JP2005515191A - 創傷の治療及び使用する組成物 - Google Patents
創傷の治療及び使用する組成物 Download PDFInfo
- Publication number
- JP2005515191A JP2005515191A JP2003546869A JP2003546869A JP2005515191A JP 2005515191 A JP2005515191 A JP 2005515191A JP 2003546869 A JP2003546869 A JP 2003546869A JP 2003546869 A JP2003546869 A JP 2003546869A JP 2005515191 A JP2005515191 A JP 2005515191A
- Authority
- JP
- Japan
- Prior art keywords
- composition
- wound
- effective amount
- pharmaceutically effective
- ions
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 206010052428 Wound Diseases 0.000 title claims abstract description 155
- 208000027418 Wounds and injury Diseases 0.000 title claims abstract description 144
- 239000000203 mixture Substances 0.000 title claims abstract description 84
- 238000000034 method Methods 0.000 claims abstract description 13
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 claims abstract description 10
- 229910001424 calcium ion Inorganic materials 0.000 claims abstract description 10
- 229910001414 potassium ion Inorganic materials 0.000 claims abstract description 9
- 229910001419 rubidium ion Inorganic materials 0.000 claims abstract description 9
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 claims abstract description 7
- 239000003937 drug carrier Substances 0.000 claims abstract description 4
- 108010000684 Matrix Metalloproteinases Proteins 0.000 claims description 27
- 102000002274 Matrix Metalloproteinases Human genes 0.000 claims description 27
- 230000035876 healing Effects 0.000 claims description 20
- 230000001684 chronic effect Effects 0.000 claims description 14
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 12
- 239000000463 material Substances 0.000 claims description 11
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 6
- 229910052725 zinc Inorganic materials 0.000 claims description 6
- 239000011701 zinc Substances 0.000 claims description 6
- 102000004310 Ion Channels Human genes 0.000 claims description 5
- 239000002202 Polyethylene glycol Substances 0.000 claims description 4
- 239000002253 acid Substances 0.000 claims description 4
- 229920001223 polyethylene glycol Polymers 0.000 claims description 4
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 3
- 239000011591 potassium Substances 0.000 claims description 3
- 229940123973 Oxygen scavenger Drugs 0.000 claims description 2
- 230000007935 neutral effect Effects 0.000 claims 2
- 230000001737 promoting effect Effects 0.000 claims 2
- 102100026802 72 kDa type IV collagenase Human genes 0.000 abstract description 24
- 101710151806 72 kDa type IV collagenase Proteins 0.000 abstract description 23
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 abstract description 7
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 abstract description 7
- 230000000694 effects Effects 0.000 abstract description 6
- 241000894006 Bacteria Species 0.000 abstract 1
- 238000001574 biopsy Methods 0.000 description 16
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 12
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 12
- 210000002744 extracellular matrix Anatomy 0.000 description 12
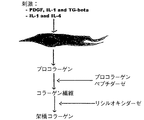
- 108010035532 Collagen Proteins 0.000 description 11
- 102000008186 Collagen Human genes 0.000 description 11
- 229920001436 collagen Polymers 0.000 description 11
- 230000029663 wound healing Effects 0.000 description 10
- 210000002950 fibroblast Anatomy 0.000 description 9
- 230000008569 process Effects 0.000 description 8
- 102000009123 Fibrin Human genes 0.000 description 7
- 108010073385 Fibrin Proteins 0.000 description 7
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 7
- 229950003499 fibrin Drugs 0.000 description 7
- UEJSSZHHYBHCEL-UHFFFAOYSA-N silver(1+) sulfadiazinate Chemical compound [Ag+].C1=CC(N)=CC=C1S(=O)(=O)[N-]C1=NC=CC=N1 UEJSSZHHYBHCEL-UHFFFAOYSA-N 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 102000005741 Metalloproteases Human genes 0.000 description 6
- 108010006035 Metalloproteases Proteins 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 206010061218 Inflammation Diseases 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 150000003431 steroids Chemical class 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000003556 assay Methods 0.000 description 4
- 210000004207 dermis Anatomy 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- ZKRFOXLVOKTUTA-KQYNXXCUSA-N 9-(5-phosphoribofuranosyl)-6-mercaptopurine Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(NC=NC2=S)=C2N=C1 ZKRFOXLVOKTUTA-KQYNXXCUSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- 101000669513 Homo sapiens Metalloproteinase inhibitor 1 Proteins 0.000 description 3
- 102100039364 Metalloproteinase inhibitor 1 Human genes 0.000 description 3
- 208000004210 Pressure Ulcer Diseases 0.000 description 3
- 241000282898 Sus scrofa Species 0.000 description 3
- 208000025865 Ulcer Diseases 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
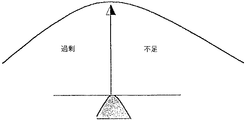
- 230000015556 catabolic process Effects 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 230000002500 effect on skin Effects 0.000 description 3
- 229940048368 flamazine Drugs 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000001508 potassium citrate Substances 0.000 description 3
- 229960002635 potassium citrate Drugs 0.000 description 3
- QEEAPRPFLLJWCF-UHFFFAOYSA-K potassium citrate (anhydrous) Chemical compound [K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O QEEAPRPFLLJWCF-UHFFFAOYSA-K 0.000 description 3
- 235000011082 potassium citrates Nutrition 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- IGLNJRXAVVLDKE-UHFFFAOYSA-N rubidium atom Chemical compound [Rb] IGLNJRXAVVLDKE-UHFFFAOYSA-N 0.000 description 3
- 210000003491 skin Anatomy 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 231100000397 ulcer Toxicity 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 206010056340 Diabetic ulcer Diseases 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 208000005230 Leg Ulcer Diseases 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 102000001938 Plasminogen Activators Human genes 0.000 description 2
- 108010001014 Plasminogen Activators Proteins 0.000 description 2
- 102000010752 Plasminogen Inactivators Human genes 0.000 description 2
- 108010077971 Plasminogen Inactivators Proteins 0.000 description 2
- 206010040943 Skin Ulcer Diseases 0.000 description 2
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 2
- 206010044546 Traumatic ulcer Diseases 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 210000004969 inflammatory cell Anatomy 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229910001410 inorganic ion Inorganic materials 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000001338 necrotic effect Effects 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 238000013310 pig model Methods 0.000 description 2
- 229940012957 plasmin Drugs 0.000 description 2
- 229940127126 plasminogen activator Drugs 0.000 description 2
- 239000002797 plasminogen activator inhibitor Substances 0.000 description 2
- 229960003975 potassium Drugs 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000007634 remodeling Methods 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- 229910052701 rubidium Inorganic materials 0.000 description 2
- FGDZQCVHDSGLHJ-UHFFFAOYSA-M rubidium chloride Chemical compound [Cl-].[Rb+] FGDZQCVHDSGLHJ-UHFFFAOYSA-M 0.000 description 2
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- GKFPPCXIBHQRQT-UHFFFAOYSA-N 6-(2-carboxy-4,5-dihydroxy-6-methoxyoxan-3-yl)oxy-4,5-dihydroxy-3-methoxyoxane-2-carboxylic acid Chemical compound OC1C(O)C(OC)OC(C(O)=O)C1OC1C(O)C(O)C(OC)C(C(O)=O)O1 GKFPPCXIBHQRQT-UHFFFAOYSA-N 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 102100033312 Alpha-2-macroglobulin Human genes 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 208000003656 Electric Burns Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 229920000219 Ethylene vinyl alcohol Polymers 0.000 description 1
- 206010063560 Excessive granulation tissue Diseases 0.000 description 1
- 206010017076 Fracture Diseases 0.000 description 1
- 206010018852 Haematoma Diseases 0.000 description 1
- 101000627872 Homo sapiens 72 kDa type IV collagenase Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 208000034693 Laceration Diseases 0.000 description 1
- AMFGWXWBFGVCKG-UHFFFAOYSA-N Panavia opaque Chemical compound C1=CC(OCC(O)COC(=O)C(=C)C)=CC=C1C(C)(C)C1=CC=C(OCC(O)COC(=O)C(C)=C)C=C1 AMFGWXWBFGVCKG-UHFFFAOYSA-N 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 108010015078 Pregnancy-Associated alpha 2-Macroglobulins Proteins 0.000 description 1
- 108010050808 Procollagen Proteins 0.000 description 1
- 108010064622 Procollagen N-Endopeptidase Proteins 0.000 description 1
- 102000015339 Procollagen N-endopeptidase Human genes 0.000 description 1
- 108010003894 Protein-Lysine 6-Oxidase Proteins 0.000 description 1
- 102100026858 Protein-lysine 6-oxidase Human genes 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108010005246 Tissue Inhibitor of Metalloproteinases Proteins 0.000 description 1
- 102000005876 Tissue Inhibitor of Metalloproteinases Human genes 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 235000004883 caffeic acid Nutrition 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- HHEAADYXPMHMCT-UHFFFAOYSA-N dpph Chemical compound [O-][N+](=O)C1=CC([N+](=O)[O-])=CC([N+]([O-])=O)=C1[N]N(C=1C=CC=CC=1)C1=CC=CC=C1 HHEAADYXPMHMCT-UHFFFAOYSA-N 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 210000001339 epidermal cell Anatomy 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- UFRKOOWSQGXVKV-UHFFFAOYSA-N ethene;ethenol Chemical compound C=C.OC=C UFRKOOWSQGXVKV-UHFFFAOYSA-N 0.000 description 1
- HDERJYVLTPVNRI-UHFFFAOYSA-N ethene;ethenyl acetate Chemical group C=C.CC(=O)OC=C HDERJYVLTPVNRI-UHFFFAOYSA-N 0.000 description 1
- 239000004715 ethylene vinyl alcohol Substances 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 210000001126 granulation tissue Anatomy 0.000 description 1
- 230000037313 granulation tissue formation Effects 0.000 description 1
- 210000004565 granule cell Anatomy 0.000 description 1
- 239000013003 healing agent Substances 0.000 description 1
- 235000012907 honey Nutrition 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 229940060367 inert ingredients Drugs 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000013383 initial experiment Methods 0.000 description 1
- 238000013101 initial test Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 230000001617 migratory effect Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 230000037311 normal skin Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 1
- 239000003642 reactive oxygen metabolite Substances 0.000 description 1
- 229940102127 rubidium chloride Drugs 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000002000 scavenging effect Effects 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 206010040872 skin infection Diseases 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 230000006492 vascular dysfunction Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 239000003878 whitfield ointment Substances 0.000 description 1
- 230000037314 wound repair Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/075—Ethers or acetals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/06—Aluminium, calcium or magnesium; Compounds thereof, e.g. clay
