EP0408469B1 - Copper-iron-cobalt-titanium alloy featuring high mechanical and electrical properties and process for the manufacture thereof - Google Patents
Copper-iron-cobalt-titanium alloy featuring high mechanical and electrical properties and process for the manufacture thereof Download PDFInfo
- Publication number
- EP0408469B1 EP0408469B1 EP90420315A EP90420315A EP0408469B1 EP 0408469 B1 EP0408469 B1 EP 0408469B1 EP 90420315 A EP90420315 A EP 90420315A EP 90420315 A EP90420315 A EP 90420315A EP 0408469 B1 EP0408469 B1 EP 0408469B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alloy
- content
- temperature
- process according
- conductivity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims description 13
- 230000008569 process Effects 0.000 title claims description 11
- 238000004519 manufacturing process Methods 0.000 title claims description 10
- 229910001069 Ti alloy Inorganic materials 0.000 title description 8
- PKZXQRCILGFEFN-UHFFFAOYSA-N [Ti].[Co].[Fe].[Cu] Chemical compound [Ti].[Co].[Fe].[Cu] PKZXQRCILGFEFN-UHFFFAOYSA-N 0.000 title description 3
- 229910045601 alloy Inorganic materials 0.000 claims description 60
- 239000000956 alloy Substances 0.000 claims description 60
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 28
- 238000001556 precipitation Methods 0.000 claims description 27
- 239000010936 titanium Substances 0.000 claims description 20
- 239000000203 mixture Substances 0.000 claims description 19
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 13
- 229910052796 boron Inorganic materials 0.000 claims description 13
- 238000010438 heat treatment Methods 0.000 claims description 12
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 10
- 229910052719 titanium Inorganic materials 0.000 claims description 10
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 239000001301 oxygen Substances 0.000 claims description 8
- 239000010941 cobalt Substances 0.000 claims description 7
- 229910017052 cobalt Inorganic materials 0.000 claims description 7
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 7
- 238000007792 addition Methods 0.000 claims description 6
- 229910052742 iron Inorganic materials 0.000 claims description 6
- 229910020598 Co Fe Inorganic materials 0.000 claims description 5
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 5
- 239000010949 copper Substances 0.000 claims description 5
- 230000009466 transformation Effects 0.000 claims description 5
- 238000005266 casting Methods 0.000 claims description 4
- 229910052802 copper Inorganic materials 0.000 claims description 4
- 229910052810 boron oxide Inorganic materials 0.000 claims description 2
- JKWMSGQKBLHBQQ-UHFFFAOYSA-N diboron trioxide Chemical compound O=BOB=O JKWMSGQKBLHBQQ-UHFFFAOYSA-N 0.000 claims description 2
- 239000012535 impurity Substances 0.000 claims description 2
- 229910020517 Co—Ti Inorganic materials 0.000 claims 1
- 239000004020 conductor Substances 0.000 claims 1
- 238000002844 melting Methods 0.000 claims 1
- 230000008018 melting Effects 0.000 claims 1
- 238000011282 treatment Methods 0.000 description 18
- 239000011777 magnesium Substances 0.000 description 9
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 7
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 229910052749 magnesium Inorganic materials 0.000 description 7
- 239000011574 phosphorus Substances 0.000 description 7
- 229910052698 phosphorus Inorganic materials 0.000 description 7
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 6
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 6
- 229910017061 Fe Co Inorganic materials 0.000 description 5
- 238000010586 diagram Methods 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229910000881 Cu alloy Inorganic materials 0.000 description 3
- 239000003708 ampul Substances 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 229910052786 argon Inorganic materials 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 238000000265 homogenisation Methods 0.000 description 3
- 229910052759 nickel Inorganic materials 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 238000005096 rolling process Methods 0.000 description 3
- 238000005482 strain hardening Methods 0.000 description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000010453 quartz Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000011265 semifinished product Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229910052582 BN Inorganic materials 0.000 description 1
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229910021248 Co2Ti Inorganic materials 0.000 description 1
- 229910000640 Fe alloy Inorganic materials 0.000 description 1
- 229910005438 FeTi Inorganic materials 0.000 description 1
- 229910005487 Ni2Si Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 229910052775 Thulium Inorganic materials 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052790 beryllium Inorganic materials 0.000 description 1
- ATBAMAFKBVZNFJ-UHFFFAOYSA-N beryllium atom Chemical compound [Be] ATBAMAFKBVZNFJ-UHFFFAOYSA-N 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003610 charcoal Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 238000005097 cold rolling Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- IYRDVAUFQZOLSB-UHFFFAOYSA-N copper iron Chemical compound [Fe].[Cu] IYRDVAUFQZOLSB-UHFFFAOYSA-N 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- -1 electrolytic Co Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 238000005098 hot rolling Methods 0.000 description 1
- 229910001338 liquidmetal Inorganic materials 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/02—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of metals or alloys
- H01B1/026—Alloys based on copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/06—Alloys based on copper with nickel or cobalt as the next major constituent
Definitions
- the present invention relates to a copper-iron-cobalt-titanium alloy, its process for manufacturing it, as well as its field of use.
- the electrical interconnection is evolving rapidly. Whether in the field of electronics (component support grids, contacts), or in the field of connectors (clips, lugs, connectors), the size of the parts carrying the electric current is constantly decreasing. On the other hand, the complexity of the form of these contacts is only increasing.
- the manufacturer of copper alloys and semi-finished products is therefore subject to the following challenge: increase the electrical and thermal conductivity of traditional alloys to limit the heating of connectors and maintain or improve the level of mechanical properties. The improvement of these mechanical properties must obviously include the ability of the alloy to be deformed in the directions parallel and perpendicular to the rolling direction.
- a ternary copper alloy with 2% nickel and 0.5% silicon has been known for a long time which has good mechanical properties (mechanical resistance 600 MPa); however, such an alloy has an electrical conductivity limited to 60% IACS because of the solubility of the Ni2Si precipitate.
- US Pat. No. 4,559,200 shows the improvements brought about by small additions of magnesium or nickel to a CuFeTi alloy. More recently, a copper-iron-cobalt-titanium alloy described in Polish patent No. 115185 has been proposed covering a wide range of compositions. These alloys can reach 85% IACS conductivity for a tensile strength of 440 MPa. However, these properties to be achieved require two heat treatments.
- Example 1 shows that this ratio is highly significant for expressing the variability of the electrical conductivity. In the field where Co / Fe is between 0.1 and 0.9 and more particularly between 0.15 and 0.45 the electrical conductivity is particularly high. It should be noted that the electrical conductivity values of Example 1 are to be considered in a relative and not absolute manner, these tests being selection tests in the laboratory, which do not necessarily reproduce exactly all the means that can be used industrially, which influences on absolute conductivity values.
- the compositions of iron, cobalt, titanium are respectively between 0.1 and 1%, between 0.05 and 0.4 and between 0.035 and 0.6%, as for the residual oxygen content it is preferably less than 20 ppm.
- Obtaining high-performance alloys requires deoxidation of the bath of the liquid alloy, in particular to have control over the composition of the bath and to prevent the addition elements, titanium in particular, from playing the role of deoxidation and are not eliminated.
- the composition is also well controlled by preparation under vacuum, the oxygen content then being very low, generally less than 0.0005%. But due to the high cost, the Applicant preferred a conventional fusion, with deoxidation of the bath.
- the Applicant has thus carried out semi-industrial tests with deoxidation of the Cu Fe Co Ti alloy bath of composition according to the invention. She observed that phosphorus, a deoxidizing agent often used in the prior art, did not lead to a very efficient alloy, so she studied and compared several deoxidizing agents (see example 2): phosphorus, magnesium and boron. The Applicant has surprisingly found that boron leads to more efficient alloys than those obtained with phosphorus or magnesium although the latter is, on the basis of thermodynamic data, the most powerful deoxidizing agent of the three.
- boron makes it possible to obtain a bath of low residual oxygen content, and, on the other hand, the boron oxide formed is easily removed from the bath, unlike the other oxides, which, among other consequences, avoids the hard spots of the alloy during cutting at high speed, and finally that the residual boron content in the alloy is very low, generally less than 0.0005%, (but nevertheless detectable); the consequence is a high level of conductivity and a relatively low temperature TM, TM being the temperature of the precipitation treatment which leads to the maximum conductivity (see FIG. 3 of Example 2); finally, note the greater fineness of dispersion of the precipitates in the case of deoxidation with boron.
- the precipitation treatment is part of the transformation phase of the alloy which, after the casting of the alloy, includes its homogenization between 800 ° C and 1000 ° C for a time between 0.1 and 10 hours, its hot rolling up to 650 ° C followed by a possible quenching which can vary from 20 ° C / min to 2000 ° C / min , its cold rolling with one or more intermediate anneals; however, the excellent cold deformability of the alloy according to the invention generally allows it to be shaped with only a thermal precipitation treatment, which constitutes an economic range.
- the properties of the semi-finished products obtained, whether electrical conductivity or mechanical characteristics, also depend on the transformation phase and in particular on the thermal precipitation treatment. With regard to the conductivity, FIG.
- the precipitation treatment takes place at a temperature between TM and Tm and preferably close to Tm to obtain "balanced" properties according to the invention:% IACS> 80 and Rm> 500 MPa.
- Tm will be, at most, 80 ° C lower than TM; another method to define Tm is to consider the slope of the% IACS curve as a function of the temperature: Tm corresponds to the temperature where the slope begins to increase appreciably and reaches for example the value of 0.3% IACS / ° C.
- the slope change area is preferred.
- Example 3 clearly shows that only the alloy according to the invention (test C ′) exhibits high properties both in conductivity and mechanical properties, but it is however necessary to note the advantage of this type of treatment for greatly increasing the characteristics. mechanics of other alloys (tests A 'and B') when average conductivities (around 70% IACS) are sufficient.
- a precipitation treatment "at low temperature”, between 350 ° and 550 ° C will give maximum mechanical resistance (tests A 'and B') while a treatment at "high temperature” between 450 ° and 650 ° C will rather lead to maximum conductivity, the common domain between 450 ° and 550 ° being that in which the mechanical and conductivity properties are "balanced".
- the duration of the precipitation treatments varies according to the technology used: from 1 hour to 10 hours in a static oven and from 10 seconds to 30 minutes in a passing oven.
- the alloy according to the invention it is possible to reinforce the mechanical properties by adding elements such as aluminum, tin, zinc, nickel, silver, chromium to the basic composition. , beryllium, rare earths.
- the total sum of these elements must be less than 1.5% if one wants to keep sufficient conductivity: these additions of elements generally reducing the electrical conductivity constitute only a secondary form of the invention.
- the invention shows that only the combination of particular means that are the composition of the alloy with a ratio Co Fe precise, the particular choice of a deoxidizing agent and a temperature range for the precipitation treatment, makes it possible to obtain both a high electrical conductivity and a high mechanical resistance.
- Example 4 illustrates well the "classic" properties of alloys of the prior art: when they have a high electrical conductivity their mechanical resistance is low and vice versa. It clearly shows the advantageous performances of the product obtained according to the invention.
- the range of production of the alloys according to the invention is particularly economical since high work hardening rates can be achieved with a single heat treatment: the precipitation heat treatment.
- the alloys of the invention are suitable for applications requiring simultaneously high conductivity and mechanical resistance, they are recommended for the manufacture of conductive elements for electronics and in connection and in particular for applications such as leadframes, springs contact, connections.
- FIG. 1 illustrates, on a diagram having the ratio Ti / (Fe + Co) on the abscissa and the electrical conductivity in% IACS on the ordinate, the results obtained for the 7 tests denoted R1 to R7, described in Example 1.
- FIG. 2 illustrates, on a diagram having the Co / Fe ratio on the abscissa and the electrical conductivity in% IACS on the ordinate, the results obtained for the 7 tests, denoted R1 to R7, described in Example 1, which allow the plotting of a curve.
- FIG. 3 illustrates, on a diagram having the temperature in ° C on the abscissa and the electrical conductivity in% IACS on the ordinate, the variations in electrical conductivity as a function of the precipitation treatment temperature for each of the three deoxidizing agents studied in Example 2 magnesium (curve A), phosphorus (curve B), boron (curve C).
- FIG. 4 illustrates, on a diagram having on the abscissa the mechanical resistance in MPa, and on the order of the electrical conductivity in% IACS, the performances of the alloy obtained according to the invention (C '), according to Polish patent n ° 115185 , (D and F) and according to American patent n ° 4559200 (E), as indicated in example 4.
- the zone (III) where the alloy obtained according to the invention is found is that of the alloys having at the same time mechanical characteristics and electrical conductivity high.
- Table 2 which follows indicates the conductivity of each alloy expressed in% IACS measured at room temperature, as a function of the precipitation temperature:
- Table 2 shows that the maximum conductivity values, expressed in% IACS and underlined in this table, are obtained for a precipitation temperature close to 560 ° C and that these maximum values are very dispersed.
- Ti / (Fe + Co) ratio of Polish patent n ° 115185 shows that, on the one hand in the range 0.25 - 1 for Ti / (Fe + Co) claimed for this report, the conductivity varies a lot for similar values (comparison of tests R1, R2, R3, R4 between them and tests R5, R6, R7 between them) and that, on the other hand this ratio Ti / (Fe + Co) does not make it possible to determine the favorable domain of high conductivities since the 7 representative points do not make it possible to draw a curve having an indisputable maximum (see FIG.
- This example illustrates a modality of the shaping of alloys produced in all points as in example 2 (test A 'of example 3 corresponds to test A of example 2, likewise for B 'and C')), except that the precipitation treatment takes place at a lower temperature (505 ° C for A ', 485 ° C for B', 475 ° C for C ') for 4 hours and the final rolling corresponds at a thickness reduction of 29%:
- the following properties are obtained:
- These alloys exhibit a hardness greater than 130 HV after 30 minutes at 450 ° C., which illustrates their excellent resistance to softening.
- FIG. 4 situates these tests in a plane having the mechanical resistance on the abscissa and the electrical conductivity on the ordinate and clearly illustrates the advantage of the invention.
- the non-comparative test F is given for information: it corresponds to test D but with a transformation range comprising two heat treatments instead of one.
Description
La présente invention concerne un alliage cuivre-fer-cobalt-titane, son procédé pour le fabriquer, ainsi que son domaine d'utilisation.
L'interconnexion électrique évolue rapidement. Que ce soit dans le domaine de l'électronique (grilles de support de composants, contacts), ou dans le domaine de la connectique (clips, cosses, connecteurs), la dimension des pièces véhiculant le courant électrique diminue sans cesse. D'autre part, la complexité de la forme de ces contacts ne fait qu'augmenter.
Le fabricant d'alliages et de demi-produits cuivreux est donc soumis au challenge suivant : augmenter la conductivité électrique et thermique des alliages traditionnels pour limiter l'échauffement des connecteurs et conserver ou améliorer le niveau des propriétés mécaniques. L'amélioration de ces propriétés mécaniques doit inclure bien évidemment l'aptitude de l'alliage à être déformé suivant les directions parallèles et perpendiculaires au sens de laminage.The present invention relates to a copper-iron-cobalt-titanium alloy, its process for manufacturing it, as well as its field of use.
The electrical interconnection is evolving rapidly. Whether in the field of electronics (component support grids, contacts), or in the field of connectors (clips, lugs, connectors), the size of the parts carrying the electric current is constantly decreasing. On the other hand, the complexity of the form of these contacts is only increasing.
The manufacturer of copper alloys and semi-finished products is therefore subject to the following challenge: increase the electrical and thermal conductivity of traditional alloys to limit the heating of connectors and maintain or improve the level of mechanical properties. The improvement of these mechanical properties must obviously include the ability of the alloy to be deformed in the directions parallel and perpendicular to the rolling direction.
Pour la connectique on emploie généralement des bronzes de 4 à 9% d'étain qui présentent d'excellentes propriétés mécaniques et une déformabilité adaptée à ce type d'application. Cependant, leur conductivité électrique qui varie de 12 à 20% IACS est insuffisante car elle limite la miniaturisation des connecteurs à cause du problème d'échauffement.
Pour l'électronique, par exemple dans le domaine des grilles de supports, on emploie généralement des alliages cuivre-fer (C19400) qui présentent une conductivité de 65% IACS. Toutefois le compromis propriétés mécaniques/tenue à l'adoucissement fait que ces alliages ne peuvent être employés lorsque les températures d'encapsulation sont trop élevées et dépassent 400°C.
Sauf mention contraire, toutes les compositions d'alliages de cette demande de brevet sont en pourcentage pondéral.
On connaît depuis longtemps un alliage ternaire de cuivre à 2% de nickel et à 0,5% de silicium qui a de bonnes propriétés mécaniques (résistance mécanique 600 MPa); cependant un tel alliage a une conductivité électrique limitée à 60% IACS à cause de la solubilité du précipité Ni₂Si.For connectors, bronzes of 4 to 9% tin are generally used which have excellent mechanical properties and a deformability adapted to this type of application. However, their electrical conductivity, which varies from 12 to 20% IACS, is insufficient because it limits the miniaturization of the connectors because of the heating problem.
For electronics, for example in the field of support grids, copper-iron alloys (C19400) are generally used which have a conductivity of 65% IACS. However, the compromise between mechanical properties and softening resistance means that these alloys cannot be used when the encapsulation temperatures are too high and exceed 400 ° C.
Unless otherwise stated, all the alloy compositions of this patent application are in percentage by weight.
A ternary copper alloy with 2% nickel and 0.5% silicon has been known for a long time which has good mechanical properties (
Par ailleurs, le brevet US 4 559 200 montre les améliorations qu'apportent de faibles ajouts de magnésium ou de nickel à un alliage CuFeTi.
Plus récemment un alliage cuivre-fer-cobalt-titane décrit dans le brevet polonais n° 115185 a été proposé couvrant une large plage de compositions. Ces alliages peuvent atteindre 85% IACS de conductivité pour une résistance à la traction de 440 MPa. Cependant ces propriétés pour être atteintes nécessitent deux traitements thermiques.Furthermore, US Pat. No. 4,559,200 shows the improvements brought about by small additions of magnesium or nickel to a CuFeTi alloy.
More recently, a copper-iron-cobalt-titanium alloy described in Polish patent No. 115185 has been proposed covering a wide range of compositions. These alloys can reach 85% IACS conductivity for a tensile strength of 440 MPa. However, these properties to be achieved require two heat treatments.
Jusqu'à présent on ne savait donc pas fabriquer de façon économique un alliage présentant à la fois de hautes caractéristiques mécaniques, typiquement une résistance mécanique supérieure à 500 MPa et une conductivité élevée, supérieure à 80% IACS.Until now, therefore, it has not been known how to economically manufacture an alloy having both high mechanical characteristics, typically a mechanical resistance greater than 500 MPa and a high conductivity greater than 80% IACS.
L'invention a pour objet un alliage de cuivre à haute résistance mécanique, nettement supérieure à 500 MPa, à conductivité supérieure à 80% IACS et présentant outre une bonne tenue à l'adoucissement, un coût de fabrication peu élevé.
Selon l'invention l'obtention de ces performances élevées résulte de la mise en oeuvre de trois types de moyens se situant à différents stades du processus d'élaboration de l'alliage et des demi-produits issus de cet alliage: il s'agit de moyens concernant la composition de l'alliage, la désoxydation du bain de l'alliage liquide qui permet d'éviter la fabrication sous vide et la température de précipitation lors de la mise en forme de l'alliage.
Plus précisément, le procédé selon l'invention est caractérisé par le fait que:
- 1) la composition de l'alliage satisfait aux conditions suivantes (composition pondérale):
- rapport Co/Fe compris entre 0,10 et 0,90
- rapport Ti/(Fe+Co) compris entre 0,30 et 1
- teneur en fer comprise entre 0,030 et 2%
- teneur en cobalt comprise entre 0,025 et 1,8%
- teneur en titane comprise entre 0,025 et 4%
- teneur en oxygène inférieure à 50 ppm
- teneur totale en autres additions éventuelles inférieure à 1,5%
- teneur en impuretés métalliques inférieure à 0,1% avec chacune d'elle inférieure à 0,015%
- reste cuivre
- 2) le bain de l'alliage liquide est désoxydé au bore
- 3) le traitement thermique de précipitation est réalisé à une température inférieure, d'au plus 80°C, à la température TM du traitement de précipitation conduisant à la conductivité maximum.
According to the invention, obtaining these high performances results from the use of three types of means located at different stages of the process for the production of the alloy and of the semi-products produced from this alloy: these are: means concerning the composition of the alloy, the deoxidation of the bath of the liquid alloy which makes it possible to avoid manufacturing under vacuum and the precipitation temperature during the forming of the alloy.
More specifically, the method according to the invention is characterized in that:
- 1) the composition of the alloy satisfies the following conditions (weight composition):
- Co / Fe ratio between 0.10 and 0.90
- Ti / (Fe + Co) ratio between 0.30 and 1
- iron content between 0.030 and 2%
- cobalt content between 0.025 and 1.8%
- titanium content between 0.025 and 4%
- oxygen content below 50 ppm
- total content of any other additions less than 1.5%
- content of metallic impurities less than 0.1% with each of them less than 0.015%
- rest copper
- 2) the bath of the liquid alloy is deoxidized with boron
- 3) the thermal precipitation treatment is carried out at a temperature lower, at most 80 ° C, than the temperature TM of the precipitation treatment leading to maximum conductivity.
Ces trois moyens vont être détaillés et situés par rapport à l'art antérieur.
En étudiant les propriétés des alliages Cu Fe Co Ti de l'art antérieur, la demanderesse a observé que la conductivité électrique variait considérablement en fonction du rapport Ti/(Fe+Co) et surtout de manière erratique comme le montre la figure 1 de l'exemple 1, ce qui ne permet donc pas de sélectionner des alliages Cu Fe Co Ti de hautes performances électriques bien que soit respecté le rapport Ti/(Fe + Co), qui traduit la stoechiométrie des précipités possibles (FeTi, Fe₂Ti, Co Ti, Co₂Ti).These three means will be detailed and located in relation to the prior art.
By studying the properties of Cu Fe Co Ti alloys of the prior art, the applicant observed that the electrical conductivity varied considerably as a function of the Ti / (Fe + Co) ratio and especially erratically as shown in FIG. 1 of l example 1, which therefore does not make it possible to select Cu Fe Co Ti alloys with high electrical performance although the Ti / (Fe + Co) ratio is respected, which translates the stoichiometry of the possible precipitates (FeTi, Fe₂Ti, Co Ti , Co₂Ti).
Poursuivant l'analyse des performances de ces alliages, la demanderesse a eu la surprise de constater que le rapport Co/Fe avait une grande influence sur la conductivité électrique de ces alliages. La figure 2 de l'exemple 1 montre que ce rapport est hautement significatif pour exprimer la variabilité de la conductivité électrique. Dans le domaine où Co/Fe est compris entre 0,1 et 0,9 et plus particulièrement entre 0,15 et 0,45 la conductivité électrique est particulièrement élevée. Il convient de noter que les valeurs de conductivité électrique de l'exemple 1 sont à considérer de manière relative et non absolue, ces essais étant des essais de sélection au laboratoire, qui ne reproduisent pas nécessairement exactement tous les moyens utilisables industriellement, ce qui influe sur les valeurs absolues de conductivité.Continuing the analysis of the performance of these alloys, the Applicant was surprised to note that the Co / Fe ratio had a great influence on the electrical conductivity of these alloys. Figure 2 of Example 1 shows that this ratio is highly significant for expressing the variability of the electrical conductivity. In the field where Co / Fe is between 0.1 and 0.9 and more particularly between 0.15 and 0.45 the electrical conductivity is particularly high. It should be noted that the electrical conductivity values of Example 1 are to be considered in a relative and not absolute manner, these tests being selection tests in the laboratory, which do not necessarily reproduce exactly all the means that can be used industrially, which influences on absolute conductivity values.
De préférence, les compositions en fer, cobalt, titane sont comprises respectivement entre 0,1 et 1%, entre 0,05 et 0,4 et entre 0,035 et 0,6%, quant à la teneur en oxygène résiduel elle est de préférence inférieure à 20 ppm.Preferably, the compositions of iron, cobalt, titanium are respectively between 0.1 and 1%, between 0.05 and 0.4 and between 0.035 and 0.6%, as for the residual oxygen content it is preferably less than 20 ppm.
C'est la précipitation d'un composé bien défini riche en fer et titane avec du cobalt qui confère à l'alliage ses propriétés exceptionnelles : résistance mécanique, conductivité, formabilité.It is the precipitation of a well defined compound rich in iron and titanium with cobalt which gives the alloy its exceptional properties: mechanical resistance, conductivity, formability.
L'obtention d'alliages performants nécessite une désoxydation du bain de l'alliage liquide, notamment pour avoir la maîtrise de la composition du bain et éviter que les éléments d'addition, le titane en particulier, ne jouent le rôle d'agent de désoxydation et ne soient éliminés.Obtaining high-performance alloys requires deoxidation of the bath of the liquid alloy, in particular to have control over the composition of the bath and to prevent the addition elements, titanium in particular, from playing the role of deoxidation and are not eliminated.
La composition est également bien contrôlée par une élaboration sous vide, la teneur en oxygène étant alors très faible, généralement inférieure à 0,0005%. Mais en raison du coût élevé, la demanderesse a préféré une fusion classique, avec désoxydation du bain.
La demanderesse a ainsi réalisé des essais semi-industriels avec désoxydation du bain d'alliage Cu Fe Co Ti de composition selon l'invention. Elle a observé que le phosphore, agent de désoxydation souvent utilisé dans l'art antérieur, ne conduisait pas à un alliage très performant, aussi a-t-elle étudié et comparé plusieurs agents de désoxydation (voir exemple 2) : le phosphore, le magnésium et le bore. La demanderesse a constaté avec surprise que le bore conduisait à des alliages plus performants que ceux obtenus avec le phosphore ou le magnésium bien que ce dernier soit, sur la base de données thermodynamiques, l'agent de désoxydation le plus puissant des trois. En effet, on constate que d'une part le bore permet d'obtenir un bain de faible teneur en oxygène résiduel, que d'autre part l'oxyde de bore formé s'élimine facilement du bain contrairement aux autres oxydes, ce qui, entre autres conséquences, évite les points durs de l'alliage lors de découpe à grande vitesse, et enfin que la teneur résiduelle en bore dans l'alliage est très faible, généralement inférieure à 0,0005%, (mais cependant détectable); la conséquence en est un niveau élevé de conductivité et une température TM relativement basse, TM étant la température du traitement de précipitation qui conduit à la conductivité maximum (voir figure 3 de l'exemple 2) ; enfin il faut noter la plus grande finesse de dispersion des précipités dans le cas de la désoxydation au bore.
Le traitement de précipitation s'insère dans la phase de transformation de l'alliage qui comporte, après la coulée de l'alliage, son homogénéisation entre 800°C et 1000°C pendant un temps compris entre 0,1 et 10 heures, son laminage à chaud jusqu'à 650°C suivi d'une trempe éventuelle pouvant varier de 20°C/min à 2000°C/min, son laminage à froid avec un ou plusieurs recuits intermédiaires ; cependant, l'excellente déformabilité à froid de l'alliage selon l'invention permet généralement sa mise en forme avec seulement un traitement thermique de précipitation, ce qui constitue une gamme économique.
Les propriétés des demi-produits obtenus qu'il s'agisse de la conductivité électrique ou des caractéristiques mécaniques, dépendent aussi de la phase de transformation et notamment du traitement thermique de précipitation.
En ce qui concerne la conductivité, la figure 3 de l'exemple 2 montre que la conductivité passe par un maximum pour une température de précipitation TM (TM=515°C pour l'essai C) et que ce maximum a une forme aplatie : la conductivité reste élevée pour un large domaine de température, entre 475 et 550°C pour l'essai C et dans ce domaine, la pente de la courbe donnant la conductivité en fonction de la température est faible et inférieure à 0,2% IACS/°C.The composition is also well controlled by preparation under vacuum, the oxygen content then being very low, generally less than 0.0005%. But due to the high cost, the Applicant preferred a conventional fusion, with deoxidation of the bath.
The Applicant has thus carried out semi-industrial tests with deoxidation of the Cu Fe Co Ti alloy bath of composition according to the invention. She observed that phosphorus, a deoxidizing agent often used in the prior art, did not lead to a very efficient alloy, so she studied and compared several deoxidizing agents (see example 2): phosphorus, magnesium and boron. The Applicant has surprisingly found that boron leads to more efficient alloys than those obtained with phosphorus or magnesium although the latter is, on the basis of thermodynamic data, the most powerful deoxidizing agent of the three. In fact, it can be seen that, on the one hand, boron makes it possible to obtain a bath of low residual oxygen content, and, on the other hand, the boron oxide formed is easily removed from the bath, unlike the other oxides, which, among other consequences, avoids the hard spots of the alloy during cutting at high speed, and finally that the residual boron content in the alloy is very low, generally less than 0.0005%, (but nevertheless detectable); the consequence is a high level of conductivity and a relatively low temperature TM, TM being the temperature of the precipitation treatment which leads to the maximum conductivity (see FIG. 3 of Example 2); finally, note the greater fineness of dispersion of the precipitates in the case of deoxidation with boron.
The precipitation treatment is part of the transformation phase of the alloy which, after the casting of the alloy, includes its homogenization between 800 ° C and 1000 ° C for a time between 0.1 and 10 hours, its hot rolling up to 650 ° C followed by a possible quenching which can vary from 20 ° C / min to 2000 ° C / min , its cold rolling with one or more intermediate anneals; however, the excellent cold deformability of the alloy according to the invention generally allows it to be shaped with only a thermal precipitation treatment, which constitutes an economic range.
The properties of the semi-finished products obtained, whether electrical conductivity or mechanical characteristics, also depend on the transformation phase and in particular on the thermal precipitation treatment.
With regard to the conductivity, FIG. 3 of Example 2 shows that the conductivity passes through a maximum for a precipitation temperature TM (TM = 515 ° C for test C) and that this maximum has a flat shape: conductivity remains high for a wide temperature range, between 475 and 550 ° C for test C and in this area, the slope of the curve giving the conductivity as a function of temperature is low and less than 0.2% IACS / ° C.
La demanderesse a observé que contrairement à ce que l'on pouvait supposer, il est avantageux de soumettre l'alliage selon l'invention à un traitement de précipitation à une température inférieure à TM : dans ce cas là, pour une perte minime en conductivité électrique les caractéristiques mécaniques augmentent de manière très significative.
Ainsi, la comparaison des exemples 2 et 3 (essais C et C') montre que la conductivité passe de 83,5 à 83% IACS (-1%) alors que la résistance mécanique passe de 488 MPa à 525 MPa (+ 7,6%).
Par température inférieure à TM, il faut entendre toute température correspondant au niveau de conductivité souhaité (> 80% IACS) ; la détermination graphique (voir figure 3) est immédiate: l'intersection de la droite d'ordonnée 80% IACS avec la courbe C détermine la température minimum Tm.
Selon l'invention le traitement de précipitation a lieu à une température comprise entre TM et Tm et de préférence proche de Tm pour obtenir des propriétés "équilibrées" selon l'invention: % IACS > 80 et Rm > 500 MPa. En général, Tm sera, au plus, inférieur de 80°C à TM ; une autre méthode pour définir Tm est de considérer la pente de la courbe %IACS en fonction de la température : Tm correspond à la température où la pente commence à augmenter sensiblement et atteint par exemple la valeur de 0,3 % IACS/°C. C'est la zone de changement de pente qui est préférée.The Applicant has observed that, contrary to what one might suppose, it is advantageous to subject the alloy according to the invention to a precipitation treatment at a temperature below TM: in this case there, for a minimal loss in conductivity electrical mechanical characteristics increase very significantly.
Thus, the comparison of examples 2 and 3 (tests C and C ') shows that the conductivity goes from 83.5 to 83% IACS (-1%) while the mechanical resistance goes from 488 MPa to 525 MPa (+ 7, 6%).
By temperature below TM, we mean any temperature corresponding to the desired conductivity level (> 80% IACS); the graphical determination (see Figure 3) is immediate: the intersection of the 80% IACS ordinate line with curve C determines the minimum temperature Tm.
According to the invention, the precipitation treatment takes place at a temperature between TM and Tm and preferably close to Tm to obtain "balanced" properties according to the invention:% IACS> 80 and Rm> 500 MPa. In general, Tm will be, at most, 80 ° C lower than TM; another method to define Tm is to consider the slope of the% IACS curve as a function of the temperature: Tm corresponds to the temperature where the slope begins to increase appreciably and reaches for example the value of 0.3% IACS / ° C. The slope change area is preferred.
L'exemple 3 montre bien que seul l'alliage selon l'invention (essai C') présente des propriétés élevées à la fois en conductivité et propriétés mécaniques mais il faut cependant noter l'intérêt de ce type de traitement pour augmenter fortement les caractéristiques mécaniques des autres alliages (essais A' et B') lorsque des conductivités moyennes (vers 70% IACS) sont suffisantes.Example 3 clearly shows that only the alloy according to the invention (test C ′) exhibits high properties both in conductivity and mechanical properties, but it is however necessary to note the advantage of this type of treatment for greatly increasing the characteristics. mechanics of other alloys (tests A 'and B') when average conductivities (around 70% IACS) are sufficient.
D'une manière plus générale, un traitement de précipitation "à basse température", entre 350° et 550°C donnera une résistance mécanique maximum (essais A' et B') alors qu'un traitement à "haute température" entre 450° et 650°C conduira plutôt à une conductivité maximum, le domaine commun entre 450° et 550° étant celui où les propriétés mécaniques et de conductivité sont "équilibrées".More generally, a precipitation treatment "at low temperature", between 350 ° and 550 ° C will give maximum mechanical resistance (tests A 'and B') while a treatment at "high temperature" between 450 ° and 650 ° C will rather lead to maximum conductivity, the common domain between 450 ° and 550 ° being that in which the mechanical and conductivity properties are "balanced".
La durée des traitements de précipitation varie selon la technologie utilisée : de 1 heure à 10 heures en four statique et de 10 secondes à 30 minutes en four à passage.
A partir de l'alliage selon l'invention, il est possible de renforcer les propriétés mécaniques en ajoutant à la composition de base des éléments tels que l'aluminium, l'étain, le zinc, le nickel, l'argent, le chrome, le beryllium, les terres rares. La somme totale de ces éléments doit être inférieure à 1,5% si l'on veut garder une conductivité suffisante : ces additions d'éléments diminuant en général la conductivité électrique ne constituent qu'une modalité secondaire de l'invention.
L'invention montre que seule la combinaison de moyens particuliers que sont la composition de l'alliage avec un rapport
Comme déjà mentionné, la gamme d'élaboration des alliages selon l'invention est particulièrement économique car des taux d'écrouissage élevés peuvent être atteints avec un seul traitement thermique: le traitement thermique de précipitation.The duration of the precipitation treatments varies according to the technology used: from 1 hour to 10 hours in a static oven and from 10 seconds to 30 minutes in a passing oven.
From the alloy according to the invention, it is possible to reinforce the mechanical properties by adding elements such as aluminum, tin, zinc, nickel, silver, chromium to the basic composition. , beryllium, rare earths. The total sum of these elements must be less than 1.5% if one wants to keep sufficient conductivity: these additions of elements generally reducing the electrical conductivity constitute only a secondary form of the invention.
The invention shows that only the combination of particular means that are the composition of the alloy with a ratio
As already mentioned, the range of production of the alloys according to the invention is particularly economical since high work hardening rates can be achieved with a single heat treatment: the precipitation heat treatment.
Les alliages de l'invention sont appropriés aux applications nécessitant simultanément une conductivité et une résistance mécanique élevées, ils sont recommandés pour la fabrication d'éléments conducteurs pour l'électronique et en connection et en particulier pour des applications telle que les leadframes, les ressorts de contact, les connexions.The alloys of the invention are suitable for applications requiring simultaneously high conductivity and mechanical resistance, they are recommended for the manufacture of conductive elements for electronics and in connection and in particular for applications such as leadframes, springs contact, connections.
La figure 1 illustre, sur un diagramme ayant en abscisse le rapport Ti/(Fe+Co) et en ordonnée la conductivité électrique en %IACS, les résultats obtenus pour les 7 essais notés R1 à R7, décrits à l'exemple 1. FIG. 1 illustrates, on a diagram having the ratio Ti / (Fe + Co) on the abscissa and the electrical conductivity in% IACS on the ordinate, the results obtained for the 7 tests denoted R1 to R7, described in Example 1.
La figure 2 illustre, sur un diagramme ayant en abscisse le rapport Co/Fe et en ordonnée la conductivité électrique en %IACS, les résultats obtenus pour les 7 essais, notés R1 à R7, décrits à l'exemple 1, qui permettent le tracé d'une courbe. FIG. 2 illustrates, on a diagram having the Co / Fe ratio on the abscissa and the electrical conductivity in% IACS on the ordinate, the results obtained for the 7 tests, denoted R1 to R7, described in Example 1, which allow the plotting of a curve.
La figure 3 illustre, sur un diagramme ayant en abscisse la température en °C et en ordonnée la conductivité électrique en %IACS, les variations de conductivité électrique en fonction de la température de traitement de précipitation pour chacun des trois agents de désoxydation étudiés à l'exemple 2 le magnésium (courbe A), le phosphore (courbe B), le bore (courbe C). FIG. 3 illustrates, on a diagram having the temperature in ° C on the abscissa and the electrical conductivity in% IACS on the ordinate, the variations in electrical conductivity as a function of the precipitation treatment temperature for each of the three deoxidizing agents studied in Example 2 magnesium (curve A), phosphorus (curve B), boron (curve C).
La figure 4 illustre, sur un diagramme ayant en abscisse la résistance mécanique en MPa, et en ordonné la conductivité électrique en %IACS, les performances de l'alliage obtenu selon l'invention (C'), selon le brevet polonais n° 115185, (D et F) et selon le brevet américain n° 4559200 (E), comme indiqué à l'exemple 4. La zone (III) où se trouve l'alliage obtenu selon l'invention est celle des alliages ayant a la fois des caractéristiques mécaniques et une conductivité électrique élevées. FIG. 4 illustrates, on a diagram having on the abscissa the mechanical resistance in MPa, and on the order of the electrical conductivity in% IACS, the performances of the alloy obtained according to the invention (C '), according to Polish patent n ° 115185 , (D and F) and according to American patent n ° 4559200 (E), as indicated in example 4. The zone (III) where the alloy obtained according to the invention is found is that of the alloys having at the same time mechanical characteristics and electrical conductivity high.
Dans cet exemple, on étudie l'influence de la composition de l'alliage sur la conductivité électrique.In this example, we study the influence of the composition of the alloy on the electrical conductivity.
Sept alliages Cu Fe Co Ti, référencés de R1 à R7, ont été élaborés au laboratoire, par fusion des éléments purs (Cu C2, Fe électrolytique, Co électrolytique, Ti 140 fourni par CEZUS), dans un creuset en nitrure de bore chauffé au four à induction. La fusion a été réalisée sous argon, à la pression atmosphérique. Ces conditions de laboratoire permettent d'élaborer des alliages Cu Fe Co Ti sans qu'il soit nécessaire de désoxyder le bain, de manière à obtenir des résultats dépendant essentiellement de la composition de l'alliage.Seven Cu Fe Co Ti alloys, referenced from R1 to R7, were developed in the laboratory, by fusion of pure elements (Cu C2, electrolytic Fe, electrolytic Co, Ti 140 supplied by CEZUS), in a boron nitride crucible heated to induction oven. The fusion was carried out under argon, at atmospheric pressure. These laboratory conditions make it possible to develop Cu Fe Co Ti alloys without it being necessary to deoxidize the bath, so as to obtain results essentially dependent on the composition of the alloy.
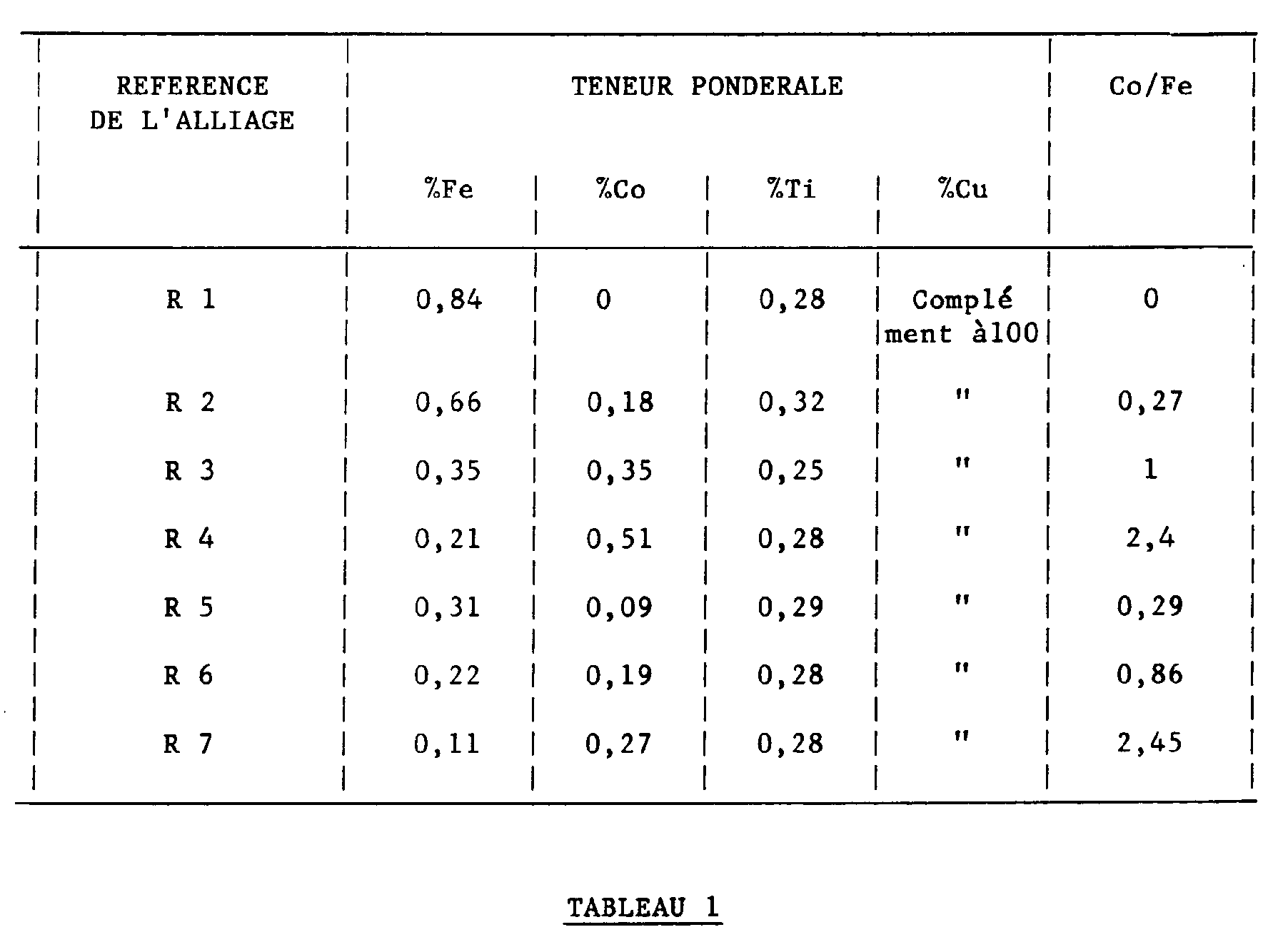
Le tableau 1 indique la composition de ces alliages
Le métal liquide est coulé dans une lingotière en cuivre refroidie à l'eau. La lingotière permet de couler des billettes d'environ 16 mm de diamètre pour 100 mm de hauteur, soit environ une charge de 180 g.
Des échantillons parallèlépipédiques de 3 x 3 x 50 mm sont ensuite découpés dans les lingots. C'est sur ces barrettes que sont effectués les différents traitements thermiques et mécaniques:
- a) d'homogénéisation: les échantillons bruts de coulée, enveloppés dans une feuille de molybdène, sont enfermés dans une ampoule à quartz sous vide. L'ampoule est ensuite placée au coeur d'un bloc d'acier chauffé par un four à résistance à la température de traitement, à 920°C. Après deux heures de maintien à cette température, l'ampoule est brisée dans une cuve d'eau.
- b) d'écrouissage: les alliages sont laminés à froid après le traitement d'homogénéisation. Ce taux d'écrouissage appliqué est d'environ 80%, soit une épaisseur finale de bande de 0,5 mm, obtenue en une dizaine de passes successives.
- c) de précipitation: les échantillons sont chauffés dans un four à résistance sous pression atmosphérique d'argon dans les conditions suivantes: chauffage depuis la température ambiante jusqu'à 200°C, palier d'une heure à cette température, montée de 200° à la température de précipitation à 200°C/heure,
maintien pendant 1 heure à la température de précipitation, puis refroidissement à 400°C/heure.
Parallelepipedic samples of 3 x 3 x 50 mm are then cut from the ingots. It is on these bars that the various thermal and mechanical treatments are carried out:
- a) homogenization: the raw casting samples, wrapped in a molybdenum sheet, are enclosed in a quartz vacuum interrupter. The bulb is then placed in the heart of a block of steel heated by an oven with resistance to the processing temperature, at 920 ° C. After two hours of maintaining at this temperature, the bulb is broken in a tank of water.
- b) work hardening: the alloys are cold rolled after the homogenization treatment. This applied work hardening rate is around 80%, ie a final strip thickness of 0.5 mm, obtained in ten successive passes.
- c) precipitation: the samples are heated in a resistance furnace under atmospheric pressure of argon under the following conditions: heating from ambient temperature to 200 ° C., one hour plateau at this temperature, rise by 200 ° at the precipitation temperature at 200 ° C / hour, holding for 1 hour at the precipitation temperature, then cooling to 400 ° C / hour.
Le Tableau 2 qui suit indique la conductivité de chaque alliage exprimé en % IACS mesurée à la température ambiante, en fonction de la température de précipitation:
Le Tableau 2 montre que les valeurs maximum de conductivité, exprimées en %IACS et soulignées dans ce tableau, sont obtenues pour une température de précipitation voisine de 560°C et que ces valeurs maximum sont très dispersées.
L'analyse de ces résultats selon le critère de l'art antérieur (rapport Ti/(Fe + Co) du brevet polonais n° 115185) montre que, d'une part dans le domaine 0,25 - 1 pour Ti/(Fe + Co) revendiqué pour ce rapport, la conductivité varie beaucoup pour des valeurs voisines (comparaison des essais R1, R2, R3, R4 entre eux et des essais R5, R6, R7 entre eux) et que, d'autre part ce rapport Ti/(Fe + Co) ne permet pas de déterminer le domaine favorable des hautes conductivités puisque les 7 points représentatifs ne permettent pas de tracer une courbe présentant de manière indiscutable un maximum (voir figure 1).
Par contre, l'analyse de ces résultats selon le critère trouvé par la demanderesse (le rapport
The analysis of these results according to the criterion of the prior art (Ti / (Fe + Co) ratio of Polish patent n ° 115185) shows that, on the one hand in the range 0.25 - 1 for Ti / (Fe + Co) claimed for this report, the conductivity varies a lot for similar values (comparison of tests R1, R2, R3, R4 between them and tests R5, R6, R7 between them) and that, on the other hand this ratio Ti / (Fe + Co) does not make it possible to determine the favorable domain of high conductivities since the 7 representative points do not make it possible to draw a curve having an indisputable maximum (see FIG. 1).
However, the analysis of these results according to the criterion found by the applicant (the report
Dans cet exemple, on étudie, dans des conditions voisines des conditions industrielles, l'influence du mode de désoxydation du bain en réalisant trois essais ayant un rapport
Ces trois agents de désoxydation sont introduits dans le bain liquide de façon à neutraliser la même quantité d'oxygène.
Compte tenu des masses atomiques du magnésium, du phosphore et du bore et si l'on se base sur une quantité de 0,06% de Mg alors il faut 0,03% de P et 0,018% de B pour neutraliser la même quantité d'oxygène. Dans un four à induction de 10 kg de capacité utile, on fait fondre à 1250°C dans un creuset en graphite, le cuivre, le fer, le cobalt, ces deux derniers étant sous forme d'alliages mère. On ajoute ensuite le bore ou le phosphore ou le magnésium et le titane également sous forme d'alliage mère puis on procède à un dégazage. Le tableau 3 indique la composition de la charge pour chaque essai:
These three deoxidizing agents are introduced into the liquid bath so as to neutralize the same amount of oxygen.
Taking into account the atomic masses of magnesium, phosphorus and boron and if one bases oneself on an amount of 0.06% of Mg then one needs 0.03% of P and 0.018% of B to neutralize the same amount of 'oxygen. In an induction furnace with a useful capacity of 10 kg, copper, iron, cobalt are melted at 1250 ° C in a graphite crucible, the latter two being in the form of master alloys. Next, boron or phosphorus or magnesium and titanium are also added in the form of a parent alloy, followed by degassing. Table 3 shows the composition of the load for each test:
Pendant toutes ces opérations, le bain est recouvert de charbon de bois. On coule à environ 1200°C. Les plateaux sont ensuite homogénéisés à 920°C pendant deux heures puis laminés à chaud en plusieurs passes. Après la dernière passe, ils sont trempés dans l'eau à environ 700°C. Après fraisage à 9 mm, les plateaux sont laminés à froid sans recuit intermédiaire jusqu'à obtenir des bandes de 0,8 mm d'épaisseur. Les alliages sont alors soumis à un traitement de précipitation pendant 4 heures à la température TM ci-dessous, comprise entre 500°C et 600°C, conduisant à la conductivité optimum (voir figure 3):
- essai A
- 575°C
- essai B
- 535°C
- essai C
- 515°C
Ce traitement thermique est suivi d'un laminage final avec réduction d'épaisseur de 44%.
On obtient des alliages présentant les caractéristiques suivantes:
et les propriétés mécaniques et propriétés de conductivité suivantes:
- test A
- 575 ° C
- test B
- 535 ° C
- test C
- 515 ° C
This heat treatment is followed by a final rolling with thickness reduction of 44%.
Alloys are obtained having the following characteristics:
and the following mechanical properties and conductivity properties:
Cet exemple illustre une modalité de la mise en forme d'alliages élaborés en tous points comme à l'exemple 2 (l'essai A' de l'exemple 3 correspond à l'essai A de l'exemple 2, de même pour B' et C')), sauf que le traitement de précipitation a lieu à plus basse température (505°C pour A', 485°C pour B', 475°C pour C') pendant 4 heures et que le laminage final correspond à une réduction d'épaisseur de 29%:
On obtient les propriétés suivantes:
The following properties are obtained:
Ces alliages présentent, après maintien de 30 minutes à 450°C une dureté supérieure à 130 HV, ce qui illustre leur excellente résistance à l'adoucissement.These alloys exhibit a hardness greater than 130 HV after 30 minutes at 450 ° C., which illustrates their excellent resistance to softening.
Cet exemple compare l'invention à l'art antérieur pour une gamme de transformation ne comportant qu'un seul traitement thermique (recuit de précipitation):
- essai C'
- : exemple 3
- essai D
- : selon brevet polonais n° 115185
- essai E
- : selon brevet américain n° 4 559 200
- test C '
- : example 3
- test D
- : according to Polish patent n ° 115185
- test E
- : according to US Patent No. 4,559,200
La figure 4 situe ces essais dans un plan ayant en abscisse la résistance mécanique et en ordonnée la conductivité électrique et illustre clairement l'intérêt de l'invention.
L'essai F, non comparatif, est donné à titre d'information: il correspond à l'essai D mais avec une gamme de transformation comportant deux traitements thermiques au lieu d'un.FIG. 4 situates these tests in a plane having the mechanical resistance on the abscissa and the electrical conductivity on the ordinate and clearly illustrates the advantage of the invention.
The non-comparative test F is given for information: it corresponds to test D but with a transformation range comprising two heat treatments instead of one.
Claims (11)
- Process for the production of a Cu-Fe-Co-Ti alloy incorporating a phase of producing the alloy and an alloy transformation phase with a precipitation heat treatment, characterized in that:a) an alloy is prepared, whose composition satisfies the following conditions (weight compositions):
Co/Fe ratio between 0.10 and 0.90
Ti/(Fe+Co) ratio between 0.30 and 1
iron content between 0.030 and 2%
cobalt content between 0.025 and 1.8%
titanium content between 0.025 and 4%
oxygen content below 50 ppm
total content of other optional additions below 1.5%
content of metallic impurities below 0.1% with each of them below 0.015%, the residue being copper;b) the molten alloy bath is deoxidized by introducing boron into it and eliminating the boron oxide formed;c) the cold drawn alloy undergoes a precipitation heat treatment at a temperature lower, by at the most 80°C, than the temperature TM leading to the maximum electrical conductivity. - Process according to claim 2, wherein the oxygen content is below 20 ppm.
- Process according to claim 2, wherein the iron content is between 0.1 and 1%.
- Process according to claim 2, wherein the cobalt content is between 0.05 and 0.4%.
- Process according to claim 2, wherein the titanium content is between 0.035 and 0.6%.
- Process according to claim 2, wherein the titanium is introduced in mother alloy form following the introduction of the boron, so as to avoid titanium losses and obviate melting and vacuum casting.
- Process according to claim 2, wherein the precipitation heat treatment is carried out at a temperature, below the temperature TM, for which the gradient of the conductivity curve in % IACS as a function of the temperature is between 0.1 and 0.3%IACS/°C.
- Alloy obtained according to any one of the claims 1 to 8.
- Alloy according to claim 9, characterized in that it contains less than 10 ppm boron.
- Application of the alloy according to any one of the claims 9 and 10 to the production of conductor elements for electronics and the connector industry and in particular component support grids, contact springs and connections.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8909906 | 1989-07-07 | ||
| FR8909906A FR2649418B1 (en) | 1989-07-07 | 1989-07-07 | COPPER-IRON-COBALT-TITANIUM ALLOY WITH HIGH MECHANICAL AND ELECTRICAL CHARACTERISTICS AND MANUFACTURING METHOD THEREOF |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0408469A1 EP0408469A1 (en) | 1991-01-16 |
| EP0408469B1 true EP0408469B1 (en) | 1993-11-24 |
Family
ID=9384052
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP90420315A Expired - Lifetime EP0408469B1 (en) | 1989-07-07 | 1990-07-04 | Copper-iron-cobalt-titanium alloy featuring high mechanical and electrical properties and process for the manufacture thereof |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US5026434A (en) |
| EP (1) | EP0408469B1 (en) |
| JP (1) | JPH0694578B2 (en) |
| KR (1) | KR940002684B1 (en) |
| DE (1) | DE69004756T2 (en) |
| ES (1) | ES2046754T3 (en) |
| FI (1) | FI95815C (en) |
| FR (1) | FR2649418B1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6282064B1 (en) * | 1994-03-15 | 2001-08-28 | International Business Machines Corporation | Head gimbal assembly with integrated electrical conductors |
| US6539609B2 (en) | 1994-07-05 | 2003-04-01 | International Business Machines Corporation | Method of forming a head gimbal assembly |
| FR2809626B1 (en) * | 2000-05-30 | 2003-03-07 | Poudres & Explosifs Ste Nale | NEEDLELESS SYRINGE WITH MULTI-DUCT EJECTOR INSULATION MEMBRANE |
| CN113265558B (en) * | 2021-03-22 | 2022-10-14 | 江西省科学院应用物理研究所 | Copper-iron alloy with excellent bending resistance and processing method thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2783143A (en) * | 1954-06-24 | 1957-02-26 | Driver Co Wilbur B | Age-hardenable, copper-base alloy |
| US4047980A (en) * | 1976-10-04 | 1977-09-13 | Olin Corporation | Processing chromium-containing precipitation hardenable copper base alloys |
| JPS6039139A (en) * | 1983-08-12 | 1985-02-28 | Mitsui Mining & Smelting Co Ltd | Softening resistant copper alloy with high conductivity |
| DE3511999A1 (en) * | 1985-04-02 | 1986-10-02 | Wieland-Werke Ag, 7900 Ulm | USE OF A COPPER-TITANIUM-COBALT ALLOY AS A MATERIAL FOR ELECTRONIC COMPONENTS |
| JPS6250426A (en) * | 1985-08-29 | 1987-03-05 | Furukawa Electric Co Ltd:The | Copper alloy for electronic appliance |
| JPH0788545B2 (en) * | 1987-04-28 | 1995-09-27 | 三菱マテリアル株式会社 | High strength and high toughness Cu alloy with little characteristic anisotropy |
-
1989
- 1989-07-07 FR FR8909906A patent/FR2649418B1/en not_active Expired - Fee Related
-
1990
- 1990-06-25 US US07/542,919 patent/US5026434A/en not_active Expired - Fee Related
- 1990-07-04 DE DE90420315T patent/DE69004756T2/en not_active Expired - Fee Related
- 1990-07-04 EP EP90420315A patent/EP0408469B1/en not_active Expired - Lifetime
- 1990-07-04 ES ES199090420315T patent/ES2046754T3/en not_active Expired - Lifetime
- 1990-07-06 FI FI903449A patent/FI95815C/en not_active IP Right Cessation
- 1990-07-06 JP JP2179393A patent/JPH0694578B2/en not_active Expired - Lifetime
- 1990-07-07 KR KR1019900010356A patent/KR940002684B1/en not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| JPH0694578B2 (en) | 1994-11-24 |
| US5026434A (en) | 1991-06-25 |
| FR2649418B1 (en) | 1991-09-20 |
| DE69004756D1 (en) | 1994-01-05 |
| ES2046754T3 (en) | 1994-02-01 |
| FR2649418A1 (en) | 1991-01-11 |
| FI903449A0 (en) | 1990-07-06 |
| FI95815C (en) | 1996-03-25 |
| DE69004756T2 (en) | 1994-05-05 |
| KR910003132A (en) | 1991-02-27 |
| KR940002684B1 (en) | 1994-03-30 |
| JPH0353036A (en) | 1991-03-07 |
| EP0408469A1 (en) | 1991-01-16 |
| FI95815B (en) | 1995-12-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JPH059502B2 (en) | ||
| CN101828240A (en) | Aluminum electric wire for automobiles and process for producing the aluminum electric wire | |
| FR2819825A1 (en) | METHOD FOR MANUFACTURING A FE-NI ALLOY STRIP | |
| EP0408469B1 (en) | Copper-iron-cobalt-titanium alloy featuring high mechanical and electrical properties and process for the manufacture thereof | |
| JP2008255416A (en) | Method for manufacturing copper material, and copper material | |
| BE1000537A4 (en) | Alloy metal copper, particularly for building components for electronic. | |
| EP1287171B1 (en) | Hardened fe-ni alloy for making integrated circuit grids and method for making same | |
| JPS6216269B2 (en) | ||
| FR2565601A1 (en) | COPPER, NICKEL, TINNEY, TITANIUM ALLOY, PROCESS FOR MANUFACTURING THE SAME, AND USE THEREOF | |
| TW524863B (en) | Copper alloy and process for making same | |
| JP2002317232A (en) | Tin based alloy containing tin-titanium based compound, production method therefor and precursory body of triniobium stannide superconducting wire rod using the alloy | |
| FR2470323A1 (en) | COPPER ALLOY TUBE FOR TRANSPORTING DRINKING WATER AND HEAT EXCHANGERS | |
| FR2661922A1 (en) | SPINODAL DECOMPOSITION COPPER ALLOYS AND PROCESS FOR OBTAINING THE SAME. | |
| EP0581647B1 (en) | Process for preparing of soft magnetic alloys with high permeability and alloys | |
| JPH0314896B2 (en) | ||
| EP0384862B1 (en) | Copper-tin alloys, partly deoxidized with magnesium or calcium, for electrical and/or thermal conductors | |
| JP6308672B2 (en) | Platinum rhodium alloy and method for producing the same | |
| JP7120389B1 (en) | Copper alloy plastic working materials, copper alloy wire rods, parts for electronic and electrical equipment, terminals | |
| JPS6017039A (en) | Copper alloy with superior heat resistance, mechanical characteristic, workability and electric conductivity | |
| JP3227072B2 (en) | Method of manufacturing aluminum alloy wire for electric conduction | |
| JP2932726B2 (en) | Manufacturing method of copper alloy wire | |
| JPH059184B2 (en) | ||
| BE437400A (en) | ||
| FR2491499A1 (en) | ANISOTROPIC MAGNETIC ALLOY AND PROCESS FOR PRODUCING THE SAME | |
| JPH09272958A (en) | Phosphor bronze low in surface cracking sensitivity and its production |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE ES GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19910128 |
|
| 17Q | First examination report despatched |
Effective date: 19930222 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE ES GB IT NL |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19931208 |
|
| REF | Corresponds to: |
Ref document number: 69004756 Country of ref document: DE Date of ref document: 19940105 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2046754 Country of ref document: ES Kind code of ref document: T3 |
|
| ITF | It: translation for a ep patent filed |
Owner name: ING. A. GIAMBROCONO & C. S.R.L. |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19990628 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 19990714 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19990721 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19990910 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000704 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000705 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010201 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20000704 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20010201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010501 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20010810 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050704 |