JP2010503699A5 - - Google Patents
Download PDFInfo
- Publication number
- JP2010503699A5 JP2010503699A5 JP2009528458A JP2009528458A JP2010503699A5 JP 2010503699 A5 JP2010503699 A5 JP 2010503699A5 JP 2009528458 A JP2009528458 A JP 2009528458A JP 2009528458 A JP2009528458 A JP 2009528458A JP 2010503699 A5 JP2010503699 A5 JP 2010503699A5
- Authority
- JP
- Japan
- Prior art keywords
- compound
- group
- poly
- therapeutic agent
- tissue
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 150000001875 compounds Chemical class 0.000 claims 51
- 239000003814 drug Substances 0.000 claims 11
- 229940124597 therapeutic agent Drugs 0.000 claims 11
- 210000001519 tissue Anatomy 0.000 claims 11
- 239000007943 implant Substances 0.000 claims 6
- 239000000203 mixture Substances 0.000 claims 6
- 230000002792 vascular Effects 0.000 claims 5
- -1 flavorings Substances 0.000 claims 4
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 claims 4
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 claims 4
- 229960002930 sirolimus Drugs 0.000 claims 4
- 125000003545 alkoxy group Chemical group 0.000 claims 3
- 239000003795 chemical substances by application Substances 0.000 claims 3
- 239000002552 dosage form Substances 0.000 claims 3
- 150000002596 lactones Chemical class 0.000 claims 3
- 229920000642 polymer Polymers 0.000 claims 3
- 230000009885 systemic effect Effects 0.000 claims 3
- 125000000217 alkyl group Chemical group 0.000 claims 2
- 230000004663 cell proliferation Effects 0.000 claims 2
- 229920001577 copolymer Polymers 0.000 claims 2
- 239000005038 ethylene vinyl acetate Substances 0.000 claims 2
- 150000004677 hydrates Chemical class 0.000 claims 2
- 239000002207 metabolite Substances 0.000 claims 2
- 239000002184 metal Substances 0.000 claims 2
- 229910052751 metal Inorganic materials 0.000 claims 2
- 150000002739 metals Chemical class 0.000 claims 2
- 238000000034 method Methods 0.000 claims 2
- 239000012038 nucleophile Substances 0.000 claims 2
- 210000000056 organ Anatomy 0.000 claims 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims 2
- 229920001490 poly(butyl methacrylate) polymer Polymers 0.000 claims 2
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 claims 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 claims 2
- 239000004926 polymethyl methacrylate Substances 0.000 claims 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 claims 2
- 239000004810 polytetrafluoroethylene Substances 0.000 claims 2
- 239000000651 prodrug Substances 0.000 claims 2
- 229940002612 prodrug Drugs 0.000 claims 2
- 150000003839 salts Chemical class 0.000 claims 2
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 claims 1
- 229920000219 Ethylene vinyl alcohol Polymers 0.000 claims 1
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 claims 1
- 206010061218 Inflammation Diseases 0.000 claims 1
- 239000004677 Nylon Substances 0.000 claims 1
- 239000004952 Polyamide Substances 0.000 claims 1
- 229920002873 Polyethylenimine Polymers 0.000 claims 1
- 239000004642 Polyimide Substances 0.000 claims 1
- 239000002253 acid Substances 0.000 claims 1
- 125000002009 alkene group Chemical group 0.000 claims 1
- 239000002260 anti-inflammatory agent Substances 0.000 claims 1
- 229940121363 anti-inflammatory agent Drugs 0.000 claims 1
- 230000001028 anti-proliverative effect Effects 0.000 claims 1
- 239000003146 anticoagulant agent Substances 0.000 claims 1
- 239000002246 antineoplastic agent Substances 0.000 claims 1
- 239000003963 antioxidant agent Substances 0.000 claims 1
- 229940127218 antiplatelet drug Drugs 0.000 claims 1
- 229960004676 antithrombotic agent Drugs 0.000 claims 1
- 125000003118 aryl group Chemical group 0.000 claims 1
- 239000011230 binding agent Substances 0.000 claims 1
- 230000010261 cell growth Effects 0.000 claims 1
- 239000000919 ceramic Substances 0.000 claims 1
- 238000000576 coating method Methods 0.000 claims 1
- 239000003086 colorant Substances 0.000 claims 1
- 239000007884 disintegrant Substances 0.000 claims 1
- 150000002118 epoxides Chemical class 0.000 claims 1
- 239000000945 filler Substances 0.000 claims 1
- 235000003599 food sweetener Nutrition 0.000 claims 1
- 239000003018 immunosuppressive agent Substances 0.000 claims 1
- 229940125721 immunosuppressive agent Drugs 0.000 claims 1
- 230000004054 inflammatory process Effects 0.000 claims 1
- 238000007918 intramuscular administration Methods 0.000 claims 1
- 238000007912 intraperitoneal administration Methods 0.000 claims 1
- 238000001990 intravenous administration Methods 0.000 claims 1
- 239000000314 lubricant Substances 0.000 claims 1
- 210000004072 lung Anatomy 0.000 claims 1
- 238000004519 manufacturing process Methods 0.000 claims 1
- 210000004877 mucosa Anatomy 0.000 claims 1
- 229920001778 nylon Polymers 0.000 claims 1
- 150000002978 peroxides Chemical class 0.000 claims 1
- 150000004965 peroxy acids Chemical class 0.000 claims 1
- 239000000106 platelet aggregation inhibitor Substances 0.000 claims 1
- 229920001432 poly(L-lactide) Polymers 0.000 claims 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims 1
- 229920000052 poly(p-xylylene) Polymers 0.000 claims 1
- 229920002401 polyacrylamide Polymers 0.000 claims 1
- 229920002647 polyamide Polymers 0.000 claims 1
- 229920000515 polycarbonate Polymers 0.000 claims 1
- 239000004417 polycarbonate Substances 0.000 claims 1
- 229920001223 polyethylene glycol Polymers 0.000 claims 1
- 229920002338 polyhydroxyethylmethacrylate Polymers 0.000 claims 1
- 229920001721 polyimide Polymers 0.000 claims 1
- 229920000193 polymethacrylate Polymers 0.000 claims 1
- 229920001296 polysiloxane Polymers 0.000 claims 1
- 229920002635 polyurethane Polymers 0.000 claims 1
- 239000004814 polyurethane Substances 0.000 claims 1
- 229920000915 polyvinyl chloride Polymers 0.000 claims 1
- 239000004800 polyvinyl chloride Substances 0.000 claims 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 claims 1
- 239000007787 solid Substances 0.000 claims 1
- 239000002904 solvent Substances 0.000 claims 1
- 239000003381 stabilizer Substances 0.000 claims 1
- 238000007920 subcutaneous administration Methods 0.000 claims 1
- 239000000829 suppository Substances 0.000 claims 1
- 239000003765 sweetening agent Substances 0.000 claims 1
- 238000002560 therapeutic procedure Methods 0.000 claims 1
- 230000000699 topical effect Effects 0.000 claims 1
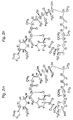
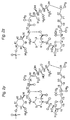
- GLSXDSUZPXGLQM-SXSWIABHSA-N C[C@H](C[C@H](CC[C@H]1O)C[C@H]1OC)[C@H](CC([C@H](C)/C=C(\C)/[C@H]([C@H](C(C(C[C@H](C)/C=C/C=C/C=C(\C)/C(/C[C@H](CC[C@H]1C)O[C@]1(C(C(N1[C@H]2CCCC1)=O)=O)O)=[O]/C)=C)=O)OC)O)=O)OC2=O Chemical compound C[C@H](C[C@H](CC[C@H]1O)C[C@H]1OC)[C@H](CC([C@H](C)/C=C(\C)/[C@H]([C@H](C(C(C[C@H](C)/C=C/C=C/C=C(\C)/C(/C[C@H](CC[C@H]1C)O[C@]1(C(C(N1[C@H]2CCCC1)=O)=O)O)=[O]/C)=C)=O)OC)O)=O)OC2=O GLSXDSUZPXGLQM-SXSWIABHSA-N 0.000 description 1
- 0 C[C@](C[C@]1C[C@@](*)[C@](*)CC1)[C@](CC([C@](C)C=C(C)C([C@](C([C@](C)C[C@](C)C=CC=CC=C(C)[C@@](*)C[C@@](CC[C@]1C)O[C@]1(C)C(C(N1[C@]2CCCC1)=O)=O)=O)OC)O)=O)OC2=O Chemical compound C[C@](C[C@]1C[C@@](*)[C@](*)CC1)[C@](CC([C@](C)C=C(C)C([C@](C([C@](C)C[C@](C)C=CC=CC=C(C)[C@@](*)C[C@@](CC[C@]1C)O[C@]1(C)C(C(N1[C@]2CCCC1)=O)=O)=O)OC)O)=O)OC2=O 0.000 description 1
Images
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US82553106P | 2006-09-13 | 2006-09-13 | |
| US60/825,531 | 2006-09-13 | ||
| PCT/US2007/078317 WO2008033956A2 (en) | 2006-09-13 | 2007-09-12 | Macrocyclic lactone compounds and methods for their use |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015017340A Division JP2015129140A (ja) | 2006-09-13 | 2015-01-30 | 大環状ラクトン化合物およびその使用方法 |
| JP2016197623A Division JP2017036302A (ja) | 2006-09-13 | 2016-10-05 | 大環状ラクトン化合物およびその使用方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010503699A JP2010503699A (ja) | 2010-02-04 |
| JP2010503699A5 true JP2010503699A5 (enExample) | 2010-11-04 |
| JP6049160B2 JP6049160B2 (ja) | 2016-12-27 |
Family
ID=39184568
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009528458A Expired - Fee Related JP6049160B2 (ja) | 2006-09-13 | 2007-09-12 | 大環状ラクトン化合物およびその使用方法 |
| JP2015017340A Withdrawn JP2015129140A (ja) | 2006-09-13 | 2015-01-30 | 大環状ラクトン化合物およびその使用方法 |
| JP2016197623A Withdrawn JP2017036302A (ja) | 2006-09-13 | 2016-10-05 | 大環状ラクトン化合物およびその使用方法 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015017340A Withdrawn JP2015129140A (ja) | 2006-09-13 | 2015-01-30 | 大環状ラクトン化合物およびその使用方法 |
| JP2016197623A Withdrawn JP2017036302A (ja) | 2006-09-13 | 2016-10-05 | 大環状ラクトン化合物およびその使用方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US7867988B2 (enExample) |
| EP (3) | EP2431036B1 (enExample) |
| JP (3) | JP6049160B2 (enExample) |
| CN (2) | CN101557814B (enExample) |
| BR (1) | BRPI0716950A2 (enExample) |
| WO (1) | WO2008033956A2 (enExample) |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8741378B1 (en) | 2001-06-27 | 2014-06-03 | Advanced Cardiovascular Systems, Inc. | Methods of coating an implantable device |
| WO2007011708A2 (en) | 2005-07-15 | 2007-01-25 | Micell Technologies, Inc. | Stent with polymer coating containing amorphous rapamycin |
| JP5756588B2 (ja) | 2005-07-15 | 2015-07-29 | ミセル テクノロジーズ、インコーポレイテッド | 制御されたモルホロジーの薬剤粉末を含むポリマーコーティング |
| CA2996768C (en) | 2006-04-26 | 2020-12-08 | Micell Technologies, Inc. | Coatings containing multiple drugs |
| US10695327B2 (en) | 2006-09-13 | 2020-06-30 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| CN101557814B (zh) | 2006-09-13 | 2015-05-20 | 万能医药公司 | 大环内酯化合物及它们的使用方法 |
| US8088789B2 (en) | 2006-09-13 | 2012-01-03 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| EP2081694B1 (en) | 2006-10-23 | 2020-05-13 | Micell Technologies, Inc. | Holder for electrically charging a substrate during coating |
| JP5603598B2 (ja) | 2007-01-08 | 2014-10-08 | ミセル テクノロジーズ、インコーポレイテッド | 生物分解層を有するステント |
| US11426494B2 (en) | 2007-01-08 | 2022-08-30 | MT Acquisition Holdings LLC | Stents having biodegradable layers |
| US9433516B2 (en) | 2007-04-17 | 2016-09-06 | Micell Technologies, Inc. | Stents having controlled elution |
| AU2008256684B2 (en) | 2007-05-25 | 2012-06-14 | Micell Technologies, Inc. | Polymer films for medical device coating |
| WO2009114010A1 (en) * | 2008-03-11 | 2009-09-17 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| BRPI0910969B8 (pt) | 2008-04-17 | 2021-06-22 | Micell Technologies Inc | dispositivo |
| US8206636B2 (en) | 2008-06-20 | 2012-06-26 | Amaranth Medical Pte. | Stent fabrication via tubular casting processes |
| US8206635B2 (en) | 2008-06-20 | 2012-06-26 | Amaranth Medical Pte. | Stent fabrication via tubular casting processes |
| US10898620B2 (en) | 2008-06-20 | 2021-01-26 | Razmodics Llc | Composite stent having multi-axial flexibility and method of manufacture thereof |
| EP2313122B1 (en) | 2008-07-17 | 2019-03-06 | Micell Technologies, Inc. | Drug delivery medical device |
| WO2011009096A1 (en) | 2009-07-16 | 2011-01-20 | Micell Technologies, Inc. | Drug delivery medical device |
| US20100086579A1 (en) * | 2008-10-03 | 2010-04-08 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| US8834913B2 (en) | 2008-12-26 | 2014-09-16 | Battelle Memorial Institute | Medical implants and methods of making medical implants |
| US20100256746A1 (en) * | 2009-03-23 | 2010-10-07 | Micell Technologies, Inc. | Biodegradable polymers |
| EP2413847A4 (en) | 2009-04-01 | 2013-11-27 | Micell Technologies Inc | COATED STENTS |
| WO2011011632A1 (en) * | 2009-07-22 | 2011-01-27 | Tufts University | Methods and compositions for modulating membrane potential to influence cell behavior |
| EP2531140B1 (en) | 2010-02-02 | 2017-11-01 | Micell Technologies, Inc. | Stent and stent delivery system with improved deliverability |
| US8795762B2 (en) | 2010-03-26 | 2014-08-05 | Battelle Memorial Institute | System and method for enhanced electrostatic deposition and surface coatings |
| US8685433B2 (en) | 2010-03-31 | 2014-04-01 | Abbott Cardiovascular Systems Inc. | Absorbable coating for implantable device |
| WO2011133655A1 (en) | 2010-04-22 | 2011-10-27 | Micell Technologies, Inc. | Stents and other devices having extracellular matrix coating |
| EP2593039B1 (en) | 2010-07-16 | 2022-11-30 | Micell Technologies, Inc. | Drug delivery medical device |
| EP2654629B1 (en) | 2010-12-20 | 2015-10-07 | Graftcraft I Göteborg AB | Removable stent and method of production |
| US8945207B2 (en) | 2010-12-20 | 2015-02-03 | Graftcraft I Göteborg Ab | Removable stent and method of production |
| US20120323311A1 (en) * | 2011-04-13 | 2012-12-20 | Micell Technologies, Inc. | Stents having controlled elution |
| WO2012166819A1 (en) | 2011-05-31 | 2012-12-06 | Micell Technologies, Inc. | System and process for formation of a time-released, drug-eluting transferable coating |
| WO2013012689A1 (en) | 2011-07-15 | 2013-01-24 | Micell Technologies, Inc. | Drug delivery medical device |
| EP2734261B1 (en) | 2011-07-18 | 2018-02-21 | Mor-Research Applications Ltd. | A device for adjusting the intraocular pressure |
| US10188772B2 (en) | 2011-10-18 | 2019-01-29 | Micell Technologies, Inc. | Drug delivery medical device |
| AU2013270798B2 (en) | 2012-06-08 | 2017-09-07 | Biotronik Ag | Rapamycin 40-O-cyclic hydrocarbon esters, compositions and methods |
| US10751450B2 (en) | 2012-06-08 | 2020-08-25 | Biotronik Ag | Rapamycin 40-O-cyclic hydrocarbon esters, compositions and methods |
| JP6330024B2 (ja) | 2013-03-12 | 2018-05-23 | マイセル・テクノロジーズ,インコーポレイテッド | 生体吸収性バイオメディカルインプラント |
| CN107823202B (zh) | 2013-03-13 | 2021-04-06 | 参天制药株式会社 | 睑板腺功能障碍的治疗剂 |
| JP2016519965A (ja) | 2013-05-15 | 2016-07-11 | マイセル・テクノロジーズ,インコーポレイテッド | 生体吸収性バイオメディカルインプラント |
| CN103622986B (zh) * | 2013-11-27 | 2014-11-05 | 广州华农大实验兽药有限公司 | 一种畜禽用抗应激平衡调节剂组合物及其制备方法与应用 |
| WO2015119653A1 (en) | 2014-02-04 | 2015-08-13 | Abbott Cardiovascular Systems Inc. | Drug delivery scaffold or stent with a novolimus and lactide based coating such that novolimus has a minimum amount of bonding to the coating |
| US11654036B2 (en) | 2020-05-26 | 2023-05-23 | Elixir Medical Corporation | Anticoagulant compounds and methods and devices for their use |
Family Cites Families (71)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA737247B (en) | 1972-09-29 | 1975-04-30 | Ayerst Mckenna & Harrison | Rapamycin and process of preparation |
| US3993749A (en) | 1974-04-12 | 1976-11-23 | Ayerst Mckenna And Harrison Ltd. | Rapamycin and process of preparation |
| US5206018A (en) | 1978-11-03 | 1993-04-27 | Ayerst, Mckenna & Harrison, Inc. | Use of rapamycin in treatment of tumors |
| US4316885A (en) | 1980-08-25 | 1982-02-23 | Ayerst, Mckenna And Harrison, Inc. | Acyl derivatives of rapamycin |
| US4650803A (en) | 1985-12-06 | 1987-03-17 | University Of Kansas | Prodrugs of rapamycin |
| US5080899A (en) | 1991-02-22 | 1992-01-14 | American Home Products Corporation | Method of treating pulmonary inflammation |
| US5078999A (en) | 1991-02-22 | 1992-01-07 | American Home Products Corporation | Method of treating systemic lupus erythematosus |
| US5120842A (en) | 1991-04-01 | 1992-06-09 | American Home Products Corporation | Silyl ethers of rapamycin |
| US5321009A (en) | 1991-04-03 | 1994-06-14 | American Home Products Corporation | Method of treating diabetes |
| DK0525960T3 (da) | 1991-06-18 | 1996-04-15 | American Home Prod | Anvendelse af rapamycin til behandling af T-celle leukæmi/lymfon |
| IL102414A (en) | 1991-07-25 | 1996-08-04 | Univ Louisville Res Found | Medicinal preparations for the treatment of ocular inflammation, containing rapamycin |
| US5286730A (en) | 1991-09-17 | 1994-02-15 | American Home Products Corporation | Method of treating immunoinflammatory disease |
| US5286731A (en) | 1991-09-17 | 1994-02-15 | American Home Products Corporation | Method of treating immunoinflammatory bowel disease |
| US5151413A (en) | 1991-11-06 | 1992-09-29 | American Home Products Corporation | Rapamycin acetals as immunosuppressant and antifungal agents |
| US5516781A (en) | 1992-01-09 | 1996-05-14 | American Home Products Corporation | Method of treating restenosis with rapamycin |
| JP3140228B2 (ja) * | 1992-02-17 | 2001-03-05 | ファイザー製薬株式会社 | 新規な大環状ラクトンおよびその生産菌 |
| US5288711A (en) | 1992-04-28 | 1994-02-22 | American Home Products Corporation | Method of treating hyperproliferative vascular disease |
| ZA935112B (en) * | 1992-07-17 | 1994-02-08 | Smithkline Beecham Corp | Rapamycin derivatives |
| GB9221220D0 (en) | 1992-10-09 | 1992-11-25 | Sandoz Ag | Organic componds |
| US5258389A (en) | 1992-11-09 | 1993-11-02 | Merck & Co., Inc. | O-aryl, O-alkyl, O-alkenyl and O-alkynylrapamycin derivatives |
| WO1994012655A1 (en) * | 1992-11-20 | 1994-06-09 | Smithkline Beecham Corporation | Process for producing rapamycin derivatives |
| US5595756A (en) * | 1993-12-22 | 1997-01-21 | Inex Pharmaceuticals Corporation | Liposomal compositions for enhanced retention of bioactive agents |
| US5362718A (en) | 1994-04-18 | 1994-11-08 | American Home Products Corporation | Rapamycin hydroxyesters |
| US5849730A (en) * | 1994-08-31 | 1998-12-15 | Pfizer Inc. | Process for preparing demethylrapamycins |
| FI971995A7 (fi) * | 1994-11-10 | 1997-05-09 | Pfizer | Makrosykliset laktoniyhdisteet ja niiden tuotantomenetelmä |
| US5561138A (en) | 1994-12-13 | 1996-10-01 | American Home Products Corporation | Method of treating anemia |
| US5496832A (en) | 1995-03-09 | 1996-03-05 | American Home Products Corporation | Method of treating cardiac inflammatory disease |
| RU2158267C2 (ru) | 1995-06-09 | 2000-10-27 | Новартис Аг | Производные рапамицина и фармацевтическая композиция на их основе |
| GB9606452D0 (en) | 1996-03-27 | 1996-06-05 | Sandoz Ltd | Organic compounds |
| AU4205597A (en) | 1996-08-20 | 1998-03-06 | Novartis Ag | Methods for prevention of cellular proliferation and restenosis |
| US6273913B1 (en) | 1997-04-18 | 2001-08-14 | Cordis Corporation | Modified stent useful for delivery of drugs along stent strut |
| US6015815A (en) * | 1997-09-26 | 2000-01-18 | Abbott Laboratories | Tetrazole-containing rapamycin analogs with shortened half-lives |
| US7399480B2 (en) | 1997-09-26 | 2008-07-15 | Abbott Laboratories | Methods of administering tetrazole-containing rapamycin analogs with other therapeutic substances using medical devices |
| US20030129215A1 (en) * | 1998-09-24 | 2003-07-10 | T-Ram, Inc. | Medical devices containing rapamycin analogs |
| US6890546B2 (en) | 1998-09-24 | 2005-05-10 | Abbott Laboratories | Medical devices containing rapamycin analogs |
| US5957975A (en) | 1997-12-15 | 1999-09-28 | The Cleveland Clinic Foundation | Stent having a programmed pattern of in vivo degradation |
| US7208010B2 (en) | 2000-10-16 | 2007-04-24 | Conor Medsystems, Inc. | Expandable medical device for delivery of beneficial agent |
| US8029561B1 (en) * | 2000-05-12 | 2011-10-04 | Cordis Corporation | Drug combination useful for prevention of restenosis |
| US20010029351A1 (en) | 1998-04-16 | 2001-10-11 | Robert Falotico | Drug combinations and delivery devices for the prevention and treatment of vascular disease |
| US20020005206A1 (en) * | 2000-05-19 | 2002-01-17 | Robert Falotico | Antiproliferative drug and delivery device |
| US6776796B2 (en) | 2000-05-12 | 2004-08-17 | Cordis Corportation | Antiinflammatory drug and delivery device |
| US20020007213A1 (en) | 2000-05-19 | 2002-01-17 | Robert Falotico | Drug/drug delivery systems for the prevention and treatment of vascular disease |
| US20050002986A1 (en) | 2000-05-12 | 2005-01-06 | Robert Falotico | Drug/drug delivery systems for the prevention and treatment of vascular disease |
| CA2408754C (en) * | 2000-05-12 | 2011-01-04 | Cordis Corporation | Delivery devices for treatment of vascular disease |
| HUP0301802A3 (en) * | 2000-06-28 | 2009-04-28 | Teva Pharma | Process for producing carvedilol, crystalline carvedilol, process for producing it and pharmaceutical composition containing it |
| WO2002056790A2 (en) | 2000-12-22 | 2002-07-25 | Avantec Vascular Corporation | Delivery of therapeutic capable agents |
| IL156887A0 (en) | 2001-02-16 | 2004-02-08 | Jomed Gmbh | Implants with fk506 |
| US20020127263A1 (en) | 2001-02-27 | 2002-09-12 | Wenda Carlyle | Peroxisome proliferator-acitvated receptor gamma ligand eluting medical device |
| US6641611B2 (en) * | 2001-11-26 | 2003-11-04 | Swaminathan Jayaraman | Therapeutic coating for an intravascular implant |
| US6805703B2 (en) | 2001-09-18 | 2004-10-19 | Scimed Life Systems, Inc. | Protective membrane for reconfiguring a workpiece |
| US7025734B1 (en) | 2001-09-28 | 2006-04-11 | Advanced Cardiovascular Systmes, Inc. | Guidewire with chemical sensing capabilities |
| WO2003064383A2 (en) * | 2002-02-01 | 2003-08-07 | Ariad Gene Therapeutics, Inc. | Phosphorus-containing compounds & uses thereof |
| BR0308053A (pt) | 2002-02-28 | 2004-12-28 | Novartis Ag | Stents revestidos com n-{5-[4-(4-metil-piperazino-metil)-benzoilamido]-2-metil fenil}-4-(3-piridil)-2-pirimidina-amina |
| US20040072857A1 (en) * | 2002-07-02 | 2004-04-15 | Jacob Waugh | Polymerized and modified rapamycins and their use in coating medical prostheses |
| BR0314013A (pt) * | 2002-09-06 | 2005-07-12 | Abbott Lab | Equipamento médico contendo inibidor de hidratação |
| US9770349B2 (en) | 2002-11-13 | 2017-09-26 | University Of Virginia Patent Foundation | Nanoporous stents with enhanced cellular adhesion and reduced neointimal formation |
| US20050033417A1 (en) * | 2003-07-31 | 2005-02-10 | John Borges | Coating for controlled release of a therapeutic agent |
| US7220755B2 (en) * | 2003-11-12 | 2007-05-22 | Biosensors International Group, Ltd. | 42-O-alkoxyalkyl rapamycin derivatives and compositions comprising same |
| US20050208095A1 (en) | 2003-11-20 | 2005-09-22 | Angiotech International Ag | Polymer compositions and methods for their use |
| WO2005063304A2 (en) * | 2003-12-24 | 2005-07-14 | Board Of Regents, The University Of Texas_System | Poly (l-glutamic acid) paramagnetic material complex and use as a biodegradable mri contrast agent |
| JP2007534337A (ja) * | 2004-04-27 | 2007-11-29 | ワイス | ラパマイシン特異的メチラーゼを用いるラパマイシンの標識 |
| US20070059336A1 (en) | 2004-04-30 | 2007-03-15 | Allergan, Inc. | Anti-angiogenic sustained release intraocular implants and related methods |
| KR20070083839A (ko) * | 2004-09-29 | 2007-08-24 | 코디스 코포레이션 | 안정한 무정형 라파마이신 유사 화합물의 약학적 투약 형태 |
| US20060129215A1 (en) * | 2004-12-09 | 2006-06-15 | Helmus Michael N | Medical devices having nanostructured regions for controlled tissue biocompatibility and drug delivery |
| JP5312018B2 (ja) | 2005-04-05 | 2013-10-09 | エリクシアー メディカル コーポレイション | 分解性の移植可能な医療装置 |
| KR20080018874A (ko) | 2005-04-27 | 2008-02-28 | 유니버시티 오브 플로리다 리서치 파운데이션, 아이엔씨. | 인간 질병과 관련된 돌연변이 단백질의 분해 촉진을 위한물질 및 방법 |
| US20070014760A1 (en) | 2005-07-18 | 2007-01-18 | Peyman Gholam A | Enhanced recovery following ocular surgery |
| US8585753B2 (en) | 2006-03-04 | 2013-11-19 | John James Scanlon | Fibrillated biodegradable prosthesis |
| US7820812B2 (en) | 2006-07-25 | 2010-10-26 | Abbott Laboratories | Methods of manufacturing crystalline forms of rapamycin analogs |
| US8088789B2 (en) * | 2006-09-13 | 2012-01-03 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| CN101557814B (zh) | 2006-09-13 | 2015-05-20 | 万能医药公司 | 大环内酯化合物及它们的使用方法 |
-
2007
- 2007-09-12 CN CN200780033959.3A patent/CN101557814B/zh not_active Expired - Fee Related
- 2007-09-12 WO PCT/US2007/078317 patent/WO2008033956A2/en not_active Ceased
- 2007-09-12 EP EP11191788.6A patent/EP2431036B1/en active Active
- 2007-09-12 JP JP2009528458A patent/JP6049160B2/ja not_active Expired - Fee Related
- 2007-09-12 EP EP07842371.2A patent/EP2083834B1/en active Active
- 2007-09-12 EP EP13181253.9A patent/EP2702993B1/en active Active
- 2007-09-12 CN CN201510178065.XA patent/CN104906087A/zh active Pending
- 2007-09-12 US US11/854,312 patent/US7867988B2/en active Active
- 2007-09-12 BR BRPI0716950-7A patent/BRPI0716950A2/pt not_active Application Discontinuation
-
2010
- 2010-12-02 US US12/959,153 patent/US20110097364A1/en not_active Abandoned
-
2011
- 2011-12-21 US US13/333,526 patent/US8367081B2/en not_active Expired - Fee Related
- 2011-12-21 US US13/333,573 patent/US8404641B2/en not_active Expired - Fee Related
-
2015
- 2015-01-30 JP JP2015017340A patent/JP2015129140A/ja not_active Withdrawn
-
2016
- 2016-10-05 JP JP2016197623A patent/JP2017036302A/ja not_active Withdrawn
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2010503699A5 (enExample) | ||
| EP2431036B1 (en) | Macrocyclic lactone compounds and methods for their use | |
| US9149470B2 (en) | Macrocyclic lactone compounds and methods for their use | |
| WO2020257283A1 (en) | Nicotinyl riboside compounds and their uses | |
| JP5627705B2 (ja) | 聴力損傷および目まいの治療用のβ−カルボリン | |
| KR20130055618A (ko) | 항재협착제 및 수송 촉진성 분자 분산제로 코팅된 카테터 풍선 | |
| CN101637624A (zh) | 含有雷帕霉素类似物的医疗装置 | |
| JP2012504654A (ja) | 多環状ラクトン化合物およびその使用方法 | |
| US20080171129A1 (en) | Drug eluting medical device using polymeric therapeutics with patterned coating | |
| WO2009114010A1 (en) | Macrocyclic lactone compounds and methods for their use | |
| US20230165839A1 (en) | Macrocyclic lactone compounds and methods for their use | |
| US20040204435A1 (en) | Alternating treatment with topoisomerase I and topoisomerase II inhibitors |