン(TiO2)、フッ化アンモニウム(NH4F)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.999Mg0.001O2を得る。
【0048】
[比較例4]
二酸化チタン(TiO2)、炭酸マグネシウム(MgCO3)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCoO2F0.002を得る。
【0049】
[比較例5]
フッ化アンモニウム(NH4F)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.998Ti0.001Mg0.001O2を得る。
【0050】
[比較例6]
炭酸マグネシウム(MgCO3)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.999Ti0.001O2F0.002を得る。
【0051】
[比較例7]
二酸化チタン(TiO2)を使用しない以外は実施例1と同様にして、比表面積が0.61m2/g、平均粒径が3.6μmの正極活物質粉末LiCo0.999Mg0.001O2F0.002を得る。
【0052】
(評価)
実施例1〜13及び比較例1〜7で得られた正極活物質粉末を用いてラミネート電池を作製し、ガス発生、電池特性(サイクル特性、高負荷特性)及び熱安定性について測定した結果を表1にまとめる。電池の膨張率、常温(25℃)での容量維持率及び高負荷容量は前記と同様に測定する。高温(60℃)での容量維持率は、60℃高温槽中で測定し、300サイクル目の容量維持率(%)を求める以外は常温(25℃)での容量維持率と同様に測定する。熱安定性については、次のように示差走査熱量測定を行い、発熱開始温度により評価する。
【0053】
(熱安定性の評価)
▲1▼正極活物質粉末90重量部と、導電剤としてのカーボン5重量部と、ポリフッ化ビニリデン5重量部とを混練してペーストを調製し、これを単極評価可能なデマンタブル式のセル正極集電体に塗布し、電解液にエチレンカーボネートを用いて二次電池を作製する。
▲2▼定電流による充放電を行いなじませた後、一定電流の下で電池電圧が4.3Vになるまで充電を行う。
▲3▼充電後、二次電池から正極を取り出し、洗浄、乾燥を行い、正極活物質を削り取る。
▲4▼正極から削り取った正極活物質5mgとエチレンカーボネート2mgをAlセルに入れ、示差走査熱量分析を行い、発熱開始温度を求める。
【0054】
示差走査熱量分析とは、基準物質と試料とを同時に一定の速度で加熱しながら両者の間に生じる温度差を測定し、試料物質の熱的特性を解析する方法であり、正極活物質について測定すると、低温部では示差走査熱量は変化しないが、ある温度以上で示差走査熱量が大きく増大する。この時の温度を発熱開始温度とし、この温度が高いほど熱安定性が良いといえる。
【0055】
表1から、比較例1〜7に比べ、実施例1〜13は正極活物質中にTi元素、アルカリ土類元素及びハロゲン元素を含むことによって、電池の膨張率が低減し、容量維持率、高負荷容量が高く、電池特性(サイクル特性、高負荷特性)が優れていることがわかる。サイクル特性については、常温(25℃)でのサイクル特性よりも、高温(60℃)でのサイクル特性において特に効果が著しいことがわかる。また、発熱開始温度は比較例に比べ高く、熱安定性にも優れていることがわかる。例えば、Ti元素、Mg元素、F元素の3種類の元素をいずれも含まない比較例1、1種類の元素のみ含む比較例2〜4、2種類の元素を含む比較例5〜7に比べ、3種類の元素を全て含む実施例1の場合、電池の膨張率は低く、且つ容量維持率、高負荷容量が高くなっている。また、発熱開始温度も高くなっている。このように、正極活物質中にTi元素、Mg元素、F元素の3種類の元素を全て含むことによって、相乗効果として正極活物質の結晶転移或いは分解がさらに抑制される結果、電池内のガス発生は著しく低減し、電池特性(サイクル特性、高負荷特性)及び熱安定性は非常に向上する。
【0056】
【表1】
【0057】
【発明の効果】
リチウムイオン二次電池の正極活物質として一般式がLivCo1−w−xTiwMxOyXz(Mは少なくとも一種以上のアルカリ土類金属元素、Xは少なくとも一種以上のハロゲン元素を示す。vは0.95≦v≦1.05、wは0<w≦0.05、xは0<x≦0.10、yは1≦y≦2.5、zは0<z≦0.10である。)で表される正極活物質を用いることにより、電池内のガス発生を低減し、電池特性(サイクル特性、高負荷特性)及び熱安定性を向上させることができる。
【図面の簡単な説明】
【図1】正極活物質中のF量(z値)と電池の膨張率の関係を示す特性図
【図2】正極活物質中のMg量(x値)と電池の膨張率の関係を示す特性図
【図3】正極活物質の比表面積と電池の膨張率の関係を示す特性図
【図4】正極活物質中のTi量(w値)と容量維持率の関係を示す特性図
【図5】正極活物質中のTi量(w値)と高負荷容量容量の関係を示す特性図
【図6】正極活物質中のLi量(v値)と高負荷容量の関係を示す特性図
【図7】正極活物質中のLi量(v値)と放電容量の関係を示す特性図[0001]
BACKGROUND OF THE INVENTION
The present invention relates to a positive electrode active material used for a lithium ion secondary battery, and in particular, a positive electrode active material that generates less gas and has excellent battery characteristics (cycle characteristics, high load characteristics) and thermal stability, and a method for producing the same. About.
