JP2010535239A - カルボキシル修飾されたフルクタンまたはその塩を含む眼科用組成物 - Google Patents
カルボキシル修飾されたフルクタンまたはその塩を含む眼科用組成物 Download PDFInfo
- Publication number
- JP2010535239A JP2010535239A JP2010520168A JP2010520168A JP2010535239A JP 2010535239 A JP2010535239 A JP 2010535239A JP 2010520168 A JP2010520168 A JP 2010520168A JP 2010520168 A JP2010520168 A JP 2010520168A JP 2010535239 A JP2010535239 A JP 2010535239A
- Authority
- JP
- Japan
- Prior art keywords
- composition
- composition according
- group
- carboxyl
- salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
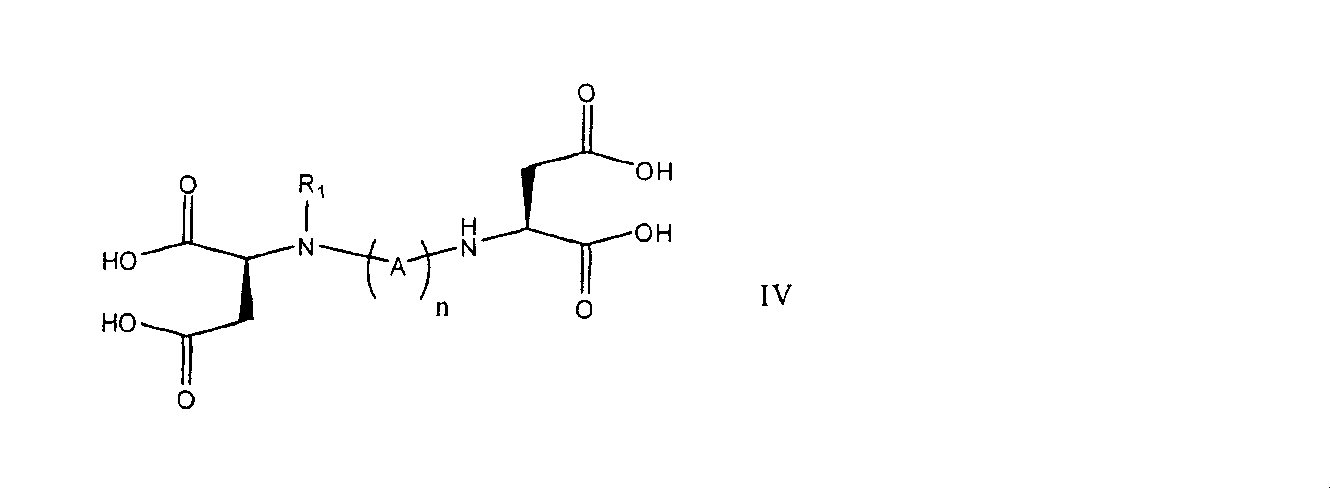
- 0 *N(***N[C@@](CC(O)=O)C(O)=O)[C@@](CC(O)=O)C(O)=O Chemical compound *N(***N[C@@](CC(O)=O)C(O)=O)[C@@](CC(O)=O)C(O)=O 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L12/00—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor
- A61L12/08—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor using chemical substances
- A61L12/14—Organic compounds not covered by groups A61L12/10 or A61L12/12
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L12/00—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor
- A61L12/08—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor using chemical substances
- A61L12/14—Organic compounds not covered by groups A61L12/10 or A61L12/12
- A61L12/141—Biguanides, e.g. chlorhexidine
- A61L12/142—Polymeric biguanides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L12/00—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor
- A61L12/08—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor using chemical substances
- A61L12/14—Organic compounds not covered by groups A61L12/10 or A61L12/12
- A61L12/143—Quaternary ammonium compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L12/00—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor
- A61L12/08—Methods or apparatus for disinfecting or sterilising contact lenses; Accessories therefor using chemical substances
- A61L12/14—Organic compounds not covered by groups A61L12/10 or A61L12/12
- A61L12/143—Quaternary ammonium compounds
- A61L12/145—Polymeric quaternary ammonium compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Ophthalmology & Optometry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Apparatus For Disinfection Or Sterilisation (AREA)
- Eyeglasses (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
Description
EDTA:エチレンジアミン四酢酸
PHMB:ポリ(ヘキサメチレンビグアニド)
Dequest(登録商標)は、ホスホン酸ヒドロキシアルキルの30質量%水溶液である。
Dequest(登録商標)PBは、カルボキシメリルイヌリンの水溶液である。
Tetronic(登録商標)1107は、BASF社から市販されている界面活性剤である。
Pluronic(登録商標)P123は、BASF社から市販されている界面活性剤である。
Pluronic(登録商標)F127は、BASF社から市販されている界面活性剤である。
Claims (13)
- カルボキシル修飾されたフルクタンまたはその塩を含んでいて、浸透圧モル濃度が200mOsmol/kg〜400mOsmol/kgの範囲である眼科用組成物。
- α-[4-トリス(2-ヒドロキシエチル)アンモニウムクロリド-2-ブテニル]ポリ[1-ジメチルアンモニウムクロリド-2-ブテニル]-ω-トリス(2-ヒドロキシエチル)アンモニウムクロリド、ハロゲン化ベンズアルコニウム、アレキシジンとその塩、ヘキサメチレンビグアニドとその塩、これらのポリマー、これらの混合物からなるグループの中から選択したカチオン性殺菌成分をさらに含む、請求項1に記載の組成物。
- 上記カチオン性殺菌成分の選択が、0.2ppm〜2ppmの割合で存在するポリ(ヘキサメチレンビグアニド)、2ppm〜15ppmの割合で存在するα-[4-トリス(2-ヒドロキシエチル)アンモニウムクロリド-2-ブテニル]ポリ[1-ジメチルアンモニウムクロリド-2-ブテニル]-ω-トリス(2-ヒドロキシエチル)アンモニウムクロリド、これらの任意の混合物からなるグループの中からなされる、請求項1または2に記載の組成物。
- 上記カルボキシル修飾されたフルクタンまたはその塩が、カルボキシアルキルイヌリンまたはその塩である、請求項1〜3のいずれか1項に記載の組成物。
- 上記カルボキシアルキルイヌリンの選択が、カルボキシメチルイヌリンとその塩、カルボキシエチルイヌリンとその塩からなるグループの中からなされる、請求項4に記載の組成物。
- 上記カルボキシル修飾されたフルクタンが、アンヒドロフルクトース単位1つにつき0.3〜3個のカルボキシル基を含む、請求項1〜5のいずれか1項に記載の組成物。
- 上記カルボキシアルキルイヌリンが、アンヒドロフルクトース単位1つにつき0.3〜3個のカルボキシル基を含む、請求項4または5に記載の組成物。
- ヒアルロン酸とアルギン酸塩からなるグループの中から選択した1種類以上のバイオポリマーとその任意の誘導体をさらに含む、請求項1〜7のいずれか1項に記載の組成物。
- デキスパンテノール、ソルビトール、これらの任意の混合物のいずれかをさらに含む、請求項1〜8のいずれか1項に記載の組成物。
- ヒドロキシプロピルメチルセルロース、プロピレングリコール、ヒドロキシプロピルグアルのいずれかをさらに含む、請求項1〜9のいずれか1項に記載の組成物。
- R1がRであり;R2とR3が、それぞれ独立にC1〜C4アルキルの中から選択され;R4がC2〜C4アルキレンであり;YがSO3 -である、請求項11に記載の組成物。
- コンタクト・レンズ再湿潤化用液滴、コンタクト・レンズ・パッケージング溶液、コンタクト・レンズ殺菌溶液からなるグループの中から選択した目のケア製品またはコンタクト・レンズ・ケア製品において請求項1〜12のいずれか1項に記載の組成物を使用する方法。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US95353507P | 2007-08-02 | 2007-08-02 | |
| US60/953,535 | 2007-08-02 | ||
| US12/180,694 | 2008-07-28 | ||
| US12/180,694 US20090036404A1 (en) | 2007-08-02 | 2008-07-28 | Ophthalmic compositions comprising a carboxyl-modified fructan or a salt thereof |
| PCT/US2008/071675 WO2009032433A1 (en) | 2007-08-02 | 2008-07-31 | Ophthalmic compositions comprising a carboxyl-modified fructan or a salt thereof |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010535239A true JP2010535239A (ja) | 2010-11-18 |
| JP2010535239A5 JP2010535239A5 (ja) | 2011-09-15 |
| JP5543346B2 JP5543346B2 (ja) | 2014-07-09 |
Family
ID=40338735
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010520168A Active JP5543346B2 (ja) | 2007-08-02 | 2008-07-31 | カルボキシル修飾されたフルクタンまたはその塩を含む眼科用組成物 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20090036404A1 (ja) |
| EP (1) | EP2178571B1 (ja) |
| JP (1) | JP5543346B2 (ja) |
| AT (1) | ATE511865T1 (ja) |
| CA (1) | CA2693492C (ja) |
| ES (1) | ES2364122T3 (ja) |
| WO (1) | WO2009032433A1 (ja) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HUE034051T2 (en) | 2007-11-27 | 2018-01-29 | Algipharma As | Use of alginate-containing oligomers to combat biofilms |
| US20100234319A1 (en) * | 2009-03-11 | 2010-09-16 | Abbott Medical Optics Inc. | Complex of Polymeric Quaternary Ammonium and Anionic Polymers as a New Antimicrobial Agent for Ophthalmic Compositions |
| US8815831B2 (en) | 2009-06-03 | 2014-08-26 | Algipharma As | Treatment of Acinetobacter with alginate oligomers and antibiotics |
| US8501200B2 (en) * | 2010-04-26 | 2013-08-06 | Bausch & Lomb Incorporated | Ophthalmic compositions with biguanide and PEG-glycerol esters |
| EP2790654A2 (en) * | 2011-12-12 | 2014-10-22 | Italmatch Chemicals S.P.A. | Cosmetic composition for skin or hair care |
| US8883705B2 (en) | 2012-10-30 | 2014-11-11 | The Clorox Company | Cationic micelles with anionic polymeric counterions systems thereof |
| US8765114B2 (en) | 2012-10-30 | 2014-07-01 | The Clorox Company | Anionic micelles with cationic polymeric counterions methods thereof |
| US8883706B2 (en) | 2012-10-30 | 2014-11-11 | The Clorox Company | Anionic micelles with cationic polymeric counterions systems thereof |
| US8728454B1 (en) | 2012-10-30 | 2014-05-20 | The Clorox Company | Cationic micelles with anionic polymeric counterions compositions thereof |
| US8728530B1 (en) | 2012-10-30 | 2014-05-20 | The Clorox Company | Anionic micelles with cationic polymeric counterions compositions thereof |
| GB201621050D0 (en) * | 2016-12-12 | 2017-01-25 | Provita Eurotech Ltd | Antimicrobial compositions |
| EP3561036B1 (en) * | 2018-04-27 | 2023-08-09 | The Procter & Gamble Company | Hard surface cleaners comprising carboxylated fructan |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001501194A (ja) * | 1996-09-23 | 2001-01-30 | フオルスカルパテント アイ ウプサラ アクチボラゲツト | ゲル形成性医薬組成物 |
| JP2005523299A (ja) * | 2002-03-18 | 2005-08-04 | ノバルティス アクチエンゲゼルシャフト | シクロフルクタン、担体および薬物を含む局所用組成物 |
| WO2006107330A1 (en) * | 2005-04-04 | 2006-10-12 | Advanced Medical Optics, Inc. | Stable ophthalmic oil-in-water emulsions with sodium hyaluronate for alleviating dry eye |
| WO2009018060A1 (en) * | 2007-08-01 | 2009-02-05 | Bausch & Lomb Incorporated | Ophthalmic compositions comprising a terpene and a natural polymer |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL80298A (en) * | 1986-10-14 | 1993-01-31 | Res & Dev Co Ltd | Eye drops |
| US5290572A (en) * | 1992-08-06 | 1994-03-01 | Deo Corporation | Opthalmic composition for treating dry eye |
| IT1273011B (it) * | 1994-07-25 | 1997-07-01 | Trhecnopharma S A | Preparato oftalmico per l'uso come lacrima artificiale |
| US5696171A (en) * | 1994-08-30 | 1997-12-09 | Allergan, Inc. | Contact lens disinfecting compositions and methods employing terpenes |
| NL1004738C2 (nl) * | 1996-12-10 | 1998-06-11 | Cooperatie Cosun U A | Fructaan-polycarbonzuur. |
| CN1769411A (zh) * | 1997-11-26 | 2006-05-10 | 先进医用光学公司 | 护理接触镜片的多用途溶液 |
| NL1009379C2 (nl) * | 1998-06-11 | 1999-12-15 | Cooperatie Cosun U A | Dispergeermiddel. |
| IT1306123B1 (it) * | 1999-04-02 | 2001-05-30 | Technopharma Sa | Soluzione oftalmica viscosizzata con azione detergente sulle lenti acontatto. |
| NL1012482C2 (nl) * | 1999-06-30 | 2001-01-03 | Co Peratie Cosun U A | Bleekactivator op basis van inuline. |
| US6315771B1 (en) * | 1999-12-09 | 2001-11-13 | Nidek Co., Ltd. | Apparatus for corneal surgery |
| WO2002034178A1 (en) * | 2000-10-20 | 2002-05-02 | Bausch & Lomb Incorporated | Method and system for improving vision |
| BRPI0312331A2 (pt) * | 2002-07-03 | 2016-06-28 | Pericor Science Inc | composições de ácido hialurônico e métodos de emprego. |
| US20040034042A1 (en) * | 2002-08-14 | 2004-02-19 | Masao Tsuji | Preservative composition |
| US20040185068A1 (en) * | 2003-03-18 | 2004-09-23 | Zhi-Jian Yu | Self-emulsifying compositions, methods of use and preparation |
| JP4954076B2 (ja) * | 2004-10-01 | 2012-06-13 | メニコン シンガポール ピーティーイー. リミテッド | コンタクトレンズ包装容器溶液 |
| JP2008519846A (ja) * | 2004-11-09 | 2008-06-12 | アドバンスト メディカル オプティクス, インコーポレーテッド | 眼科用溶液 |
-
2008
- 2008-07-28 US US12/180,694 patent/US20090036404A1/en not_active Abandoned
- 2008-07-31 AT AT08829398T patent/ATE511865T1/de not_active IP Right Cessation
- 2008-07-31 WO PCT/US2008/071675 patent/WO2009032433A1/en not_active Ceased
- 2008-07-31 JP JP2010520168A patent/JP5543346B2/ja active Active
- 2008-07-31 ES ES08829398T patent/ES2364122T3/es active Active
- 2008-07-31 CA CA2693492A patent/CA2693492C/en active Active
- 2008-07-31 EP EP08829398A patent/EP2178571B1/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001501194A (ja) * | 1996-09-23 | 2001-01-30 | フオルスカルパテント アイ ウプサラ アクチボラゲツト | ゲル形成性医薬組成物 |
| JP2005523299A (ja) * | 2002-03-18 | 2005-08-04 | ノバルティス アクチエンゲゼルシャフト | シクロフルクタン、担体および薬物を含む局所用組成物 |
| WO2006107330A1 (en) * | 2005-04-04 | 2006-10-12 | Advanced Medical Optics, Inc. | Stable ophthalmic oil-in-water emulsions with sodium hyaluronate for alleviating dry eye |
| WO2009018060A1 (en) * | 2007-08-01 | 2009-02-05 | Bausch & Lomb Incorporated | Ophthalmic compositions comprising a terpene and a natural polymer |
Also Published As
| Publication number | Publication date |
|---|---|
| US20090036404A1 (en) | 2009-02-05 |
| CA2693492A1 (en) | 2009-03-12 |
| JP5543346B2 (ja) | 2014-07-09 |
| WO2009032433A1 (en) | 2009-03-12 |
| ATE511865T1 (de) | 2011-06-15 |
| CA2693492C (en) | 2013-01-15 |
| ES2364122T3 (es) | 2011-08-25 |
| EP2178571B1 (en) | 2011-06-08 |
| EP2178571A1 (en) | 2010-04-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5543346B2 (ja) | カルボキシル修飾されたフルクタンまたはその塩を含む眼科用組成物 | |
| KR101096325B1 (ko) | 양쪽성 계면활성제 및 히아루론산을 갖는 안과용 조성물 | |
| EP2171025B1 (en) | Ophthalmic composition with hyaluronic acid | |
| US9096819B2 (en) | Ophthalmic compositions with an amphoteric surfactant and an anionic biopolymer | |
| US20090036554A1 (en) | Ophthalmic compositions comprising a terpene compound | |
| US20110046033A1 (en) | Multipurpose Lens Care Solution with Benefits to Corneal Epithelial Barrier Function | |
| US20100178317A1 (en) | Lens Care Solutions with Hyaluronic Acid | |
| US9125405B2 (en) | Contact lens solution with a tertiary amine oxide | |
| US7632794B1 (en) | Lens care solutions comprising alkyldimonium hydroxypropyl alkylglucosides | |
| US8889160B2 (en) | Ophthalmic compositions with biguanide and PEG-glycerol esters | |
| EP2262521B1 (en) | Ophthalmic compositions comprising a dipeptide with a glycine moiety | |
| EP2903655A1 (en) | Minimizing biological lipid deposits on contact lenses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110728 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110728 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20130613 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130709 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131008 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131016 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140109 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140408 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140508 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5543346 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |