EP1061069A1 - Urethanisierte Beta-Hydroxyalkylamid-Verbindung, ein Verfahren zu ihrer Herstellung sowie deren Verwendung zur Herstellung von Pulverlacken - Google Patents
Urethanisierte Beta-Hydroxyalkylamid-Verbindung, ein Verfahren zu ihrer Herstellung sowie deren Verwendung zur Herstellung von Pulverlacken Download PDFInfo
- Publication number
- EP1061069A1 EP1061069A1 EP00108883A EP00108883A EP1061069A1 EP 1061069 A1 EP1061069 A1 EP 1061069A1 EP 00108883 A EP00108883 A EP 00108883A EP 00108883 A EP00108883 A EP 00108883A EP 1061069 A1 EP1061069 A1 EP 1061069A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- diisocyanate
- hydroxyalkylamide
- urethanized
- powder coatings
- transparent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000843 powder Substances 0.000 title claims abstract description 45
- 238000000576 coating method Methods 0.000 title claims abstract description 43
- 150000001875 compounds Chemical class 0.000 title claims abstract description 39
- 238000000034 method Methods 0.000 title claims abstract description 12
- 238000002360 preparation method Methods 0.000 title claims description 10
- 238000004519 manufacturing process Methods 0.000 claims abstract description 12
- 229920000642 polymer Polymers 0.000 claims description 19
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 18
- 239000005056 polyisocyanate Substances 0.000 claims description 18
- 229920001228 polyisocyanate Polymers 0.000 claims description 18
- 229920000728 polyester Polymers 0.000 claims description 15
- 239000002253 acid Substances 0.000 claims description 14
- -1 aluminum carboxylate salts Chemical class 0.000 claims description 14
- 239000003795 chemical substances by application Substances 0.000 claims description 13
- 239000005058 Isophorone diisocyanate Substances 0.000 claims description 12
- NIMLQBUJDJZYEJ-UHFFFAOYSA-N isophorone diisocyanate Chemical compound CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1 NIMLQBUJDJZYEJ-UHFFFAOYSA-N 0.000 claims description 12
- 239000000126 substance Substances 0.000 claims description 12
- 239000000203 mixture Substances 0.000 claims description 11
- 239000003054 catalyst Substances 0.000 claims description 9
- ZFSLODLOARCGLH-UHFFFAOYSA-N isocyanuric acid Chemical compound OC1=NC(O)=NC(O)=N1 ZFSLODLOARCGLH-UHFFFAOYSA-N 0.000 claims description 8
- 239000011248 coating agent Substances 0.000 claims description 7
- 239000002904 solvent Substances 0.000 claims description 7
- 239000005057 Hexamethylene diisocyanate Substances 0.000 claims description 6
- 239000000654 additive Substances 0.000 claims description 6
- RRAMGCGOFNQTLD-UHFFFAOYSA-N hexamethylene diisocyanate Chemical compound O=C=NCCCCCCN=C=O RRAMGCGOFNQTLD-UHFFFAOYSA-N 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 6
- 125000001931 aliphatic group Chemical group 0.000 claims description 5
- 125000003118 aryl group Chemical group 0.000 claims description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 5
- 238000007872 degassing Methods 0.000 claims description 5
- 238000002844 melting Methods 0.000 claims description 5
- 230000008018 melting Effects 0.000 claims description 5
- 229920000058 polyacrylate Polymers 0.000 claims description 5
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 claims description 4
- JGCWKVKYRNXTMD-UHFFFAOYSA-N bicyclo[2.2.1]heptane;isocyanic acid Chemical compound N=C=O.N=C=O.C1CC2CCC1C2 JGCWKVKYRNXTMD-UHFFFAOYSA-N 0.000 claims description 4
- 239000000945 filler Substances 0.000 claims description 4
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 3
- 229910052782 aluminium Inorganic materials 0.000 claims description 3
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 claims description 3
- 238000006243 chemical reaction Methods 0.000 claims description 3
- 150000002430 hydrocarbons Chemical group 0.000 claims description 3
- 150000002431 hydrogen Chemical class 0.000 claims description 3
- 125000000962 organic group Chemical group 0.000 claims description 3
- 229920006395 saturated elastomer Polymers 0.000 claims description 3
- VNMOIBZLSJDQEO-UHFFFAOYSA-N 1,10-diisocyanatodecane Chemical compound O=C=NCCCCCCCCCCN=C=O VNMOIBZLSJDQEO-UHFFFAOYSA-N 0.000 claims description 2
- GFNDFCFPJQPVQL-UHFFFAOYSA-N 1,12-diisocyanatododecane Chemical compound O=C=NCCCCCCCCCCCCN=C=O GFNDFCFPJQPVQL-UHFFFAOYSA-N 0.000 claims description 2
- ZTNJGMFHJYGMDR-UHFFFAOYSA-N 1,2-diisocyanatoethane Chemical compound O=C=NCCN=C=O ZTNJGMFHJYGMDR-UHFFFAOYSA-N 0.000 claims description 2
- ZGDSDWSIFQBAJS-UHFFFAOYSA-N 1,2-diisocyanatopropane Chemical compound O=C=NC(C)CN=C=O ZGDSDWSIFQBAJS-UHFFFAOYSA-N 0.000 claims description 2
- 239000005059 1,4-Cyclohexyldiisocyanate Substances 0.000 claims description 2
- OVBFMUAFNIIQAL-UHFFFAOYSA-N 1,4-diisocyanatobutane Chemical compound O=C=NCCCCN=C=O OVBFMUAFNIIQAL-UHFFFAOYSA-N 0.000 claims description 2
- DFPJRUKWEPYFJT-UHFFFAOYSA-N 1,5-diisocyanatopentane Chemical compound O=C=NCCCCCN=C=O DFPJRUKWEPYFJT-UHFFFAOYSA-N 0.000 claims description 2
- VZDIRINETBAVAV-UHFFFAOYSA-N 2,4-diisocyanato-1-methylcyclohexane Chemical compound CC1CCC(N=C=O)CC1N=C=O VZDIRINETBAVAV-UHFFFAOYSA-N 0.000 claims description 2
- KORSJDCBLAPZEQ-UHFFFAOYSA-N dicyclohexylmethane-4,4'-diisocyanate Chemical compound C1CC(N=C=O)CCC1CC1CCC(N=C=O)CC1 KORSJDCBLAPZEQ-UHFFFAOYSA-N 0.000 claims description 2
- 125000000623 heterocyclic group Chemical group 0.000 claims description 2
- 239000010936 titanium Substances 0.000 claims description 2
- 229910052719 titanium Inorganic materials 0.000 claims description 2
- 235000014692 zinc oxide Nutrition 0.000 claims description 2
- RNWHGQJWIACOKP-UHFFFAOYSA-N zinc;oxygen(2-) Chemical class [O-2].[Zn+2] RNWHGQJWIACOKP-UHFFFAOYSA-N 0.000 claims description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N Alumina Chemical class [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims 1
- 238000010923 batch production Methods 0.000 claims 1
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 claims 1
- 239000000178 monomer Substances 0.000 description 11
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 10
- 229920001577 copolymer Polymers 0.000 description 9
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 6
- 239000004971 Cross linker Substances 0.000 description 6
- 230000002378 acidificating effect Effects 0.000 description 6
- 150000007513 acids Chemical class 0.000 description 5
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 4
- ISAOCJYIOMOJEB-UHFFFAOYSA-N benzoin Chemical compound C=1C=CC=CC=1C(O)C(=O)C1=CC=CC=C1 ISAOCJYIOMOJEB-UHFFFAOYSA-N 0.000 description 4
- 239000003999 initiator Substances 0.000 description 4
- 229920005862 polyol Polymers 0.000 description 4
- 150000003077 polyols Chemical class 0.000 description 4
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 238000007334 copolymerization reaction Methods 0.000 description 3
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 3
- 239000012948 isocyanate Substances 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- 239000000049 pigment Substances 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- XLLIQLLCWZCATF-UHFFFAOYSA-N 2-methoxyethyl acetate Chemical compound COCCOC(C)=O XLLIQLLCWZCATF-UHFFFAOYSA-N 0.000 description 2
- FFWSICBKRCICMR-UHFFFAOYSA-N 5-methyl-2-hexanone Chemical compound CC(C)CCC(C)=O FFWSICBKRCICMR-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 239000004594 Masterbatch (MB) Substances 0.000 description 2
- 244000028419 Styrax benzoin Species 0.000 description 2
- 235000000126 Styrax benzoin Nutrition 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 235000008411 Sumatra benzointree Nutrition 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 125000005907 alkyl ester group Chemical group 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229960002130 benzoin Drugs 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- GHLKSLMMWAKNBM-UHFFFAOYSA-N dodecane-1,12-diol Chemical compound OCCCCCCCCCCCCO GHLKSLMMWAKNBM-UHFFFAOYSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 235000019382 gum benzoic Nutrition 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- AOGQPLXWSUTHQB-UHFFFAOYSA-N hexyl acetate Chemical compound CCCCCCOC(C)=O AOGQPLXWSUTHQB-UHFFFAOYSA-N 0.000 description 2
- 230000003505 mutagenic effect Effects 0.000 description 2
- OKRNLSUTBJUVKA-UHFFFAOYSA-N n,n,n',n'-Tetrakis(2-hydroxyethyl)adipamide Chemical compound OCCN(CCO)C(=O)CCCCC(=O)N(CCO)CCO OKRNLSUTBJUVKA-UHFFFAOYSA-N 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- CYIDZMCFTVVTJO-UHFFFAOYSA-N pyromellitic acid Chemical compound OC(=O)C1=CC(C(O)=O)=C(C(O)=O)C=C1C(O)=O CYIDZMCFTVVTJO-UHFFFAOYSA-N 0.000 description 2
- 150000003254 radicals Chemical class 0.000 description 2
- 238000005245 sintering Methods 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 2
- 229920001187 thermosetting polymer Polymers 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 239000012463 white pigment Substances 0.000 description 2
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 description 1
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- PXGZQGDTEZPERC-UHFFFAOYSA-N 1,4-cyclohexanedicarboxylic acid Chemical compound OC(=O)C1CCC(C(O)=O)CC1 PXGZQGDTEZPERC-UHFFFAOYSA-N 0.000 description 1
- LGJCFVYMIJLQJO-UHFFFAOYSA-N 1-dodecylperoxydodecane Chemical class CCCCCCCCCCCCOOCCCCCCCCCCCC LGJCFVYMIJLQJO-UHFFFAOYSA-N 0.000 description 1
- FQXGHZNSUOHCLO-UHFFFAOYSA-N 2,2,4,4-tetramethyl-1,3-cyclobutanediol Chemical compound CC1(C)C(O)C(C)(C)C1O FQXGHZNSUOHCLO-UHFFFAOYSA-N 0.000 description 1
- OOHZIRUJZFRULE-UHFFFAOYSA-N 2,2-dimethylpropyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCC(C)(C)C OOHZIRUJZFRULE-UHFFFAOYSA-N 0.000 description 1
- YAJYJWXEWKRTPO-UHFFFAOYSA-N 2,3,3,4,4,5-hexamethylhexane-2-thiol Chemical compound CC(C)C(C)(C)C(C)(C)C(C)(C)S YAJYJWXEWKRTPO-UHFFFAOYSA-N 0.000 description 1
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 1
- XMNIXWIUMCBBBL-UHFFFAOYSA-N 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)OOC(C)(C)C1=CC=CC=C1 XMNIXWIUMCBBBL-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 1
- DABQKEQFLJIRHU-UHFFFAOYSA-N 2-Propenoic acid, 2-methyl-, 3,3,5-trimethylcyclohexyl ester Chemical compound CC1CC(OC(=O)C(C)=C)CC(C)(C)C1 DABQKEQFLJIRHU-UHFFFAOYSA-N 0.000 description 1
- KRDXTHSSNCTAGY-UHFFFAOYSA-N 2-cyclohexylpyrrolidine Chemical compound C1CCNC1C1CCCCC1 KRDXTHSSNCTAGY-UHFFFAOYSA-N 0.000 description 1
- SVONRAPFKPVNKG-UHFFFAOYSA-N 2-ethoxyethyl acetate Chemical compound CCOCCOC(C)=O SVONRAPFKPVNKG-UHFFFAOYSA-N 0.000 description 1
- FRIBMENBGGCKPD-UHFFFAOYSA-N 3-(2,3-dimethoxyphenyl)prop-2-enal Chemical compound COC1=CC=CC(C=CC=O)=C1OC FRIBMENBGGCKPD-UHFFFAOYSA-N 0.000 description 1
- DKIDEFUBRARXTE-UHFFFAOYSA-N 3-mercaptopropanoic acid Chemical compound OC(=O)CCS DKIDEFUBRARXTE-UHFFFAOYSA-N 0.000 description 1
- CCTFMNIEFHGTDU-UHFFFAOYSA-N 3-methoxypropyl acetate Chemical compound COCCCOC(C)=O CCTFMNIEFHGTDU-UHFFFAOYSA-N 0.000 description 1
- SXFJDZNJHVPHPH-UHFFFAOYSA-N 3-methylpentane-1,5-diol Chemical compound OCCC(C)CCO SXFJDZNJHVPHPH-UHFFFAOYSA-N 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical class C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- 235000009161 Espostoa lanata Nutrition 0.000 description 1
- 240000001624 Espostoa lanata Species 0.000 description 1
- PMVSDNDAUGGCCE-TYYBGVCCSA-L Ferrous fumarate Chemical compound [Fe+2].[O-]C(=O)\C=C\C([O-])=O PMVSDNDAUGGCCE-TYYBGVCCSA-L 0.000 description 1
- 238000005684 Liebig rearrangement reaction Methods 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- 241000232261 Napeanthus Species 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- ALQSHHUCVQOPAS-UHFFFAOYSA-N Pentane-1,5-diol Chemical compound OCCCCCO ALQSHHUCVQOPAS-UHFFFAOYSA-N 0.000 description 1
- 241000170793 Phalaris canariensis Species 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 229910010413 TiO 2 Inorganic materials 0.000 description 1
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- IAXXETNIOYFMLW-COPLHBTASA-N [(1s,3s,4s)-4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl] 2-methylprop-2-enoate Chemical compound C1C[C@]2(C)[C@@H](OC(=O)C(=C)C)C[C@H]1C2(C)C IAXXETNIOYFMLW-COPLHBTASA-N 0.000 description 1
- YIMQCDZDWXUDCA-UHFFFAOYSA-N [4-(hydroxymethyl)cyclohexyl]methanol Chemical compound OCC1CCC(CO)CC1 YIMQCDZDWXUDCA-UHFFFAOYSA-N 0.000 description 1
- KYIKRXIYLAGAKQ-UHFFFAOYSA-N abcn Chemical compound C1CCCCC1(C#N)N=NC1(C#N)CCCCC1 KYIKRXIYLAGAKQ-UHFFFAOYSA-N 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- ZCZSIDMEHXZRLG-UHFFFAOYSA-N acetic acid heptyl ester Natural products CCCCCCCOC(C)=O ZCZSIDMEHXZRLG-UHFFFAOYSA-N 0.000 description 1
- 150000008065 acid anhydrides Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000008064 anhydrides Chemical group 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical class C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 1
- OHJMTUPIZMNBFR-UHFFFAOYSA-N biuret Chemical compound NC(=O)NC(N)=O OHJMTUPIZMNBFR-UHFFFAOYSA-N 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 238000012662 bulk polymerization Methods 0.000 description 1
- MPMBRWOOISTHJV-UHFFFAOYSA-N but-1-enylbenzene Chemical compound CCC=CC1=CC=CC=C1 MPMBRWOOISTHJV-UHFFFAOYSA-N 0.000 description 1
- OWBTYPJTUOEWEK-UHFFFAOYSA-N butane-2,3-diol Chemical compound CC(O)C(C)O OWBTYPJTUOEWEK-UHFFFAOYSA-N 0.000 description 1
- IWTBWSGPDGPTIB-UHFFFAOYSA-N butanoyl butaneperoxoate Chemical compound CCCC(=O)OOC(=O)CCC IWTBWSGPDGPTIB-UHFFFAOYSA-N 0.000 description 1
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- LDHQCZJRKDOVOX-NSCUHMNNSA-N crotonic acid Chemical compound C\C=C\C(O)=O LDHQCZJRKDOVOX-NSCUHMNNSA-N 0.000 description 1
- VKONPUDBRVKQLM-UHFFFAOYSA-N cyclohexane-1,4-diol Chemical compound OC1CCC(O)CC1 VKONPUDBRVKQLM-UHFFFAOYSA-N 0.000 description 1
- OIWOHHBRDFKZNC-UHFFFAOYSA-N cyclohexyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OC1CCCCC1 OIWOHHBRDFKZNC-UHFFFAOYSA-N 0.000 description 1
- LSXWFXONGKSEMY-UHFFFAOYSA-N di-tert-butyl peroxide Chemical compound CC(C)(C)OOC(C)(C)C LSXWFXONGKSEMY-UHFFFAOYSA-N 0.000 description 1
- 239000012933 diacyl peroxide Substances 0.000 description 1
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- WNAHIZMDSQCWRP-UHFFFAOYSA-N dodecane-1-thiol Chemical compound CCCCCCCCCCCCS WNAHIZMDSQCWRP-UHFFFAOYSA-N 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000007720 emulsion polymerization reaction Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 238000010528 free radical solution polymerization reaction Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- JPXGPRBLTIYFQG-UHFFFAOYSA-N heptan-4-yl acetate Chemical compound CCCC(CCC)OC(C)=O JPXGPRBLTIYFQG-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 150000002432 hydroperoxides Chemical class 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229940119545 isobornyl methacrylate Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 239000003471 mutagenic agent Substances 0.000 description 1
- 231100000707 mutagenic chemical Toxicity 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- ZWWQICJTBOCQLA-UHFFFAOYSA-N o-propan-2-yl (propan-2-yloxycarbothioyldisulfanyl)methanethioate Chemical compound CC(C)OC(=S)SSC(=S)OC(C)C ZWWQICJTBOCQLA-UHFFFAOYSA-N 0.000 description 1
- HMZGPNHSPWNGEP-UHFFFAOYSA-N octadecyl 2-methylprop-2-enoate Chemical compound CCCCCCCCCCCCCCCCCCOC(=O)C(C)=C HMZGPNHSPWNGEP-UHFFFAOYSA-N 0.000 description 1
- UWBHMRBRLOJJAA-UHFFFAOYSA-N oxaluric acid Chemical compound NC(=O)NC(=O)C(O)=O UWBHMRBRLOJJAA-UHFFFAOYSA-N 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 125000005634 peroxydicarbonate group Chemical group 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- HJWLCRVIBGQPNF-UHFFFAOYSA-N prop-2-enylbenzene Chemical compound C=CCC1=CC=CC=C1 HJWLCRVIBGQPNF-UHFFFAOYSA-N 0.000 description 1
- 125000001501 propionyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000010557 suspension polymerization reaction Methods 0.000 description 1
- GJBRNHKUVLOCEB-UHFFFAOYSA-N tert-butyl benzenecarboperoxoate Chemical compound CC(C)(C)OOC(=O)C1=CC=CC=C1 GJBRNHKUVLOCEB-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- LDHQCZJRKDOVOX-UHFFFAOYSA-N trans-crotonic acid Natural products CC=CC(O)=O LDHQCZJRKDOVOX-UHFFFAOYSA-N 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
- AVWRKZWQTYIKIY-UHFFFAOYSA-N urea-1-carboxylic acid Chemical group NC(=O)NC(O)=O AVWRKZWQTYIKIY-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N urethane group Chemical group NC(=O)OCC JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/77—Polyisocyanates or polyisothiocyanates having heteroatoms in addition to the isocyanate or isothiocyanate nitrogen and oxygen or sulfur
- C08G18/78—Nitrogen
- C08G18/79—Nitrogen characterised by the polyisocyanates used, these having groups formed by oligomerisation of isocyanates or isothiocyanates
- C08G18/791—Nitrogen characterised by the polyisocyanates used, these having groups formed by oligomerisation of isocyanates or isothiocyanates containing isocyanurate groups
- C08G18/792—Nitrogen characterised by the polyisocyanates used, these having groups formed by oligomerisation of isocyanates or isothiocyanates containing isocyanurate groups formed by oligomerisation of aliphatic and/or cycloaliphatic isocyanates or isothiocyanates
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/24—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atom of at least one of the carbamate groups bound to a carbon atom of a ring other than a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/38—Low-molecular-weight compounds having heteroatoms other than oxygen
- C08G18/3819—Low-molecular-weight compounds having heteroatoms other than oxygen having nitrogen
- C08G18/3823—Low-molecular-weight compounds having heteroatoms other than oxygen having nitrogen containing -N-C=O groups
- C08G18/3825—Low-molecular-weight compounds having heteroatoms other than oxygen having nitrogen containing -N-C=O groups containing amide groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2150/00—Compositions for coatings
- C08G2150/20—Compositions for powder coatings
Definitions
- the invention relates to urethanized ⁇ -hydroxyalkylamide compounds, a process for their production, their use for the production of highly reactive powder coatings and the Powder coatings themselves.
- TGIC trigylycidyl isocyanurate
- acid-functional polyesters result in corrosion-resistant and weather-stable powder coatings.
- EP 0 536 085 describes that the production of the TGIC in solid form requires expensive processes or a relatively large and therefore likewise expensive cleaning effort.
- TGIC is also recognized by the European Community as a category II mutagen ( should be regarded as mutagenic ”) and has been labeled as toxic since May 31, 1998.
- the object of the present invention was therefore to find new crosslinkers which are in Combination with polymers containing carboxyl groups can be processed into powder coatings can and their coatings are extremely resistant to chemicals.
- urethanized ⁇ -hydroxyalkylamide compounds represent excellent crosslinkers and in combination with acidic polymers Powder coatings cause a drastic increase in chemical resistance without loss in flexibility, hardness, reactivity and weather resistance must be taken.
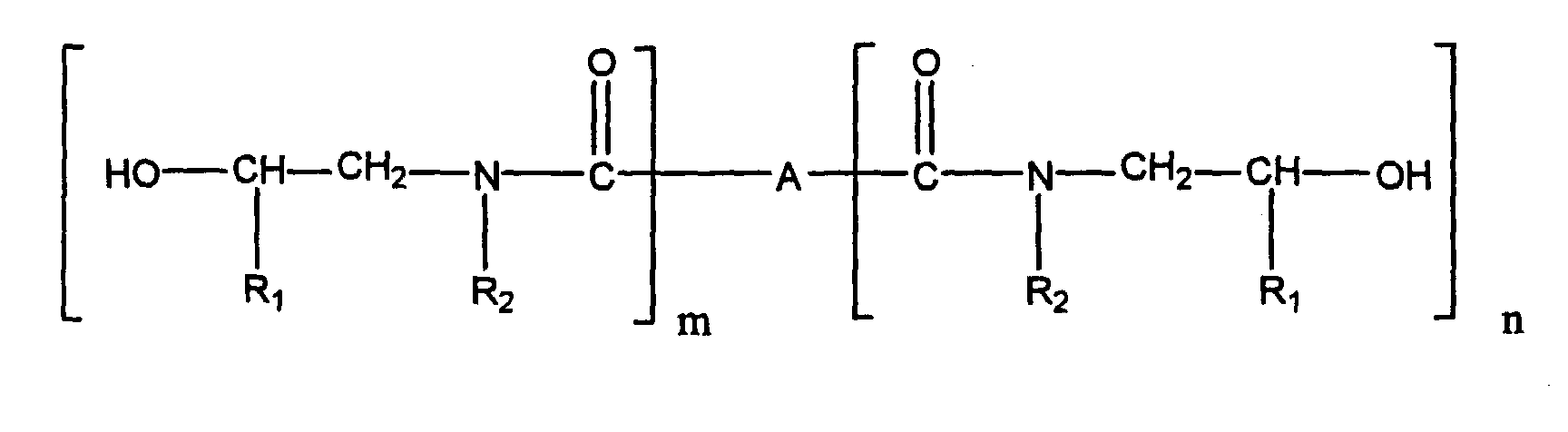
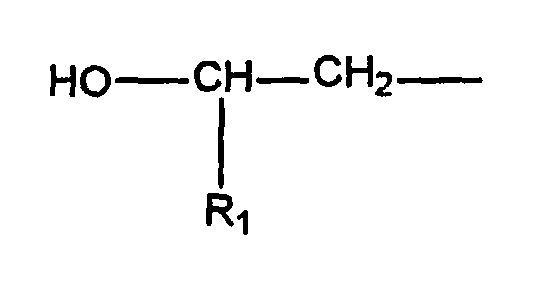
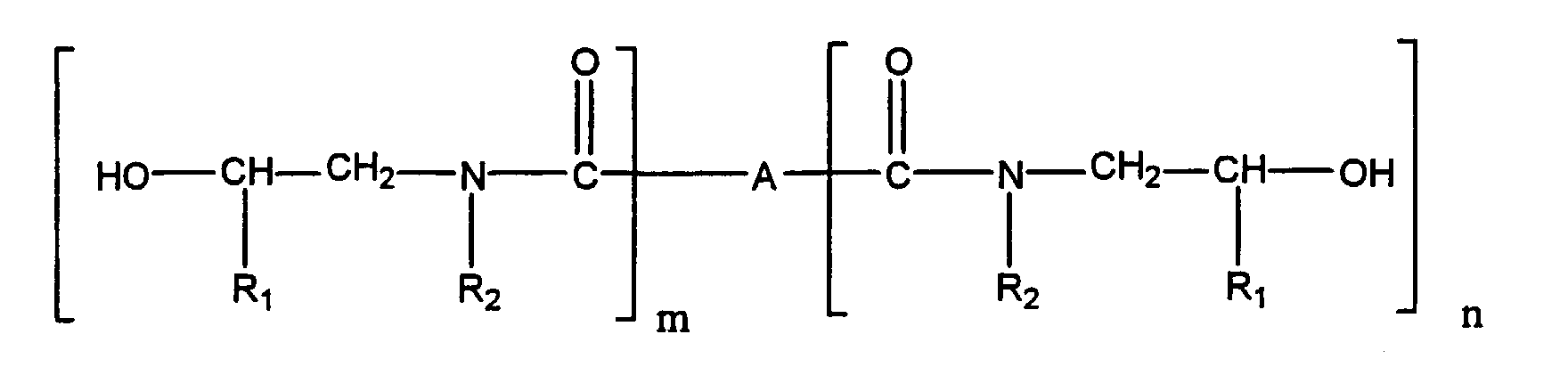
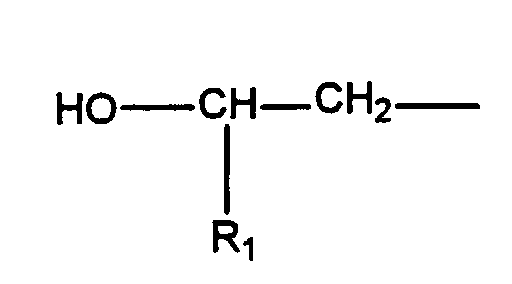
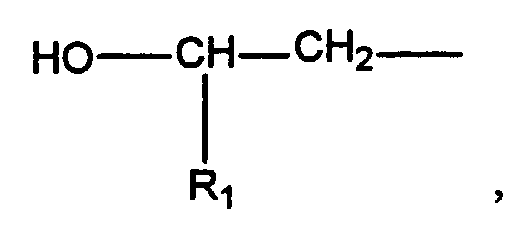
- a preferred subject of the invention are such urethanized ⁇ -hydroxyalkylamide compounds, the ⁇ -hydroxyalkylamide A) having the formula in which R 1 is hydrogen or a C 1 -C 5 alkyl group, R 2 is hydrogen, a C 1 -C 5 alkyl group or and A represents a chemical bond or a monovalent or polyvalent organic group selected from saturated, unsaturated or aromatic hydrocarbon groups or substituted hydrocarbon groups having 2 to 20 carbon atoms, m is 1 to 2, n is 0 to 2 and m + n at least 2.
- These compounds according to the invention particularly preferably have a functionality of four or more.
- Another object of the invention is the use of the urethanized ⁇ -hydroxyalkylamide compounds for the production of transparent or pigmented external resistant Powder coatings of high reactivity and hardness, excellent gloss and very good chemical resistance, made from the urethanized ⁇ -hydroxyalkylamide compound and carboxyl group-containing polymers and those in powder coating chemistry usual additives such.
- the invention also relates to transparent and pigmented powder coatings, which contain the urethanized ⁇ -hydroxyalkylamide compounds according to the invention.
- the ⁇ -hydroxyalkylamides A) are known in principle and z. B. in US 4,076,917, US 4,101,606, EP 0 322 834 and EP 0 473 380.
- the structure can be described as follows: in which R 1 is hydrogen or C 1 -C 5 alkyl, R 2 is hydrogen, C 1 -C 5 alkyl or in which R 1 has the meaning given above and A is a chemical bond or a monovalent or polyvalent organic group derived from saturated, unsaturated or aromatic hydrocarbon groups, including substituted hydrocarbon groups having 2 to 20 carbon atoms, m is 1 to 2, n is 0 to 2 and m + n is at least 2.
- the non-aromatic polyisocyanate B) with an NCO functionality 2 2 can be any aliphatic, (cyclo) aliphatic, cycloaliphatic and heterocyclic polyisocyanate with at least two isocyanate groups and mixtures thereof.
- Such polyisocyanates are used, for. B. in Houben-Weyl, Methods of Organic Chemistry, Volume 14/2, page 61 ff., And J. Liebigs Annalen der Chemie, Volume 562, pages 75 to 136.
- Representative examples of the polyisocyanates are aliphatic isocyanates such as alkylene isocyanates, e.g. B.
- ethylene diisocyanate propylene diisocyanate, tetramethylene diisocyanate, pentamethylene diisocyanate, 2-methylpentamethylene-1,5-diisocyanate (MPDI), hexamethylene diisocyanate (HDI), trimethylhexamethylene-1,6-diisocyanate (TMDI), in particular the 2,2,4- and the 2nd , 4,4-isomers and technical mixtures of both isomers, decamethylene diisocyanate and dodecamethylene diisocyanate and cycloalkylene isocyanates, e.g. B.
- MPDI 2-methylpentamethylene-1,5-diisocyanate
- HDI hexamethylene diisocyanate
- TMDI trimethylhexamethylene-1,6-diisocyanate
- decamethylene diisocyanate and dodecamethylene diisocyanate and cycloalkylene isocyanates e.g. B.
- Polyisocyanates which are obtainable by reacting polyisocyanates with themselves via isocyanate groups are advantageous, in particular isocyanurates which are formed by the reaction of three isocyanate groups.
- the polyisocyanates can also contain biuret or allophanate groups.
- the invention also relates to a process for the preparation of urethanized ⁇ -hydroxyalkylamide compound, characterized in that 65 to 96 wt .-% at least one ⁇ -hydroxyalkylamide A) with 4 to 35% by weight at least one not aromatic polyisocyanate B) are implemented, the urethanized ⁇ -hydroxyalkylamide compounds terminal hydroxyl groups and a functionality ⁇ 2 exhibit.
- the preparation of the urethanized ⁇ -hydroxyalkylamide compounds according to the invention can be done in solvent, but is preferred in bulk, so solvent-free, manufactured.
- the ⁇ -hydroxyalkylamide A) is presented and the Polyisocyanate B) added.
- the implementation can be done by determining the NCO number are followed and is finished after 30 minutes to 3 hours. To cool down, Crushing and bagging use known methods and technologies.
- Another object of the present invention is the use of Urethanized ⁇ -hydroxyalkylamide compounds according to the invention for the preparation transparent or pigmented weather-resistant powder coatings with high reactivity and Hardness and excellent gloss.
- the invention also relates to transparent or pigmented powder coatings, which the urethanized ⁇ -hydroxyalkylamide compounds according to the invention and those containing carboxyl groups Polymers and the usual in powder coating chemistry Additives such as B. pigments, fillers, leveling agents, degassing agents, if necessary Contain catalysts and other additives. Compared to ⁇ -hydroxyalkylamide crosslinkers, which have no urethane groups, are distinguished from those according to the invention Powder coatings produced coatings through a greatly improved Chemical resistance.
- any polymer can be used be that contains at least two carboxyl groups and melts at at least 60 ° C.
- the carboxyl group-containing polymers are preferably Polyester polycarboxylic acids consisting of polyols and polycarboxylic acids or their Derivatives are made.
- the melting range of these acidic polyesters is in a range of 60 to 160 ° C, preferably 80 to 120 ° C; their acid number varies from 10 to 150 mg KOH / g, preferably 30 to 60 mg KOH / g.
- the OH numbers should be less than 10 mg KOH / g.
- polyester polycarboxylic acids for the production of the polyester polycarboxylic acids to be used according to the invention are polycarboxylic acid, such as. B. oxal, adipine, 2,2,4 (2,4,4) trimethyladipine, Azelain, Sebacin, Decandicarbon, Dodecandicarbon, Fumar, Phthalic, isophthalic, terephthalic, trimellitic, pyromellitic acid are used.
- polyols used as polyols are: ethylene glycol, 1,2- and 1,3-propanediol, 1,2-, 1,3-, 1,4- and 2,3-butanediol, 1,5-pentanediol, 3-methyl-1,5-pentanediol, Neopentyl glycol, 1,12-dodecanediol, 2,2,4 (2,4,4) -trimethyl-1,6-hexanediol, Trimethylolpropane, glycerin, pentaerythritol, 1,4-bishydroxymethylcyclohexane, Cyclohexane-1,4-diol, diethylene glycol, triethylene glycol and dipropylene glycol.
- polyesters containing hydroxyl groups can also be used known processes from polycarboxylic acids and polyols are produced with Polycarboxylic acid and / or polycarboxylic acid anhydrides to the polyester polycar
- the monomers b) are preferably (cyclo) alkyl esters of acrylic or Methacrylic acid with 2 to 18 carbon atoms in the (cyclo) alkyl radical.
- suitable or preferably suitable monomers b) are ethyl (methyl) acrylate, n-propyl (meth) acrylate, Isopropyl (meth) acrylate, n-butyl (meth) acrylate, isobutyl (meth) acrylate, tert-butyl (meth) acrylate, 2-ethylhexyl (meth) acrylate, cyclohexyl methacrylate, Neopentyl methacrylate, isobornyl methacrylate, 3,3,5-trimethylcyclohexyl methacrylate and stearyl methacrylate.
- styrene vinyl toluene and ethyl styrene into consideration.
- d) are acrylic and methacrylic acid, which are also preferably used as well as crotonic acid, itaconic acid, fumaric acid, maleic acid and Citaconic acid,
- copolymerization Monomers a) to d) mentioned by customary radical polymerization processes take place, such as solution, emulsion, bead or bulk polymerization.
- the monomers are at temperatures of 60 to 160 ° C, preferably 80 up to 150 ° C, in the presence of radical formers and optionally molecular weight regulators copolymerized.
- the carboxyl-functional acrylate copolymers are prepared in inert form Solvents.
- Suitable solvents are, for example, aromatics, such as benzene, toluene, Xylene; Esters, such as ethyl acetate, butyl acetate, hexyl acetate, heptyl acetate, methyl glycol acetate, Ethyl glycol acetate, methoxypropylacetate; Ethers, such as tetrahydrofuran, Dioxane, diethylene glycol dimethyl ether; Ketones, such as acetone, methyl ethyl ketone, Methyl isobutyl ketone, methyl n-amyl ketone, methyl isoamyl ketone, or any Mixtures of such solvents.
- the copolymers can be prepared continuously or batchwise respectively. Usually in a polymerization reactor is uniform and continuously metered in the monomer mixture and the initiator and at the same time the appropriate amount of polymer continuously removed. Preferably you can chemically almost uniform copolymers are produced. Chemically close uniform Copolymers can also be made by the reaction mixture can run into a stirred tank at a constant speed, without to discharge the polymer.
- Some of the monomers can also be exemplified in solvents of the type mentioned Submit the species and the remaining monomers and auxiliaries separately or together enter this template at the reaction temperature. In general, this is done Polymerization under atmospheric pressure, however, can also be carried out at pressures up to 25 bar be performed.
- the initiators are used in amounts of 0.05 to 15% by weight, based on the total amount of monomers used.
- Suitable initiators are conventional radical initiators, such as. B. aliphatic azo compounds, such as azodiisobutyronitrile, azo-bis-2-methylvaleronitrile, 1,1'-azo-bis-1-cyclohexanenitrile and 2,2'-azo-bis-isobutyric acid alkyl ester; symmetrical diacyl peroxides, such as B. acetyl, propionyl or butyryl peroxide, with bromine, nitro, Methyl or methoxy groups substituted benzoyl peroxides, lauryl peroxides; symmetrical peroxydicarbonates, e.g. B.
- B. aliphatic azo compounds such as azodiisobutyronitrile, azo-bis-2-methylvaleronitrile, 1,1'-azo-bis-1-cyclohexanenitrile and 2,2'-azo-bis-isobutyric acid alkyl ester
- tert-butyl perbenzoate Hydroperoxides, such as for example tert-butyl hydroperoxide, cumene hydroperoxide; Dialkyl peroxides, such as Dicumyl peroxide, tert-butyl cumyl peroxide or di-tert-butyl peroxide.
- the molecular weight of the copolymers can be customary regulators in the preparation be used. Examples include mercaptopropionic acid, tert-dodecyl mercaptan, n-dodecyl mercaptan or diisopropylxanthogen disulfide.
- the Regulators can be in amounts of 0.1 to 10 wt .-%, based on the total amount of Monomers can be added.
- solutions of the copolymers obtained during the copolymerization can then fed to the evaporation or degassing process without further processing be in which the solvent, for example in an evaporation extruder or Spray dryer at approx. 120 to 160 ° C and a vacuum of 100 to 300 mbar removed and the copolymers to be used according to the invention obtained become.
- the mixing ratio of the carboxyl group-containing polymers and those of the invention urethanized ⁇ -hydroxyalkylamide compound is usually like this chosen that the ratio of carboxyl groups to hydroxyl groups 0.6: 1 to 1.6: 1 is.
- the addition of a catalyst to increase the gelling rate of the thermosetting powder coating materials is not necessary.
- the acidic polymer contains an aliphatic resin which contains residues of 1,4-cyclohexanedicarboxylic acid (CHDA) or 2,2,4,4-tetramethyl-1,3-cyclobutanediol of 1,4-CHDA or hydrogenated bisphenol A.
- CHDA 1,4-cyclohexanedicarboxylic acid
- 2,2,4,4-tetramethyl-1,3-cyclobutanediol of 1,4-CHDA or hydrogenated bisphenol A As described in WO 95/01406, catalysts composed of C 1 -C 18 zinc, aluminum or titanium carboxylate salts or aluminum or zinc oxides have an accelerating effect. They are used in amounts of 0.03 to 1.0% by weight, based on the total amount of powder.
- the urethanized ⁇ -hydroxyalkylamide compounds according to the invention are for the production of powder coatings with the appropriate carboxyl group Polymers and optionally catalysts as well as pigments and conventional auxiliaries such as fillers, degassing agents and leveling agents. All ingredient of the powder coating are homogenized in the melt. This can be done in suitable units, such as. B. heatable kneaders, but preferably by Extrude, take place, with upper temperature limits of 130 to 140 ° C not should be exceeded. The extruded mass opens up after cooling Room temperature and after suitable comminution to the ready-to-spray powder grind.
- the application of the ready-to-spray powder to suitable substrates can by the known methods, such as. B. by electrostatic or tribostatic Powder spraying, sintering, electrostatic sintering. After this The coated workpieces are applied in powder form for curing for 60 to 5 minutes heated to a temperature of 150 to 220 ° C.
- VESTANAT® T 1890 isocyanurate from IPDI, NCO number 17.1%, CREANOVA Spezialchemie GmbH
- isophorone diisocyanate IPDI, NCO number 37.8%, CREANOVA Spezialchemie GmbH
- the ⁇ -hydroxyalkylamide A) is placed in a steel pot and in a Temperature of approx. 125 ° C melted.
- the polyisocyanate B) is added. After a reaction time of about 3 hours, the product cooled and crushed.
- the acidic polyester Grilesta P 7617 from EMS-Chemie AG (Acid number 35 mg KOH / g, Tg 61 ° C, viscosity at 200 ° C 3 500 mPa ⁇ s) used.
- the crushed products i.e. H. urethanized ⁇ -hydroxyalkylamide compound, acidic polyester, leveling agent masterbatch, if necessary catalysts, are mixed with the White pigment intimately mixed in a pan mill and then in one Homogenized twin screw extruder from Berstorff up to a maximum of 130 ° C.
- the extrudate is broken and placed on a pin mill Grain size ⁇ 100 ⁇ m milled.
- the powder produced in this way is treated with an electrostatic Powder spraying system at 60 KV on degreased, possibly pre-treated iron sheets applied and in a convection oven at temperatures between 150 and Burned in at 220 ° C.
- leveling agent - a commercial copolymer of butyl acrylate and 2-ethylhexyl acrylate - in the corresponding polyester in the The melt is homogenized and crushed after solidification.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Paints Or Removers (AREA)
- Polyurethanes Or Polyureas (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Macromolecular Compounds Obtained By Forming Nitrogen-Containing Linkages In General (AREA)
Abstract
Description
und
- A
- Herstellung der erfindungsgemäßen urethanisierten β-Hydroxyalkylamid-Verbindung
- A 1
- β-Hydroxyalkylamid A)
Als β-Hydroxyalkylamid A) wurde das PRIMID XL-552 (OH-Zahl: 682 mg KOH/g, Schmelzpunkt: 125 °C, EMS-Chemie AG) eingesetzt.
| Urethanisierte β-Hydroxyalkylamide im Vergleich zum β-Hydroxyalkylamid | |||||
| Beispiele Nr. | Zusamensetzung | Kenndaten | |||
| PRIMID XL-552 [Gew.-%] | T 1890 [Gew.-%] | IPDI [Gew.-%] | OH-Zahl [mg KOH/g] | Smp. [°C} | |
| A 3.1 | 72,9 | 27,1 | - | 435 | 93 - 98 |
| A 3.2 | 80,2 | 19,8 | - | 501 | 104 - 110 |
| A 3.3 | 84,4 | 15,6 | - | 539 | 109 - 113 |
| A 3.4 | 73,9 | 24,8 | 1,3 | 441 | 90 - 95 |
| A 3.5 Vergleich | 100 | - | - | 682 | 125 |
- SD
- = Schichtdicke in µm
- HK
- = Härte nach König (sec) (DIN 53 157)
- ET
- = Tiefung nach Erichsen (DIN 53 156)
- GS
- = Gitterschnittprüfung (DIN 53 151)
- GG 60 °-Winkel
- = Messung des Glanzes n. Gardner (ASTM-D 5233)
- Imp rev.
- = Impact reverse in inch·lb
- MEK-Test
- = Methylethylketon-Test in Hüben
| Pigmentierte Pulverlacke | |||||
| Beispiel C Rezeptur | 1 | 2 | 3 | 4 | 5 Vergleich |
| Vernetzer gem. A 3 | 6,86 | 6,53 | 6,09 | 7,35 | 4,84 |
| Tabelle 1 | (1) | (2) | (3) | (4) | (5) |
| Polyester gem. B | 93,14 | 93,47 | 93,91 | 92,65 | 95,16 |
| Bemerkungen: | 35 Gew.-% TiO2 (Weißpigment), 1,0 Gew.-% Resiflow PV 88, 0,3 Gew.-% Benzoin, COOH/OH-Verhältnis = 1 : 1 | ||||
| Lack-Daten | |||||
| SD | 50 - 64 | 70 - 94 | 62 - 96 | 55 - 70 | 81 - 101 |
| HK | 174 | 192 | 195 | 192 | 193 |
| GS | 0 | 0 | 0 | 0 | 0 |
| GG 60 °-Winkel | 92 | 92 | 93 | 91 | 92 |
| ET | > 10 | > 10 | > 10 | > 10 | > 10 |
| Imp. Rev. | > 80 | > 80 | > 80 | > 80 | > 80 |
| MEK-Test | 32 | 50 | 50 | 46 | 16 |
| Härtung: | 200 °C/10 Minuten |
Claims (19)
- Urethanisierte β-Hydroxyalkylamid-Verbindungen, aufgebaut aus den Komponentenwobei die urethanisierten β-Hydroxyalkylamid-Verbindungen terminal Hydroxylgruppen tragen und eine Funktionalität ≥ 2 aufweisen.A) 65 bis 96 Gew.-% β-Hydroxyalkylamid,
undB) 4 bis 35 Gew.-%eines nicht aromatischen Polyisocyanats mit einer NCO-Funktionalität ≥ 2, - Urethanisierte β-Hydroxyalkylamid-Verbindungen nach Anspruch 1,
dadurch gekennzeichnet,
daß das β-Hydroxyalkylamid A) die Formel aufweist in der R1 Wasserstoff oder eine C1-C5-Alkylgruppe, R2 Wasserstoff, eine C1-C5-Alkylgruppe oder und A eine chemische Bindung oder eine einwertige oder mehrwertige organische Gruppe, ausgewählt aus gesättigten, ungesättigten oder aromatischen Kohlenwasserstoffgruppen, oder substituierten Kohlenwasserstoffgruppen mit 2 bis 20 Kohlenstoffatomen, bedeuten, m ist 1 bis 2, n ist 0 bis 2 und m + n ist mindestens 2. - Urethanisierte β-Hydroxyalkylamid-Verbindungen nach Anspruch 1 oder 2, wobei diese eine Funktionalität ≥ 4 aufweisen.
- Urethanisierte β-Hydroxyalkylamid-Verbindung nach mindestens einem der Ansprüche 1 bis 3,
dadurch gekennzeichnet,
daß als Polyisocyanat B) mindestens eine Verbindung aus der Gruppe der aliphatischen, (cyclo)aliphatischen, cycloaliphatischen oder heterocyclischen Polyisocyanate mit mindestens zwei Isocyanatgruppen ausgewählt wird. - Urethanisierte β-Hydroxyalkylamid-Verbindung nach mindestens einem der Ansprüche 1 bis 4,
dadurch gekennzeichnet,
daß als Polyisocyanat B) mindestens eine Verbindung ausgewählt aus Ethylendiisocyanat, Propylendiisocyanat, Tetramethylendiisocyanat, Pentamethylendiisocyanat, 2-Methylpentamethylen-1,5-diisocyanat (MPDI), Hexamethylendiisocyanat (HDI), Trimethylhexamethylen-1,6-diisoyanat (TMDI), insbesondere das 2,2,4- und das 2,4,4-Isomere und technische Gemische beider Isomeren, Decamethylendiisocyanat und Dodecamethylendiisocyanat, 1,3-Cyclopentyldiisocyanat, 1,2-Cyclohexyldiisocyanat, 1,4-Cyclohexyldiisocyanat, ω,ω' -Diisocyanato-1,4-methylcyclohexan, ω,ω' -Diisocyanato-1,3-dimethylcyclohexan, 1-Methyl-2,4-diisocyanatocyclohexan, 4,4' -Methylen-bis(cyclohexylisocyanat), Norbornandiisocyanat (NBDI), 3,3,5-Trimethyl-1-isocyanato-3-isocyanatomethylcclohexan (Isophorondiisocyanat IPDI) eingesetzt wird. - Urethanisierte β-Hydroxyalkylamid-Verbindung nach mindestens einem der Ansprüche 1 bis 5,
dadurch gekennzeichnet,
daß als Polyisocyanat B) beliebige Gemische aus 2-Methylpentamethylen-1,5-diisocyanat, 2,2,4-Trimethylhexamethylen-1,6-diisocyanat, 2,4,4-Trimethyl-hexamethylen-1,6-diisocyanat, Norbornandiisocyanat, Isophorondiisocyanat, Isocyanurat des 2-Methylpentamethylen-1,5-diisocyanat, Isocyanurat des Hexamethylendiisocyanat oder Isocyanurat des Isophorondiisocyanat eingesetzt wird. - Verfahren zur Herstellung von urethanisierten β-Hydroxyalkylamid-Verbindung,
dadurch gekennzeichnet,
daß 65 bis 96 Gew.-% mindestens eines β-Hydroxyalkylamid A) mit 4 bis 35 Gew.-% mindestens eines nicht aromatischen Polyisocyanats B) umgesetzt werden, die urethanisierten β-Hydroxyalkylamid-Verbindungen terminal Hydroxylgruppen tragen und eine Funktionalität ≥ 2 aufweisen. - Verfahren zur Herstellung der urethanisierten β-Hydroxyalkylamid-Verbindung nach Anspruch 7,
dadurch gekennzeichnet,
daß die Umsetzung der Verbindungen A) und B) lösemittelfrei in einem Batchprozeß erfolgt. - Verwendung der urethanisierten β-Hydroxyalkylamid-Verbindung nach den Ansprüchen 1 bis 8,
in Kombination mit carboxylgruppenhaltigen Polymeren zur Herstellung transparenter oder pigmentierter witterungsbeständiger Pulverlacke mit sehr guter Chemikalienresistenz. - Transparente oder pigmentierte Pulverlacke,
dadurch gekennzeichnet,
daß diese mindestens eine urethanisierte β-Hydroxyalkylamid-Verbindung gemäß den Ansprüchen 1 bis 6, in Kombination mit carboxylgruppenhaltigen Polymeren sowie weiteren Hilfs- und Zuschlagstoffe, enthalten. - Transparente oder pigmentierte Pulverlacke nach Anspruch 10,
dadurch gekennzeichnet,
daß als Hilfs- und Zuschlagstoffe Füllstoffe, Verlaufmittel, Entgasungsmittel oder Katalysatoren enthalten sind. - Transparente oder pigmentierte Pulverlacke nach den Ansprüchen 10 bis 11,
dadurch gekennzeichnet,
daß ein COOH/OH-Verhältnis von 0,6 : 1,0 bis 1,6 : 1,0 zugrunde liegt. - Transparente oder pigmentierte Pulverlacke nach Anspruch 12,
dadurch gekennzeichnet,
daß ein COOH/OH-Verhältnis von 0,8 : 1,0 bis 1,2 : 1,0 zugrunde liegt. - Transparente oder pigmentierte Pulverlacke nach Anspruch 12,
dadurch gekennzeichnet,
daß ein COOH/OH-Verhältnis von 1 : 1 bis 1 : 1 zugrunde liegt. - Transparente oder pigmentierte Pulverlacke nach den Ansprüchen 10 bis 14,
dadurch gekennzeichnet,
daß die Pulverlacke Katalysatoren in einer Konzentration von 0,03 bis 1,0 Gew.-%, bezogen auf die gesamte Pulverlackmenge, enthalten. - Transparente oder pigmentierte Pulverlacke nach Anspruch 15,
dadurch gekennzeichnet,
daß die Pulverlacke als Katalysatoren Aluminium-Carboxylat-Salze, Titan-Carboxylat-Salze, Aluminiumoxide oder Zinkoxide enthalten. - Transparente oder pigmentierte Pulverlacke nach den Ansprüchen 10 bis 16,
dadurch gekennzeichnet,
daß als carboxylgruppenhaltige Polymere Polycarboxylpolyester oder Polycarboxylpolyacrylate eingesetzt werden. - Transparente oder pigmentierte Pulverlacke nach Anspruch 17,
dadurch gekennzeichnet,
daß die Polycarboxylpolyester eine Säurezahl von 10 bis 150 mg KOH/g, und einen Schmelzpunkt von 60 bis 160 °C aufweisen. - Transparente oder pigmentierte Pulverlacke nach Anspruch 17,
dadurch gekennzeichnet,
daß die Polycarboxylpolyacrylate eine Säurezahl von 10 bis 150 mg KOH/g und einen Schmelzpunkt von 60 bis 160 °C aufweisen.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19925543 | 1999-06-04 | ||
| DE19925543A DE19925543A1 (de) | 1999-06-04 | 1999-06-04 | Urethanisierte beta-Hydroxyalkylamid-Verbindung, ein Verfahren zu ihrer Herstellung sowie deren Verwendung zur Herstellung von Pulverlacken |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1061069A1 true EP1061069A1 (de) | 2000-12-20 |
| EP1061069B1 EP1061069B1 (de) | 2003-06-04 |
Family
ID=7910192
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP00108883A Expired - Lifetime EP1061069B1 (de) | 1999-06-04 | 2000-04-26 | Urethanisierte Beta-Hydroxyalkylamid-Verbindung, ein Verfahren zu ihrer Herstellung sowie deren Verwendung zur Herstellung von Pulverlacken |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US6342576B1 (de) |
| EP (1) | EP1061069B1 (de) |
| JP (1) | JP2001011147A (de) |
| AT (1) | ATE242203T1 (de) |
| AU (1) | AU764357B2 (de) |
| DE (2) | DE19925543A1 (de) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1127905A1 (de) * | 2000-02-25 | 2001-08-29 | Degussa AG | Transparente oder pigmentierte Pulverlacke mit Vernetzern aus Hydroxyalkylamiden und blockierten, nicht aromatischen Polyisocyanaten |
| WO2008060411A3 (en) * | 2006-11-14 | 2008-08-07 | Bayer Materialscience Llc | New liquid modified diphenylmethane diisocyanates |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10035013A1 (de) * | 2000-07-19 | 2002-01-31 | Bayer Ag | Verfahren zur Herstellung von Uretdionpolyisocyanaten mit verbesserter Monomerenstabilität |
| US6503999B1 (en) * | 2001-06-04 | 2003-01-07 | Ppg Industries Ohio, Inc. | Use of beta-hydroxyalkylamide in ambient and low bake liquid coatings |
| WO2004059083A1 (ja) * | 2002-12-26 | 2004-07-15 | Canon Kabushiki Kaisha | カール低減剤、インクジェット用インク、インクジェット記録方法及びカール低減方法 |
| JP5211307B2 (ja) * | 2011-03-04 | 2013-06-12 | 東洋インキScホールディングス株式会社 | 感光性組成物 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0322834A2 (de) * | 1987-12-30 | 1989-07-05 | Ppg Industries, Inc. | Hydroxyalkylamid-Härtungsmittel für Pulverlacke |
| EP0698629A2 (de) * | 1994-08-26 | 1996-02-28 | Ems-Inventa Ag | Epoxidfreies, wärmehärtbares Beschichtungssystem |
| EP0789043A2 (de) * | 1996-02-08 | 1997-08-13 | Bayer Ag | Polyurethan-Pulvermattlacke |
-
1999
- 1999-06-04 DE DE19925543A patent/DE19925543A1/de not_active Withdrawn
-
2000
- 2000-04-26 EP EP00108883A patent/EP1061069B1/de not_active Expired - Lifetime
- 2000-04-26 DE DE50002421T patent/DE50002421D1/de not_active Expired - Fee Related
- 2000-04-26 AT AT00108883T patent/ATE242203T1/de not_active IP Right Cessation
- 2000-05-04 AU AU32523/00A patent/AU764357B2/en not_active Ceased
- 2000-05-25 US US09/577,598 patent/US6342576B1/en not_active Expired - Fee Related
- 2000-06-01 JP JP2000164803A patent/JP2001011147A/ja not_active Withdrawn
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0322834A2 (de) * | 1987-12-30 | 1989-07-05 | Ppg Industries, Inc. | Hydroxyalkylamid-Härtungsmittel für Pulverlacke |
| EP0698629A2 (de) * | 1994-08-26 | 1996-02-28 | Ems-Inventa Ag | Epoxidfreies, wärmehärtbares Beschichtungssystem |
| EP0789043A2 (de) * | 1996-02-08 | 1997-08-13 | Bayer Ag | Polyurethan-Pulvermattlacke |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1127905A1 (de) * | 2000-02-25 | 2001-08-29 | Degussa AG | Transparente oder pigmentierte Pulverlacke mit Vernetzern aus Hydroxyalkylamiden und blockierten, nicht aromatischen Polyisocyanaten |
| US6500548B2 (en) | 2000-02-25 | 2002-12-31 | Degussa Ag | Transparent or pigmented powder coating materials with crosslinkers comprising hydroxyalkylamides and blocked nonaromatic polyisocyanates |
| WO2008060411A3 (en) * | 2006-11-14 | 2008-08-07 | Bayer Materialscience Llc | New liquid modified diphenylmethane diisocyanates |
Also Published As
| Publication number | Publication date |
|---|---|
| US6342576B1 (en) | 2002-01-29 |
| AU764357B2 (en) | 2003-08-14 |
| JP2001011147A (ja) | 2001-01-16 |
| EP1061069B1 (de) | 2003-06-04 |
| DE50002421D1 (de) | 2003-07-10 |
| DE19925543A1 (de) | 2000-12-07 |
| AU3252300A (en) | 2000-12-07 |
| ATE242203T1 (de) | 2003-06-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0669351B1 (de) | Pulverlack und seine Verwendung | |
| DE3033860A1 (de) | Neue isocyanato-isocyanurate, ein verfahren zu ihrer herstellung, sowie ihre verwendung als isocyanatkomponente in polyurethanlacken | |
| DE3624775A1 (de) | Pulverlack und seine verwendung zur beschichtung von hitzeresistenten substraten | |
| EP0652263B1 (de) | Pulverlack und seine Verwendung | |
| DE69001651T2 (de) | Ueberzugszusammensetzungen, daraus hergestellte ueberzuege mit matter oberflaeche sowie blockierte polyisocyanate. | |
| EP0531862B1 (de) | Verfahren zur Herstellung von Pulverlacken und ihre Verwendung | |
| EP1061069B1 (de) | Urethanisierte Beta-Hydroxyalkylamid-Verbindung, ein Verfahren zu ihrer Herstellung sowie deren Verwendung zur Herstellung von Pulverlacken | |
| EP0555705B1 (de) | Pulverlack, ein Verfahren zu seiner Herstellung und seine Verwendung | |
| DE3541859A1 (de) | Verfahren zur herstellung von isocyanuratgruppen aufweisenden polyisocyanaten und ihre verwendung als isocyanatkomponente in polyurethanlacken | |
| DE4209035A1 (de) | Verfahren zur herstellung von hydroxyfunktionellen copolymerisaten | |
| DE19841542A1 (de) | Feste oxazolinterminierte, urethangruppenhaltige Polyaddionsverbindungen, ein Verfahren zu ihrer Herstellung sowie deren Verwendung | |
| EP0694592B1 (de) | Pulverlack und seine Verwendung | |
| DE2515485A1 (de) | Triisocyanate und ihre verwendung | |
| EP0085913B1 (de) | Reaktive carboxylgruppenhaltige Polymere, Verfahren zu ihrer Herstellung und ihre Verwendung als Bindemittelkomponente für Pulverlacke | |
| EP0686176B1 (de) | Pulverlacke auf basis carboxylgruppen enthaltender polyester und geigneter vernetzungsmittel sowie verfahren zur beschichtung von metallblechen | |
| EP0538597A1 (de) | Blockierte höherfuntionelle Polyisocyanataddukte, ein Verfahren zu ihrer Herstellung sowie deren Verwendung | |
| EP1127906B1 (de) | Transparente oder pigmentierte Pulverlacke mit Vernetzern aus Hydroxyalkylamiden und Uretdiongruppen enthaltenden Polyisocyanaten | |
| EP0519186B1 (de) | Pulverlacke | |
| DE19754748B4 (de) | Verfahren zur Herstellung eines blockierten Lackpolyisocyanats und dessen Verwendung für PUR-Lacke | |
| EP1127905B1 (de) | Transparente oder pigmentierte Pulverlacke mit Vernetzern aus Hydroxyalkylamiden und blockierten, nicht aromatischen Polyisocyanaten | |
| DE19928290A1 (de) | Epoxidterminierte, uretdiongruppenhaltige Polyadditionsverbindungen, Verfahren zu ihrer Herstellung sowie deren Verwendung | |
| DE19729242A1 (de) | Pulverisierbare Bindemittel | |
| EP1491564B1 (de) | Polyisocyanat-modifizierte Polycarbonsäuren | |
| DE3138196A1 (de) | Pulverfoermiges ueberzugsmittel und seine verwendung | |
| EP0473033A1 (de) | Pulverlacke und die Verwendung von ausgewählten Bindemittelkombinationen in Pulverlacken |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20000426 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: DEGUSSA AG |
|
| AKX | Designation fees paid |
Free format text: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| 17Q | First examination report despatched |
Effective date: 20020326 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030604 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030604 Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030604 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030604 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: CH Ref legal event code: NV Representative=s name: SCHMAUDER & PARTNER AG PATENTANWALTSBUERO |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: GERMAN |
|
| REF | Corresponds to: |
Ref document number: 50002421 Country of ref document: DE Date of ref document: 20030710 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20030723 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030904 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030904 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030904 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030904 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030915 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| ET | Fr: translation filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20040331 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20040405 Year of fee payment: 5 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20040415 Year of fee payment: 5 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040426 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040430 Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040430 |
|
| 26N | No opposition filed |
Effective date: 20040305 |
|
| BERE | Be: lapsed |
Owner name: *DEGUSSA A.G. Effective date: 20040430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050426 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050426 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050426 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20050426 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20051230 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20051230 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20080415 Year of fee payment: 9 Ref country code: DE Payment date: 20080418 Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PCAR Free format text: SCHMAUDER & PARTNER AG PATENT- UND MARKENANWAELTE VSP;ZWAENGIWEG 7;8038 ZUERICH (CH) |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: EVONIK DEGUSSA GMBH Free format text: DEGUSSA AG#BENNIGSENPLATZ 1#40474 DUESSELDORF (DE) -TRANSFER TO- EVONIK DEGUSSA GMBH#RELLINGHAUSER STRASSE 1-11#45128 ESSEN (DE) |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090430 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20091103 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090430 |