JP2008501795A - ハイブリッド幹細胞の治療的再プログラミングおよび成熟 - Google Patents
ハイブリッド幹細胞の治療的再プログラミングおよび成熟 Download PDFInfo
- Publication number
- JP2008501795A JP2008501795A JP2007527196A JP2007527196A JP2008501795A JP 2008501795 A JP2008501795 A JP 2008501795A JP 2007527196 A JP2007527196 A JP 2007527196A JP 2007527196 A JP2007527196 A JP 2007527196A JP 2008501795 A JP2008501795 A JP 2008501795A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- stem cells
- stem
- host
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 210000000130 stem cell Anatomy 0.000 title claims abstract description 239
- 230000001225 therapeutic effect Effects 0.000 title claims abstract description 52
- 230000008672 reprogramming Effects 0.000 title claims abstract description 46
- 230000035800 maturation Effects 0.000 title description 48
- 210000004027 cell Anatomy 0.000 claims abstract description 578
- 238000000034 method Methods 0.000 claims abstract description 122
- 210000001671 embryonic stem cell Anatomy 0.000 claims description 83
- 230000004927 fusion Effects 0.000 claims description 55
- 210000004602 germ cell Anatomy 0.000 claims description 44
- 239000000284 extract Substances 0.000 claims description 43
- 230000008569 process Effects 0.000 claims description 41
- 230000007159 enucleation Effects 0.000 claims description 22
- 239000000126 substance Substances 0.000 claims description 20
- 230000004936 stimulating effect Effects 0.000 claims description 17
- 230000018109 developmental process Effects 0.000 claims description 13
- 230000001605 fetal effect Effects 0.000 claims description 13
- 238000011161 development Methods 0.000 claims description 11
- 210000001778 pluripotent stem cell Anatomy 0.000 claims description 11
- 210000001988 somatic stem cell Anatomy 0.000 claims description 9
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 claims description 8
- 210000000604 fetal stem cell Anatomy 0.000 claims description 8
- 241000124008 Mammalia Species 0.000 claims description 7
- 238000012258 culturing Methods 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 210000001178 neural stem cell Anatomy 0.000 claims description 6
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 claims description 4
- RTKIYFITIVXBLE-UHFFFAOYSA-N Trichostatin A Natural products ONC(=O)C=CC(C)=CC(C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-UHFFFAOYSA-N 0.000 claims description 4
- 229940121372 histone deacetylase inhibitor Drugs 0.000 claims description 4
- 239000003276 histone deacetylase inhibitor Substances 0.000 claims description 4
- 210000001082 somatic cell Anatomy 0.000 claims description 4
- RTKIYFITIVXBLE-QEQCGCAPSA-N trichostatin A Chemical compound ONC(=O)/C=C/C(/C)=C/[C@@H](C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-QEQCGCAPSA-N 0.000 claims description 4
- 241000700605 Viruses Species 0.000 claims description 3
- 238000000520 microinjection Methods 0.000 claims description 3
- 238000007500 overflow downdraw method Methods 0.000 claims description 3
- 239000003814 drug Substances 0.000 claims description 2
- 229940124597 therapeutic agent Drugs 0.000 claims 1
- 238000002560 therapeutic procedure Methods 0.000 abstract description 15
- 230000008929 regeneration Effects 0.000 abstract description 7
- 238000011069 regeneration method Methods 0.000 abstract description 7
- 230000001172 regenerating effect Effects 0.000 abstract description 4
- 239000002609 medium Substances 0.000 description 59
- 210000004940 nucleus Anatomy 0.000 description 45
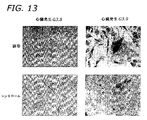
- 210000001519 tissue Anatomy 0.000 description 36
- 230000004069 differentiation Effects 0.000 description 34
- 210000001161 mammalian embryo Anatomy 0.000 description 29
- 230000013020 embryo development Effects 0.000 description 22
- 210000000287 oocyte Anatomy 0.000 description 22
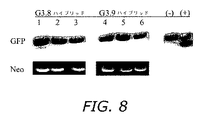
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 20
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 20
- 230000006870 function Effects 0.000 description 20
- 239000005090 green fluorescent protein Substances 0.000 description 20
- 230000008439 repair process Effects 0.000 description 18
- 210000001185 bone marrow Anatomy 0.000 description 17
- SDZRWUKZFQQKKV-JHADDHBZSA-N cytochalasin D Chemical compound C([C@H]1[C@@H]2[C@@H](C([C@@H](O)[C@H]\3[C@]2([C@@H](/C=C/[C@@](C)(O)C(=O)[C@@H](C)C/C=C/3)OC(C)=O)C(=O)N1)=C)C)C1=CC=CC=C1 SDZRWUKZFQQKKV-JHADDHBZSA-N 0.000 description 16
- 210000003754 fetus Anatomy 0.000 description 16
- 238000002360 preparation method Methods 0.000 description 16
- 241001465754 Metazoa Species 0.000 description 14
- 230000001413 cellular effect Effects 0.000 description 14
- 210000002257 embryonic structure Anatomy 0.000 description 14
- 210000000056 organ Anatomy 0.000 description 14
- 241000282414 Homo sapiens Species 0.000 description 13
- 230000005305 organ development Effects 0.000 description 13
- 238000002054 transplantation Methods 0.000 description 13
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 12
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 12
- 210000002798 bone marrow cell Anatomy 0.000 description 12
- 210000000805 cytoplasm Anatomy 0.000 description 12
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 12
- 241000699670 Mus sp. Species 0.000 description 11
- 210000002304 esc Anatomy 0.000 description 11
- 230000000670 limiting effect Effects 0.000 description 11
- 210000004789 organ system Anatomy 0.000 description 11
- 241000894007 species Species 0.000 description 11
- 102000000426 Integrin alpha6 Human genes 0.000 description 10
- 108010041100 Integrin alpha6 Proteins 0.000 description 10
- 102000016971 Proto-Oncogene Proteins c-kit Human genes 0.000 description 10
- 108010014608 Proto-Oncogene Proteins c-kit Proteins 0.000 description 10
- 210000001654 germ layer Anatomy 0.000 description 10
- 230000001939 inductive effect Effects 0.000 description 10
- 230000004048 modification Effects 0.000 description 10
- 238000012986 modification Methods 0.000 description 10
- 239000002953 phosphate buffered saline Substances 0.000 description 10
- 210000002966 serum Anatomy 0.000 description 10
- 238000011282 treatment Methods 0.000 description 10
- 239000000203 mixture Substances 0.000 description 9
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 230000032683 aging Effects 0.000 description 8
- 230000032823 cell division Effects 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 8
- 239000007995 HEPES buffer Substances 0.000 description 7
- 206010061216 Infarction Diseases 0.000 description 7
- 210000000601 blood cell Anatomy 0.000 description 7
- 238000002513 implantation Methods 0.000 description 7
- 230000007574 infarction Effects 0.000 description 7
- 210000004698 lymphocyte Anatomy 0.000 description 7
- 210000002826 placenta Anatomy 0.000 description 7
- 238000000926 separation method Methods 0.000 description 7
- 108091035539 telomere Proteins 0.000 description 7
- 210000003411 telomere Anatomy 0.000 description 7
- 102000055501 telomere Human genes 0.000 description 7
- BVIAOQMSVZHOJM-UHFFFAOYSA-N N(6),N(6)-dimethyladenine Chemical compound CN(C)C1=NC=NC2=C1N=CN2 BVIAOQMSVZHOJM-UHFFFAOYSA-N 0.000 description 6
- GBOGMAARMMDZGR-UHFFFAOYSA-N UNPD149280 Natural products N1C(=O)C23OC(=O)C=CC(O)CCCC(C)CC=CC3C(O)C(=C)C(C)C2C1CC1=CC=CC=C1 GBOGMAARMMDZGR-UHFFFAOYSA-N 0.000 description 6
- 210000004504 adult stem cell Anatomy 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 239000008004 cell lysis buffer Substances 0.000 description 6
- 238000010367 cloning Methods 0.000 description 6
- GBOGMAARMMDZGR-JREHFAHYSA-N cytochalasin B Natural products C[C@H]1CCC[C@@H](O)C=CC(=O)O[C@@]23[C@H](C=CC1)[C@H](O)C(=C)[C@@H](C)[C@@H]2[C@H](Cc4ccccc4)NC3=O GBOGMAARMMDZGR-JREHFAHYSA-N 0.000 description 6
- GBOGMAARMMDZGR-TYHYBEHESA-N cytochalasin B Chemical compound C([C@H]1[C@@H]2[C@@H](C([C@@H](O)[C@@H]3/C=C/C[C@H](C)CCC[C@@H](O)/C=C/C(=O)O[C@@]23C(=O)N1)=C)C)C1=CC=CC=C1 GBOGMAARMMDZGR-TYHYBEHESA-N 0.000 description 6
- 210000002242 embryoid body Anatomy 0.000 description 6
- 230000003394 haemopoietic effect Effects 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 230000031864 metaphase Effects 0.000 description 6
- 229960004857 mitomycin Drugs 0.000 description 6
- 210000003205 muscle Anatomy 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 210000004291 uterus Anatomy 0.000 description 6
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 5
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 5
- 102100037362 Fibronectin Human genes 0.000 description 5
- 108010067306 Fibronectins Proteins 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 5
- 241001494479 Pecora Species 0.000 description 5
- 229930182555 Penicillin Natural products 0.000 description 5
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 5
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 230000007910 cell fusion Effects 0.000 description 5
- 239000006285 cell suspension Substances 0.000 description 5
- 230000032459 dedifferentiation Effects 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- 230000001771 impaired effect Effects 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 238000012423 maintenance Methods 0.000 description 5
- 210000005087 mononuclear cell Anatomy 0.000 description 5
- 210000002894 multi-fate stem cell Anatomy 0.000 description 5
- 230000001272 neurogenic effect Effects 0.000 description 5
- 210000001672 ovary Anatomy 0.000 description 5
- 229940049954 penicillin Drugs 0.000 description 5
- 230000035935 pregnancy Effects 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- OZFAFGSSMRRTDW-UHFFFAOYSA-N (2,4-dichlorophenyl) benzenesulfonate Chemical compound ClC1=CC(Cl)=CC=C1OS(=O)(=O)C1=CC=CC=C1 OZFAFGSSMRRTDW-UHFFFAOYSA-N 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 4
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 4
- 239000012981 Hank's balanced salt solution Substances 0.000 description 4
- 108090000581 Leukemia inhibitory factor Proteins 0.000 description 4
- 108010004469 allophycocyanin Proteins 0.000 description 4
- 210000002459 blastocyst Anatomy 0.000 description 4
- 210000001172 blastoderm Anatomy 0.000 description 4
- 238000010322 bone marrow transplantation Methods 0.000 description 4
- 230000001086 cytosolic effect Effects 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 230000029087 digestion Effects 0.000 description 4
- 210000001900 endoderm Anatomy 0.000 description 4
- 230000007045 gastrulation Effects 0.000 description 4
- 210000002149 gonad Anatomy 0.000 description 4
- 239000001963 growth medium Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- 230000011987 methylation Effects 0.000 description 4
- 238000007069 methylation reaction Methods 0.000 description 4
- 210000004165 myocardium Anatomy 0.000 description 4
- 230000002611 ovarian Effects 0.000 description 4
- 210000004508 polar body Anatomy 0.000 description 4
- 238000003752 polymerase chain reaction Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 229960005322 streptomycin Drugs 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 230000008093 supporting effect Effects 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 230000035899 viability Effects 0.000 description 4
- 210000001325 yolk sac Anatomy 0.000 description 4
- 210000004340 zona pellucida Anatomy 0.000 description 4
- 102000029816 Collagenase Human genes 0.000 description 3
- 108060005980 Collagenase Proteins 0.000 description 3
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 3
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 3
- 102100032352 Leukemia inhibitory factor Human genes 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 239000004677 Nylon Substances 0.000 description 3
- 230000002293 adipogenic effect Effects 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 210000000709 aorta Anatomy 0.000 description 3
- 230000006907 apoptotic process Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004958 brain cell Anatomy 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 230000000747 cardiac effect Effects 0.000 description 3
- 230000001269 cardiogenic effect Effects 0.000 description 3
- 230000011712 cell development Effects 0.000 description 3
- 210000003855 cell nucleus Anatomy 0.000 description 3
- 238000002659 cell therapy Methods 0.000 description 3
- 230000002648 chondrogenic effect Effects 0.000 description 3
- 210000000349 chromosome Anatomy 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 229960002424 collagenase Drugs 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 3
- 210000003981 ectoderm Anatomy 0.000 description 3
- 239000003797 essential amino acid Substances 0.000 description 3
- 235000020776 essential amino acid Nutrition 0.000 description 3
- 230000004720 fertilization Effects 0.000 description 3
- 210000005260 human cell Anatomy 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 3
- 208000010125 myocardial infarction Diseases 0.000 description 3
- 229920001778 nylon Polymers 0.000 description 3
- 230000002188 osteogenic effect Effects 0.000 description 3
- 230000004792 oxidative damage Effects 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 230000000392 somatic effect Effects 0.000 description 3
- 238000000527 sonication Methods 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 230000002381 testicular Effects 0.000 description 3
- 210000001550 testis Anatomy 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 3
- PRDFBSVERLRRMY-UHFFFAOYSA-N 2'-(4-ethoxyphenyl)-5-(4-methylpiperazin-1-yl)-2,5'-bibenzimidazole Chemical compound C1=CC(OCC)=CC=C1C1=NC2=CC=C(C=3NC4=CC(=CC=C4N=3)N3CCN(C)CC3)C=C2N1 PRDFBSVERLRRMY-UHFFFAOYSA-N 0.000 description 2
- HIYAVKIYRIFSCZ-CYEMHPAKSA-N 5-(methylamino)-2-[[(2S,3R,5R,6S,8R,9R)-3,5,9-trimethyl-2-[(2S)-1-oxo-1-(1H-pyrrol-2-yl)propan-2-yl]-1,7-dioxaspiro[5.5]undecan-8-yl]methyl]-1,3-benzoxazole-4-carboxylic acid Chemical compound O=C([C@@H](C)[C@H]1O[C@@]2([C@@H](C[C@H]1C)C)O[C@@H]([C@@H](CC2)C)CC=1OC2=CC=C(C(=C2N=1)C(O)=O)NC)C1=CC=CN1 HIYAVKIYRIFSCZ-CYEMHPAKSA-N 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 241000700199 Cavia porcellus Species 0.000 description 2
- 206010053567 Coagulopathies Diseases 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 102000016911 Deoxyribonucleases Human genes 0.000 description 2
- 108010053770 Deoxyribonucleases Proteins 0.000 description 2
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 description 2
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 2
- 229920001917 Ficoll Polymers 0.000 description 2
- 229930091371 Fructose Natural products 0.000 description 2
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 2
- 239000005715 Fructose Substances 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000942967 Homo sapiens Leukemia inhibitory factor Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 102000011782 Keratins Human genes 0.000 description 2
- 108010076876 Keratins Proteins 0.000 description 2
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 2
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 108010047620 Phytohemagglutinins Proteins 0.000 description 2
- 108010059712 Pronase Proteins 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 206010067171 Regurgitation Diseases 0.000 description 2
- 239000004809 Teflon Substances 0.000 description 2
- 229920006362 Teflon® Polymers 0.000 description 2
- 206010053648 Vascular occlusion Diseases 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 2
- 210000001789 adipocyte Anatomy 0.000 description 2
- 230000009815 adipogenic differentiation Effects 0.000 description 2
- 210000000577 adipose tissue Anatomy 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 239000007640 basal medium Substances 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- HIYAVKIYRIFSCZ-UHFFFAOYSA-N calcium ionophore A23187 Natural products N=1C2=C(C(O)=O)C(NC)=CC=C2OC=1CC(C(CC1)C)OC1(C(CC1C)C)OC1C(C)C(=O)C1=CC=CN1 HIYAVKIYRIFSCZ-UHFFFAOYSA-N 0.000 description 2
- 230000012292 cell migration Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000007385 chemical modification Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000009816 chondrogenic differentiation Effects 0.000 description 2
- 230000035602 clotting Effects 0.000 description 2
- 238000003501 co-culture Methods 0.000 description 2
- 238000005056 compaction Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 238000007887 coronary angioplasty Methods 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 210000001840 diploid cell Anatomy 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 230000008143 early embryonic development Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000011067 equilibration Methods 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 239000012894 fetal calf serum Substances 0.000 description 2
- 230000004578 fetal growth Effects 0.000 description 2
- 210000002950 fibroblast Anatomy 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 210000004392 genitalia Anatomy 0.000 description 2
- 230000037442 genomic alteration Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000002710 gonadal effect Effects 0.000 description 2
- CPBQJMYROZQQJC-UHFFFAOYSA-N helium neon Chemical compound [He].[Ne] CPBQJMYROZQQJC-UHFFFAOYSA-N 0.000 description 2
- 239000000185 hemagglutinin Substances 0.000 description 2
- 230000011132 hemopoiesis Effects 0.000 description 2
- 102000046645 human LIF Human genes 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 229960003299 ketamine Drugs 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 230000008774 maternal effect Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 210000003716 mesoderm Anatomy 0.000 description 2
- 238000012009 microbiological test Methods 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 239000011824 nuclear material Substances 0.000 description 2
- 230000009818 osteogenic differentiation Effects 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 210000001322 periplasm Anatomy 0.000 description 2
- 230000035790 physiological processes and functions Effects 0.000 description 2
- 230000001885 phytohemagglutinin Effects 0.000 description 2
- -1 polyethylene Polymers 0.000 description 2
- 238000004904 shortening Methods 0.000 description 2
- 210000004267 spermatic cord Anatomy 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 210000000603 stem cell niche Anatomy 0.000 description 2
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 2
- 210000002303 tibia Anatomy 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 210000000689 upper leg Anatomy 0.000 description 2
- 208000021331 vascular occlusion disease Diseases 0.000 description 2
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 108010081589 Becaplermin Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 101100289995 Caenorhabditis elegans mac-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 206010068051 Chimerism Diseases 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- 230000035131 DNA demethylation Effects 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000976622 Homo sapiens Zinc finger protein 42 homolog Proteins 0.000 description 1
- 108010003272 Hyaluronate lyase Proteins 0.000 description 1
- 102000001974 Hyaluronidases Human genes 0.000 description 1
- MIJPAVRNWPDMOR-ZAFYKAAXSA-N L-ascorbic acid 2-phosphate Chemical compound OC[C@H](O)[C@H]1OC(=O)C(OP(O)(O)=O)=C1O MIJPAVRNWPDMOR-ZAFYKAAXSA-N 0.000 description 1
- 102000004058 Leukemia inhibitory factor Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 101000942966 Mus musculus Leukemia inhibitory factor Proteins 0.000 description 1
- 102000008730 Nestin Human genes 0.000 description 1
- 108010088225 Nestin Proteins 0.000 description 1
- 102000002584 Octamer Transcription Factor-3 Human genes 0.000 description 1
- 108010068425 Octamer Transcription Factor-3 Proteins 0.000 description 1
- NPGIHFRTRXVWOY-UHFFFAOYSA-N Oil red O Chemical compound Cc1ccc(C)c(c1)N=Nc1cc(C)c(cc1C)N=Nc1c(O)ccc2ccccc12 NPGIHFRTRXVWOY-UHFFFAOYSA-N 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 108091081062 Repeated sequence (DNA) Proteins 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 108010017842 Telomerase Proteins 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 208000036029 Uterine contractions during pregnancy Diseases 0.000 description 1
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 210000002593 Y chromosome Anatomy 0.000 description 1
- 102100023550 Zinc finger protein 42 homolog Human genes 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 238000011298 ablation treatment Methods 0.000 description 1
- 210000002718 aborted fetus Anatomy 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 210000001691 amnion Anatomy 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 210000003663 amniotic stem cell Anatomy 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001857 anti-mycotic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000002543 antimycotic Substances 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000035584 blastogenesis Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 235000011148 calcium chloride Nutrition 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000022159 cartilage development Effects 0.000 description 1
- 230000032677 cell aging Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 208000037887 cell injury Diseases 0.000 description 1
- 239000002771 cell marker Substances 0.000 description 1
- 230000011748 cell maturation Effects 0.000 description 1
- 210000005169 cell of the uterus Anatomy 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 230000004637 cellular stress Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000003399 chemotactic effect Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 210000001612 chondrocyte Anatomy 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000005138 cryopreservation Methods 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000001335 demethylating effect Effects 0.000 description 1
- 230000017858 demethylation Effects 0.000 description 1
- 238000010520 demethylation reaction Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000000408 embryogenic effect Effects 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 230000001973 epigenetic effect Effects 0.000 description 1
- 230000008472 epithelial growth Effects 0.000 description 1
- 210000004420 female germ cell Anatomy 0.000 description 1
- 230000008175 fetal development Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 238000010362 genome editing Methods 0.000 description 1
- 230000008826 genomic mutation Effects 0.000 description 1
- 210000001368 germline stem cell Anatomy 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 210000001173 gonocyte Anatomy 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 230000009067 heart development Effects 0.000 description 1
- 208000018706 hematopoietic system disease Diseases 0.000 description 1
- 229960002773 hyaluronidase Drugs 0.000 description 1
- 210000004754 hybrid cell Anatomy 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000016507 interphase Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 230000000302 ischemic effect Effects 0.000 description 1
- 210000004731 jugular vein Anatomy 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 238000002350 laparotomy Methods 0.000 description 1
- 230000003520 lipogenic effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 210000003794 male germ cell Anatomy 0.000 description 1
- 210000000953 male primordial germ cell Anatomy 0.000 description 1
- 230000010311 mammalian development Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000010297 mechanical methods and process Methods 0.000 description 1
- 230000021121 meiosis Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 210000002198 morula cell Anatomy 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- 210000000754 myometrium Anatomy 0.000 description 1
- 229910052754 neon Inorganic materials 0.000 description 1
- GKAOGPIIYCISHV-UHFFFAOYSA-N neon atom Chemical compound [Ne] GKAOGPIIYCISHV-UHFFFAOYSA-N 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000005055 nestin Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000037311 normal skin Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 230000033667 organ regeneration Effects 0.000 description 1
- 230000000888 organogenic effect Effects 0.000 description 1
- 210000005009 osteogenic cell Anatomy 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000007388 punch biopsy Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 239000003642 reactive oxygen metabolite Substances 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 210000005132 reproductive cell Anatomy 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 210000002863 seminiferous tubule Anatomy 0.000 description 1
- 210000000717 sertoli cell Anatomy 0.000 description 1
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 1
- 125000005629 sialic acid group Chemical group 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000021595 spermatogenesis Effects 0.000 description 1
- 238000009168 stem cell therapy Methods 0.000 description 1
- 238000009580 stem-cell therapy Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 210000002536 stromal cell Anatomy 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 210000003014 totipotent stem cell Anatomy 0.000 description 1
- UZKQTCBAMSWPJD-UQCOIBPSSA-N trans-Zeatin Natural products OCC(/C)=C\CNC1=NC=NC2=C1N=CN2 UZKQTCBAMSWPJD-UQCOIBPSSA-N 0.000 description 1
- UZKQTCBAMSWPJD-FARCUNLSSA-N trans-zeatin Chemical compound OCC(/C)=C/CNC1=NC=NC2=C1N=CN2 UZKQTCBAMSWPJD-FARCUNLSSA-N 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000008736 traumatic injury Effects 0.000 description 1
- 239000001226 triphosphate Substances 0.000 description 1
- 235000011178 triphosphate Nutrition 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 230000009278 visceral effect Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 239000012130 whole-cell lysate Substances 0.000 description 1
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 1
- 229960001600 xylazine Drugs 0.000 description 1
- 229940023877 zeatin Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0667—Adipose-derived stem cells [ADSC]; Adipose stromal stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0608—Germ cells
- C12N5/061—Sperm cells, spermatogonia
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
- C12N5/12—Fused cells, e.g. hybridomas
- C12N5/16—Animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/06—Anti-neoplasic drugs, anti-retroviral drugs, e.g. azacytidine, cyclophosphamide
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/70—Enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/02—Coculture with; Conditioned medium produced by embryonic cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2517/00—Cells related to new breeds of animals
- C12N2517/04—Cells produced using nuclear transfer
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2517/00—Cells related to new breeds of animals
- C12N2517/10—Conditioning of cells for in vitro fecondation or nuclear transfer
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- General Engineering & Computer Science (AREA)
- Developmental Biology & Embryology (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Heart & Thoracic Surgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Reproductive Health (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cardiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Rheumatology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/864,788 US20050090004A1 (en) | 2003-01-16 | 2004-06-08 | Stem cell maturation for all tissue lines |
| US58814604P | 2004-07-15 | 2004-07-15 | |
| PCT/US2005/005052 WO2005123123A2 (en) | 2004-06-08 | 2005-02-16 | Therapeutic reprogramming, hybrid stem cells and maturation |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008501795A true JP2008501795A (ja) | 2008-01-24 |
| JP2008501795A5 JP2008501795A5 (enExample) | 2008-04-03 |
Family
ID=35355246
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007527196A Withdrawn JP2008501795A (ja) | 2004-06-08 | 2005-02-16 | ハイブリッド幹細胞の治療的再プログラミングおよび成熟 |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP1758989A2 (enExample) |
| JP (1) | JP2008501795A (enExample) |
| AU (1) | AU2005253923A1 (enExample) |
| BR (1) | BRPI0511889A (enExample) |
| CA (1) | CA2567692A1 (enExample) |
| RU (1) | RU2006147263A (enExample) |
| WO (1) | WO2005123123A2 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008038148A2 (en) * | 2006-05-11 | 2008-04-03 | Andrew Craig Boquest | Stem cells and methods of making and using stem cells |
| US8940900B2 (en) | 2007-02-28 | 2015-01-27 | Advinus Therapeutics Private Limited | 2,2,2-tri-substituted acetamide derivatives as glucokinase activators, their process and pharmaceutical application |
| US8299115B2 (en) | 2007-06-08 | 2012-10-30 | Debnath Bhuniya | Pyrrole-2-carboxamide derivatives as glucokinase activators, their process and pharmaceutical application |
| EP2090649A1 (en) | 2008-02-13 | 2009-08-19 | Fondazione Telethon | Method for reprogramming differentiated cells |
| EP2402327B1 (en) | 2010-06-29 | 2018-03-07 | Impetis Biosciences Ltd. | Acetamide compounds as glucokinase activators, their process and medicinal applications |
| EP2796873A1 (en) * | 2013-04-25 | 2014-10-29 | QGel SA | Method for a cell-based drug screening assay and the use thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002521493A (ja) * | 1998-07-31 | 2002-07-16 | ジェンザイム コーポレーション | 間葉幹細胞移植による心機能の改善 |
| US20020136709A1 (en) * | 2000-12-12 | 2002-09-26 | Nucleus Remodeling, Inc. | In vitro-derived adult pluripotent stem cells and uses therefor |
| JP2004248505A (ja) * | 2001-09-21 | 2004-09-09 | Norio Nakatsuji | 移植抗原の一部または全てを欠除したes細胞由来の未分化な体細胞融合細胞およびその製造 |
| CA2474766A1 (en) * | 2002-01-16 | 2003-07-31 | Chauncey Sayre | Stem cell maturation for all tissue types |
| EP1513921A1 (en) * | 2002-05-31 | 2005-03-16 | Apollo Life Sciences Pty Ltd. | A method of cell therapy using fused cell hybrids |
| AU2003295444A1 (en) * | 2002-11-15 | 2004-06-15 | The Board Of Trustees Of The University Of Illinois | Methods for in vitro expansion of hematopoietic stem cells |
-
2005
- 2005-02-16 CA CA002567692A patent/CA2567692A1/en not_active Abandoned
- 2005-02-16 RU RU2006147263/14A patent/RU2006147263A/ru unknown
- 2005-02-16 AU AU2005253923A patent/AU2005253923A1/en not_active Abandoned
- 2005-02-16 WO PCT/US2005/005052 patent/WO2005123123A2/en not_active Ceased
- 2005-02-16 JP JP2007527196A patent/JP2008501795A/ja not_active Withdrawn
- 2005-02-16 BR BRPI0511889-1A patent/BRPI0511889A/pt not_active IP Right Cessation
- 2005-02-16 EP EP05723204A patent/EP1758989A2/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| WO2005123123A3 (en) | 2006-03-02 |
| RU2006147263A (ru) | 2008-07-20 |
| EP1758989A2 (en) | 2007-03-07 |
| BRPI0511889A (pt) | 2008-01-15 |
| WO2005123123A2 (en) | 2005-12-29 |
| CA2567692A1 (en) | 2005-12-29 |
| AU2005253923A1 (en) | 2005-12-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20090263357A1 (en) | Therapeutic Reprogramming, Hybrid Stem Cells and Maturation | |
| Ratajczak et al. | Pluripotent and multipotent stem cells in adult tissues | |
| Du et al. | Reviews: stem cells and female reproduction | |
| Bishop et al. | Embryonic stem cells | |
| Vaags et al. | Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential | |
| Jin et al. | Enhanced development of porcine embryos cloned from bone marrow mesenchymal stem cells | |
| JP4314372B2 (ja) | 精巣細胞由来多能性幹細胞の製造方法 | |
| US20080044392A1 (en) | Isolation of Stem Cell-Like Cells and Use Thereof | |
| Young et al. | Pluripotent stem cells, endogenous versus reprogrammed, a review | |
| WO1995010599A1 (en) | Embryonic stem cell-like cells | |
| WO2002034890A2 (en) | Pluripotential stem cells | |
| AU2006243810B2 (en) | A method for producing stem cells or stem cell-like cells from mammalian embryos | |
| JP2008501795A (ja) | ハイブリッド幹細胞の治療的再プログラミングおよび成熟 | |
| JP2003509031A (ja) | ドナー細胞又は分化細胞からの核を使用する豚のクローニング並びに多能性豚の産生 | |
| WO2006084229A2 (en) | Use of nuclear material to therapeutically reprogram differentiated cells | |
| CN1973033A (zh) | 治疗用再程序化、杂交干细胞和成熟 | |
| JP2005523685A (ja) | 体細胞由来胚幹細胞及びそれらの分化子孫 | |
| WO2006040615A2 (en) | Differenciation of stem cells for therapeutic use | |
| WO2003083088A2 (en) | Stem cell therapy | |
| US20060188491A1 (en) | Use of nuclear material to therapeutically reprogram differentiated cells | |
| JP2008528059A (ja) | 分化細胞を治療用に再プログラム化するための核の材料の使用 | |
| Kaufman et al. | Embryonic Stem Cells: Unique Potential to Treat Autoimmune Diseases | |
| WO2005040361A1 (ja) | 幹細胞の簡易調製法およびそれに使用するフィーダー細胞 | |
| Brevini et al. | Pluripotency in domestic animal cells | |
| Stewart | Stem Cells and Stem Cell therapies: the Future for Regenerative Medicine? |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20080116 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20080118 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20080116 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080215 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080215 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20080724 |