EP3006038B1 - Single doses of oritavancin for treating or preventing a bacterial infection - Google Patents
Single doses of oritavancin for treating or preventing a bacterial infection Download PDFInfo
- Publication number
- EP3006038B1 EP3006038B1 EP15195726.3A EP15195726A EP3006038B1 EP 3006038 B1 EP3006038 B1 EP 3006038B1 EP 15195726 A EP15195726 A EP 15195726A EP 3006038 B1 EP3006038 B1 EP 3006038B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- oritavancin
- bacterial infection
- prophylaxis
- subject
- staphylococcus aureus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/14—Peptides containing saccharide radicals; Derivatives thereof, e.g. bleomycin, phleomycin, muramylpeptides or vancomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
Definitions
- Oritavancin diphosphate is a semi-synthetic lipoglycopeptide derivative of a naturally occurring glycopeptide. Its structure confers potent antibacterial activity against gram-positive bacteria, including vancomycin-resistant enterococci (VRE), methicillin- and vancomycin-resistant staphylococci, and penicillin-resistant streptococci.
- VRE vancomycin-resistant enterococci
- the rapidity of its bactericidal activity against exponentially-growing S. aureus ( ⁇ 3-log reduction within 15 minutes to 2 hours against MSSA, MRSA, and VRSA) is one of the features that distinguishes it from the prototypic glycopeptide vancomycin ( McKay et al., J Antimicrob Chemother. 63(6): 1191-9 (2009), Epub 2009 Apr 15 ).
- Oritavancin like vancomycin, binds to the Acyl-D-Alanyl-D-Alanine terminus of the peptidoglycan precursor, lipid-bound N-acetyl-glucosamine-N-acetyl-muramic acid-pentapeptide ( Reynolds, Eur J Clin Microbiol Infect Dis 8(11):943-950 (1989 ); Nicas and Allen, Resistance and mechanism of action. In: Nagarajan R, editor. Glycopeptide antibiotics.
- oritavancin inhibits cell wall biosynthesis even when the substrate is the altered peptidoglycan precursor that is present in VRE and vancomycin-resistant S. aureus (VRSA).
- oritavancin antibacterial activity extends beyond that of vancomycin to include glycopeptide-resistant enterococci and staphylococci ( Ward et al., Expert Opin Investig Drugs 15:417-429 (2006 ); Scheinfeld, J Drugs Dermatol 6:97-103 (2007 )).
- Oritavancin also collapses transmembrane potential in gram positive bacteria, leading to rapid killing ( McKay et al., Poster C1-682: Oritavancin disrupts transmembrane potential and membrane integrity concomitantly with cell killing in Staphylococcus aureus and vancomycin-resistant Enterococci. 46th Intersci Conf Antimicro Agents Chemo, San Francisco, CA, Sept. 27-30, 2006 ). These multiple effects contribute to the rapid bactericidal activity of oritavancin.
- Oritavancin is in clinical development against serious gram-positive infections, where administration of the drug is via intravenous infusion using several dosages administered over a series of days.
- Mercier & Hrebickova (Expert Review of Anti-Infective Therapy 3(3): 325-332, 2005 ) reviews the efficacy of oritavancin as an antibacterial in various studies.
- Lee et al. (Surgical Infections 6(3): 283-295, 2005 ) also reviews treatments for cSSSIs, including phase III trials using oritavancin.
- the development of alternative dosing regimens for the drug could expand treatment options available to physicians.
- the present invention is directed to oritavancin or compositions containing oritavancin for medical uses in which novel dosing regimens are to be used.
- the invention provides a pharmaceutical composition comprising oritavancin, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier or diluent, for use in treating, preventing or providing prophylaxis for a bacterial infection in a subject, wherein said treating, preventing or prophylaxis is by administration of one dose of a therapeutically effective amount of said pharmaceutical composition over a course of therapy, prevention or prophylaxis to a subject having a bacterial infection, at risk of exposure to infectious bacteria, or at risk of developing a bacterial infection, which thereby treats, prevents or provides prophylaxis for said bacterial infection in said subject, wherein said one dose comprises 1200 mg oritavancin, or a pharmaceutically acceptable salt thereof, wherein the bacteria causing the bacterial infection is a bacteria selected from the group consisting of Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Staphylococcus aureus, vancomycin-inter
- the invention provides the use of oritavancin, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier or diluent in the preparation of a medicament for treating, preventing or providing prophylaxis for a bacterial infection in a subject, wherein said treating, preventing or prophylaxis is by administration of one dose of a therapeutically effective amount of said medicament over a course of therapy, prevention or prophylaxis to a subject having a bacterial infection, at risk of exposure to infectious bacteria, or at risk of developing a bacterial infection, which thereby treats, prevents or provides prophylaxis for said bacterial infection in said subject, wherein said one dose comprises at least 1200 mg oritavancin, or a pharmaceutically acceptable salt thereof wherein the bacteria causing the bacterial infection is a bacteria selected from the group consisting of Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Staphylococcus aureus, vancomycin-
- the pharmaceutical composition comprises 1200 mg oritavancin, or a pharmaceutically acceptable salt thereof.
- the pharmaceutical composition for use according to the invention is for prevention of a bacterial infection
- prevention is maintained for at least about 24, 72 or 114 hours.
- Oritavancin is to be administered in the form of a pharmaceutical composition or medicament comprising oritavancin.
- Oritavancin may be used per se in the present invention, or in the form of a pharmaceutically acceptable salt, hydrate, solvate, or mixtures thereof.
- pharmaceutically acceptable salt refers to non-toxic acid addition salts derived from inorganic and organic acids. While reference is made herein to both “oritavancin” and “a pharmaceutically acceptable salt thereof, the term “oritavancin” should be understood to include both the compound per se as well as a pharmaceutically acceptable salt, unless otherwise indicated by context, as the term “oritavancin” alone may be used for the sake of brevity.
- Acids commonly employed to form acid addition salts are inorganic acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, phosphoric acid, and the like, and organic acids such as p-toluenesulfonic acid, methanesulfonic acid, oxalic acid, p-bromophenylsulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid, acetic acid, and the like.
- Base addition salts include those derived from inorganic bases, such as ammonium or alkali or alkaline earth metal hydroxides, carbonates, bicarbonates, and the like.
- any salt for use according to this invention is not of a critical nature, so long as the salt as a whole is pharmacologically acceptable and as long as the counter-ion does not contribute undesired qualities to the salt as a whole.
- glycopeptide antibiotics including oritavancin and analogs thereof, may be found, for example, in U.S. Patent No. 5,840,684 .
- compositions for use according to the invention comprise oritavancin, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier or excipient.
- Pharmaceutically acceptable carriers and excipient are those compounds, solutions, substances or materials that can be used to produce formulations of oritavancin that are suitable for administration to a subject, such as a human.
- carriers and excipients for use in the present invention are those useful in preparing a pharmaceutical composition that is generally safe, non-toxic and neither biologically nor otherwise undesirable, and that may present pharmacologically favorable profiles, and include carriers and excipients that are acceptable for veterinary use as well as human pharmaceutical use.
- Suitable pharmaceutically acceptable carriers and excipients are well known in art and can be determined by those of skill in the art as the clinical situation warrants. The skilled artisan will understand that diluents are included within the scope of the terms carriers and excipients.
- suitable carriers and excipients include dextrose, water, glycerol, ethanol, propylene glycol, polysorbate 80 (Tween-80 TM ), poly(ethylene)glycol 300 and 400 (PEG 300 and 400), PEGylated castor oil (e.g. Cremophor EL), poloxamer 407 and 188, a cyclodextrin or a cyclodextrin derivative (including HPCD ((2-hydroxypropyl)-cyclodextrin) and (2-hydroxyethyl)-cyclodextrin; see, e.g., U.S. patent application publication 20060194717 ), hydrophilic and hydrophobic carriers, and combinations thereof.
- dextrose water, glycerol, ethanol, propylene glycol, polysorbate 80 (Tween-80 TM ), poly(ethylene)glycol 300 and 400 (PEG 300 and 400), PEGylated castor oil (e.g. Cremophor
- Hydrophobic carriers include, for example, fat emulsions, lipids, PEGylated phospholids, polymer matrices, biocompatible polymers, lipospheres, vesicles, particles, and liposomes.
- the terms specifically exclude cell culture medium. More particularly: (1) 5% (w/v) dextrose, or (2) water, may be used as a pharmaceutically acceptable carrier.
- Excipients included in a formulation have different purposes depending, for example on the nature of the drug, and the mode of administration.
- examples of generally used excipients include, without limitation: stabilizing agents, solubilizing agents and surfactants, buffers, antioxidants and preservatives, tonicity agents, bulking agents, lubricating agents, emulsifiers, suspending or viscosity agents, inert diluents, fillers, disintegrating agents, binding agents, wetting agents, lubricating agents, antibacterials, chelating agents, sweeteners, perfuming agents, flavouring agents, coloring agents, administration aids, and combinations thereof.
- compositions may contain common carriers and excipients, such as cornstarch or gelatin, lactose, sucrose, microcrystalline cellulose, kaolin, mannitol, dicalcium phosphate, sodium chloride, alginic acid, croscarmellose sodium, and sodium starch glycolate.
- common carriers and excipients such as cornstarch or gelatin, lactose, sucrose, microcrystalline cellulose, kaolin, mannitol, dicalcium phosphate, sodium chloride, alginic acid, croscarmellose sodium, and sodium starch glycolate.
- the particular carrier, diluent or excipient used will depend upon the means and purpose for which the active ingredient is being applied.
- composition compatible excipients also include tonicity agents that make the composition compatible with blood. Tonicity agents are particularly desirable in injectable formulations.
- compositions for use according to the invention are known to those skilled in the art.
- pharmaceutical compositions may be prepared following conventional techniques of the pharmaceutical chemist involving steps such as mixing, granulating, and compressing when necessary for tablet forms, or mixing, filling, and dissolving the ingredients as appropriate, to give the desired products for various routes of administration.
- the present invention facilitates methods comprising contacting bacteria with an effective amount of oritavancin, or a pharmaceutically acceptable salt thereof.
- the contacting may be carried out in vitro (in biochemical and/or cellular assays), in vivo in a non-human animal, in vivo in mammals, including humans and/or ex vivo (e.g. for sterilization purposes).
- the term "contacting” is meant to broadly refer to bringing a bacterial cell and a molecule of oritavancin into sufficient proximity such that oritavancin can exert an effect on the bacterial cell.
- Oritavancin may be transported to the location of the bacterial cell, or oritavancin may be situated in a location to which the bacterial cell travels or is brought into contact.
- contacting includes physical interaction between oritavancin and a bacterial cell, as well as interactions that do not require physical interaction.
- a "subject” means an animal, such as a mammal, including humans, other higher primates, lower primates, and animals of veterinary importance, such as dogs, cats, horses, sheep, goats, and cattle and the like, preferably a human.
- the subject may have a bacterial infection, may be at risk for developing a bacterial infection, or may be at greater risk than the general population for exposure to infectious bacteria.
- bacterial infection refers to an infection caused by a species or strain of bacteria for which the single dosing methods disclosed herein are appropriate.
- the pharmaceutical composition (or medicament) for use according to the invention may be used in the treatment of subjects having bacterial skin infections, such as complicated skin and skin structure infections (cSSSI) and complicated and uncomplicated skin and soft tissue infections (SSTI), including abscesses, ulcers, burns and cellulitis.
- the pharmaceutical composition (or medicament) for use according to the invention may also be used for treatment of deep bacterial infections, such as major abscess, infected ulcer, major burn, or deep and extensive cellulitis.
- bacterial infections that may be treated according to the present invention include blood stream infections (BSI), catheter-related blood stream infections (CRBSI), osteomyelitis, prosthetic joint infections, pneumonia (community acquired and nosicomial), joint space infections and device infections (e.g., infections associated with pace makers and internal cardiac defibrillators).
- BSI blood stream infections
- CBSI catheter-related blood stream infections
- osteomyelitis osteomyelitis
- prosthetic joint infections e.g., pneumonia
- pneumonia community acquired and nosicomial
- joint space infections e.g., infections associated with pace makers and internal cardiac defibrillators.
- device infections e.g., infections associated with pace makers and internal cardiac defibrillators.
- the pharmaceutical composition (or medicament) for use according to the invention can also be used concomitantly with surgical intervention for the bacterial infection.
- compositions may be used according to the present invention to treat bacterial infections caused by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Staphylococcus aureus, vancomycin-intermediate Staphylococcus aureus, vancomycin hetero-intermediate Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, Enterococcus faecalis or vancomycin-resistant Enterococcus faecalis.
- the pharmaceutical composition (or medicament) for use according to the invention may be used to treat a bacterial infection in a subject.
- the treatment comprises administering one dose of oritavancin, or a pharmaceutically acceptable salt thereof, formulated as a pharmaceutical composition or medicament, over a course of therapy to a subject.
- the subject has a bacterial infection, which is treated by administration of oritavancin.
- the treatment according to the present invention may be based on achieving a particular pharmacokinetic profile for oritavancin in a subject ( Bhavnani et al., Antimicrobial Agents Chemother. 50(3):994-1000 (2006 )).
- the one dose of oritavancin, or a pharmaceutically acceptable salt thereof, formulated as a pharmaceutical composition may be administered in an amount sufficient to achieve one or more of: (1) a maximum plasma concentration (C max ) of oritavancin of not less than a selected level, or (2) a minimum area under the concentration curve (AUC 0-24hr) of at least a selected level.
- a therapeutically effective minimum C max of oritavancin will vary based on the concentration of oritavancin in the formulation being administered to a subject, the means of administration, the duration of administration, the type of bacterial infection being treated and the identity of the bacteria in the infection, among other factors such as the physical characteristics of the subject. However, under most circumstances a minimum C max of not less than about 1 ⁇ g/mL should be achieved in a subject. Thus, the treatment according to the present invention may achieve a minimum C max of not less than about 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 or 100 ⁇ g/mL in the subject.
- the C max of oritavancin is not less than about 20 ⁇ g/mL. In other preferred aspects, the C max of oritavancin is not less than about 40 ⁇ g/mL, 60 ⁇ g/mL, or 80 ⁇ g/mL.
- the present invention includes achieving a minimum AUC of not less than about 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100, 105, 110, 115, 120, 125, 130, 135, 140, 145 or 150 mg*h/L ⁇ g/mL in the subject.
- the AUC 0-24hr of oritavancin is at least about 20 mg*h/L ⁇ g/mL.
- the AUC 0-24hr of oritavancin is at least about 40 mg*h/L/ ⁇ g/mL, 80 mg*h/L ⁇ g/mL, or 120 mg*h/L ⁇ g/mL.
- the dose contains 1200 mg oritavancin.
- the dosage may be administered all at once, such as with an oral formulation in a capsule, or slowly over a period of time, such as with an intravenous administration.
- the administering period can be a matter of minutes, such as about 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100, 105, 110, 115, 120 or more minutes, or a period of hours, such as about 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5 or more hours.
- the administration of the dose may be interrupted, such as where the dose is administered via intravenous infusion and the dose is divided into two or more infusion bags. Under such circumstances, the administration of the dose may be interrupted while the infusion bags are changed.
- prophylaxis for bacterial infections may be provided in a subject by administering one dose of oritavancin, or a pharmaceutically acceptable salt thereof, formulated as a pharmaceutical composition (or medicament) over a course of therapy to a subject.
- the subject is at risk of developing a bacterial infection, and administration of oritavancin provides prophylaxis for a bacterial infection in the subject.
- the prophylaxis may be about a reduction of at least about 100%, 99%, 98%, 97%, 96%, 95%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5% or 1% in the subject, versus a subject to which oritavancin has not been administered.
- the prophylaxis may last in the subject for at least about 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 22, 23 or 24 hours, or 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30 or more days after administration of oritavancin.
- prophylaxis is maintained for the duration of a surgical procedure, a dental procedure or an invasive medical procedure.
- multiple myeloma, chronic lympocytic leukemia, lymphoma people at increased occupational risk (e.g., public services workers, such a fire, water, sanitary, police, medical, and laboratory workers, hospital workers), people in closed populations (e.g., prisons, military, nursing homes) and others that have immunological deficiencies that might enhance their susceptibility to bacterial infection.
- the prevention may last in the subject for at least about 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 35, 40, 45, 50 or more days after administration of oritavancin.
- Patients were excluded from the study if they received any systemic antimicrobial agent with gram-positive coverage for more than 24 hours within the 3 days prior to enrollment (unless the gram-positive pathogen was resistant in vitro to the antimicrobial agent or the patient was clinically failing on prior therapy), had a history of severe hypersensitivity reactions to glycopeptides and any of their excipients (patients who had histamine-like infusion reactions to the glycopeptide vancomycin were not excluded) or had an anticipated need for more than 10 days of conventional antibiotic therapy. Pregnant women or women who were nursing were also excluded from the study.
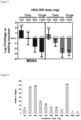
- Single intravenous doses of ORI 0.5 to 40 mg/kg were tested against one MSSA and one MRSA strain.
- Efficacy of ORI doses simulating human exposure HEQ doses, i.e. 24h AUC-matched
- HEQ doses i.e. 24h AUC-matched
- Both thighs were harvested and CFU counts were evaluated after 24h (single doses) or 72 h (HEQ doses) treatment.
- the range of doses allowed for a robust assessment of the dose-proportionality (also termed linearity) of the PK of oritavancin. Although a formal statistical assessment of dose-proportionality was not performed, the goodness-of-fit of the final population PK model was used to detect any apparent lack of dose-proportionality.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Dermatology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP24186111.1A EP4464375A3 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9331408P | 2008-08-30 | 2008-08-30 | |
| US9349708P | 2008-09-02 | 2008-09-02 | |
| EP09810708.9A EP2337575B1 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
| PCT/US2009/055466 WO2010025438A2 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09810708.9A Division-Into EP2337575B1 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
| EP09810708.9A Division EP2337575B1 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP24186111.1A Division EP4464375A3 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3006038A1 EP3006038A1 (en) | 2016-04-13 |
| EP3006038B1 true EP3006038B1 (en) | 2024-07-03 |
Family
ID=41722337
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15195726.3A Active EP3006038B1 (en) | 2008-08-30 | 2009-08-29 | Single doses of oritavancin for treating or preventing a bacterial infection |
| EP09810708.9A Revoked EP2337575B1 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
| EP24186111.1A Pending EP4464375A3 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09810708.9A Revoked EP2337575B1 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
| EP24186111.1A Pending EP4464375A3 (en) | 2008-08-30 | 2009-08-29 | Methods of treatment using single doses of oritavancin |
Country Status (18)
| Country | Link |
|---|---|
| US (2) | US8420592B2 (enExample) |
| EP (3) | EP3006038B1 (enExample) |
| JP (1) | JP5782615B2 (enExample) |
| CN (2) | CN102215858A (enExample) |
| AU (1) | AU2009285564B2 (enExample) |
| CA (1) | CA2736860C (enExample) |
| DK (2) | DK2337575T3 (enExample) |
| EA (1) | EA020490B1 (enExample) |
| ES (2) | ES2570401T3 (enExample) |
| FI (1) | FI3006038T3 (enExample) |
| HU (3) | HUE027373T2 (enExample) |
| MX (1) | MX2011002249A (enExample) |
| NL (1) | NL300834I2 (enExample) |
| NO (1) | NO2016019I2 (enExample) |
| NZ (1) | NZ591525A (enExample) |
| PL (2) | PL2337575T3 (enExample) |
| PT (1) | PT3006038T (enExample) |
| WO (1) | WO2010025438A2 (enExample) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HUE027373T2 (hu) * | 2008-08-30 | 2016-09-28 | The Medicines Co | Kezelési eljárások oritavancin egyszeri dózisainak alkalmazásával |
| PT2424559T (pt) | 2009-04-28 | 2024-04-30 | Melinta Therapeutics Llc | Métodos de tratamento de infeções bacterianas utilizando oritavancina |
| WO2014031681A1 (en) * | 2012-08-20 | 2014-02-27 | The University Of Chicago | Inhibiting gram positive bacteria |
| US20160101148A1 (en) * | 2013-04-22 | 2016-04-14 | The Medicines Company | Treatment and prevention of bacterial skin infections using oritavancin |
| US20160206688A1 (en) * | 2013-08-26 | 2016-07-21 | The Medicines Company | Methods for treating bacteremia and osteomyelitis using oritavancin |
| WO2015031579A2 (en) * | 2013-08-28 | 2015-03-05 | The Medicines Company | Methods for treating bacterial infections using oritavancin and polymyxins |
| WO2015192031A1 (en) * | 2014-06-12 | 2015-12-17 | Cem-102 Pharmaceuticals, Inc. | Compositions and methods for treating bone and joint infections |
| CN107206050A (zh) * | 2014-07-17 | 2017-09-26 | 医药公司 | 高纯度奥利万星及其生产方法 |
| KR20180111948A (ko) * | 2016-02-18 | 2018-10-11 | 멜린타 테라퓨틱스, 인크. | 오리타반신 제제 |
| WO2017179003A1 (en) | 2016-04-15 | 2017-10-19 | Lupin Limited | Topical compositions for ophthalmic and otic use |
| CN108409837B (zh) * | 2018-03-06 | 2021-09-24 | 上海来益生物药物研究开发中心有限责任公司 | 一组具有抗耐药性细菌活性的糖肽类化合物、其制备方法和应用 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5840684A (en) | 1994-01-28 | 1998-11-24 | Eli Lilly And Company | Glycopeptide antibiotic derivatives |
| US5994297A (en) * | 1997-08-22 | 1999-11-30 | Eli Lilly And Company | Therapy for Staphylococcus aureus |
| DE60016734T2 (de) * | 1999-05-03 | 2005-12-08 | Eli Lilly And Co., Indianapolis | Monatliche gaben von glykopeptid-antibiotika zur langzeitprävention von streptokokkus pneumoniae infektionen |
| ES2316445T3 (es) | 2000-05-02 | 2009-04-16 | Theravance, Inc. | Composicion que contiene una ciclodextrina y un antibiotico glicopeptidico. |
| US20030176327A1 (en) * | 2001-10-25 | 2003-09-18 | Cassell Gail Houston | Antibiotics for treating biohazardous bacterial agents |
| CN1662643A (zh) * | 2002-05-28 | 2005-08-31 | 贝克顿·迪金森公司 | 人腺泡细胞的扩增和转分化 |
| AU2003274927A1 (en) * | 2002-08-23 | 2004-03-11 | Genome Therapeutics Corporation | Methods and reagents for preventing bacteremias |
| US20040127403A1 (en) * | 2002-09-06 | 2004-07-01 | Francesco Parenti | Methods for treating and preventing Gram-positive bacteremias |
| US20070249577A1 (en) * | 2005-05-10 | 2007-10-25 | Hopkins Scott J | Method for reducing the risk of or preventing infection due to surgical or invasive medical procedures |
| US9089507B2 (en) * | 2006-09-25 | 2015-07-28 | The Medicines Company | Use of oritavancin for prevention and treatment of anthrax |
| HUE027373T2 (hu) * | 2008-08-30 | 2016-09-28 | The Medicines Co | Kezelési eljárások oritavancin egyszeri dózisainak alkalmazásával |
| PT2424559T (pt) * | 2009-04-28 | 2024-04-30 | Melinta Therapeutics Llc | Métodos de tratamento de infeções bacterianas utilizando oritavancina |
| WO2015031579A2 (en) * | 2013-08-28 | 2015-03-05 | The Medicines Company | Methods for treating bacterial infections using oritavancin and polymyxins |
-
2009
- 2009-08-29 HU HUE09810708A patent/HUE027373T2/hu unknown
- 2009-08-29 AU AU2009285564A patent/AU2009285564B2/en active Active
- 2009-08-29 CN CN2009801439475A patent/CN102215858A/zh active Pending
- 2009-08-29 ES ES09810708T patent/ES2570401T3/es active Active
- 2009-08-29 MX MX2011002249A patent/MX2011002249A/es active IP Right Grant
- 2009-08-29 JP JP2011525264A patent/JP5782615B2/ja active Active
- 2009-08-29 EP EP15195726.3A patent/EP3006038B1/en active Active
- 2009-08-29 DK DK09810708.9T patent/DK2337575T3/en active
- 2009-08-29 EP EP09810708.9A patent/EP2337575B1/en not_active Revoked
- 2009-08-29 PL PL09810708.9T patent/PL2337575T3/pl unknown
- 2009-08-29 ES ES15195726T patent/ES2994966T3/es active Active
- 2009-08-29 PT PT151957263T patent/PT3006038T/pt unknown
- 2009-08-29 WO PCT/US2009/055466 patent/WO2010025438A2/en not_active Ceased
- 2009-08-29 PL PL15195726.3T patent/PL3006038T3/pl unknown
- 2009-08-29 EP EP24186111.1A patent/EP4464375A3/en active Pending
- 2009-08-29 FI FIEP15195726.3T patent/FI3006038T3/fi active
- 2009-08-29 US US13/060,811 patent/US8420592B2/en active Active
- 2009-08-29 CA CA2736860A patent/CA2736860C/en active Active
- 2009-08-29 CN CN201610900690.5A patent/CN106620649A/zh active Pending
- 2009-08-29 NZ NZ591525A patent/NZ591525A/en unknown
- 2009-08-29 HU HUE15195726A patent/HUE068423T2/hu unknown
- 2009-08-29 EA EA201100413A patent/EA020490B1/ru unknown
- 2009-08-29 DK DK15195726.3T patent/DK3006038T3/da active
-
2013
- 2013-02-11 US US13/763,799 patent/US20130172237A1/en active Pending
-
2016
- 2016-10-06 HU HUS1600039C patent/HUS1600039I1/hu unknown
- 2016-10-11 NL NL300834C patent/NL300834I2/nl unknown
- 2016-10-11 NO NO2016019C patent/NO2016019I2/no unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3006038B1 (en) | Single doses of oritavancin for treating or preventing a bacterial infection | |
| CA2767614C (en) | Fusidic acid dosing regimens for treatment of bacterial infections | |
| US20250241897A1 (en) | Composition for eradicating helicobacter pylori | |
| EP2424559B1 (en) | Methods of treating bacterial infections using oritavancin | |
| US8450300B2 (en) | Fusidic acid dosing regimens for treatment of bacterial infections | |
| EP2068890B1 (en) | Use of oritavancin for prevention and treatment of anthrax | |
| US9775878B2 (en) | Methods for treating bacterial infections using oritavancin and polymyxins | |
| US20160101148A1 (en) | Treatment and prevention of bacterial skin infections using oritavancin | |
| AU2013202360B2 (en) | Methods of treatment using single doses of oritavancin | |
| EP3038616B1 (en) | Methods for treating bacteremia and osteomyelitis using oritavancin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2337575 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20161013 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20180727 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 17/00 20060101ALI20220328BHEP Ipc: A61P 31/04 20060101ALI20220328BHEP Ipc: A61K 38/14 20060101AFI20220328BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20220715 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| INTG | Intention to grant announced |
Effective date: 20230102 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230524 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 17/00 20060101ALI20230523BHEP Ipc: A61P 31/04 20060101ALI20230523BHEP Ipc: A61K 38/14 20060101AFI20230523BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20230607 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| INTG | Intention to grant announced |
Effective date: 20231103 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2337575 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602009065297 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: FI Ref legal event code: FGE |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 3006038 Country of ref document: PT Date of ref document: 20240823 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20240819 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20240821 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: MELINTA THERAPEUTICS, LLC |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E068423 Country of ref document: HU |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20240402162 Country of ref document: GR Effective date: 20241209 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241103 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2994966 Country of ref document: ES Kind code of ref document: T3 Effective date: 20250204 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602009065297 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240703 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20250404 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250818 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: HU Payment date: 20250822 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FI Payment date: 20250820 Year of fee payment: 17 Ref country code: ES Payment date: 20250910 Year of fee payment: 17 Ref country code: PT Payment date: 20250821 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20250826 Year of fee payment: 17 Ref country code: DE Payment date: 20250819 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 20250826 Year of fee payment: 17 Ref country code: NO Payment date: 20250821 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20250819 Year of fee payment: 17 Ref country code: IT Payment date: 20250821 Year of fee payment: 17 Ref country code: TR Payment date: 20250822 Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 1699115 Country of ref document: AT Kind code of ref document: T Effective date: 20240703 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250818 Year of fee payment: 17 Ref country code: GB Payment date: 20250818 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20250819 Year of fee payment: 17 Ref country code: FR Payment date: 20250818 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20250819 Year of fee payment: 17 Ref country code: CH Payment date: 20250901 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20250819 Year of fee payment: 17 Ref country code: CZ Payment date: 20250819 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: RO Payment date: 20250829 Year of fee payment: 17 |