EP0349734B1 - Titanium-aluminium intermetallic compound and process for its preparation - Google Patents
Titanium-aluminium intermetallic compound and process for its preparation Download PDFInfo
- Publication number
- EP0349734B1 EP0349734B1 EP19890108489 EP89108489A EP0349734B1 EP 0349734 B1 EP0349734 B1 EP 0349734B1 EP 19890108489 EP19890108489 EP 19890108489 EP 89108489 A EP89108489 A EP 89108489A EP 0349734 B1 EP0349734 B1 EP 0349734B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- atomic
- intermetallic compound
- group
- periodic table
- temperature
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C14/00—Alloys based on titanium
Definitions
- the present invention relates to a Ti-Al intermetallic compound having an improved room-temperature ductility and high-temperature oxidation resistance, and is suitable for use as a high-temperature heat-resistant strength material for aircraft turbine engines, gas turbines for power generators, automobile engines, rotation bodies and the like, and further, to a process for the preparation of this intermetallic compound.
- the Ti-Al intermetallic compound has almost the highest high-temperature specific strength among metallic materials, and furthermore, has an excellent corrosion resistance and a light weight. It was reported in Metallurgical Transaction, Vol. 6A (1975), page 1991, that a high-temperature strength of 40 kg/mm2 was obtained at 800°C, and therefore, it is considered that the Ti-Al intermetallic compound is most suitable for application to parts of gas turbines, valves and pistons of automobile engines, high-temperature dies, bearing parts and the like, due to the foregoing excellent characteristics.
- the Ti-Al intermetallic compound has a composition latitude in the phase diagram, and in the composition range of 40 to 52 atomic % of Ti and 60 to 48 atomic % of Al, an Llo structure (basically a face-centered tetragonal structure but wherein the Ti layers and Al layers are arranged alternately in the [001] direction) is formed in the thermally equilibriated state. Accordingly, an abnormal strengthening phenomenon wherein the strength is increased in the single crystal state with an increase of the temperature was found, and it is known that, even in the case of polycrystal materials, the strength is not reduced at a high temperature of up to 800°C.
- the polycrystals of the Ti-Al intermetallic compound are defective in that the ductility is low at temperatures ranging from room temperature to about 700°C.
- the compressibility is 0.4% at room temperature and about 1.1% at 700°C (see JP-B-59-581).
- FR-A-2 462 483 discloses the possibility to improve the ductility of Ti-Al alloys at room temperature. The influence of vanadium on this property has been investigated. Antimony, bismuth and carbon are mentioned in FR-A-2 462 483 as elements which improve the resistance of the Ti-Al against creep.
- the Ti-Al intermetallic compound has a light weight, a high heat-resistance, and an excellent corrosion resistance, it is suitable for a turbine blade to be used at high temperatures.
- the room-temperature ductility of the Ti-Al intermetallic compound is low (the compressibility is 0.4%), a casting or forging thereof is difficult and the safety reliability at room temperature is poor, and thus a practical utilization thereof is uncertain.
- a room-temperature ductility is necessary.
- a primary object of the present invention is to provide a Ti-Al intermetallic compound material having a room-temperature compressibility of at least 25% and an improved high-temperature oxidation resistance.
- a Ti-Al intermetallic compound comprising 40 to 52 atomic % of Ti and 48 to 60 atomic % of Al, and further, containing 10 to 3000 atomic ppm of at least one of the elements P and As (elements of the group V B of the Periodic Table) and Se and Te (elements of the group VI B of the Periodic Table), the total of added elements representing 100 %, wherein the basic crystal structure of the matrix is an ordered structure of the Llo type, the room-temperature compressibility (ductility) is high, and a good high-temperature oxidation resistance is retained.
- a process for the preparation of a Ti-Al intermetallic compound material which comprises melting and solidifying a starting material having the above-mentioned composition in an inert gas atmosphere and, if necessary, annealing the solidified product.
- a preferred Ti-Al intermetallic compound comprises 40 to 50 atomic percent of titan, 50 to 60 atomic percent of aluminum and 100 to 1000 atomic ppm of phosphor.
- Another preferred intermetallic compound comprises 45 to 50 atomic percent of titan, 50 to 55 atomic percent of aluminum and 100 to 1000 atomic ppm of phosphor.
- an intermetallic compound wherein the content of at least one of the elements phosphor and arsen (elements of the group V B of the Periodic Table) and selen and tellur (elements of the group VI B of the Periodic Table) is 10 to 1000 atomic ppm is preferred.
- a solidified product is annealed at a temperature of 900 to 1000 °C after melting and solidifying.
- at least one of the elements phosphor and arsen (elements of the group V B of the Periodic Table) and selen and tellur (elements of the group VI B of the Periodic Table) is added in an amount of 10 to 1000 atomic ppm.
- the present inventors carried out investigations into improving the ductility in the Ti-Al intermetallic compound, and as a result, found that, in the Ti-Al intermetallic compound in which at least one of the elements P and As (elements of the group V B of the periodic table) and Se and Te (elements of the group VI B of the Periodic Table) is incorporated, the compressibility is at least 25% at room temperature and about 60% at 600°C, and the ductility at temperatures ranging from room temperature to about 700°C is greatly improved.