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/30—Zinc; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/18—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/10—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing inorganic materials
- A61L2300/102—Metals or metal compounds, e.g. salts such as bicarbonates, carbonates, oxides, zeolites, silicates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US33433701P | 2001-11-29 | 2001-11-29 | |
| PCT/US2002/038175 WO2003045366A1 (en) | 2001-11-29 | 2002-11-27 | Treatment of wounds and compositions employed |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005515191A true JP2005515191A (ja) | 2005-05-26 |
| JP2005515191A5 JP2005515191A5 (enExample) | 2006-01-26 |
Family
ID=23306754
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003546869A Pending JP2005515191A (ja) | 2001-11-29 | 2002-11-27 | 創傷の治療及び使用する組成物 |
Country Status (8)
| Country | Link |
|---|---|
| US (5) | US20030133991A1 (enExample) |
| EP (1) | EP1461024A4 (enExample) |
| JP (1) | JP2005515191A (enExample) |
| AU (1) | AU2002359529B2 (enExample) |
| CA (1) | CA2468390A1 (enExample) |
| MX (1) | MXPA04005219A (enExample) |
| NZ (1) | NZ533252A (enExample) |
| WO (1) | WO2003045366A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014104064A1 (ja) * | 2012-12-26 | 2014-07-03 | 株式会社エーゼット | 創傷治癒促進剤 |
| JP2014520657A (ja) * | 2011-07-20 | 2014-08-25 | スリーエム イノベイティブ プロパティズ カンパニー | イオン担持組成物を含んだ包帯 |
| JP2014521649A (ja) * | 2011-07-28 | 2014-08-28 | スリーエム イノベイティブ プロパティズ カンパニー | 創傷治癒組成物及び使用方法 |
| WO2016199907A1 (ja) * | 2015-06-12 | 2016-12-15 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| WO2016199905A1 (ja) * | 2015-06-12 | 2016-12-15 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| WO2018105739A1 (ja) * | 2016-12-09 | 2018-06-14 | Jfeミネラル株式会社 | 亜鉛イオン徐放性に優れる無機組成物およびその製造方法 |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040023937A1 (en) * | 2002-05-24 | 2004-02-05 | Greystone Medical Group, Inc. | Anti-cancer formulation |
| JP2006511585A (ja) * | 2002-12-23 | 2006-04-06 | グレイストーン メディカル グループ、インコーポレイテッド | 慢性創傷管理における活性酸素種の減少 |
| US20050175715A1 (en) * | 2003-08-25 | 2005-08-11 | Monroe Stephen H. | Cellular depolarization and regulation of matrix metalloproteinases |
| WO2006002326A2 (en) * | 2004-06-22 | 2006-01-05 | Greystone Medical Group, Inc. | Methods for treatment of wounds using time release compositions |
| US20070212342A1 (en) * | 2005-12-20 | 2007-09-13 | Swiss-American Products, Inc. | Protease compositions for the treatment of damaged tissue |
| US8795730B2 (en) * | 2006-01-31 | 2014-08-05 | David John Vachon | Compositions and methods for promoting the healing of tissue of multicellular organisms |
| US7687650B2 (en) | 2006-02-03 | 2010-03-30 | Jr Chem, Llc | Chemical compositions and methods of making them |
| PL1993569T3 (pl) | 2006-02-03 | 2014-11-28 | Obagi Cosmeceuticals Llc | Terapia przeciw starzeniu z użyciem kompozycji miedzi i cynku |
| US7897800B2 (en) | 2006-02-03 | 2011-03-01 | Jr Chem, Llc | Chemical compositions and methods of making them |
| WO2007143586A2 (en) * | 2006-06-01 | 2007-12-13 | Pharmaionx, Inc. | Composition for wound care and method of using same |
| US7867522B2 (en) | 2006-09-28 | 2011-01-11 | Jr Chem, Llc | Method of wound/burn healing using copper-zinc compositions |
| EP2152359A2 (en) | 2007-05-25 | 2010-02-17 | Compex Medical S.A. | Wound healing electrode set |
| ITBS20070178A1 (it) * | 2007-11-15 | 2009-05-16 | Paoli Ambrosi Gianfranco De | Composizione per uso farmaceutico e/o cosmetico e/o in forma di dispositivo medico per favorire processi di cicatrizzazione, per il trattamento di cicatrici ipertrofiche e per migliorare le proprieta' biomeccaniche della cute |
| US20100260863A1 (en) * | 2007-12-11 | 2010-10-14 | Monroe Stephen H | Composition of aqueous buffer solution for the treatment of cellular environment and ion channels and methods for using same |
| US8273791B2 (en) | 2008-01-04 | 2012-09-25 | Jr Chem, Llc | Compositions, kits and regimens for the treatment of skin, especially décolletage |
| CA2750636C (en) | 2009-01-23 | 2017-07-25 | Jr Chem, Llc | Rosacea treatments and kits for performing them |
| US20110008271A1 (en) * | 2009-07-13 | 2011-01-13 | Jr Chem, Llc | Rosacea treatments using polymetal complexes |
| US8952057B2 (en) | 2011-01-11 | 2015-02-10 | Jr Chem, Llc | Compositions for anorectal use and methods for treating anorectal disorders |
| GB201116523D0 (en) * | 2011-09-23 | 2011-11-09 | Systagenix Wound Man Ip Co Bv | Wound prognosis |
| EP2896963B1 (en) | 2011-01-31 | 2019-01-30 | Woundchek Laboratories B.V. | Wound prognosis |
| US9351995B1 (en) | 2011-04-29 | 2016-05-31 | Red Oax Holdings, LLC | Compositions and methods of enhancing wound healing |
| RU2636523C1 (ru) * | 2017-02-09 | 2017-11-23 | федеральное государственное бюджетное образовательное учреждение высшего образования "Воронежский государственный медицинский университет имени Н.Н. Бурденко" Министерства здравоохранения Российской Федерации (ФГБОУ ВО ВГМУ им. Н.Н. Бурденко Минздрава России) | Лекарственное средство для ускорения заживления ран, содержащее хлорид рубидия в виде порошка |
| CN116631510B (zh) * | 2022-10-28 | 2024-01-12 | 中国人民解放军军事科学院军事医学研究院 | 一种用于鉴别诊断克罗恩病和溃疡性结肠炎的装置 |
| WO2024196897A1 (en) * | 2023-03-22 | 2024-09-26 | Courtland Imel | Novel methods for increasing healing speed of human and animal wounds |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08503462A (ja) * | 1992-11-06 | 1996-04-16 | エイチ・イー・スタンリー・ファーマシューティカルズ・インコーポレイテッド | オーク樹皮抽出物の組成物、関連する合成組成物およびこれを用いる方法 |
| WO2000054593A1 (en) * | 1999-03-12 | 2000-09-21 | Bristol-Myers Squibb Company | Iodine preparation compositon |
| WO2001082896A1 (en) * | 2000-04-28 | 2001-11-08 | Biolife, L.L.C | Hemostatic agent, method and carrier for applying a blood clotting agent |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4600711A (en) * | 1982-08-18 | 1986-07-15 | The University Of Kentucky Research Foundation | Composition for topical and infusion treatment of wounds and burns |
| DE3416777C2 (de) * | 1984-05-07 | 1986-11-20 | Gödecke AG, 1000 Berlin | Pharmazeutische topische Zubereitungen |
| AUPN104895A0 (en) * | 1995-02-10 | 1995-03-09 | Lai, John | Methods of and preparations for the treatment of ailments and disorders |
| US20020141964A1 (en) * | 2001-01-19 | 2002-10-03 | Patterson James A. | Composition for arresting the flow of blood and method |
| US6600057B2 (en) * | 2000-12-29 | 2003-07-29 | Kimberly-Clark Worldwide, Inc. | Matrix metalloproteinase inhibitors |
-
2002
- 2002-11-27 WO PCT/US2002/038175 patent/WO2003045366A1/en not_active Ceased
- 2002-11-27 JP JP2003546869A patent/JP2005515191A/ja active Pending
- 2002-11-27 US US10/305,713 patent/US20030133991A1/en not_active Abandoned
- 2002-11-27 AU AU2002359529A patent/AU2002359529B2/en not_active Ceased
- 2002-11-27 MX MXPA04005219A patent/MXPA04005219A/es unknown
- 2002-11-27 EP EP02794072A patent/EP1461024A4/en not_active Withdrawn
- 2002-11-27 NZ NZ533252A patent/NZ533252A/xx not_active IP Right Cessation
- 2002-11-27 CA CA002468390A patent/CA2468390A1/en not_active Abandoned
-
2005
- 2005-03-15 US US11/081,119 patent/US20060029682A1/en not_active Abandoned
-
2006
- 2006-09-20 US US11/523,869 patent/US20070009611A1/en not_active Abandoned
-
2007
- 2007-07-17 US US11/779,026 patent/US20070298121A1/en not_active Abandoned
-
2009
- 2009-09-23 US US12/565,244 patent/US20100196507A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08503462A (ja) * | 1992-11-06 | 1996-04-16 | エイチ・イー・スタンリー・ファーマシューティカルズ・インコーポレイテッド | オーク樹皮抽出物の組成物、関連する合成組成物およびこれを用いる方法 |
| WO2000054593A1 (en) * | 1999-03-12 | 2000-09-21 | Bristol-Myers Squibb Company | Iodine preparation compositon |
| WO2001082896A1 (en) * | 2000-04-28 | 2001-11-08 | Biolife, L.L.C | Hemostatic agent, method and carrier for applying a blood clotting agent |
Non-Patent Citations (1)
| Title |
|---|
| JPN6009029844, 医薬品添加物事典, 1994, 第1版, 第38−39頁 * |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014520657A (ja) * | 2011-07-20 | 2014-08-25 | スリーエム イノベイティブ プロパティズ カンパニー | イオン担持組成物を含んだ包帯 |
| JP2014521649A (ja) * | 2011-07-28 | 2014-08-28 | スリーエム イノベイティブ プロパティズ カンパニー | 創傷治癒組成物及び使用方法 |
| JPWO2014104064A1 (ja) * | 2012-12-26 | 2017-01-12 | 株式会社エーゼット | 創傷治癒促進剤 |
| US9962364B2 (en) | 2012-12-26 | 2018-05-08 | A-Z Ltd. | Wound healing accelerator |
| WO2014104064A1 (ja) * | 2012-12-26 | 2014-07-03 | 株式会社エーゼット | 創傷治癒促進剤 |
| JPWO2016199907A1 (ja) * | 2015-06-12 | 2018-03-29 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| JPWO2016199905A1 (ja) * | 2015-06-12 | 2017-06-22 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| KR20170142205A (ko) * | 2015-06-12 | 2017-12-27 | 제이에프이미네라르 가부시키가이샤 | 피부 창상 또는 피부 거침 치료제 |
| WO2016199905A1 (ja) * | 2015-06-12 | 2016-12-15 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| WO2016199907A1 (ja) * | 2015-06-12 | 2016-12-15 | Jfeミネラル株式会社 | 皮膚創傷または皮膚荒れ治療剤 |
| KR101889677B1 (ko) * | 2015-06-12 | 2018-08-17 | 제이에프이미네라르 가부시키가이샤 | 피부 창상 또는 피부 거침 치료제 |
| US10780114B2 (en) | 2015-06-12 | 2020-09-22 | Jfe Mineral Company, Ltd. | Therapeutic agent for skin wound or rough skin |
| US11020427B2 (en) | 2015-06-12 | 2021-06-01 | Jfe Mineral Company, Ltd. | Therapeutic agent for skin wound or rough skin |
| WO2018105739A1 (ja) * | 2016-12-09 | 2018-06-14 | Jfeミネラル株式会社 | 亜鉛イオン徐放性に優れる無機組成物およびその製造方法 |
| JPWO2018105739A1 (ja) * | 2016-12-09 | 2019-10-24 | Jfeミネラル株式会社 | 亜鉛イオン徐放性に優れる無機組成物およびその製造方法 |
| US10987380B2 (en) | 2016-12-09 | 2021-04-27 | Jfe Mineral Company, Ltd. | Hydrozincite containing zinc carbonate hydroxide hydrate and method of making |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2468390A1 (en) | 2003-06-05 |
| MXPA04005219A (es) | 2005-06-20 |
| EP1461024A4 (en) | 2009-03-25 |
| US20070298121A1 (en) | 2007-12-27 |
| US20100196507A1 (en) | 2010-08-05 |
| EP1461024A1 (en) | 2004-09-29 |
| AU2002359529A1 (en) | 2003-06-10 |
| US20070009611A1 (en) | 2007-01-11 |
| WO2003045366A1 (en) | 2003-06-05 |
| AU2002359529B2 (en) | 2008-02-21 |
| US20030133991A1 (en) | 2003-07-17 |
| US20060029682A1 (en) | 2006-02-09 |
| NZ533252A (en) | 2006-03-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2005515191A (ja) | 創傷の治療及び使用する組成物 | |
| EP3308793B1 (en) | Therapeutic agent for skin wound or rough skin | |
| US20070190178A1 (en) | Composition and method for the therapeutic modulation of matrix metalloproteinase | |
| JP6970980B2 (ja) | 慢性創傷の壊死組織除去法 | |
| CN102105177B (zh) | 治疗皮肤损伤的药物组合物、敷料和方法,用于制备所述敷料的中间组合物和方法,以及与胶原基质缔合的铈盐的用途 | |
| US20240285735A1 (en) | Methods of debridement of chronic wounds | |
| CA3012299C (en) | Debriding composition for treating wounds | |
| HK1150785B (en) | Pharmaceutical composition, dressing and method for treating skin lesion, intermediate composition and process for preparing said dressing, and use of cerium salt associated with a collagen matrix |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20051125 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20051125 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090619 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090918 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091005 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091016 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091027 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091118 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091126 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100413 |