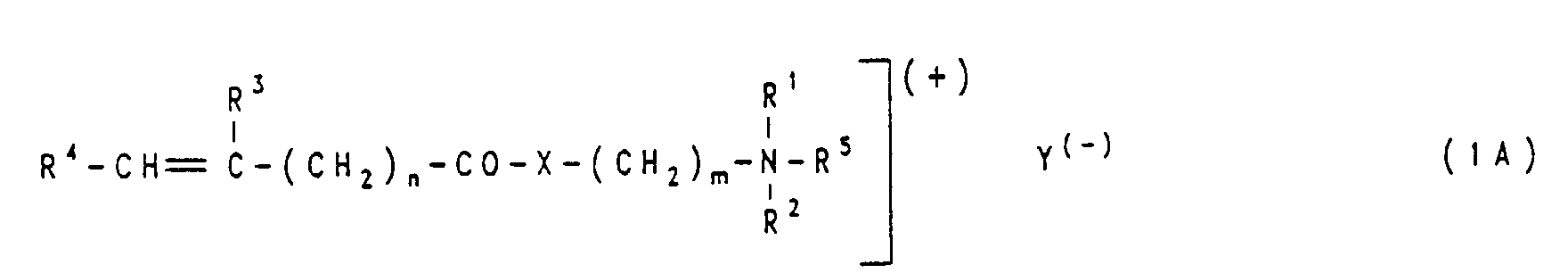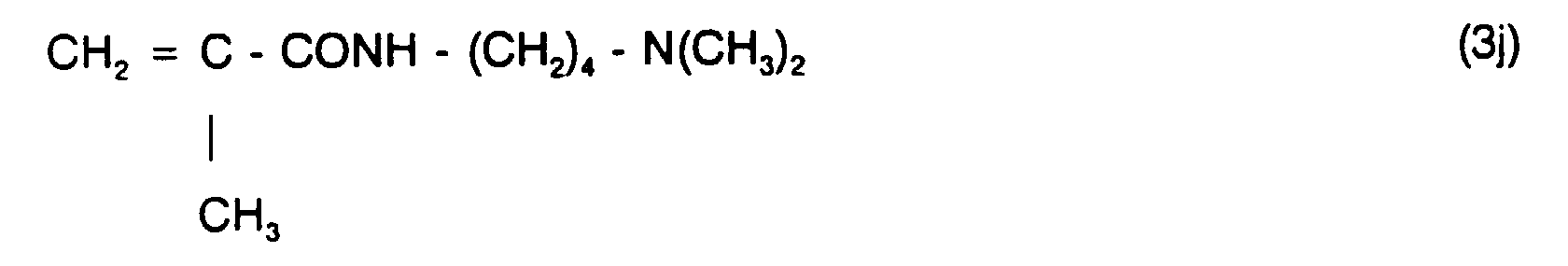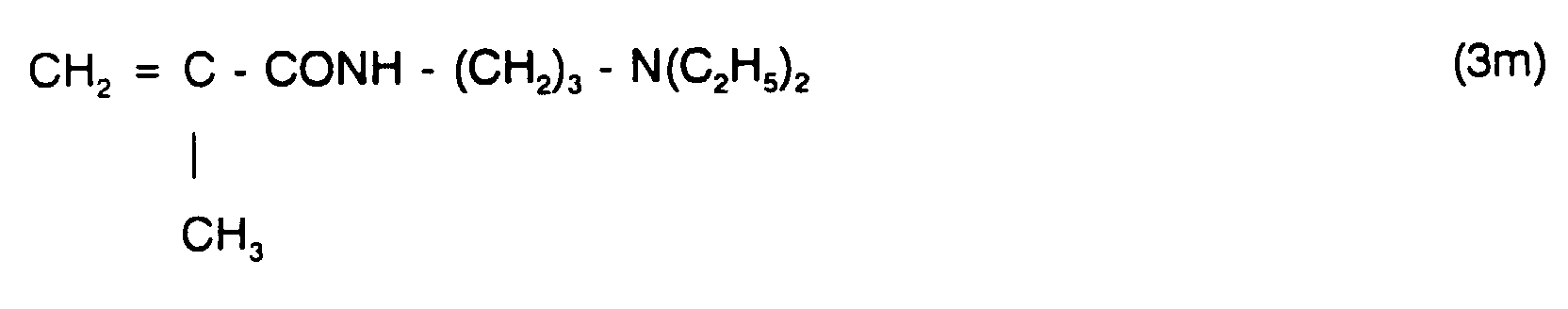EP0509397B1 - Process for dyeing cellulosic fibrous material modified with N-containing basic polymers with anionic reactive dyestuffs, and the modified cellulosic fibrous material as such - Google Patents
Process for dyeing cellulosic fibrous material modified with N-containing basic polymers with anionic reactive dyestuffs, and the modified cellulosic fibrous material as such Download PDFInfo
- Publication number
- EP0509397B1 EP0509397B1 EP92106157A EP92106157A EP0509397B1 EP 0509397 B1 EP0509397 B1 EP 0509397B1 EP 92106157 A EP92106157 A EP 92106157A EP 92106157 A EP92106157 A EP 92106157A EP 0509397 B1 EP0509397 B1 EP 0509397B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- hydrogen
- carbon atoms
- methyl
- radical
- alkyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000004043 dyeing Methods 0.000 title claims description 45
- 239000002657 fibrous material Substances 0.000 title claims description 45
- 238000000034 method Methods 0.000 title claims description 45
- 229920000642 polymer Polymers 0.000 title claims description 26
- 125000000129 anionic group Chemical group 0.000 title claims description 10
- -1 alkyl radical Chemical class 0.000 claims description 61
- 229920003043 Cellulose fiber Polymers 0.000 claims description 49
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 48
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 43
- 125000004432 carbon atom Chemical group C* 0.000 claims description 42
- 239000000975 dye Substances 0.000 claims description 40
- 239000001257 hydrogen Substances 0.000 claims description 39
- 229910052739 hydrogen Inorganic materials 0.000 claims description 39
- 239000000178 monomer Substances 0.000 claims description 31
- 229920000742 Cotton Polymers 0.000 claims description 26
- 125000000217 alkyl group Chemical group 0.000 claims description 20
- 150000001875 compounds Chemical class 0.000 claims description 19
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 17
- 150000003254 radicals Chemical class 0.000 claims description 17
- 229910052757 nitrogen Inorganic materials 0.000 claims description 16
- 150000001450 anions Chemical class 0.000 claims description 15
- 238000006116 polymerization reaction Methods 0.000 claims description 14
- 125000005842 heteroatom Chemical group 0.000 claims description 13
- 239000000985 reactive dye Substances 0.000 claims description 13
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 12
- 239000000758 substrate Substances 0.000 claims description 11
- 229920001577 copolymer Polymers 0.000 claims description 10
- 239000000835 fiber Substances 0.000 claims description 10
- 150000002431 hydrogen Chemical class 0.000 claims description 9
- 229920006395 saturated elastomer Polymers 0.000 claims description 9
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 8
- 239000003792 electrolyte Substances 0.000 claims description 7
- 239000003513 alkali Substances 0.000 claims description 6
- 239000001301 oxygen Substances 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 5
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 claims description 4
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 4
- 125000003884 phenylalkyl group Chemical group 0.000 claims description 4
- 125000001424 substituent group Chemical group 0.000 claims description 4
- 240000008564 Boehmeria nivea Species 0.000 claims description 2
- 240000000491 Corchorus aestuans Species 0.000 claims description 2
- 235000011777 Corchorus aestuans Nutrition 0.000 claims description 2
- 235000010862 Corchorus capsularis Nutrition 0.000 claims description 2
- 229920001519 homopolymer Polymers 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 239000004627 regenerated cellulose Substances 0.000 claims description 2
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 claims 2
- 125000004429 atom Chemical group 0.000 claims 1
- 230000000379 polymerizing effect Effects 0.000 claims 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 78
- 239000004744 fabric Substances 0.000 description 54
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 41
- 239000000243 solution Substances 0.000 description 37
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 22
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 16
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 15
- 238000010521 absorption reaction Methods 0.000 description 15
- 235000002639 sodium chloride Nutrition 0.000 description 12
- 239000011780 sodium chloride Substances 0.000 description 11
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 10
- 239000000203 mixture Substances 0.000 description 10
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 238000007334 copolymerization reaction Methods 0.000 description 9
- 238000011282 treatment Methods 0.000 description 9
- 239000000843 powder Substances 0.000 description 8
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 7
- 229960000583 acetic acid Drugs 0.000 description 7
- 239000007864 aqueous solution Substances 0.000 description 7
- 239000003999 initiator Substances 0.000 description 7
- FLCAEMBIQVZWIF-UHFFFAOYSA-N 6-(dimethylamino)-2-methylhex-2-enamide Chemical compound CN(C)CCCC=C(C)C(N)=O FLCAEMBIQVZWIF-UHFFFAOYSA-N 0.000 description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 6
- ROOXNKNUYICQNP-UHFFFAOYSA-N ammonium persulfate Chemical compound [NH4+].[NH4+].[O-]S(=O)(=O)OOS([O-])(=O)=O ROOXNKNUYICQNP-UHFFFAOYSA-N 0.000 description 6
- 229920002678 cellulose Polymers 0.000 description 6
- 239000001913 cellulose Substances 0.000 description 6
- 239000012362 glacial acetic acid Substances 0.000 description 6
- 238000010992 reflux Methods 0.000 description 6
- 239000004753 textile Substances 0.000 description 6
- 239000000080 wetting agent Substances 0.000 description 6
- 239000004202 carbamide Substances 0.000 description 5
- 239000012153 distilled water Substances 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 4
- GQOKIYDTHHZSCJ-UHFFFAOYSA-M dimethyl-bis(prop-2-enyl)azanium;chloride Chemical compound [Cl-].C=CC[N+](C)(C)CC=C GQOKIYDTHHZSCJ-UHFFFAOYSA-M 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 3
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 229910001870 ammonium persulfate Inorganic materials 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- 0 *C1CCCCC1 Chemical compound *C1CCCCC1 0.000 description 2
- FQLDWHZJBSAUDT-UHFFFAOYSA-N 2-ethenyl-5-methyl-4,5-dihydro-1h-imidazole Chemical compound CC1CN=C(C=C)N1 FQLDWHZJBSAUDT-UHFFFAOYSA-N 0.000 description 2
- XUGNJOCQALIQFG-UHFFFAOYSA-N 2-ethenylquinoline Chemical compound C1=CC=CC2=NC(C=C)=CC=C21 XUGNJOCQALIQFG-UHFFFAOYSA-N 0.000 description 2
- KGIGUEBEKRSTEW-UHFFFAOYSA-N 2-vinylpyridine Chemical compound C=CC1=CC=CC=N1 KGIGUEBEKRSTEW-UHFFFAOYSA-N 0.000 description 2
- KFDVPJUYSDEJTH-UHFFFAOYSA-N 4-ethenylpyridine Chemical compound C=CC1=CC=NC=C1 KFDVPJUYSDEJTH-UHFFFAOYSA-N 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 2
- LXEKPEMOWBOYRF-UHFFFAOYSA-N [2-[(1-azaniumyl-1-imino-2-methylpropan-2-yl)diazenyl]-2-methylpropanimidoyl]azanium;dichloride Chemical compound Cl.Cl.NC(=N)C(C)(C)N=NC(C)(C)C(N)=N LXEKPEMOWBOYRF-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 239000000987 azo dye Substances 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 238000010411 cooking Methods 0.000 description 2
- 150000002009 diols Chemical class 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 239000003791 organic solvent mixture Substances 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000002985 plastic film Substances 0.000 description 2
- 229920006255 plastic film Polymers 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 235000019353 potassium silicate Nutrition 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 2
- YPFDHNVEDLHUCE-UHFFFAOYSA-N propane-1,3-diol Chemical compound OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 2
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 2
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- BEQKKZICTDFVMG-UHFFFAOYSA-N 1,2,3,4,6-pentaoxepane-5,7-dione Chemical compound O=C1OOOOC(=O)O1 BEQKKZICTDFVMG-UHFFFAOYSA-N 0.000 description 1
- LZDKZFUFMNSQCJ-UHFFFAOYSA-N 1,2-diethoxyethane Chemical compound CCOCCOCC LZDKZFUFMNSQCJ-UHFFFAOYSA-N 0.000 description 1
- LGJCFVYMIJLQJO-UHFFFAOYSA-N 1-dodecylperoxydodecane Chemical compound CCCCCCCCCCCCOOCCCCCCCCCCCC LGJCFVYMIJLQJO-UHFFFAOYSA-N 0.000 description 1
- ZIMGAUCXTGSYIY-UHFFFAOYSA-N 1-ethenyl-2,4-dimethylimidazole Chemical compound CC1=CN(C=C)C(C)=N1 ZIMGAUCXTGSYIY-UHFFFAOYSA-N 0.000 description 1
- BDHGFCVQWMDIQX-UHFFFAOYSA-N 1-ethenyl-2-methylimidazole Chemical compound CC1=NC=CN1C=C BDHGFCVQWMDIQX-UHFFFAOYSA-N 0.000 description 1
- HXVJQEGYAYABRY-UHFFFAOYSA-N 1-ethenyl-4,5-dihydroimidazole Chemical compound C=CN1CCN=C1 HXVJQEGYAYABRY-UHFFFAOYSA-N 0.000 description 1
- OSSNTDFYBPYIEC-UHFFFAOYSA-N 1-ethenylimidazole Chemical compound C=CN1C=CN=C1 OSSNTDFYBPYIEC-UHFFFAOYSA-N 0.000 description 1
- CTXUTPWZJZHRJC-UHFFFAOYSA-N 1-ethenylpyrrole Chemical compound C=CN1C=CC=C1 CTXUTPWZJZHRJC-UHFFFAOYSA-N 0.000 description 1
- QYLYCIMKTCNLGY-UHFFFAOYSA-N 11-methyldodecoxycarbonyloxy 11-methyldodecyl carbonate Chemical compound CC(C)CCCCCCCCCCOC(=O)OOC(=O)OCCCCCCCCCCC(C)C QYLYCIMKTCNLGY-UHFFFAOYSA-N 0.000 description 1
- NIDVYFMEMGTAJQ-UHFFFAOYSA-N 2-(1-ethenylimidazol-4-yl)ethanol Chemical compound OCCC1=CN(C=C)C=N1 NIDVYFMEMGTAJQ-UHFFFAOYSA-N 0.000 description 1
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 1
- XMNIXWIUMCBBBL-UHFFFAOYSA-N 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)OOC(C)(C)C1=CC=CC=C1 XMNIXWIUMCBBBL-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- QMYCJCOPYOPWTI-UHFFFAOYSA-N 2-[(1-amino-1-imino-2-methylpropan-2-yl)diazenyl]-2-methylpropanimidamide;hydron;chloride Chemical compound Cl.NC(=N)C(C)(C)N=NC(C)(C)C(N)=N QMYCJCOPYOPWTI-UHFFFAOYSA-N 0.000 description 1
- WYGWHHGCAGTUCH-UHFFFAOYSA-N 2-[(2-cyano-4-methylpentan-2-yl)diazenyl]-2,4-dimethylpentanenitrile Chemical compound CC(C)CC(C)(C#N)N=NC(C)(C#N)CC(C)C WYGWHHGCAGTUCH-UHFFFAOYSA-N 0.000 description 1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 1
- WFUGQJXVXHBTEM-UHFFFAOYSA-N 2-hydroperoxy-2-(2-hydroperoxybutan-2-ylperoxy)butane Chemical compound CCC(C)(OO)OOC(C)(CC)OO WFUGQJXVXHBTEM-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- XHKSJRJFBBOFAM-UHFFFAOYSA-N 2-prop-1-en-2-yl-4,5-dihydro-1h-imidazole Chemical compound CC(=C)C1=NCCN1 XHKSJRJFBBOFAM-UHFFFAOYSA-N 0.000 description 1
- SFOHETZMLXYFPZ-UHFFFAOYSA-N 2-prop-1-en-2-ylquinoline Chemical compound C1=CC=CC2=NC(C(=C)C)=CC=C21 SFOHETZMLXYFPZ-UHFFFAOYSA-N 0.000 description 1
- YEYKMVJDLWJFOA-UHFFFAOYSA-N 2-propoxyethanol Chemical compound CCCOCCO YEYKMVJDLWJFOA-UHFFFAOYSA-N 0.000 description 1
- SLRMQYXOBQWXCR-UHFFFAOYSA-N 2154-56-5 Chemical compound [CH2]C1=CC=CC=C1 SLRMQYXOBQWXCR-UHFFFAOYSA-N 0.000 description 1
- RMRIPULFCPVFQB-UHFFFAOYSA-N 3-ethenyl-2-methyl-1h-isoquinoline Chemical compound C1=CC=C2C=C(C=C)N(C)CC2=C1 RMRIPULFCPVFQB-UHFFFAOYSA-N 0.000 description 1
- XWYGLWPLDNVHLW-UHFFFAOYSA-N 3-ethenyl-2-methylquinolin-8-ol Chemical compound C1=CC=C2C=C(C=C)C(C)=NC2=C1O XWYGLWPLDNVHLW-UHFFFAOYSA-N 0.000 description 1
- DPZYLEIWHTWHCU-UHFFFAOYSA-N 3-ethenylpyridine Chemical compound C=CC1=CC=CN=C1 DPZYLEIWHTWHCU-UHFFFAOYSA-N 0.000 description 1
- WWDWPSQOAUMNDT-UHFFFAOYSA-N 3-prop-1-en-2-ylpyridine Chemical compound CC(=C)C1=CC=CN=C1 WWDWPSQOAUMNDT-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- OPPHXULEHGYZRW-UHFFFAOYSA-N 4-methoxy-2,4-dimethyl-2-phenyldiazenylpentanenitrile Chemical compound COC(C)(C)CC(C)(C#N)N=NC1=CC=CC=C1 OPPHXULEHGYZRW-UHFFFAOYSA-N 0.000 description 1
- LNGIEJFJWSPNQW-UHFFFAOYSA-N 5-ethenyl-1-methylisoquinoline Chemical compound C1=CC=C2C(C)=NC=CC2=C1C=C LNGIEJFJWSPNQW-UHFFFAOYSA-N 0.000 description 1
- WIYVVIUBKNTNKG-UHFFFAOYSA-N 6,7-dimethoxy-3,4-dihydronaphthalene-2-carboxylic acid Chemical compound C1CC(C(O)=O)=CC2=C1C=C(OC)C(OC)=C2 WIYVVIUBKNTNKG-UHFFFAOYSA-N 0.000 description 1
- XKXGWYAQJRXDPI-UHFFFAOYSA-N 7-methyloctanoyl 7-methyloctaneperoxoate Chemical compound CC(C)CCCCCC(=O)OOC(=O)CCCCCC(C)C XKXGWYAQJRXDPI-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 239000004342 Benzoyl peroxide Substances 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- YIVJZNGAASQVEM-UHFFFAOYSA-N Lauroyl peroxide Chemical compound CCCCCCCCCCCC(=O)OOC(=O)CCCCCCCCCCC YIVJZNGAASQVEM-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- CAHJSIYBJUPLHQ-UHFFFAOYSA-N O.O.C(C(C)C)(=O)N.C(C(C)C)(=O)N Chemical compound O.O.C(C(C)C)(=O)N.C(C(C)C)(=O)N CAHJSIYBJUPLHQ-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- SJEYSFABYSGQBG-UHFFFAOYSA-M Patent blue Chemical compound [Na+].C1=CC(N(CC)CC)=CC=C1C(C=1C(=CC(=CC=1)S([O-])(=O)=O)S([O-])(=O)=O)=C1C=CC(=[N+](CC)CC)C=C1 SJEYSFABYSGQBG-UHFFFAOYSA-M 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 1
- 229920000297 Rayon Polymers 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 241001584775 Tunga penetrans Species 0.000 description 1
- YUDRVAHLXDBKSR-UHFFFAOYSA-N [CH]1CCCCC1 Chemical compound [CH]1CCCCC1 YUDRVAHLXDBKSR-UHFFFAOYSA-N 0.000 description 1
- CIUQDSCDWFSTQR-UHFFFAOYSA-N [C]1=CC=CC=C1 Chemical compound [C]1=CC=CC=C1 CIUQDSCDWFSTQR-UHFFFAOYSA-N 0.000 description 1
- 239000000980 acid dye Substances 0.000 description 1
- 150000007933 aliphatic carboxylic acids Chemical class 0.000 description 1
- 150000003973 alkyl amines Chemical class 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- RMRFFCXPLWYOOY-UHFFFAOYSA-N allyl radical Chemical compound [CH2]C=C RMRFFCXPLWYOOY-UHFFFAOYSA-N 0.000 description 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 1
- 150000004056 anthraquinones Chemical class 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- WDEQGLDWZMIMJM-UHFFFAOYSA-N benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate Chemical compound OCC1CC(O)CN1C(=O)OCC1=CC=CC=C1 WDEQGLDWZMIMJM-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- FNPHUPWGQFNFKA-UHFFFAOYSA-N bis[2-(1h-imidazol-2-yl)propan-2-yl]diazene;dihydrochloride Chemical compound Cl.Cl.N=1C=CNC=1C(C)(C)N=NC(C)(C)C1=NC=CN1 FNPHUPWGQFNFKA-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 150000004700 cobalt complex Chemical class 0.000 description 1
- 239000007859 condensation product Substances 0.000 description 1
- 238000010014 continuous dyeing Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 150000004699 copper complex Chemical class 0.000 description 1
- AOLYLEFSPFALGJ-UHFFFAOYSA-N copper formazan Chemical compound [Cu].NN=CN=N AOLYLEFSPFALGJ-UHFFFAOYSA-N 0.000 description 1
- HXCKCCRKGXHOBK-UHFFFAOYSA-N cycloheptane Chemical compound [CH]1CCCCCC1 HXCKCCRKGXHOBK-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- XXJWXESWEXIICW-UHFFFAOYSA-N diethylene glycol monoethyl ether Chemical compound CCOCCOCCO XXJWXESWEXIICW-UHFFFAOYSA-N 0.000 description 1
- 229940075557 diethylene glycol monoethyl ether Drugs 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- QZYRMODBFHTNHF-UHFFFAOYSA-N ditert-butyl benzene-1,2-dicarboperoxoate Chemical compound CC(C)(C)OOC(=O)C1=CC=CC=C1C(=O)OOC(C)(C)C QZYRMODBFHTNHF-UHFFFAOYSA-N 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-M iodide Chemical compound [I-] XMBWDFGMSWQBCA-UHFFFAOYSA-M 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000009981 jet dyeing Methods 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- FQPSGWSUVKBHSU-UHFFFAOYSA-N methacrylamide Chemical compound CC(=C)C(N)=O FQPSGWSUVKBHSU-UHFFFAOYSA-N 0.000 description 1
- DUVTXUGBACWHBP-UHFFFAOYSA-N methyl 2-(1h-benzimidazol-2-ylmethoxy)benzoate Chemical compound COC(=O)C1=CC=CC=C1OCC1=NC2=CC=CC=C2N1 DUVTXUGBACWHBP-UHFFFAOYSA-N 0.000 description 1
- ZQMHJBXHRFJKOT-UHFFFAOYSA-N methyl 2-[(1-methoxy-2-methyl-1-oxopropan-2-yl)diazenyl]-2-methylpropanoate Chemical compound COC(=O)C(C)(C)N=NC(C)(C)C(=O)OC ZQMHJBXHRFJKOT-UHFFFAOYSA-N 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- MMNOTXXCQAFCLV-UHFFFAOYSA-N n,n-dimethylmethanamine;2-methyl-n-propylprop-2-enamide;hydrochloride Chemical compound [Cl-].C[NH+](C)C.CCCNC(=O)C(C)=C MMNOTXXCQAFCLV-UHFFFAOYSA-N 0.000 description 1
- QYZFTMMPKCOTAN-UHFFFAOYSA-N n-[2-(2-hydroxyethylamino)ethyl]-2-[[1-[2-(2-hydroxyethylamino)ethylamino]-2-methyl-1-oxopropan-2-yl]diazenyl]-2-methylpropanamide Chemical compound OCCNCCNC(=O)C(C)(C)N=NC(C)(C)C(=O)NCCNCCO QYZFTMMPKCOTAN-UHFFFAOYSA-N 0.000 description 1
- CYTJMBLSQUBVMS-UHFFFAOYSA-N n-[[2-cyanopropan-2-yl(formyl)amino]hydrazinylidene]formamide Chemical compound N#CC(C)(C)N(C=O)NN=NC=O CYTJMBLSQUBVMS-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical class C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000001007 phthalocyanine dye Substances 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 239000003505 polymerization initiator Substances 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 125000001844 prenyl group Chemical group [H]C([*])([H])C([H])=C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 125000001422 pyrrolinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 239000002964 rayon Substances 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000010015 semi-continuous dyeing Methods 0.000 description 1
- 239000002453 shampoo Substances 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- KQYLUTYUZIVHND-UHFFFAOYSA-N tert-butyl 2,2-dimethyloctaneperoxoate Chemical compound CCCCCCC(C)(C)C(=O)OOC(C)(C)C KQYLUTYUZIVHND-UHFFFAOYSA-N 0.000 description 1
- GJBRNHKUVLOCEB-UHFFFAOYSA-N tert-butyl benzenecarboperoxoate Chemical compound CC(C)(C)OOC(=O)C1=CC=CC=C1 GJBRNHKUVLOCEB-UHFFFAOYSA-N 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- VZTGWJFIMGVKSN-UHFFFAOYSA-O trimethyl-[3-(2-methylprop-2-enoylamino)propyl]azanium Chemical compound CC(=C)C(=O)NCCC[N+](C)(C)C VZTGWJFIMGVKSN-UHFFFAOYSA-O 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M14/00—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials
- D06M14/18—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials using wave energy or particle radiation
- D06M14/20—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials using wave energy or particle radiation on to materials of natural origin
- D06M14/22—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials using wave energy or particle radiation on to materials of natural origin of vegetal origin, e.g. cellulose or derivatives thereof
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/58—Material containing hydroxyl groups
- D06P3/60—Natural or regenerated cellulose
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M14/00—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials
- D06M14/02—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials on to materials of natural origin
- D06M14/04—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials on to materials of natural origin of vegetal origin, e.g. cellulose or derivatives thereof
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/58—Material containing hydroxyl groups
- D06P3/60—Natural or regenerated cellulose
- D06P3/6008—Natural or regenerated cellulose using acid dyes
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/58—Material containing hydroxyl groups
- D06P3/60—Natural or regenerated cellulose
- D06P3/6025—Natural or regenerated cellulose using vat or sulfur dyes
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/58—Material containing hydroxyl groups
- D06P3/60—Natural or regenerated cellulose
- D06P3/66—Natural or regenerated cellulose using reactive dyes
Definitions
- Textile materials such as, for example, woven fabrics, knitted fabrics, yarns and threads, which consist of cellulose fibers or of a mixture of cellulose fibers with other fibers, are dyed using anionic dyes, in particular anionic fiber-reactive dyes, in accordance with the usual application techniques, for example in such a way that , in particular when using fiber-reactive dyes, the dyeing process in the presence of an alkali, such as, for example, sodium hydroxide solution, sodium carbonate and sodium silicate, and in the presence of electrolytes, such as, for example, sodium chloride or sodium sulfate, at elevated temperatures, for example at 60.degree.
- an alkali such as, for example, sodium hydroxide solution, sodium carbonate and sodium silicate
- electrolytes such as, for example, sodium chloride or sodium sulfate
- Continuous and semi-continuous dyeing methods are also used for this, such as the pad-steam process or the pad-cold retention process.
- the textile material is first padded with the aqueous liquor containing the dye (fiber-reactive dye) and the alkali required for fixation is applied to the textile material in a later, separate impregnation step.
- Suitable alkali are preferably sodium hydroxide solution, potassium hydroxide solution, sodium carbonate (in aqueous solution) and aqueous water glass solutions.
- the fiber materials dyed in this way are distinguished by a high brilliance of the dyeing and by very good fastness to use, such as, for example, wet fastness, rubbing fastness, cooking fastness and light fastness.
- the present invention therefore relates to a process for dyeing cellulose fiber materials with anionic fiber-reactive dyes, which is characterized in that the dyeing is carried out using low-electrolyte and alkali-free dyeing liquors and a modified cellulose fiber described below is used as the cellulose fiber material.
- Modified cellulose fiber materials that can be used in the dyeing process according to the invention are cellulose fiber materials onto which a polymer has been grafted that contains at least one polymerizable, N-containing, basic monomer from the group of the general formulas (1), (1A), (2 ) and (2A) as building block contains:
- Monovalent anions Y (-) are, for example, monovalent residues of a mineral acid, sulfonic acid or carboxylic acid or a residue of a polybasic mineral acid, sulfonic acid or carboxylic acid equivalent to a monovalent anion.
- Monovalent anions Y (-) are, for example, the nitrate, hydrogen sulfate, benzenesulfonate, fluoride, chloride, bromide, iodide, acetate or propionate anion or the anionic radical of another aliphatic or aromatic carboxylic acid.
- a part of a multivalent anion equivalent to a monovalent anion is, for example, half the equivalent of a sulfate anion or the third equivalent of a phosphate anion.
- Y (-) is preferably a halogen anion, such as the bromide or iodide anion and in particular the chloride anion.
- Alkyl radicals in the formulas above are, for example, the methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec.-butyl, iso-butyl, n-pentyl and n-hexyl radical .
- Cycloalkyl radicals are, for example, the cyclopentyl, cyclohexyl and cycloheptyl radical, preferably the cyclohexyl radical thereof.
- Examples of hydroxyalkyl radicals are 2-hydroxyethyl, 2-hydroxy-n-propyl, 3-hydroxy-n-propyl, 2-, 3- or 4-hydroxy-n-butyl and 2-methyl-3- hydroxy-n-propyl residue.
- R 1 and R 2 or R 6 and R 7 together with the N atom to which they are attached form a 5- or 6-membered, saturated or unsaturated, optionally interrupted by heteroatoms, these are, for example Pyrrolidinyl, piperidino, pyrrolyl, pyridinyl-piperazinyl, morpholino or thiomorpholino radical.
- Phenylalkyl radicals in which the alkyl radical can be straight-chain or branched are, for example, the benzyl, phenethyl, 3-phenyl-n-propyl, 2-phenyl-n-propyl, 3-phenyl-n-propyl, 3-phenyl-n-butyl or 4-phenyl-n-butyl radical, preferably the benzyl radical thereof.
- Examples of a radical of the formula - (CH 2 -CH 2 -O) p -phenyl are, for example, the ⁇ - (phenoxy) ethyl radical or radicals in which p represents the number 2 or 4.
- the abovementioned monomers are particularly preferred in the form of their quaternized compounds corresponding to the general formula (1A), the three alkyl radicals on the quaternary nitrogen atom preferably having the same meaning.
- Y (-) has one of the meanings mentioned above and is preferably the chloride anion.
- Preferred compounds of the general formula (2A) are those in which the radical R 7 is an allyl radical, preferably the dimethyl allyl radical.
- Substituents in the radical R 11 are, for example, alkyl radicals of 1 to 4 carbon atoms and hydroxyalkyl radicals of 2 to 4 carbon atoms.
- R 12 is preferably a hydrogen atom.
- the 5-membered, heterocyclic, saturated or unsaturated radicals representing the radical R 11 are, for example, the imidazolyl, imidazolinyl, pyrrolyl, pyrrolinyl, pyrrolidinyl and the indolyl radical.
- Five-membered ring compounds of the general formula (5) are, for example, N-vinyl-imidazole, 1-vinyl-2-imidazoline, 2-vinyl-4-methyl-imidazoline, 2-vinyl-5-methyl-imidazoline, 1-vinyl-2 -methyl imidazole, 1-vinyl-4- ( ⁇ -hydroxyethyl) imidazole, N-vinyl pyrrole, 2-isopropenyl-2-imidazoline and 1-vinyl-2,4-dimethyl-imidazole.
- Residues R 11 the Six-membered heterocyclic, saturated or unsaturated radicals are, for example, the pyrridinyl or quinolinyl radical.
- Six-membered heterocyclic compounds of the general formula (5) are, for example, 2-vinyl-pyridine, 3-vinyl-pyridine, 4-vinyl-pyridine, 3-isopropenyl-pyridine, 2-vinyl-quinoline, 2-methyl-3-vinyl- 8-hydroxy-quinoline, 2-vinyl-quinoline, 2-methyl-3-vinyl-isoquinoline, 1-methyl-5-vinyl-isoquinoline and 2-isopropenyl-quinoline.
- vinyl pyridine and its derivatives are particularly preferred.
- the polymer grafted onto the cellulose fiber material can be composed of monomeric building blocks of one or more compounds of the formulas (1), (1A), (2), (2A) and / or (5) mentioned above. It can also contain other polymerizable monomers.
- the polymer grafted onto the cellulose fiber material according to the invention contains, for example, 20 to 100 mol%, preferably 40 to 100 mol% and particularly preferably 80 to 100 mol%, of one or more compounds of the above formulas.
- the division between the monomers of the above formulas is arbitrary.
- the grafted-on polymer contains only one of the compounds of the above formulas, i.e. a cellulose fiber material with a grafted-on homopolymer is preferred.
- Copolymers which may be grafted onto the cellulose fiber material are those which, in addition to the above-mentioned monomers of the general formulas (1), (1A), (2), (2A) and / or (5), are one or more comonomers corresponding to those
- the following general formulas (6) and (7) contain:
- Monomers of the general formula (6) are, for example, acrylamide and methacrylamide.
- Compounds of the general formula (7) are, for example, the (C 1 -C 6 ) alkyl esters, in particular the methyl and ethyl esters, acrylic and methacrylic acid.
- the grafting of the mono- or copolymer onto the cellulose fiber is carried out in such a way that one or more monomers of the formulas (1), (1A), (2), (2A) and / or (5) optionally in the presence of one or more monomers of the general formula (6) and / or (7) are polymerized.
- the polymerization or copolymerization on the fiber takes place in a suitable solvent.
- a suitable solvent such are in particular water in a mixture with a water-miscible solvent which is inert to the monomers.
- Such solvents are, for example, lower alcohols, such as alkanols of 1 to 5 carbon atoms, for example methanol, ethanol, n-propanol, isopropanol, n-butanol and tert-butanol, glycols and diols, such as, for example, ethylene glycol, propylene glycol and propane-1 , 3-diol, di- and polyglycols, such as, for example, diethylene glycol and triethylene glycol, glycol ethers, such as, for example, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, ethylene glycol mono-n-propyl ether, ethylene glycol mono-n-n butyl ether, ethylene glycol dimethyl ether and ethylene glycol diethyl ether, and also ketones, such as acetone and methyl ethyl ketone
- the homopolymerization and the copolymerization in the presence of the cellulose fiber material can also be carried out in a mixture of different solvents containing water. If one or more components are used in the form of aqueous solutions, a further addition of water is generally not necessary. Water in a mixture of alcohols, such as alkanols, in particular those having 1 to 4 carbon atoms, and in a mixture with diols and / or glycols is preferred. It may be expedient to add organic solvent or solvent mixture once or in particular several times during the homo- or copolymerization.
- the pH is adjusted to values from 3 to 11.5, preferably from 3 to 8.5.
- An acid preferably an organic acid, in particular formic acid, acetic acid, glycolic acid and / or citric acid, is generally used for this.
- the homo- and copolymerization is carried out in the presence of the cellulose fiber material at room temperature (10 to 30 ° C) or at a higher temperature of up to about 160 ° C, but usually at a temperature between 40 and 100 ° C and preferably between 60 and 90 ° C, particularly preferably between 65 to 85 ° C, performed.
- Suitable polymerization initiators such as, for example, radical-forming substances, are used for this in the usual way.
- the action of high-energy radiation, such as UV rays, as well as microwaves and the action of ultrasound, can also serve to start the polymerization.
- Free-radical initiators are, for example, benzoyl peroxide, tert-butyl hydroperoxide, cumene peroxide, methyl ethyl ketone peroxide, lauryl peroxide, tert-butyl perbenzoate, di-tert-butyl perphthalate, azodiisobutyronitrile, 2,2'-azo-bis- (2 , 4-dimethylvaleronitrile), 2-phenyl-azo-2,4-dimethyl-4-methoxy-valeronitrile, 2-cyano-2-propyl-azo-formamide, azodiisobutyramide, dimethyl-, diethyl- or di-n-butyl- azobismethylvalerate, tert-butyl perneodecanoate, di-isononanoyl peroxide, tert-amyl perpivalate, Di-2-ethylhexyl peroxydicarbonate, dil
- the polymerization or copolymerization or the grafting in the absence of oxygen. This can be done in a manner known per se by purging or passing through an inert gas, such as nitrogen.
- the monomer components are used in amounts such that the polymer grafted onto the cellulose contains at least 20 mol%, preferably at least 40 mol% and particularly preferably at least 80 mol% of one or more compounds of the formulas (1), (1A), ( 2), (2A) and / or (5) in copolymerized form.
- the grafting can be carried out in such a way that the cellulose fiber material to be grafted is introduced into the polymerization vessel together with the solution of the monomer (s) and the homopolymerization or copolymerization is carried out.
- the homo- or copolymerization is usually complete after about 0.5 to 4 hours, in many cases after 0.5 to 2.5 hours.
- the cellulose fiber material is then removed from the polymerization container, freed from adhering liquid, for example by squeezing, rinsed with water and dried.
- the grafting of the cellulose fiber can also be carried out in such a way that the monomer solution is applied to the cellulose fiber material by padding, slapping, soaking or spraying, and then the homo- or copolymerization at normal temperature or elevated temperature, expediently by the action of high-energy rays or ultrasound is triggered.
- the cellulose fiber material is then stored in a moist condition for a further 2 to 36 hours, preferably 10 to 24 hours, and is then dried, after rinsing, if necessary.
- Cellulose fiber materials which are suitable for modification in the manner according to the invention are, for example, cotton and rayon as well as the linen, jute and ramie fibers and regenerated cellulose fibers, preferably the cotton fiber.
- the modified cellulose fiber materials can also be present as a proportion in blended fabrics, such as blended fabrics of such modified cellulose fibers with polyester fibers.
- the modification of the cellulose fiber according to the invention can take place in the blended fabric itself and in all processing states, such as yarn, flake, sliver and piece goods.
- JP-A-56-096 972 describes that a cotton-polyester fabric grafted with 2- (dimethylamino) ethyl methacrylate can be dyed with acid dyes, the dyed fabric having improved wash fastness.
- US Pat. No. 3,514,385 describes the grafting of textile material made from cellulose with ethylenically unsaturated monomers such as 4-vinyl-pyridine to improve general fabric properties such as strength, temperature stability, washing properties, fastness properties and others.
- EP-A2-0 265 768 impregnates textiles with cationic cellulose copolymers, for example copolymers of hydroxyethyl cellulose and dimethyldiallyl ammonium chloride, in order to improve the dyeability with anionic textile dyes.
- the present invention also relates to cellulose fiber materials which have been modified in the manner according to the invention by grafting on a copolymer of the compounds of the general formula (1) and / or (2) or their quaternized derivatives with at least one monomer of the general formulas (6) and (7) , and the process for their production.
- the dyeing of cellulose fiber materials modified in this way in the manner according to the invention is carried out analogously to the known dyeing methods for dyeing cellulose fiber materials using anionic fiber-reactive dyes, and using the temperature ranges and customary amounts of dye known to be used for this, but with the exception according to the invention that for the dyebaths and padding liquors of the invention
- Dyeing process an addition of alkaline compounds, such as are usually used for fixing fiber-reactive dyes, such as sodium carbonate, potassium carbonate, sodium hydroxide solution and water glass, can be excluded and furthermore the usual addition of electrolyte salts, in particular the migration of the dye on the fiber should increase, not or only to a small extent, ie up to a maximum of 10 g per liter of dye bath or dye liquor is required.
- the dyeing process according to the invention is accordingly carried out within a pH range from 3 to 7.5, preferably from 4.5 to 7.
- Dyeing processes which can be used according to the invention are, for example, the various extraction processes, such as dyeing on the jigger and on the reel runner or dyeing from a long or short liquor, dyeing in jet dyeing machines, dyeing using the pad-cold-dwell method or after a block-hot steam fixing process.
- the pull-out process can be carried out in the usual liquor ratio of 1: 3 to 1:20.
- the dyeing temperature can be between 30 and 90 ° C, preferably at temperatures below 60 ° C; As can be seen from the above-mentioned application of the pad-cold residence method according to the invention, dyeing is also advantageously possible at room temperature (10 to 30 ° C.).
- auxiliaries such as surfactants (wetting agents), urea and leveling aids or auxiliaries, which improve the solubility of dyes in the concentrated padding liquors, such as condensation products of formaldehyde and optionally alkyl-substituted naphthalenesulfonic acids.
- anionic dyes which have one or more sulfo and / or carboxy groups and which contain fiber-reactive groups are suitable for the dyeing methods according to the invention. They can belong to the class of azo dyes, copper complex, cobalt complex and chromium complex azo dyes, copper and nickel phthalocyanine dyes, anthraquinone, copper formazane and triphendioxazine dyes. Such dyes have been described in numerous literature and are well known to those skilled in the art.
- the dyeings of the modified cellulose fiber materials obtainable in the manner according to the invention require no further aftertreatment after removal from the dyebath or after completion of the fixation of the dye on the substrate, in particular no complicated aftertreatment process including washing.
- a final boiling treatment of the dyed substrate with a washing solution to improve the fastness properties is not necessary.
- the cotton cloth is then removed from the reaction flask, squeezed off, rinsed twice with 500 ml of water each time, squeezed out in between and then dried at 100 ° C. for 5 minutes.
- the absorption of polymer is 5.3%.
- a pH of 8.5 to 9.0 is set with 231 g of glacial acetic acid. 960 g of water and 2060 mg of 2,2'-azobis (2-amidino-propane) dihydrochloride as initiator are added; the flask is then evacuated three times, rendered inert with nitrogen and heated to 79 to 80 ° C.
- the active substance concentration of polymer is 25%.
- the K value of a 1% aqueous solution is: 112600.
- Example 2 When Example 2 is repeated, the cotton cloth is replaced by a 3.4 g, 8 x 47 cm large cellulose cloth. The absorption of polymer is 2.95%.
- Example 3 When Example 3 is repeated, the cotton cloth is replaced by a 5.7 g, equally large cloth made of a 65:35% polyester / cotton blended fabric. The absorption of polymer is 3.4%.
- the damp cloth is subjected to a microwave treatment (30 seconds with 90 watts and then 4 times 30 seconds with 720 watts). Thereafter, the damp cloth is left for 24 hours at room temperature and then dried for 5 minutes at 100 ° C. The absorption of polymer is 29.5%.
- Example 6 The monomer solution given in Example 6 is applied to a cotton cloth and squeezed off in the manner given in Example 6.
- the damp cloth is exposed to UV radiation in order to trigger the polymerization and grafting.
- the aftertreatment of the cloth is carried out as indicated in Example 6.
- the absorption of polymer is 30.8%.
- the polymer solution obtained is 38% (to determine the content, a sample is heated to 120 ° C. for 2 hours at a pressure of 200 mbar). The viscosity of the solution is measured in a DIN cup (nozzle diameter 6 mm) for 44.6 seconds. The cloth is removed and treated as in Example 1. The absorption of polymer is 4.7%.
- a cotton fabric modified in accordance with Example 1 is first boiled in water for 15 minutes and then dried. This fabric is then dyed according to a pad cold-dyeing process.
- the fabric sealed with the dye solution is wound onto a dock, wrapped in a plastic film and left at 20 ° C. for 16 hours and then rinsed with cold and hot water and dried.
- a strong, uniformly colored orange coloration is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
- Example A an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
- a cotton fabric modified in accordance with Example 1 is first boiled in water for 15 minutes and then dried. This fabric is then treated with an aqueous dye solution containing 28.5 parts of a 50% electrolyte-containing (predominantly sodium chloride) dye powder of the known dye of the formula in 1000 parts by volume Contains 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved, using a padder with a liquor absorption of 80%, based on the weight of the fabric, at 20 ° C. The padded fabric is wound on a dock, wrapped in a plastic wrap and left at 20 ° C for 16 hours and then rinsed with cold and hot water and dried.
- aqueous dye solution containing 28.5 parts of a 50% electrolyte-containing (predominantly sodium chloride) dye powder of the known dye of the formula in 1000 parts by volume Contains 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved, using a padder with a liquor absorption
- a strong, uniformly colored red color is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
- Example D an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
- a cotton fabric modified in accordance with Example 9 is first boiled in water for 15 minutes and then dried. This fabric is then dyed according to a pad cold-dyeing process.
- an aqueous dye solution containing 28.5 parts of the dye powder described in Example A, 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved in 1000 parts by volume, using a padder with a liquor absorption of 80%, based on the Weight of the fabric, applied to the fabric at 20 ° C.
- the fabric sealed with the dye solution is wound onto a dock, wrapped in a plastic film and left at 20 ° C. for 16 hours and then rinsed with cold and hot water and dried.
- a strong, uniformly colored orange coloration is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
- Example A an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
- a cotton fabric modified in accordance with Example 9 is first boiled in water for 15 minutes and then dried. This fabric is then treated with an aqueous dye solution containing 28.5 parts of the in. In 1000 parts by volume Contains dye powder described in Example D, 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved, padded at 20 ° C. using a padder with a liquor absorption of 80%, based on the weight of the fabric. The padded fabric is wound on a dock, wrapped in a plastic wrap and left at 20 ° C for 16 hours and then rinsed with cold and hot water and dried.
- a strong, uniformly colored red color is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
- Example D an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
- Coloring (AREA)
- Graft Or Block Polymers (AREA)
Description
Textilmaterialien, wie beispielsweise Gewebe, Gewirke, Garne und Fäden, die aus Cellulosefasern oder aus einem Gemisch aus Cellulosefasern mit anderen Fasern bestehen, werden unter Verwendung von anionischen Farbstoffen, insbesondere anionischen faserreaktiven Farbstoffen, gemäß den üblichen Anwendungstechniken beispielsweise in der Weise gefärbt, daß man, insbesondere bei der Anwendung von faserreaktiven Farbstoffen, den Färbeprozeß in Gegenwart eines Alkali, wie beispielsweise Natronlauge, Natriumcarbonat und Natriumsilikat, und in Anwesenheit von Elektrolyten, wie beispielsweise Natriumchlorid oder Natriumsulfat, bei erhöhten Temperaturen, wie beispielsweise bei 60°C, durchführt.
Weiterhin kommen hierfür kontinuierliche und halbkontinuierliche Färbeweisen zur Anwendung, wie beispielsweise das Klotz-Heißdampf(Pad-Steam)-Verfahren oder das Klotz-Kaltverweil-Verfahren zur Anwendung. Beim Pad-Steam-Prozeß wie auch beim Kaltverweilverfahren wird das Textilmaterial zunächst mit der den Farbstoff (faserreaktiven Farbstoff) enthaltenden wäßrigen Flotte geklotzt und das zur Fixierung benötigte Alkali in einem späteren, getrennten Imprägnierschritt auf das Textilmaterial appliziert. Als Alkali kommen vorzugsweise Natronlauge, Kalilauge, Natriumcarbonat (in wäßriger Lösung) und wäßrige Wasserglaslösungen in Betracht.Textile materials, such as, for example, woven fabrics, knitted fabrics, yarns and threads, which consist of cellulose fibers or of a mixture of cellulose fibers with other fibers, are dyed using anionic dyes, in particular anionic fiber-reactive dyes, in accordance with the usual application techniques, for example in such a way that , in particular when using fiber-reactive dyes, the dyeing process in the presence of an alkali, such as, for example, sodium hydroxide solution, sodium carbonate and sodium silicate, and in the presence of electrolytes, such as, for example, sodium chloride or sodium sulfate, at elevated temperatures, for example at 60.degree.
Continuous and semi-continuous dyeing methods are also used for this, such as the pad-steam process or the pad-cold retention process. In the pad-steam process as well as in the cold residence process, the textile material is first padded with the aqueous liquor containing the dye (fiber-reactive dye) and the alkali required for fixation is applied to the textile material in a later, separate impregnation step. Suitable alkali are preferably sodium hydroxide solution, potassium hydroxide solution, sodium carbonate (in aqueous solution) and aqueous water glass solutions.
Insbesondere bei der Anwendung von faserreaktiven Farbstoffen, die mit der Faser eine kovalente Bindung eingehen, zeichnen sich die so gefärbten Fasermaterialien durch eine hohe Brillanz der Färbung und durch sehr gute Gebrauchsechtheiten, wie beispielsweise Naßechtheiten, Reibechtheiten, Kochechtheiten und Lichtechtheiten, aus.Particularly when using fiber-reactive dyes that form a covalent bond with the fiber, the fiber materials dyed in this way are distinguished by a high brilliance of the dyeing and by very good fastness to use, such as, for example, wet fastness, rubbing fastness, cooking fastness and light fastness.
Da neben dem Fixiervorgang des faserreaktiven Farbstoffes in der wäßrigen, stark alkalischen Färbeflotte noch zusätzlich eine Hydrolysereaktion des faserreaktiven Farbstoffes ablaufen kann oder die Fixierung auf dem Fasermaterial nicht vollständig ist, müssen im Anschluß an den Färbeprozeß teilweise umfangreiche und zeitaufwendige Wasch- und Spülprozesse ausgeführt werden, wie das mehrfache Spülen mit kaltem und heißem Wasser und einer dazwischen liegenden Neutralisationsbehandlung zur Entfernung überschüssigem Alkalis auf dem gefärbten Material und des weiteren beispielsweise eine Kochwäsche mit einem nichtionogenen Waschmittel, um die guten Echtheiten der Färbung zu gewährleisten.Since, in addition to the fixing process of the fiber-reactive dye in the aqueous, strongly alkaline dye liquor, a hydrolysis reaction of the fiber-reactive dye can also take place or the fixing on the fiber material is not complete, extensive and time-consuming washing and rinsing processes have to be carried out after the dyeing process, such as multiple rinsing with cold and hot water and an intermediate neutralization treatment to remove excess alkali on the dyed material and, for example, a boil wash with a nonionic detergent to ensure the good fastness of the dyeing.
Es hat nicht an Versuchen gefehlt, diese Nachbehandlungsprozesse zu verbessern und bezüglich ihres Aufwandes zu verringern und zusätzlich die Echtheiten der Färbungen zu verbessern. So hat man gemäß der Deutschen Offenlegungsschrift 37 09 766 versucht, Cellulosefasermaterial mit faserreaktiven Farbstoffen alkalifrei zu färben, wobei das Fasermaterial vor dem Färbeprozeß einer Modifizierung durch Vorbehandlung mit einem Umsetzungsprodukt aus Polyethylenamin und einem bifunktionellen Alkylierungsmittel ausgesetzt und hierdurch kationisiert und geschlichtet wird. In ähnlicher Weise erfolgt eine Modifizierung der Cellulosefaser durch Vorbehandlung mit einem Umsetzungsprodukt aus einem Epihalogenhydrin und einem Alkylamin gemäß der Deutschen Offenlegungsschrift Nr. 38 31 464. Zwar können mit diesen so vorbehandelten Fasermaterialien mit faserreaktiven Farbstoffen Färbungen erhalten werden, die gute Gebrauchsechtheiten besitzen, jedoch entsprechen diese Echtheiten, insbesondere die Lichtechtheiten, nicht der Qualität, die die Färbungen, die mit den anfangs erwähnten konventionellen Färbeweisen erhalten werden, besitzen.There has been no shortage of attempts to improve these post-treatment processes and to reduce their expenditure and additionally to improve the fastness of the dyeings. Thus, according to German Offenlegungsschrift 37 09 766, attempts have been made to dye cellulose fiber material with fiber-reactive dyes without alkali, the fiber material being subjected to a modification by pretreatment with a reaction product of polyethylene amine and a bifunctional alkylating agent before the dyeing process, and thereby cationized and sized. The cellulose fiber is modified in a similar manner by pretreatment with a reaction product of an epihalohydrin and an alkylamine in accordance with German Offenlegungsschrift No. 38 31 464. It is true that with these fiber materials pretreated in this way, dyeings can be obtained with fiber-reactive dyes which have good fastness to use, but which correspond these fastness properties, in particular the light fastness properties, are not of the quality which the colorations obtained with the conventional dyeing methods mentioned at the beginning have.
Es wurde nunmehr gefunden, daß man in überraschender Weise Färbungen mit anionischen faserreaktiven Farbstoffen, mit guten Gebrauchsechtheiten, die denen einer nach konventioneller Färbeweise erhältlichen Färbung entsprechen, erhält, wenn man ein modifiziertes, kationisiertes Cellulosefasermaterial verwendet, bei welchem die Polymerisation von speziellen N-haltigen, basischen, olefinischen Monomeren auf dem Fasermaterial direkt ausgeführt wurde und die Monomeren aus der nachfolgenden Reihe der Monomeren ausgewählt werden; die Verwendung dieses so modifizierten Cellulosefasermaterials erlaubt es, das Färbeverfahren unter Anwendung von elektrolytarmen und alkalifreien Färbeflotten durchzuführen, weswegen auch die aufwendige Nachbehandlung der Färbungen durch Spül- und Kochprozesse entfällt.It has now been found that dyeings with anionic fiber-reactive dyes, with good fastness properties which correspond to those obtained by conventional dyeing, are obtained in a surprising manner when using a modified, cationized cellulose fiber material in which the polymerization of special N-containing , basic, olefinic monomers on the fiber material was carried out directly and the monomers are selected from the following series of monomers; the use of this modified cellulose fiber material makes it possible to carry out the dyeing process using low-electrolyte and alkali-free dyeing liquors, which is why there is no need for time-consuming post-treatment of the dyeings by rinsing and cooking processes.
Die vorliegende Erfindung betrifft deshalb ein Verfahren zum Färben von Cellulosefasermaterialien mit anionischen faserreaktiven Farbstoffen, das dadurch gekennzeichnet ist, daß man die Färbung unter Anwendung elektrolytarmer und alkalifreier Färbeflotten durchführt und als Cellulosefasermaterial eine nachfolgend beschriebene modifizierte Cellulosefaser verwendet.The present invention therefore relates to a process for dyeing cellulose fiber materials with anionic fiber-reactive dyes, which is characterized in that the dyeing is carried out using low-electrolyte and alkali-free dyeing liquors and a modified cellulose fiber described below is used as the cellulose fiber material.
Modifizierte Cellulosefasermaterialien, die erfindungsgemäß in das Färbeverfahren eingesetzt werden können, sind Cellulosefasermaterialien, auf die ein Polymerisat aufgepfropft worden ist, das mindestens ein polymerisierbares, N-haltiges, basisches Monomeres aus der Gruppe der allgemeinen Formeln (1), (1A), (2) und (2A) als Baustein enthält:
In diesen Formel bedeuten:
- R1
- ist Wasserstoff, Alkyl von 1 bis 6 C-Atomen, Cycloalkyl von 5 bis 7 C-Atomen oder Hydroxyalkyl mit einem Alkylrest von 2 bis 4 C-Atomen;
- R2
- hat eine der für R1 genannten Bedeutungen und kann zu R1 gleich oder von R1 verschieden sein; oder
- R1 und R2
- bilden zusammen mit dem N-Atom den Rest eines 1 bis 3 Alkylenreste von 1 bis 5 C-Atomen und gegebenenfalls 1 oder 2 Heterogruppen enthaltenden gesättigten, ungesättigten oder teilungesättigten, 5- oder 6-gliedrigen Ringes, wobei die Heterogruppen aus den Gruppen der Formeln - O-, -S- , -N= und -NH- ausgewählt sind;
- R3
- ist Wasserstoff oder Methyl;
- R4
- ist Wasserstoff oder Methyl;
- X
- ist Sauerstoff oder bevorzugt eine Gruppe der Formel -NH- ;
- m
- ist eine ganze Zahl von 1 bis 6, bevorzugt die Zahl 2 oder 3;
- n
- ist eine ganze Zahl von 1 bis 4 oder bevorzugt die Zahl Null;
- R5
- ist Alkyl von 1 bis 6 C-Atomen, Phenylalkyl mit einem Alkylrest von 1 bis 4 C-Atomen oder ein Rest der Formel -(CH2-CH2-O)p -phenyl , in welcher p für eine ganze Zahl von 1 bis 4 steht und phenyl der Phenylrest ist;
- R6
- ist Wasserstoff, Alkyl von 1 bis 6 C-Atomen, Cycloalkyl von 5 bis 7 C-Atomen oder Hydroxyalkyl mit einem Alkylrest von 2 bis 4 C-Atomen;
- R7
- ist Wasserstoff, Alkyl von 1 bis 6 C-Atomen, Cycloalkyl von 5 bis 7 C-Atomen oder ein Rest der allgemeinen Formel -CH2-CH=CH2 ,
-CH2-C(CH3)=CH2, -CH2-CH=CH-CH3 oder -CH2-C(CH3)=CH-CH3 ; oder - R6 und R7
- bilden zusammen mit dem N-Atom den Rest eines 1 bis 3 Alkylenreste von 1 bis 5 C-Atomen und gegebenenfalls 1 oder 2 Heterogruppen enthaltenden gesättigten, ungesättigten oder teilungesättigten, 5- oder 6-gliedrigen Ringes, wobei die Heterogruppen aus den Gruppen der Formeln -O-, -S-, -N= und -NH- ausgewählt sind;
- R8
- ist Wasserstoff oder Methyl;
- R9
- ist Wasserstoff oder Methyl;
- Y(-)
- ist ein einwertiges Anion oder ein einem einwertigen Anion äquivalenter Teil eines mehrwertigen Anions.
- R 1
- is hydrogen, alkyl of 1 to 6 carbon atoms, cycloalkyl of 5 to 7 carbon atoms or hydroxyalkyl with an alkyl radical of 2 to 4 carbon atoms;
- R 2
- has one of the meanings mentioned for R 1 and may be different to R 1 equal to or from R 1; or
- R 1 and R 2
- together with the N atom form the remainder of a 1 to 3 alkylene radicals of 1 to 5 C atoms and optionally 1 or 2 hetero groups containing saturated, unsaturated or partially unsaturated, 5- or 6-membered ring, the hetero groups from the groups of the formulas - O-, -S-, -N = and -NH- are selected;
- R 3
- is hydrogen or methyl;
- R 4
- is hydrogen or methyl;
- X
- is oxygen or preferably a group of the formula -NH-;
- m
- is an integer from 1 to 6, preferably the number 2 or 3;
- n
- is an integer from 1 to 4 or preferably the number zero;
- R 5
- is alkyl of 1 to 6 carbon atoms, phenylalkyl with an alkyl radical of 1 to 4 carbon atoms or a radical of the formula - (CH 2 -CH 2 -O) p -phenyl, in which p is an integer from 1 to 4 is and phenyl is the phenyl radical;
- R 6
- is hydrogen, alkyl of 1 to 6 carbon atoms, cycloalkyl of 5 to 7 carbon atoms or hydroxyalkyl with an alkyl radical of 2 to 4 carbon atoms;
- R 7
- is hydrogen, alkyl of 1 to 6 carbon atoms, cycloalkyl of 5 to 7 carbon atoms or a radical of the general formula -CH 2 -CH = CH 2 ,
-CH 2 -C (CH 3 ) = CH 2 , -CH 2 -CH = CH-CH 3 or -CH 2 -C (CH 3 ) = CH-CH 3 ; or - R 6 and R 7
- together with the N atom form the remainder of a 1 to 3 alkylene radicals of 1 to 5 C atoms and optionally containing 1 or 2 hetero groups of saturated, unsaturated or partially unsaturated, 5- or 6-membered ring, the hetero groups from the groups of the formulas -O-, -S-, -N = and -NH- are selected;
- R 8
- is hydrogen or methyl;
- R 9
- is hydrogen or methyl;
- Y (-)
- is a monovalent anion or a part of a polyvalent anion equivalent to a monovalent anion.
Einwertige Anionen Y(-) sind beispielsweise einwertige Reste einer Mineralsäure, Sulfonsäure oder Carbonsäure oder ein einem einwertigen Anion äquivalenter Rest einer mehrbasischen Mineralsäure, Sulfonsäure oder Carbonsäure. Einwertige Anionen Y(-) sind beispielsweise das Nitrat-, Hydrogensulfat-, Benzolsulfonat-, Fluorid-, Chlorid-, Bromid-, Jodid-, Acetat- oder Propionat-Anion oder der anionische Rest einer anderen aliphatischen oder aromatischen Carbonsäure. Ein einem einwertigen Anion äquivatenter Teil eines mehrwertigen Anions sind beispielsweise das halbe Equivalent eines Sulfatanions oder das drittel Äquivalent eines Phosphatanions. Vorzugsweise ist Y(-) ein Halogenanion, wie das Bromid- oder Jodidanion und insbesondere das Chlorid-Anion.Monovalent anions Y (-) are, for example, monovalent residues of a mineral acid, sulfonic acid or carboxylic acid or a residue of a polybasic mineral acid, sulfonic acid or carboxylic acid equivalent to a monovalent anion. Monovalent anions Y (-) are, for example, the nitrate, hydrogen sulfate, benzenesulfonate, fluoride, chloride, bromide, iodide, acetate or propionate anion or the anionic radical of another aliphatic or aromatic carboxylic acid. A part of a multivalent anion equivalent to a monovalent anion is, for example, half the equivalent of a sulfate anion or the third equivalent of a phosphate anion. Y (-) is preferably a halogen anion, such as the bromide or iodide anion and in particular the chloride anion.
Alkylreste in den obigen Formeln sind beispielsweise der Methyl-, Ethyl-, n-Propyl-, iso-Propyl-, n-Butyl-, sec.-Butyl-, iso-Butyl-, n-Pentyl- und n-Hexyl-Rest. Cycloalkylreste sind beispielsweise der Cyclopentyl-, Cyclohexyl- und Cycloheptylrest, bevorzugt hiervon der Cyclohexylrest. Hydroxyalkylreste sind beispielsweise der 2-Hydroxyethyl-, 2-Hydroxy-n-propyl-, 3-Hydroxy-n-propyl-, der 2-, 3- oder 4-Hydroxy-n-butyl- und der 2-Methyl-3-hydroxy-n-propyl-Rest. Sofern R1 und R2 bzw. R6 und R7 zusammen mit dem N-Atom, an das sie gebunden sind, einen 5- oder 6-gliedrigen, gesättigten oder ungesättigten, gegebenenfalls durch Heteroatome unterbrochene Reste bilden, so sind diese beispielsweise der Pyrrolidinyl-, Piperidino-, Pyrrolyl-, Pyridinyl-Piperazinyl-, Morpholino- oder Thiomorpholino-Rest.Alkyl radicals in the formulas above are, for example, the methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec.-butyl, iso-butyl, n-pentyl and n-hexyl radical . Cycloalkyl radicals are, for example, the cyclopentyl, cyclohexyl and cycloheptyl radical, preferably the cyclohexyl radical thereof. Examples of hydroxyalkyl radicals are 2-hydroxyethyl, 2-hydroxy-n-propyl, 3-hydroxy-n-propyl, 2-, 3- or 4-hydroxy-n-butyl and 2-methyl-3- hydroxy-n-propyl residue. If R 1 and R 2 or R 6 and R 7 together with the N atom to which they are attached form a 5- or 6-membered, saturated or unsaturated, optionally interrupted by heteroatoms, these are, for example Pyrrolidinyl, piperidino, pyrrolyl, pyridinyl-piperazinyl, morpholino or thiomorpholino radical.
Phenylalkylreste, in denen der Alkylrest geradkettig oder verzweigt sein kann, sind beispielsweise der Benzyl-, Phenethyl-, 3-Phenyl-n-propyl-, 2-Phenyl-n-propyl-, 3-Phenyl-n-propyl-, 3-Phenyl-n-butyl- oder 4-Phenyl-n-butyl-Rest, bevorzugt hiervon der Benzylrest.
Beispiele für einen Rest der Formel -(CH2-CH2-O)p-phenyl sind beispielsweise der β-(Phenoxy)-ethyl-Rest oder Reste, in welchen p für die Zahl 2 oder 4 steht.Phenylalkyl radicals in which the alkyl radical can be straight-chain or branched are, for example, the benzyl, phenethyl, 3-phenyl-n-propyl, 2-phenyl-n-propyl, 3-phenyl-n-propyl, 3-phenyl-n-butyl or 4-phenyl-n-butyl radical, preferably the benzyl radical thereof.
Examples of a radical of the formula - (CH 2 -CH 2 -O) p -phenyl are, for example, the β- (phenoxy) ethyl radical or radicals in which p represents the number 2 or 4.
Besonders geeignete und bevorzugte, olefinische, N-haltige Monomere sind beispielsweise Verbindungen der allgemeinen Formeln (3a) bis (3m):
CH2 = CH - CO - O - CH2 - CH2 - N(CH3)2 (3a)
CH2 = CH - CO - O - CH2 - CH2 - N(n-C4H9)2 (3b)
CH2 = CH - CO - O - CH2 - CH2 - CH2 - N(CH3)2 (3c)
CH 2 = CH - CO - O - CH 2 - CH 2 - N (CH 3 ) 2 (3a)
CH 2 = CH - CO - O - CH 2 - CH 2 - N (nC 4 H 9 ) 2 (3b)
CH 2 = CH - CO - O - CH 2 - CH 2 - CH 2 - N (CH 3 ) 2 (3c)
Die obengenannten Monomeren sind insbesondere in Form ihrer quaternierten Verbindungen entsprechend der allgemeinen Formel (1A) bevorzugt, wobei die drei Alkylreste am quartären Stickstoffatom bevorzugt die gleiche Bedeutung haben. Insbesondere bevorzugt sind hiervon Verbindungen der Formeln (4a) bis (4c)
Von den Verbindungen der allgemeinen Formel (2A) sind diejenigen als bevorzugt zu nennen, in welchen der Rest R7 ein Allylrest, bevorzugt der Dimethyl-allyl-Rest ist.Preferred compounds of the general formula (2A) are those in which the radical R 7 is an allyl radical, preferably the dimethyl allyl radical.
Weitere Monomere, die erfindungsgemäß zur Modifizierung der Cellulosefaser dienen können, sind beispielsweise Verbindungen der allgemeinen Formel (5)
- R10
- Wasserstoff oder Methyl ist,
- R11
- ein fünf- oder sechsgliedriger heterocyclischer Rest ist, der mindestens ein basisches Stickstoffatom enthält und der einen ankondensierten Benzolring besitzen kann und/oder durch einen oder mehrere, bevorzugt 1 oder 2, Substituenten substituiert sein kann, und
- R12
- Wasserstoff oder Methyl bedeutet.
- R 10
- Is hydrogen or methyl,
- R 11
- is a five- or six-membered heterocyclic radical which contains at least one basic nitrogen atom and which may have a fused-on benzene ring and / or may be substituted by one or more, preferably 1 or 2, substituents, and
- R 12
- Means hydrogen or methyl.
Substituenten im Rest R11 sind beispielsweise Alkylreste von 1 bis 4 C-Atomen und Hydroxyalkylreste von 2 bis 4 C-Atomen. R12 ist bevorzugt ein Wasserstoffatom. Für den Rest R11 stehende 5-gliedrige, heterocyclische, gesättigte oder ungesättigte Reste sind beispielsweise der Imidazolyl-, Imidazolinyl-, Pyrrolyl-, Pyrrolinyl-, Pyrrolidinyl- und der Indolyl-Rest.Substituents in the radical R 11 are, for example, alkyl radicals of 1 to 4 carbon atoms and hydroxyalkyl radicals of 2 to 4 carbon atoms. R 12 is preferably a hydrogen atom. The 5-membered, heterocyclic, saturated or unsaturated radicals representing the radical R 11 are, for example, the imidazolyl, imidazolinyl, pyrrolyl, pyrrolinyl, pyrrolidinyl and the indolyl radical.
Fünfgliedrige Ringverbindungen der allgemeinen Formel (5) sind beispielsweise das N-Vinyl-imidazol, 1-Vinyl-2-imidazolin, 2-Vinyl-4-methyl-imidazolin, 2-Vinyl-5-methyl-imidazolin, 1-Vinyl-2-methyl-imidazol, 1-Vinyl-4-(β-hydroxyethyl)-imidazol, N-Vinyl-pyrrol, 2-Isopropenyl-2-imidazolin und 1-Vinyl-2,4-dimethyl-imidazol. Reste R11, die sechsgliedrige heterocyclische, gesättigte oder ungesättigte Reste darstellen, sind beispielsweise der Pyrridinyl- oder Chinolinyl-Rest. Sechsgliedrige heterocyclische Verbindungen der allgemeinen Formel (5) sind beispielsweise das 2-Vinyl-pyridin, 3-Vinyl-pyridin, 4-Vinyl-pyridin, 3-Isopropenyl-pyridin, 2-Vinyl-chinolin, 2-Methyl-3-vinyl-8-hydroxy-chinolin, 2-Vinyl-chinolin, 2-Methyl-3-vinyl-isochinolin, 1-Methyl-5-vinyl-isochinolin und 2-Isopropenyl-chinolin. Hiervon sind das Vinylpyridin und dessen Derivate besonders bevorzugt.Five-membered ring compounds of the general formula (5) are, for example, N-vinyl-imidazole, 1-vinyl-2-imidazoline, 2-vinyl-4-methyl-imidazoline, 2-vinyl-5-methyl-imidazoline, 1-vinyl-2 -methyl imidazole, 1-vinyl-4- (β-hydroxyethyl) imidazole, N-vinyl pyrrole, 2-isopropenyl-2-imidazoline and 1-vinyl-2,4-dimethyl-imidazole. Residues R 11 , the Six-membered heterocyclic, saturated or unsaturated radicals are, for example, the pyrridinyl or quinolinyl radical. Six-membered heterocyclic compounds of the general formula (5) are, for example, 2-vinyl-pyridine, 3-vinyl-pyridine, 4-vinyl-pyridine, 3-isopropenyl-pyridine, 2-vinyl-quinoline, 2-methyl-3-vinyl- 8-hydroxy-quinoline, 2-vinyl-quinoline, 2-methyl-3-vinyl-isoquinoline, 1-methyl-5-vinyl-isoquinoline and 2-isopropenyl-quinoline. Of these, vinyl pyridine and its derivatives are particularly preferred.
Das auf das Cellulosefasermaterial aufgepfropfte Polymerisat kann aus monomeren Bausteinen einer oder mehrerer Verbindungen der obengenannten Formeln (1), (1A), (2), (2A) und/oder (5) aufgebaut sein. Es kann auch noch andere einpolymerisierbare Monomeren enthalten. Das auf das Cellulosefasermaterial erfindungsgemäß aufgepfropfte Polymerisat enthält beispielsweise 20 bis 100 Mol-%, vorzugsweise 40 bis 100 Mol-% und insbesondere bevorzugt 80 bis 100 Mol-%, einer oder mehrerer Verbindungen der obengenannten Formeln. Hierbei ist die Aufteilung zwischen den Monomeren der obengenannten Formeln beliebig. In der Regel enthält das aufgepfropfte Polymerisat nur eine der Verbindungen der obengenannten Formeln, d.h. ein Cellulosefasermaterial mit einem aufgepropften Homopolymerisat ist bevorzugt.The polymer grafted onto the cellulose fiber material can be composed of monomeric building blocks of one or more compounds of the formulas (1), (1A), (2), (2A) and / or (5) mentioned above. It can also contain other polymerizable monomers. The polymer grafted onto the cellulose fiber material according to the invention contains, for example, 20 to 100 mol%, preferably 40 to 100 mol% and particularly preferably 80 to 100 mol%, of one or more compounds of the above formulas. The division between the monomers of the above formulas is arbitrary. As a rule, the grafted-on polymer contains only one of the compounds of the above formulas, i.e. a cellulose fiber material with a grafted-on homopolymer is preferred.
Copolymerisate, die auf das Cellulosefasermaterial aufgepfropft sein können, sind solche, die neben den obengenannten Monomeren der allgemeinen Formeln (1), (1A), (2), (2A) und/oder (5) als ein oder mehrere Comonomere solche entsprechend den nachstehenden allgemeinen Formeln (6) und (7) enthalten:
In diesen Formeln bedeuten:
- R1 und R2
- haben eine der obengenannten Bedeutungen, wobei R1 und R2 auch zusammen mit dem N-Atom den erwähnten heterocyclischen Rest bilden können:
- R13
- ist Wasserstoff oder Methyl;
- R14
- ist Wasserstoff oder Methyl;
- R15
- ist Wasserstoff oder Methyl;
- R16
- ist Wasserstoff oder Methyl;
- R17
- ist Alkyl von 1 bis 6 C-Atomen;
- q
- ist die Zahl Null, 1, 2 oder 3.
- R 1 and R 2
- have one of the meanings given above, where R 1 and R 2 can also form the heterocyclic radical mentioned together with the N atom:
- R 13
- is hydrogen or methyl;
- R 14
- is hydrogen or methyl;
- R 15
- is hydrogen or methyl;
- R 16
- is hydrogen or methyl;
- R 17
- is alkyl of 1 to 6 carbon atoms;
- q
- is the number zero, 1, 2 or 3.
Monomere der allgemeinen Formel (6) sind beispielsweise Acrylamid und Methacrylamid. Verbindungen der allgemeinen Formel (7) sind beispielsweise die (C1-C6)-Alkyl-ester, insbesondere die Methyl- und Ethylester, der Acryl- und der Methacrylsäure.Monomers of the general formula (6) are, for example, acrylamide and methacrylamide. Compounds of the general formula (7) are, for example, the (C 1 -C 6 ) alkyl esters, in particular the methyl and ethyl esters, acrylic and methacrylic acid.
Die Pfropfung des Mono- oder Copolymeren auf die Cellulosefaser wird so durchgeführt, daß ein oder mehrere Monomeren der Formeln (1), (1A), (2), (2A) und/oder (5) gegebenenfalls in Gegenwart von einem oder mehreren Monomeren der allgemeinen Formel (6) und/oder (7) polymerisiert werden. Die Polymerisation bzw. Copolymerisation auf der Faser erfolgt in einem geeigneten Lösungsmittel. Solche sind insbesondere Wasser im Gemisch mit einem wassermischbaren Lösungsmittel, das gegenüber den Monomeren inert ist. Solche Lösungsmittel sind beispielsweise niedere Alkohole, wie Alkanole von 1 bis 5 C-Atomen, beispielsweise Methanol, Ethanol, n-Propanol, Isopropanol, n-Butanol und tert.-Butanol, Glykole und Diole, wie beispielsweise Ethylenglykol, Propylenglykol und Propan-1,3-diol, Di-und Polyglykole, wie beispielsweise Diethylenglykol und Triethylenglykol, Glykolether, wie beispielsweise Diethylenglykol-monomethylether, Diethylenglykolmonoethylether, Ethylenglykol-monomethylether, Ethylenglykol-monoethylether, Ethylenglykol-mono-n-propyl-ether, Ethylenglykol-mono-n-butyl-ether, Ethylenglykoldimethylether und Ethylenglykol-diethylether, und des weiteren Ketone, wie beispielsweise Aceton und Methyl-ethyl-keton.The grafting of the mono- or copolymer onto the cellulose fiber is carried out in such a way that one or more monomers of the formulas (1), (1A), (2), (2A) and / or (5) optionally in the presence of one or more monomers of the general formula (6) and / or (7) are polymerized. The polymerization or copolymerization on the fiber takes place in a suitable solvent. Such are in particular water in a mixture with a water-miscible solvent which is inert to the monomers. Such solvents are, for example, lower alcohols, such as alkanols of 1 to 5 carbon atoms, for example methanol, ethanol, n-propanol, isopropanol, n-butanol and tert-butanol, glycols and diols, such as, for example, ethylene glycol, propylene glycol and propane-1 , 3-diol, di- and polyglycols, such as, for example, diethylene glycol and triethylene glycol, glycol ethers, such as, for example, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, ethylene glycol mono-n-propyl ether, ethylene glycol mono-n-n butyl ether, ethylene glycol dimethyl ether and ethylene glycol diethyl ether, and also ketones, such as acetone and methyl ethyl ketone.
Die Homopolymerisation und die Copolymerisation in Gegenwart des Cellulosefasermaterials können auch in einem Gemisch verschiedener Lösungsmittel durchgeführt werden, das Wasser enthält. Falls eine oder mehrere Komponenten in Form von wäßrigen Lösungen eingesetzt werden, ist in der Regel ein weiterer Wasserzusatz nicht erforderlich. Bevorzugt ist Wasser im Gemisch von Alkoholen, wie Alkanolen, insbesondere solchen mit 1 bis 4 C-Atomen, und im Gemisch mit Diolen und/oder Glykolen. Es kann zweckmäßig sein, während der Homo- oder Copolymerisation organisches Lösungsmittel oder Lösungsmittelgemisch einmal oder insbesondere mehrmals in Anteilen nachzusetzen.The homopolymerization and the copolymerization in the presence of the cellulose fiber material can also be carried out in a mixture of different solvents containing water. If one or more components are used in the form of aqueous solutions, a further addition of water is generally not necessary. Water in a mixture of alcohols, such as alkanols, in particular those having 1 to 4 carbon atoms, and in a mixture with diols and / or glycols is preferred. It may be expedient to add organic solvent or solvent mixture once or in particular several times during the homo- or copolymerization.
Vor Beginn der Homo- bzw. Copolymerisation ist es zweckmäßig, den pH-Wert auf Werte 3 bis 11,5, vorzugsweise 3 bis 8,5, einzustellen. Hierfür wird in der Regel eine Säure, vorzugsweise eine organische Säure, insbesondere Ameisensäure, Essigsäure, Glykolsäure und/oder Zitronensäure verwendet.Before the start of homopolymerization or copolymerization, it is advisable to adjust the pH to values from 3 to 11.5, preferably from 3 to 8.5. An acid, preferably an organic acid, in particular formic acid, acetic acid, glycolic acid and / or citric acid, is generally used for this.
Die Homo- und Copolymerisation wird in Gegenwart des Cellulosefasermaterials bei Raumtemperatur (10 bis 30°C) oder bei höherer Temperatur von bis zu etwa 160°C, in der Regel jedoch bei einer Temperatur zwischen 40 und 100°C und vorzugsweise zwischen 60 und 90°C, insbesondere bevorzugt zwischen 65 bis 85°C, durchgeführt. Hierfür verwendet man in üblicher Weise geeignete Polymerisationsinitiatoren, wie beispielsweise radikalbildende Substanzen. Auch die Einwirkung energiereicher Strahlung, wie UV-Strahlen, und ebenso Mikrowellen und die Einwirkung von Ultraschall, können dazu dienen, um die Polymerisation zu starten.The homo- and copolymerization is carried out in the presence of the cellulose fiber material at room temperature (10 to 30 ° C) or at a higher temperature of up to about 160 ° C, but usually at a temperature between 40 and 100 ° C and preferably between 60 and 90 ° C, particularly preferably between 65 to 85 ° C, performed. Suitable polymerization initiators, such as, for example, radical-forming substances, are used for this in the usual way. The action of high-energy radiation, such as UV rays, as well as microwaves and the action of ultrasound, can also serve to start the polymerization.
Radikalbildende Initiatoren sind beispielsweise Benzoylperoxid, tert.-Butylhydroperoxid, Cumolperoxid, Methyl-ethyl-keton-peroxid, Laurylperoxid, tert.-Butylperbenzoat, Di-tert.-butyl-perphthalat, Azodiisobutyronitril, 2,2'-Azo-bis-(2,4-dimethylvaleronitril), 2-Phenyl-azo-2,4-dimethyl-4-methoxy-valeronitril, 2-Cyano-2-propyl-azo-formamid, Azodiisobutyramid, Dimethyl-, Diethyl- oder Di-n-butyl-azobismethylvalerat, tert.-Butyl-perneodecanoat, Di-isononanoyl-peroxid, tert.-Amylperpivalat, Di-2-ethylhexyl-peroxydicarbonat, Dilauroylperoxid, Di-isotridecylperoxydicarbonat und tert.-Butylperoxy-isopropyl-percarbonat. Vorzugsweise wird als Initiator 2,2'-Azobis-(2-amidinopropan)-dihydrochlorid, 2,2'-Azo-bis-(2-imidazol-2-yl-propan)-dihydro-chlorid, 2,2'-Azo-bis(2-carbamoyl-propan)-dihydrat oder 2,2'-Azobis-(2-methoxycarbonyl-propan) verwendet. Bezogen auf die Monomerenmenge werden 0,01 bis 2 Gew.%, vorzugsweise 0,1 bis 1 Gew.%, Initiator angewandt.Free-radical initiators are, for example, benzoyl peroxide, tert-butyl hydroperoxide, cumene peroxide, methyl ethyl ketone peroxide, lauryl peroxide, tert-butyl perbenzoate, di-tert-butyl perphthalate, azodiisobutyronitrile, 2,2'-azo-bis- (2 , 4-dimethylvaleronitrile), 2-phenyl-azo-2,4-dimethyl-4-methoxy-valeronitrile, 2-cyano-2-propyl-azo-formamide, azodiisobutyramide, dimethyl-, diethyl- or di-n-butyl- azobismethylvalerate, tert-butyl perneodecanoate, di-isononanoyl peroxide, tert-amyl perpivalate, Di-2-ethylhexyl peroxydicarbonate, dilauroyl peroxide, di-isotridecyl peroxydicarbonate and tert-butyl peroxy-isopropyl percarbonate. 2,2'-Azobis- (2-amidinopropane) dihydrochloride, 2,2'-azobis (2-imidazol-2-yl-propane) dihydrochloride, 2,2'-azo is preferred as the initiator bis (2-carbamoyl-propane) dihydrate or 2,2'-azobis (2-methoxycarbonyl-propane) used. Based on the amount of monomers, 0.01 to 2% by weight, preferably 0.1 to 1% by weight, of initiator is used.
Es ist zweckmäßig, die Polymerisation oder Copolymerisation bzw. die Pfropfung unter Ausschluß von Sauerstoff durchzuführen. Dies kann in an sich bekannter Weise durch Spülen bzw. Durchleiten eines Inertgases, wie bspw. Stickstoff, erfolgen. Die Monomerkomponenten werden in solchen Mengen eingesetzt, daß das auf die Cellulose aufgepfropfte Polymerisat mindestens 20 Mol-%, vorzugsweise mindestens 40 Mol-% und insbesondere bevorzugt mindestens 80 Mol-% einer oder mehrerer Verbindungen der Formeln (1), (1A), (2), (2A) und/oder (5) in einpolymerisierter Form enthält.It is expedient to carry out the polymerization or copolymerization or the grafting in the absence of oxygen. This can be done in a manner known per se by purging or passing through an inert gas, such as nitrogen. The monomer components are used in amounts such that the polymer grafted onto the cellulose contains at least 20 mol%, preferably at least 40 mol% and particularly preferably at least 80 mol% of one or more compounds of the formulas (1), (1A), ( 2), (2A) and / or (5) in copolymerized form.
Die Pfropfung kann erfindungsgemäß so durchgeführt werden, daß das zu pfropfende Cellulosefasermaterial in das Polymerisationsgefäß zusammen mit der Lösung des oder der Monomeren eingebracht und die Homo- bzw. Copolymerisation durchgeführt wird. Unter diesen Bedingungen ist die Homo- bzw. Copolymersation in der Regel nach etwa 0,5 bis 4 Stunden, in vielen Fällen nach 0,5 bis 2,5 Stunden, beendet.According to the invention, the grafting can be carried out in such a way that the cellulose fiber material to be grafted is introduced into the polymerization vessel together with the solution of the monomer (s) and the homopolymerization or copolymerization is carried out. Under these conditions, the homo- or copolymerization is usually complete after about 0.5 to 4 hours, in many cases after 0.5 to 2.5 hours.
Dann wird das Cellulosefasermaterial aus dem Polymerisationsbehälter entfernt, von anhaftender Flüssigkeit, bspw. durch Abquetschen, befreit, mit Wasser gespült und getrocknet.The cellulose fiber material is then removed from the polymerization container, freed from adhering liquid, for example by squeezing, rinsed with water and dried.
Die Pfropfung der Cellulosefaser kann aber auch so durchgeführt werden, daß die Monomerlösung durch Foulardieren, Pflatschen, Tränken oder Besprühen auf das Cellulosefasermaterial aufgebracht und dann die Homo- bzw. Copolymerisation bei Normaltemperatur oder erhöhter Temperatur, zweckmäßigerweise durch die Einwirkung von energiereichen Strahlen oder von Ultraschall, ausgelöst wird. Dabei ist häufig eine etwa 0,5- bis 10-minutige Bestrahlung oder Beschallung ausreichend. Danach wird das Cellulosefasermaterial noch 2 bis 36 Stunden, vorzugsweise 10 bis 24 Stunden, feucht gelagert und anschließend nach eventuellem Spülen getrocknet wird.However, the grafting of the cellulose fiber can also be carried out in such a way that the monomer solution is applied to the cellulose fiber material by padding, slapping, soaking or spraying, and then the homo- or copolymerization at normal temperature or elevated temperature, expediently by the action of high-energy rays or ultrasound is triggered. Here about 0.5 to 10 minutes of radiation or sonication is often sufficient. The cellulose fiber material is then stored in a moist condition for a further 2 to 36 hours, preferably 10 to 24 hours, and is then dried, after rinsing, if necessary.
