JP2011507933A - 免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 - Google Patents
免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 Download PDFInfo
- Publication number
- JP2011507933A JP2011507933A JP2010540130A JP2010540130A JP2011507933A JP 2011507933 A JP2011507933 A JP 2011507933A JP 2010540130 A JP2010540130 A JP 2010540130A JP 2010540130 A JP2010540130 A JP 2010540130A JP 2011507933 A JP2011507933 A JP 2011507933A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- target
- antigen
- bound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 81
- 230000001472 cytotoxic effect Effects 0.000 title claims description 14
- 231100000433 cytotoxic Toxicity 0.000 title claims description 11
- 230000000694 effects Effects 0.000 title description 26
- 230000008685 targeting Effects 0.000 claims abstract description 173
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 166
- 239000000427 antigen Substances 0.000 claims abstract description 112
- 108091007433 antigens Proteins 0.000 claims abstract description 112
- 102000036639 antigens Human genes 0.000 claims abstract description 112
- 239000012636 effector Substances 0.000 claims abstract description 73
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 64
- 230000009919 sequestration Effects 0.000 claims abstract description 21
- 230000014759 maintenance of location Effects 0.000 claims abstract description 9
- 210000004027 cell Anatomy 0.000 claims description 407
- 102100035721 Syndecan-1 Human genes 0.000 claims description 239
- 101000874179 Homo sapiens Syndecan-1 Proteins 0.000 claims description 234
- 210000004369 blood Anatomy 0.000 claims description 21
- 239000008280 blood Substances 0.000 claims description 21
- 210000002381 plasma Anatomy 0.000 claims description 19
- 238000002955 isolation Methods 0.000 claims description 15
- 201000010099 disease Diseases 0.000 claims description 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 10
- 229940124549 vasodilator Drugs 0.000 claims description 10
- 239000003071 vasodilator agent Substances 0.000 claims description 10
- 239000003446 ligand Substances 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 8
- 239000003463 adsorbent Substances 0.000 claims description 7
- 210000001519 tissue Anatomy 0.000 claims description 7
- 238000011269 treatment regimen Methods 0.000 claims description 7
- 210000000601 blood cell Anatomy 0.000 claims description 6
- 210000002919 epithelial cell Anatomy 0.000 claims description 6
- 229960001289 prazosin Drugs 0.000 claims description 5
- IENZQIKPVFGBNW-UHFFFAOYSA-N prazosin Chemical compound N=1C(N)=C2C=C(OC)C(OC)=CC2=NC=1N(CC1)CCN1C(=O)C1=CC=CO1 IENZQIKPVFGBNW-UHFFFAOYSA-N 0.000 claims description 5
- 239000003937 drug carrier Substances 0.000 claims description 4
- 210000003494 hepatocyte Anatomy 0.000 claims description 4
- 230000009885 systemic effect Effects 0.000 claims description 4
- 229960001389 doxazosin Drugs 0.000 claims description 3
- RUZYUOTYCVRMRZ-UHFFFAOYSA-N doxazosin Chemical compound C1OC2=CC=CC=C2OC1C(=O)N(CC1)CCN1C1=NC(N)=C(C=C(C(OC)=C2)OC)C2=N1 RUZYUOTYCVRMRZ-UHFFFAOYSA-N 0.000 claims description 3
- VCKUSRYTPJJLNI-UHFFFAOYSA-N terazosin Chemical compound N=1C(N)=C2C=C(OC)C(OC)=CC2=NC=1N(CC1)CCN1C(=O)C1CCCO1 VCKUSRYTPJJLNI-UHFFFAOYSA-N 0.000 claims description 3
- 229960001693 terazosin Drugs 0.000 claims description 3
- 210000001185 bone marrow Anatomy 0.000 claims description 2
- 210000004556 brain Anatomy 0.000 claims description 2
- 210000003734 kidney Anatomy 0.000 claims description 2
- 210000002216 heart Anatomy 0.000 claims 1
- 230000001172 regenerating effect Effects 0.000 claims 1
- 230000027455 binding Effects 0.000 abstract description 44
- 239000000203 mixture Substances 0.000 abstract description 9
- 210000004881 tumor cell Anatomy 0.000 description 40
- 238000011282 treatment Methods 0.000 description 34
- 206010035226 Plasma cell myeloma Diseases 0.000 description 25
- 230000035945 sensitivity Effects 0.000 description 25
- 210000004882 non-tumor cell Anatomy 0.000 description 24
- 239000003814 drug Substances 0.000 description 23
- 230000001965 increasing effect Effects 0.000 description 21
- 208000034578 Multiple myelomas Diseases 0.000 description 16
- 201000011510 cancer Diseases 0.000 description 15
- 229940127121 immunoconjugate Drugs 0.000 description 15
- 125000005647 linker group Chemical group 0.000 description 15
- 229940079593 drug Drugs 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 13
- 108060003951 Immunoglobulin Proteins 0.000 description 12
- 230000003013 cytotoxicity Effects 0.000 description 12
- 231100000135 cytotoxicity Toxicity 0.000 description 12
- 102000018358 immunoglobulin Human genes 0.000 description 12
- 235000018102 proteins Nutrition 0.000 description 11
- 108090000623 proteins and genes Proteins 0.000 description 11
- 102000004169 proteins and genes Human genes 0.000 description 11
- 229960004641 rituximab Drugs 0.000 description 11
- 239000011324 bead Substances 0.000 description 10
- RTQWWZBSTRGEAV-PKHIMPSTSA-N 2-[[(2s)-2-[bis(carboxymethyl)amino]-3-[4-(methylcarbamoylamino)phenyl]propyl]-[2-[bis(carboxymethyl)amino]propyl]amino]acetic acid Chemical compound CNC(=O)NC1=CC=C(C[C@@H](CN(CC(C)N(CC(O)=O)CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O)C=C1 RTQWWZBSTRGEAV-PKHIMPSTSA-N 0.000 description 9
- 241000699666 Mus <mouse, genus> Species 0.000 description 9
- 108090000058 Syndecan-1 Proteins 0.000 description 9
- 229960001001 ibritumomab tiuxetan Drugs 0.000 description 9
- 201000000050 myeloid neoplasm Diseases 0.000 description 9
- 108020003175 receptors Proteins 0.000 description 9
- 102000005962 receptors Human genes 0.000 description 9
- 239000003053 toxin Substances 0.000 description 9
- 231100000765 toxin Toxicity 0.000 description 9
- 108700012359 toxins Proteins 0.000 description 9
- 238000004113 cell culture Methods 0.000 description 8
- 238000003570 cell viability assay Methods 0.000 description 8
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 8
- 239000011159 matrix material Substances 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- UOWVMDUEMSNCAV-WYENRQIDSA-N rachelmycin Chemical class C1([C@]23C[C@@H]2CN1C(=O)C=1NC=2C(OC)=C(O)C4=C(C=2C=1)CCN4C(=O)C1=CC=2C=4CCN(C=4C(O)=C(C=2N1)OC)C(N)=O)=CC(=O)C1=C3C(C)=CN1 UOWVMDUEMSNCAV-WYENRQIDSA-N 0.000 description 8
- -1 CCL17 Proteins 0.000 description 7
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 7
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 7
- 229930126263 Maytansine Natural products 0.000 description 7
- 230000006907 apoptotic process Effects 0.000 description 7
- 210000003719 b-lymphocyte Anatomy 0.000 description 7
- 230000006378 damage Effects 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 210000004180 plasmocyte Anatomy 0.000 description 7
- 238000011533 pre-incubation Methods 0.000 description 7
- QWPXBEHQFHACTK-KZVYIGENSA-N (10e,12e)-86-chloro-12,14,4-trihydroxy-85,14-dimethoxy-33,2,7,10-tetramethyl-15,16-dihydro-14h-7-aza-1(6,4)-oxazina-3(2,3)-oxirana-8(1,3)-benzenacyclotetradecaphane-10,12-dien-6-one Chemical compound CN1C(=O)CC(O)C2(C)OC2C(C)C(OC(=O)N2)CC2(O)C(OC)\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 QWPXBEHQFHACTK-KZVYIGENSA-N 0.000 description 6
- 102000008186 Collagen Human genes 0.000 description 6
- 108010035532 Collagen Proteins 0.000 description 6
- 229940123237 Taxane Drugs 0.000 description 6
- 230000003833 cell viability Effects 0.000 description 6
- 229920001436 collagen Polymers 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 6
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- VWQVUPCCIRVNHF-OUBTZVSYSA-N Yttrium-90 Chemical compound [90Y] VWQVUPCCIRVNHF-OUBTZVSYSA-N 0.000 description 5
- 230000030833 cell death Effects 0.000 description 5
- 238000003501 co-culture Methods 0.000 description 5
- 239000012228 culture supernatant Substances 0.000 description 5
- 230000003394 haemopoietic effect Effects 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 229960005558 mertansine Drugs 0.000 description 5
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000000926 separation method Methods 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 230000001988 toxicity Effects 0.000 description 5
- 231100000419 toxicity Toxicity 0.000 description 5
- 230000035899 viability Effects 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 4
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 4
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 4
- 102000003705 Syndecan-1 Human genes 0.000 description 4
- 238000002835 absorbance Methods 0.000 description 4
- 230000000890 antigenic effect Effects 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 4
- 229930195731 calicheamicin Natural products 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- ANZJBCHSOXCCRQ-FKUXLPTCSA-N mertansine Chemical compound CO[C@@H]([C@@]1(O)C[C@H](OC(=O)N1)[C@@H](C)[C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(=O)CCS)CC(=O)N1C)\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 ANZJBCHSOXCCRQ-FKUXLPTCSA-N 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 239000000651 prodrug Substances 0.000 description 4
- 229940002612 prodrug Drugs 0.000 description 4
- JUJBNYBVVQSIOU-UHFFFAOYSA-M sodium;4-[2-(4-iodophenyl)-3-(4-nitrophenyl)tetrazol-2-ium-5-yl]benzene-1,3-disulfonate Chemical compound [Na+].C1=CC([N+](=O)[O-])=CC=C1N1[N+](C=2C=CC(I)=CC=2)=NC(C=2C(=CC(=CC=2)S([O-])(=O)=O)S([O-])(=O)=O)=N1 JUJBNYBVVQSIOU-UHFFFAOYSA-M 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 231100000331 toxic Toxicity 0.000 description 4
- 230000002588 toxic effect Effects 0.000 description 4
- MQLACMBJVPINKE-UHFFFAOYSA-N 10-[(3-hydroxy-4-methoxyphenyl)methylidene]anthracen-9-one Chemical compound C1=C(O)C(OC)=CC=C1C=C1C2=CC=CC=C2C(=O)C2=CC=CC=C21 MQLACMBJVPINKE-UHFFFAOYSA-N 0.000 description 3
- 102000051403 ADAMTS4 Human genes 0.000 description 3
- 108091005664 ADAMTS4 Proteins 0.000 description 3
- 241000283707 Capra Species 0.000 description 3
- 241000282693 Cercopithecidae Species 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108090000368 Fibroblast growth factor 8 Proteins 0.000 description 3
- 102000019298 Lipocalin Human genes 0.000 description 3
- 108050006654 Lipocalin Proteins 0.000 description 3
- QWPXBEHQFHACTK-UHFFFAOYSA-N Maytansinol Natural products CN1C(=O)CC(O)C2(C)OC2C(C)C(OC(=O)N2)CC2(O)C(OC)C=CC=C(C)CC2=CC(OC)=C(Cl)C1=C2 QWPXBEHQFHACTK-UHFFFAOYSA-N 0.000 description 3
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 239000000562 conjugate Substances 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 238000002823 phage display Methods 0.000 description 3
- 102000005162 pleiotrophin Human genes 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 238000005204 segregation Methods 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 210000000130 stem cell Anatomy 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 2
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 2
- 102100030988 Angiotensin-converting enzyme Human genes 0.000 description 2
- 108091023037 Aptamer Proteins 0.000 description 2
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 2
