EP1273652A1 - Fuel additive and fuel compositon containing the same - Google Patents
Fuel additive and fuel compositon containing the same Download PDFInfo
- Publication number
- EP1273652A1 EP1273652A1 EP02254662A EP02254662A EP1273652A1 EP 1273652 A1 EP1273652 A1 EP 1273652A1 EP 02254662 A EP02254662 A EP 02254662A EP 02254662 A EP02254662 A EP 02254662A EP 1273652 A1 EP1273652 A1 EP 1273652A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- oxide
- fuel
- alkylene
- amide
- fuel additive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/224—Amides; Imides carboxylic acid amides, imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/221—Organic compounds containing nitrogen compounds of uncertain formula; reaction products where mixtures of compounds are obtained
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/06—Use of additives to fuels or fires for particular purposes for facilitating soot removal
Definitions
- the present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide.
- the present invention relates to the use of the fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel, to enhance the acceleration response and the driving performance of internal combustion engines, such as gasoline or diesel engines.
- hydrocarbon-based fuels include alcohol (e.g. methanol and ethanol), ether (e.g. methyl-t-butyl ether), and ketone (e.g. acetone).
- alcohol e.g. methanol and ethanol
- ether e.g. methyl-t-butyl ether
- ketone e.g. acetone
- additives such as hydrazine or nitro compounds (for example nitromethane including nitropropane and nitroparaffin, or nitrobenzene) have been examined for automobile racing.
- nitromethane including nitropropane and nitroparaffin, or nitrobenzene have been examined for automobile racing.
- the problem with using such additives is that they often have an adverse effect on the durability of the engine and its components.
- organometallic compounds e.g. tetraethyl lead or similar lead alkyls:ferrocene, methyl cyclopentadienyl manganese tricarbonyl
- aromatic amine compounds e.g. aniline, monomethyl aniline, or dimethyl aniline
- Japanese Patent Application Number (Kokai) 58-104996 (corresponding to US Patent Number 4,409,000) describes the use of an alkyl amine or ethylene-oxide-adducted alkenyl amine as an additive in automobile fuel to clean carburetors and engines.
- solubility in water as well as the engine performance can be improved by adding fatty acid diethanol amide, fatty acid ethoxylate and alcohol ethoxylate to a liquid fuel such as gasoline or diesel fuel.
- the present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide.
- the present invention relates to the use of the fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel, to enhance the acceleration response and the driving performance of internal combustion engines, such as gasoline or diesel engines.
- the present invention relates to a fuel additive comprising an alkylene-oxide-adducted hydrocarbyl amide having from 3 to 50 moles of alkylene oxide per mole of hydrocarbyl amide.
- the present invention relates to a fuel composition
- a fuel composition comprising a major amount of a hydrocarbon boiling in the gasoline or diesel range and, from 10 to 10,000 ppm weight per weight of fuel, of the fuel additive of the present invention.
- the present invention relates to a method of operating an automobile engine with the fuel composition of the present invention.
- the present invention relates to a method of improving the acceleration performance of a gasoline automobile engine comprising additizing the fuel additive of the present invention to a gasoline and operating the engine with the additized gasoline.
- the present invention is based on the discovery that certain alkylene-oxide-adducted hydrocarbyl amides are surprisingly useful for improving the acceleration response and the driving performance of internal combustion engines when used as fuel additives in hydrocarbon-based fuels, such as gasoline fuel or diesel fuel.
- the present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide and its use as a fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel.
- hydrocarbyl refers to an organic radical primarily composed of carbon and hydrogen which may be aliphatic, alicyclic, aromatic or combinations thereof, e.g., aralkyl or alkaryl. Such hydrocarbyl groups may also contain aliphatic unsaturation, i.e., olefinic or acetylenic unsaturation, and may contain minor amounts of heteroatoms, such as oxygen or nitrogen, or halogens, such as chlorine. When used in conjunction with carboxylic fatty acids, hydrocarbyl will also include olefinic unsaturation.
- alkyl refers to both straight- and branched-chain alkyl groups.
- lower alkyl refers to alkyl groups having 1 to about 6 carbon atoms and includes primary, secondary and tertiary alkyl groups.
- Typical lower alkyl groups include, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, t-butyl, n-pentyl, n-hexyl and the like.
- alkenyl refers to an alkyl group with unsaturation.
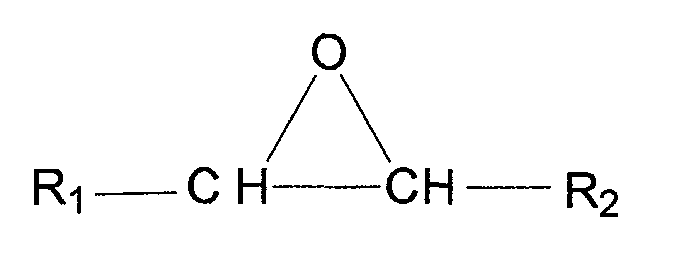
- alkylene oxide refers to a compound having the formula: wherein R 1 and R 2 are each independently hydrogen or lower alkyl having from 1 to 6 carbon atoms.
