JP2009512467A - ランセットとテスト室とを備える分析補助手段 - Google Patents
ランセットとテスト室とを備える分析補助手段 Download PDFInfo
- Publication number
- JP2009512467A JP2009512467A JP2008535945A JP2008535945A JP2009512467A JP 2009512467 A JP2009512467 A JP 2009512467A JP 2008535945 A JP2008535945 A JP 2008535945A JP 2008535945 A JP2008535945 A JP 2008535945A JP 2009512467 A JP2009512467 A JP 2009512467A
- Authority
- JP
- Japan
- Prior art keywords
- lancet
- protective cap
- needle
- tip
- analysis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000012360 testing method Methods 0.000 title claims description 77
- 230000001681 protective effect Effects 0.000 claims abstract description 89
- 238000004458 analytical method Methods 0.000 claims abstract description 35
- 239000000463 material Substances 0.000 claims abstract description 34
- 239000007788 liquid Substances 0.000 claims abstract description 28
- 238000004519 manufacturing process Methods 0.000 claims abstract description 12
- 239000003566 sealing material Substances 0.000 claims description 22
- 239000003153 chemical reaction reagent Substances 0.000 claims description 13
- 230000001954 sterilising effect Effects 0.000 claims description 12
- 239000012491 analyte Substances 0.000 claims description 7
- 238000001514 detection method Methods 0.000 claims description 7
- 238000009423 ventilation Methods 0.000 claims description 7
- 230000008859 change Effects 0.000 claims description 5
- 229920001971 elastomer Polymers 0.000 claims description 3
- 239000000806 elastomer Substances 0.000 claims description 2
- 238000011156 evaluation Methods 0.000 claims description 2
- 238000000034 method Methods 0.000 abstract description 30
- 229920003023 plastic Polymers 0.000 abstract description 24
- 239000004033 plastic Substances 0.000 abstract description 23
- 239000013013 elastic material Substances 0.000 abstract description 15
- 239000004020 conductor Substances 0.000 abstract description 4
- 239000008280 blood Substances 0.000 description 41
- 210000004369 blood Anatomy 0.000 description 41
- 238000012795 verification Methods 0.000 description 23
- 238000010586 diagram Methods 0.000 description 20
- 230000008569 process Effects 0.000 description 18
- 238000005259 measurement Methods 0.000 description 17
- 239000000523 sample Substances 0.000 description 14
- 229910052751 metal Inorganic materials 0.000 description 13
- 239000002184 metal Substances 0.000 description 13
- 230000003287 optical effect Effects 0.000 description 12
- 238000004659 sterilization and disinfection Methods 0.000 description 10
- 238000007789 sealing Methods 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 239000000126 substance Substances 0.000 description 8
- 230000009471 action Effects 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 230000004224 protection Effects 0.000 description 7
- 238000012546 transfer Methods 0.000 description 7
- 238000001746 injection moulding Methods 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- 208000027418 Wounds and injury Diseases 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- -1 polypropylene Polymers 0.000 description 5
- 229920002725 thermoplastic elastomer Polymers 0.000 description 5
- 241000894006 Bacteria Species 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 239000000919 ceramic Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 210000001124 body fluid Anatomy 0.000 description 3
- 239000010839 body fluid Substances 0.000 description 3
- 239000011162 core material Substances 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 238000002845 discoloration Methods 0.000 description 3
- 238000002848 electrochemical method Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000036512 infertility Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 239000002985 plastic film Substances 0.000 description 3
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 3
- 230000000284 resting effect Effects 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 238000009736 wetting Methods 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 239000004952 Polyamide Substances 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- 230000003187 abdominal effect Effects 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 239000012503 blood component Substances 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000000875 corresponding effect Effects 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 239000010408 film Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910002804 graphite Inorganic materials 0.000 description 2
- 239000010439 graphite Substances 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 230000005661 hydrophobic surface Effects 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 229910052741 iridium Inorganic materials 0.000 description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- 229920006255 plastic film Polymers 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920002647 polyamide Polymers 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000009979 protective mechanism Effects 0.000 description 2
- 239000012488 sample solution Substances 0.000 description 2
- 229920006344 thermoplastic copolyester Polymers 0.000 description 2
- 229920005992 thermoplastic resin Polymers 0.000 description 2
- 241001552669 Adonis annua Species 0.000 description 1
- 229910000497 Amalgam Inorganic materials 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- 229920001634 Copolyester Polymers 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 239000004433 Thermoplastic polyurethane Substances 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 229910052770 Uranium Inorganic materials 0.000 description 1
- 206010047531 Visual acuity reduced Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 238000004159 blood analysis Methods 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 230000002079 cooperative effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013039 cover film Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 210000000624 ear auricle Anatomy 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000000840 electrochemical analysis Methods 0.000 description 1
- 238000000835 electrochemical detection Methods 0.000 description 1
- 238000004049 embossing Methods 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 239000011796 hollow space material Substances 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 230000005660 hydrophilic surface Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 239000002114 nanocomposite Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920006289 polycarbonate film Polymers 0.000 description 1
- 229910021420 polycrystalline silicon Inorganic materials 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000010970 precious metal Substances 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 238000004080 punching Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- WUAPFZMCVAUBPE-UHFFFAOYSA-N rhenium atom Chemical compound [Re] WUAPFZMCVAUBPE-UHFFFAOYSA-N 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 1
- 238000007788 roughening Methods 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 229920001935 styrene-ethylene-butadiene-styrene Polymers 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- 239000005061 synthetic rubber Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 229920002397 thermoplastic olefin Polymers 0.000 description 1
- 229920002803 thermoplastic polyurethane Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- JFALSRSLKYAFGM-UHFFFAOYSA-N uranium(0) Chemical compound [U] JFALSRSLKYAFGM-UHFFFAOYSA-N 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
- 238000013022 venting Methods 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/1468—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means
- A61B5/1477—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means non-invasive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14532—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring glucose, e.g. by tissue impedance measurement
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150015—Source of blood
- A61B5/150022—Source of blood for capillary blood or interstitial fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150206—Construction or design features not otherwise provided for; manufacturing or production; packages; sterilisation of piercing element, piercing device or sampling device
- A61B5/150213—Venting means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150206—Construction or design features not otherwise provided for; manufacturing or production; packages; sterilisation of piercing element, piercing device or sampling device
- A61B5/150274—Manufacture or production processes or steps for blood sampling devices
- A61B5/150282—Manufacture or production processes or steps for blood sampling devices for piercing elements, e.g. blade, lancet, canula, needle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150358—Strips for collecting blood, e.g. absorbent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150374—Details of piercing elements or protective means for preventing accidental injuries by such piercing elements
- A61B5/150381—Design of piercing elements
- A61B5/150412—Pointed piercing elements, e.g. needles, lancets for piercing the skin
- A61B5/150435—Specific design of proximal end
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150374—Details of piercing elements or protective means for preventing accidental injuries by such piercing elements
- A61B5/150381—Design of piercing elements
- A61B5/150503—Single-ended needles
- A61B5/150511—Details of construction of shaft
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150374—Details of piercing elements or protective means for preventing accidental injuries by such piercing elements
- A61B5/150534—Design of protective means for piercing elements for preventing accidental needle sticks, e.g. shields, caps, protectors, axially extensible sleeves, pivotable protective sleeves
- A61B5/150572—Pierceable protectors, e.g. shields, caps, sleeves or films, e.g. for hygienic purposes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/151—Devices specially adapted for taking samples of capillary blood, e.g. by lancets, needles or blades
- A61B5/15101—Details
- A61B5/15103—Piercing procedure
- A61B5/15107—Piercing being assisted by a triggering mechanism
- A61B5/15113—Manually triggered, i.e. the triggering requires a deliberate action by the user such as pressing a drive button
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/151—Devices specially adapted for taking samples of capillary blood, e.g. by lancets, needles or blades
- A61B5/15186—Devices loaded with a single lancet, i.e. a single lancet with or without a casing is loaded into a reusable drive device and then discarded after use; drive devices reloadable for multiple use
- A61B5/15188—Constructional features of reusable driving devices
- A61B5/1519—Constructional features of reusable driving devices comprising driving means, e.g. a spring, for propelling the piercing unit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/151—Devices specially adapted for taking samples of capillary blood, e.g. by lancets, needles or blades
- A61B5/15186—Devices loaded with a single lancet, i.e. a single lancet with or without a casing is loaded into a reusable drive device and then discarded after use; drive devices reloadable for multiple use
- A61B5/15188—Constructional features of reusable driving devices
- A61B5/15192—Constructional features of reusable driving devices comprising driving means, e.g. a spring, for retracting the lancet unit into the driving device housing
- A61B5/15194—Constructional features of reusable driving devices comprising driving means, e.g. a spring, for retracting the lancet unit into the driving device housing fully automatically retracted, i.e. the retraction does not require a deliberate action by the user, e.g. by terminating the contact with the patient's skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/157—Devices characterised by integrated means for measuring characteristics of blood
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2562/00—Details of sensors; Constructional details of sensor housings or probes; Accessories for sensors
- A61B2562/02—Details of sensors specially adapted for in-vivo measurements
- A61B2562/0295—Strip shaped analyte sensors for apparatus classified in A61B5/145 or A61B5/157
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/1468—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means
- A61B5/1486—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means using enzyme electrodes, e.g. with immobilised oxidase
- A61B5/14865—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means using enzyme electrodes, e.g. with immobilised oxidase invasive, e.g. introduced into the body by a catheter or needle or using implanted sensors
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Surgery (AREA)
- Pathology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Hematology (AREA)
- Manufacturing & Machinery (AREA)
- Optics & Photonics (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Emergency Medicine (AREA)
- Dermatology (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Abstract
Description
1.このディスポは(血糖)測定器具の収容装置へ差し込まれ、それによって固定される。マガジン収納式のディスポのために、ディスポのマガジンが測定器具へ挿入される。
2.穿刺ユニットの駆動メカニズムに応力がかけられて、ディスポの駆動装置に結合される。
3.ディスポが測定器具に固定されたときに、ディスポの接点が測定器具にある電気供給部と接触する。
4.利用者が分析補助手段の開口部に指で、または測定が実施されるべき身体部位で接触する。
5.穿刺プロセスが作動すると針が前方に向かって動き、その際に、防護キャップを通って高い速度で開口部から外に出る。穿刺プロセス全体が数ミリ秒の単位で進行する。
6.皮膚への穿刺が完了した後、針が再び引き戻される。このとき、ランセットにある捕捉装置が防護キャップおよび場合によりその他のシール材を一緒に引き戻す。駆動装置が場合により再び結合解除される。
a)ランセットをカウンター電極として使用するときは、ランセットが、駆動ユニットに組み込まれていてよい追加のばね接点と接続される。
7.利用者は(引き続き)補助手段の開口部にその採取装置で接触しており、それにより、吸引開口部(たとえば毛管)が血液滴を採取することができる。
8.試料液移送のための手段の吸引作用により、ディスポの血液は、検証部材を備えたテスト部材があるテスト室内の個所に移送される。
9.検証部材では、検証されるべき血液成分と検証試薬との反応が行われ、この反応がたとえば測光式または電気化学式の検出によって検出される。
10.電子データから測定結果が算出され、利用者に視覚的または音響的に表示される。
11.マガジン収納式のディスポの場合、マガジンを1サイクルだけ先に進める。使い捨てディスポの場合、使用済みのディスポは廃棄されるか、または手で取り外される。
(1)ハウジング部品を射出成形する。
(2)ランセット針の埋設を含めて、本体(防護キャップ)を射出成形する(場合により「針頭部」すなわち穿刺器具により把持可能な肉厚部の生成も含む)。
(3)針先端部を軟質プラスチックで押出被覆する。
(4)たとえば電離性放射線によって「未加工ディスポ」を滅菌する。
この「未加工ディスポ」は、たとえば断裁や打抜きによって個別ディスポに分割される帯状製品として存在しているのが好ましい。
(5)ハウジングへ電極を塗布(スパッタ、プリントなど)または埋設(レーザ剥離、エッチング、射出成形など)する。
(6)検証部材を備えるテスト部材をハウジングの電極の上に入れる。
(7)テスト組立、すなわち「未加工ディスポ」をハウジングと結合。
(8)ハウジングの閉鎖と梱包。
ランセット針の先端部は、どの実施形態でも未使用状態のときに菌を通さないよう遮蔽されており、すなわち、ランセットの使用直前まで菌がランセット先端部へ入ることがない。適当な滅菌の後、ランセット先端部は長期間にわたって滅菌状態に保たれる。
ランセット先端部の滅菌状態は、たとえばランセットとテスト部材を結合するための以後の製造ステップでも保証される。このとき、デリケートな針先端部は機械的な影響(曲げなど)から守られている。
本発明によるディスポの利用者は、未使用のランセット針による意図しないけがから守られる。本来の使用者以外の人物についても同様であるのは言うまでもない。
テスト室はランセットを使用する前に密閉されている。
テスト室のすべての開口部は防護キャップだけによって、もしくはさらに別のシール材との組み合わせで閉止されている。
本発明によるディスポは従来式の製造方法で低コストに大量個数を製造することができる。
本発明によるディスポは大幅に小型化されており、したがって、コンパクトな自動式のシステムで採用するのに適している。
分析テスト部材として、電気化学式または光学式のセンサの公知のバリエーションを採用することができる。
電気化学測定の場合、ランセットを(それが導電性材料で製作されていれば)液位計および/またはカウンター電極として利用することができる。
Claims (12)
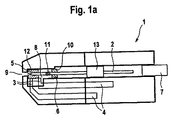
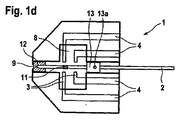
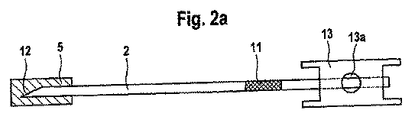
- 分析補助手段において、
i)ランセットを含んでおり、該ランセットは、
ランセット針を含んでおり、該ランセット針は先端部と、
前記ランセット針を少なくとも前記先端部の領域で全面的に取り囲む防護キャップとを備えており、
前記ランセット針は前記防護キャップに対して相対的に変位可能であり、前記ランセットは、前記ランセットが引き戻されたときに前記防護キャップを一緒に引き戻すように前記防護キャップと協働作用し、
ii)試薬系を含む室を備えたテスト部材を含んでおり、前記室は前記防護キャップで閉止された開口部を有している分析補助手段。 - 前記防護キャップの材料はエラストマーであることを特徴とする請求項1記載の分析補助手段。
- 前記防護キャップは前記針先端部を滅菌状態に取り囲むことを特徴とする請求項1または2記載の分析補助手段。
- 前記分析補助手段は試料液移送をするための手段を有していることを特徴とする請求項1〜3のいずれか1項に記載の分析補助手段。
- 試料液移送をするための前記手段は毛管間隙、毛管通路、吸引能力のある材料からなる芯材、または吸引能力のある材料からなるウェブであることを特徴とする請求項4記載の分析補助手段。
- 前記補助手段は、前記ランセットおよび前記補助手段に対して相対的に変位可能なシール材によって密閉された第2の開口部を有していることを特徴とする請求項1〜5のいずれか1項に記載の分析補助手段。
- 前記防護キャップは前記両方の開口部を密閉することを特徴とする請求項6記載の分析補助手段。
- 前記ランセットには、前記ランセットが引き戻されるときに前記ランセットにより突き破られた前記防護キャップを一緒に引き戻す捕捉装置または表面構造があることを特徴とする請求項1〜7のいずれか1項に記載の分析補助手段。
- 前記ランセットが引き戻されるときに引き戻された前記防護キャップは、前記ランセットに対して相対的に変位可能な第2のシール材を一緒に引き戻し、換気口が開口されることを特徴とする請求項8記載の分析補助手段。
- 前記ランセットは試料液位測定をするための手段を有しており、および/またはカウンター電極としての役目を果たすことを特徴とする請求項1〜9のいずれか1項に記載の分析補助手段。
- 分析補助手段を製造する方法において、
ランセット針の先端部が防護キャップの中にあるランセットを準備するステップと、
前記ランセットが引き戻されるときに前記防護キャップを一緒に引き戻す捕捉装置を前記ランセットに準備するステップと、
試薬系と室の開口部とを含む、室を備えたテスト部材を準備するステップと、
前記ランセットを滅菌するステップと、
前記ランセットを前記室へ挿入し、前記防護キャップが前記室の前記開口部を閉止するようにするステップとを有している方法。 - 分析補助手段を備える分析システムにおいて、
ランセットを含んでおり、該ランセットは、
ランセット針を含んでおり、該ランセット針は先端部と、
前記ランセット針を少なくとも前記先端部の領域で全面的に取り囲む防護キャップとを備えており、
前記ランセットが引き戻されるときに前記防護キャップを一緒に引き戻す、ランセットにある捕捉装置または表面構造と、
試薬変化が起きたときに生じる信号を検知する検出ユニットと、
前記信号に基づいて分析物の濃度を判定する評価装置とを含んでいる分析システム。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05022830A EP1776925A1 (de) | 2005-10-20 | 2005-10-20 | Analytisches Hilfsmittel mit einer Lanzette und einer Testkammer |
| PCT/EP2006/009944 WO2007045411A1 (de) | 2005-10-20 | 2006-10-14 | Analytisches hilfsmittel mit sterilschutz für die lanzette und testkammer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009512467A true JP2009512467A (ja) | 2009-03-26 |
| JP2009512467A5 JP2009512467A5 (ja) | 2009-09-24 |
Family
ID=35892414
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008535945A Pending JP2009512467A (ja) | 2005-10-20 | 2006-10-14 | ランセットとテスト室とを備える分析補助手段 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20080243032A1 (ja) |
| EP (2) | EP1776925A1 (ja) |
| JP (1) | JP2009512467A (ja) |
| CN (1) | CN101291621B (ja) |
| AT (1) | ATE474502T1 (ja) |
| DE (1) | DE502006007496D1 (ja) |
| WO (1) | WO2007045411A1 (ja) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2022105703A (ja) * | 2015-09-07 | 2022-07-14 | プラズマティカ リミテッド | 医療装置ビューポートの曇り防止 |
| US11974728B2 (en) | 2015-09-07 | 2024-05-07 | Plasmatica Ltd. | Preventing fog on a medical device viewport |
| US12070193B2 (en) | 2021-04-22 | 2024-08-27 | Plasmatica Ltd. | Multiple pumps for reducing pressure for plasma treatment |
| US12262877B2 (en) | 2015-09-07 | 2025-04-01 | Plasmatica Ltd. | Methods and systems for providing plasma treatments to optical surfaces |
| JP7660157B2 (ja) | 2011-04-29 | 2025-04-10 | ユアバイオ ヘルス, インコーポレイテッド | 流体の送達および/または受け取り |
| US12310728B2 (en) | 2010-11-09 | 2025-05-27 | Yourbio Health, Inc. | Systems and interfaces for blood sampling |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8636672B2 (en) * | 2007-02-28 | 2014-01-28 | Nipro Diagnostics, Inc. | Test strip with integrated lancet |
| US7927290B2 (en) * | 2006-01-05 | 2011-04-19 | Panasonic Corporation | Blood test apparatus |
| EP2153774A1 (en) * | 2007-04-29 | 2010-02-17 | Arkray, Inc. | Analyzing system |
| EP2226007A1 (de) * | 2009-02-19 | 2010-09-08 | Roche Diagnostics GmbH | Testelementmagazin mit abgedeckten Testfeldern |
| EP2226008A1 (de) | 2009-02-19 | 2010-09-08 | Roche Diagnostics GmbH | Verfahren zur Herstellung eines analytischen Magazins |
| EP2398388B1 (de) | 2009-02-19 | 2020-04-08 | Roche Diabetes Care GmbH | Platzsparende magazinierung analytischer hilfsmittel |
| EP2241252A1 (de) * | 2009-03-17 | 2010-10-20 | F. Hoffmann-La Roche AG | Testvorrichtung insbesondere für Blutzuckertests |
| US20130197452A1 (en) * | 2010-09-27 | 2013-08-01 | Terumo Kabushiki Kaisha | Medical instrument with attached needle |
| WO2012106060A2 (en) * | 2011-01-06 | 2012-08-09 | Pepex Biomedical, Inc. | Sensor module with enhanced capillary flow |
| WO2014200924A1 (en) | 2013-06-10 | 2014-12-18 | Facet Technologies, Llc | Lancet needle with alignment and retention notch |
| WO2016187780A1 (en) * | 2015-05-25 | 2016-12-01 | Coyote Bioscience Yixing Co., Ltd. | Device and method for sample collection |
| US10154809B2 (en) * | 2015-06-24 | 2018-12-18 | University Of Virginia Patent Foundation | Test strip device and related methods thereof |
| WO2024163311A1 (en) * | 2023-02-02 | 2024-08-08 | Siemens Healthcare Diagnostics Inc. | Laminate urinalysis assay strip and methods of producing and using same |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5125908A (en) * | 1990-10-19 | 1992-06-30 | Cohen Milton J | Hypodermic syringe with protective holder |
| JP2001133430A (ja) * | 1999-11-08 | 2001-05-18 | Arkray Inc | 体液測定装置、およびこの体液測定装置に挿着して使用する挿着体 |
| JP2003153885A (ja) * | 2001-08-29 | 2003-05-27 | F Hoffmann La Roche Ag | ランセットと試験要素を有する分析装置 |
| JP2003527897A (ja) * | 2000-03-04 | 2003-09-24 | エフ.ホフマン−ラ ロシュ アーゲー | 先端が衛生的に保護された血液ランセット |
| JP2004512129A (ja) * | 2000-10-31 | 2004-04-22 | エフ ホフマン−ラ ロッシュ アクチェン ゲゼルシャフト | 採血用システム |
| JP2004283568A (ja) * | 2003-03-20 | 2004-10-14 | F Hoffmann-La Roche Ag | 再使用防止式ランセットシステム切開補助具 |
| JP4757301B2 (ja) * | 2005-05-20 | 2011-08-24 | エフ ホフマン−ラ ロッシュ アクチェン ゲゼルシャフト | 滅菌保護部を有するランセットシステム |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE8007991U1 (de) * | 1980-03-22 | 1981-04-09 | Clinicon Mannheim GmbH, 6800 Mannheim | Blutlanzettenvorrichtung zur Entnahme von Blut für Diagnosezwecke |
| US4627445A (en) * | 1985-04-08 | 1986-12-09 | Garid, Inc. | Glucose medical monitoring system |
| US4999582A (en) * | 1989-12-15 | 1991-03-12 | Boehringer Mannheim Corp. | Biosensor electrode excitation circuit |
| US5243516A (en) * | 1989-12-15 | 1993-09-07 | Boehringer Mannheim Corporation | Biosensing instrument and method |
| US4963814A (en) * | 1989-12-15 | 1990-10-16 | Boehringer Mannheim Corporation | Regulated bifurcated power supply |
| US4999632A (en) * | 1989-12-15 | 1991-03-12 | Boehringer Mannheim Corporation | Analog to digital conversion with noise reduction |
| US5366609A (en) * | 1993-06-08 | 1994-11-22 | Boehringer Mannheim Corporation | Biosensing meter with pluggable memory key |
| US5405511A (en) * | 1993-06-08 | 1995-04-11 | Boehringer Mannheim Corporation | Biosensing meter with ambient temperature estimation method and system |
| US5352351A (en) * | 1993-06-08 | 1994-10-04 | Boehringer Mannheim Corporation | Biosensing meter with fail/safe procedures to prevent erroneous indications |
| CA2153883C (en) * | 1993-06-08 | 1999-02-09 | Bradley E. White | Biosensing meter which detects proper electrode engagement and distinguishes sample and check strips |
| DE4320463A1 (de) * | 1993-06-21 | 1994-12-22 | Boehringer Mannheim Gmbh | Blutlanzettenvorrichtung zur Entnahme von Blut für Diagnosezwecke |
| ATE227844T1 (de) * | 1997-02-06 | 2002-11-15 | Therasense Inc | Kleinvolumiger sensor zur in-vitro bestimmung |
| DE19840856B4 (de) * | 1998-09-07 | 2008-04-10 | Roche Diagnostics Gmbh | System zur Gewinnung einer Köperflüssigkeit, Lanzettenmagazin, Lanzette, Lanzettensatz, Stechhilfe und Verfahren zur Entnahme einer Lanzette aus einem Lanzettenmagazin sowie Verwendung des Systems |
-
2005
- 2005-10-20 EP EP05022830A patent/EP1776925A1/de not_active Withdrawn
-
2006
- 2006-10-14 EP EP06806285A patent/EP1940289B1/de not_active Not-in-force
- 2006-10-14 AT AT06806285T patent/ATE474502T1/de active
- 2006-10-14 JP JP2008535945A patent/JP2009512467A/ja active Pending
- 2006-10-14 DE DE502006007496T patent/DE502006007496D1/de active Active
- 2006-10-14 CN CN2006800392336A patent/CN101291621B/zh not_active Expired - Fee Related
- 2006-10-14 WO PCT/EP2006/009944 patent/WO2007045411A1/de active Application Filing
-
2008
- 2008-04-18 US US12/105,304 patent/US20080243032A1/en not_active Abandoned
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5125908A (en) * | 1990-10-19 | 1992-06-30 | Cohen Milton J | Hypodermic syringe with protective holder |
| JP2001133430A (ja) * | 1999-11-08 | 2001-05-18 | Arkray Inc | 体液測定装置、およびこの体液測定装置に挿着して使用する挿着体 |
| JP2003527897A (ja) * | 2000-03-04 | 2003-09-24 | エフ.ホフマン−ラ ロシュ アーゲー | 先端が衛生的に保護された血液ランセット |
| JP2005270679A (ja) * | 2000-03-04 | 2005-10-06 | F Hoffmann La Roche Ag | 先端が衛生的に保護された血液ランセット |
| JP2004512129A (ja) * | 2000-10-31 | 2004-04-22 | エフ ホフマン−ラ ロッシュ アクチェン ゲゼルシャフト | 採血用システム |
| JP2003153885A (ja) * | 2001-08-29 | 2003-05-27 | F Hoffmann La Roche Ag | ランセットと試験要素を有する分析装置 |
| JP2005185852A (ja) * | 2001-08-29 | 2005-07-14 | F Hoffmann La Roche Ag | ランセットと試験要素を有する分析装置を製造する方法 |
| JP2004283568A (ja) * | 2003-03-20 | 2004-10-14 | F Hoffmann-La Roche Ag | 再使用防止式ランセットシステム切開補助具 |
| JP4757301B2 (ja) * | 2005-05-20 | 2011-08-24 | エフ ホフマン−ラ ロッシュ アクチェン ゲゼルシャフト | 滅菌保護部を有するランセットシステム |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12310728B2 (en) | 2010-11-09 | 2025-05-27 | Yourbio Health, Inc. | Systems and interfaces for blood sampling |
| JP7660157B2 (ja) | 2011-04-29 | 2025-04-10 | ユアバイオ ヘルス, インコーポレイテッド | 流体の送達および/または受け取り |
| JP2022105703A (ja) * | 2015-09-07 | 2022-07-14 | プラズマティカ リミテッド | 医療装置ビューポートの曇り防止 |
| KR20230098716A (ko) * | 2015-09-07 | 2023-07-04 | 플라즈마티카 리미티드 | 의료 디바이스 뷰포트 상에서의 김서림 방지 |
| JP7345923B2 (ja) | 2015-09-07 | 2023-09-19 | プラズマティカ リミテッド | 医療装置ビューポートの曇り防止 |
| US11974728B2 (en) | 2015-09-07 | 2024-05-07 | Plasmatica Ltd. | Preventing fog on a medical device viewport |
| KR102738274B1 (ko) | 2015-09-07 | 2024-12-05 | 플라즈마티카 리미티드 | 의료 디바이스 뷰포트 상에서의 김서림 방지 |
| US12262877B2 (en) | 2015-09-07 | 2025-04-01 | Plasmatica Ltd. | Methods and systems for providing plasma treatments to optical surfaces |
| US12070193B2 (en) | 2021-04-22 | 2024-08-27 | Plasmatica Ltd. | Multiple pumps for reducing pressure for plasma treatment |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007045411A1 (de) | 2007-04-26 |
| CN101291621A (zh) | 2008-10-22 |
| ATE474502T1 (de) | 2010-08-15 |
| DE502006007496D1 (de) | 2010-09-02 |
| CN101291621B (zh) | 2010-09-01 |
| US20080243032A1 (en) | 2008-10-02 |
| HK1125019A1 (en) | 2009-07-31 |
| EP1940289B1 (de) | 2010-07-21 |
| EP1776925A1 (de) | 2007-04-25 |
| EP1940289A1 (de) | 2008-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2009512467A (ja) | ランセットとテスト室とを備える分析補助手段 | |
| US8523784B2 (en) | Analytical device with lancet and test element | |
| JP4468324B2 (ja) | 分析補助手段 | |
| JP6194307B2 (ja) | ランセット作動方法及び装置 | |
| EP1643908B1 (en) | System for withdrawing body fluid | |
| CN103607950B (zh) | 组织穿透装置 | |
| US7819822B2 (en) | Body fluid sampling device | |
| US8257276B2 (en) | Lancet device having capillary action | |
| US8628724B2 (en) | Integrated needle and test strip with aspiration apparatus and method of use | |
| JP2017140464A (ja) | 一体化ランシングデバイス | |
| US20050283094A1 (en) | Disposable lancet and lancing cap combination for increased hygiene | |
| WO2010039447A1 (en) | Method and apparatus for a fluid sampling device | |
| CN1929783A (zh) | 体液采样设备 | |
| US8172866B2 (en) | Medical aid | |
| JP2000232974A (ja) | 検体採取用具および穿刺デバイス | |
| HK1125019B (zh) | 具有刺血器和测试腔的分析辅助机构 | |
| JP2003180663A (ja) | 体液成分測定装置 | |
| HK1107238A (en) | Body fluid sampling device | |
| HK1116646A (en) | Disposable lancet and lancing cap combination for increased hygiene |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090806 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090806 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20100525 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111206 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120515 |