WO2021033768A1 - Copolymer and method for producing same, copolymer mixture, dope resin composition, and resin molded body and method for producing same - Google Patents
Copolymer and method for producing same, copolymer mixture, dope resin composition, and resin molded body and method for producing same Download PDFInfo
- Publication number
- WO2021033768A1 WO2021033768A1 PCT/JP2020/031644 JP2020031644W WO2021033768A1 WO 2021033768 A1 WO2021033768 A1 WO 2021033768A1 JP 2020031644 W JP2020031644 W JP 2020031644W WO 2021033768 A1 WO2021033768 A1 WO 2021033768A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- copolymer
- solvent
- film
- group
- cyclic
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/02—Monocarboxylic acids having less than ten carbon atoms, Derivatives thereof
- C08F20/10—Esters
- C08F20/12—Esters of monohydric alcohols or phenols
- C08F20/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F20/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F220/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F224/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a heterocyclic ring containing oxygen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2500/00—Characteristics or properties of obtained polyolefins; Use thereof
- C08F2500/26—Use as polymer for film forming
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2333/00—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Derivatives of such polymers
- C08J2333/04—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Derivatives of such polymers esters
Definitions
- the present invention relates to a copolymer and a method for producing the same, a copolymer mixture, a dope resin composition, and a resin molded product and a method for producing the same.
- Copolymers containing structural units derived from ⁇ -methylene lactone are excellent in transparency, heat resistance, and optical isotropic properties, and are expected to be applied to optical applications.
- Patent Document 1 describes that a film or the like, which is a molded product of a copolymer (resin) containing a constituent unit derived from a predetermined ⁇ -methylene lactone, is suitable for use as an optical member.
- a copolymer containing a structural unit derived from ⁇ -methylene lactone tends to have low solubility in a solvent, so that polymerization is carried out without a solvent or in a dimethyl sulfoxide (DMSO) solvent.
- DMSO dimethyl sulfoxide
- the heat of polymerization cannot be removed by polymerization without a solvent, and the solvent itself is decomposed by heating to generate harmful substances in polymerization with a DMSO solvent, and it has explosiveness under specific conditions. Therefore, there is a problem in terms of safety and it is not suitable for industrialization.
- the present invention provides a method for producing a copolymer containing a structural unit derived from ⁇ -methylenelactone by polymerization using a solvent, which can improve the transparency of the obtained copolymer.
- the main purpose is to provide.
- the present invention relates to the following methods for producing a copolymer according to [1] to [8], the copolymer according to [9] to [11], and the copolymer according to [12] to [14].
- [1] A method for producing a copolymer containing a structural unit derived from ⁇ -methylenelactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, which is ⁇ in the presence of a solvent.
- a copolymer comprising a step of polymerizing a monomer containing methylene lactone and alkyl (meth) acrylate, wherein the solvent is a solvent satisfying either the following condition (A) or the following condition (B).
- the second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, and is a mixed solvent having a boiling point of 70 to 120 ° C.
- the solvent is a solvent that satisfies the condition (B).
- the second solvent is ⁇ -butyrolactone, ⁇ -valerolactone, ⁇ -valerolactone, N, N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, N, N'-dimethylimidazolidinone.
- a dope resin composition of 5% by mass or more As a reference, a dope resin composition of 5% by mass or more.
- a resin composition containing the copolymer according to any one of [9] to [11] or the copolymer mixture according to any one of [12] to [14] is molded into a resin molded product.
- a method for producing a resin molded product comprising a step of obtaining the above.
- Production of a resin molded product comprising a step of applying the dope resin composition according to [15] and a step of removing a dispersion medium from the coated dope resin composition to obtain a resin molded product.
- the present invention there is a method for producing a copolymer containing a structural unit derived from ⁇ -methylenelactone by polymerization using a solvent, which can improve the transparency of the obtained copolymer.
- the method for producing a copolymer according to some forms facilitates a polymerization reaction under reflux.
- a copolymer obtained by such a production method and a copolymer mixture containing the copolymer.
- the copolymer mixture according to some forms tends to be excellent in reducing the processing load on the formed film, the strength of the formed film, and the like.
- a dope resin composition using the copolymer or the copolymer mixture, a resin molded product, and a method for producing the same.
- resin (composition) is a broader concept than “(co) polymer”.
- the resin may be composed of, for example, one kind or two or more kinds of (co) polymers, or may contain an additive such as an antioxidant other than the (co) polymer, if necessary. May be good.
- the method for producing a copolymer according to an embodiment is a method for producing a copolymer containing a structural unit derived from ⁇ -methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms. Is.
- the structural unit derived from ⁇ -methylene lactone is formed by the polymerization of ⁇ -methylene lactone in which a methylene group is bonded to the carbon at the ⁇ position.
- the specific structure of the structural unit derived from ⁇ -methylene lactone is not particularly limited.
- the number of ring members of the lactone is not particularly limited, but is preferably a 5-membered ring ( ⁇ -lactone) or a 6-membered ring because the ring structure is highly stable and higher surface strength can be obtained based on this high stability. ( ⁇ -Lactone).
- ⁇ -methylene lactone having a 5-membered ring or a 6-membered ring are ⁇ -methylene- ⁇ -butyrolactone and ⁇ -methylene- ⁇ -valerolactone. These may have substituents.
- the structural unit derived from ⁇ -methylene lactone is preferably a structural unit having a structure represented by the following formula (1).
- R 1 to R 4 in the formula (1) are hydrogen atoms or hydrocarbon groups having 1 to 18 carbon atoms independently of each other.
- the structural unit having the structure represented by the formula (1) can be formed by polymerizing a monomer containing ⁇ -methylene- ⁇ -butyrolactone represented by the following formula (2).
- R 1 to R 4 in the formula (2) are hydrogen atoms or hydrocarbon groups having 1 to 18 carbon atoms independently of each other.
- the hydrocarbon group is an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- the aliphatic hydrocarbon group is, for example, an alkyl group.
- the alkyl group preferably has 1 to 10 carbon atoms, and more preferably 1 to 8 carbon atoms.
- the alkyl group may be linear, branched, or cyclic. Examples of the alkyl group include a methyl group, an ethyl group, an n-propyl group, an i-propyl group, an n-butyl group, a t-butyl group, an n-pentyl group, an n-hexyl group, a cyclopentyl group, a cyclohexyl group and the like. Can be mentioned.
- the aromatic hydrocarbon group is not particularly limited, and may contain, for example, a heterocyclic structure.
- Examples of the aromatic hydrocarbon group include a phenyl group, a tolyl group, a benzyl group and the like.
- R 1 to R 4 are preferably hydrogen atoms or alkyl groups having 1 to 10 carbon atoms, more preferably all hydrogen atoms, independently of each other.
- the content of the constituent unit derived from ⁇ -methylene lactone in the copolymer is preferably 5 to 40% by mass, more preferably 7.5 to 35% by mass, and further preferably 10 from the viewpoint of further improving heat resistance and the like. ⁇ 30% by mass.
- the content of each structural unit in the copolymer can be obtained by dissolving the copolymer in a deuterated solvent, measuring 1 1 H-NMR, and calculating the area ratio of the peak corresponding to each structural unit. ..
- the structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms is formed by polymerization of alkyl (meth) acrylate.
- alkyl group having 1 to 6 carbon atoms in the alkyl (meth) acrylate include a methyl group, an ethyl group, an n-propyl group, an i-propyl group, an n-butyl group, a t-butyl group and an n-pentyl group. , N-hexyl group, cyclopentyl group, cyclohexyl group and the like. These may be used individually by 1 type, and may be used in combination of 2 or more type.
- the number of carbon atoms of the alkyl group in the alkyl (meth) acrylate is preferably 1 to 3, more preferably 1 or 2, and even more preferably 1.
- the number of carbon atoms of the alkyl group in alkyl methacrylate is preferably 1 to 3, more preferably 1 or 2, and even more preferably 1.
- the content of the structural unit derived from alkyl (meth) acrylate in the copolymer is preferably 95 to 60% by mass, more preferably 92.5 to 65% by mass from the viewpoint of further improving heat resistance, transparency and the like. , More preferably 90 to 70% by mass.
- the copolymer may contain a structural unit derived from ⁇ -methylene lactone and a structural unit of other monomers other than the structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms. .. Specific examples thereof include benzyl (meth) acrylate, chloromethyl (meth) acrylate, 2-chloroethyl (meth) acrylate, styrene, vinyltoluene, ⁇ -methylstyrene, acrylonitrile, methyl vinylketone, ethylene, and propylene. Examples thereof include structural units derived from monomers such as vinyl acetate. These may be used individually by 1 type, and may be used in combination of 2 or more type.
- the content of other structural units in the copolymer is preferably 30% by mass or less, more preferably 20% by mass or less, and further preferably 10% by mass or less.
- the method for producing a copolymer according to the present embodiment includes a step of polymerizing a monomer containing ⁇ -methylenelactone and alkyl (meth) acrylate in the presence of a solvent.
- the solvent is a solvent that satisfies either the following condition (A) or the following condition (B).
- Condition (A) At least one solvent selected from the group consisting of cyclic amides and cyclic esters.
- the second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, and is a mixed solvent having a boiling point of 70 to 120 ° C.
- the method for producing a copolymer according to the present embodiment it is possible to improve the transparency of the obtained copolymer.
- the reason why such an effect is exerted is not always clear, but the present inventors consider that the cause of coloring (decrease in transparency) is the formation of a homopolymer of ⁇ -methylene lactone.
- the homopolymer is soluble in the DMSO solvent alone, but tends to be insoluble or difficult to dissolve in the solvent satisfying the condition (A) or the solvent satisfying the condition (B). Therefore, by polymerizing the monomer containing ⁇ -methylenelactone with a solvent satisfying the condition (A) or a solvent satisfying the condition (B), the formation of a homopolymer of ⁇ -methylenelactone is suppressed. It is believed that this can reduce the coloring of the copolymer and improve the transparency.
- the method for producing a copolymer according to the present embodiment polymerizes under reflux when producing a copolymer containing a structural unit derived from ⁇ -methylenelactone.
- the reaction becomes easy, and for example, the polymerization can be carried out in a reflux state at a general polymerization temperature (for example, 70 to 120 ° C.).
- a general polymerization temperature for example, 70 to 120 ° C.
- the heat of polymerization at the time of polymerization can be slowed down and the polymerization temperature can be controlled near the boiling point, so that the polymerization can proceed safely and stably.
- the boiling point of the mixed solvent is 120 ° C.
- the polymerization rate can be easily controlled, by-products are suppressed, and the polymerization temperature does not become too high than the boiling point of the (meth) alkyl acrylate monomer. Is. Further, when the boiling point of the mixed solvent is 70 ° C. or higher, it is advantageous in terms of productivity such as the viscosity of the polymer solution and the polymerization rate.
- the solvent represented by the condition (A) is at least one solvent selected from the group consisting of cyclic amides and cyclic esters, and may be one single solvent or a mixed solvent in which two or more are combined. It may be present, but it is preferably one kind of single solvent.
- the boiling point of the solvent (the boiling point of the single solvent or the boiling point of the mixed solvent) is preferably more than 200 ° C. and 300 ° C. or lower because the content of the solvent (compound) in the copolymer mixture can be easily controlled.
- cyclic amide examples include N-methylpyrrolidone (NMP), N, N'-dimethylimidazolidinone (DMI) and the like.
- NMP N-methylpyrrolidone
- DMI N, N'-dimethylimidazolidinone
- the cyclic amide is preferably NMP because of its high versatility.
- cyclic ester examples include ⁇ -butyrolactone (GBL), ⁇ -valerolactone, ⁇ -valerolactone and the like.
- GBL ⁇ -butyrolactone
- ⁇ -valerolactone ⁇ -valerolactone
- the cyclic ester is preferably GBL from the viewpoint of high versatility.
- the polymerization temperature and the polymerization time vary depending on the type of the monomer used, the ratio of use and the like, but the polymerization temperature is preferably 0 to 150 ° C., more preferably 50. It is about 150 ° C., more preferably 60 to 140 ° C.
- the polymerization time is preferably 0.5 to 20 hours, more preferably 1 to 10 hours.
- the solvent represented by the condition (B) is a mixed solvent composed of a first solvent having a boiling point of less than 100 ° C. and a second solvent having a boiling point of 100 ° C. or higher, and the first solvent is a ketone and chloride. At least one selected from the group consisting of alkyl, the second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, with a boiling point (of the mixed solvent) of 70-120. It is a mixed solvent at ° C.
- the first solvent is a solvent having a boiling point of less than 100 ° C., and is at least one solvent selected from the group consisting of ketones and alkyl chlorides.
- a first solvent include ketones such as acetone (ACE) and methyl ethyl ketone (MEK), and alkyl chlorides such as dichloromethane, chloroform, 1,2-dichloroethane, and 1,1-dichloroethane.
- ACE acetone
- MEK methyl ethyl ketone
- alkyl chlorides such as dichloromethane, chloroform, 1,2-dichloroethane, and 1,1-dichloroethane.
- the first solvent one type may be used alone, or two or more types may be used in combination.
- the first solvent is preferably acetone.
- the second solvent is a solvent having a boiling point of 100 ° C. or higher, and is at least one solvent selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides.
- a second solvent include cyclic ketones such as cyclohexanone (anone) and cyclopentanone, cyclic esters such as ⁇ -butyrolactone (GBL), ⁇ -valerolactone and ⁇ -valerolactone, N, N-.
- Examples thereof include amides such as dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone (NMP), N, N'-dimethylimidazolidinone (DMI), and sulfoxides such as dimethyl sulfoxide.
- the second solvent one type may be used alone, or two or more types may be used in combination.
- One aspect of the second solvent is preferably cyclic ketones, more preferably cyclohexanone.
- Another aspect of the second solvent is preferably at least one selected from the group consisting of cyclic esters, amides, and sulfoxides, more preferably ⁇ -butyrolactone, ⁇ -valerolactone, ⁇ -valerolactone, N, N. -Dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, N, N'-dimethylimidazolidinone, and at least one selected from the group consisting of dimethyl sulfoxide
- the combination of the first solvent and the second solvent may be a combination of acetone and cyclohexanone. According to the studies by the present inventors, it has been found that acetone and cyclohexanone tend to be difficult to dissolve the copolymers by themselves, but the above-mentioned copolymers are very easily dissolved. Therefore, by using such a mixed solvent, it becomes easy to carry out the polymerization reaction under reflux, and it becomes possible to improve the transparency of the obtained copolymer.
- the above-mentioned copolymer consists of an alkyl chloride having a boiling point of less than 100 ° C. as a first solvent and a group consisting of a cyclic ester, an amide, and a sulfoxide having a boiling point of 100 ° C. or higher as a second solvent. It tends to be easily soluble in at least one solvent of choice. Therefore, the combination of the first solvent and the second solvent is an alkyl chloride having a boiling point of less than 100 ° C. and a solvent having a boiling point of 100 ° C. or higher, from cyclic ketones, cyclic esters, amides, and sulfoxides.
- It may be a combination with at least one solvent selected from the above group, a solvent having a boiling point of less than 100 ° C., and at least one solvent selected from the group consisting of ketone and alkyl chloride, and a cyclic ester. It may be a combination with at least one solvent selected from the group consisting of, amide, and sulfoxide.
- the boiling point of the mixed solvent is 70 to 120 ° C, preferably 75 to 115 ° C, and more preferably 80 to 110 ° C.
- the boiling point of the mixed solvent means a value measured by the method described in Examples.
- the mixing ratio of the first solvent and the second solvent is not particularly limited as long as the boiling point of the mixed solvent is 70 to 120 ° C., and can be adjusted at any ratio.
- the mass ratio of the first solvent to the second solvent is preferably 1/9 or more, more preferably 2/8 or more, and preferably 2/8 or more. It is 9/1 or less, more preferably 8/2 or less, further preferably 7/3 or less, particularly preferably 6/4 or less, and most preferably 5/5 or less.
- the polymerization temperature and the polymerization time differ depending on the type of the monomer used, the ratio of use, etc., but the polymerization temperature is easy to control the polymerization rate and is a by-product.
- the temperature is preferably 120 ° C. or lower, and from the viewpoint of productivity such as the viscosity of the polymerization solution and the polymerization rate, it is preferable. It is 70 ° C. or higher.
- the polymerization temperature is more preferably 75 to 115 ° C, still more preferably 80 to 110 ° C.
- the polymerization time is preferably 0.5 to 20 hours, more preferably 1 to 10 hours.
- the method of charging each monomer component ( ⁇ -methylenelactone, alkyl (meth) acrylate, other monomer, etc.) into the reactor is not particularly limited, and before charging the polymerization initiator.
- a method in which the entire amount of the monomer is added a method in which the entire amount of the monomer is continuously added dropwise at the same time as the polymerization initiator is added, and a part of the monomer is first added and the remaining monomer is added after the polymerization is started.
- a polymerization initiator When polymerizing the monomer, a polymerization initiator may be added if necessary.
- the polymerization initiator include cumene hydroperoxide, diisopropylbenzene hydroperoxide, di-t-butyl peroxide, lauroyl peroxide, benzoyl peroxide, t-butylperoxyisopropyl carbonate, and t-amylperoxy-2.
- Organic peroxides such as -ethylhexanoate, t-butylperoxy-2-ethylhexanoate; 2,2'-azobis (isobutyronitrile), 1,1'-azobis (cyclohexanecarbonitrile), Examples thereof include azo compounds such as 2,2'-azobis (2,4-dimethylvaleronitrile) and dimethyl-2,2'-azobis (2-methylpropionate).
- the content ratio of the polymerization initiator may be appropriately set according to the combination of the monomers used, the reaction conditions, etc., and is not particularly limited, but is preferably 10 to 10000% by mass, more preferably, with respect to all the monomers. Is 100 to 3000 mass ppm, more preferably 300 to 2000 mass ppm.
- a chain transfer agent When polymerizing the monomer, a chain transfer agent may be added if necessary.
- the chain transfer agent include monofunctional thiol compounds such as n-dodecyl mercaptoethanol and ⁇ -mercaptopropionic acid; bifunctional thiol compounds such as biterminal mercapto-modified polysiloxane; side chain polyfunctional mercapto having mercapto-modified side chains. Examples include modified polysiloxane.
- the content ratio of the chain transfer agent may be appropriately set according to the combination of monomers to be used, reaction conditions, etc., and is not particularly limited, but is preferably 10 to 10000 mass ppm, more preferably, with respect to all the monomers. Is 100 to 3000 mass ppm.
- the concentration of the copolymer in the polymerization reaction mixture is 90% by mass or less, more preferably 70. It is mass% or less, more preferably 50 mass% or less. Further, if the concentration of the copolymer in the polymerization reaction mixture is too low, the productivity is lowered. Therefore, it is preferable to control the concentration of the polymer in the polymerization reaction mixture to be 10% by mass or more, more preferably. It is 20% by mass or more.
- the polymerization reaction mixture obtained through the polymerization step usually contains a solvent in addition to the target copolymer.
- the method for separating the copolymer from the solvent is not particularly limited, and examples thereof include a method by reprecipitation, a devolatilizer consisting of a heat exchanger and a devolatilization tank, and a method of desolving the copolymer using a vented extruder. Be done.
- the copolymer according to one embodiment contains a structural unit derived from ⁇ -methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, and has a thickness of 100 ⁇ m when formed into a film.
- the internal haze per hit is less than 2.5%.
- the copolymer of this embodiment can be a copolymer obtained by the above-mentioned production method. Since the above-mentioned production method can improve the transparency of the obtained copolymer, by using the production method, the internal haze per 100 ⁇ m thickness of the film and the time of the film are obtained. It may be possible to set the internal b * value or the like per 100 ⁇ m of the thickness of the L * a * b * color system within a predetermined range.
- the copolymer has an internal haze of less than 2.5% per 100 ⁇ m thickness when made into a film.
- the internal haze is preferably 2.0% or less, more preferably 1.5% or less, still more preferably 1.0% or less, and particularly preferably 0.8% or less.
- the internal haze per 100 ⁇ m thickness of the copolymer as a film can be measured by, for example, the method described in Examples. Further, the temperature at which the copolymer is hot-press molded may be, for example, 200 to 270 ° C., more specifically 240 ° C.
- the copolymer according to another embodiment contains a structural unit derived from ⁇ -methylene lactone and a structural unit derived from an alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, and has a weight average molecular weight (Mw). Is 200,000 or more and 1,000,000 or less.
- the copolymer is in a state in which the solvent used in the above production method is substantially free.
- substantially free means that the content of the solvent may be less than 10 parts by mass based on the total amount of the copolymer.
- the solvent content can be measured by, for example, the method described in Examples using a film formed from a copolymer or a copolymer mixture.
- the internal b * value per 100 ⁇ m of the thickness of the L * a * b * color system when formed into a film is preferably less than 1.6.
- the internal b * value is more preferably 1.2 or less, further preferably 0.8 or less, particularly preferably 0.6 or less, and most preferably 0.4 or less.
- the internal b * value per 100 ⁇ m of the thickness of the L * a * b * color system when made into a film of a copolymer can be measured by, for example, the method described in Examples. Further, the temperature at which the copolymer is hot-press molded may be, for example, 200 to 270 ° C., more specifically 240 ° C.
- the weight average molecular weight (Mw) of the copolymer is preferably 100,000 or more, more preferably 150,000 or more, further preferably 200,000 or more, particularly preferably 220,000 or more, and most preferably 240000 or more.
- the weight average molecular weight (Mw) of the copolymer is preferably 1,000,000 or less, more preferably 750000 or less, still more preferably 500,000 or less.
- the Mw of the copolymer can be measured, for example, by the method described in Examples.
- the number average molecular weight (Mn) of the copolymer is preferably 20,000 or more, more preferably 50,000 or more, and further preferably 100,000 or more.
- the number average molecular weight (Mn) of the copolymer is preferably 500,000 or less, more preferably 400,000 or less, still more preferably 300,000 or less.
- the Mn of the copolymer can be measured by, for example, the method described in Examples.
- the dispersity (Mw / Mn) of the copolymer is preferably 3.0 or less, more preferably 2.8 or less, and further preferably 2.5 or less.
- the copolymer has a yellowness (YI) measured in accordance with JIS Z 8729 when a 15% chloroform solution of the copolymer is used, preferably 5 or less, more preferably 3 or less, still more preferable. Is less than or equal to 1. When the YI of the copolymer is in such a range, a low-colored resin molded product can be obtained.
- YI yellowness
- the glass transition temperature (Tg) measured in accordance with JIS K 7121 of the copolymer is preferably 110 ° C. or higher, more preferably 115 ° C. or higher, still more preferably 115 ° C. or higher, from the viewpoint of further improving heat resistance and the like. It is 120 ° C. or higher.
- the upper limit of the glass transition temperature of the copolymer is not particularly limited, but can be, for example, 160 ° C. or lower.
- the 5% weight loss temperature of the copolymer is preferably 280 ° C. or higher, more preferably 290 ° C. or higher, still more preferably 300 ° C. or higher, from the viewpoint of further improving heat resistance and the like.
- the upper limit of the 5% weight loss temperature of the copolymer is not particularly limited, but can be, for example, 400 ° C. or lower.
- the 5% weight loss temperature can be measured, for example, by the method described in Examples.
- the copolymer mixture includes a copolymer containing a structural unit derived from ⁇ -methylenelactone and a structural unit derived from an alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, and a cyclic amide. It contains a cyclic ester and at least one compound selected from the group consisting of cyclic ketones.
- One aspect of the copolymer mixture contains the above-mentioned copolymer and at least one compound selected from the group consisting of cyclic amides, cyclic esters, and cyclic ketones.
- the content of the compound is preferably 10 to 3000 mass ppm based on the total amount of the copolymer.
- the content of the compound is more preferably 200 mass ppm or more, further preferably 300 mass ppm or more, still more preferably 2500 mass ppm or less, still more preferably 2000 mass ppm or less, based on the total amount of the copolymer. is there.
- the content of the compound When the content of the compound is in such a range, the processability of the resin, the reduction of the processing load on the formed film, the strength of the formed film and the like tend to be excellent.
- the content of the compound can be measured by, for example, the method described in Examples using a film formed from a copolymer or a mixture of copolymers.
- the copolymer mixture is obtained by leaving the solvent (compound) so that the content of the compound is within a predetermined range when the copolymer is separated from the solvent in the above-mentioned method for producing a copolymer. It can also be obtained by adding a compound so that the content of the compound is within a predetermined range in the copolymer after isolation.
- the doped resin composition contains the above-mentioned copolymer and a dispersion medium.
- the doped resin composition can be suitably used for producing a resin molded product.
- an alkyl chloride solvent such as chloroform and dichloromethane
- an aromatic solvent such as toluene, xylene and benzene
- an alcohol solvent such as methanol, ethanol, isopropanol, n-butanol and 2-butanol.
- These may be used individually by 1 type, and may be used in combination of 2 or more type. However, when two or more kinds are used in combination, the solvent represented by the above condition (B) is excluded.
- the boiling point of the mixed solvent is preferably 70 to 120 ° C.
- the combination of the first solvent and the second solvent of the mixed solvent, the boiling point of the mixed solvent, the mixing ratio of the first solvent and the second solvent, and the like are determined by the above-mentioned first solvent and the second solvent.
- the combination is the same as the boiling point of the mixed solvent, the mixing ratio of the first solvent and the second solvent, and the like. Therefore, a duplicate description will be omitted here.
- the first solvent may be further added to the above-mentioned mixed solvent.
- the boiling point of the dispersion medium when the first solvent is further added to the above-mentioned mixed solvent is preferably 30 to 110 ° C, more preferably 40 to 100 ° C.
- the content of the copolymer in the dope resin composition is 5% by mass or more, preferably 10% by mass or more, more preferably 10% by mass or more, based on the total amount of the dope resin composition, from the viewpoint of efficiently producing the resin molded product. Is 15% by mass or more, more preferably 20% by mass or more.
- the content of the copolymer is preferably 60% by mass or less, more preferably 50% by mass, based on the total amount of the doped resin composition, from the viewpoint of ensuring fluidity in order to stably produce in the production equipment. Hereinafter, it is more preferably 40% by mass or less.
- the doped resin composition may contain other polymers in the resin molded product described later.
- the content of the other polymer is preferably 0 to 50% by mass, more preferably 0 to 40% by mass, still more preferably 0 to 30% by mass, and particularly preferably 0 to 0 to 50% by mass based on the total amount of the doped resin composition. It is 20% by mass, most preferably 0 to 10% by mass.
- the doped resin composition may contain other additives in the resin molded product described later.
- the doped resin composition can contain one or more other additives.
- the content of the other additives is preferably 0 to 5% by mass, more preferably 0 to 2% by mass, still more preferably 0 to 0.5% by mass, based on the total amount of the doped resin composition.
- the yellowness (YI) measured in accordance with JIS Z 8729 of the doped resin composition is preferably 5 or less, more preferably 3 or less, still more preferably 1 from the viewpoint of obtaining a low-colored resin molded product. It is as follows.
- the viscosity of the doped resin composition at 25 ° C. is preferably 0.001 Pa ⁇ s or more, more preferably 0.01 Pa ⁇ s or more, still more preferably 0.1 Pa ⁇ s, from the viewpoint of improving the productivity of the resin molded product.
- the above is preferably 10 Pa ⁇ s or less, more preferably 5 Pa ⁇ s or less, still more preferably 1 Pa ⁇ s or less.
- the viscosity at 25 ° C. can be measured by, for example, the method described in Examples.
- the haze measured in accordance with JIS K7136 of the doped resin composition is preferably 5 or less, more preferably 3 or less, and further preferably 1 or less from the viewpoint of obtaining a highly transparent resin molded product.
- the resin molded product according to one embodiment contains the above-mentioned copolymer or the above-mentioned copolymer mixture as a main component.
- the resin molded product according to the present embodiment is a resin composition containing the above-mentioned copolymer or the above-mentioned copolymer mixture, or a dope resin composition containing the above-mentioned copolymer or the above-mentioned copolymer mixture. Can be manufactured using.
- the content of the copolymer or the copolymer mixture is preferably 50 to 100% by mass, more preferably 60 to 100% by mass, still more preferably 70 to 100% by mass, particularly preferably 70 to 100% by mass, based on the total amount of the resin molded product. Is 80 to 100% by mass, most preferably 90 to 100% by mass.
- the content of the copolymer or the copolymer mixture in the resin molded product is 50% by mass or more, a resin molded product having better transparency can be obtained.
- the resin molded product may contain a polymer (other polymer) other than the above-mentioned copolymer.
- other polymers include olefin polymers such as polyethylene, polypropylene, ethylene-propylene copolymer, and poly (4-methyl-1-pentene); halogen-containing polymers such as vinyl chloride and vinyl chlorinated resin; Acrylic polymers such as polymethyl methacrylate; styrene polymers such as polystyrene, styrene-methyl methacrylate copolymer, styrene-acrylonitrile copolymer, acrylonitrile-butadiene-styrene block copolymer; polyethylene terephthalate, polybutylene terephthalate, Polyesters such as polyethylene naphthalate; polyamides such as nylon 6, nylon 66, nylon 610; polyacetals; polycarbonates; polyphenylene oxides; polyphenylene sulfides; polyether ether keto
- the content of the other polymer is preferably 0 to 50% by mass, more preferably 0 to 40% by mass, still more preferably 0 to 30% by mass, particularly, based on the total amount of the resin molded product (resin composition). It is preferably 0 to 20% by mass, and most preferably 0 to 10% by mass.
- the resin molded product may contain other additives.
- Other additives include, for example, antioxidants such as hindered phenol-based, phosphorus-based and sulfur-based; stabilizers such as light-resistant stabilizers, weather-resistant stabilizers and heat stabilizers; reinforcing materials such as glass fibers and carbon fibers.
- Ultraviolet absorbers such as phenylsalicylate, (2,2'-hydroxy-5-methylphenyl) benzotriazole, 2-hydroxybenzophenone; near infrared absorbers; tris (dibromopropyl) phosphate, triallyl phosphate, antimony oxide Flame retardants such as; Antistatic agents such as anionic, cationic and nonionic surfactants; Colorants such as inorganic pigments, organic pigments and dyes; Organic fillers or inorganic fillers; Resin modifiers; Organic fillers or Inorganic fillers; plasticizers; lubricants; antistatic agents; flame retardants; fluidizing agents; compatibilizers and the like.
- the resin molded product may contain one or more other additives.
- the content of the other additives is preferably 0 to 5% by mass, more preferably 0 to 2% by mass, still more preferably 0 to 0.5% by mass, based on the total amount of the resin molded product.
- the resin molded body is preferably a film-shaped resin molded body or a sheet-shaped resin molded body and a planar resin molded body.
- the film-shaped resin molded body (film) means that the film thickness is less than 350 ⁇ m
- the sheet-shaped resin molded body (sheet) means that the film thickness is 350 ⁇ m or more. ..
- One aspect of the method for producing a resin molded product includes a step of molding a resin composition containing the above-mentioned copolymer or the above-mentioned copolymer mixture to obtain a resin molded product.
- the method for molding the resin composition is not particularly limited, and examples thereof include conventionally known methods such as a melt extrusion method, a calendar method, and a compression molding method. Among these, the method for molding the resin composition is preferably a melt extrusion method.
- the resin composition may contain the above-mentioned other polymer, the above-mentioned other additive, etc. in addition to the above-mentioned copolymer or the above-mentioned copolymer mixture according to the desired resin molded product. Good.
- the content of the copolymer or copolymer mixture, other polymer, other additives, etc. in the resin composition may be the same as the content of each component exemplified in the resin molded product.
- melt extrusion method examples include a T-die method, an inflation method, and the like.
- the molding temperature of the resin molded product is preferably 150 to 350 ° C., more preferably 200 to 300 ° C.
- Another aspect of the method for producing a resin molded product includes a step of applying the above-mentioned dope resin composition and a step of removing a dispersion medium from the coated dope resin composition to obtain a resin molded product.
- the method for applying the doped resin composition is not particularly limited, and examples thereof include conventionally known methods such as a solution casting method (solution casting method).
- a solution casting method solution casting method
- an apparatus such as a drum type casting machine, a band type casting machine, or a spin coater can be used.
- the method for removing the dispersion medium from the doped resin composition is not particularly limited, and examples thereof include a method of heating the doped resin composition to volatilize the dispersion medium.
- the heating temperature can be appropriately set according to the dispersion medium used.
- the film-shaped resin molded body can be made into a stretched film by stretching.
- the film is preferably a stretched film because of its excellent flexibility and, in some cases, the ability to impart a phase difference.
- a conventionally known stretching method can be applied, and examples thereof include uniaxial stretching such as free width uniaxial stretching and constant width uniaxial stretching; biaxial stretching such as sequential biaxial stretching and simultaneous biaxial stretching. Be done.
- the method of stretching the film is preferably biaxial stretching in that the bending resistance in any two orthogonal directions in the film plane is improved.
- the stretching temperature when stretching the film is preferably near the glass transition temperature of the above-mentioned copolymer. More specifically, it is preferably (glass transition temperature -30) ° C to (glass transition temperature +100) ° C, more preferably (glass transition temperature -20) ° C to (glass transition temperature +50) ° C, and even more preferably (glass).
- the transition temperature is ⁇ 10) ° C. to (glass transition temperature +30) ° C.
- the stretching ratio when stretching the film may be, for example, in the range of 1.05 to 10 times in each of the vertical and horizontal directions.
- the film thickness of the film-shaped resin molded product (film) is preferably 1 ⁇ m or more and less than 350 ⁇ m, and more preferably 10 ⁇ m or more and 300 ⁇ m or less.
- the film thickness of the sheet-shaped resin molded product (sheet) is preferably 350 ⁇ m or more and 10 mm or less, and more preferably 350 ⁇ m or more and 5 mm or less.
- the total light transmittance of the film measured by a method according to JIS K7136 is preferably 85% or more, more preferably 88% or more, still more preferably 90% or more, and particularly preferably 90% or more. It is 92% or more.
- the total light transmittance is a measure of transparency, and when it is 85% or more, the transparency of the film can be sufficient.
- the elastic modulus of the film is preferably 4 GPa or more, more preferably 4.5 GPa or more, and further preferably 5 GPa or more from the viewpoint of further improving the strength of the film.
- the upper limit of the elastic modulus of the film is not particularly limited, but can be, for example, 15 GPa or less.
- the elastic modulus of the film can be measured, for example, by the method described in Examples.
- the Young's modulus of the film is preferably 4 GPa or more, more preferably 4.5 GPa or more, and further preferably 5 GPa or more from the viewpoint of further improving the strength of the film.
- the upper limit of the Young's modulus of the film is not particularly limited, but can be, for example, 15 GPa or less.
- the Young's modulus of the film can be measured, for example, by the method described in Examples.
- the pencil hardness of the film is preferably H or more, more preferably 2H or more, and further preferably 3H or more from the viewpoint of further improving the strength of the film.
- the film breaks into the bent portion even if the number of times of bending exceeds 100,000 in a bending test (foldable test) in which the film is repeatedly bent and returned in a U shape under predetermined conditions.
- the predetermined condition can be, for example, the condition described in the embodiment.
- the resin molded product of the present embodiment can be applied to various uses, for example, it can be suitably applied to optical applications.
- applications include light guide members, film applications, lenses (optical lenses, etc.), covers, foam applications (for example, cushioning materials, heat insulating / heat insulating materials, vibration damping materials, soundproofing materials, sealing materials). , Packing material, etc.).
- the resin molded product of the present embodiment can be suitably used for optical applications.
- the resin molded product of the present embodiment is excellent in transparency, heat resistance, flexibility, and surface hardness, it can be suitably used for flexible display applications, and in particular, It can be more preferably used as the outermost cover window.
- the flexible display include a thin and bendable flexible type organic EL display, a smartphone that can be folded or rolled up, and the like.
- the film-shaped resin molded body has a low phase difference, it can also be used as a protective film or the like for each layer of a flexible display. Further, it is possible to manufacture a polarizing plate or a touch panel using a film-shaped resin molded body.
- a film-shaped resin molded body (film)
- it may be used as a laminated body having another layer such as a hard coat layer, for example.
- the cover window for the flexible display formed from the film can be arranged on the surface of the flexible display via, for example, an adhesive layer or the like.
- part means “part by mass”.
- various physical properties were measured and evaluated as follows.
- Example A [Polymerization reaction rate and polymer composition analysis]
- the amount of unreacted monomer in the obtained polymerization reaction solution was gas chromatographed (manufactured by Shimadzu Corporation, device name: GC). -2014) was used for measurement and determination.
- Weight average molecular weight and number average molecular weight The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined by polystyrene conversion using gel permeation chromatography (GPC). The equipment and measurement conditions used for the measurement are as follows.
- ML content in copolymer The ML content (content of the structural unit derived from ⁇ -methylene lactone) in the copolymer was determined by 1 1 H-NMR. Specifically, using deuterated DMSO or deuterated chloroform as a deuterated solvent, 1 H-NMR measurement was performed using a nuclear magnetic resonance spectrometer (AV300M manufactured by BRUKER), and the area ratio of the obtained 1 H-NMR profile was obtained. I asked for it.
- Glass transition temperature (Tg) The glass transition temperature of the copolymer was determined in accordance with the provisions of JIS K 7121. Specifically, using a differential scanning calorimeter (manufactured by Rigaku, Thermo plus EVO DSC-8230), a sample of about 10 mg was heated from room temperature to 200 ° C. (heating rate 20 ° C./min) under a nitrogen gas atmosphere. The DSC curve obtained was evaluated by the starting point method. As a reference, ⁇ -alumina was used.
- the 5% weight loss temperature of the copolymer was determined in accordance with JIS K 7120. Specifically, using a differential differential thermal balance device (manufactured by Rigaku, Thermo plus2 Tg-8120), a sample of about 10 mg was heated from room temperature to 400 ° C. at 10 ° C./min under a nitrogen gas atmosphere. At this time, it was determined by measuring the temperature at the time when the mass of the sample being heated decreased by 5%.
- the thickness of the film was determined by a digital micrometer (manufactured by Mitutoyo).
- Total light transmittance of film The total light transmittance of the film was determined in accordance with the regulations of JIS K7361. Specifically, the measurement was performed using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.).
- the Young's modulus of the film was evaluated with respect to the stretched film (thickness 4 ⁇ m) by a method compliant with ISO-14577-1 using an ultrafine hardness tester (Fisher Instruments HM-2000). The evaluation was carried out with the unstretched film fixed to the glass substrate.
- Example A1 A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube, 9 parts of methyl methacrylate (MMA), 0.75 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and N-methylpyrrolidone as a solvent ( 10 parts of NMP) was charged, and the temperature was raised to 105 ° C. through nitrogen. Then, 0.003 parts of t-amylperoxyisononanoate (manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”) was added as a polymerization initiator, and 0.2 parts of NMP.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- NMP N-methylpyrrolidone
- Solution polymerization was carried out at 105-115 ° C. for 6 hours while dropping 0.005 part of the initiator 570 and 0.25 part of ML diluted in 1 at a constant rate over 2 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.8% and 99.3%, respectively.
- the obtained polymerization reaction solution was vacuum dried (1 mmHg) at 240 ° C. for 2 hours to obtain a white copolymer.
- Table 1 shows the physical characteristics of the obtained copolymer.
- the obtained copolymer was heat-press molded at 240 ° C.
- Weight average molecular weight and number average molecular weight of copolymer The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined in the same manner as the weight average molecular weight and the number average molecular weight of the copolymer of ⁇ Example A>.
- ML content in copolymer The ML content in the copolymer (content of the structural unit derived from ⁇ -methylenelactone) was determined in the same manner as the ML content in the copolymer of ⁇ Example A>.
- the internal haze of the copolymer was determined in accordance with JIS K7136. Specifically, an unstretched film obtained by hot-press molding the copolymer at 240 ° C. and 40 MPa for 10 minutes was prepared, and an optical path length was prepared using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.). A 10 mm quartz cell was filled with 1,2,3,4-tetrahydronaphthalene (tetralin), and a film was immersed therein for measurement, and the internal haze value per 100 ⁇ m was calculated.
- a haze meter Nippon Denshoku Kogyo Co., Ltd.
- solvent (compound) content in copolymer or copolymer mixture The solvent (compound) content in the copolymer or the copolymer mixture is determined by gas chromatography (manufactured by Shimadzu Corporation, device name: GC-2014) after dissolving the copolymer or the copolymer mixture in dimethylacetamide. It was determined by measuring using.
- Viscosity of Doping Resin Composition The viscosity of the doped resin composition was measured at 25 ° C. using a BHII type viscometer (manufactured by Toki Sangyo).
- the yellowness (YI) of the doped resin composition was determined in accordance with the provisions of JIS Z 8729. Specifically, the measurement was performed using a quartz cell having an optical path length of 10 mm in a transmission mode of a spectrocolor difference meter (manufactured by Nippon Denshoku Kogyo: Coloreter ZE6000).
- the haze of the doped resin composition was determined in accordance with the regulations of JIS K7136. Specifically, it was measured using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.) using a quartz cell having an optical path length of 10 mm.
- Total light transmittance of film The total light transmittance of the film was determined in the same manner as the total light transmittance of the film of ⁇ Example A>.
- the in-plane retardation Re and the thickness direction retardation Rth with respect to light having a wavelength of 589 nm of the stretched film were measured using a fully automatic birefringence meter (“KOBRA-WR” manufactured by Oji Measuring Instruments) under the condition of an incident angle of 40 °. .. Specifically, the refractive index in the slow axis direction in the plane of the film is nx, the refractive index in the phase advance axis direction in the plane of the film is ny, the refractive index in the thickness direction of the film is nz, and the thickness of the film. In-plane retardation Re and thickness direction retardation Rth were obtained from the following equations, respectively.
- in-plane retardation Re (nx-ny) x d
- thickness direction retardation Rth [(nx + ny) /2-nz] ⁇ d
- Test Example B2 Polymerization, reprecipitation, and drying were carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to ⁇ -butyrolactone (GBL) to obtain 6.5 parts of a white copolymer.
- Table 2 shows the physical characteristics of the obtained copolymer.
- Test Example B3 Polymerization, reprecipitation, and drying were carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to dimethyl sulfoxide (DMSO) to obtain 6 parts of a white copolymer.
- Table 2 shows the physical characteristics of the obtained copolymer.
- Test Example B4 Polymerization was carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to toluene. However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
- Test Example B5 Polymerization was carried out in the same manner as in Test Example B1 except that a solvent such as NMP was not used. However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
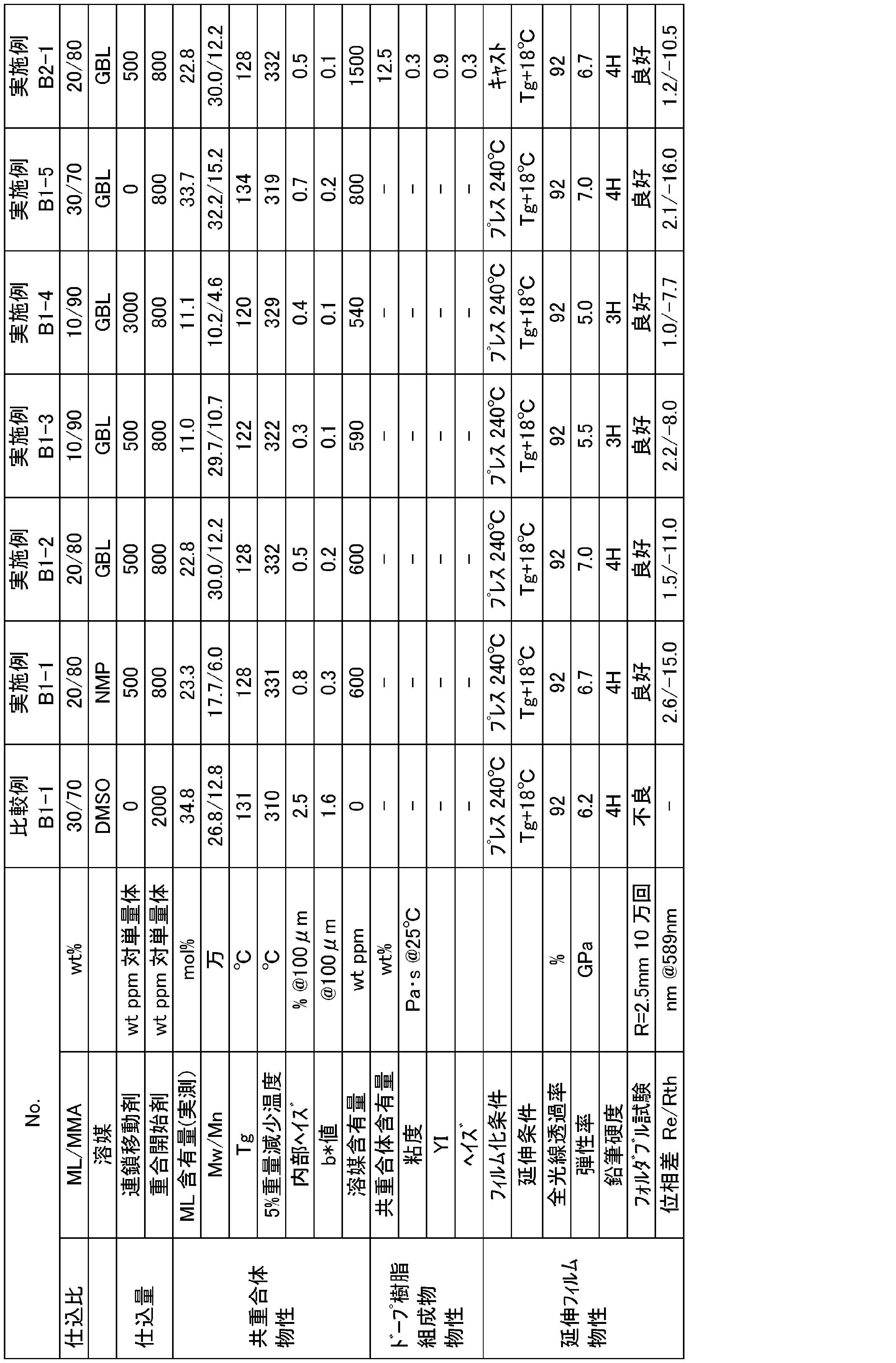
- Example B1-1 ⁇ Synthesis of copolymer by stirring polymerization and preparation of film>
- Example B1-1 In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 8 parts of methyl methacrylate (MMA), 1.5 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and n- as a chain transfer agent.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- n- n- as a chain transfer agent
- nDM dodecyl mercaptan
- NMP N-methylpyrrolidone
- initiator 570 t-amylperoxyisononanoate (manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”) was added as a polymerization initiator, and 0.2 parts of NMP. 0.005 part of the initiator diluted with 570 and 0.5 part of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. After the dropping, 4 parts of NMP was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.5%, respectively.
- the obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer.
- Table 3 shows the physical characteristics of the obtained copolymer.
- Example B1-1 was hot press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 ⁇ m.
- the obtained unstretched film was cut into a size of 96 mm ⁇ 96 mm, and 300% / min at a stretching temperature (146 ° C.) of Tg + 18 ° C. using a sequential biaxial stretching machine (X6-S manufactured by Toyo Seiki Seisakusho).
- a stretched film having a thickness of 40 ⁇ m is obtained by sequentially biaxially stretching the film so that the stretching ratio becomes 2.0 times in the vertical direction (MD direction) and the horizontal direction (TD direction) at the stretching speed of. Obtained.
- Table 3 shows the physical characteristics of the obtained stretched film.
- Example B1-2 A copolymer and a stretched film having a thickness of 40 ⁇ m were obtained in the same manner as in Example B1-1 except that the solvent was changed from NMP to ⁇ -butyrolactone (GBL).
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.5% and 99.0%, respectively.
- Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- Example B1-3 A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube was charged with 9 parts of MMA, 0.75 parts of ML, 0.005 parts of nDM, and 10 parts of GBL as a solvent. The temperature was raised to 105 ° C. through nitrogen. After that, 0.003 parts of the initiator 570 was added, and 0.005 parts of the initiator 570 diluted with 0.2 parts of GBL and 0.25 parts of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. did. After the dropping, 4 parts of GBL was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.4% and 99.2%, respectively.
- the obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 ⁇ m.
- Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- Example B1-4 The same as in Example B1-3 except that nDM was changed from 0.005 part to 0.03 part, GBL was changed from 10 part to 15 part as a solvent, and 4 parts of GBL added after the dropping was not added. , A copolymer and a stretched film having a thickness of 40 ⁇ m were obtained. The conversion rates of MMA and ML calculated from the amount of unreacted monomer in the polymerization reaction solution were 98.0% and 98.5%, respectively. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- Example B1-5 A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube was charged with 7 parts of MMA, 2.2 parts of ML, and 10 parts of GBL as a solvent, and the temperature was raised to 105 ° C. through nitrogen. I warmed it up. After that, 0.003 parts of the initiator 570 was added, and 0.005 parts of the initiator 570 diluted with 0.2 parts of GBL and 0.8 parts of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. did. After the dropping, 4 parts of GBL was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.2%, respectively.
- the obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 ⁇ m.
- Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- the obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 ⁇ m.
- Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- Example B2-1 A dope resin composition having a solid content of 12.5% by mass is mixed with 1 part of the copolymer obtained in Example B1-2 and 7 parts of dichloromethane as a dispersion medium, shaken for 1 minute, and then stirred and mixed for 60 minutes. Was produced.
- the viscosity of the doped resin composition was 0.3 Pa ⁇ s, YI was 0.9, and haze was 0.3%.
- the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
- the doped resin composition was dropped onto the PET film and spread over a film thickness of 800 ⁇ m using an applicator. Then, the PET film was placed in a dryer and dried at 40 ° C. for 30 minutes and 60 ° C. for 30 minutes, and then the applied film was peeled off from PET. Wide mountain-shaped clips were attached vertically so that the obtained film would not curl, and the film was hung in a dryer and then dried at 100 ° C. for 12 hours to obtain an unstretched cast film having a thickness of 160 ⁇ m. The obtained unstretched cast film was sequentially stretched using a biaxial stretching machine in the same manner as in Example B1-1 to obtain a stretched film having a thickness of 40 ⁇ m. Table 3 shows the physical characteristics of the obtained stretched film.
- the reaction rate at the time of the polymerization reaction in the stirring polymerization and the content of the specific monomer unit in the copolymer are the reaction rate at the time of the polymerization reaction in the stirring polymerization of ⁇ Test Example B, Example B, and Comparative Example B> and It was determined in the same manner as the content of the specific monomer unit in the copolymer.
- Weight average molecular weight and number average molecular weight of copolymer The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined in the same manner as the weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer of ⁇ Example A>.
- ML content in copolymer The ML content in the copolymer (content of the structural unit derived from ⁇ -methylenelactone) was determined in the same manner as the ML content in the copolymer of ⁇ Example A>.
- solvent (compound) content in copolymer The solvent (compound) content in the copolymer was determined in the same manner as the solvent (compound) content in the copolymer of ⁇ Test Example B, Example B, and Comparative Example B>.
- Viscosity of Doping Resin Composition The viscosity of the doped resin composition was determined in the same manner as the viscosity of the doped resin composition of ⁇ Test Example B, Example B, and Comparative Example B>.
- the yellowness (YI) of the doped resin composition was determined in the same manner as the yellowness (YI) of the doped resin composition of ⁇ Test Example B, Example B, and Comparative Example B>.
- the haze of the doped resin composition was determined in the same manner as the haze of the doped resin composition of ⁇ Test Example B, Example B, and Comparative Example B>.
- Total light transmittance of film The total light transmittance of the film was determined in the same manner as the total light transmittance of the film of ⁇ Example A>.
- Test Example C2 Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to acetone (ACE). However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
- ACE acetone
- Test Example C3 Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to cyclohexanone (anone). However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
- Test Example C4 Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to a mixed solvent (1: 1 (mass ratio)) of acetone (ACE) and cyclohexanone (anone). After the polymerization, it was diluted with chloroform, added to methanol and reprecipitated, and a white solid was taken out. Then, it was vacuum dried at 240 ° C. for 1 hour to obtain about 6 parts of a white copolymer. Table 4 shows the physical characteristics of the obtained copolymer.
- Example C1-1 ⁇ Synthesis of copolymer by stirring polymerization and preparation of film> (Example C1-1)
- a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube 7.5 parts of methyl methacrylate (MMA), 2 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and n- as a chain transfer agent.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- n- chain transfer agent
- nDM dodecyl mercaptan
- 10 parts of a mixed solvent in which acetone (ACE) and cyclohexanone (anone) were mixed at a ratio of 1: 1 (mass ratio) were charged, and the temperature was raised to 70 ° C. through nitrogen. I warmed it up.
- AIBN 0.004 parts of AIBN was added as a polymerization initiator, and 0.011 parts of the initiator AIBN and 0 were diluted with a mixed solvent in which 0.2 parts of ACE and anon were mixed at a ratio of 1: 1 (mass ratio). .5 parts of ML was added dropwise at 70-75 ° C. over 3 hours at a constant rate. After the dropping, a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 1: 1 (mass ratio) was added, and solution stirring polymerization was further carried out at 70 to 75 ° C. for 4 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 92.1% and 95.5%, respectively.
- the obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer.
- Table 5 shows the physical characteristics of the obtained copolymer.
- Example C1-1 was heat-press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 ⁇ m.
- the obtained unstretched film was cut into a size of 96 mm ⁇ 96 mm, and 300% / min at a stretching temperature (146 ° C.) of Tg + 18 ° C. using a sequential biaxial stretching machine (X6-S manufactured by Toyo Seiki Seisakusho).
- a stretched film having a thickness of 40 ⁇ m is obtained by sequentially biaxially stretching the film so that the stretching ratio becomes 2.0 times in the vertical direction (MD direction) and the horizontal direction (TD direction) at the stretching speed of. Obtained.
- Table 5 shows the physical characteristics of the obtained stretched film.
- Example C1-2 In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 6 parts of methyl methacrylate (MMA), 3 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and n-dodecyl mercaptan as a chain transfer agent. 0.005 part of (nDM) and 10 parts of a mixed solvent prepared by mixing acetone (ACE) and cyclohexanone (anone) as a solvent at a ratio of 3: 7 (mass ratio) were charged, and the temperature was raised to 85 ° C. through nitrogen. It was.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- n-dodecyl mercaptan n-dodecyl mercaptan
- initiator 575 t-amylperoxy2-ethylhexanoate (manufactured by Alchema Yoshitomi, Luperox® 575, hereinafter also referred to as "initiator 575") was added, and 0.2 parts of ACE were added.
- 0.009 parts of initiator 575 and 1 part of ML diluted with a mixed solvent mixed with anon at a ratio of 3: 7 (mass ratio) were added dropwise at 85 to 90 ° C. over 3 hours at a constant rate.
- a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 3: 7 (mass ratio) was added, and solution stirring polymerization was further carried out at 85 to 90 ° C.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 93.1% and 95.5%, respectively.
- the obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer.
- Table 5 shows the physical characteristics of the obtained copolymer.
- Example C1-2 was heat-press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 ⁇ m.
- the obtained unstretched film was cut into a size of 96 mm ⁇ 96 mm, and 300% / min at a stretching temperature (146 ° C.) of Tg + 18 ° C. using a sequential biaxial stretching machine (X6-S manufactured by Toyo Seiki Seisakusho).
- a stretched film having a thickness of 40 ⁇ m is obtained by sequentially biaxially stretching the film so that the stretching ratio becomes 2.0 times in the vertical direction (MD direction) and the horizontal direction (TD direction) at the stretching speed of. Obtained.
- Table 5 shows the physical characteristics of the obtained stretched film.
- Example C1-3 A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube, containing 6 parts of methyl methacrylate (MMA), 3 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and acetone (ACE) as a solvent. 10 parts of a mixed solvent in which ⁇ -butyrolactone (GBL) was mixed at a ratio of 2: 8 (mass ratio) was charged, and the temperature was raised to 100 ° C. while passing nitrogen through the mixed solvent.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- ACE acetone
- t-amylperoxyisononanoate manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”
- ACE a polymerization initiator
- GBL were diluted in a mixed solvent mixed at a ratio of 2: 8 (mass ratio), and 0.009 parts of the initiator 570 and 1 part of ML were added dropwise at 100 to 110 ° C. over 3 hours at a constant rate.
- Example C1-3 was heat-press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 ⁇ m.
- the obtained unstretched film was cut into a size of 96 mm ⁇ 96 mm, and 300% / min at a stretching temperature (146 ° C.) of Tg + 18 ° C. using a sequential biaxial stretching machine (X6-S manufactured by Toyo Seiki Seisakusho).
- a stretched film having a thickness of 40 ⁇ m is obtained by sequentially biaxially stretching the film so that the stretching ratio becomes 2.0 times in the vertical direction (MD direction) and the horizontal direction (TD direction) at the stretching speed of. Obtained.
- Table 5 shows the physical characteristics of the obtained stretched film.
- Example C1-4 A reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 5 parts of methyl methacrylate (MMA), 3.2 parts of ⁇ -methylene- ⁇ -butyrolactone (ML), and acetone (ACE) as a solvent. ) And N, N-dimethylacetamide (DMAc) in a ratio of 3: 7 (mass ratio), 10 parts of a mixed solvent was charged, and the temperature was raised to 85 ° C. while passing nitrogen through the mixed solvent.
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- ACE acetone
- Example C1-4 was hot press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 ⁇ m.
- the obtained unstretched film was cut into a size of 96 mm ⁇ 96 mm, and 300% / min at a stretching temperature (146 ° C.) of Tg + 18 ° C. using a sequential biaxial stretching machine (X6-S manufactured by Toyo Seiki Seisakusho).
- a stretched film having a thickness of 40 ⁇ m is obtained by sequentially biaxially stretching the film so that the stretching ratio becomes 2.0 times in the vertical direction (MD direction) and the horizontal direction (TD direction) at the stretching speed of. Obtained.
- Table 5 shows the physical characteristics of the obtained stretched film.
- the obtained polymerization reaction solution was used in the same manner as in Example C1-1 to obtain a copolymer and a stretched film having a thickness of 40 ⁇ m.
- Table 5 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
- Example C2-1 ⁇ Preparation of Doping Resin Composition and Preparation of Film> (Example C2-1)
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- n- a chain transfer agent
- MMA methyl methacrylate
- ML ⁇ -methylene- ⁇ -butyrolactone
- nDM dodecyl mercaptan
- ACE acetone
- cyclohexanone anone
- 0.004 parts of the initiator 575 was added, and 0.009 parts of the initiator 575 and 0.5 were diluted with a mixed solvent in which 0.2 parts of ACE and anon were mixed at a ratio of 3: 7 (mass ratio).
- the ML of the part was added dropwise at 85 to 90 ° C. over 3 hours at a constant rate.
- a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 3: 7 (mass ratio) was added, and solution stirring polymerization was further carried out at 85 to 90 ° C. for 4 hours.
- the conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 93.1% and 97.5%, respectively.
- the obtained polymerization reaction solution was diluted with 55 parts of a mixed solvent in which ACE and anon were mixed at a ratio of 3: 7 (mass ratio) to prepare a dope resin composition having a copolymer content of 12.5% by mass.
- the doped resin composition was pressure filtered through a 3 ⁇ m membrane filter.
- the physical characteristics of the doped resin composition are shown in Table 5. When the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
- Example C2-1 the doped resin composition of Example C2-1 was applied to a glass substrate and vacuum dried at 150 ° C. for 2 hours to obtain an unstretched cast film having a thickness of 120 ⁇ m.
- the obtained unstretched cast film was sequentially stretched using a biaxial stretching machine in the same manner as in Example C1-1 to obtain a stretched film having a thickness of 30 ⁇ m.
- Table 5 shows the physical characteristics of the obtained stretched film.
- Example C2-2 A dope resin composition having a solid content of 12.5% by mass is mixed with 1 part of the copolymer obtained in Example C1-2 and 7 parts of dichloromethane as a dispersion medium, shaken for 1 minute, and then stirred and mixed for 60 minutes. Was produced.
- the physical characteristics of the doped resin composition are shown in Table 5. When the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
- Example C2-2 the doped resin composition of Example C2-2 was added dropwise to the PET film, and the film was spread over a film thickness of 800 ⁇ m using an applicator. Then, the PET film was placed in a dryer and dried at 40 ° C. for 30 minutes and 60 ° C. for 30 minutes, and then the applied film was peeled off from PET. Wide mountain-shaped clips were attached vertically so that the obtained film would not curl, and the film was hung in a dryer and then dried at 100 ° C. for 12 hours to obtain an unstretched cast film having a thickness of 160 ⁇ m. The obtained unstretched cast film was sequentially stretched using a biaxial stretching machine in the same manner as in Example C1-1 to obtain a stretched film having a thickness of 40 ⁇ m. Table 5 shows the physical characteristics of the obtained stretched film.
Abstract
Description
[1]α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体の製造方法であって、溶媒の存在下、α-メチレンラクトン及び(メタ)アクリル酸アルキルを含む単量体を重合させる工程を備え、溶媒が、下記条件(A)又は下記条件(B)のいずれかを満たす溶媒である、共重合体の製造方法。
条件(A):環状アミド及び環状エステルからなる群より選ばれる少なくとも1種の溶媒である。
条件(B):100℃未満の沸点を有する第1の溶媒及び100℃以上の沸点を有する第2の溶媒からなる混合溶媒であって、第1の溶媒がケトン及び塩化アルキルからなる群より選ばれる少なくとも1種であり、第2の溶媒が環状ケトン、環状エステル、アミド、及びスルホキシドからなる群より選ばれる少なくとも1種であり、沸点が70~120℃である混合溶媒である。
[2]溶媒が、条件(A)を満たす溶媒である、[1]に記載の共重合体の製造方法。
[3]溶媒が、条件(B)を満たす溶媒である、[1]に記載の共重合体の製造方法。
[4]第1の溶媒がアセトンである、[3]に記載の共重合体の製造方法。
[5]第2の溶媒が環状ケトンである、[3]又は[4]に記載の共重合体の製造方法。
[6]環状ケトンがシクロヘキサノンである、[5]に記載の共重合体の製造方法。
[7]第2の溶媒が環状エステル、アミド、及びスルホキシドからなる群より選ばれる少なくとも1種である、[3]又は[4]に記載の共重合体の製造方法。
[8]第2の溶媒がγ-ブチロラクトン、γ-バレロラクトン、δ-バレロラクトン、N,N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、N-メチルピロリドン、N,N’-ジメチルイミダゾリジノン、及びジメチルスルホキシドからなる群より選ばれる少なくとも1種である、[7]に記載の共重合体の製造方法。
[9]α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含み、フィルムにしたときの厚さ100μm当たりの内部ヘイズが2.5%未満である、共重合体。
[10]α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含み、かつ重量平均分子量が200000以上1000000以下である、共重合体。
[11]フィルムにしたときのL*a*b*表色系の厚さ100μm当たりの内部b*値が1.6未満である、[9]又は[10]に記載の共重合体。
[12][9]~[11]のいずれかに記載の共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有する、共重合体混合物。
[13]α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有する、共重合体混合物。
[14]化合物の含有量が、共重合体の全量を基準として、10~3000質量ppmである、[12]又は[13]に記載の共重合体混合物。
[15][9]~[11]のいずれかに記載の共重合体と、分散媒とを含有するドープ樹脂組成物であって、共重合体の含有量が、ドープ樹脂組成物の全量を基準として、5質量%以上である、ドープ樹脂組成物。
[16][9]~[11]のいずれかに記載の共重合体又は[12]~[14]のいずれかに記載の共重合体混合物を含有する、樹脂成形体。
[17][9]~[11]のいずれかに記載の共重合体又は[12]~[14]のいずれかに記載の共重合体混合物を含有する樹脂組成物を成形して樹脂成形体を得る工程を備える、樹脂成形体の製造方法。
[18][15]に記載のドープ樹脂組成物を塗工する工程と、塗工されたドープ樹脂組成物から分散媒を除去して樹脂成形体を得る工程とを備える、樹脂成形体の製造方法。 The present invention relates to the following methods for producing a copolymer according to [1] to [8], the copolymer according to [9] to [11], and the copolymer according to [12] to [14]. Provided are a mixture, a dope resin composition according to [15], a resin molded product according to [16], and a method for producing the resin molded product according to [17] and [18].
[1] A method for producing a copolymer containing a structural unit derived from α-methylenelactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, which is α in the presence of a solvent. -Production of a copolymer comprising a step of polymerizing a monomer containing methylene lactone and alkyl (meth) acrylate, wherein the solvent is a solvent satisfying either the following condition (A) or the following condition (B). Method.
Condition (A): At least one solvent selected from the group consisting of cyclic amides and cyclic esters.
Condition (B): A mixed solvent consisting of a first solvent having a boiling point of less than 100 ° C. and a second solvent having a boiling point of 100 ° C. or higher, and the first solvent is selected from the group consisting of ketones and alkyl chlorides. The second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, and is a mixed solvent having a boiling point of 70 to 120 ° C.
[2] The method for producing a copolymer according to [1], wherein the solvent is a solvent that satisfies the condition (A).
[3] The method for producing a copolymer according to [1], wherein the solvent is a solvent that satisfies the condition (B).
[4] The method for producing a copolymer according to [3], wherein the first solvent is acetone.
[5] The method for producing a copolymer according to [3] or [4], wherein the second solvent is a cyclic ketone.
[6] The method for producing a copolymer according to [5], wherein the cyclic ketone is cyclohexanone.
[7] The method for producing a copolymer according to [3] or [4], wherein the second solvent is at least one selected from the group consisting of cyclic esters, amides, and sulfoxides.
[8] The second solvent is γ-butyrolactone, γ-valerolactone, δ-valerolactone, N, N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, N, N'-dimethylimidazolidinone. The method for producing a copolymer according to [7], which is at least one selected from the group consisting of dimethyl sulfoxide and dimethyl sulfoxide.
[9] Containing a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, the internal haze per 100 μm thickness when made into a film is 2. Copolymer, less than 5%.
[10] A copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms and having a weight average molecular weight of 200,000 or more and 1,000,000 or less. ..
[11] The copolymer according to [9] or [10], wherein the L * a * b * color system has an internal b * value of less than 1.6 per 100 μm of thickness when formed into a film.
[12] A copolymer mixture containing the copolymer according to any one of [9] to [11] and at least one compound selected from the group consisting of cyclic amides, cyclic esters, and cyclic ketones. ..
[13] Consists of a copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from an alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, a cyclic amide, a cyclic ester, and a cyclic ketone. A copolymer mixture containing at least one compound selected from the group.
[14] The copolymer mixture according to [12] or [13], wherein the content of the compound is 10 to 3000 mass ppm based on the total amount of the copolymer.
[15] A dope resin composition containing the copolymer according to any one of [9] to [11] and a dispersion medium, wherein the content of the copolymer is the total amount of the dope resin composition. As a reference, a dope resin composition of 5% by mass or more.
[16] A resin molded product containing the copolymer according to any one of [9] to [11] or the copolymer mixture according to any one of [12] to [14].
[17] A resin composition containing the copolymer according to any one of [9] to [11] or the copolymer mixture according to any one of [12] to [14] is molded into a resin molded product. A method for producing a resin molded product, comprising a step of obtaining the above.
[18] Production of a resin molded product comprising a step of applying the dope resin composition according to [15] and a step of removing a dispersion medium from the coated dope resin composition to obtain a resin molded product. Method.
一実施形態に係る共重合体の製造方法は、α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体の製造方法である。 [Method for producing copolymer]
The method for producing a copolymer according to an embodiment is a method for producing a copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms. Is.
条件(A):環状アミド及び環状エステルからなる群より選ばれる少なくとも1種の溶媒である。
条件(B):100℃未満の沸点を有する第1の溶媒及び100℃以上の沸点を有する第2の溶媒からなる混合溶媒であって、第1の溶媒がケトン及び塩化アルキルからなる群より選ばれる少なくとも1種であり、第2の溶媒が環状ケトン、環状エステル、アミド、及びスルホキシドからなる群より選ばれる少なくとも1種であり、沸点が70~120℃である混合溶媒である。 The method for producing a copolymer according to the present embodiment includes a step of polymerizing a monomer containing α-methylenelactone and alkyl (meth) acrylate in the presence of a solvent. The solvent is a solvent that satisfies either the following condition (A) or the following condition (B).
Condition (A): At least one solvent selected from the group consisting of cyclic amides and cyclic esters.
Condition (B): A mixed solvent consisting of a first solvent having a boiling point of less than 100 ° C. and a second solvent having a boiling point of 100 ° C. or higher, and the first solvent is selected from the group consisting of ketones and alkyl chlorides. The second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, and is a mixed solvent having a boiling point of 70 to 120 ° C.
一実施形態に係る共重合体は、α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含み、フィルムにしたときの厚さ100μm当たりの内部ヘイズが2.5%未満である。本実施形態の共重合体は、上記製造方法によって得られる共重合体であり得る。上記製造方法は、得られる共重合体の透明性を改善することが可能であることから、当該製造方法を用いることによって、フィルムにしたときの厚さ100μm当たりの内部ヘイズ、フィルムにしたときのL*a*b*表色系の厚さ100μm当たりの内部b*値等を所定の範囲にすることが可能となり得る。 [Copolymer]
The copolymer according to one embodiment contains a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, and has a thickness of 100 μm when formed into a film. The internal haze per hit is less than 2.5%. The copolymer of this embodiment can be a copolymer obtained by the above-mentioned production method. Since the above-mentioned production method can improve the transparency of the obtained copolymer, by using the production method, the internal haze per 100 μm thickness of the film and the time of the film are obtained. It may be possible to set the internal b * value or the like per 100 μm of the thickness of the L * a * b * color system within a predetermined range.
一実施形態に係る共重合体混合物は、α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有する。共重合体混合物の一態様は、上述の共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有するものである。 [Copolymer mixture]
The copolymer mixture according to one embodiment includes a copolymer containing a structural unit derived from α-methylenelactone and a structural unit derived from an alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, and a cyclic amide. It contains a cyclic ester and at least one compound selected from the group consisting of cyclic ketones. One aspect of the copolymer mixture contains the above-mentioned copolymer and at least one compound selected from the group consisting of cyclic amides, cyclic esters, and cyclic ketones.
一実施形態に係るドープ樹脂組成物は、上述の共重合体と、分散媒とを含有する。ドープ樹脂組成物は、樹脂成形体の製造に好適に用いることができる。 [Doping resin composition]
The doped resin composition according to one embodiment contains the above-mentioned copolymer and a dispersion medium. The doped resin composition can be suitably used for producing a resin molded product.
一実施形態に係る樹脂成形体は、上述の共重合体又は上述の共重合体混合物を主成分として含有する。本実施形態に係る樹脂成形体は、上述の共重合体若しくは上述の共重合体混合物を含有する樹脂組成物、又は、上述の共重合体若しくは上述の共重合体混合物を含有するドープ樹脂組成物を用いて製造することができる。 [Resin molded product and its manufacturing method]
The resin molded product according to one embodiment contains the above-mentioned copolymer or the above-mentioned copolymer mixture as a main component. The resin molded product according to the present embodiment is a resin composition containing the above-mentioned copolymer or the above-mentioned copolymer mixture, or a dope resin composition containing the above-mentioned copolymer or the above-mentioned copolymer mixture. Can be manufactured using.
[重合反応率及び重合体組成分析]
重合反応時の反応率及び共重合体中の特定単量体単位の含有率は、得られた重合反応液中の未反応単量体の量をガスクロマトグラフィー(島津製作所製、装置名:GC-2014)を用いて測定して求めた。 <Example A>
[Polymerization reaction rate and polymer composition analysis]
For the reaction rate during the polymerization reaction and the content of the specific monomer unit in the copolymer, the amount of unreacted monomer in the obtained polymerization reaction solution was gas chromatographed (manufactured by Shimadzu Corporation, device name: GC). -2014) was used for measurement and determination.
共重合体の重量平均分子量(Mw)及び数平均分子量(Mn)は、ゲル浸透クロマトグラフィー(GPC)を用いて、ポリスチレン換算により求めた。測定に用いた装置及び測定条件は、以下のとおりである。
システム:東ソー製GPCシステムHLC-8220
測定側カラム構成:
・ガードカラム(東ソー製、TSKguardcolumn SuperHZ-L)
・分離カラム(東ソー製、TSKgel SuperHZM-M) 2本直列接続
リファレンス側カラム構成:
・リファレンスカラム(東ソー製、TSKgel SuperH-RC)
展開溶媒:クロロホルム(和光純薬工業製、特級)
展開溶媒の流量:0.6mL/分
標準試料:TSK標準ポリスチレン(東ソー製、PS-オリゴマーキット)
カラム温度:40℃ [Weight average molecular weight and number average molecular weight]
The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined by polystyrene conversion using gel permeation chromatography (GPC). The equipment and measurement conditions used for the measurement are as follows.
System: Tosoh GPC system HLC-8220
Measurement side column configuration:
・ Guard column (manufactured by Tosoh, TSKguardcolum SuperHZ-L)
-Separation column (manufactured by Tosoh, TSKgel SuperHZM-M) 2 series connection Reference side column configuration:
・ Reference column (manufactured by Tosoh, TSKgel SuperH-RC)
Developing solvent: Chloroform (manufactured by Wako Pure Chemical Industries, Ltd., special grade)
Flow rate of developing solvent: 0.6 mL / min Standard sample: TSK standard polystyrene (Tosoh, PS-oligomer kit)
Column temperature: 40 ° C
共重合体中のML含有量(α-メチレンラクトン由来の構成単位の含有量)は、1H-NMRにより求めた。具体的には、重溶媒として重DMSO又は重クロロホルムを使用し、核磁気共鳴分光計(BRUKER製、AV300M)を用いて1H-NMR測定を行い、得られた1H-NMRプロファイルの面積比から求めた。 [ML content in copolymer]
The ML content (content of the structural unit derived from α-methylene lactone) in the copolymer was determined by 1 1 H-NMR. Specifically, using deuterated DMSO or deuterated chloroform as a deuterated solvent, 1 H-NMR measurement was performed using a nuclear magnetic resonance spectrometer (AV300M manufactured by BRUKER), and the area ratio of the obtained 1 H-NMR profile was obtained. I asked for it.
共重合体のガラス転移温度は、JIS K 7121の規定に準拠して求めた。具体的には、示差走査熱量計(リガク製、Thermo plus EVO DSC-8230)を用い、窒素ガス雰囲気下、約10mgのサンプルを常温から200℃まで昇温(昇温速度20℃/分)して得られたDSC曲線から、始点法により評価した。リファレンスには、α-アルミナを用いた。 [Glass transition temperature (Tg)]
The glass transition temperature of the copolymer was determined in accordance with the provisions of JIS K 7121. Specifically, using a differential scanning calorimeter (manufactured by Rigaku, Thermo plus EVO DSC-8230), a sample of about 10 mg was heated from room temperature to 200 ° C. (heating rate 20 ° C./min) under a nitrogen gas atmosphere. The DSC curve obtained was evaluated by the starting point method. As a reference, α-alumina was used.
共重合体の5%重量減少温度はJIS K 7120の規定に準拠して求めた。具体的には、差動型示差熱天秤装置(リガク製、Thermo plus2 Tg-8120)を用い、窒素ガス雰囲気下、約10mgのサンプルを常温から400℃まで10℃/分で昇温した。このとき、昇温中のサンプルの質量が5%減少した時点での温度を測定することにより求めた。 [5% weight loss temperature]
The 5% weight loss temperature of the copolymer was determined in accordance with JIS K 7120. Specifically, using a differential differential thermal balance device (manufactured by Rigaku, Thermo plus2 Tg-8120), a sample of about 10 mg was heated from room temperature to 400 ° C. at 10 ° C./min under a nitrogen gas atmosphere. At this time, it was determined by measuring the temperature at the time when the mass of the sample being heated decreased by 5%.
フィルムの厚さは、デジマチックマイクロメーター(ミツトヨ製)により求めた。 [Film thickness]
The thickness of the film was determined by a digital micrometer (manufactured by Mitutoyo).
フィルムの全光線透過率はJIS K7361の規定に準拠して求めた。具体的には、ヘイズメーター(日本電色工業製、NDH-1001DP)を用いて測定した。 [Total light transmittance of film]
The total light transmittance of the film was determined in accordance with the regulations of JIS K7361. Specifically, the measurement was performed using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.).
延伸フィルムを90mm×20mmの大きさに切り出して試験片とし、温度25℃、相対湿度50%の雰囲気下で、JIS K7127に準拠し、オートグラフ(島津製作所製:AG-X)を用いて引張試験を実施した。条件は引張速度を歪0.5%まで0.25mm/分、それ以降は1mm/分とし、チャック間距離を55mm、変位計での測定する標線間隔を25mmとして、25℃で3回試験を行い、その平均値を測定値とした。変位は非接触伸び幅計(島津製作所製:TRViewX)を用いて計測し、弾性率は歪が0.05%から0.25%までの間の傾きとして評価した。 [Film tensile test (modulus measurement)]
The stretched film is cut into a size of 90 mm × 20 mm to make a test piece, and is tensioned using an autograph (manufactured by Shimadzu Corporation: AG-X) in an atmosphere of a temperature of 25 ° C. and a relative humidity of 50% in accordance with JIS K7127. The test was carried out. The conditions are that the tensile speed is 0.25 mm / min up to a strain of 0.5%, then 1 mm / min, the distance between the chucks is 55 mm, the marked line interval measured by the displacement meter is 25 mm, and the test is performed three times at 25 ° C. Was performed, and the average value was used as the measured value. The displacement was measured using a non-contact elongation width meter (manufactured by Shimadzu Corporation: TRViewX), and the elastic modulus was evaluated as a slope between 0.05% and 0.25% of strain.
フィルムのヤング率は、延伸フィルム(厚さ4μm)に対して、超微小硬度計(フィッシャーインストルメンツ製、フィッシャースコープHM-2000)を用い、ISO-14577-1に準拠した方法により評価した。評価は、未延伸フィルムをガラス基板に固定した状態で実施した。測定条件は、四角錐型のビッカース圧子(対面角a=136°)を使用し、最大試験荷重3mN;荷重付加時のアプリケーション時間20秒;クリープ時間5秒;荷重減少時のアプリケーション時間20秒;測定温度室温(25℃)とし、3回測定した値を平均化して求めた。 [Young's modulus of film]
The Young's modulus of the film was evaluated with respect to the stretched film (thickness 4 μm) by a method compliant with ISO-14577-1 using an ultrafine hardness tester (Fisher Instruments HM-2000). The evaluation was carried out with the unstretched film fixed to the glass substrate. The measurement conditions were a square cone-shaped Vickers indenter (face-to-face angle a = 136 °), maximum test load 3 mN; application time 20 seconds when load was applied; creep time 5 seconds; application time 20 seconds when load was reduced; The measurement temperature was room temperature (25 ° C.), and the values measured three times were averaged and obtained.
フィルムの鉛筆硬度は、JIS-S-6006が規定する試験用鉛筆を用い、安田精機製作所製 鉛筆引っかき硬度試験機No.533を用いて、JIS K5600-5-4(1999)に準拠して、750g荷重下で評価を行い、傷がつかない最も高い鉛筆の硬度を鉛筆硬度とした。 [Pencil hardness of film]
For the pencil hardness of the film, use a test pencil specified by JIS-S-6006, and use a pencil scratch hardness tester No. 1 manufactured by Yasuda Seiki Seisakusho. Evaluation was performed using 533 under a load of 750 g in accordance with JIS K5600-5-4 (1999), and the highest hardness of the pencil that was not scratched was defined as the pencil hardness.
延伸フィルムを15mm×80mmの大きさに切り出して試験片とし、Tension-FreeFolding Clamshell-type(ユアサシステム機器製、DMLHP-CS)にテープで固定した。また、試験片を長辺の半分の位置で折り曲げ、折り畳まれた状態の試験片の長辺の両端部間の距離が5mmとなり、試験片の折り曲げ部分の曲率半径が2.5mmとなるように折り畳まれた状態を設定した。その後、25℃の環境下で、平坦に開いた状態から折り畳まれた状態にすることを1回の屈曲として、1分間に30回の屈曲回数で、10万回屈曲を繰り返した。試験後の折り畳まれた部分のフィルムが破断していなかった場合を「良好」、破断していた場合を「不良」として評価した。 [Film foldable test]
The stretched film was cut into a size of 15 mm × 80 mm to obtain a test piece, and taped to Tension-FreeFolding Clamshell-type (manufactured by Yuasa System Co., Ltd., DMLHP-CS). Further, the test piece is bent at the half position of the long side so that the distance between both ends of the long side of the folded test piece is 5 mm and the radius of curvature of the bent portion of the test piece is 2.5 mm. The folded state was set. Then, in an environment of 25 ° C., changing from a flat open state to a folded state was regarded as one bending, and bending was repeated 100,000 times with 30 bending times per minute. The case where the film in the folded portion after the test was not broken was evaluated as "good", and the case where the film was broken was evaluated as "bad".
撹拌装置、温度センサー、冷却管、窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)9部、α-メチレン-γ-ブチロラクトン(ML)0.75部、溶媒としてN-メチルピロリドン(NMP)10部を仕込み、これに窒素を通じつつ105℃まで昇温させた。その後、重合開始剤としてt-アミルパーオキシイソノナノエート(アルケマ吉富製、ルペロックス(登録商標)570、以下「開始剤570」ともいう。)を0.003部加えるとともに、0.2部のNMPで希釈した0.005部の開始剤570と0.25部のMLを2時間かけて一定速度で滴下しながら105-115℃で溶液重合を6時間行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ98.8%、99.3%であった。次に得られた重合反応液を240℃で2時間真空乾燥(1mmHg)することで、白色の共重合体を得た。得られた共重合体の物性を表1に示す。次に得られた共重合体を240℃で熱プレス成形して、厚さ約160μmの未延伸プレスフィルムを得た後、得られた未延伸キャストフィルムを96mm×96mmの大きさに切り出し、逐次二軸延伸機(東洋精機製作所製、X6-S)を用いて、延伸温度140℃(Tg+18℃)にて300%/分の延伸速度で縦方向(MD方向)及び横方向(TD方向)の順にそれぞれ延伸倍率が2.0倍となるように逐次二軸延伸を行い、冷却することにより、厚さ40μmの延伸フィルムを得た。得られた延伸フィルムの物性を表1に示す。 (Example A1)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube, 9 parts of methyl methacrylate (MMA), 0.75 parts of α-methylene-γ-butyrolactone (ML), and N-methylpyrrolidone as a solvent ( 10 parts of NMP) was charged, and the temperature was raised to 105 ° C. through nitrogen. Then, 0.003 parts of t-amylperoxyisononanoate (manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”) was added as a polymerization initiator, and 0.2 parts of NMP. Solution polymerization was carried out at 105-115 ° C. for 6 hours while dropping 0.005 part of the initiator 570 and 0.25 part of ML diluted in 1 at a constant rate over 2 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.8% and 99.3%, respectively. Next, the obtained polymerization reaction solution was vacuum dried (1 mmHg) at 240 ° C. for 2 hours to obtain a white copolymer. Table 1 shows the physical characteristics of the obtained copolymer. Next, the obtained copolymer was heat-press molded at 240 ° C. to obtain an unstretched press film having a thickness of about 160 μm, and then the obtained unstretched cast film was cut into a size of 96 mm × 96 mm and sequentially. Using a biaxial stretching machine (manufactured by Toyo Seiki Seisakusho, X6-S), the stretching speed is 300% / min at a stretching temperature of 140 ° C. (Tg + 18 ° C.) in the longitudinal direction (MD direction) and the transverse direction (TD direction). Biaxial stretching was carried out in sequence so that the stretching ratio was 2.0 times, and the film was cooled to obtain a stretched film having a thickness of 40 μm. Table 1 shows the physical characteristics of the obtained stretched film.
[静置重合における重合反応率]
静置重合における重合反応率は、重合液をクロロホルムで希釈後、メタノール中に滴下して再沈殿により共重合体を取り出し、共重合体を240℃で1時間乾燥した後に得られた共重合体量により簡易的に求めた。 <Test Example B, Example B, and Comparative Example B>
[Polymerization reaction rate in static polymerization]
The polymerization reaction rate in the static polymerization was determined by diluting the polymerization solution with chloroform, dropping it in methanol, removing the copolymer by reprecipitation, and drying the copolymer at 240 ° C. for 1 hour. It was simply calculated by the amount.
撹拌重合における重合反応時の反応率及び共重合体中の特定単量体単位の含有率は、得られた重合反応液中の未反応単量体の量をガスクロマトグラフィー(島津製作所製、装置名:GC-2014)を用いて測定して求めた。 [Polymerization reaction rate in agitation polymerization and composition analysis of copolymer]
For the reaction rate during the polymerization reaction in the stirring polymerization and the content of the specific monomer unit in the copolymer, the amount of unreacted monomer in the obtained polymerization reaction solution was gas chromatographed (manufactured by Shimadzu Corporation, apparatus). Name: GC-2014) was used for measurement.
共重合体の重量平均分子量(Mw)及び数平均分子量(Mn)は、<実施例A>の共重合体の重量平均分子量及び数平均分子量と同様にして求めた。 [Weight average molecular weight and number average molecular weight of copolymer]
The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined in the same manner as the weight average molecular weight and the number average molecular weight of the copolymer of <Example A>.
共重合体中のML含有量(α-メチレンラクトン由来の構成単位の含有量)は、<実施例A>の共重合体中のML含有量と同様にして求めた。 [ML content in copolymer]
The ML content in the copolymer (content of the structural unit derived from α-methylenelactone) was determined in the same manner as the ML content in the copolymer of <Example A>.
共重合体のガラス転移温度は、<実施例A>の共重合体のガラス転移温度と同様にして求めた。 [Copolymer glass transition temperature (Tg)]
The glass transition temperature of the copolymer was determined in the same manner as the glass transition temperature of the copolymer of <Example A>.
共重合体の5%重量減少温度は、<実施例A>の共重合体の5%重量減少温度と同様にして求めた。 [5% weight loss temperature of copolymer]
The 5% weight loss temperature of the copolymer was determined in the same manner as the 5% weight loss temperature of the copolymer of <Example A>.