Cellulosefasermaterialien, die in erfindungsgemäßer Weise zur Modifizierung geeignet sind, sind beispielsweise Baumwolle und Zellwolle sowie die Leinen-, Jute-und Ramie-Fasern und regenerierte Cellulosefasern, vorzugsweise die Baumwollfaser. Die modifizierten Cellulosefasermaterialien können auch als Anteil in Mischgeweben vorliegen, wie beispielsweise Mischgewebe solcher modifizierter Cellosefasern mit Polyesterfasern. Die erfindungsgemäße Modifizierung der Cellulosefaser kann in dem Mischgewebe selbst erfolgen sowie in allen Verarbeitungszuständen, so als Garn, Flocke, Kammzug und Stückware. Es ist insbesondere als Substrat ein Cellulosefasermaterial geeignet, das in der vorstehenden Weise modifiziert, d.h. auf das Polymerisat gepfropft worden ist, und auf das insbesondere 0,5 bis 40 Gew.-%, vorzugsweise 2 bis 25 Gew.-% und insbesondere bevorzugt 4 bis 15 Gew.-% Polymerisat, bezogen auf die Cellulosefaser, aufgepfropft worden ist.Cellulose fiber materials which are suitable for modification in the manner according to the invention are, for example, cotton and rayon as well as the linen, jute and ramie fibers and regenerated cellulose fibers, preferably the cotton fiber. The modified cellulose fiber materials can also be present as a proportion in blended fabrics, such as blended fabrics of such modified cellulose fibers with polyester fibers. The modification of the cellulose fiber according to the invention can take place in the blended fabric itself and in all processing states, such as yarn, flake, sliver and piece goods. A cellulose fiber material which is modified in the above manner, i.e. has been grafted onto the polymer and onto which in particular 0.5 to 40% by weight, preferably 2 to 25% by weight and particularly preferably 4 to 15% by weight of polymer, based on the cellulose fiber, has been grafted.
Trockene Cellulose bzw. deren Derivate wurde bereits nach den Angaben der US-A 4 464 523 mit N,N-Diallyl-N,N-dialkyl-ammoniumhalogeniden als Monomeren durch Polymerisation modifiziert, wobei solche modifizierten Celluloseabkömmlinge als Hilfsmittel in Shampoos und andere Haarbehandlungsmittel Verwendung fanden. Der Einsatz von solchermaßen modifizierten Cellulosefasermaterialien, wie von solchermaßen modifizierter Baumwolle, als Substrat zum Färben mit anionischen, insbesondere faserreaktiven, Farbstoffen ist jedoch noch nicht bekannt. Insbesondere ist die Modifizierung durch Aufpfropfen der Polymerisate, einschließlich Copolymerisate, von Verbindungen der allgemeinen Formeln (1), (1A), (2) und/oder (2A) neu.According to US Pat. No. 4,464,523, dry cellulose or its derivatives has already been modified by polymerization using N, N-diallyl-N, N-dialkylammonium halides as monomers, such modified cellulose derivatives being used as auxiliaries in shampoos and other hair treatment agents found. However, the use of cellulose fiber materials modified in this way, such as cotton modified in this way, as a substrate for dyeing with anionic, in particular fiber-reactive, dyes is not yet known. In particular, the modification by grafting on the polymers, including copolymers, of compounds of the general formulas (1), (1A), (2) and / or (2A) is new.
In der JP-A-56-096 972 wird beschrieben, daß ein mit 2-(Dimethylamino)ethylmethacrylat gepfropftes Baumwoll-Polyester-Gewebe mit Säurefarbstoffen gefärbt werden kann, wobei das gefärbte Gewebe eine verbesserte Waschechtheit aufweist.JP-A-56-096 972 describes that a cotton-polyester fabric grafted with 2- (dimethylamino) ethyl methacrylate can be dyed with acid dyes, the dyed fabric having improved wash fastness.
In der US-A-3 514 385 wird die Pfropfung von Textilmaterial aus Cellulose mit ethylenisch ungesättigten Monomeren wie 4-Vinyl-pyridin zur Verbesserung allgemeiner Gewebeeigenschaften wie Festigkeit, Temperaturstabilität, Wascheigenschaften, Echtheiten u.a. beschrieben.
In der EP-A2-0 265 768 werden Textilien mit kationischen Cellulosecopolymeren, beispielsweise Copolymeren aus Hydroxyethylcellulose und Dimethyldiallyl-ammoniumchlorid, imprägniert, um die Anfärbbarkeit mit anionischen Textilfarbstoffen zu verbessern.US Pat. No. 3,514,385 describes the grafting of textile material made from cellulose with ethylenically unsaturated monomers such as 4-vinyl-pyridine to improve general fabric properties such as strength, temperature stability, washing properties, fastness properties and others.
EP-A2-0 265 768 impregnates textiles with cationic cellulose copolymers, for example copolymers of hydroxyethyl cellulose and dimethyldiallyl ammonium chloride, in order to improve the dyeability with anionic textile dyes.
Die vorliegende Erfindung betrifft auch Cellulosefasermaterialien, die in erfindungsgemäßer Weise durch Aufpfropfen eines Copolymerisats der Verbindungen der allgemeinen Formel (1) und/oder (2) bzw. deren quarternierten Derivate mit mindestens einem Monomeren der allgemeinen Formeln (6) und (7) modifiziert wurden, sowie das Verfahren zu deren Herstellung.The present invention also relates to cellulose fiber materials which have been modified in the manner according to the invention by grafting on a copolymer of the compounds of the general formula (1) and / or (2) or their quaternized derivatives with at least one monomer of the general formulas (6) and (7) , and the process for their production.
Das Färben solchermaßen modifizierter Cellulosefasermaterialien in erfindungsgemäßer Weise erfolgt analog den bekannten Färbeweisen zum Färben von Cellulosefasermaterialien unter Anwendung von anionischen faserreaktiven Farbstoffen, und unter Anwendung der hierfür bekanntermaßen eingesetzten Temperaturbereiche und üblichen Farbstoffmengen, jedoch mit der erfindungsgemäßen Ausnahme, daß für die Färbebäder und Klotzflotten der erfindungsgemäßen Färbeverfahren ein Zusatz von alkalisch wirkenden Verbindungen, wie sie üblicherweise zur Fixierung von faserreaktiven Farbstoffen benutzt werden, wie beispielsweise Natriumcarbonat, Kaliumcarbonat, Natronlauge und Wasserglas, ausgeschlossen werden kann und des weiteren der übliche Zusatz an Elektrolytsalzen, die insbesondere die Migration des Farbstoffes auf der Faser erhöhen sollen, nicht oder nur in geringem Maße, d.h. bis zu höchstens 10 g pro Liter Färbebad oder Färbeflotte, erforderlich ist. Das erfindungsgemäße Färbeverfahren erfolgt demgemäß innerhalb eines pH-Bereiches von 3 bis 7,5, vorzugsweise von 4,5 bis 7.The dyeing of cellulose fiber materials modified in this way in the manner according to the invention is carried out analogously to the known dyeing methods for dyeing cellulose fiber materials using anionic fiber-reactive dyes, and using the temperature ranges and customary amounts of dye known to be used for this, but with the exception according to the invention that for the dyebaths and padding liquors of the invention Dyeing process, an addition of alkaline compounds, such as are usually used for fixing fiber-reactive dyes, such as sodium carbonate, potassium carbonate, sodium hydroxide solution and water glass, can be excluded and furthermore the usual addition of electrolyte salts, in particular the migration of the dye on the fiber should increase, not or only to a small extent, ie up to a maximum of 10 g per liter of dye bath or dye liquor is required. The dyeing process according to the invention is accordingly carried out within a pH range from 3 to 7.5, preferably from 4.5 to 7.
Färbeverfahren, die erfindungsgemäß eingesetzt werden können, sind beispielsweise die verschiedenen Ausziehverfahren, wie das Färben auf dem Jigger und auf der Haspelkufe oder das Färben aus langer oder kurzer Flotte, das Färben in Jet-Färbemaschinen, das Färben nach dem Klotz-Kaltverweil-Verfahren oder nach einem Klotz-Heißdampf-Fixierverfahren. Beim Ausziehverfahren kann man im üblichen Flottenverhältnis von 1 : 3 bis 1 : 20 arbeiten. Die Färbetemperatur kann zwischen 30 und 90°C betragen, bevorzugt liegt sie bei Temperaturen unterhalb 60°C; wie sich aus der oben erwähnten erfindungsgemäßen Anwendung des Klotz-Kaltverweil-Verfahrens ergibt, ist das Färben auch vorteilhaft bei Raumtemperatur (10 bis 30°C) möglich.Dyeing processes which can be used according to the invention are, for example, the various extraction processes, such as dyeing on the jigger and on the reel runner or dyeing from a long or short liquor, dyeing in jet dyeing machines, dyeing using the pad-cold-dwell method or after a block-hot steam fixing process. The pull-out process can be carried out in the usual liquor ratio of 1: 3 to 1:20. The dyeing temperature can be between 30 and 90 ° C, preferably at temperatures below 60 ° C; As can be seen from the above-mentioned application of the pad-cold residence method according to the invention, dyeing is also advantageously possible at room temperature (10 to 30 ° C.).
In den erfindungsgemäßen Färbeprozessen können darüber hinaus die üblichen Hilfsmittel, wie Tenside (Netzmittel), Harnstoff und Egalisierhilfsmittel oder Hilfsmittel, die die Löslichkeit von Farbstoffen in den konzentrierten Klotzflotten verbessern, wie beispielsweise Kondensationsprodukte aus Formaldehyd und gegebenenfalls alkylsubstituierten Naphthalinsulfonsäuren, eingesetzt werden.In the dyeing processes according to the invention, the usual auxiliaries, such as surfactants (wetting agents), urea and leveling aids or auxiliaries, which improve the solubility of dyes in the concentrated padding liquors, such as condensation products of formaldehyde and optionally alkyl-substituted naphthalenesulfonic acids.
Für die erfindungsgemäße Färbeweise sind alle anionischen Farbstoffe, die eine oder mehrere Sulfo- und/oder Carboxygruppen besitzen und die faserreaktive Gruppen enthalten, geeignet. Sie können sowohl der Klasse der Azofarbstoffe, der Kupferkomplex-, Kobaltkomplex- und Chromkomplex-Azofarbstoffe, der Kupfer- und Nickelphthalocyanin-Farbstoffe, der Anthrachinon-, Kupferformazan- und Triphendioxazinfarbstoffe angehören. Solche Farbstoffe sind zahlreich in der Literatur beschrieben und dem Fachmann allseits geläufig.All anionic dyes which have one or more sulfo and / or carboxy groups and which contain fiber-reactive groups are suitable for the dyeing methods according to the invention. They can belong to the class of azo dyes, copper complex, cobalt complex and chromium complex azo dyes, copper and nickel phthalocyanine dyes, anthraquinone, copper formazane and triphendioxazine dyes. Such dyes have been described in numerous literature and are well known to those skilled in the art.
Die in erfindungsgemäßer Weise erhältlichen Färbungen der modifizierten Cellulosefasermaterialien benötigen nach der Entnahme aus dem Färbebad bzw. nach Beendigung der Fixierung des Farbstoffes auf dem Substrat keine weitere Nachbehandlung, insbesondere keinen aufwendigen Nachbehandlungsprozeß unter Einbeziehung einer Wäsche. In der Regel genügt ein übliches ein- oder mehrmaliges Spülen des gefärbten Substrates mit kaltem, warmem oder heißem Wasser, das gegebenenfalls ein nichtionogenes Netzmittel enthalten kann. Eine Kochendbehandlung des gefärbten Substrates mit einer Waschlösung zur Verbesserung der Echtheitseigenschaften ist nicht erforderlich.The dyeings of the modified cellulose fiber materials obtainable in the manner according to the invention require no further aftertreatment after removal from the dyebath or after completion of the fixation of the dye on the substrate, in particular no complicated aftertreatment process including washing. As a rule, it is sufficient to rinse the colored substrate once or several times with cold, warm or hot water, which may optionally contain a nonionic wetting agent. A final boiling treatment of the dyed substrate with a washing solution to improve the fastness properties is not necessary.
Die nachstehenden Beispiele dienen zur Erläuterung der Erfindung. Die darin genannten Teile sind Gewichtsteile, die Prozentangaben stellen Gewichtsprozente dar, sofern nicht anders vermerkt. Gewichtsteile beziehen sich zu Volumenteilen wie Kilogramm zu Liter.The following examples serve to illustrate the invention. The parts mentioned are parts by weight, the percentages are percentages by weight, unless stated otherwise. Parts by weight relate to parts by volume such as kilograms to liters.
In einem 5-Liter-Dreihalskolben, der mit einem Ankerrührer, Stickstoffeinleitungsrohr und Rückflußkühler versehen ist, wird ein 2,8 g schweres,
8 x 30 cm großes Baumwolltuch befestigt. Dann werden
- 1000 ml destilliertes Wasser,
- 113,3 g Kochsalz,
- 573 mg Ethylendiamintetraessigsäure-dinatriumsalz und
- 2372,5 mg Dimethyl-diallyl-ammoniumchlorid als 61%ige wäßrige Lösung vorgelegt, der Kolben dreimal evakuiert, mit Stickstoff inertisiert und unter 380 bis 400 mbar auf 80°C geheizt. Bei dieser Temperatur werden unter Rühren 64,5 g einer 5,25%igen Ammoniumpersulfat-Lösung zudosiert; es wird eine Stunde geheizt, und sodann werden weitere 54 g einer 12,5%igen Ammoniumpersulfat-Lösung zudosiert und eine Stunde bei 80°C geheizt. Nach Zugabe von weiteren 118 g einer wäßrigen Ammoniumpersulfat-Lösung wird die Polymerisation bei 80 bis 83°C, während 2 Stunden durchgeführt.
8 x 30 cm cotton cloth attached. Then be
- 1000 ml of distilled water,
- 113.3 g of table salt,
- 573 mg disodium salt of ethylenediaminetetraacetic acid and
- 2372.5 mg of dimethyldiallylammonium chloride as 61% aqueous solution, the flask was evacuated three times, inerted with nitrogen and heated to 80 ° C. under 380 to 400 mbar. At this temperature, 64.5 g of a 5.25% ammonium persulfate solution are metered in with stirring; it is heated for one hour, and then another 54 g of a 12.5% strength ammonium persulfate solution are metered in and heated at 80 ° C. for one hour. After adding a further 118 g of an aqueous ammonium persulfate solution, the polymerization is carried out at 80 to 83 ° C. for 2 hours.
Anschließend wird das Baumwolltuch aus dem Reaktionskolben entnommen, abgequetscht, zweimal mit je 500 ml Wasser gespült, zwischendurch abgequetscht und danach 5 Minuten bei 100°C getrocknet. Die Aufnahme an Polymer beträgt 5,3 %.The cotton cloth is then removed from the reaction flask, squeezed off, rinsed twice with 500 ml of water each time, squeezed out in between and then dried at 100 ° C. for 5 minutes. The absorption of polymer is 5.3%.
In einem 5-Liter-Dreihalskolben, der mit einem Ankerrührer, Gaseinleitungsrohr und Rückflußkühler versehen ist, wird ein 5,4 g schweres und 8 x 60 cm großes Baumwolltuch befestigt, und
- 90 g NaCl, gelöst in 100 ml Wasser,
- 130,9 g Dimethylaminopropylmethacrylamid,
- 319,5 g Ethanol und
- 407 mg Ethylendiamintetraessigsäure-dinatriumsalz
- 90 g NaCl, dissolved in 100 ml water,
- 130.9 g dimethylaminopropyl methacrylamide,
- 319.5 g of ethanol and
- 407 mg disodium salt of ethylenediaminetetraacetic acid
Mit 231 g Eisessig wird ein pH-Wert von 8,5 bis 9,0 eingestellt. 960 g Wasser und 2060 mg 2,2'-Azo-bis(2-amidino-propan)-dihydrochlorid als Initiator werden zugegeben; anschließend wird der Kolben dreimal evakuiert, mit Stickstoff inertisiert und auf 79 bis 80°C geheizt.A pH of 8.5 to 9.0 is set with 231 g of glacial acetic acid. 960 g of water and 2060 mg of 2,2'-azobis (2-amidino-propane) dihydrochloride as initiator are added; the flask is then evacuated three times, rendered inert with nitrogen and heated to 79 to 80 ° C.
Sobald die Innentemperatur von 80°C erreicht ist, werden nacheinander
- 45,8 g Ethanol,
- 38,5 g Ethanol,
- 93 g Ethanol und schließlich nochmals
- 322,5 g Ethanol zugegeben.
- 45.8 g ethanol,
- 38.5 g of ethanol,
- 93 g of ethanol and finally again
- 322.5 g of ethanol were added.
Zwischen jeder Zugabe wird 15 Minuten nachgerührt und am Ende 2 h bei 80°C auspolymerisiert. Danach wird das Baumwolltuch aus dem Kolben entnommen, dreimal mit je 200 ml Wasser gespült, wobei zwischendurch abgequetscht wird, und anschließend 5 Minuten getrocknet. Die Aufnahme an Polymer beträgt 4,9 %.Between each addition, stirring is continued for 15 minutes and polymerization is complete at 80 ° C. for 2 hours. The cotton cloth is then removed from the flask, rinsed three times with 200 ml of water each time, being squeezed in between, and then dried for 5 minutes. The absorption of polymer is 4.9%.
Die Wirksubstanzkonzentration an Polymerisat beträgt 25 %. Der K-Wert einer 1%igen wäßrigen Lösung beträgt: 112600.The active substance concentration of polymer is 25%. The K value of a 1% aqueous solution is: 112600.
Bei einer Wiederholung des Beispiels 2 wird das Baumwolltuch durch ein 3,4 g schweres, 8 x 47 cm großes Zellwolltuch ersetzt. Die Aufnahme an Polymer beträgt 2,95 %.When Example 2 is repeated, the cotton cloth is replaced by a 3.4 g, 8 x 47 cm large cellulose cloth. The absorption of polymer is 2.95%.
Bei einer Wiederholung des Beispiels 3 wird das Baumwolltuch durch ein 5,7 g schweres gleichgroßes Tuch aus einem 65:35 %-Polyester/Baumwoll-Mischgewebe ersetzt. Die Aufnahme an Polymer beträgt 3,4 %.When Example 3 is repeated, the cotton cloth is replaced by a 5.7 g, equally large cloth made of a 65:35% polyester / cotton blended fabric. The absorption of polymer is 3.4%.
In einem 3-Liter-Dreihalskolben, der mit einem Ankerrührer, Gaseinleitungsrohr und Rückflußkühler versehen ist, wird eine Lösung aus
- 200 g destilliertem Wasser,
- 56,7 g Kochsalz,
- 287 mg Ethylendiamintetraessigsäuredinatriumsalz,
- 250 g Ethanol und
- 1210 g Dimethyl-diallylammoniumchlorid als 61 % wäßrige Lösung vorgelegt.
- 200 g of distilled water,
- 56.7 g of table salt,
- 287 mg of ethylenediaminetetraacetic acid disodium salt,
- 250 g ethanol and
- 1210 g of dimethyl-diallylammonium chloride presented as a 61% aqueous solution.
Dann wird mit 9,5 g Eisessig ein pH-Wert von 3,7 bis 3,8 eingestellt und mit 368,8 g Ethanol verdünnt. Der Gehalt an monomerer Wirksubstanz beträgt 34,7 %.Then a pH of 3.7 to 3.8 is adjusted with 9.5 g of glacial acetic acid and diluted with 368.8 g of ethanol. The content of monomeric active substance is 34.7%.
827 g dieser 34,7%igen Monomerlösung werden in ein Becherglas gefüllt. In die Lösung wird ein 100 x 25 cm großes, 11,6 g schweres Baumwollgewebe getaucht und 30 Minuten mit einer Ultraschallquelle beschallt. Dann wird das Baumwolltuch abgequetscht und feucht 24 h bei Raumtemperatur gelagert. Die Aufnahme an Polymer beträgt 37 %.827 g of this 34.7% monomer solution are placed in a beaker. A 100 x 25 cm, 11.6 g cotton fabric is dipped into the solution and sonicated for 30 minutes with an ultrasound source. Then the cotton cloth is squeezed off and stored moist at room temperature for 24 hours. The uptake of polymer is 37%.
Ein Lösung aus
- 200 ml Wasser,
- 18,1 g Kochsalz,
- 206,18 18 g Dimethylaminopropylmethacrylamid,
- 63,9 g Ethanol und
- 81,4 mg Ethylendiamintetraessigsäure-dinatriumsalz
- 200 ml water,
- 18.1 g of table salt,
- 206.18 18 g dimethylaminopropyl methacrylamide,
- 63.9 g of ethanol and
- 81.4 mg disodium salt of ethylenediaminetetraacetic acid
Das feuchte Tuch wird einer Mikrowellenbehandlung unterzogen (30 Sekunden mit 90 Watt und anschließend 4 mal 30 Sekunden mit 720 Watt). Danach läßt man das feuchte Stofftuch 24 h bei Zimmertemperatur verweilen und trocknet es anschließend 5 Minuten bei 100°C. Die Aufnahme an Polymer beträgt 29,5 %.The damp cloth is subjected to a microwave treatment (30 seconds with 90 watts and then 4 times 30 seconds with 720 watts). Thereafter, the damp cloth is left for 24 hours at room temperature and then dried for 5 minutes at 100 ° C. The absorption of polymer is 29.5%.
Die in Beispiel 6 angegebene Monomerlösung wird in der in Beispiel 6 angegebenen Weise auf ein Baumwolltuch aufgebracht und abgequetscht. Das feuchte Tuch wird einer UV-Bestrahlung ausgesetzt, um die Polymerisation und Pfropfung auszulösen. Die Nachbehandlung des Tuches erfolgt wie in Beispiel 6 angegeben. Die Aufnahme an Polymer beträgt 30,8 %.The monomer solution given in Example 6 is applied to a cotton cloth and squeezed off in the manner given in Example 6. The damp cloth is exposed to UV radiation in order to trigger the polymerization and grafting. The aftertreatment of the cloth is carried out as indicated in Example 6. The absorption of polymer is 30.8%.
In einem 5-Liter-Dreihalskolben, der mit einem Ankerrührer, Gaseinleitungsrohr und Rückflußkühler versehen ist, befindet sich ein 5,7 g schweres und 8 x 60 cm großes Stofftuch aus einem 30:70%-Baumwolle/Polyester-Mischgewebe.
800 g destilliertes Wasser werden zugegeben, 127,5 g NaCl darin gelöst und danach 1452 g Dimethylaminopropylmethyacrylamid, 713,6 g Ethanol und 573 mg Ethylendiamintetraessigsäure-dinatriumsalz zugesetzt. Mit 246,4 g Eisessig wird ein pH-Wert von 8 bis 9 eingestellt.In a 5 liter three-necked flask, which is equipped with an anchor stirrer, gas inlet pipe and reflux condenser, there is a 5.7 g heavy and 8 x 60 cm large cloth made from a 30: 70% cotton / polyester blend.
800 g of distilled water are added, 127.5 g of NaCl are dissolved therein, and then 1452 g of dimethylaminopropylmethyacrylamide, 713.6 g of ethanol and 573 mg of ethylenediaminetetraacetic acid disodium salt are added. A pH of 8 to 9 is adjusted with 246.4 g of glacial acetic acid.
Nun werden 2,9 g 2,2'-Azobis-2-amidinopropan-dihydrochlorid und 290,5 g Wasser hinzugefügt. Diese Monomerlösung wird 1 h bei 20 bis 25°C gerührt. Das Tuch wird abgequetscht und in 2 Teile geteilt. Je ein Teil wird den folgenden Behandlungen unterworfen:
- I. Der eine Teil des Tuches wird einer Mikrowellenbehandlung während 30 Sekunden mit 90 Watt und 2 Minuten lang mit 720 Watt ausgesetzt. Danach wird es 5 Minuten bei 100°C getrocknet. Die Aufnahme an Polymer beträgt 7,9 % des Warengewichts.
- II. Der andere Teil des Tuches wird 15 Minuten einer UV-Bestrahlung ausgesetzt und anschließend ebenfalls 5 Minuten bei 100°C getrocknet. Die Aufnahme an Polymerisat beträgt 9,11 % des Warengewichts.
- I. One part of the cloth is subjected to microwave treatment for 30 seconds with 90 watts and for 2 minutes with 720 watts. Then it is dried for 5 minutes at 100 ° C. The absorption of polymer is 7.9% of the product weight.
- II. The other part of the cloth is exposed to UV radiation for 15 minutes and then also dried at 100 ° C. for 5 minutes. The absorption of polymer is 9.11% of the product weight.
In einem 5-Liter-Dreihalskolben, der mit einem Ankerrührer, Gaseinleitungsrohr und Rückflußkühler versehen ist, befindet sich ein 5,7 g schweres, 8 x 60 cm großes Stofftuch aus Baumwolle. 200 ml destilliertes Wasser werden hinzugegeben, 127,5 g NaCl darin gelöst und danach 1186,3 g Dimethyl-diallyl-ammoniumchlorid als 61,2%ige wäßrige Lösung, 1452 g Trimethylammoniumpropyl-methacrylamid, 573 mg Ethylendiamintetraessigsäuredinatriumsalz und 450 g Ethanol nacheinander hinzugesetzt. Mit 1,0 g Eisessig wird ein pH-Wert von 6,0 eingestellt. Nach der Zugabe von 76,9 g Ethanol werden 2,9 g 2,2'-Azobis-(2-amidinopropan)-hydrochlorid als Initiator zugesetzt, und unter Darüberleiten von Stickstoff wird auf eine Innentemperatur von 78 bis 80°C geheizt. Sobald diese Innentemperatur erreicht ist, beginnt die Polymerisation und Pfropfung unter Temperaturanstieg auf 84°C.In a 5 liter three-necked flask, which is equipped with an anchor stirrer, gas inlet pipe and reflux condenser, there is a 5.7 g, 8 x 60 cm cotton cloth. 200 ml of distilled water are added, 127.5 g of NaCl are dissolved therein and then 1186.3 g of dimethyldiallylammonium chloride as a 61.2% aqueous solution, 1452 g of trimethylammonium propyl methacrylamide, 573 mg of ethylenediaminetetraacetic acid disodium salt and 450 g of ethanol are added in succession. A pH of 6.0 is set with 1.0 g of glacial acetic acid. After the addition of 76.9 g of ethanol, 2.9 g of 2,2'-azobis (2-amidinopropane) hydrochloride are added as the initiator, and the mixture is heated to an internal temperature of 78 to 80 ° C. while passing nitrogen over it. As soon as this internal temperature is reached, the polymerization and grafting begins with an increase in temperature to 84 ° C.
Nach Beendigung der Polymerisation werden nacheinander 64,5 g, 54,2 g, 118,4 g und 453 g Ethanol jeweils in Abständen von 10 bis 15 Minuten zugefügt. Nach der Zugabe des letzten Anteils wird 1 h bei 77 bis 80°C nachgerührt. Danach wird das Tuch entnommen und wie in Beispiel 1 nachbehandelt. Die Aufnahme an Polymer beträgt 14,6 %.After the end of the polymerization, 64.5 g, 54.2 g, 118.4 g and 453 g of ethanol are added in succession at intervals of 10 to 15 minutes. After the last portion has been added, the mixture is stirred at 77 to 80 ° C. for 1 h. The cloth is then removed and treated as in Example 1. The absorption of polymer is 14.6%.
In einem 5-Liter-Dreihalskolben, der mit einem Ankerrührer, Gaseinleitungsrohr und Rückflußkühler versehen ist, befindet sich ein 5,6 g schweres und 8 x 60 cm großes Stofftuch aus Baumwolle. 100 g destilliertes Wasser, 127,5 g NaCl,
- 1452 g Trimethylammoniumpropylmethacrylamidchlorid als 50%ige wäßrige Lösung,
- 726 g Dimethylaminopropylmethacrylamid,
- 573 mg Ethylendiamintetraessigsäuredinatriumsalz, 450 g Ethanol und
- 638,2 g Eisessig werden nacheinander zugegeben. Danach fügt man 2,9 g 2,2'-Azobis-(-2-amidinopropandihydrochlorid) als Initiator hinzu und heizt unter Darüberleiten von Stickstoff auf eine Temperatur von 80°C. Die Polymerisation beginnt, sobald die Temperatur von 80°C erreicht ist, und steigt dann noch bis auf 89°C. Danach wird Ethanol in Anteilen von 64,5 g, 54,2 g, 118,4 g und
- 400 g Ethanol hinzugefügt; nach jeder Zugabe wird 10 Minuten nachgerührt, und am Ende der Zugabe wird bei 78 bis 80°C während 2 h nachgeheizt.
- 1452 g of trimethylammonium propyl methacrylamide chloride as a 50% aqueous solution,
- 726 g of dimethylaminopropyl methacrylamide,
- 573 mg of ethylenediaminetetraacetic acid disodium salt, 450 g of ethanol and
- 638.2 g of glacial acetic acid are added in succession. Thereafter, 2.9 g of 2,2'-azobis - (- 2-amidinopropane dihydrochloride) are added as an initiator and the mixture is heated to a temperature of 80 ° C. while passing nitrogen over it. The polymerization begins as soon as the temperature of 80 ° C is reached, and then rises to 89 ° C. Thereafter, ethanol in proportions of 64.5 g, 54.2 g, 118.4 g and
- 400 g of ethanol added; after each addition, stirring is continued for 10 minutes, and at the end of the addition the mixture is heated at 78 to 80 ° C for 2 h.
Die erhaltene Polymerisatlösung ist 38%ig (zur Gehaltsbestimmung wird eine Probe 2 h lang bei einem Druck von 200 mbar auf 120°C erhitzt). Für die Viskosität der Lösung werden im DIN-Becher (Düsedurchmesser 6 mm) 44,6 Sekunden gemessen. Das Tuch wird entnommen und wie in Beispiel 1 nachbehandelt. Die Aufnahme an Polymer beträgt 4,7 %.The polymer solution obtained is 38% (to determine the content, a sample is heated to 120 ° C. for 2 hours at a pressure of 200 mbar). The viscosity of the solution is measured in a DIN cup (nozzle diameter 6 mm) for 44.6 seconds. The cloth is removed and treated as in Example 1. The absorption of polymer is 4.7%.
Ein gemäß Beispiel 1 modifiziertes Baumwollgewebe wird zunächst 15 Minuten in Wasser kochend behandelt und anschließend getrocknet. Dieses Gewebe wird sodann gemäß einem Klotz-Kaltverweil-Färbeverfahren gefärbt. Hierzu wird eine wäßrige Farbstofflösung, die in 1000 Vol.-Teilen 28,5 Teile eines 50%igen elektrolythaltigen (vorwiegend natriumchloridhaltigen) Farbstoffpulvers des bekannten Farbstoffes der Formel
Es wird eine farbstarke, gleichmäßig gefärbte orange Färbung erhalten, die gute Allgemeinechtheiten, insbesondere gute Reib- und Lichtechtheiten, besitzt.A strong, uniformly colored orange coloration is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
10 Teile des gemäß Beispiel 1 modifizierten und anschließend durch 15-minütige kochende Behandlung in Wasser nachbehandelte, getrocknete Baumwollgewebe wird in 200 Vol.-Teile einer wäßrigen Farbstofflösung gegeben, die 0,2 Teile des im Beispiel A beschriebenen Farbstoffpulvers gelöst enthält. Dieses Färbebad wird innerhalb von 30 Minuten auf 60°C geheizt, und der Färbeprozeß wird bei dieser Temperatur 60 Minuten weitergeführt. Anschließend wird das Gewebe aus dem Bad entnommen und mit kaltem und mit heißem Wasser gespült und getrocknet.10 parts of the dried cotton fabric modified according to Example 1 and then aftertreated by water for 15 minutes in water are placed in 200 parts by volume of an aqueous dye solution which contains 0.2 part of the dye powder described in Example A in solution. This dye bath is heated to 60 ° C. in the course of 30 minutes and the dyeing process is continued at this temperature for 60 minutes. The tissue is then removed from the bath and rinsed with cold and hot water and dried.
Es wird, wie im Beispiel A, eine orange Färbung mit guten Allgemeinechtheiten, wie insbesondere guten Reib- und Lichtechtheiten, erhalten.As in Example A, an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
Ein gemäß Beispiel 1 modifiziertes Baumwollgewebe wird zunächst 15 Minuten in Wasser kochend behandelt und anschließend getrocknet. Dieses Gewebe wird sodann mit einer wäßrigen Farbstofflösung, die in 1000 Vol.-Teilen 28,5 Teile eines 50%igen elektrolythaltigen (vorwiegend natriumchloridhaltigen) Farbstoffpulvers des bekannten Farbstoffes der Formel
Es wird eine farbstarke, gleichmäßig gefärbte rote Färbung erhalten, die gute Allgemeinechtheiten, insbesondere gute Reib- und Lichtechtheiten, besitzt.A strong, uniformly colored red color is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
10 Teile des gemäß Beispiel 1 modifizierten und anschließend durch 15-minütige kochende Behandlung in Wasser nachbehandelte, getrocknete Baumwollgewebe wird in 200 Vol.-Teile einer wäßrigen Farbstofflösung gegeben, die 0,2 Teile des im Beispiel D beschriebenen Farbstoffpulvers gelöst enthält. Dieses Färbebad wird innerhalb von 30 Minuten auf 60°C geheizt und der Färbeprozeß bei dieser Temperatur 60 Minuten weitergeführt. Anschließend wird das Gewebe aus dem Bad entnommen und mit kaltem und mit heißem Wasser gespült und getrocknet.10 parts of the dried cotton fabric modified according to Example 1 and then aftertreated by 15 minutes of boiling treatment in water are added to 200 parts by volume of an aqueous dye solution which contains 0.2 part of the dye powder described in Example D in solution. This dye bath is heated to 60 ° C. within 30 minutes and the dyeing process is continued at this temperature for 60 minutes. The tissue is then removed from the bath and rinsed with cold and hot water and dried.
Es wird, wie im Beispiel D, eine orange Färbung mit guten Allgemeinechtheiten, wie insbesondere guten Reib- und Lichtechtheiten, erhalten.As in Example D, an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
Ein gemäß Beispiel 9 modifiziertes Baumwollgewebe wird zunächst 15 Minuten in Wasser kochend behandelt und anschließend getrocknet. Dieses Gewebe wird sodann gemäß einem Klotz-Kaltverweil-Färbeverfahren gefärbt. Hierzu wird eine wäßrige Farbstofflösung, die in 1000 Vol.-Teilen 28,5 Teile des in Beispiel A beschriebenen Farbstoffpulvers, 100 Teile Harnstoff und 3 Teile eines handelsüblichen nichtionogenen Benetzungsmittels gelöst enthielt, mittels eines Foulards mit einer Flottenaufnahme von 80 %, bezogen auf das Gewicht des Gewebes, bei 20°C auf das Gewebe aufgebracht. Das mit der Farbstofflösung geklotzte Gewebe wird auf eine Docke aufgewickelt, in eine Plastikfolie gehüllt und während 16 Stunden bei 20°C liegen lassen und danach mit kaltem und mit heißem Wasser gespült und getrocknet.A cotton fabric modified in accordance with Example 9 is first boiled in water for 15 minutes and then dried. This fabric is then dyed according to a pad cold-dyeing process. For this purpose, an aqueous dye solution containing 28.5 parts of the dye powder described in Example A, 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved in 1000 parts by volume, using a padder with a liquor absorption of 80%, based on the Weight of the fabric, applied to the fabric at 20 ° C. The fabric sealed with the dye solution is wound onto a dock, wrapped in a plastic film and left at 20 ° C. for 16 hours and then rinsed with cold and hot water and dried.
Es wird eine farbstarke, gleichmäßig gefärbte orange Färbung erhalten, die gute Allgemeinechtheiten, insbesondere gute Reib- und Lichtechtheiten, besitzt.A strong, uniformly colored orange coloration is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
10 Teile des gemäß Beispiel 9 modifizierten und anschließend durch 15-minütige kochende Behandlung in Wasser nachbehandelte, getrocknete Baumwollgewebe wird in 200 Vol.-Teile einer wäßrigen Farbstofflösung gegeben, die 0,2 Teile des im Beispiel A beschriebenen Farbstoffpulvers gelöst enthält. Dieses Färbebad wird innerhalb von 30 Minuten auf 60°C geheizt, und der Färbeprozeß wird bei dieser Temperatur 60 Minuten weitergeführt. Anschließend wird das Gewebe aus dem Bad entnommen und mit kaltem und mit heißem Wasser gespült und getrocknet.10 parts of the dried cotton fabric modified according to Example 9 and subsequently aftertreated by water for 15 minutes in water are placed in 200 parts by volume of an aqueous dye solution which contains 0.2 part of the dye powder described in Example A in solution. This dye bath is heated to 60 ° C. in the course of 30 minutes and the dyeing process is continued at this temperature for 60 minutes. The tissue is then removed from the bath and rinsed with cold and hot water and dried.
Es wird, wie im Beispiel A, eine orange Färbung mit guten Allgemeinechtheiten, wie insbesondere guten Reib- und Lichtechtheiten, erhalten.As in Example A, an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
Ein gemäß Beispiel 9 modifiziertes Baumwollgewebe wird zunächst 15 Minuten in Wasser kochend behandelt und anschließend getrocknet. Dieses Gewebe wird sodann mit einer wäßrigen Farbstofflösung, die in 1000 Vol.-Teilen 28,5 Teile des in Beispiel D beschriebenen Farbstoffpulvers, 100 Teile Harnstoff und 3 Teile eines handelsüblichen nichtionogenen Benetzungsmittels gelöst enthält, mittels eines Foulards mit einer Flottenaufnahme von 80 %, bezogen auf das Gewicht des Gewebes, bei 20°C geklotzt. Das geklotzte Gewebe wird auf eine Docke aufgewickelt, in eine Plastikfolie gehüllt und während 16 Stunden bei 20°C liegen lassen und danach mit kaltem und mit heißem Wasser gespült und getrocknet.A cotton fabric modified in accordance with Example 9 is first boiled in water for 15 minutes and then dried. This fabric is then treated with an aqueous dye solution containing 28.5 parts of the in. In 1000 parts by volume Contains dye powder described in Example D, 100 parts of urea and 3 parts of a commercially available nonionic wetting agent dissolved, padded at 20 ° C. using a padder with a liquor absorption of 80%, based on the weight of the fabric. The padded fabric is wound on a dock, wrapped in a plastic wrap and left at 20 ° C for 16 hours and then rinsed with cold and hot water and dried.
Es wird eine farbstarke, gleichmäßig gefärbte rote Färbung erhalten, die gute Allgemeinechtheiten, insbesondere gute Reib- und Lichtechtheiten, besitzt.A strong, uniformly colored red color is obtained which has good general fastness properties, in particular good fastness to rubbing and light.
10 Teile des gemäß Beispiel 9 modifizierten und anschließend durch 15-minütige kochende Behandlung in Wasser nachbehandelte, getrocknete Baumwollgewebe wird in 200 Vol.-Teile einer wäßrigen Farbstofflösung gegeben, die 0,2 Teile des im Beispiel D beschriebenen Farbstoffpulvers gelöst enthält. Dieses Färbebad wird innerhalb von 30 Minuten auf 60°C geheizt und der Färbeprozeß bei dieser Temperatur 60 Minuten weitergeführt. Anschließend wird das Gewebe aus dem Bad entnommen und mit kaltem und mit heißem Wasser gespült und getrocknet.10 parts of the dried cotton fabric modified according to Example 9 and then aftertreated by water for 15 minutes in water are placed in 200 parts by volume of an aqueous dye solution which contains 0.2 part of the dye powder described in Example D. This dye bath is heated to 60 ° C. within 30 minutes and the dyeing process is continued at this temperature for 60 minutes. The tissue is then removed from the bath and rinsed with cold and hot water and dried.
Es wird, wie im Beispiel D, eine orange Färbung mit guten Allgemeinechtheiten, wie insbesondere guten Reib- und Lichtechtheiten, erhalten.As in Example D, an orange coloration with good general fastness properties, in particular good fastness to rubbing and light, is obtained.
Claims (13)
- A process for dyeing cellulose fiber materials with anionic fiber-reactive dyes using aqueous dyeing solutions and padding liquors in which the dye is in solution, which comprises using a substrate comprising a cellulose fiber material onto which a polymer has been grafted by polymerization of at least one compound conforming to at least one of the following formulae (1), (1A), (2), (2A) and (5)
- The process of claim 1, wherein the substrate used is a grafted cellulose fiber material onto which 0.5 to 40% by weight, preferably 2 to 25% by weight, particularly preferably 4 to 15% by weight, of polymer has been grafted.
- The process of claim 1 or 2, wherein the substrate used is a grafted cellulose fiber material onto which has been grafted a copolymer which in addition to one or more monomers of the formulae (1), (1A), (2), (2A) and (5) mentioned in claim 1 contains one or more other polymerizable monomers, in particular one or more compounds conforming to the formulae (6) and/or (7)
- The process of one or more of claims 1 to 3, wherein the substrate used is a cellulose fiber material with a grafted-on copolymer which contains at least 20 mol%, preferably at least 40 mol%, particularly preferably at least 80 mol%, of one or more monomers of the formulae (1), (1A), (2), (2A) and/or (5) mentioned in claim 1.
- The process of one or more of claims 1 to 4, wherein the dyeing of the substrate is carried out at a temperature between 10 and 30°C.
- The process of one or more of claims 1 to 4, wherein the dyeing is carried out at a temperature of 30 to 60°C.
- The process of any one of claims 1 to 6, wherein the substrate used is a cellulose fiber material whose grafted-on polymer contains a compound of the formula (1) and/or (1A) where X is a group of the formula -NH- and/or a compound of the formula (2) and/or (2A) where R7 is an optionally methyl-substituted allyl group.
- The process of claim 1, wherein the substrate used is a cellulose fiber material onto which has been grafted a homopolymer.
- The process of one or more of claims 1 to 8, wherein the grafted cellulose fiber material is used in fiber form, in particular in the form of grafted linen, jute or ramie fibers or in the form of fibers of grafted, regenerated cellulose, particularly preferably as fibers of grafted cotton.
- A cellulose fiber material which has been modified by grafting with a copolymer of at least one N-containing basic monomer conforming to at least one of the formulae (1), (1A), (2), (2A) and (5)
- The modified cellulose fiber material of claim 10, wherein the grafted-on copolymer contains at least 20 mol%, preferably at least 40 mol%, particularly preferably at least 80 mol%, of one or more monomers of at least one of the formulae (1), (1A), (2), (2A) and (5).
- The modified cellulose fiber material of either of claims 10 or 11, containing 0.5 to 40% by weight, preferably 2 to 25% by weight, particularly preferably 4 to 15% by weight, of the copolymer graft.
- A process for preparing the modified cellulose fiber materials as claimed in any one of claims 10-12, which comprises polymerizing at least one N-containing basic monomer of the formulae (1), (1A), (2), (2A) and (5) mentioned and defined in claim 10 onto the cellulose fiber material in the presence of a comonomer conforming to the formula (6) and/or (7) mentioned and defined in claim 10.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE4112227 | 1991-04-15 | ||
| DE4112227 | 1991-04-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0509397A1 EP0509397A1 (en) | 1992-10-21 |
| EP0509397B1 true EP0509397B1 (en) | 1997-03-12 |
Family
ID=6429594
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP92106157A Expired - Lifetime EP0509397B1 (en) | 1991-04-15 | 1992-04-09 | Process for dyeing cellulosic fibrous material modified with N-containing basic polymers with anionic reactive dyestuffs, and the modified cellulosic fibrous material as such |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP0509397B1 (en) |
| JP (1) | JPH05140880A (en) |
| KR (1) | KR920020011A (en) |
| BR (1) | BR9201379A (en) |
| DE (1) | DE59208153D1 (en) |
| TR (1) | TR25952A (en) |
| TW (1) | TW201803B (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2126794T3 (en) * | 1994-01-29 | 1999-04-01 | Dystar Textilfarben Gmbh & Co | AMINATED CELLULOSIC SYNTHETIC FIBERS. |
| DE4422758A1 (en) * | 1994-06-29 | 1996-01-04 | Hoechst Ag | Aminated viscose fibres, dyeable in absence of salt or alkali |
| WO2013141222A1 (en) * | 2012-03-19 | 2013-09-26 | Jsr株式会社 | Resist pattern forming method and photoresist composition |
| JP7079624B2 (en) * | 2018-03-07 | 2022-06-02 | 株式会社日本触媒 | Textile Material Printing or Dyeing Composition, Method for Producing Textile Material with Processing Layer for Printing or Dyeing, and Method for Producing Textile Material for Printing or Dyeing. |
| CN110041463B (en) * | 2018-12-28 | 2021-06-04 | 福建清源科技有限公司 | Modified tamarind gum polymer and preparation method and application thereof |
| GR1010528B (en) * | 2022-05-09 | 2023-08-29 | Ιδρυμα Τεχνολογιας Και Ερευνας / Ινστιτουτο Επιστημων Χημικης Μηχανικης (Ιτε / Ιεχμη), | Method of improving the dyeing capacity on cotton fabrics |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3709766A1 (en) * | 1987-03-25 | 1988-10-06 | Hoechst Ag | METHOD FOR ALKALI-FREE DYEING WITH REACTIVE DYES |
| DE3831464A1 (en) * | 1988-09-16 | 1990-03-29 | Hoechst Ag | METHOD FOR ALKALI-FREE DYEING AND PRINTING OF CELLULOSE FIBERS |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3514385A (en) * | 1966-07-19 | 1970-05-26 | Du Pont | Process for radiation grafting onto a partially swollen cellulosic substrate |
| JPS5696972A (en) * | 1979-12-28 | 1981-08-05 | Toray Industries | Enhancement of dyeing fastness |
| US4737156A (en) * | 1986-10-27 | 1988-04-12 | National Starch And Chemical Corporation | Fabric treatment with a composition comprising a cellulose graft copolymer |
-
1992
- 1992-04-07 TW TW081102649A patent/TW201803B/zh active
- 1992-04-09 EP EP92106157A patent/EP0509397B1/en not_active Expired - Lifetime
- 1992-04-09 DE DE59208153T patent/DE59208153D1/en not_active Expired - Fee Related
- 1992-04-13 TR TR92/0332A patent/TR25952A/en unknown
- 1992-04-14 JP JP4094595A patent/JPH05140880A/en not_active Withdrawn
- 1992-04-14 KR KR1019920006212A patent/KR920020011A/en not_active Application Discontinuation
- 1992-04-14 BR BR929201379A patent/BR9201379A/en not_active Application Discontinuation
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3709766A1 (en) * | 1987-03-25 | 1988-10-06 | Hoechst Ag | METHOD FOR ALKALI-FREE DYEING WITH REACTIVE DYES |
| DE3831464A1 (en) * | 1988-09-16 | 1990-03-29 | Hoechst Ag | METHOD FOR ALKALI-FREE DYEING AND PRINTING OF CELLULOSE FIBERS |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0509397A1 (en) | 1992-10-21 |
| KR920020011A (en) | 1992-11-20 |
| JPH05140880A (en) | 1993-06-08 |
| TR25952A (en) | 1993-11-01 |
| BR9201379A (en) | 1992-12-01 |
| TW201803B (en) | 1993-03-11 |
| DE59208153D1 (en) | 1997-04-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0155232B1 (en) | Process for dyeing cellulosic textile materials | |
| US4602055A (en) | Process for pad dyeing cellulosic textile materials | |
| EP0245202B1 (en) | Process for printing or dyeing cellulosic textiles | |
| EP0363319B1 (en) | Water soluble or water dispersable graft polymers, their manufacture and their use | |
| US5002587A (en) | Copolymers which are water-soluble or dispersible in water, their preparation and use | |
| EP0509397B1 (en) | Process for dyeing cellulosic fibrous material modified with N-containing basic polymers with anionic reactive dyestuffs, and the modified cellulosic fibrous material as such | |
| US4264324A (en) | After treatment of cellulosic textiles dyed with fiber-reactive dyes | |
| DE1420333A1 (en) | Dye-absorbing copolymers, processes for the production of the same as well as molded articles produced therefrom | |
| US4810254A (en) | Wet-fastness properties of sulphur dyestuffs dyeings on cellulose treated with poly-di-allyl-ammonium salt | |
| US5230711A (en) | Dyeing of cellulose with soluble sulphur dyes without reducing agent | |
| EP0812949A2 (en) | Process for the treatment of dyed fibrous cellulosic materials | |
| EP0250365B1 (en) | Process for the after-treatment of dyed cellulosic fibrous material | |
| US2932550A (en) | Acid pretreatment of polyacrylonitrile-type fibers and the treated fibers | |
| DE2738497C3 (en) | Process for dyeing and, if necessary, the simultaneous finishing of textiles | |
| US2732317A (en) | Modified cellulose and method of | |
| US3481682A (en) | Modifying keratinic fibers with unsaturated sulfonic acids and blending fibers so modified with fibers having different dye affinity to obtain products which are differentially dyeable | |
| KR100266246B1 (en) | Process for the fixation of dyes containing at least one polymerisable double bond by means of ionising radiation | |
| US4414000A (en) | Process for the continuous or semicontinuous dyeing of voluminous cellulose fabrics with azo developing dyestuffs using acrylamide polymers | |
| US3486838A (en) | Modification of textile material with methylolated lactams | |
| US4735628A (en) | Wet fast dyed cellulosic materials | |
| JPS6160194B2 (en) | ||
| JPH0146635B2 (en) | ||
| DE1469618A1 (en) | Process for improving the fastness properties of dyed cellulose fiber material | |
| JPH0440476B2 (en) | ||
| DE2011073B2 (en) | Process for the production of textile material modified by graft copolymerization |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR GB IT LI PT |
|
| 17P | Request for examination filed |
Effective date: 19921125 |
|
| 17Q | First examination report despatched |
Effective date: 19951108 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE FR GB IT LI PT |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 59208153 Country of ref document: DE Date of ref document: 19970417 |
|
| ITF | It: translation for a ep patent filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19970515 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19980318 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19980326 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 19980327 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19980330 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19980408 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19980508 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990409 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990430 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990430 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990430 |
|
| BERE | Be: lapsed |
Owner name: HOECHST A.G. Effective date: 19990430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19991031 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19990409 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19991231 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000201 |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: MM4A Free format text: LAPSE DUE TO NON-PAYMENT OF FEES Effective date: 19991031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050409 |