[0002]
[Prior art]
In recent years, lithium-ion secondary batteries having high energy density have been adopted as batteries incorporated in electronic devices such as portable personal computers and video cameras. The lithium ion secondary battery includes a positive electrode plate in which a positive electrode active material such as lithium cobalt composite oxide is held on a positive electrode current collector that is a support, and a negative electrode active material such as lithium metal that is a support. It consists of a negative electrode plate held by a current collector, a nonaqueous electrolyte solution made of an organic solvent in which a lithium salt such as LiPF 6 is dissolved, and a separator that is interposed between the positive electrode plate and the negative electrode plate to prevent short-circuiting of both electrodes. . Among these, a laminated battery stored in a battery case of a metal-laminated resin film or a battery stored in a thin metal case is wound with a positive electrode plate, a negative electrode plate, and a separator formed into a thin sheet shape. Compared with a battery housed in a metal case of a mold, there is a problem that it easily expands due to gas generation in the battery, heat generation or external heating, and the battery pack case storing the battery also expands and deforms.
[0003]
Conventionally, when LiCoO 2 is used as the positive electrode active material of a lithium ion secondary battery, when the charging voltage is increased for the purpose of improving the discharge capacity, crystal transition of the positive electrode active material or decomposition of the positive electrode active material occurs. Oxygen from the cobalt acid is released, and this oxygen oxidizes and decomposes the nonaqueous electrolytic solution. As a result, gas is generated in the battery, and the above problem occurs in the laminated battery and the like.
[0004]
Similarly, when the charging voltage is increased for the purpose of improving the discharge capacity, the battery characteristics (cycle characteristics, high load characteristics) and thermal stability are also reduced along with the crystal transition or decomposition of the positive electrode active material. In addition, the positive electrode active material LiCoO 2 has low conductivity, and therefore the conductivity is improved by coating the conductive carbon. However, when the contact with the carbon is poor, it causes the cycle deterioration. It was.
[0005]
[Problems to be solved by the invention]
The present invention has been made in view of the above-described circumstances. A positive electrode active material capable of reducing gas generation in a lithium ion secondary battery and improving battery characteristics (cycle characteristics, high load characteristics) and thermal stability, and a method for producing the same The purpose is to provide.
[0006]
[Means for Solving the Problems]
The present inventors have result of intensive studies to solve the above problems, the general formula as a cathode active material for a lithium ion secondary battery Li v Co 1-w-x Ti w M x O y X z (M is at least One or more alkaline earth metal elements, X represents at least one halogen element, v is 0.95 ≦ v ≦ 1.05, w is 0 <w ≦ 0.05, x is 0 <x ≦ 0. 10 and y are 1 ≦ y ≦ 2.5 and z is 0 <z ≦ 0.10.) By using a positive electrode active material represented by the following formula, the present invention can be solved. It came to complete.
[0007]
That is, the positive electrode active material for a lithium ion secondary battery of the present invention, the general formula Li v Co 1-w-x Ti w M x O y X z (M is at least one or more alkaline earth metal elements, X is At least one halogen element, v is 0.95 ≦ v ≦ 1.05, w is 0 <w ≦ 0.05, x is 0 <x ≦ 0.10, and y is 1 ≦ y ≦ 2.5. , Z is 0 <z ≦ 0.10), and the alkaline earth metal element M is preferably Be, Mg, Ca, Sr, or Ba, and particularly Mg, Ca Ba is preferred. Further, as the halogen element X, F, Cl, Br, and I are preferable, and F and Cl are particularly preferable. The Li amount (v value) in the composition affects the discharge capacity and high load capacity of the lithium ion secondary battery, and is preferably in the range of 0.95 ≦ v ≦ 1.05. In addition, Ti amount (w value), M amount (x value) and X amount (z value) in the composition contribute to gas generation, battery characteristics (cycle characteristics, high load characteristics) and thermal stability of lithium ion secondary batteries. The range of 0 <w ≦ 0.05, 0 <x ≦ 0.10, and 0 <z ≦ 0.10 is preferable, and 0.0001 ≦ w ≦ 0.05, 0 <x ≦ 0. A range of 01, 0.001 ≦ z ≦ 0.07 is more preferable. The amount of O (y value) in the composition varies depending on the method of introducing a halogen element into the positive electrode active material, and is in the range of 1 ≦ y ≦ 2.5.
[0008]
The positive electrode active material for a lithium ion secondary battery of the present invention has a specific surface area in the range of 0.2 to 2.0 m 2 / g. The specific surface area of the positive electrode active material greatly affects the gas generation of the lithium ion secondary battery. In particular, in the case of the positive electrode active material of the present invention represented by the above general formula, the specific surface area is 0.2 to 2.0 m 2 / Gas generation can be greatly reduced in the range of g. More preferably, it is the range of 0.4-0.8 m < 2 > / g.
