EP0102930B2 - Wetting agent and its use as mercerising aid - Google Patents
Wetting agent and its use as mercerising aid Download PDFInfo
- Publication number
- EP0102930B2 EP0102930B2 EP83810399A EP83810399A EP0102930B2 EP 0102930 B2 EP0102930 B2 EP 0102930B2 EP 83810399 A EP83810399 A EP 83810399A EP 83810399 A EP83810399 A EP 83810399A EP 0102930 B2 EP0102930 B2 EP 0102930B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- component
- wetting agent
- agent according
- wetting
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000080 wetting agent Substances 0.000 title claims description 53
- 238000009992 mercerising Methods 0.000 title claims 2
- SVTBMSDMJJWYQN-UHFFFAOYSA-N 2-methylpentane-2,4-diol Chemical compound CC(O)CC(C)(C)O SVTBMSDMJJWYQN-UHFFFAOYSA-N 0.000 claims description 10
- 239000007864 aqueous solution Substances 0.000 claims description 9
- 150000001875 compounds Chemical class 0.000 claims description 7
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- RGHNJXZEOKUKBD-SQOUGZDYSA-N Gluconic acid Natural products OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- 239000000835 fiber Substances 0.000 claims description 4
- 229920002866 paraformaldehyde Polymers 0.000 claims description 4
- 159000000000 sodium salts Chemical class 0.000 claims description 4
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 claims description 3
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 claims description 3
- 229930040373 Paraformaldehyde Natural products 0.000 claims description 3
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 claims description 3
- 239000000174 gluconic acid Substances 0.000 claims description 3
- 235000012208 gluconic acid Nutrition 0.000 claims description 3
- 239000000463 material Substances 0.000 claims description 3
- 229910052783 alkali metal Chemical group 0.000 claims description 2
- 150000001340 alkali metals Chemical group 0.000 claims description 2
- 239000008139 complexing agent Substances 0.000 claims description 2
- 239000004205 dimethyl polysiloxane Substances 0.000 claims description 2
- 235000013870 dimethyl polysiloxane Nutrition 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims description 2
- 229920001296 polysiloxane Polymers 0.000 claims description 2
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims 1
- 229910019142 PO4 Inorganic materials 0.000 claims 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims 1
- 230000003165 hydrotropic effect Effects 0.000 claims 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims 1
- 239000010452 phosphate Substances 0.000 claims 1
- HEMHJVSKTPXQMS-UHFFFAOYSA-M sodium hydroxide Inorganic materials [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 26
- 238000005517 mercerization Methods 0.000 description 18
- YIWUKEYIRIRTPP-UHFFFAOYSA-N 2-ethylhexan-1-ol Chemical compound CCCCC(CC)CO YIWUKEYIRIRTPP-UHFFFAOYSA-N 0.000 description 13
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical class OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 12
- -1 aliphatic alcohols Chemical class 0.000 description 12
- 239000006260 foam Substances 0.000 description 12
- 239000000203 mixture Substances 0.000 description 11
- 125000004432 carbon atom Chemical group C* 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 238000009736 wetting Methods 0.000 description 9
- 229920000742 Cotton Polymers 0.000 description 8
- 150000003014 phosphoric acid esters Chemical group 0.000 description 8
- 239000003513 alkali Substances 0.000 description 7
- 239000004744 fabric Substances 0.000 description 7
- 238000011084 recovery Methods 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 239000002657 fibrous material Substances 0.000 description 5
- 238000005187 foaming Methods 0.000 description 5
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 4
- 150000001298 alcohols Chemical class 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 229920002678 cellulose Polymers 0.000 description 4
- 235000010980 cellulose Nutrition 0.000 description 4
- 150000002191 fatty alcohols Chemical class 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- ZWRUINPWMLAQRD-UHFFFAOYSA-N nonan-1-ol Chemical compound CCCCCCCCCO ZWRUINPWMLAQRD-UHFFFAOYSA-N 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- BBMCTIGTTCKYKF-UHFFFAOYSA-N 1-heptanol Chemical compound CCCCCCCO BBMCTIGTTCKYKF-UHFFFAOYSA-N 0.000 description 2
- 239000004721 Polyphenylene oxide Substances 0.000 description 2
- 229920000297 Rayon Polymers 0.000 description 2
- BNMJSBUIDQYHIN-UHFFFAOYSA-N butyl dihydrogen phosphate Chemical compound CCCCOP(O)(O)=O BNMJSBUIDQYHIN-UHFFFAOYSA-N 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 239000007859 condensation product Substances 0.000 description 2
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Chemical compound CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 2
- WMFOQBRAJBCJND-UHFFFAOYSA-M lithium hydroxide Inorganic materials [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 239000002964 rayon Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- KWMLJOLKUYYJFJ-GASJEMHNSA-N (2xi)-D-gluco-heptonic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C(O)=O KWMLJOLKUYYJFJ-GASJEMHNSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- HLZKNKRTKFSKGZ-UHFFFAOYSA-N 1-Tetradecanol Natural products CCCCCCCCCCCCCCO HLZKNKRTKFSKGZ-UHFFFAOYSA-N 0.000 description 1
- AEQDJSLRWYMAQI-UHFFFAOYSA-N 2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline Chemical compound C1CN2CC(C(=C(OC)C=C3)OC)=C3CC2C2=C1C=C(OC)C(OC)=C2 AEQDJSLRWYMAQI-UHFFFAOYSA-N 0.000 description 1
- PQSMEVPHTJECDZ-UHFFFAOYSA-N 2,3-dimethylheptan-2-ol Chemical compound CCCCC(C)C(C)(C)O PQSMEVPHTJECDZ-UHFFFAOYSA-N 0.000 description 1
- MHGOKSLTIUHUBF-UHFFFAOYSA-N 2-ethylhexyl sulfate Chemical compound CCCCC(CC)COS(O)(=O)=O MHGOKSLTIUHUBF-UHFFFAOYSA-N 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000012766 Cannabis sativa ssp. sativa var. sativa Nutrition 0.000 description 1
- 235000012765 Cannabis sativa ssp. sativa var. spontanea Nutrition 0.000 description 1
- 229920003043 Cellulose fiber Polymers 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 1
- 240000006240 Linum usitatissimum Species 0.000 description 1
- 235000004431 Linum usitatissimum Nutrition 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- HRKAMJBPFPHCSD-UHFFFAOYSA-N Tri-isobutylphosphate Chemical compound CC(C)COP(=O)(OCC(C)C)OCC(C)C HRKAMJBPFPHCSD-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000009120 camo Nutrition 0.000 description 1
- 235000005607 chanvre indien Nutrition 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 238000010411 cooking Methods 0.000 description 1
- WKUTWFJCICCFBL-UHFFFAOYSA-L disodium;2-methylpropyl phosphate Chemical compound [Na+].[Na+].CC(C)COP([O-])([O-])=O WKUTWFJCICCFBL-UHFFFAOYSA-L 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 238000007730 finishing process Methods 0.000 description 1
- 239000011487 hemp Substances 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N n-hexadecyl alcohol Natural products CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N n-octadecyl alcohol Natural products CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940055577 oleyl alcohol Drugs 0.000 description 1
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 150000003839 salts Chemical group 0.000 description 1
- ZJFVCVZCBZUNLZ-UHFFFAOYSA-M sodium bis(2-ethoxyethyl) phosphate Chemical compound [Na+].P(=O)(OCCOCC)(OCCOCC)[O-] ZJFVCVZCBZUNLZ-UHFFFAOYSA-M 0.000 description 1
- 239000000176 sodium gluconate Substances 0.000 description 1
- 235000012207 sodium gluconate Nutrition 0.000 description 1
- 229940005574 sodium gluconate Drugs 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 239000004753 textile Substances 0.000 description 1
- SJAUZDFPAWWLCG-UHFFFAOYSA-N tris(2-ethoxyethyl) phosphate Chemical compound CCOCCOP(=O)(OCCOCC)OCCOCC SJAUZDFPAWWLCG-UHFFFAOYSA-N 0.000 description 1
- 210000002268 wool Anatomy 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M11/00—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with inorganic substances or complexes thereof; Such treatment combined with mechanical treatment, e.g. mercerising
- D06M11/32—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with inorganic substances or complexes thereof; Such treatment combined with mechanical treatment, e.g. mercerising with oxygen, ozone, ozonides, oxides, hydroxides or percompounds; Salts derived from anions with an amphoteric element-oxygen bond
- D06M11/36—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with inorganic substances or complexes thereof; Such treatment combined with mechanical treatment, e.g. mercerising with oxygen, ozone, ozonides, oxides, hydroxides or percompounds; Salts derived from anions with an amphoteric element-oxygen bond with oxides, hydroxides or mixed oxides; with salts derived from anions with an amphoteric element-oxygen bond
- D06M11/38—Oxides or hydroxides of elements of Groups 1 or 11 of the Periodic Table
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S516/00—Colloid systems and wetting agents; subcombinations thereof; processes of
- Y10S516/01—Wetting, emulsifying, dispersing, or stabilizing agents
- Y10S516/03—Organic sulfoxy compound containing
Definitions
- the present invention relates to wetting agents which can be used in particular as mercerizing aids.
