WO2024257553A1 - 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 - Google Patents
結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 Download PDFInfo
- Publication number
- WO2024257553A1 WO2024257553A1 PCT/JP2024/018482 JP2024018482W WO2024257553A1 WO 2024257553 A1 WO2024257553 A1 WO 2024257553A1 JP 2024018482 W JP2024018482 W JP 2024018482W WO 2024257553 A1 WO2024257553 A1 WO 2024257553A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polyester resin
- crystalline polyester
- mol
- coating film
- aqueous dispersion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/16—Dicarboxylic acids and dihydroxy compounds
- C08G63/18—Dicarboxylic acids and dihydroxy compounds the acids or hydroxy compounds containing carbocyclic rings
- C08G63/181—Acids containing aromatic rings
- C08G63/183—Terephthalic acids
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D167/00—Coating compositions based on polyesters obtained by reactions forming a carboxylic ester link in the main chain; Coating compositions based on derivatives of such polymers
- C09D167/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/02—Emulsion paints including aerosols
Definitions
- the present invention relates to a crystalline polyester resin aqueous dispersion. More specifically, the present invention relates to a crystalline polyester resin aqueous dispersion suitable for use as a paint for cans, and even more specifically, to a crystalline polyester resin aqueous dispersion suitable for coating cans that contain beverages or foods (hereinafter collectively referred to as "food and beverages"), a paint composition containing the same, and a coating film made from the coating composition and a metal can having the coating film.
- beverages or foods hereinafter collectively referred to as "food and beverages”
- Metal cans such as beverage cans and food cans are coated with an organic resin to prevent corrosion of the metal by food (corrosion resistance) and to preserve the flavor and taste of the contents (flavor).
- This coating is subjected to high-stress processing such as necking and threading during the molding process of the mouth of a bottle can. Therefore, the coating needs to be durable against such post-processing (processability). It is also required to have adhesion to metal materials and hardenability.
- the can may be subjected to high temperature and high humidity conditions such as retort sterilization, and even in such cases, the coating must not only maintain adhesion to the metal material, but also not turn white (retort resistance).
- epoxy-based paints such as epoxy-phenolic paints, epoxy-amino-based paints, and epoxy-acrylic paints
- polyester-based paints such as polyester-phenolic paints, polyester-amino-based paints, and polyester-isocyanate-based paints
- vinyl chloride-based paints have been widely used as paints that have the above-mentioned corrosion resistance, flavor, and can withstand the molding process of cans.
- bisphenol A a raw material for epoxy resins
- vinyl chloride-based paints have problems with stabilizers and the generation of dioxins during incineration.
- Formaldehyde which is used as a raw material for phenolic resins and amino resins, and remains in the paint, is known to be harmful to the human body, including being carcinogenic, and to have a negative effect on the flavor of the contents.
- isocyanate resins are known to be harmful to the human body, including being carcinogenic.
- a resin composition for metal containers or metal lids has been proposed, for example, in which an acrylic-modified polyester resin obtained by graft-polymerizing a polymerizable unsaturated monomer component onto a polyester resin having an ethylenic double bond at the resin end, and a ⁇ -hydroxyalkylamide crosslinking agent are dispersed in an aqueous medium (Patent Document 1).
- Patent Document 2 proposes a method for producing an aqueous dispersion of crystalline polyester resin that is used to produce toner for developing electrostatic images.
- the crystalline polyester resin described in Patent Document 2 is intended for the manufacture of toner for developing electrostatic images, and it was found that when used as a resin composition for metal containers or metal lids, the retort resistance is poor due to its low melting point. Furthermore, when the melting point of the crystalline polyester resin is increased in order to improve retort resistance, the solvent solubility deteriorates, resulting in the problem that a crystalline polyester resin aqueous dispersion cannot be obtained.
- the object of the present invention is to provide an aqueous dispersion of crystalline polyester resin which is substantially free of curing agents and thus eliminates harmful substances such as bisphenol A and formaldehyde, has excellent solvent solubility and therefore requires a relatively small amount of organic solvent when dispersing in water, and is capable of forming a coating film with excellent processability and retort resistance properties, as well as a paint composition, coating film, and metal can containing the same.
- the object of the present invention is to provide an aqueous dispersion based on crystalline polyester resin which combines excellent retort resistance and solvent solubility, as well as a paint composition, coating film, and metal can containing the same.
- the present inventors have conducted extensive research to achieve this object, and have found that by using specific polyvalent carboxylic acid components and polyhydric alcohol components of the crystalline polyester resin, the planarity and symmetry of the molecule can be partially broken while maintaining high crystallinity to a certain extent, and as a result, the coating film obtained by the crystalline polyester resin aqueous dispersion can satisfy both retort resistance and processability, and can also satisfy the solvent solubility of the resin when it is dispersed in water. Furthermore, the acid value and melting point of the crystalline polyester resin are also important, and it has been found that by setting these within specific ranges, retort resistance, processability, and solvent solubility can be achieved at a higher level.
- a crystalline polyester resin aqueous dispersion comprising the following crystalline polyester resin (A): Crystalline polyester resin (A): A crystalline polyester resin having a polycarboxylic acid component and a polyhydric alcohol component as copolymerization components, containing 75 mol% or more of terephthalic acid as the polycarboxylic acid component and 55 mol% or more of 1,6-hexanediol as the polyhydric alcohol component, having an acid value of 180 eq/ton or more, and having a melting point of 120 to 160°C.
- a coating composition comprising the crystalline polyester resin aqueous dispersion according to [1] or [2], wherein the curing agent content is less than 1 part by mass per 100 parts by mass of the crystalline polyester resin (A) (solid content) in the aqueous dispersion.
- a metal can having the coating film according to [4].
- the crystalline polyester resin aqueous dispersion of the present invention has excellent solvent solubility when dispersed in water because the crystalline polyester resin in the aqueous dispersion is configured as described above, and can form a coating film with excellent processability and retort resistance properties even without containing a curing agent, and can eliminate harmful substances such as bisphenol A and formaldehyde. Therefore, the crystalline polyester resin aqueous dispersion of the present invention is suitable for coating compositions, coating films, and metal cans for use in beverage cans and food cans.
- the crystalline polyester resin (A) of the present invention has a chemical structure that can be obtained by a polycondensation product of a polycarboxylic acid and a polyhydric alcohol, and the polycarboxylic acid and the polyhydric alcohol each consist of one or more selected components.
- the copolymerization amount of terephthalic acid When the total amount of the polyvalent carboxylic acid components used in the present invention is taken as 100 mol%, the copolymerization amount of terephthalic acid must be 75 mol% or more, preferably 80 mol% or more, and more preferably 85 mol% or more. By making it 75 mol% or more, the crystallinity and glass transition temperature of the obtained polyester will be high, and the retort resistance of the coating film will be good.
