CN114907221A - 一种氘代氨溴索衍生物及其制备和用途 - Google Patents
一种氘代氨溴索衍生物及其制备和用途 Download PDFInfo
- Publication number
- CN114907221A CN114907221A CN202110164994.0A CN202110164994A CN114907221A CN 114907221 A CN114907221 A CN 114907221A CN 202110164994 A CN202110164994 A CN 202110164994A CN 114907221 A CN114907221 A CN 114907221A
- Authority
- CN
- China
- Prior art keywords
- deuterated
- pharmaceutically acceptable
- acceptable salt
- ambroxol
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- JBDGDEWWOUBZPM-XYPYZODXSA-N ambroxol Chemical class NC1=C(Br)C=C(Br)C=C1CN[C@@H]1CC[C@@H](O)CC1 JBDGDEWWOUBZPM-XYPYZODXSA-N 0.000 title claims abstract description 20
- 238000002360 preparation method Methods 0.000 title claims abstract description 8
- 150000001875 compounds Chemical class 0.000 claims abstract description 23
- 150000003839 salts Chemical class 0.000 claims abstract description 15
- 239000003814 drug Substances 0.000 claims description 15
- 229910052805 deuterium Inorganic materials 0.000 claims description 7
- 239000012453 solvate Substances 0.000 claims description 6
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 claims description 6
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 6
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical group Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 239000001257 hydrogen Substances 0.000 claims description 4
- 239000000126 substance Substances 0.000 claims description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 3
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 claims description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 3
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 claims description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 claims description 3
- 229910019142 PO4 Inorganic materials 0.000 claims description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 claims description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 3
- 229940077388 benzenesulfonate Drugs 0.000 claims description 3
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 claims description 3
- 229940049920 malate Drugs 0.000 claims description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 3
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 3
- 239000000463 material Substances 0.000 claims description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 3
- 239000010452 phosphate Substances 0.000 claims description 3
- 229940095064 tartrate Drugs 0.000 claims description 3
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 claims description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 claims description 2
- 239000004480 active ingredient Substances 0.000 claims description 2
- 125000004429 atom Chemical group 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 2
- 239000008194 pharmaceutical composition Substances 0.000 claims description 2
- WCYWZMWISLQXQU-FIBGUPNXSA-N trideuteriomethane Chemical group [2H][C]([2H])[2H] WCYWZMWISLQXQU-FIBGUPNXSA-N 0.000 claims description 2
- 230000000510 mucolytic effect Effects 0.000 claims 1
- 239000003172 expectorant agent Substances 0.000 abstract description 3
- 230000003419 expectorant effect Effects 0.000 abstract description 2
- 229940066493 expectorants Drugs 0.000 abstract 1
- 229940079593 drug Drugs 0.000 description 14
- 229960000985 ambroxol hydrochloride Drugs 0.000 description 12
- QNVKOSLOVOTXKF-UHFFFAOYSA-N 4-[(2-amino-3,5-dibromophenyl)methylamino]cyclohexan-1-ol;hydron;chloride Chemical compound Cl.NC1=C(Br)C=C(Br)C=C1CNC1CCC(O)CC1 QNVKOSLOVOTXKF-UHFFFAOYSA-N 0.000 description 11
- 238000006243 chemical reaction Methods 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 229960005174 ambroxol Drugs 0.000 description 8
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 7
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 241000700159 Rattus Species 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 229940090044 injection Drugs 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 206010036790 Productive cough Diseases 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- XUKUURHRXDUEBC-SXOMAYOGSA-N (3s,5r)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-SXOMAYOGSA-N 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 206010062717 Increased upper airway secretion Diseases 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 208000026435 phlegm Diseases 0.000 description 3
- 210000002381 plasma Anatomy 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 208000024794 sputum Diseases 0.000 description 3
- 210000003802 sputum Anatomy 0.000 description 3
- 238000013112 stability test Methods 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 206010006451 bronchitis Diseases 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 229940125904 compound 1 Drugs 0.000 description 2
- -1 compound ambroxol hydrochloride Chemical class 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000012065 filter cake Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 210000004907 gland Anatomy 0.000 description 2
- 229940093181 glucose injection Drugs 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- IMLXLGZJLAOKJN-UHFFFAOYSA-N 4-aminocyclohexan-1-ol Chemical compound NC1CCC(O)CC1 IMLXLGZJLAOKJN-UHFFFAOYSA-N 0.000 description 1
- 208000030090 Acute Disease Diseases 0.000 description 1
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 description 1
- ARMLBUAMNBQDHJ-UHFFFAOYSA-N BrC=1C=C2CN(C=NC2=C(C=1)Br)C1CCC(CC1)O Chemical compound BrC=1C=C2CN(C=NC2=C(C=1)Br)C1CCC(CC1)O ARMLBUAMNBQDHJ-UHFFFAOYSA-N 0.000 description 1
- 208000014085 Chronic respiratory disease Diseases 0.000 description 1
- 239000005696 Diammonium phosphate Substances 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 235000010678 Paulownia tomentosa Nutrition 0.000 description 1
- 240000002834 Paulownia tomentosa Species 0.000 description 1
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 description 1
- 241001620634 Roger Species 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- WEVYAHXRMPXWCK-UHFFFAOYSA-N acetonitrile Substances CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229960000508 bedaquiline Drugs 0.000 description 1
- QUIJNHUBAXPXFS-XLJNKUFUSA-N bedaquiline Chemical compound C1([C@H](C2=CC3=CC(Br)=CC=C3N=C2OC)[C@@](O)(CCN(C)C)C=2C3=CC=CC=C3C=CC=2)=CC=CC=C1 QUIJNHUBAXPXFS-XLJNKUFUSA-N 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- OJGDCBLYJGHCIH-UHFFFAOYSA-N bromhexine Chemical class C1CCCCC1N(C)CC1=CC(Br)=CC(Br)=C1N OJGDCBLYJGHCIH-UHFFFAOYSA-N 0.000 description 1
- 201000009267 bronchiectasis Diseases 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000012295 chemical reaction liquid Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 210000004081 cilia Anatomy 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- MNNHAPBLZZVQHP-UHFFFAOYSA-N diammonium hydrogen phosphate Chemical compound [NH4+].[NH4+].OP([O-])([O-])=O MNNHAPBLZZVQHP-UHFFFAOYSA-N 0.000 description 1
- 229910000388 diammonium phosphate Inorganic materials 0.000 description 1
- 235000019838 diammonium phosphate Nutrition 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 229940072185 drug for treatment of tuberculosis Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- VFRSADQPWYCXDG-LEUCUCNGSA-N ethyl (2s,5s)-5-methylpyrrolidine-2-carboxylate;2,2,2-trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.CCOC(=O)[C@@H]1CC[C@H](C)N1 VFRSADQPWYCXDG-LEUCUCNGSA-N 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000008098 formaldehyde solution Substances 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- ZFGMDIBRIDKWMY-PASTXAENSA-N heparin Chemical compound CC(O)=N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O[C@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@@H](O[C@@H]3[C@@H](OC(O)[C@H](OS(O)(=O)=O)[C@H]3O)C(O)=O)O[C@@H]2O)CS(O)(=O)=O)[C@H](O)[C@H]1O ZFGMDIBRIDKWMY-PASTXAENSA-N 0.000 description 1
- 229960001008 heparin sodium Drugs 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 208000030603 inherited susceptibility to asthma Diseases 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000011866 long-term treatment Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000003580 lung surfactant Substances 0.000 description 1
- 229940066294 lung surfactant Drugs 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000009115 maintenance therapy Methods 0.000 description 1
- 230000007257 malfunction Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229940066491 mucolytics Drugs 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- YTJSFYQNRXLOIC-UHFFFAOYSA-N octadecylsilane Chemical group CCCCCCCCCCCCCCCCCC[SiH3] YTJSFYQNRXLOIC-UHFFFAOYSA-N 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C215/00—Compounds containing amino and hydroxy groups bound to the same carbon skeleton
- C07C215/42—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having amino groups or hydroxy groups bound to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton
- C07C215/44—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having amino groups or hydroxy groups bound to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton bound to carbon atoms of the same ring or condensed ring system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/10—Expectorants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/12—Mucolytics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B59/00—Introduction of isotopes of elements into organic compounds ; Labelled organic compounds per se
- C07B59/001—Acyclic or carbocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/02—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton by reactions involving the formation of amino groups from compounds containing hydroxy groups or etherified or esterified hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C249/00—Preparation of compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C249/02—Preparation of compounds containing nitrogen atoms doubly-bound to a carbon skeleton of compounds containing imino groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/05—Isotopically modified compounds, e.g. labelled
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Pulmonology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
技术领域
本发明属于药物化学技术领域,具体涉及一种化合物及其制备和用途。
背景技术
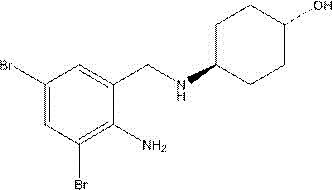
氨溴索又称溴环己基胺醇,为反式-4-[(2-氨基-3,5-二溴苄基)胺基]环己醇,其分子式为C13H18Br2N2O,其结构式为:
氨溴索为溴己新的代谢产物,本品为粘液溶解剂,能增加呼吸道粘膜浆液腺的分泌,减少粘液腺分泌,从而降低痰液粘度;还可促进肺表面活性物质的分泌,增加支气管纤毛运动,使痰液易于咳出。本品从血液至组织的分布快且显著,肺脏为主要靶器管。血浆半衰期为7-12小时,无累积效应。氨溴索主要在肝脏代谢,大约90%经肾脏排除。适用于伴有痰液分泌不正常及排痰功能不良的急性、慢性呼吸道疾病,例如慢性支气管炎急性加重、喘息型支气管炎、支气管扩张及支气管哮喘的祛痰治疗。术后肺部并发症的预防性治疗。早产儿及新生儿婴儿呼吸窘迫综合症(IRDS)的治疗。
4-(6,8-二溴-3,4-二氢喹唑啉-3-基)-环己醇(杂质B)是氨溴索主要的降解杂质。临床滴注氨溴索注射液,关于是否可以用葡萄糖注射液作为溶媒,曾经有过反复的意见。2013年3月,因临床反应,氨溴索注射液用5%葡萄糖注射液稀释,有微粒析出的现象。当时生产氨溴索注射液(商品名,沐舒坦)的勃林格殷格翰(中国)公司做了一个实验,将沐舒坦与来自不同国家不同生产商、不同批次的5%葡萄糖溶液混合,检出了一定数量的降解产物B-是由盐酸氨溴索与葡萄糖溶液中普遍存在的微量甲醛发生反应后形成的。于是该公司发表了关于“盐酸氨溴索注射液与5%葡萄糖注射液不宜混合使用”致经销商/医院的沟通信。同年12月该公司又发布信息:之前公司在进一步毒理学实验尚未完成的情况下,本着严谨科学的态度,建议慎用盐酸氨溴索与5%葡萄糖溶液混合使用。而最新完成的毒理学研究显示,没有发现任何证据表明该降解产物会对患者安全性带来风险。基于目前为止的所有证据,勃林格殷格翰公司确认沐舒坦(盐酸氨溴索注射液)可以安全地与5%葡萄糖溶液配伍使用。在中国现行产品说明书中,盐酸氨溴索注射液(15mg/2ml)被批准可以与溶媒(生理盐水、林格氏液、5%葡萄糖溶液、5%果糖溶液)混合后静脉给药。但是还有可能存在一定的风险。以盐酸氨溴索为起始原料,与甲醛经环合,氧化两步反应合成了此有关物质。
氘代药物是指将药物分子中的部分氢原子替换为氘。由于氘在药物分子中形状和体积与氢接近,氘代药物一般会保留原来药物的生物活性和选择性。由于C-D键比C-H键更稳定,使得氘代药物在化学反应过程中,C-D键更不容易断裂,其半衰期会延长。
由于生物系统的代谢过程复杂,药物在生物体内的药代动力学性质受到多方面因素影响,也表现出相应的复杂性。与相应的非氘代药物相比,氘代药物药代动力学性质的变化表现出极大的偶然性和不可预测性。某些位点的氘代非但不能延长半衰期,反而可能会使其缩短(Scott L.Harbeson,Roger D.Tung.Deuterium in Drug Discovery andDevelopment,P405-406。),劣化其药代动力学性质;另一方面,药物分子上某些位置的氢因为空间位阻等原因也不易被氘代,因此,药物的氘代并非随心所欲,可氘代的位点是不可预期的。
本发明人期望通过对氨溴索进行氘代,得到一类稳定性好、祛痰、药代动力学性质良好、降低有毒副作用的代谢产物的氘代药物。
发明内容
本发明的目的在于提供一种具有祛痰生物活性的氘代氨溴索衍生物。
其中R1和R2分别独立地选自氢和氘、CH3、CD3,且所述R1和R2中至少含有一个D原子。
进一步地,所述的立体化学异构体为(1r,4r)。
进一步地,所述的药学上可接受的盐为盐酸盐、硫酸盐、枸橼酸盐、苯磺酸盐、氢溴酸盐、氢氟酸盐、磷酸盐、乙酸盐、丙酸盐、丁二酸盐、草酸盐、苹果酸盐、琥珀酸盐、富马酸盐、马来酸盐、酒石酸盐或三氟乙酸盐;优选为盐酸盐。
进一步地,所述的化合物为:
本发明还提供了上述的氘代氨溴索衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐在制备祛痰药物中的用途。
一种药物组合物,它是以上述的氘代氨溴索衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐为活性成分,加上药学上可接受的辅料制备而成的制剂。
本发明中,“治疗”也包括复发性(relapse)预防或阶段性(phase)预防,以及急性或慢性体征、症状和/或功能失常的治疗。治疗可以是对症治疗,例如抑制症状。它可以在短期内实现,在中期内调整,或者可以说是长期治疗,例如在维持疗法里面。
所述“预防”包括延迟和/或阻止病症、疾病或病况和/或其伴发症状的发作;防止对象染上病症、疾病或病况;或降低对象染上病症、疾病或病况的风险的方法。
本发明中,“药学上可接受的”是指某载体、运载物、稀释剂、辅料,和/或所形成的盐通常在化学上或物理上与构成某药物剂型的其它成分相兼容,并在生理上与受体相兼容。
本发明中所述盐可以是化合物的盐酸盐、硫酸盐、枸橼酸盐、苯磺酸盐、氢溴酸盐、氢氟酸盐、磷酸盐、乙酸盐、丙酸盐、丁二酸盐、草酸盐、苹果酸盐、琥珀酸盐、富马酸盐、马来酸盐、酒石酸盐或三氟乙酸盐。
显然,根据本发明的上述内容,按照本领域的普通技术知识和惯用手段,在不脱离本发明上述基本技术思想前提下,还可以做出其它多种形式的修改、替换或变更。
具体实施方式
以下通过实施例形式的具体实施方式,对本发明的上述内容再作进一步的详细说明。但不应将此理解为本发明上述主题的范围仅限于以下的实例。凡基于本发明上述内容所实现的技术均属于本发明的范围。
除特别标注的外,本发明所用试剂和测试设备均为常规的市售试剂和设备。
实施例1、(1r,4r)-4-((2-(氨基-D)-3,5-二溴苄基)氨基)环己-1-醇(化合物1)
第一步:合成(1r,4r)-4-((((E)-2-(氨基-D)-3,5-二溴亚苄基)氨基)环己-1-醇(1-2)
向干燥洁净的250ml反应瓶中加入2-(一氘代-氨基)-3,5-二溴苯甲醛25g(0.089mol),反式-4-氨基环己醇10.3g(0.089mol),无水乙醇150ml,启动搅拌,升温至回流,保温反应12小时。TCL监控,反应毕,然后降温至20~30℃,向反应体系中加入纯化水75ml,析出大量固体,过滤,滤饼干燥得浅黄色固体33.5g,收率:99.4%,HPLC纯度97.6%。
第二步:合成(1r,4r)-4-((2-(氨基-D)-3,5-二溴苄基)氨基)环己-1-醇(化合物1)
向干燥洁净500ml反应瓶中加入中间体 32.5g(0.086mol),无水甲醇130ml,启动搅拌,升温至40~60℃,保温30min,然后降温至20~30℃,向反应液中滴加硼氢化钠水溶液(NaBH4:3.25g 0.086mol;H2O:29.25g配制溶液),滴加完毕后,保温反应3小时,然后加入水,再加入二氯甲烷萃取(3*100ml),有机相合并,减压浓缩后加入丙酮,加入盐酸成盐,析出大量固体,过滤,滤饼干燥得浅黄色至类白色固体(HPLC纯度99.2%)。
本发明的其他化合物可用类似的方法制备得到。
以下通过试验例的方式来说明本发明的有益效果。
试验例1、本发明化合物的药学稳定性实验
(1)高效照高效波相色谱法测定,用十八烷基硅烷键合硅胶为填充剂,以0.01mol/L磷酸氢二铵溶液(用磷酸调节pH值至7.0)-乙腈(50:50)为流动相,检测波长为248nm。盐酸氨溴索与杂质B(相对保留时间约为0.8)的分离度应大于4.0。
(2)取盐酸氨溴索约5mg,加甲醇0.2ml溶解,再加甲醛溶液(1-100)40μl,摇匀,置60℃水浴中加热5分钟,氮气吹干。残渣加水5ml溶解,再加流动相稀释至20,摇匀。
(4)取上述供试品溶液20μl分别注入液相色谱仪,记录色谱图,以归一化法计算杂质B的含量。
体外药学稳定性试验结果见表1。
表1、体外药学稳定性试验结果
| 杂质B | 主峰(%) | |
| 盐酸氨溴索 | 43.8% | 56.2% |
| 实施例1化合物 | 11.6% | 88.4% |
| 实施例2化合物 | 0.05% | 99.5% |
如上表所示,实施例1和实施例2制备的化合物稳定性较盐酸氨溴索好。显示了本发明的氘代化合物都比非氘代的化合物盐酸氨溴索好,表明本发明化合物体外有更好的稳定性和安全性。
试验例2、本发明化合物的大鼠药代动力学
1)实验材料及仪器:
Agilent 1100高效液相色谱系统,API4000三重四极杆质谱仪,配有电喷雾离子化(ESI源),Analyst 1.4(美国ABI公司) 。
高速冷冻离心机,购自Thermo Fisher Scientific
分析天平,购自赛多利斯,SECURA225D-1CN
健康SPF级SD大鼠12只,雄性,动物合格证编号:42000600002461
2)实验方法及结果
将12只SD大鼠(180-250g)分为A、B两组,每组6只,禁食过夜(自由饮水)后,分别灌胃给药56.25mg;于给药前及给药后0.5、1、1.5、2、3、4、6、8、12、24h断尾取血约0.5ml,入放入装有肝素钠的1.5ml的EP管中,4℃离心5min分离血浆,于-20℃保存待测。然后采用LC/MS/MS法测定血浆中的待测化合物浓度。
表2、本发明化合物的大鼠药代动力学参数
从表2可以看出本发明化合物的暴露量(AUC)显著高于贝达喹啉,显示了更好的药代动力学。预期在临床上可以减小使用剂量,成为更安全,病人依从性更好的抗结核病药物。
综上,本发明提供的氘代氨溴索化合物具有更好的代谢稳定性和药代动力学,能够用于制备更安全有效的祛痰治疗的药物。应用前景优良。
Claims (6)
2.根据权利要求1所述的化合物或其化学立体异构体、溶剂化合物或药学上可接受的盐,所述的化学异构体为(1r,4r)。
3.根据权利要求1所述的化合物或其化学立体异构体、溶剂化合物或药学上可接受的盐,其特征在于:所述的药学上可接受的盐为盐酸盐、硫酸盐、枸橼酸盐、苯磺酸盐、氢溴酸盐、氢氟酸盐、磷酸盐、乙酸盐、丙酸盐、丁二酸盐、草酸盐、苹果酸盐、琥珀酸盐、富马酸盐、马来酸盐、酒石酸盐或三氟乙酸盐;优选为盐酸盐。
5.权利要求1~4任一项所述的氘代的氨溴索衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐在制备黏痰溶解药物中的用途。
6.一种药物组合物,其特征在于:它是以权利要求1~4任一项所述的氘代的氨溴索衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐为活性成分,加上药学上可接受的辅料制备而成的制剂。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110164994.0A CN114907221A (zh) | 2021-02-06 | 2021-02-06 | 一种氘代氨溴索衍生物及其制备和用途 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110164994.0A CN114907221A (zh) | 2021-02-06 | 2021-02-06 | 一种氘代氨溴索衍生物及其制备和用途 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN114907221A true CN114907221A (zh) | 2022-08-16 |
Family
ID=82761407
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110164994.0A Pending CN114907221A (zh) | 2021-02-06 | 2021-02-06 | 一种氘代氨溴索衍生物及其制备和用途 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114907221A (zh) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023076997A1 (en) * | 2021-10-28 | 2023-05-04 | Zywie, Llc | Modified forms of ambroxol for therapeutic use |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110740983A (zh) * | 2017-02-07 | 2020-01-31 | Stc.Unm公司 | 用于持久自噬诱导的同位素增强的氨溴索 |
-
2021
- 2021-02-06 CN CN202110164994.0A patent/CN114907221A/zh active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110740983A (zh) * | 2017-02-07 | 2020-01-31 | Stc.Unm公司 | 用于持久自噬诱导的同位素增强的氨溴索 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023076997A1 (en) * | 2021-10-28 | 2023-05-04 | Zywie, Llc | Modified forms of ambroxol for therapeutic use |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20190231687A1 (en) | Endoxifen methods and compositions in the treatment of psychiatric and neurodegenerative diseases | |
| RU2685236C2 (ru) | Вдыхаемые частицы, содержащие тиотропий | |
| JP2003534273A (ja) | 新規組成物 | |
| JP2003534272A (ja) | 新規方法 | |
| US11572334B2 (en) | Methods for making and using endoxifen | |
| JP2000516262A (ja) | エレトリプタンヘミスルフェートおよびカフェインを含有する医薬組成物 | |
| KR20070100735A (ko) | 약학적 화합물 및 조성물 | |
| EP3828193A1 (en) | Crystal form of lanosterol prodrug compound and application thereof | |
| US20120164075A1 (en) | Endoxifen methods and compositions in the treatment of mammalian diseases | |
| US11970446B2 (en) | Crystalline salt forms of mesembrine | |
| CN114907221A (zh) | 一种氘代氨溴索衍生物及其制备和用途 | |
| CA3070043A1 (en) | Amorphous form of vilanterol trifenatate and processes for the preparation thereof | |
| JP2007527922A (ja) | 実質的に純粋なトルテロジンタルトレート及びその調製方法 | |
| US9896423B2 (en) | Deuterium substituted 1-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]piperazine compound or derivatives thereof, and pharmaceutical composition and use thereof | |
| CN108159026B (zh) | 一种稳定的吸入用盐酸氨溴索溶液及其制备方法 | |
| TW201231465A (en) | Process for the preparation of new tiotropium salts, tiotropium salts as such and pharmaceutical compositions thereof | |
| RU2440972C2 (ru) | Кристаллический сульфат левосальбутамола, способ его получения и фармацевтическая композиция, содержащая его | |
| CN110693861A (zh) | 一种硫酸特布他林雾化吸入用溶液制剂及其制备方法 | |
| JP4727990B2 (ja) | モルヒネ−6−グルクロニドの塩 | |
| CN111821309B (zh) | 一种具有改良溶出速度的达芦那韦组合物 | |
| RU2768482C1 (ru) | Фармацевтическая композиция агониста каппа-опиоидного рецептора | |
| US20220016078A1 (en) | Methods Of Treating Kidney Stones | |
| WO2023174090A1 (zh) | 达比加群酯共晶及其制备方法 | |
| WO2022001889A1 (zh) | 去甲曲马多的盐及其用途 | |
| TW200837067A (en) | Ascomycin and pimecrolimus having reduced levels of desmethylascomycin and 32-deoxy-32-epichloro-desmethylascomycin respectively, and methods for preparation thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |