CN116425607A - 一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 - Google Patents
一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 Download PDFInfo
- Publication number
- CN116425607A CN116425607A CN202310060760.0A CN202310060760A CN116425607A CN 116425607 A CN116425607 A CN 116425607A CN 202310060760 A CN202310060760 A CN 202310060760A CN 116425607 A CN116425607 A CN 116425607A
- Authority
- CN
- China
- Prior art keywords
- trans
- alkene
- cis
- chromium
- intermediate alkyne
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- -1 trans-form alkene Chemical class 0.000 title claims abstract description 28
- 238000000034 method Methods 0.000 title claims abstract description 27
- 150000001345 alkine derivatives Chemical class 0.000 title claims abstract description 21
- 238000005984 hydrogenation reaction Methods 0.000 title claims description 4
- 238000006243 chemical reaction Methods 0.000 claims abstract description 32
- 239000003054 catalyst Substances 0.000 claims abstract description 23
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims abstract description 20
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 16
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 15
- 239000001257 hydrogen Substances 0.000 claims abstract description 15
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 12
- 239000002808 molecular sieve Substances 0.000 claims abstract description 12
- PNIOSOLFWMPMMJ-UHFFFAOYSA-N N[Cr]=C Chemical compound N[Cr]=C PNIOSOLFWMPMMJ-UHFFFAOYSA-N 0.000 claims abstract description 11
- 125000004122 cyclic group Chemical group 0.000 claims abstract description 11
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 claims abstract description 11
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims abstract description 10
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims abstract description 9
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims abstract description 9
- 229910052804 chromium Inorganic materials 0.000 claims abstract description 9
- 239000011651 chromium Substances 0.000 claims abstract description 9
- 239000011777 magnesium Substances 0.000 claims abstract description 9
- 229910052749 magnesium Inorganic materials 0.000 claims abstract description 9
- 239000002904 solvent Substances 0.000 claims abstract description 8
- 239000005046 Chlorosilane Substances 0.000 claims abstract description 7
- KOPOQZFJUQMUML-UHFFFAOYSA-N chlorosilane Chemical compound Cl[SiH3] KOPOQZFJUQMUML-UHFFFAOYSA-N 0.000 claims abstract description 7
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical group C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 claims description 17
- 230000002194 synthesizing effect Effects 0.000 claims description 12
- 239000005051 trimethylchlorosilane Substances 0.000 claims description 8
- DCFKHNIGBAHNSS-UHFFFAOYSA-N chloro(triethyl)silane Chemical compound CC[Si](Cl)(CC)CC DCFKHNIGBAHNSS-UHFFFAOYSA-N 0.000 claims description 6
- 125000004076 pyridyl group Chemical group 0.000 claims description 4
- 125000001544 thienyl group Chemical group 0.000 claims description 4
- BMQDAIUNAGXSKR-UHFFFAOYSA-N (3-hydroxy-2,3-dimethylbutan-2-yl)oxyboronic acid Chemical compound CC(C)(O)C(C)(C)OB(O)O BMQDAIUNAGXSKR-UHFFFAOYSA-N 0.000 claims description 3
- 125000003118 aryl group Chemical group 0.000 claims description 3
- 125000002541 furyl group Chemical group 0.000 claims description 3
- 150000002431 hydrogen Chemical class 0.000 claims description 3
- 238000003756 stirring Methods 0.000 claims description 3
- JSQJUDVTRRCSRU-UHFFFAOYSA-N tributyl(chloro)silane Chemical compound CCCC[Si](Cl)(CCCC)CCCC JSQJUDVTRRCSRU-UHFFFAOYSA-N 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 2
- 125000003342 alkenyl group Chemical group 0.000 claims description 2
- 125000003545 alkoxy group Chemical group 0.000 claims description 2
- 150000005840 aryl radicals Chemical class 0.000 claims description 2
- 229910052799 carbon Inorganic materials 0.000 claims description 2
- 150000002148 esters Chemical class 0.000 claims description 2
- 150000008282 halocarbons Chemical class 0.000 claims description 2
- 229910052710 silicon Inorganic materials 0.000 claims description 2
- 239000010703 silicon Substances 0.000 claims description 2
- 125000004426 substituted alkynyl group Chemical group 0.000 claims description 2
- 125000003368 amide group Chemical group 0.000 claims 1
- 229910052751 metal Inorganic materials 0.000 abstract description 7
- 239000002184 metal Substances 0.000 abstract description 7
- 239000000758 substrate Substances 0.000 abstract description 6
- 238000006555 catalytic reaction Methods 0.000 abstract description 3
- 239000012298 atmosphere Substances 0.000 abstract description 2
- 239000000654 additive Substances 0.000 abstract 1
- 239000003638 chemical reducing agent Substances 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- JRXXLCKWQFKACW-UHFFFAOYSA-N biphenylacetylene Chemical group C1=CC=CC=C1C#CC1=CC=CC=C1 JRXXLCKWQFKACW-UHFFFAOYSA-N 0.000 description 26
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 18
- 230000015572 biosynthetic process Effects 0.000 description 9
- 238000003786 synthesis reaction Methods 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- BJIOGJUNALELMI-ONEGZZNKSA-N trans-isoeugenol Chemical compound COC1=CC(\C=C\C)=CC=C1O BJIOGJUNALELMI-ONEGZZNKSA-N 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 238000010898 silica gel chromatography Methods 0.000 description 4
- BJIOGJUNALELMI-UHFFFAOYSA-N trans-isoeugenol Natural products COC1=CC(C=CC)=CC=C1O BJIOGJUNALELMI-UHFFFAOYSA-N 0.000 description 4
- PJANXHGTPQOBST-QXMHVHEDSA-N cis-stilbene Chemical compound C=1C=CC=CC=1/C=C\C1=CC=CC=C1 PJANXHGTPQOBST-QXMHVHEDSA-N 0.000 description 3
- 150000002391 heterocyclic compounds Chemical class 0.000 description 3
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 3
- RUVINXPYWBROJD-ONEGZZNKSA-N trans-anethole Chemical compound COC1=CC=C(\C=C\C)C=C1 RUVINXPYWBROJD-ONEGZZNKSA-N 0.000 description 3
- QVJGHZTXDKQLRT-UHFFFAOYSA-N 1-methoxy-4-prop-1-ynylbenzene Chemical compound COC1=CC=C(C#CC)C=C1 QVJGHZTXDKQLRT-UHFFFAOYSA-N 0.000 description 2
- BJEHEPQJTRPTDU-UHFFFAOYSA-N 2-methoxy-4-prop-1-ynylphenol Chemical compound COC1=CC(C#CC)=CC=C1O BJEHEPQJTRPTDU-UHFFFAOYSA-N 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 239000003480 eluent Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000011259 mixed solution Substances 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- DZGHBGLILAEHOR-QXMHVHEDSA-N (z)-dodec-6-ene Chemical compound CCCCC\C=C/CCCCC DZGHBGLILAEHOR-QXMHVHEDSA-N 0.000 description 1
- GDHNBPHYVRHYCC-PLNGDYQASA-N 1,3-dimethoxy-5-[(z)-2-(4-methoxyphenyl)ethenyl]benzene Chemical compound C1=CC(OC)=CC=C1\C=C/C1=CC(OC)=CC(OC)=C1 GDHNBPHYVRHYCC-PLNGDYQASA-N 0.000 description 1
- GOPIJZBYBMAVGB-UHFFFAOYSA-N 1,3-dimethoxy-5-[2-(4-methoxyphenyl)ethynyl]benzene Chemical group C1=CC(OC)=CC=C1C#CC1=CC(OC)=CC(OC)=C1 GOPIJZBYBMAVGB-UHFFFAOYSA-N 0.000 description 1
- YMZAAGCDWVIPNL-UHFFFAOYSA-N 1-chloro-4-(2-phenylethynyl)benzene Chemical compound C1=CC(Cl)=CC=C1C#CC1=CC=CC=C1 YMZAAGCDWVIPNL-UHFFFAOYSA-N 0.000 description 1
- TTYKTMUIQGPMMH-SREVYHEPSA-N 1-chloro-4-[(z)-2-phenylethenyl]benzene Chemical compound C1=CC(Cl)=CC=C1\C=C/C1=CC=CC=C1 TTYKTMUIQGPMMH-SREVYHEPSA-N 0.000 description 1
- MUOZIRLLUPEXED-UHFFFAOYSA-N 2-hex-1-ynylnaphthalene Chemical compound C1=CC=CC2=CC(C#CCCCC)=CC=C21 MUOZIRLLUPEXED-UHFFFAOYSA-N 0.000 description 1
- BOKCJGOOHNNDCL-UHFFFAOYSA-N 3-(2-phenylethynyl)aniline Chemical compound NC1=CC=CC(C#CC=2C=CC=CC=2)=C1 BOKCJGOOHNNDCL-UHFFFAOYSA-N 0.000 description 1
- ONMWLTAZXNKXQX-UHFFFAOYSA-N 3-(2-phenylethynyl)phenol Chemical compound OC1=CC=CC(C#CC=2C=CC=CC=2)=C1 ONMWLTAZXNKXQX-UHFFFAOYSA-N 0.000 description 1
- ZLYFXWKSLUXFMU-UHFFFAOYSA-N 3-(2-phenylethynyl)pyridine Chemical compound C1=CC=CC=C1C#CC1=CC=CN=C1 ZLYFXWKSLUXFMU-UHFFFAOYSA-N 0.000 description 1
- CVWNFWSBDGLRPO-UHFFFAOYSA-N 3-(2-phenylethynyl)thiophene Chemical compound S1C=CC(C#CC=2C=CC=CC=2)=C1 CVWNFWSBDGLRPO-UHFFFAOYSA-N 0.000 description 1
- STNDIHAVRCIBCC-KTKRTIGZSA-N 3-[(Z)-2-phenylethenyl]aniline Chemical compound NC=1C=C(\C=C/C2=CC=CC=C2)C=CC=1 STNDIHAVRCIBCC-KTKRTIGZSA-N 0.000 description 1
- XBHJTSIYYWRJFQ-MDZDMXLPSA-N 3-[(e)-2-phenylethenyl]phenol Chemical compound OC1=CC=CC(\C=C\C=2C=CC=CC=2)=C1 XBHJTSIYYWRJFQ-MDZDMXLPSA-N 0.000 description 1
- RMSGACVDMOLUPL-CMDGGOBGSA-N 3-[(e)-2-phenylethenyl]pyridine Chemical compound C=1C=CC=CC=1/C=C/C1=CC=CN=C1 RMSGACVDMOLUPL-CMDGGOBGSA-N 0.000 description 1
- KLNYYUBWOQQZAE-VOTSOKGWSA-N 3-[(e)-2-phenylethenyl]thiophene Chemical compound C1=CSC=C1/C=C/C1=CC=CC=C1 KLNYYUBWOQQZAE-VOTSOKGWSA-N 0.000 description 1
- LGZKGOGODCLQHG-CYBMUJFWSA-N 5-[(2r)-2-hydroxy-2-(3,4,5-trimethoxyphenyl)ethyl]-2-methoxyphenol Chemical compound C1=C(O)C(OC)=CC=C1C[C@@H](O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-CYBMUJFWSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- QNVSXXGDAPORNA-UHFFFAOYSA-N Resveratrol Natural products OC1=CC=CC(C=CC=2C=C(O)C(O)=CC=2)=C1 QNVSXXGDAPORNA-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- LUKBXSAWLPMMSZ-OWOJBTEDSA-N Trans-resveratrol Chemical compound C1=CC(O)=CC=C1\C=C\C1=CC(O)=CC(O)=C1 LUKBXSAWLPMMSZ-OWOJBTEDSA-N 0.000 description 1
- LLAXRYBKRTYKLH-UHFFFAOYSA-N [4-(2-phenylethynyl)phenyl]methanol Chemical compound C1=CC(CO)=CC=C1C#CC1=CC=CC=C1 LLAXRYBKRTYKLH-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 125000005103 alkyl silyl group Chemical group 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229940011037 anethole Drugs 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- RIOXQFHNBCKOKP-UHFFFAOYSA-N benomyl Chemical compound C1=CC=C2N(C(=O)NCCCC)C(NC(=O)OC)=NC2=C1 RIOXQFHNBCKOKP-UHFFFAOYSA-N 0.000 description 1
- MITFXPHMIHQXPI-UHFFFAOYSA-N benzoxaprofen Natural products N=1C2=CC(C(C(O)=O)C)=CC=C2OC=1C1=CC=C(Cl)C=C1 MITFXPHMIHQXPI-UHFFFAOYSA-N 0.000 description 1