共重合体の内部ヘイズはJIS K7136の規定に準拠して求めた。具体的には、共重合体を240℃、40MPaで10分間熱プレス成形して得られた未延伸フィルムを用意し、ヘイズメーター(日本電色工業製、NDH-1001DP)を用いて、光路長10mmの石英セルに1,2,3,4-テトラヒドロナフタリン(テトラリン)を満たし、その中にフィルムを浸漬して測定し、100μm当たりの内部ヘイズ値として算出した。 [Internal haze of copolymer]
The internal haze of the copolymer was determined in accordance with JIS K7136. Specifically, an unstretched film obtained by hot-press molding the copolymer at 240 ° C. and 40 MPa for 10 minutes was prepared, and an optical path length was prepared using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.). A 10 mm quartz cell was filled with 1,2,3,4-tetrahydronaphthalene (tetralin), and a film was immersed therein for measurement, and the internal haze value per 100 μm was calculated.
共重合体を240℃、40MPaで10分間熱プレス成形して得られた未延伸フィルムを用意し、分光光度計(日本電色工業製、Colormeter ZE6000)を用いて、光路長10mmの石英セルに1,2,3,4-テトラヒドロナフタリン(テトラリン)を満たし、その中にフィルムを浸漬して測定し、L*a*b*表色系の厚さ100μm当たりのb*値として算出した。 [Internal b * value of copolymer]
An unstretched film obtained by hot-press molding the copolymer at 240 ° C. and 40 MPa for 10 minutes was prepared, and a spectrophotometer (Colormeter ZE6000 manufactured by Nippon Denshoku Kogyo Co., Ltd.) was used to form a quartz cell having an optical path length of 10 mm. It was filled with 1,2,3,4-tetrahydronaphthalene (tetralin), and the film was immersed in the film for measurement, and calculated as the b * value per 100 μm of the thickness of the L * a * b * color system.
共重合体又は共重合体混合物中の溶媒(化合物)含有量は、共重合体又は共重合体混合物をジメチルアセトアミドに溶解させた後、ガスクロマトグラフィー(島津製作所製、装置名:GC-2014)を用いて測定して求めた。 [Solvent (compound) content in copolymer or copolymer mixture]
The solvent (compound) content in the copolymer or the copolymer mixture is determined by gas chromatography (manufactured by Shimadzu Corporation, device name: GC-2014) after dissolving the copolymer or the copolymer mixture in dimethylacetamide. It was determined by measuring using.
ドープ樹脂組成物の粘度は、BHII型粘度計(東機産業製)を用いて25℃にて測定した。 [Viscosity of Doping Resin Composition]
The viscosity of the doped resin composition was measured at 25 ° C. using a BHII type viscometer (manufactured by Toki Sangyo).
ドープ樹脂組成物の黄色度(YI)はJIS Z 8729の規定に準拠して求めた。具体的には分光色差計(日本電色工業製:Colormeter ZE6000)の透過モードで、光路長10mmの石英セルを用いて測定した。 [Yellowness (YI) of Doping Resin Composition]
The yellowness (YI) of the doped resin composition was determined in accordance with the provisions of JIS Z 8729. Specifically, the measurement was performed using a quartz cell having an optical path length of 10 mm in a transmission mode of a spectrocolor difference meter (manufactured by Nippon Denshoku Kogyo: Coloreter ZE6000).
ドープ樹脂組成物のヘイズはJIS K7136の規定に準拠して求めた。具体的には、ヘイズメーター(日本電色工業製、NDH-1001DP)を用いて、光路長10mmの石英セルを用いて測定した。 [Haze of Doping Resin Composition]
The haze of the doped resin composition was determined in accordance with the regulations of JIS K7136. Specifically, it was measured using a haze meter (NDH-1001DP, manufactured by Nippon Denshoku Kogyo Co., Ltd.) using a quartz cell having an optical path length of 10 mm.
フィルムの厚さは、<実施例A>のフィルムの厚さと同様にして求めた。 [Film thickness]
The film thickness was determined in the same manner as the film thickness of <Example A>.
フィルムの全光線透過率は、<実施例A>のフィルムの全光線透過率と同様にして求めた。 [Total light transmittance of film]
The total light transmittance of the film was determined in the same manner as the total light transmittance of the film of <Example A>.
<実施例A>のフィルムの引張試験と同様にしてフィルムの引張試験(弾性率測定)を行い、弾性率を測定した。 [Film tensile test (modulus measurement)]
A tensile test (measurement of elastic modulus) of the film was performed in the same manner as the tensile test of the film of <Example A>, and the elastic modulus was measured.
フィルムの鉛筆硬度は、<実施例A>のフィルムの鉛筆硬度と同様にして求めた。 [Pencil hardness of film]
The pencil hardness of the film was determined in the same manner as the pencil hardness of the film of <Example A>.
<実施例A>のフィルムのフォルダブル試験と同様にしてフィルムのフォルダブル試験を行い、<実施例A>と同様に、試験後の折り畳まれた部分のフィルムの破断の有無を評価した。 [Film foldable test]
The film foldable test was performed in the same manner as in the film foldable test of <Example A>, and the presence or absence of breakage of the film in the folded portion after the test was evaluated in the same manner as in <Example A>.
延伸フィルムの波長589nmの光に対する面内位相差Re及び厚さ方向位相差Rthを、全自動複屈折計(王子計測機器製「KOBRA-WR」)を用いて入射角40°の条件で測定した。具体的には、フィルムの面内における遅相軸方向の屈折率をnx、フィルムの面内における進相軸方向の屈折率をny、フィルムの厚さ方向の屈折率をnz、フィルムの厚さをdとして、下記式から面内位相差Re及び厚さ方向位相差Rthをそれぞれ求めた。なお、下記の実施例においては、フィルムの厚さdを40μmとして、面内位相差Re及び厚さ方向位相差Rthを求めた。
面内位相差Re=(nx-ny)×d
厚さ方向位相差Rth=[(nx+ny)/2-nz]×d [Film phase difference]
The in-plane retardation Re and the thickness direction retardation Rth with respect to light having a wavelength of 589 nm of the stretched film were measured using a fully automatic birefringence meter (“KOBRA-WR” manufactured by Oji Measuring Instruments) under the condition of an incident angle of 40 °. .. Specifically, the refractive index in the slow axis direction in the plane of the film is nx, the refractive index in the phase advance axis direction in the plane of the film is ny, the refractive index in the thickness direction of the film is nz, and the thickness of the film. In-plane retardation Re and thickness direction retardation Rth were obtained from the following equations, respectively. In the following examples, the in-plane retardation Re and the thickness direction retardation Rth were determined with the film thickness d being 40 μm.
In-plane phase difference Re = (nx-ny) x d
Thickness direction phase difference Rth = [(nx + ny) /2-nz] × d
(試験例B1)
密閉できる反応容器内に、メタクリル酸メチル(MMA)7部、α-メチレン-γ-ブチロラクトン(ML)3部、溶媒としてN-メチルピロリドン(NMP)10部、及び開始剤としてアゾビスイソブチロニトリル(AIBN)を0.03部仕込み、これに窒素を2分間バブリングして容器内を窒素置換後、蓋を締めて密閉した。その後、反応容器を75℃のオイルバスに2時間漬けて重合を行った。重合後クロロホルムで希釈した後、メタノール中に加えて再沈殿を行い、白色固体を取り出した。その後240℃で1時間真空乾燥をして、白色の共重合体を約6部得た。得られた共重合体の物性を表2に示す。 <Synthesis of copolymer by static polymerization>
(Test Example B1)
In a hermetically sealed reaction vessel, 7 parts of methyl methacrylate (MMA), 3 parts of α-methylene-γ-butyrolactone (ML), 10 parts of N-methylpyrrolidone (NMP) as a solvent, and azobisisobutyro as an initiator. 0.03 part of nitrile (AIBN) was charged, nitrogen was bubbled to this for 2 minutes, the inside of the container was replaced with nitrogen, and the lid was closed to seal the container. Then, the reaction vessel was immersed in an oil bath at 75 ° C. for 2 hours for polymerization. After the polymerization, it was diluted with chloroform, added to methanol and reprecipitated, and a white solid was taken out. Then, it was vacuum dried at 240 ° C. for 1 hour to obtain about 6 parts of a white copolymer. Table 2 shows the physical characteristics of the obtained copolymer.
溶媒をNMPからγ-ブチロラクトン(GBL)に変更した以外は、試験例B1と同様にして重合、再沈殿、乾燥を行い、白色の共重合体を6.5部得た。得られた共重合体の物性を表2に示す。 (Test Example B2)
Polymerization, reprecipitation, and drying were carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to γ-butyrolactone (GBL) to obtain 6.5 parts of a white copolymer. Table 2 shows the physical characteristics of the obtained copolymer.
溶媒をNMPからジメチルスルホキシド(DMSO)に変更した以外は、試験例B1と同様にして重合、再沈殿、乾燥を行い、白色の共重合体を6部得た。得られた共重合体の物性を表2に示す。 (Test Example B3)
Polymerization, reprecipitation, and drying were carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to dimethyl sulfoxide (DMSO) to obtain 6 parts of a white copolymer. Table 2 shows the physical characteristics of the obtained copolymer.
溶媒をNMPからトルエンに変更した以外は、試験例B1と同様にして重合を行った。しかし、重合の途中で固体が析出し固化したため重合を終了した。 (Test Example B4)
Polymerization was carried out in the same manner as in Test Example B1 except that the solvent was changed from NMP to toluene. However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
NMP等の溶媒を用いなかった以外は、試験例B1と同様にして重合を行った。しかし、重合の途中で固体が析出し固化したため重合を終了した。 (Test Example B5)
Polymerization was carried out in the same manner as in Test Example B1 except that a solvent such as NMP was not used. However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
(実施例B1-1)
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)8部、α-メチレン-γ-ブチロラクトン(ML)1.5部、連鎖移動剤としてn-ドデシルメルカプタン(nDM)0.005部、及び溶媒としてN-メチルピロリドン(NMP)10部を仕込み、これに窒素を通じつつ105℃まで昇温させた。その後、重合開始剤としてt-アミルパーオキシイソノナノエート(アルケマ吉富製、ルペロックス(登録商標)570、以下「開始剤570」ともいう。)を0.003部加えるとともに、0.2部のNMPで希釈した0.005部の開始剤570及び0.5部のMLを105~115℃で2時間かけて一定速度で滴下した。滴下後、4部のNMPを加え、さらに105~115℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ99.1%、99.5%であった。得られた重合反応液を240℃で2時間真空乾燥(133Pa(1mmHg))することで、白色の共重合体を得た。得られた共重合体の物性を表3に示す。 <Synthesis of copolymer by stirring polymerization and preparation of film>
(Example B1-1)
In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 8 parts of methyl methacrylate (MMA), 1.5 parts of α-methylene-γ-butyrolactone (ML), and n- as a chain transfer agent. 0.005 part of dodecyl mercaptan (nDM) and 10 parts of N-methylpyrrolidone (NMP) as a solvent were charged, and the temperature was raised to 105 ° C. through nitrogen. Then, 0.003 parts of t-amylperoxyisononanoate (manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”) was added as a polymerization initiator, and 0.2 parts of NMP. 0.005 part of the initiator diluted with 570 and 0.5 part of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. After the dropping, 4 parts of NMP was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.5%, respectively. The obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer. Table 3 shows the physical characteristics of the obtained copolymer.
溶媒をNMPからγ-ブチロラクトン(GBL)に変更した以外は、実施例B1-1と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。なお、重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ98.5%、99.0%であった。得られた共重合体の物性及び延伸フィルムの物性を表3に示す。 (Example B1-2)
A copolymer and a stretched film having a thickness of 40 μm were obtained in the same manner as in Example B1-1 except that the solvent was changed from NMP to γ-butyrolactone (GBL). The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.5% and 99.0%, respectively. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、MMAを9部、MLを0.75部、nDMを0.005部、及び溶媒としてGBLを10部仕込み、これに窒素を通じつつ105℃まで昇温させた。その後開始剤570を0.003部加えるとともに、0.2部のGBLで希釈した0.005部の開始剤570及び0.25部のMLを105~115℃で2時間かけて一定速度で滴下した。滴下後4部のGBLを加え、さらに105~115℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ98.4%、99.2%であった。得られた重合反応液を実施例B1-1と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。得られた共重合体の物性及び延伸フィルムの物性を表3に示す。 (Example B1-3)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube was charged with 9 parts of MMA, 0.75 parts of ML, 0.005 parts of nDM, and 10 parts of GBL as a solvent. The temperature was raised to 105 ° C. through nitrogen. After that, 0.003 parts of the initiator 570 was added, and 0.005 parts of the initiator 570 diluted with 0.2 parts of GBL and 0.25 parts of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. did. After the dropping, 4 parts of GBL was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 98.4% and 99.2%, respectively. The obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 μm. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
nDMを0.005部から0.03部、溶媒としてGBLを10部から15部に変更し、滴下後に加えていた4部のGBLを加えなかった以外は、実施例B1-3と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。なお、重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ98.0%、98.5%であった。得られた共重合体の物性及び延伸フィルムの物性を表3に示す。 (Example B1-4)
The same as in Example B1-3 except that nDM was changed from 0.005 part to 0.03 part, GBL was changed from 10 part to 15 part as a solvent, and 4 parts of GBL added after the dropping was not added. , A copolymer and a stretched film having a thickness of 40 μm were obtained. The conversion rates of MMA and ML calculated from the amount of unreacted monomer in the polymerization reaction solution were 98.0% and 98.5%, respectively. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、MMAを7部、MLを2.2部、及び溶媒としてGBLを10部仕込み、これに窒素を通じつつ105℃まで昇温させた。その後開始剤570を0.003部加えるとともに、0.2部のGBLで希釈した0.005部の開始剤570及び0.8部のMLを105~115℃で2時間かけて一定速度で滴下した。滴下後4部のGBLを加え、さらに105~115℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ99.1%、99.2%であった。得られた重合反応液を実施例B1-1と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。得られた共重合体の物性及び延伸フィルムの物性を表3に示す。 (Example B1-5)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube was charged with 7 parts of MMA, 2.2 parts of ML, and 10 parts of GBL as a solvent, and the temperature was raised to 105 ° C. through nitrogen. I warmed it up. After that, 0.003 parts of the initiator 570 was added, and 0.005 parts of the initiator 570 diluted with 0.2 parts of GBL and 0.8 parts of ML were added dropwise at 105 to 115 ° C. over 2 hours at a constant rate. did. After the dropping, 4 parts of GBL was added, and solution stirring polymerization was further carried out at 105 to 115 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.2%, respectively. The obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 μm. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、MMAを7部、MLを3部、及び溶媒としてDMSOを10部仕込み、これに窒素を通じつつ105℃まで昇温させた。その後、開始剤570を0.02部加え、105~115℃で6時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ99.1%、99.5%であった。得られた重合反応液を実施例B1-1と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。得られた共重合体の物性及び延伸フィルムの物性を表3に示す。 (Comparative Example B1-1)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube is charged with 7 parts of MMA, 3 parts of ML, and 10 parts of DMSO as a solvent, and the temperature is raised to 105 ° C. through nitrogen. It was. Then, 0.02 part of the initiator 570 was added, and solution stirring polymerization was carried out at 105 to 115 ° C. for 6 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.5%, respectively. The obtained polymerization reaction solution was used in the same manner as in Example B1-1 to obtain a copolymer and a stretched film having a thickness of 40 μm. Table 3 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
(実施例B2-1)
実施例B1-2で得られた共重合体1部及び分散媒としてのジクロロメタン7部を混合し、1分間手振り後、60分間撹拌混合して、固形分12.5質量%のドープ樹脂組成物を作製した。ドープ樹脂組成物の粘度は0.3Pa・s、YIは0.9、ヘイズは0.3%であった。ドープ樹脂組成物を目視で確認したところ、均一に分散しており、その後一晩静置してもドープ樹脂組成物の外観に変化は見られなかった。 <Preparation of Doping Resin Composition and Preparation of Film>
(Example B2-1)
A dope resin composition having a solid content of 12.5% by mass is mixed with 1 part of the copolymer obtained in Example B1-2 and 7 parts of dichloromethane as a dispersion medium, shaken for 1 minute, and then stirred and mixed for 60 minutes. Was produced. The viscosity of the doped resin composition was 0.3 Pa · s, YI was 0.9, and haze was 0.3%. When the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
[混合溶媒の沸点測定]
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた1Lのセパラブルフラスコに、500mlの混合溶媒を加え、これに窒素を通じつつ撹拌しながら昇温を行い、冷却管から還流がかかったときの内温を、混合溶媒の沸点として測定した。 <Test Example C, Example C, and Comparative Example C>
[Measurement of boiling point of mixed solvent]
500 ml of a mixed solvent was added to a 1 L separable flask equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube, and the temperature was raised while stirring while passing nitrogen through the flask, and reflux was applied from the cooling tube. The internal temperature at that time was measured as the boiling point of the mixed solvent.
静置重合における重合反応率は、<試験例B、実施例B、及び比較例B>の静置重合における重合反応率と同様にして簡易的に求めた。 [Polymerization reaction rate in static polymerization]
The polymerization reaction rate in the static polymerization was simply determined in the same manner as the polymerization reaction rate in the static polymerization of <Test Example B, Example B, and Comparative Example B>.
撹拌重合における重合反応時の反応率及び共重合体中の特定単量体単位の含有率は、<試験例B、実施例B、及び比較例B>の撹拌重合における重合反応時の反応率及び共重合体中の特定単量体単位の含有率と同様にして求めた。 [Polymerization reaction rate in agitation polymerization and composition analysis of copolymer]
The reaction rate at the time of the polymerization reaction in the stirring polymerization and the content of the specific monomer unit in the copolymer are the reaction rate at the time of the polymerization reaction in the stirring polymerization of <Test Example B, Example B, and Comparative Example B> and It was determined in the same manner as the content of the specific monomer unit in the copolymer.
共重合体の重量平均分子量(Mw)及び数平均分子量(Mn)は、<実施例A>の共重合体の重量平均分子量(Mw)及び数平均分子量(Mn)と同様にして求めた。 [Weight average molecular weight and number average molecular weight of copolymer]
The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer were determined in the same manner as the weight average molecular weight (Mw) and the number average molecular weight (Mn) of the copolymer of <Example A>.
共重合体中のML含有量(α-メチレンラクトン由来の構成単位の含有量)は、<実施例A>の共重合体中のML含有量と同様にして求めた。 [ML content in copolymer]
The ML content in the copolymer (content of the structural unit derived from α-methylenelactone) was determined in the same manner as the ML content in the copolymer of <Example A>.
共重合体のガラス転移温度は、<実施例A>の共重合体のガラス転移温度と同様にして求めた。 [Copolymer glass transition temperature (Tg)]
The glass transition temperature of the copolymer was determined in the same manner as the glass transition temperature of the copolymer of <Example A>.
共重合体の5%重量減少温度は、<実施例A>の共重合体の5%重量減少温度と同様にして求めた。 [5% weight loss temperature of copolymer]
The 5% weight loss temperature of the copolymer was determined in the same manner as the 5% weight loss temperature of the copolymer of <Example A>.
共重合体の内部ヘイズは、<試験例B、実施例B、及び比較例B>の共重合体の内部ヘイズと同様にして求めた。 [Internal haze of copolymer]
The internal haze of the copolymer was determined in the same manner as the internal haze of the copolymer of <Test Example B, Example B, and Comparative Example B>.
共重合体の内部b*値は、<試験例B、実施例B、及び比較例B>の共重合体の内部b*値と同様にして求めた。 [Internal b * value of copolymer]
Internal b * values of the copolymers were determined in the same manner as the internal b * value of the copolymer of <Test Example B, Example B, and Comparative Example B>.
共重合体中の溶媒(化合物)含有量は、<試験例B、実施例B、及び比較例B>の共重合体中の溶媒(化合物)含有量と同様にして求めた。 [Solvent (compound) content in copolymer]
The solvent (compound) content in the copolymer was determined in the same manner as the solvent (compound) content in the copolymer of <Test Example B, Example B, and Comparative Example B>.
ドープ樹脂組成物の粘度は、<試験例B、実施例B、及び比較例B>のドープ樹脂組成物の粘度と同様にして求めた。 [Viscosity of Doping Resin Composition]
The viscosity of the doped resin composition was determined in the same manner as the viscosity of the doped resin composition of <Test Example B, Example B, and Comparative Example B>.
ドープ樹脂組成物の黄色度(YI)は、<試験例B、実施例B、及び比較例B>のドープ樹脂組成物の黄色度(YI)と同様にして求めた。 [Yellowness (YI) of Doping Resin Composition]
The yellowness (YI) of the doped resin composition was determined in the same manner as the yellowness (YI) of the doped resin composition of <Test Example B, Example B, and Comparative Example B>.
ドープ樹脂組成物のヘイズは、<試験例B、実施例B、及び比較例B>のドープ樹脂組成物のヘイズと同様にして求めた。 [Haze of Doping Resin Composition]
The haze of the doped resin composition was determined in the same manner as the haze of the doped resin composition of <Test Example B, Example B, and Comparative Example B>.
フィルムの厚さは、<実施例A>のフィルムの厚さと同様にして求めた。 [Film thickness]
The film thickness was determined in the same manner as the film thickness of <Example A>.
フィルムの全光線透過率は、<実施例A>のフィルムの全光線透過率と同様にして求めた。 [Total light transmittance of film]
The total light transmittance of the film was determined in the same manner as the total light transmittance of the film of <Example A>.
<実施例A>のフィルムの引張試験と同様にしてフィルムの引張試験(弾性率測定)を行い、弾性率を測定した。 [Film tensile test (modulus measurement)]
A tensile test (measurement of elastic modulus) of the film was performed in the same manner as the tensile test of the film of <Example A>, and the elastic modulus was measured.
フィルムの鉛筆硬度は、<実施例A>のフィルムの鉛筆硬度と同様にして求めた。 [Pencil hardness of film]
The pencil hardness of the film was determined in the same manner as the pencil hardness of the film of <Example A>.
<実施例A>のフィルムのフォルダブル試験と同様にしてフィルムのフォルダブル試験を行い、<実施例A>と同様に、試験後の折り畳まれた部分のフィルムの破断の有無を評価した。 [Film foldable test]
The film foldable test was performed in the same manner as in the film foldable test of <Example A>, and the presence or absence of breakage of the film in the folded portion after the test was evaluated in the same manner as in <Example A>.
フィルムの位相差は、<試験例B、実施例B、及び比較例B>のフィルムの位相差と同様にして求めた。 [Film phase difference]
The phase difference of the film was determined in the same manner as the phase difference of the films of <Test Example B, Example B, and Comparative Example B>.
(試験例C1)
密閉できる反応容器内に、メタクリル酸メチル(MMA)7部、α-メチレン-γ-ブチロラクトン(ML)3部、溶媒としてトルエン(tol)10部、及び開始剤としてアゾビスイソブチロニトリル(AIBN)を0.03部仕込み、これに窒素を2分間バブリングして容器内を窒素置換後、蓋を締めて密閉した。その後、反応容器を75℃のオイルバスに2時間漬けて重合を行った。しかし重合の途中で固体が析出し固化したため重合を終了した。重合後クロロホルムで希釈した後、メタノール中に加えて再沈殿を行い、白色固体を取り出した。 <Synthesis of copolymer by static polymerization>
(Test Example C1)
In a hermetically sealed reaction vessel, 7 parts of methyl methacrylate (MMA), 3 parts of α-methylene-γ-butyrolactone (ML), 10 parts of toluene (tor) as the solvent, and azobisisobutyronitrile (AIBN) as the initiator. ) Was charged, and nitrogen was bubbled to this for 2 minutes to replace the inside of the container with nitrogen, and then the lid was closed to seal the container. Then, the reaction vessel was immersed in an oil bath at 75 ° C. for 2 hours for polymerization. However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization. After the polymerization, it was diluted with chloroform, added to methanol and reprecipitated, and a white solid was taken out.
溶媒をトルエンからアセトン(ACE)に変更した以外は、試験例C1と同様にして重合を行った。しかし、重合の途中で固体が析出し固化したため重合を終了した。 (Test Example C2)
Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to acetone (ACE). However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
溶媒をトルエンからシクロヘキサノン(アノン)に変更した以外は、試験例C1と同様にして重合を行った。しかし、重合の途中で固体が析出し固化したため重合を終了した。 (Test Example C3)
Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to cyclohexanone (anone). However, the polymerization was terminated because the solid was precipitated and solidified during the polymerization.
溶媒をトルエンからアセトン(ACE)とシクロヘキサノン(アノン)との混合溶媒(1:1(質量比))に変更した以外は、試験例C1と同様にして重合を行った。重合後クロロホルムで希釈した後、メタノール中に加えて再沈殿を行い、白色固体を取り出した。その後240℃で1時間真空乾燥をして、白色の共重合体を約6部得た。得られた共重合体の物性を表4に示す。 (Test Example C4)
Polymerization was carried out in the same manner as in Test Example C1 except that the solvent was changed from toluene to a mixed solvent (1: 1 (mass ratio)) of acetone (ACE) and cyclohexanone (anone). After the polymerization, it was diluted with chloroform, added to methanol and reprecipitated, and a white solid was taken out. Then, it was vacuum dried at 240 ° C. for 1 hour to obtain about 6 parts of a white copolymer. Table 4 shows the physical characteristics of the obtained copolymer.
(実施例C1-1)
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)7.5部、α-メチレン-γ-ブチロラクトン(ML)2部、連鎖移動剤としてn-ドデシルメルカプタン(nDM)0.005部、及び溶媒としてアセトン(ACE)とシクロヘキサノン(アノン)とを1:1(質量比)で混合した混合溶媒10部を仕込み、これに窒素を通じつつ70℃まで昇温させた。その後、重合開始剤としてAIBNを0.004部加えるとともに、0.2部のACEとアノンとを1:1(質量比)で混合した混合溶媒で希釈した0.011部の開始剤AIBN及び0.5部のMLを70~75℃で3時間かけて一定速度で滴下した。滴下後、4部のACEとアノンとを1:1(質量比)で混合した混合溶媒を加え、さらに70~75℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ92.1%、95.5%であった。得られた重合反応液を240℃で2時間真空乾燥(133Pa(1mmHg))することで、白色の共重合体を得た。得られた共重合体の物性を表5に示す。 <Synthesis of copolymer by stirring polymerization and preparation of film>
(Example C1-1)
In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 7.5 parts of methyl methacrylate (MMA), 2 parts of α-methylene-γ-butyrolactone (ML), and n- as a chain transfer agent. 0.005 part of dodecyl mercaptan (nDM) and 10 parts of a mixed solvent in which acetone (ACE) and cyclohexanone (anone) were mixed at a ratio of 1: 1 (mass ratio) were charged, and the temperature was raised to 70 ° C. through nitrogen. I warmed it up. Then, 0.004 parts of AIBN was added as a polymerization initiator, and 0.011 parts of the initiator AIBN and 0 were diluted with a mixed solvent in which 0.2 parts of ACE and anon were mixed at a ratio of 1: 1 (mass ratio). .5 parts of ML was added dropwise at 70-75 ° C. over 3 hours at a constant rate. After the dropping, a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 1: 1 (mass ratio) was added, and solution stirring polymerization was further carried out at 70 to 75 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 92.1% and 95.5%, respectively. The obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer. Table 5 shows the physical characteristics of the obtained copolymer.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)6部、α-メチレン-γ-ブチロラクトン(ML)3部、連鎖移動剤としてn-ドデシルメルカプタン(nDM)0.005部、及び溶媒としてアセトン(ACE)とシクロヘキサノン(アノン)とを3:7(質量比)で混合した混合溶媒10部を仕込み、これに窒素を通じつつ85℃まで昇温させた。その後、t-アミルパーオキシ2-エチルヘキサノエート(アルケマ吉富製、ルペロックス(登録商標)575、以下「開始剤575」ともいう。)を0.004部加えるとともに、0.2部のACEとアノンとを3:7(質量比)で混合した混合溶媒で希釈した0.009部の開始剤575及び1部のMLを85~90℃で3時間かけて一定速度で滴下した。滴下後、4部のACEとアノンとを3:7(質量比)で混合した混合溶媒を加え、さらに85~90℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ93.1%、95.5%であった。得られた重合反応液を240℃で2時間真空乾燥(133Pa(1mmHg))することで、白色の共重合体を得た。得られた共重合体の物性を表5に示す。 (Example C1-2)
In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 6 parts of methyl methacrylate (MMA), 3 parts of α-methylene-γ-butyrolactone (ML), and n-dodecyl mercaptan as a chain transfer agent. 0.005 part of (nDM) and 10 parts of a mixed solvent prepared by mixing acetone (ACE) and cyclohexanone (anone) as a solvent at a ratio of 3: 7 (mass ratio) were charged, and the temperature was raised to 85 ° C. through nitrogen. It was. Then, 0.004 parts of t-amylperoxy2-ethylhexanoate (manufactured by Alchema Yoshitomi, Luperox® 575, hereinafter also referred to as "initiator 575") was added, and 0.2 parts of ACE were added. 0.009 parts of initiator 575 and 1 part of ML diluted with a mixed solvent mixed with anon at a ratio of 3: 7 (mass ratio) were added dropwise at 85 to 90 ° C. over 3 hours at a constant rate. After the dropping, a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 3: 7 (mass ratio) was added, and solution stirring polymerization was further carried out at 85 to 90 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 93.1% and 95.5%, respectively. The obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer. Table 5 shows the physical characteristics of the obtained copolymer.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)6部、α-メチレン-γ-ブチロラクトン(ML)3部、及び溶媒としてアセトン(ACE)とγ‐ブチロラクトン(GBL)を2:8(質量比)で混合した混合溶媒10部を仕込み、これに窒素を通じつつ100℃まで昇温させた。その後、重合開始剤としてt-アミルパーオキシイソノナノエート(アルケマ吉富製、ルペロックス(登録商標)570、以下「開始剤570」ともいう。)を0.004部加えるとともに、0.2部のACEとGBLとを2:8(質量比)で混合した混合溶媒で希釈した0.009部の開始剤570及び1部のMLを100~110℃で3時間かけて一定速度で滴下した。滴下後、4部のACEとGBLとを2:8(質量比)で混合した混合溶媒を加え、さらに100~110℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ92.2%、96.5%であった。得られた重合反応液を240℃で2時間真空乾燥(133Pa(1mmHg))することで、白色の共重合体を得た。得られた共重合体の物性を表5に示す。 (Example C1-3)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube, containing 6 parts of methyl methacrylate (MMA), 3 parts of α-methylene-γ-butyrolactone (ML), and acetone (ACE) as a solvent. 10 parts of a mixed solvent in which γ-butyrolactone (GBL) was mixed at a ratio of 2: 8 (mass ratio) was charged, and the temperature was raised to 100 ° C. while passing nitrogen through the mixed solvent. After that, 0.004 parts of t-amylperoxyisononanoate (manufactured by Alchema Yoshitomi, Luperox (registered trademark) 570, hereinafter also referred to as “initiator 570”) was added as a polymerization initiator, and 0.2 parts of ACE was added. And GBL were diluted in a mixed solvent mixed at a ratio of 2: 8 (mass ratio), and 0.009 parts of the initiator 570 and 1 part of ML were added dropwise at 100 to 110 ° C. over 3 hours at a constant rate. After the dropping, a mixed solvent in which 4 parts of ACE and GBL were mixed at a ratio of 2: 8 (mass ratio) was added, and solution stirring polymerization was further carried out at 100 to 110 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 92.2% and 96.5%, respectively. The obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer. Table 5 shows the physical characteristics of the obtained copolymer.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)5部、α-メチレン-γ-ブチロラクトン(ML)3.2部、及び溶媒としてアセトン(ACE)とN,N-ジメチルアセトアミド(DMAc)とを3:7(質量比)で混合した混合溶媒10部を仕込み、これに窒素を通じつつ85℃まで昇温させた。その後、開始剤575を0.004部加えるとともに、0.2部のACEとDMAcとを3:7(質量比)で混合した混合溶媒で希釈した0.009部の開始剤575及び1.8部のMLを85~90℃で3時間かけて一定速度で滴下した。滴下後、4部のACEとDMAcとを3:7(質量比)で混合した混合溶媒を加え、さらに85~90℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ90.2%、95.5%であった。得られた重合反応液を240℃で2時間真空乾燥(133Pa(1mmHg))することで、白色の共重合体を得た。得られた共重合体の物性を表5に示す。 (Example C1-4)
A reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 5 parts of methyl methacrylate (MMA), 3.2 parts of α-methylene-γ-butyrolactone (ML), and acetone (ACE) as a solvent. ) And N, N-dimethylacetamide (DMAc) in a ratio of 3: 7 (mass ratio), 10 parts of a mixed solvent was charged, and the temperature was raised to 85 ° C. while passing nitrogen through the mixed solvent. Then, 0.004 parts of the initiator 575 was added, and 0.009 parts of the initiator 575 and 1.8 were diluted with a mixed solvent in which 0.2 parts of ACE and DMAc were mixed at a ratio of 3: 7 (mass ratio). The ML of the part was added dropwise at a constant rate at 85 to 90 ° C. over 3 hours. After the dropping, a mixed solvent in which 4 parts of ACE and DMAc were mixed at a ratio of 3: 7 (mass ratio) was added, and solution stirring polymerization was further carried out at 85 to 90 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 90.2% and 95.5%, respectively. The obtained polymerization reaction solution was vacuum dried (133 Pa (1 mmHg)) at 240 ° C. for 2 hours to obtain a white copolymer. Table 5 shows the physical characteristics of the obtained copolymer.
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、MMAを7部、MLを3部、及び溶媒としてDMSOを10部仕込み、これに窒素を通じつつ105℃まで昇温させた。その後、開始剤570を0.02部加え、105~110℃で6時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ99.1%、99.5%であった。得られた重合反応液を実施例C1-1と同様にして、共重合体及び厚さ40μmの延伸フィルムを得た。得られた共重合体の物性及び延伸フィルムの物性を表5に示す。 (Comparative Example 1-1)
A reactor equipped with a stirrer, a temperature sensor, a cooling tube, and a nitrogen introduction tube is charged with 7 parts of MMA, 3 parts of ML, and 10 parts of DMSO as a solvent, and the temperature is raised to 105 ° C. through nitrogen. It was. Then, 0.02 part of the initiator 570 was added, and solution stirring polymerization was carried out at 105 to 110 ° C. for 6 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 99.1% and 99.5%, respectively. The obtained polymerization reaction solution was used in the same manner as in Example C1-1 to obtain a copolymer and a stretched film having a thickness of 40 μm. Table 5 shows the physical characteristics of the obtained copolymer and the physical characteristics of the stretched film.
(実施例C2-1)
撹拌装置、温度センサー、冷却管、及び窒素導入管を備えた反応器に、メタクリル酸メチル(MMA)7.5部、α-メチレン-γ-ブチロラクトン(ML)2部、連鎖移動剤としてn-ドデシルメルカプタン(nDM)0.005部、及び溶媒としてアセトン(ACE)とシクロヘキサノン(アノン)とを3:7(質量比)で混合した混合溶媒10部を仕込み、これに窒素を通じつつ85℃まで昇温させた。その後、開始剤575を0.004部加えるとともに、0.2部のACEとアノンとを3:7(質量比)で混合した混合溶媒で希釈した0.009部の開始剤575及び0.5部のMLを85~90℃で3時間かけて一定速度で滴下した。滴下後、4部のACEとアノンとを3:7(質量比)で混合した混合溶媒を加え、さらに85~90℃で4時間溶液撹拌重合を行った。重合反応液中の未反応単量体量より算出したMMA、MLの転化率はそれぞれ93.1%、97.5%であった。得られた重合反応液をACEとアノンとを3:7(質量比)で混合した混合溶媒55部で希釈し、共重合体含有量12.5質量%のドープ樹脂組成物を作製した。ドープ樹脂組成物は、3μmのメンブレンフィルターで加圧濾過した。ドープ樹脂組成物の物性を表5に示す。ドープ樹脂組成物を目視で確認したところ、均一に分散しており、その後一晩静置してもドープ樹脂組成物の外観に変化は見られなかった。 <Preparation of Doping Resin Composition and Preparation of Film>
(Example C2-1)
In a reactor equipped with a stirrer, temperature sensor, cooling tube, and nitrogen introduction tube, 7.5 parts of methyl methacrylate (MMA), 2 parts of α-methylene-γ-butyrolactone (ML), and n- as a chain transfer agent. 0.005 part of dodecyl mercaptan (nDM) and 10 parts of a mixed solvent in which acetone (ACE) and cyclohexanone (anone) were mixed at a ratio of 3: 7 (mass ratio) were charged, and the temperature was raised to 85 ° C. through nitrogen. I warmed it up. Then, 0.004 parts of the initiator 575 was added, and 0.009 parts of the initiator 575 and 0.5 were diluted with a mixed solvent in which 0.2 parts of ACE and anon were mixed at a ratio of 3: 7 (mass ratio). The ML of the part was added dropwise at 85 to 90 ° C. over 3 hours at a constant rate. After the dropping, a mixed solvent in which 4 parts of ACE and Anon were mixed at a ratio of 3: 7 (mass ratio) was added, and solution stirring polymerization was further carried out at 85 to 90 ° C. for 4 hours. The conversion rates of MMA and ML calculated from the amount of unreacted monomers in the polymerization reaction solution were 93.1% and 97.5%, respectively. The obtained polymerization reaction solution was diluted with 55 parts of a mixed solvent in which ACE and anon were mixed at a ratio of 3: 7 (mass ratio) to prepare a dope resin composition having a copolymer content of 12.5% by mass. The doped resin composition was pressure filtered through a 3 μm membrane filter. The physical characteristics of the doped resin composition are shown in Table 5. When the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
実施例C1-2で得られた共重合体1部及び分散媒としてのジクロロメタン7部を混合し、1分間手振り後、60分間撹拌混合して、固形分12.5質量%のドープ樹脂組成物を作製した。ドープ樹脂組成物の物性は表5に示す。ドープ樹脂組成物を目視で確認したところ、均一に分散しており、その後一晩静置してもドープ樹脂組成物の外観に変化は見られなかった。 (Example C2-2)
A dope resin composition having a solid content of 12.5% by mass is mixed with 1 part of the copolymer obtained in Example C1-2 and 7 parts of dichloromethane as a dispersion medium, shaken for 1 minute, and then stirred and mixed for 60 minutes. Was produced. The physical characteristics of the doped resin composition are shown in Table 5. When the dope resin composition was visually confirmed, it was uniformly dispersed, and no change was observed in the appearance of the dope resin composition even after being allowed to stand overnight.
Claims (18)
- α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体の製造方法であって、
溶媒の存在下、前記α-メチレンラクトン及び前記(メタ)アクリル酸アルキルを含む単量体を重合させる工程を備え、
前記溶媒が、下記条件(A)又は下記条件(B)のいずれかを満たす溶媒である、共重合体の製造方法。
条件(A):環状アミド及び環状エステルからなる群より選ばれる少なくとも1種の溶媒である。
条件(B):100℃未満の沸点を有する第1の溶媒及び100℃以上の沸点を有する第2の溶媒からなる混合溶媒であって、前記第1の溶媒がケトン及び塩化アルキルからなる群より選ばれる少なくとも1種であり、前記第2の溶媒が環状ケトン、環状エステル、アミド、及びスルホキシドからなる群より選ばれる少なくとも1種であり、沸点が70~120℃である混合溶媒である。 A method for producing a copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms.
A step of polymerizing a monomer containing the α-methylene lactone and the alkyl (meth) acrylate in the presence of a solvent is provided.
A method for producing a copolymer, wherein the solvent is a solvent that satisfies either the following condition (A) or the following condition (B).
Condition (A): At least one solvent selected from the group consisting of cyclic amides and cyclic esters.
Condition (B): A mixed solvent composed of a first solvent having a boiling point of less than 100 ° C. and a second solvent having a boiling point of 100 ° C. or higher, from the group in which the first solvent is composed of a ketone and an alkyl chloride. It is at least one selected, and the second solvent is at least one selected from the group consisting of cyclic ketones, cyclic esters, amides, and sulfoxides, and is a mixed solvent having a boiling point of 70 to 120 ° C. - 前記溶媒が、前記条件(A)を満たす溶媒である、請求項1に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 1, wherein the solvent is a solvent that satisfies the condition (A).
- 前記溶媒が、前記条件(B)を満たす溶媒である、請求項1に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 1, wherein the solvent is a solvent that satisfies the condition (B).
- 前記第1の溶媒がアセトンである、請求項3に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 3, wherein the first solvent is acetone.
- 前記第2の溶媒が環状ケトンである、請求項3又は4に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 3 or 4, wherein the second solvent is a cyclic ketone.
- 前記環状ケトンがシクロヘキサノンである、請求項5に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 5, wherein the cyclic ketone is cyclohexanone.
- 前記第2の溶媒が環状エステル、アミド、及びスルホキシドからなる群より選ばれる少なくとも1種である、請求項3又は4に記載の共重合体の製造方法。 The method for producing a copolymer according to claim 3 or 4, wherein the second solvent is at least one selected from the group consisting of cyclic esters, amides, and sulfoxides.
- 前記第2の溶媒がγ-ブチロラクトン、γ-バレロラクトン、δ-バレロラクトン、N,N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、N-メチルピロリドン、N,N’-ジメチルイミダゾリジノン、及びジメチルスルホキシドからなる群より選ばれる少なくとも1種である、請求項7に記載の共重合体の製造方法。 The second solvent is γ-butyrolactone, γ-valerolactone, δ-valerolactone, N, N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, N, N'-dimethylimidazolidinone, and The method for producing a copolymer according to claim 7, which is at least one selected from the group consisting of dimethyl sulfoxide.
- α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含み、
フィルムにしたときの厚さ100μm当たりの内部ヘイズが2.5%未満である、共重合体。 It contains a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms.
A copolymer having an internal haze of less than 2.5% per 100 μm thickness when made into a film. - α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含み、かつ重量平均分子量が200000以上1000000以下である、共重合体。 A copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms and having a weight average molecular weight of 200,000 or more and 1,000,000 or less.
- フィルムにしたときのL*a*b*表色系の厚さ100μm当たりの内部b*値が1.6未満である、請求項9又は10に記載の共重合体。 The copolymer according to claim 9 or 10, wherein the L * a * b * color system has an internal b * value of less than 1.6 per 100 μm of thickness when made into a film.
- 請求項9~11のいずれか一項に記載の共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有する、共重合体混合物。 A copolymer mixture containing the copolymer according to any one of claims 9 to 11 and at least one compound selected from the group consisting of cyclic amides, cyclic esters, and cyclic ketones.
- α-メチレンラクトン由来の構成単位及び炭素数1~6のアルキル基を有する(メタ)アクリル酸アルキル由来の構成単位を含む共重合体と、環状アミド、環状エステル、及び環状ケトンからなる群より選ばれる少なくとも1種の化合物とを含有する、共重合体混合物。 Selected from the group consisting of a copolymer containing a structural unit derived from α-methylene lactone and a structural unit derived from an alkyl (meth) acrylate having an alkyl group having 1 to 6 carbon atoms, a cyclic amide, a cyclic ester, and a cyclic ketone. A copolymer mixture containing at least one compound.
- 前記化合物の含有量が、前記共重合体の全量を基準として、10~3000質量ppmである、請求項12又は13に記載の共重合体混合物。 The copolymer mixture according to claim 12 or 13, wherein the content of the compound is 10 to 3000 mass ppm based on the total amount of the copolymer.
- 請求項9~11のいずれか一項に記載の共重合体と、分散媒とを含有するドープ樹脂組成物であって、
前記共重合体の含有量が、前記ドープ樹脂組成物の全量を基準として、5質量%以上である、ドープ樹脂組成物。 A dope resin composition containing the copolymer according to any one of claims 9 to 11 and a dispersion medium.
A dope resin composition in which the content of the copolymer is 5% by mass or more based on the total amount of the dope resin composition. - 請求項9~11のいずれか一項に記載の共重合体又は請求項12~14のいずれか一項に記載の共重合体混合物を含有する、樹脂成形体。 A resin molded product containing the copolymer according to any one of claims 9 to 11 or the copolymer mixture according to any one of claims 12 to 14.
- 請求項9~11のいずれか一項に記載の共重合体又は請求項12~14のいずれか一項に記載の共重合体混合物を含有する樹脂組成物を成形して樹脂成形体を得る工程を備える、樹脂成形体の製造方法。 A step of molding a resin composition containing the copolymer according to any one of claims 9 to 11 or the copolymer mixture according to any one of claims 12 to 14 to obtain a resin molded product. A method for producing a resin molded product.
- 請求項15に記載のドープ樹脂組成物を塗工する工程と、
塗工された前記ドープ樹脂組成物から前記分散媒を除去して樹脂成形体を得る工程とを備える、樹脂成形体の製造方法。 The step of applying the doped resin composition according to claim 15, and
A method for producing a resin molded product, comprising a step of removing the dispersion medium from the coated dope resin composition to obtain a resin molded product.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202080059114.7A CN114341210A (en) | 2019-08-22 | 2020-08-21 | Copolymer and method for producing same, copolymer mixture, doped resin composition, and resin molded article and method for producing same |
| JP2021540990A JP7474771B2 (en) | 2019-08-22 | 2020-08-21 | Copolymer and method for producing same, copolymer mixture, dope resin composition, resin molded body and method for producing same |
| KR1020227006248A KR20220042168A (en) | 2019-08-22 | 2020-08-21 | Copolymer and its manufacturing method, copolymer mixture, dope resin composition, and resin molded object and its manufacturing method |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019-152204 | 2019-08-22 | ||
| JP2019152204 | 2019-08-22 | ||
| JP2019185398 | 2019-10-08 | ||
| JP2019-185398 | 2019-10-08 | ||
| JP2020056024 | 2020-03-26 | ||
| JP2020-056024 | 2020-03-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021033768A1 true WO2021033768A1 (en) | 2021-02-25 |
Family
ID=74660895
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/031644 WO2021033768A1 (en) | 2019-08-22 | 2020-08-21 | Copolymer and method for producing same, copolymer mixture, dope resin composition, and resin molded body and method for producing same |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP7474771B2 (en) |
| KR (1) | KR20220042168A (en) |
| CN (1) | CN114341210A (en) |
| WO (1) | WO2021033768A1 (en) |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08231648A (en) * | 1995-01-17 | 1996-09-10 | Degussa Ag | Production of copolymer for molding material for producing casting glass or for producing molding with hot shape retentivity |
| JPH0912645A (en) * | 1995-06-30 | 1997-01-14 | Mitsubishi Rayon Co Ltd | Clear heat-resistant resin |
| JP2009041007A (en) * | 2007-07-18 | 2009-02-26 | Toray Ind Inc | Optically isotropic acrylic resin film and its manufacturing method |
| JP2009517538A (en) * | 2005-11-30 | 2009-04-30 | ゼネラル・エレクトリック・カンパニイ | Tulipaline copolymer |
| JP2009227787A (en) * | 2008-03-21 | 2009-10-08 | Mitsubishi Rayon Co Ltd | Method for producing methyl methacrylate-based copolymer, and method for producing plastic optical fiber |
| JP2010179640A (en) * | 2009-02-09 | 2010-08-19 | Nippon Shokubai Co Ltd | Thermoplastic resin molded body, and image display apparatus provided with the same |
| JP2011046805A (en) * | 2009-08-26 | 2011-03-10 | Teijin Ltd | Transparent resin composition |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0912644A (en) * | 1995-06-29 | 1997-01-14 | Mitsubishi Rayon Co Ltd | Clear heat-resistant resin |
| JP4240204B2 (en) * | 2003-03-07 | 2009-03-18 | 日産化学工業株式会社 | Positive photosensitive resin composition |
| CN101360768A (en) * | 2005-11-30 | 2009-02-04 | 通用电气公司 | Tulipalin copolymers |
| JP2008163187A (en) * | 2006-12-28 | 2008-07-17 | Toray Ind Inc | Thermoplastic polymer and its production method |
| WO2008081863A1 (en) * | 2006-12-29 | 2008-07-10 | Nippon Shokubai Co., Ltd. | Polymer, and sheet-like molded thermoplastic resin article for optical purposes |
| TW201538543A (en) | 2014-02-03 | 2015-10-16 | Kuraray Co | Copolymer and molded article |
-
2020
- 2020-08-21 CN CN202080059114.7A patent/CN114341210A/en active Pending
- 2020-08-21 KR KR1020227006248A patent/KR20220042168A/en active Search and Examination
- 2020-08-21 WO PCT/JP2020/031644 patent/WO2021033768A1/en active Application Filing
- 2020-08-21 JP JP2021540990A patent/JP7474771B2/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08231648A (en) * | 1995-01-17 | 1996-09-10 | Degussa Ag | Production of copolymer for molding material for producing casting glass or for producing molding with hot shape retentivity |
| JPH0912645A (en) * | 1995-06-30 | 1997-01-14 | Mitsubishi Rayon Co Ltd | Clear heat-resistant resin |
| JP2009517538A (en) * | 2005-11-30 | 2009-04-30 | ゼネラル・エレクトリック・カンパニイ | Tulipaline copolymer |
| JP2009041007A (en) * | 2007-07-18 | 2009-02-26 | Toray Ind Inc | Optically isotropic acrylic resin film and its manufacturing method |
| JP2009227787A (en) * | 2008-03-21 | 2009-10-08 | Mitsubishi Rayon Co Ltd | Method for producing methyl methacrylate-based copolymer, and method for producing plastic optical fiber |
| JP2010179640A (en) * | 2009-02-09 | 2010-08-19 | Nippon Shokubai Co Ltd | Thermoplastic resin molded body, and image display apparatus provided with the same |
| JP2011046805A (en) * | 2009-08-26 | 2011-03-10 | Teijin Ltd | Transparent resin composition |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7474771B2 (en) | 2024-04-25 |
| CN114341210A (en) | 2022-04-12 |
| KR20220042168A (en) | 2022-04-04 |
| JPWO2021033768A1 (en) | 2021-02-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3054322B1 (en) | Resin composition for optical film, optical film formed using same, and polarizing plate and image display device comprising same | |
| US20180312682A1 (en) | Optical Material | |
| US7985803B2 (en) | Resin composition for optical material | |
| JP5291361B2 (en) | Resin composition for optical materials | |
| JP5770374B2 (en) | Resin composition, optical film formed using the same, polarizing plate including the same, and liquid crystal display device | |
| JP2013152473A (en) | Optical film and liquid crystal display including the same | |
| JP5600843B2 (en) | Optical film resin composition, polarizer protective film including the same, and liquid crystal display device | |
| US11225540B2 (en) | Copolymer and optical film using same | |
| JPWO2015098980A1 (en) | Optical thermoplastic resin and molded body | |
| US9315639B2 (en) | Optical films, phase difference films, and LCD comprising the same | |
| KR20080110012A (en) | Optical films comprising (meth)acrylic resins and method for preparing the same | |
| JP2008179813A (en) | Planar thermoplastic resin molded article | |
| US20230295358A1 (en) | Glutarimide resin | |
| WO2021033768A1 (en) | Copolymer and method for producing same, copolymer mixture, dope resin composition, and resin molded body and method for producing same | |
| JP4479409B2 (en) | Transparency resin composition and optical film | |
| JP2022120507A (en) | Copolymer and method for producing the same, and film | |
| JP2020105260A (en) | Polymer and optical film including the same | |
| JP3878688B2 (en) | Heat resistant film | |
| JP2023044845A (en) | Copolymer and method for producing the same, dope liquid, and film | |
| JP5580514B2 (en) | Method for producing retardation film | |
| JP2022083035A (en) | Method for producing copolymer | |
| JP2005010294A (en) | Multilayer product | |
| JP3994827B2 (en) | Method for producing transparent stretched film | |
| JP2019035018A (en) | Thermoplastic resin composition, optical film, and polarizing plate | |
| JP2022140384A (en) | Copolymer particles and method for producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20854884 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2021540990 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20227006248 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 20854884 Country of ref document: EP Kind code of ref document: A1 |