EP3592304B1 - Combined stent reperfusion system - Google Patents
Combined stent reperfusion system Download PDFInfo
- Publication number
- EP3592304B1 EP3592304B1 EP18771178.3A EP18771178A EP3592304B1 EP 3592304 B1 EP3592304 B1 EP 3592304B1 EP 18771178 A EP18771178 A EP 18771178A EP 3592304 B1 EP3592304 B1 EP 3592304B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- balloon
- stent
- catheter
- lumen
- occlusion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/9517—Instruments specially adapted for placement or removal of stents or stent-grafts handle assemblies therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A61M25/0032—Multi-lumen catheters with stationary elements characterized by at least one unconventionally shaped lumen, e.g. polygons, ellipsoids, wedges or shapes comprising concave and convex parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1011—Multiple balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/104—Balloon catheters used for angioplasty
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12027—Type of occlusion
- A61B17/1204—Type of occlusion temporary occlusion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
- A61B17/12109—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12136—Balloons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00017—Electrical control of surgical instruments
- A61B2017/00022—Sensing or detecting at the treatment site
- A61B2017/00084—Temperature
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00234—Surgical instruments, devices or methods for minimally invasive surgery
- A61B2017/00238—Type of minimally invasive operation
- A61B2017/00243—Type of minimally invasive operation cardiac
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/06—Measuring instruments not otherwise provided for
- A61B2090/064—Measuring instruments not otherwise provided for for measuring force, pressure or mechanical tension
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0008—Rounded shapes, e.g. with rounded corners elliptical or oval
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0001—Means for transferring electromagnetic energy to implants
- A61F2250/0002—Means for transferring electromagnetic energy to implants for data transfer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0067—Means for introducing or releasing pharmaceutical products into the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A61M2025/0037—Multi-lumen catheters with stationary elements characterized by lumina being arranged side-by-side
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/105—Balloon catheters with special features or adapted for special applications having a balloon suitable for drug delivery, e.g. by using holes for delivery, drug coating or membranes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1061—Balloon catheters with special features or adapted for special applications having separate inflations tubes, e.g. coaxial tubes or tubes otherwise arranged apart from the catheter tube
Definitions
- MI myocardial infarction
- ischemic cardiomyopathy are the most common causes of cardiac morbidity and mortality.
- Microvascular obstruction and no reflow are the principal causes of post-MI heart failure, adverse LV remodelling, scar/aneurysm formation and arrhythmias.
- WO 01/70325 A2 discloses an emboli protection system providing one or more inflatable blocking ballons for isolation of a section of a blood vessel to prevent migration of emboli from the section during an interventional procedure, and fluid infusion and evacuation ports for flushing emboli from the isolated section.
- One embodiment provides for a distal blocking balloon catheter, over which an interventional device can be introduced, and a proximal blocking balloon catheter to be introduced over the interventional device for isolating a portion of a blood vessel to be treated.
- the blocking balloons can be perforated to provide the infusion ports, and thrombolytic inflation fluid may be used to break down and dissolve thrombus and plaque in the isolated portion of the blood vessel.
- the interventional therapeutic device such as an angioplasty balloon catheter may be incorporated into the distal blocking balloon catheter, or the distal and blocking proximal balloon catheters can be incorporated with the interventional therapeutic device together in one device.
- Infusion ports may also be provided in either or both of the distal and proximal blocking catheters.
- the present invention provides a technology that combines the delivery of a coronary stent with a device for treating microvascular obstructions while avoiding reperfusion injuries.
- the invention is defined by a combined stent delivery and occlusion device according to independent claim 1 and by a combined stent delivery and occlusion device according to independent claim 2.
- One aspect of the disclosure pertains to the placement of a stent using an occlusion and perfusion catheter to diagnose and treat microvascular obstruction/ no reflow, and to avoid reperfusion injury.
- a catheter is provided with a stent placed over a balloon delivery device and is used for revascularizing the heart and/or other organs including, but not limited to, the brain, lungs, kidneys, muscles, intestines etc.
- the catheter is configured to be placed over a pressure/temperature-sensing guidewire to allow for real-time measurement of distal vessel pressure and temperatures, i.e. distal to the balloon delivery device.
- the measurement technology may be mounted directly to the delivery catheter.
- the catheter has an infusion lumen, which can infuse cardioprotective or therapeutic agents into the coronary circulation.
- Another aspect of the disclosure is a device that can infuse cardio-protective and/or therapeutic agents into the microcirculation before a stent delivery balloon is collapsed.
- the stent balloon while inflated, acts as an occlusion balloon.
- the catheter lumen is available to deliver a cardio-protective agent to reduce the potential negative effect of the reintroduction of blood flow when the balloon is deflated. After deflation, the stent remains in place to promote continued epicardial perfusion of the coronary tree.
- the combined stent delivery and occlusion device comprises a stent delivery balloon with an occlusion balloon. These two balloons may have different properties.
- the stent delivery balloon and the occlusion balloon may be mounted on a catheter shaft. They may be fixed longitudinally to the shaft or may be mounted such that the longitudinal position is adjustable to offer more accurate placement.
- Another aspect of the disclosure which is not covered by the invention is a method of reperfusing using a catheter having a stent delivery balloon and an occlusion balloon.
- the method begins by placing a catheter into the artery, preferably over a rapid exchange wire with pressure and temperature-sensing capabilities at a distal end of the guide wire.
- the occlusion balloon is then inflated to avoid reperfusion.
- the stent is then delivered by inflating the stent delivery balloon. Once the stent is in place, the stent delivery balloon is deflated.
- the occlusion balloon remains inflated to prevent reperfusion from occurring.
- a cardio-protective agent is then infused through the infusion lumen of the catheter.
- the effect of the cardio-protective agent is measured with the pressure/temperature sensor. Once the cardio-protective effect is achieved, the occlusion balloon is deflated. After the blood reperfuses, the degree of microvascular damage can be measured and potentially treated.
- Figure 1 shows a single balloon embodiment 10 of a delivery device of the invention.
- the delivery device 10 includes a manifold 12 at a proximal end that includes an infusion port 14 and a stent balloon inflation port 16.
- the manifold tapers to a flexible catheter 20 that proximally contains two lumens - an infusion lumen 22 that is in fluid communication with the infusion port 14 and an inflation lumen 24 that is in fluid communication with the balloon inflation port 16.
- Figure 2 is a cross section of the catheter 20 taken along section lines A-A of Figure 1.
- Figure 2 shows the infusion lumen 22 and the inflation lumen 24.
- FIG. 1 there is shown a therapeutic agent port 30 that leads to a therapeutic agent lumen 32 in the catheter 20.
- Figure 3 shows a cross section of the catheter 20 taken along section lines B-B of Figure 1 . It can thus be seen that distal of the therapeutic agent port, the catheter has three lumens, an infusion lumen 22, an inflation lumen 24 and a therapeutic agent lumen 32.
- a balloon 40 with a stent 42 Distal of the therapeutic agent port 30 is a balloon 40 with a stent 42.
- the balloon 40 is in fluid communication with the inflation lumen 24 such that fluid passing distally through the inflation lumen 24 terminates in the balloon 40.
- a stent 42 surrounds the balloon 40 and is expanded thereby when the balloon 40 is inflated.
- the stent 42 due to its memory properties, remains expanded after the balloon 40 deflates. Thus, deflating balloon 40 results in separation of the stent 42.
- distal of the balloon 40 is the distal end 50 of the catheter 20.
- the distal end 50 includes an open end of the infusion lumen 22.
- the delivery device 10 involves routing the catheter 20 over a guide wire (not shown) to the target site.
- the infusion lumen 22 is used as a guidewire lumen while the device 10 is being advanced to the target site.
- the guidewire includes a pressure and temperature sensor to provide real-time measurement of distal vessel pressures and temperatures at a location distal of the balloon delivery device.
- the balloon 40 is inflated causing the stent 42 to expand against the native tissue.
- the inflation of the balloon 40 also results in an occlusion of the vessel.
- a cardio-protective agent is infused via the infusion port 14 and through the infusion lumen 22, exiting the lumen 22 at the distal end 50 of the catheter, downstream of the occlusion balloon 40.
- the cardio-protective agent reduces the potential negative effects of reintroducing blood flow when the balloon 40 is deflated.
- the balloon 40 is deflated, allowing normal blood reperfusion of the coronary circulation.
- the stent 42 remains in place and secures continued epicardial perfusion of the coronary tree. After blood reperfusion is complete, the degree of microvascular damage can be measured and potentially treated.
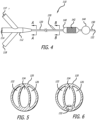
- FIG. 4 shows a dual balloon embodiment 110 of a delivery device of the invention.
- the delivery device 110 includes a manifold 112 at a proximal end that includes an infusion port 114, a stent balloon inflation port 116, and an occlusion balloon inflation port 118.
- the manifold tapers to a flexible catheter 120 that proximally contains three lumens - an infusion lumen 122 that is in fluid communication with the infusion port 114, a stent balloon inflation lumen 124 that is in fluid communication with the stent balloon inflation port 116, and an occlusion balloon inflation lumen 126 that is in fluid communication with the occlusion balloon inflation port 118.
- Figure 5 is a cross section of the catheter 120 taken along section lines A-A of Figure 4.
- Figure 5 shows the infusion lumen 122 and the inflation lumen 124.
- FIG. 6 shows a therapeutic agent port 130 that leads to a therapeutic agent lumen 132 in the catheter 20.
- Figure 6 shows a cross section of the catheter 20 taken along section lines B-B of Figure 4 . It can thus be seen that distal of the therapeutic agent port, the catheter has four lumens, the infusion lumen 122, the inflation lumen 124, the occlusion lumen 126, and an therapeutic agent lumen 132.
- a balloon 140 with a stent 142 Distal of the therapeutic agent port 130 is a balloon 140 with a stent 142.
- the balloon 140 is in fluid communication with the inflation lumen 124 such that fluid passing distally through the inflation lumen 124 terminates in the balloon 140.
- a stent 142 surrounds the balloon 140 and is expanded thereby when the balloon 140 in inflated.
- the stent 142 due to its memory properties, remains expanded after the balloon 140 deflates. Thus, deflating balloon 140 results in separation of the stent 142.
- the occlusion balloon 144 is in fluid communication with the occlusion lumen 126 such that fluid passing distally through the occlusion lumen 126 terminates in the balloon 144.
- the distal end 150 of the catheter 120 includes an open end of the infusion lumen 122.
- the delivery device 110 involves routing the catheter 120 over a guide wire (not shown) to the target site.
- the infusion lumen 122 is used as a guidewire lumen while the device 110 is being advanced to the target site.
- the guidewire includes a pressure and temperature sensor to provide real-time measurement of distal vessel pressures and temperatures at a location distal of the balloon delivery device.
- the occlusion balloon 144 is inflated to occlude the vessel and prevent reperfusion.
- the stent balloon 140 is inflated causing the stent 142 to expand against the native tissue.
- the stent balloon 140 is then deflated, separating the stent 142 from the device.
- a cardio-protective agent is infused via the infusion port 114 and through the infusion lumen 122, exiting the lumen 122 at the distal end 150 of the catheter, downstream of the occlusion balloon 144.
- the cardio-protective agent reduces the potential negative effects of reintroducing blood flow when the balloon 144 is deflated.
- the occlusion balloon 144 is deflated, allowing normal blood reperfusion of the coronary circulation.
- the stent 142 remains in place and secures continued epicardial perfusion of the coronary tree. After blood reperfusion is complete, the degree of microvascular damage can be measured and potentially treated.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Anesthesiology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Transplantation (AREA)
- Physics & Mathematics (AREA)
- Geometry (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Surgery (AREA)
- Reproductive Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762473740P | 2017-03-20 | 2017-03-20 | |
| PCT/US2018/023422 WO2018175485A1 (en) | 2017-03-20 | 2018-03-20 | Combined stent reperfusion system |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3592304A1 EP3592304A1 (en) | 2020-01-15 |
| EP3592304A4 EP3592304A4 (en) | 2020-05-27 |
| EP3592304B1 true EP3592304B1 (en) | 2023-09-13 |
Family
ID=63584822
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18771178.3A Active EP3592304B1 (en) | 2017-03-20 | 2018-03-20 | Combined stent reperfusion system |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US10952883B2 (enExample) |
| EP (1) | EP3592304B1 (enExample) |
| JP (1) | JP2020511290A (enExample) |
| CN (1) | CN111093568B (enExample) |
| AU (1) | AU2018237167A1 (enExample) |
| CA (1) | CA3057463A1 (enExample) |
| WO (1) | WO2018175485A1 (enExample) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11433183B2 (en) | 2018-03-09 | 2022-09-06 | Corflow Therapeutics Ag | System for diagnosing and treating microvascular obstructions |
| CA3010447A1 (en) | 2016-01-04 | 2017-07-13 | Corflow Therapeutics Ag | System and methods for treating mvo |
| WO2019060421A1 (en) | 2017-09-19 | 2019-03-28 | Jon Helge Hoem | INTRACORONARY MICROVASCULAR OBSTRUCTION CHARACTERIZATION (MVO) AND MYOCARDIAL INFARCTION |
| WO2019232452A1 (en) | 2018-05-31 | 2019-12-05 | Bernard Andre | Microfluidic coronary circulatory model |
| ES2964535T3 (es) | 2018-09-21 | 2024-04-08 | Corflow Therapeutics Ag | Aparato para la evaluación de la disfunción microvascular |
| US12115327B2 (en) * | 2019-03-05 | 2024-10-15 | Measurement Specialties, Inc. | Sensor device and methods of operation for a catheter based treatment of myocardial microvascular obstruction |
| WO2021035190A1 (en) | 2019-08-21 | 2021-02-25 | Corflow Therapeutics Ag | Controlled-flow infusion catheter and method |
| CN110681034B (zh) * | 2019-11-06 | 2025-03-25 | 上海慧达医疗器械有限公司 | 循环式药物灌注导管 |
| US20210260349A1 (en) * | 2020-02-24 | 2021-08-26 | Transit Scientific, LLC | Balloon-assisted infusion techniques |
| AU2021274173A1 (en) | 2020-05-22 | 2022-12-22 | Corflow Therapeutics Ag | Controlled flow infusion microvascular dysfunction diagnostic and therapy |
| AU2022206116A1 (en) | 2021-01-11 | 2023-07-20 | Corflow Therapeutics Ag | Apparatus and method for determining and/or treating microvascular obstruction |
| EP4218625B1 (en) | 2022-02-01 | 2025-04-02 | Albert-Ludwigs-Universität Freiburg | A dual catheter arrangement and system for reperfusion of an ischemic tissue region via a coronary vessel |
| EP4501387A1 (en) | 2023-07-31 | 2025-02-05 | Albert-Ludwigs-Universität Freiburg | A dual catheter arrangement and system for reperfusion of an ischemic tissue region via a coronary vessel |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015108928A1 (en) * | 2014-01-14 | 2015-07-23 | Volcano Corporation | Systems and methods for evaluating hemodialysis arteriovenous fistula maturation |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4961738A (en) * | 1987-01-28 | 1990-10-09 | Mackin Robert A | Angioplasty catheter with illumination and visualization within angioplasty balloon |
| JPH02297381A (ja) * | 1988-10-05 | 1990-12-07 | Abiomed Lp | 心機能助成気嚢及びその挿入方法 |
| AU5751290A (en) * | 1989-06-27 | 1991-01-03 | C.R. Bard Inc. | Coaxial ptca catheter with anchor joint |
| US5439446A (en) * | 1994-06-30 | 1995-08-08 | Boston Scientific Corporation | Stent and therapeutic delivery system |
| DE69534748T2 (de) * | 1994-09-02 | 2006-11-02 | Volcano Corp. (n.d, Ges.d.Staates Delaware), Rancho Cordova | Ultraminiatur-druckfühler und leitdraht hierfür |
| EP0917886B1 (en) * | 1997-10-23 | 2003-10-01 | Schneider (Europe) GmbH | Seal for catheter assembly with dilation and occlusion balloon |
| EP0920882A3 (en) * | 1997-12-04 | 2000-01-05 | Schneider Inc. | Balloon dilatation-drug delivery catheter and stent deployment-drug delivery catheter in rapid exchange configuration |
| US6295990B1 (en) * | 1998-02-03 | 2001-10-02 | Salient Interventional Systems, Inc. | Methods and systems for treating ischemia |
| US6517515B1 (en) * | 1998-03-04 | 2003-02-11 | Scimed Life Systems, Inc. | Catheter having variable size guide wire lumen |
| US6575966B2 (en) * | 1999-08-23 | 2003-06-10 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US6485500B1 (en) * | 2000-03-21 | 2002-11-26 | Advanced Cardiovascular Systems, Inc. | Emboli protection system |
| US20050015048A1 (en) * | 2003-03-12 | 2005-01-20 | Chiu Jessica G. | Infusion treatment agents, catheters, filter devices, and occlusion devices, and use thereof |
| US7250041B2 (en) * | 2003-03-12 | 2007-07-31 | Abbott Cardiovascular Systems Inc. | Retrograde pressure regulated infusion |
| US8403976B2 (en) * | 2004-04-08 | 2013-03-26 | Contego Medical Llc | Percutaneous transluminal angioplasty device with integral embolic filter |
| US7955371B2 (en) | 2004-05-12 | 2011-06-07 | Medtronic Vascular, Inc. | System and method for stent deployment and infusion of a therapeutic agent into tissue adjacent to the stent ends |
| IES20040803A2 (en) * | 2004-12-01 | 2006-06-14 | Medtronic Vascular Connaught | Drug delivery device |
| US20060142632A1 (en) * | 2004-12-29 | 2006-06-29 | Attila Meretei | Systems and methods for removing plaque from a blood vessel |
| US7837650B1 (en) | 2004-12-30 | 2010-11-23 | Advanced Cardiovascular Systems, Inc. | Method and apparatus to prevent reperfusion injury |
| JP2008173137A (ja) * | 2005-03-03 | 2008-07-31 | Goodman Co Ltd | ダブルバルーンカテーテル |
| US20070100279A1 (en) * | 2005-11-03 | 2007-05-03 | Paragon Intellectual Properties, Llc | Radiopaque-balloon microcatheter and methods of manufacture |
| JP5066992B2 (ja) * | 2007-04-18 | 2012-11-07 | 株式会社カネカ | バルーンカテーテル |
| CN201058169Y (zh) * | 2007-05-24 | 2008-05-14 | 席刚明 | 一种同轴球囊溶栓导管 |
| US8216209B2 (en) | 2007-05-31 | 2012-07-10 | Abbott Cardiovascular Systems Inc. | Method and apparatus for delivering an agent to a kidney |
| US20100042198A1 (en) * | 2008-08-18 | 2010-02-18 | Burton David G | Single piece double wall dilation balloon catheter |
| EP3093039A1 (en) * | 2008-11-03 | 2016-11-16 | Advanced Catheter Therapies, Inc. | Occlusion perfusion catheter |
| EP2413787B1 (en) * | 2009-03-31 | 2017-07-26 | St. Jude Medical Coordination Center BVBA | Sensor guide wire |
| US8740961B2 (en) * | 2009-08-13 | 2014-06-03 | Richard Eustis Fulton, III | Method for treating a target site in a vascular body channel |
| WO2011056588A1 (en) * | 2009-10-26 | 2011-05-12 | Poiesis Medical, Llc | A method for manufacturing a balloon encapsulated catheter tip |
| US20160082178A1 (en) * | 2009-12-02 | 2016-03-24 | Renovorx, Inc. | Angiographic methods for identification of feeder vessels |
| US8706209B2 (en) * | 2010-02-05 | 2014-04-22 | 3Dt Holdings, Llc | Devices, systems, and methods for measuring parallel tissue conductance, luminal cross-sectional areas, fluid velocity, and/or determining plaque vulnerability using temperature |
| EP2661304A1 (en) * | 2010-10-18 | 2013-11-13 | Cardiosonic Ltd. | Therapeutics reservoir |
| US9084877B2 (en) * | 2010-11-04 | 2015-07-21 | The Children's Hospital Of Philadelphia | Magnetic targeting device, system and method |
| JP2012200573A (ja) * | 2011-03-28 | 2012-10-22 | Kobe Univ | 2バルーンカテーテル |
| US9533124B2 (en) * | 2011-04-14 | 2017-01-03 | Abbott Cardiovascular Systems Inc. | Reperfusion injury devices |
| WO2013055896A1 (en) * | 2011-10-14 | 2013-04-18 | Acist Medical Systems, Inc. | Device and methods for measuring and treating an anatomical structure |
| JP5891046B2 (ja) * | 2012-01-23 | 2016-03-22 | テルモ株式会社 | バルーンおよびバルーンカテーテル |
| EP2938252B1 (en) * | 2012-12-31 | 2019-05-15 | Volcano Corporation | Intravascular device and production method thereof |
| US11330989B2 (en) * | 2014-06-16 | 2022-05-17 | Medtronic Vascular, Inc. | Microcatheter sensor design for mounting sensor to minimize induced strain |
| JP2016168151A (ja) * | 2015-03-12 | 2016-09-23 | 株式会社東海メディカルプロダクツ | バルーンカテーテル |
| CA3010447A1 (en) | 2016-01-04 | 2017-07-13 | Corflow Therapeutics Ag | System and methods for treating mvo |
| CN106310495A (zh) * | 2016-08-22 | 2017-01-11 | 苗立夫 | 一种灌注扩张球囊系统 |
-
2018
- 2018-03-20 EP EP18771178.3A patent/EP3592304B1/en active Active
- 2018-03-20 WO PCT/US2018/023422 patent/WO2018175485A1/en not_active Ceased
- 2018-03-20 US US15/926,911 patent/US10952883B2/en active Active
- 2018-03-20 CN CN201880026524.4A patent/CN111093568B/zh active Active
- 2018-03-20 AU AU2018237167A patent/AU2018237167A1/en not_active Abandoned
- 2018-03-20 JP JP2020501414A patent/JP2020511290A/ja active Pending
- 2018-03-20 CA CA3057463A patent/CA3057463A1/en active Pending
-
2021
- 2021-03-19 US US17/207,194 patent/US20210228387A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015108928A1 (en) * | 2014-01-14 | 2015-07-23 | Volcano Corporation | Systems and methods for evaluating hemodialysis arteriovenous fistula maturation |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2018237167A1 (en) | 2019-10-24 |
| JP2020511290A (ja) | 2020-04-16 |
| US20210228387A1 (en) | 2021-07-29 |
| EP3592304A4 (en) | 2020-05-27 |
| US20180280172A1 (en) | 2018-10-04 |
| US10952883B2 (en) | 2021-03-23 |
| EP3592304A1 (en) | 2020-01-15 |
| CN111093568B (zh) | 2022-03-15 |
| CA3057463A1 (en) | 2018-09-27 |
| CN111093568A (zh) | 2020-05-01 |
| WO2018175485A1 (en) | 2018-09-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3592304B1 (en) | Combined stent reperfusion system | |
| JP3683587B2 (ja) | 心臓手術中の人工心肺ポンプサポートを提供するためのカテーテル装置 | |
| US20200337564A1 (en) | Catheter System and Method For Occluding A Body Vessel | |
| JP3992734B2 (ja) | 心臓を停止させるための血管内システム | |
| US4456000A (en) | Expandable occlusion apparatus | |
| JP6099861B2 (ja) | 体内管、特に冠静脈洞内に導入するためのバルーン・カテーテル | |
| US4581017A (en) | Catheter systems | |
| US4413989A (en) | Expandable occlusion apparatus | |
| US20100305678A1 (en) | Thrombectomy and Balloon Angioplasty/Stenting Device | |
| WO1999029227A2 (en) | Endolumenal aortic isolation assembly and method | |
| JP3804351B2 (ja) | バルーンカテーテル | |
| US20110224606A1 (en) | Method and apparatus for remote ischemic conditioning during revascularization | |
| US20040162519A1 (en) | Aortic occlusion balloon cannula | |
| US11602618B2 (en) | Device forming an infusion catheter for treating at least one partial or total obstruction in a passage, such as a body passage | |
| WO2023238117A1 (en) | Device and method for perfusing a subject's heart via the coronary sinus | |
| CN215606012U (zh) | 一种可调节双球囊肺动脉止血器 | |
| EP4501387A1 (en) | A dual catheter arrangement and system for reperfusion of an ischemic tissue region via a coronary vessel | |
| JP2001104485A (ja) | 上行大動脈用オクリュージョンカテーテル | |
| CN112971964A (zh) | 一种用于心脏手术的球囊和消融装置 | |
| CA2225327A1 (en) | A perfusion balloon catheter | |
| JP2006175246A (ja) | バルーンカテーテル |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20191008 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20200424 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61F 2/958 20130101AFI20200420BHEP Ipc: A61B 17/12 20060101ALI20200420BHEP Ipc: A61M 25/00 20060101ALI20200420BHEP Ipc: A61B 90/00 20160101ALI20200420BHEP Ipc: A61M 25/10 20130101ALI20200420BHEP Ipc: A61F 2/95 20130101ALI20200420BHEP Ipc: A61B 17/00 20060101ALI20200420BHEP |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20220104 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20230406 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602018057531 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231214 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231213 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231214 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1610523 Country of ref document: AT Kind code of ref document: T Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240113 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240113 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240115 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602018057531 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| 26N | No opposition filed |
Effective date: 20240614 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240320 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20240331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240320 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250221 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250317 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250326 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20180320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20180320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230913 |