WO2016039048A1 - Pyridine compound and use thereof - Google Patents
Pyridine compound and use thereof Download PDFInfo
- Publication number
- WO2016039048A1 WO2016039048A1 PCT/JP2015/072141 JP2015072141W WO2016039048A1 WO 2016039048 A1 WO2016039048 A1 WO 2016039048A1 JP 2015072141 W JP2015072141 W JP 2015072141W WO 2016039048 A1 WO2016039048 A1 WO 2016039048A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- substituted
- unsubstituted
- aryl
- alkyl
- Prior art date
Links
- 0 Cc(c(C)nc(C)c1*ON=C(*)I)c1O* Chemical compound Cc(c(C)nc(C)c1*ON=C(*)I)c1O* 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/50—1,3-Diazoles; Hydrogenated 1,3-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/54—1,3-Diazines; Hydrogenated 1,3-diazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/80—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,2
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/68—One oxygen atom attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D451/00—Heterocyclic compounds containing 8-azabicyclo [3.2.1] octane, 9-azabicyclo [3.3.1] nonane, or 3-oxa-9-azatricyclo [3.3.1.0<2,4>] nonane ring systems, e.g. tropane or granatane alkaloids, scopolamine; Cyclic acetals thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D453/00—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids
Definitions
- the present invention relates to pyridine compounds and uses such as agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides.
- This application claims priority based on Japanese Patent Application No. 2014-184671 filed in Japan on September 10, 2014, the contents of which are incorporated herein by reference.
- control agents In the cultivation of agricultural and horticultural crops, many control agents have been proposed for crop diseases. Most of the proposed control agents have insufficient control efficacy, their use is limited by the emergence of drug-resistant pathogens, cause phytotoxicity and contamination of plants, or toxicity to human and fish And the impact on the environment is not enough. Therefore, there is a strong demand for the emergence of a control agent that can be safely used with few such drawbacks.
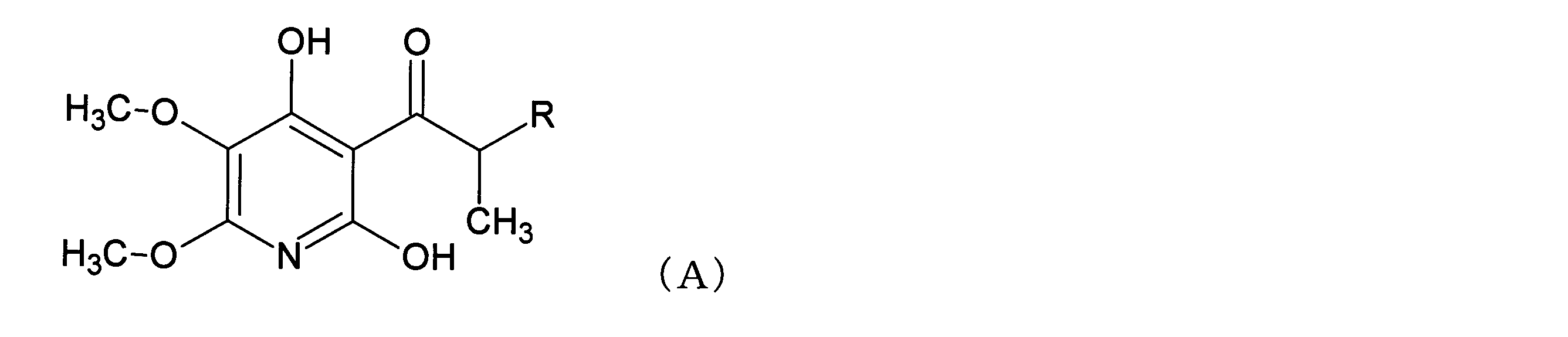
- Patent Document 1 discloses a pyridine compound represented by the formula (A) or the formula (B). According to Patent Document 1, this pyridine compound seems to be useful as a complex II inhibitor of an electron transport system.
- Non-Patent Document 1 discloses a pyridone compound represented by the formula (C). According to Non-Patent Document 1, this pyridone compound seems to be useful as an antimalarial agent.
- An object of the present invention is to provide a novel pyridine compound, an agricultural and horticultural fungicide, a pest control agent, and an insecticide or acaricide.
- R 1 is hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ⁇ 6 alkoxy group, an unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
- R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, unsubstituted or C3-8 cycloalkyl group substituted with G 2 , C1-6 alkoxy group unsubstituted or substituted with G 1 , formyl group, formyloxy group, C1-6 alkyl unsubstituted or substituted with G 1 carbonyloxy group, an unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, (unsubstituted or C1 ⁇ 6 alkoxyimino substituted with G 1) -C1 ⁇ 6 alkyl group, a cyano group, or a halogeno group, Indicates.
- R 1 and R 2 may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which each of R 1 and R 2 is bonded.
- R 4 is a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ⁇ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ⁇ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2
- G a are independently a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, no showing a substituted by a substituted or G 1 a C2 ⁇ 6 alkynyl, unsubstituted or C3 ⁇ 8 cycloalkyl group substituted by G 2 or unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, .
- G b represents a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ⁇ 6 alkynyl group substituted by one, unsubstituted or C3 ⁇ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ⁇ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
- T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C ( ⁇ O) —N (G b ) —.
- * Indicates the bonding position of the group represented by the formula (II).
- Q represents any one of organic groups represented by formula (III) to formula (XII).
- Ar 1 represents an unsubstituted or C6 ⁇ 10 aryl group substituted by G 2 or unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 2,.
- Ar 2 is unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, unsubstituted or C6 ⁇ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ⁇ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
- Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 .
- Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G 2 .
- Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 .
- R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group.
- Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded.
- X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by NR c .
- R c is hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 1-6 alkylcarbonyl group, an unsubstituted or C6 ⁇ 10 aryl group substituted with G 2, unsubstituted or C6 ⁇ 10 arylsulfonyl group substituted with G 2, substituted with unsubstituted or G 1
- a C1-6 alkoxycarbonyl group or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 is shown.
- A is unsubstituted or C1 ⁇ C6 alkylene group substituted with G 3, unsubstituted or C2 ⁇ C6 alkenylene group substituted with G 3, unsubstituted or C2 ⁇ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group.
- B a is unsubstituted or C1 ⁇ C6 alkylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkenylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ⁇ C6 alkyleneoxy C1 ⁇ 6 alkylene group substituted with G 4, unsubstituted or C3 ⁇ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4
- a C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown.
- R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group.
- B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached.
- B b is unsubstituted or C1 ⁇ C6 alkylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkenylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkynylene substituted with G 4 group, an unsubstituted or C3 ⁇ C6 cycloalkylene group substituted by G 4, unsubstituted or C4 ⁇ C6 cycloalkenylene group substituted with G 4, unsubstituted or 3-6 membered substituted by G 4 A heterocyclylene group or a carbonyl group is shown.
- T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
- T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ⁇ 10 aryl C1 ⁇ 6 alkyl)-C1 ⁇ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ⁇ 10 aryl C1 which is substituted by G 2 ⁇ shows the 6 alkoxy)-C1 ⁇ 6 alkyl group or a (unsubstituted or C6 ⁇ 10 aryl C1-6 alkyl substituted with G 2) -3 ⁇ 6-membered heterocyclyl group.
- T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group. * Indicates the bonding position of the organic group represented by formula (III) to formula (XII).
- G 1 is a hydroxyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxycarbonyloxy group, cyano A group or a halogeno group.
- R 1, R 2, R 3 , R 4, R a, R c when it is the group more than two substituted by G 1 of G a or G b, G 1 according the mutual May be the same or different.
- G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 alkoxyC1 ⁇ 6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1 ⁇ 6 haloalkoxy group, C1-6 haloalkoxy C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxy carbonyloxy group, C1 ⁇ 6 alkoxycarbonylamino group, an unsubstituted or
- G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group or a halogeno group. If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
- G 3 is a C1-6 alkyl group, C1-6 alkoxy group, formyl group, C1-6 alkylcarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, halogeno group, C1-6 alkylene group, or oxo group Indicates.
- G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ⁇ 6 alkoxy C1 ⁇ 6 alkoxy group, substituted with C2 ⁇ 6 alkenyloxy group, C1 ⁇ 6 alkoxycarbonyl group, an unsubstituted or C6 ⁇ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ⁇ 10 aryl C1 ⁇ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ⁇
- a pest control agent containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
- An insecticide or acaricide containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2] above, and tautomers and salts thereof.
- An ectoparasite control agent comprising, as an active ingredient, at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
- the pyridine compound according to the present invention is a novel compound that has effects such as pest control, bactericidal, acaricidal, insecticidal and the like, does not cause phytotoxicity on plants, and has little toxicity to human fish and environmental impacts. . In particular, it exhibits an excellent control effect against wheat diseases.
- the pyridine compound according to the present invention is useful as an active ingredient of agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides.
- the pyridine compound according to the present invention includes a compound represented by formula (I) (hereinafter sometimes referred to as compound (I)), a tautomer of compound (I), and a salt of compound (I). It is.
- R 1 is hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ⁇ 6 alkoxy group, an unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
- the C1-6 alkyl group may be linear or branched if it has 3 or more carbon atoms.
- Examples of the C1-6 alkyl group include methyl group, ethyl group, n-propyl group, i-propyl group, n-butyl group, s-butyl group, i-butyl group, t-butyl group, n-pentyl group, n -Hexyl group, i-pentyl group, neopentyl group, 2-methylbutyl group, 2,2-dimethylpropyl group, i-hexyl group and the like.
- C2-6 alkenyl groups include vinyl, 1-propenyl, 2-propenyl (allyl), 1-butenyl, 2-butenyl, 3-butenyl, 1-methylvinyl (isopropenyl) 1-methyl-2-propenyl group, 2-methyl-2-propenyl group, 1-pentenyl group, 2-pentenyl group, 3-pentenyl group, 4-pentenyl group, 1-methyl-2-butenyl group, 2- Examples thereof include a methyl-2-butenyl group, a 1-hexenyl group, a 2-hexenyl group, a 3-hexenyl group, a 4-hexenyl group, and a 5-hexenyl group.
- C1-6 alkoxy groups include methoxy, ethoxy, n-propoxy, n-butoxy, n-pentyloxy, n-hexyloxy, i-propoxy, i-butoxy, s-butoxy , T-butoxy group, i-hexyloxy group and the like.
- Examples of the C6-10 aryl group include a phenyl group, a naphthyl group, an azulenyl group, an indenyl group, an indanyl group, and a tetralinyl group.
- the halogeno group include a fluoro group, a chloro group, a bromo group, and an iodo group.
- the substituent G 1 is a hydroxyl group, a C1-6 alkoxy group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkoxycarbonyl group, a formyloxy group, a C1-6 alkylcarbonyloxy group, a C1-6 alkoxycarbonyloxy group. , A cyano group, or a halogeno group.
- R 1 , R 2 , R 3 , R 4 , R a , R c , G a , or G b are groups substituted with G 1
- G 1 May be the same or different.
- the C1-6 alkoxy group and the halogeno group in the substituent G 1 are as described above.
- Examples of the C1-6 alkoxy group include a methoxymethoxy group and a methoxyethoxy group.
- Examples of the C1-6 alkoxycarbonyl group include a methoxycarbonyl group, an ethoxycarbonyl group, an n-propoxycarbonyl group, an i-propoxycarbonyl group, and a t-butoxycarbonyl group.
- Examples of the C1-6 alkylcarbonyloxy group include an acetyloxy group, a propionyloxy group, and a butyryloxy group.
- Examples of the C1-6 alkoxycarbonyloxy group include a methoxycarbonyloxy group, an ethoxycarbonyloxy group, an n-propoxycarbonyloxy group, an i-propoxycarbonyloxy group, an n-butoxycarbonyloxy group, and a t-butoxycarbonyloxy group. be able to.
- Substituent G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 Alkoxy C1-6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 6 alkoxycarbonyloxy group, C1-6 alkoxycarbonylamino group
- the -6 alkoxycarbonyloxy group, the C6-10 aryl group, and the halogeno group are as described above.
- Examples of the C2-6 alkynyl group include ethynyl group, 1-propynyl group, 2-propynyl group, 1-butynyl group, 2-butynyl group, 3-butynyl group, 1-methyl-2-propynyl group, 2-methyl-3 -Butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 2-methyl-3-pentynyl, 1-hexynyl, 1,1 And -dimethyl-2-butynyl group.
- Examples of the C3-8 cycloalkyl group include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cycloheptyl group, and a 2-adamantyl group.
- the C1-6 alkoxy C1-6 alkyl group is the above-described C1-6 alkoxy group substituted on the C1-6 alkyl group already described.
- C1-6 alkoxy C1-6 alkyl group includes methoxymethyl group, ethoxymethyl group, methoxyethyl group, ethoxyethyl group, methoxy-n-propyl group, ethoxymethyl group, ethoxyethyl group, n-propoxymethyl group, i -Propoxyethyl group, s-butoxymethyl group, t-butoxyethyl group and the like can be mentioned.
- the C3-8 cycloalkyl C1-6 alkoxy group is the above-described C3-6 cycloalkyl group substituted on the C1-6 alkoxy group already described.
- Examples of the C3-8 cycloalkyl C1-6 alkoxy group include a cyclopropylmethoxy group, a cyclobutylmethoxy group, a cyclopentylmethoxy group, a cyclohexylmethoxy group, and a 2- (cyclopropyl) -ethoxy group.
- the C1-6 alkylcarbonyl group is a group in which the above C1-6 alkyl group is bonded to a carbonyl group.
- Examples of the C1-6 alkylcarbonyl group include an acetyl group, a propionyl group, a butyryl group, an isobutyryl group, and a pivaloyl group.
- the C1-6 alkoxycarbonylamino group is a group in which the above-described C1-6 alkoxycarbonyl group is substituted on the amino group.
- Examples of the C1-6 alkoxycarbonylamino group include a methoxycarbonylamino group, an ethoxycarbonylamino group, an n-propoxycarbonylamino group, an i-propoxycarbonylamino group, an n-butoxycarbonylamino group, and a t-butoxycarbonylamino group. be able to.
- the C6-10 aryl C1-6 alkyl group is obtained by substituting the above-mentioned C6-10 aryl group for the C1-6 alkyl group already described. Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
- the C6-10 aryloxy group is a group in which the above-described C6-10 aryl group is substituted on the hydroxyl group.
- Examples of the C6-10 aryloxy group include a phenoxy group and a naphthoxy group.
- the C6-10 aryl C1-6 alkoxy group is the above-described C1-6 alkoxy group substituted by the above-mentioned C6-10 aryl group.
- Examples of the C6-10 aryl C1-6 alkoxy group include a benzyloxy group.
- the 3- to 6-membered heterocyclyl group includes 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom, and a sulfur atom as ring constituent atoms.
- the heterocyclyl group may be monocyclic or polycyclic. In the polycyclic heterocyclyl group, when at least one ring is heterocyclyl, the remaining ring may be a saturated alicyclic ring, an unsaturated alicyclic ring, or an aromatic ring.
- Examples of the 3- to 6-membered heterocyclyl group include a 3- to 6-membered saturated heterocyclyl group, a 5- to 6-membered heteroaryl group, and a 5- to 6-membered partially unsaturated heterocyclyl group.
- Examples of the 3- to 6-membered saturated heterocyclyl group include aziridinyl group, oxiranyl group, azetidinyl group, oxetanyl group, pyrrolidinyl group, tetrahydrofuranyl group, thiazolidinyl group, piperidyl group, piperazinyl group, morpholinyl group, tetrahydropyranyl group, dioxolanyl group, dioxanyl Examples include groups.
- Examples of 5-membered heteroaryl groups include pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl, etc. Can do.
- Examples of the 6-membered heteroaryl group include a pyridyl group, a pyrazinyl group, a pyrimidinyl group, a pyridanidyl group, and a triazinyl group.
- the 3- to 6-membered heterocyclyloxy group is a hydroxyl group substituted with a 3- to 6-membered heterocyclyl group.
- Examples of the 3- to 6-membered heterocyclyloxy group include a pyrazolyloxy group and a pyridyloxy group.
- the 3- to 6-membered heterocyclyloxy group is preferably a 5- to 6-membered heterocyclyloxy group.
- the C1-6 alkylthio group is obtained by substituting a C1-6 alkyl group for an SH group.
- Examples of the C1-6 alkylthio group include methylthio group, ethylthio group, n-propylthio group, n-butylthio group, n-pentylthio group, n-hexylthio group, i-propylthio group, i-butylthio group and the like.
- the C1-6 alkylsulfinyl group is a C1-6 alkyl group bonded to a sulfinyl group.
- Examples of the C1-6 alkylsulfinyl group include a methylsulfinyl group, an ethylsulfinyl group, and a t-butylsulfinyl group.
- the C1-6 alkylsulfonyl group is a sulfonyl group having a C1-6 alkyl group bonded thereto.
- Examples of the C1-6 alkylsulfonyl group include a methylsulfonyl group, an ethylsulfonyl group, and a t-butylsulfonyl group.
- the C6-10 arylthio group is a group in which a C6-10 aryl group is substituted on the SH group.
- Examples of the C6-10 arylthio group include a phenylthio group and a naphthylthio group.
- the C6-10 arylsulfinyl group is a sulfinyl group substituted with a C6-10 aryl group.
- Examples of the C6-10 arylsulfinyl group include a phenylsulfinyl group and a naphthylsulfinyl group.
- the C6-10 arylsulfonyl group is a sulfonyl group substituted with a C6-10 aryl group.
- Examples of the C6-10 arylsulfonyl group include a phenylsulfonyl group and a naphthylsulfonyl group.
- the C6-10 arylamino group is a group in which an amino group is substituted with a C6-10 aryl group.
- Examples of the C6-10 arylamino group include a phenylamino group.
- the C1-6 alkylene group is a divalent group formed by removing two hydrogen atoms from the C1-6 alkane.
- Examples of the C1-6 alkylene group include a methylene group, an ethylene group (dimethylene group), a trimethylene group, a tetramethylene group, and a propane-1,2-diyl group (that is, a propylene group).
- the C1-6 alkylenedioxy group is a divalent group formed by replacing two hydrogen atoms in a C1-6 alkane with an oxy group. Examples of the C1-6 alkylenedioxy group include a methylenedioxy group (—OCH 2 O—), an ethylenedioxy group (—OCH 2 CH 2 O—), and a trimethylenedioxy group.
- Examples of the C6-10 aryl group substituted with a C1-6 alkylenedioxy group as G 2 include a 2,3-dihydro-benzo [1,4] dioxyl group, a benzo [1,3] dioxolyl group, and the like. Can do.
- the C1-6 haloalkyl group, the C2-6 haloalkenyl group, the C2-6 haloalkynyl group, the C1-6 haloalkoxy group, and the C1-6 haloalkylenedioxy group are the C1-6 alkyl groups, C2-6 A halogeno group is substituted on a 6 alkenyl group, a C2-6 alkynyl group, a C1-6 alkoxy group, and a C1-6 alkylenedioxy group.
- C1-6 haloalkyl group includes a fluoromethyl group, a chloromethyl group, a bromomethyl group, a difluoromethyl group, a dichloromethyl group, a dibromomethyl group, a trifluoromethyl group, a trichloromethyl group, a tribromomethyl group, a 1-chloroethyl group, 2,2,2-trifluoroethyl group, 2,2,2-trichloroethyl group, pentafluoroethyl group, 4-fluorobutyl group, 4-chlorobutyl group, 3,3,3-trifluoropropyl group, 2, Examples include 2,2-trifluoro-1-trifluoromethylethyl group, perfluorohexyl group, perchlorohexyl group, 2,4,6-trichlorohexyl group and the like.
- Examples of the C2-6 haloalkenyl group include a 2-chloro-1-propenyl group and a 2-fluoro-1-butenyl group.
- Examples of the C2-6 haloalkynyl group include a 4,4-dichloro-1-butynyl group, a 4-fluoro-1-pentynyl group, and a 5-bromo-2-pentynyl group.
- C1-6 haloalkoxy groups include chloromethoxy, dichloromethoxy, difluoromethoxy, trichloromethoxy, trifluoromethoxy, 1-fluoroethoxy, 1,1-difluoroethoxy, 2,2,2- Trifluoroethoxy group, 1,1,2,2-tetrafluoroethoxy group, pentafluoroethoxy group, 2,2,3,4,4,4-hexafluoro-butoxy group, 1-bromo-1,1,2 , 2-tetrafluoroethoxy group and the like.
- C1-6 alkoxy C1-6 haloalkyl group, C1-6 haloalkoxy C1-6 haloalkoxy group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, and C1-6 haloalkylsulfonyl group are the same as those described above, C1 A halogeno group is substituted on a -6 alkoxy C1-6 alkyl group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkylthio group, a C1-6 alkylsulfinyl group, and a C1-6 alkylsulfonyl group.
- Examples of the C1-6 alkoxy C1-6 haloalkyl group include a difluoro (methoxy) methyl group and a 1,1,1,3,3,3-hexafluoro-2-methoxypropan-2-yl group.
- Examples of the C1-6 haloalkoxy group include a difluoro (methoxy) methoxy group and a 1,2,2-trifluoro-2- (trifluoromethoxy) ethoxy group.
- Examples of the C1-6 haloalkylthio group include a trifluoromethylthio group and a 2,2,2-trifluoroethylthio group.
- Examples of the C1-6 haloalkylsulfinyl group include a trifluoromethylsulfinyl group and a 2,2,2-trifluoroethylsulfinyl group.
- Examples of the C1-6 haloalkylsulfonyl group include a trifluoromethylsulfonyl group and a 2,2,2-trifluoroethylsulfonyl group.
- the substituent G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group, or a halogeno group. These are as already described. If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
- R 1 Hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1 (preferably unsubstituted), unsubstituted or C1 ⁇ 6 alkenyl group substituted with G 1 (preferably C1 ⁇ 6 alkoxycarbonyl group)
- G 1 preferably C1 ⁇ 6 alkoxycarbonyl group
- a C6-10 aryl group preferably a phenyl group
- G 2 preferably a C1-6 alkoxy group
- G 2 preferably a C1-6 alkoxy group
- cyano group or a halogeno group An unsubstituted C1-6 alkyl group or a halogeno group is more preferred.
- R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 3-8 cycloalkyl group substituted with G 2 , an unsubstituted group Or a C1-6 alkoxy group substituted with G 1 , a formyl group, a formyloxy group, an unsubstituted or C1-6 alkylcarbonyloxy group substituted with G 1 , an unsubstituted or C6 substituted with G 2 A 10 aryl group, (unsubstituted or G 1 -substituted C1-6 alkoxyimino) -C1-6 alkyl group, cyano group, or halogeno group;
- a C1-6 alkyl group, a C3-8 cycloalkyl group, a C1-6 alkoxy group, a C1-6 alkylcarbonyloxy group, a C6-10 aryl group, a halogeno group, and a substituent G 1 , G 2 is as described above.
- Examples of the (C1-6 alkoxyimino) -C1-6 alkyl group include a methoxyimino-methyl group and a 1- (ethoxyimino) -ethyl group.
- R 1 and R 2 may together form a 5-6 membered ring with the carbon atom to which each of R 1 and R 2 is attached.
- Examples of such a 5- to 6-membered ring include a cyclopentene ring and a cyclohexene ring.
- R 2 An unsubstituted or G 1 -substituted C1-6 alkyl group (preferably unsubstituted) is preferred.
- R 3 A hydrogen atom, a C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group), A C1-6 alkoxy group which is unsubstituted or substituted with G 1 (preferably unsubstituted), Unsubstituted C3-8 cycloalkyl group (preferably 3-4 cycloalkyl group), (Unsubstituted or G 1 -substituted C 1-6 alkoxyimino) -C 1-6 alkyl group (preferably unsubstituted), Preferably a halogeno group, a cyano group or a formyl group, An unsubstituted or substituted C1-6 alkyl group substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group), an unsubstituted C3-8 cycloalkyl group (preferably a 3-4 cycloalkyl group) or a halogeno group
- R 4 is a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ⁇ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ⁇ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2
- R 4 C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C6-10 aryl C1-6 alkyl group, C1-6 alkylcarbonyl group, C1-6 alkoxy
- the carbonyl group, C1-6 alkylsulfonyl group, substituent G 1 and substituent G 2 are as described above.
- the 3- to 6-membered heterocyclyl C1-6 alkyl group is obtained by substituting a 3- to 6-membered heterocyclyl group for a C1-6 alkyl group.
- the 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc. Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
- the C6-10 arylcarbonyl group is obtained by bonding a C6-10 aryl group to a carbonyl group. Examples of the C6-10 arylcarbonyl group include a benzoyl group.
- Examples of the C2-6 alkenyloxycarbonyl group include a vinyloxycarbonyl group, a 1-propenyloxycarbonyl group, and a 2-propenyloxycarbonyl group (allyloxycarbonyl group).
- Examples of the C1-6 alkylaminocarbonyl group include a methylaminocarbonyl group and a dimethylaminocarbonyl group.
- Examples of the (C1-6 alkylthio) carbonyl group include (methylthio) carbonyl group and (ethylthio) carbonyl group.
- Examples of the C1-6 alkylamino (thiocarbonyl) group include a methylamino (thiocarbonyl) group and a dimethylamino (thiocarbonyl) group.
- G a are independently a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, no showing a substituted by a substituted or G 1 a C2 ⁇ 6 alkynyl, unsubstituted or C3 ⁇ 8 cycloalkyl group substituted by G 2 or unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, .
- G b represents a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ⁇ 6 alkynyl group substituted by one, unsubstituted or C3 ⁇ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ⁇ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
- T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C ( ⁇ O) —N (G b ) —.
- * Indicates the bonding position of the group represented by the formula (II).
- Examples of the group represented by the formula (II) include the following.
- R 4 is a hydrogen atom, A C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkylcarbonyloxy group, a C1-6 alkoxy group, a C1-6 alkoxyC1-6 alkoxy group), A C2-6 alkenyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted), C6-10 aryl C1-6 alkyl group (preferably benzyl group) which is unsubstituted or substituted with G 2 (preferably C1-6 alkyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group) , A C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted), A C1-6 alkoxycarbonyl group which is unsubstituted or substituted with G 1 (preferably a halogeno group), An unsubstituted or G 1 substituted (C1-6 alkylthi
- [Q] Q represents any one of organic groups represented by the formulas (III) to (XII). Note that * indicates the bonding position of the organic group represented by the formulas (III) to (XII).
- R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group.
- the C1-6 alkyl group, the C6-10 aryl group, the substituent G 1 and the substituent G 2 in R a are as described above.
- Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded.
- the 5- or 6-membered ring which is formed by Ar 1 and R a may be mentioned cyclopentene ring, a cyclohexene ring.
- R a is preferably an unsubstituted alkyl group or a hydrogen atom.
- X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by the formula: NR c .
- R c is a hydrogen atom, an unsubstituted or C1 ⁇ 6 alkyl group substituted with G 1, unsubstituted or C2 ⁇ 6 alkenyl group substituted with G 1, substituted by unsubstituted or G 1 have been C1 ⁇ 6 alkyl group, unsubstituted or C6 ⁇ 10 aryl group substituted with G 2, unsubstituted or C6 ⁇ 10 arylsulfonyl group substituted with G 2 or unsubstituted or G 1, A C1-6 alkoxycarbonyl group substituted with or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 .
- C 1-6 alkyl group, C6-10 aryl group, C1-6 alkylcarbonyl group, C6-10 arylcarbonyl group, C6-10 arylsulfonyl group, C1-6 alkoxycarbonyl group, substituent G 1 and substituent in R c G 2 is as already described.
- Examples of the C3-8 cycloalkyloxycarbonyl group include a cyclopropyloxycarbonyl group and a cyclohexyloxycarbonyl group.
- X is preferably an oxygen atom or a divalent group represented by NR c , and more preferably an oxygen atom.
- A is unsubstituted or C1 ⁇ C6 alkylene group substituted with G 3, unsubstituted or C2 ⁇ C6 alkenylene group substituted with G 3, unsubstituted or C2 ⁇ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group.
- the C1-C6 alkylene group for A is as described above.
- the C2-C6 alkenylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkene.
- Examples of the C2 to C6 alkenylene group include an ethenylene group, a propenylene group, and a butenylene group.
- the C2-C6 alkynylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkyne.
- Examples of the C2 to C6 alkynylene group include an ethynylene group, a propynylene group, a butynylene group, and the like.
- Examples of the C1-C6 alkyleneoxy group include a methyleneoxy group (—CH 2 O—) and an ethyleneoxy group (—CH 2 CH 2 O—).
- Examples of the oxy C1-C6 alkylene group include an oxymethylene group (—OCH 2 —) and an oxyethylene group (—OCH 2 CH 2 —).
- G 3 is a C1-6 alkyl group, C1-6 alkoxy group, formyl group, C1-6 alkylcarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, halogeno group, C1-6 alkylene group, or oxo group Indicates.
- the C1-6 alkyl group, C1-6 alkoxy group, C1-6 alkylcarbonyl group, C1-6 alkylcarbonyloxy group, halogeno group, and C1-6 alkylene group are as described above.
- A is a C1-C6 alkylene group which is unsubstituted or substituted with G 3 (preferably a C1-6 alkyl group, C1-6 alkylene group), unsubstituted or G 3 (preferably C1-6 6 alkyl group) is preferably a C2-C6 alkenylene group substituted with an unsubstituted C2-C6 alkynylene group, An unsubstituted or C1-C6 alkylene group substituted with a C1-6 alkylene group and an unsubstituted C2-C6 alkenylene group are more preferred.
- the C1-C6 alkylene group substituted with a C1-6 alkylene group is, for example, a divalent group as shown below (* indicates a bonding position).
- Ar 1 represents an unsubstituted or C6 ⁇ 10 aryl group substituted by G 2 or unsubstituted or 3-10 membered heterocyclyl group which is substituted by G 2,.
- the C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
- the 3- to 10-membered heterocyclyl group is a cyclic group containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom and a sulfur atom as constituent atoms of the ring.
- the heterocyclyl group may be monocyclic or polycyclic. In the polycyclic heterocyclyl group, when at least one ring is heterocyclyl, the remaining ring may be a saturated alicyclic ring, an unsaturated alicyclic ring, or an aromatic ring.
- Examples of the 3- to 10-membered heterocyclyl group include a 3- to 10-membered saturated heterocyclyl group, a 5- to 10-membered heteroaryl group, and a 5- to 6-membered partially unsaturated heterocyclyl group.
- Ar 1 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group) or an unsubstituted or G 2 -substituted 5- to 10-membered heteroaryl group (preferably Is preferably a 5- to 6-membered heteroaryl group, more preferably a pyridyl group, a piperidyl group or a pyrazolyl group.
- G 2 in Ar 1 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group)
- An oxy group preferably a phenoxy group
- a 3-6 membered heterocyclyloxy group preferably a pyridyl group
- unsubstituted or substituted with G 21 preferably a C1-6 haloalkyl group, a C1-6 halogeno group
- Ar 2 is unsubstituted or C6 ⁇ 10 aryl group substituted by G 2, unsubstituted or C6 ⁇ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ⁇ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
- the C6-10 aryl group, C6-10 aryloxy group, 3-6 membered heterocyclyl group, 3-6 membered heterocyclyloxy group, and substituent G 2 are as described above.
- the 3- to 6-membered heterocyclylthio group is a group in which a 3- to 6-membered heterocyclyl group is substituted for the SH group.
- Examples of the 3- to 6-membered heterocyclylthio group include “5- to 6-membered heteroarylthio groups” such as a pyrazolylthio group and a pyridylthio group.
- Ar 2 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group), an unsubstituted or G 2 -substituted C 6-10 aryloxy group (preferably phenoxy group), an unsubstituted or 5-6 membered heteroaryl group substituted with G 2 (preferably pyridyl group, piperidyl group, pyrazolyl group), or a 5-6 membered heteroaryl which is substituted by unsubstituted or G 2
- An aryloxy group preferably a pyridyloxy group is preferable.
- G 2 in Ar 2 is, C1 ⁇ 6 alkyl group, C1 ⁇ 6 haloalkyl group, C1 ⁇ 6 alkoxy group, C1 ⁇ 6 haloalkoxy group, an unsubstituted or G 21 (preferably C1 ⁇ 6 haloalkyl group, C1 ⁇ 6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group) An oxy group (preferably a phenoxy group), a 3-6 membered heterocyclyloxy group (preferably a pyridyl group) which is unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 halogeno group), a cyano group , C1-6 haloalkylenedi
- Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 .
- the C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
- Ar 3 is preferably a phenyl group substituted with G 2 .
- G 2 in Ar 3 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), unsubstituted or G 21 (preferably a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, a halogeno group A C6-10 aryloxy group (preferably a phenoxy group) substituted with a cyano group, a C6-10 aryl C1-6 alkyl group unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group) (preferably preferably benzyl group), C6 ⁇ 10 aryl substituted with unsubstituted or G 21 (preferably
- Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G2.
- the C6-10 arylene group is a divalent group in which two hydrogen atoms in the C6 to C10 aromatic ring are removed.
- Examples of the C6 to C10 arylene group in Ar 4 include a phenylene group and a naphthylene group.
- Ar 4 is preferably an unsubstituted phenylene group.
- Q is (VIII)
- R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
- Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 .
- the 3- to 6-membered heterocyclyl group in Ar 5 and the substituent G 2 are as described above.
- Ar 5 is preferably a 5- to 6-membered heteroaryl group (preferably a pyrazolyl group) which is unsubstituted or substituted with G 2 (preferably a C 1-6 haloalkyl group).
- B a is unsubstituted or C1 ⁇ C6 alkylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkenylene group substituted with G 4, unsubstituted or C2 ⁇ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ⁇ C6 alkyleneoxy C1 ⁇ 6 alkylene group substituted with G 4, unsubstituted or C3 ⁇ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4
- a C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown.
- R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group.
- C1 ⁇ C6 alkylene group, C2 ⁇ C6 alkenylene group, and C2 ⁇ C6 alkynylene radicals are those as already mentioned.
- the C1-C6 alkyl group and the C1-C6 alkoxycarbonyl group in R d are as described above.
- the C1 to C6 alkyleneoxy C1 to 6 alkylene group is a group formed by bonding two C1 to 6 alkylene groups via an oxygen atom. Examples of the C1-C6 alkyleneoxy C1-6 alkylene group include a methyleneoxymethylene group, a methyleneoxyethylene group, and an ethyleneoxymethylene group.
- the C3-C6 cycloalkylene group is a divalent group formed by removing two hydrogen atoms from a C3-C6 cycloalkane.
- Examples of the C3-C6 cycloalkylene group include a cyclopropylene group (1,2-cyclopropylene group), a cyclobutylene group (1,2-cyclobutylene group, 1,3-cyclobutylene group), a cyclopentylene group (1,2 -Cyclopentylene group or 1,3-cyclopentylene group), cyclohexylene group (1,2-cyclohexylene group, 1,3-cyclohexylene group, or 1,4-cyclohexylene group).
- the C4-C6 cycloalkenylene group is a divalent group formed by removing two hydrogen atoms from a C4-C6 cycloalkene.