- BJIOGJUNALELMI-ARJAWSKDSA-N cis-isoeugenol Chemical compound COC1=CC(\C=C/C)=CC=C1O BJIOGJUNALELMI-ARJAWSKDSA-N 0.000 description 1
- LGZKGOGODCLQHG-UHFFFAOYSA-N combretastatin Natural products C1=C(O)C(OC)=CC=C1CC(O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-UHFFFAOYSA-N 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- UHOVQNZJYSORNB-MZWXYZOWSA-N deuterated benzene Substances [2H]C1=C([2H])C([2H])=C([2H])C([2H])=C1[2H] UHOVQNZJYSORNB-MZWXYZOWSA-N 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000002778 food additive Substances 0.000 description 1
- 235000013373 food additive Nutrition 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000011572 manganese Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 229910052755 nonmetal Inorganic materials 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- RUVINXPYWBROJD-UHFFFAOYSA-N para-methoxyphenyl Natural products COC1=CC=C(C=CC)C=C1 RUVINXPYWBROJD-UHFFFAOYSA-N 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- 229940016667 resveratrol Drugs 0.000 description 1
- 235000021283 resveratrol Nutrition 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical group C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- PJANXHGTPQOBST-VAWYXSNFSA-N trans-stilbene Chemical group C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- RPNIKWRXCAOXLD-UHFFFAOYSA-N trimethyl(oct-1-ynyl)silane Chemical compound CCCCCCC#C[Si](C)(C)C RPNIKWRXCAOXLD-UHFFFAOYSA-N 0.000 description 1
- DWVPGZOBZDSBQL-ZHACJKMWSA-N trimethyl-[(e)-oct-1-enyl]silane Chemical compound CCCCCC\C=C\[Si](C)(C)C DWVPGZOBZDSBQL-ZHACJKMWSA-N 0.000 description 1
- UZRJWRFLYPENFH-UHFFFAOYSA-N trimethyl-[4-(2-phenylethynyl)phenyl]silane Chemical group C1=CC([Si](C)(C)C)=CC=C1C#CC1=CC=CC=C1 UZRJWRFLYPENFH-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C5/00—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms
- C07C5/02—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms by hydrogenation
- C07C5/08—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms by hydrogenation of carbon-to-carbon triple bonds
- C07C5/09—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms by hydrogenation of carbon-to-carbon triple bonds to carbon-to-carbon double bonds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/16—Catalysts comprising hydrides, coordination complexes or organic compounds containing coordination complexes
- B01J31/22—Organic complexes
- B01J31/2265—Carbenes or carbynes, i.e.(image)
- B01J31/2269—Heterocyclic carbenes
- B01J31/2273—Heterocyclic carbenes with only nitrogen as heteroatomic ring members, e.g. 1,3-diarylimidazoline-2-ylidenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C17/00—Preparation of halogenated hydrocarbons
- C07C17/35—Preparation of halogenated hydrocarbons by reactions not affecting the number of carbon or of halogen atoms in the reaction
- C07C17/354—Preparation of halogenated hydrocarbons by reactions not affecting the number of carbon or of halogen atoms in the reaction by hydrogenation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/68—Preparation of compounds containing amino groups bound to a carbon skeleton from amines, by reactions not involving amino groups, e.g. reduction of unsaturated amines, aromatisation, or substitution of the carbon skeleton
- C07C209/70—Preparation of compounds containing amino groups bound to a carbon skeleton from amines, by reactions not involving amino groups, e.g. reduction of unsaturated amines, aromatisation, or substitution of the carbon skeleton by reduction of unsaturated amines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/17—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrogenation of carbon-to-carbon double or triple bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C37/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom of a six-membered aromatic ring
- C07C37/001—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom of a six-membered aromatic ring by modification in a side chain
- C07C37/003—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom of a six-membered aromatic ring by modification in a side chain by hydrogenation of an unsaturated part
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C41/00—Preparation of ethers; Preparation of compounds having groups, groups or groups
- C07C41/01—Preparation of ethers
- C07C41/18—Preparation of ethers by reactions not forming ether-oxygen bonds
- C07C41/20—Preparation of ethers by reactions not forming ether-oxygen bonds by hydrogenation of carbon-to-carbon double or triple bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/28—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group
- C07C67/283—Preparation of carboxylic acid esters by modifying the hydroxylic moiety of the ester, such modification not being an introduction of an ester group by hydrogenation of unsaturated carbon-to-carbon bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/06—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom containing only hydrogen and carbon atoms in addition to the ring nitrogen atom
- C07D213/127—Preparation from compounds containing pyridine rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/06—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom containing only hydrogen and carbon atoms in addition to the ring nitrogen atom
- C07D213/16—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom containing only hydrogen and carbon atoms in addition to the ring nitrogen atom containing only one pyridine ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/36—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/08—Hydrogen atoms or radicals containing only hydrogen and carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/0803—Compounds with Si-C or Si-Si linkages
- C07F7/0805—Compounds with Si-C or Si-Si linkages comprising only Si, C or H atoms
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2231/00—Catalytic reactions performed with catalysts classified in B01J31/00
- B01J2231/60—Reduction reactions, e.g. hydrogenation
- B01J2231/64—Reductions in general of organic substrates, e.g. hydride reductions or hydrogenations
- B01J2231/641—Hydrogenation of organic substrates, i.e. H2 or H-transfer hydrogenations, e.g. Fischer-Tropsch processes
- B01J2231/645—Hydrogenation of organic substrates, i.e. H2 or H-transfer hydrogenations, e.g. Fischer-Tropsch processes of C=C or C-C triple bonds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2531/00—Additional information regarding catalytic systems classified in B01J31/00
- B01J2531/60—Complexes comprising metals of Group VI (VIA or VIB) as the central metal
- B01J2531/62—Chromium
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/09—Geometrical isomers
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
Abstract
本发明公开了一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法,该方法以环状(烷基)(氨基)卡宾铬络合物为催化剂,镁单质为还原剂,氯硅烷和分子筛为添加剂,四氢呋喃作为溶剂,在氢气氛围下与中间炔烃在25~100℃反应,制备顺式或反式烯烃。该方法具有反应条件温和、催化剂金属廉价和丰产、底物适用范围广、操作简单、选择性高等优点。
Description
技术领域
本发明是关于制备顺式或反式烯烃化合物的方法,该方法利用不同的环状(烷基)(氨基)卡宾铬络合物为催化剂,在氢气氛围条件下选择性氢化中间炔烃,分别得到相应的顺式或反式烯烃。
背景技术
烯烃是一类重要的有机化合物,在材料、食品和制药等领域具有重要的应用。目前,市场上许多畅销的药品和食品添加剂含有烯烃结构。如白藜芦醇,利卡灵-A,苯烯莫德,异丁香酚,茴香脑分子中含有反式烯烃结构;康普瑞汀分子中含有顺式烯烃结构。通过过渡金属催化剂高选择性半还原中间炔烃合成顺式和反式烯烃是最实用和高效的方法之一,受到大力发展并逐渐应用到工业生产中。中间炔烃的半还原通常需要在氢气,计量的酸或其他氢源的条件下实现。与其他氢源相比较,氢气作为氢源有着原子经济性高,反应环保,体系干净等优点。虽然已有炔烃半氢化的方法可以得到相应的顺式或反式烯烃化合物,但是合成方法主要依赖昂贵金属催化剂(Pd,Ru,Rh,Ir)。对于常见廉价金属催化剂,例如:铜在催化合成顺式烯烃需要8-10 MPa的高压(Chem.Eur.J.2015,21, 15934–15938,Synthesis2017,49, 2470–2482);铬催化合成顺式二苯乙烯衍生物存在Z/E选择性差的缺点(1/1至 10/1摩尔比) (ChemCatChem2020,12, 1–6);采用锰催化剂只能合成含有芳基的顺式烯烃,而且存在底物受限的缺点(Org. Lett.2020,22, 5423−5428);铁催化剂在合成顺式烯烃时,对于杂环化合物几乎不反应或产率较低(J.Am.Chem.Soc.2019,141, 17452−17458,ChemSusChem2019,12, 3864 – 3870);镍催化剂在合成顺式烯烃时也需要120℃的高温(ChemSusChem2019,12, 3363 – 3369)。对于反式烯烃的合成更具有挑战性,常常面临过氢化产物的生成和选择性差的问题。例如:银催化合成反式烯烃需要150℃高温(J.Am.Chem.Soc.2015,137, 14598−14601);镍催化和非金属催化合成反式烯烃则存在需要昂贵和具有毒性的氘代苯(氘代甲苯)作为溶剂或140℃高温,不利于大量的制备(J.Am.Chem.Soc.2020,142, 5396−5407,Nature Chemistry2013,5, 718–723,Chem.Eur.J.2015,21, 3495 – 3501);铁催化合成反式烯烃对于杂环化合物比较受限,且选择性较差(Angew.Chem.Int.Ed.2013,52, 14131 –14134);钴催化合成芳基烷基的反式烯烃,底物只能局限在烷基硅取代的炔烃,不能适用于其他烷基取代的炔烃(J.Am.Chem.Soc.2016,138, 13700−13705)。从之前的报道来看,采用廉价金属催化剂制备顺式或反式烯烃,反应条件比较苛刻,Z/E选择性差,杂环化合物不兼容,烷基烷基取代的中间炔烃均不能以很好的选择性得到反式炔烃。从上可见,高选择性合成指定构型烯烃结构的方法具有广阔的应用前景。采用廉价金属催化剂,反应条件温和,底物适用性范围广,高选择性的制备顺式或反式烯烃具有十分重要的意义。
发明内容
如前所述,虽然已有方法制备顺式或反式烯烃化合物,但是其具有局限性。本发明的目的是提供一种反应条件温和、以廉价金属为催化剂、底物适用范围广、操作简单、立体选择性高、反应体系干净的制备顺式或反式烯烃化合物的方法。
针对上述目的,本发明采用的技术方案是:将下面反应式所示的中间炔烃化合物、环状(烷基)(氨基)卡宾铬络合物催化剂、镁、氯硅烷以及分子筛加入四氢呋喃中,通入氢气,在25~100℃下搅拌反应,得到反应式所示的Z-3或E-3烯烃化合物。该方法具有如下反应通式:
式中,R1,R2代表连在炔碳上的C1~C20烷基、呋喃基,噻吩基,吡啶基,芳基(芳基上含C1~C10烷基、烷氧基、酰胺基、酯基、卤代烃基、呋喃基,噻吩基,吡啶基,烯基,硅基,频哪醇硼酸酯基,氨基,羟基,取代炔基)任意一种或任意两种取代的组合。
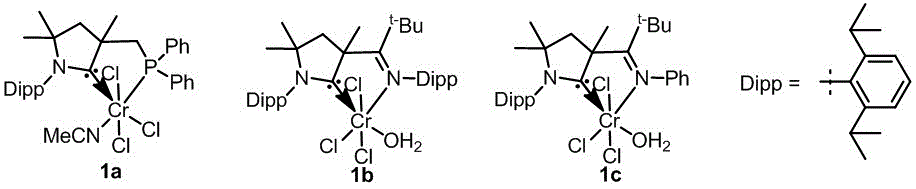
上述环状(烷基)(氨基)卡宾铬络合物催化剂的结构式如下所示:
上述环状(烷基)(氨基)卡宾铬络合物催化剂的加入量为中间炔烃化合物摩尔量的0.5%~10%。
上述镁的加入量为中间炔烃化合物毫摩尔量的1~3倍。
上述的氯硅烷为三甲基氯硅烷、三乙基氯硅烷或三丁基基氯硅烷中任意一种。氯硅烷的加入量为中间炔烃化合物摩尔量的0.2~2倍。
上述的分子筛为4Å分子筛或5Å分子筛中任意一种。
上述的有机溶剂为四氢呋喃。
上述方法中,通入氢气的压力为0.1~6 MPa。
上述方法中,在25~100℃下搅拌反应12~24小时。
顺式烯烃的合成步骤:
在有搅拌子的反应管中依次加入36 mg (0.2 mmol) 1,2-二苯乙炔、7 mg (0.01mmol)环状(烷基)(氨基)卡宾铬络合物催化剂1b、10 mg (0.4 mmol)镁,22 mg (0.1 mmol)三甲基氯硅烷,25 mg 4 Å MS,2 mL四氢呋喃,将反应管放入高压釜中,通入5 MPa的氢气。反应体系放置于40℃搅拌24 h。反应用盐酸水溶液淬灭,用乙酸乙酯萃取,萃取液在减压下除去挥发性溶剂。再经硅胶柱层析得到顺式烯烃化合物。
反式烯烃的合成步骤:
在有搅拌子的反应管中依次加入36 mg (0.2 mmol) 1,2-二苯乙炔、6 mg (0.01mmol)环状(烷基)(氨基)卡宾铬络合物催化剂1a,10 mg (0.4 mmol)镁,11 mg (0.05mmol)三甲基氯硅烷,2 mL四氢呋喃,将反应管防入高压釜中,通入1 MPa的氢气。反应体系放置于100℃搅拌24 h。反应用盐酸水溶液淬灭,用乙酸乙酯萃取,萃取液在减压下除去挥发性溶剂。再经硅胶柱层析得到反式烯烃化合物。
与已有的方法比较,本发明具有如下的优点:反应条件温和,廉价金属催化剂,反应快速且产率较高,底物适用范围广,产物E/Z选择性高,反应体系干净,适合顺式或反式烯烃化合物的生产。
具体实施方式
以下的实施例,在于详细的说明本发明而非限制本发明。
实施例1~23
实施例1
在有搅拌子的反应管中依次加入36 mg (0.2 mmol) 1,2-二苯乙炔、7 mg (0.01mmol)环状(烷基)(氨基)卡宾铬络合物催化剂1b、10 mg (0.4 mmol)镁,22 mg (0.1 mmol)三甲基氯硅烷,25 mg 4 Å MS,2 mL四氢呋喃,将反应管放入高压釜中,通入5 MPa的氢气。反应体系放置于40℃搅拌24 h。反应用盐酸水溶液淬灭,用乙酸乙酯萃取,萃取液在减压下除去挥发性溶剂。再经硅胶柱层析(洗脱剂为石油醚与乙酸乙酯体积比100:1的混合液),得到顺式-1,2二苯乙烯,产率88%,Z/E=93:7。
对比实施例1
用等摩尔量的1c替换实施例1中的1b,其他步骤与实施例1相同,得到顺式-1,2二苯乙烯,产率90%,Z/E=70:30。
对比实施例1
用等体积三乙基氯硅烷替换实施例1中的三甲基氯硅烷,其他步骤与实施例1相同,得到顺式-1,2二苯乙烯,产率83%,Z/E=92:8。
对比实施例1
用等体积三丁基基氯硅烷替换实施例1中的三甲基氯硅烷,其他步骤与实施例1相同,得到顺式-1,2二苯乙烯,产率85%,Z/E=90:10。
对比实施例1
用等质量的5Å分子筛替换实施例1中的4Å分子筛,其他步骤与实施例1相同,得到顺式-1,2二苯乙烯,产率81%,Z/E=93:7。
实施例2
本实施例中,用等摩尔4-(苯乙炔基)氯苯替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到4-氯-顺-二苯乙烯,产率86%,Z/E=97:3。
实施例3
本实施例中,用等摩尔4-(苯乙炔基)苯基特戊酸酯替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到4-特戊酰氧基-顺-二苯乙烯,产率92%,Z/E=97:3。
实施例4
本实施例中,用等摩尔4-(苯乙炔基)苯甲醇替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到4-羟甲基-顺-二苯乙烯,产率72%,Z/E=99:1。
实施例5
本实施例中,用等摩尔3-(苯乙炔基)苯胺替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到3-氨基-顺-二苯乙烯,产率88%,Z/E>99:1。
实施例6
本实施例中,用等摩尔2-(己-1-炔基)萘替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到顺-2-(1-己烯基)萘,产率90%,Z/E=98:2。
实施例7
本实施例中,用等摩尔6-十二炔替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到顺-6-十二烯,产率88%,Z/E=99:1。
实施例8
本实施例中,用等摩尔4-甲氧基苯丙炔替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到顺式-茴香烯,产率96%,Z/E=95:5。
实施例9
本实施例中,用等摩尔3-甲氧基-4-羟基苯丙炔替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到顺式-异丁香酚,产率84%,Z/E=96:4。
实施例10
本实施例中,用等摩尔1-(3,5-二甲氧基苯基)-2-(4-甲氧基苯基)乙炔替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到3,4',5-三甲氧基-顺-二苯乙烯,产率88%,Z/E=99:1。
实施例11
本实施例中,用等摩尔(E)-4,4'-(hex-3-ene-3,4-diyl)bis(hex-1-yn-1-ylbenzene)替换实施例1中的1,2-二苯乙炔,其他步骤与实施例1相同,得到1-((Z)-hex-1-en-1-yl)-4-((E)-4-(4-(hex-1-yn-1-yl)phenyl)hex-3-en-3-yl)benzene,产率44%,Z/E=99:1。
实施例12
在有搅拌子的反应管中依次加入36 mg (0.2 mmol) 1,2-二苯乙炔、6 mg (0.01mmol)环状(烷基)(氨基)卡宾铬络合物催化剂1a,10 mg (0.4 mmol)镁,11 mg (0.05mmol)三甲基氯硅烷,2 mL四氢呋喃,将反应管防入高压釜中,通入1 MPa的氢气。反应体系放置于100℃搅拌24 h。反应用盐酸水溶液淬灭,用乙酸乙酯萃取,萃取液在减压下除去挥发性溶剂。再经硅胶柱层析(洗脱剂为石油醚与乙酸乙酯体积比100:1的混合液),得到反式-1,2二苯乙烯,产率85%,E/Z=99:1。
实施例13
本实施例中,用等摩尔3-(苯乙炔基)苯酚替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-3-羟基二苯乙烯,产率87%,E/Z=90:10。
实施例14
本实施例中,用等摩尔4-(三甲硅基)二苯乙炔替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-4-(三甲基硅基)二苯乙烯,产率87%,E/Z=90:10。
实施例15
本实施例中,用等摩尔4-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)二苯乙炔替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-4-(频哪醇硼酸酯基)二苯乙烯,产率65%,E/Z=86:14。
实施例16
本实施例中,用等摩尔3-(3-(苯乙炔基)苯基)呋喃替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-3-(3-呋喃基)二苯乙烯,产率95%,E/Z=98:2。
实施例17
本实施例中,用等摩尔3-(苯乙炔基)噻吩替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-3-苯乙烯基噻吩,产率84%,E/Z=92:8。
实施例18
本实施例中,用等摩尔3-(苯乙炔基)吡啶替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-3-苯乙烯基吡啶,产率39%,E/Z=94:6。
实施例19
本实施例中,用等摩尔1,3-二甲基-5-(辛-1-炔基)苯替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反-1,3-二甲基-5-(辛-1-烯基)苯,产率85%,E/Z=99:1。
实施例20
本实施例中,用等摩尔4-甲氧基苯丙炔替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反式-茴香脑,产率92%,E/Z=97:3。
实施例21
本实施例中,用等摩尔3-甲氧基-4-羟基苯丙炔替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反式-异丁香酚,产率87%,E/Z=98:2。
实施例22
本实施例中,用等摩尔(E)-4,4'-(hex-3-ene-3,4-diyl)bis(hex-1-yn-1-ylbenzene)替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到(E)-4,4'-((E)-hex-3-ene-3,4-diyl)bis(((E)-hex-1-en-1-yl)benzene), 产率76%,E/Z=99:1。
实施例23
本实施例中,用等摩尔1-三甲基硅基-1-辛炔替换实施例14中的1,2-二苯乙炔,其他步骤与实施例14相同,得到反式-1-三甲基硅基-1-辛烯, 产率78%,E/Z=96:4。
Claims (8)
2.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:所述通入氢气的压力为0.1~6MPa。
3.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:所述环状(烷基)(氨基)卡宾铬络合物催化剂的加入量为中间炔烃化合物摩尔量的0.5%~10%。
4.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:所述镁的加入量为中间炔烃化合物摩尔量的1~3倍。
5.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:所述氯硅烷为三甲基氯硅烷(TMSCl)、三乙基氯硅烷或三丁基氯硅烷。氯硅烷的加入量为中间炔烃化合物摩尔量的0.2~2倍。
7.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:溶剂为四氢呋喃。
8.根据权利要求1所述的以铬催化中间炔烃选择性氢化合成顺式或反式烯烃的方法,其特征在于:在25~100℃下搅拌反应12~24小时。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310060760.0A CN116425607A (zh) | 2023-01-18 | 2023-01-18 | 一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310060760.0A CN116425607A (zh) | 2023-01-18 | 2023-01-18 | 一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116425607A true CN116425607A (zh) | 2023-07-14 |
Family
ID=87078491
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310060760.0A Pending CN116425607A (zh) | 2023-01-18 | 2023-01-18 | 一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116425607A (zh) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120130086A1 (en) * | 2010-03-03 | 2012-05-24 | Sk Global Chemical Co., Ltd. | Highly Active and Selective Ethylene Oligomerization Catalyst and Method of Preparing Hexene or Octene Using the Same |
| CN112661653A (zh) * | 2020-12-28 | 2021-04-16 | 四川大学 | 一种环状(烷基)(氨基)卡宾铬络合物催化还原硝基化合物制备胺的方法 |
| CN113443952A (zh) * | 2021-07-15 | 2021-09-28 | 南通大学 | 水供氢铱催化炔烃半还原选择性合成顺、反式烯烃的方法 |
-
2023
- 2023-01-18 CN CN202310060760.0A patent/CN116425607A/zh active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120130086A1 (en) * | 2010-03-03 | 2012-05-24 | Sk Global Chemical Co., Ltd. | Highly Active and Selective Ethylene Oligomerization Catalyst and Method of Preparing Hexene or Octene Using the Same |
| CN112661653A (zh) * | 2020-12-28 | 2021-04-16 | 四川大学 | 一种环状(烷基)(氨基)卡宾铬络合物催化还原硝基化合物制备胺的方法 |
| CN113443952A (zh) * | 2021-07-15 | 2021-09-28 | 南通大学 | 水供氢铱催化炔烃半还原选择性合成顺、反式烯烃的方法 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Rainier et al. | Aluminum-and boron-mediated C-glycoside synthesis from 1, 2-anhydroglycosides | |
| US20130018207A1 (en) | Frustrated Lewis Pair Compositions | |
| CN113563370B (zh) | 一种壳聚糖负载铜材料催化制备α位有取代基的β-硼基酮的制备方法 | |
| Mori et al. | Pd (0)–polyethyleneimine complex as a partial hydrogenation catalyst of alkynes to alkenes | |
| JP6200417B2 (ja) | ヒドロキシフェニルシクロヘキサノール化合物の製造方法 | |
| CN116425607A (zh) | 一种铬催化中间炔烃选择性氢化制备顺式或反式烯烃的方法 | |
| CN115785135B (zh) | 多取代异戊二烯基硼酸酯及其合成方法 | |
| US11873265B2 (en) | Method for preparing benzyl amine compound | |
| CN103748065B (zh) | 2-烯基胺化合物的制造方法 | |
| CN113443952B (zh) | 水供氢铱催化炔烃半还原选择性合成顺、反式烯烃的方法 | |
| JPS63230694A (ja) | 少なくとも1個のシクロアルキル環を含有するシランまたはシロキサン化合物の製法 | |
| WO2021138908A1 (zh) | γ-戊内酯的制备方法 | |
| CN102950025B (zh) | 一种亚胺不对称加氢催化剂及其用途 | |
| CN112390831B (zh) | 三碟烯环金属钯化合物及用途 | |
| KR102712847B1 (ko) | 신규 구조의 유기인계 촉매 및 이를 이용한 선형 디니트릴의 제조방법 | |
| CN112574092B (zh) | 一种制备2-二芳基甲基取代吲哚类化合物的绿色新方法 | |
| CN112645784B (zh) | 一种简单高效的二芳香炔烃合成方法 | |
| KR20230039328A (ko) | 리간드 화합물, 이의 제조방법 및 이를 이용한 α,β-불포화 카르복실레이트의 제조방법 | |
| JP3003268B2 (ja) | アルコキシアルカジエンの製造方法 | |
| JP2023135313A (ja) | ラクトン類製造用触媒及びラクトン類の製造方法 | |
| CN118164888A (zh) | 一种[2.2]对环芳烷手性二铑(ii)金属配合物及其制备和应用 | |
| CN113683474A (zh) | 一种降冰片烷类化合物的合成方法 | |
| CN118791344A (zh) | 一种醇脱羟基羰基化合成1,2-二芳基酮的方法 | |
| CN115784895A (zh) | 一种芳基硝基化合物非金属还原制备芳胺化合物的方法 | |
| CN115677451A (zh) | 一种制备反式二苄基乙烯类化合物的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |