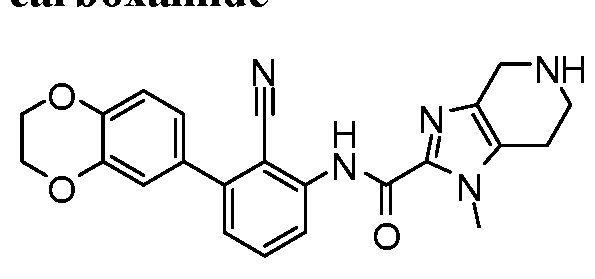
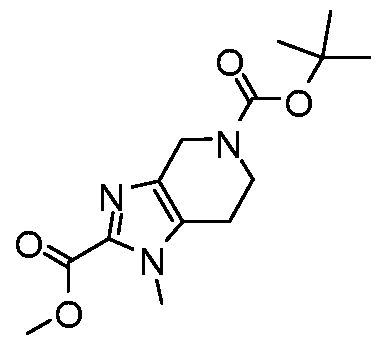
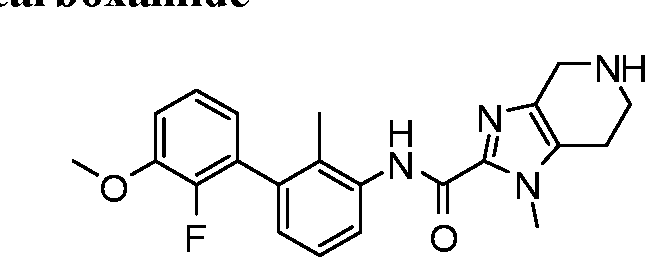
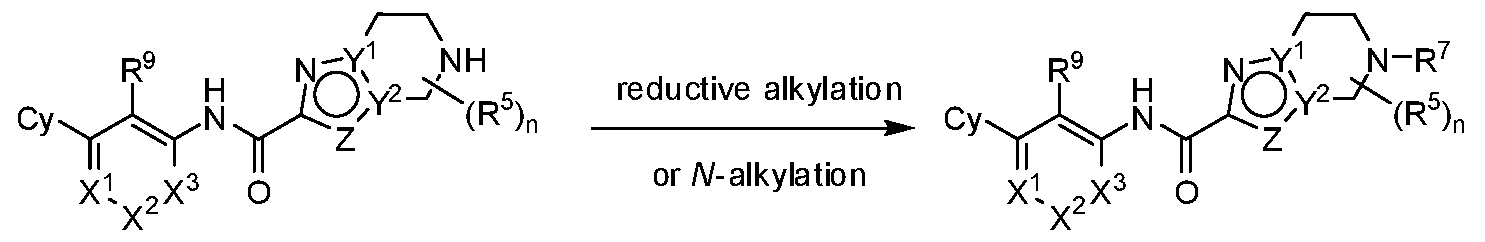WO2017222976A1 - Heterocyclic compounds as immunomodulators - Google Patents
Heterocyclic compounds as immunomodulators Download PDFInfo
- Publication number
- WO2017222976A1 WO2017222976A1 PCT/US2017/038120 US2017038120W WO2017222976A1 WO 2017222976 A1 WO2017222976 A1 WO 2017222976A1 US 2017038120 W US2017038120 W US 2017038120W WO 2017222976 A1 WO2017222976 A1 WO 2017222976A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- cycloalkyl
- membered heterocycloalkyl
- aryl
- membered heteroaryl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- YLVKIWGFEKBGDI-UHFFFAOYSA-N CC#Cc(c(-c(cc1)cc2c1OCCO2)ccc1)c1NC(c1nc(CNCC2)c2[n]1C)=O Chemical compound CC#Cc(c(-c(cc1)cc2c1OCCO2)ccc1)c1NC(c1nc(CNCC2)c2[n]1C)=O YLVKIWGFEKBGDI-UHFFFAOYSA-N 0.000 description 1
- ZKAHJQDDNLLCLY-UHFFFAOYSA-N CC(C)(C)OC(N(CC1)Cc2c1nc(C(Nc1cccc(Br)c1Cl)=O)[s]2)=O Chemical compound CC(C)(C)OC(N(CC1)Cc2c1nc(C(Nc1cccc(Br)c1Cl)=O)[s]2)=O ZKAHJQDDNLLCLY-UHFFFAOYSA-N 0.000 description 1
- ZEMWGYSXQDCQCN-UHFFFAOYSA-N C[n]1c(C(Nc(cccc2-c(cccc3OC)c3F)c2C#N)=O)nc2c1CCN(CC(O)=O)C2 Chemical compound C[n]1c(C(Nc(cccc2-c(cccc3OC)c3F)c2C#N)=O)nc2c1CCN(CC(O)=O)C2 ZEMWGYSXQDCQCN-UHFFFAOYSA-N 0.000 description 1
- FLTOYURMNUSNEB-UHFFFAOYSA-N Cc(c(-c(cc1)cc2c1OCCO2)ncc1)c1NC(c1nc(CCNC2)c2[s]1)=O Chemical compound Cc(c(-c(cc1)cc2c1OCCO2)ncc1)c1NC(c1nc(CCNC2)c2[s]1)=O FLTOYURMNUSNEB-UHFFFAOYSA-N 0.000 description 1
- NDPCCNFWJYWCCB-UHFFFAOYSA-N Cc(c(-c1ccccc1)ccc1)c1NC(c1nc(CCN(CC(O)=O)C2)c2[s]1)=O Chemical compound Cc(c(-c1ccccc1)ccc1)c1NC(c1nc(CCN(CC(O)=O)C2)c2[s]1)=O NDPCCNFWJYWCCB-UHFFFAOYSA-N 0.000 description 1
- ZUAKEFAXYDFLER-UHFFFAOYSA-N Nc(c(Br)ccc1)c1NC(c1nc(CCNC2)c2[s]1)=O Chemical compound Nc(c(Br)ccc1)c1NC(c1nc(CCNC2)c2[s]1)=O ZUAKEFAXYDFLER-UHFFFAOYSA-N 0.000 description 1
- MBMHUQLUMDNICN-UHFFFAOYSA-N O=C(c1nc(CCNC2)c2[s]1)Nc(cccc1-c(cc2)cc3c2OCCO3)c1Cl Chemical compound O=C(c1nc(CCNC2)c2[s]1)Nc(cccc1-c(cc2)cc3c2OCCO3)c1Cl MBMHUQLUMDNICN-UHFFFAOYSA-N 0.000 description 1
- IYNNZNGQWAYLRK-UHFFFAOYSA-N O=C(c1nc(CCNC2)c2[s]1)Nc(cccc1-c2ccccc2)c1Cl Chemical compound O=C(c1nc(CCNC2)c2[s]1)Nc(cccc1-c2ccccc2)c1Cl IYNNZNGQWAYLRK-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D515/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00
- C07D515/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00 in which the condensed system contains two hetero rings
- C07D515/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4985—Pyrazines or piperazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D513/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00
- C07D513/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00 in which the condensed system contains two hetero rings
- C07D513/04—Ortho-condensed systems
Definitions
- the present application is concerned with pharmaceutically active compounds.
- the disclosure provides compounds as well as their compositions and methods of use.
- the compounds modulate PD-1/PD-L1 protein/protein interaction and are useful in the treatment of various diseases including infectious diseases and cancer.
- the immune system plays an important role in controlling and eradicating diseases such as cancer.
- cancer cells often develop strategies to evade or to suppress the immune system in order to favor their growth.
- One such mechanism is altering the expression of co-stimulatory and co-inhibitory molecules expressed on immune cells (Postow et al, J. Clinical Oncology 2015, 1 -9).
- Blocking the signaling of an inhibitory immune checkpoint, such as PD-1 has proven to be a promising and effective treatment modality.
- PD-1 Programmed cell death-1
- CD279 is a cell surface receptor expressed on activated T cells, natural killer T cells, B cells, and macrophages (Greenwald et al, Annu. Rev. Immunol 2005, 23 :515-548; Okazaki and Honjo, Trends Immunol 2006,
- PD- 1 functions as an intrinsic negative feedback system to prevent the activation of T-cells, which in turn reduces autoimmunity and promotes self-tolerance.
- PD- 1 is also known to play a critical role in the suppression of antigen-specific T cell response in diseases like cancer and viral infection (Sharpe et al, Nat Immunol 2007 8, 239-245; Postow et al, J. Clinical Oncol 2015, 1 -9).
- the structure of PD-1 consists of an extracellular immunoglobulin variable-like domain followed by a transmembrane region and an intracellular domain (Parry et al, Mol Cell Biol 2005, 9543-9553).
- the intracellular domain contains two phosphorylation sites located in an immunoreceptor tyrosine-based inhibitory motif and an immunoreceptor tyrosine-based switch motif, which suggests that PD-1 negatively regulates T cell receptor- mediated signals.
- PD-1 has two ligands, PD-L1 and PD-L2 (Parry et al, Mol Cell Biol 2005, 9543-9553; Latchman et al, Nat Immunol 2001 , 2, 261-268), and they differ in their expression patterns.
- PD-L1 protein is upregulated on macrophages and dendritic cells in response to lipopolysaccharide and GM-CSF treatment, and on T cells and B cells upon T cell receptor and B cell receptor signaling. PD-L1 is also highly expressed on almost all tumor cells, and the expression is further increased after IFN- ⁇ treatment (Iwai et al, PNAS2002, 99(19): 12293-7; Blank et al, Cancer Res 2004, 64(3): 1140-5).
- tumor PD- Ll expression status has been shown to be prognostic in multiple tumor types (Wang et al, Eur J Surg Oncol 2015; Huang et al, Oncol Rep 2015; Sabatier et al, Oncotarget 2015, 6(7): 5449-5464).
- PD-L2 expression in contrast, is more restricted and is expressed mainly by dendritic cells (Nakae et al, J Immunol 2006, 177:566-73).
- Ligation of PD-1 with its ligands PD-L1 and PD-L2 on T cells delivers a signal that inhibits IL-2 and IFN- ⁇ production, as well as cell proliferation induced upon T cell receptor activation (Carter et al, Eur J Immunol 2002, 32(3):634-43; Freeman et al, J Exp Med 2000, 192(7): 1027-34).
- the mechanism involves recruitment of SHP-2 or SHP-1 phosphatases to inhibit T cell receptor signaling such as Syk and Lck phosphorylation (Sharpe et al, Nat Immunol 2007, 8, 239-245).
- Activation of the PD-1 signaling axis also attenuates PKC- ⁇ activation loop phosphorylation, which is necessary for the activation of NF- ⁇ and API pathways, and for cytokine production such as IL-2, IFN- ⁇ and TNF (Sharpe et al, Nat Immunol 2007, 8, 239-245; Carter et al, Eur J Immunol 2002, 32(3):634-43; Freeman et al, J Exp Med 2000,
- PD-1 -deficient mice have been shown to develop lupus-like glomerulonephritis and dilated cardiomyopathy (Nishimura et al, Immunity 1999, 11 : 141-151; Nishimura et al, Science 2001, 291 :319-322).
- LCMV model of chronic infection it has been shown that PD-1/PD-L1 interaction inhibits activation, expansion and acquisition of effector functions of virus-specific CD8 T cells (Barber et al, Nature 2006, 439, 682-7).
- the present disclosure further provides a pharmaceutical composition
- a pharmaceutical composition comprising a compound of the disclosure, or a pharmaceutically acceptable salt or a stereoisomer thereof, and at least one pharmaceutically acceptable carrier or excipient.
- the present disclosure further provides methods of modulating or inhibiting PD- 1/PD-Ll protein/protein interaction, which comprises administering to an individual a compound of the disclosure, or a pharmaceutically acceptable salt or a stereoisomer thereof.
- the present disclosure further provides methods of treating a disease or disorder in a patient comprising administering to the patient a therapeutically effective amount of a compound of the disclosure, or a pharmaceutically acceptable salt or a stereoisomer thereof.
- G 1 is NR 6 and G 2 is CPJR 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-10 cycloalkyl, C3- 10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ oaryl, Ce- ⁇ aryl-Ci-4 alkyl-, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OR 10 , Ci-4 haloalkyl, Ci-4 haloalkoxy, NH 2 , -NHR 10 , -NR 10 R 10 , NHOR 10 , C(0)R 10 , C(O)NR 10 R 10 , C(0)OR 10 ,
- each R 10 is independently selected from H, Ci-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, C1-4 alkoxy, C3-10 cycloalkyl, C3-10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ aryl, Ce- ⁇ aryl-Ci-4 alkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4- 10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the C1-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, Ci-4 alkoxy, C3-10 cycloalkyl, C3-io cycloalkyl-Ci-4
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered
- NR a C( NCN)NR a R a , NR a S(0)R a , NR a S(0) 2 R a , NR a S(0) 2 NR a R a , S(0)R a , S(0)NR a R a , S(0) 2 R a , and S(0) 2 NR a R a , wherein the Ci-e alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce-io aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 4 , R 5 , R 6 , R 7 and R 8 are each optional
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R B substituents;
- R 5 substituents attached to the same carbon atom taken together with the carbon atom to which they are attached, form a C3-6 cycloalkyl ring or 4-, 5-, 6- or 7- membered heterocycloalkyl ring, wherein the C3-6 cycloalkyl ring and 4-, 5-, 6- or 7- membered heterocycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R B substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NO2, OR 11 , SR 1 1 , NH 2 , NHR 1 1 , NR N R N , NHOR 1 1 , C(0)R N , C(0)NR N R N , C(0)OR N , OC(0)R N , OC(0)NR N R N , NR N C(0)R N , NRHC ⁇ OR!! ⁇ RHC ⁇
- each R 1 1 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- each R A is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R a are each optionally substituted with 1 , 2 or 3 R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- Ci-e alkyl, Ci-e haloalkyl, phenyl, C3-6 cycloalkyl, 5-6 membered heteroaryl, and 4-6 membered heterocycloalkyl of R n is optionally substituted with 1, 2 or 3 R q substituents;
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, C6-io aryl, 5-10 membered heteroaryl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NH 2 , NHOR e , OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , OC(0)R e , OC(0)NR e R e , NHR e , NR e R e , NR e C(0)R e , NR e C(0)NR e R e , NR e
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R e are each optionally substituted with 1, 2 or 3 independently selected R f substituents;
- each R g is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- R a substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, 7-, 8-, 9- or 10-membered heterocycloalkyl group optionally substituted with 1, 2 or 3 R h substituents independently selected from Ci-6 alkyl, Ci-6 haloalkyl, C3-10 cycloalkyl, 4-7 membered heterocycloalkyl, Ce- ⁇ aryl, 5-6 membered heteroaryl, Ce- ⁇ aryl- C1-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-6 membered heteroaryl)-Ci-4 alkyl-, (4-7 membered heterocycloalkyl)-Ci-4 alkyl-, C 1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, halo, CN, OR 1 , SR NHOR 1 , C(0)R ⁇ C ⁇ NR , C(0)OR ⁇ OC(0)R ⁇
- heterocycloalkyl)-Ci-4 alkyl- of R h are each optionally substituted by 1, 2, or 3 RJ substituents independently selected from C1-4 alkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 5- or 6-membered heteroaryl, 4-6 membered heterocycloalkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, halo, Ci-4 haloalkyl, Ci-4haloalkoxy, CN, NHOR k , OR k , SR k , C(0)R k , C(0)NR k R k , C(0)OR k , OC(0)R k , OC(0)NR k R k , NHR k , NR k R k , NR k C(0)R k , NR k C(0)NR k R k , NR k C(0)OR k , NR k C(0)OR k , NR k C(0)
- each R 1 or R k is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloal
- R e substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R 1 substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R k substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R° substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R r substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- each R° or R r is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C1-4 haloalkyl, C2-4 alkenyl, and C2-4 alkynyl, wherein the C1-4 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C2-4 alkenyl, and C2-4 alkynyl of R 1 , R k , R° or R r are each optionally substituted with 1, 2 or 3 R q substituents;
- each R 3 ⁇ 4 is independently selected from OH, CN, -COOH, NH2, halo, Ci-6 haloalkyl, Ci-6 alkyl, Ci-6 alkoxy, Ci-6 haloalkoxy, Ci-6 alkylthio, phenyl, 5-6 membered heteroaryl, 4-6 membered heterocycloalkyl, C3-6 cycloalkyl, NHR 12 and NR 12 R 12 , wherein the Ci-6 alkyl, phenyl, C3-6 cycloalkyl, 4-6 membered heterocycloalkyl, and 5-6 membered heteroaryl of R q are each optionally substituted with halo, OH, CN, -COOH, NH2, C1-4 alkoxy, C1-4 haloalkyl, Ci-4 haloalkoxy, phenyl, C3-10 cycloalkyl, 5-6 membered heteroaryl and 4-6 membered heterocycloalkyl and each R 12 is independently Ci-6 alkyl
- n is an integer of 1, 2, 3 or 4.
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-10 cycloalkyl, C3- 10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ aryl, Ce- ⁇ aryl-Ci-4 alkyl-, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OR 10 , Ci-4 haloalkyl, Ci-4 haloalkoxy, NH 2 , -NHR 10 , -NR 10 R 10 , NHOR 10 , C(0)R 10 , C(O)NR 10 R 10 , C(0)OR 10 ,
- each R 10 is independently selected from H, Ci-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, Cw alkoxy, C3-10 cycloalkyl, C3-10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ aryl, Ce- ⁇ aryl-Ci-4 alkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4- 10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the C1-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, Ci-4 alkoxy, C3-10 cycloalkyl, C3-10 cycloalkyl-Ci-4 alkyl-, wherein the C1-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, Ci-4 alkoxy, C3-10 cycloalkyl, C3-10 cycloalkyl
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered
- NR a C( NCN)NR a R a , NR a S(0)R a , NR a S(0) 2 R a , NR a S(0) 2 NR a R a , S(0)R a , S(0)NR a R a , S(0) 2 R a , and S(0) 2 NR a R a , wherein the Ci-e alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce-io aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 4 , R 5 , R 6 , R 7 and R 8 are each optional
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 member
- each R 11 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-C 1-4 alkyl-, C3-io cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 11 are each optionally substituted with 1 , 2 or 3 R b substituents;
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R a are each optionally substituted with 1 , 2 or 3 R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, C6-io aryl, 5-10 membered heteroaryl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NH 2 , NHOR e
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R e are each optionally substituted with 1, 2 or 3 independently selected R f substituents;
- each R g is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- R a substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, 7-, 8-, 9- or 10-membered heterocycloalkyl group optionally substituted with 1, 2 or 3 R h substituents independently selected from Ci-6 alkyl, Ci-6 haloalkyl, C3-10 cycloalkyl, 4-7 membered heterocycloalkyl, Ce- ⁇ aryl, 5-6 membered heteroaryl, Ce- ⁇ aryl- Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-6 membered heteroaryl)-Ci-4 alkyl-, (4-7 membered heterocycloalkyl)-Ci-4 alkyl-, C 1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, halo, CN, OR 1 , SR, NHOR, C(0)R, C C N , C(0)OR, OC(0)R,
- heterocycloalkyl)-Ci-4 alkyl- of R h are each optionally substituted by 1, 2, or 3 Ri substituents independently selected from C1-4 alkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 5- or 6-membered heteroaryl, 4-6 membered heterocycloalkyl, C2-4 alkenyl, C2-4 alkynyl, halo, C1-4 haloalkyl, Ci-4haloalkoxy, CN, NHOR k , OR k , SR k , C(0)R k , C(0)NR k R k , C(0)OR k , OC(0)R k ,
- each R 1 or R k is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalky
- R e substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R 1 substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R k substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R° substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R r substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- each R° or R r is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C1-4 haloalkyl, C2-4 alkenyl, and C2-4 alkynyl, wherein the C1-4 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C2-4 alkenyl, and C2-4 alkynyl of R° or R r are each optionally substituted with 1, 2 or 3 R q substituents;
- each R q is independently selected from OH, CN, -COOH, NH2, halo, C 1-6 haloalkyl, Ci-6 alkyl, Ci-6 alkoxy, Ci-6 haloalkoxy, Ci-6 alkylthio, phenyl, 5-6 membered heteroaryl, 4-6 membered heterocycloalkyl, C3-6 cycloalkyl, NHR 12 and NR 12 R 12 , wherein the Ci-6 alkyl, phenyl, C3-6 cycloalkyl, 4-6 membered heterocycloalkyl, and 5-6 membered heteroaryl of R q are each optionally substituted with halo, OH, CN, -COOH, NH2, C1-4 alkoxy, C1-4 haloalkyl, Ci-4 haloalkoxy, phenyl, C3-10 cycloalkyl, 5-6 membered heteroaryl and 4-6 membered heterocycloalkyl and each R 12 is independently Ci-6 alkyl
- n is an integer of 1, 2, 3 or 4.
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-10 cycloalkyl, C3- 10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ aryl, Ce- ⁇ aryl-Ci-4 alkyl-, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OR 10 , Ci-4 haloalkyl, Ci-4 haloalkoxy, NH 2 , -NHR 10 , -NR 10 R 10 , NHOR 10 , C(0)R 10 , C(O)NR 10 R 10 , C(0)OR 10 ,
- each R 10 is independently selected from H, C1-4 alkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, C1-4 alkoxy, C3-10 cycloalkyl, C3-10 cycloalkyl-Ci-4 alkyl-, Ce- ⁇ aryl, Ce- ⁇ aryl-Ci-4 alkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4- 10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the C1-4 alkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, Ci-4 alkoxy, C3-10 cycloalkyl, C3-io cycl
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 5 substituents attached to the same carbon atom taken together with the carbon atom to which they are attached, form a C3-6 cycloalkyl ring or 4-, 5-, 6- or 7- membered heterocycloalkyl ring, wherein the C3-6 cycloalkyl ring and 4-, 5-, 6- or 7- membered heterocycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NO2, OR 11 , SR 11 , NH2, NHR 11 , NR n R n , NHOR 11 , C(0)R n , C(0)NR n R n , C(0)OR n , OC(0)R n , OC(0)NR n R n , NR n C(0)R n , NR H C
- each R 11 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-C 1-4 alkyl-, C3-io cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 11 are each optionally substituted with 1 , 2 or 3 R b substituents;
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R a are each optionally substituted with 1 , 2 or 3 R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- Ci-e alkyl, Ci-e haloalkyl, phenyl, C3-6 cycloalkyl, 5-6 membered heteroaryl, and 4-6 membered heterocycloalkyl of R n is optionally substituted with 1, 2 or 3 R q substituents;
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, C6-io aryl, 5-10 membered heteroaryl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NH2, NHOR e , OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , OC(0)R e , OC(0)NR e R e , NHR e , NR e R e , NR e C(0)R e , NR e C(0)NR e R e , NR e C(0)
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R e are each optionally substituted with 1, 2 or 3 independently selected R f substituents;
- each R is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- cycloalkyl-C 1-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R p are each optionally substituted with 1, 2 or 3 R q substituents; or any two R a substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, 7-, 8-, 9- or 10-membered heterocycloalkyl group optionally substituted with 1, 2 or 3 R h substituents independently selected from Ci-6 alkyl, Ci-6 haloalkyl, C3-10 cycloalkyl, 4-7 membered heterocycloalkyl, Ce- ⁇ aryl, 5-6 membered heteroaryl, Ce- ⁇ aryl- Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-6 membered heteroaryl)-Ci-4 alkyl-, (4-7 membered heterocycloal
- heterocycloalkyl)-Ci-4 alkyl- of R h are each optionally substituted by 1, 2, or 3 RJ substituents independently selected from C1-4 alkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 5- or 6-membered heteroaryl, 4-6 membered heterocycloalkyl, C2-4 alkenyl, C2-4 alkynyl, halo, C1-4 haloalkyl, Ci-4haloalkoxy, CN, NHOR k , 0R k , SR k , C(0)R k , C(0)NR k R k , C(0)OR k , OC(0)R k ,
- each R 1 or R k is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl
- R e substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R 1 substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents, or 1, 2, or 3 independently selected R q substituents; or any two R k substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents, or 1, 2, or 3 independently selected R q substituents; or any two R° substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents, or 1, 2, or 3 independently selected R q substituents; or any two R r substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycl
- each R q is independently selected from OH, CN, -COOH, NH2, halo, C 1-6 haloalkyl, Ci-6 alkyl, Ci-6 alkoxy, Ci-6 haloalkoxy, Ci-6 alkylthio, phenyl, 5-6 membered heteroaryl, 4-6 membered heterocycloalkyl, C3-6 cycloalkyl, NHR 12 and NR 12 R 12 , wherein the Ci-6 alkyl, phenyl, C3-6 cycloalkyl, 4-6 membered heterocycloalkyl, and 5-6 membered heteroaryl of R q are each optionally substituted with halo, OH, CN, -COOH, NH2, C1-4 alkoxy, C1-4 haloalkyl, Ci-4 haloalkoxy, phenyl, C3-10 cycloalkyl, 5-6 membered heteroaryl and 4-6 membered heterocycloalkyl and each R 12 is independently Ci-6 alkyl
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- each R 10 is independently selected from H and C1-4 alkyl optionally substituted with 1 or 2 groups independently selected from halo, OH, CN and C1-4 alkoxy; and wherein the C1-4 alkyl, C3-6 cycloalkyl, C 2 -4 alkenyl, C 2 -4 alkynyl and C1-4 alkoxy of R 1 , R 2 and R 3 are each optionally substituted with 1 or 2 substituents independently selected from halo, OH, CN and C1-4 alkoxy;
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C 2 -6 alkenyl, C 2 -6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 5 substituents attached to the same carbon atom taken together with the carbon atom to which they are attached, form a C3-6 cycloalkyl ring or 4-, 5-, 6- or 7- membered heterocycloalkyl ring, wherein the C3-6 cycloalkyl ring and 4-, 5-, 6- or 7- membered heterocycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, N0 2 , OR 11 , SR.
- each R 11 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-C 1-4 alkyl-, C3-io cycloalkyl-Ci-4 alkyl-, (5-10 member
- each R a is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R a are each optionally substituted with 1 , 2 or 3 R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- Ci-e alkyl, Ci-e haloalkyl, phenyl, C3-6 cycloalkyl, 5-6 membered heteroaryl, and 4-6 membered heterocycloalkyl of R n is optionally substituted with 1, 2 or 3 R q substituents;
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, C6-io aryl, 5-10 membered heteroaryl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NH 2 , NHOR e , OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , OC(0)R e , OC(0)NR e R e , NHR e , NR e R e , NR e C(0)R e , NR e C(0)NR e R e , NR e
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C 2 -6 alkenyl, C 2 -6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C 2 -6 alkenyl, C 2 -6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R e are each optionally substituted with 1, 2 or 3 independently selected R f substituents;
- each R g is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R p is optionally substituted with 1, 2 or 3 R q substituents;
- R a substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, 7-, 8-, 9- or 10-membered heterocycloalkyl group optionally substituted with 1, 2 or 3 R h substituents independently selected from Ci-6 alkyl, Ci-6 haloalkyl, C3-10 cycloalkyl, 4-7 membered heterocycloalkyl, Ce- ⁇ aryl, 5-6 membered heteroaryl, C3-10 cycloalkyl-C 1-4 alkyl-, (5-6 membered heteroaryl)-C 1-4 alkyl-, (4-7 membered
- R c substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R e substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R 1 substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R k substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R° substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- R r substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents;
- each R 1 , R k , R° or R r is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C1-4 haloalkyl, C 2 -4 alkenyl, and C 2 -4 alkynyl, wherein the C1-4 alkyl, Ci-6 haloalkyl, C3-6 cycloalkyl, Ce- ⁇ aryl, 4-6 membered heterocycloalkyl, 5 or 6-membered heteroaryl, C2-4 alkenyl, and C2-4 alkynyl of R 1 , R k , R° or R r are each optionally substituted with 1, 2 or 3 R q substituents;
- each R 3 ⁇ 4 is independently selected from OH, CN, -COOH, NH2, halo, Ci-6 haloalkyl, Ci-6 alkyl, Ci-6 alkoxy, Ci-6 haloalkoxy, Ci-6 alkylthio, phenyl, 5-6 membered heteroaryl, 4-6 membered heterocycloalkyl, C3-6 cycloalkyl, NHR 12 and NR 12 R 12 , wherein the Ci-6 alkyl, phenyl, C3-6 cycloalkyl, 4-6 membered heterocycloalkyl, and 5-6 membered heteroaryl of R q are each optionally substituted with halo, OH, CN, -COOH, NH2, C1-4 alkoxy, C1-4 haloalkyl, Ci-4 haloalkoxy, phenyl, C3-10 cycloalkyl and 4-6 membered heterocycloalkyl and each R 12 is independently Ci-6 alkyl;
- n is an integer of 1, 2, 3 or 4.
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- each R 10 is independently selected from H and C1-4 alkyl optionally substituted with 1 or 2 groups independently selected from halo, OH, CN and C1-4 alkoxy; and wherein the C1-4 alkyl, C3-6 cycloalkyl, C2-4 alkenyl, C2-4 alkynyl and C1-4 alkoxy of R 1 , R 2 and R 3 are each optionally substituted with 1 or 2 substituents independently selected from halo, OH, CN and C1-4 alkoxy;
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-C 1-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered
- NR a C( NCN)NR a R a , NR a S(0)R a , NR a S(0) 2 R a , NR a S(0) 2 NR a R a , S(0)R a , S(0)NR a R a , S(0)2R a , and S(0)2NR a R a , wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce-io aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 4 , R 5 , R 6 , R 7 and R 8 are each optionally substitute
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 5 substituents attached to the same carbon atom taken together with the carbon atom to which they are attached, form a C3-6 cycloalkyl ring or 4-, 5-, 6- or 7- membered heterocycloalkyl ring, wherein the C3-6 cycloalkyl ring and 4-, 5-, 6- or 7- membered heterocycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-14 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-14 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NO2, OR 11 , SR 11 , NH2, NHR 11 , NR n R n , NHOR 11 , C(0)R n , C(0)NR n R n , C(0)OR n , OC(0)R n , OC(0)NR n R n , NR n C(0)R n , NRHC ⁇
- each R 11 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-C 1-4 alkyl-, C3-io cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R 11 are each optionally substituted with 1 , 2 or 3 R b substituents;
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-io aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R a are each optionally substituted with 1 , 2 or 3 R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- Ci-e alkyl, Ci-e haloalkyl, phenyl, C3-6 cycloalkyl, 5-6 membered heteroaryl, and 4-6 membered heterocycloalkyl of R n is optionally substituted with 1, 2 or 3 R q substituents;
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, C6-io aryl, 5-10 membered heteroaryl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl- , C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, (4-10 membered heterocycloalkyl)-Ci- 4 alkyl-, CN, NH2, NHOR e , OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , OC(0)R e , OC(0)NR e R e , NHR e , NR e R e , NR e C(0)R e , NR e C(0)NR e R e , NR e C(0)
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl- , (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R e are each optionally substituted with 1, 2 or 3 independently selected R f substituents;
- each R is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl- and (4-10 membered heterocycloalkyl)-Ci-4 alkyl- of R p is optionally substituted with 1, 2 or 3 R q substituents;
- R a substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, 7-, 8-, 9- or 10-membered heterocycloalkyl group optionally substituted with 1, 2 or 3 R h substituents independently selected from Ci-6 alkyl, Ci-6 haloalkyl, C3-10 cycloalkyl, 4-7 membered heterocycloalkyl, Ce- ⁇ aryl, 5-6 membered heteroaryl, C3-10 cycloalkyl-C 1-4 alkyl-, (5-6 membered heteroaryl)-C 1-4 alkyl-, (4-7 membered
- R c substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R e substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents;
- R 1 substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1 , 2, or 3 independently selected R h substituents, or 1 , 2, or 3 independently selected R q substituents; or any two R k substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents, or 1, 2, or 3 independently selected R q substituents; or any two R° substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group optionally substituted with 1, 2, or 3 independently selected R h substituents, or 1, 2, or 3 independently selected R q substituents; or any two R r substituents together with the nitrogen atom to which they are attached form a 4-, 5-, 6-, or 7-member
- each R q is independently selected from OH, CN, -COOH, NH2, halo, C 1-6 haloalkyl, Ci-6 alkyl, Ci-6 alkoxy, Ci-6 haloalkoxy, Ci-6 alkylthio, phenyl, 5-6 membered heteroaryl, 4-6 membered heterocycloalkyl, C3-6 cycloalkyl, NHR 12 and NR 12 R 12 , wherein the Ci-6 alkyl, phenyl, C3-6 cycloalkyl, 4-6 membered heterocycloalkyl, and 5-6 membered heteroaryl of R q are each optionally substituted with halo, OH, CN, -COOH, NH2, C1-4 alkoxy, C1-4 haloalkyl, Ci-4 haloalkoxy, phenyl, C3-10 cycloalkyl and 4-6 membered heterocycloalkyl and each R 12 is independently Ci-6 alkyl;
- n is an integer of 1, 2, 3 or 4.
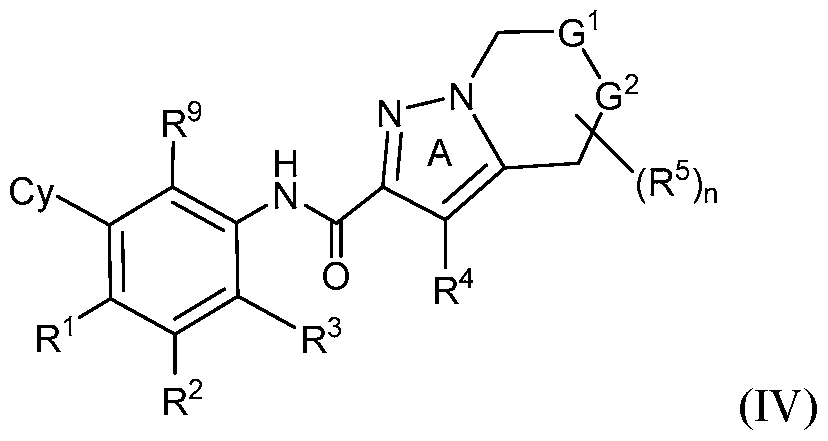
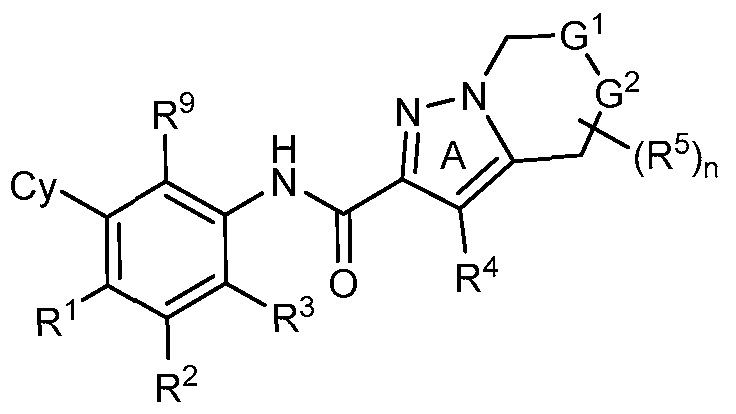
- rovided herein is a compound having Formula (IV):
- R 1 , R 2 and R 3 are each independently selected from H, Ci-4 alkyl, C3-6 cycloalkyl, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OH, C1-4 alkoxy, Ci-4 haloalkyl, Ci- 4 haloalkoxy, NH 2 , -NH-Ci-4 alkyl, -N(Ci- 4 alkyl) 2 , C(0)R 10 , C(O)NR 10 R 10 , C(0)OR 10 , OC(0)R 10 , OC(O)NR 10 R 10 , NR 10 C(O)R 10 , NR 10 C(O)OR 10 , NR 10 S(O)R 10 , NR 10 S(O) 2 R 10 , NR 10 S(O) 2 NR 10 R 10 , S(0)R 10 , S(O)NR 10 R 10 , S(0) 2 R 10 R 10 , and S(O) 2 NR 10 R 10 , wherein
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-6 cycloalkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, halo, CN, OH, C1-4 alkoxy, Ci-4 haloalkyl, Ci- 4 haloalkoxy, NH 2 , -NH-Ci-4 alkyl, and -N(Ci-4 alkyl) 2 .
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, halo, CN, OH, C1-4 alkoxy, Ci-4 haloalkyl, or C1-4 haloalkoxy.
- R 1 is H
- R 2 is H or halo
- R 3 is H
- R 1 , R 2 , and R 3 are H.
- Cy is phenyl, 5- or 6-membered heteroaryl, C3-6 cycloalkyl or
- 5- or 6-membered heterocycloalkyl each of which is optionally substituted with 1 to 5 independently selected R 8 substituents; or two adjacent R 8 substituents on the Cy ring, taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or
- 6- membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents.
- Cy is phenyl optionally substituted with 1 to 5 R 8 substituents.
- Cy is 5- or 6-membered heteroaryl optionally substituted with 1 to 5 independently selected R 8 substituents.
- Cy is C3-6 cycloalkyl optionally substituted with 1 to 5 independently selected R 8 substituents.
- Cy is 5- or 6-membered heterocycloalkyl optionally substituted with 1 to 5 independently selected R 8 substituents.
- Cy is phenyl, 2-thiophenyl, 3-thiophenyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 3,6-dihydro-2H-pyran-4-yl, cyclohexyl, cyclohexenyl, 2,3-dihydro-l,4- benzodioxin-6-yl, l,3-benzodioxin-5-yl, 2-methylindazol-6-yl or 1 -methylindazol-4-yl, each of which is optionally substituted with 1 to 5 R 8 substituents.
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-e haloalkoxy, CN, NO2, OR 11 , SR 11 , NH2, NHR 11 , NR n R n , NHOR 11 , C(0)R n ,
- Ci-e alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R 9 are each optionally substituted with 1, 2 or 3 R b substituents.
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, CN, NO2, or NH2, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R 9 are each optionally substituted with 1, 2 or 3 R b substituents.
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, CN, NO2, and NH 2 .
- R 9 is halo, Ci-6 alkyl, or CN.
- R 9 is CH3, CN or halo. In some embodiments, R 9 is CH3. In other embodiments, R 9 is CN. Yet in certain embodiments, R 9 is halo such as F, CI or Br.
- Z is S, CR 4 , NR 4 , or N and R 4 is independently H or Ci-6 alkyl.
- Z is S, CH, NCH3 or N.
- Z is S.
- Z is CH.
- Z is N(Ci-6 alkyl) such as NCH3.
- Z is N.
- Y 1 is C or N and Y 2 is C.
- Y 1 is C and Y 2 is N.
- Y 1 is C, Y 2 is N and Z is N;
- Y 1 is C, Y 2 is N and Z is CR 4 ;
- Y 1 is C, Y 2 is C and Z is S; or
- Y 1 is C, Y 2 is C and Z is O.
- Y 1 is N, Y 2 is C and Z is N. In certain embodiments, Y 1 is N, Y 2 is C and Z is CR 4 . In certain embodiments, Y 1 is C, Y 2 is N and Z is N. In some embodiments, Y 1 is C, Y 2 is N and Z is CR 4 . In some embodiments, Y 1 is C, Y 2 is C and Z is S. Yet in some embodiments, Y 1 is C, Y 2 is C and Z is O.
- R 5 is H.
- G 1 is NR 6 and G 2 is CR 7 R 7 . In some embodiments, G 1 is CR 6 R 6 and G 2 is NR 7 . In some embodiments, R 6 is H or Ci-6 alkyl optionally substituted with 1, 2 or 3 R b substituents. In some embodiments, R 7 is H or Ci-6 alkyl optionally substituted with 1, 2 or 3 R b substituents.
- R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci-ehaloalkoxy, CN, OH, NH 2 , N0 2 , NHOR c , OR c , SR C , C(0)R c , C(0)NR c R c , C(0)OR c , OC(0)R c , OC(0)NR c R c , NHR C , NR C R C , NR c C(0)R c , NR c C(0)OR c , , c C(0)OR c ,
- Ci-6 alkyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R b are each further optionally substituted with 1-3 independently selected R d substituents.
- R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci-ehaloalkoxy, CN, OH, NH2, NO2, OR c , SR C , C(0)R c , C(0)NR c R c , C(0)OR c , NHR C , NR C R C , and NR c C(0)R c ; wherein the Ci-e alkyl, Ci-e haloalkyl, and Ci-e haloalkoxy of R b are each further optionally substituted with 1-3 independently selected R d substituents.
- R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci-e haloalkoxy, CN, OH, NH 2 , OR c , C(0)R c , C(0)NR c R c , and C(0)OR c .
- R b substituent is independently selected from Ci-6 alkyl, CN, OH, and C(0)OR c .
- R b is Ci-6 alkyl such as methyl.
- R b is CN.
- R b is OH.
- R b is C(0)OR c such as C(0)OH or C(0)0(Ci-e alkyl).
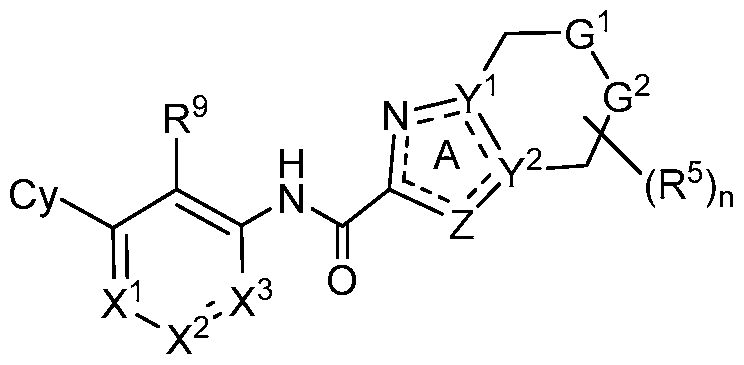
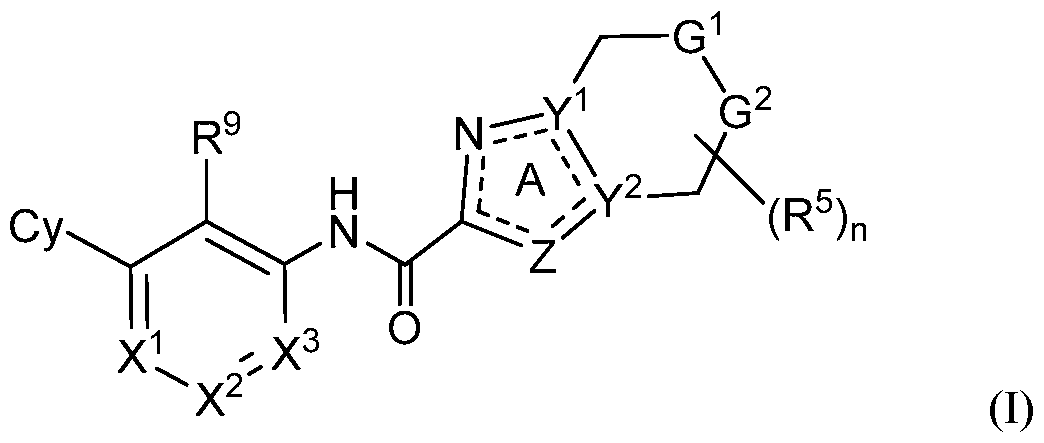
- provided herein is a compound of Formula I, or a
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is O, S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously N;
- Cy is Ce-10 aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-6 cycloalkyl, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OH, Ci-4 alkoxy, Ci-4 haloalkyl, Ci-4 haloalkoxy, NH2, -NH- Ci-4 alkyl, -N(Ci- 4 alkyl) 2 , C(0)R 10 , C(O)NR 10 R 10 , C(0)OR 10 , OC(0)R 10 , OC(O)NR 10 R 10 , NR 10 C(O)R 10 , NR 10 C(O)OR 10 , NR 10 S(O)R 10 , NR 10 S(O) 2 R 10 , NR 10 S(O) 2 NR 10 R 10 , S(0)R 10 , S(O)NR 10 R 10 , S(0) 2 R 10 R 10 , and S(O) 2 NR 10 R 10 , wherein each R 10 is independently selected
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C 2 -6 alkenyl, C 2 -e alkynyl, Ci-e haloalkyl, Ci-e haloalkoxy, CN, N0 2 , OR a , SR a , C(0)R a , C(0)NR a R a , C(0)OR a , OC(0)R a , OC(0)NR a R a , NHR a , NR a R a , NR a C(0)R a , NR a C(0)OR a , NR a S(0)R a , NR a S(0) 2 R a , NR a S(0) 2 NR a R a , S(0)R a , S(0)NR a R a , S(0) 2 R a , and S(0) 2 NR a R a , wherein the Ci
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, CN, N0 2 , OR 11 , SR 11 , NH 2 , NHR 11 , NR n R n , NHOR 11 , C(0)R n , C(0)NR n R n , C(0)OR n , OC(0)R n , OC(0)NR n R n , NR n C(0)R n , NR n C(0)OR n , NR ⁇ C ⁇ NR 11 !*.
- Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R 9 are each optionally substituted with 1, 2 or 3 R b substituents;
- each R 11 is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- each R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci- ehaloalkoxy, CN, OH, NH 2 , N0 2 , NHOR c , OR c , SR C , C(0)R c , C(0)NR c R c , C(0)OR c , OC(0)R c , OC(0)NR c R c , NHR C , NR C R C , NR c C(0)R c , NR c C(0)OR c , NR c C(0)NR c R c ,
- Ci-6 alkyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R b are each further optionally substituted with 1-3 independently selected R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl, wherein the Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl of R c are each optionally substituted with 1, 2 or 3 R f substituents independently selected from Ci-6 alkyl, Ci-e haloalkyl, C2-6 alkenyl, C2-6 alkynyl, halo, CN, OR g , SR g , C(0)R g , C(0)NR g R g , C(0)OR g , OC(0)R g , OC(0)NR g R g , NHR g , NR g R g , NR g C(0)R g , NR g C(0)NR g R g , NR g C(0)OR g , S(0)R g
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, CN, NH 2 , OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , OC(0)R e , OC(0)NR e R e , NHR e , NR e R e , NR e C(0)R e , NR e C(0)NR e R e , NR e C(0)OR e , S(0)R e , S(0)NR e R e , S(0) 2 R e , NR e S(0) 2 R e , NR e S(0) 2 NR e R e , and S(0) 2 NR e R e ;
- each R e is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-;
- each R g is independently selected from H, Ci-6 alkyl, Ci-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, Ce- ⁇ aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered
- heterocycloalkyl Ce- ⁇ aryl-Ci-4 alkyl-, C3-10 cycloalkyl-Ci-4 alkyl-, (5-10 membered heteroaryl)-Ci-4 alkyl-, and (4-10 membered heterocycloalkyl)-Ci-4 alkyl-;
- z is a single bond or a double bond to maintain ring A being aromatic; and the subscript n is an integer of 1 , 2, 3 or 4.
- provided herein is a compound of Formula I, or a
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is Ce- ⁇ aryl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C3-6 cycloalkyl, C 2 -4 alkenyl, C 2 -4 alkynyl, halo, CN, OH, Ci-4 alkoxy, Ci-4 haloalkyl, Ci-4 haloalkoxy, NH 2 , -NH- Ci-4 alkyl, and -N(Ci- 4 alkyl) 2 ;
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-e haloalkyl, Ci-e haloalkoxy, CN, NO2, OR a , SR a , C(0)R a ,
- Ci-e alkyl, C2-6 alkenyl, and C2-6 alkynyl of R 4 , R 5 , R 6 , R 7 and R 8 are each optionally substituted with 1, 2, 3, 4 or 5 R b substituents;
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused phenyl ring, a fused 5-, 6- or 7-membered heterocycloalkyl ring, a fused 5- or 6-membered heteroaryl ring or a fused C3-6 cycloalkyl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused phenyl ring, fused 5-, 6- or 7-membered heterocycloalkyl ring, fused 5- or 6-membered heteroaryl ring and fused C3-6 cycloalkyl ring are each optionally substituted with 1, 2 or 3 independently selected R b substituents;
- R 9 is halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, Ci-6 haloalkoxy, CN, NO2, or NH2, wherein the Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R 9 are each optionally substituted with 1, 2 or 3 R b substituents;
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- each R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci- 6 haloalkoxy, CN, OH, NH2, NO2, OR c , SR C , C(0)R c , C(0)NR c R c , C(0)OR c , NHR C , NR C R C , and NR c C(0)R c ; wherein the Ci-6 alkyl, Ci-6 haloalkyl, and Ci-6 haloalkoxy of R b are each further optionally substituted with 1-3 independently selected R d substituents;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- each R d is independently selected from Ci-6 alkyl, Ci-6 haloalkyl, halo, CN, NH2, OR e , SR e , C(0)R e , C(0)NR e R e , C(0)OR e , NHR e , NR e R e , and NR e C(0)R e ;
- each R e is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- n is an integer of 1 or 2.
- provided herein is a compound of Formula I, or a
- G 1 is NR 6 and G 2 is CR 7 R 7 ;
- G 1 is CR 6 R 6 and G 2 is NR 7 ;
- X 1 is N or CR 1 ;
- X 2 is N or CR 2 ;
- X 3 is N or CR 3 ;
- Z is S, N, NR 4 or CR 4 ;
- Y 1 and Y 2 are each independently N or C, provided Y 1 and Y 2 are not simultaneously
- Cy is phenyl, C3-10 cycloalkyl, 5- to 14-membered heteroaryl, or 4- to 10-membered heterocycloalkyl, each of which is optionally substituted with 1 to 5 independently selected R 8 substituents;
- R 1 , R 2 and R 3 are each independently selected from H, C1-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, halo, CN, OH, C1-4 alkoxy, Ci-4 haloalkyl, or Ci-4 haloalkoxy;
- R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from H, halo, Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-e haloalkyl, Ci-e haloalkoxy, CN, NO2, OR a , and C(0)OR a , wherein the Ci-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl of R 4 , R 5 , R 6 , R 7 and R 8 are each optionally substituted with 1 or 2 R b substituents;
- R 8 substituents on the Cy ring taken together with the atoms to which they are attached, form a fused 5-, 6- or 7-membered heterocycloalkyl ring, or a fused 5- or 6-membered heteroaryl ring, wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring each have 1-4 heteroatoms as ring members selected from N, O and S and wherein the fused 5-, 6- or 7-membered heterocycloalkyl ring and fused 5- or 6-membered heteroaryl ring are each optionally substituted with 1 or 2 independently selected R b substituents;
- R 9 is halo, Ci-e alkyl, or CN
- each R a is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- each R b substituent is independently selected from halo, Ci-6 alkyl, Ci-6 haloalkyl, Ci- e haloalkoxy, CN, OH, NH2, OR c , C(0)R c , C(0)NR c R c , and C(0)OR c ;
- each R c is independently selected from H, Ci-6 alkyl, C 1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
- n is an integer of 1 or 2.
- compounds of Formula (I) or any subformulas as disclosed herein when Cy is phenyl, R 8 is not 4-aminopiperidin-l-yl, optionally substituted with 1-5 independently selected R b substituents. In some embodiments, compounds of Formula (I) or any subformulas as disclosed herein, when Cy is phenyl, R 8 is not -NHC(0)R a , wherein R a is 5- or 6-membered heteroaryl, or 2-pyridon-3-yl, each of which is optionally substituted with 1 -5 independently selected R d substituents.
- R 8 is not (10-membered bicyclic heteroaryl)-NH-, optionally substituted with 1-5 independently selected R d substituents.
- embodiments of the compounds of Formula (I) can be combined in any suitable combination.
- Ci-6 alkyl is specifically intended to individually disclose (without limitation) methyl, ethyl, C3 alkyl, C4 alkyl, C5 alkyl and Ce alkyl.
- n-membered typically describes the number of ring-forming atoms in a moiety where the number of ring-forming atoms is n.
- piperidinyl is an example of a 6-membered heterocycloalkyl ring
- pyrazolyl is an example of a 5-membered heteroaryl ring
- pyridyl is an example of a 6-membered heteroaryl ring
- 1,2,3,4-tetrahydro-naphthalene is an example of a 10-membered cycloalkyl group.
- each linking substituent include both the forward and backward forms of the linking substituent.
- -NR(CR'R") n - includes both -NR(CR'R") n - and -(CR'R")nNR- and is intended to disclose each of the forms individually.
- the Markush variables listed for that group are understood to be linking groups. For example, if the structure requires a linking group and the Markush group definition for that variable lists “alkyl” or "aryl” then it is understood that the "alkyl” or “aryl” represents a linking alkylene group or arylene group, respectively.
- substituted means that an atom or group of atoms formally replaces hydrogen as a "substituent" attached to another group.
- substituted refers to any level of substitution, e.g. , mono-, di-, tri-, tetra- or penta-substitution, where such substitution is permitted.
- the substituents are independently selected, and substitution may be at any chemically accessible position. It is to be understood that substitution at a given atom is limited by valency. It is to be understood that substitution at a given atom results in a chemically stable molecule.
- optionally substituted means unsubstituted or substituted.
- substituted means that a hydrogen atom is removed and replaced by a substituent.
- a single divalent substituent e.g., oxo, can replace two hydrogen atoms.
- Cn-m indicates a range which includes the endpoints, wherein n and m are integers and indicate the number of carbons. Examples include Ci-4, Ci-6 and the like.
- alkyl employed alone or in combination with other terms, refers to a saturated hydrocarbon group that may be straight-chained or branched.
- Cn-m alkyl refers to an alkyl group having n to m carbon atoms.
- An alkyl group formally corresponds to an alkane with one C-H bond replaced by the point of attachment of the alkyl group to the remainder of the compound.
- the alkyl group contains from 1 to 6 carbon atoms, from 1 to 4 carbon atoms, from 1 to 3 carbon atoms, or 1 to 2 carbon atoms.
- alkyl moieties include, but are not limited to, chemical groups such as methyl, ethyl, ft-propyl, isopropyl, w-butyl, fert-butyl, isobutyl, sec-butyl; higher homologs such as 2- methyl-1 -butyl, w-pentyl, 3-pentyl, w-hexyl, 1 ,2,2-trimethylpropyl and the like.
- alkenyl employed alone or in combination with other terms, refers to a straight-chain or branched hydrocarbon group corresponding to an alkyl group having one or more double carbon-carbon bonds.
- An alkenyl group formally corresponds to an alkene with one C-H bond replaced by the point of attachment of the alkenyl group to the remainder of the compound.
- Cn-m alkenyl refers to an alkenyl group having n to m carbons. In some embodiments, the alkenyl moiety contains 2 to 6, 2 to 4, or 2 to 3 carbon atoms.
- Example alkenyl groups include, but are not limited to, ethenyl, w-propenyl, isopropenyl, n- butenyl, seobutenyl and the like.
- alkynyl employed alone or in combination with other terms, refers to a straight-chain or branched hydrocarbon group corresponding to an alkyl group having one or more triple carbon-carbon bonds.
- An alkynyl group formally corresponds to an alkyne with one C-H bond replaced by the point of attachment of the alkyl group to the remainder of the compound.
- Cn-m alkynyl refers to an alkynyl group having n to m carbons.
- Example alkynyl groups include, but are not limited to, ethynyl, propyn-l-yl, propyn-2-yl and the like.
- the alkynyl moiety contains 2 to 6, 2 to 4, or 2 to 3 carbon atoms.
- alkylene employed alone or in combination with other terms, refers to a divalent alkyl linking group.
- An alkylene group formally corresponds to an alkane with two C-H bond replaced by points of attachment of the alkylene group to the remainder of the compound.
- Cn-m alkylene refers to an alkylene group having n to m carbon atoms.
- alkylene groups include, but are not limited to, ethan-l,2-diyl, propan-l ,3-diyl, propan-l,2-diyl, butan-l ,4-diyl, butan-l,3-diyl, butan-l,2-diyl, 2-methyl-propan-l,3-diyl and the like.
- alkoxy employed alone or in combination with other terms, refers to a group of formula -O-alkyl, wherein the alkyl group is as defined above.
- Cn-m alkoxy refers to an alkoxy group, the alkyl group of which has n to m carbons.
- Example alkoxy groups include methoxy, ethoxy, propoxy (e.g. , w-propoxy and isopropoxy), i-butoxy and the like.
- the alkyl group has 1 to 6, 1 to 4, or 1 to 3 carbon atoms.
- amino refers to a group of formula -NH2.
- cyano or "nitrile” refers to a group of formula -C ⁇ N, which also may be written as -CN.
- halo refers to fluoro, chloro, bromo and iodo.
- halo refers to a halogen atom selected from F, CI, or Br.
- halo groups are F.
- haloalkyl refers to an alkyl group in which one or more of the hydrogen atoms has been replaced by a halogen atom.
- Cn-m haloalkyl refers to a Cn-m alkyl group having n to m carbon atoms and from at least one up to ⁇ 2(n to m)+l ⁇ halogen atoms, which may either be the same or different.
- the halogen atoms are fluoro atoms.
- the haloalkyl group has 1 to 6 or 1 to 4 carbon atoms.
- Example haloalkyl groups include CF3, C2F5, CHF2, CCh, CHCI2, C2CI5 and the like.
- the haloalkyl group is a fluoroalkyl group.
- haloalkoxy refers to a group of formula -O-haloalkyl, wherein the haloalkyl group is as defined above.
- Cn-m haloalkoxy refers to a haloalkoxy group, the haloalkyl group of which has n to m carbons.
- Example haloalkoxy groups include trifluoromethoxy and the like. In some embodiments, the haloalkoxy group has 1 to 6, 1 to 4, or 1 to 3 carbon atoms.
- oxo refers to an oxygen atom as a divalent substituent, forming a carbonyl group when attached to carbon, or attached to a heteroatom forming a sulfoxide or sulfone group, or an N-oxide group.
- aromatic refers to a carbocycle or heterocycle having one or more polyunsaturated rings having aromatic character (i. e. , having (4n + 2) delocalized ⁇ (pi) electrons where n is an integer).
- aryl employed alone or in combination with other terms, refers to an aromatic hydrocarbon group, which may be monocyclic or poly cyclic (e.g. , having 2 fused rings).
- Cn- m aryl refers to an aryl group having from n to m ring carbon atoms.
- Aryl groups include, e.g. , phenyl, naphthyl, and the like. In some embodiments, aryl groups have from 6 to about 10 carbon atoms. In some embodiments aryl groups have 6 carbon atoms. In some embodiments aryl groups have 10 carbon atoms. In some embodiments, the aryl group is phenyl. In some embodiments, the aryl group is naphthyl.
- heteroaryl or “heteroaromatic,” employed alone or in combination with other terms, refers to a monocyclic or poly cyclic aromatic heterocycle having at least one heteroatom ring member selected from sulfur, oxygen and nitrogen.
- the heteroaryl ring has 1 , 2, 3 or 4 heteroatom ring members independently selected from nitrogen, sulfur and oxygen.
- any ring-forming ⁇ in a heteroaryl moiety can be an N-oxide.
- the heteroaryl has 5-14 ring atoms including carbon atoms and 1 , 2, 3 or 4 heteroatom ring members independently selected from nitrogen, sulfur and oxygen.
- the heteroaryl has 5-10 ring atoms including carbon atoms and 1 , 2, 3 or 4 heteroatom ring members independently selected from nitrogen, sulfur and oxygen. In some embodiments, the heteroaryl has 5-6 ring atoms and 1 or 2 heteroatom ring members independently selected from nitrogen, sulfur and oxygen. In some embodiments, the heteroaryl is a five-membered or six-membered heteroaryl ring. In other embodiments, the heteroaryl is an eight-membered, nine-membered or ten-membered fused bicyclic heteroaryl ring.
- Example heteroaryl groups include, but are not limited to, pyridinyl (pyridyl), pyrimidinyl, pyrazinyl, pyridazinyl, pyrrolyl, pyrazolyl, azolyl, oxazolyl, thiazolyl, imidazolyl, furanyl, thiophenyl, quinolinyl, isoquinolinyl, naphthyridinyl (including 1,2-, 1,3-, 1,4-, 1,5-, 1,6-, 1,7-, 1,8-, 2,3- and 2,6-naphthyridine), indolyl, indazolyl, benzothiophenyl, benzofuranyl, benzisoxazolyl, imidazo[l,2-Z>]thiazolyl, purinyl, and the like.
- pyridinyl pyridyl
- pyrimidinyl pyrazinyl
- a five-membered heteroaryl ring is a heteroaryl group having five ring atoms wherein one or more (e.g. , 1, 2 or 3) ring atoms are independently selected from N, O and S.
- Exemplary five-membered ring heteroaryls include thienyl, furyl, pyrrolyl, imidazolyl, thiazolyl, oxazolyl, pyrazolyl, isothiazolyl, isoxazolyl, 1,2,3-triazolyl, tetrazolyl, 1,2,3- thiadiazolyl, 1,2,3-oxadiazolyl, 1 ,2,4-triazolyl, 1 ,2,4-thiadiazolyl, 1,2,4-oxadiazolyl, 1,3,4- triazolyl, 1,3,4-thiadiazolyl and 1,3,4-oxadiazolyl.
- a six-membered heteroaryl ring is a heteroaryl group having six ring atoms wherein one or more (e.g. , 1, 2 or 3) ring atoms are independently selected from N, O and S.
- Exemplary six-membered ring heteroaryls are pyridyl, pyrazinyl, pyrimidinyl, triazinyl and pyridazinyl.
- cycloalkyl employed alone or in combination with other terms, refers to a non-aromatic hydrocarbon ring system (monocyclic, bicyclic or poly cyclic), including cyclized alkyl and alkenyl groups.
- Cn-m cycloalkyl refers to a cycloalkyl that has n to m ring member carbon atoms.
- Cycloalkyl groups can include mono- or poly cyclic (e.g. , having 2, 3 or 4 fused rings) groups and spirocycles. Cycloalkyl groups can have 3, 4, 5, 6 or 7 ring-forming carbons (C3-7).
- the cycloalkyl group has 3 to 6 ring members, 3 to 5 ring members, or 3 to 4 ring members. In some embodiments, the cycloalkyl group is monocyclic. In some embodiments, the cycloalkyl group is monocyclic or bicyclic. In some embodiments, the cycloalkyl group is a C3-6 monocyclic cycloalkyl group. Ring- forming carbon atoms of a cycloalkyl group can be optionally oxidized to form an oxo or sulfido group. Cycloalkyl groups also include cycloalkylidenes.
- cycloalkyl is cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl. Also included in the definition of cycloalkyl are moieties that have one or more aromatic rings fused (i.e. , having a bond in common with) to the cycloalkyl ring, e.g., benzo or thienyl derivatives of cyclopentane, cyclohexane and the like.
- a cycloalkyl group containing a fused aromatic ring can be attached through any ring-forming atom including a ring-forming atom of the fused aromatic ring.
- cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclopentenyl, cyclohexenyl, cyclohexadienyl, cycloheptatrienyl, norbornyl, norpinyl, norcamyl, bicyclo[l . l . l]pentanyl, bicyclo[2.1.1]hexanyl, and the like.
- the cycloalkyl group is cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- heterocycloalkyl refers to a non-aromatic ring or ring system, which may optionally contain one or more alkenylene groups as part of the ring structure, which has at least one heteroatom ring member independently selected from nitrogen, sulfur oxygen and phosphorus, and which has 4-10 ring members, 4-7 ring members, or 4-6 ring members. Included within the term “heterocycloalkyl” are monocyclic 4-, 5-, 6- and 7-membered heterocycloalkyl groups. Heterocycloalkyl groups can include mono- or bicyclic (e.g., having two fused or bridged rings) ring systems.
- the heterocycloalkyl group is a monocyclic group having 1, 2 or 3 heteroatoms independently selected from nitrogen, sulfur and oxygen. Ring- forming carbon atoms and heteroatoms of a heterocycloalkyl group can be optionally oxidized to form an oxo or sulfido group or other oxidized linkage (e.g., C(O), S(O), C(S) or S(0)2, N-oxide etc.) or a nitrogen atom can be quaternized.
- the heterocycloalkyl group can be attached through a ring-forming carbon atom or a ring-forming heteroatom. In some embodiments, the heterocycloalkyl group contains 0 to 3 double bonds. In some
- the heterocycloalkyl group contains 0 to 2 double bonds. Also included in the definition of heterocycloalkyl are moieties that have one or more aromatic rings fused (i.e., having a bond in common with) to the heterocycloalkyl ring, e.g., benzo or thienyl derivatives of piperidine, morpholine, azepine, etc.
- a heterocycloalkyl group containing a fused aromatic ring can be attached through any ring-forming atom including a ring-forming atom of the fused aromatic ring.
- heterocycloalkyl groups include azetidinyl, azepanyl, dihydrobenzofuranyl, dihydrofuranyl, dihydropyranyl, dihydrobenzodioxinyl, benzodioxinyl, morpholino, 3-oxa-9-azaspiro[5.5]undecanyl, l-oxa-8-azaspiro[4.5]decanyl, piperidinyl, piperazinyl, oxopiperazinyl, pyranyl, pyrrolidinyl, quinuclidinyl,
- the definitions or embodiments refer to specific rings (e.g. , an azetidine ring, a pyridine ring, etc.). Unless otherwise indicated, these rings can be attached to any ring member provided that the valency of the atom is not exceeded. For example, an azetidine ring may be attached at any position of the ring, whereas an azetidin-3-yl ring is attached at the 3 -position.
- the compounds described herein can be asymmetric (e.g. , having one or more stereocenters). All stereoisomers, such as enantiomers and diastereomers, are intended unless otherwise indicated.
- One method includes fractional recrystallization using a chiral resolving acid which is an optically active, salt-forming organic acid.
- Suitable resolving agents for fractional recrystallization methods are, e.g. , optically active acids, such as the D and L forms of tartaric acid, diacetyltartaric acid, dibenzoyltartaric acid, mandelic acid, malic acid, lactic acid or the various optically active camphorsulfonic acids such as ⁇ - camphorsulfonic acid.
- Other resolving agents suitable for fractional crystallization methods include stereoisomerically pure forms of a-methylbenzylamine (e.g.
- Resolution of racemic mixtures can also be carried out by elution on a column packed with an optically active resolving agent (e.g., dinitrobenzoylphenylglycine).
- an optically active resolving agent e.g., dinitrobenzoylphenylglycine
- Suitable elution solvent composition can be determined by one skilled in the art.
- the compounds of the invention have the (i?)-configuration. In other embodiments, the compounds have the ( ⁇ -configuration. In compounds with more than one chiral centers, each of the chiral centers in the compound may be independently (R) or (S), unless otherwise indicated.
- Tautomeric forms result from the swapping of a single bond with an adjacent double bond together with the concomitant migration of a proton.
- Tautomeric forms include prototropic tautomers which are isomeric protonation states having the same empirical formula and total charge.
- Example prototropic tautomers include ketone - enol pairs, amide - imidic acid pairs, lactam - lactim pairs, enamine - imine pairs, and annular forms where a proton can occupy two or more positions of a heterocyclic system, e.g.
- Tautomeric forms can be in equilibrium or sterically locked into one form by appropriate substitution.
- Compounds of the invention can also include all isotopes of atoms occurring in the intermediates or final compounds.
- Isotopes include those atoms having the same atomic number but different mass numbers.
- isotopes of hydrogen include tritium and deuterium.
- One or more constituent atoms of the compounds of the invention can be replaced or substituted with isotopes of the atoms in natural or non-natural abundance.
- the compound includes at least one deuterium atom.
- one or more hydrogen atoms in a compound of the present disclosure can be replaced or substituted by deuterium.
- the compound includes two or more deuterium atoms.
- the compound includes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1 or 12 deuterium atoms. Synthetic methods for including isotopes into organic compounds are known in the art.
- compound as used herein is meant to include all stereoisomers, geometric isomers, tautomers and isotopes of the structures depicted.
- the term is also meant to refer to compounds of the inventions, regardless of how they are prepared, e.g., synthetically, through biological process (e.g., metabolism or enzyme conversion), or a combination thereof.
- All compounds, and pharmaceutically acceptable salts thereof can be found together with other substances such as water and solvents (e.g. , hydrates and solvates) or can be isolated.
- solvents e.g. , hydrates and solvates
- the compounds described herein and salts thereof may occur in various forms and may, e.g. , take the form of solvates, including hydrates.
- the compounds may be in any solid state form, such as a polymorph or solvate, so unless clearly indicated otherwise, reference in the specification to compounds and salts thereof should be understood as encompassing any solid state form of the compound.
- the compounds of the invention, or salts thereof are substantially isolated.
- substantially isolated is meant that the compound is at least partially or substantially separated from the environment in which it was formed or detected.
- Partial separation can include, e.g. , a composition enriched in the compounds of the invention.
- Substantial separation can include compositions containing at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 97%, or at least about 99% by weight of the compounds of the invention, or salt thereof.
- phrases "pharmaceutically acceptable” is employed herein to refer to those compounds, materials, compositions and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
- ambient temperature and “room temperature,” as used herein, are understood in the art, and refer generally to a temperature, e.g. , a reaction temperature, that is about the temperature of the room in which the reaction is carried out, e.g. , a temperature from about 20 °C to about 30 °C.
- the present invention also includes pharmaceutically acceptable salts of the compounds described herein.
- pharmaceutically acceptable salts refers to derivatives of the disclosed compounds wherein the parent compound is modified by converting an existing acid or base moiety to its salt form.
- examples of pharmaceutically acceptable salts include, but are not limited to, mineral or organic acid salts of basic residues such as amines; alkali or organic salts of acidic residues such as carboxylic acids; and the like.
- the pharmaceutically acceptable salts of the present invention include the non-toxic salts of the parent compound formed, e.g. , from non-toxic inorganic or organic acids.
- the pharmaceutically acceptable salts of the present invention can be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods.
- such salts can be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropriate base or acid in water or in an organic solvent, or in a mixture of the two; generally, non-aqueous media like ether, ethyl acetate, alcohols (e.g. , methanol, ethanol, iso-propanol or butanol) or acetonitrile (MeCN) are preferred.
- non-aqueous media like ether, ethyl acetate, alcohols (e.g. , methanol, ethanol, iso-propanol or butanol) or acetonitrile (MeCN) are preferred.
- suitable salts are found in Remington 's Pharmaceutical Sciences, 17 th Ed., (Mack Publishing Company, Easton, 1985), p. 1418, Berge et al, J. Pharm. Scl , 1977, 66( ⁇ ), 1 -19 and in Stahl et al
- Suitable solvents can be substantially non-reactive with the starting materials (reactants), the intermediates or products at the temperatures at which the reactions are carried out, e.g. , temperatures which can range from the solvent's freezing temperature to the solvent's boiling temperature.
- a given reaction can be carried out in one solvent or a mixture of more than one solvent.
- suitable solvents for a particular reaction step can be selected by the skilled artisan.
- Reactions can be monitored according to any suitable method known in the art.
- product formation can be monitored by spectroscopic means, such as nuclear magnetic resonance spectroscopy (e.g. , 3 ⁇ 4 or 1 C), infrared spectroscopy, spectrophotometry (e.g. , UV -visible), mass spectrometry or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC).
- spectroscopic means such as nuclear magnetic resonance spectroscopy (e.g. , 3 ⁇ 4 or 1 C), infrared spectroscopy, spectrophotometry (e.g. , UV -visible), mass spectrometry or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC).
- HPLC high performance liquid chromatography
- TLC thin layer chromatography
- Compound of formula 1-7 can be synthesized using a process shown in Scheme 1.
- a palladium-catalyzed cross-coupling reaction of halo-substituted aromatic amine 1-1 with a suitable coupling reagent 1-2 (where M is, e.g., -B(OH)2) under standard conditions (such as Suzuki coupling reaction, e.g. , in the presence of a palladium catalyst and a suitable base) can produce compound 1-3.
- M is, e.g., -B(OH)2
- Suzuki coupling reaction e.g., in the presence of a palladium catalyst and a suitable base
- the reaction of aromatic amine 1-3 with an acid of formula 1-4 using a coupling reagent such as, but not limited to, HATU can give the amide 1-5, which can be deprotected under acidic conditions (e.g.
- the R 7 group can be introduced either by direct alkylation with an alkyl halide or reductive alkylation with an aldehyde or a ketone to give the desired product of formula 1-7.
- compound of formula 2-7 can be synthesized using a process shown in Scheme 2.
- the reaction of halo-substituted aromatic amine 2-1 with an ester of formula 2-2 in the presence of a suitable base such as, but not limited to, potassium fert-butoxide or sodium hydride can furnish the amide 2-3.
- the Boc protecting group in compound 2-3 can be removed under acidic conditions (e.g., hydrochloric acid or trifluoroacetic acid) to provide the free amine of formula 2-4.
- the Cy ring can be installed by the cross-coupling of compound 2-4 with a suitable coupling reagent 2-5 (where M is, e.g., -B(OH)2) under standard conditions (such as Suzuki coupling reaction, e.g.
- R 7 group can be introduced either by direct alkylation with an alkyl halide or reductive alkylation with an aldehyde or a ketone to give the desired product of formula 2-7.
- Ester of formula 3-3 can be synthesized using a process shown in Scheme 3.
- the free amine group in compound 3-1 can be protected with Boc to give the compound of formula 3- 2.
- Compound 3-2 can be deprotonated by a strong base such as, but not limited to, n-butyl lithium or lithium bis(trimethylsilyl)amide to generate the corresponding aryl lithium intermediate, which can further react with a chloroformate or carbon dioxide to give the desired ester or acid of formula 3-3.
- a strong base such as, but not limited to, n-butyl lithium or lithium bis(trimethylsilyl)amide to generate the corresponding aryl lithium intermediate, which can further react with a chloroformate or carbon dioxide to give the desired ester or acid of formula 3-3.
- Compounds of the present disclosure can inhibit the activity of PD-1/PD-L1 protein/protein interaction and, thus, are useful in treating diseases and disorders associated with activity of PD-1 and the diseases and disorders associated with PD-L1 including its interaction with other proteins such as PD-1 and B7-1 (CD80).
- the compounds of the present disclosure demonstrate better efficacy and favorable safety and toxicity profiles in animal studies.
- the compounds of the present disclosure, or pharmaceutically acceptable salts or stereoisomers thereof are useful for therapeutic administration to enhance, stimulate and/or increase immunity in cancer or chronic infection, including enhancement of response to vaccination.
- the present disclosure provides a method for inhibiting or blocking the PD-1/PD-L1 protein/protein interaction.
- the method includes administering to an individual or a patient a compound of Formula (I) or any of the formulas as described herein or of a compound as recited in any of the claims and described herein, or a pharmaceutically acceptable salt or a stereoisomer thereof.
- the compounds of the present disclosure can be used alone, in combination with other agents or therapies or as an adjuvant or neoadjuvant for the treatment of diseases or disorders, including cancer or infection diseases.
- any of the compounds of the disclosure including any of the embodiments thereof, may be used.
- the compounds of the present disclosure inhibit the PD-1/PD-L1 protein/protein interaction, resulting in a PD-1 pathway blockade.
- the blockade of PD-1 can enhance the immune response to cancerous cells and infectious diseases in mammals, including humans.
- the present disclosure provides treatment of an individual or a patient in vivo using a compound of Formula (I) or a salt or stereoisomer thereof such that growth of cancerous tumors is inhibited.
- a compound of Formula (I) or of any of the formulas as described herein, or a compound as recited in any of the claims and described herein, or a salt or stereoisomer thereof, can be used to inhibit the growth of cancerous tumors.
- a compound of Formula (I) or of any of the formulas as described herein, or a compound as recited in any of the claims and described herein, or a salt or stereoisomer thereof can be used in conjunction with other agents or standard cancer treatments, as described below.
- the present disclosure provides a method for inhibiting growth of tumor cells in vitro. The method includes contacting the tumor cells in vitro with a compound of Formula (I) or of any of the formulas as described herein, or of a compound as recited in any of the claims and described herein, or of a salt or stereoisomer thereof.
- the present disclosure provides a method for inhibiting growth of tumor cells in an individual or a patient.
- the method includes administering to the individual or patient in need thereof a therapeutically effective amount of a compound of Formula (I) or of any of the formulas as described herein, or of a compound as recited in any of the claims and described herein, or a salt or a stereoisomer thereof.
- a method for treating cancer includes administering to a patient in need thereof, a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a salt thereof.
- cancers include those whose growth may be inhibited using compounds of the disclosure and cancers typically responsive to immunotherapy.
- the present disclosure provides a method of enhancing, stimulating and/or increasing the immune response in a patient.
- the method includes administering to the patient in need thereof a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a salt thereof.
- cancers that are treatable using the compounds of the present disclosure include, but are not limited to, bone cancer, pancreatic cancer, skin cancer, cancer of the head or neck, cutaneous or intraocular malignant melanoma, uterine cancer, ovarian cancer, rectal cancer, cancer of the anal region, stomach cancer, testicular cancer, uterine cancer, carcinoma of the fallopian tubes, carcinoma of the endometrium, endometrial cancer, carcinoma of the cervix, carcinoma of the vagina, carcinoma of the vulva, Hodgkin's Disease, non-Hodgkin's lymphoma, cancer of the esophagus, cancer of the small intestine, cancer of the endocrine system, cancer of the thyroid gland, cancer of the parathyroid gland, cancer of the adrenal gland, sarcoma of soft tissue, cancer of the urethra, cancer of the penis, chronic or acute leukemias including acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leuk
- cancers treatable with compounds of the present disclosure include melanoma (e.g., metastatic malignant melanoma), renal cancer (e.g. clear cell carcinoma), prostate cancer (e.g. hormone refractory prostate adenocarcinoma), breast cancer, colon cancer and lung cancer (e.g. non-small cell lung cancer). Additionally, the disclosure includes refractory or recurrent malignancies whose growth may be inhibited using the compounds of the disclosure.
- melanoma e.g., metastatic malignant melanoma
- renal cancer e.g. clear cell carcinoma
- prostate cancer e.g. hormone refractory prostate adenocarcinoma
- breast cancer e.g. hormone refractory prostate adenocarcinoma
- colon cancer e.g. non-small cell lung cancer
- lung cancer e.g. non-small cell lung cancer
- cancers that are treatable using the compounds of the present disclosure include, but are not limited to, solid tumors (e.g. , prostate cancer, colon cancer, esophageal cancer, endometrial cancer, ovarian cancer, uterine cancer, renal cancer, hepatic cancer, pancreatic cancer, gastric cancer, breast cancer, lung cancer, cancers of the head and neck, thyroid cancer, glioblastoma, sarcoma, bladder cancer, etc.), hematological cancers (e.g.
- lymphoma leukemia such as acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), DLBCL, mantle cell lymphoma, Non-Hodgkin lymphoma (including relapsed or refractory NHL and recurrent follicular), Hodgkin lymphoma or multiple myeloma) and combinations of said cancers.
- ALL acute lymphoblastic leukemia
- AML acute myelogenous leukemia
- CLL chronic lymphocytic leukemia
- CML chronic myelogenous leukemia
- DLBCL mantle cell lymphoma
- Non-Hodgkin lymphoma including relapsed or refractory NHL and recurrent follicular
- Hodgkin lymphoma or multiple myeloma and combinations of said cancers.
- PD-1 pathway blockade with compounds of the present disclosure can also be used for treating infections such as viral, bacteria, fungus and parasite infections.
- the present disclosure provides a method for treating infections such as viral infections. The method includes administering to a patient in need thereof, a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, a salt thereof.
- viruses causing infections treatable by methods of the present disclosure include, but are not limit to, human immunodeficiency virus, human papillomavirus, influenza, hepatitis A, B, C or D viruses, adenovirus, pox vims, herpes simpl ex viruses, human cytomegalovirus, severe acute respirator ⁇ ' syndrome virus, eboia virus, and measles virus.
- viruses causing infections treatable by methods of the present disclosure include, but are not limit to, hepatitis (A, B, or C), herpes virus (e.g., VZV, HSV-1, HAV-6, HSV-II, and CMV, Epstein Barr virus), adenovirus, influenza virus, fiaviviruses, echovirus, rhinovirus, coxsackie virus, cornovirus, respiratory syncytial virus, mumpsvirus, rotavirus, measles virus, rubella virus, parvovirus, vaccinia virus, HTLV virus, dengue virus, papillomavirus, molluscum virus, poliovirus, rabies virus, JC virus and arboviral encephalitis virus.
- herpes virus e.g., VZV, HSV-1, HAV-6, HSV-II, and CMV, Epstein Barr virus
- adenovirus e.g., adenovirus
- influenza virus e.g., V
- the present disclosure provides a method for treating bacterial infections.
- the method includes administering to a patient in need thereof, a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a salt thereof.
- Non-limiting examples of pathogenic bacteria causing infections treatable by methods of the disclosure include chlamydia, rickettsial bacteria, mycobacteria, staphylococci, streptococci, pneumonococci, meningococci and conococci, klebsiella, proteus, serratia, pseudomonas, legionella, diphtheria, salmonella, bacilli, cholera, tetanus, botulism, anthrax, plague, leptospirosis, and Lyme's disease bacteria.
- the present disclosure provides a method for treating fungus infections.
- the method includes administering to a patient in need thereof, a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a salt thereof.
- pathogenic fungi causing infections treatable by methods of the disclosure include Candida (albicans, krusei, glabrata, tropicalis, etc.), Cryptococcus neoformans, Aspergillus
- Genus Mucorales micor, absidia, rhizophus
- Sporothrix schenkii Blastomyces dermatitidis
- Paracoccidioides brasiliensis Coccidioides immitis
- Histoplasma capsulatum Histoplasma capsulatum.
- the present disclosure provides a method for treating parasite infections.
- the method includes administering to a patient in need thereof, a therapeutically effective amount of a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a salt thereof.
- Non-limiting examples of pathogenic parasites causing infections treatable by methods of the disclosure include Entamoeba histolytica, Balantidium coli, Naegleriafowleri, Acanthamoeba sp., Giardia lambia, Cryptosporidium sp., Pneumocystis carinii, Plasmodium vivax, Babesia microti, Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondi, and Nippostrongylus brasiliensis.
- mice preferably mice, rats, other rodents, rabbits, dogs, cats, swine, cattle, sheep, horses, or primates, and most preferably humans.
- terapéuticaally effective amount refers to the amount of active compound or pharmaceutical agent that elicits the biological or medicinal response in a tissue, system, animal, individual or human that is being sought by a researcher, veterinarian, medical doctor or other clinician.
- treating refers to one or more of (1) inhibiting the disease; e.g. , inhibiting a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e. , arresting further development of the pathology and/or symptomatology); and (2) ameliorating the disease; e.g., ameliorating a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e. , reversing the pathology and/or symptomatology) such as decreasing the severity of disease.
- the compounds of the invention are useful in preventing or reducing the risk of developing any of the diseases referred to herein; e.g. , preventing or reducing the risk of developing a disease, condition or disorder in an individual who may be predisposed to the disease, condition or disorder but does not yet experience or display the pathology or symptomatology of the disease.
- Cancer cell growth and survival can be impacted by multiple signaling pathways.
- Targeting more than one signaling pathway may reduce the likelihood of drug-resistance arising in a cell population, and/or reduce the toxicity of treatment.
- the compounds of the present disclosure can be used in combination with one or more other enzyme/protein/receptor inhibitors for the treatment of diseases, such as cancer or infections.
- diseases such as cancer or infections.
- cancers include solid tumors and liquid tumors, such as blood cancers.
- infections include viral infections, bacterial infections, fungus infections or parasite infections.
- the compounds of the present disclosure can be combined with one or more inhibitors of the following kinases for the treatment of cancer: Aktl, Akt2, Akt3, TGF-PR, PKA, PKG, PKC, CaM-kinase, phosphorylase kinase, MEKK, ERK, MAPK, mTOR, EGFR, HER2, HER3, HER4, INS-R, IGF-1R, IR-R, PDGFaR, PDGFPR, CSFIR, KIT, FLK-II, KDR/FLK-1, FLK-4, flt-1, FGFR1, FGFR2, FGFR3, FGFR4, c-Met, Ron, Sea, TRKA, TRKB, TRKC, FLT3, VEGFR/Flt2, Flt4, EphAl, EphA2, EphA3, EphB2, EphB4, Tie2, Src, Fyn, Lck, Fgr, Btk, Fak, SYK,
- the compounds of the present disclosure can be combined with one or more of the following inhibitors for the treatment of cancer or infections.
- inhibitors that can be combined with the compounds of the present disclosure for treatment of cancer and infections include an FGFR inhibitor (FGFR1, FGFR2, FGFR3 or FGFR4, e.g., INCB54828, INCB62079 and INCB63904), a JAK inhibitor (JAK1 and/or JAK2, e.g., ruxolitinib, baricitinib or INCB39110), an IDO inhibitor (e.g., epacadostat and NLG919), an LSD1 inhibitor (e.g., INCB59872 and I CB60003), a TDO inhibitor, a PI3K-delta inhibitor (e.g., INCB50797 and I CB50465), a PI3K-gamma inhibitor such as a PI3K-gamma selective inhibitor, a Pirn inhibitor,
- FGFR inhibitor FG
- immune checkpoint inhibitors include inhibitors against immune checkpoint molecules such as CD27, CD28, CD40, CD122, CD96, CD73, CD47, OX40, GITR, CSF1R, JAK, PI3K delta, PI3K gamma, TAM, arginase, CD137 (also known as 4-1BB), ICOS, A2AR, B7-H3, B7-H4, BTLA, CTLA-4, LAG3, TIM3, VISTA, PD-1 , PD-L1 and PD-L2.
- immune checkpoint inhibitors include inhibitors against immune checkpoint molecules such as CD27, CD28, CD40, CD122, CD96, CD73, CD47, OX40, GITR, CSF1R, JAK, PI3K delta, PI3K gamma, TAM, arginase, CD137 (also known as 4-1BB), ICOS, A2AR, B7-H3, B7-H4, BTLA, CTLA-4, LAG3,
- the immune checkpoint molecule is a stimulatory checkpoint molecule selected from CD27, CD28, CD40, ICOS, OX40, GITR and CD137.
- the immune checkpoint molecule is an inhibitory checkpoint molecule selected from A2AR, B7-H3, B7-H4, BTLA, CTLA-4, IDO, KIR, LAG3, PD-1, TIM3, and VISTA.
- the compounds provided herein can be used in combination with one or more agents selected from KIR inhibitors, TIGIT inhibitors, LAIRl inhibitors, CD 160 inhibitors, 2B4 inhibitors and TGFR beta inhibitors.
- the inhibitor of an immune checkpoint molecule is anti-PDl antibody, anti-PD-Ll antibody, or anti-CTLA-4 antibody.
- the inhibitor of an immune checkpoint molecule is an inhibitor of PD-1 , e.g., an anti-PD-1 monoclonal antibody.
- the anti-PD-1 monoclonal antibody is nivolumab, pembrolizumab (also known as MK-3475), pidilizumab, SHR-1210, PDR001 , or AMP-224.
- the anti-PD-1 monoclonal antibody is nivolumab or pembrolizumab.
- the anti-PDl antibody is pembrolizumab.
- the anti PD-1 antibody is SHR-1210.
- the inhibitor of an immune checkpoint molecule is an inhibitor of PD-L1 , e.g., an anti-PD-Ll monoclonal antibody.
- the anti-PD-Ll monoclonal antibody is BMS-935559, MEDI4736, MPDL3280A (also known as RG7446), or MSB0010718C.
- the anti-PD-Ll monoclonal antibody is BMS-935559, MEDI4736, MPDL3280A (also known as RG7446), or MSB0010718C.
- the anti-PD-Ll monoclonal antibody is
- the inhibitor of an immune checkpoint molecule is an inhibitor of CTLA-4, e.g., an anti-CTLA-4 antibody.
- the anti-CTLA-4 antibody is ipilimumab.
- the inhibitor of an immune checkpoint molecule is an inhibitor of LAG3, e.g., an anti-LAG3 antibody.
- the anti-LAG3 antibody is BMS-986016 or LAG525.
- the inhibitor of an immune checkpoint molecule is an inhibitor of GITR, e.g., an anti-GITR antibody.
- the anti-GITR antibody is TRX518 or MK-4166.
- the inhibitor of an immune checkpoint molecule is an inhibitor of OX40, e.g., an anti-OX40 antibody or OX40L fusion protein.
- OX40 e.g., an anti-OX40 antibody or OX40L fusion protein.
- the anti-OX40 antibody is MEDI0562.
- the OX40L fusion protein is MEDI6383.
- the agent is an alkylating agent, a proteasome inhibitor, a corticosteroid, or an immunomodulatory agent.
- an alkylating agent include cyclophosphamide (CY), melphalan (MEL), and bendamustine.
- the proteasome inhibitor is carfilzomib.
- the corticosteroid is dexamethasone (DEX).
- the immunomodulatory agent is lenalidomide (LEN) or pomalidomide (POM).
- the compounds of the present disclosure can further be used in combination with other methods of treating cancers, for example by chemotherapy, irradiation therapy, tumor- targeted therapy, adjuvant therapy, immunotherapy or surgery.
- immunotherapy include cytokine treatment (e.g., interferons, GM-CSF, G-CSF, IL-2), CRS-207
- the compounds can be administered in combination with one or more anti-cancer drugs, such as a chemotherapeutics.
- Example chemotherapeutics include any of: abarelix, aldesleukin, alemtuzumab, alitretinoin, allopurinol, altretamine, anastrozole, arsenic trioxide, asparaginase, azacitidine, bevacizumab, bexarotene, baricitinib, bleomycin, bortezombi, bortezomib, busulfan intravenous, busulfan oral, calusterone, capecitabine, carboplatin, carmustine, cetuximab, chlorambucil, cisplatin, cladribine, clofarabine, cyclophosphamide, cytarabine, dacarbazine, dactinomycin, dalteparin sodium, dasatinib, daunorubicin, decitabine, denileukin, denileukin diftitox, dexrazox
- anti-cancer agent(s) include antibody therapeutics such as trastuzumab (Herceptin), antibodies to costimulatory molecules such as CTLA-4 (e.g., ipilimumab), 4- 1BB, antibodies to PD-1 and PD-L1 , or antibodies to cytokines (IL-10, TGF- ⁇ , etc.).
- Herceptin antibodies to costimulatory molecules
- CTLA-4 e.g., ipilimumab
- 4- 1BB antibodies to PD-1 and PD-L1
- cytokines IL-10, TGF- ⁇ , etc.
- antibodies to PD-1 and/or PD-L1 that can be combined with compounds of the present disclosure for the treatment of cancer or infections such as viral, bacteria, fungus and parasite infections include, but are not limited to, nivolumab, pembrolizumab, MPDL3280A, MEDI-4736 and SHR-1210.
- the compounds of the present disclosure can further be used in combination with one or more anti-inflammatory agents, steroids, immunosuppressants or therapeutic antibodies.
- the compounds of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or salts thereof can be combined with another immunogenic agent, such as cancerous cells, purified tumor antigens (including recombinant proteins, peptides, and carbohydrate molecules), cells, and cells transfected with genes encoding immune stimulating cytokines.
- tumor vaccines include peptides of melanoma antigens, such as peptides of gplOO, MAGE antigens, Trp-2, MARTI and/or tyrosinase, or tumor cells transfected to express the cytokine GM-CSF.
- tumor vaccines include the proteins from viruses implicated in human cancers such as Human Papilloma Viruses (HPV), Hepatitis Viruses (HBV and HCV) and Kaposi's Herpes Sarcoma Virus (KHSV).
- HPV Human Papilloma Viruses
- HBV and HCV Hepatitis Viruses
- KHSV Kaposi's Herpes Sarcoma Virus
- the compounds of the present disclosure can be used in combination with tumor specific antigen such as heat shock proteins isolated from tumor tissue itself.
- the compounds of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or salts thereof can be combined with dendritic cells immunization to activate potent anti-tumor responses.
- the compounds of the present disclosure can be used in combination with bispecific macrocyclic peptides that target Fe alpha or Fe gamma receptor-expressing effectors cells to tumor cells.
- the compounds of the present disclosure can also be combined with macrocyclic peptides that activate host immune responsiveness.
- the compounds of the present disclosure can be used in combination with bone marrow transplant for the treatment of a variety of tumors of hematopoietic origin.
- the compounds of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or salts thereof can be used in combination with vaccines, to stimulate the immune response to pathogens, toxins, and self antigens.
- pathogens for which this therapeutic approach may be particularly useful include pathogens for which there is currently no effective vaccine, or pathogens for which conventional vaccines are less than completely effective. These include, but are not limited to, HIV, Hepatitis (A, B, & C), Influenza, Herpes, Giardia, Malaria, Leishmania, Staphylococcus aureus, Pseudomonas Aeruginosa.
- Viruses causing infections treatable by methods of the present disclosure include, but are not limit to human papillomavirus, influenza, hepatitis A, B, C or D viruses, adenovirus, poxvirus, herpes simplex viruses, human c tomegalovirus, severe acute respiratory syndrome virus, ebola virus, measles virus, herpes virus (e.g., VZV, HSV-1, HAV-6, HSV-II, and CMV, Epstein Barr virus), flaviviruses, echovirus, rhinovirus, coxsackie virus, cornovirus, respiratory syncytial virus, mumpsvirus, rotavirus, measles virus, rubella virus, parvovirus, vaccinia virus, HTLV virus, dengue virus, papillomavirus, molluscum virus, poliovirus, rabies virus, JC virus and arboviral encephalitis virus.
- human papillomavirus influenza, hepatitis A
- Pathogenic bacteria causing infections treatable by methods of the disclosure include, but are not limited to, chlamydia, rickettsial bacteria, mycobacteria, staphylococci, streptococci, pneumonococci, meningococci and conococci, klebsiella, proteus, serratia, pseudomonas, legionella, diphtheria, salmonella, bacilli, cholera, tetanus, botulism, anthrax, plague, leptospirosis, and Lyme's disease bacteria.
- Pathogenic fungi causing infections treatable by methods of the disclosure include, but are not limited to, Candida (albicans, krusei, glabrata, tropicalis, etc.), Cryptococcus neoformans, Aspergillus (fumigatus, niger, etc.), Genus Mucorales (mucor, absidia, rhizophus), Sporothrix schenkii, Blastomyces dermatitidis, Paracoccidioides brasiliensis, Coccidioides immitis and Histoplasma capsulatum.
- Candida albicans, krusei, glabrata, tropicalis, etc.
- Cryptococcus neoformans Aspergillus (fumigatus, niger, etc.)
- Genus Mucorales micor, absidia, rhizophus
- Sporothrix schenkii Blastomyces dermatitidis
- Paracoccidioides brasiliensis C
- Pathogenic parasites causing infections treatable by methods of the disclosure include, but are not limited to, Entamoeba histolytica, Balantidium coli, Naegleriafowleri, Acanthamoeba sp., Giardia lambia, Cryptosporidium sp., Pneumocystis carinii, Plasmodium vivax, Babesia microti, Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondi, and Nippostrongylus brasiliensis.
- more than one pharmaceutical agent When more than one pharmaceutical agent is administered to a patient, they can be administered simultaneously, separately, sequentially, or in combination (e.g. , for more than two agents).
- the compounds of the present disclosure can be administered in the form of pharmaceutical compositions.
- a composition comprising a compound of Formula (I) or any of the formulas as described herein, a compound as recited in any of the claims and described herein, or a pharmaceutically acceptable salt thereof, or any of the embodiments thereof, and at least one pharmaceutically acceptable carrier or excipient.
- These compositions can be prepared in a manner well known in the pharmaceutical art, and can be administered by a variety of routes, depending upon whether local or systemic treatment is indicated and upon the area to be treated.
- Administration may be topical (including transdermal, epidermal, ophthalmic and to mucous membranes including intranasal, vaginal and rectal delivery), pulmonary (e.g. , by inhalation or insufflation of powders or aerosols, including by nebulizer; intratracheal or intranasal), oral or parenteral.
- Parenteral administration includes intravenous, intraarterial, subcutaneous, intraperitoneal intramuscular or injection or infusion; or intracranial, e.g. , intrathecal or intraventricular, administration.
- Parenteral administration can be in the form of a single bolus dose, or may be, e.g. , by a continuous perfusion pump.
- Pharmaceutical compositions and formulations for topical administration may include transdermal patches, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders.
- compositions which contain, as the active ingredient, the compound of the present disclosure or a pharmaceutically acceptable salt thereof, in combination with one or more pharmaceutically acceptable carriers or excipients.
- the composition is suitable for topical administration.
- the active ingredient is typically mixed with an excipient, diluted by an excipient or enclosed within such a carrier in the form of, e.g. , a capsule, sachet, paper, or other container.
- the excipient when it serves as a diluent, it can be a solid, semi-solid, or liquid material, which acts as a vehicle, carrier or medium for the active ingredient.
- the compositions can be in the form of tablets, pills, powders, lozenges, sachets, cachets, elixirs, suspensions, emulsions, solutions, syrups, aerosols (as a solid or in a liquid medium), ointments containing, e.g. , up to 10% by weight of the active compound, soft and hard gelatin capsules, suppositories, sterile injectable solutions and sterile packaged powders.
- the active compound can be milled to provide the appropriate particle size prior to combining with the other ingredients. If the active compound is substantially insoluble, it can be milled to a particle size of less than 200 mesh. If the active compound is substantially water soluble, the particle size can be adjusted by milling to provide a substantially uniform distribution in the formulation, e.g., about 40 mesh.
- the compounds of the invention may be milled using known milling procedures such as wet milling to obtain a particle size appropriate for tablet formation and for other formulation types.
- Finely divided (nanoparticulate) preparations of the compounds of the invention can be prepared by processes known in the art see, e.g., WO 2002/000196.
- excipients include lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia, calcium phosphate, alginates, tragacanth, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, water, syrup and methyl cellulose.
- the formulations can additionally include: lubricating agents such as talc, magnesium stearate and mineral oil; wetting agents; emulsifying and suspending agents; preserving agents such as methyl- and propylhydroxy-benzoates; sweetening agents; and flavoring agents.
- the compositions of the invention can be formulated so as to provide quick, sustained or delayed release of the active ingredient after administration to the patient by employing procedures known in the art.
- the pharmaceutical composition comprises silicified microcrystalline cellulose (SMCC) and at least one compound described herein, or a pharmaceutically acceptable salt thereof.
- SMCC silicified microcrystalline cellulose
- the silicified microcrystalline cellulose comprises about 98% microcrystalline cellulose and about 2% silicon dioxide w/w.
- the composition is a sustained release composition comprising at least one compound described herein, or a pharmaceutically acceptable salt thereof, and at least one pharmaceutically acceptable carrier or excipient.
- the composition comprises at least one compound described herein, or a pharmaceutically acceptable salt thereof, and at least one component selected from microcrystalline cellulose, lactose monohydrate, hydroxypropyl methylcellulose and polyethylene oxide.
- the composition comprises at least one compound described herein, or a pharmaceutically acceptable salt thereof, and microcrystalline cellulose, lactose monohydrate and hydroxypropyl methylcellulose.
- the composition comprises at least one compound described herein, or a pharmaceutically acceptable salt thereof, and microcrystalline cellulose, lactose monohydrate and polyethylene oxide.
- the composition further comprises magnesium stearate or silicon dioxide.
- the microcrystalline cellulose is Avicel PH102TM.
- the lactose monohydrate is Fast-flo 316TM.
- the hydroxypropyl methylcellulose is hydroxypropyl methylcellulose 2208 K4M (e.g. , Methocel K4 M
- the polyethylene oxide is polyethylene oxide WSR 1105 (e.g. , Poly ox WSR 1105TM).
- a wet granulation process is used to produce the composition. In some embodiments, a dry granulation process is used to produce the composition.
- compositions can be formulated in a unit dosage form, each dosage containing from about 5 to about 1,000 mg (1 g), more usually about 100 mg to about 500 mg, of the active ingredient. In some embodiments, each dosage contains about 10 mg of the active ingredient. In some embodiments, each dosage contains about 50 mg of the active ingredient. In some embodiments, each dosage contains about 25 mg of the active ingredient.
- unit dosage forms refers to physically discrete units suitable as unitary dosages for human subjects and other mammals, each unit containing a predetermined quantity of active material calculated to produce the desired therapeutic effect, in association with a suitable
- the components used to formulate the pharmaceutical compositions are of high purity and are substantially free of potentially harmful contaminants (e.g., at least National Food grade, generally at least analytical grade, and more typically at least pharmaceutical grade).
- the composition is preferably manufactured or formulated under Good Manufacturing Practice standards as defined in the applicable regulations of the U.S. Food and Drug Administration.
- suitable formulations may be sterile and/or substantially isotonic and/or in full compliance with all Good
- the active compound may be effective over a wide dosage range and is generally administered in a therapeutically effective amount. It will be understood, however, that the amount of the compound actually administered will usually be determined by a physician, according to the relevant circumstances, including the condition to be treated, the chosen route of administration, the actual compound administered, the age, weight, and response of the individual patient, the severity of the patient's symptoms and the like.
- the therapeutic dosage of a compound of the present invention can vary according to, e.g., the particular use for which the treatment is made, the manner of administration of the compound, the health and condition of the patient, and the judgment of the prescribing physician.
- the compounds of the invention can be provided in an aqueous physiological buffer solution containing about 0.1 to about 10% w/v of the compound for parenteral administration.
- Some typical dose ranges are from about 1 ⁇ g/kg to about 1 g/kg of body weight per day. In some embodiments, the dose range is from about 0.01 mg/kg to about 100 mg/kg of body weight per day.
- the dosage is likely to depend on such variables as the type and extent of progression of the disease or disorder, the overall health status of the particular patient, the relative biological efficacy of the compound selected, formulation of the excipient, and its route of administration. Effective doses can be extrapolated from dose-response curves derived from in vitro or animal model test systems.
- the principal active ingredient is mixed with a pharmaceutical excipient to form a solid preformulation composition containing a homogeneous mixture of a compound of the present invention.
- a solid preformulation composition containing a homogeneous mixture of a compound of the present invention.
- the active ingredient is typically dispersed evenly throughout the composition so that the composition can be readily subdivided into equally effective unit dosage forms such as tablets, pills and capsules.
- This solid preformulation is then subdivided into unit dosage forms of the type described above containing from, e.g., about 0.1 to about 1000 mg of the active ingredient of the present invention.
- the tablets or pills of the present invention can be coated or otherwise compounded to provide a dosage form affording the advantage of prolonged action.
- the tablet or pill can comprise an inner dosage and an outer dosage component, the latter being in the form of an envelope over the former.
- the two components can be separated by an enteric layer which serves to resist disintegration in the stomach and permit the inner component to pass intact into the duodenum or to be delayed in release.
- enteric layers or coatings such materials including a number of polymeric acids and mixtures of polymeric acids with such materials as shellac, cetyl alcohol and cellulose acetate.
- liquid forms in which the compounds and compositions of the present invention can be incorporated for administration orally or by injection include aqueous solutions, suitably flavored syrups, aqueous or oil suspensions, and flavored emulsions with edible oils such as cottonseed oil, sesame oil, coconut oil, or peanut oil, as well as elixirs and similar pharmaceutical vehicles.
- compositions for inhalation or insufflation include solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders.
- the liquid or solid compositions may contain suitable pharmaceutically acceptable excipients as described supra.
- the compositions are administered by the oral or nasal respiratory route for local or systemic effect.
- Compositions can be nebulized by use of inert gases. Nebulized solutions may be breathed directly from the nebulizing device or the nebulizing device can be attached to a face mask, tent, or intermittent positive pressure breathing machine. Solution, suspension, or powder compositions can be administered orally or nasally from devices which deliver the formulation in an appropriate manner.
- Topical formulations can contain one or more conventional carriers.
- ointments can contain water and one or more hydrophobic carriers selected from, e.g., liquid paraffin, poly oxy ethylene alkyl ether, propylene glycol, white Vaseline, and the like.
- Carrier compositions of creams can be based on water in combination with glycerol and one or more other components, e.g., glycerinemonostearate, PEG-glycerinemonostearate and cetylstearyl alcohol.
- Gels can be formulated using isopropyl alcohol and water, suitably in combination with other components such as, e.g., glycerol, hydroxy ethyl cellulose, and the like.
- topical formulations contain at least about 0.1, at least about 0.25, at least about 0.5, at least about 1, at least about 2 or at least about 5 wt % of the compound of the invention.
- the topical formulations can be suitably packaged in tubes of, e.g., 100 g which are optionally associated with instructions for the treatment of the select indication, e.g. , psoriasis or other skin condition.
- compositions can be administered to a patient already suffering from a disease in an amount sufficient to cure or at least partially arrest the symptoms of the disease and its complications. Effective doses will depend on the disease condition being treated as well as by the judgment of the attending clinician depending upon factors such as the severity of the disease, the age, weight and general condition of the patient and the like.
- compositions administered to a patient can be in the form of pharmaceutical compositions described above. These compositions can be sterilized by conventional sterilization techniques, or may be sterile filtered. Aqueous solutions can be packaged for use as is, or lyophilized, the lyophilized preparation being combined with a sterile aqueous carrier prior to administration.
- the pH of the compound preparations typically will be between 3 and 11, more preferably from 5 to 9 and most preferably from 7 to 8. It will be understood that use of certain of the foregoing excipients, carriers or stabilizers will result in the formation of pharmaceutical salts.
- the therapeutic dosage of a compound of the present invention can vary according to, e.g., the particular use for which the treatment is made, the manner of administration of the compound, the health and condition of the patient, and the judgment of the prescribing physician.
- the compounds of the invention can be provided in an aqueous physiological buffer solution containing about 0.1 to about 10% w/v of the compound for parenteral administration.
- Some typical dose ranges are from about 1 ⁇ g/kg to about 1 g/kg of body weight per day. In some embodiments, the dose range is from about 0.01 mg/kg to about 100 mg/kg of body weight per day.
- the dosage is likely to depend on such variables as the type and extent of progression of the disease or disorder, the overall health status of the particular patient, the relative biological efficacy of the compound selected, formulation of the excipient, and its route of administration. Effective doses can be extrapolated from dose-response curves derived from in vitro or animal model test systems.
- the compounds of the present disclosure can further be useful in investigations of biological processes in normal and abnormal tissues.
- another aspect of the present invention relates to labeled compounds of the invention (radio-labeled, fluorescent-labeled, etc.) that would be useful not only in imaging techniques but also in assays, both in vitro and in vivo, for localizing and quantitating PD-1 or PD-Ll protein in tissue samples, including human, and for identifying PD-Ll ligands by inhibition binding of a labeled compound.
- the present invention includes PD-1/PD-L1 binding assays that contain such labeled compounds.
- the present invention further includes isotopically-substituted compounds of the disclosure.
- An “isotopically-substituted” compound is a compound of the invention where one or more atoms are replaced or substituted by an atom having an atomic mass or mass number different from the atomic mass or mass number typically found in nature (i.e. , naturally occurring). It is to be understood that a “radio-labeled” is a compound that has incorporated at least one isotope that is radioactive (e.g., radionuclide). Suitable
- radionuclides that may be incorporated in compounds of the present invention include but are not limited to 3 ⁇ 4 (also written as T for tritium), n C, 1 C, 14 C, 1 N, 15 N, 15 0, 17 0, 18 0, 18 F, 35 S, 6C1, 82 Br, 75 Br, 76 Br, 77 Br, 12 I, 124 I, 125 I and 1 l l.
- the radionuclide that is incorporated in the instant radio-labeled compounds will depend on the specific application of that radio-labeled compound. For example, for in vitro PD-Ll protein labeling and competition assays, compounds that incorporate H, 14 C, 82 Br, 125 I, 1 1 1, 5 S or will generally be most useful.
- n C, 18 F, 125 I, 12 I, 124 I, 1 X I, 75 Br, 76 Br or 77 Br will generally be most useful.
- the radionuclide is selected from the group consisting of H, 14 C, 125 1, 5 S and 82 Br. Synthetic methods for incorporating radio-isotopes into organic compounds are known in the art.
- a labeled compound of the invention can be used in a screening assay to identify and/or evaluate compounds.
- a newly synthesized or identified compound i.e. , test compound
- a test compound which is labeled can be evaluated for its ability to bind a PD- Ll protein by monitoring its concentration variation when contacting with the PD-Ll protein, through tracking of the labeling.
- a test compound (labeled) can be evaluated for its ability to reduce binding of another compound which is known to bind to a PD-Ll protein (i.e., standard compound). Accordingly, the ability of a test compound to compete with the standard compound for binding to the PD-L1 protein directly correlates to its binding affinity.
- the standard compound is labeled and test compounds are unlabeled. Accordingly, the concentration of the labeled standard compound is monitored in order to evaluate the competition between the standard compound and the test compound, and the relative binding affinity of the test compound is thus ascertained.
- kits useful useful, e.g., in the treatment or prevention of diseases or disorders associated with the activity of PD-L1 including its interaction with other proteins such as PD-1 and B7-1 (CD80), such as cancer or infections, which include one or more containers containing a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula (I), or any of the embodiments thereof.
- kits can further include one or more of various conventional pharmaceutical kit components, such as, e.g. , containers with one or more pharmaceutically acceptable carriers, additional containers, etc., as will be readily apparent to those skilled in the art.
- Instructions, either as inserts or as labels, indicating quantities of the components to be administered, guidelines for administration, and/or guidelines for mixing the components, can also be included in the kit.
- Step 2 tert-butyl 2-( ⁇ [2-cyano-3-(2, 3-dihydro-l, 4-benzodioxin-6- yl)phenyl]amino ⁇ carbonyl)- -dihydro[ 1, 3] thiazolo [5, 4-c]pyridine-5(4H)-carboxylate
- Step 1 tert-butyl 2- ⁇ [(3-bromo-2-cyanophenyl)amino]carbonyl ⁇ -6, 7- dihydrof 1, 3 ] thiazolo [ 5, 4-c ]pyridine-5( 4H)-carboxylate
- Step 3 N-(2-cyanobiphenyl-3-yl)-4, 5, 6, 7-tetrahydro[ 1, 3 Jthiazolof 5, 4-c ]pyridine-2- carboxamide
- This compound was prepared using similar procedures as described for Example 1 with 5-(tert-butoxycarbonyl)-4,5,6,7-tetrahydropyrazolo[l,5-a]pyrazine-2-carboxylic acid (AstaTech, Cat#: 74720) replacing 5-(tert-butoxycarbonyl)-4,5,6,7- tetrahydro[l,3]thiazolo[5,4-c]pyridine-2-carboxylic acid in Step 2.
- Step 1 tert-butyl l-methyl-l, 4, 6, 7-tetrahydro-5H-imidazo[4,5-c]pyridine-5-carboxylate
- Step 3 tert-butyl 2-( ⁇ [2-cyano-3-(2, 3-dihydro-l, 4-benzodioxin-6- yl)phenyl]amino ⁇ carbonyl)-l-methyl-l, 4, 6, 7-tetrahydro-5H-imidazo[4, 5-c]pyridine-5- carboxylate
- Step 4 N-[2-cyano-3-(2, 3-dihydro-l, 4-benzodioxin-6-yl)phenyl]-l-methyl-4, 5, 6, 7- tetrahydro-lH-imidazo[ 4, 5-c Jpyridine-2-carboxamide
- Step 1 3-(2, 3-dihydrobenzo [b ][ l,4]dioxin-6- l)-2-methylaniline
- Step 2 tert-butyl 2-(3-(2, 3-dihydrobenzo[b] [l, 4]dioxin-6-yl)-2-methylphenylcarbamoyl)-6, 7- dihydrothiazolof 5,
- Step 3 N-[3-(2, 3-dihydro-l,4-benzodioxin-6-yl)-2-methylphenyl]-4,5, 6, 7- tetrahydrof 1, 3 ] thiazolo [ 5, 4-c ]pyridine-2-carboxamide
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and (2- fluoro-3-methoxyphenyl)boronic acid replacing phenylboronic acid in Step 3.
- LC-MS calculated for C21H21FN3O2S (M+H) + : m/z 398.1; found 398.2.
- This compound was prepared using similar procedures as described for Example 12, starting with 2'-fluoro-3'-methoxy-2-methylbiphenyl-3-amine, prepared using similar procedures for the synthesis of 2-amino-6-(2,3-dihydro-l,4-benzodioxin-6-yl)benzonitrile in Example 1, Step 1.
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and (1- methyl- lH-indazol-4-yl)boronic acid ⁇ Combi-Blocks; cat#BB-9017) replacing phenylboronic acid in Step 3.
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and [2- fluoro-3-(hydroxymethyl)phenyl]boronic acid ⁇ Combi-Blocks, Cat#: BB-6579) replacing phenylboronic acid in Step 3.
- LC-MS calculated for C21H21FN3O2S (M+H) + : m/z 398.1; found 398.2.
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and indazole-4-boronic acid hydrochloride ⁇ Aldrich, Cat#: 709379) replacing phenylboronic acid in Step 3.
- LC-MS calculated for C21H20N5OS (M+H) + : m/z 390.1; found 390.2.
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and 2- methyl-2H-indazol-6-ylboronic acid pinacol ester ⁇ Combi-Blocks, Cat#:PN-9131) replacing phenylboronic acid in Step 3.
- LC-MS calculated for C22H22N5OS (M+H) + : m/z 404.2; found 404.2.
- This compound was prepared using similar procedures as described for Example 2 with 3-bromo-2-methylaniline replacing 2-amino-6-bromobenzonitrile in Step 1, and 2- (cyanomethyl)phenylboronic acid ⁇ Combi-Blocks, Cat#:BB-2136) replacing phenylboronic acid in Step 3.
- Step 1 tert-butyl 2-(3-bromo-2-chlorophenylcarbamoyl)-6, 7-dihydrothiazolo[5,4-c]pyridine- 5( 4H)-carboxylate
- Step 2 N-( 3-bromo-2-chlorophenyl)-4, 5, 6, 7-tetrahydrothiazolo[ 5, 4-c ]pyridine-2- carboxamide
- Ci3Hi 2 BrClN 3 OS (M+H) + : m/z 372.0; found 372.0.
- Step 3 N-(2-chloro-2'-fluoro-3'-methoxybiphenyl-3-yl)-4, 5, 6, 7-tetrahydro[l, 3]thiazolo[5, 4- c ]pyridine-2-carboxamide
- Example 1 This compound was prepared using similar procedures as described for Example 12, Step 3 to 4, starting with 5-tert-butyl 2-methyl l-methyl-6,7-dihydro-lH-imidazo[4,5- c]pyridine-2,5(4H)-dicarboxylate from Example 12, Step 2 and 2-amino-6-(l-methyl-lH- indazol-4-yl)benzonitrile, prepared using similar procedures for the synthesis of 2-amino-6- (2,3-dihydro-l,4-benzodioxin-6-yl)benzonitrile in.
- the assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 ⁇ . Inhibitors were first serially diluted in DMSO and then added to the plate wells before the addition of other reaction components. The final concentration of DMSO in the assay was 1%. The assays were carried out at 25° C in the PBS buffer (pH 7.4) with 0.05% Tween-20 and 0.1% BSA.
- Recombinant human PD-L1 protein (19-238) with a His- tag at the C-terminus was purchased from AcroBiosy stems (PD1-H5229).
- Recombinant human PD-1 protein (25-167) with Fc tag at the C-terminus was also purchased from
- PD-L1 and PD-1 proteins were diluted in the assay buffer and ⁇ 0 ⁇ . was added to the plate well. Plates were centrifuged and proteins were
- Example 1 Data obtained for the Example compounds using the PD-l/PD-Ll homogenous time- resolved fluorescence (HTRF) binding assay described in Example A is provided in Table 1.
- Table 1 Data obtained for the Example compounds using the PD-l/PD-Ll homogenous time- resolved fluorescence (HTRF) binding assay described in Example A is provided in Table 1.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Virology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Plural Heterocyclic Compounds (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
Description
Claims
Priority Applications (30)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| NZ749960A NZ749960B2 (en) | 2017-06-19 | Heterocyclic compounds as immunomodulators | |
| UAA201900525A UA125391C2 (en) | 2016-06-20 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| SM20220392T SMT202200392T1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| DK17734589.9T DK3472167T3 (en) | 2016-06-20 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| CR20190028A CR20190028A (en) | 2016-06-19 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| EA201990074A EA201990074A1 (en) | 2016-09-19 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| MX2018016273A MX389513B (en) | 2016-06-20 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS. |
| MA45392A MA45392B1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds used as immunomodulators |
| EP22184212.3A EP4137489A1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| MYPI2018002678A MY197280A (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| SI201731245T SI3472167T1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| SG11201811414TA SG11201811414TA (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| CA3028685A CA3028685A1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| KR1020197001807A KR102685249B1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic Compounds as Immunomodulators |
| BR112018076534-1A BR112018076534A2 (en) | 2016-06-20 | 2017-06-19 | heterocyclic compounds as immunomodulators |
| CN201780049752.9A CN109890819B (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| EP17734589.9A EP3472167B1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| IL263825A IL263825B (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| LTEPPCT/US2017/038120T LT3472167T (en) | 2016-06-20 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| HRP20221030TT HRP20221030T1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| ES17734589T ES2927984T3 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| JP2018566514A JP7000357B2 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| PL17734589.9T PL3472167T3 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| MDE20190506T MD3472167T2 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| RS20220907A RS63673B1 (en) | 2016-06-20 | 2017-06-19 | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| AU2017281285A AU2017281285C1 (en) | 2016-06-20 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
| PH12018502710A PH12018502710A1 (en) | 2016-06-20 | 2018-12-20 | Heterocyclic compounds as immunomodulators |
| CONC2019/0000386A CO2019000386A2 (en) | 2016-06-20 | 2019-01-15 | Heterocyclic compounds as immunomodulators |
| AU2021250978A AU2021250978B2 (en) | 2016-06-20 | 2021-10-15 | Heterocyclic compounds as immunomodulators |
| JP2021209731A JP7394824B2 (en) | 2016-06-20 | 2021-12-23 | Heterocyclic compounds as immunomodulators |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662352485P | 2016-06-20 | 2016-06-20 | |
| US62/352,485 | 2016-06-20 | ||
| US201662396353P | 2016-09-19 | 2016-09-19 | |
| US62/396,353 | 2016-09-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017222976A1 true WO2017222976A1 (en) | 2017-12-28 |
Family
ID=59258383
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2017/038120 Ceased WO2017222976A1 (en) | 2016-06-19 | 2017-06-19 | Heterocyclic compounds as immunomodulators |
Country Status (32)
| Country | Link |
|---|---|
| US (4) | US20170362253A1 (en) |
| EP (2) | EP3472167B1 (en) |
| JP (2) | JP7000357B2 (en) |
| KR (1) | KR102685249B1 (en) |
| CN (1) | CN109890819B (en) |
| AU (2) | AU2017281285C1 (en) |
| BR (1) | BR112018076534A2 (en) |
| CA (1) | CA3028685A1 (en) |
| CL (1) | CL2018003701A1 (en) |
| CO (1) | CO2019000386A2 (en) |
| DK (1) | DK3472167T3 (en) |
| EC (1) | ECSP19003773A (en) |
| ES (1) | ES2927984T3 (en) |
| HR (1) | HRP20221030T1 (en) |
| HU (1) | HUE060256T2 (en) |
| IL (1) | IL263825B (en) |
| LT (1) | LT3472167T (en) |
| MD (1) | MD3472167T2 (en) |
| MX (2) | MX389513B (en) |
| MY (1) | MY197280A (en) |
| PE (1) | PE20190731A1 (en) |
| PH (1) | PH12018502710A1 (en) |
| PL (1) | PL3472167T3 (en) |
| PT (1) | PT3472167T (en) |
| RS (1) | RS63673B1 (en) |
| SG (2) | SG10202012828TA (en) |
| SI (1) | SI3472167T1 (en) |
| SM (1) | SMT202200392T1 (en) |
| TW (1) | TWI771305B (en) |
| UA (1) | UA125391C2 (en) |
| WO (1) | WO2017222976A1 (en) |
| ZA (1) | ZA202405294B (en) |
Cited By (92)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018119224A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Tetrahydro imidazo[4,5-c]pyridine derivatives as pd-l1 internalization inducers |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019032547A1 (en) | 2017-08-08 | 2019-02-14 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| US10308644B2 (en) | 2016-12-22 | 2019-06-04 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2019149183A1 (en) * | 2018-02-05 | 2019-08-08 | 上海和誉生物医药科技有限公司 | Biaryl derivative, preparation method thereof and pharmaceutical application thereof |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| WO2019165043A2 (en) | 2018-02-22 | 2019-08-29 | Chemocentryx, Inc. | Indane-amines as pd-l1 antagonists |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2019217821A1 (en) * | 2018-05-11 | 2019-11-14 | Incyte Corporation | Tetrahydro-imidazo[4,5-c]pyridine derivatives as pd-l1 immunomodulators |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020088357A1 (en) | 2018-11-02 | 2020-05-07 | 上海再极医药科技有限公司 | Diphenyl-like compound, intermediate thereof, preparation method therefor, pharmaceutical composition thereof and uses thereof |
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| US10669271B2 (en) | 2018-03-30 | 2020-06-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2020156564A1 (en) * | 2019-02-02 | 2020-08-06 | 南京明德新药研发有限公司 | Vinylpyridine carboxamide compound as pd-l1 immunomodulator |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| US10793565B2 (en) | 2016-12-22 | 2020-10-06 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| WO2021007386A1 (en) | 2019-07-10 | 2021-01-14 | Chemocentryx, Inc. | Indanes as pd-l1 inhibitors |
| WO2021011891A1 (en) | 2019-07-18 | 2021-01-21 | Gilead Sciences, Inc. | Long-acting formulations of tenofovir alafenamide |
| US10919852B2 (en) | 2017-07-28 | 2021-02-16 | Chemocentryx, Inc. | Immunomodulator compounds |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021076691A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of pd-l1 diseases |
| WO2021076688A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of pd-l1 diseases |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2021136354A1 (en) | 2020-01-03 | 2021-07-08 | 上海翰森生物医药科技有限公司 | Biphenyl derivative inhibitor, preparation method therefor and use thereof |
| WO2021138512A1 (en) | 2020-01-03 | 2021-07-08 | Incyte Corporation | Combination therapy comprising a2a/a2b and pd-1/pd-l1 inhibitors |
| WO2021147940A1 (en) * | 2020-01-21 | 2021-07-29 | 上海华汇拓医药科技有限公司 | Pd-1/pd-l1 inhibitor, preparation method therefor, and use thereof |
| JP2021520342A (en) * | 2018-04-03 | 2021-08-19 | ベータ ファーマシューティカルズ カンパニー リミテッド | Immunomodulators, compositions and methods thereof |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| WO2021226206A2 (en) | 2020-05-05 | 2021-11-11 | Teon Therapeutics, Inc. | Cannabinoid receptor type 2 (cb2) modulators and uses thereof |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US11266643B2 (en) | 2019-05-15 | 2022-03-08 | Chemocentryx, Inc. | Triaryl compounds for treatment of PD-L1 diseases |
| WO2022052926A1 (en) | 2020-09-09 | 2022-03-17 | 广州再极医药科技有限公司 | Aromatic ethylene compound and preparation method therefor, and intermediate, pharmaceutical composition, and application thereof |
| RU2768830C9 (en) * | 2018-02-05 | 2022-06-17 | Aббиско Тхерaпеутицс Цо., Лтд. | Biaryl derivative, method for production thereof and pharmaceutical application thereof |
| WO2022147092A1 (en) | 2020-12-29 | 2022-07-07 | Incyte Corporation | Combination therapy comprising a2a/a2b inhibitors, pd-1/pd-l1 inhibitors, and anti-cd73 antibodies |
| US11401279B2 (en) | 2019-09-30 | 2022-08-02 | Incyte Corporation | Pyrido[3,2-d]pyrimidine compounds as immunomodulators |
| US11407749B2 (en) | 2015-10-19 | 2022-08-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11426364B2 (en) | 2016-06-27 | 2022-08-30 | Chemocentryx, Inc. | Immunomodulator compounds |
| US11465981B2 (en) | 2016-12-22 | 2022-10-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11485708B2 (en) | 2019-06-20 | 2022-11-01 | Chemocentryx, Inc. | Compounds for treatment of PD-L1 diseases |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| WO2022261301A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-cancer agents |
| WO2022261310A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-body drug conjugates |
| US11535615B2 (en) | 2015-12-22 | 2022-12-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| US11572366B2 (en) | 2015-11-19 | 2023-02-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2023034530A1 (en) | 2021-09-02 | 2023-03-09 | Teon Therapeutics, Inc. | Methods of improving growth and function of immune cells |
| US11608337B2 (en) | 2016-05-06 | 2023-03-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11613536B2 (en) | 2016-08-29 | 2023-03-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2023081730A1 (en) | 2021-11-03 | 2023-05-11 | Teon Therapeutics, Inc. | 4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide derivatives as cannabinoid cb2 receptor modulators for the treatment of cancer |
| WO2023097211A1 (en) | 2021-11-24 | 2023-06-01 | The University Of Southern California | Methods for enhancing immune checkpoint inhibitor therapy |
| US11673894B2 (en) | 2018-02-27 | 2023-06-13 | Incyte Corporation | Imidazopyrimidines and triazolopyrimidines as A2A / A2B inhibitors |
| US11673883B2 (en) | 2016-05-26 | 2023-06-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11718605B2 (en) | 2016-07-14 | 2023-08-08 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11753406B2 (en) | 2019-08-09 | 2023-09-12 | Incyte Corporation | Salts of a PD-1/PD-L1 inhibitor |
| US11760756B2 (en) | 2020-11-06 | 2023-09-19 | Incyte Corporation | Crystalline form of a PD-1/PD-L1 inhibitor |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| US11866434B2 (en) | 2020-11-06 | 2024-01-09 | Incyte Corporation | Process for making a PD-1/PD-L1 inhibitor and salts and crystalline forms thereof |
| US11866451B2 (en) | 2019-11-11 | 2024-01-09 | Incyte Corporation | Salts and crystalline forms of a PD-1/PD-L1 inhibitor |
| US11873309B2 (en) | 2016-06-20 | 2024-01-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11873304B2 (en) | 2018-05-18 | 2024-01-16 | Incyte Corporation | Fused pyrimidine derivatives as A2A/A2B inhibitors |
| WO2024015372A1 (en) | 2022-07-14 | 2024-01-18 | Teon Therapeutics, Inc. | Adenosine receptor antagonists and uses thereof |
| US11884665B2 (en) | 2019-01-29 | 2024-01-30 | Incyte Corporation | Pyrazolopyridines and triazolopyridines as A2A / A2B inhibitors |
| US11999740B2 (en) | 2018-07-05 | 2024-06-04 | Incyte Corporation | Fused pyrazine derivatives as A2A / A2B inhibitors |
| WO2025080593A1 (en) | 2023-10-09 | 2025-04-17 | Incyte Corporation | Combination therapy using a kras g12d inhibitor and pd-1 inhibitor or pd-l1 inhibitor |
| WO2025122695A1 (en) | 2023-12-06 | 2025-06-12 | Incyte Corporation | Combination therapy comprising dgk inhibitors and pd-1/pd-l1 inhibitors |
| US12466822B2 (en) | 2016-12-22 | 2025-11-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2025240243A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with bulevirtide and an inhibitory nucleic acid targeting hepatitis b virus |
| WO2025240244A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies comprising bulevirtide and lonafarnib for use in the treatment of hepatitis d virus infection |
| WO2025240246A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
| WO2025240242A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
Families Citing this family (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012088266A2 (en) | 2010-12-22 | 2012-06-28 | Incyte Corporation | Substituted imidazopyridazines and benzimidazoles as inhibitors of fgfr3 |
| HUE042374T2 (en) | 2012-06-13 | 2019-06-28 | Incyte Holdings Corp | Substituted tricyclic compounds as fgfr inhibitors |
| KR102469849B1 (en) | 2013-04-19 | 2022-11-23 | 인사이트 홀딩스 코포레이션 | Bicyclic heterocycles as fgfr inhibitors |
| US10851105B2 (en) | 2014-10-22 | 2020-12-01 | Incyte Corporation | Bicyclic heterocycles as FGFR4 inhibitors |
| AU2016219822B2 (en) | 2015-02-20 | 2020-07-09 | Incyte Holdings Corporation | Bicyclic heterocycles as FGFR inhibitors |
| MA41551A (en) | 2015-02-20 | 2017-12-26 | Incyte Corp | BICYCLIC HETEROCYCLES USED AS FGFR4 INHIBITORS |
| WO2018124766A2 (en) | 2016-12-28 | 2018-07-05 | 주식회사 녹십자랩셀 | Chimeric antigen receptor and natural killer cells expressing same |
| US11130740B2 (en) | 2017-04-25 | 2021-09-28 | Arbutus Biopharma Corporation | Substituted 2,3-dihydro-1H-indene analogs and methods using same |
| AR111960A1 (en) | 2017-05-26 | 2019-09-04 | Incyte Corp | CRYSTALLINE FORMS OF A FGFR INHIBITOR AND PROCESSES FOR ITS PREPARATION |
| US11649294B2 (en) | 2017-11-14 | 2023-05-16 | GC Cell Corporation | Anti-HER2 antibody or antigen-binding fragment thereof, and chimeric antigen receptor comprising same |
| CA3082328A1 (en) | 2017-11-14 | 2019-05-23 | Green Cross Lab Cell Corporation | Anti-her2 antibody or antigen-binding fragment thereof, and chimeric antigen receptor comprising same |
| US12083118B2 (en) | 2018-03-29 | 2024-09-10 | Arbutus Biopharma Corporation | Substituted 1,1′-biphenyl compounds, analogues thereof, and methods using same |
| EP4309737A3 (en) | 2018-05-04 | 2024-03-27 | Incyte Corporation | Solid forms of an fgfr inhibitor and processes for preparing the same |
| EP3788046B1 (en) | 2018-05-04 | 2025-12-10 | Incyte Corporation | Salts of an fgfr inhibitor |
| EP3810109B1 (en) | 2018-05-31 | 2024-08-07 | Peloton Therapeutics, Inc. | Compounds and compositions for inhibiting cd73 |
| SG11202101632XA (en) | 2018-09-10 | 2021-03-30 | Kaken Pharmaceutical Co Ltd | Novel heteroaromatic amide derivative and medicine containing same |
| WO2020068729A1 (en) | 2018-09-25 | 2020-04-02 | Incyte Corporation | Pyrazolo[4,3-d]pyrimidine compounds as alk2 abd/or fgfr modulators |
| US12331320B2 (en) | 2018-10-10 | 2025-06-17 | The Research Foundation For The State University Of New York | Genome edited cancer cell vaccines |
| US11066404B2 (en) | 2018-10-11 | 2021-07-20 | Incyte Corporation | Dihydropyrido[2,3-d]pyrimidinone compounds as CDK2 inhibitors |
| CR20210336A (en) | 2018-12-20 | 2021-12-06 | Incyte Corp | IMIDAZOPYRIDAZINE AND IMIDAZOPYRIDINE COMPOUNDS AS ACTIVIN RECEPTOR KINASE-2 INHIBITORS |
| JP2022519772A (en) | 2019-02-15 | 2022-03-24 | インサイト・コーポレイション | Cyclin-dependent kinase 2 biomarker and its use |
| WO2020168197A1 (en) | 2019-02-15 | 2020-08-20 | Incyte Corporation | Pyrrolo[2,3-d]pyrimidinone compounds as cdk2 inhibitors |
| US11472791B2 (en) | 2019-03-05 | 2022-10-18 | Incyte Corporation | Pyrazolyl pyrimidinylamine compounds as CDK2 inhibitors |
| US11628162B2 (en) | 2019-03-08 | 2023-04-18 | Incyte Corporation | Methods of treating cancer with an FGFR inhibitor |
| WO2020205560A1 (en) | 2019-03-29 | 2020-10-08 | Incyte Corporation | Sulfonylamide compounds as cdk2 inhibitors |
| US11440914B2 (en) | 2019-05-01 | 2022-09-13 | Incyte Corporation | Tricyclic amine compounds as CDK2 inhibitors |
| US11447494B2 (en) | 2019-05-01 | 2022-09-20 | Incyte Corporation | Tricyclic amine compounds as CDK2 inhibitors |
| US11591329B2 (en) | 2019-07-09 | 2023-02-28 | Incyte Corporation | Bicyclic heterocycles as FGFR inhibitors |
| BR112022002698A2 (en) | 2019-08-14 | 2022-07-19 | Incyte Corp | IMIDAZOLYL PYRIMIDYNYLAMINE COMPOUNDS AS CDK2 INHIBITORS |
| US12122767B2 (en) | 2019-10-01 | 2024-10-22 | Incyte Corporation | Bicyclic heterocycles as FGFR inhibitors |
| AU2020364007A1 (en) | 2019-10-11 | 2022-04-28 | Incyte Corporation | Bicyclic amines as CDK2 inhibitors |
| WO2021076602A1 (en) | 2019-10-14 | 2021-04-22 | Incyte Corporation | Bicyclic heterocycles as fgfr inhibitors |
| WO2021076728A1 (en) | 2019-10-16 | 2021-04-22 | Incyte Corporation | Bicyclic heterocycles as fgfr inhibitors |
| WO2021093817A1 (en) * | 2019-11-15 | 2021-05-20 | 杭州和正医药有限公司 | Immunomodulatory compounds, composition and application thereof |
| KR20220131900A (en) | 2019-12-04 | 2022-09-29 | 인사이트 코포레이션 | Derivatives of FGFR inhibitors |
| JP7720840B2 (en) | 2019-12-04 | 2025-08-08 | インサイト・コーポレイション | Tricyclic heterocycles as FGFR inhibitors |
| CN110950886A (en) * | 2019-12-13 | 2020-04-03 | 苏州莱克施德药业有限公司 | Method for synthesizing 1-methyl-imidazole-2-methyl formate derivative |
| WO2021139647A1 (en) * | 2020-01-07 | 2021-07-15 | 上海和誉生物医药科技有限公司 | Biphenyl fluorine double bond derivative, preparation method therefor, and pharmaceutical application thereof |
| IL294526B1 (en) | 2020-01-10 | 2025-10-01 | Incyte Corp | Tricyclic compounds as inhibitors of kras |
| US12012409B2 (en) | 2020-01-15 | 2024-06-18 | Incyte Corporation | Bicyclic heterocycles as FGFR inhibitors |
| US11530218B2 (en) | 2020-01-20 | 2022-12-20 | Incyte Corporation | Spiro compounds as inhibitors of KRAS |
| TW202140487A (en) | 2020-02-06 | 2021-11-01 | 美商英塞特公司 | Salts and solid forms and processes of preparing a pi3k inhibitor |
| JP2023516441A (en) | 2020-03-06 | 2023-04-19 | インサイト・コーポレイション | Combination therapy comprising an AXL/MER inhibitor and a PD-1/PD-L1 inhibitor |
| IL297165A (en) | 2020-04-16 | 2022-12-01 | Incyte Corp | Fused tricyclic kras inhibitors |
| US11739102B2 (en) | 2020-05-13 | 2023-08-29 | Incyte Corporation | Fused pyrimidine compounds as KRAS inhibitors |
| TWI895442B (en) | 2020-06-12 | 2025-09-01 | 美商英塞特公司 | Imidazopyridazine compounds and uses thereof |
| WO2021257863A1 (en) | 2020-06-19 | 2021-12-23 | Incyte Corporation | Pyrrolotriazine compounds as jak2 v617f inhibitors |
| US11691971B2 (en) | 2020-06-19 | 2023-07-04 | Incyte Corporation | Naphthyridinone compounds as JAK2 V617F inhibitors |
| US11767323B2 (en) | 2020-07-02 | 2023-09-26 | Incyte Corporation | Tricyclic pyridone compounds as JAK2 V617F inhibitors |
| IL299612A (en) | 2020-07-02 | 2023-03-01 | Incyte Corp | Tricyclic urea compounds as jak2 v617f inhibitors |
| US11661422B2 (en) | 2020-08-27 | 2023-05-30 | Incyte Corporation | Tricyclic urea compounds as JAK2 V617F inhibitors |
| US11999752B2 (en) | 2020-08-28 | 2024-06-04 | Incyte Corporation | Vinyl imidazole compounds as inhibitors of KRAS |
| US11767320B2 (en) | 2020-10-02 | 2023-09-26 | Incyte Corporation | Bicyclic dione compounds as inhibitors of KRAS |
| WO2022140231A1 (en) | 2020-12-21 | 2022-06-30 | Incyte Corporation | Deazaguaine compounds as jak2 v617f inhibitors |
| US11958861B2 (en) | 2021-02-25 | 2024-04-16 | Incyte Corporation | Spirocyclic lactams as JAK2 V617F inhibitors |
| WO2022204112A1 (en) | 2021-03-22 | 2022-09-29 | Incyte Corporation | Imidazole and triazole kras inhibitors |
| CA3215903A1 (en) | 2021-04-12 | 2022-10-20 | Incyte Corporation | Combination therapy comprising an fgfr inhibitor and a nectin-4 targeting agent |
| WO2022261159A1 (en) | 2021-06-09 | 2022-12-15 | Incyte Corporation | Tricyclic heterocycles as fgfr inhibitors |
| EP4352059A1 (en) | 2021-06-09 | 2024-04-17 | Incyte Corporation | Tricyclic heterocycles as fgfr inhibitors |
| US11981671B2 (en) | 2021-06-21 | 2024-05-14 | Incyte Corporation | Bicyclic pyrazolyl amines as CDK2 inhibitors |
| US12441727B2 (en) | 2021-07-07 | 2025-10-14 | Incyte Corporation | Tricyclic compounds as inhibitors of KRAS |
| JP2024529347A (en) | 2021-07-14 | 2024-08-06 | インサイト・コーポレイション | Tricyclic Compounds as Inhibitors of KRAS |
| CA3229855A1 (en) | 2021-08-31 | 2023-03-09 | Incyte Corporation | Naphthyridine compounds as inhibitors of kras |
| WO2023049697A1 (en) | 2021-09-21 | 2023-03-30 | Incyte Corporation | Hetero-tricyclic compounds as inhibitors of kras |
| WO2023056421A1 (en) | 2021-10-01 | 2023-04-06 | Incyte Corporation | Pyrazoloquinoline kras inhibitors |
| KR20240101561A (en) | 2021-10-14 | 2024-07-02 | 인사이트 코포레이션 | Quinoline compounds as inhibitors of KRAS |
| CA3239205A1 (en) | 2021-11-22 | 2023-05-25 | Incyte Corporation | Combination therapy comprising an fgfr inhibitor and a kras inhibitor |
| US11976073B2 (en) | 2021-12-10 | 2024-05-07 | Incyte Corporation | Bicyclic amines as CDK2 inhibitors |
| AU2022418585A1 (en) | 2021-12-22 | 2024-07-11 | Incyte Corporation | Salts and solid forms of an fgfr inhibitor and processes of preparing thereof |
| EP4493558A1 (en) | 2022-03-17 | 2025-01-22 | Incyte Corporation | Tricyclic urea compounds as jak2 v617f inhibitors |
| TW202402279A (en) | 2022-06-08 | 2024-01-16 | 美商英塞特公司 | Tricyclic triazolo compounds as dgk inhibitors |
| WO2024015731A1 (en) | 2022-07-11 | 2024-01-18 | Incyte Corporation | Fused tricyclic compounds as inhibitors of kras g12v mutants |
| US20240190876A1 (en) | 2022-10-21 | 2024-06-13 | Incyte Corporation | Tricyclic Urea Compounds As JAK2 V617F Inhibitors |
| US20240217989A1 (en) | 2022-11-18 | 2024-07-04 | Incyte Corporation | Heteroaryl Fluoroalkenes As DGK Inhibitors |
| US20240270739A1 (en) | 2023-01-12 | 2024-08-15 | Incyte Corporation | Heteroaryl Fluoroalkenes As DGK Inhibitors |
| WO2024191996A1 (en) | 2023-03-13 | 2024-09-19 | Incyte Corporation | Bicyclic ureas as kinase inhibitors |
| WO2024220532A1 (en) | 2023-04-18 | 2024-10-24 | Incyte Corporation | Pyrrolidine kras inhibitors |
| WO2025043151A2 (en) | 2023-08-24 | 2025-02-27 | Incyte Corporation | Bicyclic dgk inhibitors |
| US20250163079A1 (en) | 2023-11-01 | 2025-05-22 | Incyte Corporation | Kras inhibitors |
| TW202523667A (en) | 2023-12-05 | 2025-06-16 | 美商英塞特公司 | Tricyclic triazolo compounds as dgk inhibitors |
| WO2025129002A1 (en) | 2023-12-13 | 2025-06-19 | Incyte Corporation | Bicyclooctane kras inhibitors |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002000196A2 (en) | 2000-06-28 | 2002-01-03 | Smithkline Beecham P.L.C. | Wet milling process |
| WO2009039397A2 (en) * | 2007-09-20 | 2009-03-26 | Cgi Pharmaceuticals, Inc. | Substituted amides, methods of making, use thereof for the treatment of diseases such as cancer |
| WO2013157021A1 (en) * | 2012-04-20 | 2013-10-24 | Advinus Therapeutics Limited | Bicyclic compounds, compositions and medicinal applications thereof |
Family Cites Families (356)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3272781A (en) | 1963-08-07 | 1966-09-13 | American Potash & Chem Corp | Boroureas of phosphinoborine polymers |
| FR1425700A (en) | 1965-02-22 | 1966-01-24 | Basf Ag | Compounds forming metal complexes and method of preparing and using them |
| US4208328A (en) | 1978-04-27 | 1980-06-17 | General Electric Company | Alkyl 3,5-dihydroxy-4-(2-benzothiazolyl)benzoates |
| US4789711A (en) | 1986-12-02 | 1988-12-06 | Ciba-Geigy Corporation | Multifunctional epoxide resins |
| DE3828535A1 (en) | 1988-08-23 | 1990-03-08 | Basf Ag | BENZIMIDAZOLE-2-CARBON-ACIDANILIDE, THEIR USE AS ANTI-LIGHTING AGENT FOR ORGANIC MATERIAL AND ORGANIC MATERIAL STABILIZED THEREOF |
| US5077164A (en) | 1989-06-21 | 1991-12-31 | Minolta Camera Kabushiki Kaisha | Photosensitive member containing an azo dye |
| EP0644460B1 (en) | 1993-09-20 | 1999-12-08 | Fuji Photo Film Co., Ltd. | Positive working photoresist composition |
| JP3461397B2 (en) | 1995-01-11 | 2003-10-27 | 富士写真フイルム株式会社 | Positive photoresist composition |
| WO1998027108A2 (en) | 1996-12-16 | 1998-06-25 | Fujisawa Pharmaceutical Co., Ltd. | New amide compounds and their use as nitric oxide synthase inhibitors |
| JPH10316853A (en) | 1997-05-15 | 1998-12-02 | Sumitomo Bakelite Co Ltd | Resin composition for interlaminar insulating membrane for multilayer interconnection of semiconductor, and production of the insulating membrane |
| WO1999018096A1 (en) | 1997-10-02 | 1999-04-15 | Merck & Co., Inc. | Inhibitors of prenyl-protein transferase |
| AU2745899A (en) | 1998-03-05 | 1999-09-20 | Nissan Chemical Industries Ltd. | Anilide compounds and herbicide |
| JP2000128987A (en) | 1998-10-28 | 2000-05-09 | Sumitomo Bakelite Co Ltd | Polybenzoxazole precursor and polybenzoxazole |
| JP2000128984A (en) | 1998-10-28 | 2000-05-09 | Sumitomo Bakelite Co Ltd | Polybenzoxazole precursor and resin |
| JP2000128986A (en) | 1998-10-28 | 2000-05-09 | Sumitomo Bakelite Co Ltd | Polybenzoxazole precursor and polybenzoxazole |
| US6297351B1 (en) | 1998-12-17 | 2001-10-02 | Sumitomo Bakelite Company Limited | Polybenzoxazole resin and precursor thereof |
| HUP0104987A3 (en) | 1998-12-18 | 2002-09-30 | Axys Pharmaceuticals Inc South | Benzimidazole or indole derivatives protease inhibitors, and pharmaceutical compositions containing them |
| JP2000212281A (en) | 1999-01-27 | 2000-08-02 | Sumitomo Bakelite Co Ltd | Polybenzoxazole precursor and polybenzoxazole resin |
| WO2001007409A1 (en) | 1999-07-23 | 2001-02-01 | Astrazeneca Uk Limited | Carbazole derivatives and their use as neuropeptide y5 receptor ligands |
| JP2001114893A (en) | 1999-10-15 | 2001-04-24 | Sumitomo Bakelite Co Ltd | Polybenzoxazole resin and its precursor |
| US6372907B1 (en) | 1999-11-03 | 2002-04-16 | Apptera Corporation | Water-soluble rhodamine dye peptide conjugates |
| JP2001163975A (en) | 1999-12-03 | 2001-06-19 | Sumitomo Bakelite Co Ltd | Polybenzoxazole resin and its precursor |
| RU2223761C2 (en) | 1999-12-27 | 2004-02-20 | Джапан Тобакко Инк. | Ring-condensed compounds and their using as medicinal agents |
| ATE372337T1 (en) | 2000-02-01 | 2007-09-15 | Abbott Gmbh & Co Kg | HETEROCYCLIC COMPOUNDS AND THEIR APPLICATION AS PARP INHIBITORS |
| US6521618B2 (en) | 2000-03-28 | 2003-02-18 | Wyeth | 3-cyanoquinolines, 3-cyano-1,6-naphthyridines, and 3-cyano-1,7-naphthyridines as protein kinase inhibitors |
| EP1268478B1 (en) | 2000-03-31 | 2007-05-02 | Ortho-McNeil Pharmaceutical, Inc. | Phenyl-substituted imidazopyridines |
| EP1278734A2 (en) | 2000-04-24 | 2003-01-29 | Merck Frosst Canada & Co. | Method of treatment using phenyl and biaryl derivatives as prostaglandin e inhibitors and compounds useful therefore |
| WO2002014321A1 (en) | 2000-08-11 | 2002-02-21 | The Regents Of The University Of California | Use of stat-6 inhibitors as therapeutic agents |
| EP1343785A2 (en) | 2000-12-13 | 2003-09-17 | Basf Aktiengesellschaft | Use of substituted imidazoazines, novel imidazoazines, methods for the production thereof, and agents containing these compounds |
| AU2002239344A1 (en) | 2000-12-15 | 2002-06-24 | Glaxo Group Limited | Pyrazolopyridines |
| SE0100567D0 (en) | 2001-02-20 | 2001-02-20 | Astrazeneca Ab | Compounds |
| EP1373240B1 (en) | 2001-03-14 | 2005-06-15 | Eli Lilly And Company | Retinoid x receptor modulators |
| EP1372643A1 (en) | 2001-03-30 | 2004-01-02 | Smithkline Beecham Corporation | Pyrazolopyridines, process for their preparation and use as therapeutic compounds |
| EP1377575B1 (en) | 2001-04-10 | 2006-07-05 | SmithKline Beecham Corporation | Antiviral pyrazolopyridine compounds |
| JP2002316966A (en) | 2001-04-19 | 2002-10-31 | Ueno Seiyaku Oyo Kenkyusho:Kk | Binaphthol derivative and method for producing the same |
| EP1385847B1 (en) | 2001-04-27 | 2005-06-01 | SmithKline Beecham Corporation | Pyrazolo[1,5-a]pyridine derivatives |
| AR035543A1 (en) | 2001-06-26 | 2004-06-16 | Japan Tobacco Inc | THERAPEUTIC AGENT FOR HEPATITIS C THAT INCLUDES A CONDENSED RING COMPOUND, CONDENSED RING COMPOUND, PHARMACEUTICAL COMPOSITION THAT UNDERSTANDS, BENZIMIDAZOL, THIAZOL AND BIFENYL COMPOUNDS USED AS INTERMEDIARY COMPARTMENTS OF COMPARTMENTS |
| US20040214834A1 (en) | 2001-09-07 | 2004-10-28 | Kristjan Gudmunsson | Pyrazolo-pyridines for the treatment of herpes infections |
| TWI331526B (en) | 2001-09-21 | 2010-10-11 | Bristol Myers Squibb Pharma Co | Lactam-containing compounds and derivatives thereof as factor xa inhibitors |
| AU2002334969A1 (en) | 2001-10-09 | 2003-04-22 | Sylvie Barchechath | Use of stat-6 inhibitors as therapeutic agents |
| BR0213208A (en) | 2001-10-09 | 2004-08-31 | Upjohn Co | Substituted arylsulfonyl tetrahydro- and hexahydro-carbazoles compounds, their compositions and uses |
| JP4488740B2 (en) | 2001-11-13 | 2010-06-23 | ダナ−ファーバー キャンサー インスティテュート,インコーポレイテッド | Agents that modulate immune cell activation and methods of use thereof |
| JP4024579B2 (en) | 2002-01-22 | 2007-12-19 | 住友ベークライト株式会社 | Plastic optical waveguide material and optical waveguide |
| US7273885B2 (en) | 2002-04-11 | 2007-09-25 | Vertex Pharmaceuticals Incorporated | Inhibitors of serine proteases, particularly HCV NS3-NS4A protease |
| CN100343255C (en) | 2002-04-23 | 2007-10-17 | 盐野义制药株式会社 | Pyrazolo (1,5-a) pyrimidine derivative and nad(p)h oxidase inhibitor containing the same |
| JPWO2004007472A1 (en) | 2002-07-10 | 2005-11-17 | 小野薬品工業株式会社 | CCR4 antagonist and pharmaceutical use thereof |
| AU2003249244A1 (en) | 2002-07-15 | 2004-02-02 | Combinatorx, Incorporated | Methods for the treatment of neoplasms |
| JP2004059761A (en) | 2002-07-30 | 2004-02-26 | Sumitomo Bakelite Co Ltd | Polybenzoxazole resin, its precursor, and optical waveguide material and optical waveguide using these |
| WO2004035522A1 (en) | 2002-08-30 | 2004-04-29 | Bf Research Institute, Inc. | Diagnostic probes and remedies for diseases with accumulation of prion protein, and stains for prion protein |
| JP2004091369A (en) * | 2002-08-30 | 2004-03-25 | Sumitomo Pharmaceut Co Ltd | New biphenyl compounds |
| US7153863B2 (en) | 2002-10-03 | 2006-12-26 | Smithkline Beecham Corporation | Therapeutic compounds based on pyrazolopyridline derivatives |
| JP2006504755A (en) | 2002-10-15 | 2006-02-09 | スミスクライン ビーチャム コーポレーション | Pyridazine compounds as GSK-3 inhibitors |
| MXPA05006828A (en) | 2002-12-23 | 2005-09-08 | Wyeth Corp | Antibodies against pd-1 and uses therefor. |
| KR100624406B1 (en) | 2002-12-30 | 2006-09-18 | 삼성에스디아이 주식회사 | Biphenyl Derivatives and Organic Electroluminescent Devices Employing the Same |
| US7320989B2 (en) | 2003-02-28 | 2008-01-22 | Encysive Pharmaceuticals, Inc. | Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-II receptor antagonists |
| US7078419B2 (en) | 2003-03-10 | 2006-07-18 | Boehringer Ingelheim Pharmaceuticals, Inc. | Cytokine inhibitors |
| TW200505902A (en) | 2003-03-20 | 2005-02-16 | Schering Corp | Cannabinoid receptor ligands |
| JP4595288B2 (en) | 2003-03-25 | 2010-12-08 | 住友ベークライト株式会社 | Polybenzoxazole resin, precursor thereof, optical waveguide material using the same, and optical waveguide |
| JP2006522789A (en) | 2003-04-11 | 2006-10-05 | グレンマーク・ファーマシューティカルズ・エス・エー | Novel heterocyclic compounds useful for the treatment of inflammatory and allergic disorders; methods for their preparation and pharmaceutical compositions |
| BRPI0410439A (en) | 2003-05-19 | 2006-06-06 | Irm Llc | immunosuppressive compounds and compositions |
| JP2005002330A (en) | 2003-05-19 | 2005-01-06 | Sumitomo Electric Ind Ltd | Optical resin material, optical element, optical module, fluorinated polymer precursor and fluorinated polymer |
| US7405295B2 (en) | 2003-06-04 | 2008-07-29 | Cgi Pharmaceuticals, Inc. | Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of Bruton's tyrosine kinase by such compounds |
| US20060183746A1 (en) | 2003-06-04 | 2006-08-17 | Currie Kevin S | Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of Bruton's tyrosine kinase by such compounds |
| WO2005005429A1 (en) | 2003-06-30 | 2005-01-20 | Cellular Genomics, Inc. | Certain heterocyclic substituted imidazo[1,2-a]pyrazin-8-ylamines and methods of inhibition of bruton’s tyrosine kinase by such compounds |
| US7276608B2 (en) | 2003-07-11 | 2007-10-02 | Bristol-Myers Squibb Company | Tetrahydroquinoline derivatives as cannabinoid receptor modulators |
| WO2005004863A1 (en) | 2003-07-11 | 2005-01-20 | Merck Patent Gmbh | Benzimidazole carboxamides as raf kinase inhibitors |
| WO2005012221A1 (en) | 2003-08-04 | 2005-02-10 | Ono Pharmaceutical Co., Ltd. | Diphenyl ether compound, process for producing the same, and use |
| WO2005014543A1 (en) | 2003-08-06 | 2005-02-17 | Japan Tobacco Inc. | Condensed ring compound and use thereof as hcv polymerase inhibitor |
| US7504401B2 (en) | 2003-08-29 | 2009-03-17 | Locus Pharmaceuticals, Inc. | Anti-cancer agents and uses thereof |
| CN102060806A (en) | 2003-09-11 | 2011-05-18 | iTherX药品公司 | Cytokine inhibitors |
| CN1863774B (en) | 2003-10-08 | 2010-12-15 | Irm责任有限公司 | Compounds and compositions as protein kinase inhibitors |
| JPWO2005040135A1 (en) | 2003-10-24 | 2007-03-08 | 小野薬品工業株式会社 | Anti-stress drugs and their medicinal uses |
| US20050288295A1 (en) | 2003-11-11 | 2005-12-29 | Currie Kevin S | Certain imidazo[1,2-a]pyrazin-8-ylamines, method of making, and method of use thereof |
| JP2007534652A (en) | 2003-12-23 | 2007-11-29 | ビーエーエスエフ アクチェンゲゼルシャフト | 3-Trifluoromethylpicolinic acid anilide and its use as a fungicide |
| EP1715867A4 (en) | 2004-02-12 | 2009-04-15 | Merck & Co Inc | BIPYRIDYL AMIDES AS MODULATORS OF GLUTAMATE METABOTROPIC REPEATER-5 |
| JPWO2005077948A1 (en) | 2004-02-16 | 2008-01-10 | 第一製薬株式会社 | Antifungal heterocyclic compounds |
| GB0403864D0 (en) | 2004-02-20 | 2004-03-24 | Ucl Ventures | Modulator |
| JP2005248082A (en) | 2004-03-05 | 2005-09-15 | Sumitomo Electric Ind Ltd | Method for producing polybenzoxazole resin precursor and method for producing polybenzoxazole resin |
| AU2005220882A1 (en) | 2004-03-08 | 2005-09-22 | Georgia State University Research Foundation, Inc | Novel dicationic imidazo(1,2-a)pyridines and 5,6,7,8-tetrahydro-imidazo(1,2a)pyridines as antiprotozoal agents |
| WO2005086904A2 (en) | 2004-03-08 | 2005-09-22 | Amgen Inc. | Therapeutic modulation of ppar (gamma) activity |
| CN1972914A (en) | 2004-03-31 | 2007-05-30 | 詹森药业有限公司 | Non-imidazole heterocyclic compounds as histamine H3 receptor ligands |
| JP2005290301A (en) | 2004-04-02 | 2005-10-20 | Sumitomo Electric Ind Ltd | Method for producing polybenzoxazole resin precursor and method for producing polybenzoxazole resin |
| CN1937994A (en) | 2004-04-06 | 2007-03-28 | 宝洁公司 | Keratin dyeing compounds, keratin dyeing compositions comprising them and their use |
| JP4879165B2 (en) | 2004-04-20 | 2012-02-22 | トランス テック ファーマ,インコーポレイテッド | Substituted thiazole and pyrimidine derivatives as modulators of melanocortin receptors |
| DE102004021716A1 (en) | 2004-04-30 | 2005-12-01 | Grünenthal GmbH | Substituted imidazo [1,2-a] pyridine compounds and drugs containing substituted imidazo [1,2-a] pyridine compounds |
| WO2005108387A2 (en) | 2004-05-03 | 2005-11-17 | Boehringer Ingelheim Pharmaceuticals, Inc. | Cytokine inhibitors |
| TW200626142A (en) | 2004-09-21 | 2006-08-01 | Glaxo Group Ltd | Chemical compounds |
| WO2006034337A2 (en) | 2004-09-23 | 2006-03-30 | Wyeth | Carbazole and cyclopentaindole derivatives to treat infection with hepatitis c virus |
| ATE479687T1 (en) | 2004-10-15 | 2010-09-15 | Takeda Pharmaceutical | KINASE INHIBITORS |
| AU2005304473A1 (en) | 2004-11-10 | 2006-05-18 | Cgi Pharmaceuticals, Inc. | Imidazo[1 , 2-a] pyrazin-8-ylamines useful as modulators of kinase activity |
| DE102004054665A1 (en) | 2004-11-12 | 2006-05-18 | Bayer Cropscience Gmbh | Substituted bicyclic and tricyclic pyrazole derivatives Methods for the preparation and use as herbicides and plant growth regulators |
| CA2599987A1 (en) | 2005-03-03 | 2006-09-08 | Sirtris Pharmaceuticals, Inc. | Fused heterocyclic compounds and their use as sirtuin modulators |
| CN101223141A (en) | 2005-03-10 | 2008-07-16 | Cgi药品股份有限公司 | Certain substituted amides, method of making, and method of use thereof |
| JP2006290883A (en) | 2005-03-17 | 2006-10-26 | Nippon Nohyaku Co Ltd | Substituted heterocyclic carboxylic acid anilide derivatives, intermediates thereof, agricultural and horticultural agents, and methods of use thereof |
| JP4361545B2 (en) | 2005-05-09 | 2009-11-11 | 小野薬品工業株式会社 | Cancer treatment method using human monoclonal antibody and anti-PD-1 antibody alone or in combination with other immunotherapy against Programmed Death 1 (PD-1) |
| MX2007014510A (en) | 2005-05-20 | 2008-02-05 | Array Biopharma Inc | Raf inhibitor compounds and methods of use thereof. |
| HRP20151102T1 (en) | 2005-07-01 | 2015-11-20 | E. R. Squibb & Sons, L.L.C. | HUMAN MONOCLONAL ANTIBODIES FOR LIGAND PROGRAMMED DEATH 1 (PD-L1) |
| US20080220968A1 (en) | 2005-07-05 | 2008-09-11 | Ge Healthcare Bio-Sciences Ab | [1, 2, 4] Triazolo [1, 5-A] Pyrimidine Derivatives as Chromatographic Adsorbent for the Selective Adsorption of Igg |
| WO2007034282A2 (en) | 2005-09-19 | 2007-03-29 | Pfizer Products Inc. | Diaryl-imidazole compounds condensed with a heterocycle as c3a receptor antagonists |
| US7723336B2 (en) | 2005-09-22 | 2010-05-25 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| US20070078136A1 (en) | 2005-09-22 | 2007-04-05 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| AU2006307314C1 (en) | 2005-10-25 | 2011-08-25 | Shionogi & Co., Ltd. | Aminodihydrothiazine derivative |
| AU2006316322B2 (en) | 2005-11-22 | 2011-08-25 | Merck Canada Inc. | Tricyclic compounds useful as inhibitors of kinases |
| WO2007067711A2 (en) | 2005-12-08 | 2007-06-14 | Amphora Discovery Corporation | Certain chemical entities, compositions, and methods for modulating trpv1 |
| US20090281120A1 (en) | 2005-12-12 | 2009-11-12 | Ono Pharmaceutical Co., Ltd | Bicyclic heterocyclic compound |
| US20090281075A1 (en) | 2006-02-17 | 2009-11-12 | Pharmacopeia, Inc. | Isomeric purinones and 1h-imidazopyridinones as pkc-theta inhibitors |
| WO2007096764A2 (en) | 2006-02-27 | 2007-08-30 | Glenmark Pharmaceuticals S.A. | Bicyclic heteroaryl derivatives as cannabinoid receptor modulators |
| JPWO2007102531A1 (en) | 2006-03-08 | 2009-07-23 | 武田薬品工業株式会社 | Concomitant medication |
| AU2007233709A1 (en) | 2006-03-31 | 2007-10-11 | Novartis Ag | Organic compounds |
| WO2008118122A2 (en) | 2006-05-08 | 2008-10-02 | Molecular Neuroimaging, Llc | Compounds and amyloid probes thereof for therapeutic and imaging uses |
| WO2007146712A2 (en) | 2006-06-09 | 2007-12-21 | Kemia, Inc. | Therapy using cytokine inhibitors |
| US20080280891A1 (en) | 2006-06-27 | 2008-11-13 | Locus Pharmaceuticals, Inc. | Anti-cancer agents and uses thereof |
| BRPI0713187A2 (en) | 2006-07-20 | 2012-10-16 | Mehmet Kahraman | method of inhibiting rho kinase, method of treating rho kinase mediated disease, compound and pharmaceutical composition |
| DE102006035018B4 (en) | 2006-07-28 | 2009-07-23 | Novaled Ag | Oxazole triplet emitter for OLED applications |
| WO2008021745A2 (en) | 2006-08-16 | 2008-02-21 | Itherx Pharmaceuticals, Inc. | Hepatitis c virus entry inhibitors |
| TWI389895B (en) | 2006-08-21 | 2013-03-21 | Infinity Discovery Inc | Compounds and methods for inhibiting the interaction of bcl proteins with binding partners |
| WO2008027812A2 (en) | 2006-08-28 | 2008-03-06 | Forest Laboratories Holdings Limited | Imidazopyridine and imidazopyrimidine derivatives |
| US20100160292A1 (en) | 2006-09-11 | 2010-06-24 | Cgi Pharmaceuticals, Inc | Kinase Inhibitors, and Methods of Using and Identifying Kinase Inhibitors |
| CN101573348A (en) | 2006-09-11 | 2009-11-04 | 矩阵实验室有限公司 | Dibenzofuran derivatives as PDE-4 and PDE-10 inhibitors |
| AR063707A1 (en) | 2006-09-11 | 2009-02-11 | Cgi Pharmaceuticals Inc | CERTAIN AMIDAS REPLACED, THE USE OF THE SAME FOR THE TREATMENT OF DISEASES MEDIATED BY THE INHIBITION OF THE ACTIVITY OF BTK AND PHARMACEUTICAL COMPOSITIONS THAT UNDERSTAND THEM. |
| US7838523B2 (en) | 2006-09-11 | 2010-11-23 | Cgi Pharmaceuticals, Inc. | Certain substituted amides, method of making, and method of use thereof |
| AR063706A1 (en) | 2006-09-11 | 2009-02-11 | Cgi Pharmaceuticals Inc | CERTAIN AMIDAS REPLACED, THE USE OF THE SAME FOR THE TREATMENT OF DISEASES MEDIATED BY THE INHIBITION OF THE ACTIVITY OF BTK AND PHARMACEUTICAL COMPOSITIONS THAT UNDERSTAND THEM. |
| FR2906250B1 (en) | 2006-09-22 | 2008-10-31 | Sanofi Aventis Sa | DERIVATIVES OF 2-ARYL-6PHENYL-IMIDAZO (1,2-A) PYRIDINES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| CA2667644A1 (en) | 2006-10-27 | 2008-05-15 | Wyeth | Tricyclic compounds as matrix metalloproteinase inhibitors |
| AR063628A1 (en) | 2006-11-08 | 2009-02-04 | Bristol Myers Squibb Co | USEFUL PIRIDINONE COMPOUNDS FOR CANCER TREATMENT |
| GB0623209D0 (en) | 2006-11-21 | 2007-01-03 | F2G Ltd | Antifungal agents |
| WO2008064318A2 (en) | 2006-11-22 | 2008-05-29 | University Of Medicine And Dentistry Of New Jersey | Peripheral opioid receptor active compounds |
| WO2008064317A1 (en) | 2006-11-22 | 2008-05-29 | University Of Medicine And Dentistry Of New Jersey | Lipophilic opioid receptor active compounds |
| US20100004301A1 (en) | 2006-12-14 | 2010-01-07 | Benjamin Pelcman | Benzoxazoles Useful in the Treatment of Inflammation |
| US8513270B2 (en) | 2006-12-22 | 2013-08-20 | Incyte Corporation | Substituted heterocycles as Janus kinase inhibitors |
| US8338437B2 (en) | 2007-02-28 | 2012-12-25 | Methylgene Inc. | Amines as small molecule inhibitors |
| EP1964840A1 (en) | 2007-02-28 | 2008-09-03 | sanofi-aventis | Imidazo[1,2-a]pyridines and their use as pharmaceuticals |
| EP1964841A1 (en) | 2007-02-28 | 2008-09-03 | sanofi-aventis | Imidazo[1,2-a]azine and their use as pharmaceuticals |
| JP2008218327A (en) | 2007-03-07 | 2008-09-18 | Hitachi Ltd | Electrolyte, electrolyte membrane, membrane electrode assembly using the same, fuel cell power source and fuel cell power source system |
| JP2010120852A (en) * | 2007-03-09 | 2010-06-03 | Daiichi Sankyo Co Ltd | New diamide derivative |
| ES2388454T3 (en) | 2007-03-22 | 2012-10-15 | Astrazeneca Ab | Quinoline derivatives for the treatment of inflammatory diseases |
| ES2476605T3 (en) | 2007-04-24 | 2014-07-15 | Shionogi & Co., Ltd. | Aminohydrotiazine derivatives substituted with cyclic groups |
| JP5383483B2 (en) | 2007-04-24 | 2014-01-08 | 塩野義製薬株式会社 | Pharmaceutical composition for the treatment of Alzheimer's disease |
| WO2008134553A1 (en) | 2007-04-26 | 2008-11-06 | Xenon Pharmaceuticals Inc. | Methods of using bicyclic compounds in treating sodium channel-mediated diseases |
| WO2008141249A1 (en) | 2007-05-10 | 2008-11-20 | Acadia Pharmaceuticals Inc. | Imidazol (1,2-a)pyridines and related compounds with activity at cannabinoid cb2 receptors |
| NZ582150A (en) | 2007-06-18 | 2012-08-31 | Msd Oss Bv | Antibodies to human programmed death receptor pd-1 |
| WO2009027733A1 (en) | 2007-08-24 | 2009-03-05 | Astrazeneca Ab | (2-pyridin-3-ylimidazo[1,2-b]pyridazin-6-yl) urea derivatives as antibacterial agents |
| AU2008302746B2 (en) | 2007-09-20 | 2014-07-03 | Amgen Inc. | 1-(4-(benzylbenzamid0) -benzyl) azetidine-3-carboxylic acid derivatives and related compounds as SlP receptor modulators for the treatment of immune disorders |
| DE102007048716A1 (en) | 2007-10-11 | 2009-04-23 | Merck Patent Gmbh | Imidazo [1,2-a] pyrimidine derivatives |
| TW200932219A (en) | 2007-10-24 | 2009-08-01 | Astellas Pharma Inc | Oxadiazolidinedione compound |
| MX2010004491A (en) | 2007-10-25 | 2010-06-21 | Astrazeneca Ab | Pyridine and pyrazine derivatives - 083. |
| US7868001B2 (en) | 2007-11-02 | 2011-01-11 | Hutchison Medipharma Enterprises Limited | Cytokine inhibitors |
| WO2009062059A2 (en) | 2007-11-08 | 2009-05-14 | Pharmacopeia, Inc. | Isomeric purinones and 1h-imidazopyridinones as pkc-theta inhibitors |
| EP2231143B1 (en) | 2007-12-13 | 2013-07-03 | Merck Sharp & Dohme Corp. | 5H-pyrido[4,3-b]indoles as INHIBITORS OF JANUS KINASES |
| RU2364597C1 (en) | 2007-12-14 | 2009-08-20 | Андрей Александрович Иващенко | HETEROCYCLIC INHIBITORS OF Hh-SYGNAL CASCADE, BASED ON THEM MEDICINAL COMPOSITIONS AND METHOD OF TREATING DISEASES INDUCED BY ABBARANT ACTIVITY OF Hh-SIGNAL SYSTEM |
| EA201000949A1 (en) | 2007-12-19 | 2011-02-28 | Зингента Партисипейшнс Аг | INSECTICIDE CONNECTIONS |
| CA2710016A1 (en) | 2007-12-21 | 2009-07-02 | The University Of Sydney | Translocator protein ligands |
| AU2008345225A1 (en) | 2007-12-21 | 2009-07-09 | University Of Rochester | Method for altering the lifespan of eukaryotic organisms |
| ES2548774T3 (en) | 2008-01-18 | 2015-10-20 | Eisai R&D Management Co., Ltd. | Condensed aminodihydrotiazine derivative |
| WO2009096202A1 (en) | 2008-01-31 | 2009-08-06 | Konica Minolta Holdings, Inc. | Halogenated polycyclic aromatic compound and method for producing the same |
| BRPI0908529A2 (en) | 2008-02-26 | 2015-09-29 | Novartis Ag | organic compost |
| EP2095818A1 (en) | 2008-02-29 | 2009-09-02 | AEterna Zentaris GmbH | Use of LHRH antagonists at non-castrating doses |
| WO2009114335A2 (en) | 2008-03-12 | 2009-09-17 | Merck & Co., Inc. | Pd-1 binding proteins |
| FR2928921B1 (en) | 2008-03-21 | 2010-04-23 | Sanofi Aventis | POLYSUBSTITUTED DERIVATIVES OF 2-ARYL-6-PHENYL-IMIDAZO-1,2-A! PYRIDINES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| FR2928922B1 (en) | 2008-03-21 | 2010-04-23 | Sanofi Aventis | DERIVATIVES OF POLYSUBSTITUTED 2-ARYL-6-PHENYL-IMIDAZO-1,2-A! PYRIDINES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| FR2928924B1 (en) | 2008-03-21 | 2010-04-23 | Sanofi Aventis | POLYSUBSTITUTED DERIVATIVES OF 6-HETEROARYL-IMIDAZO-1,2-A! PYRIDINES, THEIR PREPARATION AND THEIR THERAPEUTIC APPLICATION |
| EP2271646A1 (en) | 2008-03-31 | 2011-01-12 | Takeda Pharmaceutical Company Limited | Apoptosis signal-regulating kinase 1 inhibitors |
| KR101034351B1 (en) | 2008-05-14 | 2011-05-16 | 한국화학연구원 | Pyridine derivatives or pharmaceutically acceptable salts thereof substituted with novel benzoxazoles, preparation methods thereof, and pharmaceutical compositions for the prevention and treatment of abnormal cell growth diseases containing the same as active ingredients |
| CA2724842A1 (en) | 2008-05-19 | 2009-11-26 | Sunovion Pharmaceuticals Inc. | Imidazo[1,2-a]pyridine compounds |
| JP2011521960A (en) | 2008-05-29 | 2011-07-28 | サートリス ファーマシューティカルズ, インコーポレイテッド | Imidazopyridine and related analogs as sirtuin modulators |
| JP5502858B2 (en) | 2008-06-05 | 2014-05-28 | グラクソ グループ リミテッド | 4-Carboxamide indazole derivatives useful as inhibitors of PI3 kinase |
| US8476430B2 (en) | 2008-07-24 | 2013-07-02 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| US9540322B2 (en) | 2008-08-18 | 2017-01-10 | Yale University | MIF modulators |
| US9643922B2 (en) | 2008-08-18 | 2017-05-09 | Yale University | MIF modulators |
| JP2011231017A (en) | 2008-09-09 | 2011-11-17 | Nissan Chem Ind Ltd | Process for producing optically active epoxy compound and optically active sulfoxide compound, ligand and complex for use in the process, and process for producing the complex |
| AU2009296392B2 (en) | 2008-09-26 | 2016-06-02 | Dana-Farber Cancer Institute, Inc. | Human anti-PD-1, PD-L1, and PD-L2 antibodies and uses therefor |
| EP2365970B1 (en) | 2008-11-12 | 2018-03-21 | Gilead Connecticut, Inc. | Pyridazinones and their use as btk inhibitors |
| ES2403633T3 (en) | 2008-12-04 | 2013-05-20 | Proximagen Limited | Imidazopyridine Compounds |
| KR20250091300A (en) | 2008-12-09 | 2025-06-20 | 제넨테크, 인크. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| WO2010080474A1 (en) | 2008-12-19 | 2010-07-15 | Bristol-Myers Squibb Company | Carbazole and carboline kinase inhibitors |
| US8084620B2 (en) | 2008-12-19 | 2011-12-27 | Bristol-Myers Squibb Company | Carbazole carboxamide compounds useful as kinase inhibitors |
| TW201035100A (en) | 2008-12-19 | 2010-10-01 | Cephalon Inc | Pyrrolotriazines as ALK and JAK2 inhibitors |
| JP5624275B2 (en) | 2008-12-22 | 2014-11-12 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| US8541424B2 (en) | 2008-12-23 | 2013-09-24 | Abbott Laboratories | Anti-viral compounds |
| JP5578490B2 (en) | 2008-12-26 | 2014-08-27 | 味の素株式会社 | Pyrazolopyrimidine compounds |
| JP5844159B2 (en) | 2009-02-09 | 2016-01-13 | ユニヴェルシテ デクス−マルセイユUniversite D’Aix−Marseille | PD-1 antibody and PD-L1 antibody and use thereof |
| JP2010202530A (en) | 2009-02-27 | 2010-09-16 | Tokyo Institute Of Technology | Heterocycle-containing aromatic compound, and optical material |
| KR20100101054A (en) | 2009-03-07 | 2010-09-16 | 주식회사 메디젠텍 | Composition for treating or preventing nuclear export of gsk3- mediated disease including compound for inhibiting nuclear export of gsk3 |
| CN102574786A (en) | 2009-04-02 | 2012-07-11 | 默克雪兰诺有限公司 | Dihydroorotate dehydrogenase inhibitors |
| WO2010119264A1 (en) | 2009-04-16 | 2010-10-21 | Centro Nacional De Investigaciones Oncólogicas (Cnio) | Imidazopyrazines for use as kinase inhibitors |
| US8338441B2 (en) | 2009-05-15 | 2012-12-25 | Gilead Sciences, Inc. | Inhibitors of human immunodeficiency virus replication |
| WO2011002635A1 (en) | 2009-06-30 | 2011-01-06 | Siga Technologies, Inc. | Treatment and prevention of dengue virus infections |
| US8993604B2 (en) | 2009-06-30 | 2015-03-31 | Siga Technologies, Inc. | Treatment and prevention of dengue virus infections |
| TWI625121B (en) | 2009-07-13 | 2018-06-01 | 基利科學股份有限公司 | Inhibitor of kinases that regulate apoptosis signaling |
| JP2011057661A (en) | 2009-08-14 | 2011-03-24 | Bayer Cropscience Ag | Pesticidal carboxamides |
| UA108363C2 (en) | 2009-10-08 | 2015-04-27 | IMINOTIADIASIADIOXIDE OXIDES AS BACE INHIBITORS, COMPOSITIONS THEREOF AND THEIR APPLICATIONS | |
| WO2011047129A1 (en) | 2009-10-15 | 2011-04-21 | Southern Research Institute | Treatment of neurodegenerative diseases, causation of memory enhancement, and assay for screening compounds for such |
| DK2488525T3 (en) | 2009-10-16 | 2018-08-06 | Melinta Therapeutics Inc | ANTIMICROBIAL COMPOUNDS AND PROCEDURES FOR PREPARING AND USING THEREOF |
| WO2011050245A1 (en) | 2009-10-23 | 2011-04-28 | Yangbo Feng | Bicyclic heteroaryls as kinase inhibitors |
| JP2013512251A (en) | 2009-11-24 | 2013-04-11 | アンプリミューン、インコーポレーテッド | Simultaneous inhibition of PD-L1 / PD-L2 |
| EP2518072A4 (en) | 2009-12-24 | 2014-06-04 | Ajinomoto Kk | Imidazopyridazine compounds |
| US20130022629A1 (en) * | 2010-01-04 | 2013-01-24 | Sharpe Arlene H | Modulators of Immunoinhibitory Receptor PD-1, and Methods of Use Thereof |
| WO2011097607A1 (en) | 2010-02-08 | 2011-08-11 | Southern Research Institute | Anti-viral treatment and assay to screen for anti-viral agent |
| TW201136919A (en) | 2010-03-02 | 2011-11-01 | Merck Sharp & Amp Dohme Corp | Inhibitors of hepatitis C virus NS5B polymerase |
| ES2862931T3 (en) | 2010-03-04 | 2021-10-08 | Merck Sharp & Dohme | Catechol O-methyl transferase inhibitors and their use in the treatment of psychotic disorders |
| RU2576662C2 (en) | 2010-03-18 | 2016-03-10 | Энститю Пастер Корея | Antiinfective compounds |
| US8410117B2 (en) | 2010-03-26 | 2013-04-02 | Hoffmann-La Roche Inc. | Imidazopyrimidine derivatives |
| EP2582668B1 (en) | 2010-06-16 | 2016-01-13 | Bristol-Myers Squibb Company | Carboline carboxamide compounds useful as kinase inhibitors |
| CA2802344C (en) | 2010-06-18 | 2023-06-13 | The Brigham And Women's Hospital, Inc. | Bi-specific antibodies against tim-3 and pd-1 for immunotherapy in chronic immune conditions |
| US8907053B2 (en) | 2010-06-25 | 2014-12-09 | Aurigene Discovery Technologies Limited | Immunosuppression modulating compounds |
| CN102295642B (en) | 2010-06-25 | 2016-04-06 | 中国人民解放军军事医学科学院毒物药物研究所 | 2-Aryimidazole is [1,2-a] pyridine-3-acetamide, Preparation Method And The Use also |
| EP2402345A1 (en) | 2010-06-29 | 2012-01-04 | Basf Se | Pyrazole fused bicyclic compounds |
| CN101891895B (en) | 2010-07-28 | 2011-11-30 | 南京航空航天大学 | Benzothiazole derivatives metal coordination polymer based on bridged bis-salicylaldehyde structure as well as manufacture method and application thereof |
| WO2012016133A2 (en) | 2010-07-29 | 2012-02-02 | President And Fellows Of Harvard College | Ros1 kinase inhibitors for the treatment of glioblastoma and other p53-deficient cancers |
| US8633200B2 (en) | 2010-09-08 | 2014-01-21 | Bristol-Myers Squibb Company | Inhibitors of human immunodeficiency virus replication |
| CN101993415B (en) | 2010-09-15 | 2013-08-14 | 北京韩美药品有限公司 | Compound as Hedgehog path inhibitor, medicine composition containing same and application thereof |
| EP2624837A4 (en) | 2010-10-04 | 2014-03-26 | Inst Hepatitis & Virus Res | Novel inhibitors of secretion of hepatitis b virus antigens |
| WO2012052745A1 (en) | 2010-10-21 | 2012-04-26 | Centro Nacional De Investigaciones Oncológicas (Cnio) | Combinations of pi3k inhibitors with a second anti -tumor agent |
| EP2444084A1 (en) | 2010-10-21 | 2012-04-25 | Centro Nacional de Investigaciones Oncológicas (CNIO) | Use of PI3K inibitors for the treatment of obesity |
| CN103282034A (en) | 2010-11-18 | 2013-09-04 | 利亘制药公司 | Use of hematopoietic growth factor mimetics |
| UY33808A (en) | 2010-12-17 | 2012-07-31 | Syngenta Participations Ag | INSECTICIDE COMPOUNDS |
| TWI617559B (en) | 2010-12-22 | 2018-03-11 | 江蘇恆瑞醫藥股份有限公司 | 2-arylimidazo[1,2-b]pyridazine, 2-phenylimidazo[1,2-a]pyridine, and 2-phenylimidazo[1,2-a]pyrazine derivatives |
| PE20140471A1 (en) | 2011-01-04 | 2014-04-13 | Novartis Ag | MODULATORS OF THE COMPLEMENT ROUTE AND USES OF THEM |
| US9018395B2 (en) | 2011-01-27 | 2015-04-28 | Université de Montréal | Pyrazolopyridine and pyrazolopyrimidine derivatives as melanocortin-4 receptor modulators |
| WO2012125886A1 (en) | 2011-03-17 | 2012-09-20 | Bristol-Myers Squibb Company | Pyrrolopyridazine jak3 inhibitors and their use for the treatment of inflammatory and autoimmune diseases |
| US9464065B2 (en) | 2011-03-24 | 2016-10-11 | The Scripps Research Institute | Compounds and methods for inducing chondrogenesis |
| AU2012243329B2 (en) | 2011-04-13 | 2015-09-17 | Merck Sharp & Dohme Corp. | 5-substituted iminothiazines and their mono-and dioxides as BACE inhibitors,compositions,and their use |
| CN102796103A (en) | 2011-05-23 | 2012-11-28 | 南京英派药业有限公司 | 6-(aryl formyl) imidazo [1,2-a] pyrimidine and 6-(aryl formyl) [1,2,4] triazol [4,3-a] pyrimidine serving as Hedgehog inhibitors and application thereof |
| EP2713722B1 (en) | 2011-05-31 | 2017-03-15 | Receptos, LLC | Novel glp-1 receptor stabilizers and modulators |
| GB201109763D0 (en) | 2011-06-10 | 2011-07-27 | Ucl Business Plc | Compounds |
| WO2012175991A1 (en) | 2011-06-24 | 2012-12-27 | Pharminox Limited | Fused pentacyclic anti - proliferative compounds |
| CA2841111A1 (en) | 2011-07-08 | 2013-01-17 | Novartis Ag | Novel pyrrolo pyrimidine derivatives |
| EP2548877A1 (en) | 2011-07-19 | 2013-01-23 | MSD Oss B.V. | 4-(5-Membered fused pyridinyl)benzamides as BTK-inhibitors |
| WO2013033901A1 (en) | 2011-09-08 | 2013-03-14 | Merck Sharp & Dohme Corp. | Heterocyclic-substituted benzofuran derivatives and methods of use thereof for the treatment of viral diseases |
| WO2013040528A1 (en) | 2011-09-16 | 2013-03-21 | Microbiotix, Inc. | Antimicrobial compounds |
| WO2013043521A1 (en) | 2011-09-22 | 2013-03-28 | Merck Sharp & Dohme Corp. | Pyrazolopyridyl compounds as aldosterone synthase inhibitors |
| JP6040677B2 (en) | 2011-09-29 | 2016-12-07 | 東洋インキScホールディングス株式会社 | Resin composition for solar cell encapsulant |
| IN2014DN03206A (en) | 2011-10-13 | 2015-05-22 | Novartis Ag | |
| EP2768833A4 (en) | 2011-10-20 | 2015-04-15 | Sirtris Pharmaceuticals Inc | Substituted bicyclic aza-heterocycles and analogues as sirtuin modulators |
| AU2012324517A1 (en) | 2011-10-21 | 2014-05-22 | Torrent Pharmaceuticals Limited | Novel substituted imidazopyrimidines as Gpbar1 receptor modulators |
| WO2013120040A1 (en) | 2012-02-10 | 2013-08-15 | Children's Medical Center Corporation | Targeted pathway inhibition to improve muscle structure, function and activity in muscular dystrophy |
| US9034882B2 (en) | 2012-03-05 | 2015-05-19 | Bristol-Myers Squibb Company | Inhibitors of human immunodeficiency virus replication |
| EP2824099A4 (en) | 2012-03-09 | 2015-11-11 | Carna Biosciences Inc | NEW TRIAZINE DERIVATIVE |
| PH12014500842A1 (en) | 2012-04-20 | 2014-06-09 | Gilead Sciences Inc | Benzothiazol-6-yl acetic acid derivatives and their use for treating an hiv infection |
| US20150105433A1 (en) | 2012-04-27 | 2015-04-16 | The Uab Research Foundation | TREATING VIRAL INFECTIONS HAVING VIRAL RNAs TRANSLATED BY A NON-IRES MEDIATED MECHANISM |
| BR112014029578B1 (en) | 2012-06-18 | 2019-10-22 | Sumitomo Chemical Co | fused heterocyclic compound, pest control composition and method |
| WO2014007228A1 (en) | 2012-07-03 | 2014-01-09 | 小野薬品工業株式会社 | Compound having agonistic activity on somatostatin receptor, and use thereof for medical purposes |
| ES2689429T3 (en) | 2012-07-13 | 2018-11-14 | Ucb Biopharma Sprl | Imidazopyridine derivatives as modulators of TNF activity |
| GB201212513D0 (en) | 2012-07-13 | 2012-08-29 | Ucb Pharma Sa | Therapeutic agents |
| JP2015178457A (en) | 2012-07-25 | 2015-10-08 | 杏林製薬株式会社 | Pyrazolopyridine derivative and pharmacologically permissible salt of the same |
| WO2014039595A1 (en) | 2012-09-06 | 2014-03-13 | Bristol-Myers Squibb Company | Imidazopyridazine jak3 inhibitors and their use for the treatment of inflammatory and autoimmune diseases |
| JP6666147B2 (en) | 2012-09-26 | 2020-03-13 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | Cyclic ether pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| WO2014061693A1 (en) | 2012-10-17 | 2014-04-24 | 塩野義製薬株式会社 | Novel non-aromatic carbocyclic or non-aromatic heterocyclic derivative |
| US9163027B2 (en) | 2012-11-21 | 2015-10-20 | Stategics, Inc. | Substituted triazolo-pyrimidine compounds for modulating cell proliferation differentiation and survival |
| JP6037804B2 (en) | 2012-12-03 | 2016-12-07 | 富士フイルム株式会社 | Gas separation membrane |
| HUE050215T2 (en) | 2013-01-15 | 2020-11-30 | Incyte Holdings Corp | Thiazolecarboxamide and pyridinecarboxamide compounds are useful as Pim kinase inhibitors |
| BR112015016315A2 (en) | 2013-01-22 | 2017-07-11 | Hoffmann La Roche | fluoro- [1,3] oxazine as bace1 inhibitors |
| CN103933036B (en) | 2013-01-23 | 2017-10-13 | 中国人民解放军军事医学科学院毒物药物研究所 | 2 Aryimidazoles simultaneously the acetamide derivative of [1,2 α] pyridine 3 prepare preventing and treating PTSD medicine in purposes |
| WO2014121085A1 (en) | 2013-01-31 | 2014-08-07 | Thomas Jefferson University | Pd-l1 and pd-l2-based fusion proteins and uses thereof |
| PT2963037T (en) | 2013-02-27 | 2019-04-23 | Mochida Pharm Co Ltd | Novel pyrazole derivative |
| AP2015008716A0 (en) | 2013-03-08 | 2015-09-30 | Amgen Inc | Perfluorinated cyclopropyl fused 1,3-oxazin-2-amine compounds as beta-secretase inhibitors and methods of use |
| CN104045552B (en) | 2013-03-13 | 2019-06-11 | 江苏先声药业有限公司 | Medicinal compound as neuroprotective agent |
| AU2014231768A1 (en) | 2013-03-13 | 2015-09-24 | Australian Nuclear Science And Technology Organisation | Transgenic non-human organisms with non-functional TSPO genes |
| ES2623904T3 (en) | 2013-03-14 | 2017-07-12 | VIIV Healthcare UK (No.5) Limited | Human immunodeficiency virus replication inhibitors |
| EA201591610A1 (en) | 2013-03-14 | 2015-12-30 | Курадев Фарма Прайвит Лтд. | KINURENIN PATH INHIBITORS |
| US10273243B2 (en) | 2013-03-14 | 2019-04-30 | The Trustees Of Columbia University In The City Of New York | 4-phenylpiperidines, their preparation and use |
| JP6562898B2 (en) | 2013-03-14 | 2019-08-21 | セルタクシス,インコーポレイテッド | Inhibitor of leukotriene A4 hydrolase |
| US9308236B2 (en) | 2013-03-15 | 2016-04-12 | Bristol-Myers Squibb Company | Macrocyclic inhibitors of the PD-1/PD-L1 and CD80(B7-1)/PD-L1 protein/protein interactions |
| WO2014181287A1 (en) | 2013-05-09 | 2014-11-13 | Piramal Enterprises Limited | Heterocyclyl compounds and uses thereof |
| CA2916298C (en) | 2013-06-26 | 2021-10-12 | Abbvie Inc. | Primary carboxamides as btk inhibitors |
| MX2015017821A (en) | 2013-07-02 | 2016-04-15 | Syngenta Participations Ag | Pesticidally active bi- or tricyclic heterocycles with sulfur containing substituents. |
| TWI641595B (en) | 2013-07-17 | 2018-11-21 | 日商大塚製藥股份有限公司 | Cyanotriazole compounds |
| CA2918910A1 (en) | 2013-07-25 | 2015-01-29 | Dana-Farber Cancer Institute, Inc. | Inhibitors of transcription factors and uses thereof |
| EP2835375A1 (en) | 2013-08-09 | 2015-02-11 | Fundació Institut Català d'Investigació Química | Bis-salphen compounds and carbonaceous material composites comprising them |
| KR101715090B1 (en) | 2013-08-28 | 2017-03-13 | 한국화학연구원 | Novel compound or pharmaceutically acceptable salt thereof and pharmaceutical composition for prevention or treatment of disease caused by influenza virus infection containing the same as an active ingredient |
| EA029901B1 (en) | 2013-09-04 | 2018-05-31 | Бристол-Майерс Сквибб Компани | Compounds useful as immunomodulators |
| CN105849092A (en) | 2013-09-06 | 2016-08-10 | 奥瑞基尼探索技术有限公司 | 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as immunomodulators |
| BR112016004889B1 (en) | 2013-09-06 | 2023-10-03 | Aurigene Discovery Technologies Limited | 1,2,4-OXADIAZOLE DERIVATIVES AS IMMUNOMODULATORS |
| WO2015036927A1 (en) | 2013-09-10 | 2015-03-19 | Aurigene Discovery Technologies Limited | Immunomodulating peptidomimetic derivatives |
| JP6336870B2 (en) | 2013-09-30 | 2018-06-06 | 日本ポリプロ株式会社 | Biphenol compound, olefin polymerization catalyst using the same, and process for producing olefin polymer |
| FR3012140B1 (en) | 2013-10-18 | 2016-08-26 | Arkema France | UNIT AND PROCESS FOR THE PURIFICATION OF RAW METHYL METHACRYLATE |
| GB201321743D0 (en) | 2013-12-09 | 2014-01-22 | Ucb Pharma Sa | Therapeutic agents |
| GB201321736D0 (en) | 2013-12-09 | 2014-01-22 | Ucb Pharma Sa | Therapeutic agents |
| GB201321733D0 (en) | 2013-12-09 | 2014-01-22 | Ucb Pharma Sa | Therapeutic agents |
| GB201321746D0 (en) | 2013-12-09 | 2014-01-22 | Ucb Pharma Sa | Therapeutic agents |
| WO2015095337A2 (en) | 2013-12-18 | 2015-06-25 | The Rockefeller University | PYRAZOLO[1,5-a]PYRIMIDINECARBOXAMIDE DERIVATIVES FOR TREATING COGNITIVE IMPAIRMENT |
| BR112016017527B1 (en) | 2014-01-03 | 2021-01-05 | Bayer Animal Health Gmbh | compounds, compound uses, pharmaceutical compositions, process for producing compositions and non-therapeutic method to control pests |
| WO2015120364A1 (en) | 2014-02-10 | 2015-08-13 | Merck Sharp & Dohme Corp. | Antibodies that bind to human tau and assay for quantifying human tau using the antibodies |
| EP3110805B1 (en) | 2014-02-25 | 2019-12-11 | Achillion Pharmaceuticals, Inc. | Compounds for treatment of complement mediated disorders |
| US9394365B1 (en) | 2014-03-12 | 2016-07-19 | Yeda Research And Development Co., Ltd | Reducing systemic regulatory T cell levels or activity for treatment of alzheimer's disease |
| JP6490464B2 (en) | 2014-03-26 | 2019-03-27 | 三井化学株式会社 | Transition metal compound, catalyst for olefin polymerization, and process for producing olefin polymer |
| PT3125883T (en) | 2014-04-04 | 2020-10-12 | Iomet Pharma Ltd | Indole derivatives for use in medicine |
| US9850225B2 (en) | 2014-04-14 | 2017-12-26 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2015175678A1 (en) | 2014-05-14 | 2015-11-19 | President And Fellows Of Harvard College | Organic light-emitting diode materials |
| CN106065009B (en) | 2014-06-28 | 2019-03-01 | 广东东阳光药业有限公司 | Application as the compound of hepatitis c inhibitor and its in drug |
| CN104211726B (en) | 2014-08-11 | 2017-06-16 | 中南民族大学 | The tooth double-core titanium complex of non-luxuriant class three, Preparation method and use |
| JP2017530959A (en) | 2014-09-17 | 2017-10-19 | エピザイム,インコーポレイティド | CARM1 inhibitors and uses thereof |
| WO2016041511A1 (en) | 2014-09-19 | 2016-03-24 | Yen-Ta Lu | Benzo-heterocyclic compounds and their applications |
| ES2907622T3 (en) | 2014-10-06 | 2022-04-25 | Merck Patent Gmbh | Heteroaryl Compounds as BTK Inhibitors and Uses of These |
| JP2017537940A (en) | 2014-12-10 | 2017-12-21 | マサチューセッツ インスティテュート オブ テクノロジー | Fused 1,3-azole derivatives useful for the treatment of proliferative diseases |
| JP6853619B2 (en) | 2015-01-16 | 2021-03-31 | 大塚製薬株式会社 | Pharmaceutical use of cyanotriazole compounds |
| DE112016000383A5 (en) | 2015-01-20 | 2017-10-05 | Cynora Gmbh | Organic molecules, in particular for use in optoelectronic components |
| EP3261442B1 (en) | 2015-01-20 | 2020-04-01 | Merck Sharp & Dohme Corp. | Iminothiadiazine dioxides bearing an amine-linked substituent as bace inhibitors, compositions, and their use |
| WO2016156282A1 (en) | 2015-04-02 | 2016-10-06 | Bayer Cropscience Aktiengesellschaft | Novel triazole compounds for controlling phytopathogenic harmful fungi |
| WO2017035405A1 (en) | 2015-08-26 | 2017-03-02 | Achillion Pharmaceuticals, Inc. | Amino compounds for treatment of immune and inflammatory disorders |
| US10745382B2 (en) | 2015-10-15 | 2020-08-18 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| MA52119A (en) | 2015-10-19 | 2018-08-29 | Ncyte Corp | HETEROCYCLIC COMPOUNDS USED AS IMMUNOMODULATORS |
| US10633370B2 (en) | 2015-10-21 | 2020-04-28 | University of Pittsburgh—of the Commonwealth System of Higher Education | Phenyl indole allosteric inhibitors of p97 ATPase |
| US9603950B1 (en) | 2015-10-25 | 2017-03-28 | Institute Of Nuclear Energy Research | Compounds of imaging agent with HDAC inhibitor for treatment of Alzheimer syndrome and method of synthesis thereof |
| KR101717601B1 (en) | 2015-11-10 | 2017-03-20 | 한국화학연구원 | Novel compound or pharmaceutically acceptable salt thereof and pharmaceutical composition for prevention or treatment of disease caused by influenza virus infection containing the same as an active ingredient |
| PT3377488T (en) | 2015-11-19 | 2022-11-21 | Incyte Corp | Heterocyclic compounds as immunomodulators |
| MA44075A (en) | 2015-12-17 | 2021-05-19 | Incyte Corp | N-PHENYL-PYRIDINE-2-CARBOXAMIDE DERIVATIVES AND THEIR USE AS MODULATORS OF PROTEIN / PROTEIN PD-1 / PD-L1 INTERACTIONS |
| SI3394033T1 (en) | 2015-12-22 | 2021-03-31 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2017107052A1 (en) | 2015-12-22 | 2017-06-29 | Merck Sharp & Dohme Corp. | Soluble guanylate cyclase stimulators |
| AU2016378482A1 (en) | 2015-12-22 | 2018-07-12 | Synthon B.V. | Pharmaceutical composition comprising amorphous lenalidomide and an antioxidant |
| BR112018012926B1 (en) | 2015-12-22 | 2022-08-16 | Syngenta Participations Ag | PYRAZOLE DERIVATIVES ACTIVE IN TERMS OF PESTICIDES, PESTICIDE COMPOSITION, METHOD FOR PEST CONTROL, METHOD FOR THE PROTECTION OF VEGETABLE PROPAGATION MATERIAL FROM PEST ATTACK AND COATED VEGETABLE PROPAGATION MATERIAL |
| SG10202111399YA (en) | 2015-12-22 | 2021-11-29 | Immatics Biotechnologies Gmbh | Peptides and combination of peptides for use in immunotherapy against breast cancer and other cancers |
| KR101653560B1 (en) | 2016-02-02 | 2016-09-12 | 한국화학연구원 | Novel compound or pharmaceutically acceptable salt thereof and pharmaceutical composition for prevention or treatment of disease caused by influenza virus infection containing the same as an active ingredient |
| PE20190377A1 (en) | 2016-04-22 | 2019-03-08 | Incyte Corp | FORMULATIONS OF AN LSD INHIBITOR 1 |
| WO2017192961A1 (en) | 2016-05-06 | 2017-11-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| EP3464279B1 (en) | 2016-05-26 | 2021-11-24 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| DK3471755T3 (en) | 2016-06-20 | 2020-05-18 | Elanco Us Inc | PEGYLED PIG INTERFERON AND PROCEDURES FOR USING IT |
| PL3472167T3 (en) | 2016-06-20 | 2022-12-19 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP7042812B2 (en) | 2016-06-20 | 2022-03-28 | ノバルティス アーゲー | Crystal form of triazolopyrimidine compound |
| EP3808748A1 (en) | 2016-06-21 | 2021-04-21 | X4 Pharmaceuticals, Inc. | Substituted piperidines as cxcr4-inhibitors |
| EP3484866B1 (en) | 2016-07-14 | 2022-09-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| AU2017305399A1 (en) | 2016-08-03 | 2019-01-31 | Arising International, Inc. | Symmetric or semi-symmetric compounds useful as immunomodulators |
| ES2941716T3 (en) | 2016-08-29 | 2023-05-25 | Incyte Corp | Heterocyclic compounds as immunomodulators |
| SG11201901390TA (en) | 2016-08-30 | 2019-03-28 | Tetraphase Pharmaceuticals Inc | Tetracycline compounds and methods of treatment |
| TWI795381B (en) | 2016-12-21 | 2023-03-11 | 比利時商健生藥品公司 | Pyrazole derivatives as malt1 inhibitors |
| US10858364B2 (en) | 2016-12-21 | 2020-12-08 | Acerta Pharma B.V. | Imidazopyrazine inhibitors of Bruton's tyrosine kinase |
| EP3558973B1 (en) | 2016-12-22 | 2021-09-15 | Incyte Corporation | Pyridine derivatives as immunomodulators |
| US20180177784A1 (en) | 2016-12-22 | 2018-06-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| KR102696516B1 (en) | 2016-12-22 | 2024-08-22 | 인사이트 코포레이션 | Benzoxazole derivatives as immunomodulators |
| MY197501A (en) | 2016-12-22 | 2023-06-19 | Incyte Corp | Tetrahydro imidazo[4,5-c]pyridine derivatives as pd-l1 internalization inducers |
| PE20191541A1 (en) | 2016-12-22 | 2019-10-23 | Calithera Biosciences Inc | COMPOSITIONS AND METHODS TO INHIBIT THE ACTION OF ARGINASE |
| US20180179201A1 (en) | 2016-12-22 | 2018-06-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2018119286A1 (en) | 2016-12-22 | 2018-06-28 | Incyte Corporation | Bicyclic heteroaromatic compounds as immunomodulators |
| JOP20180040A1 (en) | 2017-04-20 | 2019-01-30 | Gilead Sciences Inc | Pd-1/pd-l1 inhibitors |
| CN111225896B (en) | 2017-07-28 | 2024-03-26 | 凯莫森特里克斯股份有限公司 | Immunomodulatory compounds |
| KR102670486B1 (en) | 2017-08-08 | 2024-05-28 | 케모센트릭스, 인크. | Macrocyclic immunomodulators |
| EP3669872A4 (en) | 2017-08-18 | 2021-05-05 | Shanghai Ennovabio Pharmaceuticals Co., Ltd. | Compound having pd-l1 inhibitory activity, preparation method therefor and use thereof |
| EP4212529B1 (en) | 2018-03-30 | 2025-01-29 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP2021520342A (en) | 2018-04-03 | 2021-08-19 | ベータ ファーマシューティカルズ カンパニー リミテッド | Immunomodulators, compositions and methods thereof |
| CA3093130C (en) | 2018-04-19 | 2023-10-17 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| FI4219492T3 (en) | 2018-05-11 | 2025-02-17 | Incyte Corp | Heterocyclic compounds as immunomodulators |
| JP7158577B2 (en) | 2018-10-24 | 2022-10-21 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1/PD-L1 inhibitor |
| SG11202105850YA (en) | 2018-11-02 | 2021-07-29 | Shanghai Maxinovel Pharmaceuticals Co Ltd | Diphenyl-like compound, intermediate thereof, preparation method therefor, pharmaceutical composition thereof and uses thereof |
| US11596692B1 (en) | 2018-11-21 | 2023-03-07 | Incyte Corporation | PD-L1/STING conjugates and methods of use |
| SG11202108367XA (en) | 2019-01-31 | 2021-08-30 | Betta Pharmaceuticals Co Ltd | Immunomodulators, compositions and methods thereof |
| GB201911210D0 (en) | 2019-08-06 | 2019-09-18 | Amlo Biosciences Ltd | Clinical management of oropharyngeal squamous cell carcinoma |
| CA3150434A1 (en) | 2019-08-09 | 2021-02-18 | Incyte Corporation | Salts of a pd-1/pd-l1 inhibitor |
| WO2021053207A1 (en) | 2019-09-20 | 2021-03-25 | Transgene | Combination of a poxvirus encoding hpv polypeptides and il-2 with an anti-pd-l1 antibody |
| AR120109A1 (en) | 2019-09-30 | 2022-02-02 | Incyte Corp | PYRIDO[3,2-D]PYRIMIDINE COMPOUNDS AS IMMUNOMODULATORS |
| IL292524A (en) | 2019-11-11 | 2022-06-01 | Incyte Corp | Salts and crystalline forms of pd-1/pd-l1 inhibitor |
| KR20230006568A (en) | 2020-05-04 | 2023-01-10 | 비욘드스프링 파마수티컬스, 인코포레이티드. | Triple combination therapy to enhance cancer cell death in cancers with low immunogenicity |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| PE20231438A1 (en) | 2020-11-06 | 2023-09-14 | Incyte Corp | PROCESS FOR MAKING A PD-1/PD-L1 INHIBITOR AND SALTS AND CRYSTALLINE FORMS THEREOF |
| WO2022099075A1 (en) | 2020-11-06 | 2022-05-12 | Incyte Corporation | Crystalline form of a pd-1/pd-l1 inhibitor |
| WO2022133176A1 (en) | 2020-12-18 | 2022-06-23 | Incyte Corporation | Oral formulation for a pd-l1 inhibitor |
| US20230149409A1 (en) | 2021-09-24 | 2023-05-18 | Incyte Corporation | Treatment of human papillomavirus-associated cancers by pd-l1 inhibitors |
-
2017
- 2017-06-19 PL PL17734589.9T patent/PL3472167T3/en unknown
- 2017-06-19 UA UAA201900525A patent/UA125391C2/en unknown
- 2017-06-19 SI SI201731245T patent/SI3472167T1/en unknown
- 2017-06-19 BR BR112018076534-1A patent/BR112018076534A2/en not_active Application Discontinuation
- 2017-06-19 MD MDE20190506T patent/MD3472167T2/en unknown
- 2017-06-19 RS RS20220907A patent/RS63673B1/en unknown
- 2017-06-19 CA CA3028685A patent/CA3028685A1/en active Pending
- 2017-06-19 AU AU2017281285A patent/AU2017281285C1/en active Active
- 2017-06-19 EP EP17734589.9A patent/EP3472167B1/en active Active
- 2017-06-19 SM SM20220392T patent/SMT202200392T1/en unknown
- 2017-06-19 HR HRP20221030TT patent/HRP20221030T1/en unknown
- 2017-06-19 PE PE2018003268A patent/PE20190731A1/en unknown
- 2017-06-19 SG SG10202012828TA patent/SG10202012828TA/en unknown
- 2017-06-19 EP EP22184212.3A patent/EP4137489A1/en active Pending
- 2017-06-19 ES ES17734589T patent/ES2927984T3/en active Active
- 2017-06-19 CN CN201780049752.9A patent/CN109890819B/en active Active
- 2017-06-19 MY MYPI2018002678A patent/MY197280A/en unknown
- 2017-06-19 SG SG11201811414TA patent/SG11201811414TA/en unknown
- 2017-06-19 US US15/626,496 patent/US20170362253A1/en not_active Abandoned
- 2017-06-19 DK DK17734589.9T patent/DK3472167T3/en active
- 2017-06-19 WO PCT/US2017/038120 patent/WO2017222976A1/en not_active Ceased
- 2017-06-19 KR KR1020197001807A patent/KR102685249B1/en active Active
- 2017-06-19 JP JP2018566514A patent/JP7000357B2/en active Active
- 2017-06-19 IL IL263825A patent/IL263825B/en unknown
- 2017-06-19 HU HUE17734589A patent/HUE060256T2/en unknown
- 2017-06-19 TW TW106120373A patent/TWI771305B/en active
- 2017-06-19 LT LTEPPCT/US2017/038120T patent/LT3472167T/en unknown
- 2017-06-19 PT PT177345899T patent/PT3472167T/en unknown
- 2017-06-19 MX MX2018016273A patent/MX389513B/en unknown
-
2018
- 2018-02-27 US US15/906,538 patent/US20190040082A1/en not_active Abandoned
- 2018-10-25 US US16/170,898 patent/US20190062345A1/en not_active Abandoned
- 2018-12-19 MX MX2022000789A patent/MX2022000789A/en unknown
- 2018-12-19 CL CL2018003701A patent/CL2018003701A1/en unknown
- 2018-12-20 PH PH12018502710A patent/PH12018502710A1/en unknown
-
2019
- 2019-01-15 CO CONC2019/0000386A patent/CO2019000386A2/en unknown
- 2019-01-18 EC ECSENADI20193773A patent/ECSP19003773A/en unknown
-
2020
- 2020-11-19 US US16/952,872 patent/US11873309B2/en active Active
-
2021
- 2021-10-15 AU AU2021250978A patent/AU2021250978B2/en active Active
- 2021-12-23 JP JP2021209731A patent/JP7394824B2/en active Active
-
2024
- 2024-07-08 ZA ZA2024/05294A patent/ZA202405294B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002000196A2 (en) | 2000-06-28 | 2002-01-03 | Smithkline Beecham P.L.C. | Wet milling process |
| WO2009039397A2 (en) * | 2007-09-20 | 2009-03-26 | Cgi Pharmaceuticals, Inc. | Substituted amides, methods of making, use thereof for the treatment of diseases such as cancer |
| WO2013157021A1 (en) * | 2012-04-20 | 2013-10-24 | Advinus Therapeutics Limited | Bicyclic compounds, compositions and medicinal applications thereof |
Non-Patent Citations (32)
| Title |
|---|
| "Remington's Pharmaceutical Sciences", 1985, MACK PUBLISHING COMPANY, pages: 1418 |
| BARBER ET AL., NATURE, vol. 439, 2006, pages 682 - 7 |
| BERGE ET AL., J. PHARM. SCI., vol. 66, no. 1, 1977, pages 1 - 19 |
| BLANK ET AL., CANCER RES, vol. 64, no. 3, 2004, pages 1140 - 5 |
| BLOM ET AL.: "Optimizing Preparative LC-MS Configurations and Methods for Parallel Synthesis Purification", J. COMBI. CHEM., vol. 5, 2003, pages 670 - 83 |
| BLOM ET AL.: "Preparative LC-MS Purification: Improved Compound Specific Method Optimization", J. COMBI. CHEM, vol. 6, 2004, pages 874 - 883 |
| BLOM: "Two-Pump At Column Dilution Configuration for Preparative LC-MS", J. COMBI. CHEM., vol. 4, 2002, pages 295 - 301 |
| CARTER ET AL., EUR J IMMUNOL, vol. 32, no. 3, 2002, pages 634 - 43 |
| FREEMAN ET AL., J EXP MED, vol. 192, no. 7, 2000, pages 1027 - 34 |
| GREENWALD ET AL., ANNU. REV. IMMUNOL, vol. 23, 2005, pages 515 - 548 |
| HUANG ET AL., ONCOL REP, 2015 |
| IWAI ET AL., PNAS, vol. 99, no. 19, 2002, pages 12293 - 7 |
| KOCIENSKI: "Protecting Groups", 2007, THIEME |
| LATCHMAN ET AL., NAT IMMUNOL, vol. 2, 2001, pages 261 - 268 |
| M. A. POSTOW ET AL: "Immune Checkpoint Blockade in Cancer Therapy", JOURNAL OF CLINICAL ONCOLOGY, vol. 33, no. 17, 20 January 2015 (2015-01-20), US, pages 1974 - 1982, XP055320016, ISSN: 0732-183X, DOI: 10.1200/JCO.2014.59.4358 * |
| NAKAE ET AL., J IMMUNOL, vol. 177, 2006, pages 566 - 73 |
| NISHIMURA ET AL., IMMUNITY, vol. 11, 1999, pages 141 - 151 |
| NISHIMURA ET AL., SCIENCE, vol. 291, 2001, pages 319 - 322 |
| OKAZAKI; HONJO, TRENDS IMMUNOL, 2006, pages 195 - 201 |
| PARRY ET AL., MOL CELL BIOL, 2005, pages 9543 - 9553 |
| PETURSSION ET AL.: "Protecting Groups in Carbohydrate Chemistry", J. CHEM. EDUC., vol. 74, no. 11, 1997, pages 1297 |
| POSTOW ET AL., J. CLINICAL ONCOL, 2015, pages 1 - 9 |
| POSTOW ET AL., J. CLINICAL ONCOLOGY, 2015, pages 1 - 9 |
| ROBERTSON: "Protecting Group Chemistry", 2000, OXFORD UNIVERSITY PRESS |
| SABATIER ET AL., ONCOTARGET, vol. 6, no. 7, 2015, pages 5449 - 5464 |
| SHARPE ET AL., NAT IMMUNOL, vol. 8, 2007, pages 239 - 245 |
| SMITH ET AL.: "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure", 2007, WILEY |
| STAHL ET AL.: "Handbook of Pharmaceutical Salts: Properties, Selection, and Use", 2002, WILEY |
| WANG ET AL., EUR J SURG ONCOL, 2015 |
| WUTS ET AL.: "Protective Groups in Organic Synthesis", 2006, WILEY |
| YOUNG WENDY B ET AL: "Discovery of highly potent and selective Bruton's tyrosine kinase inhibitors: Pyridazinone analogs with improved metabolic stability", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 26, no. 2, January 2016 (2016-01-01), pages 575 - 579, XP029380242, ISSN: 0960-894X, DOI: 10.1016/J.BMCL.2015.11.076 * |
| YOUNG, W.B. ET AL.: "Potent and selective Bruton's tyrosine kinase inhibbitors: Discovery of GDC-0834", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 25, 2015, pages 1333 - 1337, XP002772334 * |
Cited By (163)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11407749B2 (en) | 2015-10-19 | 2022-08-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11572366B2 (en) | 2015-11-19 | 2023-02-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11866435B2 (en) | 2015-12-22 | 2024-01-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11535615B2 (en) | 2015-12-22 | 2022-12-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11608337B2 (en) | 2016-05-06 | 2023-03-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11673883B2 (en) | 2016-05-26 | 2023-06-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11873309B2 (en) | 2016-06-20 | 2024-01-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11793771B2 (en) | 2016-06-27 | 2023-10-24 | Chemocentryx, Inc. | Immunomodulator compounds |
| US11426364B2 (en) | 2016-06-27 | 2022-08-30 | Chemocentryx, Inc. | Immunomodulator compounds |
| US11718605B2 (en) | 2016-07-14 | 2023-08-08 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11613536B2 (en) | 2016-08-29 | 2023-03-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| US11274285B2 (en) | 2016-10-14 | 2022-03-15 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the Hepatitis B virus genome |
| US11787793B2 (en) | 2016-12-22 | 2023-10-17 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10308644B2 (en) | 2016-12-22 | 2019-06-04 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10793565B2 (en) | 2016-12-22 | 2020-10-06 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US12466822B2 (en) | 2016-12-22 | 2025-11-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11566026B2 (en) | 2016-12-22 | 2023-01-31 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11465981B2 (en) | 2016-12-22 | 2022-10-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| EP4223369A1 (en) * | 2016-12-22 | 2023-08-09 | Incyte Corporation | Immunomodulator compounds and methods of use |
| US10800768B2 (en) | 2016-12-22 | 2020-10-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11339149B2 (en) | 2016-12-22 | 2022-05-24 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10806785B2 (en) | 2016-12-22 | 2020-10-20 | Incyte Corporation | Immunomodulator compounds and methods of use |
| WO2018119224A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Tetrahydro imidazo[4,5-c]pyridine derivatives as pd-l1 internalization inducers |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| EP4026835A2 (en) | 2017-04-20 | 2022-07-13 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US11708326B2 (en) | 2017-07-28 | 2023-07-25 | Chemocentryx, Inc. | Immunomodulator compounds |
| US10919852B2 (en) | 2017-07-28 | 2021-02-16 | Chemocentryx, Inc. | Immunomodulator compounds |
| US10392405B2 (en) | 2017-08-08 | 2019-08-27 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| WO2019032547A1 (en) | 2017-08-08 | 2019-02-14 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| US11059834B2 (en) | 2017-08-08 | 2021-07-13 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| US11691985B2 (en) | 2017-08-08 | 2023-07-04 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| EP3750887A4 (en) * | 2018-02-05 | 2021-10-20 | Abbisko Therapeutics Co., Ltd. | BIARYLE DERIVATIVE, ITS PREPARATION PROCESS, AND ITS PHARMACEUTICAL APPLICATION |
| WO2019149183A1 (en) * | 2018-02-05 | 2019-08-08 | 上海和誉生物医药科技有限公司 | Biaryl derivative, preparation method thereof and pharmaceutical application thereof |
| RU2768830C2 (en) * | 2018-02-05 | 2022-03-24 | Аббиско Тхерапеутицс Цо., Лтд. | Biaryl derivative, method for production thereof and pharmaceutical application thereof |
| RU2768830C9 (en) * | 2018-02-05 | 2022-06-17 | Aббиско Тхерaпеутицс Цо., Лтд. | Biaryl derivative, method for production thereof and pharmaceutical application thereof |
| US11459339B2 (en) | 2018-02-05 | 2022-10-04 | Abbisko Therapeutics Co., Ltd. | Biaryl derivative, preparation method thereof and pharmaceutical application thereof |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US11555029B2 (en) | 2018-02-13 | 2023-01-17 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| EP4227302A1 (en) | 2018-02-13 | 2023-08-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US10710986B2 (en) | 2018-02-13 | 2020-07-14 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| US12338233B2 (en) | 2018-02-13 | 2025-06-24 | Gilead Sciences, Inc. | PD-1/Pd-L1 inhibitors |
| US10568874B2 (en) | 2018-02-22 | 2020-02-25 | Chemocentryx, Inc. | Indane-amines as PD-L1 antagonists |
| US11759458B2 (en) | 2018-02-22 | 2023-09-19 | Chemocentryx, Inc. | Indane-amines as PD-L1 antagonists |
| US11135210B2 (en) | 2018-02-22 | 2021-10-05 | Chemocentryx, Inc. | Indane-amines as PD-L1 antagonists |
| WO2019165043A2 (en) | 2018-02-22 | 2019-08-29 | Chemocentryx, Inc. | Indane-amines as pd-l1 antagonists |
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| US11673894B2 (en) | 2018-02-27 | 2023-06-13 | Incyte Corporation | Imidazopyrimidines and triazolopyrimidines as A2A / A2B inhibitors |
| US10669271B2 (en) | 2018-03-30 | 2020-06-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US12247026B2 (en) | 2018-03-30 | 2025-03-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11124511B2 (en) | 2018-03-30 | 2021-09-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP2021520342A (en) * | 2018-04-03 | 2021-08-19 | ベータ ファーマシューティカルズ カンパニー リミテッド | Immunomodulators, compositions and methods thereof |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| US11292812B2 (en) | 2018-04-06 | 2022-04-05 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| US11149052B2 (en) | 2018-04-06 | 2021-10-19 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| US11788077B2 (en) | 2018-04-12 | 2023-10-17 | Precision Biosciences, Inc. | Polynucleotides encoding optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| US11142750B2 (en) | 2018-04-12 | 2021-10-12 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| EP4600247A2 (en) | 2018-04-19 | 2025-08-13 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US10899735B2 (en) | 2018-04-19 | 2021-01-26 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| CN112752756A (en) * | 2018-05-11 | 2021-05-04 | 因赛特公司 | Tetrahydro-imidazo [4,5-c ] pyridine derivatives as PD-L1 immunomodulators |
| CN112752756B (en) * | 2018-05-11 | 2024-06-25 | 因赛特公司 | Tetrahydro-imidazo[4,5-c]pyridine derivatives as PD-L1 immunomodulators |
| US10906920B2 (en) | 2018-05-11 | 2021-02-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP7328995B2 (en) | 2018-05-11 | 2023-08-17 | インサイト・コーポレイション | Tetrahydro-imidazo[4,5-C]pyridine derivatives as PD-L1 immunomodulators |
| JP2021524842A (en) * | 2018-05-11 | 2021-09-16 | インサイト・コーポレイションIncyte Corporation | Tetrahydro-imidazole [4,5-C] pyridine derivative as PD-L1 immunomodulator |
| EP4520328A3 (en) * | 2018-05-11 | 2025-04-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| AU2019267824B2 (en) * | 2018-05-11 | 2023-10-05 | Incyte Corporation | Tetrahydro-imidazo(4,5-C)pyridine derivatives as PD-L1 immunomodulators |
| JP2023162216A (en) * | 2018-05-11 | 2023-11-08 | インサイト・コーポレイション | Tetrahydro-imidazo[4,5-C]pyridine derivatives as PD-L1 immunomodulators |
| US10618916B2 (en) | 2018-05-11 | 2020-04-14 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US12187743B2 (en) | 2018-05-11 | 2025-01-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| KR102806565B1 (en) | 2018-05-11 | 2025-05-14 | 인사이트 코포레이션 | Tetrahydro-imidazo[4,5-c]pyridine derivatives as PD-L1 immunomodulators |
| TWI821288B (en) * | 2018-05-11 | 2023-11-11 | 美商英塞特公司 | Heterocyclic compounds as immunomodulators |
| EP4219492A1 (en) * | 2018-05-11 | 2023-08-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11414433B2 (en) | 2018-05-11 | 2022-08-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2019217821A1 (en) * | 2018-05-11 | 2019-11-14 | Incyte Corporation | Tetrahydro-imidazo[4,5-c]pyridine derivatives as pd-l1 immunomodulators |
| KR20210018282A (en) * | 2018-05-11 | 2021-02-17 | 인사이트 코포레이션 | Tetrahydro-imidazo[4,5-c]pyridine derivatives as PD-L1 immunomodulators |
| US11873304B2 (en) | 2018-05-18 | 2024-01-16 | Incyte Corporation | Fused pyrimidine derivatives as A2A/A2B inhibitors |
| US11999740B2 (en) | 2018-07-05 | 2024-06-04 | Incyte Corporation | Fused pyrazine derivatives as A2A / A2B inhibitors |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US10774071B2 (en) | 2018-07-13 | 2020-09-15 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| EP4234030A2 (en) | 2018-07-13 | 2023-08-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US12269812B2 (en) | 2018-07-13 | 2025-04-08 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| US11236085B2 (en) | 2018-10-24 | 2022-02-01 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| EP4371987A1 (en) | 2018-10-31 | 2024-05-22 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020088357A1 (en) | 2018-11-02 | 2020-05-07 | 上海再极医药科技有限公司 | Diphenyl-like compound, intermediate thereof, preparation method therefor, pharmaceutical composition thereof and uses thereof |
| US11884665B2 (en) | 2019-01-29 | 2024-01-30 | Incyte Corporation | Pyrazolopyridines and triazolopyridines as A2A / A2B inhibitors |
| US12152013B2 (en) | 2019-02-02 | 2024-11-26 | Novaonco Js Therapeutics Co., Ltd. | Vinylpyridine carboxamide compound as PD-L1 immunomodulator |
| KR20210124329A (en) * | 2019-02-02 | 2021-10-14 | 메드샤인 디스커버리 아이엔씨. | Vinyl pyridine carboxamide compound as a PD-L1 immunomodulator |
| KR102668124B1 (en) | 2019-02-02 | 2024-05-23 | 노바온코 제이에스 테라퓨틱스 컴퍼니 리미티드 | Vinyl pyridine carboxamide compounds, PD-L1 immunomodulators |
| WO2020156564A1 (en) * | 2019-02-02 | 2020-08-06 | 南京明德新药研发有限公司 | Vinylpyridine carboxamide compound as pd-l1 immunomodulator |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| US11766447B2 (en) | 2019-03-07 | 2023-09-26 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| US12318403B2 (en) | 2019-03-07 | 2025-06-03 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides and prodrugs thereof |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| EP4458416A2 (en) | 2019-04-17 | 2024-11-06 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| US11266643B2 (en) | 2019-05-15 | 2022-03-08 | Chemocentryx, Inc. | Triaryl compounds for treatment of PD-L1 diseases |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| US11485708B2 (en) | 2019-06-20 | 2022-11-01 | Chemocentryx, Inc. | Compounds for treatment of PD-L1 diseases |
| WO2021007386A1 (en) | 2019-07-10 | 2021-01-14 | Chemocentryx, Inc. | Indanes as pd-l1 inhibitors |
| US11872217B2 (en) | 2019-07-10 | 2024-01-16 | Chemocentryx, Inc. | Indanes as PD-L1 inhibitors |
| WO2021011891A1 (en) | 2019-07-18 | 2021-01-21 | Gilead Sciences, Inc. | Long-acting formulations of tenofovir alafenamide |
| US11753406B2 (en) | 2019-08-09 | 2023-09-12 | Incyte Corporation | Salts of a PD-1/PD-L1 inhibitor |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| US12247038B2 (en) | 2019-09-30 | 2025-03-11 | Incyte Corporation | Pyrido[3,2-d]pyrimidine compounds as immunomodulators |
| US11401279B2 (en) | 2019-09-30 | 2022-08-02 | Incyte Corporation | Pyrido[3,2-d]pyrimidine compounds as immunomodulators |
| EP4458975A2 (en) | 2019-09-30 | 2024-11-06 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021076688A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of pd-l1 diseases |
| WO2021076691A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of pd-l1 diseases |
| US11866429B2 (en) | 2019-10-16 | 2024-01-09 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of PD-L1 diseases |
| US12371433B2 (en) | 2019-10-16 | 2025-07-29 | Amgen Inc. | Heteroaryl-biphenyl amines for the treatment of PD-L1 diseases |
| US11713307B2 (en) | 2019-10-16 | 2023-08-01 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of PD-L1 diseases |
| US11866451B2 (en) | 2019-11-11 | 2024-01-09 | Incyte Corporation | Salts and crystalline forms of a PD-1/PD-L1 inhibitor |
| US12410418B2 (en) | 2019-12-06 | 2025-09-09 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| EP4567109A2 (en) | 2019-12-06 | 2025-06-11 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2021136354A1 (en) | 2020-01-03 | 2021-07-08 | 上海翰森生物医药科技有限公司 | Biphenyl derivative inhibitor, preparation method therefor and use thereof |
| WO2021138512A1 (en) | 2020-01-03 | 2021-07-08 | Incyte Corporation | Combination therapy comprising a2a/a2b and pd-1/pd-l1 inhibitors |
| WO2021147940A1 (en) * | 2020-01-21 | 2021-07-29 | 上海华汇拓医药科技有限公司 | Pd-1/pd-l1 inhibitor, preparation method therefor, and use thereof |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| WO2021226206A2 (en) | 2020-05-05 | 2021-11-11 | Teon Therapeutics, Inc. | Cannabinoid receptor type 2 (cb2) modulators and uses thereof |
| WO2022052926A1 (en) | 2020-09-09 | 2022-03-17 | 广州再极医药科技有限公司 | Aromatic ethylene compound and preparation method therefor, and intermediate, pharmaceutical composition, and application thereof |
| US12404272B2 (en) | 2020-11-06 | 2025-09-02 | Incyte Corporation | Process for making a PD-1/PD-L1 inhibitor and salts and crystalline forms thereof |
| US11866434B2 (en) | 2020-11-06 | 2024-01-09 | Incyte Corporation | Process for making a PD-1/PD-L1 inhibitor and salts and crystalline forms thereof |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| US11760756B2 (en) | 2020-11-06 | 2023-09-19 | Incyte Corporation | Crystalline form of a PD-1/PD-L1 inhibitor |
| US12084443B2 (en) | 2020-11-06 | 2024-09-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| WO2022147092A1 (en) | 2020-12-29 | 2022-07-07 | Incyte Corporation | Combination therapy comprising a2a/a2b inhibitors, pd-1/pd-l1 inhibitors, and anti-cd73 antibodies |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| US11957693B2 (en) | 2021-06-11 | 2024-04-16 | Gilead Sciences, Inc. | Combination MCL-1 inhibitors with anti-cancer agents |
| WO2022261301A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-cancer agents |
| WO2022261310A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-body drug conjugates |
| US11931424B2 (en) | 2021-06-11 | 2024-03-19 | Gilead Sciences, Inc. | Combination MCL-1 inhibitors with anti-body drug conjugates |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2023034530A1 (en) | 2021-09-02 | 2023-03-09 | Teon Therapeutics, Inc. | Methods of improving growth and function of immune cells |
| WO2023081730A1 (en) | 2021-11-03 | 2023-05-11 | Teon Therapeutics, Inc. | 4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide derivatives as cannabinoid cb2 receptor modulators for the treatment of cancer |
| WO2023097211A1 (en) | 2021-11-24 | 2023-06-01 | The University Of Southern California | Methods for enhancing immune checkpoint inhibitor therapy |
| WO2024015372A1 (en) | 2022-07-14 | 2024-01-18 | Teon Therapeutics, Inc. | Adenosine receptor antagonists and uses thereof |
| US12497383B2 (en) | 2023-06-12 | 2025-12-16 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of PD-L1 diseases |
| WO2025080593A1 (en) | 2023-10-09 | 2025-04-17 | Incyte Corporation | Combination therapy using a kras g12d inhibitor and pd-1 inhibitor or pd-l1 inhibitor |
| WO2025122695A1 (en) | 2023-12-06 | 2025-06-12 | Incyte Corporation | Combination therapy comprising dgk inhibitors and pd-1/pd-l1 inhibitors |
| WO2025240243A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with bulevirtide and an inhibitory nucleic acid targeting hepatitis b virus |
| WO2025240246A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
| WO2025240242A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
| WO2025240244A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies comprising bulevirtide and lonafarnib for use in the treatment of hepatitis d virus infection |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2021250978B2 (en) | Heterocyclic compounds as immunomodulators | |
| US11866435B2 (en) | Heterocyclic compounds as immunomodulators | |
| US11407749B2 (en) | Heterocyclic compounds as immunomodulators | |
| US10793565B2 (en) | Heterocyclic compounds as immunomodulators | |
| EP3452476B1 (en) | Heterocyclic compounds as immunomodulators | |
| WO2018119221A1 (en) | Pyridine derivatives as immunomodulators | |
| WO2017087777A1 (en) | Heterocyclic compounds as immunomodulators | |
| HK40089511A (en) | Heterocyclic compounds as immunomodulators | |
| HK40006033B (en) | Heterocyclic compounds as immunomodulators | |
| HK40006033A (en) | Heterocyclic compounds as immunomodulators | |
| NZ788114A (en) | Heterocyclic compounds as immunomodulators | |
| HK40014225B (en) | Triazolo[1,5-a]pyridine derivatives as immunomodulators | |
| HK40014225A (en) | Triazolo[1,5-a]pyridine derivatives as immunomodulators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17734589 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2018566514 Country of ref document: JP Kind code of ref document: A Ref document number: 3028685 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112018076534 Country of ref document: BR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: NC2019/0000386 Country of ref document: CO |
|
| ENP | Entry into the national phase |
Ref document number: 20197001807 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017734589 Country of ref document: EP Effective date: 20190121 |
|
| ENP | Entry into the national phase |
Ref document number: 2017281285 Country of ref document: AU Date of ref document: 20170619 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 112018076534 Country of ref document: BR Kind code of ref document: A2 Effective date: 20181219 |
|
| WWP | Wipo information: published in national office |
Ref document number: NC2019/0000386 Country of ref document: CO |
|
| WWG | Wipo information: grant in national office |
Ref document number: NC2019/0000386 Country of ref document: CO |
|
| WWG | Wipo information: grant in national office |
Ref document number: 749960 Country of ref document: NZ |