- Mercerization is a common step in the processing of cellulosic fiber materials and concerns the treatment of these materials in aqueous alkaline liquors.
- CH-A-192 832 describes e.g. Sulfuric acid esters of ethylene oxide adducts of aliphatic alcohols, which increase the wetting ability of the mercerizing liquors. According to CH-A-271 087, the wetting capacity of these liquors is increased by a mixture of alkyl sulfuric acids with certain alcohols and ethers. It is also known from DE-A-1 048 865 to use the sulfuric acid esters according to CH-A-192 832 in a mixture with a branched fatty alcohol as a wetting agent in the mercerization of cotton yarn.
- Wetting agents have now been found which, when used in mercerizing liquors, overcome the disadvantages mentioned and are notable for good wetting action and very little foaming.
- the wetting agents according to the invention always contain a C S- C 18 alcohol, for example 2-ethyl-n- hexanol, and optionally a hydrotopizing agent, for example 2,4-dihydroxy-2-methylpentane.
- the wetting agents known from BE-A-698 817 have an inadequate wetting action, which manifests itself in the relatively low shrinking speeds of the cotton.
- the present invention furthermore relates to the use of the wetting agents in the mercerization of cellulosic fiber materials and the mercerizing baths containing the wetting agents.
- component (a) in the wetting agents according to the invention there are sulfuric acid esters of fatty alcohols of the formula into consideration.
- the alkyl radical R 1 in this formula contains 8 or 9 carbon atoms and is derived from octyl or nonyl alcohol.
- sulfuric acid esters of branched isomers of the alcohols mentioned e.g. 2-ethylhexanol or trimethylhexanol.
- the sulfuric acid esters mentioned can be used alone or as a (technical) mixture with one another in the wetting agents according to the invention.
- the phosphoric acid esters of a monoalcohol used as component (b) can be used as monoesters of the formula or as a mixture of the esters of the formulas (2a) and (2b).
- the radicals R 2 contain 4 to 6 carbon atoms and are derived from butyl, amyl and hexyl alcohol.
- the phosphoric acid esters can be used alone or as a (technical) mixture with one another.
- Monobutyl phosphate is particularly suitable.
- component (c) hexanol, heptanol and octyl, nonyl and decyl alcohol, and also lauryl, myristyl, cetyl, stearyl and oleyl alcohol can also be used. These can also be used in the form of condensation products with paraformaldehyde [(CH 2 O) x ].
- Component (c), like components (a) and (b), can be used in the form of (technical) mixtures of the alcohols in question.
- Another component that can be used in combination with the components (a) to (d) mentioned (component (e)) is an adduct of one.
- Organopolysiloxane preferably dimethylpolysiloxane and ethylene or propylene oxide.
- Such adducts can by the likely formula are represented, in which q is 3 to 50, preferably 3 to 25, r 2 or 3, s 0 to 15, t 1 to 25, X1 3 to 10 and R 3 alkyl having 1 to 4 carbon atoms, preferably methyl.
- Such polyether siloxanes are e.g. in DE-B-1 719 238 and in US-A-2 834 748, 3 389 160 and 3 505 377.
- polyether siloxanes that can be used as optional component (s) correspond to the likely formula wherein R 3 and R 4 are each alkyl having 1 to 4 carbon atoms, preferably methyl, a '1 to 20, b' 2 to 20, c '1 to 50, d' 1 or 2, preferably 1, and m 2 to 5 is.
- siloxane compounds are described in DE-B-1 795 557.
- siloxane compounds which have a cloud point of 20 to 70, in particular 25 to 50 ° C.
- SILICONSURFACTANT L 546 @ (trademark) is suitable, for example, as a commercially available component (e) which corresponds to the likely formula (3) and has a cloud point of 32 ° C.
- the wetting agents according to the invention can contain further components, e.g. Contain complexing agent (component (f)).
- component (f) Contain complexing agent
- Suitable components (f) preferably correspond to the formula wherein R 1 and R 2 independently of one another are -CH 2 0H, -CHO or -C0 2 M, M is hydrogen or an alkali metal, preferably sodium, and X1 is 2 to 5.
- Particularly preferred compounds of the formula (5) are hydroxycarboxylic acids of the formula wherein M and X1 have the meanings given, or a lactone of these hydroxycarboxylic acids. Gluconic acid and glucoheptanoic acid or their sodium salts show particularly good results.
- Suitable hydrotroping agents preferably correspond to the formula wherein R 3 and R 4 are independently hydrogen or alkyl having 1 to 4 carbon atoms, especially methyl, and x 2 is 1 to 5.
- X2 1 and R 3 and R 4 are independently hydrogen or methyl.
- a particularly valuable compound is 2-methyl-2,4-pentanediol.
- wetting agents according to the invention which contain both component (f) and component (d) lead to particularly good results.
- the combination of gluconic acid (sodium salt) / 2,4-dihydroxy-2-methylpentane should be mentioned in particular.
- Components (a), (b), (c) and (d) optionally (e) and / or (f) are preferably used in the form of an aqueous solution.
- the aqueous solutions of the novel wetting agent 5-50% of component (a), 0, 6 -30% of component (b), 0.5-5% of component (c), 5-15% of component (d ) and optionally 0.1-1% of component (e) and / or 0.1-5% of component (f).
- wetting agents which contain 25-40% of component (a), 0.5-20% of component (b), 0.5-2% of component (c), 10-15% of the component in aqueous solution (d) and optionally 0.1-0.5% of component (e) and / or 2-4% of component (f).
- Particularly preferred wetting agents contain 30-35% of component (a), 0.5-10% of component (b), 1-1.5% of component (c), 10-15% of component (d) in aqueous solution. , 0.1-0.5% of component (e) and 2-4% of component (f).
- the wetting agents according to the invention are suitable as auxiliaries in finishing processes of cellulose-containing fiber materials. In particular, they prove to be valuable wetting agents in the mercerization of these fiber materials.
- 1-20 preferably 2.5-10 g of the above-mentioned aqueous solutions per liter of mercerizing liquor are used.
- Cellulose fibers for example, have a higher gloss during mercerization. At the same time, their dye absorption capacity and tear resistance are improved.