- fuel or "hydrocarbon-based fuel” refers to normally liquid hydrocarbons having boiling points in the range of gasoline and diesel fuels.
- the present invention involves a fuel additive comprising an alkylene-oxide-adducted hydrocarbyl amide having from 3 to 50 moles, preferably from 3 to 20 moles, more preferably from 4 to 15 moles, of alkylene oxide per mole of hydrocarbyl amide.
- the alkylene-oxide-adducted hydrocarbyl amide of the present invention is derived from an alkyl amide having from 4 to 75, preferably from 8 to 22, carbon atoms or alkenyl amide with at least one or two points of unsaturation having from 4 to 75, preferably from 8 to 22, carbon atoms.
- alkyl amides suitable for the present invention include, but are not limited to, octyl amide (capryl amide), nonyl amide, decyl amide (caprin amide), undecyl amide dodecyl amide (lauryl amide), tridecyl amide, teradecyl amide (myristyl amide), pentadecyl amide, hexadecyl amide (palmityl amide), heptadecyl amide, octadecyl amide (stearyl amide), nonadecyl amide, eicosyl amide (alkyl amide), or docosyl amide (behenyl amide).
- alkenyl amides include, but are not limited to, palmitoolein amide, oleyl amide, isooleyl amide, elaidyl amide, linolyl amide, linoleyl amide.
- the alkyl or alkenyl amide is a coconut oil fatty acid amide.
- the alkylene oxide adducted to the hydrocarbyl amide of the present invention is derived from an alkylene group having from 2 to 5 carbon atoms.
- the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, and pentylene oxide. Ethylene oxide and propylene oxide are particularly preferred.
- mixtures of alkylene oxides are desirable in which, for example, a mixture of ethylene oxide and propylene oxide may be used to form the alkylene-oxide-adducted hydrocarbyl amide of the present invention.
- a respective molar ratio of from 1:5 to 5:1 may be used in the case of a mixture of ethylene oxide and propylene oxide.
- a desirable number of moles of the alkylene oxide to be adducted to the hydrocarbyl amide will be in the range of from 3 to 50 moles of alkylene oxide per 1 mole of hydrocarbyl amide. More preferably, the range of from 3 to 20 moles is particularly desirable. Most preferably, the range of from 4 to 15 moles is most preferable as a molar range of the additive.
- the alkylene-oxide adducted hydrocarbon amide is derived from an alkylene-oxide-adduction reaction involving a coconut oil fatty acid amide with ethylene oxide and propylene oxide.
- the alkylene-oxide adducted hydrocarbyl amides useful as fuel additives in the present invention can be also a mixed product wherein various types and different moles of alkylene oxide and can be adducted to various types of hydrocarbyl amides.
- the present invention provides for a method of operating gasoline engine automobiles wherein an automobile equipped with a gasoline engine is operated with the fuel composition of the present invention.
- the method of operating gasoline engine automobiles is preferred when the amount of alkylene oxide is from 3 to 20 moles per mole of hydrocarbyl amide and the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- the present invention further provides for a method of improving the driving and acceleration performance of internal combustion engines, such as a gasoline or diesel engines in automobiles, by using the fuel additive described herein.
- the fuel additive of the present invention improves acceleration performance of internal combustion engines when the fuel additive is added to a low boiling point hydrocarbon-based fuel like gasoline, and the driving performance is also improved when the additive is added to other hydrocarbon-based fuel like a diesel fuel, alcohol fuel or ether fuel.
- the method of improving acceleration performance in gasoline engine automobiles is preferred when the amount of alkylene oxide is from 3 to 20 moles per mole of hydrocarbyl amide and the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- the amount of fuel additive of the present invention added in a hydrocarbon-based fuel will typically be in a range of from 10 to 10,000 ppm weight per weight (active component ratio). More preferably, the desired range is from 10 to 5,000 ppm weight per weight, while a range of from 10 to 1,000 ppm weight per weight is most preferable.
- the fuel additive of the present invention is normally supplied as an organic solvent solution with an effective fuel additive content of at least 30 weight %, based on the amount of the fuel additive and organic solvent solution.
- a concentrated fuel additive product may be prepared which contained at least 30 weight % of the active component. This product can be added according to any selected method including adding it into the fuel tank of a fuel station or into the fuel tank of a passenger car.
- the fuel additive of the present invention can also be combined with one, two, or more other additives publicly known to be used in hydrocarbon-based fuels.
- additives include, but are not limited to, deposit control additives such as detergents or dispersants, corrosion inhibitors, oxidation inhibitors, metal deactivators, corrosion inhibitors, demulsifiers, static electricity preventing agents, anti-coagulation agents, anti-knock agents, oxygenates, flow improvers, pour point depressants, cetane improvers and auxiliary-solution agents.
- a Toyota Camry 1800cc, 5MT (Type E-SV40, provided with Knock Sensor, type 4S-FE engine), mounted on a chassis dynamometer, was operated at a constant speed of 20 km/hr.
- the acceleration measurement was initiated by fully opening the throttle and measuring the amount of time required for the vehicle speed to reach 110 km/hr with the transmission locked in fourth gear. This measurement was repeated 10 times using the same fuel and the median value obtained from these 10 measurements was determined as the acceleration time period.
- the entire test procedure was executed within a single day.
- the gasoline used have the following specifications: density (at 15°C): 0.7389 g/cm 3 , Reid vapor pressure: 60.5 Kpa, octane number: 90.2 (RON), 82.3 (MON), aromatics (volume %): 29.9, olefin (volume %): 15.6, 10% distillation temperature (°C): 50.0, 50% distillation temperature (°C): 92.0, 90% distillation temperature (°C): 169.5.
- the fuel composition was adjusted by adding 100mg/L of 5 moles of oleyl amide-ethylene oxide (fuel additive) to this gasoline.
- Example 2 The test was carried out as described in Example 1, using four moles of propylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) was added at a concentration of 100 mg/L to this gasoline in order to prepare a fuel composition containing the fuel additive.
- fuel additive propylene oxide adducted coconut oil fatty acid di-ethanol amide
- Example 2 The test was carried out as described in Example 1, using ten moles of propylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) was added to provide a concentration of 100 mg/L in this gasoline in order to prepare a fuel composition containing the fuel additive.
- fuel additive propylene oxide adducted coconut oil fatty acid di-ethanol amide
- Example 2 The test was carried out as described in Example 1 except the gasoline used had the following specifications: density 9 at 15°C: 0.7303 g/cm 3 , Reid vapor pressure: 60.2Kpa, octane number: 92.1 (RON), aromatics (volume %): 23.19, olefin (volume %) 19, 10% distillation temperature (°C): 54.3, 50% distillation temperature (°C): 86.2, 90% distillation temperature (°C): 158.1 and using four moles of propylene oxide and two moles of ethylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) were added to provide a concentration of 100 mg/L in this gasoline.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Combustion & Propulsion (AREA)
- Liquid Carbonaceous Fuels (AREA)
Abstract
Description
- The present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide. In a further aspect the present invention relates to the use of the fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel, to enhance the acceleration response and the driving performance of internal combustion engines, such as gasoline or diesel engines.
- In order to increase engine output power and acceleration response of spark ignition engines in automobiles, various types of oxygen-containing additives for hydrocarbon-based fuel have been investigated. These hydrocarbon-based fuels include alcohol (e.g. methanol and ethanol), ether (e.g. methyl-t-butyl ether), and ketone (e.g. acetone). In addition, the use of additives, such as hydrazine or nitro compounds (for example nitromethane including nitropropane and nitroparaffin, or nitrobenzene) have been examined for automobile racing. However, the problem with using such additives is that they often have an adverse effect on the durability of the engine and its components.
- It is also known that organometallic compounds (e.g. tetraethyl lead or similar lead alkyls:ferrocene, methyl cyclopentadienyl manganese tricarbonyl ), as well as aromatic amine compounds (e.g. aniline, monomethyl aniline, or dimethyl aniline) can be used as anti-knocking agents. However, it has been confirmed that these compounds dramatically reduce the operating efficiency of three-way catalysts due to catalyst poisoning.
- Japanese Patent Application Number (Kokai) 58-104996 (corresponding to US Patent Number 4,409,000) describes the use of an alkyl amine or ethylene-oxide-adducted alkenyl amine as an additive in automobile fuel to clean carburetors and engines.
- According to European Patent Number 0869163 A1 it is possible to reduce friction in gasoline engines by adding N,N-bis(hydroxyalkyl) alkyl amine to gasoline.
- According to PCT Patent Publication 2001-502374 (WO-98/17746), solubility in water as well as the engine performance can be improved by adding fatty acid diethanol amide, fatty acid ethoxylate and alcohol ethoxylate to a liquid fuel such as gasoline or diesel fuel.
- The present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide. In a further aspect the present invention relates to the use of the fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel, to enhance the acceleration response and the driving performance of internal combustion engines, such as gasoline or diesel engines.
- In its broadest aspect, the present invention relates to a fuel additive comprising an alkylene-oxide-adducted hydrocarbyl amide having from 3 to 50 moles of alkylene oxide per mole of hydrocarbyl amide.
- In another aspect, the present invention relates to a fuel composition comprising a major amount of a hydrocarbon boiling in the gasoline or diesel range and, from 10 to 10,000 ppm weight per weight of fuel, of the fuel additive of the present invention.
- In still another aspect, the present invention relates to a method of operating an automobile engine with the fuel composition of the present invention.
- In a further aspect, the present invention relates to a method of improving the acceleration performance of a gasoline automobile engine comprising additizing the fuel additive of the present invention to a gasoline and operating the engine with the additized gasoline.
- Among other factors, the present invention is based on the discovery that certain alkylene-oxide-adducted hydrocarbyl amides are surprisingly useful for improving the acceleration response and the driving performance of internal combustion engines when used as fuel additives in hydrocarbon-based fuels, such as gasoline fuel or diesel fuel.
- As stated above, the present invention relates to a fuel additive containing an alkylene-oxide-adducted hydrocarbyl amide and its use as a fuel additive in a hydrocarbon-based fuel, such as gasoline fuel or diesel fuel.
- Prior to discussing the present invention in detail, the following terms will have the following meanings unless expressly stated to the contrary.
- The term "hydrocarbyl" refers to an organic radical primarily composed of carbon and hydrogen which may be aliphatic, alicyclic, aromatic or combinations thereof, e.g., aralkyl or alkaryl. Such hydrocarbyl groups may also contain aliphatic unsaturation, i.e., olefinic or acetylenic unsaturation, and may contain minor amounts of heteroatoms, such as oxygen or nitrogen, or halogens, such as chlorine. When used in conjunction with carboxylic fatty acids, hydrocarbyl will also include olefinic unsaturation.
- The term "alkyl" refers to both straight- and branched-chain alkyl groups.
- The term "lower alkyl" refers to alkyl groups having 1 to about 6 carbon atoms and includes primary, secondary and tertiary alkyl groups. Typical lower alkyl groups include, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, t-butyl, n-pentyl, n-hexyl and the like.
- The term "alkenyl" refers to an alkyl group with unsaturation.
-
- The term "fuel" or "hydrocarbon-based fuel" refers to normally liquid hydrocarbons having boiling points in the range of gasoline and diesel fuels.
- In its broadest aspect, the present invention involves a fuel additive comprising an alkylene-oxide-adducted hydrocarbyl amide having from 3 to 50 moles, preferably from 3 to 20 moles, more preferably from 4 to 15 moles, of alkylene oxide per mole of hydrocarbyl amide.
- The alkylene-oxide-adducted hydrocarbyl amide of the present invention is derived from an alkyl amide having from 4 to 75, preferably from 8 to 22, carbon atoms or alkenyl amide with at least one or two points of unsaturation having from 4 to 75, preferably from 8 to 22, carbon atoms. Examples of desirable alkyl amides suitable for the present invention include, but are not limited to, octyl amide (capryl amide), nonyl amide, decyl amide (caprin amide), undecyl amide dodecyl amide (lauryl amide), tridecyl amide, teradecyl amide (myristyl amide), pentadecyl amide, hexadecyl amide (palmityl amide), heptadecyl amide, octadecyl amide (stearyl amide), nonadecyl amide, eicosyl amide (alkyl amide), or docosyl amide (behenyl amide). Examples of desirable alkenyl amides include, but are not limited to, palmitoolein amide, oleyl amide, isooleyl amide, elaidyl amide, linolyl amide, linoleyl amide. Preferably, the alkyl or alkenyl amide is a coconut oil fatty acid amide.
- The alkylene oxide adducted to the hydrocarbyl amide of the present invention is derived from an alkylene group having from 2 to 5 carbon atoms. Preferably, the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, and pentylene oxide. Ethylene oxide and propylene oxide are particularly preferred. In addition, mixtures of alkylene oxides are desirable in which, for example, a mixture of ethylene oxide and propylene oxide may be used to form the alkylene-oxide-adducted hydrocarbyl amide of the present invention. A respective molar ratio of from 1:5 to 5:1 may be used in the case of a mixture of ethylene oxide and propylene oxide.
- A desirable number of moles of the alkylene oxide to be adducted to the hydrocarbyl amide will be in the range of from 3 to 50 moles of alkylene oxide per 1 mole of hydrocarbyl amide. More preferably, the range of from 3 to 20 moles is particularly desirable. Most preferably, the range of from 4 to 15 moles is most preferable as a molar range of the additive.
- Preferably, the alkylene-oxide adducted hydrocarbon amide is derived from an alkylene-oxide-adduction reaction involving a coconut oil fatty acid amide with ethylene oxide and propylene oxide. However, the alkylene-oxide adducted hydrocarbyl amides useful as fuel additives in the present invention can be also a mixed product wherein various types and different moles of alkylene oxide and can be adducted to various types of hydrocarbyl amides.
- The present invention provides for a method of operating gasoline engine automobiles wherein an automobile equipped with a gasoline engine is operated with the fuel composition of the present invention. The method of operating gasoline engine automobiles is preferred when the amount of alkylene oxide is from 3 to 20 moles per mole of hydrocarbyl amide and the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- The present invention further provides for a method of improving the driving and acceleration performance of internal combustion engines, such as a gasoline or diesel engines in automobiles, by using the fuel additive described herein.
- The fuel additive of the present invention improves acceleration performance of internal combustion engines when the fuel additive is added to a low boiling point hydrocarbon-based fuel like gasoline, and the driving performance is also improved when the additive is added to other hydrocarbon-based fuel like a diesel fuel, alcohol fuel or ether fuel. The method of improving acceleration performance in gasoline engine automobiles is preferred when the amount of alkylene oxide is from 3 to 20 moles per mole of hydrocarbyl amide and the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- The amount of fuel additive of the present invention added in a hydrocarbon-based fuel will typically be in a range of from 10 to 10,000 ppm weight per weight (active component ratio). More preferably, the desired range is from 10 to 5,000 ppm weight per weight, while a range of from 10 to 1,000 ppm weight per weight is most preferable.
- The fuel additive of the present invention is normally supplied as an organic solvent solution with an effective fuel additive content of at least 30 weight %, based on the amount of the fuel additive and organic solvent solution.
- Although no particular limitations are imposed on the method used to add a fuel additive of the present invention to a hydrocarbon-based fuel, a concentrated fuel additive product may be prepared which contained at least 30 weight % of the active component. This product can be added according to any selected method including adding it into the fuel tank of a fuel station or into the fuel tank of a passenger car.
- The fuel additive of the present invention can also be combined with one, two, or more other additives publicly known to be used in hydrocarbon-based fuels. Such additives include, but are not limited to, deposit control additives such as detergents or dispersants, corrosion inhibitors, oxidation inhibitors, metal deactivators, corrosion inhibitors, demulsifiers, static electricity preventing agents, anti-coagulation agents, anti-knock agents, oxygenates, flow improvers, pour point depressants, cetane improvers and auxiliary-solution agents.
- The invention will be further illustrated by the following examples, which set forth particularly advantageous method embodiments. While the examples are provided to illustrate the present invention, they are not intended to limit it. The present invention has been described with reference to specific embodiments and it is intended to cover those various changes and substitutions that may be made by those skilled in the art without departing from the spirit and scope of the appended claims.
- A Toyota Camry 1800cc, 5MT (Type E-SV40, provided with Knock Sensor, type 4S-FE engine), mounted on a chassis dynamometer, was operated at a constant speed of 20 km/hr. The acceleration measurement was initiated by fully opening the throttle and measuring the amount of time required for the vehicle speed to reach 110 km/hr with the transmission locked in fourth gear. This measurement was repeated 10 times using the same fuel and the median value obtained from these 10 measurements was determined as the acceleration time period. In addition, in order to minimize the influence of ambient conditions (temperature, pressure, etc.) on engine performance, the entire test procedure was executed within a single day.
- The gasoline used have the following specifications: density (at 15°C): 0.7389 g/cm3, Reid vapor pressure: 60.5 Kpa, octane number: 90.2 (RON), 82.3 (MON), aromatics (volume %): 29.9, olefin (volume %): 15.6, 10% distillation temperature (°C): 50.0, 50% distillation temperature (°C): 92.0, 90% distillation temperature (°C): 169.5. The fuel composition was adjusted by adding 100mg/L of 5 moles of oleyl amide-ethylene oxide (fuel additive) to this gasoline.
- Gasoline containing the above described fuel additive and gasoline without the fuel additive (same as the above) were then tested in accordance with the test procedures described herein above. Table 1 shows the results.
Test Fuel Oil Acceleration Time Period (20-110km/hours) Gasoline with No Additive 24.91 seconds (Comparative Example) Fuel Composition 24.69 seconds containing Additive - From the different acceleration time periods shown in Table 1, it is clear that the acceleration performance was improved by the fuel additive of the present invention. Although the difference in the acceleration time period indicated in Table 1 is not dramatic (less than 1%) with the fuel additive of the present invention, this is a distinct difference, particularly in case of cars needing to attain a high speed, such as racing cars, etc. Furthermore, in addition to the importance of acceleration for racing cars, even a small improvement in acceleration performance can be very important for passenger cars driving on public roads where it may be necessary to suddenly accelerate in order to avoid an accident, etc., as a result of a sudden event.
- The test was carried out as described in Example 1, using four moles of propylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) was added at a concentration of 100 mg/L to this gasoline in order to prepare a fuel composition containing the fuel additive.
- Gasoline containing the above described fuel additive and gasoline without the fuel additive (same as the above) were then tested in the acceleration evaluation test in accordance with the test procedures described in Example 1. Table 2 shows the results of the test.
Test Fuel Oil Acceleration Time Period (20-110km/hours) Gasoline with No Additive 24.51 seconds (Comparative Example) Fuel Composition 24.38 seconds containing Additive - As shown by the results in Table 2, the acceleration performance was clearly improved when the fuel additive of the present invention was employed in the fuel.
- The test was carried out as described in Example 1, using ten moles of propylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) was added to provide a concentration of 100 mg/L in this gasoline in order to prepare a fuel composition containing the fuel additive.
- Gasoline containing the above described fuel additive and gasoline without fuel additive (same as the above) were then tested in accordance with the test procedures described previously. Table 3 shows the results of the test.
Test Fuel Oil Acceleration Time Period (20-110km/hours) Gasoline with No Additive 24.85 seconds (Comparative Example) Fuel Composition 24.74 seconds containing Additive - As shown by the results in Table 3, the acceleration performance was clearly improved when the fuel additive of the present invention was employed in the fuel.
- The test was carried out as described in Example 1 except the gasoline used had the following specifications: density 9 at 15°C: 0.7303 g/cm3, Reid vapor pressure: 60.2Kpa, octane number: 92.1 (RON), aromatics (volume %): 23.19, olefin (volume %) 19, 10% distillation temperature (°C): 54.3, 50% distillation temperature (°C): 86.2, 90% distillation temperature (°C): 158.1 and using four moles of propylene oxide and two moles of ethylene oxide adducted coconut oil fatty acid di-ethanol amide (fuel additive) were added to provide a concentration of 100 mg/L in this gasoline.
- Gasoline containing the above described fuel additive and gasoline without fuel additive (same as the above) were then tested in accordance with the test procedures described previously. Table 4 shows the results of the test.
Test Fuel Oil Acceleration Time Period (20-110km/hours) Gasoline with No Additive 23.96 seconds (Comparative Example) Fuel Composition 23.75 seconds containing Additive - As shown by the results in Table 4, the acceleration performance was clearly improved when the fuel additive of the present invention was employed in the fuel.
Claims (15)
- A fuel additive comprising an alkylene-oxide-adducted hydrocarbyl amide having from 3 to 50 moles of alkylene oxide per mole of hydrocarbyl amide.
- The fuel additive according to Claim 1, wherein the alkylene-oxide-adducted hydrocarbyl amide has from 3 to 20 moles of alkylene oxide per mole of hydrocarbyl amide.
- The fuel additive according to Claim 2, wherein the alkylene-oxide-adducted hydrocarbyl amide has from 4 to 15 moles of alkylene oxide per mole of hydrocarbyl amide.
- A fuel additive according to Claim 1, wherein the alkylene-oxide-adducted hydrocarbyl amide is derived from an alkyl or alkenyl amide having from 4 to 75 carbon atoms.
- A fuel additive according to Claim 4, wherein the alkylene-oxide-adducted hydrocarbyl amide is derived from an alkyl or alkenyl amide having from 8 to 22 carbon atoms.
- A fuel additive according to Claim 5, wherein the alkyl or alkenyl amide is a coconut oil fatty acid amide.
- A fuel additive according to Claim 1, wherein the alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- A fuel additive according to Claim 7, wherein the alkylene oxide is elected from the group consisting of ethylene oxide, propylene oxide, or a mixture thereof.
- A fuel additive according to Claim 1, wherein the alkylene-oxide-adducted hydrocarbyl amide is derived from an alkylene-oxide-adduction reaction involving a coconut oil fatty acid amide with ethylene oxide and propylene oxide.
- A fuel additive according to claim 1, wherein the amount of alkylene oxide is from 3 to 20 moles per mole of hydrocarbyl amide and said alkylene oxide is selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, pentylene oxide, or mixtures thereof.
- A fuel composition for automotive fuels comprising a major amount of hydrocarbon boiling in the gasoline or diesel range and, from 10 to 10,000 ppm weight per weight of fuel, of a fuel additive as claimed in any preceding claim.
- The fuel composition according to Claim 10, wherein the alkylene-oxide-adducted hydrocarbyl amide is in the range of from 10 to 5,000 ppm weight per weight of fuel.
- The fuel composition according to Claim 12, wherein the alkylene-oxide-adducted hydrocarbyl amide is in the range of from 10 to 10,000 ppm weight per weight of fuel.
- A method of operating gasoline automobile engines comprising operating said engine with the fuel composition according to Claim 11, 12 or 13.
- A method of improving the acceleration performance of gasoline automobile engines comprising additizing the fuel additive of any one of claims 1 to 10 to a gasoline and operating said engine with said gasoline.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001206390 | 2001-07-06 | ||
| JP2001206390 | 2001-07-06 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1273652A1 true EP1273652A1 (en) | 2003-01-08 |
| EP1273652B1 EP1273652B1 (en) | 2006-11-29 |
Family
ID=19042532
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP02254662A Expired - Fee Related EP1273652B1 (en) | 2001-07-06 | 2002-07-03 | Fuel additive and fuel compositon containing the same |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20030046861A1 (en) |
| EP (1) | EP1273652B1 (en) |
| KR (1) | KR100822381B1 (en) |
| CA (1) | CA2390822A1 (en) |
| DE (1) | DE60216370T2 (en) |
| SG (1) | SG119156A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1435385A1 (en) * | 2003-01-06 | 2004-07-07 | Chevron Texaco Japan Ltd. | Fuel additive composition and fuel composition containing the same |
| EP1731591A2 (en) * | 2005-05-13 | 2006-12-13 | Chevron Oronite Company LLC | A fuel compostion containing an alkylene oxide-adducted hydrocarbyl amide having reduced amine by-products |
| WO2011163122A1 (en) * | 2010-06-21 | 2011-12-29 | Shell Oil Company | Fuel composition and its use |
| WO2015059206A1 (en) * | 2013-10-24 | 2015-04-30 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2949733A1 (en) * | 2014-05-28 | 2015-12-02 | Shell Internationale Research Maatschappij B.V. | Gasoline compositions comprising oxanilide uv filter compounds |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7790924B2 (en) * | 2004-11-19 | 2010-09-07 | Chevron Oronite Company Llc | Process for preparing alkylene oxide-adducted hydrocarbyl amides |
| US20060277820A1 (en) * | 2005-06-13 | 2006-12-14 | Puri Suresh K | Synergistic deposit control additive composition for gasoline fuel and process thereof |
| US20060277819A1 (en) * | 2005-06-13 | 2006-12-14 | Puri Suresh K | Synergistic deposit control additive composition for diesel fuel and process thereof |
| US8222180B2 (en) * | 2005-08-01 | 2012-07-17 | Indian Oil Corporation Limited | Adsorbent composition for removal of refractory sulphur compounds from refinery streams and process thereof |
| KR20190119422A (en) | 2018-04-12 | 2019-10-22 | 강정남 | A chemical additives for diesel fuels using nanomaterials |
| KR102139822B1 (en) * | 2018-11-28 | 2020-07-30 | 주식회사 카라 | A fuel component inspection and component controlling device of automobile |
| KR102034851B1 (en) | 2018-12-21 | 2019-10-21 | 주식회사 동이기술 | Phase stabilizing composition for alternative fuel of Automobile gasoline, alternative fuel of Automobile gasoline comprising the same and manufacturing method of the alternative fuel of Automobile gasoline |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1112754A (en) * | 1965-03-22 | 1968-05-08 | Armour & Co | Novel amino amides and their use in hydrocarbon fuels |
| EP0012345A1 (en) * | 1978-12-16 | 1980-06-25 | Bayer Ag | Fuel oils and their application |
| US4398919A (en) * | 1981-11-04 | 1983-08-16 | Akzona Incorporated | Polyethoxylated compounds as coal-water slurry surfactants |
| DE3709195A1 (en) * | 1987-02-10 | 1988-08-18 | Guenther Dr Boehmke | Storage-stable emulsifiers |
| WO1998016599A1 (en) * | 1996-10-11 | 1998-04-23 | Infineum Holdings Bv | Fuel compositions |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2854437A1 (en) * | 1978-12-16 | 1980-06-26 | Bayer Ag | FUELS, METHOD FOR THEIR PRODUCTION AND THEIR USE |
| US4389322A (en) * | 1979-11-16 | 1983-06-21 | Mobil Oil Corporation | Friction reducing additives and compositions thereof |
| US4409000A (en) * | 1981-12-14 | 1983-10-11 | The Lubrizol Corporation | Combinations of hydroxy amines and carboxylic dispersants as fuel additives |
| US4729769A (en) * | 1986-05-08 | 1988-03-08 | Texaco Inc. | Gasoline compositions containing reaction products of fatty acid esters and amines as carburetor detergents |
| US6312481B1 (en) * | 1994-09-22 | 2001-11-06 | Shell Oil Company | Fuel compositions |
-
2002
- 2002-05-08 US US10/142,750 patent/US20030046861A1/en not_active Abandoned
- 2002-06-17 CA CA002390822A patent/CA2390822A1/en not_active Abandoned
- 2002-07-03 SG SG200204016A patent/SG119156A1/en unknown
- 2002-07-03 DE DE60216370T patent/DE60216370T2/en not_active Expired - Lifetime
- 2002-07-03 EP EP02254662A patent/EP1273652B1/en not_active Expired - Fee Related
- 2002-07-05 KR KR1020020038912A patent/KR100822381B1/en active IP Right Grant
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1112754A (en) * | 1965-03-22 | 1968-05-08 | Armour & Co | Novel amino amides and their use in hydrocarbon fuels |
| EP0012345A1 (en) * | 1978-12-16 | 1980-06-25 | Bayer Ag | Fuel oils and their application |
| US4398919A (en) * | 1981-11-04 | 1983-08-16 | Akzona Incorporated | Polyethoxylated compounds as coal-water slurry surfactants |
| DE3709195A1 (en) * | 1987-02-10 | 1988-08-18 | Guenther Dr Boehmke | Storage-stable emulsifiers |
| WO1998016599A1 (en) * | 1996-10-11 | 1998-04-23 | Infineum Holdings Bv | Fuel compositions |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1435385A1 (en) * | 2003-01-06 | 2004-07-07 | Chevron Texaco Japan Ltd. | Fuel additive composition and fuel composition containing the same |
| SG122811A1 (en) * | 2003-01-06 | 2006-06-29 | Chevrontexaco Japan Ltd | Fuel additive composition and fuel composition containing the same |
| US8388704B2 (en) | 2003-01-06 | 2013-03-05 | Chevron Texaco Japan Limited | Fuel additive composition and fuel composition containing the same |
| EP1731591A2 (en) * | 2005-05-13 | 2006-12-13 | Chevron Oronite Company LLC | A fuel compostion containing an alkylene oxide-adducted hydrocarbyl amide having reduced amine by-products |
| EP1731591A3 (en) * | 2005-05-13 | 2007-07-25 | Chevron Oronite Company LLC | A fuel compostion containing an alkylene oxide-adducted hydrocarbyl amide having reduced amine by-products |
| US7744661B2 (en) | 2005-05-13 | 2010-06-29 | Chevron Oronite Company Llc | Fuel composition containing an alkylene oxide-adducted hydrocarbyl amide having reduced amine by-products |
| WO2011163122A1 (en) * | 2010-06-21 | 2011-12-29 | Shell Oil Company | Fuel composition and its use |
| WO2015059206A1 (en) * | 2013-10-24 | 2015-04-30 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2949733A1 (en) * | 2014-05-28 | 2015-12-02 | Shell Internationale Research Maatschappij B.V. | Gasoline compositions comprising oxanilide uv filter compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1273652B1 (en) | 2006-11-29 |
| DE60216370D1 (en) | 2007-01-11 |
| CA2390822A1 (en) | 2003-01-06 |
| SG119156A1 (en) | 2006-02-28 |
| US20030046861A1 (en) | 2003-03-13 |
| DE60216370T2 (en) | 2007-03-15 |
| KR20030005050A (en) | 2003-01-15 |
| KR100822381B1 (en) | 2008-04-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3796355B2 (en) | Gasoline composition containing an ignition modifier | |
| EP1273652B1 (en) | Fuel additive and fuel compositon containing the same | |
| EP1435386B1 (en) | Use of a fuel additive composition for improving acceleration of a gasoline engine | |
| EP1013746B1 (en) | Fuels with enhanced lubricity | |
| US4236898A (en) | Friction modifier for gasoline | |
| US6488723B2 (en) | Motor fuel additive composition and method for preparation thereof | |
| EP1435385B1 (en) | Fuel additive composition and fuel composition containing the same | |
| US3707362A (en) | Method and composition for optimizing air-fuel ratio distribution in internal combustion engines | |
| WO1991013949A1 (en) | Motor fuel additive composition and method for preparation thereof | |
| RU2139914C1 (en) | Ashless high-octane motor gasoline additive | |
| US6589302B1 (en) | Friction modifier for poor lubricity fuels | |
| EP0634472A1 (en) | Compositions for control of deposits, exhaust emissions and/or fuel consumption in internal combustion engines | |
| US6423107B1 (en) | Detergent compositions for gasoline-type fuels that contain polytetrahydrofuran derivatives | |
| US4895578A (en) | Hydrocarbon fuel detergent | |
| JP2002338974A (en) | Fuel oil composition for gasoline engine | |
| RU2264434C2 (en) | Multifunctional additive for production of motor car gasolines and internal combustion engine fuel based on gasoline containing multifunctional additive | |
| RU2337129C1 (en) | Ash-free high-octane additive to automobile gasoline | |
| Hamid et al. | Effect of MTBE blending on the properties of gasoline | |
| RU2132359C1 (en) | Multifunctional additive for preparing automobile gasolines | |
| CN103602355B (en) | For the treatment of the fuel dope of the inside deposition thing of fuel injector | |
| JP2002356683A (en) | Fuel composition for gasoline engine | |
| RU2256694C1 (en) | Multifunctional gasoline additive | |
| CN100432196C (en) | Gasoline compositions | |
| RU2213126C1 (en) | Gasoline additive, fuel composition | |
| RU2241739C1 (en) | Multifunctional additive to a fuel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| 17P | Request for examination filed |
Effective date: 20030430 |
|
| AKX | Designation fees paid |
Designated state(s): DE FR GB NL |
|
| 17Q | First examination report despatched |
Effective date: 20041124 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB NL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 60216370 Country of ref document: DE Date of ref document: 20070111 Kind code of ref document: P |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20070830 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60216370 Country of ref document: DE Representative=s name: HASELTINE LAKE LLP, DE |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20200612 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20200624 Year of fee payment: 19 Ref country code: NL Payment date: 20200615 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20200624 Year of fee payment: 19 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60216370 Country of ref document: DE Representative=s name: HL KEMPNER PATENTANWALT, RECHTSANWALT, SOLICIT, DE |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 60216370 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20210801 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20210703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210703 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210801 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210731 |