[0009]
The method for producing a positive electrode active material for a lithium ion secondary battery according to the present invention includes firing a raw material mixture obtained by mixing a lithium compound, a cobalt compound, a titanium compound, a compound containing an alkaline earth metal element, and a compound containing a halogen element. It is characterized by crushing.
[0010]
DETAILED DESCRIPTION OF THE INVENTION
As shown below, the synthesis of the positive electrode active material for lithium ion secondary battery of the present invention is a raw material in which a lithium compound, a cobalt compound, a titanium compound, a compound containing an alkaline earth metal element, and a compound containing a halogen element are mixed. This is done by calcining the mixture after firing.
[0011]
As a compound containing a lithium compound, a cobalt compound, a titanium compound, and an alkaline earth metal element, oxides, hydroxides, carbonates, nitrates, sulfates, acetates, halides, and the like are used. As the compound, alkali metal halides, ammonium salts and the like are used. For example, Li 2 O, LiOH, Li 2 CO 3 , LiHCO 3 , LiNO 3 , Li 2 SO 4 , Li (CH 3 COO), LiCl, or the like is used as the lithium compound, and Co 3 O is used as the cobalt compound. 4 , Co 2 O 3 , CoCO 3 , Co (NO 3 ) 2 , CoCl 2 and the like are preferably used. As the titanium compound, TiO 2 , Ti (NO 3 ) 4 , Ti (SO 4 ) 2 or the like is used, and as the compound containing an alkaline earth metal element, MgCO 3 , Mg (NO 3 ) 2 , MgCl 2 , CaCO 3 , Ca (NO 3 ) 2 , CaCl 2 , BaCO 3 , Ba (NO 3 ) 2 , BaSO 4, etc. are used, and compounds containing halogen elements include LiF, LiCl, LiBr, LiI, NH 4 F, NH 4 Cl, NH 4 Br, NH 4 I and the like are preferably used. In mixing these raw materials, the powdery raw materials may be mixed as they are, or may be mixed as a slurry using water or an organic solvent. The slurry mixture is dried to obtain a raw material mixture.
[0012]
The raw material mixture thus obtained is fired in the temperature range of 500 to 1000 ° C. for 1 to 24 hours in air or in a weakly oxidizing atmosphere. Preferably, baking is performed at a temperature range of 800 to 1000 ° C. for 6 to 12 hours. When the firing temperature is less than 500 ° C., unreacted raw materials remain in the positive electrode active material, and the original characteristics of the positive electrode active material cannot be utilized. On the other hand, when the temperature exceeds 1000 ° C., the particle size of the positive electrode active material becomes too large and the battery characteristics deteriorate. When the firing time is less than 1 hour, the diffusion reaction between the raw material particles does not proceed. When 24 hours have elapsed, the diffusion reaction is almost completed, and thus no further firing is necessary.
[0013]
The fired product obtained by the above firing is pulverized using a roughing machine, and the positive electrode of the present invention has a specific surface area of 0.2 to 2.0 m 2 / g and an average particle size of 1.0 to 12.0 μm. Get the active material.
[0014]
In the lithium ion secondary battery using the positive electrode active material of the present invention, the oxidative decomposition reaction of the electrolytic solution is suppressed, and the amount of gas generated in the battery is reduced. Characteristics, high load characteristics) and thermal stability are also improved.
[0015]
Next, a lithium ion secondary battery is produced using the positive electrode active material of the present invention, and the results of measuring gas generation, battery characteristics (cycle characteristics, high load characteristics) and thermal stability will be described.
[0016]
(Production of lithium ion secondary battery)
A paste is prepared by kneading 90 parts by weight of a positive electrode active material powder, 5 parts by weight of acetylene black as a conductive agent, and 5 parts by weight of polyvinylidene fluoride, applying this to a positive electrode current collector, and drying to form a positive electrode plate And Further, natural graphite is used for the negative electrode, a porous propylene film is used for the separator, and a solution in which LiPF 6 is dissolved at a concentration of 1 mol / l in a mixed solvent of ethylene carbonate: diethyl carbonate = 1: 1 (volume ratio) is used as the electrolyte. To produce a lithium ion secondary battery. Here, a thin sheet of a positive electrode plate, a negative electrode plate and a separator is wound to produce a laminated battery housed in a battery case of a metal laminated resin film.
[0017]
(Evaluation of gas generation)
The general formula is LiCo 0.998 Ti 0.001 Mg 0.001 O 2 F z , LiCo 0.999 Ti 0.001 O 2 F z , LiCo 0.999 Mg 0.001 O 2 F z and LiCoO 2 F z A laminate battery is manufactured using various positive electrode active materials represented by the following formula, and is charged at a constant current up to 4.2 V at a charging load of 0.5 C, and then stored at 80 ° C. for 2 days. ) Is obtained from the following equation (where 1C is a current load that completes charging or discharging in one hour).
Expansion coefficient of battery = {(battery thickness after storage at 80 ° C.−battery thickness before measurement) / battery thickness before measurement} × 100
[0018]
FIG. 1 shows the relationship between the amount of F (z value) in the positive electrode active material and the expansion coefficient of the battery. As is clear from this figure, the expansion rate of the battery using the positive electrode active material LiCo 0.998 Ti 0.001 Mg 0.001 O 2 F z (1a) of the present invention is such that the z value is 0 <z ≦ 0. It is low in the range of .10, particularly very low in the range of 0.001 ≦ z ≦ 0.07, indicating that the amount of gas generated in the battery is reduced. Further, a positive electrode active material LiCo 0.999 Ti 0.001 O 2 F z (1b) not containing Mg element, a positive electrode active material LiCo 0.999 Mg 0.001 O 2 F z (1c) not containing Ti element, and It can be seen that the expansion coefficient is very low as compared with the battery using the positive electrode active material LiCoO 2 F z (1d) containing neither Mg element nor Ti element. Thus, by including all three types of elements, Ti element, Mg element and F element, in the positive electrode active material, the expansion coefficient of the battery is greatly reduced. Similar characteristics are also exhibited when an alkaline earth metal element other than Mg and a halogen element other than F are included.
[0019]
In the same manner as above, the general formulas are LiCo 0.999-x Ti 0.001 Mg x O 2 F 0.002 , LiCo 0.999-x Ti 0.001 Mg x O 2 , LiCo 1-x Mg x O. A laminate battery is produced using various positive electrode active materials represented by 2 F 0.002 and LiCo 1-x Mg x O 2 , and the expansion coefficient of the battery is obtained. FIG. 2 shows the relationship between the amount of Mg (x value) in the positive electrode active material and the expansion coefficient of the battery. As is clear from this figure, the expansion coefficient of the battery using the positive electrode active material LiCo 0.999-x Ti 0.001 Mg x O 2 F 0.002 (2a) of the present invention is such that the x value is 0 <x It is found that the amount of gas generated in the battery is reduced because the amount is less in the range of ≦ 0.10, particularly in the range of 0 <x ≦ 0.01. The positive electrode active material LiCo 0.999-x Ti 0.001 Mg containing no F element x O 2 (2b), not containing Ti element cathode active material LiCo 1-x Mg x O 2 F 0.002 (2c ) and compared to a battery using both containing no cathode active material LiCo 1-x Mg x O 2 (2d) the F element and the Ti element, the expansion ratio is seen to be very low. Thus, by including all three types of elements, Ti element, Mg element and F element, in the positive electrode active material, the expansion coefficient of the battery is greatly reduced. Similar characteristics are also exhibited when an alkaline earth metal element other than Mg and a halogen element other than F are included.
[0020]
This is because the lattice constant is reduced by dissolving an alkaline earth metal element in the positive electrode active material, the volume change associated with charge / discharge is reduced, and the stress applied to the particles is alleviated. It is considered that the amount of gas generated in the battery is reduced as a result of suppressing the decomposition of the electrolyte and the oxidative decomposition reaction of the electrolyte. Furthermore, it is thought that the amount of gas is reduced because the alkaline earth metal element and the halogen element that are not in solid solution react with each other and the positive electrode active material is coated to reduce the influence on the negative electrode. It is done. Further, regarding the Mg amount (x value) in the positive electrode active material, if the x value exceeds 0.10, not only the expansion coefficient of the battery increases but also the discharge capacity decreases, so 0 <x ≦ 0. A range of 10 is preferred.
[0021]
Next, a laminate battery is manufactured using various positive electrode active materials LiCo 0.998 Ti 0.001 Mg 0.001 O 2 F 0.002 having different specific surface areas, and the expansion coefficient (%) of the battery is similarly obtained. . FIG. 3 shows the relationship between the specific surface area of the positive electrode active material and the expansion coefficient of the battery. As is clear from this figure, the expansion coefficient of the battery is small at a specific surface area of 2.0 m 2 / g or less, particularly very low at 0.8 m 2 / g or less, and the amount of gas generated in the battery is small. It can be seen that it is reduced. When the specific surface area is larger than 2.0 m 2 / g, it is considered that the reactivity of the oxidative decomposition reaction of the electrolytic solution occurring on or near the surface of the positive electrode active material increases, and as a result, the amount of gas generated in the battery increases. On the other hand, when the specific surface area is smaller than 0.4 m 2 / g, the particle size of the positive electrode active material becomes too large and the battery characteristics are deteriorated.
[0022]
(Evaluation of cycle characteristics)
Laminated batteries were prepared using various positive electrode active materials represented by the general formula LiCo 0.999-w Ti w Mg 0.001 O 2 F 0.002 , and charged at a normal temperature (25 ° C.) of 0. After charging at a constant current up to 4.2 V at 5 C, 500 cycles of charge and discharge discharging to 2.75 V at 1.0 C are performed, and the capacity retention rate (%) at the 500th cycle is obtained from the following equation.
Capacity retention rate = (discharge capacity at 500th cycle / discharge capacity at the first cycle) × 100
[0023]
FIG. 4 shows the relationship between the amount of Ti (w value) in the positive electrode active material and the capacity retention rate. As is apparent from this figure, the capacity retention ratio is high when the w value is in the range of 0 <w ≦ 0.05, particularly very high in the range of 0.0001 ≦ w ≦ 0.05. It turns out that it is excellent.
[0024]
(Evaluation of high load characteristics)
Laminate batteries were prepared using various positive electrode active materials represented by the general formula LiCo 0.999-w Ti w Mg 0.001 O 2 F 0.002 , and the voltage was set to 4.2 V at a charging load of 2.0 C. After current charging, the discharge capacity when discharged to 2.75 V at 2.0 C is obtained as the high load capacity (mAh / g).
[0025]
FIG. 5 shows the relationship between the amount of Ti (w value) in the positive electrode active material and high load capacity. As is apparent from this figure, the high load capacity is high when the w value is in the range of 0 <w ≦ 0.05, particularly very high in the range of 0.0001 ≦ w ≦ 0.05. It is understood that is superior.
[0026]
By including Ti element in the positive electrode active material in this manner, it is considered that the battery characteristics (cycle characteristics, high load characteristics) are improved as a result of suppressing crystal transition or decomposition of the positive electrode active material.
[0027]
Similarly, a laminated battery is manufactured using a positive electrode active material represented by a general formula of Li v Co 0.998 Ti 0.001 Mg 0.001 O 2 F 0.002 , and has a high load capacity (mAh / g). Ask for. FIG. 6 shows the relationship between the Li amount (v value) in the positive electrode active material and the high load capacity. From this figure, it can be seen that the high load capacity decreases when the v value becomes larger than 1.05.
[0028]
FIG. 7 shows the relationship between the Li amount (v value) in the positive electrode active material and the discharge capacity when discharged at a normal current density (0.25 C). From this figure, it can be seen that the discharge capacity decreases when the v value becomes smaller than 0.95.
[0029]
Accordingly, considering both the high load capacity and the normal discharge capacity, the v value needs to be set in the range of 0.95 ≦ v ≦ 1.05.
[0030]
Examples of the present invention will be described below, but it goes without saying that the present invention is not limited to specific examples.
[0031]
【Example】
[Example 1]
Lithium carbonate (Li 2 CO 3 ), cobalt trioxide (Co 3 O 4 ), titanium dioxide (TiO 2 ), magnesium carbonate (MgCO 3 ), and ammonium fluoride (NH 4 F), v = 1.0, Weigh so that w = 0.001, x = 0.001, z = 0.002 and dry mix. The obtained raw material mixture was calcined in the air at 900 ° C. for 10 hours, and then pulverized using a raking machine to obtain a positive electrode active material powder having a specific surface area of 0.63 m 2 / g and an average particle size of 3.5 μm. LiCo 0.998 Ti 0.001 Mg 0.001 O 2 F 0.002 is obtained.
[0032]
The specific surface area is a value measured by a constant pressure BET one-point method using nitrogen gas adsorption, and the average particle diameter is a value obtained by measuring the specific surface area by an air permeation method and obtaining the average value of the particle diameters of primary particles. It is a value measured using a Fisher sub-sieve sizer (FSSS).
[0033]
[Example 2]
The positive electrode active material powder LiCo 0.994 Ti 0.005 Mg 0. 0 having a specific surface area of 0.65 m 2 / g and an average particle size of 3.4 μm was the same as in Example 1 except that w = 0.005 . 001 O 2 F 0.002 is obtained.
[0034]
[Example 3]
The positive electrode active material powder LiCo 0.997 Ti 0.001 Mg 0. 0 having a specific surface area of 0.63 m 2 / g and an average particle size of 3.3 μm was the same as in Example 1 except that x = 0.002 . 002 O 2 F 0.002 is obtained.
[0035]
[Example 4]
A positive electrode active material powder LiCo 0.994 Ti 0.001 Mg 0. 0 having a specific surface area of 0.63 m 2 / g and an average particle size of 3.3 μm, except that x = 0.005 . 005 O 2 F 0.002 is obtained.
[0036]
[Example 5]
The positive electrode active material powder LiCo 0.989 Ti 0.001 Mg 0. 0 having a specific surface area of 0.63 m 2 / g and an average particle size of 3.3 μm was the same as in Example 1 except that x = 0.01 . 010 O 2 F 0.002 is obtained.
[0037]
[Example 6]
A positive electrode active material powder LiCo 0.998 Ti 0.001 Mg 0 .0 having a specific surface area of 0.62 m 2 / g and an average particle size of 3.5 μm, except that z = 0.006 . 001 O 2 F 0.006 is obtained.
[0038]
[Example 7]
The positive electrode active material powder LiCo 0.998 Ti 0.001 Mg 0 .0 having a specific surface area of 0.62 m 2 / g and an average particle size of 3.5 μm was obtained in the same manner as in Example 1 except that z = 0.01 . 001 O 2 F 0.01 is obtained.
[0039]
[Example 8]
Positive electrode active material powder having a specific surface area of 0.59 m 2 / g and an average particle size of 3.7 μm, as in Example 1, except that calcium carbonate (CaCO 3 ) is used instead of magnesium carbonate (MgCO 3 ). LiCo 0.998 Ti 0.001 Ca 0.001 O 2 F 0.002 is obtained.
[0040]
[Example 9]
Positive electrode active material powder having a specific surface area of 0.62 m 2 / g and an average particle size of 3.5 μm, as in Example 1, except that barium carbonate (BaCO 3 ) is used instead of magnesium carbonate (MgCO 3 ). LiCo 0.998 Ti 0.001 Ba 0.001 O 2 F 0.002 is obtained.
[0041]
[Example 10]
A positive electrode having a specific surface area of 0.61 m 2 / g and an average particle diameter of 3.6 μm, as in Example 1, except that ammonium chloride (NH 4 Cl) is used instead of ammonium fluoride (NH 4 F). An active material powder LiCo 0.998 Ti 0.001 Mg 0.001 O 2 Cl 0.002 is obtained.
[0042]
[Example 11]
A specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm were used in the same manner as in Example 1 except that ammonium bromide (NH 4 Br) was used instead of ammonium fluoride (NH 4 F). A positive electrode active material powder LiCo 0.998 Ti 0.001 Mg 0.001 O 2 Br 0.002 is obtained.
[0043]
[Example 12]
A specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm were used in the same manner as in Example 1 except that ammonium iodide (NH 4 I) was used instead of ammonium fluoride (NH 4 F). Positive electrode active material powder LiCo 0.998 Ti 0.001 Mg 0.001 O 2 I 0.002 is obtained.
[0044]
[Example 13]
Cathode active material powder having a specific surface area of 0.62 m 2 / g and an average particle diameter of 3.5 μm, as in Example 1, except that strontium carbonate (SrCO 3 ) is used instead of magnesium carbonate (MgCO 3 ). LiCo 0.998 Ti 0.001 Sr 0.001 O 2 F 0.002 is obtained.
[0045]
[Comparative Example 1]
The specific surface area was 0.61 m 2 / g and the average particle size was 3 as in Example 1, except that titanium dioxide (TiO 2 ), magnesium carbonate (MgCO 3 ), and ammonium fluoride (NH 4 F) were not used. A positive electrode active material powder LiCoO 2 of 6 μm is obtained.
[0046]
[Comparative Example 2]
Positive electrode active material powder having a specific surface area of 0.61 m 2 / g and an average particle diameter of 3.6 μm, as in Example 1, except that magnesium carbonate (MgCO 3 ) and ammonium fluoride (NH 4 F) are not used. LiCo 0.999 Ti 0.001 O 2 is obtained.
[0047]
[Comparative Example 3]
Cathode active material powder having a specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm in the same manner as in Example 1 except that titanium dioxide (TiO 2 ) and ammonium fluoride (NH 4 F) are not used. LiCo 0.999 Mg 0.001 O 2 is obtained.
[0048]
[Comparative Example 4]
Cathode active material powder LiCoO 2 having a specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm in the same manner as in Example 1 except that titanium dioxide (TiO 2 ) and magnesium carbonate (MgCO 3 ) are not used. F 0.002 is obtained.
[0049]
[Comparative Example 5]
A positive electrode active material powder LiCo 0.998 Ti 0. 0 having a specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm in the same manner as in Example 1 except that ammonium fluoride (NH 4 F) is not used . 001 Mg 0.001 O 2 is obtained.
[0050]
[Comparative Example 6]
A positive electrode active material powder LiCo 0.999 Ti 0.001 O having a specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm, as in Example 1, except that magnesium carbonate (MgCO 3 ) is not used. 2 F 0.002 is obtained.
[0051]
[Comparative Example 7]
A positive electrode active material powder LiCo 0.999 Mg 0.001 O having a specific surface area of 0.61 m 2 / g and an average particle size of 3.6 μm, as in Example 1, except that titanium dioxide (TiO 2 ) is not used. 2 F 0.002 is obtained.
[0052]
(Evaluation)
Laminated batteries were prepared using the positive electrode active material powders obtained in Examples 1 to 13 and Comparative Examples 1 to 7, and the results of gas generation, battery characteristics (cycle characteristics, high load characteristics) and thermal stability were measured. Table 1 summarizes. The expansion coefficient of the battery, the capacity retention ratio at normal temperature (25 ° C.), and the high load capacity are measured in the same manner as described above. The capacity maintenance rate at high temperature (60 ° C.) is measured in a high temperature bath at 60 ° C., and is measured in the same manner as the capacity maintenance rate at room temperature (25 ° C.) except that the capacity maintenance rate (%) at the 300th cycle is obtained. . About thermal stability, differential scanning calorimetry is performed as follows, and it evaluates with heat generation start temperature.
[0053]
(Evaluation of thermal stability)
(1) A demountable cell positive electrode capable of preparing a paste by kneading 90 parts by weight of a positive electrode active material powder, 5 parts by weight of carbon as a conductive agent, and 5 parts by weight of polyvinylidene fluoride, and capable of evaluating this as a single electrode A secondary battery is manufactured by applying to a current collector and using ethylene carbonate as an electrolytic solution.
{Circle around (2)} After charging / discharging with a constant current and charging, charging is performed under a constant current until the battery voltage reaches 4.3V.
(3) After charging, the positive electrode is taken out from the secondary battery, washed and dried, and the positive electrode active material is scraped off.
(4) 5 mg of the positive electrode active material and 2 mg of ethylene carbonate scraped from the positive electrode are placed in an Al cell, and differential scanning calorimetry is performed to determine the heat generation start temperature.
[0054]
Differential scanning calorimetry is a method for analyzing the thermal characteristics of a sample material by measuring the temperature difference between the reference material and the sample while heating them simultaneously at a constant rate. Then, the differential scanning calorific value does not change in the low temperature part, but the differential scanning calorific value greatly increases above a certain temperature. The temperature at this time is defined as the heat generation start temperature, and it can be said that the higher the temperature, the better the thermal stability.
[0055]
From Table 1, compared with Comparative Examples 1-7, Examples 1-13 contain the Ti element, alkaline-earth element, and a halogen element in a positive electrode active material, the expansion coefficient of a battery reduces, a capacity maintenance rate, It can be seen that the high load capacity is high and the battery characteristics (cycle characteristics, high load characteristics) are excellent. Regarding the cycle characteristics, it can be seen that the effect is particularly remarkable in the cycle characteristics at a high temperature (60 ° C.) than the cycle characteristics at a normal temperature (25 ° C.). Moreover, it can be seen that the heat generation start temperature is higher than that of the comparative example and is excellent in thermal stability. For example, Comparative Example 1 that does not include any of the three elements of Ti element, Mg element, and F element, Comparative Examples 2 to 4 that include only one kind of element, and Comparative Examples 5 to 7 that include two kinds of elements, In the case of Example 1 including all three types of elements, the expansion coefficient of the battery is low, and the capacity maintenance ratio and the high load capacity are high. Moreover, the heat generation start temperature is also high. Thus, by including all three kinds of elements of Ti element, Mg element and F element in the positive electrode active material, the crystal transition or decomposition of the positive electrode active material is further suppressed as a synergistic effect. Occurrence is remarkably reduced, and battery characteristics (cycle characteristics, high load characteristics) and thermal stability are greatly improved.
[0056]
[Table 1]
[0057]
【The invention's effect】
General formula Li as a cathode active material for a lithium ion secondary battery v Co 1-w-x Ti w M x O y X z (M is at least one or more alkaline earth metal elements, X is at least one kind of halogen element V is 0.95 ≦ v ≦ 1.05, w is 0 <w ≦ 0.05, x is 0 <x ≦ 0.10, y is 1 ≦ y ≦ 2.5, and z is 0 <z. By using the positive electrode active material represented by ≦ 0.10, gas generation in the battery can be reduced, and battery characteristics (cycle characteristics, high load characteristics) and thermal stability can be improved.
[Brief description of the drawings]
FIG. 1 is a characteristic diagram showing the relationship between the amount of F (z value) in the positive electrode active material and the expansion coefficient of the battery. FIG. 2 shows the relationship between the amount of Mg (x value) in the positive electrode active material and the expansion coefficient of the battery. Fig. 3 is a characteristic diagram showing the relationship between the specific surface area of the positive electrode active material and the expansion coefficient of the battery. Fig. 4 is a characteristic diagram showing the relationship between the amount of Ti (w value) in the positive electrode active material and the capacity retention rate. 5] Characteristic diagram showing the relationship between the amount of Ti (w value) in the positive electrode active material and high load capacity capacity. [Fig. 6] Characteristic diagram showing the relationship between the amount of Li (v value) in the positive electrode active material and high load capacity. FIG. 7 is a characteristic diagram showing the relationship between the Li amount (v value) in the positive electrode active material and the discharge capacity.
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001218250A JP3695366B2 (en) | 2001-03-21 | 2001-07-18 | Positive electrode active material for lithium ion secondary battery and method for producing the same |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001-80781 | 2001-03-21 | ||
| JP2001080781 | 2001-03-21 | ||
| JP2001218250A JP3695366B2 (en) | 2001-03-21 | 2001-07-18 | Positive electrode active material for lithium ion secondary battery and method for producing the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2002352802A JP2002352802A (en) | 2002-12-06 |
| JP3695366B2 true JP3695366B2 (en) | 2005-09-14 |
Family
ID=26611677
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001218250A Expired - Fee Related JP3695366B2 (en) | 2001-03-21 | 2001-07-18 | Positive electrode active material for lithium ion secondary battery and method for producing the same |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP3695366B2 (en) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4193481B2 (en) * | 2002-12-10 | 2008-12-10 | ソニー株式会社 | Positive electrode active material, method for producing the same, and nonaqueous electrolyte secondary battery |
| JP4276469B2 (en) * | 2003-05-19 | 2009-06-10 | 日本化学工業株式会社 | Method for producing lithium cobalt oxide |
| JP4502664B2 (en) * | 2004-02-24 | 2010-07-14 | 三洋電機株式会社 | Non-aqueous electrolyte secondary battery |
| KR101320391B1 (en) | 2011-07-18 | 2013-10-23 | 삼성에스디아이 주식회사 | Positive active material for lithium secondary battery, preparing method thereof, positive electrode including the same, and lithium secondary battery employing the same |
| JP5897357B2 (en) * | 2012-03-01 | 2016-03-30 | 日本化学工業株式会社 | Lithium secondary battery positive electrode active material manufacturing method, lithium secondary battery positive electrode active material, and lithium secondary battery |
| JP5897356B2 (en) * | 2012-03-01 | 2016-03-30 | 日本化学工業株式会社 | Method for producing positive electrode active material for lithium secondary battery |
| CN116759629A (en) * | 2016-07-05 | 2023-09-15 | 株式会社半导体能源研究所 | Method for producing positive electrode active material, and lithium ion secondary battery |
| US11094927B2 (en) | 2016-10-12 | 2021-08-17 | Semiconductor Energy Laboratory Co., Ltd. | Positive electrode active material particle and manufacturing method of positive electrode active material particle |
| CN112201778A (en) | 2017-05-12 | 2021-01-08 | 株式会社半导体能源研究所 | Positive electrode active material particles |
| KR102591354B1 (en) | 2017-05-19 | 2023-10-19 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material, method for manufacturing positive electrode active material, and secondary battery |
| US20200176770A1 (en) * | 2017-06-26 | 2020-06-04 | Semiconductor Energy Laboratory Co., Ltd. | Method for manufacturing positive electrode active material, and secondary battery |
| JP6885802B2 (en) * | 2017-06-26 | 2021-06-16 | 株式会社半導体エネルギー研究所 | Secondary battery |
| JP7234902B2 (en) * | 2019-11-01 | 2023-03-08 | 株式会社豊田中央研究所 | Self-supporting microporous layer |
-
2001
- 2001-07-18 JP JP2001218250A patent/JP3695366B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2002352802A (en) | 2002-12-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8066913B2 (en) | Li-Ni composite oxide particles for non-aqueous electrolyte secondary cell, process for producing the same, and non-aqueous electrolyte secondary cell | |
| JP3777988B2 (en) | Positive electrode active material for lithium secondary battery and method for producing the same | |
| JP2000077071A (en) | Nonaqueous electrolyte secondary battery | |
| JP2000182618A (en) | Positive electrode active material for lithium secondary battery | |
| JP3695366B2 (en) | Positive electrode active material for lithium ion secondary battery and method for producing the same | |
| US20150037677A1 (en) | Positive electrode active substance particles for non-aqueous electrolyte secondary batteries and process for producing the same, and non-aqueous electrolyte secondary battery | |
| JP2002124258A (en) | Lithium manganate particle powder and its manufacturing method | |
| JP3468098B2 (en) | Method for producing positive electrode active material for lithium secondary battery | |
| JP2019114454A (en) | Method of manufacturing positive electrode material for non-aqueous secondary battery | |
| JP4168609B2 (en) | Positive electrode active material for lithium ion secondary battery | |
| JP4055414B2 (en) | Positive electrode active material for lithium ion secondary battery | |
| JP3793054B2 (en) | Nonaqueous electrolyte secondary battery | |
| JP4055269B2 (en) | Manganese oxide and method for producing the same, lithium manganese composite oxide using manganese oxide, and method for producing the same | |
| JP3695365B2 (en) | Cathode active material for lithium ion secondary battery | |
| JP2004006293A (en) | Positive electrode material, its manufacturing method, and battery using it | |
| JP2003146662A (en) | Lithium-nickel-manganese complex oxide, method for manufacturing the same and use of the same | |
| JP3746099B2 (en) | Cathode active material for lithium battery and method for producing the same | |
| JP2000311675A (en) | Nonaqueous electrolyte secondary battery | |
| WO2011135953A1 (en) | Electrode active material and non-aqueous electrolyte secondary battery provided with same | |
| JPH1173960A (en) | Positive electrode active material for nonaqueous electrolyte secondary battery and the nonaquoues electrolyte secondary battery | |
| JP2002343356A (en) | Lithium manganese double oxide particle, its manufacturing method and secondary battery | |
| JPH05198300A (en) | Manufacture of nonaqueous electrolyte lithium secondary battery and positive electrode active material used for it | |
| JP2003109593A (en) | Nonaqueous electrolyte secondary battery and manufacturing method of the same | |
| JP2001196062A (en) | Lithium manganate mixture and lithium secondary battery using the same | |
| JP4240853B2 (en) | Positive electrode active material for lithium ion secondary battery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20040412 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20040803 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20040824 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20040824 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20041207 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20050607 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20050620 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 3695366 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: R3D04 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20090708 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20090708 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20090708 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20100708 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20100708 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110708 Year of fee payment: 6 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110708 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110708 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120708 Year of fee payment: 7 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120708 Year of fee payment: 7 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120708 Year of fee payment: 7 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120708 Year of fee payment: 7 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130708 Year of fee payment: 8 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130708 Year of fee payment: 8 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |