EP2886629B1 - Procédé d'hydrodesulfuration de coupes d'hydrocarbures - Google Patents
Procédé d'hydrodesulfuration de coupes d'hydrocarbures Download PDFInfo
- Publication number
- EP2886629B1 EP2886629B1 EP14306951.6A EP14306951A EP2886629B1 EP 2886629 B1 EP2886629 B1 EP 2886629B1 EP 14306951 A EP14306951 A EP 14306951A EP 2886629 B1 EP2886629 B1 EP 2886629B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- stage
- metal
- weight
- hydrocarbons
- cut
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 150000002430 hydrocarbons Chemical class 0.000 title claims description 72
- 229930195733 hydrocarbon Natural products 0.000 title claims description 70
- 238000000034 method Methods 0.000 title claims description 49
- 239000004215 Carbon black (E152) Substances 0.000 title claims description 24
- 239000000203 mixture Substances 0.000 claims description 76
- 239000003054 catalyst Substances 0.000 claims description 73
- 229910052751 metal Inorganic materials 0.000 claims description 57
- 239000002184 metal Substances 0.000 claims description 57
- 238000009835 boiling Methods 0.000 claims description 53
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 45
- 229910052717 sulfur Inorganic materials 0.000 claims description 44
- 239000011593 sulfur Substances 0.000 claims description 43
- 239000001257 hydrogen Substances 0.000 claims description 36
- 229910052739 hydrogen Inorganic materials 0.000 claims description 36
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 35
- 239000007788 liquid Substances 0.000 claims description 27
- 150000001336 alkenes Chemical class 0.000 claims description 26
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 claims description 18
- 238000004523 catalytic cracking Methods 0.000 claims description 18
- 238000005984 hydrogenation reaction Methods 0.000 claims description 14
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 13
- 229910052750 molybdenum Inorganic materials 0.000 claims description 13
- 239000011733 molybdenum Substances 0.000 claims description 13
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 12
- 239000010941 cobalt Substances 0.000 claims description 12
- 229910017052 cobalt Inorganic materials 0.000 claims description 12
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 12
- 238000004821 distillation Methods 0.000 claims description 12
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 10
- 229910052698 phosphorus Inorganic materials 0.000 claims description 10
- 239000011574 phosphorus Substances 0.000 claims description 10
- 229910052799 carbon Inorganic materials 0.000 claims description 8
- 238000004939 coking Methods 0.000 claims description 7
- 150000001993 dienes Chemical class 0.000 claims description 7
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 7
- 229910052721 tungsten Inorganic materials 0.000 claims description 7
- 239000010937 tungsten Substances 0.000 claims description 7
- 229910052759 nickel Inorganic materials 0.000 claims description 6
- 238000004230 steam cracking Methods 0.000 claims description 6
- 229910000037 hydrogen sulfide Inorganic materials 0.000 claims description 4
- 238000004519 manufacturing process Methods 0.000 claims description 4
- 238000011144 upstream manufacturing Methods 0.000 claims 1
- 239000003502 gasoline Substances 0.000 description 55
- 239000003921 oil Substances 0.000 description 13
- 150000003464 sulfur compounds Chemical class 0.000 description 13
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 11
- 239000007789 gas Substances 0.000 description 11
- 238000000926 separation method Methods 0.000 description 11
- 238000006243 chemical reaction Methods 0.000 description 8
- 238000006477 desulfuration reaction Methods 0.000 description 8
- 230000023556 desulfurization Effects 0.000 description 8
- 239000000446 fuel Substances 0.000 description 8
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 8
- 239000003350 kerosene Substances 0.000 description 7
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 7
- 230000000737 periodic effect Effects 0.000 description 7
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 6
- 230000003197 catalytic effect Effects 0.000 description 6
- 238000005987 sulfurization reaction Methods 0.000 description 6
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 150000005673 monoalkenes Chemical class 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- LSDPWZHWYPCBBB-UHFFFAOYSA-N Methanethiol Chemical compound SC LSDPWZHWYPCBBB-UHFFFAOYSA-N 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- WQOXQRCZOLPYPM-UHFFFAOYSA-N dimethyl disulfide Chemical compound CSSC WQOXQRCZOLPYPM-UHFFFAOYSA-N 0.000 description 4
- 230000008030 elimination Effects 0.000 description 4
- 238000003379 elimination reaction Methods 0.000 description 4
- 230000006641 stabilisation Effects 0.000 description 4
- 238000011105 stabilization Methods 0.000 description 4
- 230000007704 transition Effects 0.000 description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 3
- 229910052794 bromium Inorganic materials 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 229910044991 metal oxide Inorganic materials 0.000 description 3
- 150000004706 metal oxides Chemical class 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- URLKBWYHVLBVBO-UHFFFAOYSA-N Para-Xylene Chemical group CC1=CC=C(C)C=C1 URLKBWYHVLBVBO-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000001833 catalytic reforming Methods 0.000 description 2
- 238000005336 cracking Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000011066 ex-situ storage Methods 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 239000000295 fuel oil Substances 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 235000012245 magnesium oxide Nutrition 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- XTQHKBHJIVJGKJ-UHFFFAOYSA-N sulfur monoxide Chemical class S=O XTQHKBHJIVJGKJ-UHFFFAOYSA-N 0.000 description 2
- 229910052815 sulfur oxide Inorganic materials 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 239000000809 air pollutant Substances 0.000 description 1
- 231100001243 air pollutant Toxicity 0.000 description 1
- QGAVSDVURUSLQK-UHFFFAOYSA-N ammonium heptamolybdate Chemical compound N.N.N.N.N.N.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.[Mo].[Mo].[Mo].[Mo].[Mo].[Mo].[Mo] QGAVSDVURUSLQK-UHFFFAOYSA-N 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical compound [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 description 1
- 229910001981 cobalt nitrate Inorganic materials 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 239000010730 cutting oil Substances 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- -1 extrudates Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000004231 fluid catalytic cracking Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical class [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 238000004525 petroleum distillation Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 238000010517 secondary reaction Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/02—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing
- C10G45/04—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/02—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing
- C10G45/04—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used
- C10G45/06—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used containing nickel or cobalt metal, or compounds thereof
- C10G45/08—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used containing nickel or cobalt metal, or compounds thereof in combination with chromium, molybdenum, or tungsten metals, or compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G65/00—Treatment of hydrocarbon oils by two or more hydrotreatment processes only
- C10G65/02—Treatment of hydrocarbon oils by two or more hydrotreatment processes only plural serial stages only
- C10G65/04—Treatment of hydrocarbon oils by two or more hydrotreatment processes only plural serial stages only including only refining steps
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G67/00—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only
- C10G67/02—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/104—Light gasoline having a boiling range of about 20 - 100 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/1044—Heavy gasoline or naphtha having a boiling range of about 100 - 180 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/1048—Middle distillates
- C10G2300/1051—Kerosene having a boiling range of about 180 - 230 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/1048—Middle distillates
- C10G2300/1055—Diesel having a boiling range of about 230 - 330 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1037—Hydrocarbon fractions
- C10G2300/1048—Middle distillates
- C10G2300/1059—Gasoil having a boiling range of about 330 - 427 °C
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/20—Characteristics of the feedstock or the products
- C10G2300/201—Impurities
- C10G2300/202—Heteroatoms content, i.e. S, N, O, P
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/02—Gasoline
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/04—Diesel oil
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/06—Gasoil
Definitions
- the present invention relates to a process for the simultaneous production of two cuts of hydrocarbons with low sulfur contents.
- the process makes it possible to desulfurize jointly (as a mixture) a gasoline cut containing olefins and a cut heavier than the gasoline cut so as to subsequently produce a desulfurized gasoline cut with a limited loss of octane number and a heavy cut also desulfurized.
- the present invention is particularly interesting for producing two desulfurized cuts which can be sent respectively to the gasoline pool and to the diesel, kerosene and/or fuel oil pool.
- Sulfur in fuels is an undesirable impurity because it is converted to sulfur oxides when these products are burned.
- Sulfur oxides are unwanted air pollutants that can further deactivate most catalysts that have been developed for catalytic converters used in cars to catalyze the conversion of harmful exhaust gases. Therefore, it is desirable to reduce the sulfur content of products used in gasoline and diesel fuel compositions to the lowest possible levels.
- Catalytic cracking gasoline is the essential product of FCC (FCC "Fluid Catalytic Cracking” according to Anglo-Saxon terminology) obtained with a yield of around 50% and represents approximately 25 to 30% of the gasoline pool of refineries. 'Western Europe.
- FCC Fluid Catalytic Cracking
- the main negative characteristic of these FCC gasolines compared to commercial fuels is their high sulfur contents and thus constitute the main vector of the presence of sulfur in fuels.
- hydrocarbons produced from catalytic cracking processes are conventionally treated by hydrotreating.
- the hydrotreating process includes contacting the hydrocarbon feed with hydrogen in the presence of a catalyst so as to convert the sulfur contained in the impurities into hydrogen sulfide, which can then be separated and converted in elemental sulfur.
- Hydrotreating processes can result in partial destruction of feedstock olefins by converting them to saturated hydrocarbons through hydrogenation. This destruction of olefins by hydrogenation is not desirable in the case of cracked gasolines because it results in costly hydrogen consumption and a significant reduction in the octane number of hydrodesulfurized gasolines.
- the residual sulfur compounds generally present in desulfurized gasoline can be separated into two distinct families: the non-hydrodesulfurized sulfur compounds present in the feed and the sulfur compounds formed in the hydrodesulfurization reactor by secondary reactions known as recombination.
- the majority compounds are the mercaptans resulting from the addition of the H 2 S formed in the reactor to the mono-olefins present in the feed. Reducing the content of recombinant mercaptans can be achieved by catalytic hydrodesulphurization but at the cost of saturation of a significant part of the mono-olefins, which then leads to a sharp reduction in the octane number of the gasoline. as well as overconsumption of hydrogen.
- the document process EP 902 078 thus treats a distillate resulting from an atmospheric distillation step.
- This type of distillate contains practically no compounds olefinic hydrocarbons unlike the feed treated in the present invention, one of the cuts which compose it contains a significant content of olefins, typically greater than 20% by weight relative to the total weight of said cut.
- the majority of recombinant sulfur compounds encountered in the process of the document EP 902 078 are therefore not mercaptans resulting from the addition of the H 2 S formed in the reactor to the mono-olefins present in the feed, but probably the result of the addition of the H 2 S formed to olefins resulting cracking reactions induced by the high temperature necessary for very deep desulphurization of the feed.
- the solution recommended by the patent EP 902 078 consists of carrying out extensive hydrodesulfurization at high temperature in a first reactor followed by gentler hydrodesulfurization in a second reactor which makes it possible to eliminate any recombination mercaptans and/or olefins which would have been produced in the first reactor.
- This way of operating is unsuitable for a feed containing gasoline from a conversion unit with a high olefin content because it risks causing significant hydrogenation of said olefins during the first step, thus inducing a loss of octane number. unwanted.
- the document US 2013/0087484 describes a process for producing p-xylene from a mixture of naphtha and light cutting oil (LCO, “Light Cycle Oil” according to Anglo-Saxon terminology) from a catalytic cracking unit.
- the process comprises a step of hydrodesulfurization of said mixture followed by fractionation of the desulfurized effluent into three cuts, namely, a light C 2 -C 4 cut, a naphtha cut and a heavy cut.
- the intermediate naphtha cut is processed in a catalytic reforming unit to produce aromatics and the heavy cut is hydrocracked to give an aromatics-rich effluent which is recycled to the fractionation column.
- the second hydrodesulfurization step is carried out at a temperature lower by at least 10°C, preferably by at least 20°C, than that of the first hydrodesulfurization step.
- the prior art also includes the document FR 2811328 which teaches a process for hydrodesulfurization of a gasoline cut which can be a mixture of gasolines coming from different conversion processes such as steam cracking, coking or visbreaking processes or even gasolines directly resulting from the atmospheric distillation of petroleum.
- An aim of the invention is to propose a hydrodesulfurization process which can respond to the problems of overcapacity of gasoline hydrodesulfurization units.
- the boiling temperatures can fluctuate by plus or minus 5°C compared to the values mentioned.
- the inventors have surprisingly observed that it is possible to jointly hydrodesulfurize a mixture containing a gasoline cut and a distillate cut loaded with sulfur and with a low olefin content in order to produce a gasoline cut with a low sulfur content, in particular in mercaptans, without significant loss of octane number and a cut of distillate depleted in sulfur which can then be upgraded to the diesel and/or kerosene pool or as fuel for maritime use.
- the treatment in the first hydrodesulfurization step of the hydrocarbon mixture leads surprisingly to limit the formation of recombinant mercaptans, reaction products of the addition of H 2 S with the olefins, and thus to obtain at the end of the process a gasoline cut having a very low mercaptan content.
- the second hydrodesulfurization step is carried out under conditions which then favor the hydroconversion of the more refractory sulfur compounds which essentially come from the distillate cut.
- the process according to the invention responds well to the problem of overcapacity of gasoline hydrodesulphurization units to the extent that these same units can now be used to jointly desulphurize gasoline cuts and middle distillate cuts which are bases for the formulation of fuels.
- the invention therefore relates to a process implementing at least two successive hydrodesulphurization stages of a mixture of hydrocarbons consisting of a first and a second hydrocarbon fraction with an intermediate stage of elimination of the hydrogen sulfide (H 2 S) formed in the first hydrodesulfurization step and with a reaction temperature in the second hydrodesulfurization step which is higher than that in the first hydrodesulfurization step.
- H 2 S hydrogen sulfide
- the catalyst of step a) is a hydrodesulfurization catalyst which comprises a Group VIII metal chosen from nickel and cobalt and a Group VIB metal chosen from molybdenum and tungsten.
- the catalyst of step c) is also a hydrodesulfurization catalyst which comprises a metal from group VIII chosen from nickel and cobalt and a metal from group VIB chosen from molybdenum and tungsten.
- the first fraction of hydrocarbons containing olefins is an olefinic gasoline cut from a catalytic cracking, steam cracking, coking, visbreaking unit.
- the second hydrocarbon fraction of the mixture treated by the process according to the invention is a light oil cut from a catalytic cracking unit (LCO or "Light Cycle Oil” according to Anglo-Saxon terminology).
- LCO catalytic cracking unit
- the feed for the process according to the invention is a mixture containing a catlaytic cracked gasoline cut and an LCO light oil cut.
- said mixture is the product of a distillation of an effluent from a catalytic cracking unit.
- the light fraction of the LCO i.e. the compounds having a boiling point lower than 300°C, and very preferably lower than 265°C, is used in mixture with the catalytic cracking gasoline.
- the first fraction or hydrocarbon cut represents between 30 and 70% by weight of the mixture.
- the first fraction of the mixture is a heavy fraction of a catalytic cracked gasoline and the second fraction is a cut of light LCO oil.
- the heavy fraction of catalytically cracked gasoline is obtained by distillation of a catalytically cracked gasoline cut into two fractions, a light C5- fraction comprising hydrocarbons having a number of carbon atoms of between 2 and 5 atoms and a heavy C6+ fraction comprising hydrocarbons having a number of atoms of carbon greater than or equal to 6.
- said gasoline cut is treated in a step of selective hydrogenation of the diolefins.
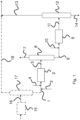
- the first cut of hydrocarbons treated by the process according to the invention is sent via line 1 to a first hydrodesulphurization reactor 2.
- This first cut of hydrocarbons is combined (mixed) with a second cut of hydrocarbons supplied by line 3.
- the mixture which is made up of two fractions, is then treated in the first hydrodesulfurization reactor 2.
- the first hydrocarbon cut an olefinic gasoline cut from a catalytic cracking, steam cracking, coking, visbreaking unit.
- the gasoline cut is a catalytic cracked gasoline.
- the gasoline cut has an initial boiling temperature of between 35°C and 100°C and a final boiling temperature of between 130 and 200°C, preferably between 150 and 170°C and more preferably between 155 and 165°C.
- the olefin content of the first cut (or first fraction making up the mixture) is between 20 and 80% by weight of said cut.
- the second hydrocarbon cut has an initial boiling temperature of approximately 160°C and the final boiling temperature of between 260 and 340°C and comprises a fraction of at least 10% by weight of hydrocarbons having a temperature boiling temperature between 220°C and its final boiling temperature.
- This second hydrocarbon cut is a light oil cut from a catalytic cracking unit (LCO or “Light Cycle Oil” according to Anglo-Saxon terminology).
- LCO catalytic cracking unit
- This second cut has an olefin content lower than that of the first cut and a total sulfur content higher than that of the first cut.
- said second fraction comprises at least 10% by weight of hydrocarbons having a boiling temperature range between 220°C and the final boiling temperature of the mixture.
- the first hydrodesulfurization step converts part of the sulfur present in the mixture into hydrogen sulfide (H 2 S). It consists of passing the mixture of hydrocarbons to be treated in the presence of hydrogen (supplied via line 4), over a hydrodesulphurization catalyst, at a temperature between 200°C and 400°C, preferably between 250°C. C and 340°C and at a pressure of between 1 and 10 MPa, preferably between 1.5 and 4 MPa.
- the liquid space velocity is generally between 1 and 10 h -1 , preferably between 2 and 5 h -1 and the H 2 /HC ratio is between 50 Nliters/liter (l/l) and 500 Nliters/liter, preferably between 100 Nliters/liter and 450 Nliters/liter, and more preferably between 150 Nliters/liter and 400 Nliters/liter.
- the H 2 /HC ratio is the ratio between the volume flow rate of hydrogen under 1 atmosphere and at 0°C and the volume flow rate of hydrocarbons.
- the effluent resulting from this hydrodesulfurization step withdrawn by line 5 comprises the mixture of partially desulfurized hydrocarbons, the residual hydrogen and the H 2 S produced by decomposition of sulfur compounds.
- This hydrodesulfurization step is carried out for example in a fixed bed or moving bed reactor.
- the catalyst used during the first hydrodesulfurization step of the hydrodesulfurization process according to the invention comprises an active metal phase deposited on a support, said active phase comprising at least one metal from group VIII of the periodic table of elements (groups 8, 9 and 10 according to the new notation of the periodic classification of the elements: Handbook of Chemistry and Physics, 76th edition, 1995-1996 ) and at least one metal from group VIB of the periodic table of elements (group 6 according to the new notation of the periodic table of elements: Handbook of Chemistry and Physics, 76th edition, 1995-1996 ).
- the active phase of said catalyst further comprises phosphorus.
- the catalyst of the first hydrodesulfurization step may also additionally contain one or more organic compounds.
- the content of metal(s) from group VIB in said catalyst of the first hydrodesulfurization step is between 4 and 40% by weight of oxide(s) of metal(s) from group VIB, preferably between 8 and 35% by weight of metal oxide(s) from group VIB, very preferably between 10 and 30% by weight of metal oxide(s) from group VIB relative to the total weight of the catalyst.
- the Group VIB metal is molybdenum or tungsten or a mixture of these two elements, and more preferably the Group VIB metal consists solely of molybdenum or tungsten.
- the Group VIB metal is most preferably molybdenum.
- the content of metal(s) from Group VIII in said catalyst of the first hydrodesulfurization step is between 1.5 and 9% by weight of oxide(s) of metal(s) from Group VIII, of preferably between 2 and 8% by weight of oxide(s) of metal(s) from Group VIII relative to the total weight of the catalyst.
- the group VIII metal is a non-noble metal from group VIII of the periodic table of elements.
- said Group VIII metal is cobalt or nickel or a mixture of these two elements, and more preferably the Group VIII metal consists solely of cobalt or nickel.
- the Group VIII metal is most preferably cobalt.
- the molar ratio of metal(s) from group VIII to metal(s) from group VIB in the catalyst in oxide form is between 0.1 and 0.8, very preferably between 0.2 and 0.6, and so even more preferred between 0.3 and 0.5.
- the phosphorus content of the catalyst of the first hydrodesulfurization step is preferably between 0.1 and 20% by weight of P 2 O 5 , more preferably between 0.2 and 15% by weight of P 2 O 5 , very preferably between 0.3 and 10% by weight of P 2 O 5 relative to the total weight of the catalyst.
- the molar ratio of phosphorus to metal(s) of group VIB in the catalyst of the first hydrodesulfurization step is greater than or equal to 0.05, preferably greater than or equal to 0.1, more preferably between 0.15 and 0.6, even more preferably between 0.15 and 0.5.
- the support of the catalyst of the first hydrodesulfurization step on which the active phase is deposited is advantageously formed of at least one porous solid in oxide form chosen from the group consisting of aluminas, silicas, silica-alumina or even titanium or magnesium oxides used alone or mixed with alumina or silica-alumina. It is preferably chosen from the group consisting of silicas, transition aluminas and silica-alumina. More preferably, said support consists solely of a transition alumina or of a mixture of transition aluminas.
- the specific surface area of the catalyst is generally between 100 and 400 m 2 /g, preferably between 150 and 300 m 2 /g.
- the catalyst of the first hydrodesulfurization step is advantageously in the form of beads, extrudates, pellets, or irregular and non-spherical agglomerates whose specific shape can result from a crushing step.
- said support is in the form of balls or extrudates.
- the catalyst of the first hydrodesulfurization step is preferably used at least partly in its sulfurized form.
- Sulfurization consists of passing a charge containing at least one sulfur compound, which once decomposed leads to the fixation of sulfur on the catalyst.
- This charge can be gaseous or liquid, for example hydrogen containing H 2 S, or a liquid containing at least one sulfur compound.
- the sulfurization step can be carried out in situ, that is to say within the process according to the invention, or ex situ, that is to say in a unit dedicated to the sulfurization of catalysts.
- the process comprises a step where the H 2 S is at least partly eliminated from the effluent obtained at the end of the first hydrodesulfurization step.
- This step can be carried out using any techniques known to those skilled in the art. It can be carried out directly in the conditions in which the effluent is found at the end of this step or after the conditions have been changed in order to facilitate the elimination of at least part of the H 2 S.
- a gas/liquid separation following which the liquid effluent is sent to a stripping column while the gaseous effluent is sent to a stage of amine wash.
- the effluent from the reactor of the first hydrodesulphurization stage is sent via line 5 to a stripping column 6 which makes it possible to separate at the top of the column a gaseous flow 7 containing hydrogen and H 2 S and at the bottom an effluent containing a mixture of hydrocarbons 8 partially desulfurized and freed of H 2 S.
- a mixture of hydrocarbons is obtained having a total sulfur content of between 100 and 1000 ppm by weight, preferably between 200 and 500 ppm by weight.
- the effluent comprising the partially desulfurized hydrocarbon mixture is treated in an additional hydrodesulfurization step (HDS) aimed at improving the final desulfurization rate.
- This second step aims to transform the refractory sulfur compounds present in the mixture and which are essentially provided by the second cut implemented in the process according to the invention.
- the effluent is sent via line 8 to a hydrodesulfurization reactor 9 and is brought into contact with a hydrodesulfurization catalyst in the presence of hydrogen supplied by line 10.
- the temperature of the second HDS stage is higher than that of the first HDS stage, preferably higher by at least 5°C and even more preferably by at least 10°C.
- the second hydrodesulfurization step uses a catalyst having a selectivity in hydrodesulfurization with respect to the hydrogenation of olefins greater than the catalyst of the first hydrodesulfurization step.
- the catalyst suitable for this second hydrodesulfurization step comprises at least one metal from group VIII (groups 8, 9 and 10 according to the new notation of the periodic classification of the elements: Handbook of Chemistry and Physics, 76th edition, 1995-1996 ) and at least one metal from group VIB (group 6 according to the new notation of the periodic classification of the elements: Handbook of Chemistry and Physics, 76th edition, 1995-1996 ) on an appropriate support.
- the Group VIII metal content expressed as oxide is generally between 0.5 and 15% by weight, preferably between 1 and 10% by weight relative to the total weight of catalyst.
- the metal content of group VIB is generally between 1.5 and 60% by weight, preferably between 3 and 50% by weight per contribution to the total weight of catalyst.
- the Group VIII metal is preferably cobalt and the Group VIB metal is generally molybdenum or tungsten.
- the catalyst for the second hydrodesulfurization step further comprises phosphorus.
- the phosphorus content of said catalyst is preferably between 0.1 and 20% by weight of P 2 O 5 , more preferably between 0.2 and 15% by weight of P 2 O 5 , very preferably between 0.3 and 10% by weight of P 2 O 5 relative to the total weight of the catalyst.
- the catalyst further comprises one or more organic compounds.
- the catalyst support is usually a porous solid, such as for example alumina, silica-alumina or other porous solids, such as for example magnesia, silica or titanium oxide, alone or in combination. mixture with alumina or silica-alumina.
- a porous solid such as for example alumina, silica-alumina or other porous solids, such as for example magnesia, silica or titanium oxide, alone or in combination. mixture with alumina or silica-alumina.
- the catalyst according to the invention preferably has a specific surface area less than 200 m 2 /g, more preferably less than 180 m 2 /g, and very preferably less than 150 m 2 /g.
- the catalyst of the second hydrodesulfurization step is preferably used at least partly in its sulfurized form.
- Sulfurization consists of passing the charge containing at least one sulfur compound, which once decomposed leads to the fixation of sulfur on the catalyst.
- This charge can be gaseous or liquid, for example hydrogen containing H 2 S, or a liquid containing at least one sulfur compound.
- the sulfurization step can be carried out in situ, that is to say within the process according to the invention, or ex situ, that is to say in a unit dedicated to the sulfurization of catalysts.
- the desulfurized effluent has a total sulfur content generally less than 50 ppm by weight, preferably less than 30 ppm by weight and has a mercaptan content generally less than 10 ppm by weight.
- the effluent which is withdrawn from the second hydrodesulfurization reactor 9 is sent via line 11 to a separation unit 12.
- the effluent from the reactor is first sent to a balloon gas/liquid separation allowing the separation of a gas rich in H 2 S from the liquid effluent.
- This liquid effluent is then sent to a stabilization column in order to eliminate the last traces of solubilized H 2 S and produce a stabilized column bottom product, that is to say whose vapor pressure has been corrected by elimination of the lightest hydrocarbon compounds.
- the gas/liquid separation and stabilization steps are classic steps for those skilled in the art and are not shown on the figure 1 .
- the separation or distillation step consists of separating the stabilized effluent containing the mixture of hydrocarbons into at least two hydrocarbon cuts, namely a cut light hydrocarbons and a heavy hydrocarbon cut both desulfurized.
- the cutting point is generally between 160°C and 220°C, limits included.
- the separation unit used is a distillation column configured to separate at the top of the column a light desulphurized cut 13, equivalent to a gasoline cut and at the bottom a heavy desulphurized cut 14 equivalent to a distillate cut.
- the gasoline cut is sent to the gasoline pool and the desulfurized distillate cut is sent to the diesel, kerosene or fuel oil pool.
- the sulfur content in the desulfurized light cut is less than 50 ppm by weight, preferably less than 30 ppm by weight and even more preferably less than 10 ppm by weight.
- the sulfur content in the desulfurized heavy cut is less than 50 ppm by weight, optionally less than 30 ppm by weight or even less than 10 ppm by weight.
- distillate cut is recovered at the bottom, the gasoline cut is withdrawn laterally several trays below the top tray while the lightest compounds are eliminated at the top of the column in the gaseous effluent.
- the effluent from the stabilization column containing the desulfurized hydrocarbon mixture is separated into three cuts.
- the two cutting points will generally be around 160°C and around 220°C.
- the three hydrocarbon cuts have a total sulfur content of less than 50 ppm by weight, preferably less than 30 ppm by weight and even more preferably less than 10 ppm by weight.
- a first cut of gasoline type hydrocarbons is sent to a pretreatment reactor 15 before being mixed with a second cut of hydrocarbons.
- the hydrocarbon feedstock is preferably a catalytically cracked gasoline cut which generally contains diolefins at a content of between 0.1 and 3% by weight.
- the pretreatment consists of a step of selective hydrogenation of the diolefins into corresponding mono-olefins, which is carried out in the presence of a catalyst and hydrogen.
- the catalyst for selective hydrogenation of diolefins suitable for pretreatment comprises at least one metal from group VIB and at least one metal from group VIII deposited on a porous support described in the patent applications FR 2 988 732 And EP 2 161 076 of the plaintiff.
- the catalytic selective hydrogenation reaction is generally carried out in the presence of hydrogen, at a temperature between 80°C and 220°C, and preferably between 90°C and 200°C, with a liquid space velocity (LHSV) comprised between 1 and 10 h -1 , the unit of liquid space velocity being the liter of charge per liter of catalyst and per hour (l/lh).
- the operating pressure is between 0.5 MPa and 5 MPa, preferably between 1 and 4 MPa.
- the gasoline produced contains less than 0.5% by weight of diolefins, and preferably less than 0.25% by weight of diolefins.
- the first pretreated hydrocarbon cut is directed via line 16 to a separation column 17 (splitter according to Anglo-Saxon terminology) which is designed to split said pretreated charge respectively into a light fraction C5 - and a heavy fraction C6+.
- the light fraction is advantageously sent to the gasoline pool via line 18, while the heavy C6+ fraction entering line 1 is hydrodesulfurized by the process described above, that is to say mixed with a cut of middle distillate at low olefin content.
- An ⁇ hydrodesulfurization catalyst is obtained by impregnation “without excess solution” of a transition alumina in the form of beads with a specific surface area of 130 m 2 /g and a pore volume of 0.9 ml/g, by a aqueous solution containing molybdenum and cobalt in the form of ammonium heptamolybdate and cobalt nitrate.
- the catalyst is then dried and calcined in air at 500°C.
- the cobalt and molybdenum content of the ⁇ catalyst is 3% by weight of CoO and 10% by weight of MoOs.
- the catalyst is first sulfurized by treatment for 4 hours under a pressure of 3.4 MPa at 350°C, in contact with a charge consisting of 2% by weight of sulfur in the form of dimethyldisulfide in n-heptane.
- the treated feed C is a catalytic cracked gasoline whose initial boiling point is 61°C and the final point is 162°C. Its sulfur content is 765 ppm by weight and its bromine index (IBr) is 75.9 g/100 g, which corresponds approximately to 42% by weight of olefins.
- This charge C is treated with the catalyst ⁇ , under a pressure of 2 MPa, a volume ratio of hydrogen to charge to be treated (H 2 /HC) of 300 NI/l and a VVH of 4 h -1 .
- H 2 /HC volume ratio of hydrogen to charge to be treated
- VVH VVH of 4 h -1
- the effluent is cooled and the hydrogen rich in H 2 S is separated from the liquid gasoline, and the gasoline is subjected to a stripping treatment by injection of a flow of hydrogen in order to eliminate the residual traces of H 2 S dissolved in the desulfurized gasoline.
- Table 1 shows the influence of the temperature involved on the desulfurization rates and the RON index of the desulfurized effluents.
- Table 1 Analysis of desulfurized gasoline Temperature in the hydrodesulfurization reactor, 285 °C Temperature in the hydrodesulfurization reactor, 295 °C Mercaptans, ppm weight 16 7 Total sulfur, ppm weight 25 12 Desulfurization rate, % 96.7 98.4 RON loss 6.9 8.4
- a ⁇ hydrodesulfurization catalyst in the form of extrudates with a specific surface area of 180 m 2 /g whose content (weight of oxide(s) relative to the total weight of the catalyst) in cobalt, molybdenum and phosphorus are respectively 4.4% by weight of CoO and 21.3% by weight of MoO 3 and 6.0% by weight of P 2 O 5 are placed in a fixed bed tubular hydrodesulfurization reactor.
- the catalyst is first sulfurized by treatment for 4 hours under a pressure of 2 MPa at 350°C, in contact with a filler consisting of 2% by weight of sulfur in the form of dimethyldisulfide in n-heptane.
- the treated feed D has an initial boiling point of 160°C and an end point of 269°C. Its sulfur content is 5116 ppm by weight and its bromine index (IBr) is 19.5 g/100 g which corresponds approximately to 10% by weight of olefins.
- the fraction of charge D having a boiling point between 220°C and 269°C is 26.3% by weight.
- Charge D is treated with catalyst ⁇ , at a temperature of 300°C, under a pressure of 2 MPa, with a volume ratio of hydrogen to charge to be treated (H 2 /HC) of 300 NI/I and a VVH of 4 h -1 .
- H 2 /HC volume ratio of hydrogen to charge to be treated
- the effluent is cooled, the hydrogen rich in H 2 S is separated from the liquid effluent, and the effluent is subjected to a stripping treatment by injection of a flow of hydrogen in order to eliminate the residual traces of dissolved H 2 S before being analyzed.
- Table 2 shows the desulfurization rate and the sulfur and mercaptan content of the desulfurized effluent.
- Table2 Analysis Mercaptans, ppm weight 12 Total sulfur, ppm weight 34 Desulfurization rate, % 99.3
- a charge E tested in Example 3 is a mixture containing 50% by weight of charge C and 50% by weight of charge D.
- the initial boiling point of the mixture is 61°C and the final point is 269°C. vs.
- Its sulfur content is 2512 ppm by weight and its bromine index (IBr) is 53.4 g/100 g which corresponds approximately to 29.2% by weight of olefins.
- This charge E is first treated on the catalyst ⁇ , at a temperature of 330°C, under a pressure of 2 MPa, with a volume ratio of hydrogen to charge to be treated (H 2 /HC) of 300 NI/I and a VVH from 4 a.m. -1 .
- H 2 /HC volume ratio of hydrogen to charge to be treated
- VVH VVH from 4 a.m. -1 .