- the Ti content is adjusted to 40 to 52 atomic % to obtain a single phase of the Ti-Al intermetallic compound or a composition comprising a matrix of the Ti-Al intermetallic compound and a minor amount of a second phase of Ti3Al. If the Ti content is outside the above-mentioned range, an incorporation of another second phase occurs and good results cannot be attained. More specifically, if the Ti content is lower than 40 atomic %, Al2Ti or Al3Ti is present as the second phase and the presence of these compounds is not preferable, since they are brittle. If the Ti content exceeds 52 atomic %, the amount of Ti3Al as the second phase is increased. The high-temperature strength of Ti3Al is lower than that of TiAl, and therefore, from the viewpoint of the high-temperature strength, a large proportion of Ti3Al is not preferable.
- the Ti content is from 40 to 50 atomic %, a single phase (Llo type ordered structure) of the Ti-Al intermetallic compound is obtained, and if the Ti content is higher than 50 atomic % and up to 52 atomic %, Ti3Al (DO19 type ordered structure) is partially included as the second phase in the above-mentioned single phase.
- the room-temperature ductility is improved when compared to that of the compound composed solely of the single phase, under some heating conditions.
- the Ti content is 40 to 45 atomic %, an incorporation of Al2Ti as the second phase becomes possible under some casting or forging conditions, and the improvement of the ductility is reduced. Therefore, in the present invention, in view of the microstructure, preferably the lower limit of the Ti content is 45 atomic %.
- an element of the group V B of the Periodic Table (P or As) and/or an element of the group VI B of the Periodic Table (Se or Te) is incorporated in an amount of 10 to 3000 atomic ppm.
- the stacking fault energy is reduced and twinning easily occurs during plastic deformation, with the result that the room-temperature ductility is improved. This effect is enhanced with an increase of the content of the additive element, as shown in Fig. 1.
- the element of the group V B of the Periodic Table (P or As Sb) or the element of the group VI B of the Periodic Table (Se or Te) is bonded to Ti to form a compound such as TiP, TiAs, TiSe, TiSe2 or TiTe2 in the grain boundary and the matrix, this compound acts as the initiation point of a fracture, with the result that not only the room-temperature ductility but also the workability is lowered. If the content of the additive element is lower than 10 atomic ppm, the above-mentioned object cannot be obtained.
- TiO2 is generally formed in the outermost layer. Since TiO2 has an oxygen-depleted structure in which some of the lattice positions to be inherently occupied by O atoms are vacant in the crystal lattice, external oxygen atoms are diffused in the interior of the material through such oxygen-vacant positions and the oxidation is thus advanced inward. In TiO2 , Ti has a tetravalent positive charge and O has a divalent negative charge.
- the concentration of the oxygen vacancy is reduced to maintain the charge balance in the interior, the paths of diffusion of external oxygen atoms through TiO2 are reduced, and the oxidation is suppressed.
- the effect of suppressing the oxidation by the element of the group V B of the Periodic Table and/or the element of the group VI B of the Periodic Table is enhanced with an increase of the content of the additive element.
- the oxidation-suppressing effect is not satisfactory. If the content of the additive element exceeds 3000 atomic ppm, the content exceeds the dissolution limit in TiO2 and the additive element is concentrated at the interface between the TiO2 oxidation scale and the TiAl matrix to form a compound such as TiP, TiAs, TiSe, TiSe2 or TiTe2 at the interface, with the result that a breakaway of the oxidation layer occurs there and the oxidation rate is greatly increased.
- the content of the element of the group V B of the Periodic Table (P or As) and/or the element of the group VI B of the Periodic Table (Se or Te) in the Ti-Al intermetallic compound is adjusted to 10 to 3000 atomic ppm.
- the content of the additive element is up to 1000 atomic ppm, the effect whereby oxidation is effectively suppressed at temperatures of up to 800°C can be obtained.
- Bi has an effect of improving the oxidation resistance, but Bi increases the specific gravity and reduces the specific strength, and therefore, the material is disadvantageous as a high-temperature light-weight construction material. Accordingly, Bi is excluded from the element of the group V B of the Periodic Table.
- the reason why S is excluded from the element of the group VI B of the Periodic Table is that the bonding between Ti and S is too strong and causes premature breakaway of the TiO2 oxidation scale. Po is excluded for the same reason as described above with respect to Bi.
- the room-temperature ductility and the high-temperature oxidation resistance can be further improved.
- a mixture formed by adding 10 to 3000 atomic ppm of at least one element selected from the group consisting of P, As, Se and Te, optionally together with Mn and Si, to 40 to 52 atomic % of Ti and 48 to 60 atomic % of Al is once placed under vacuum (under a pressure lower than 10 ⁇ 6 Torr), and then the atmosphere is replaced by Ar gas and the mixture is made molten at a temperature higher than the melting point and ranging from 1400 to 1500°C, to minimize a reaction with a crucible, and then the melt is solidified.
- a room-temperature ductility can be obtained in the as-solidified state, but if the solidification product is annealed in the above-mentioned inert gas atmosphere, to obtain a uniform microstructure, the ductility is further improved.
- the so-obtained Ti-Al intermetallic compound having the element of the group V B of the Periodic Table (P or As) and/or the element of the group VI B of the Periodic Table (Se or Te) incorporated therein has a compressibility of at least 25% at room temperature and a compressibility of about 60% at 600°C, and the ductility is improved at temperatures ranging from room temperature to about 800°C. Since the tertiary element-free Ti-Al intermetallic compound has a compressibility of 0.4% at room-temperature and a compressibility of 1.1% at 700°C (see Japanese Unexamined Patent Publication No. 58-123847), it is obvious that the performance is greatly improved according to the present invention. Moreover, the high-temperature oxidation resistance is greatly improved compared with that of the tertiary element-free Ti-Al intermetallic compound and the Mn-added Ti-Al intermetallic compound.
- the improvement of the room-temperature compressibility is caused by a reduction of the stacking fault energy of the Ti-Al intermetallic compound by the addition of the tertiary element such as the element of the group V B of the Periodic Table (P or As) or the element of the group VI B of the Periodic Table (Se or Te).
- the tertiary element such as the element of the group V B of the Periodic Table (P or As) or the element of the group VI B of the Periodic Table (Se or Te).
- the high-temperature oxidation resistance is improved by preventing a permeation of oxygen by forming an oxide film on the surface of a material.
- oxidation is advanced by a diffusion of oxygen through oxygen ion-vacancies in TiO 2-x formed on the surface of the sample, and accordingly, in order to improve the high-temperature oxidation resistance, the concentration of the oxygen ion-vacancies must be reduced and the rate of the inward diffusion of oxygen must be suppressed.
- the reason why the high-temperature oxidation resistance is improved in the alloy of the present invention is considered to be because the element of the group V B of the Periodic Table (P or As) or the element of the group VI B of the Periodic Table (Se or Te) has a valence electron number of 5 or 6 respectively, larger than the valence electron number of Ti, i.e., 4, and therefore the tertiary element reduces the concentration of oxygen ion-vacancies in the TiO 2-x layer formed on the surface and suppresses the inward diffusion of oxygen, whereby the growth rate of the oxide layer TiO 2-x formed on the Ti-Al intermetallic compound in a high-temperature oxidizing atmosphere is reduced.
- a mixture comprising 50 atomic % of pure sponge titanium and 50 atomic % of Al, in which 94 atomic ppm (100 weight ppm) of Se or 58 atomic ppm (100 weight ppm) of Te was incorporated, was once placed under vacuum (pressure lower than 10 ⁇ 6 Torr) in a vacuum melting furnace, the atmosphere was replaced by Ar gas, and the mixture was heated at 1500°C, made molten, and then solidified. The solidified product was then annealed at 1000°C, and a heating time of 72 hours. The results are shown in Tables 1 and 2.
- the samples of the present invention had a greatly improved yield stress under compression deformation and the room-temperature compressibility was greatly improved compared with that of the tertiary element-free Ti-Al intermetallic compound. Furthermore, the yield stress and room-temperature compressibility of the samples of the present invention were comparable to those of the Ti-Al intermetallic compound having 2% by weight of Mn added thereto.
- the amount increased by oxidation in the Mn-added Ti-Al intermetallic compound was much larger than in the tertiary element-free Ti-Al intermetallic compound, but in the Se- or Te-added Ti-Al intermetallic compound, the amount increased by oxidation was much smaller, and it was confirmed that the oxidation resistance was remarkably improved.
- a mixture comprising 50 atomic % (63.9% by weight) of sponge Ti having a purity of 99.8% by weight and 50 atomic % (36.0% by weight) of Al having a purity of 99.99% by weight, in which 500 weight ppm of P was incorporated, was once placed under vacuum (pressure lower than 10 ⁇ 6 Torr) in a vacuum melting furnace, the atmosphere was replaced by Ar gas, and the mixture was heated at 1500°C, made molten, and then solidified. A part of the solidified product was then annealed at 1000°C for 72 hours.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Turbine Rotor Nozzle Sealing (AREA)
- Manufacture Of Alloys Or Alloy Compounds (AREA)
Description
- The present invention relates to a Ti-Aℓ intermetallic compound having an improved room-temperature ductility and high-temperature oxidation resistance, and is suitable for use as a high-temperature heat-resistant strength material for aircraft turbine engines, gas turbines for power generators, automobile engines, rotation bodies and the like, and further, to a process for the preparation of this intermetallic compound.
- The Ti-Aℓ intermetallic compound has almost the highest high-temperature specific strength among metallic materials, and furthermore, has an excellent corrosion resistance and a light weight. It was reported in Metallurgical Transaction, Vol. 6A (1975), page 1991, that a high-temperature strength of 40 kg/mm² was obtained at 800°C, and therefore, it is considered that the Ti-Aℓ intermetallic compound is most suitable for application to parts of gas turbines, valves and pistons of automobile engines, high-temperature dies, bearing parts and the like, due to the foregoing excellent characteristics.
- The Ti-Aℓ intermetallic compound has a composition latitude in the phase diagram, and in the composition range of 40 to 52 atomic % of Ti and 60 to 48 atomic % of Aℓ, an Llo structure (basically a face-centered tetragonal structure but wherein the Ti layers and Aℓ layers are arranged alternately in the [001] direction) is formed in the thermally equilibriated state. Accordingly, an abnormal strengthening phenomenon wherein the strength is increased in the single crystal state with an increase of the temperature was found, and it is known that, even in the case of polycrystal materials, the strength is not reduced at a high temperature of up to 800°C. Nevertheless, the polycrystals of the Ti-Aℓ intermetallic compound are defective in that the ductility is low at temperatures ranging from room temperature to about 700°C. For example, in the case of a composition of 48 atomic % of Ti and 52 atomic % of Aℓ, the compressibility is 0.4% at room temperature and about 1.1% at 700°C (see JP-B-59-581).
- The difficulties encountered in the development of a Ti-Al intermetallic compound as a practical material are mainly concerned with how to maintain a good room-temperature ductility, and it has been confirmed that an addition of Mn is effective for this purpose (see JP-A-61-41740).
- It has been reported, however, that the addition of Mn leads to a lowering of the high-temperature oxidation resistance (Tsurumi et al., Symposium of Japanese Association of Metals, Plastic Deformation of Ordered Alloys and Intermetallic Compounds, page 13, July 16, 1988).
- FR-A-2 462 483 discloses the possibility to improve the ductility of Ti-Al alloys at room temperature. The influence of vanadium on this property has been investigated. Antimony, bismuth and carbon are mentioned in FR-A-2 462 483 as elements which improve the resistance of the Ti-Al against creep.
- Further, since the Ti-Al intermetallic compound has a light weight, a high heat-resistance, and an excellent corrosion resistance, it is suitable for a turbine blade to be used at high temperatures. However since the room-temperature ductility of the Ti-Al intermetallic compound is low (the compressibility is 0.4%), a casting or forging thereof is difficult and the safety reliability at room temperature is poor, and thus a practical utilization thereof is uncertain. Moreover, as a practical material for designing, a room-temperature ductility is necessary.
- Therefore, a primary object of the present invention is to provide a Ti-Al intermetallic compound material having a room-temperature compressibility of at least 25% and an improved high-temperature oxidation resistance.
- More specifically, in accordance with the present invention, there is provided a Ti-Al intermetallic compound comprising 40 to 52 atomic % of Ti and 48 to 60 atomic % of Al, and further, containing 10 to 3000 atomic ppm of at least one of the elements P and As (elements of the group V B of the Periodic Table) and Se and Te (elements of the group VI B of the Periodic Table), the total of added elements representing 100 %, wherein the basic crystal structure of the matrix is an ordered structure of the Llo type, the room-temperature compressibility (ductility) is high, and a good high-temperature oxidation resistance is retained.
- Furthermore, in accordance with the present invention, there is provided a process for the preparation of a Ti-Al intermetallic compound material, which comprises melting and solidifying a starting material having the above-mentioned composition in an inert gas atmosphere and, if necessary, annealing the solidified product.
- A preferred Ti-Al intermetallic compound comprises 40 to 50 atomic percent of titan, 50 to 60 atomic percent of aluminum and 100 to 1000 atomic ppm of phosphor. Another preferred intermetallic compound comprises 45 to 50 atomic percent of titan, 50 to 55 atomic percent of aluminum and 100 to 1000 atomic ppm of phosphor. Furthermore, an intermetallic compound wherein the content of at least one of the elements phosphor and arsen (elements of the group V B of the Periodic Table) and selen and tellur (elements of the group VI B of the Periodic Table) is 10 to 1000 atomic ppm is preferred.
- In the preferred process of the present invention, a solidified product is annealed at a temperature of 900 to 1000 °C after melting and solidifying. In a further preferred process of the present invention, at least one of the elements phosphor and arsen (elements of the group V B of the Periodic Table) and selen and tellur (elements of the group VI B of the Periodic Table) is added in an amount of 10 to 1000 atomic ppm.
- Figure 1 illustrates the relationship between the amount added of phosphorus (P) and the compressibility in the Ti-Al intermetallic compound; and,
- Fig. 2 shows a stress-strain curve illustrating the results of the room temperature compression test of the materials of the present invention.
- The present inventors carried out investigations into improving the ductility in the Ti-Al intermetallic compound, and as a result, found that, in the Ti-Al intermetallic compound in which at least one of the elements P and As (elements of the group V B of the periodic table) and Se and Te (elements of the group VI B of the Periodic Table) is incorporated, the compressibility is at least 25% at room temperature and about 60% at 600°C, and the ductility at temperatures ranging from room temperature to about 700°C is greatly improved. Since in the tertiary element-free Ti-Al intermetallic compound (comprising 48 atomic % of Ti and 52 atomic % of Al), the compressibility is 0.4% and 1.1% at 700°C (see JP-A-62-215),
it is considered that this remarkable performance is due to the incorporation of the above-mentioned tertiary component. Furthermore, it was found that the high-temperature oxidation resistance is greatly improved when compared to that of the tertiary element-free Ti-Al intermetallic compound and the Mn-added Ti-Al intermetallic compound. - The present invention will now be described in detail. In the present invention, the Ti content is adjusted to 40 to 52 atomic % to obtain a single phase of the Ti-Al intermetallic compound or a composition comprising a matrix of the Ti-Al intermetallic compound and a minor amount of a second phase of Ti₃Al. If the Ti content is outside the above-mentioned range, an incorporation of another second phase occurs and good results cannot be attained. More specifically, if the Ti content is lower than 40 atomic %, Al₂Ti or Al₃Ti is present as the second phase and the presence of these compounds is not preferable, since they are brittle. If the Ti content exceeds 52 atomic %, the amount of Ti₃Al as the second phase is increased. The high-temperature strength of Ti₃Al is lower than that of TiAl, and therefore, from the viewpoint of the high-temperature strength, a large proportion of Ti₃Al is not preferable.
- Namely, if the Ti content is from 40 to 50 atomic %, a single phase (Llo type ordered structure) of the Ti-Al intermetallic compound is obtained, and if the Ti content is higher than 50 atomic % and up to 52 atomic %, Ti₃Al (DO₁₉ type ordered structure) is partially included as the second phase in the above-mentioned single phase. In the compound having this microstructure, the room-temperature ductility is improved when compared to that of the compound composed solely of the single phase, under some heating conditions. If the Ti content is 40 to 45 atomic %, an incorporation of Al₂Ti as the second phase becomes possible under some casting or forging conditions, and the improvement of the ductility is reduced. Therefore, in the present invention, in view of the microstructure, preferably the lower limit of the Ti content is 45 atomic %.
- In the present invention, an element of the group V B of the Periodic Table (P or As) and/or an element of the group VI B of the Periodic Table (Se or Te) is incorporated in an amount of 10 to 3000 atomic ppm.
- If the element of the group V B of the Periodic Table (P or As) and/or the compound of the group VI B of the Periodic Table (Se or Te) is present in the Ti-Al intermetallic compound, the stacking fault energy is reduced and twinning easily occurs during plastic deformation, with the result that the room-temperature ductility is improved. This effect is enhanced with an increase of the content of the additive element, as shown in Fig. 1.
- Nevertheless, if the content of the additive element exceeds 3000 atomic ppm, the element of the group V B of the Periodic Table (P or As Sb) or the element of the group VI B of the Periodic Table (Se or Te) is bonded to Ti to form a compound such as TiP, TiAs, TiSe, TiSe₂ or TiTe₂ in the grain boundary and the matrix, this compound acts as the initiation point of a fracture, with the result that not only the room-temperature ductility but also the workability is lowered. If the content of the additive element is lower than 10 atomic ppm, the above-mentioned object cannot be obtained.
- If the Ti-Al intermetallic compound is oxidized at high temperatures in an oxidizing atmosphere, TiO₂ is generally formed in the outermost layer. Since TiO₂ has an oxygen-depleted structure in which some of the lattice positions to be inherently occupied by O atoms are vacant in the crystal lattice, external oxygen atoms are diffused in the interior of the material through such oxygen-vacant positions and the oxidation is thus advanced inward. In TiO₂ , Ti has a tetravalent positive charge and O has a divalent negative charge. Accordingly, if the element of the group V B of the Periodic Table (P or As) having a pentuvalent positive charge and/or the element of the group VI B of the Periodic Table (Se or Te) having a hexavalent positive charge is present in TiO₂ , the concentration of the oxygen vacancy is reduced to maintain the charge balance in the interior, the paths of diffusion of external oxygen atoms through TiO₂ are reduced, and the oxidation is suppressed. The effect of suppressing the oxidation by the element of the group V B of the Periodic Table and/or the element of the group VI B of the Periodic Table is enhanced with an increase of the content of the additive element. If the content of the additive element is lower than 10 atomic ppm, the oxidation-suppressing effect is not satisfactory. If the content of the additive element exceeds 3000 atomic ppm, the content exceeds the dissolution limit in TiO₂ and the additive element is concentrated at the interface between the TiO₂ oxidation scale and the TiAl matrix to form a compound such as TiP, TiAs, TiSe, TiSe₂ or TiTe₂ at the interface, with the result that a breakaway of the oxidation layer occurs there and the oxidation rate is greatly increased. For the above-mentioned reasons, in the present invention, the content of the element of the group V B of the Periodic Table (P or As) and/or the element of the group VI B of the Periodic Table (Se or Te) in the Ti-Al intermetallic compound is adjusted to 10 to 3000 atomic ppm.
- If the content of the additive element is up to 1000 atomic ppm, the effect whereby oxidation is effectively suppressed at temperatures of up to 800°C can be obtained.
- Bi has an effect of improving the oxidation resistance, but Bi increases the specific gravity and reduces the specific strength, and therefore, the material is disadvantageous as a high-temperature light-weight construction material. Accordingly, Bi is excluded from the element of the group V B of the Periodic Table. The reason why S is excluded from the element of the group VI B of the Periodic Table is that the bonding between Ti and S is too strong and causes premature breakaway of the TiO₂ oxidation scale. Po is excluded for the same reason as described above with respect to Bi.
- If 0.01 to 3 atomic % of Mn and 0.01 to 1 atomic % of Si are incorporated in the Ti-Al intermetallic compound in combination with the element of the group V B of the Periodic Table (P or As) and/or the element of the group VI B of the Periodic Table (Se or Te), the room-temperature ductility and the high-temperature oxidation resistance can be further improved.
- According to the process for the preparation of the Ti-Al intermetallic compound of the present invention, a mixture formed by adding 10 to 3000 atomic ppm of at least one element selected from the group consisting of P, As, Se and Te, optionally together with Mn and Si, to 40 to 52 atomic % of Ti and 48 to 60 atomic % of Al is once placed under vacuum (under a pressure lower than 10⁻⁶ Torr), and then the atmosphere is replaced by Ar gas and the mixture is made molten at a temperature higher than the melting point and ranging from 1400 to 1500°C, to minimize a reaction with a crucible, and then the melt is solidified. A room-temperature ductility can be obtained in the as-solidified state, but if the solidification product is annealed in the above-mentioned inert gas atmosphere, to obtain a uniform microstructure, the ductility is further improved.
- The so-obtained Ti-Al intermetallic compound having the element of the group V B of the Periodic Table (P or As) and/or the element of the group VI B of the Periodic Table (Se or Te) incorporated therein has a compressibility of at least 25% at room temperature and a compressibility of about 60% at 600°C, and the ductility is improved at temperatures ranging from room temperature to about 800°C. Since the tertiary element-free Ti-Al intermetallic compound has a compressibility of 0.4% at room-temperature and a compressibility of 1.1% at 700°C (see Japanese Unexamined Patent Publication No. 58-123847), it is obvious that the performance is greatly improved according to the present invention. Moreover, the high-temperature oxidation resistance is greatly improved compared with that of the tertiary element-free Ti-Al intermetallic compound and the Mn-added Ti-Al intermetallic compound.
- The reasons why the room-temperature compressibility and the high-temperature oxidation resistance are improved by incorporation of at least one element selected from the group consisting of P, As, Se and Te in the Ti-Al intermetallic compound will now be described.
- It is considered that the improvement of the room-temperature compressibility is caused by a reduction of the stacking fault energy of the Ti-Al intermetallic compound by the addition of the tertiary element such as the element of the group V B of the Periodic Table (P or As) or the element of the group VI B of the Periodic Table (Se or Te). This reduction of the stacking fault energy facilitates twinning, especially crossing of twins, resulting in improved ductility. By electron microscope observation or in-situ high voltage electron microscope observation, it has been confirmed that, in the tertiary element-free Ti-Al intermetallic compound, twinning does not occur, but in the tertiary element-incorporated Ti-Al intermetallic compound, twinning easily occurs and the plastic deformation is advanced. By electron microscope observations, it was confirmed that this crossing of twins does not produce dislocation pile ups at the twin boundary during plastic deformation, and instead mobile dislocations are formed by a dislocation reaction to increase the ductility.
- The high-temperature oxidation resistance is improved by preventing a permeation of oxygen by forming an oxide film on the surface of a material. In the case of the Ti-Al intermetallic compound, it is considered that oxidation is advanced by a diffusion of oxygen through oxygen ion-vacancies in TiO2-x formed on the surface of the sample, and accordingly, in order to improve the high-temperature oxidation resistance, the concentration of the oxygen ion-vacancies must be reduced and the rate of the inward diffusion of oxygen must be suppressed.
- The reason why the high-temperature oxidation resistance is improved in the alloy of the present invention is considered to be because the element of the group V B of the Periodic Table (P or As) or the element of the group VI B of the Periodic Table (Se or Te) has a valence electron number of 5 or 6 respectively, larger than the valence electron number of Ti, i.e., 4, and therefore the tertiary element reduces the concentration of oxygen ion-vacancies in the TiO2-x layer formed on the surface and suppresses the inward diffusion of oxygen, whereby the growth rate of the oxide layer TiO2-x formed on the Ti-Al intermetallic compound in a high-temperature oxidizing atmosphere is reduced.
- The present invention will now be described in detail with reference to the following examples, that by no means limit the scope of the invention.
- A mixture comprising 50 atomic % of pure sponge titanium and 50 atomic % of Al, in which 94 atomic ppm (100 weight ppm) of Se or 58 atomic ppm (100 weight ppm) of Te was incorporated, was once placed under vacuum (pressure lower than 10⁻⁶ Torr) in a vacuum melting furnace, the atmosphere was replaced by Ar gas, and the mixture was heated at 1500°C, made molten, and then solidified. The solidified product was then annealed at 1000°C, and a heating time of 72 hours. The results are shown in Tables 1 and 2.
- As apparent from the results shown in Tables 1 and 2, the samples of the present invention had a greatly improved yield stress under compression deformation and the room-temperature compressibility was greatly improved compared with that of the tertiary element-free Ti-Al intermetallic compound. Furthermore, the yield stress and room-temperature compressibility of the samples of the present invention were comparable to those of the Ti-Al intermetallic compound having 2% by weight of Mn added thereto. With respect to the oxidation resistance, the amount increased by oxidation in the Mn-added Ti-Al intermetallic compound was much larger than in the tertiary element-free Ti-Al intermetallic compound, but in the Se- or Te-added Ti-Al intermetallic compound, the amount increased by oxidation was much smaller, and it was confirmed that the oxidation resistance was remarkably improved.
- A mixture comprising 50 atomic % (63.9% by weight) of sponge Ti having a purity of 99.8% by weight and 50 atomic % (36.0% by weight) of Al having a purity of 99.99% by weight, in which 500 weight ppm of P was incorporated, was once placed under vacuum (pressure lower than 10⁻⁶ Torr) in a vacuum melting furnace, the atmosphere was replaced by Ar gas, and the mixture was heated at 1500°C, made molten, and then solidified. A part of the solidified product was then annealed at 1000°C for 72 hours.
- A test piece having a diameter of 5 mm and a height of 5 mm was cut from the obtained sample, and the room-temperature compressibility test was carried out. The results are shown in the stress-strain curve of Fig. 2. From Fig. 2, it is seen that, in the P-added Ti-Al sample, the room-temperature ductility was greatly improved compared to the P-free Ti-Al sample.
-
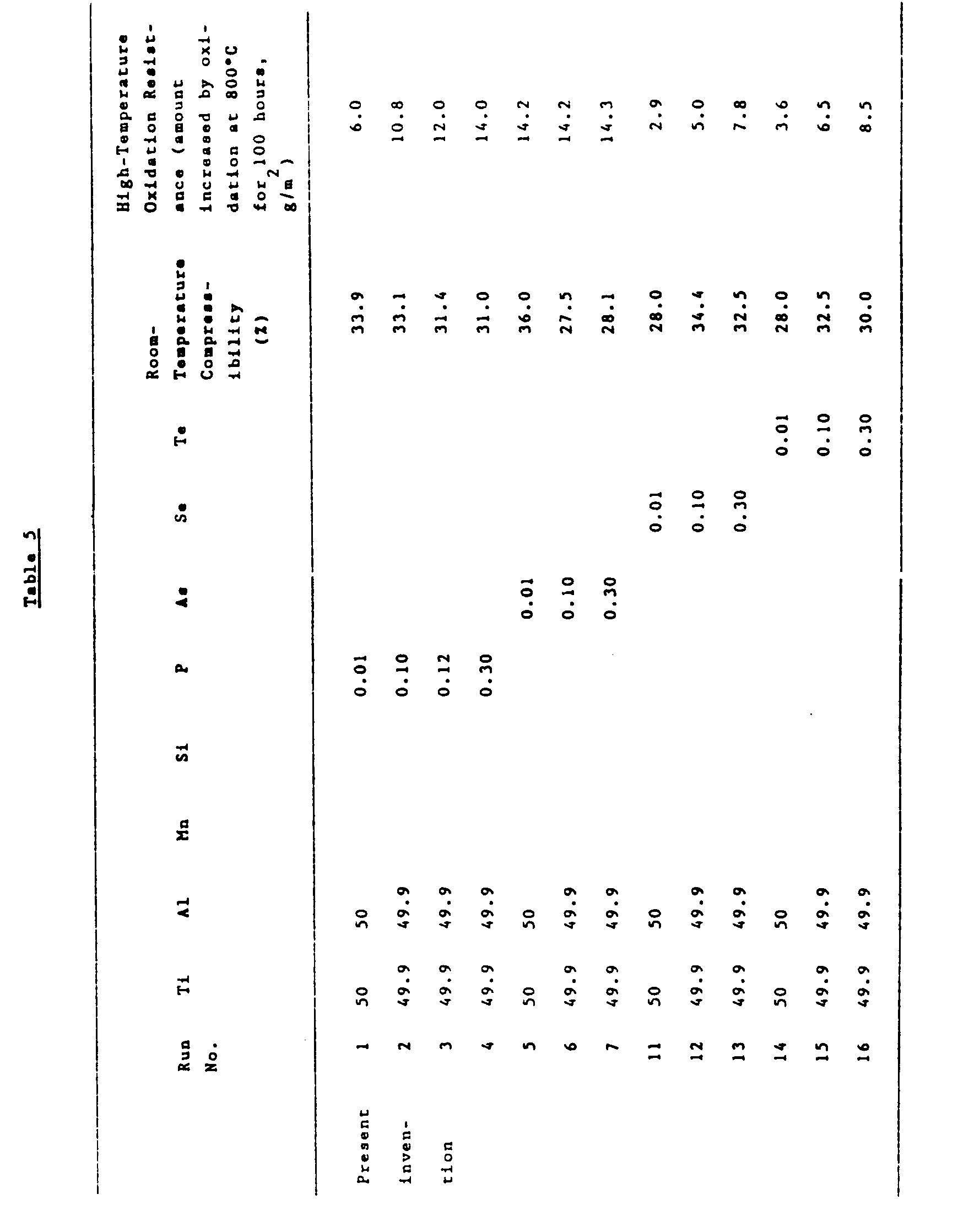
- Materials comprising Ti and Al in amounts shown in Table 5, in which the element of the group V B of the Periodic Table, the element of the group VI B of the Periodic Table Si and Mn were incorporated as shown in Table 5, were treated in the same manner as described in Example 1. The results are shown in Table 5.
-
Claims (7)
- A Ti-Al intermetallic compound which comprises 40 to 52 atomic % of Ti, 48 to 60 atomic % of Al, optionally 0.01 to 3 atomic % of Mn and 0.01 to 1 atomic % of Si and which contains 10 to 3000 atomic ppm of at least one of the elements P and As (elements of the group V B of the Periodic Table) and Se and Te (elements of the group VI B of the Periodic Table, the total of added elements representing 100%.
- A Ti-Al intermetallic compound as set forth in claim 1, which comprises 40 to 50 atomic % of Ti and 50 to 60 atomic % of Al and contains 100 to 1000 atomic ppm of P.
- A Ti-Al intermetallic compound as set forth in claim 1, which comprises 45 to 50 atomic % of Ti and 50 to 55 atomic % of Al and contains 100 to 1000 atomic ppm of P.
- A Ti-Al intermetallic compound as set forth in claim 1, wherein the content of at least one of the elements P and As (elements of the group V B of the Periodic Table and Se and Te (elements of the group VI B of the Periodic Table is 10 to 1000 atomic ppm.
- A process for the preparation of a Ti-Al intermetallic compound, which comprises adding 10 to 3000 atomic ppm of at least one of the elements P and As (elements of the group V B of the Periodic Table) and Se and Te (elements of the group VI B of the Periodic Table) to a mixture comprising 40 to 52 atomic % of Ti and 48 to 60 atomic % of Al, and melting and solidifying the mixture in an inert gas atmosphere.
- A process according to claim 5, wherein after melting and solidifying , the solidified product is annealed at a temperature of 900 to 1000°C.
- A process according to claim 5 or 6, wherein at least one of the elements P and As (element of the group V B of the Periodic Table) and Se and Te (elements of the group VI B of the Periodic Table) is added in an amount of 10 to 1000 atomic ppm.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP11624488 | 1988-05-13 | ||
| JP116244/88 | 1988-05-13 | ||
| JP317687/88 | 1988-12-16 | ||
| JP63317687A JP2711558B2 (en) | 1988-12-16 | 1988-12-16 | TiA intermetallic compound and method for producing the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0349734A1 EP0349734A1 (en) | 1990-01-10 |
| EP0349734B1 true EP0349734B1 (en) | 1994-08-31 |
Family
ID=26454617
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19890108489 Expired - Lifetime EP0349734B1 (en) | 1988-05-13 | 1989-05-11 | Titanium-aluminium intermetallic compound and process for its preparation |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0349734B1 (en) |
| DE (1) | DE68917815T2 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE59106459D1 (en) * | 1990-05-04 | 1995-10-19 | Asea Brown Boveri | High temperature alloy for machine components based on doped titanium aluminide. |
| EP0464366B1 (en) * | 1990-07-04 | 1994-11-30 | Asea Brown Boveri Ag | Process for producing a work piece from an alloy based on titanium aluminide containing a doping material |
| US5908516A (en) * | 1996-08-28 | 1999-06-01 | Nguyen-Dinh; Xuan | Titanium Aluminide alloys containing Boron, Chromium, Silicon and Tungsten |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3203794A (en) * | 1957-04-15 | 1965-08-31 | Crucible Steel Co America | Titanium-high aluminum alloys |
| US4294615A (en) * | 1979-07-25 | 1981-10-13 | United Technologies Corporation | Titanium alloys of the TiAl type |
-
1989
- 1989-05-11 EP EP19890108489 patent/EP0349734B1/en not_active Expired - Lifetime
- 1989-05-11 DE DE1989617815 patent/DE68917815T2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE68917815T2 (en) | 1995-01-05 |
| DE68917815D1 (en) | 1994-10-06 |
| EP0349734A1 (en) | 1990-01-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA1066922A (en) | Heat-resistant allow for welded structures | |
| EP0549286B1 (en) | High temperature resistant Ni-Cr alloy | |
| Liu et al. | Ordered intermetallics | |
| CA2016007C (en) | Gamma titanium aluminum alloys modified by chromium and tantalum and method of preparation | |
| Taub et al. | Ductility in boron-doped, nickel-base L12 alloys processed by rapid solidification | |
| JPH0225534A (en) | Titanium-aluminum alloy | |
| JPH11501364A (en) | Corrosion resistant iron aluminide with improved mechanical properties and corrosion resistance | |
| JPH10259435A (en) | Iridium-based alloy | |
| US5167732A (en) | Nickel aluminide base single crystal alloys | |
| Takasugi et al. | Defect structures in Co-rich Co3Ti intermetallic compound | |
| EP2078763A1 (en) | Ni-based compound superalloy having excellent oxidation resistance, process for production thereof, and heat-resistant structural material | |
| JPH09165634A (en) | Heat resistant titanium alloy | |
| JP2001049375A (en) | Al alloy having excellent vibration absorption performance and method for producing the same | |
| US5348594A (en) | Ti-Al intermetallic compound with Se | |
| Bahadur | Enhancement of high temperature strength and room temperature ductility of iron aluminides by alloying | |
| EP0349734B1 (en) | Titanium-aluminium intermetallic compound and process for its preparation | |
| JP3894987B2 (en) | Heat-resistant platinum material | |
| EP0398264B1 (en) | Precipitation hardening type nickel base single crystal cast alloy | |
| EP0413524B1 (en) | Titanium-aluminium based lightweight, heat resisting material | |
| JP2711558B2 (en) | TiA intermetallic compound and method for producing the same | |
| JP2003138334A (en) | Ni-BASED ALLOY HAVING EXCELLENT HIGH TEMPERATURE OXIDATION RESISTANCE AND HIGH TEMPERATURE DUCTILITY | |
| JP3332615B2 (en) | TiAl-based intermetallic compound-based alloy and method for producing the same | |
| JPH0250933A (en) | Ti-Al intermetallic compound containing P and its manufacturing method | |
| JPH01287243A (en) | Ti-Al intermetallic compound containing Mn and Nb and its manufacturing method | |
| US5215605A (en) | Niobium-aluminum-titanium intermetallic compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB |
|
| 17P | Request for examination filed |
Effective date: 19900709 |
|
| 17Q | First examination report despatched |
Effective date: 19921005 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB |
|
| REF | Corresponds to: |
Ref document number: 68917815 Country of ref document: DE Date of ref document: 19941006 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19980505 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19980511 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19980515 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990511 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19990511 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000301 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |