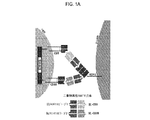
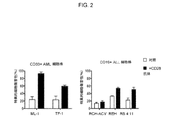
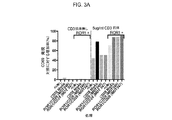
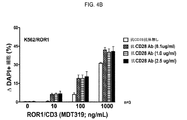
JP2019532017A - がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 - Google Patents
がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 Download PDFInfo
- Publication number
- JP2019532017A JP2019532017A JP2019501628A JP2019501628A JP2019532017A JP 2019532017 A JP2019532017 A JP 2019532017A JP 2019501628 A JP2019501628 A JP 2019501628A JP 2019501628 A JP2019501628 A JP 2019501628A JP 2019532017 A JP2019532017 A JP 2019532017A
- Authority
- JP
- Japan
- Prior art keywords
- seq
- group
- bdc
- epitopes
- cancer antigen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000027455 binding Effects 0.000 title claims abstract description 295
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 289
- 201000011510 cancer Diseases 0.000 title claims abstract description 258
- 230000001747 exhibiting effect Effects 0.000 title 1
- 239000000427 antigen Substances 0.000 claims abstract description 180
- 102000036639 antigens Human genes 0.000 claims abstract description 180
- 108091007433 antigens Proteins 0.000 claims abstract description 180
- 230000005931 immune cell recruitment Effects 0.000 claims abstract description 55
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 130
- 210000004027 cell Anatomy 0.000 claims description 116
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 108
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 108
- 101001103039 Homo sapiens Inactive tyrosine-protein kinase transmembrane receptor ROR1 Proteins 0.000 claims description 89
- 101001103036 Homo sapiens Nuclear receptor ROR-alpha Proteins 0.000 claims description 89
- 102100039615 Inactive tyrosine-protein kinase transmembrane receptor ROR1 Human genes 0.000 claims description 89
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 claims description 76
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 claims description 76
- 108090000623 proteins and genes Proteins 0.000 claims description 65
- 238000000034 method Methods 0.000 claims description 56
- 239000000203 mixture Substances 0.000 claims description 56
- 102000004169 proteins and genes Human genes 0.000 claims description 56
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 claims description 51
- 102100024952 Protein CBFA2T1 Human genes 0.000 claims description 51
- 108010074708 B7-H1 Antigen Proteins 0.000 claims description 49
- 238000011282 treatment Methods 0.000 claims description 47
- 210000002865 immune cell Anatomy 0.000 claims description 46
- 230000008685 targeting Effects 0.000 claims description 33
- -1 MUC-CD Proteins 0.000 claims description 26
- 230000003213 activating effect Effects 0.000 claims description 26
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims description 24
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims description 24
- 101000998120 Homo sapiens Interleukin-3 receptor subunit alpha Proteins 0.000 claims description 22
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 claims description 22
- 102100033493 Interleukin-3 receptor subunit alpha Human genes 0.000 claims description 22
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 22
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 claims description 20
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 20
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 20
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 claims description 18
- 230000001225 therapeutic effect Effects 0.000 claims description 18
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 17
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 17
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 claims description 16
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 claims description 16
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 claims description 16
- 230000028993 immune response Effects 0.000 claims description 16
- 230000003252 repetitive effect Effects 0.000 claims description 16
- 210000002540 macrophage Anatomy 0.000 claims description 14
- 238000012544 monitoring process Methods 0.000 claims description 14
- 230000001629 suppression Effects 0.000 claims description 14
- 102100037589 OX-2 membrane glycoprotein Human genes 0.000 claims description 12
- 101001098352 Homo sapiens OX-2 membrane glycoprotein Proteins 0.000 claims description 11
- 239000012190 activator Substances 0.000 claims description 10
- 210000000822 natural killer cell Anatomy 0.000 claims description 10
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 claims description 8
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 claims description 8
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 8
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 8
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 claims description 8
- 101150013553 CD40 gene Proteins 0.000 claims description 7
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 claims description 7
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 claims description 7
- 102000003735 Mesothelin Human genes 0.000 claims description 7
- 108090000015 Mesothelin Proteins 0.000 claims description 7
- 102100022748 Wilms tumor protein Human genes 0.000 claims description 7
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 claims description 7
- 230000004936 stimulating effect Effects 0.000 claims description 6
- 101000621309 Homo sapiens Wilms tumor protein Proteins 0.000 claims description 5
- 206010062016 Immunosuppression Diseases 0.000 claims description 5
- 230000001506 immunosuppresive effect Effects 0.000 claims description 5
- 102100038080 B-cell receptor CD22 Human genes 0.000 claims description 4
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 claims description 4
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 claims description 4
- 101001109503 Homo sapiens NKG2-C type II integral membrane protein Proteins 0.000 claims description 4
- 101001136592 Homo sapiens Prostate stem cell antigen Proteins 0.000 claims description 4
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 claims description 4
- 102100022683 NKG2-C type II integral membrane protein Human genes 0.000 claims description 4
- 102100036735 Prostate stem cell antigen Human genes 0.000 claims description 4
- 101001051488 Takifugu rubripes Neural cell adhesion molecule L1 Proteins 0.000 claims description 4
- 102000006815 folate receptor Human genes 0.000 claims description 4
- 108020005243 folate receptor Proteins 0.000 claims description 4
- 229960004641 rituximab Drugs 0.000 claims description 4
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 claims description 3
- 102100027207 CD27 antigen Human genes 0.000 claims description 3
- 102100038078 CD276 antigen Human genes 0.000 claims description 3
- 102100035793 CD83 antigen Human genes 0.000 claims description 3
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 claims description 3
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 claims description 3
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 claims description 3
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 claims description 3
- 101000914496 Homo sapiens T-cell antigen CD7 Proteins 0.000 claims description 3
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 3
- 102000017578 LAG3 Human genes 0.000 claims description 3
- 102100027208 T-cell antigen CD7 Human genes 0.000 claims description 3
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 claims description 3
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 3
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 3
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 3
- 229960002450 ofatumumab Drugs 0.000 claims description 3
- 230000000638 stimulation Effects 0.000 claims description 3
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 claims description 2
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 claims description 2
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 claims description 2
- 108700012439 CA9 Proteins 0.000 claims description 2
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 claims description 2
- 101000914324 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 5 Proteins 0.000 claims description 2
- 101000914321 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 7 Proteins 0.000 claims description 2
- 101000617725 Homo sapiens Pregnancy-specific beta-1-glycoprotein 2 Proteins 0.000 claims description 2
- 101000610551 Homo sapiens Prominin-1 Proteins 0.000 claims description 2
- 101000884271 Homo sapiens Signal transducer CD24 Proteins 0.000 claims description 2
- 102100022019 Pregnancy-specific beta-1-glycoprotein 2 Human genes 0.000 claims description 2
- 102100040120 Prominin-1 Human genes 0.000 claims description 2
- 102100038081 Signal transducer CD24 Human genes 0.000 claims description 2
- 108700020467 WT1 Proteins 0.000 claims description 2
- 101150084041 WT1 gene Proteins 0.000 claims description 2
- 229940022353 herceptin Drugs 0.000 claims description 2
- 229940126530 T cell activator Drugs 0.000 claims 2
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 claims 2
- 102100023990 60S ribosomal protein L17 Human genes 0.000 claims 1
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 claims 1
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 claims 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 claims 1
- 102100025390 Integrin beta-2 Human genes 0.000 claims 1
- 102000040856 WT1 Human genes 0.000 claims 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 67
- 235000018102 proteins Nutrition 0.000 description 53
- 230000006044 T cell activation Effects 0.000 description 38
- 150000001413 amino acids Chemical group 0.000 description 37
- 235000001014 amino acid Nutrition 0.000 description 33
- 229940024606 amino acid Drugs 0.000 description 23
- 230000014509 gene expression Effects 0.000 description 22
- 230000000694 effects Effects 0.000 description 20
- 238000006467 substitution reaction Methods 0.000 description 19
- 108010007707 Hepatitis A Virus Cellular Receptor 2 Proteins 0.000 description 16
- 230000004913 activation Effects 0.000 description 16
- 231100000135 cytotoxicity Toxicity 0.000 description 15
- 230000003013 cytotoxicity Effects 0.000 description 15
- 150000003839 salts Chemical class 0.000 description 15
- 229920001223 polyethylene glycol Polymers 0.000 description 14
- 102100020862 Lymphocyte activation gene 3 protein Human genes 0.000 description 13
- 101710092458 Lymphocyte activation gene 3 protein Proteins 0.000 description 13
- 108091008874 T cell receptors Proteins 0.000 description 13
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 13
- 238000013459 approach Methods 0.000 description 13
- 230000006690 co-activation Effects 0.000 description 13
- 239000003814 drug Substances 0.000 description 13
- 210000004881 tumor cell Anatomy 0.000 description 13
- 239000002202 Polyethylene glycol Substances 0.000 description 12
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical group [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 12
- 239000000872 buffer Substances 0.000 description 12
- 108020004414 DNA Proteins 0.000 description 11
- 239000012634 fragment Substances 0.000 description 11
- 238000004519 manufacturing process Methods 0.000 description 11
- 230000008093 supporting effect Effects 0.000 description 11
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 10
- 102000005962 receptors Human genes 0.000 description 10
- 108020003175 receptors Proteins 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 9
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 9
- 230000008901 benefit Effects 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 150000007523 nucleic acids Chemical class 0.000 description 8
- 230000002829 reductive effect Effects 0.000 description 8
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 7
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 7
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 7
- 230000008859 change Effects 0.000 description 7
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 7
- 235000014304 histidine Nutrition 0.000 description 7
- 229960002885 histidine Drugs 0.000 description 7
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 230000011664 signaling Effects 0.000 description 7
- 238000001890 transfection Methods 0.000 description 7
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 6
- 230000000139 costimulatory effect Effects 0.000 description 6
- 230000001461 cytolytic effect Effects 0.000 description 6
- 230000001086 cytosolic effect Effects 0.000 description 6
- 238000012217 deletion Methods 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 230000002163 immunogen Effects 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000003381 stabilizer Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 5
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 5
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 5
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 5
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 5
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 5
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 5
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 5
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 5
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 210000004899 c-terminal region Anatomy 0.000 description 5
- 239000000833 heterodimer Substances 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 230000006320 pegylation Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- 238000011160 research Methods 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 4
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- 102100031351 Galectin-9 Human genes 0.000 description 4
- 101710121810 Galectin-9 Proteins 0.000 description 4
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 4
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 4
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 229930195725 Mannitol Natural products 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 4
- 239000000443 aerosol Substances 0.000 description 4
- 125000001931 aliphatic group Chemical group 0.000 description 4
- 230000001093 anti-cancer Effects 0.000 description 4
- 229960003008 blinatumomab Drugs 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 238000001802 infusion Methods 0.000 description 4
- 238000001361 intraarterial administration Methods 0.000 description 4
- 238000007918 intramuscular administration Methods 0.000 description 4
- 238000007912 intraperitoneal administration Methods 0.000 description 4
- 238000007913 intrathecal administration Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 210000000265 leukocyte Anatomy 0.000 description 4
- 239000000594 mannitol Substances 0.000 description 4
- 235000010355 mannitol Nutrition 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 description 4
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 4
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000011321 prophylaxis Methods 0.000 description 4
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 150000005846 sugar alcohols Chemical class 0.000 description 4
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 238000013268 sustained release Methods 0.000 description 4
- 239000012730 sustained-release form Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 206010000830 Acute leukaemia Diseases 0.000 description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- 102000004082 Calreticulin Human genes 0.000 description 3
- 108090000549 Calreticulin Proteins 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 3
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 3
- 229920002261 Corn starch Polymers 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 3
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 3
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 3
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 3
- 102000003960 Ligases Human genes 0.000 description 3
- 108090000364 Ligases Proteins 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 108091008043 NK cell inhibitory receptors Proteins 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 206010057249 Phagocytosis Diseases 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 3
- 108091008035 T cell costimulatory receptors Proteins 0.000 description 3
- 102100033117 Toll-like receptor 9 Human genes 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 229940124691 antibody therapeutics Drugs 0.000 description 3
- 102000025171 antigen binding proteins Human genes 0.000 description 3
- 108091000831 antigen binding proteins Proteins 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 229960003121 arginine Drugs 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 230000005812 autoimmune toxicity Effects 0.000 description 3
- 231100001152 autoimmune toxicity Toxicity 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 239000004067 bulking agent Substances 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 239000002738 chelating agent Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000008120 corn starch Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 229940014259 gelatin Drugs 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 229960002449 glycine Drugs 0.000 description 3
- 229940093915 gynecological organic acid Drugs 0.000 description 3
- 210000002443 helper t lymphocyte Anatomy 0.000 description 3
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 230000005934 immune activation Effects 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000000099 in vitro assay Methods 0.000 description 3
- 230000015788 innate immune response Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 230000002601 intratumoral effect Effects 0.000 description 3
- 229960003646 lysine Drugs 0.000 description 3
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000008782 phagocytosis Effects 0.000 description 3
- 102000054765 polymorphisms of proteins Human genes 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 229950008882 polysorbate Drugs 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- 238000001542 size-exclusion chromatography Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 239000011593 sulfur Substances 0.000 description 3
- 229910052717 sulfur Inorganic materials 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 239000013598 vector Substances 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- 108010032595 Antibody Binding Sites Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 102100040839 C-type lectin domain family 6 member A Human genes 0.000 description 2
- 101710125370 C-type lectin domain family 6 member A Proteins 0.000 description 2
- 102100040840 C-type lectin domain family 7 member A Human genes 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 102000000529 Costimulatory and Inhibitory T-Cell Receptors Human genes 0.000 description 2
- 108010041504 Costimulatory and Inhibitory T-Cell Receptors Proteins 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 206010014733 Endometrial cancer Diseases 0.000 description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 2
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 2
- 101001027081 Homo sapiens Killer cell immunoglobulin-like receptor 2DL1 Proteins 0.000 description 2
- 101000945371 Homo sapiens Killer cell immunoglobulin-like receptor 2DL2 Proteins 0.000 description 2
- 101000945331 Homo sapiens Killer cell immunoglobulin-like receptor 2DL4 Proteins 0.000 description 2
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 2
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 2
- 101000934372 Homo sapiens Macrosialin Proteins 0.000 description 2
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 2
- 101000589305 Homo sapiens Natural cytotoxicity triggering receptor 2 Proteins 0.000 description 2
- 101000763579 Homo sapiens Toll-like receptor 1 Proteins 0.000 description 2
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 2
- 101000669460 Homo sapiens Toll-like receptor 5 Proteins 0.000 description 2
- 101000669406 Homo sapiens Toll-like receptor 6 Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 102100022338 Integrin alpha-M Human genes 0.000 description 2
- 102100037363 Killer cell immunoglobulin-like receptor 2DL1 Human genes 0.000 description 2
- 102100033599 Killer cell immunoglobulin-like receptor 2DL2 Human genes 0.000 description 2
- 102100033633 Killer cell immunoglobulin-like receptor 2DL4 Human genes 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 2
- 102100025136 Macrosialin Human genes 0.000 description 2
- 206010027406 Mesothelioma Diseases 0.000 description 2
- 230000006051 NK cell activation Effects 0.000 description 2
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 2
- 102100032851 Natural cytotoxicity triggering receptor 2 Human genes 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 102100025831 Scavenger receptor cysteine-rich type 1 protein M130 Human genes 0.000 description 2
- 208000003837 Second Primary Neoplasms Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000020385 T cell costimulation Effects 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- 108010060818 Toll-Like Receptor 9 Proteins 0.000 description 2
- 102000002689 Toll-like receptor Human genes 0.000 description 2
- 108020000411 Toll-like receptor Proteins 0.000 description 2
- 102100027010 Toll-like receptor 1 Human genes 0.000 description 2
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 2
- 102100039357 Toll-like receptor 5 Human genes 0.000 description 2
- 102100039387 Toll-like receptor 6 Human genes 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 229960003767 alanine Drugs 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000036436 anti-hiv Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 239000000305 astragalus gummifer gum Substances 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N biotin Natural products N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 201000003232 brachydactyly type C Diseases 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 230000005880 cancer cell killing Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 238000002659 cell therapy Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003638 chemical reducing agent Substances 0.000 description 2
- 235000013330 chicken meat Nutrition 0.000 description 2
- 208000011654 childhood malignant neoplasm Diseases 0.000 description 2
- 201000010276 collecting duct carcinoma Diseases 0.000 description 2
- 230000004154 complement system Effects 0.000 description 2
- 239000006184 cosolvent Substances 0.000 description 2
- 206010052015 cytokine release syndrome Diseases 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 108010025838 dectin 1 Proteins 0.000 description 2
- 239000003405 delayed action preparation Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- MTZQAGJQAFMTAQ-UHFFFAOYSA-N ethyl benzoate Chemical compound CCOC(=O)C1=CC=CC=C1 MTZQAGJQAFMTAQ-UHFFFAOYSA-N 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- 230000037406 food intake Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 235000004554 glutamine Nutrition 0.000 description 2
- 229960002743 glutamine Drugs 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 201000005787 hematologic cancer Diseases 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 230000006054 immunological memory Effects 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 229960003136 leucine Drugs 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 229920001427 mPEG Polymers 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 235000006109 methionine Nutrition 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 2
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 2
- 229960002216 methylparaben Drugs 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- PJUIMOJAAPLTRJ-UHFFFAOYSA-N monothioglycerol Chemical compound OCC(O)CS PJUIMOJAAPLTRJ-UHFFFAOYSA-N 0.000 description 2
- DAZSWUUAFHBCGE-KRWDZBQOSA-N n-[(2s)-3-methyl-1-oxo-1-pyrrolidin-1-ylbutan-2-yl]-3-phenylpropanamide Chemical compound N([C@@H](C(C)C)C(=O)N1CCCC1)C(=O)CCC1=CC=CC=C1 DAZSWUUAFHBCGE-KRWDZBQOSA-N 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 108091027963 non-coding RNA Proteins 0.000 description 2
- 102000042567 non-coding RNA Human genes 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- 229920001983 poloxamer Polymers 0.000 description 2
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 2
- 238000003752 polymerase chain reaction Methods 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 2
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 2
- 229960003415 propylparaben Drugs 0.000 description 2
- 238000001742 protein purification Methods 0.000 description 2
- 210000003289 regulatory T cell Anatomy 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 210000003296 saliva Anatomy 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000001797 sucrose acetate isobutyrate Substances 0.000 description 2
- 235000010983 sucrose acetate isobutyrate Nutrition 0.000 description 2
- UVGUPMLLGBCFEJ-SWTLDUCYSA-N sucrose acetate isobutyrate Chemical compound CC(C)C(=O)O[C@H]1[C@H](OC(=O)C(C)C)[C@@H](COC(=O)C(C)C)O[C@@]1(COC(C)=O)O[C@@H]1[C@H](OC(=O)C(C)C)[C@@H](OC(=O)C(C)C)[C@H](OC(=O)C(C)C)[C@@H](COC(C)=O)O1 UVGUPMLLGBCFEJ-SWTLDUCYSA-N 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 229940074410 trehalose Drugs 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 239000000811 xylitol Substances 0.000 description 2
- 235000010447 xylitol Nutrition 0.000 description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 2
- 229960002675 xylitol Drugs 0.000 description 2
- ZJIFDEVVTPEXDL-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) hydrogen carbonate Chemical compound OC(=O)ON1C(=O)CCC1=O ZJIFDEVVTPEXDL-UHFFFAOYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- AGBQKNBQESQNJD-SSDOTTSWSA-N (R)-lipoic acid Chemical compound OC(=O)CCCC[C@@H]1CCSS1 AGBQKNBQESQNJD-SSDOTTSWSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- HNSDLXPSAYFUHK-UHFFFAOYSA-N 1,4-bis(2-ethylhexyl) sulfosuccinate Chemical compound CCCCC(CC)COC(=O)CC(S(O)(=O)=O)C(=O)OCC(CC)CCCC HNSDLXPSAYFUHK-UHFFFAOYSA-N 0.000 description 1
- VOJUXHHACRXLTD-UHFFFAOYSA-N 1,4-dihydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC(O)=C21 VOJUXHHACRXLTD-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- HTCSFFGLRQDZDE-UHFFFAOYSA-N 2-azaniumyl-2-phenylpropanoate Chemical compound OC(=O)C(N)(C)C1=CC=CC=C1 HTCSFFGLRQDZDE-UHFFFAOYSA-N 0.000 description 1
- HZLCGUXUOFWCCN-UHFFFAOYSA-N 2-hydroxynonadecane-1,2,3-tricarboxylic acid Chemical compound CCCCCCCCCCCCCCCCC(C(O)=O)C(O)(C(O)=O)CC(O)=O HZLCGUXUOFWCCN-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- QXZGLTYKKZKGLN-UHFFFAOYSA-N 4-(2,5-dioxopyrrolidin-1-yl)oxy-4-oxobutanoic acid Chemical compound OC(=O)CCC(=O)ON1C(=O)CCC1=O QXZGLTYKKZKGLN-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 244000303258 Annona diversifolia Species 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 102100027205 B-cell antigen receptor complex-associated protein alpha chain Human genes 0.000 description 1
- 101710095183 B-cell antigen receptor complex-associated protein alpha chain Proteins 0.000 description 1
- 102100027203 B-cell antigen receptor complex-associated protein beta chain Human genes 0.000 description 1
- 101710166261 B-cell antigen receptor complex-associated protein beta chain Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 102100021992 CD209 antigen Human genes 0.000 description 1
- 102100038077 CD226 antigen Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010058019 Cancer Pain Diseases 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 108010077544 Chromatin Proteins 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 108700010070 Codon Usage Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102000000989 Complement System Proteins Human genes 0.000 description 1
- 108010069112 Complement System Proteins Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000938605 Crocodylia Species 0.000 description 1
- HEBKCHPVOIAQTA-QWWZWVQMSA-N D-arabinitol Chemical compound OC[C@@H](O)C(O)[C@H](O)CO HEBKCHPVOIAQTA-QWWZWVQMSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 108010008165 Etanercept Proteins 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- 108010040721 Flagellin Proteins 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 1
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 1
- 108010093488 His-His-His-His-His-His Proteins 0.000 description 1
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000884298 Homo sapiens CD226 antigen Proteins 0.000 description 1
- 101100166600 Homo sapiens CD28 gene Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101000945340 Homo sapiens Killer cell immunoglobulin-like receptor 2DS1 Proteins 0.000 description 1
- 101000945339 Homo sapiens Killer cell immunoglobulin-like receptor 2DS2 Proteins 0.000 description 1
- 101000945351 Homo sapiens Killer cell immunoglobulin-like receptor 3DL1 Proteins 0.000 description 1
- 101000945492 Homo sapiens Killer cell immunoglobulin-like receptor 3DS1 Proteins 0.000 description 1
- 101000971538 Homo sapiens Killer cell lectin-like receptor subfamily F member 1 Proteins 0.000 description 1
- 101000971533 Homo sapiens Killer cell lectin-like receptor subfamily G member 1 Proteins 0.000 description 1
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 1
- 101000979342 Homo sapiens Nuclear factor NF-kappa-B p105 subunit Proteins 0.000 description 1
- 101000638251 Homo sapiens Tumor necrosis factor ligand superfamily member 9 Proteins 0.000 description 1
- 238000012450 HuMAb Mouse Methods 0.000 description 1
- 101001057750 Human cytomegalovirus (strain AD169) Uncharacterized protein IRL2 Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 102100035678 Interferon gamma receptor 1 Human genes 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- 101150069255 KLRC1 gene Proteins 0.000 description 1
- 101150074862 KLRC3 gene Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 102100033631 Killer cell immunoglobulin-like receptor 2DS1 Human genes 0.000 description 1
- 102100033630 Killer cell immunoglobulin-like receptor 2DS2 Human genes 0.000 description 1
- 102100033627 Killer cell immunoglobulin-like receptor 3DL1 Human genes 0.000 description 1
- 102100034833 Killer cell immunoglobulin-like receptor 3DS1 Human genes 0.000 description 1
- 102100021458 Killer cell lectin-like receptor subfamily F member 1 Human genes 0.000 description 1
- 102100021457 Killer cell lectin-like receptor subfamily G member 1 Human genes 0.000 description 1
- 238000012449 Kunming mouse Methods 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- 239000004395 L-leucine Substances 0.000 description 1
- 235000019454 L-leucine Nutrition 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 description 1
- 102100025354 Macrophage mannose receptor 1 Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 208000003445 Mouth Neoplasms Diseases 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- CBQJSKKFNMDLON-JTQLQIEISA-N N-acetyl-L-phenylalanine Chemical group CC(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 CBQJSKKFNMDLON-JTQLQIEISA-N 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 108091008877 NK cell receptors Proteins 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 102100022701 NKG2-E type II integral membrane protein Human genes 0.000 description 1
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 1
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 1
- 102100021462 Natural killer cells antigen CD94 Human genes 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 1
- 101710187081 OX-2 membrane glycoprotein Proteins 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 108010067902 Peptide Library Proteins 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- JVWLUVNSQYXYBE-UHFFFAOYSA-N Ribitol Natural products OCC(C)C(O)C(O)CO JVWLUVNSQYXYBE-UHFFFAOYSA-N 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 201000010208 Seminoma Diseases 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108010029180 Sialic Acid Binding Ig-like Lectin 3 Proteins 0.000 description 1
- 102000001555 Sialic Acid Binding Ig-like Lectin 3 Human genes 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- UQZIYBXSHAGNOE-USOSMYMVSA-N Stachyose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](CO[C@@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H](CO)O2)O1 UQZIYBXSHAGNOE-USOSMYMVSA-N 0.000 description 1
- 108091081024 Start codon Proteins 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 238000012338 Therapeutic targeting Methods 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100032101 Tumor necrosis factor ligand superfamily member 9 Human genes 0.000 description 1
- 101710165474 Tumor necrosis factor receptor superfamily member 5 Proteins 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 229960003697 abatacept Drugs 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000033289 adaptive immune response Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 201000005188 adrenal gland cancer Diseases 0.000 description 1
- 208000024447 adrenal gland neoplasm Diseases 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940040563 agaric acid Drugs 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- AGBQKNBQESQNJD-UHFFFAOYSA-N alpha-Lipoic acid Natural products OC(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 230000001745 anti-biotin effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 238000002617 apheresis Methods 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229960001716 benzalkonium Drugs 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000007413 biotinylation Methods 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000009702 cancer cell proliferation Effects 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229920006317 cationic polymer Polymers 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000007958 cherry flavor Substances 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 210000003483 chromatin Anatomy 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 238000010835 comparative analysis Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 229940099112 cornstarch Drugs 0.000 description 1
- 108091008034 costimulatory receptors Proteins 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 150000003999 cyclitols Chemical class 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 210000005220 cytoplasmic tail Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000012350 deep sequencing Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 239000000412 dendrimer Substances 0.000 description 1
- 229920000736 dendritic polymer Polymers 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 229940043237 diethanolamine Drugs 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000000375 direct analysis in real time Methods 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 229960000878 docusate sodium Drugs 0.000 description 1
- 231100000276 dose-dependent cytotoxicity Toxicity 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000012063 dual-affinity re-targeting Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 229940073621 enbrel Drugs 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- FBPFZTCFMRRESA-GUCUJZIJSA-N galactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-GUCUJZIJSA-N 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 230000008073 immune recognition Effects 0.000 description 1
- 230000037189 immune system physiology Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000012482 interaction analysis Methods 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 201000004962 larynx cancer Diseases 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 1
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 1
- 150000002632 lipids Chemical group 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 238000011528 liquid biopsy Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 201000010453 lymph node cancer Diseases 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000007898 magnetic cell sorting Methods 0.000 description 1
- 125000005439 maleimidyl group Chemical group C1(C=CC(N1*)=O)=O 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 125000000311 mannosyl group Chemical group C1([C@@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229940100630 metacresol Drugs 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000002297 mitogenic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000005170 neoplastic cell Anatomy 0.000 description 1
- 230000000802 nitrating effect Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229960003301 nivolumab Drugs 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 230000005868 ontogenesis Effects 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000007968 orange flavor Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229940065514 poly(lactide) Drugs 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 108091033319 polynucleotide Chemical group 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Chemical group 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229940116317 potato starch Drugs 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000004845 protein aggregation Effects 0.000 description 1
- 230000004853 protein function Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000001698 pyrogenic effect Effects 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- HEBKCHPVOIAQTA-ZXFHETKHSA-N ribitol Chemical compound OC[C@H](O)[C@H](O)[C@H](O)CO HEBKCHPVOIAQTA-ZXFHETKHSA-N 0.000 description 1
- 229940100486 rice starch Drugs 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- UQZIYBXSHAGNOE-XNSRJBNMSA-N stachyose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@@H]3[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O3)O)O2)O)O1 UQZIYBXSHAGNOE-XNSRJBNMSA-N 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000008362 succinate buffer Substances 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 229940035024 thioglycerol Drugs 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical class CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
- 150000004043 trisaccharides Chemical class 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 229940045136 urea Drugs 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 201000011531 vascular cancer Diseases 0.000 description 1
- 206010055031 vascular neoplasm Diseases 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
- 239000009637 wintergreen oil Substances 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2815—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2866—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for cytokines, lymphokines, interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/626—Diabody or triabody
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662362397P | 2016-07-14 | 2016-07-14 | |
| US62/362,397 | 2016-07-14 | ||
| US201762480230P | 2017-03-31 | 2017-03-31 | |
| US62/480,230 | 2017-03-31 | ||
| PCT/US2017/042264 WO2018014001A1 (en) | 2016-07-14 | 2017-07-14 | Multiple bi-specific binding domain constructs with different epitope binding to treat cancer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2019532017A true JP2019532017A (ja) | 2019-11-07 |
| JP2019532017A5 JP2019532017A5 (enExample) | 2020-08-20 |
Family
ID=60952209
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019501628A Withdrawn JP2019532017A (ja) | 2016-07-14 | 2017-07-14 | がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190233534A1 (enExample) |
| EP (1) | EP3484516A4 (enExample) |
| JP (1) | JP2019532017A (enExample) |
| AU (1) | AU2017297603A1 (enExample) |
| CA (1) | CA3030841A1 (enExample) |
| WO (1) | WO2018014001A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023519932A (ja) * | 2020-03-31 | 2023-05-15 | フレッド ハッチンソン キャンサー センター | 抗cd33抗体及びその使用 |
| JP2023520399A (ja) * | 2020-03-31 | 2023-05-17 | フレッド ハッチンソン キャンサー センター | ヒト抗cd33抗体及びその使用 |
| JP2023548443A (ja) * | 2020-10-30 | 2023-11-16 | ジャナックス セラピューティクス,インク. | Cd28およびpd-l1を標的とする多特異性抗体ならびにその使用方法 |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018218207A1 (en) * | 2017-05-26 | 2018-11-29 | Fred Hutchinson Cancer Research Center | Anti-cd33 antibodies and uses thereof |
| MA52970A (fr) * | 2018-06-21 | 2021-04-28 | Regeneron Pharma | Anticorps anti-psma x anti-cd28 bispécifiques et leurs utilisations |
| US11608376B2 (en) | 2018-12-21 | 2023-03-21 | Hoffmann-La Roche Inc. | Tumor-targeted agonistic CD28 antigen binding molecules |
| CN109485734B (zh) * | 2018-12-30 | 2020-05-12 | 广州百暨基因科技有限公司 | 一种靶向bcma和cd19的双特异性嵌合抗原受体及其应用 |
| WO2020150513A1 (en) * | 2019-01-17 | 2020-07-23 | Fred Hutchinson Cancer Research Center | Methods to enhance the selectivity and effectiveness of cancer treatments |
| MX2021012386A (es) * | 2019-04-09 | 2022-01-18 | Sanofi Sa | Proteínas de union triespecíficas, métodos y usos de las mismas. |
| US11613576B2 (en) | 2019-04-09 | 2023-03-28 | Sanofi | Trispecific binding proteins, methods, and uses thereof |
| JP7137696B2 (ja) | 2019-05-14 | 2022-09-14 | プロヴェンション・バイオ・インコーポレイテッド | 1型糖尿病を予防するための方法および組成物 |
| CN120775051A (zh) | 2019-05-21 | 2025-10-14 | 诺华股份有限公司 | Cd19结合分子及其用途 |
| US20220251192A1 (en) * | 2019-06-26 | 2022-08-11 | Memorial Sloan Kettering Cancer Center | Anti-cd33 antibodies for treating cancer |
| KR20220042131A (ko) * | 2019-06-27 | 2022-04-04 | 베르조 테라퓨틱스, 아이엔씨. | 골수성 세포 염증성 표현형을 조절하기 위한 항-lrrc25 조성물 및 방법, 그리고 이의 용도 |
| US20230151094A1 (en) * | 2020-03-31 | 2023-05-18 | Fred Hutchinson Cancer Center | Chimeric antigen receptors targeting cd33 |
| KR20230092863A (ko) | 2020-06-11 | 2023-06-26 | 프로벤션 바이오, 인코포레이티드 | 제1형 당뇨병을 예방하기 위한 방법 및 조성물 |
| US11919958B2 (en) | 2020-08-19 | 2024-03-05 | Xencor, Inc. | Anti-CD28 compositions |
| PE20250740A1 (es) * | 2021-02-02 | 2025-03-13 | Numab Therapeutics AG | Anticuerpos multiespecificos con especificidad para ror1 y cd3 |
| CN119110809A (zh) | 2022-02-23 | 2024-12-10 | Xencor股份有限公司 | 抗CD28 x抗PSMA抗体 |
Family Cites Families (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| US5571894A (en) | 1991-02-05 | 1996-11-05 | Ciba-Geigy Corporation | Recombinant antibodies specific for a growth factor receptor |
| WO1992016221A1 (en) | 1991-03-15 | 1992-10-01 | Synergen, Inc. | Pegylation of polypeptides |
| GB9114948D0 (en) | 1991-07-11 | 1991-08-28 | Pfizer Ltd | Process for preparing sertraline intermediates |
| FI941572A7 (fi) | 1991-10-07 | 1994-05-27 | Oncologix Inc | Anti-erbB-2-monoklonaalisten vasta-aineiden yhdistelmä ja käyttömenete lmä |
| ATE503496T1 (de) | 1992-02-06 | 2011-04-15 | Novartis Vaccines & Diagnostic | Biosynthetisches bindeprotein für tumormarker |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| HUP0301208A2 (hu) | 1999-02-12 | 2003-08-28 | Genetics Institute, Llc | B7-molekulákkal reagáló humanizált immunglobulin és az ezekkel végzett kezelési eljárások |
| CN1371416B (zh) | 1999-08-24 | 2012-10-10 | 梅达里克斯公司 | 人ctla-4抗体及其应用 |
| WO2001054732A1 (en) | 2000-01-27 | 2001-08-02 | Genetics Institute, Llc. | Antibodies against ctla4 (cd152), conjugates comprising same, and uses thereof |
| EP1354600A1 (en) | 2002-04-19 | 2003-10-22 | Affimed Therapeutics AG | Antibody combination useful for tumor therapy |
| EP1639013B1 (en) | 2003-07-02 | 2012-09-12 | Innate Pharma | Pan-kir2dl NK-receptor antibodies and their use in diagnostic and therapy |
| US7288638B2 (en) | 2003-10-10 | 2007-10-30 | Bristol-Myers Squibb Company | Fully human antibodies against human 4-1BB |
| DK1791865T3 (da) | 2004-06-29 | 2010-11-01 | Immunocore Ltd | Celler der udtrykker en modificeret T-cellerecptor |
| EP1752471B9 (en) | 2005-01-05 | 2009-04-15 | f-star Biotechnologische Forschungs- und Entwicklungsges.m.b.H. | Synthetic immunoglobulin domains with binding properties engineered in regions of the molecule different from the complementarity determining regions |
| AU2006244885B2 (en) | 2005-05-09 | 2011-03-31 | E. R. Squibb & Sons, L.L.C. | Human monoclonal antibodies to programmed death 1(PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| WO2008103849A2 (en) | 2007-02-21 | 2008-08-28 | The Regents Of The University Of California | Methods and compounds for lymphoma cell detection and isolation |
| EP1829895A1 (en) | 2006-03-03 | 2007-09-05 | f-star Biotechnologische Forschungs- und Entwicklungsges.m.b.H. | Bispecific molecule binding TLR9 and CD32 and comprising a T cell epitope for treatment of allergies |
| EA200970477A1 (ru) | 2006-11-15 | 2009-12-30 | Медарекс, Инк. | Человеческие моноклональные антитела к btla и способы применения |
| WO2008076868A2 (en) | 2006-12-18 | 2008-06-26 | Abbott Laboratories | Methods and compositions related to modulation of receptor tyrosine kinase orphan receptor-1 (ror-1) |
| US8163279B2 (en) | 2007-04-13 | 2012-04-24 | Stemline Therapeutics, Inc. | IL3Rα antibody conjugates and uses thereof |
| US8242247B2 (en) | 2007-12-21 | 2012-08-14 | Hoffmann-La Roche Inc. | Bivalent, bispecific antibodies |
| US9266967B2 (en) | 2007-12-21 | 2016-02-23 | Hoffmann-La Roche, Inc. | Bivalent, bispecific antibodies |
| US8227577B2 (en) | 2007-12-21 | 2012-07-24 | Hoffman-La Roche Inc. | Bivalent, bispecific antibodies |
| US20090162359A1 (en) | 2007-12-21 | 2009-06-25 | Christian Klein | Bivalent, bispecific antibodies |
| WO2010008069A1 (ja) | 2008-07-18 | 2010-01-21 | 国立大学法人名古屋大学 | 細胞増殖阻害剤 |
| PL2342226T3 (pl) | 2008-09-26 | 2017-01-31 | Dana-Farber Cancer Institute, Inc. | Ludzkie przeciwciała anty-PD-1, PD-L1 i PD-L2 oraz ich zastosowania |
| US8709411B2 (en) | 2008-12-05 | 2014-04-29 | Novo Nordisk A/S | Combination therapy to enhance NK cell mediated cytotoxicity |
| PT2376535T (pt) | 2008-12-09 | 2017-06-23 | Hoffmann La Roche | Anticorpos anti-pd-l1 e a sua utilização para a melhoria do funcionamento das células t |
| SG175004A1 (en) | 2009-04-02 | 2011-11-28 | Roche Glycart Ag | Multispecific antibodies comprising full length antibodies and single chain fab fragments |
| DK2417156T3 (en) | 2009-04-07 | 2015-03-02 | Roche Glycart Ag | Trivalent, bispecific antibodies |
| EP2421899B1 (en) | 2009-04-23 | 2016-06-08 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Anti-human ror1 antibodies |
| AU2010252284A1 (en) | 2009-05-27 | 2011-11-17 | F. Hoffmann-La Roche Ag | Tri- or tetraspecific antibodies |
| US9676845B2 (en) | 2009-06-16 | 2017-06-13 | Hoffmann-La Roche, Inc. | Bispecific antigen binding proteins |
| US8703132B2 (en) | 2009-06-18 | 2014-04-22 | Hoffmann-La Roche, Inc. | Bispecific, tetravalent antigen binding proteins |
| WO2011054007A1 (en) | 2009-11-02 | 2011-05-05 | Oxford Biotherapeutics Ltd. | Ror1 as therapeutic and diagnostic target |
| ES2635316T3 (es) | 2009-12-18 | 2017-10-03 | Kancera Ab | Anticuerpos contra ROR1 que pueden inducir muerte celular de LLC |
| US20110165161A1 (en) * | 2009-12-23 | 2011-07-07 | Shih-Yao Lin | Anti-epcam antibodies that induce apoptosis of cancer cells and methods using same |
| WO2011100538A1 (en) | 2010-02-11 | 2011-08-18 | Alexion Pharmaceuticals, Inc. | Therapeutic methods using an ti-cd200 antibodies |
| US9242014B2 (en) | 2010-06-15 | 2016-01-26 | The Regents Of The University Of California | Receptor tyrosine kinase-like orphan receptor 1 (ROR1) single chain Fv antibody fragment conjugates and methods of use thereof |
| EP3219731A1 (en) | 2010-10-01 | 2017-09-20 | Oxford BioTherapeutics Ltd | Anti-ror1 antibodies |
| HUE048639T2 (hu) | 2010-10-27 | 2020-08-28 | Amgen Res Munich Gmbh | Eljárás DLBCL kezelésére |
| WO2012075158A1 (en) * | 2010-12-01 | 2012-06-07 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Chimeric rabbit/human ror1 antibodies |
| GB201020995D0 (en) | 2010-12-10 | 2011-01-26 | Bioinvent Int Ab | Biological materials and uses thereof |
| PT2663579T (pt) | 2011-01-14 | 2017-07-28 | Univ California | Terapêutica de anticorpos contra a proteína r0r-1 e métodos para sua utilização |
| US20140242081A1 (en) * | 2011-07-18 | 2014-08-28 | Micromet Ag | Dosing regimens for treatment of cea-expressing cancers |
| AR091649A1 (es) | 2012-07-02 | 2015-02-18 | Bristol Myers Squibb Co | Optimizacion de anticuerpos que se fijan al gen de activacion de linfocitos 3 (lag-3) y sus usos |
| DK3824905T3 (da) * | 2012-08-20 | 2025-03-17 | Fred Hutchinson Cancer Center | Fremgangsmåde og sammensætninger til cellulær immunterapi |
| US20140302037A1 (en) | 2013-03-15 | 2014-10-09 | Amgen Inc. | BISPECIFIC-Fc MOLECULES |
| US20140308285A1 (en) | 2013-03-15 | 2014-10-16 | Amgen Inc. | Heterodimeric bispecific antibodies |
| EP2789630A1 (en) | 2013-04-09 | 2014-10-15 | EngMab AG | Bispecific antibodies against CD3e and ROR1 |
| CN119775416A (zh) | 2013-12-24 | 2025-04-08 | 杨森制药公司 | 抗vista抗体及片段 |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| AU2015266880B2 (en) * | 2014-05-29 | 2019-03-21 | Macrogenics, Inc. | Tri-Specific Binding Molecules and methods of use thereof |
| CU24597B1 (es) * | 2014-11-26 | 2022-05-11 | Xencor Inc | Anticuerpos biespecíficos heterodiméricos que se unen a cd3 y cd20 |
-
2017
- 2017-07-14 WO PCT/US2017/042264 patent/WO2018014001A1/en not_active Ceased
- 2017-07-14 US US16/317,782 patent/US20190233534A1/en not_active Abandoned
- 2017-07-14 AU AU2017297603A patent/AU2017297603A1/en not_active Abandoned
- 2017-07-14 JP JP2019501628A patent/JP2019532017A/ja not_active Withdrawn
- 2017-07-14 EP EP17828588.8A patent/EP3484516A4/en active Pending
- 2017-07-14 CA CA3030841A patent/CA3030841A1/en not_active Abandoned
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023519932A (ja) * | 2020-03-31 | 2023-05-15 | フレッド ハッチンソン キャンサー センター | 抗cd33抗体及びその使用 |
| JP2023520399A (ja) * | 2020-03-31 | 2023-05-17 | フレッド ハッチンソン キャンサー センター | ヒト抗cd33抗体及びその使用 |
| JP2023548443A (ja) * | 2020-10-30 | 2023-11-16 | ジャナックス セラピューティクス,インク. | Cd28およびpd-l1を標的とする多特異性抗体ならびにその使用方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2017297603A1 (en) | 2019-02-14 |
| EP3484516A1 (en) | 2019-05-22 |
| US20190233534A1 (en) | 2019-08-01 |
| EP3484516A4 (en) | 2020-03-18 |
| WO2018014001A1 (en) | 2018-01-18 |
| CA3030841A1 (en) | 2018-01-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2019532017A (ja) | がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 | |
| JP2023518049A (ja) | 新規な抗原結合ドメインおよびそれを組み込んだ合成抗原受容体 | |
| EP3820496A2 (en) | Fusion constructs and methods of using thereof | |
| IL262321B1 (en) | Compositions and methods for selective protein expression | |
| JP2021524269A (ja) | メソテリン及びcd137結合分子 | |
| US20250064934A1 (en) | Genetically engineered cells having anti-cd19 / anti-cd22 chimeric antigen receptors, and uses thereof | |
| IL297916A (en) | Compositions and methods for tcr reprogramming using CD70-specific fusion proteins | |
| TW202504914A (zh) | 嵌合抗原受體及其用途 | |
| WO2022063291A1 (en) | Fibronectin extra domain b (edb) -specific car-t for cancer | |
| WO2025106626A1 (en) | Genetically engineered cells expressing c-x-c chemokine receptor type 4, and uses thereof | |
| US20250090582A1 (en) | Genetically engineered cells having anti-cd133 / anti-egfr chimeric antigen receptors, and uses thereof | |
| CN114685659B (zh) | Cd22特异性人源化抗体及利用其的嵌合抗原受体 | |
| AU2021305234A1 (en) | Fusion constructs and methods of using thereof | |
| US20230381317A1 (en) | Methods for controlled activation and/or expansion of genetically engineered cells using polyethylene glycol (peg) receptors | |
| WO2025059589A1 (en) | Immune cell engaging molecules | |
| AU2023375625A1 (en) | Genetically engineered cells having anti-nectin4 chimeric antigen receptors, and uses thereof | |
| US20230212286A1 (en) | Antibodies specific for cd22 and uses thereof | |
| WO2025207798A1 (en) | Genetically engineered cells having anti-cd123 chimeric antigen receptors, and uses thereof | |
| WO2025207795A1 (en) | Genetically engineered cells having anti-cd33 / anti-cd123 chimeric antigen receptors, and uses thereof | |
| WO2025245169A1 (en) | Immunotherapy cells equipped with a collagen-targeting payload | |
| WO2026022767A1 (ko) | B7-h3을 표적하는 항체, 및 이를 포함하는 키메라 항원 수용체 | |
| HK40067100A (zh) | Cd22特异性人源化抗体及利用其的嵌合抗原受体 | |
| WO2020150513A1 (en) | Methods to enhance the selectivity and effectiveness of cancer treatments | |
| HK40056378B (zh) | 人源化抗claudin18.2嵌合抗原受体及其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200709 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200709 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20210816 |