EP2986693B1 - Schmiermittelzusammensetzung auf der basis von metallnanopartikeln - Google Patents
Schmiermittelzusammensetzung auf der basis von metallnanopartikeln Download PDFInfo
- Publication number
- EP2986693B1 EP2986693B1 EP14718967.4A EP14718967A EP2986693B1 EP 2986693 B1 EP2986693 B1 EP 2986693B1 EP 14718967 A EP14718967 A EP 14718967A EP 2986693 B1 EP2986693 B1 EP 2986693B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- dispersant
- lubricant composition
- daltons
- composition according
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 189
- 239000000314 lubricant Substances 0.000 title claims description 72
- 239000002082 metal nanoparticle Substances 0.000 title description 45
- 239000002270 dispersing agent Substances 0.000 claims description 99
- 239000002105 nanoparticle Substances 0.000 claims description 59
- 239000002199 base oil Substances 0.000 claims description 51
- 230000001050 lubricating effect Effects 0.000 claims description 51
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical group O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 claims description 48
- 239000000654 additive Substances 0.000 claims description 35
- 230000005540 biological transmission Effects 0.000 claims description 24
- 230000000996 additive effect Effects 0.000 claims description 22
- 229920000642 polymer Polymers 0.000 claims description 21
- 239000003921 oil Substances 0.000 claims description 19
- 150000001875 compounds Chemical class 0.000 claims description 18
- 229920013639 polyalphaolefin Polymers 0.000 claims description 17
- -1 MoSe2 Inorganic materials 0.000 claims description 15
- 239000012141 concentrate Substances 0.000 claims description 14
- YLZKDFKNMPCZCH-UHFFFAOYSA-J [W](S)(S)(S)S Chemical compound [W](S)(S)(S)S YLZKDFKNMPCZCH-UHFFFAOYSA-J 0.000 claims description 13
- 229910052751 metal Inorganic materials 0.000 claims description 12
- 230000001603 reducing effect Effects 0.000 claims description 12
- 239000003963 antioxidant agent Substances 0.000 claims description 11
- 239000002184 metal Substances 0.000 claims description 10
- 229910052723 transition metal Inorganic materials 0.000 claims description 10
- 150000003624 transition metals Chemical class 0.000 claims description 10
- 150000002148 esters Chemical class 0.000 claims description 9
- 239000007787 solid Substances 0.000 claims description 9
- 229920005862 polyol Polymers 0.000 claims description 8
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 7
- 229910052721 tungsten Inorganic materials 0.000 claims description 7
- 239000010937 tungsten Substances 0.000 claims description 7
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 6
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 6
- 229920001577 copolymer Polymers 0.000 claims description 6
- 239000002245 particle Substances 0.000 claims description 6
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 6
- 239000005977 Ethylene Substances 0.000 claims description 5
- 230000003078 antioxidant effect Effects 0.000 claims description 5
- 229910052750 molybdenum Inorganic materials 0.000 claims description 5
- 239000011733 molybdenum Substances 0.000 claims description 5
- 229910052757 nitrogen Inorganic materials 0.000 claims description 5
- 230000003647 oxidation Effects 0.000 claims description 5
- 238000007254 oxidation reaction Methods 0.000 claims description 5
- 239000002530 phenolic antioxidant Substances 0.000 claims description 5
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 4
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 claims description 4
- 229910052798 chalcogen Inorganic materials 0.000 claims description 4
- 150000001787 chalcogens Chemical group 0.000 claims description 4
- CWQXQMHSOZUFJS-UHFFFAOYSA-N molybdenum disulfide Chemical compound S=[Mo]=S CWQXQMHSOZUFJS-UHFFFAOYSA-N 0.000 claims description 4
- 229910052982 molybdenum disulfide Inorganic materials 0.000 claims description 4
- 229910052718 tin Inorganic materials 0.000 claims description 4
- 239000011135 tin Substances 0.000 claims description 4
- 229910052719 titanium Inorganic materials 0.000 claims description 4
- 239000010936 titanium Substances 0.000 claims description 4
- 238000004627 transmission electron microscopy Methods 0.000 claims description 4
- 229910052725 zinc Inorganic materials 0.000 claims description 4
- 239000011701 zinc Substances 0.000 claims description 4
- 229910052726 zirconium Inorganic materials 0.000 claims description 4
- 239000004711 α-olefin Substances 0.000 claims description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 3
- 229910052684 Cerium Inorganic materials 0.000 claims description 3
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 3
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 3
- 229910052782 aluminium Inorganic materials 0.000 claims description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 3
- ZMIGMASIKSOYAM-UHFFFAOYSA-N cerium Chemical compound [Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce] ZMIGMASIKSOYAM-UHFFFAOYSA-N 0.000 claims description 3
- 229910052735 hafnium Inorganic materials 0.000 claims description 3
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 claims description 3
- 229910052738 indium Inorganic materials 0.000 claims description 3
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 claims description 3
- 229910052758 niobium Inorganic materials 0.000 claims description 3
- 239000010955 niobium Substances 0.000 claims description 3
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 claims description 3
- 229910052697 platinum Inorganic materials 0.000 claims description 3
- 229920000768 polyamine Polymers 0.000 claims description 3
- 150000003077 polyols Chemical class 0.000 claims description 3
- 229910052702 rhenium Inorganic materials 0.000 claims description 3
- WUAPFZMCVAUBPE-UHFFFAOYSA-N rhenium atom Chemical compound [Re] WUAPFZMCVAUBPE-UHFFFAOYSA-N 0.000 claims description 3
- 238000004901 spalling Methods 0.000 claims description 3
- 229910052715 tantalum Inorganic materials 0.000 claims description 3
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 claims description 3
- 238000009826 distribution Methods 0.000 claims description 2
- 238000002173 high-resolution transmission electron microscopy Methods 0.000 claims description 2
- 229910003090 WSe2 Inorganic materials 0.000 claims 2
- 229910052961 molybdenite Inorganic materials 0.000 claims 2
- 229910016021 MoTe2 Inorganic materials 0.000 claims 1
- 229910020042 NbS2 Inorganic materials 0.000 claims 1
- 229910020039 NbSe2 Inorganic materials 0.000 claims 1
- 229910020046 NbTe2 Inorganic materials 0.000 claims 1
- 229910004211 TaS2 Inorganic materials 0.000 claims 1
- 229910004214 TaSe2 Inorganic materials 0.000 claims 1
- 229910003092 TiS2 Inorganic materials 0.000 claims 1
- 229910010322 TiS3 Inorganic materials 0.000 claims 1
- 229910006247 ZrS2 Inorganic materials 0.000 claims 1
- 239000004411 aluminium Substances 0.000 claims 1
- 125000004433 nitrogen atom Chemical group N* 0.000 claims 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 claims 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 claims 1
- 125000004432 carbon atom Chemical group C* 0.000 description 20
- 238000012360 testing method Methods 0.000 description 19
- ITRNXVSDJBHYNJ-UHFFFAOYSA-N tungsten disulfide Chemical compound S=[W]=S ITRNXVSDJBHYNJ-UHFFFAOYSA-N 0.000 description 19
- 229960002317 succinimide Drugs 0.000 description 18
- 125000004429 atom Chemical group 0.000 description 17
- 229920002367 Polyisobutene Polymers 0.000 description 14
- 229910003472 fullerene Inorganic materials 0.000 description 13
- 238000000034 method Methods 0.000 description 13
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical compound C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 12
- 239000012208 gear oil Substances 0.000 description 9
- 239000003607 modifier Substances 0.000 description 9
- 239000002086 nanomaterial Substances 0.000 description 9
- 229910052717 sulfur Inorganic materials 0.000 description 9
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 8
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 8
- 238000002835 absorbance Methods 0.000 description 8
- 238000005461 lubrication Methods 0.000 description 8
- 239000011593 sulfur Substances 0.000 description 8
- 239000008186 active pharmaceutical agent Substances 0.000 description 7
- 230000000052 comparative effect Effects 0.000 description 7
- 125000000217 alkyl group Chemical group 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000008021 deposition Effects 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 5
- 239000006228 supernatant Substances 0.000 description 5
- SXYOAESUCSYJNZ-UHFFFAOYSA-L zinc;bis(6-methylheptoxy)-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [Zn+2].CC(C)CCCCCOP([S-])(=S)OCCCCCC(C)C.CC(C)CCCCCOP([S-])(=S)OCCCCCC(C)C SXYOAESUCSYJNZ-UHFFFAOYSA-L 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 229920000089 Cyclic olefin copolymer Polymers 0.000 description 4
- 238000013019 agitation Methods 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 125000002947 alkylene group Chemical group 0.000 description 3
- 239000002518 antifoaming agent Substances 0.000 description 3
- 150000004770 chalcogenides Chemical class 0.000 description 3
- 238000002485 combustion reaction Methods 0.000 description 3
- 238000005260 corrosion Methods 0.000 description 3
