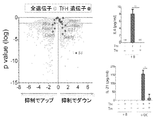
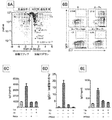
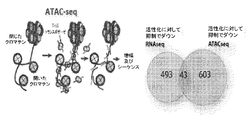
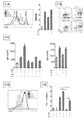
JP2018538280A - 免疫応答を調節するための組成物及び方法 - Google Patents
免疫応答を調節するための組成物及び方法 Download PDFInfo
- Publication number
- JP2018538280A JP2018538280A JP2018526676A JP2018526676A JP2018538280A JP 2018538280 A JP2018538280 A JP 2018538280A JP 2018526676 A JP2018526676 A JP 2018526676A JP 2018526676 A JP2018526676 A JP 2018526676A JP 2018538280 A JP2018538280 A JP 2018538280A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- tfr
- tfh
- cell
- subject
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0637—Immunosuppressive T lymphocytes, e.g. regulatory T cells or Treg
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/26—Lymph; Lymph nodes; Thymus; Spleen; Splenocytes; Thymocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/22—Immunosuppressive or immunotolerising
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55522—Cytokines; Lymphokines; Interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55522—Cytokines; Lymphokines; Interferons
- A61K2039/55527—Interleukins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/244—Interleukins [IL]
- C07K16/248—IL-6
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2302—Interleukin-2 (IL-2)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2306—Interleukin-6 (IL-6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2321—Interleukin-21 (IL-21)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/50—Cell markers; Cell surface determinants
- C12N2501/515—CD3, T-cell receptor complex
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Biochemistry (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Mycology (AREA)
- Cell Biology (AREA)
- General Engineering & Computer Science (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Virology (AREA)
- Developmental Biology & Embryology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562258876P | 2015-11-23 | 2015-11-23 | |
| US62/258,876 | 2015-11-23 | ||
| US201662300339P | 2016-02-26 | 2016-02-26 | |
| US62/300,339 | 2016-02-26 | ||
| PCT/US2016/063604 WO2017091729A1 (en) | 2015-11-23 | 2016-11-23 | Compositions and methods for modulating an immune response |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2018538280A true JP2018538280A (ja) | 2018-12-27 |
| JP2018538280A5 JP2018538280A5 (enExample) | 2020-01-09 |
Family
ID=58763732
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018526676A Abandoned JP2018538280A (ja) | 2015-11-23 | 2016-11-23 | 免疫応答を調節するための組成物及び方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US11028364B2 (enExample) |
| EP (1) | EP3380197A4 (enExample) |
| JP (1) | JP2018538280A (enExample) |
| AU (1) | AU2016361569A1 (enExample) |
| CA (1) | CA3005899A1 (enExample) |
| WO (1) | WO2017091729A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022196699A1 (ja) * | 2021-03-16 | 2022-09-22 | 国立大学法人大阪大学 | SARS-CoV-2ウイルスに特異的な濾胞性ヘルパーT細胞(Tfh) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2531216B1 (en) * | 2010-02-04 | 2019-03-27 | The Trustees Of The University Of Pennsylvania | Icos critically regulates the expansion and function of inflammatory human th17 cells |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010003002A2 (en) * | 2008-07-02 | 2010-01-07 | Board Of Regents, The University Of Texas System | Modulation of follicular helper t cells |
| WO2014074852A1 (en) * | 2012-11-09 | 2014-05-15 | President And Fellows Of Harvard College | Compositions and methods for modulating an immune response |
-
2016
- 2016-11-23 EP EP16869273.9A patent/EP3380197A4/en not_active Withdrawn
- 2016-11-23 AU AU2016361569A patent/AU2016361569A1/en not_active Abandoned
- 2016-11-23 US US15/778,524 patent/US11028364B2/en active Active
- 2016-11-23 JP JP2018526676A patent/JP2018538280A/ja not_active Abandoned
- 2016-11-23 CA CA3005899A patent/CA3005899A1/en not_active Abandoned
- 2016-11-23 WO PCT/US2016/063604 patent/WO2017091729A1/en not_active Ceased
-
2021
- 2021-05-07 US US17/314,438 patent/US20220010275A1/en not_active Abandoned
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022196699A1 (ja) * | 2021-03-16 | 2022-09-22 | 国立大学法人大阪大学 | SARS-CoV-2ウイルスに特異的な濾胞性ヘルパーT細胞(Tfh) |
Also Published As
| Publication number | Publication date |
|---|---|
| CA3005899A1 (en) | 2017-06-01 |
| WO2017091729A1 (en) | 2017-06-01 |
| AU2016361569A1 (en) | 2018-06-21 |
| EP3380197A4 (en) | 2019-08-07 |
| US11028364B2 (en) | 2021-06-08 |
| US20180340147A1 (en) | 2018-11-29 |
| EP3380197A1 (en) | 2018-10-03 |
| US20220010275A1 (en) | 2022-01-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10577586B2 (en) | Compositions and methods for modulating an immune response | |
| JP2021506304A (ja) | 人工抗原提示細胞および使用方法 | |
| CN113383071A (zh) | 用于t细胞工程化的组合物和方法 | |
| JP2019531056A (ja) | 融合タンパク質を使用したtcrの再プログラム化のための組成物及び方法 | |
| JP2022521738A (ja) | 負荷可能な抗原提示ポリペプチドを含む操作された赤血球系細胞および使用方法 | |
| KR20230018376A (ko) | 키메라 항원 수용체를 발현하는 바이러스 특이적 면역세포를 사용한 암의 치료 및 예방 | |
| JP5016489B2 (ja) | 抗原提示ヒトγδT細胞の調製及び免疫療法における使用 | |
| AU2023221021A1 (en) | Compositions and methods for antigen-specific t cell expansion | |
| US20220010275A1 (en) | Compositions and methods for modulating an immune response | |
| Xia et al. | Tracking ex vivo-expanded CD4+ CD25+ and CD8+ CD25+ regulatory T cells after infusion to prevent donor lymphocyte infusion-induced lethal acute graft-versus-host disease | |
| Pothoven et al. | Rapamycin-conditioned donor dendritic cells differentiate CD4+ CD25+ Foxp3+ T cells in vitro with TGF-β1 for islet transplantation | |
| JP2024512368A (ja) | T細胞機能障害を評価および治療するための組成物および方法 | |
| CN114269907B (zh) | 细胞治疗方法 | |
| CN118459604B (zh) | 一种同时靶向bcma和cd19的car-t细胞及其应用 | |
| WO1996016674A1 (en) | In vitro t-lymphopoiesis system | |
| WO2024124222A1 (en) | T cell manufacturing compositions and methods | |
| US20240226155A1 (en) | Methods and Products for Reducing HIV Reservoirs | |
| US20240400992A1 (en) | Compositions and methods for antigen-specific t cell expansion | |
| TWI904120B (zh) | Cd19和cd22嵌合抗原受體及其用途 | |
| EP3941487B1 (en) | Cd28 t cell cultures, compositions, and methods of using thereof | |
| WO2023019128A1 (en) | Optimizing t cell differentiation state with micrornas | |
| WO2025035125A1 (en) | Suppression of type 1 diabetes by intrathymic il-4 | |
| Kilgore | Early Determinants of CD8 T Cell Fate in the Response to Subunit Immunization | |
| JP2024521711A (ja) | 改善された免疫細胞集団を産生する方法 | |
| Buckle | Antigen-specific peptide immunotherapy for treatment and prevention of type 1 diabetes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191120 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20191120 |
|
| A762 | Written abandonment of application |
Free format text: JAPANESE INTERMEDIATE CODE: A762 Effective date: 20200924 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20200925 |