- polycarboxylic acid components other than terephthalic acid used in the present invention are not particularly limited, but for example, the following polycarboxylic acids or their esters and anhydrides can be used.
- the polyvalent carboxylic acid component other than terephthalic acid used in the present invention can be a dicarboxylic acid (a) (hereinafter referred to as component (a)) that has a ring structure and, if the ring is a naphthalene ring, has carboxylic acid groups at the 2- and 6-positions, and if the ring is a cyclohexyl ring, has carboxylic acid groups at the 1- and 4-positions. It is preferable that the carboxylic acid group of component (a) is directly bonded to the ring structure.
- component (a) include 2,6-naphthalenedicarboxylic acid and 1,4-cyclohexanedicarboxylic acid. These can be used alone or in combination of two or more kinds.
- the polyvalent carboxylic acid component used in the present invention preferably contains component (a).
- component (a) the solvent solubility can be improved without significantly impairing the crystallinity of the polyester resin.
- a dicarboxylic acid having a naphthalene ring as a ring structure and carboxylic acid groups at the 2- and 6-positions is more preferable, since it has less steric hindrance and is particularly easy to maintain crystallinity.
- the copolymerization ratio (mol%) of component (a) is preferably 5 to 20 mol%, more preferably 8 to 18%, and even more preferably 10 to 15 mol%.
- the solvent solubility of the resulting polyester is good, making it possible to obtain a stable crystalline polyester resin aqueous dispersion.
- a moderate glass transition temperature is obtained, making it possible to achieve both retort resistance and processability of the coating film.
- the polyvalent carboxylic acid component other than terephthalic acid used in the present invention includes a dicarboxylic acid (b) (hereinafter referred to as component (b)) having a ring structure and excluding component (a).
- component (b) dicarboxylic acid
- the ring structure of component (b) is preferably a benzene ring, a furan ring, a naphthalene ring, or a cyclohexyl ring.
- the carboxylic acid group of component (b) is preferably directly bonded to the ring structure.
- component (b) examples include isophthalic acid, 1,2-naphthalenedicarboxylic acid, 1,3-naphthalenedicarboxylic acid, 1,4-naphthalenedicarboxylic acid, 1,5-naphthalenedicarboxylic acid, 1,6-naphthalenedicarboxylic acid, 1,7-naphthalenedicarboxylic acid, 1,8-naphthalenedicarboxylic acid, 2,3-naphthalenedicarboxylic acid, 2,7-naphthalenedicarboxylic acid, sodium 5-sulfoisophthalate, 2,5-furandicarboxylic acid, 1,2-cyclohexanedicarboxylic acid, 1,3-cyclohexanedicarboxylic acid, and hexahydrophthalic acid. These may be used alone or in combination.
- the (b) component is an optional component, and when the total amount of the polyvalent carboxylic acid components is taken as 100 mol%, the copolymerization ratio of the (b) component is preferably 20 mol% or less. More preferably, it is 15 mol% or less, and even more preferably, it is 10 mol% or less, and it may be 0 mol%. By making it 20 mol% or less, the retort resistance of the obtained polyester is improved.
- the total copolymerization ratio (mol %) of components (a) and (b) is preferably 5 to 20 mol %, more preferably 8 to 18 mol %, and even more preferably 10 to 15 mol %, assuming that the total amount of polyvalent carboxylic acid components is 100 mol %.
- the solvent solubility of the resulting polyester is improved.
- the retort resistance of the resulting polyester is improved.
- the polycarboxylic acid components other than terephthalic acid used in the present invention may be aliphatic polycarboxylic acids, alicyclic polycarboxylic acids, aromatic polycarboxylic acids, etc. other than component (a) or (b).
- aliphatic polycarboxylic acids include fumaric acid, adipic acid, sebacic acid, malonic acid, succinic acid, etc.
- alicyclic polycarboxylic acids and aromatic polycarboxylic acids include those with three or more functional groups. These may be used alone or in combination.
- the polyhydric alcohol component used in the present invention contains 1,6-hexanediol.
- the linear chain structure and alkyl chain length of 1,6-hexanediol contribute to high crystallinity that satisfies solvent solubility, retort resistance, and processability.
- the copolymerization ratio of 1,6-hexanediol must be 55 mol% or more, more preferably 60 mol% or more, even more preferably 65 mol% or more, and particularly preferably 70 mol% or more.
- the crystallinity of the resulting polyester is high, and in addition to improving retort resistance, processability and solvent solubility are improved.
- It is also preferably 90 mol% or less, and even more preferably 80 mol% or less. By making it 90 mol% or less, the solvent solubility of the resulting polyester is improved, and a stable crystalline polyester resin water dispersion can be obtained.
- the polyhydric alcohol component other than 1,6-hexanediol used in the present invention is not particularly limited, but specific examples include polyhydric alcohol (c) having a side chain (hereinafter referred to as component (c)).
- Component (c) is preferably a diol having a side chain.
- the side chain in component (c) refers to an atom or atomic group that branches off from a main chain that has a hydrocarbon group (carbon chain) connecting two hydroxyl groups.
- the side chain in component (c) is preferably an alkyl group.
- the number of carbon atoms in the alkyl group is preferably 1 to 50, more preferably 2 to 40, and even more preferably 3 to 35.
- the number of side chains may be 1 or 2 or more.
- the alkyl group may be linear or may have a side chain.
- the component (c) include 1,2-propylene glycol, 1,2-butanediol, 1,3-butanediol, 2-methyl-1,3-propanediol, neopentyl glycol, 3-methyl-1,5-pentanediol, 2-methyl-1,3-hexanediol, 2-methyl-2-ethyl-1,3-propanediol, 2,2-diethyl-1,3-propanediol, 2-ethyl-2-n-propyl-1,3-propanediol, 2,2-di-n-propyl-1,3-propanediol, 2-n-butyl-2-ethyl-1,3-propanediol, 2,2-di-n-butyl-1,3-propanediol, 2,2-di-n-butyl-1,3-propane
- Component (c) is an optional component, and when the total amount of the polyhydric alcohol components is taken as 100 mol%, the copolymerization ratio of component (c) is preferably 20 mol% or less. More preferably, it is 15 mol% or less, and even more preferably, it is 10 mol% or less, and it may even be 0 mol%. By making it 20 mol% or less, it is possible to achieve both solvent solubility and retort resistance of the resulting polyester.
- the polyhydric alcohol component other than 1,6-hexanediol used in the present invention includes a linear polyhydric alcohol (d) (hereinafter referred to as component (d)).
- component (d) examples include ethylene glycol, 1,3-propylene glycol, 1,4-butanediol, 1,5-pentanediol, 1,8-octanediol, etc., and these can be used alone or in combination of two or more.
- Component (d) is an optional component, and when the total amount of the polyhydric alcohol components is taken as 100 mol%, the copolymerization ratio of component (d) is preferably 40 mol% or less. More preferably, it is 30 mol% or less, and even more preferably, it is 20 mol% or less, and it may even be 0 mol%. By making it 20 mol% or less, it is possible to achieve both solvent solubility and retort resistance of the resulting polyester.
- the polyhydric alcohol component other than 1,6-hexanediol used in the present invention includes polyhydric alcohol (e) having an aromatic ring skeleton or an alicyclic skeleton (hereinafter referred to as component (e)).
- component (e) examples include 1,4-cyclohexanedimethanol, tricyclodecane dimethanol, hydroquinone, catechol, resorcinol, etc., and these can be used alone or in combination of two or more.
- Component (e) is an optional component, and when the total amount of the polyhydric alcohol components is taken as 100 mol%, the copolymerization ratio of component (e) is preferably 30 mol% or less, more preferably 25 mol% or less, and even more preferably 20 mol% or less, and may even be 0 mol%. By keeping it 30 mol% or less, it is possible to achieve both solvent solubility and retort resistance of the resulting polyester.
- the copolymerization ratio is preferably less than 5 mol%, more preferably less than 1 mol%, and most preferably does not contain isosorbide. By making it less than 5 mol%, the solvent solubility and retort resistance of the resulting polyester can be improved.
- the polyvalent carboxylic acid component and polyhydric alcohol component constituting the crystalline polyester resin (A) in the present invention can be made from raw materials derived from biomass resources.
- Biomass resources include the stored energy of sunlight converted into starch or cellulose by the photosynthetic action of plants, the bodies of animals that grow by eating plants, and products made by processing plants or animals.
- biomass resources are plant resources, including, for example, wood, rice straw, rice husks, rice bran, old rice, corn, sugar cane, cassava, sago palm, soybean pulp, corn cobs, tapioca waste, bagasse, vegetable oil residue, potatoes, buckwheat, soybeans, oils and fats, waste paper, papermaking residues, marine product residues, livestock waste, sewage sludge, and food waste. More preferred are corn, sugar cane, cassava, and sago palm.
- polycarboxylic acid raw materials derived from biomass resources include adipic acid, sebacic acid, fumaric acid, itaconic acid, terephthalic acid, and 2,5-furandicarboxylic acid. These may be used alone or as a mixture of two or more.
- polyhydric alcohol raw materials derived from biomass resources include ethylene glycol, 1,2-propylene glycol, 1,3-propylene glycol, neopentyl glycol, 1,4-butanediol, and 1,4-cyclohexanedimethanol. These may be used alone or as a mixture of two or more.
- the trifunctional or higher polyhydric alcohol component glycerin, trimethylolethane, trimethylolpropane, mannitol, sorbitol, and pentaerythritol can be mentioned, and one or more of these can be used.
- the crystalline polyester resin (A) has a branched structure, the processability of the resulting coating film becomes good.
- the crystalline polyester resin (A) of the present invention can be given an acid value by any method.
- Methods for giving an acid value include a method of adding a compound having a polyvalent carboxylic anhydride group in the molecule in the later stage of polycondensation, and a method of giving a high acid value to the prepolymer (oligomer) stage and then polycondensing this to obtain a polyester resin having an acid value.
- the former method of adding a reaction is preferred because of the ease of operation and the ease of obtaining the target acid value.
- carboxylic acid monoanhydrides include phthalic anhydride, succinic anhydride, maleic anhydride, trimellitic anhydride, itaconic anhydride, and citraconic anhydride, and one or more of these can be selected and used.
- trimellitic anhydride is preferred from the standpoint of versatility and economy.
- carboxylic acid polyanhydrides include pyromellitic anhydride, 1,2,3,4-butane tetracarboxylic acid dianhydride, 1,2,3,4-cyclopentane tetracarboxylic acid dianhydride, 3,3',4,4'-benzophenone tetracarboxylic acid dianhydride, 2,3,6,7-naphthalene tetracarboxylic acid dianhydride, ethylene glycol bistrimellitate dianhydride, and 2,2',3,3'-biphenyl tetracarboxylic acid dianhydride, and one or more of these can be selected and used.
- ethylene glycol bistrimellitate dianhydride is preferred from the standpoint of versatility and economy.
- the compound having a polyvalent carboxylic acid anhydride group in the molecule to impart the acid value can be a carboxylic acid monoanhydride or a carboxylic acid polyanhydride, which can be used alone or in combination.
- the acid value of the crystalline polyester resin (A) in the present invention must be 180 eq/ton or more, preferably 200 eq/ton or more, more preferably 220 eq/ton or more, and even more preferably 250 eq/ton or more.
- the acid value at or above the lower limit, sufficient water dispersion stability can be ensured.
- There is no particular upper limit for the acid value but in order to reduce the amount of unreacted acid components and oligomers during the acid addition reaction, a value of 400 eq/ton or less is preferred.
- the concentration of the metal sulfonate salt in the crystalline polyester resin (A) is preferably less than 50 eq/ton. More preferably, it is 20 eq/ton or less, even more preferably, it is 10 eq/ton or less, and particularly preferably, it is 5 eq/ton. By making it less than 50 eq/ton, the hydrophilicity of the resulting polyester is reduced and the retort resistance is improved.
- the polyhydric alcohol component is distilled off from the esterified product obtained in the esterification reaction under reduced pressure at a temperature of 220 to 280°C, and the polycondensation reaction is continued until the desired molecular weight is reached.
- the reaction temperature for polycondensation is preferably 220 to 280°C, more preferably 240 to 275°C.
- the degree of reduced pressure is preferably 130 Pa or less. If the degree of reduced pressure is insufficient, the polycondensation time tends to be long, which is not preferable. It is preferable to gradually reduce the pressure from atmospheric pressure to 130 Pa or less over a period of 30 to 180 minutes.
- organic titanate compounds such as tetrabutyl titanate, or organic tin compounds such as germanium dioxide, antimony oxide, and tin octylate. From the standpoint of reaction activity, organic titanate compounds are preferred, and from the standpoint of resin coloration, germanium dioxide is preferred.
- the glass transition temperature of the crystalline polyester resin (A) in the present invention is preferably 10°C or higher, more preferably 15°C or higher, from the viewpoint of water resistance, particularly retort resistance of the coating film. Also, from the viewpoint of processability, it is preferably 40°C or lower, more preferably 35°C or lower.
- crystallinity refers to the melting point (Tm) exhibited by the polyester resin when it is measured under the above-mentioned conditions. Also, high crystallinity means that the polyester resin has a high melting point.
- the former method (a) is preferable from the viewpoint of film-forming properties.
- the temperature at which the crystalline polyester resin (A) is dissolved is preferably 40 to 160°C, more preferably 50 to 140°C, even more preferably 60 to 130°C, and most preferably 70 to 100°C. If the temperature is less than 40°C, the crystalline polyester resin (A) may not be sufficiently dissolved, and the entanglements between molecular chains may not be sufficiently disentangled, and if the temperature exceeds 160°C, there is an increased risk of deterioration of the crystalline polyester resin (A).
- Examples of organic solvents in which the crystalline polyester resin (A) can be dissolved by heating in the temperature range of 40 to 160°C include methyl ethyl ketone, cyclohexanone, dimethylacetamide, dimethylformamide, N-methylpyrrolidone, tetrahydrofuran, 1,4-dioxane, 1,3-dioxane, 1,3-dioxolane, 1,2-hexanediol, methyl cellosolve, butyl cellosolve, ethyl carbitol, butyl carbitol, propylene glycol monomethyl ether, propylene glycol monopropyl ether, propylene glycol monobutyl ether, triethylene glycol monobutyl ether, etc.
- the average particle size is less than 30 nm, there is a tendency for the film-forming properties to improve significantly, but this is undesirable as it makes it easier for fusion and aggregation to occur between the dispersed particles, resulting in a high possibility of thickening and poor dispersion.
- the crystalline polyester resin aqueous dispersion of the present invention is preferably prepared with a resin solids concentration of 10 to 45% by mass. More preferably, it is 15 to 40% by mass, and even more preferably, it is in the range of 20 to 35% by mass. If the resin solids concentration exceeds 45% by mass, the viscosity of the aqueous dispersion increases and aggregation between the resin particles becomes more likely to occur, resulting in a significant decrease in dispersion stability. Furthermore, if it is less than 10% by mass, it is difficult to say that it is practical from both the perspective of production and application.
- the coating composition of the present invention contains at least the above-mentioned crystalline polyester resin aqueous dispersion.
- the crystalline polyester (A) in the aqueous dispersion is contained as a base component.
- the component with the highest content (mass ratio) among the solid components (non-volatile components excluding volatile substances such as water and organic solvents) that form the coating film in the coating composition is defined as the base component.
- the coating composition of the present invention is capable of forming a coating film using only the crystalline polyester resin aqueous dispersion without the addition of a curing agent. Therefore, it is preferable that the coating composition of the present invention does not substantially contain a curing agent, that is, the curing agent content is preferably less than 1 part by mass (solids content equivalent) per 100 parts by mass (solids content equivalent) of the crystalline polyester resin (A) in the aqueous dispersion.
- the content of the curing agent is preferably less than 1 part by mass per 100 parts by mass of the crystalline polyester resin (A) (solid content). Less than 0.5 parts by mass is more preferable, less than 0.1 parts by mass is even more preferable, and it is most preferable that no curing agent is included. If the content of the curing agent is higher than the above range, not only is it less economical, but there is also a risk of reduced processability due to self-condensation reactions between the curing agents, volatilization of the blocking agent, generation of harmful outgassing such as formaldehyde, and poor stability during long-term storage.
- curing agent refers to a known curing agent that reacts with polyester resin to form a crosslinked structure.
- the form of the crosslinked structure include a reaction in which the unsaturated double bonds in the polyester resin react through a radical addition reaction, a cationic addition reaction, or an anionic addition reaction to generate an intermolecular carbon-carbon bond, or a condensation reaction, a polyaddition reaction, or an ester exchange reaction with a polyvalent carboxylic acid group or a polyhydric alcohol group in the polyester resin to form an intermolecular bond.
- curing agents include phenolic resins, amino resins, isocyanate compounds, epoxy compounds, ⁇ -hydroxylamide compounds, and unsaturated bond-containing resins.
- the coating composition of the present invention is ideal for coating food and beverage cans.
- various additives may be blended depending on the purpose.
- Plasticizers to improve solubility in organic solvents, leveling agents and surfactants to improve application properties, coating film smoothness, and appearance, lubricants to prevent scratches on the coating film, and even coloring pigments, and in some cases polyester resins other than the crystalline polyester resin of the present invention, and resins other than polyester resins, such as acrylic resin emulsions and polyurethane resin emulsions, can be blended within a range that does not impair the purpose of the present invention, such as food hygiene and flavor properties.
- the coating composition of the present invention can be blended with other resins for the purpose of modifying the coating film, such as by imparting flexibility or adhesion.
- other resins include amorphous polyesters, crystalline polyesters, ethylene-polymerizable unsaturated carboxylic acid copolymers, and ethylene-polymerizable carboxylic acid copolymer ionomers. Blending at least one resin selected from these may impart flexibility and/or adhesion to the coating film.
- the coating composition of the present invention can be applied to metal substrates for cans, such as aluminum, stainless steel, and tinplate, using a gravure roll coater, comma coater, or spray method.
- a gravure roll coater, comma coater, or spray method There are no particular limitations on the film thickness, but a dry film thickness of 3 to 18 ⁇ m is usually preferred, and a range of 5 to 15 ⁇ m is also preferred.
- the coating film is usually baked at a temperature in the range of about 180 to 260°C for about 20 seconds to 1 hour, and preferably at a temperature in the range of about 200 to 240°C for about 30 seconds to 10 minutes.
- the coating film made of the crystalline polyester resin aqueous dispersion of the present invention is baked within the above range and then aged. By aging, crystallization in the coating film is further promoted, resulting in good retort resistance.
- the coating film of the present invention is a substrate coated with a crystalline polyester resin water dispersion (two layers: substrate/crystalline polyester resin water dispersion). It may also have a configuration in which a layer made of another resin is superimposed on either the top or bottom of the crystalline polyester resin water dispersion layer. In that case, the layer made of another resin is referred to as a coating layer.
- the coating film of the present invention can be obtained by laminating the crystalline polyester resin water dispersion of the present invention on various substrates according to a conventional method, and further laminating another resin layer on top of it.
- the metal can of the present invention has the above-mentioned coating film.
- Metal cans can be obtained by coating one or both sides, and if necessary, the end faces, of a metal plate made of a metal material that can be used for, for example, beverage cans, canned food cans, their lids, caps, etc.
- the metal material include tinplate, tin-free steel, aluminum, etc.
- Metal plates made of these metal materials may be used that have been previously treated with phosphate, chromate chromate, chromate phosphate, or other anticorrosive treatments using rust-preventive agents, or that have been subjected to surface treatments aimed at improving the adhesion of the coating film.
- ⁇ Crystalline polyester resin (A)> Measurement of resin composition A crystalline polyester resin sample was dissolved in deuterated chloroform and subjected to 1H-NMR analysis using a nuclear magnetic resonance (NMR) device 400-MR manufactured by VARIAN Corp. The molar ratio was calculated from the integral value ratio.
- NMR nuclear magnetic resonance
- Tm melting point
- Tg glass transition temperature Measurement was performed using a differential scanning calorimeter (DSC) DSC-220 manufactured by Seiko Instruments Inc.
- the aging treatment of the crystalline polyester resin was performed under the aging treatment conditions of 100°C x 30 hours, and 5 mg of the sample of the crystalline polyester resin after the treatment was placed in an aluminum clamped lid type container and sealed, and nitrogen gas was flowed at 30 ml/min to create a nitrogen atmosphere, and then the sample was cooled to -50°C using liquid nitrogen, and then heated to 200°C at 20°C/min.
- the temperature at the apex of the maximum peak of the heat of fusion obtained in this process was determined as the melting point (Tm, unit: °C).
- Average particle size The average particle size of the polyester resin water dispersion was measured.
- a laser diffraction/scattering particle size distribution measuring device (Beckman Coulter Counter LS13 320) was used. The particle distribution was prepared on a volume basis using this device, and the average diameter value was taken as the average particle size.
- test piece A crystalline polyester resin aqueous dispersion was applied to one side of a tinplate (JIS G 3303 (2008) SPTE, 70 mm x 150 mm x 0.3 mm) using a bar coater so that the film thickness after drying would be 10 ⁇ 2 ⁇ m, and the coating was baked under baking conditions of 200°C x 30 seconds, and then aging treatment was performed at 80°C x 1 hour. This was used as a test piece (hereinafter referred to as the test piece).
- a direct current voltage of 5.0 V was applied between the aluminum plate electrode and the uncoated part on the back side of the test plate, and the electric current value was measured.
- a smaller electric current value means better bending characteristics. (judgement) ⁇ : Less than 0.5 mA ⁇ : 0.5 mA or more and less than 1.0 mA ⁇ : 1.0 mA or more and less than 2.0 mA ⁇ : 2.0 mA or more
- Synthesis of crystalline polyester resin (a) 650 parts by mass of terephthalic acid, 870 parts by mass of 1,6-hexanediol, 50 parts by mass of 1,4-cyclohexanedimethanol, and 0.4 parts by mass (0.03 mol% relative to the total acid component) of tetra-n-butyl titanate (hereinafter sometimes abbreviated as TBT) as a catalyst were charged into a 3L four-neck flask, and the temperature was gradually raised to 240 ° C. over 3 hours, while carrying out an esterification reaction.
- TBT tetra-n-butyl titanate
- the resulting crystalline polyester resin (a) had a reduced viscosity of 0.60 dl/g, a glass transition temperature (Tg) of 15° C., a crystalline melting point (Tm) of 155° C. and an acid value of 220 eq/ton.
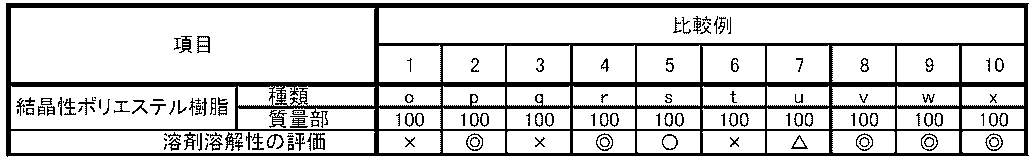
- compositions and properties of each crystalline polyester resin are shown in Table 1.
- crystalline polyester resin water dispersion (B-1) Manufacture of crystalline polyester resin water dispersion (B-1)
- the crystalline polyester resin (a) was dispersed in water. 20 parts by mass of the crystalline polyester resin (a) and 10 parts by mass of cyclohexanone were charged into a reaction vessel equipped with a stirrer, a condenser, and a thermometer, and the resin was dissolved at 130 ° C. for 2 hours. After dissolution, the temperature was lowered to 100 ° C., and 5 parts by mass of 2-propanol and 0.5 parts by mass of dimethylaminoethanol were added and stirred for 30 minutes. Next, 65 parts of hot water were added and stirred for 1 hour. The stirring speed was 200 rpm. The internal temperature was then lowered to room temperature to obtain a crystalline polyester resin water dispersion (B-1).
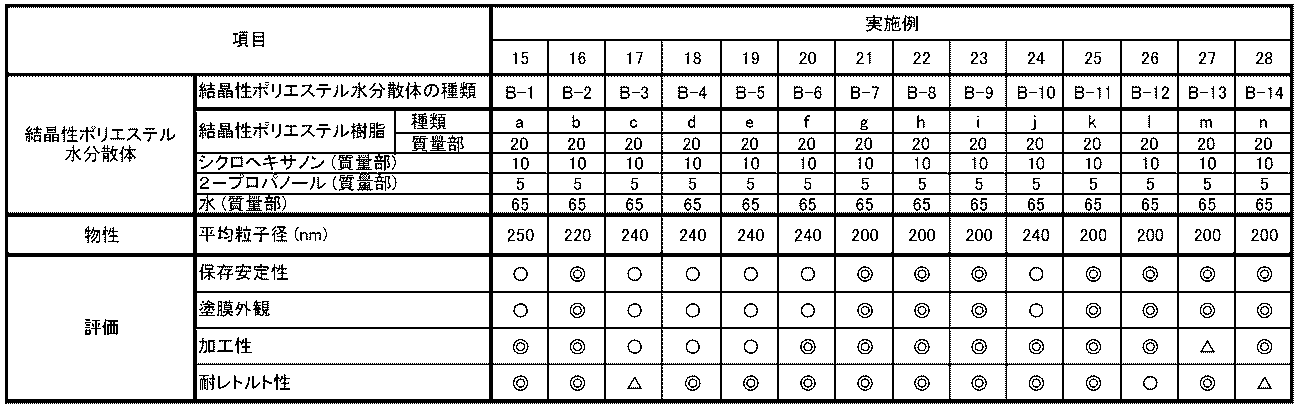
- the solvent solubility of the crystalline polyester resins of Examples 1 to 14 was excellent or good.
- the crystalline polyester resin aqueous dispersions of Examples 15 to 28, which used the crystalline polyester resins of Examples 1 to 14, respectively were excellent or good in terms of storage stability, coating film appearance when made into a coating film, processability, and retort resistance.
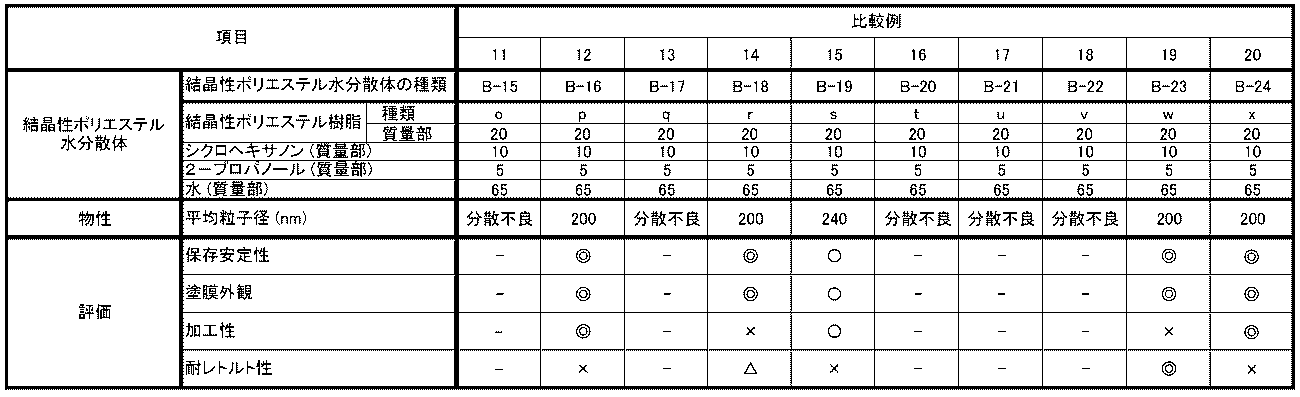
- the crystalline polyester resin of Comparative Example 1 had a high melting point, and therefore was poor in solvent solubility, and could not be used to make an aqueous dispersion in Comparative Example 11.
- the melting point of the crystalline polyester resin was lowered by using 1,7-heptanediol instead of 1,6-hexanediol, and an aqueous dispersion was made using it in Comparative Example 12, but the retort resistance of the coating film was significantly poor.
- Comparative Example 3 the melting point of the crystalline polyester resin was higher because 1,4-butanediol was used instead of 1,6-hexanediol, and therefore the solvent solubility was poor, and it could not be used to make an aqueous dispersion in Comparative Example 13.
- isophthalic acid was used instead of terephthalic acid, which probably caused the main chain of the crystalline polyester resin to bend more than necessary, and the coating film of the aqueous dispersion using it in Comparative Example 14 had significantly poor processability.
- Comparative Example 5 the melting point of the crystalline polyester resin was low, and therefore the coating film of the aqueous dispersion using it in Comparative Example 15 had significantly poor retort resistance.
- Comparative Examples 6 and 7 the copolymerization ratio of 1,6-hexanediol in the polyhydric alcohol component was low, resulting in poor solvent solubility, and in Comparative Examples 16 and 17, it was not possible to use them to make an aqueous dispersion.
- Comparative Example 8 the acid value of the crystalline polyester resin was low, and in Comparative Example 18, it was not possible to use them to make an aqueous dispersion.
- Comparative Example 9 the content of terephthalic acid in the crystalline polyester resin was low, resulting in a high glass transition temperature, and in Comparative Example 19, the processability of the coating film of the aqueous dispersion using it was significantly poor.
- Comparative Example 10 the content of terephthalic acid in the crystalline polyester resin was low, resulting in a low glass transition temperature, and in Comparative Example 20, the retort resistance of the coating film of the aqueous dispersion using it was significantly poor.
- the crystalline polyester resin of the present invention has excellent solvent solubility, and when used in a coating film, the aqueous dispersion using this resin has excellent coating film appearance, processability, and retort resistance, making it suitable as a base agent for paints applied to metal food and beverage cans, etc.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dispersion Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Polyesters Or Polycarbonates (AREA)
Abstract
Description
[1]下記の結晶性ポリエステル樹脂(A)を含むことを特徴とする結晶性ポリエステル樹脂水分散体。
結晶性ポリエステル樹脂(A):多価カルボン酸成分と多価アルコール成分を共重合成分とし、多価カルボン酸成分として、テレフタル酸を75モル%以上含有し、多価アルコール成分として、1,6-ヘキサンジオールを55モル%以上含有し、酸価が180eq/ton以上、かつ融点が120~160℃である結晶性ポリエステル樹脂。
[2]前記結晶性ポリエステル樹脂(A)のスルホン酸金属塩の濃度が50eq/ton未満である請求項1に記載の結晶性ポリエステル樹脂水分散体。
[3][1]または[2]に記載の結晶性ポリエステル樹脂水分散体を含有し、前記水分散体中の結晶性ポリエステル樹脂(A)(固形分)の100質量部に対し、硬化剤含有量が1質量部未満である塗料組成物。
[4][3]に記載の塗料組成物から得られる塗膜。
[5][4]に記載の塗膜を有する金属缶。
本発明の結晶性ポリエステル樹脂(A)は、多価カルボン酸と多価アルコールとの重縮合物によって得ることのできる化学構造からなり、多価カルボン酸と多価アルコールはそれぞれ1種または2種以上の選択された成分からなるものである。
本発明の結晶性ポリエステル樹脂水分散体は、結晶性ポリエステル樹脂(A)を結晶性ポリエステル樹脂(A)が溶解する水溶性有機溶剤に溶解し、必要に応じて塩基性化合物、水を逐次加え分散する方法(a)、結晶性ポリエステル樹脂(A)と水、結晶性ポリエステル樹脂(A)を溶解する水溶性有機溶剤、必要に応じて塩基性化合物を加え、加熱し分散する方法(b)等により作成することができる。また、有機溶剤を減量したい場合、あるいは完全に除去して水分散体化したい場合は100℃以下の沸点を有する有機溶剤を用いて分散した後、加熱、もしくは減圧下で溶剤を抜き取ることも可能である。結晶性ポリエステル樹脂の場合は、造膜性の点から前者の方法(a)で行うことが好ましい。
(1)樹脂組成の測定
結晶性ポリエステル樹脂の試料を、重クロロホルムに溶解し、VARIAN社製 核磁気共鳴(NMR)装置400-MRを用いて、1H-NMR分析を行った。その積分値比より、モル比を求めた。
結晶性ポリエステル樹脂の試料0.1gをフェノール/テトラクロロエタン(質量比6/4)の混合溶媒25ccに溶解し、30℃でウベローデ粘度菅を用いて測定した。
セイコーインスツルメンツ(株)製の示差走査型熱量計(DSC)DSC-220を用いて測定した。エージング処理条件100℃×30時間として結晶性ポリエステル樹脂のエージング処理を行い、処理後の結晶性ポリエステル樹脂の試料5mgをアルミニウム製の抑え蓋型容器に入れて密封し、窒素ガスを30ml/minで流し、窒素雰囲気下とした後、液体窒素を用いて-50℃まで冷却し、次いで200℃まで20℃/分にて昇温させた。この過程にて得られる融解熱の最大ピークの頂点の温度を融点(Tm、単位:℃)として求めた。また、前記測定装置を用いて、同条件で200℃まで昇温した後、-50℃まで急冷し、再度200℃まで20℃/分にて昇温させた。この過程にて得られる吸熱曲線において、吸熱ピークが出る前のベースラインと、吸熱ピークに向かう接線との交点の温度をガラス転移温度(Tg、単位:℃)とした。
結晶性ポリエステル樹脂の試料0.2gを40mlのクロロホルムに溶解し、0.01Nの水酸化カリウムエタノール溶液で滴定し、ポリエステル樹脂106gあたりの当量(eq/ton)を求めた。指示薬にはフェノールフタレインを用いた。
結晶性ポリエステル樹脂2gをシクロヘキサノン8gに溶解させ、100℃で3時間加温(静置)した時の結晶性ポリエステル樹脂の溶解状態を目視で以下のように判定した。
(判定)
◎:ほぼ全て溶解(80%以上溶解)
○:わずかに溶け残りあり(70%以上溶解)
△:少量の溶け残りあり(60%以上溶解)
×:溶け残りあり(50%未満)
ポリエステル樹脂水分散体の平均粒子径を測定した。測定には、レーザー回折・散乱法粒度分布測定装置(ベックマン社製コールターカウンターLS13 320)を用いた。そして、本装置により粒子分布を体積基準で作製し、平均径の値を平均粒子径とした。
ポリエステル樹脂水分散体を25℃で24時間静置した後の分散状態を目視で以下のように判定した。
(判定)
◎:外観変化なし
○:沈降物あり
×:固化している
ブリキ板(JIS G 3303(2008) SPTE、70mm×150mm×0.3mm)の片面にバーコーターで、結晶性ポリエステル樹脂水分散体を乾燥後の膜厚が10±2μmになるように塗装し、焼付条件200℃×30秒間として焼き付け、次に80℃×1hrのエージング処理を行った。これを試験片とした(以下、試験片という)。
得られた試験片の塗膜外観を目視で以下のように判定した。
(判定)
◎:ひび割れおよび凝集物の発生なし。
○:ひび割れまたは凝集物が出ている。
△:塗膜が一部剥がれている。
×:塗膜が全体的に剥がれている。
得られた試験片を、塗膜が外側となる方向に180°折り曲げ加工を施し、折り曲げ部に発生する塗膜の割れについて、通電値を測定することにより評価した。なお、折り曲げ加工は、間に何も挟み込まず(いわゆる0T)に折り曲げた。アルミ板製の電極(幅20mm、奥行き50mm、厚さ0.5mm)の上に1%NaCl水溶液に浸したスポンジ(幅20mm、奥行き50mm、厚さ10mm)を載せたものを用意し、スポンジの20mmの辺と平行になるように試験片の折り曲げ部の中央部付近をスポンジに接触させた。アルミ板電極と試験板の裏面の非塗装部との間に5.0Vの直流電圧をかけ、通電値を測定した。通電値が小さい方が折り曲げ特性が良好であることを意味する。
(判定)
◎:0.5mA未満
○:0.5mA以上1.0mA未満
△:1.0mA以上2.0mA未満
×:2.0mA以上
試験片を立ててステンレスカップに入れ、これにイオン交換水を試験片の半分の高さになるまで注ぎ、これをレトルト試験機(トミー工業(株)製 ES-315)の圧力釜の中に設置し、125℃×30分のレトルト処理を行なった。処理後の評価は一般的に塗膜に対してより厳しい条件にさらされることになると思われる蒸気接触部分で行い、硬化膜の白化、ブリスターの状態を目視で以下のように判定した。
(判定)
◎:良好(白化、ブリスターともになし)
○:わずかに白化はあるがブリスターはない
△:若干の白化および/または若干のブリスターがある
×:著しい白化および/または著しいブリスターがある
テレフタル酸650質量部、1,6-ヘキサンジオール870質量部、1,4-シクロヘキサンジメタノール50質量部、触媒としてテトラ-n-ブチルチタネート(以下、TBTと略記する場合がある)0.4質量部(全酸成分に対して0.03モル%)を3L四つ口フラスコに仕込み、3時間かけて240℃まで徐々に昇温しながら、エステル化反応を行った。エステル化反応終了後、系内を徐々に減圧していき、1時間かけて10mmHgまで減圧重合を行うとともに温度を250℃まで昇温し、さらに1mmHg以下の真空下で90分間後期重合を行なった。重縮合反応終了後、窒素雰囲気下で210℃に冷却し、次いで無水トリメリット酸を所定量投入し、窒素雰囲気下、220℃、30分攪拌を継続することで実施した。反応終了後、これを取り出し結晶性ポリエステル樹脂(a)を得た。得られた結晶性ポリエステル樹脂(a)の還元粘度は0.60dl/g、ガラス転移温度(Tg)は15℃、結晶融点(Tm)は155℃、酸価は220eq/tonであった。
結晶性ポリエステル樹脂(a)の合成と同様に、結晶性ポリエステル樹脂(b)~(f)、(n)~(u)、(x)は直接エステル化法にて、結晶性ポリエステル樹脂(g)~(m)、(v)~(w)はエステル交換法にて、但し仕込み組成を変更して、表1に示されるような樹脂組成で、それぞれ製造した。
得られた結晶性ポリエステル樹脂(a)~(n)、(о)~(x)を使用して溶剤溶解性の評価を実施した。それぞれの評価結果を表2,表3に示す。
以下の手順に従い、結晶性ポリエステル樹脂(a)の水分散化を行った。攪拌機、コンデンサー、温度計を具備した反応容器に結晶性ポリエステル樹脂(a)20質量部、シクロヘキサノン10質量部を仕込み、130℃にて2時間かけて樹脂を溶解した。溶解後に100℃まで降温し、2-プロパノール5質量部、ジメチルアミノエタノール0.5質量部を投入し30分撹拌を行った。次いで温水65部を投入し、1時間攪拌を行った。攪拌速度は200rpmとした。その後内温を室温まで降温し、結晶性ポリエステル樹脂水分散体(B-1)を得た。
結晶性ポリエステル樹脂水分散体(B-1)の製造と同様にして、結晶性ポリエステル樹脂(b)~(n)、(о)~(x)をそれぞれ使用して、結晶性ポリエステル樹脂水分散体(B-2)~(B-14)、(B-15)~(B-24)を得た。
得られた結晶性ポリエステル樹脂水分散体(B-1)~(B-14)、(B-15)~(B-24)を使用して各種特性評価を実施した。それぞれの評価結果を表4、表5に示す。
Claims (5)
- 下記の結晶性ポリエステル樹脂(A)を含むことを特徴とする結晶性ポリエステル樹脂水分散体。
結晶性ポリエステル樹脂(A):多価カルボン酸成分と多価アルコール成分を共重合成分とし、多価カルボン酸成分として、テレフタル酸を75モル%以上含有し、多価アルコール成分として、1,6-ヘキサンジオールを55モル%以上含有し、酸価が180eq/ton以上、かつ融点が120~160℃である結晶性ポリエステル樹脂。 - 前記結晶性ポリエステル樹脂(A)のスルホン酸金属塩の濃度が50eq/ton未満である請求項1に記載の結晶性ポリエステル樹脂水分散体。
- 請求項1または2に記載の結晶性ポリエステル樹脂水分散体を含有し、前記水分散体中の結晶性ポリエステル樹脂(A)(固形分)の100質量部に対し、硬化剤含有量が1質量部未満である塗料組成物。
- 請求項3に記載の塗料組成物から得られる塗膜。
- 請求項4に記載の塗膜を有する金属缶。
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202480014481.3A CN120752282A (zh) | 2023-06-13 | 2024-05-20 | 结晶性聚酯树脂水分散体、涂料组合物、涂膜及金属罐 |
| JP2024543996A JP7598568B1 (ja) | 2023-06-13 | 2024-05-20 | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2023097261 | 2023-06-13 | ||
| JP2023-097261 | 2023-06-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024257553A1 true WO2024257553A1 (ja) | 2024-12-19 |
Family
ID=93852061
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2024/018482 Pending WO2024257553A1 (ja) | 2023-06-13 | 2024-05-20 | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024257553A1 (ja) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS53105593A (en) * | 1977-02-25 | 1978-09-13 | Nippon Synthetic Chem Ind Co Ltd:The | Production of thermosetting polyester resin |
| JPS6268816A (ja) * | 1985-09-16 | 1987-03-28 | チバ−ガイギ− アクチエンゲゼルシヤフト | 枝分れしたポリエステル、その製造方法、粉体被覆組成物及び熱溶融型接着剤 |
| JPH04335059A (ja) * | 1991-05-10 | 1992-11-24 | Teijin Ltd | ポリエステル水分散体及びこれを塗布した易接着性ポリエステルフイルム |
| JPH07505419A (ja) * | 1992-04-03 | 1995-06-15 | ビーエーエスエフ アクチェンゲゼルシャフト | オレフィン系不飽和モノマーからなるヒドロキシル基含有プレポリマーをベースとするポリエステルおよび電子写真トナー用の結合剤としてのその使用 |
| JP2007277497A (ja) * | 2006-04-12 | 2007-10-25 | Toyobo Co Ltd | ポリエステル樹脂水分散体 |
| JP2013247177A (ja) * | 2012-05-24 | 2013-12-09 | Goo Chemical Co Ltd | リフトオフ法用レジスト剤 |
| JP2018123249A (ja) | 2017-02-02 | 2018-08-09 | コニカミノルタ株式会社 | 結晶性ポリエステル樹脂水性分散体の製造方法、静電荷像現像用トナーの製造方法 |
| JP2020079393A (ja) | 2018-11-13 | 2020-05-28 | 東洋製罐グループホールディングス株式会社 | 耐レトルト白化性に優れた水性塗料組成物及び塗装金属基体 |
| WO2022071219A1 (ja) * | 2020-09-30 | 2022-04-07 | 東洋紡株式会社 | グラフト変性生分解性ポリエステル樹脂水系分散体 |
-
2024
- 2024-05-20 WO PCT/JP2024/018482 patent/WO2024257553A1/ja active Pending
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS53105593A (en) * | 1977-02-25 | 1978-09-13 | Nippon Synthetic Chem Ind Co Ltd:The | Production of thermosetting polyester resin |
| JPS6268816A (ja) * | 1985-09-16 | 1987-03-28 | チバ−ガイギ− アクチエンゲゼルシヤフト | 枝分れしたポリエステル、その製造方法、粉体被覆組成物及び熱溶融型接着剤 |
| JPH04335059A (ja) * | 1991-05-10 | 1992-11-24 | Teijin Ltd | ポリエステル水分散体及びこれを塗布した易接着性ポリエステルフイルム |
| JPH07505419A (ja) * | 1992-04-03 | 1995-06-15 | ビーエーエスエフ アクチェンゲゼルシャフト | オレフィン系不飽和モノマーからなるヒドロキシル基含有プレポリマーをベースとするポリエステルおよび電子写真トナー用の結合剤としてのその使用 |
| JP2007277497A (ja) * | 2006-04-12 | 2007-10-25 | Toyobo Co Ltd | ポリエステル樹脂水分散体 |
| JP2013247177A (ja) * | 2012-05-24 | 2013-12-09 | Goo Chemical Co Ltd | リフトオフ法用レジスト剤 |
| JP2018123249A (ja) | 2017-02-02 | 2018-08-09 | コニカミノルタ株式会社 | 結晶性ポリエステル樹脂水性分散体の製造方法、静電荷像現像用トナーの製造方法 |
| JP2020079393A (ja) | 2018-11-13 | 2020-05-28 | 東洋製罐グループホールディングス株式会社 | 耐レトルト白化性に優れた水性塗料組成物及び塗装金属基体 |
| WO2022071219A1 (ja) * | 2020-09-30 | 2022-04-07 | 東洋紡株式会社 | グラフト変性生分解性ポリエステル樹脂水系分散体 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2022168910A1 (ja) | ポリエステル樹脂組成物、水分散体、塗料組成物および塗膜 | |
| JP7517628B1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| WO2022168912A1 (ja) | ポリエステル樹脂組成物、水分散体、塗料組成物および塗膜 | |
| WO2022168911A1 (ja) | ポリエステル樹脂組成物、水分散体、塗料組成物および塗膜 | |
| JP7598568B1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| JP7548472B1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| JP7598567B1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| WO2024257553A1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| WO2024157685A1 (ja) | 結晶性ポリエステル樹脂水分散体、塗料組成物、塗膜及び金属缶 | |
| JP7415282B2 (ja) | 共重合ポリエステルおよび水分散体 | |
| WO2024157682A1 (ja) | 結晶性ポリエステル樹脂、塗料組成物、塗膜及び金属缶 | |
| WO2025220398A1 (ja) | 結晶性ポリエステル樹脂 | |
| WO2022202831A1 (ja) | ポリエステル樹脂組成物、水分散体、塗料組成物および塗膜 | |
| WO2023013474A1 (ja) | ポリエステル樹脂組成物、水分散体、塗料組成物および塗膜 | |
| WO2023199973A1 (ja) | ポリエステル樹脂水性分散体、水性接着剤、水性塗料、水性インキおよびコーティング剤 | |
| WO2025164268A1 (ja) | ポリエステル樹脂、ポリエステル樹脂組成物、塗料組成物、塗膜及び金属缶 | |
| CN115777000A (zh) | 用于工业涂料的无定形共聚酯树脂和用于使用此类涂料组合物涂覆金属表面的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2024543996 Country of ref document: JP |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 24823175 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202480014481.3 Country of ref document: CN |
|
| WWP | Wipo information: published in national office |
Ref document number: 202480014481.3 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2024823175 Country of ref document: EP |