- 239000013078 crystal Substances 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 229910001092 metal group alloy Inorganic materials 0.000 description 3
- 229910000000 metal hydroxide Inorganic materials 0.000 description 3
- 150000004692 metal hydroxides Chemical class 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 229910016001 MoSe Inorganic materials 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- 238000003917 TEM image Methods 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 230000007797 corrosion Effects 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000012990 dithiocarbamate Substances 0.000 description 2
- 150000002118 epoxides Chemical class 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- KHYKFSXXGRUKRE-UHFFFAOYSA-J molybdenum(4+) tetracarbamodithioate Chemical class C(N)([S-])=S.[Mo+4].C(N)([S-])=S.C(N)([S-])=S.C(N)([S-])=S KHYKFSXXGRUKRE-UHFFFAOYSA-J 0.000 description 2
- 239000010705 motor oil Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229910052714 tellurium Chemical group 0.000 description 2
- PORWMNRCUJJQNO-UHFFFAOYSA-N tellurium atom Chemical group [Te] PORWMNRCUJJQNO-UHFFFAOYSA-N 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- KWKAKUADMBZCLK-UHFFFAOYSA-N 1-octene Chemical compound CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 description 1
- SPSPIUSUWPLVKD-UHFFFAOYSA-N 2,3-dibutyl-6-methylphenol Chemical compound CCCCC1=CC=C(C)C(O)=C1CCCC SPSPIUSUWPLVKD-UHFFFAOYSA-N 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- RREANTFLPGEWEN-MBLPBCRHSA-N 7-[4-[[(3z)-3-[4-amino-5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidin-2-yl]imino-5-fluoro-2-oxoindol-1-yl]methyl]piperazin-1-yl]-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(\N=C/3C4=CC(F)=CC=C4N(CN4CCN(CC4)C=4C(=CC=5C(=O)C(C(O)=O)=CN(C=5C=4)C4CC4)F)C\3=O)=NC=2)N)=C1 RREANTFLPGEWEN-MBLPBCRHSA-N 0.000 description 1
- 241000234282 Allium Species 0.000 description 1
- 235000002732 Allium cepa var. cepa Nutrition 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 239000005069 Extreme pressure additive Substances 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- XQVWYOYUZDUNRW-UHFFFAOYSA-N N-Phenyl-1-naphthylamine Chemical class C=1C=CC2=CC=CC=C2C=1NC1=CC=CC=C1 XQVWYOYUZDUNRW-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- GLOYGJPNNKTDIG-UHFFFAOYSA-N SC=1N=NSC=1S Chemical class SC=1N=NSC=1S GLOYGJPNNKTDIG-UHFFFAOYSA-N 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical group [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- BTHAQRDGBHUQMR-UHFFFAOYSA-N [S]P(=O)=O Chemical compound [S]P(=O)=O BTHAQRDGBHUQMR-UHFFFAOYSA-N 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 239000007866 anti-wear additive Substances 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- DKVNPHBNOWQYFE-UHFFFAOYSA-N carbamodithioic acid Chemical compound NC(S)=S DKVNPHBNOWQYFE-UHFFFAOYSA-N 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000004517 catalytic hydrocracking Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical class C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 1
- 150000004659 dithiocarbamates Chemical class 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 229920001038 ethylene copolymer Polymers 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 125000004404 heteroalkyl group Chemical group 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000003760 magnetic stirring Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- AFFLGGQVNFXPEV-UHFFFAOYSA-N n-decene Natural products CCCCCCCCC=C AFFLGGQVNFXPEV-UHFFFAOYSA-N 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 150000002898 organic sulfur compounds Chemical class 0.000 description 1
- 150000002903 organophosphorus compounds Chemical class 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920005652 polyisobutylene succinic anhydride Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000003134 recirculating effect Effects 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Chemical group 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 239000004071 soot Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- WMYJOZQKDZZHAC-UHFFFAOYSA-H trizinc;dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S WMYJOZQKDZZHAC-UHFFFAOYSA-H 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M161/00—Lubricating compositions characterised by the additive being a mixture of a macromolecular compound and a non-macromolecular compound, each of these compounds being essential
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M141/00—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential
- C10M141/06—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential at least one of them being an organic nitrogen-containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M171/00—Lubricating compositions characterised by purely physical criteria, e.g. containing as base-material, thickener or additive, ingredients which are characterised exclusively by their numerically specified physical properties, i.e. containing ingredients which are physically well-defined but for which the chemical nature is either unspecified or only very vaguely indicated
- C10M171/06—Particles of special shape or size
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
- C10M2201/066—Molybdenum sulfide
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2203/00—Organic non-macromolecular hydrocarbon compounds and hydrocarbon fractions as ingredients in lubricant compositions
- C10M2203/10—Petroleum or coal fractions, e.g. tars, solvents, bitumen
- C10M2203/102—Aliphatic fractions
- C10M2203/1025—Aliphatic fractions used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
- C10M2205/028—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers containing aliphatic monomers having more than four carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
- C10M2205/028—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers containing aliphatic monomers having more than four carbon atoms
- C10M2205/0285—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers containing aliphatic monomers having more than four carbon atoms used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/04—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing aromatic monomers, e.g. styrene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/026—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings with tertiary alkyl groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/2805—Esters used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/283—Esters of polyhydroxy compounds
- C10M2207/2835—Esters of polyhydroxy compounds used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2209/00—Organic macromolecular compounds containing oxygen as ingredients in lubricant compositions
- C10M2209/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2209/08—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate type
- C10M2209/084—Acrylate; Methacrylate
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/064—Di- and triaryl amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/28—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/06—Groups 3 or 13
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/08—Groups 4 or 14
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/10—Groups 5 or 15
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/12—Groups 6 or 16
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/14—Group 7
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/16—Groups 8, 9, or 10
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/02—Viscosity; Viscosity index
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/055—Particles related characteristics
- C10N2020/06—Particles of special shape or size
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/06—Oiliness; Film-strength; Anti-wear; Resistance to extreme pressure
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
- C10N2040/044—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives for manual transmissions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2050/00—Form in which the lubricant is applied to the material being lubricated
- C10N2050/015—Dispersions of solid lubricants
Definitions
- the present invention is applicable to the field of lubricants, and more particularly to the field of lubricants for motor vehicles, particularly in the field of lubricants for transmission members of motor vehicles.
- the invention relates to a lubricating composition comprising metal nanoparticles. More particularly, the invention relates to a lubricant composition comprising a dispersant of high weight average molecular weight and metal nanoparticles.
- the lubricant composition according to the invention simultaneously has good stability and good anti-peeling properties.
- the present invention also relates to a method for reducing the peeling of a mechanical part employing this lubricating composition.
- the present invention also relates to a concentrate type composition of additives comprising a dispersant of high weight average molecular weight and metal nanoparticles.
- the transmission components of motor vehicles operate under heavy load and high speeds.
- the oils for these transmission members must therefore be particularly effective in protecting the parts against wear and fatigue, and in particular protect the gear teeth against the phenomenon of chipping.
