EP0474545B1 - Verfahren zum Süssen in einem festen Bett von sauren Petroleumdestillaten mit einem Siedebereich zwischen 125 und 350 Grad - Google Patents
Verfahren zum Süssen in einem festen Bett von sauren Petroleumdestillaten mit einem Siedebereich zwischen 125 und 350 Grad Download PDFInfo
- Publication number
- EP0474545B1 EP0474545B1 EP91402340A EP91402340A EP0474545B1 EP 0474545 B1 EP0474545 B1 EP 0474545B1 EP 91402340 A EP91402340 A EP 91402340A EP 91402340 A EP91402340 A EP 91402340A EP 0474545 B1 EP0474545 B1 EP 0474545B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- distillate
- water
- distillates
- process according
- catalyst
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims abstract description 44
- 230000008569 process Effects 0.000 title claims abstract description 39
- 239000002253 acid Substances 0.000 title claims abstract description 22
- 239000003209 petroleum derivative Substances 0.000 title claims abstract description 6
- 238000009835 boiling Methods 0.000 title 1
- 239000003054 catalyst Substances 0.000 claims abstract description 29
- 238000007254 oxidation reaction Methods 0.000 claims abstract description 17
- 230000003647 oxidation Effects 0.000 claims abstract description 15
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 claims abstract description 10
- 239000007800 oxidant agent Substances 0.000 claims abstract description 8
- 239000003637 basic solution Substances 0.000 claims abstract description 7
- 230000009467 reduction Effects 0.000 claims abstract description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 19
- 238000005406 washing Methods 0.000 claims description 10
- 150000003839 salts Chemical class 0.000 claims description 7
- 239000013522 chelant Substances 0.000 claims description 6
- 229910052751 metal Inorganic materials 0.000 claims description 6
- 239000002184 metal Substances 0.000 claims description 6
- 239000004927 clay Substances 0.000 claims description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 4
- 238000006243 chemical reaction Methods 0.000 claims description 4
- 229910052760 oxygen Inorganic materials 0.000 claims description 4
- 239000001301 oxygen Substances 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 claims description 3
- 230000036571 hydration Effects 0.000 claims description 3
- 238000006703 hydration reaction Methods 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 2
- 230000002745 absorbent Effects 0.000 claims description 2
- 239000002250 absorbent Substances 0.000 claims description 2
- MPMSMUBQXQALQI-UHFFFAOYSA-N cobalt phthalocyanine Chemical compound [Co+2].C12=CC=CC=C2C(N=C2[N-]C(C3=CC=CC=C32)=N2)=NC1=NC([C]1C=CC=CC1=1)=NC=1N=C1[C]3C=CC=CC3=C2[N-]1 MPMSMUBQXQALQI-UHFFFAOYSA-N 0.000 claims description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 claims description 2
- 239000011159 matrix material Substances 0.000 claims description 2
- 239000011707 mineral Substances 0.000 claims description 2
- 239000007787 solid Substances 0.000 claims description 2
- 238000005194 fractionation Methods 0.000 claims 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 claims 1
- 230000002378 acidificating effect Effects 0.000 abstract description 4
- 150000008044 alkali metal hydroxides Chemical class 0.000 abstract description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 11
- 150000002989 phenols Chemical class 0.000 description 7
- LSDPWZHWYPCBBB-UHFFFAOYSA-N Methanethiol Chemical compound SC LSDPWZHWYPCBBB-UHFFFAOYSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000003350 kerosene Substances 0.000 description 6
- 150000007513 acids Chemical class 0.000 description 5
- 239000010779 crude oil Substances 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 4
- 238000004821 distillation Methods 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 230000003197 catalytic effect Effects 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 239000002574 poison Substances 0.000 description 3
- 231100000614 poison Toxicity 0.000 description 3
- 230000008901 benefit Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000006386 neutralization reaction Methods 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- KFVINGKPXQSPNP-UHFFFAOYSA-N 4-amino-2-[2-(diethylamino)ethyl]-n-propanoylbenzamide Chemical compound CCN(CC)CCC1=CC(N)=CC=C1C(=O)NC(=O)CC KFVINGKPXQSPNP-UHFFFAOYSA-N 0.000 description 1
- 101000780028 Homo sapiens Natriuretic peptides A Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102100034296 Natriuretic peptides A Human genes 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 229910001854 alkali hydroxide Inorganic materials 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000004523 catalytic cracking Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 125000005608 naphthenic acid group Chemical group 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 150000007965 phenolic acids Chemical class 0.000 description 1
- 235000009048 phenolic acids Nutrition 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 230000036632 reaction speed Effects 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G27/00—Refining of hydrocarbon oils in the absence of hydrogen, by oxidation
- C10G27/04—Refining of hydrocarbon oils in the absence of hydrogen, by oxidation with oxygen or compounds generating oxygen
- C10G27/10—Refining of hydrocarbon oils in the absence of hydrogen, by oxidation with oxygen or compounds generating oxygen in the presence of metal-containing organic complexes, e.g. chelates, or cationic ion-exchange resins
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G19/00—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G27/00—Refining of hydrocarbon oils in the absence of hydrogen, by oxidation
- C10G27/04—Refining of hydrocarbon oils in the absence of hydrogen, by oxidation with oxygen or compounds generating oxygen
Definitions
- the present invention relates to a fixed-bed softening process for acid petroleum distillates with cutting temperatures of between approximately 125 and approximately 350 ° C.
- This process is particularly intended to soften distillates and, more specifically, kerosenes, with an acid number higher than 0.03 mg of KOH per gram, for which it is difficult, with conventional processes, to obtain distillates.
- conform to the specifications required for their use namely, mainly their acid number, their mercaptan content and their color.
- the acidity of the distillates is measured by an acid number, which is determined by standard ASTM D 3242 and which effectively corresponds to the additional acidity of the naphthenic and carboxylic acids and of the phenol compounds present in these distillates.
- the first step consists in reducing, at least partially, the acidity of the distillates before the oxidation reaction of the mercaptans, either by prewashing them with basic solutions, in order to dissolve in acids the phenolic acids and compounds, generators of poisons of the catalyst, either by filtering them in order to trap these acids or these phenol compounds on the filter and thus to purify the distillates.
- This step is well described in American patents no. 3,398,086, 4,033,860 and 4,121,999 and in French patent no. 2,392,103.
- the second step consists in oxidizing the mercaptans still present in these distillates, by putting them in the presence of a metallic phthalocyanine deposited on a support, an oxidizing agent and a basic solution, often alkaline, with a pH generally between 9 and 14.
- the third stage corresponds to a finishing treatment of the softened distillates, comprising washing with water, to extract the residual alkaline compounds and possibly certain soluble salts of oxidation products of the residual phenol compounds, then drying, by passage over a salt filter, and a discoloration, by passing over a clay filter, which fixes the oxidation products still present, color generators.
- EP-A-0 376 774 describes a process for softening an petroleum cut, in which the catalytic oxidation reaction of the mercaptans is carried out in the absence of an aqueous phase and in the presence of an oxidizing agent and in which the water molecules formed during this reaction are removed from the catalytic support by periodic washing of the latter using a water-miscible solvent.
- the problems posed by high acidity petroleum cuts are however not mentioned in this document.
- the aim of the present invention is therefore a softening process which makes it easier to treat, industrially, distillates and, in particular, kerosene with a high acid number, in the absence of basic solutions such as soda in any of the processing steps, with a view to simultaneously obtaining the acidity, mercaptan content and color characteristics targeted for the sweetened distillates.
- the present invention also aims to reduce the number of treatment steps and, therefore, the number of reactors, while limiting the number of stops of the unit for washing deposits on the supported catalyst.
- the object of the present invention is therefore a process for softening acid petroleum distillates, with cutting temperatures of between approximately 125 and approximately 350 ° C., the acid number of which is greater than or equal to 0.03 mg of KOH per gram, characterized in that the reduction of the acidity and the oxidation of the mercaptans necessary for the softening of these distillates are carried out jointly in a single step, by passing these distillates, in the presence of an oxidizing agent, but in the absence of a basic solution, in particular of alkali hydroxide, on an oxidation catalyst with a specific surface of between 1 and 10 m2 / g and, preferably, between 2 and 6 m2 / g, and of which the microporous volume is between 0.01 and 0.10 cm3 / g and, preferably, between 0.02 and 0.05 cm3 / g, at a temperature between 30 and 80 ° C and, preferably between 35 and 45 ° C, in the presence of an oxidizing agent corresponding to an oxygen supply,
- an excess of oxygen, relative to the stoichiometry, is particularly necessary for the distillates with high acid number, but this action will preferably be combined with the temperature and the spatial reaction speed, in order to avoid a deterioration in the color of the distillates softened by the process according to the invention.
- the surface properties of the support of the oxidation catalyst make it possible for these compounds to pass through the support, hitherto considered to be poisons of the catalyst.
- Another unexpected advantage of the invention is therefore to reduce the frequency of the periodic catalyst washing operations in order to restore its activity, the active sites no longer being covered with deposits.
- not only does such a method limit the number of reactors, but it allows the operator to reduce the capacity of the softening reactor, by operating at an hourly space velocity at least twice as fast as that used in the conventional processes.
- a preferred oxidation catalyst is that consisting of a solid absorbent support impregnated with a metal chelate, which comprises, in% by weight, between 0.05 and 5% of metal chelate, between 5 and 35% of pyrolyzed carbon and between 60 and 90% of a mineral matrix whose hydration rate is between 1 and 20% and preferably between 2 and 10%.
- the metal chelate is a cobalt phthalocyanine.
- the distillates treated by this process usually have a drying effect on the supported catalyst, which, at over time, deactivates the supported catalyst.
- This is, in particular, the case for the catalyst described in European patent No. 252,853.
- the Applicant has chosen to add water, continuously or batchwise, to the catalyst, preferably by injecting water into the distillate to be treated before entering the reactor, in order to maintain its hydration rate between 1 and 20%.
- water continuously or batchwise, to the catalyst, preferably by injecting water into the distillate to be treated before entering the reactor, in order to maintain its hydration rate between 1 and 20%.
- the quantity of water injected will be 0.05 to 0.20 times the volume of catalyst loaded in the reactor and this for a short period of time, between 10 and 30 minutes, without stop the softening reaction, unlike conventional methods.
- the method according to the invention makes it possible to reduce the acid number from 50 to 100%, for distillates whose initial acid number is greater than 0.03 mg of KOH / g.
- This will be particularly the case for kerosene from the distillation of crude oils, such as "Zuluf” and "Heavy Iran", for which the steps of prewash with soda and washing with water, according to the processes known, have hitherto been essential for obtaining sweetened distillates conforming to the specifications required for marketing.
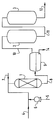
- the method according to the invention can be implemented in an industrial unit such as that shown schematically in the attached single figure.
- This unit includes a reactor 1, in which the fixed bed of the oxidation catalyst is installed, a salt filter 2, for drying the softened distillate, and a clay 3 to discolor it.
- the reactor 1 is supplied at its upper part by a pipe 4 for supplying the distillate, a water inlet pipe 5, on which will advantageously be placed a pump 6 for regulating the quantity of water to be injected continuously or discontinuously, and a pipe 7 for injecting air or an oxidizing agent.
- An outlet pipe for the softened distillate 8 is placed at the bottom of the reactor 1; it leads to a decanter 9 intended for the elimination of water by a line 14.
- a pipe 10 evacuates the softened distillate towards the top of the salt filter 2 and the pipe 11 from the bottom of the salt filter 2 routes it to the top of the clay filter 3.
- the latter has at its base a line 12 for evacuating the softened distillate in accordance with the specifications required for its marketing.
- process A The purpose of the present example is to study the respective performances of the process of the invention, called process A, and of a conventional process in three stages using sodium hydroxide, called process T (control process).
- catalyst A The catalysts used in processes A and T will be called catalyst A and catalyst T respectively. Their characteristics are given in Table 1 below:
- the oxidizing agent is oxygen in the air.
- process A leads to a reduction in acidity, a reduction in the mercaptan content and a color of the sweetened distillate as good as with the process T, but with twice the hourly space velocity and a lower number of treatment steps (no more prewash with soda, nor washing with water of the softened product), without the use of soda, which avoids recycling problems of the latter.
- Operating at an hourly space velocity twice as high in process A as in process T will allow the manufacturer to limit the capacity of the softening reactor.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Seasonings (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Paper (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Catalysts (AREA)
- Lubricants (AREA)
Claims (7)
- Verfahren zur Aufbereitung von sauren Eröldestillaten mit einem Siedebereich zwischen etwa 125°C und etwa 350°C und mit einer Säurezahl größer als oder gleich 0,03 mg KOH/g im Festbett, dadurch gekennzeichnet, daß die zur Aufbereitung der Destillate erforderliche Aciditätsverringerung und nötige Mercaptanoxydation gemeinsam in einem einzigen Schritt durchgeführt werden, wobei das Destillat in Gegenwart eines Oxydationsmittels, aber in Abwesenheit einer basischen Lösung, insbesondere einer Alkalihydroxyd - Lösung, über einen Oxydationskatalysator mit einer spezifischen Oberfläche zwischen 1 und 10 m²/Gramm, vorzugsweise zwischen 2 und 6 m² /Gramm, und mit einem Mikroporenvolumen zwischen 0,01 und 0,10 cm³/Gramm, vorzugsweise zwischen 0,02 und 0,05 cm³/Gramm, bei einer Temperatur zwischen 30 und 80°C, vorzugsweise zwischen 35 und 45°C, mit einer Luftmenge im Reaktor, welche einer Sauerstoffzugabe zwischen dem 0,9- und 2fachen, vorzugsweise zwischen dem 1- und 1,4fachen der für die Reaktion erforderlichen stöchiometrischen Menge entspricht, unter einem Druck zwischen 1 und 30 Bar, vorzugsweise zwischen 2 und 15 Bar, und mit einer stündlichen Raumgeschwindigkeit des zu behandelnden Destillats zwischen 0,5 und 6 v.v.h., vorzugsweise zwischen 1 und 4 v.v.h., geleitet wird.
- Verfahren nach Anspruch 1, dadurch gekennzeichnet, daß der Oxydationskatalysator aus einem absorbierenden festen Support besteht, welcher mit einem Metallchelat imprägniert ist, und folgende Zusammensetzung aufweist:
zwischen 0,05 und 10 Gew.-% Metallchelat
zwischen 5 und 35 Gew.-% pyrolisierter Kohlenstoff
zwischen 60 und 90 Gew.-% mineralische Matrix mit einem Hydratationsgrad zwischen 1 und 20 %. - Verfahren nach Anspruch 1 oder 2, dadurch gekennzeichnet, daß es sich beim Metallchelat um ein Kobaltphthalocyanin handelt.
- Verfahren nach Anspruch 1, 2 oder 3, dadurch gekennzeichnet, daß zum Rehydratisieren des Katalysators dem Reaktor kontinuierlich oder diskontinuierlich Wasser zugeführt wird, vorzugsweise durch Auflösung des Wassers in dem zu behandelnden Destillat.
- Verfahren nach einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, daß zwischen 100 und 500 ppm Wasser kontinuierlich in das Destillat eingespritzt werden.
- Verfahren nach einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, daß ein Wasservolumen, welches 5 bis 20 % des Katalysatorvolumens darstellt, in weniger als 30 Minuten diskontinuierlich eingespritzt wird.
- Verfahren nach einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, daß das aufbereitete Destillat anschließend einer Endbehandlung unterworfen wird, welche darin besteht, daß es ein Salfilter und dann ein Tonfilter ohne Phase des Waschens mit Wasser durchströmt.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9010942A FR2666344B1 (fr) | 1990-09-03 | 1990-09-03 | Procede d'adoucissement en lit fixe de distillats petroliers acides de temperatures de coupe comprises entre environ 125 et environ 350 degre c. |
| FR9010942 | 1990-09-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0474545A1 EP0474545A1 (de) | 1992-03-11 |
| EP0474545B1 true EP0474545B1 (de) | 1994-05-04 |
Family
ID=9400029
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91402340A Expired - Lifetime EP0474545B1 (de) | 1990-09-03 | 1991-09-02 | Verfahren zum Süssen in einem festen Bett von sauren Petroleumdestillaten mit einem Siedebereich zwischen 125 und 350 Grad |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US5382354A (de) |
| EP (1) | EP0474545B1 (de) |
| KR (1) | KR920006485A (de) |
| CN (1) | CN1028765C (de) |
| AT (1) | ATE105325T1 (de) |
| DE (1) | DE69101893T2 (de) |
| DK (1) | DK0474545T3 (de) |
| ES (1) | ES2053297T3 (de) |
| FR (1) | FR2666344B1 (de) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2947185B1 (fr) | 2009-06-26 | 2011-07-01 | Total Sa | Procede de traitement de gaz acides |
| CA2781361A1 (en) * | 2009-12-04 | 2011-06-09 | Exxonmobil Research And Engineering Company | Method for increasing color quality and stability of fuel field of the invention |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0013257B1 (de) * | 1978-12-22 | 1983-06-22 | Ciba-Geigy Ag | Verfahren zur Erhöhung der Lichtechtheit, Fluoreszenzpolarisation und Fluoreszenzquantenausbeute von Cyaninfarbstoffen, stabilisierte Cyaninfarbstoffzubereitung, Verfahren zu ihrer Herstellung und ihre Verwendung |
| IS1740B (is) * | 1982-02-05 | 1999-12-31 | Albright & Wilson Uk Limited | Samsetning á hreinsivökva |
| MX167884B (es) * | 1983-12-22 | 1993-04-20 | Albright & Wilson | Composicion detergente liquida |
| FR2601263B1 (fr) * | 1986-07-11 | 1988-11-25 | Total France | Nouveau produit composite catalytique pour l'oxydation des mercaptans et son utilisation pour l'adoucissement des coupes petrolieres. |
| GB8724254D0 (en) * | 1987-10-15 | 1987-11-18 | Unilever Plc | Hair treatment product |
| FR2640636B1 (fr) * | 1988-12-21 | 1991-04-05 | Total France | Procede d'adoucissement en lit fixe de coupes petrolieres |
| FR2651791B1 (fr) * | 1989-09-08 | 1994-05-20 | Total France Cie Raffinage Distr | Procede d'adoucissement en lit fixe de coupes petrolieres. |
-
1990
- 1990-09-03 FR FR9010942A patent/FR2666344B1/fr not_active Expired - Lifetime
-
1991
- 1991-09-02 DK DK91402340.3T patent/DK0474545T3/da active
- 1991-09-02 AT AT9191402340T patent/ATE105325T1/de not_active IP Right Cessation
- 1991-09-02 EP EP91402340A patent/EP0474545B1/de not_active Expired - Lifetime
- 1991-09-02 ES ES91402340T patent/ES2053297T3/es not_active Expired - Lifetime
- 1991-09-02 DE DE69101893T patent/DE69101893T2/de not_active Expired - Fee Related
- 1991-09-02 KR KR1019910015253A patent/KR920006485A/ko not_active Ceased
- 1991-09-03 CN CN91108731A patent/CN1028765C/zh not_active Expired - Fee Related
- 1991-11-13 US US07/753,114 patent/US5382354A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| ATE105325T1 (de) | 1994-05-15 |
| DK0474545T3 (da) | 1994-06-13 |
| DE69101893T2 (de) | 1994-11-10 |
| CN1060105A (zh) | 1992-04-08 |
| DE69101893D1 (de) | 1994-06-09 |
| FR2666344A1 (fr) | 1992-03-06 |
| KR920006485A (ko) | 1992-04-27 |
| US5382354A (en) | 1995-01-17 |
| FR2666344B1 (fr) | 1992-12-18 |
| EP0474545A1 (de) | 1992-03-11 |
| CN1028765C (zh) | 1995-06-07 |
| ES2053297T3 (es) | 1994-07-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6673236B2 (en) | Method for the production of hydrocarbon fuels with ultra-low sulfur content | |
| US5239096A (en) | Degumming process for plant oils | |
| RU2037516C1 (ru) | Способ очистки глицеридного масла | |
| EP0578194B1 (de) | Verfahren zur Herstellung von Phenol und Methylethylketon | |
| CH665219A5 (fr) | Procede d'esterification de graisses et d'huiles, et preparation enzymatique par la mise en oeuvre du procede. | |
| EP0474545B1 (de) | Verfahren zum Süssen in einem festen Bett von sauren Petroleumdestillaten mit einem Siedebereich zwischen 125 und 350 Grad | |
| CH667671A5 (fr) | Procede pour la fabrication d'une preparation enzymatique pour interesterification. | |
| FR2486072A1 (fr) | Procede pour la fabrication de l'acide b-hydroxybutyrique et de ses oligocondensats | |
| US1533031A (en) | Process of regenerating decolorizing carbon | |
| JPS6369891A (ja) | 精製油の製造方法 | |
| CN101469279A (zh) | 一种脱除烃原料中镍和钒的方法 | |
| EP0712392B1 (de) | Verfahren zur herstellung von cumenhydroperoxyd | |
| EP0416979B1 (de) | Verfahren zum Süssen von Petroleumschnitten im festen Bett | |
| US2230582A (en) | Method for the preparation of high grade fatty acids | |
| BE496062A (de) | ||
| FR2478644A1 (fr) | Procede de preparation de malathion | |
| EP0376774B1 (de) | Fettbettverfahren für das Süssen von Erdölfraktionen | |
| US1770166A (en) | Method of treating adsorbents | |
| KR20050103308A (ko) | 방법 | |
| JPH0234692A (ja) | メチルエステルの製造方法 | |
| US5118857A (en) | Polar solvent treatment of n-dodecyl mercaptoethanol | |
| US2720539A (en) | Refining naphthenic acids | |
| SU952950A1 (ru) | Способ депарафинизации нефтепродуктов | |
| SU1346659A1 (ru) | Способ получени светлых горных восков | |
| JPH0225449A (ja) | 酢酸アリルの製造法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES GB GR IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19920402 |
|
| 17Q | First examination report despatched |
Effective date: 19930413 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES GB GR IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 105325 Country of ref document: AT Date of ref document: 19940515 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 69101893 Country of ref document: DE Date of ref document: 19940609 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2053297 Country of ref document: ES Kind code of ref document: T3 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: FG4A Free format text: 3011701 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19940812 |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 91402340.3 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19960801 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19960826 Year of fee payment: 6 Ref country code: DK Payment date: 19960826 Year of fee payment: 6 Ref country code: DE Payment date: 19960826 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19960827 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 19960829 Year of fee payment: 6 Ref country code: AT Payment date: 19960829 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19960903 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 19960918 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19970822 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19970828 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970902 Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970902 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970902 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970903 Ref country code: ES Free format text: LAPSE BECAUSE OF THE APPLICANT RENOUNCES Effective date: 19970903 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 |
|
| BERE | Be: lapsed |
Owner name: S.A. TOTAL RAFFINAGE DISTRIBUTION Effective date: 19970930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980603 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 91402340.3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980902 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990401 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19980902 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19990401 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20001009 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050902 |