- 102000055105 BH3 Interacting Domain Death Agonist Human genes 0.000 description 2
- 108700000712 BH3 Interacting Domain Death Agonist Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 2
- 101710132601 Capsid protein Proteins 0.000 description 2
- 239000012624 DNA alkylating agent Substances 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 102000001398 Granzyme Human genes 0.000 description 2
- 108060005986 Granzyme Proteins 0.000 description 2
- 235000005612 Grewia tenax Nutrition 0.000 description 2
- 244000041633 Grewia tenax Species 0.000 description 2
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 2
- 102100021866 Hepatocyte growth factor Human genes 0.000 description 2
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 2
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 2
- 108010084592 Saporins Proteins 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- BIWLZRYQMYYVBY-UHFFFAOYSA-N [3-(2,5-dioxopyrrolidin-1-yl)pyridin-2-yl] propanedithioate Chemical compound CCC(=S)SC1=NC=CC=C1N1C(=O)CCC1=O BIWLZRYQMYYVBY-UHFFFAOYSA-N 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 239000002160 alpha blocker Substances 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 230000005756 apoptotic signaling Effects 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000025084 cell cycle arrest Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 230000000536 complexating effect Effects 0.000 description 2
- 238000010668 complexation reaction Methods 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 210000002889 endothelial cell Anatomy 0.000 description 2
- 238000007667 floating Methods 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 239000007850 fluorescent dye Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 210000000232 gallbladder Anatomy 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 210000004408 hybridoma Anatomy 0.000 description 2
- 230000005847 immunogenicity Effects 0.000 description 2
- 229940051026 immunotoxin Drugs 0.000 description 2
- 230000002637 immunotoxin Effects 0.000 description 2
- 239000002596 immunotoxin Substances 0.000 description 2
- 231100000608 immunotoxin Toxicity 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 2
- 208000025113 myeloid leukemia Diseases 0.000 description 2
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical class N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000002203 pretreatment Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- 238000009738 saturating Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 231100000057 systemic toxicity Toxicity 0.000 description 2
- 125000003831 tetrazolyl group Chemical group 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 125000003396 thiol group Chemical group [H]S* 0.000 description 2
- 150000003573 thiols Chemical class 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical class CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- CZGRVRBQKQOWIY-UHFFFAOYSA-N 2-(2,5-dioxopyrrolidin-1-yl)-1-[(2,5-dioxopyrrol-1-yl)methyl]-2-sulfocyclohexane-1-carboxylic acid Chemical compound C1CCCC(S(O)(=O)=O)(N2C(CCC2=O)=O)C1(C(=O)O)CN1C(=O)C=CC1=O CZGRVRBQKQOWIY-UHFFFAOYSA-N 0.000 description 1
- LKDMKWNDBAVNQZ-UHFFFAOYSA-N 4-[[1-[[1-[2-[[1-(4-nitroanilino)-1-oxo-3-phenylpropan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-oxobutanoic acid Chemical compound OC(=O)CCC(=O)NC(C)C(=O)NC(C)C(=O)N1CCCC1C(=O)NC(C(=O)NC=1C=CC(=CC=1)[N+]([O-])=O)CC1=CC=CC=C1 LKDMKWNDBAVNQZ-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 239000005541 ACE inhibitor Substances 0.000 description 1
- 208000036832 Adenocarcinoma of ovary Diseases 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 101800002638 Alpha-amanitin Proteins 0.000 description 1
- 108020004491 Antisense DNA Proteins 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 239000000592 Artificial Cell Substances 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000028564 B-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 231100000699 Bacterial toxin Toxicity 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 102100032366 C-C motif chemokine 7 Human genes 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 108090000397 Caspase 3 Proteins 0.000 description 1
- 108090000567 Caspase 7 Proteins 0.000 description 1
- 102100029855 Caspase-3 Human genes 0.000 description 1
- 102100038902 Caspase-7 Human genes 0.000 description 1
- 108090000538 Caspase-8 Proteins 0.000 description 1
- 102100026548 Caspase-8 Human genes 0.000 description 1
- 108090000566 Caspase-9 Proteins 0.000 description 1
- 102100026550 Caspase-9 Human genes 0.000 description 1
- 102100025975 Cathepsin G Human genes 0.000 description 1
- 108090000617 Cathepsin G Proteins 0.000 description 1
- 108010055166 Chemokine CCL5 Proteins 0.000 description 1
- 102000012422 Collagen Type I Human genes 0.000 description 1
- 108010022452 Collagen Type I Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102100023688 Eotaxin Human genes 0.000 description 1
- 229930189413 Esperamicin Natural products 0.000 description 1
- 101710082714 Exotoxin A Proteins 0.000 description 1
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102100034221 Growth-regulated alpha protein Human genes 0.000 description 1
- 108090000100 Hepatocyte Growth Factor Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000797762 Homo sapiens C-C motif chemokine 5 Proteins 0.000 description 1
- 101000797758 Homo sapiens C-C motif chemokine 7 Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000978392 Homo sapiens Eotaxin Proteins 0.000 description 1
- 101001069921 Homo sapiens Growth-regulated alpha protein Proteins 0.000 description 1
- 101000898034 Homo sapiens Hepatocyte growth factor Proteins 0.000 description 1
- 101001076408 Homo sapiens Interleukin-6 Proteins 0.000 description 1
- 101100041816 Homo sapiens SCD gene Proteins 0.000 description 1
- 101000868152 Homo sapiens Son of sevenless homolog 1 Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 108010028275 Leukocyte Elastase Proteins 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 240000001427 Mallotus nudiflorus Species 0.000 description 1
- 102100030417 Matrilysin Human genes 0.000 description 1
- 108090000855 Matrilysin Proteins 0.000 description 1
- 241001441512 Maytenus serrata Species 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- 102100030335 Midkine Human genes 0.000 description 1
- 108010092801 Midkine Proteins 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 102100033174 Neutrophil elastase Human genes 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061328 Ovarian epithelial cancer Diseases 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 102000016387 Pancreatic elastase Human genes 0.000 description 1
- 108010067372 Pancreatic elastase Proteins 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 231100000742 Plant toxin Toxicity 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 108010039491 Ricin Proteins 0.000 description 1
- RXGJTYFDKOHJHK-UHFFFAOYSA-N S-deoxo-amaninamide Natural products CCC(C)C1NC(=O)CNC(=O)C2Cc3c(SCC(NC(=O)CNC1=O)C(=O)NC(CC(=O)N)C(=O)N4CC(O)CC4C(=O)NC(C(C)C(O)CO)C(=O)N2)[nH]c5ccccc35 RXGJTYFDKOHJHK-UHFFFAOYSA-N 0.000 description 1
- 101150097713 SCD1 gene Proteins 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 231100000632 Spindle poison Toxicity 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 102100028897 Stearoyl-CoA desaturase Human genes 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 241000133426 Streptomyces zelensis Species 0.000 description 1
- 108050006774 Syndecan Proteins 0.000 description 1
- 101710090322 Truncated surface protein Proteins 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 241000863480 Vinca Species 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 239000004007 alpha amanitin Substances 0.000 description 1
- 229940124308 alpha-adrenoreceptor antagonist Drugs 0.000 description 1
- CIORWBWIBBPXCG-SXZCQOKQSA-N alpha-amanitin Chemical compound O=C1N[C@@H](CC(N)=O)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@@H]([C@@H](C)[C@@H](O)CO)C(=O)N[C@@H](C2)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@H]1C[S@@](=O)C1=C2C2=CC=C(O)C=C2N1 CIORWBWIBBPXCG-SXZCQOKQSA-N 0.000 description 1
- CIORWBWIBBPXCG-UHFFFAOYSA-N alpha-amanitin Natural products O=C1NC(CC(N)=O)C(=O)N2CC(O)CC2C(=O)NC(C(C)C(O)CO)C(=O)NC(C2)C(=O)NCC(=O)NC(C(C)CC)C(=O)NCC(=O)NC1CS(=O)C1=C2C2=CC=C(O)C=C2N1 CIORWBWIBBPXCG-UHFFFAOYSA-N 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003972 antineoplastic antibiotic Substances 0.000 description 1
- 239000003816 antisense DNA Substances 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 239000004019 antithrombin Substances 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 108010044540 auristatin Proteins 0.000 description 1
- 239000000688 bacterial toxin Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 201000001531 bladder carcinoma Diseases 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- 229950007296 cantuzumab mertansine Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000009643 clonogenic assay Methods 0.000 description 1
- 231100000096 clonogenic assay Toxicity 0.000 description 1
- 238000011198 co-culture assay Methods 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 210000002249 digestive system Anatomy 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- XEYBHCRIKKKOSS-UHFFFAOYSA-N disodium;azanylidyneoxidanium;iron(2+);pentacyanide Chemical compound [Na+].[Na+].[Fe+2].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].[O+]#N XEYBHCRIKKKOSS-UHFFFAOYSA-N 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 125000002228 disulfide group Chemical group 0.000 description 1
- 125000005414 dithiopyridyl group Chemical group 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229960005501 duocarmycin Drugs 0.000 description 1
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 description 1
- 229930184221 duocarmycin Natural products 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- LJQQFQHBKUKHIS-WJHRIEJJSA-N esperamicin Chemical compound O1CC(NC(C)C)C(OC)CC1OC1C(O)C(NOC2OC(C)C(SC)C(O)C2)C(C)OC1OC1C(\C2=C/CSSSC)=C(NC(=O)OC)C(=O)C(OC3OC(C)C(O)C(OC(=O)C=4C(=CC(OC)=C(OC)C=4)NC(=O)C(=C)OC)C3)C2(O)C#C\C=C/C#C1 LJQQFQHBKUKHIS-WJHRIEJJSA-N 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 230000003325 follicular Effects 0.000 description 1
- 201000003444 follicular lymphoma Diseases 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 125000002485 formyl group Chemical class [H]C(*)=O 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 201000007487 gallbladder carcinoma Diseases 0.000 description 1
- 102000013069 gamma-Crystallins Human genes 0.000 description 1
- 108010079934 gamma-Crystallins Proteins 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 229960003297 gemtuzumab ozogamicin Drugs 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 230000011132 hemopoiesis Effects 0.000 description 1
- 102000050954 human SDC1 Human genes 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- APFVFJFRJDLVQX-AHCXROLUSA-N indium-111 Chemical compound [111In] APFVFJFRJDLVQX-AHCXROLUSA-N 0.000 description 1
- 229940055742 indium-111 Drugs 0.000 description 1
- 230000004941 influx Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 108010028309 kalinin Proteins 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 108010057670 laminin 1 Proteins 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 201000005243 lung squamous cell carcinoma Diseases 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000000302 molecular modelling Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- DIOQZVSQGTUSAI-UHFFFAOYSA-N n-butylhexane Natural products CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 231100000065 noncytotoxic Toxicity 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 208000013371 ovarian adenocarcinoma Diseases 0.000 description 1
- 201000006588 ovary adenocarcinoma Diseases 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 1
- 230000003836 peripheral circulation Effects 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000003123 plant toxin Substances 0.000 description 1
- 239000003058 plasma substitute Substances 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000011363 radioimmunotherapy Methods 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 238000006268 reductive amination reaction Methods 0.000 description 1
- 201000010174 renal carcinoma Diseases 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 210000005122 simple epithelium Anatomy 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 229940083618 sodium nitroprusside Drugs 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000005127 stratified epithelium Anatomy 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 125000005413 thiopyridyl group Chemical group 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- PXSOHRWMIRDKMP-UHFFFAOYSA-N triaziquone Chemical compound O=C1C(N2CC2)=C(N2CC2)C(=O)C=C1N1CC1 PXSOHRWMIRDKMP-UHFFFAOYSA-N 0.000 description 1
- 230000010304 tumor cell viability Effects 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 238000007039 two-step reaction Methods 0.000 description 1
- 208000010570 urinary bladder carcinoma Diseases 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 230000000304 vasodilatating effect Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960005502 α-amanitin Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6891—Pre-targeting systems involving an antibody for targeting specific cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Nanotechnology (AREA)
- Cell Biology (AREA)
- Medical Informatics (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biotechnology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1663007P | 2007-12-26 | 2007-12-26 | |
| US1661307P | 2007-12-26 | 2007-12-26 | |
| PCT/EP2008/068268 WO2009080831A1 (en) | 2007-12-26 | 2008-12-23 | Method of decreasing cytotoxic side-effects and improving efficacy of immunoconjugates |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014241327A Division JP2015038158A (ja) | 2007-12-26 | 2014-11-28 | 免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011507933A true JP2011507933A (ja) | 2011-03-10 |
| JP2011507933A5 JP2011507933A5 (OSRAM) | 2012-02-16 |
Family
ID=40459778
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010540130A Pending JP2011507933A (ja) | 2007-12-26 | 2008-12-23 | 免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 |
| JP2014241327A Pending JP2015038158A (ja) | 2007-12-26 | 2014-11-28 | 免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014241327A Pending JP2015038158A (ja) | 2007-12-26 | 2014-11-28 | 免疫複合体の細胞傷害性副作用の低減及び有効性の改善方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9011864B2 (OSRAM) |
| EP (1) | EP2238169A1 (OSRAM) |
| JP (2) | JP2011507933A (OSRAM) |
| AR (1) | AR069979A1 (OSRAM) |
| TW (1) | TWI524897B (OSRAM) |
| WO (1) | WO2009080831A1 (OSRAM) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016510597A (ja) * | 2013-03-07 | 2016-04-11 | ベイラー カレッジ オブ メディスンBaylor College Of Medicine | がんにおけるcd138の標的化 |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
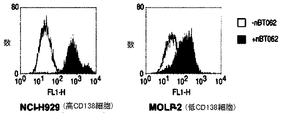
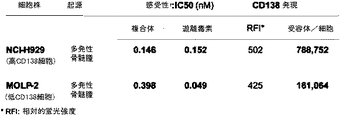
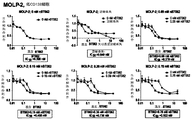
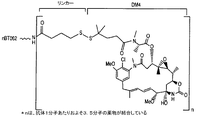
| US20060045877A1 (en) * | 2004-08-30 | 2006-03-02 | Goldmakher Viktor S | Immunoconjugates targeting syndecan-1 expressing cells and use thereof |
| MX2010007102A (es) * | 2007-12-26 | 2011-07-01 | Biotest Ag | Inmunoconjugados dirigidos contra cd138 y sus usos. |
| WO2009080829A1 (en) * | 2007-12-26 | 2009-07-02 | Biotest Ag | Agents targeting cd138 and uses thereof |
| US9446146B2 (en) * | 2007-12-26 | 2016-09-20 | Biotest Ag | Methods and agents for improving targeting of CD138 expressing tumor cells |
| AU2013201618B2 (en) * | 2009-05-06 | 2016-06-02 | Biotest Ag | Uses of immunoconjugates targeting CD138 |
| CA2761120A1 (en) * | 2009-05-06 | 2010-11-11 | Biotest Ag | Uses of immunoconjugates targeting cd138 |
| EP2589609A1 (en) | 2011-11-03 | 2013-05-08 | Pierre Fabre Medicament | Antigen binding protein and its use as addressing product for the treatment of cancer |
| KR20140100571A (ko) | 2011-12-08 | 2014-08-14 | 바이오테스트 아게 | Cd138을 타겟팅하는 면역접합체의 용도 |
| WO2014089177A2 (en) | 2012-12-04 | 2014-06-12 | Massachusetts Institute Of Technology | Compounds, conjugates and compositions of epipolythiodiketopiperazines and polythiodiketopiperazines |
| US10208125B2 (en) | 2013-07-15 | 2019-02-19 | University of Pittsburgh—of the Commonwealth System of Higher Education | Anti-mucin 1 binding agents and uses thereof |
| EP3736292B1 (en) | 2013-12-17 | 2024-05-08 | Genentech, Inc. | Anti-cd3 antibodies and methods of use |
| AU2015282627B2 (en) | 2014-06-30 | 2020-04-02 | Glykos Finland Oy | Saccharide derivative of a toxic payload and antibody conjugates thereof |
| CN107708741A (zh) * | 2015-06-12 | 2018-02-16 | 免疫医疗公司 | 用嵌合抗原受体(car)构建体和表达car构建体的t细胞(car‑t)或nk细胞(car‑nk)进行的疾病疗法 |
| US10323094B2 (en) | 2015-06-16 | 2019-06-18 | Genentech, Inc. | Humanized and affinity matured antibodies to FcRH5 and methods of use |
| EP3310811B1 (en) | 2015-06-16 | 2021-06-16 | Genentech, Inc. | Anti-cd3 antibodies and methods of use |
| US10918627B2 (en) | 2016-05-11 | 2021-02-16 | Massachusetts Institute Of Technology | Convergent and enantioselective total synthesis of Communesin analogs |
| WO2018093821A1 (en) | 2016-11-15 | 2018-05-24 | Genentech, Inc. | Dosing for treatment with anti-cd20/anti-cd3 bispecific antibodies |
| WO2018112334A1 (en) | 2016-12-16 | 2018-06-21 | Bluefin Biomedicine, Inc. | Anti-cub domain-containing protein 1 (cdcp1) antibodies, antibody drug conjugates, and methods of use thereof |
| WO2018209239A1 (en) | 2017-05-11 | 2018-11-15 | Massachusetts Institute Of Technology | Potent agelastatin derivatives as modulators for cancer invasion and metastasis |
| AU2018345637A1 (en) | 2017-10-02 | 2020-03-05 | Visterra, Inc. | Antibody molecules to CD138 and uses thereof |
| US10640508B2 (en) | 2017-10-13 | 2020-05-05 | Massachusetts Institute Of Technology | Diazene directed modular synthesis of compounds with quaternary carbon centers |
| AR115360A1 (es) | 2018-02-08 | 2021-01-13 | Genentech Inc | Moléculas de unión al antígeno y métodos de uso |
| EP3962528A4 (en) * | 2019-04-29 | 2023-01-11 | Immunogen, Inc. | Therapeutic combinations comprising anti-cd123 immunoconjugates |
| US11535634B2 (en) | 2019-06-05 | 2022-12-27 | Massachusetts Institute Of Technology | Compounds, conjugates, and compositions of epipolythiodiketopiperazines and polythiodiketopiperazines and uses thereof |
| TW202112822A (zh) | 2019-06-17 | 2021-04-01 | 美商威特拉公司 | 針對cd138之人類化抗體分子及其用途 |
| US11845799B2 (en) | 2019-12-13 | 2023-12-19 | Genentech, Inc. | Anti-Ly6G6D antibodies and methods of use |
| EP4240766A2 (en) | 2020-11-04 | 2023-09-13 | Genentech, Inc. | Subcutaneous dosing of anti-cd20/anti-cd3 bispecific antibodies |
| JP7402381B2 (ja) | 2020-11-04 | 2023-12-20 | ジェネンテック, インコーポレイテッド | 抗cd20/抗cd3二重特異性抗体による処置のための投与 |
| US12030888B2 (en) | 2021-02-24 | 2024-07-09 | Massachusetts Institute Of Technology | Himastatin derivatives, and processes of preparation thereof, and uses thereof |
| JP2023546368A (ja) | 2021-05-14 | 2023-11-02 | ジェネンテック, インコーポレイテッド | モスネツズマブおよびポラツズマブベドチンを用いたcd20陽性増殖性障害の処置のための方法 |
| EP4508086A1 (en) | 2022-04-13 | 2025-02-19 | Genentech, Inc. | Pharmaceutical compositions of mosunetuzumab and methods of use |
| GB202313214D0 (en) * | 2023-08-30 | 2023-10-11 | Iksuda Therapeutics Ltd | Co-administration of antibody-drug conjugate |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01501476A (ja) * | 1986-10-09 | 1989-05-25 | ネオルックス コーポレイション | 抗体、抗体断片、ホルモン並びに他の標的剤、およびそれらの配合体を標的とする改良方法 |
| US20050271653A1 (en) * | 2002-04-23 | 2005-12-08 | Meir Strahilevitz | Methods and devices for targeting a site in a mammal and for removing species from a mammal |
| US20060045877A1 (en) * | 2004-08-30 | 2006-03-02 | Goldmakher Viktor S | Immunoconjugates targeting syndecan-1 expressing cells and use thereof |
Family Cites Families (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3896111A (en) | 1973-02-20 | 1975-07-22 | Research Corp | Ansa macrolides |
| US4151042A (en) | 1977-03-31 | 1979-04-24 | Takeda Chemical Industries, Ltd. | Method for producing maytansinol and its derivatives |
| US4169888A (en) | 1977-10-17 | 1979-10-02 | The Upjohn Company | Composition of matter and process |
| US4137230A (en) | 1977-11-14 | 1979-01-30 | Takeda Chemical Industries, Ltd. | Method for the production of maytansinoids |
| US4265814A (en) | 1978-03-24 | 1981-05-05 | Takeda Chemical Industries | Matansinol 3-n-hexadecanoate |
| US4307016A (en) | 1978-03-24 | 1981-12-22 | Takeda Chemical Industries, Ltd. | Demethyl maytansinoids |
| JPS5562090A (en) | 1978-10-27 | 1980-05-10 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4256746A (en) | 1978-11-14 | 1981-03-17 | Takeda Chemical Industries | Dechloromaytansinoids, their pharmaceutical compositions and method of use |
| JPS55164687A (en) | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS5566585A (en) | 1978-11-14 | 1980-05-20 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55102583A (en) | 1979-01-31 | 1980-08-05 | Takeda Chem Ind Ltd | 20-acyloxy-20-demethylmaytansinoid compound |
| JPS55162791A (en) | 1979-06-05 | 1980-12-18 | Takeda Chem Ind Ltd | Antibiotic c-15003pnd and its preparation |
| JPS55164685A (en) | 1979-06-08 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55164686A (en) | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4309428A (en) | 1979-07-30 | 1982-01-05 | Takeda Chemical Industries, Ltd. | Maytansinoids |
| JPS5645483A (en) | 1979-09-19 | 1981-04-25 | Takeda Chem Ind Ltd | C-15003phm and its preparation |
| EP0028683A1 (en) | 1979-09-21 | 1981-05-20 | Takeda Chemical Industries, Ltd. | Antibiotic C-15003 PHO and production thereof |
| JPS5645485A (en) | 1979-09-21 | 1981-04-25 | Takeda Chem Ind Ltd | Production of c-15003pnd |
| US4444887A (en) | 1979-12-10 | 1984-04-24 | Sloan-Kettering Institute | Process for making human antibody producing B-lymphocytes |
| WO1982001188A1 (en) | 1980-10-08 | 1982-04-15 | Takeda Chemical Industries Ltd | 4,5-deoxymaytansinoide compounds and process for preparing same |
| US4313946A (en) | 1981-01-27 | 1982-02-02 | The United States Of America As Represented By The Secretary Of Agriculture | Chemotherapeutically active maytansinoids from Trewia nudiflora |
| US4315929A (en) | 1981-01-27 | 1982-02-16 | The United States Of America As Represented By The Secretary Of Agriculture | Method of controlling the European corn borer with trewiasine |
| JPS57192389A (en) | 1981-05-20 | 1982-11-26 | Takeda Chem Ind Ltd | Novel maytansinoid |
| US4761111A (en) | 1981-09-18 | 1988-08-02 | Brown Andrew M | Automobile lifting and towing equipment |
| US4418064A (en) | 1982-09-29 | 1983-11-29 | The United States Of America As Represented By The Secretary Of Agriculture | Chemotherapeutically active maytansinoids: treflorine, trenudine, and N-methyltrenudone |
| GB8607679D0 (en) | 1986-03-27 | 1986-04-30 | Winter G P | Recombinant dna product |
| US5034223A (en) * | 1986-10-09 | 1991-07-23 | Neorx Corporation | Methods for improved targeting of antibody, antibody fragments, hormones and other targeting agents, and conjugates thereof |
| US5053394A (en) | 1988-09-21 | 1991-10-01 | American Cyanamid Company | Targeted forms of methyltrithio antitumor agents |
| US5612016A (en) * | 1988-04-01 | 1997-03-18 | Immunomedics, Inc. | Conjugates of antibodies and bifunctional ligands |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5208020A (en) | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| ATE356869T1 (de) | 1990-01-12 | 2007-04-15 | Amgen Fremont Inc | Bildung von xenogenen antikörpern |
| AU648313B2 (en) | 1990-04-25 | 1994-04-21 | Pharmacia & Upjohn Company | Novel CC-1065 analogs |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| DE69233482T2 (de) | 1991-05-17 | 2006-01-12 | Merck & Co., Inc. | Verfahren zur Verminderung der Immunogenität der variablen Antikörperdomänen |
| US5998656A (en) | 1991-09-23 | 1999-12-07 | Florida State University | C10 tricyclic taxanes |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US6080777A (en) | 1992-01-31 | 2000-06-27 | The Trustees Of Columbia University In The City Of New York | Taxol as a radiation sensitizer |
| US5200534A (en) | 1992-03-13 | 1993-04-06 | University Of Florida | Process for the preparation of taxol and 10-deacetyltaxol |
| DE69231123T2 (de) | 1992-03-25 | 2001-02-15 | Immunogen Inc | Konjugaten von Zell-bindender Mittel und Derivaten von CC-1065 |
| US6005079A (en) | 1992-08-21 | 1999-12-21 | Vrije Universiteit Brussels | Immunoglobulins devoid of light chains |
| US5639641A (en) | 1992-09-09 | 1997-06-17 | Immunogen Inc. | Resurfacing of rodent antibodies |
| US5703247A (en) | 1993-03-11 | 1997-12-30 | Virginia Tech Intellectual Properties, Inc. | 2-Debenzoyl-2-acyl taxol derivatives and methods for making same |
| US5475011A (en) | 1993-03-26 | 1995-12-12 | The Research Foundation Of State University Of New York | Anti-tumor compounds, pharmaceutical compositions, methods for preparation thereof and for treatment |
| US6740734B1 (en) | 1994-01-14 | 2004-05-25 | Biovitrum Ab | Bacterial receptor structures |
| SE9400088D0 (sv) | 1994-01-14 | 1994-01-14 | Kabi Pharmacia Ab | Bacterial receptor structures |
| US5773001A (en) | 1994-06-03 | 1998-06-30 | American Cyanamid Company | Conjugates of methyltrithio antitumor agents and intermediates for their synthesis |
| US5763477A (en) | 1994-07-22 | 1998-06-09 | Dr. Reddy's Research Foundation | Taxane derivatives from 14-β-hydroxy-10 deacetylbaccatin III |
| EP0815096A4 (en) | 1995-03-10 | 1998-07-08 | Hauser Chemical Res Inc | EPOXIDE OF CEPHALOMANNIN, ITS ANALOGS, AND PROCESS FOR PREPARING THE SAME |
| ATE390933T1 (de) | 1995-04-27 | 2008-04-15 | Amgen Fremont Inc | Aus immunisierten xenomäusen stammende menschliche antikörper gegen il-8 |
| AU2466895A (en) | 1995-04-28 | 1996-11-18 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5712374A (en) | 1995-06-07 | 1998-01-27 | American Cyanamid Company | Method for the preparation of substantiallly monomeric calicheamicin derivative/carrier conjugates |
| US5714586A (en) | 1995-06-07 | 1998-02-03 | American Cyanamid Company | Methods for the preparation of monomeric calicheamicin derivative/carrier conjugates |
| US5916771A (en) | 1996-10-11 | 1999-06-29 | Abgenix, Inc. | Production of a multimeric protein by cell fusion method |
| EP2314625B1 (en) | 1996-12-03 | 2014-05-07 | Amgen Fremont Inc. | Transgenic mammals having human Ig loci including plural VH and Vkappa regions and antibodies produced therefrom |
| SI0970126T1 (en) | 1997-04-14 | 2001-08-31 | Micromet Ag | Novel method for the production of antihuman antigen receptors and uses thereof |
| US6235883B1 (en) | 1997-05-05 | 2001-05-22 | Abgenix, Inc. | Human monoclonal antibodies to epidermal growth factor receptor |
| US6962702B2 (en) | 1998-06-22 | 2005-11-08 | Immunomedics Inc. | Production and use of novel peptide-based agents for use with bi-specific antibodies |
| US6087362A (en) * | 1999-03-16 | 2000-07-11 | Pentech Pharmaceuticals, Inc. | Apomorphine and sildenafil composition |
| PT1242401E (pt) | 1999-11-24 | 2007-03-30 | Immunogen Inc | Agentes citotóxicos compreendendo taxanos e o seu uso terapêutico |
| US6333410B1 (en) | 2000-08-18 | 2001-12-25 | Immunogen, Inc. | Process for the preparation and purification of thiol-containing maytansinoids |
| US6441163B1 (en) | 2001-05-31 | 2002-08-27 | Immunogen, Inc. | Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents |
| NZ603111A (en) | 2001-08-03 | 2014-05-30 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US6716821B2 (en) | 2001-12-21 | 2004-04-06 | Immunogen Inc. | Cytotoxic agents bearing a reactive polyethylene glycol moiety, cytotoxic conjugates comprising polyethylene glycol linking groups, and methods of making and using the same |
| US6534660B1 (en) | 2002-04-05 | 2003-03-18 | Immunogen, Inc. | CC-1065 analog synthesis |
| US6756397B2 (en) | 2002-04-05 | 2004-06-29 | Immunogen, Inc. | Prodrugs of CC-1065 analogs |
| HUE057124T2 (hu) | 2002-05-02 | 2022-04-28 | Wyeth Holdings Llc | Calicheamicin származék - hordozó konjugátumok |
| US20050276812A1 (en) | 2004-06-01 | 2005-12-15 | Genentech, Inc. | Antibody-drug conjugates and methods |
| US6596757B1 (en) | 2002-05-14 | 2003-07-22 | Immunogen Inc. | Cytotoxic agents comprising polyethylene glycol-containing taxanes and their therapeutic use |
| US7390898B2 (en) | 2002-08-02 | 2008-06-24 | Immunogen Inc. | Cytotoxic agents containing novel potent taxanes and their therapeutic use |
| US20040126379A1 (en) | 2002-08-21 | 2004-07-01 | Boehringer Ingelheim International Gmbh | Compositions and methods for treating cancer using cytotoxic CD44 antibody immunoconjugates and chemotherapeutic agents |
| JP5425365B2 (ja) | 2003-01-22 | 2014-02-26 | グリカート バイオテクノロジー アクチェンゲゼルシャフト | 増加したFcレセプター結合親和性およびエフェクター機能を有する抗体を作製するための融合構築物およびその使用 |
| US7449303B2 (en) | 2003-05-02 | 2008-11-11 | Health Research, Inc. | Use of JAG2 expression in diagnosis of plasma cell disorders |
| US7276497B2 (en) | 2003-05-20 | 2007-10-02 | Immunogen Inc. | Cytotoxic agents comprising new maytansinoids |
| AU2004258955C1 (en) | 2003-07-21 | 2012-07-26 | Immunogen, Inc. | A CA6 antigen-specific cytotoxic conjugate and methods of using the same |
| MX2007011064A (es) | 2005-03-23 | 2008-02-19 | Genmab As | Anticuerpos contra cd38 para tratamiento de mieloma multiple. |
| WO2006113623A2 (en) | 2005-04-15 | 2006-10-26 | Immunogen, Inc. | Elimination of heterogeneous or mixed cell population in tumors |
| JP2009518025A (ja) | 2005-12-06 | 2009-05-07 | ドマンティス リミテッド | 細胞表面標的に対して結合特異性を有する二重特異性リガンドおよびその使用方法 |
| EP2015772A2 (de) | 2006-05-03 | 2009-01-21 | Elke Pogge Von Strandmann | Mittel zur behandlung von malignen erkrankungen |
-
2008
- 2008-12-23 AR ARP080105722A patent/AR069979A1/es unknown
- 2008-12-23 EP EP08864283A patent/EP2238169A1/en not_active Ceased
- 2008-12-23 WO PCT/EP2008/068268 patent/WO2009080831A1/en not_active Ceased
- 2008-12-23 JP JP2010540130A patent/JP2011507933A/ja active Pending
- 2008-12-23 US US12/342,180 patent/US9011864B2/en not_active Expired - Fee Related
- 2008-12-24 TW TW097150429A patent/TWI524897B/zh not_active IP Right Cessation
-
2014
- 2014-11-28 JP JP2014241327A patent/JP2015038158A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01501476A (ja) * | 1986-10-09 | 1989-05-25 | ネオルックス コーポレイション | 抗体、抗体断片、ホルモン並びに他の標的剤、およびそれらの配合体を標的とする改良方法 |
| US20050271653A1 (en) * | 2002-04-23 | 2005-12-08 | Meir Strahilevitz | Methods and devices for targeting a site in a mammal and for removing species from a mammal |
| US20060045877A1 (en) * | 2004-08-30 | 2006-03-02 | Goldmakher Viktor S | Immunoconjugates targeting syndecan-1 expressing cells and use thereof |
Non-Patent Citations (8)
| Title |
|---|
| JPN6013024655; Cancer Immunology Immunotherapy Vol.47,No.1, 1998, p39-46 * |
| JPN6013024658; Cancer Research Vol.49,No.6, 1989, p1587-1594 * |
| JPN6013024661; Blood Vol.104,No.12, 2004, p3688-3696 * |
| JPN6013024664; Cancer Vol.94,No.Suppl.4, 2002, p1202-1209 * |
| JPN6013024667; International Journal of Cancer Vol.83,No.4, 1999, p571-576 * |
| JPN6014031680; International Journal of Hematology. Supplement Vol.76,No.Suppl.1, 2002, p106 * |
| JPN6014031682; Blood Vol.95,No.2, 2000, p388-392 * |
| JPN6014031684; Haematologica Vol.89,No.3, 2004, p370-371 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016510597A (ja) * | 2013-03-07 | 2016-04-11 | ベイラー カレッジ オブ メディスンBaylor College Of Medicine | がんにおけるcd138の標的化 |
| JP2019010117A (ja) * | 2013-03-07 | 2019-01-24 | ベイラー カレッジ オブ メディスンBaylor College Of Medicine | がんにおけるcd138の標的化 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2009080831A1 (en) | 2009-07-02 |
| TWI524897B (zh) | 2016-03-11 |
| EP2238169A1 (en) | 2010-10-13 |
| US20090181038A1 (en) | 2009-07-16 |
| JP2015038158A (ja) | 2015-02-26 |
| TW200934509A (en) | 2009-08-16 |
| US9011864B2 (en) | 2015-04-21 |
| AR069979A1 (es) | 2010-03-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9011864B2 (en) | Method of decreasing cytotoxic side-effects and improving efficacy of immunoconjugates | |
| JP5990365B2 (ja) | Cd138を標的とする剤及びその使用 | |
| AU2010244428B2 (en) | Uses of immunoconjugates targeting CD138 | |
| AU2008339911B2 (en) | Immunoconjugates targeting CD138 and uses thereof | |
| TWI450726B (zh) | 用於改良靶定於表現cd138之腫瘤細胞的方法及藥劑 | |
| US8840898B2 (en) | Immunoconjugates targeting syndecan-1 expressing cells and use thereof | |
| RU2632108C2 (ru) | Применения иммуноконъюгатов, мишенью которых является cd138 | |
| AU2013201618B2 (en) | Uses of immunoconjugates targeting CD138 | |
| HK1149032B (en) | Methods and agents for improving targeting of cd138 expressing tumor cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111222 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111222 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130528 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130827 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130903 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130927 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131004 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131028 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140729 |