- the fibers are mixed with concentrated alkalis (approx. 22-28%), e.g. treated aqueous solutions of lithium, sodium or potassium hydroxide or mixtures of these hydroxides.
- concentrated alkalis approximately 22-28%
- the fibers can be subjected to simultaneous stretching, which further increases the gloss effect.
- the temperature of the mercerizing liquors is preferably about 5 to 20 ° C.
- the temperature of the mercerizing liquors is preferably about 5 to 20 ° C.
- the main cellulose-containing fiber materials are cotton and blended fabrics with regenerated celluloses such as Cell wool and rayon (rayon) in question.
- regenerated celluloses such as Cell wool and rayon (rayon) in question.
- the mercerization of blended fabrics made from native (e.g. cotton, as well as hemp and flax) and regenerated celluloses places high demands on the concentration and composition of the mercerizing liquors due to the divergent properties of these components. For this reason, mixed fabrics are almost exclusively dry mercerized, which prevents additional (critical) contact of the mixed fabrics with cooking or mesh baths.
- the mercerization is carried out as yarn or piece mercerization.
- a detailed description of these processes as well as the mercerization in general is e.g. in Lindner, Textile auxiliaries and washing raw materials,ticianliche Verlagsgesellschaft, Stuttgart, 1964, Volume 2, page 1474 ff.
- wetting agents according to the invention in particular soche, which contain components (a) to (5), also enable quick and trouble-free recovery of the alkali from the (mercerizing) washing baths following the mercerizing bath.
- soche which contain components (a) to (5)
- wetting agents according to the invention also enable quick and trouble-free recovery of the alkali from the (mercerizing) washing baths following the mercerizing bath.
- these wetting agents have an extremely low tendency to foam.
- the concentrated liquor obtained can then be reused in the mercerizing bath.
- the wetting agents according to the invention are thus at least on a par with the prior art with regard to their wetting action, but also had the great advantage that they e.g. with the lye recovery mentioned hardly tend to foam.
- a cotton strand (weight: 1 g, length 24 cm), weighted with a weight of 33 g, is immersed in the filled cylinder and then the change in length of the strand is measured at equidistant time intervals.
- the rate of shrinkage that can be determined from this is a measure of the effectiveness of the wetting agents used in the mercerizing liquor.
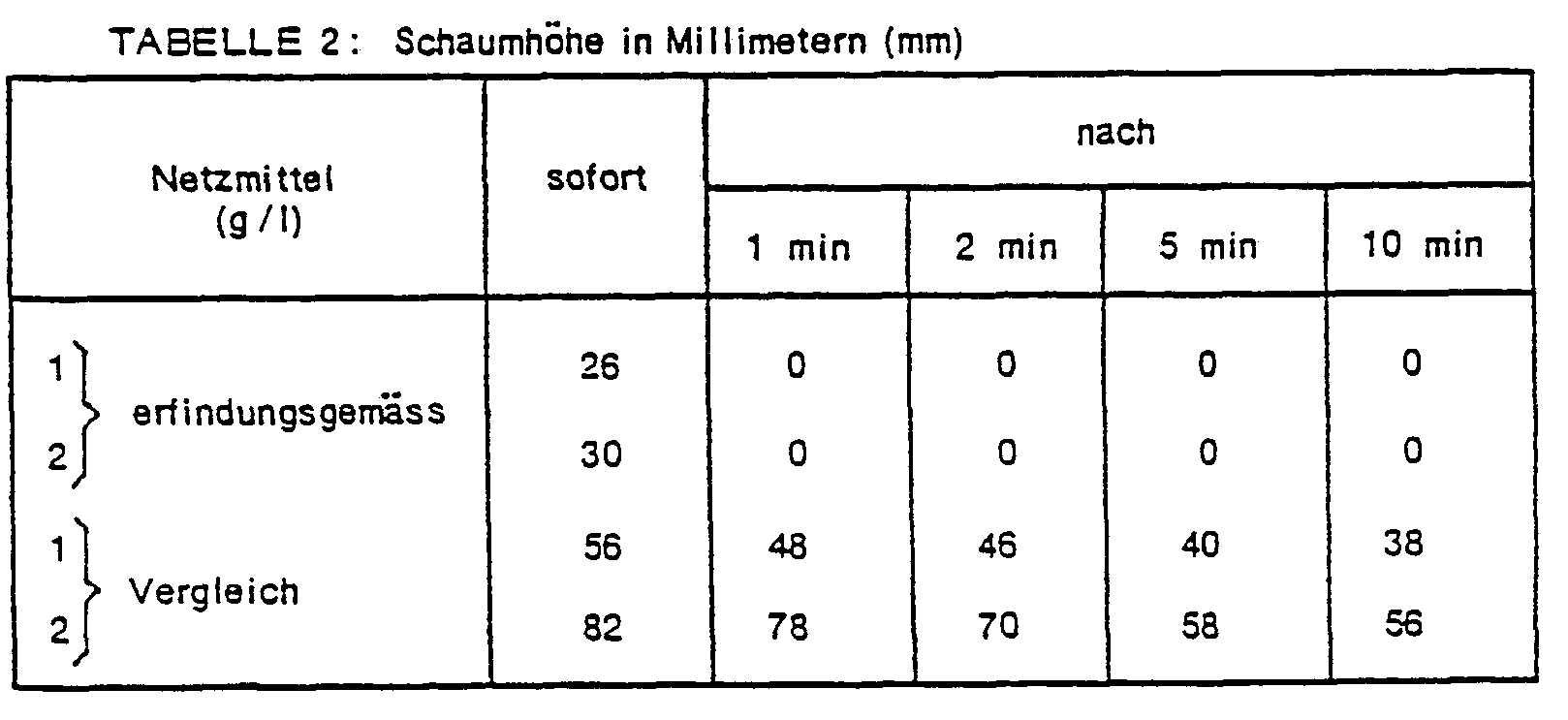
- This example shows the foam behavior of the mercerizing liquors in the application using the glass cylinder shake test.
- the wetting agent according to the invention completely suppresses foam formation.
- the foam development in the lye recovery is significantly reduced by the wetting agent according to the invention compared to the comparison wetting agent.
- This example shows the effectiveness of the wetting agents according to the invention in strongly alkaline mercerizing liquors.
- Desized cotton fabric (square meter weight 180 g) is attached to a pin frame without tension and immersed for 1 minute in a mercerizing liquor containing 311.9 g sodium hydroxide solution per liter (which corresponds to a 25% solution) and 6 g of a wetting agent from (a) 80 % of a 40% aqueous solution of 2-ethylhexyl sulfate (sodium salt), (b) 3% of a 50% aqueous solution of monobutyl phosphate, (c) 1.3% 2-ethyl-n-hexanol, (e) 0.5 % Siloconsurfactant L 546 "", (f) 3.5% sodium gluconate and (d) 11.7% 2,4-dihydroxy-2-methylpentane.
- the temperature of the mercerizing liquor is 18 ° C.
- the mixture is then leached with hot water (70 ° C.) for 1 minute and treated with cold water which contains 5 ml / l of acetic acid (40%).
- the fabric is then rinsed, squeezed off on a padder and dried in a drying cabinet at 100%. The gloss and the dyeability of the fabric are significantly improved.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Chemical Or Physical Treatment Of Fibers (AREA)
Description
Die vorliegende Erfindung betrifft Netzmittel, die insbesondere als Mercerisierhilfsmittel verwendet werden können.The present invention relates to wetting agents which can be used in particular as mercerizing aids.
Die Mercerisation ist ein üblicher Schritt bei der Verarbeitung von cellulosehaltigen Fasermaterialien und betrifft die Behandlung dieser Materialien in wässrigen alkalischen Flotten.Mercerization is a common step in the processing of cellulosic fiber materials and concerns the treatment of these materials in aqueous alkaline liquors.
Für die Mercerisation geeignete Netzmittel sind schon seit langem bekannt. CH-A-192 832 beschreibtz.B. Schwefelsäureester von Aethylenoxidaddukten aliphatischer Alkohole, die die Netzfähigkeit der Mercerisierflotten erhöhen. Gemäss CH-A-271 087 wird das Netzvermögen dieser Flotten durch eine Mischung von Alkylschwefelsäuren mit gewissen Alkoholen und Aethern erhöht. Ferner ist aus DE-A-1 048 865 bekannt, die Schwefelsäureester gemäss CH-A-192 832 im Gemisch mit einem verzweigten Fettalkohol als Netzmittel bei der Mercerisation von Baumwollgarn einzusetzen.Wetting agents suitable for mercerization have been known for a long time. CH-A-192 832 describes e.g. Sulfuric acid esters of ethylene oxide adducts of aliphatic alcohols, which increase the wetting ability of the mercerizing liquors. According to CH-A-271 087, the wetting capacity of these liquors is increased by a mixture of alkyl sulfuric acids with certain alcohols and ethers. It is also known from DE-A-1 048 865 to use the sulfuric acid esters according to CH-A-192 832 in a mixture with a branched fatty alcohol as a wetting agent in the mercerization of cotton yarn.
Die diese Netzmittel enthaltenden strak alkalischen Mercerisierflotten besitzen jedoch ein in vielen Fällen noch unzureichendes Netzvermögen. Dazu kommt, dass insbesondere bei tieferen Temperaturen und hohen Laugenkonzentrationen diese Flotten zur Gelbildung neigen können. Ihr grösster Nachteil ist aber in der z.T. sehr starken Schaumbildung während der Mercerisation zu sehen, was das Arbeiten insbesondere auf Garnmercerisiermaschinen sehr beeinträchtigen kann. Ferner stört die Bildung voluminöser Schaumschichten auch die Aufkonzentrierung und Rückgewinnung der Alkalilauge aus den auf das Mercerisierbad folgenden Waschbädern.However, the strictly alkaline mercerizing liquors containing these wetting agents have in many cases still insufficient wetting capacity. In addition, these liquors can tend to gel, especially at lower temperatures and high alkali concentrations. Your biggest disadvantage is in part very strong foam formation can be seen during the mercerization, which can impair working particularly on yarn mercerization machines. Furthermore, the formation of voluminous foam layers also interferes with the concentration and recovery of the alkali lye from the wash baths following the mercerizing bath.
Unzureichende Netzwirkung und insbesondere zu starke Schaumbilding sind Nachteile, die sich vor allem in der Garnmercerisation und bei den modernen, schnell arbeitenden Mercerisiermaschinen auf drastische Weise bemerkbar machen. Da die zu mercerisierende Ware mit hoher Geschwindigkeit durch die Flotte bewegt wird, muss sie innerhalb einer kürzeren Zeit benetzt werden, als das bei langsamer arbeitenden Maschinen der Fall ist. Die höhere Durchlaufgeschwindigkeit des zu mercerisierenden Materials gibt in der Regel Anlass zu vermehrter Schaumbilding auf der Flotte. Bei der Garnmercerisation verhindert dies nicht nur die schnelle, sondern auch die gleichmässige Benetzung des Garns. Gleichzeitig steigt die Konzentration an Mercerisierlauge in den der Mercerisierflotte nachfolgenden Waschbädern schneller an. Dies bedeutet, dass bei kontinuierlicher Arbeitsweise pro Zeiteinheit eine grössere Menge an Lauge aus diesen Waschbädern zurückgewonnen werden muss. Die Durchführung sowohl der Mercerisation als auch der Laugenrückgewinnung innherhalb einer kürzeren Zeit ist aber nur dann möglich, wenn sich die Schaumbildung in Grenzen hält und das verwendete Netzmittel eine gute Netzwirkung besitzt.Inadequate wetting and, in particular, excessive foaming are disadvantages that are particularly noticeable in yarn mercerization and in modern, fast-working mercerizing machines. Since the goods to be mercerized are moved through the fleet at high speed, they have to be wetted in a shorter time than is the case with slower machines. The higher throughput speed of the material to be mercerized usually gives rise to increased foaming on the liquor. In yarn mercerization, this not only prevents the yarn from wetting quickly, but also evenly. At the same time, the concentration of mercerizing liquor in the wash baths following the mercerizing liquor increases more rapidly. This means that with continuous operation, a larger amount of lye must be recovered from these wash baths per unit of time. However, it is only possible to carry out both the mercerization and the alkali recovery within a shorter time if the foam formation is limited and the wetting agent used has a good wetting effect.
Es wurden nun Netzmittel gefunden, die, in Mercerisierflotten eingesetzt, die genannten Nachteile überwinden und sich durch eine gute Netwirkung und sehr geringe Schaumbilding auszeichnen.Wetting agents have now been found which, when used in mercerizing liquors, overcome the disadvantages mentioned and are notable for good wetting action and very little foaming.
Insbesondere offenbart BE-A-698 817 schaumarme Netzmittel zur Verwendung als Mercerisiermittel, die
- mindestens ein anionisches Tensid auf der Basis von Sulfaten oder Sulfonaten, vorzugsweise sulfonierte, aliphatische Kohlenwasserstoffe mit 6-18 Kohlenstoffatomen, z.B. Kokosfett-Dinatriumsulfonat, Schwefelsäureester eines techischen Octanolgemisches oder des 2-Aethyl-n-hexanols,
- mindestens einen sekundären Phosphorsäureester eines aliphatischen Alkohols in Salzform, z.B. Diisopropytphosphat-Natriumsatz oder Di(ß-äthoxyäthyl)-phosphat-Natriumsalz,
- gegebenenfalls eine tertiären Phosphorsäureester eines aliphatischen Alkohols, z.B. Triisobutylphosphat oder Tri(ß-äthoxyäthyl)-phosphat und
- gegebenenfalls einen primären Phosphorsäureester, z.B. Monoisobutylphosphat-Dinatriumsalz enthalten.
- at least one anionic surfactant based on sulfates or sulfonates, preferably sulfonated, aliphatic hydrocarbons with 6-18 carbon atoms, for example coconut oil disodium sulfonate, sulfuric acid esters of a technical octanol mixture or 2-ethyl-n-hexanol,
- at least one secondary phosphoric acid ester of an aliphatic alcohol in salt form, for example sodium diisopropyphosphate or di (β-ethoxyethyl) phosphate sodium salt,
- optionally a tertiary phosphoric acid ester of an aliphatic alcohol, for example triisobutyl phosphate or tri (β-ethoxyethyl) phosphate and
- optionally contain a primary phosphoric acid ester, for example monoisobutyl phosphate disodium salt.
Im Gegensatz dazu enthalten die erfindungsgemässen Netzmittel neben einem Schwefelsäureester eines Fettalkohols, z.B. des 2-Aethyl-n-hexanols, und einem Phosphorsäureester eines Monoalkohols, z.B. des Butanols oder Isobutanols stets einen CS-C18 Alkohol, z.B. 2-Aethyl-n-hexanol, und gegebenenfalls ein Hydrotopierungsmittel, z.B. 2,4-Dihydroxy-2-methyl-pentan.In contrast, in addition to a sulfuric acid ester of a fatty alcohol, for example 2-ethyl-n-hexanol, and a phosphoric acid ester of a monoalcohol, for example butanol or isobutanol, the wetting agents according to the invention always contain a C S- C 18 alcohol, for example 2-ethyl-n- hexanol, and optionally a hydrotopizing agent, for example 2,4-dihydroxy-2-methylpentane.
Die aus BE-A-698 817 bekannten Netzmittel weisen eine unzureichende Netzwirkung auf, die sich in relativ niederen Schrumpfgeschwindigkeiten der Baumwolle äussert.The wetting agents known from BE-A-698 817 have an inadequate wetting action, which manifests itself in the relatively low shrinking speeds of the cotton.
Gegenstand der vorliegenden Erfindung sind daher Netzmittel, die
- (a) Schwefelsäureester eines Fettalkohols mit 8 oder 9 Kohlenstoffatomen,
- (b) Phosphorsäureester eines Monoalkohols mit 4 bis 6 Kohlenstoffatomen,
- (c) Alkohole mit 6 bis 18 Kohlenstoffatomen oder deren Kondensationsprodukte mit Paraformaldehyd und gegebenenfalls
- (d) ein Hydrotropierungsmittel enthalten.
- (a) sulfuric acid esters of a fatty alcohol with 8 or 9 carbon atoms,
- (b) phosphoric acid esters of a monoalcohol with 4 to 6 carbon atoms,
- (c) alcohols with 6 to 18 carbon atoms or their condensation products with paraformaldehyde and optionally
- (d) contain a hydrotroping agent.
Gegenstand der vorliegenden Erfindung ist ferner die Verwendung der Netzmittel bei der Mercerisation cellulosehlatiger Fasermaterialien sowie die die Netzmittel enthaltenden Mercerisierbäder.The present invention furthermore relates to the use of the wetting agents in the mercerization of cellulosic fiber materials and the mercerizing baths containing the wetting agents.
Als Komponente (a) in den erfindungsgemässen Netzmitteln kommen Schwefelsäureester von Fettalkoholen der Formel
Besonders gute Ergebnisse werden mit den Schwefelsäureestern von verzweigten Isomeren der genannten Alkohole erzielt, wie z.B. 2-Aethylhexanol oder Trimethylhexanol. Die genannten Schwefelsäureester können allein ode als (technisches) Gemisch untereinander in den erfindungsgemässen Netzmitteln eingesetzt werden.Particularly good results are achieved with the sulfuric acid esters of branched isomers of the alcohols mentioned, e.g. 2-ethylhexanol or trimethylhexanol. The sulfuric acid esters mentioned can be used alone or as a (technical) mixture with one another in the wetting agents according to the invention.
Die als Komponente (b) eingesetzten Phosphorsäureester eines Monoalkohols können als Monoester der Formel
Darin enthalten die Reste R2 4 bis 6 Kohlenstoffatome und leiten sich vom Butyl-, Amyl- und Hexylalkohol ab. Die Phosphorsäureester können allein oder auch als (technisches) Gemisch untereinander verwendet werden. Besonders geeignet ist Monobutylphosphat.The radicals R 2 contain 4 to 6 carbon atoms and are derived from butyl, amyl and hexyl alcohol. The phosphoric acid esters can be used alone or as a (technical) mixture with one another. Monobutyl phosphate is particularly suitable.
Als Komponente (c) können Hexanol, Heptanol sowie Octyl-, Nonyl- und Decylalkohol ferner auch Lauryl-, Myristyl-, Cetyl-, Stearyl- und Oleyl-alkohol verwendet werden. Diese können auch in Form von Kondensationsprodukten mit Paraformaldehyd [(CH2O)x] verwendet werden. Die Komponente (c) kann wie die Komponenten (a) und (b) in Form von (technischen) Gemischen der in Frage kommenden Alkohole zur Anwendung kommen.As component (c), hexanol, heptanol and octyl, nonyl and decyl alcohol, and also lauryl, myristyl, cetyl, stearyl and oleyl alcohol can also be used. These can also be used in the form of condensation products with paraformaldehyde [(CH 2 O) x ]. Component (c), like components (a) and (b), can be used in the form of (technical) mixtures of the alcohols in question.
Eine weitere Komponente, die in Kombination mit den genannten Komponenten (a) bis (d) verwendet werden kann (Komponente (e)), ist ein Addukt aus einem. Organopolysiloxan, vorzugsweise Dimethylpolysiloxan und Aethylen- oder Propylenoxid. Solche Addukte können durch die wahrscheinliche Formel
Derartige Polyäthersiloxane sind z.B. in DE-B-1 719 238 sowie in US-A-2 834 748, 3 389 160 und 3 505 377 beschrieben.Such polyether siloxanes are e.g. in DE-B-1 719 238 and in US-A-2 834 748, 3 389 160 and 3 505 377.
Weitere Polyäthersiloxane, welche als fakultative Komponente (e) verwendet werden können, entsprechen der wahrscheinlichen Formel
Derartige Siloxanverbindungen snd in DE-B-1 795 557 beschrieben.Such siloxane compounds are described in DE-B-1 795 557.
Bevorzugt sind nun solche Siloxanverbindungen, die einen Trübungspunkt von 20 bis 70, insbesondere von 25 bis 50°C aufweisen.Preference is now given to those siloxane compounds which have a cloud point of 20 to 70, in particular 25 to 50 ° C.
Als handelsübliche Komponente (e), welche der wahrscheinlichen Formel (3) entspricht, und einen Trübungspunkt von 32°C aufweist, eignet sich z.B. SILICONSURFACTANT L 546@ (Handelsmarke).SILICONSURFACTANT L 546 @ (trademark) is suitable, for example, as a commercially available component (e) which corresponds to the likely formula (3) and has a cloud point of 32 ° C.
Die erfindungsgemässen Netzmittel können weitere Komponenten, wie z.B. Komplexierungsmittel (Komponente (f)) enthalten.The wetting agents according to the invention can contain further components, e.g. Contain complexing agent (component (f)).
Geeignete Komponenten (f) entsprechen vorzugsweise der Formel
Besonders bevorzugte Verbindungen der Formel (5) sind Hydroxycarbonsäuren der Formel
Geeignete Hydrotropierungsmittel (Komponente (d)) ensprechen vorzugsweise der Formel
In bevorzugten Verbindungen der Formel (7) ist X2 1 und R3 und R4 unabhängig voneinander Wasserstoff oder Methyl. Als besonders wertvolle Verbindung ist z.B. das 2-Methyl-2,4-pentandiol zu nennen.In preferred compounds of formula (7) X2 1 and R 3 and R 4 are independently hydrogen or methyl. A particularly valuable compound is 2-methyl-2,4-pentanediol.
Erfindungsgemässe Netzmittel, die sowohl die Komponente (f) als auch die Komponente (d) enthalten, führen zu besonders guten Resultaten. Insbesondere ist in diesem Zusammenhang die Kombination Gluconsäure (Natriumsalz)/2,4-Dihydroxy-2-methyl-pentan zu erwähnen.Wetting agents according to the invention which contain both component (f) and component (d) lead to particularly good results. In this context, the combination of gluconic acid (sodium salt) / 2,4-dihydroxy-2-methylpentane should be mentioned in particular.
Die Komponenten (a), (b), (c) und (d) gegebenenfalls (e) und/oder (f) werden vorzugsweise in Form einer wässrigen Lösung verwendet.Components (a), (b), (c) and (d) optionally (e) and / or (f) are preferably used in the form of an aqueous solution.
Vorzugsweise enthalten die wässrigen Lösungen der erfindungsgemässen Netzmittel 5-50% der Komponente (a), 0,6―30% der Komponente (b), 0,5-5% der Komponente (c), 5-15% der Komponente (d) und gegebenenfalls 0,1-1% der Komponente (e) und/oder 0,1-5% der Komponente (f).Preferably, the aqueous solutions of the novel wetting agent 5-50% of component (a), 0, 6 -30% of component (b), 0.5-5% of component (c), 5-15% of component (d ) and optionally 0.1-1% of component (e) and / or 0.1-5% of component (f).
Sehr geeignet sind solche Netzmittel, die in wässriger Lösung 25-40% der Komponente (a), 0,5-20% der Komponente (b), 0,5-2% der Komponente (c), 10-15% der Komponente (d) und gegebenenfalls 0,1-0,5% der Komponente (e) und/oder 2-4% der Komponente (f) enthalten.Very suitable are those wetting agents which contain 25-40% of component (a), 0.5-20% of component (b), 0.5-2% of component (c), 10-15% of the component in aqueous solution (d) and optionally 0.1-0.5% of component (e) and / or 2-4% of component (f).
Besonders bevorzugte Netzmittel enthalten in wässriger Lösung 30-35% der Komponente (a), 0,5-10% der Komponente (b), 1-1,5% der Komponente (c), 10-15% der Komponente (d), 0,1-0,5% der Komponente (e) und 2-4% der Komponente (f).Particularly preferred wetting agents contain 30-35% of component (a), 0.5-10% of component (b), 1-1.5% of component (c), 10-15% of component (d) in aqueous solution. , 0.1-0.5% of component (e) and 2-4% of component (f).
Die erfindungsgemässen Netzmittel eignen sich als Hilfsmittel bei Veredlungsprozessen cellulosehaltiger Fasermaterialien. Insbesondere erweisen sie sich als wertvolle Netzmittel bei der Mercerisation dieser Fasermaterialien. Zu diesem Zweck werden 1-20, vorzugsweise 2,5-10 g der oben genannten wässrigen Lösungen pro Liter Mercerisierflotte verwendet.The wetting agents according to the invention are suitable as auxiliaries in finishing processes of cellulose-containing fiber materials. In particular, they prove to be valuable wetting agents in the mercerization of these fiber materials. For this purpose 1-20, preferably 2.5-10 g of the above-mentioned aqueous solutions per liter of mercerizing liquor are used.
Cellulosefasern erhalten bei der Mercerisation beispielsweise einen höheren Glanz. Gleichzeitig werden ihre Farbstoffaufnahmefähigkeit sowie die Reissfestigkeit verbessert. Die Fasern werden dazu mit konzentrierten Laugen (etwa 22-28%), wie z.B. wässrigen Lösungen von Lithium-, Natrium- oder Kaliumhydroxid oder Mischungen dieser Hydroxide behandelt. Dabei können die Fasern einer gleichzeitigen Streckung unterworfen werden, wodurch sich eine weiter Steigerung des Glanzeffektes erreichen lässt.Cellulose fibers, for example, have a higher gloss during mercerization. At the same time, their dye absorption capacity and tear resistance are improved. For this purpose, the fibers are mixed with concentrated alkalis (approx. 22-28%), e.g. treated aqueous solutions of lithium, sodium or potassium hydroxide or mixtures of these hydroxides. The fibers can be subjected to simultaneous stretching, which further increases the gloss effect.
Vorzugsweise beträgt die Temperatur der Mercerisierflotten etwa 5 bis 20°C. Je nachdem, ob die Fasern in trockenem, vorgekochtem oder vorgenetztem Zustand mercerisiert werden, spricht man von der Trocken- oder Nassmercerisation. Besonders die Trockenmercerisation macht verständlicherweise die Verwendung von sehr wirksamen Netzmitteln erforderlich.The temperature of the mercerizing liquors is preferably about 5 to 20 ° C. Depending on whether the fibers are mercerized in the dry, pre-cooked or pre-wetted state, one speaks of the dry or wet mercerization. It is understandable that dry mercerization in particular requires the use of very effective wetting agents.
Als cellulosehaltige Fasermaterialien kommen vor allem Baumwolle und Mischgewebe mit Regeneratcellulosen wie z.B. Zellwollen und Kunstseiden (Reyon) in Frage. Die Mercerisation von Mischgeweben aus nativen (z.B. Baumwolle, sowie Hanf und Flachs) und regenerierten Cellulosen stellt aber auf Grund der auseinanderstrebenden Eigenschaften dieser Komponenten hohe Anforderungen an die Konzentration sowie Zusammensetzung der Mercerisierlaugen. Deshalb wird Mischgewebe fast ausschliesslich trocken mercerisiert, wodurch ein zusätzlicher (kritischer) Kontakt des Mischgewebes mit Koch- bzw. Netzbädern vermieden wird.The main cellulose-containing fiber materials are cotton and blended fabrics with regenerated celluloses such as Cell wool and rayon (rayon) in question. However, the mercerization of blended fabrics made from native (e.g. cotton, as well as hemp and flax) and regenerated celluloses places high demands on the concentration and composition of the mercerizing liquors due to the divergent properties of these components. For this reason, mixed fabrics are almost exclusively dry mercerized, which prevents additional (critical) contact of the mixed fabrics with cooking or mesh baths.
Technisch wird die Mercerisation als Garn- oder Stückmercerisation durchgeführt. Eine detaillierte Beschreibung dieser Verfahren sowie auch der Mercerisation im allgemeinen ist z.B. in Lindner, Textilhilfsmittel und Waschrohstoffe, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1964, Band 2, Seite 1474 ff gegeben.Technically, the mercerization is carried out as yarn or piece mercerization. A detailed description of these processes as well as the mercerization in general is e.g. in Lindner, Textile auxiliaries and washing raw materials, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1964, Volume 2, page 1474 ff.
Erfindungsgemässe Netzmittel, insbesondere soche, welche die Komponente (a) bis (5) enthalten, ermöglichen aber auch eine schnelle und störungsfreie Rückgewinnung de Lauge aus den auf das Mercerisierbad folgenden (Mercerisier-) Waschbädern. Beim Abdestillieren des Wassers aus diesen Bädern, wodurch eine Aufkonzentrierung der Lauge auf den Laugengehalt des Mercerisierbades erreicht wird, ziegen diese Netzmittel eine ausserordentlich geringe Tendenz zur Schaumbildung. Die erhaltene konzentrierte Lauge kann dann im Mercerisierbad wieder verwendet werden.However, wetting agents according to the invention, in particular soche, which contain components (a) to (5), also enable quick and trouble-free recovery of the alkali from the (mercerizing) washing baths following the mercerizing bath. When the water is distilled off from these baths, as a result of which the alkali is concentrated to the alkali content of the mercerizing bath these wetting agents have an extremely low tendency to foam. The concentrated liquor obtained can then be reused in the mercerizing bath.
Die erfindungsgemässen Netzmittel zeigen sich also hinsichtlich ihrer Netzwirkung dem stand der Technik mindestens ebenbürtig, wiesen aber darüber hinaus den grossen Vorteil auf, dass sie z.B. bei der erwähnten Laugenrückgewinnung kaum zur Schaumbilding neigen.The wetting agents according to the invention are thus at least on a par with the prior art with regard to their wetting action, but also had the great advantage that they e.g. with the lye recovery mentioned hardly tend to foam.
Die folgenden Beispiele erläutern die Erfindung, ohne sie darauf zu beschränken. Teile und Prozente beziehen sich auf das Gewicht, sofern nicht anders angegeben.The following examples illustrate the invention without restricting it. Parts and percentages are by weight unless otherwise specified.
In diesem Beispiel wird die Schrumpfgeschwindigkeit von Baumwolle in Mercerisierlaugen gemäss DIN 53987 (Entwurf Juli 1973) bestimmt. Danach wird ein Glaszylinder mit 150 ml einer auf 15°C abgekühlten Natronlauge (24%) gefüllt, die 5 g/I es Netzmittels der nachfolgenden Zusammensetzung enthält:
- (a) 23,75% Schwefelsäureester des 2-Aethyl-n-hexanols,
- (b) 25,0% Phosphorsäureester des Butanols,
- (c) 1,0% 2-Aethylhexanol und
- (e) 0,25% Siliconsurfactant L 546@ (Handelsmarke) sowie 50,0% Wasser.
- (a) 23.75% sulfuric acid ester of 2-ethyl-n-hexanol,
- (b) 25.0% phosphoric acid ester of butanol,
- (c) 1.0% 2-ethylhexanol and
- (e) 0.25% Siliconsurfactant L 546 @ (trademark) and 50.0% water.
Ein Baumwollstrang (Gewicht: 1 g, Länge 24 cm), der mit einem Gewicht von 33 g beschwert ist, wird in den gefüllten Zylinder eingetaucht und dann die Längenänderung des Stranges in äquidistanten Zeitintervallen gemessen. Die daraus ermittelbare Schrumpfgeschwindigkeit ist ein Mass für die Wirksamkeit der in der Mercerisierlauge verwendeten Netzmittel.A cotton strand (weight: 1 g, length 24 cm), weighted with a weight of 33 g, is immersed in the filled cylinder and then the change in length of the strand is measured at equidistant time intervals. The rate of shrinkage that can be determined from this is a measure of the effectiveness of the wetting agents used in the mercerizing liquor.
Zum Vergleich wird eine Mercerisierlauge verwendet, die als Netzmittel nur 5 g/I des Schwefelsäureesters des 2-Aethyl-n-hexanols enthält, und die Schrumpfgeschwindigkeit eines gleichen Baumwollstrangs bestimmt. Die Ergebnisse sind in den Tabellen 1a) und 1b) zusammengefasst.
Durch Verwendung von z.B. 5 g/I des erfindungsgemässen Netzmittels erhält man nach bereits 30 Sekunden eine höhere Schrumpfung als mit dem Vergleichsnetzmittel nach 50 Sekunden.By using e.g. 5 g / l of the wetting agent according to the invention gives a higher shrinkage after only 30 seconds than with the comparison wetting agent after 50 seconds.
Dieses Beispiel zeigt das Schaumverhalten der Mercerisierflotten in der Applikation anhand des Glaszylinderschütteltests.This example shows the foam behavior of the mercerizing liquors in the application using the glass cylinder shake test.
100 ml Natronlauge (24%), die 1 bzw. 2 g/1 de in Beispiel 1 verwendeten Netzmittels enthält, werden in einen Glaszylinder eingefüllt und auf einen Temperatur von 25°C gebracht. Der Glaszylinder wird dann 1 Minute kräftig geschüttelt und die Höhe des entstandenen Schaums nach bestimmten Zeiten gemessen.100 ml of sodium hydroxide solution (24%), which contains 1 or 2 g / 1 de wetting agent used in Example 1, are introduced into a glass cylinder and brought to a temperature of 25 ° C. The glass cylinder is then shaken vigorously for 1 minute and the height of the foam formed is measured after certain times.
Zum Vergleich wird auch das Schaumverhalten einer Mercerisierflotte untersucht, die anstelle des oben verwendeten Netzmittels 1 bzw. 2 g/I des Schwefelsäureesters des 2-Aethyl-n-hexanols enthält. Die Ergebnisse sind aus der Tabelle 2 ersichtlich.
Während das Vergleichsnetzmittel z.T. sehr grosse Schaumhöhen zulässt, unterdrückt das erfindungsgemässe Netzmittel die Schaumbilding vollständig.While the comparison wetting agent partly permits very large foam heights, the wetting agent according to the invention completely suppresses foam formation.
Dieses Beispiel zeigt das Schaumverhalten eines nach der Mercerisierung verwendeten Waschbades bei der Laugenrückgewinnung.
- Aus 500 ml verdünnter Mercerisierlauge (15%), die
- 300 ml Natronlauge (24%)
- 5 g/I Netzmittel gemäss Beispiel 1 und
- 192,5 ml Wasser
- enthält, wird am Rotationsverdampfer bei etwa 10-20 mbar solange Wasser abdestilliert, bis die Laugenkonzentration wieder 24% beträgt. Während der Destillation wird die Höhe des entstandenen Schaums in Abhängigkeit von der Zeit gemessen.
- From 500 ml of diluted mercerizing solution (15%), the
- 300 ml sodium hydroxide solution (24%)
- 5 g / l of wetting agent according to Example 1 and
- 192.5 ml of water
- contains, water is distilled off on a rotary evaporator at about 10-20 mbar until the alkali concentration is again 24%. During the distillation, the amount of foam produced is measured as a function of time.
Zum Vergleich wird dieser Vorgang mit 500 ml Mercerisierlauge, die 5 g/fl des Schwefelsäureesters des 2-Aethyl-n-hexanols als Netzmittel enthält, wiederholt.
Die Schaumentwicklung bei der Laugenrückgewinnung wird durch das erfindungsgemässe Netzmittel gemessen am Vergleichsnetzmittel deutlich herabgesetzt.The foam development in the lye recovery is significantly reduced by the wetting agent according to the invention compared to the comparison wetting agent.
Dieses Beispiel zeigt die Wirksamkeit der erfindungsgemässen Netzmittel in stark alkalischen Mercerisierflotten.This example shows the effectiveness of the wetting agents according to the invention in strongly alkaline mercerizing liquors.
Entschlichtetes Baumwollgewebe (Quadratmetergewicht 180 g) wird ohne Spannung auf einem Nadelrahmen befestigt und während 1 Minute in eine Mercerisierflotte getaucht, die pro Liter 311,9 g Natronlauge (was einer 25%igen Lösung entspricht) und 6 g eines Netzmittels aus (a) 80% einer 40%igen wässrigen Lösung von 2-Aethylhexylsulfat (Natriumsalz), (b) 3% einer 50%igen wässrigen Lösung von Monobutylphosphat, (c) 1,3% 2-Aethyl-n-hexanol, (e) 0,5% Siloconsurfactant L 546'"', (f) 3,5% Natriumgluconat und (d) 11,7% 2,4-Dihydroxy-2-methyl-pentan enthält. Die Temperatur der Mercerisierflotte beträgt 18°C.Desized cotton fabric (square meter weight 180 g) is attached to a pin frame without tension and immersed for 1 minute in a mercerizing liquor containing 311.9 g sodium hydroxide solution per liter (which corresponds to a 25% solution) and 6 g of a wetting agent from (a) 80 % of a 40% aqueous solution of 2-ethylhexyl sulfate (sodium salt), (b) 3% of a 50% aqueous solution of monobutyl phosphate, (c) 1.3% 2-ethyl-n-hexanol, (e) 0.5 % Siloconsurfactant L 546 "", (f) 3.5% sodium gluconate and (d) 11.7% 2,4-dihydroxy-2-methylpentane. The temperature of the mercerizing liquor is 18 ° C.
Anschliessend wird 1 Minute mit heissem Wasser (70°C) entlaugt und mit kaltem Wasser, das 5 ml/I Essigsäure (40%) enthält, behandelt. Das Gewebe wird dann nachgespült, auf einem Foulard abgequetscht und im Trockenschrank bei 100% getrocknet. Der Glanz und die Anfärbbarkeit des Gewebes sind deutlich verbessert.The mixture is then leached with hot water (70 ° C.) for 1 minute and treated with cold water which contains 5 ml / l of acetic acid (40%). The fabric is then rinsed, squeezed off on a padder and dried in a drying cabinet at 100%. The gloss and the dyeability of the fabric are significantly improved.
Claims (12)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH533682 | 1982-09-08 | ||
| CH5336/82 | 1982-09-08 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0102930A1 EP0102930A1 (en) | 1984-03-14 |
| EP0102930B1 EP0102930B1 (en) | 1986-10-15 |
| EP0102930B2 true EP0102930B2 (en) | 1990-09-19 |
Family
ID=4291874
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83810399A Expired - Lifetime EP0102930B2 (en) | 1982-09-08 | 1983-09-02 | Wetting agent and its use as mercerising aid |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US4494952A (en) |
| EP (1) | EP0102930B2 (en) |
| BR (1) | BR8304842A (en) |
| CA (1) | CA1217006A (en) |
| DE (1) | DE3366978D1 (en) |
| ZA (1) | ZA836657B (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4844710A (en) * | 1986-12-08 | 1989-07-04 | Ciba-Geigy Corporation | Aqueous textile assistant of high storage stability and hard water resistance |
| EP0638685B1 (en) * | 1993-08-10 | 1998-12-23 | Ciba SC Holding AG | Welling agent for mercerising |
| ES2182880T3 (en) * | 1994-08-11 | 2003-03-16 | Ciba Sc Holding Ag | COMPOSITIONS OF MULTIFUNCTIONAL TEXTILE AGENTS. |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH192832A (en) * | 1936-03-24 | 1937-09-15 | Chem Fab Vormals Sandoz | Process for increasing the wetting and penetrating effect of alkalizing liquids by at least 15 o Bé. |
| CH271087A (en) | 1948-12-15 | 1950-10-15 | Ciba Geigy | Process for increasing the wetting ability of mercerising liquors. |
| BE495124A (en) * | 1949-04-13 | |||
| BE536296A (en) | 1954-03-22 | |||
| CH345323A (en) * | 1956-04-11 | 1960-03-31 | Ciba Geigy | Preparation for increasing the wetting power of mercerising liquors |
| DE1795557C3 (en) | 1961-05-27 | 1974-10-03 | Th. Goldschmidt Ag, 4300 Essen | Process for the preparation of polyalkyl silicic acid esters with a polymer distribution which approximates the statistical equilibrium. Eliminated from: 1445364 |
| BE666745A (en) | 1964-07-14 | 1966-01-12 | ||
| GB1143206A (en) | 1965-02-08 | 1969-02-19 | Union Carbide Corp | Siloxane-oxyalkylene co-polymers |
| US3449261A (en) * | 1966-02-01 | 1969-06-10 | Fmc Corp | Non-foaming wetting agents |
| US3505377A (en) | 1966-08-12 | 1970-04-07 | Union Carbide Corp | Siloxane-oxyalkylene copolymer foam stabilizers |
| DE1619040B2 (en) * | 1967-02-02 | 1974-01-24 | Farbwerke Hoechst Ag, Vormals Meister Lucius & Bruening, 6000 Frankfurt | Wetting agent for alkaline baths |
| BE698817A (en) * | 1967-05-22 | 1967-11-03 | ||
| DE2231148A1 (en) * | 1972-06-26 | 1974-01-31 | Hoechst Ag | LAUGATING AND MERCERIZING SOLUTIONS |
-
1983
- 1983-08-29 US US06/527,600 patent/US4494952A/en not_active Expired - Lifetime
- 1983-09-02 EP EP83810399A patent/EP0102930B2/en not_active Expired - Lifetime
- 1983-09-02 DE DE8383810399T patent/DE3366978D1/en not_active Expired
- 1983-09-06 CA CA000436122A patent/CA1217006A/en not_active Expired
- 1983-09-06 BR BR8304842A patent/BR8304842A/en not_active IP Right Cessation
- 1983-09-07 ZA ZA836657A patent/ZA836657B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| BR8304842A (en) | 1984-04-24 |
| DE3366978D1 (en) | 1986-11-20 |
| CA1217006A (en) | 1987-01-27 |
| EP0102930A1 (en) | 1984-03-14 |
| EP0102930B1 (en) | 1986-10-15 |
| ZA836657B (en) | 1984-04-25 |
| US4494952A (en) | 1985-01-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE2133898A1 (en) | Process for cleaning textile fabrics | |
| EP0420802B1 (en) | Aqueous, storage stable, low foaming wetting agent | |
| DE2943754A1 (en) | FOAM ABSORBERS AND THEIR USE FOR DEFOAMING AQUEOUS SYSTEMS | |
| DE2641263A1 (en) | DETACHING MEANS AND METHODS FOR CLEANING AND, IF NECESSARY, COLORING TEXTILE MATERIALS | |
| DE2418351A1 (en) | PROCESS AND COMPOSITION FOR TEXTILE BLEACHING | |
| EP0170144B1 (en) | Washing process for sensitive textiles | |
| EP1149945A1 (en) | Composition for the pretreatment of fibrous materials | |
| EP0513571A1 (en) | Improvements for the application by spraying of aqueous treating liquids on textile materials | |
| EP0102930B2 (en) | Wetting agent and its use as mercerising aid | |
| EP0274350B1 (en) | Aqueous storage-stable auxiliary textile agent resistant to hard water | |
| EP0210132B1 (en) | Aqueous, alkaline, silicate-containing composition for the bleaching of cellulosic fibres in the presence of percompounds | |
| EP0589978B1 (en) | Use of special alkyl glycosides as auxiliaries in the pretreatment of textiles | |
| DE1005923B (en) | Process for cleaning, in particular for boiling and boiling, cellulose fiber fibers | |
| DE1245898C2 (en) | Low-foaming, leveling wetting agent | |
| DE2841445A1 (en) | DETERGENTS AND DETERGENTS | |
| EP0112801A1 (en) | Magnesium complexes of oligomeric phosphonic-acid esters, process for their preparation and their use as stabilizers in bleaching baths containing alcaline peroxide | |
| EP0460036B1 (en) | Single-bath degumming and decolorizing of natural silks | |
| DE2326364A1 (en) | SOLVENT BLEACHING PROCESS | |
| EP0205088A2 (en) | Washing process for delicate textiles | |
| DE2113211C3 (en) | Mixture of surfactants | |
| DE2412175C3 (en) | Process for finishing textiles during dry cleaning | |
| DE3230101A1 (en) | Alkali-dry process for natural cellulose fibres and their blends with synthetic fibres | |
| EP0716180A1 (en) | Wetting agent for mercerizing | |
| DE694406C (en) | Process to increase the wetting ability of mercerising liquors | |
| CH671668B5 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19830905 |
|
| AK | Designated contracting states |
Designated state(s): BE CH DE FR GB IT LI |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE FR GB IT LI |
|
| REF | Corresponds to: |
Ref document number: 3366978 Country of ref document: DE Date of ref document: 19861120 |
|
| ITF | It: translation for a ep patent filed | ||
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: HUELS AKTIENGESELLSCHAFT Effective date: 19870703 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 19900919 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): BE CH DE FR GB IT LI |
|
| ITF | It: translation for a ep patent filed | ||
| EN3 | Fr: translation not filed ** decision concerning opposition | ||
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: BR |
|
| ITTA | It: last paid annual fee | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PUE Owner name: CIBA-GEIGY AG TRANSFER- CIBA SC HOLDING AG |
|
| BECN | Be: change of holder's name |
Effective date: 19961129 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Free format text: CIBA SC HOLDING AG TRANSFER- CIBA SPECIALTY CHEMICALS HOLDING INC. |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20010727 Year of fee payment: 19 Ref country code: CH Payment date: 20010727 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20010810 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20010823 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20011119 Year of fee payment: 19 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020902 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020930 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020930 |
|
| BERE | Be: lapsed |
Owner name: *CIBA SPECIALTY CHEMICALS HOLDING INC. Effective date: 20020930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030401 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020902 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030603 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |