EP2869968B1 - Article abrasif pour opérations de meulage à faible vitesse - Google Patents
Article abrasif pour opérations de meulage à faible vitesse Download PDFInfo
- Publication number
- EP2869968B1 EP2869968B1 EP13812896.2A EP13812896A EP2869968B1 EP 2869968 B1 EP2869968 B1 EP 2869968B1 EP 13812896 A EP13812896 A EP 13812896A EP 2869968 B1 EP2869968 B1 EP 2869968B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- bond material
- weight percent
- bonded abrasive
- abrasive
- oxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000463 material Substances 0.000 claims description 183
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 69
- 150000001875 compounds Chemical class 0.000 claims description 63
- 229910052814 silicon oxide Inorganic materials 0.000 claims description 61
- 239000002245 particle Substances 0.000 claims description 41
- 229910000272 alkali metal oxide Inorganic materials 0.000 claims description 38
- JKWMSGQKBLHBQQ-UHFFFAOYSA-N diboron trioxide Chemical compound O=BOB=O JKWMSGQKBLHBQQ-UHFFFAOYSA-N 0.000 claims description 34
- 230000008859 change Effects 0.000 claims description 33
- 229910052810 boron oxide Inorganic materials 0.000 claims description 28
- 229910000287 alkaline earth metal oxide Chemical class 0.000 claims description 23
- KKCBUQHMOMHUOY-UHFFFAOYSA-N Na2O Inorganic materials [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 claims description 22
- FUJCRWPEOMXPAD-UHFFFAOYSA-N Li2O Inorganic materials [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 claims description 21
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 18
- 229910052751 metal Inorganic materials 0.000 claims description 16
- 239000002184 metal Substances 0.000 claims description 16
- XUCJHNOBJLKZNU-UHFFFAOYSA-M dilithium;hydroxide Chemical compound [Li+].[Li+].[OH-] XUCJHNOBJLKZNU-UHFFFAOYSA-M 0.000 claims description 8
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical compound [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 claims description 8
- 239000000377 silicon dioxide Substances 0.000 claims description 3
- 229910052593 corundum Inorganic materials 0.000 claims 1
- 229910001845 yogo sapphire Inorganic materials 0.000 claims 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 37
- ODINCKMPIJJUCX-UHFFFAOYSA-N Calcium oxide Chemical compound [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 17
- 238000000034 method Methods 0.000 description 16
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 15
- 239000000292 calcium oxide Substances 0.000 description 15
- 235000019589 hardness Nutrition 0.000 description 15
- 229910001947 lithium oxide Inorganic materials 0.000 description 15
- CHWRSCGUEQEHOH-UHFFFAOYSA-N potassium oxide Chemical compound [O-2].[K+].[K+] CHWRSCGUEQEHOH-UHFFFAOYSA-N 0.000 description 15
- 239000000203 mixture Substances 0.000 description 10
- 238000012360 testing method Methods 0.000 description 10
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 9
- 239000000395 magnesium oxide Substances 0.000 description 9
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 9
- 239000006061 abrasive grain Substances 0.000 description 7
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical compound O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 description 6
- -1 for example Chemical class 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 239000011147 inorganic material Substances 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 238000005245 sintering Methods 0.000 description 4
- 229910019114 CoAl2O4 Inorganic materials 0.000 description 3
- 239000003082 abrasive agent Substances 0.000 description 3
- 229910010272 inorganic material Inorganic materials 0.000 description 3
- 239000011368 organic material Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 229910001950 potassium oxide Inorganic materials 0.000 description 3
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- QVQLCTNNEUAWMS-UHFFFAOYSA-N barium oxide Chemical compound [Ba]=O QVQLCTNNEUAWMS-UHFFFAOYSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 239000002826 coolant Substances 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 229910003460 diamond Inorganic materials 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- AKUNKIJLSDQFLS-UHFFFAOYSA-M dicesium;hydroxide Chemical compound [OH-].[Cs+].[Cs+] AKUNKIJLSDQFLS-UHFFFAOYSA-M 0.000 description 2
- FZFYOUJTOSBFPQ-UHFFFAOYSA-M dipotassium;hydroxide Chemical compound [OH-].[K+].[K+] FZFYOUJTOSBFPQ-UHFFFAOYSA-M 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 229910001092 metal group alloy Inorganic materials 0.000 description 2
- 239000005445 natural material Substances 0.000 description 2
- UFQXGXDIJMBKTC-UHFFFAOYSA-N oxostrontium Chemical compound [Sr]=O UFQXGXDIJMBKTC-UHFFFAOYSA-N 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 238000007493 shaping process Methods 0.000 description 2
- 229910001948 sodium oxide Inorganic materials 0.000 description 2
- 239000002023 wood Substances 0.000 description 2
- 229910000851 Alloy steel Inorganic materials 0.000 description 1
- 229910052582 BN Inorganic materials 0.000 description 1
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 description 1
- 241001251094 Formica Species 0.000 description 1
- 229910001209 Low-carbon steel Inorganic materials 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- QXJJQWWVWRCVQT-UHFFFAOYSA-K calcium;sodium;phosphate Chemical compound [Na+].[Ca+2].[O-]P([O-])([O-])=O QXJJQWWVWRCVQT-UHFFFAOYSA-K 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- KZHJGOXRZJKJNY-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Si]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O KZHJGOXRZJKJNY-UHFFFAOYSA-N 0.000 description 1
- YWEUIGNSBFLMFL-UHFFFAOYSA-N diphosphonate Chemical compound O=P(=O)OP(=O)=O YWEUIGNSBFLMFL-UHFFFAOYSA-N 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 238000007542 hardness measurement Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000007373 indentation Methods 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229910052863 mullite Inorganic materials 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 238000013001 point bending Methods 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 229910000601 superalloy Inorganic materials 0.000 description 1
- 239000013077 target material Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/04—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic
- B24D3/06—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic metallic or mixture of metals with ceramic materials, e.g. hard metals, "cermets", cements
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/04—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic
- B24D3/14—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic ceramic, i.e. vitrified bondings
- B24D3/18—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic ceramic, i.e. vitrified bondings for porous or cellular structure
Definitions
- abrasive articles and particularly bonded abrasive articles suitable for conducting lower speed grinding operations.
- Such an bonded abrasive article is disclosed for example in US 2008/222967 A1 .
- Abrasive tools are generally formed to have abrasive grains contained within a bond material for material removal applications.
- Superabrasive grains e.g., diamond or cubic boron nitride (CBN)
- CBN cubic boron nitride
- MCA microcrystalline alpha-alumina
- the bond material can be organic materials, such as a resin, or an inorganic material, such as a glass or vitrified material.
- bonded abrasive tools using a vitrified bond material and containing MCA grains or superabrasive grains are commercially useful for grinding.
- bonded abrasive tools particularly those utilizing a vitrified bond material, require high temperature forming processes, oftentimes on the order of 1100°C or greater, which can have deleterious effects on abrasive grains of MCA.
- the bond material can react with the abrasive grains, particularly MCA grains, and damage the integrity of the abrasives, reducing the grain sharpness and performance properties.
- the industry has migrated toward reducing the formation temperatures necessary to form the bond material in order to curb the high temperature degradation of the abrasive grains during the forming process.
- the industry continues to demand improved performance of such bonded abrasive articles.
- bonded abrasive articles which may be suitable for grinding and shaping of workpieces.
- the bonded abrasive articles of embodiments herein can incorporate abrasive particles within a vitreous bond material.
- Suitable applications for use of the bonded abrasive articles of the embodiments herein include grinding operations including for example, centerless grinding, cylindrical grinding, crankshaft grinding, various surface grinding operations, bearing and gear grinding operations, creepfeed grinding, and various toolroom applications.
- the method of forming a bonded abrasive article of an embodiment can be initiated by forming a mixture of suitable compounds and components to form a bond material.
- the bond can be formed of compounds of inorganic material, such as oxide compounds.
- one suitable oxide material can include silicon oxide (SiO 2 ).
- the bond material can be formed from not greater than about 55 wt% silicon oxide for the total weight of the bond material. In other embodiments, the content of silicon oxide can be less, such as not greater than about 54 wt%, not greater than about 53 wt%, not greater than about 52 wt%, or even not greater than about 51 wt%.
- the bond material may be formed from at least about 45 wt%, such as at least about 46 wt%, on the order of at least about 47 wt%, at least about 48 wt%, or even at least about 49 wt% silicon oxide for the total weight of the bond material. It will be appreciated that the amount of silicon oxide can be within a range between any of the minimum and maximum percentages noted above.
- the bond material can also incorporate a certain content of aluminum oxide (Al 2 O 3 ).
- the bond material can include at least about 12 wt% aluminum oxide for the total weight of the bond material.
- the amount of aluminum oxide can be at least about 14 wt%, at least about 15 wt%, or even at least about 16 wt%.
- the bond material may include an amount of aluminum oxide that is not greater than about 23 wt%, not greater than about 21 wt%, not greater than about 20 wt%, not greater than about 19 wt%, or even not greater than about 18 wt% for the total weight of the bond. It will be appreciated that the amount of aluminum oxide can be within a range between any of the minimum and maximum percentages noted above.
- the bond material can be formed from a particular ratio between the amount of silicon oxide as measured in weight percent versus the amount of aluminum oxide as measured in weight percent.
- the ratio of silica to alumina can be described by dividing the weight percent of silicon oxide by the weight percent of aluminum oxide within the bond material.
- the ratio of silicon oxide to aluminum oxide can be not greater than about 3.2. In other instances, the ratio of silicon oxide to aluminum oxide within the bond material can be not greater than about 3.1, not greater than about 3.0, or even not greater than about 2.9.
- the bond material can be formed, in certain instances, such that the ratio of weight percent of silicon oxide to the weight percent of aluminum oxide is at least about 2.2, such as at least about 2.3, such as on the order of at least about 2.4, at least about 2.5, at least about 2.6, or even at least about 2.7. It will be appreciated that the total amount of aluminum oxide and silicon oxide can be within a range between any of the minimum and maximum values noted above.
- the bond material is formed from a certain content of boron oxide (B 2 O 3 ).
- the bond material incorporates an amount of boron oxide not greater than about 17 wt%, or even not greater than about 16 wt%.
- the bond material can be formed from at least about 11 wt%, such as at least about 12 wt%, at least about 13 wt%, or even at least about 14 wt% boron oxide for the total weight of the bond material. It will be appreciated that the amount of boron oxide can be within a range between any of the minimum and maximum percentages noted above.
- the bond material can be formed such that the total content (i.e. sum) of the weight percent of boron oxide and weight percent of silicon oxide within the bond material can be not greater than about 70 wt% for the total weight of the bond material. In other instances, the total content of silicon oxide and boron oxide can be not greater than about 69 wt%, such as not greater than about 68 wt%, not greater than about 67 wt%, or even not greater than about 66 wt%.
- the total weight percent content of silicon oxide and boron oxide can be at least about 55 wt%, such as at least about 58 wt%, at least about 60 wt%, at least about 62 wt%, at least about 63 wt%, at least about 64 wt%, or even at least about 65 wt% for the total weight of the bond material. It will be appreciated that the total weight percent of silicon oxide and boron oxide within the bond material can be within a range between any of the minimum and maximum percentages noted above.
- the amount of silicon oxide can be greater than the amount of boron oxide within the bond material, as measured in weight percent.
- the amount of silicon oxide can be at least about 1.5 times greater, at least about 1.7 times greater, at least about 1.8 times greater, at least about 1.9 times greater, at least about 2.0 times greater, or even at least about 2.5 times greater than the amount of boron oxide.
- the bond material can include an amount of silicon oxide that is not greater than about 5 times greater, such as not more than about 4 times greater, not more than about 3.8 times greater, or even not more than about 3.5 times greater. It will be appreciated that the difference in the amount of silicon oxide as compared to the amount of boron oxide can be within a range between any of the minimum and maximum values noted above.
- the bond material can be formed from at least one alkali oxide compound (R 2 O), wherein R represents a metal selected from Group IA elements in the Periodic Table of Elements.
- R represents a metal selected from Group IA elements in the Periodic Table of Elements.
- the bond material can be formed from an alkaline oxide compound (R 2 O) from the group of compounds including lithium oxide (Li 2 O), sodium oxide (Na 2 O), potassium oxide (K 2 O), and cesium oxide (Cs 2 O), and a combination thereof.
- the bond material can be formed from a total content of alkali oxide compounds of not greater than about 20 wt% for the total weight of the bond material.
- the total content of alkali oxide compounds can be not greater than about 19 wt%, not greater than about 18 wt%, not greater than about 17 wt%, not greater than about 16 wt%, or even not greater than about 15 wt%.
- the total content of alkali oxide compounds within the bond material can be at least about 10 wt%, such as at least about 12 wt%, at least about 13 wt%, or even at least about 14 wt%. It will be appreciated that the bond material can include a total content of alkali oxide compounds within a range between any of the minimum and maximum percentages noted above.
- the bond material can be formed from not greater than about 3 individual alkali oxide compounds (R 2 O) as noted above. In fact, certain bond materials may incorporate not greater than about 2 alkali oxide compounds within the bond material.
- the bond material can be formed such that the individual content of any of the alkali oxide compounds is not greater than one half of the total content (in weight percent) of alkali oxide compounds within the bond material.
- the amount of sodium oxide can be greater than the content (weight percent) of lithium oxide or potassium oxide.
- the total content of sodium oxide as measured in weight percent can be greater than the sum of the contents of lithium oxide and potassium oxide as measured in weight percent.
- the amount of lithium oxide can be greater than the content of potassium oxide.
- the total amount of alkali oxide compounds as measured in weight percent forming the bond material can be less than the amount (as measured in weight percent) of boron oxide within the bond material.
- the total weight percent of alkali oxide compounds as compared to the total weight percent of boron oxide within the bond material can be within a range between about 0.9 to 1.5, such as within a range between about 0.9 and 1.3, or even within a range between about 0.9 and about 1.1.
- the bond material can be formed from a certain amount of alkali earth compounds (RO), wherein R represents an element from Group IIA of the Periodic Table of Elements.
- R represents an element from Group IIA of the Periodic Table of Elements.
- the bond material can incorporate alkaline earth oxide compounds such as calcium oxide (CaO), magnesium oxide (MgO), barium oxide (BaO), or even strontium oxide (SrO).
- the bond material can contain not greater than about 3.0 wt% alkaline earth oxide compounds for the total weight of the bond material.
- the bond material may contain less alkaline earth oxide compounds, such as on the order of not greater than about 2.8 wt%, not greater than about 2.2 wt%, not greater than about 2.0 wt%, or not greater than about 1.8 wt%. Still, according to one embodiment, the bond material may contain a content of one or more alkaline earth oxide compounds of at least about 0.5 wt%, such as at least about 0.8 wt%, at least about 1.0 wt%, or even at least about 1.4 wt% for the total weight of the bond material. It will be appreciated that the amount of alkaline earth oxide compounds within the bond material can be within a range between any of the minimum and maximum percentages noted above.
- the bond material can be formed from not greater than about 3 different alkaline earth oxide compounds.
- the bond material may contain not greater than 2 different alkaline earth oxide compounds.
- the bond material can be formed from 2 alkaline earth oxide compounds consisting of calcium oxide and magnesium oxide.
- the bond material can include an amount of calcium oxide that is greater than an amount of magnesium oxide. Furthermore, the amount of calcium oxide within the bond material may be greater than the content of any of the other alkaline earth oxide compounds present within the bond material.
- the bond material can be formed from a combination of alkali oxide compounds and alkaline earth oxide compounds such that the total content is not greater than about 20 wt% for the total weight of the bond material.
- the total content of alkali oxide compounds and alkaline earth oxide compounds within the bond material can be not greater than about 19 wt%, such as not greater than about 18 wt%, or even not greater than about 17 wt%.
- the total content of alkali oxide compounds and alkaline earth compounds present within the bond material is at least about 14 wt%, at least about 15 wt%, or even at least about 16 wt%. It will be appreciated that the bond material can have a total content of alkali oxide compounds and alkaline earth oxide compounds within a range between any of the minimum and maximum percentages noted above.
- the bond material can be formed such that the content of alkali oxide compounds present within the bond material is greater than the total content of alkaline earth oxide compounds.
- the bond material may be formed such that the ratio of total content (in weight percent) of alkali oxide compounds as compared to the total weight percent of alkaline earth oxide compounds (R 2 O:RO) is within a range between about 5:1 and about 15:1.
- the ratio of total weight percent of alkali oxide compounds to total weight percent of alkaline earth oxide compounds present within the bond material can be within a range between about 6:1 and about 14:1, such as within a range between about 7:1 and about 12:1, or even with a range between about 8:1 and about 10:1.
- the bond material can be formed from not greater than about 3 wt% phosphorous oxide for the total weight of the bond material.
- the bond material may contain not greater than about 2.5 wt%, such as not greater than about 2.0 wt%, not greater than about 1.5 wt%, not greater than about 1.0 wt%, not greater than about 0.8 wt%, not greater than about 0.5 wt%, or even not greater than about 0.2 wt% phosphorous oxide for the total weight of the bond material.
- the bond material may be essentially free of phosphorous oxide. Suitable contents of phosphorous oxide can facilitate certain characteristics and grinding performance properties as described herein.
- the bond material can be formed from not greater than a composition comprising not greater than about 1 wt% of certain oxide compounds, including for example, oxide compounds such as MnO 2 , ZrSiO 2 , CoAl 2 O 4 , and MgO.
- oxide compounds such as MnO 2 , ZrSiO 2 , CoAl 2 O 4 , and MgO.
- the bond material can be essentially free of the above identified oxide compounds.
- the process of forming the bonded abrasive article can further include the incorporation of a certain type of abrasive particles.
- the abrasive particles can include microcrystalline alumina (MCA).
- MCA microcrystalline alumina
- the abrasive particles can consist essentially of microcrystalline alumina.
- the abrasive particles can have an average particle size that is not greater than about 1050 microns.
- the average particle size of the abrasive particles can be less, such as on the order of not greater than 800 microns, not greater than about 600 microns, not greater than about 400 microns, not greater than about 250 microns, not greater than about 225 microns, not greater than about 200 microns, not greater than about 175 microns, not greater than about 150 microns, or even not greater than about 100 microns.
- the average particle size of the abrasive particles can be at least about 1 micron, such as at least about 5 microns, at least about 10 microns, at least about 20 microns, at least about 30 microns, or even at least about 50 microns, at least about 60 microns, at least about 70 microns, or even at least about 80 microns. It will be appreciated that the average particle size of the abrasive particles can be in a range between any of the minimum and maximum values noted above.
- microcrystalline alumina can be formed of grains having an average grain size that is sub-micron sized.
- the average grain size of a microcrystalline alumina can be not greater than about 1 micron, such as not greater than about 0.5 microns, not greater than about 0.2 microns, not greater than about 0.1 microns, not greater than about 0.08 microns, not greater than about 0.05 microns, or even not greater than about 0.02 microns.
- formation of the mixture which includes abrasive particles and bond material can further include the addition of other components, such as fillers, pore formers, and materials suitable for forming the finally-formed bonded abrasive article.
- pore forming materials can include but are not limited to bubble alumina, bubble mullite, hollow spheres including hollow glass spheres, hollow ceramic spheres, or hollow polymer spheres, polymer or plastic materials, organic compounds, fibrous materials including strands and/or fibers of glass, ceramic, or polymers.
- Other suitable pore forming materials can include naphthalene, PDB, shells, wood, and the like.
- the filler can include one or more inorganic materials, including for example oxides, and particularly may include crystalline or amorphous phases of zirconia, silica, titania, and a combination thereof.
- the mixture can be shaped. Suitable shaping processes can include pressing operations and/or molding operations and a combination thereof.
- the mixture can be shaped by cold pressing the mixture within a mold to form a green body.
- the green body can be sintered at a particular temperature to facilitate forming an abrasive article having a vitreous phase bond material.
- the sintering operation can be conducted at a sintering temperature that is less than about 1000° C.
- the sintering temperature can be less than about 980° C, such as less than about 950° C, and particularly within a range between about 800° C and 950° C. It will be appreciated that particularly low sintering temperatures may be utilized with the above-noted bond components such that excessively high temperatures are avoided and thus limiting the degradation of the abrasive particles during the forming process.
- the bonded abrasive body comprises a bond material having a vitreous phase material.
- the bond material can be a single phase vitreous material.
- the finally-formed bonded abrasive body can have a particular content of bond material, abrasive particles, and porosity.
- the body of the bonded abrasive article of the present invention has a porosity of at least about 42 vol% for the total volume of the bonded abrasive body.
- the amount of porosity can be greater such as at least about 43 vol%, such as at least about 44 vol%, at least about 45 vol%, at least about 46 vol%, at least about 48 vol%, or even at least about 50 vol% for the total volume of the bonded abrasive body.
- the bonded abrasive body has a porosity that is not greater than about 70 vol%, such as not greater than about 65 vol%, not greater than about 62 vol%, not greater than about 60 vol%, not greater than about 56 vol%, not greater than about 52 vol%, or even not greater than about 50 vol%.
- the bonded abrasive body may include a porosity of about 46% to about 50% of a total volume of the bonded abrasive body, such as a porosity of about 46% to about 48% of a total volume of the bonded abrasive body. It will be appreciated that the bonded abrasive body can have a porosity within a range between any of the minimum and maximum percentages noted above.
- the bonded abrasive body can have at least about 35 vol% abrasive particles for the total volume of the bonded abrasive body.
- the total content of abrasive particles can be greater, such as at least about 37 vol%, or even at least about 39 vol%.
- the bonded abrasive body can be formed such that it has not greater than about 50 vol% abrasive particles, such as not greater than about 48 vol%, or even not greater than about 46 vol% for the total volume of the bonded abrasive body. It will be appreciated that the content of abrasive particles within the bonded abrasive body can be within a range between any of the minimum and maximum percentages noted above.
- the bonded abrasive body is formed such that it contains a minor content (vol%) of bond material as compared to the content of porosity and abrasive particles.
- the bonded abrasive body can have not greater than about 15 vol% bond material for the total volume of the bonded abrasive body.
- the bonded abrasive body can be formed such that it contains not greater than about 14 vol%, not greater than about 13 vol%, or even not greater than about 12 vol% for the total volume of the bonded abrasive body.
- the bonded abrasive body can be formed such that it contains at least about 7 vol%, such as at least about 8 vol%, on the order of at least about 9 vol%, or even at least about 10 vol% bond material for the total volume of the bonded abrasive body.
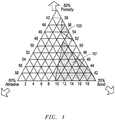
- FIG. 1 includes a diagram of phases present within a particular bonded abrasive article according to an embodiment.
- FIG. 1 includes vol% bond, vol% abrasive particles, and vol% porosity.
- the shaded region 101 represents a conventional bonded abrasive article suitable for grinding applications, while the shaded region 103 represents the phase contents of a bonded abrasive article according to an embodiment herein.
- phase content of the conventional bonded abrasive articles is significantly different from the phase content of a bonded abrasive article of an embodiment.
- conventional bonded abrasive articles typically have a maximum porosity within a range between approximately 40 vol% and 51 vol%, an abrasive particle content of approximately 42 vol% to 50 vol%, and a bond content of approximately 9 to 20 vol%.
- Conventional bonded abrasive articles typically have a maximum porosity content of 50 vol% or less because grinding applications require a bonded abrasive body having sufficient strength to deal with the excessive forces encountered during grinding, and highly porous bonded abrasive bodies have not previously been able to withstand said forces.
- a bonded abrasive article can have a considerably greater porosity than the conventional bonded abrasive articles.
- one bonded abrasive article of an embodiment can have a porosity content within a range between about 51 vol% and about 58 vol% for the total volume of the bonded abrasive body.
- a bonded abrasive article of an embodiment not covered by the scope of the present invention can have an abrasive particle content within a range between about 40 vol% and about 42 vol%, and a particularly low bond content within a range between approximately 2 vol% and about 9 vol% for the total volume of the bonded abrasive article.
- the bonded abrasive bodies of the embodiments herein can have particular characteristics unlike conventional bonded abrasive bodies.
- the bonded abrasive articles herein can have a particular content of porosity, abrasive particles, and bond, while demonstrating particular mechanical characteristics making them suitable for particular applications, such as grinding applications.
- the bonded abrasive body can have a particular modulus of rupture (MOR), which can correspond to a particular modulus of elasticity (MOE).
- MOR modulus of rupture
- MOE modulus of elasticity
- the bonded abrasive body can have a MOR of at least 45 MPa for a MOE of at least about 40 GPa.
- the MOR can be at least about 46 MPa, such as at least about 47 MPa, at least about 48 MPa, at least about 49 MPa, or even at least about 50 MPa for a MOE of 40 GPa. Still, the bonded abrasive body may have an MOR that is not greater than about 70 MPa, such as not greater than about 65 MPa, or not greater than about 60 MPa for a MOE of 40 GPa. It will be appreciated that the MOR can be within a range between any of minimum and maximum values given above.
- the MOR can be at least about 45 MPa.
- the MOR can be at least about 46 MPa, such as at least about 47 MPa, at least about 48 MPa, at least about 49 MPa, or even at least about 50 MPa.
- the MOR may be not greater than about 70 MPa, not greater than about 65 MPa, or not greater than about 60 MPa for a MOE of 45 GPa. It will be appreciated that the MOR can be within a range between any of minimum and maximum values given above.
- MOR can be measured using a standard 3 point bending test on a sample of size 4"x1"x0.5", where the load is applied across the 1"x0.5" plane, generally in accordance with ASTM D790, with the exception of the sample size.
- the failure load can be recorded and calculated back to MOR using standard equations.
- MOE can be calculated through measurement of natural frequency of the composites using a GrindoSonic instrument or similar equipment, as per standard practices in the abrasive grinding wheel industry.
- the bonded abrasive body can have a strength ratio, which is a measure of the MOR divided by the MOE.
- the strength ratio (MOR/MOE) of a particular bonded abrasive body can be at least about 0.8.
- the strength ratio can be at least about 0.9, such as at least about 1.0, at least about 1.05, at least about 1.10.
- the strength ratio may be not greater than about 3.00, such as not greater than about 2.50, not greater than about 2.00, not greater than about 1.70, not greater than about 1.50, not greater than about 1.40, or not greater than about 1.30. It will be appreciated that the strength ratio of the bonded abrasive bodies can be within a range between any of the minimum and maximum values noted above.
- the bonded abrasive body can be suitable for use in particular grinding operations.
- the bonded abrasive bodies of embodiments herein are suitable in grinding operations.
- the bonded abrasive bodies can be utilized without damaging the workpiece and providing suitable or improved grinding performance.
- references herein to the grinding capabilities of the bonded abrasive body can relate to grinding operations such as centerless grinding, cylindrical grinding, crankshaft grinding, various surface grinding operations, bearing and gear grinding operations, creepfeed grinding, and various toolroom grinding processes.
- suitable workpieces for the grinding operations can include inorganic or organic materials.
- the workpiece can include a metal, metal alloy, plastic, or natural material.
- the workpiece can include a ferrous metal, non-ferrous metal, metal alloy, metal superalloy, and a combination thereof.
- the workpiece can include an organic material, including for example, a polymer material.
- the workpiece may be a natural material, including for example, wood.
- wheel sizes of these abrasive articles may range from greater than about 11.43 cm (4.5 inches) to about 137.16 cm (54 inches) in diameter.
- Typical stock removal amounts may range from about 0.000254 cm (0.0001 inches) to about 1.27 cm (0.500 inches), depending on the application.
- the bonded abrasive body is capable of grinding workpieces at particularly high removal rates.
- the bonded abrasive body can conduct a grinding operation at a material removal rate of at least about 0.4 in 3 /min/in (258 mm 3 /min/mm).
- the material removal rate can be at least about 0.45 in 3 /min/in (290 mm 3 /min/mm), such as at least about 0.5 in 3 /min/in (322 mm 3 /min/mm), at least about 0.55 in 3 /min/in (354 mm 3 /min/mm), or even at least about 0.6 in 3 /min/in (387 mm 3 /min/mm).
- the material removal rate for certain bonded abrasive bodies may be not greater than about 1.5 in 3 /min/in (967 mm 3 /min/mm), such as not greater than about 1.2 in 3 /min/in (774 mm 3 /min/mm), not greater than about 1.0 in 3 /min/in (645 mm 3 /min/mm), or even not greater than about 0.9 in 3 /min/in (580 mm 3 /min/mm). It will be appreciated that the bonded abrasive bodies of the present application can grind a workpiece at the material removal rates within a range between any of the minimum and maximum values noted above.
- the bonded abrasive bodies of the present application can grind at a particular depth of cut (DOC).
- DOC depth of cut
- the depth of cut achieved by the bonded abrasive body can be at least about 0.003 inches (0.0762 millimeters).
- the bonded abrasive body is capable of achieving a depth of cut during grinding operations of at least about 0.004 inches (0.102 millimeters), such as at least about 0.0045 inches (0.114 millimeters), at least about 0.005 inches (0.127 millimeters), or even at least about 0.006 inches (0.152 millimeters).
- the depth of cut for grinding operations utilizing the bonded abrasive bodies herein may not be greater than about 0.01 inches (0.254 millimeters), or not great than about 0.009 inches (0.229 millimeters). It will be appreciated that the depth of cut can be within a range between any of the minimum and maximum values noted above.
- the bonded abrasive body can grind a workpiece at a maximum power that does not exceed about 10 Hp (7.5 kW), while the grinding parameters noted above are utilized.
- the maximum power during grinding operations may be not greater than about 9 Hp (6.8 kW), such as not greater than about 8 Hp (6.0 kW), or even not greater than about 7.5 Hp (5.6 kW).
- the bonded abrasive articles of the embodiments herein have superior corner holding ability, particularly as compared to conventional bonded abrasive articles.
- the bonded abrasive body can have a corner holding factor of not greater than about 0.1778 cm (0.07 inches) at an in-feed rate (Z'w) of not greater than 4.572 cm/min (1.8 inches/min), which corresponds to 0.006477 cm/sec,rad (0.00255 inches/sec,rad).
- an in-feed rate (Z'w) of 2.54 cm/min (1.0 inches/min) corresponds to 0.003607 cm/sec,rad (0.00142 inches/sec,rad)
- an in-feed rate (Z'w) of 3.556 cm/min (1.4 inches/min) correspond to 0.005029 cm/sec,rad (0.00198 inches/sec,rad).
- the corner holding factor is a measure of a change in radius after conducting 5 grinds on a workpiece of 4330V, which is a NiCrMoV hardened and tempered high strength steel alloy at a particular in-feed rate (Z'w).
- the bonded abrasive article demonstrates a corner holding factor that is not greater than about 0.1524 cm (0.06 inches), such as not greater than about 0.127 cm (0.05 inches), not greater than about 0.1016 cm (0.04 inches), for an in-feed rate (Z'w) of not greater than about 4.572 cm/min (1.80 inches/min).
- an abrasive article may include a bonded abrasive body having abrasive particles contained within a bond material.
- the bonded abrasive body may include an abrasive particle-to-bond material interfacial modulus of elasticity (MOE) of at least about 225 GPa.
- MOE interfacial modulus of elasticity
- the bonded abrasive body may be configured to grind a workpiece comprising metal at a speed of less than about 60 m/s.
- the abrasive particle-to-bond material interfacial MOE may be at least about 250 GPa, such as at least about 275 GPa, or even at least about 300 GPa.
- the abrasive particle-to-bond material interfacial MOE may be no greater than about 350 GPa, such as no greater than about 325 GPa, or even no greater than about 320 GPa.
- an abrasive article may include a bonded abrasive body having abrasive particles contained within a bond material.
- the bonded abrasive body may include an abrasive particle-to-bond material interfacial hardness of at least about 13 GPa.
- the bonded abrasive body may be configured to grind a workpiece comprising metal at a speed of less than about 60 m/s.
- the abrasive particle-to-bond material interfacial hardness may be at least about 14 GPa, or even at least about 15 GPa.
- the abrasive particle-to-bond material interfacial hardness may be no greater than about 20 GPa, such as no greater than about 18 GPa, or even no greater than about 16 GPa.
- the bonded abrasive body may include a surface finish of not greater than about 3.175 ⁇ m (125 micro-inch).
- the bonded abrasive body may perform at an in-feed rate (Z'w) of at least about 2.54 cm/min (1.0 inches/min).
- Z'w may be not greater than about 3.556 cm/min (1.4 inches/min), not greater than about 4.572 cm/min (1.8 inches/min), not greater than about 5.08 cm/min (2.0 inches/min), or even 5.588 cm/min (2.2 inches/min).
- the bonded abrasive body may include a material removal rate of at least about 3.851 cm 3 /min (0.235 in 3 /min).
- Embodiments of an abrasive article may include a bonded abrasive body having abrasive particles contained within a bond material.
- the bonded abrasive body may include a grinding factor defined as a change of x-axis radius over a change in in-feed rate.
- the grinding factor may be not greater than about 0.040 min.
- the bonded abrasive body may be configured to grind a workpiece comprising metal at a speed of less than about 60 m/s.
- the grinding factor may be not greater than about 0.035 min, such as a grinding factor not greater than about 0.030 min, or even a grinding factor not greater than about 0.028 min.
- the bonded abrasive body may include an x-axis corner holding factor of not greater than about 0.2032 cm (0.080 inches).
- the x-axis corner holding factor may be not greater than about 0.1778 cm (0.070 inches), such as not greater than about 0.1524 cm (0.060 inches), not greater than about 0.127 cm (0.050 inches), or even not greater than about 0.1067 cm (0.042 inches).
- the corner holding factor may be expressed as a percentage change in the radius of a wheel.
- the change in x-axis radius of the wheel is 2%, 1.7%, 1.4% and 1.2%, respectively.
- the bonded abrasive body may have a change in x-axis radius of no greater than 3%.
- the bonded abrasive body may have a change in x-axis radius of no greater than 2.5%, such as no greater than about 2%, no greater than about 1.7%, no greater than about 1.5%, or even no greater than about 1.3%.
- bonded abrasive body may include a grinding factor defined as a change of y-axis radius over a change in in-feed rate.
- the grinding factor may be not greater than about 0.018 min.
- Other examples of the grinding factor may be not greater than about 0.016 min, such as a grinding factor not greater than about 0.014 min, a grinding factor not greater than about 0.012 min, or even a grinding factor not greater than about 0.010 min.
- the bonded abrasive body may include a y-axis corner holding factor of not greater than about 0.08382 cm (0.033 inches), such as not greater than about 0.0762 cm (0.030 inches), not greater than about 0.0635 cm (0.025 inches), or even not greater than about 0.06096 cm (0.024 inches).
- the corner holding factor may be expressed as a percentage change in the radius of a wheel.
- the change in x-axis radius of the wheel is 0.86%, 0.71% and 0.69%, respectively.
- the bonded abrasive body may have a change in y-axis radius of no greater than about 1%.
- the bonded abrasive body may have a change in x-axis radius of no greater than about 0.9%, such as no greater than about 0.8%, or even no greater than about 0.7%.
- abrasive article may include the body requiring at least about 3% fewer dressings than a conventional OD abrasive grinding wheel, such as at least about 4% , at least about 5%, or even at least about 6% fewer dressings than a conventional OD abrasive grinding wheel.
- the body may require at least about 5% less cycle time than a conventional OD abrasive grinding wheel.
- the body may require at least about 10% less cycle time, such as at least about 15%, or even at least about 18% less cycle time than a conventional OD abrasive grinding wheel.
- Embodiments of the abrasive article may have a bonded abrasive body that can be configured to grind a workpiece comprising metal at a speed of less than about 55 m/s.
- the speed may be less than about 50 m/s, such as less than about 45 m/s, or even less than about 40 m/s.
- the speed may be at least about 35 m/s, such as at least about 40 m/s, at least about 45 m/s, or even at least about 50 m/s.
- the abrasive article may have a body including a wheel having an outer diameter in a range of about 60.96 cm (24 inches) to about 76.2 cm (30 inches), such as about 45.72 cm (18 inches) to about 76.2 cm (30 inches), about 25.4 cm (10 inches) to about 91.44 cm (36 inches), or even about 12.7 cm (5 inches) to about 137.16 cm (54 inches).
- abrasive article may include a bond material that includes a single phase vitreous material.
- Some versions of the bonded abrasive body may include a porosity of at least about 42 vol% of the total volume of the bonded abrasive body, such as a porosity of not greater than about 70 vol%.
- the bonded abrasive body may include at least about 35 vol% abrasive particles of the total volume of the bonded abrasive body. In another example, the bonded abrasive body may include not greater than about 15 vol% bond material of the total volume of the bonded abrasive body.
- the bond material may be formed from not greater than about 20 wt% boron oxide (B 2 O 3 ) for the total weight of the bond material.
- the bond material may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent aluminum oxide (Al 2 O 3 ), (SiO 2 :Al 2 O 3 ), of not greater than about 3.2.
- the bond material may be formed from not greater than about 3.0 wt% phosphorous oxide (P 2 O 5 ).
- the bond material may be essentially free of phosphorus oxide (P 2 O 5 ).
- the bond material may be formed from an alkaline earth oxide compound (RO).
- a total amount of alkaline earth oxide compound (RO) present in the bond material may be not greater than about 3.0 wt%.
- the bond material may be formed from not greater than about 3 different alkaline earth oxide compounds (RO) selected from the group of calcium oxide (CaO), magnesium oxide (MgO), barium oxide (BaO), strontium oxide (SrO).
- the bond material also may include an alkali oxide compound (R 2 O) selected from the group of compounds consisting of lithium oxide (Li 2 O), sodium oxide (Na 2 O), potassium oxide (K 2 O), and cesium oxide (Cs 2 O) and a combination thereof.
- the bond material may be formed from a total amount of alkali oxide compound (R 2 O) not greater than about 20 wt%.
- the bond material may include not greater than about 3 alkali oxide compounds (R 2 O).
- a content (wt%) of any alkali oxide compound present within the bond material may be not greater than half of a total content (wt%) of alkali oxides.
- the bond material is formed from not greater than about 55 wt% silicon oxide (SiO 2 ).
- the bond material may be formed from at least about 12 wt% aluminum oxide (Al 2 O 3 ).
- the bond material also may be formed from at least one alkali oxide compound (R 2 O) and at least one alkaline earth oxide compound (RO), wherein the total content of the alkali oxide compound and the alkaline earth oxide compound is not greater than about 20 wt%.
- Some examples of the bond may be formed from boron oxide (B 2 O 3 ) and silicon oxide (SiO 2 ), wherein the total content of boron oxide and silicon oxide may be not greater than about 70 wt%.
- the content of silicon oxide (SiO 2 ) may be greater than the content of boron oxide.
- the bond may be formed from a composition comprising not greater than about 1 wt% of oxide compounds selected from the group consisting of MnO 2 , ZrSiO 2 , CoAl 2 O 4 , and MgO.
- the bond may be formed from a composition essentially free of oxide compounds selected from the group consisting of MnO 2 , ZrSiO 2 , CoAl 2 O 4 , and MgO.
- the bonded abrasive body may be sintered at a temperature of not greater than about 1000°C.
- Embodiments of the bond material may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent aluminum oxide (Al 2 O 3 ) (SiO 2 :Al 2 O 3 ) of about 2.4 to about 3.5.
- the bond material may include a trace amount ( ⁇ 1%) of each of Fe 2 O 3 , TiO 2 and Mg, and combinations thereof.
- the bond material may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent CaO (SiO 2 :CaO) of about 32 to about 52.
- the bond material also may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent Li 2 O (SiO 2 : Li 2 O) of about 9.6 to about 26.
- the bond material may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent Na 2 O(SiO 2 :Na 2 O) of about 4.8 to about 10.4.
- the bond material may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent K 2 O(SiO 2 :K 2 O) of about 9.6 to about 26.
- the bond material also may include a ratio of weight percent silicon oxide (SiO 2 ) to weight percent B 2 O 3 (SiO 2 :B 2 O 3 ) of about 2.8 to about 5.2.
- Embodiments of the bond material may include a ratio of weight percent aluminum oxide (Al 2 O 3 ) to weight percent CaO (Al 2 O 3 :CaO) of about 10 to about 20.
- the bond material may include a ratio of weight percent aluminum oxide (Al 2 O 3 ) to weight percent Li 2 O (Al 2 O 3 : Li 2 O) of about 3 to about 10.
- the bond material also may include a ratio of weight percent aluminum oxide (Al 2 O 3 ) to weight percent Na 2 O (Al 2 O 3 :Na 2 O) of about 1.5 to about 4.
- An example of the bond material may include a ratio of weight percent aluminum oxide (Al 2 O 3 ) to weight percent K 2 O (Al 2 O 3 :K 2 O) of about 3 to about 10.
- the bond material also may include a ratio of weight percent aluminum oxide (Al 2 O 3 ) to weight percent B 2 O 3 (Al 2 O 3 :B 2 O 3 ) of about 0.9 to about 2.
- the bond material may include a ratio of weight percent CaO to weight percent Li 2 O (CaO: Li 2 O) of about 0.2 to about 0.75.
- the bond material may include a ratio of weight percent CaO to weight percent Na 2 O (CaO:Na 2 O) of about 0.1 to about 0.3.
- the bond material also may include a ratio of weight percent CaO to weight percent K 2 O (CaO:K 2 O) of about 0.2 to about 0.75.
- the bond material may include a ratio of weight percent CaO to weight percent B 2 O 3 (CaO:B 2 O 3 ) of about 0.16 to about 0.15.
- the bond material can include a ratio of weight percent Li 2 O to weight percent Na 2 O (Li 2 O:Na 2 O) of about 0.2 to about 1.
- the bond material can include a ratio of weight percent Li 2 O to weight percent K 2 O (Li 2 O:K 2 O) of about 0.4 to about 2.5.
- the bond material also can include a ratio of weight percent Li 2 O to weight percent B 2 O 3 (Li 2 O:B 2 O 3 ) of about 0.12 to about 0.5.
- a particular embodiment of the bond material may include a ratio of weight percent Na 2 O to weight percent K 2 O (Na 2 O:K 2 O) of about 1 to about 5.
- the bond material also may include a ratio of weight percent Na 2 O to weight percent B 2 O 3 (Na 2 O:B 2 O 3 ) of about 0.3 to about 1.
- the bond material can include a ratio of weight percent K 2 O to weight percent B 2 O 3 (K 2 O:B 2 O 3 ) of about 0.12 to about 0.5.
- the abrasive article may include a bonded abrasive body having abrasive particles contained within a bond material formed from not greater than about 20 wt% boron oxide (B 2 O 3 ), having a ratio of weight percent silica (SiO 2 ): weight percent alumina (Al 2 O 3 ) of not greater than about 3.2 (by weight percent) and not greater than about 3.0 wt% phosphorous oxide (P 2 O 5 ), wherein the bonded abrasive body has a porosity of at least about 42 vol% of the total volume of the bonded abrasive body.
- the bonded abrasive body may be capable of grinding a workpiece comprising metal at a speed of less than about 60 m/s.
- Embodiments of a method of grinding an abrasive article may include forming a bonded abrasive body with abrasive particles contained within a bond material, such that the bonded abrasive body comprises an abrasive particle-to-bond material interfacial modulus of elasticity (MOE) of at least about 225 GPa.
- the method may include grinding a workpiece comprising metal with the bonded abrasive body at a speed of less than about 60 m/s.
- Another embodiment of a method of grinding an abrasive article may include forming a bonded abrasive body having abrasive particles contained within a bond material, such that the bonded abrasive body comprises an abrasive particle-to-bond material interfacial hardness of at least about 13 GPa.
- the method may include grinding a workpiece comprising metal with the bonded abrasive body at a speed of less than about 60 m/s.
- Still another embodiment of the method of grinding an abrasive article can include forming a bonded abrasive body having abrasive particles contained within a bond material, such that the bonded abrasive body comprises a grinding factor defined as a change of x-axis radius over a change in in-feed rate, and the grinding factor is not greater than about 0.040 min for an in-feed rate (Z'w) of at least about 2.54 cm/min (1.0 inches/min).
- the method may include grinding a workpiece comprising metal with the bonded abrasive body at a speed of less than about 60 m/s.
- a method of grinding an abrasive article also can include forming a bonded abrasive body having abrasive particles contained within a bond material, such that the bonded abrasive body comprises a grinding factor defined as a change of y-axis radius over a change in in-feed rate, and the grinding factor is not greater than about 0.018 min for an in-feed rate (Z'w) of at least about 2.54 cm/min (1.0 inches/min).
- the method may include grinding a workpiece comprising metal with the bonded abrasive body at a speed of less than about 60 m/s.
- Still another method of grinding an abrasive article can include forming a bonded abrasive body having abrasive particles contained within a bond material formed from not greater than about 20 wt% boron oxide (B 2 O 3 ), having a ratio of weight percent silica (SiO 2 ): weight percent alumina (Al 2 O 3 ) of not greater than about 3.2 (by weight percent) and not greater than about 3.0 wt% phosphorous oxide (P 2 O 5 ), wherein the bonded abrasive body has a porosity of at least about 42 vol% of the total volume of the bonded abrasive body.
- the method may include grinding a workpiece comprising metal with the bonded abrasive body at a speed of less than about 60 m/s.
- the life or performance of a wheel in OD grinding applications may be dependent on the number of grinds it can sustain, or the number of parts that can be ground before the wheel loses its form or corner holding ability, which will also impact the part quality.
- the life of the wheel also may relate to the dressing frequency needed to generate a fresh surface for the subsequent grinding operation.
- the form holding or corner holding ability of the wheel also may be related to the ability of the bond to hold the grain and retain its goodness for the efficient grinding operation.
- abrasive wheels having 38A fused alumina abrasive particles with different bonds were tested.
- the test device was an MTS Nanoindenter XP, using a Berkovich-type indenter tip. For each sample, indents were attempted at 20 locations along a double line (see FIG.
- FIGS. 3 and 4 depict a comparison of the modulus of elasticity (MOE) and hardness, respectively, for three different bonds.
- Plots 1301, 1302, and 1303 represent the MOE of the abrasive, bond, and abrasive-to-bond interface, respectively, of a sample of the bonded abrasive articles formed according to an embodiment herein.
- This sample had a range of bond content of approximately 7 vol% to approximately 12 vol% of a total volume of the bonded abrasive body.
- this sample had a range of porosity of approximately 46 vol% to approximately 50 vol% of a total volume of the bonded abrasive body.
- a first conventional sample CS1 produced MOE values 1305, 1306, and 1307 for its abrasive, bond, and abrasive-to-bond interface, respectively.
- Sample CS1 is a bonded abrasive article commercially available as VS product from Saint Gobain Corporation.
- a second conventional sample CS2 is a bonded abrasive article commercially available as VH product from Saint Gobain Corporation.
- Sample CS2 produced MOE values 1310, 1311, and 1312 for its abrasive, bond, and abrasive-to-bond interface, respectively.
- the interface MOE 1303 of the embodiment significantly outperformed the interface MOEs 1307 and 1312 of conventional samples CS1 and CS2, respectively.
- Such results show a remarkable improvement in the MOE of the abrasive-to-bond interface of bonded abrasive articles formed according to the embodiments herein over state-of-the-art conventional bonded abrasive articles.
- plots 1401, 1402, and 1403 represent the hardness of the abrasive, bond, and abrasive-to-bond interface, respectively, of the sample of the bonded abrasive articles formed according to the embodiment of FIG. 3 .
- the first conventional sample CS1 produced hardness values 1405, 1406, and 1407 for its abrasive, bond, and abrasive-to-bond interface, respectively.
- Sample CS1 is the same as that disclosed above for FIG. 3 .
- the second conventional sample CS2 produced hardness values 1410, 1411, and 1412 for its abrasive, bond, and abrasive-to-bond interface, respectively.
- Sample CS2 is the same as that disclosed above for FIG. 3 .
- the interface hardness 1403 of the embodiment significantly outperformed the interface hardnesses 1407 and 1412 of conventional samples CS1 and CS2, respectively.
- Such results show a remarkable improvement in the hardness of the abrasive-to-bond interface of bonded abrasive articles formed according to the embodiments herein over state-of-the-art conventional bonded abrasive articles.
- the new bond has higher modulus and hardness. This is particularly significant for the weaker parts in the abrasive wheels (the bond and interface).
- the improvement in modulus and hardness of interface can help to strengthen the interface and shows that it has better connectivity with the abrasives. These designs are helpful for improving life of abrasive wheels under aggressive grinding conditions.
- the four samples were tested on a Bryant grinder in a corner holding configuration.
- the wheel speed was 50.36 m/s.
- the test material speed was 1.15 m/sec.
- the grinding mode was external plunge with a 0.254 cm (0.100-inch) width of grind.
- Each wheel was dressed with the help of a reverse plated diamond roll.
- the infeed rates were adjusted to give target material removal rates (Z'W) of 645, 903 and 1161 mm 3 /min/mm (1.0, 1.4 and 1.8 inch 3 /min/inch).
- Five consecutive radial grinds without dressing were performed on each of the test wheels at the target feed rates. Surface finish and waviness were obtained from the work material after the last grind.
- the test wheel was used to grind a Formica blank that records the wheel profile. The measurements were obtained from the blank.
- FIG. 6 includes plots of surface finish Ra versus in-feed rate (Z'w) for the three conventional bonded abrasive articles 1600, 1601 and 1602 and the embodiment of the bonded abrasive article 1605.
- the embodiment of the bonded abrasive body 1605 comprises a surface finish of not greater than about 2.159 ⁇ m (85 micro-inch) at an in-feed rate (Z'w) of 3.556 cm/min (1.4 inches/min).
- the articles 1600, 1601 and 1602 all exhibited surface finishes of at least about 3.175 ⁇ m (125 micro-inch) at an in-feed rate (Z'w) of 3.556 cm/min (1.4 inches/min).
- FIG. 7 includes plots of material removal in 5 grinds versus in-feed rate (Z'w) for the same three conventional bonded abrasive articles 1700, 1701 and 1702 and the embodiment of the bonded abrasive article 1705.
- the bonded abrasive body 1705 included a material removal rate of at least about 3.949 cm 3 /min (0.241 in 3 /min) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- the conventional articles 1700, 1701 and 1702 all exhibited material removal rates of no greater than about 3.851 cm 3 /min (0.235 in 3 /min) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- FIG. 5 A schematic diagram of the corner wear or change in radius measurements is shown in FIG. 5 .
- Dimension 1500 represents the original dimension (i.e., the axial width of 2.2225 cm (0.875 inches)) of a sample along the x-axis, while dimension 1501 represents the post-grinding dimension of the sample along the x-axis.
- dimension 1502 represents the original dimension (i.e., the diameter of 17.78 cm (7 inches)) of a sample along the y-axis, while dimension 1503 represents the post-grinding dimension of the sample along the y-axis.
- FIG. 8 includes plots of change in x-axis radius versus in-feed rate (Z'w) demonstrating a corner holding factor for the same three conventional bonded abrasive articles 1800, 1801 and 1802 and the embodiment of the bonded abrasive article 1805.
- the embodiment of the bonded abrasive body 1805 included an x-axis corner holding factor of about 0.1067 cm (0.042 inches) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- the conventional articles 1800, 1801 and 1802 all exhibited x-axis corner holding factors of at least about 0.2032 cm (0.080 inches) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- the bonded abrasive body 1805 included a grinding factor defined as a change of x-axis radius over a change in in-feed rate.
- the grinding factors are essentially the average slopes of the lines in FIG. 8 .
- 0.023 in/0.80 in/min grinding factor of about 0.029 min.
- articles 1800, 1801 and 1802 had a grinding factor of at least about 0.050 min.
- FIG. 9 includes plots of change in y-axis radius versus in-feed rate (Z'w) demonstrating a corner holding factor for the same three conventional bonded abrasive articles 1900, 1901 and 1902 and the embodiment of the bonded abrasive article 1905.
- the body 1905 exhibited a y-axis corner holding factor of about 0.06096 cm (0.024 inches) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- the articles 1900, 1901 and 1902 had y-axis corner holding factors of at least about 0.08382 cm (0.033 inches) at an in-feed rate (Z'w) of 4.572 cm/min (1.8 inches/min).
- grinding factors also were calculated based on FIG. 9 .
- 0.008 in/0.80 in/min grinding factor of about 0.01 min.
- articles 1900, 1901 and 1902 had a grinding factor of at least about 0.0188 min.
- the change in the corner radius along both the x-axis and the y-axis shows that a product with a bond in accordance with an embodiment herein shows the least amount of corner wear at all material removal rates compared to products made with conventional bond systems.
- Table 2 contains more details regarding the test conditions used in Example 3.
- Table 2 Test Conditions Units (if any) Machine Cincinnati Viking Centerless series Coolant Type Crystall 9951 Dresser RPM 4200 Radial Depth/Pass cm (in) 0.00254 (0.001) Comps (passes) / Dress 1 Total Dress Depth cm (in) 0.00254 (0.001) Wheel speed rpm 2700 Infeed Rates (R1) cm/min (in/min) 2.79908 (1.102) Infeed Rates (R2) cm/min (in/min) 1.89992 (0.748) Infeed Rates (Finish) cm/min (in/min) 0.8001 (0.315)
- FIG. 10 includes a chart of parts per dress for a conventional bonded abrasive article 2000 and the embodiment of the bonded abrasive article 2005.
- Article 2005 showed significant improvement in parts per dress (about 7% improved) with a good surface finish or form, compared to article 2000.
- FIG. 11 is a chart of cycle time for the conventional bonded abrasive article 2100 and the embodiment of the bonded abrasive article 2105.
- Article 2105 showed a significant (approximately 18%) improvement over article 2100.
- the foregoing embodiments are directed to abrasive products, and particularly bonded abrasive products, which represent a departure from the state-of-the-art.
- the bonded abrasive products of the embodiments herein utilize a combination of features that facilitate improved grinding performance.
- the bonded abrasive bodies of the embodiments herein utilize a particular amount and type of abrasive particles, particular amount and type of bond material, and have a particular amount of porosity.
- the bonded abrasives of the present embodiments are capable of operating at lower speeds during grinding operations despite having significantly higher porosity than conventional grinding wheels.
- the bonded abrasive bodies of the embodiments herein demonstrated a capability of operating at wheel speeds of less than about 60 m/s, while also demonstrating improved material removal rates, improved corner holding ability, and suitable surface finish as compared to state-of-the-art grinding wheels.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Ceramic Engineering (AREA)
- Inorganic Chemistry (AREA)
- Polishing Bodies And Polishing Tools (AREA)
Claims (14)
- Article abrasif comprenant :un corps abrasif lié ayant des particules abrasives d'alumine microcristalline contenues dans un matériau de liaison, dans lequel le corps abrasif lié comprend un module d'élasticité interfaciale (MOE) de particules abrasives à matériau de liaison d'au moins 225 GPa ; etdans lequel le corps abrasif lié comprend une teneur en liant d'au moins 7 % en volume et non supérieure à 12 % en volume pour un volume total du corps abrasif lié, une teneur en porosité d'au moins 42 % en volume et non supérieure à 70 % en volume pour le volume total du corps abrasif lié et une teneur en particules abrasives d'au moins 35 % en volume et non supérieure à 50 % en volume pour le volume total du corps abrasif lié ; etdans lequel le matériau de liaison comprend une teneur totale en une combinaison de composés d'oxyde alcalin (R2O) et de composés d'oxyde alcalino-terreux (RO) d'au moins 14 % en poids pour un poids total de matériau de liaison et une teneur en un oxyde de bore (B2O3) d'au moins 11 % en poids et non supérieure à 17 % en poids du poids total du matériau de liaison.
- Article abrasif selon la revendication 1, dans lequel le corps abrasif lié est configuré pour meuler une pièce à usiner comprenant du métal à une vitesse inférieure à environ 60 m/s.
- Article abrasif selon la revendication 1, dans lequel le corps abrasif lié comprend une dureté interfaciale de particules abrasives à matériau de liaison d'au moins 13 GPa et non supérieure à 20 GPa.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison est formé d'au moins un composé d'oxyde alcalin (R20) et d'au moins un composé d'oxyde alcalino-terreux (RO), dans lequel une teneur totale du composé d'oxyde alcalin et du composé d'oxyde alcalino-terreux ne dépasse pas 20 % en poids pour un poids total du matériau de liaison.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids d'oxyde de silicium (SiO2) et le pourcentage en poids de Li2O (SiO2: Li2O) de 9,6 à 26.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids d'oxyde de silicium (SiO2) et le pourcentage en poids de Na2O (SiO2:Na2O) de 4,8 à 10,4.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids d'oxyde de silicium (SiO2) et le pourcentage en poids de K2O (SiO2:K2O) de 9,6 à 26.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport du pourcentage en poids d'oxyde de silicium (SiO2) au pourcentage en poids de B2O3 (SiO2:B2O3) de 2,8 à 5,2.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids de Li2O au pourcentage en poids de Na2O (Li2O:Na2O) de 0,2 à 1.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids de Li2O et le pourcentage en poids de K2O (Li2O:K2O) de 0,4 à 2,5.
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport entre le pourcentage en poids de Na2O et le pourcentage en poids de K2O (Na2O:K2O) de 1 à 5.
- Article abrasif selon la revendication 1, dans lequel le corps abrasif lié comprend un facteur de meulage défini comme un changement de largeur axiale sur un changement de vitesse d'alimentation et le facteur de meulage n'est pas supérieur à 0,040 min pour une vitesse d'alimentation (Z'w) d'au moins environ 2,54 cm/min (1,0 pouce/min).
- Article abrasif selon la revendication 1, dans lequel le corps abrasif lié comprend un facteur de meulage défini comme un changement de diamètre sur un changement de vitesse d'alimentation et le facteur de meulage n'est pas supérieur à 0,018 min pour une vitesse d'alimentation (Z'w) d'au moins environ 2,54 cm/min (1,0 pouce/min).
- Article abrasif selon la revendication 1, dans lequel le matériau de liaison comprend un rapport du pourcentage en poids de silice au pourcentage en poids d'alumine, (SiO2:Al2O3), non supérieur à 3,2 et pas plus de 3,0% en poids d'oxyde de phosphore (P2O5).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP21174364.6A EP3900878A3 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261668860P | 2012-07-06 | 2012-07-06 | |
| US201261677655P | 2012-07-31 | 2012-07-31 | |
| PCT/US2013/049251 WO2014008356A1 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP21174364.6A Division EP3900878A3 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2869968A1 EP2869968A1 (fr) | 2015-05-13 |
| EP2869968A4 EP2869968A4 (fr) | 2016-06-08 |
| EP2869968B1 true EP2869968B1 (fr) | 2021-05-19 |
Family
ID=49877453
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP21174364.6A Pending EP3900878A3 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
| EP13812896.2A Active EP2869968B1 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP21174364.6A Pending EP3900878A3 (fr) | 2012-07-06 | 2013-07-03 | Article abrasif pour opérations de meulage à faible vitesse |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20140007517A1 (fr) |
| EP (2) | EP3900878A3 (fr) |
| JP (1) | JP5921772B2 (fr) |
| KR (1) | KR101704416B1 (fr) |
| CN (1) | CN104640675B (fr) |
| AR (1) | AR091657A1 (fr) |
| BR (1) | BR112015000164B1 (fr) |
| IL (1) | IL236438B (fr) |
| IN (1) | IN2015DN00417A (fr) |
| MX (1) | MX2015000143A (fr) |
| RU (1) | RU2603515C2 (fr) |
| TW (1) | TWI535535B (fr) |
| WO (1) | WO2014008356A1 (fr) |
| ZA (1) | ZA201500148B (fr) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104937113B (zh) | 2013-01-25 | 2021-07-30 | 巴塞罗那大学 | 用于预测由基于抗精神病药物的治疗诱导的锥体外系症状(eps)的发病的方法 |
| CN107107314B (zh) | 2014-12-30 | 2022-07-01 | 圣戈班磨料磨具有限公司 | 研磨制品及其形成方法 |
| US9982175B2 (en) * | 2014-12-30 | 2018-05-29 | Saint-Gobain Abrasives, Inc. | Abrasive articles and methods for forming same |
| IL298939A (en) | 2020-07-10 | 2023-02-01 | Saint Gobain Abrasives Inc | Bonded abrasive and method for its production |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008112899A2 (fr) * | 2007-03-14 | 2008-09-18 | Saint-Gobain Abrasives, Inc. | Article abrasif lié et procédé de fabrication |
| US20080222965A1 (en) * | 2007-03-14 | 2008-09-18 | Saint-Gobain Abrasives, Inc. | Bonded abrasive article and method of making |
| US20110131889A1 (en) * | 2009-12-02 | 2011-06-09 | Saint-Gobain Abrasives, Inc. | Bonded abrasive article and method of forming |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5035723A (en) * | 1989-04-28 | 1991-07-30 | Norton Company | Bonded abrasive products containing sintered sol gel alumina abrasive filaments |
| US5203886A (en) * | 1991-08-12 | 1993-04-20 | Norton Company | High porosity vitrified bonded grinding wheels |
| US5401284A (en) * | 1993-07-30 | 1995-03-28 | Sheldon; David A. | Sol-gel alumina abrasive wheel with improved corner holding |
| US5536283A (en) * | 1993-07-30 | 1996-07-16 | Norton Company | Alumina abrasive wheel with improved corner holding |
| US5738697A (en) * | 1996-07-26 | 1998-04-14 | Norton Company | High permeability grinding wheels |
| US5863308A (en) * | 1997-10-31 | 1999-01-26 | Norton Company | Low temperature bond for abrasive tools |
| JP4582671B2 (ja) * | 1997-12-16 | 2010-11-17 | 株式会社ディスコ | 研削ホイール及び該研削ホイールを搭載した研削装置 |
| US6056795A (en) * | 1998-10-23 | 2000-05-02 | Norton Company | Stiffly bonded thin abrasive wheel |
| JP2000271854A (ja) * | 1999-03-25 | 2000-10-03 | Hitachi Ltd | 加工方法及びその装置並びに半導体基板の加工方法 |
| US6123744A (en) * | 1999-06-02 | 2000-09-26 | Milacron Inc. | Vitreous bond compositions for abrasive articles |
| CA2402279C (fr) * | 2000-03-23 | 2006-01-31 | Saint-Gobain Abrasives, Inc. | Outils abrasifs colles vitrifies |
| US7544114B2 (en) * | 2002-04-11 | 2009-06-09 | Saint-Gobain Technology Company | Abrasive articles with novel structures and methods for grinding |
| US6988937B2 (en) * | 2002-04-11 | 2006-01-24 | Saint-Gobain Abrasives Technology Company | Method of roll grinding |
| RU2240914C1 (ru) * | 2003-04-23 | 2004-11-27 | Федеральное государственное унитарное предприятие "НПО "ТЕХНОМАШ" | Абразивный инструмент |
| DE602004010849T3 (de) * | 2003-12-23 | 2014-01-09 | Diamond Innovations, Inc. | Verfahren zum schleifen von rollen |
| TWI470069B (zh) * | 2011-03-31 | 2015-01-21 | Saint Gobain Abrasives Inc | 用於高速磨削操作之磨料物品 |
-
2013
- 2013-07-01 TW TW102123548A patent/TWI535535B/zh not_active IP Right Cessation
- 2013-07-02 AR ARP130102363 patent/AR091657A1/es unknown
- 2013-07-03 IN IN417DEN2015 patent/IN2015DN00417A/en unknown
- 2013-07-03 US US13/934,714 patent/US20140007517A1/en not_active Abandoned
- 2013-07-03 WO PCT/US2013/049251 patent/WO2014008356A1/fr active Application Filing
- 2013-07-03 EP EP21174364.6A patent/EP3900878A3/fr active Pending
- 2013-07-03 CN CN201380042495.8A patent/CN104640675B/zh active Active
- 2013-07-03 KR KR1020157002204A patent/KR101704416B1/ko active IP Right Grant
- 2013-07-03 EP EP13812896.2A patent/EP2869968B1/fr active Active
- 2013-07-03 JP JP2015520675A patent/JP5921772B2/ja active Active
- 2013-07-03 RU RU2015102911/02A patent/RU2603515C2/ru not_active IP Right Cessation
- 2013-07-03 BR BR112015000164-5A patent/BR112015000164B1/pt active IP Right Grant
- 2013-07-03 MX MX2015000143A patent/MX2015000143A/es unknown
-
2014
- 2014-12-24 IL IL236438A patent/IL236438B/en active IP Right Grant
-
2015
- 2015-01-09 ZA ZA2015/00148A patent/ZA201500148B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008112899A2 (fr) * | 2007-03-14 | 2008-09-18 | Saint-Gobain Abrasives, Inc. | Article abrasif lié et procédé de fabrication |
| US20080222965A1 (en) * | 2007-03-14 | 2008-09-18 | Saint-Gobain Abrasives, Inc. | Bonded abrasive article and method of making |
| US20110131889A1 (en) * | 2009-12-02 | 2011-06-09 | Saint-Gobain Abrasives, Inc. | Bonded abrasive article and method of forming |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2603515C2 (ru) | 2016-11-27 |
| AR091657A1 (es) | 2015-02-18 |
| IN2015DN00417A (fr) | 2015-06-19 |
| US20140007517A1 (en) | 2014-01-09 |
| JP5921772B2 (ja) | 2016-05-24 |
| IL236438B (en) | 2018-01-31 |
| CN104640675A (zh) | 2015-05-20 |
| MX2015000143A (es) | 2015-05-07 |
| BR112015000164A2 (pt) | 2017-06-27 |
| ZA201500148B (en) | 2015-12-23 |
| KR20150036225A (ko) | 2015-04-07 |
| KR101704416B1 (ko) | 2017-02-08 |
| EP2869968A1 (fr) | 2015-05-13 |
| JP2015521963A (ja) | 2015-08-03 |
| CN104640675B (zh) | 2017-05-24 |
| EP3900878A2 (fr) | 2021-10-27 |
| WO2014008356A1 (fr) | 2014-01-09 |
| BR112015000164B1 (pt) | 2021-01-12 |
| EP2869968A4 (fr) | 2016-06-08 |
| EP3900878A3 (fr) | 2022-03-09 |
| TWI535535B (zh) | 2016-06-01 |
| IL236438A0 (en) | 2015-02-26 |
| TW201402279A (zh) | 2014-01-16 |
| RU2015102911A (ru) | 2016-08-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8721751B2 (en) | Bonded abrasive article and method of forming | |
| US8999026B2 (en) | Bonded abrasive article and method of forming | |
| EP2869968B1 (fr) | Article abrasif pour opérations de meulage à faible vitesse | |
| CA2830841C (fr) | Article abrasif pour des operations de fraisage a haute vitesse | |
| EP3683018B1 (fr) | Procédé de formation d'un corps abrasif lié | |
| JPH04322969A (ja) | ダイヤモンド砥石のツルーイング・ドレッシング方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20150122 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20160506 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C09K 3/14 20060101ALI20160429BHEP Ipc: C09C 1/68 20060101ALI20160429BHEP Ipc: B24D 3/02 20060101ALI20160429BHEP Ipc: B24D 3/06 20060101ALI20160429BHEP Ipc: B24D 3/20 20060101AFI20160429BHEP Ipc: C09G 1/02 20060101ALI20160429BHEP |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190108 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602013077560 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: B24D0003200000 Ipc: B24D0003180000 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B24D 3/06 20060101ALI20201030BHEP Ipc: B24D 3/18 20060101AFI20201030BHEP Ipc: B24D 3/02 20060101ALI20201030BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20201204 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602013077560 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1393526 Country of ref document: AT Kind code of ref document: T Effective date: 20210615 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210819 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210820 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210919 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210819 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210920 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602013077560 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20210731 |
|
| 26N | No opposition filed |
Effective date: 20220222 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210731 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210731 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210919 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210703 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210703 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210731 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 1393526 Country of ref document: AT Kind code of ref document: T Effective date: 20210519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20130703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230530 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20230622 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20230622 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602013077560 Country of ref document: DE Representative=s name: KRAUS & LEDERER PARTGMBB, DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240620 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20240619 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CZ Payment date: 20240625 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20240619 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210519 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20240619 Year of fee payment: 12 |