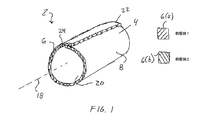
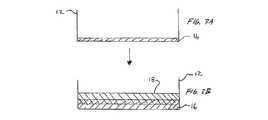
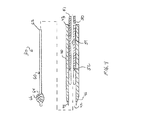
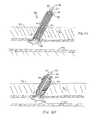
JP2011502582A - 血管穿刺を塞ぐ装置および方法 - Google Patents
血管穿刺を塞ぐ装置および方法 Download PDFInfo
- Publication number
- JP2011502582A JP2011502582A JP2010532298A JP2010532298A JP2011502582A JP 2011502582 A JP2011502582 A JP 2011502582A JP 2010532298 A JP2010532298 A JP 2010532298A JP 2010532298 A JP2010532298 A JP 2010532298A JP 2011502582 A JP2011502582 A JP 2011502582A
- Authority
- JP
- Japan
- Prior art keywords
- carrier
- adhesive layer
- layer component
- puncture
- hydrogel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 55
- 230000000903 blocking effect Effects 0.000 title claims 3
- 230000002792 vascular Effects 0.000 title description 2
- 239000012790 adhesive layer Substances 0.000 claims abstract description 117
- 239000000017 hydrogel Substances 0.000 claims abstract description 65
- 239000002243 precursor Substances 0.000 claims abstract description 63
- 238000011065 in-situ storage Methods 0.000 claims abstract description 28
- 239000000853 adhesive Substances 0.000 claims abstract description 23
- 230000001070 adhesive effect Effects 0.000 claims abstract description 22
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 9
- 239000011148 porous material Substances 0.000 claims abstract 2
- 239000000463 material Substances 0.000 claims description 53
- 229920000642 polymer Polymers 0.000 claims description 33
- 239000012530 fluid Substances 0.000 claims description 29
- 239000012190 activator Substances 0.000 claims description 13
- 229910021538 borax Inorganic materials 0.000 claims description 12
- 235000010339 sodium tetraborate Nutrition 0.000 claims description 12
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 claims description 12
- 239000003814 drug Substances 0.000 claims description 9
- 230000023597 hemostasis Effects 0.000 claims description 9
- 229940124597 therapeutic agent Drugs 0.000 claims description 6
- 238000004891 communication Methods 0.000 claims description 5
- 229920001184 polypeptide Polymers 0.000 claims description 5
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 5
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 5
- 239000003002 pH adjusting agent Substances 0.000 claims description 4
- 239000012876 carrier material Substances 0.000 claims description 3
- 238000003780 insertion Methods 0.000 claims description 2
- 230000037431 insertion Effects 0.000 claims description 2
- 230000001225 therapeutic effect Effects 0.000 claims description 2
- 239000000032 diagnostic agent Substances 0.000 claims 3
- 229940039227 diagnostic agent Drugs 0.000 claims 3
- 150000004677 hydrates Chemical class 0.000 claims 1
- 230000014759 maintenance of location Effects 0.000 abstract 1
- 210000004204 blood vessel Anatomy 0.000 description 43
- 239000010410 layer Substances 0.000 description 43
- 239000000243 solution Substances 0.000 description 15
- 229920001223 polyethylene glycol Polymers 0.000 description 10
- 238000007789 sealing Methods 0.000 description 10
- 239000008280 blood Substances 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 9
- 208000014674 injury Diseases 0.000 description 9
- 239000000565 sealant Substances 0.000 description 8
- 230000008733 trauma Effects 0.000 description 8
- 239000007864 aqueous solution Substances 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 210000002445 nipple Anatomy 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 210000001124 body fluid Anatomy 0.000 description 4
- 239000010839 body fluid Substances 0.000 description 4
- 238000004132 cross linking Methods 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- VVPXCQJEIWWMEH-UHFFFAOYSA-N 2-(2,5-dioxopyrrolidin-1-yl)pentanedioic acid Chemical compound OC(=O)CCC(C(O)=O)N1C(=O)CCC1=O VVPXCQJEIWWMEH-UHFFFAOYSA-N 0.000 description 3
- QXZGLTYKKZKGLN-UHFFFAOYSA-N 4-(2,5-dioxopyrrolidin-1-yl)oxy-4-oxobutanoic acid Chemical compound OC(=O)CCC(=O)ON1C(=O)CCC1=O QXZGLTYKKZKGLN-UHFFFAOYSA-N 0.000 description 3
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 3
- 229920000954 Polyglycolide Polymers 0.000 description 3
- 206010052428 Wound Diseases 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 229940024606 amino acid Drugs 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 230000035876 healing Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 229920000747 poly(lactic acid) Polymers 0.000 description 3
- 230000001737 promoting effect Effects 0.000 description 3
- 210000005166 vasculature Anatomy 0.000 description 3
- PVVTWNMXEHROIA-UHFFFAOYSA-N 2-(3-hydroxypropyl)-1h-quinazolin-4-one Chemical compound C1=CC=C2NC(CCCO)=NC(=O)C2=C1 PVVTWNMXEHROIA-UHFFFAOYSA-N 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 206010018852 Haematoma Diseases 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 230000000994 depressogenic effect Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- -1 for example Substances 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 230000000269 nucleophilic effect Effects 0.000 description 2
- 239000004633 polyglycolic acid Substances 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229920002994 synthetic fiber Polymers 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 206010003162 Arterial injury Diseases 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 229920002201 Oxidized cellulose Polymers 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000002313 adhesive film Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229960005188 collagen Drugs 0.000 description 1
- 239000000515 collagen sponge Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000000881 depressing effect Effects 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 210000001105 femoral artery Anatomy 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 230000002439 hemostatic effect Effects 0.000 description 1
- 230000000887 hydrating effect Effects 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000009740 moulding (composite fabrication) Methods 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229940107304 oxidized cellulose Drugs 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 229940098458 powder spray Drugs 0.000 description 1
- 238000010944 pre-mature reactiony Methods 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 239000003106 tissue adhesive Substances 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 239000005526 vasoconstrictor agent Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00491—Surgical glue applicators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/92—Stents in the form of a rolled-up sheet expanding after insertion into the vessel, e.g. with a spiral shape in cross-section
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0015—Medicaments; Biocides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0031—Hydrogels or hydrocolloids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00004—(bio)absorbable, (bio)resorbable or resorptive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00477—Coupling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00491—Surgical glue applicators
- A61B2017/00495—Surgical glue applicators for two-component glue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A61B2017/00646—Type of implements
- A61B2017/0065—Type of implements the implement being an adhesive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A61B2017/00646—Type of implements
- A61B2017/00654—Type of implements entirely comprised between the two sides of the opening
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/22—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for
- A61B2017/22038—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for with a guide wire
- A61B2017/22047—Means for immobilising the guide wire in the patient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0076—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof multilayered, e.g. laminated structures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/04—Materials for stopping bleeding
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Cardiology (AREA)
- Epidemiology (AREA)
- Materials Engineering (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Dispersion Chemistry (AREA)
- Surgical Instruments (AREA)
- Materials For Medical Uses (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US98515007P | 2007-11-02 | 2007-11-02 | |
| PCT/US2008/082102 WO2009059217A2 (en) | 2007-11-02 | 2008-10-31 | Apparatus and methods for sealing a vascular puncture |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011502582A true JP2011502582A (ja) | 2011-01-27 |
| JP2011502582A5 JP2011502582A5 (enExample) | 2011-12-22 |
Family
ID=40591779
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010532298A Pending JP2011502582A (ja) | 2007-11-02 | 2008-10-31 | 血管穿刺を塞ぐ装置および方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8852230B2 (enExample) |
| EP (1) | EP2209426A4 (enExample) |
| JP (1) | JP2011502582A (enExample) |
| WO (1) | WO2009059217A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023544891A (ja) * | 2020-10-13 | 2023-10-25 | マサチューセッツ インスチテュート オブ テクノロジー | 生体接着材及び生体接着材で組織を接着するための低侵襲方法 |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2209426A4 (en) | 2007-11-02 | 2015-04-22 | Incept Llc | APPARATUS AND METHODS FOR CLOSING VASCULAR PERFORATION |
| JP5418883B2 (ja) * | 2009-03-10 | 2014-02-19 | ニプロ株式会社 | 内視鏡下手術用デバイス |
| JP2013516279A (ja) | 2010-01-06 | 2013-05-13 | セント ジュード メディカル インコーポレイテッド | 経皮穿刺部位を封止する方法およびシステム |
| CA2730598C (en) * | 2010-03-16 | 2018-03-13 | Confluent Surgical, Inc. | Modulating drug release rate by controlling the kinetics of the ph transition in hydrogels |
| WO2012047981A2 (en) * | 2010-10-07 | 2012-04-12 | Boston Scientific Scimed, Inc. | Biodegradable adhesive film for vascular closure |
| CA2824964C (en) | 2011-01-19 | 2019-01-08 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US9820728B2 (en) | 2011-01-19 | 2017-11-21 | Access Closure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US20130046376A1 (en) | 2011-06-24 | 2013-02-21 | Ali Hassan | Method and devices for flow occlusion during device exchanges |
| WO2012178133A1 (en) | 2011-06-24 | 2012-12-27 | Accessclosure, Inc. | Transapical closure devices and methods for use |
| US10434292B2 (en) | 2011-06-24 | 2019-10-08 | Access Closure | Method and devices for flow occlusion during device exchanges |
| US8758427B2 (en) | 2011-12-02 | 2014-06-24 | Vascular Solutions, Inc. | Elongated expandable member for occluding varicose veins |
| WO2013142515A1 (en) | 2012-03-23 | 2013-09-26 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US9757105B2 (en) | 2012-03-23 | 2017-09-12 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US8721680B2 (en) | 2012-03-23 | 2014-05-13 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US9532785B2 (en) | 2012-05-09 | 2017-01-03 | Access Closure, Inc. | Method and devices for flow occlusion during device exchanges |
| US9211374B2 (en) * | 2012-05-25 | 2015-12-15 | Robert F. Wallace | Therapeutic implantable device |
| US20140135830A1 (en) * | 2012-11-14 | 2014-05-15 | St. Jude Medical Puerto Rico Llc | Dual delivery systems, devices, and related methods for bioadhesives |
| US10842969B2 (en) | 2013-10-25 | 2020-11-24 | Mercator Medsystems, Inc. | Systems and methods of treating malacia by local delivery of hydrogel to augment tissue |
| US20160045300A1 (en) * | 2014-05-16 | 2016-02-18 | Terumo Kabushiki Kaisha | Method and apparatus for treating urethral stricture |
| US10076644B2 (en) | 2014-05-16 | 2018-09-18 | Terumo Kabushiki Kaisha | Method and apparatus for treating urethral stricture |
| AU2015266837B2 (en) | 2014-05-29 | 2019-04-04 | Access Closure, Inc. | Chitosan and polyethylene glycol copolymers and methods and devices for using same for sealing a vascular puncture |
| EP3164083A1 (en) * | 2014-07-02 | 2017-05-10 | The Cleveland Clinic Foundation | Anastomosis devices and methods of using same |
| US9943314B2 (en) | 2015-04-14 | 2018-04-17 | Teleflex Innovations S.À.R.L. | Magnetically-driven delivery assembly and method |
| EP3288626B8 (en) | 2015-04-27 | 2025-12-10 | Tulavi Therapeutics, Inc. | Substances and compositions for use in blocking nerve regeneration |
| WO2017139487A1 (en) | 2016-02-09 | 2017-08-17 | Northwind Medical, Inc. | Methods, agents, and devices for local neuromodulation of autonomic nerves |
| CA3031761A1 (en) | 2016-06-29 | 2018-01-04 | Tulavi Therapeutics, Inc. | Treatment of sepsis and related inflammatory conditions by local neuromodulation of the autonomic nervous system |
| TWI644657B (zh) * | 2017-05-05 | 2018-12-21 | 張復龍 | Drainage line and its preparation method |
| CN107174319B (zh) * | 2017-06-15 | 2024-02-23 | 无锡圣诺亚科技有限公司 | 脏器小结节穿刺定位器 |
| CN112638437B (zh) | 2018-07-02 | 2023-12-08 | 图拉维治疗股份有限公司 | 原位形成神经帽的方法和装置 |
| US20210315587A1 (en) | 2018-07-02 | 2021-10-14 | Tulavi Therapeutics, Inc. | Methods and devices for in situ formed nerve cap with rapid release |
| CN115087403B (zh) * | 2019-12-19 | 2025-10-31 | 巴德外周血管股份有限公司 | 具有胸膜接近衬套的用于在穿通胸膜层中使用的导引器套管 |
| US20240066178A1 (en) * | 2022-08-30 | 2024-02-29 | Ethicon, Inc. | Sealants for tissue closure |
| US20250049984A1 (en) * | 2023-08-11 | 2025-02-13 | Ethicon, Inc. | Absorbable Dressings for Sealing Sensitive Neural Tissue and Dura Matter |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006095098A (ja) * | 2004-09-29 | 2006-04-13 | Terumo Corp | 投与器具 |
| US20060099238A1 (en) * | 2004-11-05 | 2006-05-11 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| JP2006524111A (ja) * | 2003-04-22 | 2006-10-26 | サブ−キュー・インコーポレーテッド | 固定し引き出す技術により穿刺部位を閉塞するシステム |
| JP2007203294A (ja) * | 1995-12-07 | 2007-08-16 | Bristol Myers Squibb Co | 二又はそれ以上の種類の液体成分を塗布するためのシステム及び装置 |
| JP2008518743A (ja) * | 2004-11-05 | 2008-06-05 | アクセスクロージャー,インク. | 脈管穿刺のシーリング装置 |
Family Cites Families (154)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2115492A (en) | 1936-04-27 | 1938-04-26 | Searle & Co | Pharmaceutical products adapted for injection into the human body |
| US3765419A (en) | 1971-05-14 | 1973-10-16 | Int Paper Co | Amylose acetate |
| US4002173A (en) | 1974-07-23 | 1977-01-11 | International Paper Company | Diester crosslinked polyglucan hydrogels and reticulated sponges thereof |
| US4327709A (en) | 1978-03-06 | 1982-05-04 | Datascope Corp. | Apparatus and method for the percutaneous introduction of intra-aortic balloons into the human body |
| US4260077A (en) | 1979-10-04 | 1981-04-07 | Aelco Corporation | Dual separable dispenser |
| US4362150A (en) | 1980-09-10 | 1982-12-07 | Kontron Cardiovascular Inc. | Percutaneous intra-aortic balloon apparatus |
| DE3265697D1 (en) | 1981-02-05 | 1985-10-03 | Nippon Oil Co Ltd | Process for preparing a hydrogel |
| US4734097A (en) | 1981-09-25 | 1988-03-29 | Nippon Oil Company, Ltd. | Medical material of polyvinyl alcohol and process of making |
| JPS5930881A (ja) | 1982-08-13 | 1984-02-18 | Nippon Oil Co Ltd | 保冷用ゲルの製造法 |
| US4540404A (en) | 1983-01-19 | 1985-09-10 | Datascope Corp. | Balloon catheter with intrinsic introducer for percutaneous insertion into a blood vessel over a guide wire, and method of use |
| JPS6144825A (ja) | 1984-08-09 | 1986-03-04 | Unitika Ltd | 止血剤 |
| US4738658A (en) | 1986-09-19 | 1988-04-19 | Aries Medical Incorporated | Tapered hemostatic device for use in conjunction with a catheter for alleviating blood leakage and method for using same |
| USRE34866E (en) | 1987-02-17 | 1995-02-21 | Kensey Nash Corporation | Device for sealing percutaneous puncture in a vessel |
| US4852568A (en) | 1987-02-17 | 1989-08-01 | Kensey Nash Corporation | Method and apparatus for sealing an opening in tissue of a living being |
| US4890612A (en) | 1987-02-17 | 1990-01-02 | Kensey Nash Corporation | Device for sealing percutaneous puncture in a vessel |
| US4838280A (en) | 1988-05-26 | 1989-06-13 | Haaga John R | Hemostatic sheath for a biopsy needle and method of use |
| US5550187A (en) | 1988-11-21 | 1996-08-27 | Collagen Corporation | Method of preparing crosslinked biomaterial compositions for use in tissue augmentation |
| US5643464A (en) | 1988-11-21 | 1997-07-01 | Collagen Corporation | Process for preparing a sterile, dry crosslinking agent |
| NL8901350A (nl) | 1989-05-29 | 1990-12-17 | Wouter Matthijs Muijs Van De M | Afsluitsamenstel. |
| US5487897A (en) | 1989-07-24 | 1996-01-30 | Atrix Laboratories, Inc. | Biodegradable implant precursor |
| US5104375A (en) | 1989-10-16 | 1992-04-14 | Johnson & Johnson Medical, Inc. | Locking holder for a pair of syringes and method of use |
| US5061274A (en) | 1989-12-04 | 1991-10-29 | Kensey Nash Corporation | Plug device for sealing openings and method of use |
| EP0609212A4 (en) | 1989-12-04 | 1995-04-19 | Kensey Nash Corp | PLUG DEVICE FOR SEALING OPENINGS AND METHOD FOR USE. |
| US5391183A (en) | 1990-09-21 | 1995-02-21 | Datascope Investment Corp | Device and method sealing puncture wounds |
| EP0476178A1 (en) | 1990-09-21 | 1992-03-25 | Bioplex Medical B.V. | Device for placing styptic material on perforated blood vessels |
| US5108421A (en) | 1990-10-01 | 1992-04-28 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5192300A (en) | 1990-10-01 | 1993-03-09 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5221259A (en) | 1990-12-27 | 1993-06-22 | Novoste Corporation | Wound treating device and method of using same |
| US5419765A (en) | 1990-12-27 | 1995-05-30 | Novoste Corporation | Wound treating device and method for treating wounds |
| US5230310A (en) * | 1991-03-29 | 1993-07-27 | Mazda Motor Corporation | Combustion chamber of internal combustion engine |
| NL9101051A (nl) | 1991-06-18 | 1993-01-18 | Ashridge Ag | Sluitinrichting voor een bloedvat of dergelijke. |
| US5104389A (en) | 1991-06-27 | 1992-04-14 | Cordis Corporation | Medical instrument valve with foam partition member having vapor permeable skin |
| US5259835A (en) | 1991-08-29 | 1993-11-09 | Tri-Point Medical L.P. | Wound closure means and method using flowable adhesive |
| US5290310A (en) | 1991-10-30 | 1994-03-01 | Howmedica, Inc. | Hemostatic implant introducer |
| US5282827A (en) | 1991-11-08 | 1994-02-01 | Kensey Nash Corporation | Hemostatic puncture closure system and method of use |
| US5258042A (en) | 1991-12-16 | 1993-11-02 | Henry Ford Health System | Intravascular hydrogel implant |
| US6056768A (en) | 1992-01-07 | 2000-05-02 | Cates; Christopher U. | Blood vessel sealing system |
| US6699261B1 (en) | 1992-01-07 | 2004-03-02 | Cch Associates, Inc. | Blood vessel sealing system |
| US6818008B1 (en) | 1992-01-07 | 2004-11-16 | Cch Associates, Inc. | Percutaneous puncture sealing method |
| JP3116287B2 (ja) * | 1992-02-03 | 2000-12-11 | 株式会社リコー | 光学式情報記録再生装置 |
| GB9206504D0 (en) | 1992-03-25 | 1992-05-06 | Jevco Ltd | Heteromorphic sponges as wound implants |
| US6350274B1 (en) | 1992-05-11 | 2002-02-26 | Regen Biologics, Inc. | Soft tissue closure systems |
| US5326350A (en) | 1992-05-11 | 1994-07-05 | Li Shu Tung | Soft tissue closure systems |
| US5413571A (en) | 1992-07-16 | 1995-05-09 | Sherwood Medical Company | Device for sealing hemostatic incisions |
| US5292332A (en) | 1992-07-27 | 1994-03-08 | Lee Benjamin I | Methods and device for percutanceous sealing of arterial puncture sites |
| US5443481A (en) | 1992-07-27 | 1995-08-22 | Lee; Benjamin I. | Methods and device for percutaneous sealing of arterial puncture sites |
| IL106660A (en) | 1992-08-13 | 1997-04-15 | Adcock Ingram Ltd | Hydrogel composition comprising polyvinyl alcohol polymer and its production |
| US5306254A (en) | 1992-10-01 | 1994-04-26 | Kensey Nash Corporation | Vessel position locating device and method of use |
| US5334216A (en) | 1992-12-10 | 1994-08-02 | Howmedica Inc. | Hemostatic plug |
| US5514158A (en) | 1992-12-28 | 1996-05-07 | Kanesaka; Nozomu | Sealing device for a percutaneous puncture |
| US5320639A (en) | 1993-03-12 | 1994-06-14 | Meadox Medicals, Inc. | Vascular plug delivery system |
| US5951583A (en) | 1993-05-25 | 1999-09-14 | Vascular Solutions, Inc. | Thrombin and collagen procoagulant and process for making the same |
| US5383896A (en) | 1993-05-25 | 1995-01-24 | Gershony; Gary | Vascular sealing device |
| US6017359A (en) | 1993-05-25 | 2000-01-25 | Vascular Solutions, Inc. | Vascular sealing apparatus |
| US5626601A (en) | 1995-10-27 | 1997-05-06 | Gary Gershony | Vascular sealing apparatus and method |
| US5409703A (en) | 1993-06-24 | 1995-04-25 | Carrington Laboratories, Inc. | Dried hydrogel from hydrophilic-hygroscopic polymer |
| US5725551A (en) | 1993-07-26 | 1998-03-10 | Myers; Gene | Method and apparatus for arteriotomy closure |
| US5486195A (en) | 1993-07-26 | 1996-01-23 | Myers; Gene | Method and apparatus for arteriotomy closure |
| US5403291A (en) | 1993-08-02 | 1995-04-04 | Quinton Instrument Company | Catheter with elongated side holes |
| US5431639A (en) | 1993-08-12 | 1995-07-11 | Boston Scientific Corporation | Treating wounds caused by medical procedures |
| WO1995008289A2 (en) | 1993-09-16 | 1995-03-30 | Scimed Life Systems, Inc. | Percutaneous repair of cardiovascular anomalies and repair compositions |
| US5383899A (en) | 1993-09-28 | 1995-01-24 | Hammerslag; Julius G. | Method of using a surface opening adhesive sealer |
| US5843124A (en) | 1993-09-28 | 1998-12-01 | Hemodynamics, Inc. | Surface opening adhesive sealer |
| AT400675B (de) | 1993-10-18 | 1996-02-26 | Immuno Ag | Spritzengarnitur zur aufbewahrung und applikation eines mehrkomponentenmaterials, spritzenvorrichtung und betätigungseinrichtung hiefür sowie verfahren zum herstellen einer befüllten, sterilen spritzenvorrichtung |
| US5437292A (en) | 1993-11-19 | 1995-08-01 | Bioseal, Llc | Method for sealing blood vessel puncture sites |
| US5728122A (en) | 1994-01-18 | 1998-03-17 | Datascope Investment Corp. | Guide wire with releaseable barb anchor |
| US5795331A (en) | 1994-01-24 | 1998-08-18 | Micro Therapeutics, Inc. | Balloon catheter for occluding aneurysms of branch vessels |
| DE69534780T2 (de) | 1994-04-08 | 2006-10-05 | Qlt Usa Inc., Fort Collins | Flüssige Zusammensetzungen zur Arzneistoffabgabe |
| US6302898B1 (en) | 1994-06-24 | 2001-10-16 | Advanced Closure Systems, Inc. | Devices for sealing punctures in body vessels |
| US6033401A (en) | 1997-03-12 | 2000-03-07 | Advanced Closure Systems, Inc. | Vascular sealing device with microwave antenna |
| US5709934A (en) | 1994-11-22 | 1998-01-20 | Tissue Engineering, Inc. | Bipolymer foams having extracellular matrix particulates |
| WO1996022123A1 (en) | 1995-01-18 | 1996-07-25 | Medchem Products, Inc. | Apparatus and method for applying a hemostatic agent onto a tissue |
| US5580923A (en) | 1995-03-14 | 1996-12-03 | Collagen Corporation | Anti-adhesion films and compositions for medical use |
| US5900245A (en) | 1996-03-22 | 1999-05-04 | Focal, Inc. | Compliant tissue sealants |
| US5785679A (en) | 1995-07-19 | 1998-07-28 | Endotex Interventional Systems, Inc. | Methods and apparatus for treating aneurysms and arterio-venous fistulas |
| US5731368A (en) | 1995-09-01 | 1998-03-24 | Union Carbide Chemicals & Plastics Technology Corporation | Aoueous vinyl polymer dispersions |
| US5752974A (en) | 1995-12-18 | 1998-05-19 | Collagen Corporation | Injectable or implantable biomaterials for filling or blocking lumens and voids of the body |
| DE19612628A1 (de) | 1996-03-29 | 1997-10-02 | Hoechst Ag | Verfahren zur Herstellung von porösen hydrophilen, hochquellfähigen Hydrogelen |
| US5728133A (en) | 1996-07-09 | 1998-03-17 | Cardiologics, L.L.C. | Anchoring device and method for sealing percutaneous punctures in vessels |
| US5836970A (en) | 1996-08-02 | 1998-11-17 | The Kendall Company | Hemostatic wound dressing |
| US6093388A (en) | 1996-08-12 | 2000-07-25 | Btg International Limited | Mannose-6-phosphate composition and its use in treating fibrotic disorders |
| US6063061A (en) | 1996-08-27 | 2000-05-16 | Fusion Medical Technologies, Inc. | Fragmented polymeric compositions and methods for their use |
| AU4648697A (en) | 1996-09-23 | 1998-04-14 | Chandrashekar Pathak | Methods and devices for preparing protein concentrates |
| US7009034B2 (en) | 1996-09-23 | 2006-03-07 | Incept, Llc | Biocompatible crosslinked polymers |
| WO1998030252A1 (en) | 1997-01-09 | 1998-07-16 | Cohesion Technologies, Inc. | Methods and apparatuses for making swellable uniformly shaped devices from polymeric materials |
| US5718916A (en) | 1997-02-03 | 1998-02-17 | Scherr; George H. | Alginate foam products |
| US6045570A (en) | 1997-02-11 | 2000-04-04 | Biointerventional Corporation | Biological sealant mixture and system for use in percutaneous occlusion of puncture sites and tracts in the human body and method |
| US5782860A (en) | 1997-02-11 | 1998-07-21 | Biointerventional Corporation | Closure device for percutaneous occlusion of puncture sites and tracts in the human body and method |
| US5951589A (en) | 1997-02-11 | 1999-09-14 | Biointerventional Corporation | Expansile device for use in blood vessels and tracts in the body and tension application device for use therewith and method |
| US6056769A (en) | 1997-02-11 | 2000-05-02 | Biointerventional Corporation | Expansile device for use in blood vessels and tracts in the body and tension application device for use therewith and method |
| US6371975B2 (en) | 1998-11-06 | 2002-04-16 | Neomend, Inc. | Compositions, systems, and methods for creating in situ, chemically cross-linked, mechanical barriers |
| US6271278B1 (en) | 1997-05-13 | 2001-08-07 | Purdue Research Foundation | Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength, and superabsorbent properties |
| US6162241A (en) | 1997-08-06 | 2000-12-19 | Focal, Inc. | Hemostatic tissue sealants |
| EP0955902B1 (en) | 1997-10-31 | 2004-10-13 | St. Jude Medical Puerto Rico B.V. | Hemostatic puncture closure device |
| WO1999023952A1 (en) | 1997-11-12 | 1999-05-20 | William Dubrul | Biological passageway occlusion removal |
| US5948829A (en) | 1997-11-25 | 1999-09-07 | Kimberly-Clark Worldwide, Inc. | Process for preparing an absorbent foam |
| US5941847A (en) | 1998-02-06 | 1999-08-24 | Medela Holding Ag | Breast shield with vacuum isolation element |
| EP1054635B1 (en) | 1998-02-10 | 2010-01-06 | Artemis Medical, Inc. | Occlusion, anchoring, tensioning or flow direction apparatus |
| US6280467B1 (en) * | 1998-02-26 | 2001-08-28 | World Medical Manufacturing Corporation | Delivery system for deployment and endovascular assembly of a multi-stage stented graft |
| US6315753B1 (en) | 1998-05-01 | 2001-11-13 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6048358A (en) | 1998-07-13 | 2000-04-11 | Barak; Shlomo | Method and apparatus for hemostasis following arterial catheterization |
| JP2003521270A (ja) * | 1998-08-04 | 2003-07-15 | フュージョン メディカル テクノロジーズ, インコーポレイテッド | 経皮組織路閉塞アセンブリおよび方法 |
| US6613070B2 (en) | 1998-08-04 | 2003-09-02 | Baxter International Inc. | System and method for sealing vascular penetrations with hemostatic gels |
| US20020015724A1 (en) | 1998-08-10 | 2002-02-07 | Chunlin Yang | Collagen type i and type iii hemostatic compositions for use as a vascular sealant and wound dressing |
| US6703047B2 (en) | 2001-02-02 | 2004-03-09 | Incept Llc | Dehydrated hydrogel precursor-based, tissue adherent compositions and methods of use |
| US6514534B1 (en) | 1998-08-14 | 2003-02-04 | Incept Llc | Methods for forming regional tissue adherent barriers and drug delivery systems |
| US6818018B1 (en) | 1998-08-14 | 2004-11-16 | Incept Llc | In situ polymerizable hydrogels |
| US6605294B2 (en) | 1998-08-14 | 2003-08-12 | Incept Llc | Methods of using in situ hydration of hydrogel articles for sealing or augmentation of tissue or vessels |
| US7347850B2 (en) * | 1998-08-14 | 2008-03-25 | Incept Llc | Adhesion barriers applicable by minimally invasive surgery and methods of use thereof |
| US6152943A (en) | 1998-08-14 | 2000-11-28 | Incept Llc | Methods and apparatus for intraluminal deposition of hydrogels |
| US6179862B1 (en) | 1998-08-14 | 2001-01-30 | Incept Llc | Methods and apparatus for in situ formation of hydrogels |
| EP1104324B8 (en) | 1998-08-14 | 2011-06-22 | Incept Llc | Apparatus for in situ formation of hydrogels |
| US6994686B2 (en) | 1998-08-26 | 2006-02-07 | Neomend, Inc. | Systems for applying cross-linked mechanical barriers |
| US6458147B1 (en) | 1998-11-06 | 2002-10-01 | Neomend, Inc. | Compositions, systems, and methods for arresting or controlling bleeding or fluid leakage in body tissue |
| GB9819461D0 (en) | 1998-09-08 | 1998-10-28 | Univ Strathclyde | Hydrogels |
| AUPP633798A0 (en) | 1998-10-02 | 1998-10-29 | White, Geoffrey H. | Device for the occlusion of a puncture in a bodily duct |
| US6022361A (en) | 1998-10-09 | 2000-02-08 | Biointerventional Corporation | Device for introducing and polymerizing polymeric biomaterials in the human body and method |
| US6949114B2 (en) | 1998-11-06 | 2005-09-27 | Neomend, Inc. | Systems, methods, and compositions for achieving closure of vascular puncture sites |
| US7279001B2 (en) | 1998-11-06 | 2007-10-09 | Neomend, Inc. | Systems, methods, and compositions for achieving closure of vascular puncture sites |
| US6830756B2 (en) | 1998-11-06 | 2004-12-14 | Neomend, Inc. | Systems, methods, and compositions for achieving closure of vascular puncture sites |
| WO2000030553A1 (en) | 1998-11-20 | 2000-06-02 | Medical Industries Corp. | Hemostatic agent inserting device |
| EP1137373A4 (en) | 1998-12-04 | 2004-05-19 | Chandrashekhar P Pathak | BIOCOMPATIBLE, CROSSLINKED POLYMERS |
| SE518736C2 (sv) | 1999-08-30 | 2002-11-12 | Sca Hygiene Prod Ab | Absorberande, öppencellingt skummaterial med god vätskelagringsförmåga samt absorberande struktur i ett absorberande alster |
| US6984219B2 (en) | 1999-09-23 | 2006-01-10 | Mark Ashby | Depth and puncture control for blood vessel hemostasis system |
| US20020120228A1 (en) | 2000-06-08 | 2002-08-29 | Yuh-Fun Maa | Powder compositions |
| US6890342B2 (en) | 2000-08-02 | 2005-05-10 | Loma Linda University | Method and apparatus for closing vascular puncture using hemostatic material |
| US8083768B2 (en) * | 2000-12-14 | 2011-12-27 | Ensure Medical, Inc. | Vascular plug having composite construction |
| US6537569B2 (en) | 2001-02-14 | 2003-03-25 | Microvention, Inc. | Radiation cross-linked hydrogels |
| US6569185B2 (en) | 2001-02-15 | 2003-05-27 | Scimed Life Systems Inc | Continuous infusion technique for arterial sealing |
| US6608117B1 (en) | 2001-05-11 | 2003-08-19 | Nanosystems Research Inc. | Methods for the preparation of cellular hydrogels |
| US6863680B2 (en) | 2001-11-08 | 2005-03-08 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| WO2002100245A2 (en) | 2001-06-08 | 2002-12-19 | Morris Innovative Research, Inc. | Method and apparatus for sealing access |
| US20030014075A1 (en) | 2001-07-16 | 2003-01-16 | Microvention, Inc. | Methods, materials and apparatus for deterring or preventing endoleaks following endovascular graft implanation |
| KR100947468B1 (ko) * | 2001-07-26 | 2010-03-17 | 쿠크 바이오텍, 인코포레이티드 | 혈관 폐쇄 부재 및 전달 장치 |
| JP2003043994A (ja) * | 2001-07-27 | 2003-02-14 | Canon Inc | アクティブマトリックス型ディスプレイ |
| US6592608B2 (en) | 2001-12-07 | 2003-07-15 | Biopsy Sciences, Llc | Bioabsorbable sealant |
| US6557273B2 (en) | 2001-09-28 | 2003-05-06 | Joseph Paul Polifroni | Layered arch support and method of manufacture |
| FR2838748B1 (fr) | 2002-04-17 | 2004-07-09 | Urgo Laboratoires | Nouvelles compositions adhesives thermofusibles hydrophiles |
| AU2003234159A1 (en) | 2002-04-22 | 2003-11-03 | Purdue Research Foundation | Hydrogels having enhanced elasticity and mechanical strength properties |
| ATE266969T1 (de) | 2002-06-12 | 2004-06-15 | Radi Medical Systems | Verschlussvorrichtung |
| WO2003105697A1 (en) * | 2002-06-14 | 2003-12-24 | Loma Linda University Medical Center | Vascular wound closure device and method |
| US7303575B2 (en) | 2002-08-01 | 2007-12-04 | Lumen Biomedical, Inc. | Embolism protection devices |
| US20040147016A1 (en) | 2002-09-30 | 2004-07-29 | Rowley Jonathan A. | Programmable scaffold and methods for making and using the same |
| US20040063206A1 (en) | 2002-09-30 | 2004-04-01 | Rowley Jon A. | Programmable scaffold and method for making and using the same |
| US8709038B2 (en) | 2002-12-20 | 2014-04-29 | Boston Scientific Scimed, Inc. | Puncture hole sealing device |
| US6863924B2 (en) | 2002-12-23 | 2005-03-08 | Kimberly-Clark Worldwide, Inc. | Method of making an absorbent composite |
| US8545830B2 (en) | 2003-03-24 | 2013-10-01 | University Of Tennessee Research Foundation | Multi-functional polymeric materials and their uses |
| US20040230289A1 (en) * | 2003-05-15 | 2004-11-18 | Scimed Life Systems, Inc. | Sealable attachment of endovascular stent to graft |
| US7331979B2 (en) | 2003-06-04 | 2008-02-19 | Access Closure, Inc. | Apparatus and methods for sealing a vascular puncture |
| US8157850B2 (en) * | 2003-07-29 | 2012-04-17 | Boston Scientific Scimed, Inc. | Device and method for loading a luminal graft for endoluminal delivery |
| US20070179600A1 (en) * | 2004-10-04 | 2007-08-02 | Gil Vardi | Stent graft including expandable cuff |
| US8790632B2 (en) | 2004-10-07 | 2014-07-29 | Actamax Surgical Materials, Llc | Polymer-based tissue-adhesive form medical use |
| EP2209426A4 (en) | 2007-11-02 | 2015-04-22 | Incept Llc | APPARATUS AND METHODS FOR CLOSING VASCULAR PERFORATION |
| CA2977830C (en) | 2009-05-04 | 2019-09-17 | Incept, Llc | Biomaterials for track and puncture closure |
-
2008
- 2008-10-31 EP EP08845014.3A patent/EP2209426A4/en not_active Withdrawn
- 2008-10-31 WO PCT/US2008/082102 patent/WO2009059217A2/en not_active Ceased
- 2008-10-31 JP JP2010532298A patent/JP2011502582A/ja active Pending
-
2010
- 2010-04-29 US US12/770,573 patent/US8852230B2/en active Active
-
2014
- 2014-09-15 US US14/487,030 patent/US20150005816A1/en not_active Abandoned
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007203294A (ja) * | 1995-12-07 | 2007-08-16 | Bristol Myers Squibb Co | 二又はそれ以上の種類の液体成分を塗布するためのシステム及び装置 |
| JP2006524111A (ja) * | 2003-04-22 | 2006-10-26 | サブ−キュー・インコーポレーテッド | 固定し引き出す技術により穿刺部位を閉塞するシステム |
| JP2006095098A (ja) * | 2004-09-29 | 2006-04-13 | Terumo Corp | 投与器具 |
| US20060099238A1 (en) * | 2004-11-05 | 2006-05-11 | Accessclosure, Inc. | Apparatus and methods for sealing a vascular puncture |
| JP2008518743A (ja) * | 2004-11-05 | 2008-06-05 | アクセスクロージャー,インク. | 脈管穿刺のシーリング装置 |
| JP2008518742A (ja) * | 2004-11-05 | 2008-06-05 | アクセスクロージャー,インク. | 脈管穿刺を閉塞するための器具 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023544891A (ja) * | 2020-10-13 | 2023-10-25 | マサチューセッツ インスチテュート オブ テクノロジー | 生体接着材及び生体接着材で組織を接着するための低侵襲方法 |
| US12402870B2 (en) | 2020-10-13 | 2025-09-02 | Massachusetts Institute Of Technology | Bioadhesive materials and minimally invasive methods for adhering tissues with bioadhesive materials |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2009059217A2 (en) | 2009-05-07 |
| EP2209426A4 (en) | 2015-04-22 |
| US20150005816A1 (en) | 2015-01-01 |
| EP2209426A2 (en) | 2010-07-28 |
| US8852230B2 (en) | 2014-10-07 |
| WO2009059217A3 (en) | 2009-07-23 |
| US20100274280A1 (en) | 2010-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2011502582A (ja) | 血管穿刺を塞ぐ装置および方法 | |
| JP6186536B1 (ja) | 血管の壁の貫通部を封止する器具 | |
| CA2583238C (en) | Apparatus for sealing a vascular puncture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111031 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111031 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121031 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130611 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140430 |