- the phenomenon of wear corresponds to the abrasion and tearing of metal at the surface during friction between moving parts.
- the peeling phenomenon differs from the phenomenon of wear. It corresponds to a deterioration of the parts by fatigue and occurs after a long time of aging, preceding the visible deteriorations. It is known that this phenomenon starts with the initiation of cracks to a certain depth below the surface, these cracks propagating, and when normal cracks on the surface are created, scales break off abruptly.
- the prevention of this phenomenon involves a reduction of contact stresses thanks to an appropriate geometry of the parts, and by the reduction of friction, avoiding adhesion.
- Lubricant is involved in this prevention process, mainly by the physicochemical reactivity of its additives.
- Sulfur, phosphorus, phospho sulfur or borated antiwear and extreme pressure additives are known to give the transmission oils peel protection properties.
- the other additives present in the lubricant can also have an impact, positive or negative, on the propagation of cracks inside the parts and thus on the phenomenon of chipping.
- synchronizers In manual transmissions, the presence of synchronizers induces additional constraints. Indeed, these members comprise a cone and ring device between which the friction must be precisely controlled. Thus, the friction must be sufficient for the synchronization of speeds, but it is necessary that the cone and the ring can then disengage, at the risk of blocking the synchronizer.
- the level of friction can be adjusted by adding friction modifiers in these gearbox oils.
- WO 2007/035626 discloses a lubricating composition comprising metal nanoparticles, especially based on lithium, potassium, sodium, copper, magnesium, calcium, barium or mixtures thereof.
- compositions comprising at least one base oil, at least one dispersant and nanoparticles of metal hydroxides in the form of crystals. These compositions are used for lubricating combustion engines and for neutralizing the acids formed during combustion.
- the document US2006 / 0100292 A1 discloses a method of manufacturing a fat in which at least one base oil, at least one dispersant and nanoparticles of metal hydroxides in the form of crystals are mixed. This process has the advantage of reducing foam formation, deriving environmental risks and reducing reaction time.
- the document US2009 / 0203563 discloses a process for the manufacture of an overbased or neutral detergent. This method uses a surfactant and an organic medium with a composition comprising at least one base oil, at least one dispersant and nanoparticles of metal hydroxides in the form of crystals.
- WO2011 / 081538 A1 discloses a process for producing molybdenum disulfide and tungsten particles, the method of passing and pressing between glue-coated plates a mixture of molybdenum disulfide and tungsten. This document does not describe lubricating compositions.
- the document CN 101691517 describes a motor oil comprising a dispersant and tungsten disulfide nanoparticles, to improve the life of the engine and reduce fuel consumption.
- the nanoparticle content of tungsten disulfide ranges from 15 to 34%. Such a content may cause instability of the composition and is therefore not compatible with a lubricant composition, especially for transmissions.
- no indication is given in this document as to any anti-peeling properties of the oil, particularly vis-à-vis the transmission members of a motor vehicle.
- the document EP 1 953 196 discloses a dispersion of metal nanoparticles, in particular metal oxides based on zinc, zirconium, cerium, titanium, aluminum, indium or tin in an organic solvent and in the presence of a polymeric dispersant of PIBSA type (polyisobutenylsuccinic anhydride, or in terminology Anglo-Saxon polyisobutenyl succinic anhydride).
- PIBSA type polyisobutenylsuccinic anhydride, or in terminology Anglo-Saxon polyisobutenyl succinic anhydride.
- An object of the present invention is to provide a lubricant composition overcoming all or in part the aforementioned drawbacks.
- Another object of the invention is to provide a lubricating composition which is stable and easy to implement.
- Another object of the present invention is to provide a lubrication process including reducing the spalling phenomena of mechanical parts, and more particularly motor vehicle transmission members.
- the subject of the invention is thus a lubricating composition
- the weight average molecular weight of the dispersant is evaluated according to the ASTM D5296 standard.
- the Applicant has found that the presence of a dispersant having a weight average molecular weight greater than or equal to 2000 Daltons in a lubricating composition comprising at least one base oil and metal nanoparticles makes it possible at the same time to improve the stability of the lubricating composition, and to give said composition very good anti-peeling properties.
- the present invention makes it possible to formulate lubricating compositions comprising a reduced content of metal nanoparticles and which nevertheless have remarkable anti-peeling properties.
- lubricant compositions according to the invention by the use of lubricant compositions according to the invention, the risk of residual deposition of metal nanoparticles on mechanical parts, and more particularly on transmission members of motor vehicles, is significantly reduced or even eliminated.
- the lubricant compositions according to the invention have improved storage stability and a viscosity that does not vary or is very little.
- the lubricant compositions according to the invention retain satisfactory friction properties.
- the lubricating composition consists essentially of at least one base oil, at least one dispersant having a weight average molecular weight greater than or equal to 2000 Daltons and at least one metal nanoparticle in a weight content of from 0 , 01 to 2% relative to the total weight of the lubricating composition.
- the invention also relates to a transmission oil comprising a lubricating composition as defined above.
- the invention also relates to the use of a lubricant composition as defined above for the lubrication of gearboxes or bridges, preferably motor vehicle gearboxes, advantageously for the lubrication of manual gearboxes.
- the invention also relates to the use of a lubricant composition as defined above for reducing the peeling of a mechanical part, preferably of a transmission member, more preferably of a gearbox, further more preferably of a manual gearbox.
- the invention also relates to a method for reducing the peeling of a mechanical part, preferably of a transmission member, advantageously a gearbox or a bridge, comprising at least the contacting of the part. mechanical with a lubricating composition as defined above.
- the invention also relates to a composition of additive concentrate type comprising at least one dispersant having a weight average molecular weight greater than or equal to 2000 Daltons and nanoparticles of tungsten bisulfide.
- the lubricant composition according to the invention comprises metal nanoparticles in a content by weight ranging from 0.01 to 2% relative to the total weight of the lubricating composition.
- metal nanoparticles especially metal particles, generally solid, whose average size is less than or equal to 600 nm.
- the metal nanoparticles consist of at least 80% by weight of at least one metal, or at least 80% by weight of at least one metal alloy or at least 80% by weight of at least one metal chalcogenide, especially transition metal, with respect to the total mass of the nanoparticle.
- the metal nanoparticles consist of at least 90% by weight with at least one metal, or at least 90% by weight of at least one metal alloy or at least 90% by weight of at least one metal chalcogenide, especially transition metal, with respect to the total mass of the nanoparticle.
- the metal nanoparticles consist of at least 99% by weight with at least one metal, or at least 99% by weight of at least one metal alloy or at least 99% by weight of at least one metal chalcogenide, especially transition metal, relative to the total mass of the nanoparticle, the remaining 1% being impurities.
- the metal of which the metallic nanoparticle is constituted can be chosen from the group formed by tungsten, molybdenum, zirconium, hafnium, platinum, rhenium, titanium, tantalum, niobium, zinc, cerium, aluminum, indium and tin.
- the metal nanoparticles can have the shape of spheres, lamellae, fibers, tubes, fullerene type structures.
- the metal nanoparticles used in the compositions according to the invention are solid metal nanoparticles having a fullerene type structure (in English term fullerene-like).
- MX n metal nanoparticles
- M represents a transition metal
- X a chalcogen a transition metal
- M is selected from the group consisting of tungsten, molybdenum, zirconium, hafnium, platinum, rhenium, titanium, tantalum and niobium.
- M is selected from the group consisting of molybdenum and tungsten.
- M is tungsten
- X is selected from the group consisting of oxygen, sulfur, selenium and tellurium.
- X is selected from sulfur or tellurium.
- X is sulfur
- the metal nanoparticles according to the invention are chosen from the group formed by MoS 2 , MoSe 2 , MoTe 2 , WS 2 , WSe 2 , ZrS 2 , ZrSe 2 , HfS 2 , HfSe 2 , PtS 2 , ReS 2 , ReSe 2 , TiS 3 , ZrS 3 , ZrSe 3 , HfS 3 , HfSe 3 , TiS 2 , TaS 2 , TaSe 2 , NbS 2 , NbSe 2 and NbTe 2 .
- the metal nanoparticles according to the invention are chosen from the group formed by WS 2 , WSe 2 , MoS 2 and MoSe 2 , preferentially WS 2 and MoS 2 , preferentially WS 2 .
- the nanoparticles according to the invention advantageously have a fullerene type structure.
- fullerene denotes a closed convex polyhedron nanostructure composed of carbon atoms.
- Fullerenes are similar to graphite, composed of linked hexagonal ring sheets, but they contain pentagonal, and sometimes heptagonal rings, which prevent the structure from being flat.
- fullerene-type structures were not limited to carbonaceous materials, but was likely to occur in all nanoparticles of sheet-like materials, particularly for nanoparticles including chalcogen and transition. These structures are similar to that of carbon fullerenes and are called inorganic fullerenes or fullerene type structure (in English term "Inorganic Fullerene like materials", also referred to as "IF"). Fullerene type structures are described in particular by Tenne, R., Margulis, L., M. Hodes Genut, G. Nature 1992, 360, 444 . The document EP 0580 019 describes in particular these structures and their method of synthesis.
- the metal nanoparticles are closed structures, spherical type, more or less perfect according to the synthetic methods used.
- the nanoparticles according to the invention are concentric polyhedra with a multilayer structure or in sheets. We speak of structure in “onions” or “polyhedron nested”.
- concentric polyhedron having a multilayer structure or sheets more particularly means substantially spherical polyhedra whose different layers are several spheres having the same center.
- the multilayer structure or in sheets of the nanoparticles according to the invention can in particular be determined by transmission electron microscopy (TEM or TEM).
- the metal nanoparticles are multilayer metal nanoparticles comprising from 2 to 500 layers, preferably from 20 to 200 layers, advantageously from 20 to 100 layers.
- the number of layers of the nanoparticles according to the invention can in particular be determined by transmission electron microscopy.
- the average size of the metal nanoparticles according to the invention ranges from 5 to 600 nm, preferably from 20 to 400 nm, advantageously from 50 to 200 nm.
- the size of the metal nanoparticles according to the invention can be determined using images obtained by transmission electron micrograph or by high resolution transmission electron microscopy.
- the average particle size can be determined from the measurement of the size of at least 50 solid particles visualized on transmission electron micrographs.
- the median value of the measured size distribution histogram of the solid particles is the average size of the solid particles used in the lubricating composition according to the invention.
- the average diameter of the primary metal nanoparticles according to the invention ranges from 10 to 100 nm, preferably from 30 to 70 nm.
- the average diameter of the nanoparticles according to the invention can in particular be determined by transmission electron microscopy.
- the content by weight of metal nanoparticles ranges from 0.05 to 2%, preferably from 0.1 to 1%, advantageously from 0.1 to 0.5% relative to the total weight of the lubricating composition.
- NanoLub Gear Oil Concentrate product marketed by Nanomaterials, in the form of a dispersion of multilayer nanoparticles of tungsten bisulphide in a mineral oil or of PAO type ( Poly Alfa Olefin).
- the lubricant composition according to the invention comprises at least one dispersant having a weight average molecular weight greater than or equal to 2000 Daltons.
- the weight average molecular weight of the dispersant is evaluated according to the ASTM D5296 standard.
- dispersant within the meaning of the present invention is meant more particularly any compound which ensures the suspension suspension of the metal nanoparticles.
- the dispersant may be chosen from compounds comprising at least one succinimide group, polyolefins, olefin copolymers (OCP), copolymers comprising at least one styrene unit and polyacrylates.
- the dispersant according to the invention is chosen from compounds comprising at least one succinimide group.
- the dispersant may be chosen from compounds comprising at least one substituted succinimide group or compounds comprising at least two substituted succinimide groups, the succinimide groups being connected at their atom-bearing apices. nitrogen with a polyamine group.
- substituted succinimide group in the sense of the present invention is meant a succinimide group of which at least one of the carbon peaks is substituted by a hydrocarbon group comprising from 8 to 400 carbon atoms.
- the dispersant is chosen from polyisobutylene succinimide-polyamine.
- the dispersant is a substituted succinimide of formula (I) or a substituted succinimide of formula (II) in which R 2 represents a polyisobutylene group.
- the dispersant is a substituted succinimide of formula (II) in which R 2 represents a polyisobutylene group.
- the dispersant according to the invention has a weight average molecular weight ranging from 2000 to 15000 Daltons, preferably ranging from 2500 to 10,000 Daltons, advantageously from 3000 to 7000 Daltons.
- the dispersant has, in addition, a number-average molecular mass greater than or equal to 1000 Daltons, preferably ranging from 1000 to 5000 Daltons, more preferably from 1800 to 3500 Daltons, advantageously from 1800 to 3000 Daltons.
- the number-average molecular mass of the dispersant is evaluated according to the ASTM D5296 standard.
- the content by weight of dispersant having a weight average molecular weight greater than or equal to 2000 Daltons ranges from 0.1 to 10%, preferably from 0.1 to 5%, advantageously from 0.1 to 3% relative to the total weight of the lubricating composition.
- OLOA 13000 As an example of dispersant according to the invention, mention may be made of OLOA 13000 from the company Oronite.
- the lubricant compositions according to the invention may contain any type of mineral lubricating base, synthetic or natural, animal or vegetable adapted (s) to their use.
- the base oil (s) used in the lubricant compositions according to the present invention may be oils of mineral or synthetic origin of groups I to V according to the classes defined in the API classification (or their equivalents according to the ATIEL classification) as summarized. below, alone or mixed.
- the base oil (s) used in the lubricant compositions according to the invention may be chosen from the oils of synthetic origin of group VI according to the ATIEL classification.
- the API classification is defined in American Petroleum Institute 1509 "Engine Oil Licensing and Certification System" 17th Edition, September 2012 .
- the mineral base oils according to the invention include any type of base obtained by atmospheric and vacuum distillation of crude oil, followed by refining operations such as solvent extraction, deasphalting, solvent dewaxing, hydrotreatment, hydrocracking and hydroisomerization, hydrofinishing.
- the base oils of the lubricating compositions according to the present invention may also be synthetic oils, such as polyalphaolefins (PAO) or certain esters of carboxylic acids and alcohols, in particular polyol esters.
- PAO polyalphaolefins
- certain esters of carboxylic acids and alcohols in particular polyol esters.
- the polyalphaolefins used as base oils are obtained from monomers having from 4 to 32 carbon atoms (for example octene, decene), and have a viscosity at 100 ° C. of between 1.5 and 15 cSt measured according to ASTM D445. Mixtures of synthetic and mineral oils can also be used.
- a particular lubricating base for producing the lubricating compositions according to the invention must have properties, in particular viscosity, viscosity index, sulfur, oxidation resistance, suitable for use in a gearbox, in particular in a gearbox of motor vehicles, especially in a manual gearbox.
- the base oil has a flash point greater than or equal to 150 ° C, preferably greater than or equal to 170 ° C, even more preferably greater than or equal to 190 ° C.
- the base oil is selected from the group consisting of Group I bases, Group II bases, Group III bases, Group IV bases, API Group V bases (or their equivalents according to the ATIEL classification) and their mixtures.
- the base oil can be selected from the bases of group VI of the ATIEL classification.
- the base oil is selected from the group consisting of Group III base, Group IV base, Group V base of the API classification and mixtures thereof.
- the base oil is a mixture of Group IV and Group V bases of the API classification.
- the base oil is chosen from polyalphaolefins (PAO) and esters, preferably polyol esters or mixtures thereof.
- the base oil is a mixture of at least one polyalphaolefin and at least one ester, preferably a polyol ester.
- the base oil or the base oils may be at least 50% by weight, based on the total weight of the lubricating composition, preferably at least 60%, or at least 70%. Typically, they represent (s) between 75 and 99.89% by weight, relative to the total weight of the lubricating compositions according to the invention.
- the lubricant compositions according to the invention have a kinematic viscosity at 100 ° C., measured according to the ASTM D445 standard of between 4 and 41 cSt, according to classification SAE J 306, preferably between 4.1 and 32.5 cSt.
- Preferred grades are all grades between SAE 75W and SAE 140, including SAE 75W, SAE 75W-80 and SAE 75W-90 grades.
- the lubricant compositions according to the invention have a viscosity index (VI) greater than 95 (measured according to ASTM 2270).
- the subject of the invention is a transmission oil comprising a lubricant composition according to the invention.
- the lubricating compositions according to the invention may also contain any type of additive suitable for use in transmission oil formulations, for example one or more additives chosen from the additional dispersants, the viscosity index improving polymers, the antioxidants, corrosion inhibitors, friction modifiers or defoamers, alone or in mixtures, present at the usual levels required for the application.
- additives chosen from the additional dispersants, the viscosity index improving polymers, the antioxidants, corrosion inhibitors, friction modifiers or defoamers, alone or in mixtures, present at the usual levels required for the application.
- the additional dispersants are selected from dispersants other than dispersants having a weight average molecular weight greater than or equal to 2000 Daltons.
- the additional dispersants may be selected from the groups formed by the different succinimides of the compounds of formula (I) or (II) having a weight average molecular weight greater than or equal to 2000 Daltons or the Mannich bases.
- the lubricant composition according to the invention may further comprise at least one additional additive chosen from viscosity index improving polymers, antioxidants and mixtures thereof.
- the viscosity index improving polymers may be chosen from polymers other than the dispersant according to the invention.
- the viscosity index improver polymers may be selected from the group of shear stable polymers, preferably from the group consisting of ethylene and alpha-olefin copolymers, especially ethylene / propylene copolymers.
- the additional additive is a viscosity index improving polymer selected from ethylene and alpha-olefin copolymers.
- the antioxidants may be chosen from aminated antioxidants, preferably diphenylamines, in particular dialkylphenylamines, such as octadiphenylamines, phenyl-alpha-naphthylamines, phenolic antioxidants (dibutylhydroxytoluene BHT and derivatives) or sulfur-containing antioxidants (sulfurized phenates). .
- aminated antioxidants preferably diphenylamines, in particular dialkylphenylamines, such as octadiphenylamines, phenyl-alpha-naphthylamines, phenolic antioxidants (dibutylhydroxytoluene BHT and derivatives) or sulfur-containing antioxidants (sulfurized phenates).
- the additional additive is an antioxidant selected from dialkyphenylamines, phenolic antioxidants, taken alone and mixtures thereof.
- the friction modifiers may be compounds providing metal elements different from the metal nanoparticles according to the invention or an ashless compound.
- the compounds providing metal elements mention may be made of transition metal complexes such as Mo, Sb, Sn, Fe, Cu, Zn, the ligands of which may be hydrocarbon compounds containing oxygen, nitrogen, sulfur or phosphorus, such as dithiocarbamates or dithiophosphates of molybdenum.
- the ashless friction modifiers are of organic origin and can be selected from monoesters of fatty acids and polyols, alkoxylated amines, fatty alkoxylated amines, amine phosphates, fatty alcohols, fatty epoxides, borate fatty epoxides, fatty amines or fatty acid glycerol esters.
- fatty or "fatty (s)" is intended to mean a hydrocarbon group comprising from 8 to 24 carbon atoms.
- the additional additive is a friction modifier selected from molybdenum dithiocarbamates, amine phosphates and fatty alcohols, alone or in admixture.
- the anti-corrosion additives can be chosen from phenol derivatives, in particular ethoxylated and substituted alkyl phenol derivatives in the ortho position.
- the corrosion inhibitors may be derivatives of dimercaptothiadiazole.
- the additional additive comprises a mixture of an antioxidant and a viscosity index improving polymer selected from the group consisting of alpha ethylene copolymers. olefins, especially ethylene / propylene copolymers.
- the additional additive comprises a blend of an aminated antioxidant, a phenolic antioxidant and a viscosity index improving polymer selected from ethylene and polyethylene copolymers. alpha-olefin.
- the mass ratio ranges from 1/50 to 10/1, preferably from 1/50 to 5/1, more preferably from 1/30 to 5/1. preferably from 1/10 to 5/1.
- nanoparticles of tungsten disulfide and for the dispersant also applies to the above composition.
- the nanoparticles of tungsten bisulfide have a fullerene type structure.
- nanoparticles of tungsten disulfide for the dispersant and for the additional additive also applies to the above composition.
- the nanoparticles of tungsten bisulfide have a fullerene type structure.
- the composition of the additive concentrate type according to the invention may be added at least one base oil to obtain a lubricant composition according to the invention.
- the base oil is base is selected from the group consisting of Group III base, Group IV bases, Group V bases of the API classification and mixtures thereof.
- the base oil is a mixture of Group IV and Group V bases of the API classification, preferably the base oil is chosen from polyalphaolefins (PAO) and esters and their mixture.
- PAO polyalphaolefins
- the base oil is a mixture of at least one polyalphaolefin and at least one ester, preferably a polyol ester.
- the lubricant composition according to the invention can lubricate at least one mechanical part or a mechanical member, in particular bearings, gears, universal joints, transmissions, the piston / piston / sleeve system, the camshafts, the clutch , manual or automatic gearboxes, bridges, rockers, crankcases, etc.
- the lubricant composition according to the invention can lubricate a mechanical part or a metallic member of the transmissions, the clutch, the manual or automatic gearboxes, preferably manual.
- the subject of the invention is also a process for reducing the peeling of a mechanical part, preferably of a transmission member, advantageously of a gearbox or of a bridge, comprising at least the contacting of the mechanical part with a lubricant composition as defined above or obtained from the concentrate type of additive composition as defined above.
- the set of characteristics and preferences presented for the lubricant composition also applies to the method of reducing peeling of a mechanical part according to the invention.
- the invention also relates to the use of a lubricant composition according to the invention for the lubrication of gearboxes or bridges, preferably gearboxes of motor vehicles.
- the invention relates to the use of a lubricant composition according to the invention for the lubrication of manual transmissions of motor vehicles.
- the set of characteristics and preferences presented for the lubricant composition also applies to the use for the lubrication of the gearboxes according to the invention.
- the subject of the invention is also the use of a lubricant composition according to the invention for reducing the peeling of a mechanical part, preferably of a transmission member, more preferably of a gearbox or of a gearbox. 'A bridge.
- the invention relates to the use of a lubricant composition according to the invention for reducing the spalling of a manual gearbox.
- the set of characteristics and preferences presented for the lubricating composition also applies to the use for the reduction of flaking according to the invention.
- the figure 1 shows a power recirculation bench comprising a false gearbox (111), an electric motor (112), a torque meter (113), a coupling device (114), a gearbox comprising the torque to be tested (115), a differential (116), a secondary shaft (117), a primary shaft (118), a scale detection system (119), a fifth gear (120), a reverse gear ( 121), the fourth gear (122), the third gear (123), the second gear (124), the first gear (125) and a drive belt (126).
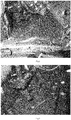
- the figure 2 is a photograph of a gearbox case after 600h test bench power recirculation with a composition according to the invention.
- the figure 3 is a photograph of a gearbox case after 400h test bench power recirculation with a composition outside the invention.
- compositions L 1 to L 5 are described in Table I; the percentages given correspond to mass percentages.
- Table I ⁇ / b> compositions L 1 (comparative) L 2 (comparative) L 3 (comparative) L 4 (invention) L 5 (invention) Group III base oil 89 89 89 89 89 Nanoparticles of tungsten disulfide (NanoLub Gear Oil Concentrate) 1 1 1 1 1 Dispersant 1 10 Dispersant 2 10 Dispersant 3 10 Dispersant 4 10 Dispersant 5 10
- the lubricating compositions according to the invention L 4 and L 5 comprising 0.2% by weight of nanoparticles of tungsten bisulfide and a dispersant having a weight average molecular mass greater than or equal to 2000 Daltons, exhibit stability. improved over lubricating compositions comprising 0.2% by weight of tungsten disulfide nanoparticles and a dispersant having a weight average molecular weight of less than 2000 Daltons.
- the aim here is to evaluate the impact of the combination of tungsten disulfide nanoparticles and a dispersant having a weight average molecular weight greater than or equal to 2000 Daltons on the friction properties of a lubricating composition by a Cameron Plint laboratory test Friction using a Cameron-Plint TE-77 alternative tribometer.
- compositions L 6 to L 7 are described in Table III; the percentages given correspond to mass percentages. ⁇ b> Table III ⁇ / b> compositions L 6 (comparative) L 7 (invention) Base oil 1 62.95 61.95 Base oil 2 15 15 Polymer 1 10 10 Polymer 2 5 5 Defoamers 0.05 0.05 Nanoparticles of tungsten bisulphide (NanoLub Gear Oil Concentrate marketed by Nanomaterials) 2 dispersed 2 Friction modifier 0.5 0.5 Package of additives 1 6.5 3.5 Kinematic viscosity at 100 ° C measured according to ASTM D445 (mm 2 / s) 14.5 14.5
- Composition L 6 is a lubricant composition conventionally used for lubricating transmissions, and in particular gearboxes for motor vehicles.
- compositions L 6 and L 7 The kinematic viscosity at 100 ° C of compositions L 6 and L 7 was adjusted to be identical, in particular by the content of base oils 1, so that these two compositions can be compared.
- the coefficient of friction of each composition was evaluated by a Cameron Plint Laboratory Scratch Test using a Cameron-Plint TE-77 alternative tribometer.
- the test bench consists of a cylinder / plane tribometer immersed in the lubricant composition to be tested. The coefficient of friction is followed during the test by measuring the tangential force on the normal effort.
- a cylinder (SKF 100C6) of length 10 mm and diameter 7 mm is applied to the steel plane immersed in the lubricant composition to be tested, the temperature of the lubricant composition is fixed at each test.
- a sinusoidal reciprocating motion is applied with a defined frequency. Each measurement is performed for a duration of 100 seconds during the test.
- the average coefficient of friction at 60 ° C. was measured under different loads ranging from 300 MPa to 650 MPa and at different speeds ranging from 70 mm / s to 550 mm / s.
- the average coefficient of friction at 100 ° C was measured under different loads ranging from 300 MPa to 650 MPa and at different speeds ranging from 70 mm / s to 550 mm / s.
- the average coefficient of friction under a load of 640 MPa was measured at different temperatures ranging from 60 ° C to 140 ° C and at different speeds ranging from 70 mm / s to 550 mm / s.
- the aim here is to evaluate the anti-peeling properties of a lubricant composition according to the invention by the implementation of a bench test with recirculation of power.
- the lubricant composition according to the invention L 8 and the composition L 9 outside the invention whose compositions are described in Table V have been prepared; the percentages given correspond to mass percentages.
- Table V ⁇ / b> compositions L 8 (invention) L 9 (comparative) Base oil 1 62.45 62.45 Base oil 2 15 15 Polymer 1 10 10 Polymer 2 5 5 Defoamers 0.05 0.05 Nanoparticles of tungsten bisulphide (NanoLub Gear Oil Concentrate marketed by Nanomaterials) 2 dispersed 2 Package of additives 1 3.5 3.5 Package of additives 2 4
- the base oils 1 and 2 are identical to those described in Example 2.
- the package of additives 2 (Anglamol 2190 from Lubrizol company) comprises a zinc dithiophosphate as a friction modifier.
- the recirculating power bench is shown in figure 1 .
- a box Renault JR5 speed is installed in a power recirculation loop and charge by a torsion system, the box being engaged on the report of 3rd.
- the machine is operated using an electric motor to obtain a rotation speed of 3000 rpm under a torque of 148 Nm at the input of the gearbox.
- the evaluation criterion, and therefore the critical part to evaluate (because of the load supported), is the pinion gear of the secondary shaft.
- the gearbox is inspected at regular intervals of about 150h after dismantling and visual quotation.
- the visual quotation is carried out on the quotation "Chrysler" to detect the presence of scales on the toothing of the pinion, with in addition a permanent vibratory follow-up to detect the appearance of chipping in the gearbox in operation.
- Vibration monitoring consists of placing an accelerometer near the test piece and recording the intensity of the vibrations during operation. In case of degradation of a room, the vibratory intensity increases. Just set a threshold to stop the device and check the appearance of scales on the toothing.
- shaft bearings and the pinion 3 rd are normally replaced every 150 hours.
- the test is stopped when a flake of maximum 12mm 2 is observed and / or when 80mm 2 of flaked surface in total is observed and / or at 312h when no flaking has appeared at this time.
- the lubricant composition according to the invention L 10 and the composition L 11 outside the invention whose compositions are described in Table VI have been prepared; the percentages given correspond to mass percentages.
- Table VI ⁇ / b> compositions L 10 (invention) L 11 (comparative) Base oil 1 60.45 62.45 Base oil 2 15 15 Polymer 1 10 10 Polymer 2 5 5 Defoamers 0.05 0.05 Nanoparticles of tungsten bisulphide (NanoLub Gear Oil Concentrate marketed by Nanomaterials) 4 4 dispersed 2 Package of additives 1 3.5 3.5
- the base oils 1 and 2 are identical to those described in Example 2.
- the figure 2 shows that no excessive deposition (200) of nanoparticles of tungsten disulfide was observed in the casing after testing with the composition according to the invention L 10 .
- composition L 11 shows an excessive deposition (300) of nanoparticles of tungsten disulfide in the housing after testing with the composition L 11 , thus potentially causing a risk of clogging the lubrication holes of the bearings or synchronizers.
- the above examples show that the lubricant compositions according to the invention have both good stability over time and good anti-peeling properties, while maintaining satisfactory friction reducing properties.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Lubricants (AREA)
Claims (15)
- Schmiermittelzusammensetzung, welche mindestens ein Grundöl, mindestens ein Dispersionsmittel mit einem gewichtsmittleren Molargewicht größer oder gleich 2000 Daltons mit einem Gewichtsgehalt von 0,1 bis 10% bezogen auf das Gesamtgewicht der Schmiermittelzusammensetzung aufweist, wobei das Dispersionsmittel ausgewählt ist aus den Verbindungen, die mindestens eine Succinimid-Gruppe und metallische Nanopartikel mit einem Gewichtsgehalt von 0,01 bis 2%, bezogen auf das Gesamtgewicht der Schmiermittelzusammensetzung, aufweisen, wobei die metallischen Nanopartikel konzentrische Polyeder mit einer Mehrlagen- oder Schichten-Struktur sind, wobei die besagten metallischen, festen Nanopartikel eine Struktur des Fulleren-Typs haben und wiedergegeben sind durch die Formel MXn, worin M ein Übergangsmetall wiedergibt, X ein Chalkogen wiedergibt, mit n=2 oder n=3 In Abhängigkeit vom Oxidationszustand des Übergangsmetalls M.
- Schmiermittel gemäß Anspruch 1, wobei das Metall, wodurch das metallische Partikel aufgebaut ist, ausgewählt ist aus der Gruppe gebildet aus Wolfram, Molybdän, Zirkonium, Hafnium, Platin, Rhenium, Titan, Tantal, Niob, Zink, Cer, Aluminium, Indium und Zinn.
- Schmiermittelzusammensetzung gemäß Anspruch 1 oder 2, wobei die metallischen Nanopartikel ausgewählt sind aus der Gruppe gebildet aus MoS2, MoSe2, MoTe2, WS2, WSe2, ZrS2, ZrSe2, HfS2, HfSe2, PtS2, ReS2, ReSe2, TiS3, ZrS3, ZrSe3, HfS3, HfSe3, TiS2, TaS2, TaSe2, NbS2, NbSe2 und NbTe2, vorzugsweise aus MoS2, MoSe2, WS2, WSe2, vorteilhafterweise aus WS2.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei der Gewichtsgehalt von metallischen Nanopartikeln von 0,05% bis 2% geht, vorzugsweise von 0,1% bis 1%, vorteilhafterweise von 0,1% bis 0,5% bezogen auf das Gesamtgewicht der Schmiermittelzusammensetzung.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei die mittlere Größe der metallischen Nanopartikel von 5 bis 600 nm geht, vorzugsweise von 20 bis 400 nm, vorteilhafterweise von 50 bis 200 nm, wobei die mittlere Größe der metallischen Nanopartikel ermittelt ist unter Hilfe von Bildern, die mittels Transmissionselektronenmikroskopie oder mittels hochauflösender Transmissionselektronenmikroskopie erhalten werden, wobei die mittlere Größe der Medianwert des Histogramms der Verteilung der gemessenen Größen der festen Partikel ist.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei das Dispersionsmittel ausgewählt ist aus den Verbindungen, welche mindestens eine substituierte Succinimid-Gruppe aufweisen oder aus den Verbindungen, welche mindestens zwei substituierte Succinimid-Gruppen aufweisen, wobei die Succinimid-Gruppen verbunden sind auf Niveau ihres Kohlenstoffscheitels, welcher ein Stickstoffatom von einer Polyamin-Gruppe trägt.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei das Dispersionsmittel ein gewichtsmittleres Molargewicht hat, das von 2000 bis 15000 Daltons geht, vorzugsweise von 2500 bis 10000 Daltons, vorteilhafterweise von 3000 bis 7000 Daltons.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei das Dispersionsmittel weiterhin ein Molekulargewichtszahlenmittel größer oder gleich 1000 Daltons hat, das vorzugsweise von 1000 bis 5000 Daltons geht, bevorzugter von 1800 bis 3500 Daltons, vorteilhafterweise von 1800 bis 3000 Daltons.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei der Gewichtsgehalt an Dispersionsmittel von 0,1 bis 5% geht, vorteilhafterweise von 0,1 bis 3%, bezogen auf das Gesamtgewicht der Schmiermittelzusammensetzung.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, wobei das Grundöl ausgewählt ist aus den Polyalphaolefinen oder den Estern, vorzugsweise den Polyolestern oder deren Mischungen.
- Schmiermittelzusammensetzung gemäß irgendeinem der vorangegangenen Ansprüche, welche weiterhin mindestens einen supplementären Zusatzstoff aufweist ausgewählt aus den Polymeren, die den Viskositätsindex verbessern, und den Antioxidantien oder deren Mischungen, wobei die Polymere, die den Viskositätsindex verbessern, ausgewählt sind aus den Copolymeren von Ethylen und alpha-Olefinen, insbesondere den Copolymeren von Ethylen und Propylen, wobei der supplementäre Zusatzstoff vorzugsweise ein Antioxidans ist ausgewählt aus den Dialkylphenylaminen, den phenolischen Antioxidantien oder deren Mischungen.
- Verwendung einer Schmiermittelzusammensetzung gemäß irgendeinem der Ansprüche 1 bis 11 in einem Getriebeöl.
- Verwendung einer Schmiermittelzusammensetzung gemäß irgendeinem der Ansprüche 1 bis 11 für die Schmierung von Schaltgetrieben oder Schaltbrücken, vorzugsweise von Schaltgetrieben von Kraftfahrzeugen, wobei die Schaltgetriebe vorzugsweise manuelle Schaltgetriebe sind.
- Verwendung einer Schmiermittelzusammensetzung gemäß irgendeinem der Ansprüche 1 bis 11 zur Reduktion des Abplatzens eines mechanischen Bauteils, vorzugsweise eines Getriebeorgans, bevorzugter eines Schaltgetriebes oder einer Schaltbrücke, vorteilhafterweise eines manuellen Schaltgetriebes.
- Zusammensetzung des Typs Additivkonzentrat, welches Wolfram-Bisulfur-Nanopartikel mit einem Gewichtsgehalt, der von 1 bis 15% bezogen auf das Gesamtgewicht der Zusammensetzung geht, und mindestens ein Dispersionsmittel, das ein gewichtsmittleres Molargewicht größer oder gleich 2000 Dalton hat mit einem Gewichtsgehalt, der von 5 bis 99% bezogen auf das Gesamtgewicht der Zusammensetzung geht, wobei das Dispersionsmittel ausgewählt ist aus den Verbindungen, die mindestens eine Succinimid-Gruppe aufweisen.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1353561A FR3004723B1 (fr) | 2013-04-19 | 2013-04-19 | Composition lubrifiante a base de nanoparticules metalliques |
| PCT/EP2014/058013 WO2014170485A1 (fr) | 2013-04-19 | 2014-04-18 | Composition lubrifiante a base de nanoparticules metalliques |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2986693A1 EP2986693A1 (de) | 2016-02-24 |
| EP2986693B1 true EP2986693B1 (de) | 2019-09-18 |
Family
ID=48979929
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP14718967.4A Active EP2986693B1 (de) | 2013-04-19 | 2014-04-18 | Schmiermittelzusammensetzung auf der basis von metallnanopartikeln |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20160075965A1 (de) |
| EP (1) | EP2986693B1 (de) |
| JP (1) | JP6440685B2 (de) |
| KR (1) | KR102154097B1 (de) |
| CN (1) | CN105247022B (de) |
| BR (1) | BR112015026415B1 (de) |
| ES (1) | ES2753261T3 (de) |
| FR (1) | FR3004723B1 (de) |
| MX (1) | MX2015014698A (de) |
| WO (1) | WO2014170485A1 (de) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3018079B1 (fr) | 2014-02-28 | 2017-06-23 | Total Marketing Services | Composition lubrifiante a base de nanoparticules metalliques |
| KR102633391B1 (ko) * | 2015-05-04 | 2024-02-06 | 픽셀리전트 테크놀로지스 엘엘씨 | 나노-첨가제를 이용한 개량 윤활제 |
| US20180291305A1 (en) * | 2015-10-06 | 2018-10-11 | Hindustan Petroleum Corporation Limited | Nano Suspension Lubricants |
| CN108602670B (zh) * | 2016-01-05 | 2023-01-13 | 纳米技术工业解决方案公司 | 水基纳米颗粒分散体 |
| BE1023466B1 (nl) * | 2016-01-29 | 2017-03-29 | Vdv Lubricants Nv | Smeermiddelsamenstelling en toepassingen daarvan |
| KR101899198B1 (ko) * | 2016-02-23 | 2018-09-17 | 주식회사 울산항업 | 나노 크기의 이황화텅스텐 분말을 포함하는 엔진 복원 첨가제 및 이의 제조방법 |
| TWI614333B (zh) * | 2016-09-19 | 2018-02-11 | 甘美繡 | 用於機件之表面改質的方法 |
| CN106544101A (zh) * | 2016-10-31 | 2017-03-29 | 苏州宇希新材料科技有限公司 | 一种耐磨润滑油的制备方法 |
| CN107267260A (zh) * | 2017-05-10 | 2017-10-20 | 蚌埠精工制药机械有限公司 | 一种用于钢管弯曲的润滑剂 |
| EP3743489B1 (de) | 2018-01-23 | 2021-08-18 | Evonik Operations GmbH | Polymere-anorganische nanopartikelzusammensetzungen, herstellungsverfahren dafür und deren verwendung als schmiermitteladditive |
| US11180712B2 (en) | 2018-01-23 | 2021-11-23 | Evonik Operations Gmbh | Polymeric-inorganic nanoparticle compositions, manufacturing process thereof and their use as lubricant additives |
| WO2019145287A1 (en) | 2018-01-23 | 2019-08-01 | Evonik Oil Additives Gmbh | Polymeric-inorganic nanoparticle compositions, manufacturing process thereof and their use as lubricant additives |
| EP4007802A1 (de) | 2019-08-01 | 2022-06-08 | King Abdullah University of Science and Technology | Polyphenolumhüllte nanoteilchen, diese enthaltende schmiermittelzusammensetzung und verfahren zu ihrer herstellung |
| EP3839016A1 (de) * | 2019-12-20 | 2021-06-23 | Total Marketing Services | Schmiermittelzusammensetzung für getriebe |
| EP3839022A1 (de) * | 2019-12-20 | 2021-06-23 | Total Marketing Services | Schmiermittelzusammensetzung zur verbesserung des kraftstoffverbrauchs und zur verringerung der reibung |
| CN111979016A (zh) * | 2020-08-03 | 2020-11-24 | 容嘉和 | 一种引擎机油添加剂 |
| WO2022241683A1 (zh) * | 2021-05-19 | 2022-11-24 | 安美科技股份有限公司 | 纤维润滑剂及其制备方法 |
| JP2023038798A (ja) * | 2021-09-07 | 2023-03-17 | 三菱重工業株式会社 | 被膜形成方法及び潤滑油組成物 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3842009A (en) * | 1970-10-19 | 1974-10-15 | American Metal Climax Inc | Molybdenum disulfide containing lubricant |
| GB2018285A (en) * | 1978-04-03 | 1979-10-17 | Atlantic Richfield Co | Improved solid particles-containing lubricating oil composition and method for using same |
| US4715972A (en) * | 1986-04-16 | 1987-12-29 | Pacholke Paula J | Solid lubricant additive for gear oils |
| ATE420940T1 (de) * | 2002-10-01 | 2009-01-15 | Lubrizol Corp | Herstellung von schmiermitteln aus metallhydroxid enthaltenden entwässerten emulsionen |
| US7759294B2 (en) * | 2003-10-24 | 2010-07-20 | Afton Chemical Corporation | Lubricant compositions |
| US20070049505A1 (en) * | 2005-08-24 | 2007-03-01 | Baker Mark R | Controlled release of additive gel(s) for functional fluids |
| WO2007035626A2 (en) * | 2005-09-20 | 2007-03-29 | The Lubrizol Corporation | Method of lubricating an internal combustion engine |
| EP1976962A2 (de) * | 2005-12-20 | 2008-10-08 | The Lubrizol Corporation | Verfahren zur herstellung eines überalkalisierten oder neutralen detergens |
| US8741821B2 (en) * | 2007-01-03 | 2014-06-03 | Afton Chemical Corporation | Nanoparticle additives and lubricant formulations containing the nanoparticle additives |
| FR2949786B1 (fr) * | 2009-09-10 | 2013-07-05 | Total Raffinage Marketing | Composition de graisse. |
| CN101691517B (zh) * | 2009-09-29 | 2012-12-19 | 中南大学 | 一种车用机油添加剂及机油 |
| PL218093B1 (pl) * | 2009-12-30 | 2014-10-31 | Inst Obróbki Plastycznej | Sposób wytwarzania smarów nanokompozytowych grafenopodobnych i zespół do wytwarzania smarów nanokompozytowych grafenopodobnych |
| US9228151B1 (en) * | 2012-11-07 | 2016-01-05 | Rand Innovations, Llc | Lubricant additive composition, lubricant, and method of preparing the same |
-
2013
- 2013-04-19 FR FR1353561A patent/FR3004723B1/fr active Active
-
2014
- 2014-04-18 WO PCT/EP2014/058013 patent/WO2014170485A1/fr active Application Filing
- 2014-04-18 ES ES14718967T patent/ES2753261T3/es active Active
- 2014-04-18 MX MX2015014698A patent/MX2015014698A/es unknown
- 2014-04-18 US US14/784,466 patent/US20160075965A1/en not_active Abandoned
- 2014-04-18 JP JP2016508188A patent/JP6440685B2/ja active Active
- 2014-04-18 KR KR1020157032959A patent/KR102154097B1/ko active IP Right Grant
- 2014-04-18 BR BR112015026415-8A patent/BR112015026415B1/pt active IP Right Grant
- 2014-04-18 CN CN201480030620.8A patent/CN105247022B/zh active Active
- 2014-04-18 EP EP14718967.4A patent/EP2986693B1/de active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| BR112015026415A2 (pt) | 2017-07-25 |
| JP6440685B2 (ja) | 2018-12-19 |
| CN105247022A (zh) | 2016-01-13 |
| ES2753261T3 (es) | 2020-04-07 |
| US20160075965A1 (en) | 2016-03-17 |
| KR102154097B1 (ko) | 2020-09-09 |
| FR3004723A1 (fr) | 2014-10-24 |
| CN105247022B (zh) | 2019-09-10 |
| JP2016515663A (ja) | 2016-05-30 |
| EP2986693A1 (de) | 2016-02-24 |
| BR112015026415B1 (pt) | 2020-12-15 |
| KR20160018490A (ko) | 2016-02-17 |
| MX2015014698A (es) | 2016-03-07 |
| FR3004723B1 (fr) | 2016-04-15 |
| WO2014170485A1 (fr) | 2014-10-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2986693B1 (de) | Schmiermittelzusammensetzung auf der basis von metallnanopartikeln | |
| EP2844726B1 (de) | Schmiermittelzusammensetzung für einen motor | |
| CA2821567C (fr) | Composition de graisse comprenant un dithiocarbamate de molybdene et du graphite | |
| EP2652100B1 (de) | Schmierfettzusammensetzung | |
| EP3110929B1 (de) | Schmiermittelzusammensetzung auf basis von metallnanopartikeln | |
| CA2771772C (fr) | Composition de graisse | |
| EP3027719A1 (de) | Schmiermittelzusammensetzungen für getriebe | |
| EP2935542A1 (de) | Schmiermittelzusammensetzung aus polyglycerolether | |
| WO2013164459A1 (fr) | Lubrifiant moteur pour vehicules a motorisation hybride ou micro-hybride | |
| EP2788462B1 (de) | Motorschmiermittel für hybrid -oder mikrohybridkraftfahrzeuge |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20151112 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: BOUFFET, ALAIN |
|
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20180810 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20190409 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602014053813 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1181310 Country of ref document: AT Kind code of ref document: T Effective date: 20191015 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191218 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191218 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191219 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2753261 Country of ref document: ES Kind code of ref document: T3 Effective date: 20200407 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1181310 Country of ref document: AT Kind code of ref document: T Effective date: 20190918 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200120 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200224 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602014053813 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG2D | Information on lapse in contracting state deleted |
Ref country code: IS |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200119 |
|
| 26N | No opposition filed |
Effective date: 20200619 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200430 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200418 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200418 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190918 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230419 |
|
| P02 | Opt-out of the competence of the unified patent court (upc) changed |
Effective date: 20230526 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602014053813 Country of ref document: DE Owner name: TOTALENERGIES ONETECH, FR Free format text: FORMER OWNER: TOTAL MARKETING SERVICES, PUTEAUX, FR |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20240418 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: PD Owner name: TOTALENERGIES ONETECH; FR Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), OTHER; FORMER OWNER NAME: TOTALENERGIES MARKETING SERVICES Effective date: 20240423 Ref country code: BE Ref legal event code: HC Owner name: TOTALENERGIES MARKETING SERVICES; FR Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CHANGE OF OWNER(S) NAME; FORMER OWNER NAME: TOTAL MARKETING SERVICES Effective date: 20240423 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240419 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20240418 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240524 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20240424 Year of fee payment: 11 Ref country code: FR Payment date: 20240425 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20240418 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: PD Owner name: TOTALENERGIES ONETECH; FR Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT; FORMER OWNER NAME: TOTALENERGIES MARKETING SERVICES Effective date: 20240926 Ref country code: NL Ref legal event code: HC Owner name: TOTALENERGIES MARKETING SERVICES; FR Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CHANGE OF OWNER(S) NAME; FORMER OWNER NAME: TOTAL MARKETING SERVICES Effective date: 20240926 |