- the feed E used in Example 3 is treated in a first hydrodesulfurization step on the catalyst ⁇ , at a temperature of 260°C, under a pressure of 2 MPa, with a volume ratio of hydrogen to feed to be treated (H 2 /HC) of 300 NI/I and a VVH of 4 h -1 .
- H 2 /HC volume ratio of hydrogen to feed to be treated
- VVH volume ratio of hydrogen to feed to be treated
- the feedstock F is then treated in a second hydrodesulfurization step on the ⁇ catalyst, at a temperature of 280°C, under a pressure of 2 MPa, with a volume ratio of hydrogen to feedstock to be treated (H 2 /HC) of 300. l/l and a VVH of 4 h -1 .
- H 2 /HC volume ratio of hydrogen to feedstock to be treated
- VVH volume ratio of hydrogen to feedstock to be treated
- the effluent from the second hydrodesulfurization stage is then separated into two cuts: a first cut (petrol cut) with a final boiling point of 160°C and a second cut with an initial point of 160°C.
- a first cut petrol cut
- a second cut with an initial point of 160°C.
- Table 4 References 1st cut 2nd cut 61°C-160°C 160°C-269°C Mercaptans, ppm weight 7 11 Total sulfur, ppm weight 9 42 Desulfurization rate, % 98.8 99.2 RON loss 5.9 Not applicable
- Example 4 shows that it is possible, from a mixture of hydrocarbons comprising at least a first cut of hydrocarbons having a boiling temperature of between 61° and 160°C and whose olefin content is of 42% by weight and a second cut of hydrocarbons having a boiling point between 160° and 269°C of which the fraction having a boiling point greater than 220°C is 26.3%, to obtain two desulphurized cuts whose sulfur content are respectively less than 10 ppm by weight of sulfur for the desulphurized cut having a boiling temperature between 61°C and 160°C and less than 50 ppm by weight of sulfur for the desulphurized cut having a temperature of boiling between 160° and 269°C while limiting the loss of RON index linked in particular to the hydrogenation of part of the olefins present in the mixture.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Catalysts (AREA)
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1362892A FR3014896B1 (fr) | 2013-12-18 | 2013-12-18 | Procede d'hydrodesulfuration de coupes d'hydrocarbures |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2886629A1 EP2886629A1 (fr) | 2015-06-24 |
| EP2886629B1 true EP2886629B1 (fr) | 2023-11-01 |
Family
ID=50179804
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP14306951.6A Active EP2886629B1 (fr) | 2013-12-18 | 2014-12-04 | Procédé d'hydrodesulfuration de coupes d'hydrocarbures |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US9505993B2 (zh) |
| EP (1) | EP2886629B1 (zh) |
| KR (1) | KR102276776B1 (zh) |
| CN (1) | CN104726132B (zh) |
| ES (1) | ES2968680T3 (zh) |
| FR (1) | FR3014896B1 (zh) |
| PL (1) | PL2886629T3 (zh) |
| RU (1) | RU2652982C2 (zh) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3056599B1 (fr) * | 2016-09-26 | 2018-09-28 | IFP Energies Nouvelles | Procede de traitement d'une essence par separation en trois coupes. |
| FR3099172B1 (fr) * | 2019-07-23 | 2021-07-16 | Ifp Energies Now | Procede de traitement d'une essence par separation en trois coupes |
| FR3099174B1 (fr) * | 2019-07-23 | 2021-11-12 | Ifp Energies Now | Procédé de production d'une essence a basse teneur en soufre et en mercaptans |
| FR3142487A1 (fr) * | 2022-11-30 | 2024-05-31 | IFP Energies Nouvelles | Procédé d’hydrodésulfuration de finition des essences mettant en œuvre un catalyseur à base de métaux du groupe VIB et VIII et du phosphore sur support alumine à faible surface spécifique |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3265610A (en) * | 1963-12-18 | 1966-08-09 | Inst Francais Du Petrole | Combined process for hydrocracking of hydrocarbons |
| US3968026A (en) * | 1975-04-28 | 1976-07-06 | Gulf Research & Development Company | Hydrodesulfurization process with parallel first stages in series with a unified second stage |
| US4016070A (en) * | 1975-11-17 | 1977-04-05 | Gulf Research & Development Company | Multiple stage hydrodesulfurization process with extended downstream catalyst life |
| US5346609A (en) * | 1991-08-15 | 1994-09-13 | Mobil Oil Corporation | Hydrocarbon upgrading process |
| JP4050364B2 (ja) * | 1997-09-11 | 2008-02-20 | 日揮株式会社 | 石油の処理方法および石油の処理装置 |
| FR2811328B1 (fr) | 2000-07-06 | 2002-08-23 | Inst Francais Du Petrole | Procede comprenant deux etapes d'hydrodesulfuration d'essence et une elimination intermediaire de l'h2s forme au cours de la premiere etape |
| US6623622B2 (en) * | 2000-10-10 | 2003-09-23 | Exxonmobil Research And Engineering Company | Two stage diesel fuel hydrotreating and stripping in a single reaction vessel |
| FR2837831B1 (fr) | 2002-03-29 | 2005-02-11 | Inst Francais Du Petrole | Procede de production d'hydrocarbures a faible teneur en soufre et en mercaptans |
| US20040129606A1 (en) * | 2003-01-07 | 2004-07-08 | Catalytic Distillation Technologies | HDS process using selected naphtha streams |
| US8435400B2 (en) * | 2005-12-16 | 2013-05-07 | Chevron U.S.A. | Systems and methods for producing a crude product |
| FR2935389B1 (fr) | 2008-09-04 | 2012-05-11 | Inst Francais Du Petrole | Procede d'hydrogenation selective mettant en oeuvre un catalyseur sulfure de composition specifique |
| CN101942331B (zh) * | 2009-07-09 | 2013-06-19 | 中国石油化工股份有限公司 | 汽油和柴油组合加氢方法 |
| US8617384B2 (en) | 2011-10-07 | 2013-12-31 | Uop Llc | Integrated catalytic cracking gasoline and light cycle oil hydroprocessing to maximize p-xylene production |
| FR2988732B1 (fr) | 2012-03-29 | 2015-02-06 | IFP Energies Nouvelles | Procede d'hydrogenation selective d'une essence |
| US9365781B2 (en) * | 2012-05-25 | 2016-06-14 | E I Du Pont De Nemours And Company | Process for direct hydrogen injection in liquid full hydroprocessing reactors |
-
2013
- 2013-12-18 FR FR1362892A patent/FR3014896B1/fr active Active
-
2014
- 2014-12-04 EP EP14306951.6A patent/EP2886629B1/fr active Active
- 2014-12-04 ES ES14306951T patent/ES2968680T3/es active Active
- 2014-12-04 PL PL14306951.6T patent/PL2886629T3/pl unknown
- 2014-12-15 RU RU2014150770A patent/RU2652982C2/ru active
- 2014-12-17 KR KR1020140182363A patent/KR102276776B1/ko active IP Right Grant
- 2014-12-17 US US14/572,946 patent/US9505993B2/en active Active
- 2014-12-18 CN CN201410787367.2A patent/CN104726132B/zh active Active
Also Published As
| Publication number | Publication date |
|---|---|
| FR3014896B1 (fr) | 2018-07-27 |
| ES2968680T3 (es) | 2024-05-13 |
| PL2886629T3 (pl) | 2024-03-25 |
| US9505993B2 (en) | 2016-11-29 |
| KR20150071665A (ko) | 2015-06-26 |
| US20150166907A1 (en) | 2015-06-18 |
| RU2652982C2 (ru) | 2018-05-04 |
| RU2014150770A (ru) | 2016-07-10 |
| FR3014896A1 (fr) | 2015-06-19 |
| KR102276776B1 (ko) | 2021-07-12 |
| CN104726132A (zh) | 2015-06-24 |
| EP2886629A1 (fr) | 2015-06-24 |
| CN104726132B (zh) | 2018-12-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1923452B1 (fr) | Procédé de désulfuration profonde des essences de craquage avec une faible perte en indice d'octane | |
| EP2169032B1 (fr) | Catalyseur permettant de décomposer ou d'hydrogéner au moins partiellement les composes soufres insaturés | |
| EP3018189B1 (fr) | Procede de conversion de charges petrolieres comprenant une etape de viscoreduction, une etape de maturation et une etape de separation des sediments pour la production de fiouls a basse teneur en sediments | |
| EP1849850B1 (fr) | Procédé de désulfuration d'essences oléfiniques comprenant au moins deux étapes distinctes d'hydrodésulfuration | |
| EP3299441B1 (fr) | Procede de traitement d'une essence par separation en trois coupes | |
| EP2816094B1 (fr) | Procédé de production d'une essence à basse teneur en soufre et en mercaptans | |
| EP1369468B1 (fr) | Procédé de production d'hydrocarbures à faible teneur en soufre et en azote | |
| EP2886629B1 (fr) | Procédé d'hydrodesulfuration de coupes d'hydrocarbures | |
| EP1354930A1 (fr) | Procédé de production d'hydocarbures à faible teneur en soufre et en mercaptans | |
| EP2644683A1 (fr) | Procédé d'hydrogenation selective d'une essence | |
| EP3228683B1 (fr) | Procede de traitement d'une essence | |
| EP3312260B1 (fr) | Procede d'hydrodesulfuration d'une essence olefinique | |
| EP1661965B1 (fr) | Procédé d'hydrotraitement d'une essence oléfinique comprenant une étape d'hydrogénation sélective | |
| WO2016096364A1 (fr) | Procede d'adoucissement en composes du type sulfure d'une essence olefinique | |
| WO2014013154A1 (fr) | Procede de desulfuration d'une essence | |
| EP1370627B1 (fr) | Procede de production d'essence a faible teneur en soufre | |
| FR2997415A1 (fr) | Procede de production d'une essence a basse teneur en soufre | |
| FR3099175A1 (fr) | Procédé de production d'une essence a basse teneur en soufre et en mercaptans | |
| EP4004159A1 (fr) | Procédé de production d'une essence a basse teneur en soufre et en mercaptans | |
| WO2015165664A1 (fr) | Procédé de production d'une essence a basse teneur en soufre et en mercaptans | |
| EP3283601B1 (fr) | Procede d'adoucissement en composes du type sulfure d'une essence olefinique | |
| WO2021013525A1 (fr) | Procede de traitement d'une essence par separation en trois coupes | |
| FR3130831A1 (fr) | Procédé de production d'une coupe essence légère à basse teneur en soufre | |
| WO2023117531A1 (fr) | Procede de traitement d'une essence contenant des composes soufres | |
| WO2024115276A1 (fr) | Catalyseur d'hydrodesulfuration de finition comprenant un metal du groupe vib, un metal du groupe viii et du phosphore sur support alumine alpha |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20141204 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| R17P | Request for examination filed (corrected) |
Effective date: 20160104 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: IFP ENERGIES NOUVELLES |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190425 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20230606 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602014088728 Country of ref document: DE |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20231226 Year of fee payment: 10 Ref country code: FR Payment date: 20231226 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240202 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240301 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1627227 Country of ref document: AT Kind code of ref document: T Effective date: 20231101 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240119 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240301 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240202 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240201 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240301 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20231227 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2968680 Country of ref document: ES Kind code of ref document: T3 Effective date: 20240513 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240201 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20231229 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602014088728 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20231204 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20231231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231101 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20231204 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |