JP2014227355A - Biofilm formation inhibitor - Google Patents
Biofilm formation inhibitor Download PDFInfo
- Publication number
- JP2014227355A JP2014227355A JP2013106254A JP2013106254A JP2014227355A JP 2014227355 A JP2014227355 A JP 2014227355A JP 2013106254 A JP2013106254 A JP 2013106254A JP 2013106254 A JP2013106254 A JP 2013106254A JP 2014227355 A JP2014227355 A JP 2014227355A
- Authority
- JP
- Japan
- Prior art keywords
- biofilm formation
- natto
- formation inhibitor
- mutans
- culture
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Abstract
Description
本発明は、バイオフィルム形成抑制剤に関する。さらに詳しくは、納豆、納豆分離菌または納豆分離菌培養液を有効成分とするバイオフィルム形成抑制剤に関する。 The present invention relates to a biofilm formation inhibitor. More specifically, the present invention relates to a biofilm formation inhibitor containing as an active ingredient natto, natto isolate, or natto isolate culture solution.
う蝕(虫歯)はう蝕原性菌であるStreptococcus mutans(以下、S. mutansと示す場合がある)やStreptococcus sobrinus(以下、S.sobrinusと示す場合がある)が歯の表面に付着・増殖し、う蝕誘発性のバイオフィルムを形成することによって引き起こされる。
近年、高齢者等において誤嚥性肺炎等のう蝕を起因とする疾病が増加しており、う蝕の予防において有用な剤の提供が望まれている。
Caries (decayed teeth) are cariogenic bacteria Streptococcus mutans (hereinafter sometimes referred to as S. mutans) and Streptococcus sobrinus (hereinafter sometimes referred to as S. sobrinus) adhere to and grow on the tooth surface. It is caused by forming a caries-induced biofilm.
In recent years, diseases caused by dental caries such as aspiration pneumonia have increased in elderly people and the like, and provision of a useful agent for prevention of dental caries is desired.
う蝕予防剤として、従来、う蝕原性菌に対する殺菌剤や抗菌剤等が使用されてきた。しかし、これらの殺菌剤や抗菌剤は、日和見菌感染症等に対し免疫機能を示す、有用な口腔常在菌にも作用してしまうという問題があった。そこで、口腔常在菌に影響を及ぼさない、新たなう蝕予防剤の提供が望まれている。 Conventionally, bactericides and antibacterial agents against cariogenic bacteria have been used as caries preventive agents. However, these bactericides and antibacterial agents have a problem that they also act on useful oral resident bacteria that exhibit immune functions against opportunistic bacterial infections and the like. Therefore, it is desired to provide a new caries preventive agent that does not affect oral bacteria.
う蝕原性菌であるS.mutansに特異的に作用するものとして、例えば、特許文献1では、ササゲ、小豆、紫花豆、白花豆、クロインゲンマメ、キントキマメ等の豆類を粉砕し、水、親水性の有機溶媒等で抽出した豆類の抽出物が、S.mutansのクオラムセンシング(細菌密度依存的遺伝子発現制御系)を制御する物質として挙げられており、S.mutansによるバイオフィルム形成を抑制することが開示されている。
特許文献2では、ヒト口腔内及び漬物から単離した乳酸菌の菌株を、S.mutansの生育やバイオフィルム形成を抑制する口腔用組成物や口腔内バイオフィルム形成抑制剤等とすることが開示されている。
S. cariogenic bacteria For example, in Patent Document 1, beans that are pulverized with beans such as cowpea, red beans, purple flower beans, white flower beans, black bean, kintokimame, and the like are extracted with water, a hydrophilic organic solvent, or the like. Extract of S. It is listed as a substance that controls mutans quorum sensing (bacterial density-dependent gene expression control system). Inhibiting biofilm formation by mutans is disclosed.
In
また、特許文献3では、ナットウキナーゼに、ミュータンス菌(S.mutans)だけでなく、複数の口腔内病原菌種を含むバイオフィルムを除去し、口臭を改善する効果があること、このナットウキナーゼを含むナットウエキスと、ポリオキシエチレン硬化ヒマシ油を必須の成分としてなる口腔用組成物は歯石除去効果および口臭改善効果が高いこと等が開示されている。
なお、特許文献4おいても豆類に納豆菌にて発酵させてできた納豆の粘質およびその成分がS.mutans(ミュータンス菌)の増殖、酸産生、産生した酸による歯の侵食、歯石形成能等を抑制することが記載されているが、この文献では、これらの具体的な効果は開示されていない。
Patent Document 3 discloses that nattokinase has an effect of improving bad breath by removing not only mutans (S. mutans) but also a plurality of oral pathogenic bacterial species, and nattokinase containing nattokinase. It has been disclosed that an oral composition comprising an extract and polyoxyethylene hydrogenated castor oil as essential components has a high tartar removal effect and a bad breath improving effect.
In Patent Document 4, the stickiness of natto produced by fermenting beans with natto bacteria and its components are S. cerevisiae. Although it is described that the growth of mutans (mutans bacteria), acid production, tooth erosion by the produced acid, calculus formation ability, etc. are suppressed, these specific effects are not disclosed in this document. .
これらの文献に示されるように、様々な成分がう蝕原性菌であるS.mutansに特異的に作用するものとして開示されているが、より安全かつ効果的にう蝕の予防が可能な剤の提供が望まれている。 As shown in these documents, various components are cariogenic bacteria. Although it is disclosed as acting specifically on mutans, it is desired to provide an agent capable of preventing caries more safely and effectively.
口腔常在菌に影響を及ぼさない、新たなう蝕予防剤の提供を課題とする。 It is an object to provide a new caries preventive agent that does not affect oral bacteria.
本発明者らは、上記課題を解決するために鋭意研究を行った結果、納豆、納豆分離菌、または納豆分離菌培養濾液を作用させることで、う蝕原性菌によるバイオフィルムの形成を抑制できることを見出し、本発明を完成するに至った。これにより、安全かつ効果的にう蝕、う蝕を起因とする疾病等の予防、治療が可能となる。 As a result of intensive research to solve the above problems, the present inventors have suppressed the formation of biofilms by cariogenic bacteria by acting natto, natto isolate, or natto isolate culture filtrate. The present inventors have found that this can be done and have completed the present invention. This makes it possible to prevent and treat caries caused by caries and caries safely and effectively.
すなわち、本発明は次の(1)〜(4)のバイオフィルム形成抑制剤等に関する。
(1)納豆、納豆分離菌、及び納豆分離菌培養濾液からなる群から選ばれるいずれかひとつ以上を有効成分として含むバイオフィルム形成抑制剤。
(2)納豆分離菌培養濾液に含まれる10kDa未満の低分子画分を有効成分とする上記(1)に記載のバイオフィルム形成抑制剤。
(3)う蝕原性菌によるバイオフィルムの形成を抑制する上記(1)または(2)に記載のバイオフィルム形成抑制剤。
(4)う蝕原性菌がStreptococcus mutansまたはStreptococcus sobrinusである上記(3)に記載のバイオフィルム形成抑制剤。
That is, the present invention relates to the following biofilm formation inhibitors (1) to (4).
(1) A biofilm formation inhibitor comprising as an active ingredient at least one selected from the group consisting of natto, natto isolates, and natto isolate culture filtrate.
(2) The biofilm formation inhibitor as described in (1) above, wherein the active ingredient is a low molecular fraction of less than 10 kDa contained in the natto isolate bacterial culture filtrate.
(3) The biofilm formation inhibitor according to (1) or (2), which suppresses biofilm formation by cariogenic bacteria.
(4) The biofilm formation inhibitor according to (3), wherein the cariogenic bacterium is Streptococcus mutans or Streptococcus sobrinus.
本発明によって、S.mutans等のう蝕原性菌に特異的に作用するバイオフィルム形成抑制剤を提供することにより、う蝕、う蝕を起因とする疾病等の予防、治療が可能となる。本発明のバイオフィルム形成抑制剤は、有効成分が食品を由来とするものであるため安全性が高く、安価に提供することができる。 In accordance with the present invention, S.M. By providing a biofilm formation inhibitor that specifically acts on cariogenic bacteria such as mutans, it becomes possible to prevent and treat caries and diseases caused by caries. The biofilm formation inhibitor of the present invention has high safety and can be provided at low cost because the active ingredient is derived from food.
本発明の「バイオフィルム形成抑制剤」とは、う蝕原性菌によって形成される、う蝕誘発性のバイオフィルムの形成を抑制する剤のことをいう。
ここで、う蝕原性菌にはS.mutans、S.sobrinus等が挙げられる。本発明の「バイオフィルム形成抑制剤」は、これらのう蝕原性菌や、う蝕原性菌を含む口腔内の細菌叢によるバイオフィルムの形成を抑制する剤であればよい。
本発明の「バイオフィルム形成抑制剤」は、このバイオフィルムの形成を抑制する剤であれば良いが、バイオフィルムの形成を抑制する効果とともにう蝕原性菌の成育を抑制する効果を有する剤であってもよい。
The “biofilm formation inhibitor” of the present invention refers to an agent that suppresses the formation of caries-induced biofilms formed by cariogenic bacteria.
Here, the cariogenic bacteria include S. cerevisiae. mutans, S.M. and sobrinus. The “biofilm formation inhibitor” of the present invention may be any agent that suppresses the formation of biofilms due to these cariogenic bacteria and oral flora containing cariogenic bacteria.
The “biofilm formation inhibitor” of the present invention may be any agent that suppresses the formation of this biofilm, but has the effect of suppressing the growth of cariogenic bacteria as well as the effect of suppressing the formation of biofilm. It may be.
本発明の「バイオフィルム形成抑制剤」は、納豆、納豆分離菌、納豆分離菌培養濾液から選ばれるいずれかひとつ以上を有効成分とする剤であればよく、これらの有効成分を二つ以上組み合わせて含む剤であってもよい。また、納豆分離菌培養濾液に含まれる10kDa未満の低分子画分を有効成分とする剤であってもよい。さらに、これらの有効成分以外に、ヒト等の動物が安全に摂取できるその他の成分、薬学的に許容される担体等を含むものであってもよい。 The “biofilm formation inhibitor” of the present invention may be any agent containing at least one selected from natto, natto isolates, and natto isolate culture filtrate, and a combination of two or more of these active components May be included. Moreover, the agent which uses as an active ingredient the low molecular fraction of less than 10 kDa contained in a natto isolate bacterial culture filtrate may be sufficient. Furthermore, in addition to these active ingredients, other ingredients that can be safely ingested by animals such as humans, pharmaceutically acceptable carriers, and the like may be included.
このような本発明の「バイオフィルム形成抑制剤」は、そのまま食品、薬品等とすることもでき、食品、薬品等の有効成分とすることもできる。また、液状、粉状、ペースト状、液体、固体等、様々な形態で利用することができる。例えば歯磨剤、洗口剤、タブレット、口中清涼剤、チューインガム、飴等に調製することができ、歯磨剤とする場合も練歯磨剤、液状歯磨剤、粉歯磨剤等の様々な形態のものとできる。 Such a “biofilm formation inhibitor” of the present invention can be used as it is as a food, medicine or the like, or can be used as an active ingredient such as food or medicine. Further, it can be used in various forms such as liquid, powder, paste, liquid and solid. For example, it can be prepared into dentifrices, mouthwashes, tablets, mouth fresheners, chewing gums, wrinkles, etc., and also in dentifrices, liquid dentifrices, powder dentifrices, etc. it can.
以下、実施例、試験例をあげて本発明をさらに詳細に説明するが、本発明はこれらに限定されるものではない。 EXAMPLES Hereinafter, although an Example and a test example are given and this invention is demonstrated further in detail, this invention is not limited to these.
バイオフィルム形成抑制剤の調製
1.納豆
市販の5種類(3社)の納豆A〜Eをそれぞれ滅菌蒸留水に10w/v%となるように加え、ブレンダー(Waring社 Commercial Blendor(Model 31BL92))にて10分間撹拌し、それぞれバイオフィルム形成抑制剤とした。
1. Preparation of biofilm formation inhibitor Natto 5 kinds (3 companies) of commercially available natto A to E were added to each sterilized distilled water so as to be 10 w / v%, and stirred for 10 minutes with a blender (Waring Commercial Blender (Model 31BL92)). It was set as the film formation inhibitor.
2.納豆分離菌
上記1.にて調製したバイオフィルム形成抑制剤(5種類)を滅菌蒸留水で適宜段階希釈し、BHI寒天培地(Brain Heart Infusion培地,BD社)へ塗布した。37℃、好気条件で2日間培養した後、生育したコロニーを釣菌し、BHI液体培地(Brain Heart Infusion培地,BD社)へ懸濁し、同条件で2日間培養した。その後、同様にBHI寒天培地へ塗布し、培養し、生育したコロニーを釣菌し、BHI液体培地で培養するという工程を2回繰り返して純化し、BHI液体培地に含まれた状態の各納豆分離菌(生菌)を、由来とする納豆の種類(納豆A〜E)に合わせて納豆分離菌a〜eとし、それぞれバイオフィルム形成抑制剤とした。
2. Natto isolates above 1. The biofilm formation inhibitors (5 types) prepared in step 1 were appropriately diluted with sterilized distilled water and applied to a BHI agar medium (Brain Heart Infusion medium, BD). After culturing at 37 ° C. for 2 days under aerobic conditions, the grown colonies were picked and suspended in a BHI liquid medium (Brain Heart Infusion medium, BD), and cultured under the same conditions for 2 days. After that, similarly applied to BHI agar medium, cultured, and the grown colonies were picked and cultured in BHI liquid medium and purified twice repeatedly to separate each natto in the state contained in BHI liquid medium. Bacteria (live bacteria) were made into natto isolates a to e according to the type of natto (natto A to E) from which they were derived, and were used as biofilm formation inhibitors.
3.培養濾液
上記2.にて調製した納豆分離菌a〜eを、BHI液体培地(BRAIN HEART INFUSION培地,BD社)へ懸濁し、さらに37℃、5%CO2で1晩培養した後、遠心分離(6,000RPM,15MIN)し、上清部分を滅菌フィルター(ポアサイズ0.2MM)処理して菌体を完全に除去した。これを由来とする納豆の種類(納豆A〜E)に合わせ培養濾液a〜eとし、それぞれバイオフィルム形成抑制剤とした。
3. Culture filtrate Above 2. The natto isolates a to e prepared in Step 1 were suspended in a BHI liquid medium (BRAIN HEART INFUSION medium, BD), further cultured overnight at 37 ° C. and 5% CO 2 , and then centrifuged (6,000 RPM, 15 MIN), and the supernatant was treated with a sterilizing filter (pore size 0.2 MM) to completely remove the cells. It was set as culture filtrate ae according to the kind (natto AE) of natto derived from this, and it was set as the biofilm formation inhibitor, respectively.
4.低分子画分
上記3.にて調製した培養濾液a〜e各12mlを、それぞれ限外濾過膜(Amicon ultra−15 Filter Unit 10,000NMWL,ミリポア社)に供し、遠心分離を行った。限外濾過後の10kDa未満の各画分を滅菌水にて12mlにメスアップしたものをそれぞれ低分子画分a〜eとし、バイオフィルム形成抑制剤とした。
なお、限外濾過後の10kDa以上の各画分も滅菌水にて12mlにメスアップし、下記試験例における試料とした。
4). Low molecular fraction 3. 12 ml of each of the culture filtrates a to e prepared in the above were applied to ultrafiltration membranes (Amicon ultra-15 Filter Unit 10,000 NMWL, Millipore) and centrifuged. Each of the fractions of less than 10 kDa after ultrafiltration was made up to 12 ml with sterilized water, and the low molecular fractions a to e were used as biofilm formation inhibitors.
Each fraction of 10 kDa or more after ultrafiltration was also made up to 12 ml with sterilized water, and used as a sample in the following test example.
[試験例1]
バイオフィルム形成抑制剤の効果の検討
上記実施例と同様に調製した各バイオフィルム形成抑制剤(納豆A〜E、納豆分離菌a〜e、培濾濾液a〜e、低分子画分a〜e)とう蝕原性菌(S.mutans)との共培養試験により、バイオフィルム形成抑制剤の効果を検討した。
[Test Example 1]
Examination of effect of biofilm formation inhibitor Each biofilm formation inhibitor (natto A to E, natto isolates a to e, culture filtrate a to e, low molecular fraction a to e prepared in the same manner as the above examples. ) The effect of the biofilm formation inhibitor was examined by a co-culture test with cariogenic bacteria (S. mutans).
1.納豆、納豆分離菌との共培養試験
上記実施例と同様に調製した各バイオフィルム形成抑制剤(納豆A〜Eまたは納豆分離菌a〜eのいずれか一種)とう蝕原性菌(S.mutans)との共培養試験により、う蝕原性菌によるバイオフィルムの形成の有無を検討した。
実施例の1.、実施例の2.と同様の方法によって調製した各バイオフィルム形成抑制剤(納豆A〜Eまたは納豆分離菌a〜eのいずれか一種)を使用した。また、B.subtilisの培養液としてBHI液体培地、37℃、好気条件にて1晩培養した後の定常期細胞を含む、B.subtilisの培養液を使用した。
う蝕原性菌(S.mutans)は、BHI液体培地(BRAIN HEART INFUSION培地,BD社)にて5%CO2、37℃で1晩培養した後の定常期細胞を含む、S.mutans培養液を使用した。
共培養のための培地には、2倍量のTryptic soy broth without dextrose培地(BD社)にglucoseを0.5%添加したものを使用した。
共培養のための培地160μL、S.mutans培養液20μLと、バイオフィルム形成抑制剤(納豆A〜Eまたは納豆分離菌a〜eのいずれか一種)20μLを混合し、96ウェルプレートに分注し、37℃、5%CO2の条件で20時間共培養した。
なお、バイオフィルム形成抑制剤の代わりにB.subtilisの培養液、または滅菌蒸留水を使用したものをControlとした。
これを蒸留水150μLで2回洗浄し、サフラニン50μLで10分染色した。その後、蒸留水150μLでさらに2回洗浄し、70%EtOHで色素抽出を行った後、プレートリーダーで吸光度(492nm)を測定し、吸光度値によりバイオフィルム量を評価した。
1. Co-culture test with natto and natto isolates Each biofilm formation inhibitor (any one of natto A to E or natto isolates a to e) and cariogenic bacteria (S. mutans) prepared in the same manner as in the above examples In the co-culture test, the presence or absence of biofilm formation by cariogenic bacteria was examined.
Example 1 Example 2 Each biofilm formation inhibitor (any one of natto A to E or natto isolates a to e) prepared by the same method was used. B. B. subtilis culture medium containing BHI liquid medium at 37 ° C. under aerobic conditions and stationary phase cells after overnight culture. Subtilis broth was used.
C. cariogenic bacteria (S. mutans) include S. mutans, including stationary phase cells after overnight culture at 37 ° C. with 5% CO 2 in BHI liquid medium (BRAIN HEART INFUSION medium, BD). Mutans broth was used.
As a medium for co-culture, a double volume of Tryptic soy broth with outextrose medium (BD) containing 0.5% glucose was used.
160 μL of medium for co-culture, S. 20 μL of mutans culture solution and 20 μL of biofilm formation inhibitor (Natto A to E or Natto isolates a to e) are mixed and dispensed into a 96-well plate at 37 ° C. with 5% CO 2 . For 20 hours.
In place of the biofilm formation inhibitor, B.I. A subtilis culture solution or sterilized distilled water was used as Control.
This was washed twice with 150 μL of distilled water and stained with 50 μL of safranin for 10 minutes. Thereafter, the plate was further washed twice with 150 μL of distilled water, dye extraction was performed with 70% EtOH, absorbance (492 nm) was measured with a plate reader, and the amount of biofilm was evaluated based on the absorbance value.
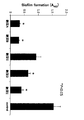
図1にバイオフィルム形成抑制剤(納豆A〜E)と共培養した場合の、バイオフィルムの形成抑制効果を示した。また、図2にバイオフィルム形成抑制剤(納豆分離菌a〜e)と共培養した場合の、バイオフィルムの形成抑制効果を示した。
その結果、図1、図2に示されるように、いずれのバイオフィルム形成抑制剤(納豆A〜E、納豆分離菌a〜e)も、う蝕原性菌(S.mutans)によるバイオフィルムの形成を有意に抑制することが確認できた。
FIG. 1 shows the biofilm formation inhibitory effect when co-cultured with biofilm formation inhibitors (natto A to E). Moreover, the biofilm formation inhibitory effect at the time of carrying out a coculture with the biofilm formation inhibitor (natto isolation bacteria ae) in FIG. 2 was shown.
As a result, as shown in FIG. 1 and FIG. 2, any biofilm formation inhibitor (natto A to E, natto isolates a to e) is a biofilm produced by cariogenic bacteria (S. mutans). It was confirmed that the formation was significantly suppressed.
2.培養濾液、低分子画分との共培養試験
上記実施例と同様に調製した各バイオフィルム形成抑制剤(培養濾液a〜eまたは低分子画分a〜eのいずれか一種)とう蝕原性菌(S.mutans)との共培養試験により、う蝕原性菌によるバイオフィルムの形成の有無を検討した。
実施例の3.、実施例の4.と同様の方法によって調製した各バイオフィルム形成抑制剤(培養濾液a〜eまたは低分子画分a〜eのいずれか一種)を使用した。また、B.subtilisの培養液としてBHI液体培地、37℃、好気条件にて1晩培養した後の定常期細胞を含む、B.subtilisの培養液を使用した。
う蝕原性菌(S.mutans)は、BHI液体培地(BRAIN HEART INFUSION培地,BD社)にて5%CO2、37℃で1晩培養した後の定常期細胞を含む、S.mutans培養液を使用した。
共培養のための培地には、2倍量のTryptic soy broth without dextrose培地(BD社)にglucoseを0.5%添加したものを使用した。
共培養のための培地80μL、S.mutans培養液20μLと、バイオフィルム形成抑制剤(培養濾液a〜eまたは低分子画分a〜eのいずれか一種)100μLを混合し、96ウェルプレートに分注した後、37℃、5%CO2の条件で20時間共培養した。
なお、バイオフィルム形成抑制剤の代わりにB.subtilisの培養液、これを実施例の4.と同様の方法で限外濾過した10kDa未満の画分、10kDa以上の画分、または滅菌蒸留水を使用したものをControlとした。
これを蒸留水150μLで2回洗浄し、サフラニン50μLで10分染色した。その後、蒸留水150μLでさらに2回洗浄し、70%EtOHで色素抽出を行った後、プレートリーダーで吸光度(492nm)を測定し、吸光度値によりバイオフィルム量を評価した。
2. Co-culture test with culture filtrate and low molecular fractions Each biofilm formation inhibitor (any one of culture filtrates a to e or low molecular fractions a to e) and cariogenic bacteria prepared in the same manner as in the above examples. The presence or absence of biofilm formation by cariogenic bacteria was examined by a co-culture test with (S. mutans).
Example 3 Example 4 Each biofilm formation inhibitor (any one of culture filtrates a to e or low molecular fractions a to e) prepared by the same method as above was used. B. B. subtilis culture medium containing BHI liquid medium at 37 ° C. under aerobic conditions and stationary phase cells after overnight culture. Subtilis broth was used.
C. cariogenic bacteria (S. mutans) include S. mutans, including stationary phase cells after overnight culture at 37 ° C. with 5% CO 2 in BHI liquid medium (BRAIN HEART INFUSION medium, BD). Mutans broth was used.
As a medium for co-culture, a double volume of Tryptic soy broth with outextrose medium (BD) containing 0.5% glucose was used.
80 μL of medium for co-culture, S. 20 μL of mutans culture solution and 100 μL of biofilm formation inhibitor (any one of culture filtrates a to e or low molecular fractions a to e) were mixed and dispensed into a 96-well plate, followed by 37 ° C., 5% CO Co-cultured for 20 hours under the conditions of 2 .
In place of the biofilm formation inhibitor, B.I. Subtilis broth, which was used in Example 4 A fraction using a fraction less than 10 kDa that was ultrafiltered in the same manner as described above and a fraction using 10 kDa or more, or using sterile distilled water was defined as Control.
This was washed twice with 150 μL of distilled water and stained with 50 μL of safranin for 10 minutes. Thereafter, the plate was further washed twice with 150 μL of distilled water, dye extraction was performed with 70% EtOH, absorbance (492 nm) was measured with a plate reader, and the amount of biofilm was evaluated based on the absorbance value.
図3にバイオフィルム形成抑制剤(培養濾液a〜e)と共培養した場合の、バイオフィルムの形成抑制効果を示した。なお、比較として、培養濾液a〜eを95度、15分で加熱したものを上記2.と同様に共培養した場合の結果(図3、Heat)も示した。
その結果、図3に示されるように、いずれのバイオフィルム形成抑制剤(培養濾液a〜e)も、う蝕原性菌(S.mutans)によるバイオフィルムの形成を有意に抑制することが確認できた。一方、培養濾液a〜eを95度、15分で加熱したものも、加熱しないバイオフィルム形成抑制剤(培養濾液a〜e)と比べてバイオフィルムの形成抑制効果は低下するものの、コントロールと比べてバイオフィルムの形成抑制効果を示すことが確認できた。
FIG. 3 shows the biofilm formation inhibitory effect when co-cultured with biofilm formation inhibitors (culture filtrates a to e). For comparison, the culture filtrates a to e heated at 95 ° C. for 15 minutes were subjected to the above 2. The results when co-cultured in the same manner as in (Fig. 3, Heat) are also shown.
As a result, as shown in FIG. 3, it was confirmed that any of the biofilm formation inhibitors (culture filtrates a to e) significantly suppressed the formation of biofilms by cariogenic bacteria (S. mutans). did it. On the other hand, the culture filtrates a to e heated at 95 ° C. for 15 minutes also have a lower biofilm formation inhibitory effect than the non-heated biofilm formation inhibitor (culture filtrate a to e), but compared with the control. It was confirmed that the biofilm formation inhibitory effect was exhibited.
図4にバイオフィルム形成抑制剤(低分子画分a〜e)のうち、バイオフィルム形成抑制剤(低分子画分e)と共培養した場合のバイオフィルムの形成抑制効果を示した。なお、比較としてバイオフィルム形成抑制剤である培養濾液e、培養濾液eから分画した10kDa以上の高分子画分を上記2.と同様に共培養した場合の結果も示した。
その結果、図4に示されるように、バイオフィルム形成抑制剤(低分子画分e)は、バイオフィルム形成抑制剤(培養濾液e)と同様にう蝕原性菌(S.mutans)によるバイオフィルムの形成を有意に抑制することが確認できた。一方、培養濾液eから分画した10kDa以上の高分子画分は、バイオフィルムの形成抑制効果を有しないことが確認できた。
なお、他のバイオフィルム形成抑制剤(低分子画分a〜d)もバイオフィルム形成抑制剤(培養濾液e)と同様にう蝕原性菌(S.mutans)によるバイオフィルムの形成を有意に抑制することが確認できた。一方、培養濾液a〜dから分画した10kDa以上の高分子画分はいずれもバイオフィルムの形成抑制効果を有しないことが確認できた。
FIG. 4 shows the biofilm formation inhibitory effect when co-cultured with the biofilm formation inhibitor (low molecular fraction e) among the biofilm formation inhibitors (low molecular fractions a to e). For comparison, the culture filtrate e, which is a biofilm formation inhibitor, and the polymer fraction of 10 kDa or higher fractionated from the culture filtrate e are prepared in the above 2. The results when co-cultured in the same manner as above are also shown.
As a result, as shown in FIG. 4, the biofilm formation inhibitor (low molecular fraction e) is biodegradable by cariogenic bacteria (S. mutans) in the same manner as the biofilm formation inhibitor (culture filtrate e). It was confirmed that the film formation was significantly suppressed. On the other hand, it was confirmed that the polymer fraction of 10 kDa or higher fractionated from the culture filtrate e had no biofilm formation inhibitory effect.
In addition, other biofilm formation inhibitors (low molecular fractions a to d) also significantly formed biofilms with cariogenic bacteria (S. mutans) in the same manner as biofilm formation inhibitors (culture filtrate e). It was confirmed that it was suppressed. On the other hand, it was confirmed that none of the polymer fractions of 10 kDa or higher fractionated from the culture filtrates a to d had a biofilm formation inhibitory effect.
[試験例2]
バイオフィルム形成抑制剤によるう蝕原性菌生育抑制効果の検討
上記実施例と同様に調製した各バイオフィルム形成抑制剤(培養濾液a〜e)とう蝕原性菌(S.mutans)との共培養試験により、う蝕原性菌の成育抑制効果を検討した。
実施例の3.と同様の方法によって調製した各バイオフィルム形成抑制剤(培養濾液a〜e)を使用した。う蝕原性菌(S.mutans)は、BHI液体培地(BRAIN HEART INFUSION培地,BD社)にて5%CO2、37℃で1晩培養した後の定常期細胞を含む、S.mutans培養液を使用した。共培養のための生育測定用培地には、2倍量のTryptic soy broth without dextrose培地(BD社)にglucoseを0.5%添加したものを使用した。
生育測定用培地80μl、バイオフィルム抑制剤100μl、S.mutans培養液20μlを混合し、96ウェルプレートに分注し、37℃、5%CO2の条件で16時間培養した。培養後600nmでの吸光度値を測定することで生育量を評価した。バイオフィルム形成抑制剤の代わりに滅菌蒸留水を使用したものをControlとした。
[Test Example 2]
Examination of growth inhibitory effect of cariogenic bacteria by biofilm formation inhibitor Co-combination of biofilm formation inhibitors (culture filtrates a to e) and cariogenic bacteria (S. mutans) prepared in the same manner as in the above examples. The growth inhibitory effect of cariogenic bacteria was examined by a culture test.
Example 3 Each biofilm formation inhibitor (culture filtrates a to e) prepared by the same method was used. C. cariogenic bacteria (S. mutans) include S. mutans, including stationary phase cells after overnight culture at 37 ° C. with 5% CO 2 in BHI liquid medium (BRAIN HEART INFUSION medium, BD). Mutans broth was used. As a medium for growth measurement for co-culture, a double volume of Tryptic soy broth outdextrose medium (BD) with 0.5% glucose added was used.
80 μl of growth measuring medium, 100 μl of biofilm inhibitor, 20 μl of mutans culture solution was mixed, dispensed into a 96-well plate, and cultured at 37 ° C. and 5% CO 2 for 16 hours. After cultivation, the amount of growth was evaluated by measuring the absorbance value at 600 nm. What used sterilized distilled water instead of the biofilm formation inhibitor was set as Control.
図5に示されるように、バイオフィルム形成抑制剤(培養濾液c)は、う蝕原性菌によるバイオフィルムの形成抑制に作用するのみならず、う蝕原性菌(S.mutans)の生育抑制にも作用することが確認できた。
一方、バイオフィルム形成抑制剤(培養濾液a、b、d、e)は、う蝕原性菌(S.mutans)の生育は抑制しないことから、う蝕原性菌の成育には作用せず、う蝕原性菌によるバイオフィルムの形成に特異的に作用する剤であることが確認できた。
As shown in FIG. 5, the biofilm formation inhibitor (culture filtrate c) not only acts on the suppression of biofilm formation by cariogenic bacteria, but also grows cariogenic bacteria (S. mutans). It was confirmed that it also acts on suppression.
On the other hand, biofilm formation inhibitors (culture filtrates a, b, d, e) do not suppress the growth of cariogenic bacteria (S. mutans) and therefore do not act on the growth of cariogenic bacteria. It was confirmed that the agent acts specifically on the formation of biofilms by cariogenic bacteria.
[試験例3]
ナットウキナーゼによるう蝕原性菌に対する作用の検討
試験例1、試験例2と同様の方法により、ナットウキナーゼにおける、う蝕原生菌に対するバイオフィルム形成抑制効果、またはう蝕原生菌の生育抑制効果の有無を検討した。ナットウキナーゼはWako純薬工業のものを使用した。
[Test Example 3]
Examination of the effect of nattokinase on cariogenic bacteria According to the same method as in Test Example 1 and Test Example 2, whether nattokinase has a biofilm formation inhibitory effect on protozoal cariogens or a growth inhibitory effect on protozoal cariogens investigated. Nattokinase was from Wako Pure Chemical Industries.
図6にナットウキナーゼによるバイオフィルムの形成抑制効果を示した。
その結果、図6に示されるように、終濃度が2mg/ml、または200μg/mlとなるようにナットウキナーゼを添加した場合には、う蝕原性菌(S.mutans)によるバイオフィルムの形成を有意に抑制したが、20μg/ml等の低濃度では、バイオフィルムの形成を抑制できないことが確認できた。
また、図7にナットウキナーゼによるう蝕原性菌の成育抑制効果の検討を示したが、いずれの濃度においても、う蝕原性菌の成育は抑制しないことが確認できた。
FIG. 6 shows the biofilm formation inhibitory effect of nattokinase.
As a result, as shown in FIG. 6, when nattokinase was added so that the final concentration was 2 mg / ml or 200 μg / ml, biofilm formation by cariogenic bacteria (S. mutans) was prevented. Although significantly suppressed, it was confirmed that biofilm formation could not be suppressed at a low concentration such as 20 μg / ml.
Moreover, although examination of the growth inhibitory effect of the cariogenic bacterium by nattokinase was shown in FIG. 7, it was confirmed that the growth of the cariogenic bacterium was not suppressed at any concentration.
本発明のS.mutans等のう蝕原性菌に特異的に作用するバイオフィルム形成抑制剤を提供することにより、う蝕、う蝕を起因とする疾病等の予防、治療が可能となる。本発明のバイオフィルム形成抑制剤は、有効成分が食品由来とするものであるため安全性が高く、安価に提供することができる。 S. of the present invention. By providing a biofilm formation inhibitor that specifically acts on cariogenic bacteria such as mutans, it becomes possible to prevent and treat caries and diseases caused by caries. The biofilm formation inhibitor of the present invention has high safety and can be provided at low cost because the active ingredient is derived from food.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013106254A JP2014227355A (en) | 2013-05-20 | 2013-05-20 | Biofilm formation inhibitor |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013106254A JP2014227355A (en) | 2013-05-20 | 2013-05-20 | Biofilm formation inhibitor |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2014227355A true JP2014227355A (en) | 2014-12-08 |
| JP2014227355A5 JP2014227355A5 (en) | 2016-06-09 |
Family
ID=52127543
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013106254A Pending JP2014227355A (en) | 2013-05-20 | 2013-05-20 | Biofilm formation inhibitor |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2014227355A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019065124A1 (en) * | 2017-09-26 | 2019-04-04 | 池田食研株式会社 | Composition to inhibit proliferation of oral bacteria |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005210979A (en) * | 2004-01-30 | 2005-08-11 | Asama Chemical Co Ltd | Antimicrobial agent and food preservative comprising the same |
| JP2006182662A (en) * | 2004-12-27 | 2006-07-13 | Lion Corp | Dentifrice composition |
| JP2007117064A (en) * | 2005-10-25 | 2007-05-17 | Shunji Ishii | Streptococcus mutans-suppressing effect by natto mucus |
| JP2007320926A (en) * | 2006-06-02 | 2007-12-13 | Sanei Gen Ffi Inc | Plaque formation inhibitor, or cariostatic agent |
| JP2009149535A (en) * | 2007-12-19 | 2009-07-09 | Lion Corp | Oral composition |
| JP2011126819A (en) * | 2009-12-18 | 2011-06-30 | Lion Corp | Composition for oral cavity |
| WO2012151555A1 (en) * | 2011-05-04 | 2012-11-08 | President And Fellows Of Harvard College | Methods and coatings for treating biofilms |
| JP2013240313A (en) * | 2012-05-21 | 2013-12-05 | Masakatsu Ito | Germicidal agent bacillus natto |
-
2013
- 2013-05-20 JP JP2013106254A patent/JP2014227355A/en active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005210979A (en) * | 2004-01-30 | 2005-08-11 | Asama Chemical Co Ltd | Antimicrobial agent and food preservative comprising the same |
| JP2006182662A (en) * | 2004-12-27 | 2006-07-13 | Lion Corp | Dentifrice composition |
| JP2007117064A (en) * | 2005-10-25 | 2007-05-17 | Shunji Ishii | Streptococcus mutans-suppressing effect by natto mucus |
| JP2007320926A (en) * | 2006-06-02 | 2007-12-13 | Sanei Gen Ffi Inc | Plaque formation inhibitor, or cariostatic agent |
| JP2009149535A (en) * | 2007-12-19 | 2009-07-09 | Lion Corp | Oral composition |
| JP2011126819A (en) * | 2009-12-18 | 2011-06-30 | Lion Corp | Composition for oral cavity |
| WO2012151555A1 (en) * | 2011-05-04 | 2012-11-08 | President And Fellows Of Harvard College | Methods and coatings for treating biofilms |
| JP2013240313A (en) * | 2012-05-21 | 2013-12-05 | Masakatsu Ito | Germicidal agent bacillus natto |
Non-Patent Citations (5)
| Title |
|---|
| BEVERAGE JAPAN, vol. 204, JPN6017029955, 1998, pages 44 - 6, ISSN: 0003616238 * |
| BULL. TOKYO DENT. COLL., vol. 44(1), JPN6017029958, 2003, pages 9 - 16, ISSN: 0003616239 * |
| ニューフードインダストリー, vol. 54(12), JPN6017012857, 2012, pages 35 - 9, ISSN: 0003537831 * |
| 日本農芸化学会誌, vol. 73(12), JPN6017012859, 1999, pages 1289 - 91, ISSN: 0003537830 * |
| 食の科学, vol. 289, JPN6017012858, 2002, pages 16 - 22, ISSN: 0003537829 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019065124A1 (en) * | 2017-09-26 | 2019-04-04 | 池田食研株式会社 | Composition to inhibit proliferation of oral bacteria |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Arslan et al. | Antimicrobial activity of poplar propolis on mutans streptococci and caries development in rats | |
| JP5365166B2 (en) | Oral composition containing lactic acid bacteria | |
| US10857090B2 (en) | Oral compositions | |
| Chen et al. | Antibacterial activity of viable and heat‐killed probiotic strains against oral pathogens | |
| Higuchi et al. | Effects of Lactobacillus salivarius WB21 combined with green tea catechins on dental caries, periodontitis, and oral malodor | |
| TW201922224A (en) | Antibacterial cosmetic composition comprising Lactobacillus plantarum culture | |
| CN103298924A (en) | Probiotic composition for oral health | |
| KR101998210B1 (en) | A composition containing tea lactic acid bacteria for exfoliating skin | |
| Llena et al. | Antimicrobial efficacy of the supernatant of Streptococcus dentisani against microorganisms implicated in root canal infections | |
| Roh et al. | Antimicrobial activity of Korean propolis extracts on oral pathogenic microorganisms | |
| Lin et al. | Inhibitory effect of Lactobacillus paracasei subsp. paracasei NTU 101 on rat dental caries | |
| Xi et al. | The effect of polyphenol-containing solutions on in situ biofilm formation on enamel and dentin | |
| CN107073053B (en) | Growth promoter for nonpathogenic resident bacteria in oral cavity, flora improving agent in oral cavity, and oral composition | |
| Talebi Ardakani et al. | Antibacterial effect of Iranian green-tea-containing mouthrinse vs chlorhexidine 0.2%: an in vitro study. | |
| Singhal et al. | Antimicrobial and antibiofilm effect of cranberry extract on Streptococcus mutans and Lactobacillus acidophilus: An in vitro study | |
| KR102095339B1 (en) | Novel lactobacillus reuteri and composition for preventing or treating periodontal diseases comprising the same | |
| KR20160098669A (en) | Mouth cleaner comprising citrus or lime extract and manufacturing method thereof | |
| JP2014227355A (en) | Biofilm formation inhibitor | |
| JP2010064961A (en) | Agent for adjusting normal oral bacterial flora and method for adjusting normal oral bacterial flora using the same | |
| Lesan et al. | The effect of probiotic yoghurt on the frequency of salivary candida | |
| JP7445910B2 (en) | Oral compositions, oral care products, and foods containing oral resident bacteria regulators | |
| JP2021020869A (en) | Oral indigenous bacteria growth promoter, and oral composition | |
| KR101921309B1 (en) | Composition for preventing or improving dental caries comprising weissella cibaria strain | |
| KR102293592B1 (en) | Skin external composition comprising extract of Martensia jejuensis and functional food comprising extract of Martensia jejuensis | |
| KR100508600B1 (en) | Antimicrobial composition and dental article comprising green tea polyphenol |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160413 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160413 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170419 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170609 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20170809 |