- Examples of the C4 to C6 cycloalkenylene group include a cyclobutenylene group (1,2-cyclobutenylene group, 1,3-cyclobutenylene group, or 3,4-cyclobutenylene group), a cyclopentenylene group (1,2-cyclopentenylene group).
- a 3- to 6-membered heterocyclylene group is a divalent group formed by removing two hydrogen atoms from a heteroalicyclic compound.
- the heteroalicyclic compound is a non-aromatic compound containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom, and a sulfur atom as constituent atoms of the ring.
- Examples of the 3- to 6-membered heterocyclylene group include a 3- to 6-membered saturated heterocyclylene group or a 5- to 6-membered partially unsaturated heterocyclylene group.
- Examples of the 3- to 6-membered heterocyclylene group include dihydrofurylene group, tetrahydrofurylene group, pyrrolinylene group, pyrrolidinylene group, pyrazolinylene group, pyrazolidinylene group, imidazolinylene group, imidazolidinylene group, oxazolinylene group, oxazolidinylene group, Examples include thiazolinylene group, thiazolidinylene group, isoxazolidinylene group, isothiazolidinylene group, dihydropyranylene group, tetrahydropyranylene group, piperidinylene group, piperazinylene group, morpholinylene group and the like.
- the 3-6 membered heterocyclylene group may
- B a divalent groups represented by substituted C1 ⁇ C6 alkylene or NR d in unsubstituted or G 4 is preferred.
- G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ⁇ 6 alkoxy C1 ⁇ 6 alkoxy group, substituted with C2 ⁇ 6 alkenyloxy group, C1 ⁇ 6 alkoxycarbonyl group, an unsubstituted or C6 ⁇ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ⁇ 10 aryl C1 ⁇ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ⁇
- the C2-6 alkenyloxy group is a hydroxyl group substituted with a C2-6 alkenyl group.
- Examples of the C2-6 alkenyloxy group include a vinyloxy group, a 1-propenyloxy group, and a 2-propenyloxy group (allyloxy group).
- the C2-6 alkenyloxy C1-6 alkyl group is a C1-6 alkyl group substituted with a C2-6 alkenyloxy group.
- Examples of the C2-6 alkenyloxy C1-6 alkyl group include a vinyloxymethyl group and an allyloxymethyl group.
- Examples of the C3-8 cycloalkyl C1-6 alkyl group include a cyclopropylmethyl group and a cyclopentylmethyl group.
- Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
- the 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc.
- Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
- Examples of the C3-8 cycloalkyloxy C1-6 alkyl group include a cyclopropyloxymethyl group and a cyclohexyloxymethyl group.
- Examples of the C6-10 aryloxy C1-6 alkyl group include a phenoxymethyl group and a naphthyloxymethyl group.
- the 3- to 6-membered heterocyclyloxy C1-6 alkyl group is preferably a 5- to 6-membered heteroarylmethyl group such as a pyrazolyloxymethyl group and a pyridyloxymethyl group.
- the C1-6 alkylidene group include a methylidene group and a propane-2-ylidene group.
- G 4 is preferably a C 1-6 alkyl group, an oxo group, or a C 1-6 alkoxyimino group.
- B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached.
- the 5- or 6-membered ring formed by B a and R a may be mentioned cyclopentene ring, a cyclohexene ring.
- T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
- T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ⁇ 10 aryl C1 ⁇ 6 alkyl)-C1 ⁇ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ⁇ 10 aryl C1 which is substituted by G 2 ⁇ shows the 6 alkoxy)-C1 ⁇ 6 alkyl group or a (unsubstituted or C6 ⁇ 10 aryl C1-6 alkyl substituted with G 2) -3 ⁇ 6-membered heterocyclyl group.
- T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
- Examples of the “(C6-10 aryl C1-6 alkoxyimino) -methyl group” in T 1 include a benzyloxyimino-methyl group.
- Examples of the “1- (C6-10 aryl C1-6 alkoxyimino) -ethyl group” for T 1 include a 1- (benzyloxyimino) -ethyl group.
- Examples of the “C6-10 aryl C1-6 alkoxy group” for T 1 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
- Examples of the “(C6-10 aryl C1-6 alkyl) -amino group” for T 2 include a benzylamino group.
- Examples of the “(C6-10 aryl C1-6 alkyl) -formyl-amino group” at T 2 include a benzyl-formylamino group.
- Examples of the “(C6-10 aryl C1-6 alkyl) -C1-6 alkylcarbonyl-amino group” for T 2 include a benzyl-acetylamino group.
- Examples of the “(C6-10 aryl C1-6 alkoxy) -C1-6 alkyl group” for T 2 include a benzyloxymethyl group, a 2-benzyloxy-1-methylpropyl group, and the like.
- Examples of the “(C6-10 aryl C1-6 alkyl) -3-6 membered heterocyclyl group” for T 2 include a 1-benzyl-pyrrolidin-3-yl group.
- Examples of the “C6-10 aryl C1-6 alkoxy group” for T 3 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
- Examples of the “C6-10 arylaminocarbonyl-C1-6 alkyl group” for T 3 include a 1-phenylcarbamoyl-2-methylpropyl group.
- the pyridine compound according to the present invention includes hydrates, various solvates and crystal polymorphs. Furthermore, the pyridine compound according to the present invention includes stereoisomers based on asymmetric carbon atoms, double bonds, etc., and mixtures and tautomers thereof.
- the present invention is a compound represented by the same structural formula, but all compounds such as optical isomers, diastereoisomers, geometrical isomers, etc. having different spatial arrangements of atoms or substituents in the structure. Includes stereoisomers.
- the stereoisomer may be a single substance or a mixture.
- the salt of the compound (I) according to the present invention is not particularly limited as long as it is an agro-horticulturally acceptable salt.
- salts of inorganic acids such as hydrochloric acid and sulfuric acid
- salts of organic acids such as acetic acid and lactic acid
- salts of alkali metals such as lithium, sodium and potassium
- salts of alkaline earth metals such as calcium and magnesium
- iron and copper And salts of organic metals such as ammonia, triethylamine, tributylamine, pyridine, hydrazine, and the like.
- the salt of compound (I) can be obtained from compound (I) by a known method.
- R ⁇ 4a> shows organic groups other than a hydrogen atom and an allyl group among said R ⁇ 4 >.
- R 1 to R 3 and Q have the same meaning as defined above.
- the compound represented by the formula (A) is a compound in which R 4 is an organic group other than a hydrogen atom and an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (A)). ).
- the compound represented by the formula (B) is a compound in which R 4 is a hydrogen atom in the compound (I) (hereinafter sometimes referred to as the compound (A)), and the compound (B) is And can be a production intermediate in the production of compound (A).
- Compound (A) can be produced from compound (B) by using a reagent represented by the formula: R 4a -L, a reagent represented by the formula: (R 4a ) 2 O, and the like.
- L represents a leaving group such as a halogeno group.
- the compound represented by the formula (C) is a compound in which R 4 is an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (C)), and the compound (C) is And can be a production intermediate in the production of compound (B).
- the compound (B) can be produced from the compound (C) by an ordinary allyl group deprotection method, for example, a catalytic reduction method or a palladium catalyst (a method using tetrakistriphenylphosphine palladium or dibenzylideneacetone palladium).
- an ordinary allyl group deprotection method for example, a catalytic reduction method or a palladium catalyst (a method using tetrakistriphenylphosphine palladium or dibenzylideneacetone palladium).
- the agricultural and horticultural fungicide, pesticide, and insecticides or acaricide of the present invention are compound (I), a tautomer of compound (I), or a salt of compound (I) (hereinafter referred to as "this” It may contain at least one selected from “inventive compounds”) as an active ingredient.
- Agricultural and horticultural fungicides of the present invention include a wide variety of filamentous fungi, such as algae (Oomycetes), Ascomycetes, incomplete fungi (Deuteromycetes), basidiomycetes, mating It can be used for controlling plant diseases derived from fungi belonging to fungi (Zygomycetes).
- filamentous fungi such as algae (Oomycetes), Ascomycetes, incomplete fungi (Deuteromycetes), basidiomycetes, mating It can be used for controlling plant diseases derived from fungi belonging to fungi (Zygomycetes).
- Rust disease Puccinia arachidis
- withering disease Pythium debaryanum
- rust spot disease Alternaria alternata
- white silk disease Sclerotium rolfsii
- cucumber: powdery mildew Sphaerotheca fuliginea
- Downy mildew Pseudoperonospora cubensis
- vine blight Mycosphaerella melonis
- vine split disease Fusarium oxysporum
- mycorrhizal disease Sclerotinia sclerotiorum
- gray mold disease Botrytis cinerea
- anthracnose disease Coldletotrichum orbiculare
- black star disease Cladosporium cucumerinum
- brown spot disease Corynespora cassicola
- seedling blight Pythium debaryanam, Rhizoctonia solani Kuhn
- Tomato Gray mold disease (Botrytis cinerea), leaf mold disease (Cladosporium fulvum), plague (Phytophthora infestans), half body wilt disease (Verticillium albo-atrum), powdery mildew (Oidium neolycopersici), ring crest Diseases (Alternaria solani), Subtilis (Pseudocercospora fuligena), etc.
- cabbage root-knot disease (Plasmodiophora brassicae), soft rot disease (Erwinia carotovora), black rot disease (Xanthomonas campesrtis pv. campestris), black spot bacterial disease (Pseudomonas syringae pv. maculicalas, Pseudomonas syringae pv. alisalensis), downy mildew (Peronospora parasitica), mycorrhizal disease (Sclerotinia sclerotiorum), black soot disease (Alternaria brassicicola), gray mold disease (Botrytis cinerea), etc.
- Kidney Sclerotinia sclerotiorum, gray mold Diseases (Botrytis cinerea), anthrax (Colletotrichum lindemuthianum), keratosis (Phaeoisariopsis griseola), etc.
- Apples powdery mildew (Podosphaera leucotricha), black spot disease (Venturia inaequalis), monilinia disease (Monilinia mali), black spot disease (Mycosphaerella pomi), rot disease (Valsa mali), spotted leaf disease (Alternaria mali), red star disease (Gymnosporang) yamadae), ring rot (Botryosphaeria berengeriana), anthracnose (Glomerella cingulata, Colletotrichum acutatum), brown spot (Diplocarpon mali), soot spot (Zygophiala jamaicensis), soot spot (Gloeodes pomigena), purple coat rot (Helicobasidium mompa), gray mold disease (Botrytis cinerea), etc.
- Ume black rot (Cladosporium carpophilum), gray mold disease (Botrytis cinerea), gray star disease (Monilinia mumecola), etc.
- Oysters powdery mildew (Phyllactinia kakicola), anthrax Diseases such as Gloeosporium kaki, Cercospora kaki, peaches: Monilinia fructicola, black scab (Cladosporium carpophilum), homopsis spoilage (Phomopsis sp.), Perforated bacteria (Xanthomonas campestris pv.
- Pruni and other almonds Monilinia laxa, spot disease (Stigmina carpophila), black spot disease (Cladosporium carpophilum), leaf blight disease (Polystigma rubrum), spotted leaf disease (Alternaria alternata), anthrax Sugar beetle (Colletotrichum gloeospoides), etc.
- Sweet potato Monilinia fructicola, anthracnose (Colletotrichum acutatum), black spot (Alternaria sp.), Larvae nuclear disease (Monilinia kusanoi), etc.
- Grapes Gray mold disease (Botrytis) cinerea), powdery mildew (Uncinula necator), late rot (Glomerella cingulata, Colletotrichum acutatum), downy mildew (Plasmopara viticola), black mildew (Elsinoe ampelina), brown spot (Pseudocercospora vitis), black rot ( Guignardia bidwellii), white rot (Coniella castaneicola), etc.
- Pear Venturia nashicola, Red Star Disease (Gymnosporangium asiaticum), Black Spot Disease (Alternaria kikuchiana), Ring Ring Disease (Botryosphaeria berengeriana) Powdery mildew (Phyllactinia mali), head blight (Phomopsis fukushii), brown spot (Stemphylium vesicarium), anthracnose (Glomerella cingulata), etc.
- Rice Rice blast (Pyricularia oryzae), blight (Rhizoctonia solani), idiot seedling (Gibberella fujikuroi), sesame leaf blight (Cochliobolus miyabeanus), seedling blight (Pythium graminicolum , White leaf blight (Xanthomonas oryzae), seedling blight (Burkholderia plantarii), brown streak (Acidovorax avenae), blight blight (Burkholderia glumae), streak blight (Cercospora oryzae), rice leaf blight (Ustilaginoidea) virens), brown rice (Alternaria alternata, Curvularia intermedia), belly black rice (Alternaria padwickii), red rice (Epicoccam purpurascenns), etc.
- Tobacco Sclerotinia sclerotiorum, powdery mildew (Erysiphe cichoracearum), plague ), Etc.
- Tulip Gray mold disease (Botrytis cinerea)
- Sunflower Downy mildew (Plasmopara halstedii), Mycorrhizal disease (Sclerotinia sclerotiorum), etc.
- Bentgrass Snow rot (Sclerotinia borealis), Large patch (Rhizoctonia solani) , Dollar spot (Sclerotinia homoeocarpa), blast (Pyricularia sp.), Red blight (Pythium aphanidermatum), anthracnose (Colletotrichum graminicola) Orchardgrass: powdery mildew (Erysiphe graminis), etc.
- Soybean Purpura (Cercospora kikuchii), downy mildew (Peronospora manshurica), stem blight (Phytophthora sojae), rust (Phakopsora pachyrhizi), sclerotia (Sclerotinia sclerotiorum) Anthracnose (Colletotrichum truncatum), gray mold (Botrytis cinerea), etc.
- Potato Phytophthora infestans, summer plague (Aleternaria solani), black rot (Thanatephorus cucumeris), etc.
- Banana Panama disease (Fusarium oxysporum), Shiga (Mycosphaerella fijiensis, Mycosphaerella musicola), etc.
- Rapeseed Sclerotinia sclerotiorum, root rot (Phoma lingam), black spot (Alternaria brassicae), etc.
- Coffee Rust (Hemileia vastatrix), Anthrax (Colletotrichum coffeanum) Brown eye disease (Cercospora coffeicola), etc.
- the insecticide or acaricide of the present invention is excellent in controlling pests such as various agricultural pests and mites that affect the growth of plants.
- the insecticide or acaricide of the present invention is effective not only for susceptible strains but also for pests of strains that have developed resistance to conventional agents such as organophosphorus agents and carbamate agents.
- Representative examples of pests of resistant strains include diamondback moth, planthopper, leafhopper and aphids.
- the pyridine compound according to the present invention is effective at all stages of development of organisms to be controlled, and exhibits an excellent control effect on eggs, nymphs, larvae and adults such as mites and insects.
- the pest control agent of the present invention is effective for controlling pests other than agricultural pests and mites.
- pests include ectoparasites and sanitary pests.
- the agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention include grains; vegetables; root vegetables; potatoes; trees such as fruit trees, tea, coffee, cacao; Turf; preferably used for plants such as cotton.
- the agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention are plant parts such as leaves, stems, stalks, flowers, buds, fruits, seeds, sprout, roots, tubers, It can be applied to tuberous roots, shoots and cuttings.
- GMO genetically modified organisms
- the agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention are seed treatments and foliage that are used to control various diseases occurring in agricultural and horticultural crops including flowers, turf, and grass. It can be used for spraying, soil application, water surface application, etc.
- the agricultural and horticultural fungicide, pest control agent, and insecticide or acaricide of the present invention are other agricultural and horticultural agents having effects such as bactericidal, insecticidal / miticidal, nematicidal, soil-killing insect pests; You may mix or use together with a regulator, a synergist, a fertilizer, a soil conditioner, animal feed, etc. An example is shown below.
- Nucleic acid biosynthesis inhibitors (A) RNA polymerase I inhibitor: benalaxyl, benalaxyl-M, furaxyl, metalaxyl, metalaxyl-M; oxadixil; cloziracone, off-race; (B) adenosine deaminase inhibitor: bupilimate, dimethylylmol, ethylimol; (C) DNA / RNA synthesis inhibitors: Himexazole, octirinone; (D) DNA topoisomerase II inhibitor: oxophosphate;
- Mitotic fission inhibitor and cell division inhibitor (A) ⁇ -tubulin polymerization inhibitor: benomyl, carbendazim, chlorphenazole, fuberidazole, thiabendazole; thiophanate, thiophanate-methyl; dietofencarb; zoxamide; ethaboxam; (B) Cell division inhibitor: Penciclone; (C) Delocalization inhibitor of spectrin-like protein: fluopicolide;
- Respiratory inhibitor (A) Complex I NADH oxidoreductase inhibitor: diflumetrim; tolfenpyrad; (B) Complex II succinate dehydrogenase inhibitors: benodanyl, flutolanil, mepronil; isofetamide; fluopyram; fenfram, flumecyclox; carboxin, oxycarboxyl; tifluzamide; , Furametopyr, isopyrazam, penflufen, penthiopyrad, sedaxane; boscalid; (C) Complex III ubiquinol oxidase Qo inhibitor: azoxystrobin, cumoxystrobin, cumethoxystrobin, enoxastrobin, fluphenoxystrobin, picoxystrobin, pyroxystrobin; Piramethostrobin, triclopyricarb; Cresoxime-methyl, trifloxystrobin; Dimoxystrobin, Phenaminestrobin, Metominostrobin
- Signaling inhibitor (A) Signaling inhibitor: quinoxyphene, proquinazide; (B) MAP • histidine kinase inhibitor in osmotic signal transduction: fenpiclonil, fludioxonil; clozolimate, iprodione, procymidone, vinclozolin;
- Lipid and cell membrane synthesis inhibitors (A) Phospholipid biosynthesis, methyltransferase-inhibitors: edifenphos, iprobenphos, pyrazophos; isoprothiolane; (B) lipid peroxidants: biphenyl, chloroneb, dichlorane, kinden, technazene, tolcrofosmethyl; etridiazole; (C) Agents that act on cell membranes: iodocarb, propamocarb, propamocarb hydrochloride, propamocarbfocetylate, prothiocarb; (D) Microorganisms that disturb the cell membrane of pathogenic bacteria: Bacillus subtilis, Bacillus subtilis QST713 strain, Bacillus subtilis FZB24 strain, Bacillus subtilis MBI600 strain, Bacillus subtilis D747 strain; (E) Agents that disrupt the cell membrane: an extract of Goseika Yupte (Tea) P
- Cell membrane sterol biosynthesis inhibitors (A) Demethylation inhibitor at the C14 position in sterol biosynthesis: Trifolin; Triflumizole, biniconazole; Azaconazole, viteltanol, bromconazole, cyproconazole, diclobutrazole, difenoconazole, diniconazole, diniconazole-M, epoxiconazole, etaconazole, fenbuconazole, fluquinconazole, flusilazole, flutriazole), fluconazole, fluconazole- Cis, hexaconazole, imibenconazole, ipconazole, metconazole, microbutanyl, penconazole, propiconazole, quinconazole, cimeconazole, tebuconazole, tetraconazole, triadimethone, triadimenol, triticonazole; prothio
- Trehalase inhibitor Validamycin
- B chitin synthase inhibitor: polyoxin, polyoxolim
- C Cellulose synthase inhibitor: dimethomorph, fulmorph, pyrimorph
- Bench Avaricarb Iprovaricarb, Toluprocarb, Variphenate; Mandipropamide
- Melanin biosynthesis inhibitor (a) Reductase inhibitor of melanin biosynthesis: Fusaride; Pyroxylone; Tricyclazole; (B) Dehydrase inhibitor of melanin biosynthesis: carpropamide; diclocimet; phenoxanyl;
- Agents having multiple action points copper (copper salt), Bordeaux liquid, copper hydroxide, copper naphthalate, copper oxide, copper oxychloride, copper sulfate, sulfur, sulfur products, calcium polysulfide; farbum, mancozeb, maneb, Mankappa, methylam, polycarbamate, propineb, thiram, dineb, ziram; captan, captahol, phorpet; chlorothalonil; diclofluuride, tolylfluanid; guazatine, iminotadine acetate, iminoctadine albecate; anilazine; dithianone; Fluorimide;
- Acetylcholinesterase inhibitor (A) Carbamate series: Alanicarb, Aldicarb, Bengiocarb, Benfuracarb, Butcarboxyme, Butoxycarboxyme, Carbaryl, Carbofuran, Carbosulfan, Ethiophenecarb, Fenobucarb, Formethanate, Furatiocarb, Isoprocarb, Methiocarb, Mesomil, Oxamyl, Pirimicarb, Propoxycarb Thiodicarb, thiophanox, triazamate, trimetacarb, XMC, xylylcarb; phenothiocarb, MIPC, MPMC, MTMC, aldoxicarb, alixicarb, aminocarb, bufencarb, cloetocarb, metam sodium, promecarb;
- GABA-agonist chloride channel antagonists chlordane, endosulfan, etiprole, fipronil, pyrafluprole, pyriprole; camfechlor, heptachlor, dienochlor; (3) Sodium channel modulators: Acrinatrin, d-cis-trans allethrin, d-transarethrin, bifenthrin, bioareslin, bioareslin isomers, violesmethrin, cycloprotorin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda- Cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, ciphenothrin [(1R) -trans is
- Nicotinic acetylcholine receptor agonists acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, nithiazine, thiacloprid, thiamethoxam, sulfoxafurol, nicotine; (5) Nicotinic acetylcholine receptor allosteric modulators: spinetoram, spinosad; (6) Chloride channel activator: abamectin, emamectin benzoate, repimectin, milbemectin; ivermectin, selamectin, doramectin, eprinomectin, moxidectin, milbemycin, milbemycin oxime; (7) Juvenile hormone-like substances: hydroprene, quinoprene, mesoprene, phenoxycarb, pyriproxyfen; geofenolan, ep
- Mite growth inhibitor clofentezin, diflovidazine, hexythiazox, etoxazole;
- Microbial-derived insect midgut mesentery Bacillus thuringiensis subsp. Isla elensis, Bacillus sphaericus, Bacillus thuringiensis subsp. Aisawai, Bacillus thuringiensis subsp. Kurstaki, Bacillus thuringiensis subsp.
- Crop proteins Cry1Ab, Cry1Ac, Cry1Fa, Cry1A.105, Cry2Ab, Vip3A, mCry3A, Cry3Ab, Cry3Bb, Cry34Ab1 / Cry35Ab1; (12) Mitochondrial ATP biosynthetic enzyme inhibitors: diafenthiuron, azocyclotin, cyhexatin, phenbutasine oxide, propargite, tetradiphone; (13) Oxidative phosphorylation uncoupler: chlorfenapyr, sulframide, DNOC; binapacryl, dinobutone, dinocup; (14) Nicotinic acetylcholine receptor channel blockers: bensultap, cartap hydrochloride; nereistoxin; thiosultap monosodium salt, thiocyclam; (15) Chitin synthesis inhibitor: bistrifluron, chlorfluazuron, diflu
- the compound of the present invention is excellent in the control effect of ectoparasites that are harmful to humans. In addition, it is a highly safe compound due to its low phytotoxicity and low toxicity to fish and warm-blooded animals. Therefore, it is useful as an active ingredient of an ectoparasite control agent.
- ectoparasites include ticks, lice and fleas.
- host animals to be treated with the ectoparasite control agent of the present invention include pets such as dogs and cats; pets; domestic animals such as cattle, horses, pigs, and sheep; It is done. In addition, a bee is mentioned.
- Ectoparasites parasitize in and on host animals, particularly warm-blooded animals.
- the ectoparasite control agent of the present invention can be applied by a known veterinary technique (topical, oral, parenteral or subcutaneous administration).
- the method it is administered orally to animals by tablet, capsule, feed mixing, etc .; it is administered to animals by immersion liquid, suppository, injection (intramuscular, subcutaneous, intravenous, intraperitoneal, etc.); A method of locally administering an aqueous solution by spraying, pour-on, spot-on, etc .; kneading an ectoparasite-controlling agent into a resin, shaping the kneaded product into an appropriate shape such as a collar or ear tag, and applying it to an animal A method of wearing and administering locally;
- the agricultural and horticultural fungicide, pesticide, and insecticide or acaricide of the present invention are not particularly limited depending on the dosage form.
- dosage forms such as wettable powder, emulsion, powder, granule, aqueous solvent, suspension, granular wettable powder, and tablet can be exemplified.
- the preparation method to a formulation is not specifically limited, A well-known preparation method can be employ
- the pharmaceutical formulation shown below is only an example, and can be modified without departing from the gist of the present invention, and the present invention is not limited by the following pharmaceutical examples. “Parts” means “parts by weight” unless otherwise specified.
- Formulation 1 wettable powder
- Compound of the present invention 40 parts Diatomaceous earth 53 parts Higher alcohol sulfate 4 parts Alkyl naphthalene sulfonate 3 parts The above is uniformly mixed and finely pulverized to obtain a wettable powder of 40% active ingredient.
- Formulation 4 Granules
- Compound of the present invention 5 parts Clay 73 parts Bentonite 20 parts Dioctyl sulfosuccinate sodium salt 1 part Potassium phosphate 1 part
- the above components are pulverized and mixed well, mixed well with water, granulated and dried, and 5% active ingredient. Get the granules.
- Step 2 Synthesis of ethyl 4-allyloxy-2,5,6-trimethylnicotinate 12.2 g of ethyl 4-hydroxy-2,5,6-trimethylnicotinate was dissolved in 120 ml of acetonitrile. To this solution, 16.1 g of potassium carbonate was added, and then 14.1 g of allyl bromide was added, and the mixture was heated to reflux for 8 hours. The reaction was then cooled to room temperature, poured into water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 10.0 g (yield 77%) of the target compound.
- Step 3 Synthesis of (4-allyloxy-2,5,6-trimethylpyridin-3-yl) -methanol 2.8 g of lithium aluminum hydride was added to 300 ml of tetrahydrofuran. To this solution, 10.0 g of ethyl 4-methoxy-2,5,6-trimethylnicotinate was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was ice-cooled, and water was added until unreacted lithium sodium hydride was decomposed. Subsequently, it was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 7.9 g (yield 97%) of the target compound.
- Step 4 Synthesis of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine (7.9 g of (4-methoxy-2,5,6-trimethylpyridin-3-yl) -methanol in dichloromethane Dissolved in 100 ml. To this solution, 9.0 g of thionyl chloride was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was poured into saturated aqueous sodium hydrogen carbonate, and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate.
- the resulting reaction solution was poured into ice water and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 7.39 g (yield 82%) of the target compound.
- Step 6 Synthesis of 2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] methyl] pyridin-4-ol 4-allyloxy-2,3,6- 0.5 g of trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] pyridine was dissolved in 10 ml of methanol. To this solution, 0.03 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate were added, and the mixture was stirred at room temperature for 2 hours. The reaction solution was poured into ice water and then extracted with chloroform.
- intersecting two lines representing the carbon-nitrogen double bond in the oxime part in the chemical formula indicate that E orientation and / or Z orientation exist.
- Step 4 3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-pyridin-4-ol
- methanol 20 ml
- Step 4 Synthesis of 4-allyloxy-3-[(E) -3-bromoprop-1-enyl] -2,5,6-trimethylpyridine
- E -3- (4-allyloxy-2,5,6 -Trimethyl-3-pyridyl) prop-2-en-1-ol 3.58 g was dissolved in 70 ml of dichloromethane. To this solution, 5.34 g of carbon tetrabromide and 4.22 g of triphenylphosphine were added under ice cooling, and the mixture was stirred overnight at room temperature. The solvent was distilled off from the obtained reaction solution under reduced pressure.
- Step 6 Synthesis of 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol 4-Allyloxy-3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridine (0.52 g) in 10 ml of methanol Dissolved in. To this solution were added 0.01 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate, and the mixture was stirred at room temperature for 2 hours.
- Step 2 Synthesis of [3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethyl-4-pyridyl] methyl carbonate
- Me is a methyl group
- Et is an ethyl group
- n Pr is a normal propyl group
- i Pr is an isopropyl group
- c Pr is a cyclopropyl group
- n Bu is a normal butyl group
- t Bu is a tertiary butyl group
- n Hex is a normal hexyl group
- c Hex is a cyclohexyl group
- n Non is a normal nonyl group
- Ph is a phenyl group
- Py is a pyridyl group
- Ac is an acyl group.
- Table 1 shows substituents in the compound represented by the formula (I-1).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- E the case where the double bond of the imine in the compound is in the E configuration
- Z the case where the imine is in the Z configuration
- EZ the case where both the configurations are mixed is indicated as “EZ”.
- A-12 and A-13 in Table 1 are stereoisomers.
- A-17 and A-18, A-21 and A-22, and A-23 and A-24 are stereoisomers.
- Table 2 shows substituents in the compound represented by the formula (I-2).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- E The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
- Table 3 shows substituents in the compound represented by the formula (I-3).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- E The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
- Table 4 shows substituents in the compound represented by the formula (I-4).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- E the case where the double bond of the imine in the compound is in the E configuration
- Z the case where it is in the Z configuration as “Z”
- EZ both configurations are mixed as “EZ”.
- Table 5 shows substituents in the compound represented by the formula (I-5).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- B b is an asymmetric partial structure
- a carbon marked with * in the structural formula represents a carbon bonded to X
- a carbon marked with ** represents a carbon bonded to Ar 2 .
- E the double bond in A
- Z the case where it is Z configuration is expressed as “Z”.
- Table 6 shows substituents in the compound represented by the formula (I-6). The case where the double bond of the imine in the compound is in the E configuration is indicated as “E”, the case where the imine is in the Z configuration is indicated as “Z”, and the case where both the configurations are mixed is indicated as “EZ”.
- Table 7 shows the substituents in the compound represented by the formula (I-7).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- Table 8 shows the substituents in the compound represented by the formula (I-8).
- E the case where the double bond of the imine in the compound is in the E configuration
- Z the case where it is in the Z configuration
- EZ the case where both configurations are mixed as “EZ”.
- Table 9 shows substituents in the compound represented by the formula (I-9).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- the carbon marked with * in the structural formula represents a carbon bonded to X.
- Table 10 shows the substituents in the compound represented by the formula (I-10).
- E the case where the double bond of the imine in the compound is in the E configuration
- Z the case where it is in the Z configuration
- EZ the case where both configurations are mixed as “EZ”.
- Table 11 shows substituents in the compound represented by the formula (I-11). Note that the carbon marked with * in the structural formula at T 2 represents carbon bonded to oxygen.
- Table 12 shows substituents in the compound represented by the formula (I-12).
- Table 14 shows substituents in the compound represented by the formula (I-13).
- Table 15 shows substituents in the compound represented by the formula (I-14).
- the carbon marked with * in the structural formula of Ar 3 represents carbon bonded to an adjacent group.
- Table 16 shows substituents in the compound represented by Formula (I-15).
- A is an asymmetric partial structure
- the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
- Table 1 shows the 1 H-NMR data for some of the compounds described in Tables 1 to 16 above.
- Wheat powdery mildew control test-1 An emulsion of 5% active ingredient was prepared by mixing and dissolving 5 parts of the compound of the present invention, 1.5 parts of polyoxyethylene sorbitan monolaurate and 93.5 parts of dimethylformamide. The emulsion was diluted with water so that the compound of the present invention was 100 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C.
- control value 100 ⁇ ⁇ area where lesions appear (treated area) / area where lesions appear (untreated area) ⁇ ⁇ 100
- the compounds shown in Table 18 were subjected to wheat powdery mildew control test. All the compounds had a control value of 75% or more.
- Test Example 2 Wheat red rust control test-1 An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 100 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section).
- Test Example 3 Wheat powdery mildew control test-2 An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C. Four days later, the appearance of lesions on the leaves was examined (treatment area). In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
- Test Example 4 Wheat red rust control test-2 An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section). In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
- the compound shown in Table 21 was subjected to a wheat red rust control test. All the compounds showed a control value of 75% or more.
- Test Example 5 Efficacy confirmation test against Japanese scallop
- an emulsion of 5% active ingredient was prepared.
- the emulsion was diluted with water so that the compound of the present invention was 125 ppm.
- Cabbage leaves were immersed in the diluted solution for 30 seconds.
- the cabbage leaves were placed in a petri dish and 5 second instar larvae were released.
- the petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%.
- life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
- Test Example 6 Efficacy confirmation test for cotton aphids Cucumbers were raised in 3-inch pots, and cotton aphid nymphs were inoculated on the first true leaves.
- An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cucumber seedlings.
- the cucumber was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, cotton aphids were evaluated for life and death, and the insecticidal rate was calculated. The test was performed in duplicate.
- the compounds shown in Table 23 were tested for efficacy against cotton aphids. All compounds had an insecticidal rate of 100%.
- Test Example 7 Efficacy confirmation test against nymph spider mung bean seedlings were grown in 3 inch pots, and 10 Kanzawa spider mite female adults were inoculated on primary leaves.
- An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings.
- the green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 3 days had passed since the spraying, the life and death of adults were determined, and the insecticidal rate was calculated. The test was performed in duplicate.
- Test Example 8 Efficacy Confirmation Test for Ayayoto An emulsion prepared by the same method as Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm. Corn leaves were immersed in the diluent for 30 seconds. This corn leaf was put into a petri dish and 5 larvae of the second instar larvae were released. The petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 6 days had passed since the release, life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
- Test Example 9 Efficacy confirmation test for bean aphids Cowpeas were raised in 3-inch pots, and bean aphid nymphs were inoculated on the primary leaves.
- An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cowpea seedlings.
- the cowpea was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, the aphid of the bean aphid was determined and the insecticidal rate was calculated. The test was performed in duplicate.
- the compounds shown in Table 26 were tested for efficacy against bean aphids. All compounds had an insecticidal rate of 80% or more.
- Test Example 10 Efficacy Confirmation Test for Kanzawa Spider Mite Green beans were bred in 3 sized pots, and 10 adult female Kanzawa spider mites were inoculated on the primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings. The green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 10 days have passed since spraying, adults were determined to be alive or dead, and the insecticidal rate was calculated. The test was performed in duplicate.
- the pyridine compound according to the present invention is a novel compound that has effects such as pest control, bactericidal, acaricidal, insecticidal and the like, does not cause phytotoxicity on plants, and has little toxicity to human fish and environmental impacts. . In particular, it exhibits an excellent control effect against wheat diseases.
- the pyridine compound according to the present invention is useful as an active ingredient of agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides, and is industrially useful.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Environmental Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Plant Pathology (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pyridine Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
The present invention provides a compound which is represented by formula (I-1) (wherein R4 represents a hydrogen atom, a C1-6 alkyl group or the like; Ar1 represents a C6-10 aryl group, a 3-6 membered heterocyclyl group or the like; A represents a C1-C6 alkylene group, a C2-C6 alkenylene group or the like; Ra represents a hydrogen atom, a C1-6 alkyl group, C6-10 aryl group or an amino group; and Ar1 and Ra may combine with each other to form a 5- or 6-membered ring together with a carbon atom to which Ar1 and Ra are bonded) or the like, or a tautomer or salt of the compound. The present invention also provides a bactericide for agricultural and horticultural use, a pest control agent, and an insecticidal or acaricidal agent, each of which contains, as an active ingredient, at least one substance selected from the group consisting of the compound, and a tautomer and salt of the compound.
Description
本発明は、ピリジン化合物、並びに農園芸用殺菌剤、有害生物防除剤、および殺虫または殺ダニ剤などの用途に関する。
本願は、2014年9月10日に、日本に出願された特願2014-184671号に基づき優先権を主張し、その内容をここに援用する。 The present invention relates to pyridine compounds and uses such as agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides.
This application claims priority based on Japanese Patent Application No. 2014-184671 filed in Japan on September 10, 2014, the contents of which are incorporated herein by reference.
本願は、2014年9月10日に、日本に出願された特願2014-184671号に基づき優先権を主張し、その内容をここに援用する。 The present invention relates to pyridine compounds and uses such as agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides.
This application claims priority based on Japanese Patent Application No. 2014-184671 filed in Japan on September 10, 2014, the contents of which are incorporated herein by reference.
農園芸作物の栽培に当り、作物の病害に対して多数の防除薬剤が提案されている。提案されている防除薬剤のほとんどは、その防除効力が不十分であったり、薬剤耐性の病原菌の出現によりその使用が制限されたり、植物体に薬害や汚染を生じさせたり、若しくは人畜魚類に対する毒性や環境への影響が大きかったりなどで、十分に満足できるものでない。そのため、かかる欠点の少ない安全に使用できる防除薬剤の出現が強く要望されている。
In the cultivation of agricultural and horticultural crops, many control agents have been proposed for crop diseases. Most of the proposed control agents have insufficient control efficacy, their use is limited by the emergence of drug-resistant pathogens, cause phytotoxicity and contamination of plants, or toxicity to human and fish And the impact on the environment is not enough. Therefore, there is a strong demand for the emergence of a control agent that can be safely used with few such drawbacks.
ところで、特許文献1には、式(A)若しくは式(B)で表されるピリジン化合物が開示されている。特許文献1によれば、このピリジン化合物は電子伝達系の複合体II阻害剤として有用であるらしい。
Incidentally, Patent Document 1 discloses a pyridine compound represented by the formula (A) or the formula (B). According to Patent Document 1, this pyridine compound seems to be useful as a complex II inhibitor of an electron transport system.
本発明の課題は、新規なピリジン化合物、ならびに農園芸用殺菌剤、有害生物防除剤、および殺虫または殺ダニ剤を提供することである。
An object of the present invention is to provide a novel pyridine compound, an agricultural and horticultural fungicide, a pest control agent, and an insecticide or acaricide.
上記課題を解決するために検討した結果、以下の態様を包含する本発明を完成するに至った。
As a result of studies to solve the above problems, the present invention including the following aspects has been completed.
〔1〕式(I)で表される化合物、またはその互変異性体若しくは塩。
[1] A compound represented by the formula (I), or a tautomer or salt thereof.
式(I)中、
R1は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、無置換の若しくはG2で置換されたC6~10アリール基、シアノ基またはハロゲノ基を示す。
R2およびR3は、それぞれ独立に、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、ホルミル基、ホルミルオキシ基、無置換の若しくはG1で置換されたC1~6アルキルカルボニルオキシ基、無置換の若しくはG2で置換されたC6~10アリール基、(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基、シアノ基、またはハロゲノ基を示す。
ここで、R1とR2は、相互に繋がって、R1およびR2のそれぞれが結合する炭素原子とともに5~6員環を形成してもよい。
R4は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC2~6アルキニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG2で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルC1~6アルキル基、ホルミル基、無置換の若しくはG1で置換されたC1~6アルキルカルボニル基、無置換の若しくはG2で置換されたC6~10アリールカルボニル基、無置換の若しくはG1で置換されたC1~6アルコキシカルボニル基、無置換の若しくはG1で置換されたC2~6アルケニルオキシカルボニル基、無置換の若しくはG1で置換されたC1~6アルキルスルホニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノカルボニル基、無置換の若しくはG1で置換された(C1~6アルキルチオ)カルボニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノ(チオカルボニル)基、または式(II)で表される有機基を示す。 In formula (I),
R 1 is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ~ 6 alkoxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or C3-8 cycloalkyl group substituted with G 2 , C1-6 alkoxy group unsubstituted or substituted with G 1 , formyl group, formyloxy group, C1-6 alkyl unsubstituted or substituted with G 1 carbonyloxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, (unsubstituted or C1 ~ 6 alkoxyimino substituted with G 1) -C1 ~ 6 alkyl group, a cyano group, or a halogeno group, Indicates.
Here, R 1 and R 2 may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which each of R 1 and R 2 is bonded.
R 4 is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2 A 3-6 membered heterocyclyl C1-6 alkyl group, a formyl group, a C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 , a C6-10 arylcarbonyl group which is unsubstituted or substituted with G 2 , unsubstituted or C1 ~ 6 alkoxycarbonyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyloxycarbonyl group substituted with G 1, unsubstituted or with G 1 Conversion has been C1 ~ 6 alkylsulfonyl group, an unsubstituted or C1 ~ 6 alkylaminocarbonyl group substituted with G 1, unsubstituted or substituted with G 1 (C1 ~ 6 alkylthio) carbonyl group, the unsubstituted Or a C1-6 alkylamino (thiocarbonyl) group substituted by G 1 or an organic group represented by the formula (II).
R1は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、無置換の若しくはG2で置換されたC6~10アリール基、シアノ基またはハロゲノ基を示す。
R2およびR3は、それぞれ独立に、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、ホルミル基、ホルミルオキシ基、無置換の若しくはG1で置換されたC1~6アルキルカルボニルオキシ基、無置換の若しくはG2で置換されたC6~10アリール基、(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基、シアノ基、またはハロゲノ基を示す。
ここで、R1とR2は、相互に繋がって、R1およびR2のそれぞれが結合する炭素原子とともに5~6員環を形成してもよい。
R4は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC2~6アルキニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG2で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルC1~6アルキル基、ホルミル基、無置換の若しくはG1で置換されたC1~6アルキルカルボニル基、無置換の若しくはG2で置換されたC6~10アリールカルボニル基、無置換の若しくはG1で置換されたC1~6アルコキシカルボニル基、無置換の若しくはG1で置換されたC2~6アルケニルオキシカルボニル基、無置換の若しくはG1で置換されたC1~6アルキルスルホニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノカルボニル基、無置換の若しくはG1で置換された(C1~6アルキルチオ)カルボニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノ(チオカルボニル)基、または式(II)で表される有機基を示す。 In formula (I),
R 1 is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ~ 6 alkoxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or C3-8 cycloalkyl group substituted with G 2 , C1-6 alkoxy group unsubstituted or substituted with G 1 , formyl group, formyloxy group, C1-6 alkyl unsubstituted or substituted with G 1 carbonyloxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, (unsubstituted or C1 ~ 6 alkoxyimino substituted with G 1) -C1 ~ 6 alkyl group, a cyano group, or a halogeno group, Indicates.
Here, R 1 and R 2 may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which each of R 1 and R 2 is bonded.
R 4 is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2 A 3-6 membered heterocyclyl C1-6 alkyl group, a formyl group, a C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 , a C6-10 arylcarbonyl group which is unsubstituted or substituted with G 2 , unsubstituted or C1 ~ 6 alkoxycarbonyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyloxycarbonyl group substituted with G 1, unsubstituted or with G 1 Conversion has been C1 ~ 6 alkylsulfonyl group, an unsubstituted or C1 ~ 6 alkylaminocarbonyl group substituted with G 1, unsubstituted or substituted with G 1 (C1 ~ 6 alkylthio) carbonyl group, the unsubstituted Or a C1-6 alkylamino (thiocarbonyl) group substituted by G 1 or an organic group represented by the formula (II).
式(II)中、Gaは、それぞれ独立に、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、または無置換のもしくはG2で置換されたC6~10アリール基を示す。
Wherein (II), G a are independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, no showing a substituted by a substituted or G 1 a C2 ~ 6 alkynyl, unsubstituted or C3 ~ 8 cycloalkyl group substituted by G 2 or unsubstituted or C6 ~ 10 aryl group substituted by G 2, .
式(II)中、Gbは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、無置換のもしくはG2で置換されたC6~10アリール基、または無置換のもしくはG2で置換された3~6員ヘテロシクリル基を示す。
Wherein (II), G b represents a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ~ 6 alkynyl group substituted by one, unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
式(II)中、Tは、酸素原子、オキシカルボニル基、カルボニルオキシ基、オキシカルボニルオキシ基、硫黄原子、(チオ)カルボニル基、カルボニル(チオ)基、(チオ)カルボニルオキシ基、オキシカルボニル(チオ)基、または-O-C(=O)-N(Gb)-で表される二価の基を示す。
*は式(II)で表される基の結合位置を示す。 In formula (II), T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C (═O) —N (G b ) —.
* Indicates the bonding position of the group represented by the formula (II).
*は式(II)で表される基の結合位置を示す。 In formula (II), T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C (═O) —N (G b ) —.
* Indicates the bonding position of the group represented by the formula (II).
Qは、式(III)~式(XII)で表される有機基のいずれかを示す。
Q represents any one of organic groups represented by formula (III) to formula (XII).
式(III)~式(XII)中、
Ar1は、無置換の若しくはG2で置換されたC6~10アリール基、または無置換の若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Ar2は、無置換の若しくはG2で置換されたC6~10アリール基、無置換の若しくはG2で置換されたC6~10アリールオキシ基、無置換の若しくはG2で置換された3~6員ヘテロシクリル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルオキシ基、または無置換の若しくはG2で置換された3~6員ヘテロシクリルチオ基を示す。
Ar3は、無置換若しくはG2で置換されたC6~10アリール基を示す。
Ar4は、無置換若しくはG2で置換されたC6~10アリーレン基を示す。
Ar5は、無置換若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Raは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG2で置換されたC6~10アリール基、またはアミノ基を示す。ここで、Ar1とRaは、相互に繋がって、Ar1とRaが結合する炭素原子とともに5~6員環を形成してもよい。
Xは、酸素原子、硫黄原子、スルフィニル基、スルホニル基、またはNRcで表される二価の基を示す。Rcは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、無置換のもしくはG2で置換されたC6~10アリールカルボニル基、無置換のもしくはG2で置換されたC6~10アリールスルホニル基、無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基、または無置換のもしくはG1で置換されたC3~8シクロアルキルオキシカルボニル基を示す。
Aは、無置換のもしくはG3で置換されたC1~C6アルキレン基、無置換のもしくはG3で置換されたC2~C6アルケニレン基、無置換のもしくはG3で置換されたC2~C6アルキニレン基、無置換のもしくはG3で置換されたC1~C6アルキレンオキシ基、無置換のもしくはG3で置換されたオキシC1~C6アルキレン基、またはカルボニル基を示す。
Baは、無置換の若しくはG4で置換されたC1~C6アルキレン基、無置換の若しくはG4で置換されたC2~C6アルケニレン基、無置換の若しくはG4で置換されたC2~C6アルキニレン基、無置換の若しくはG4で置換されたC1~C6アルキレンオキシC1~6アルキレン基、無置換の若しくはG4で置換されたC3~C6シクロアルキレン基、無置換の若しくはG4で置換されたC4~C6シクロアルケニレン基、無置換の若しくはG4で置換された3~6員ヘテロシクリレン基、またはNRdで表される二価の基を示す。Rdは、水素原子、C1~6アルキル基、またはC1~6アルコキシカルボニル基を示す。また、式(V)中、BaとRaは、相互に繋がって、BaとRaが結合する炭素原子とともに5~6員環を形成してもよい。
Bbは、無置換のもしくはG4で置換されたC1~C6アルキレン基、無置換のもしくはG4で置換されたC2~C6アルケニレン基、無置換のもしくはG4で置換されたC2~C6アルキニレン基、無置換のもしくはG4で置換されたC3~C6シクロアルキレン基、無置換のもしくはG4で置換されたC4~C6シクロアルケニレン基、無置換のもしくはG4で置換された3~6員ヘテロシクリレン基、またはカルボニル基を示す。
T1は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-メチル基、1-(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-エチル基、または無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基を示す。
T2は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-ホルミル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ)-C1~6アルキル基、または(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基を示す。
T3は、無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基、または無置換のもしくはG2で置換されたC6~10アリールアミノカルボニル-C1~6アルキル基を示す。
*は、式(III)~式(XII)で表される有機基の結合位置を示す。 In formula (III) to formula (XII),
Ar 1 represents an unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 2,.
Ar 2 is unsubstituted or C6 ~ 10 aryl group substituted by G 2, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ~ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 .
Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G 2 .
Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 .
R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group. Here, Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded.
X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by NR c . R c is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 1-6 alkylcarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted with G 2, unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 2, substituted with unsubstituted or G 1 A C1-6 alkoxycarbonyl group or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 is shown.
A is unsubstituted or C1 ~ C6 alkylene group substituted with G 3, unsubstituted or C2 ~ C6 alkenylene group substituted with G 3, unsubstituted or C2 ~ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group.
B a is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ~ C6 alkyleneoxy C1 ~ 6 alkylene group substituted with G 4, unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4 A C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown. R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group. In the formula (V), B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached.
B b is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, unsubstituted or C4 ~ C6 cycloalkenylene group substituted with G 4, unsubstituted or 3-6 membered substituted by G 4 A heterocyclylene group or a carbonyl group is shown.
T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ~ 10 aryl C1 ~ 6 alkyl)-C1 ~ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ~ 10 aryl C1 which is substituted by G 2 ~ shows the 6 alkoxy)-C1 ~ 6 alkyl group or a (unsubstituted or C6 ~ 10 aryl C1-6 alkyl substituted with G 2) -3 ~ 6-membered heterocyclyl group.
T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
* Indicates the bonding position of the organic group represented by formula (III) to formula (XII).
Ar1は、無置換の若しくはG2で置換されたC6~10アリール基、または無置換の若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Ar2は、無置換の若しくはG2で置換されたC6~10アリール基、無置換の若しくはG2で置換されたC6~10アリールオキシ基、無置換の若しくはG2で置換された3~6員ヘテロシクリル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルオキシ基、または無置換の若しくはG2で置換された3~6員ヘテロシクリルチオ基を示す。
Ar3は、無置換若しくはG2で置換されたC6~10アリール基を示す。
Ar4は、無置換若しくはG2で置換されたC6~10アリーレン基を示す。
Ar5は、無置換若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Raは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG2で置換されたC6~10アリール基、またはアミノ基を示す。ここで、Ar1とRaは、相互に繋がって、Ar1とRaが結合する炭素原子とともに5~6員環を形成してもよい。
Xは、酸素原子、硫黄原子、スルフィニル基、スルホニル基、またはNRcで表される二価の基を示す。Rcは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、無置換のもしくはG2で置換されたC6~10アリールカルボニル基、無置換のもしくはG2で置換されたC6~10アリールスルホニル基、無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基、または無置換のもしくはG1で置換されたC3~8シクロアルキルオキシカルボニル基を示す。
Aは、無置換のもしくはG3で置換されたC1~C6アルキレン基、無置換のもしくはG3で置換されたC2~C6アルケニレン基、無置換のもしくはG3で置換されたC2~C6アルキニレン基、無置換のもしくはG3で置換されたC1~C6アルキレンオキシ基、無置換のもしくはG3で置換されたオキシC1~C6アルキレン基、またはカルボニル基を示す。
Baは、無置換の若しくはG4で置換されたC1~C6アルキレン基、無置換の若しくはG4で置換されたC2~C6アルケニレン基、無置換の若しくはG4で置換されたC2~C6アルキニレン基、無置換の若しくはG4で置換されたC1~C6アルキレンオキシC1~6アルキレン基、無置換の若しくはG4で置換されたC3~C6シクロアルキレン基、無置換の若しくはG4で置換されたC4~C6シクロアルケニレン基、無置換の若しくはG4で置換された3~6員ヘテロシクリレン基、またはNRdで表される二価の基を示す。Rdは、水素原子、C1~6アルキル基、またはC1~6アルコキシカルボニル基を示す。また、式(V)中、BaとRaは、相互に繋がって、BaとRaが結合する炭素原子とともに5~6員環を形成してもよい。
Bbは、無置換のもしくはG4で置換されたC1~C6アルキレン基、無置換のもしくはG4で置換されたC2~C6アルケニレン基、無置換のもしくはG4で置換されたC2~C6アルキニレン基、無置換のもしくはG4で置換されたC3~C6シクロアルキレン基、無置換のもしくはG4で置換されたC4~C6シクロアルケニレン基、無置換のもしくはG4で置換された3~6員ヘテロシクリレン基、またはカルボニル基を示す。
T1は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-メチル基、1-(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-エチル基、または無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基を示す。
T2は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-ホルミル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ)-C1~6アルキル基、または(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基を示す。
T3は、無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基、または無置換のもしくはG2で置換されたC6~10アリールアミノカルボニル-C1~6アルキル基を示す。
*は、式(III)~式(XII)で表される有機基の結合位置を示す。 In formula (III) to formula (XII),
Ar 1 represents an unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 2,.
Ar 2 is unsubstituted or C6 ~ 10 aryl group substituted by G 2, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ~ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 .
Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G 2 .
Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 .
R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group. Here, Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded.
X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by NR c . R c is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 1-6 alkylcarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted with G 2, unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 2, substituted with unsubstituted or G 1 A C1-6 alkoxycarbonyl group or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 is shown.
A is unsubstituted or C1 ~ C6 alkylene group substituted with G 3, unsubstituted or C2 ~ C6 alkenylene group substituted with G 3, unsubstituted or C2 ~ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group.
B a is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ~ C6 alkyleneoxy C1 ~ 6 alkylene group substituted with G 4, unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4 A C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown. R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group. In the formula (V), B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached.
B b is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, unsubstituted or C4 ~ C6 cycloalkenylene group substituted with G 4, unsubstituted or 3-6 membered substituted by G 4 A heterocyclylene group or a carbonyl group is shown.
T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ~ 10 aryl C1 ~ 6 alkyl)-C1 ~ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ~ 10 aryl C1 which is substituted by G 2 ~ shows the 6 alkoxy)-C1 ~ 6 alkyl group or a (unsubstituted or C6 ~ 10 aryl C1-6 alkyl substituted with G 2) -3 ~ 6-membered heterocyclyl group.
T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
* Indicates the bonding position of the organic group represented by formula (III) to formula (XII).
G1は、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、シアノ基、またはハロゲノ基を示す。R1、R2、R3、R4、Ra、Rc、Ga、またはGbのうちの2つ以上がG1で置換された前記の基である場合、係るG1は、相互に同じであっても異なってもよい。
G 1 is a hydroxyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxycarbonyloxy group, cyano A group or a halogeno group. R 1, R 2, R 3 , R 4, R a, R c, when it is the group more than two substituted by G 1 of G a or G b,, G 1 according the mutual May be the same or different.
G2は、C1~6アルキル基、C2~6アルケニル基、C2~6アルキニル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C1~6アルコキシC1~6ハロアルキル基、C2~6ハロアルケニル基、C2~6ハロアルキニル基、水酸基、C1~6アルコキシ基、C3~8シクロアルキルC1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6ハロアルコキシ基、C1~6ハロアルコキシC1~6ハロアルコキシ基、ホルミル基、C1~6アルキルカルボニル基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、C1~6アルコキシカルボニルアミノ基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシ基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシ基、C1~6アルキルチオ基、C1~6アルキルスルフィニル基、C1~6アルキルスルホニル基、C1~6ハロアルキルチオ基、C1~6ハロアルキルスルフィニル基、C1~6ハロアルキルスルホニル基、ペンタフルオロスルファニル基、無置換の若しくはG21で置換されたC6~10アリールチオ基、無置換の若しくはG21で置換されたC6~10アリールスルフィニル基、無置換の若しくはG21で置換されたC6~10アリールスルホニル基、無置換の若しくはG21で置換されたC6~10アリールアミノ基、ニトロ基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、またはC1~6ハロアルキレンジオキシ基を示す。R1、R2、R3、R4、Ra、Rc、Ga、Gb、Ar1、Ar2、Ar3、Ar4、Ar5、T1、T2、またはT3のうちの2つ以上がG2で置換された前記の基である場合、係るG2は、相互に同じであっても異なってもよい。
G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 alkoxyC1 ~ 6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1 ~ 6 haloalkoxy group, C1-6 haloalkoxy C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxy carbonyloxy group, C1 ~ 6 alkoxycarbonylamino group, an unsubstituted or G 21 Substituted C6 ~ 10 aryl group, an unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkyl group substituted with G 21, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 21, unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxy group substituted by G 21, unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 21, unsubstituted or 3-6 membered heterocyclyloxy group which is substituted by G 21 C1-6 alkylthio group, C1-6 alkylsulfinyl group, C1-6 alkylsulfonyl group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, C1-6 haloalkylsulfonyl group, pentafluorosulfanyl group, unsubstituted or C6 ~ 10 arylthio group substituted with G 21, C6 substituted with unsubstituted or G 21 ~ 10 arylsulfinyl group, C6-10 arylsulfonyl group unsubstituted or substituted with G 21 , C6-10 arylamino group unsubstituted or substituted with G 21 , nitro group, cyano group, halogeno group, C1— A 6 alkylene group, a C1-6 alkylenedioxy group, or a C1-6 haloalkylenedioxy group; Of R 1 , R 2 , R 3 , R 4 , R a , R c , G a , G b , Ar 1 , Ar 2 , Ar 3 , Ar 4 , Ar 5 , T 1 , T 2 , or T 3 If more than one is a said group substituted with G 2, G 2 according may be the same or different from each other.
G21は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、C6~10アリール基またはハロゲノ基を示す。G2またはG4のうちの2つ以上がG21で置換された前記の基である場合、係るG21は、相互に同じであっても異なってもよい。
G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group or a halogeno group. If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
G3は、C1~6アルキル基、C1~6アルコキシ基、ホルミル基、C1~6アルキルカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、ハロゲノ基、C1~6アルキレン基、またはオキソ基を示す。
G 3 is a C1-6 alkyl group, C1-6 alkoxy group, formyl group, C1-6 alkylcarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, halogeno group, C1-6 alkylene group, or oxo group Indicates.
G4は、C1~6アルキル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C2~6アルケニルオキシC1~6アルキル基、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C2~6アルケニルオキシ基、C1~6アルコキシカルボニル基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、オキソ基、C3~8シクロアルキルC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルC1~6アルキル基、C3~8シクロアルキルオキシC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシC1~6アルキル基、C1~6アルキリデン基、C1~6アルコキシイミノ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシイミノ基、またはC1~6アルキルヒドラジノ基を示す。
ただし、Qが式(VII)または(VIII)の場合、R1は水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ~ 6 alkoxy C1 ~ 6 alkoxy group, substituted with C2 ~ 6 alkenyloxy group, C1 ~ 6 alkoxycarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ~ 10 aryl C1 ~ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ~ 6 alkyl substituted with unsubstituted or G 21 Group, C3 ~ 8 cycloalkyloxy C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryloxy C1 ~ 6 alkyl group substituted with G 21, unsubstituted or 3-6 membered heterocyclyl substituted with G 21 oxy C1 ~ 6 alkyl group, C1 ~ 6 alkylidene group, C1 ~ 6 alkoxyimino group, the unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxyimino group substituted by G 21 or C1 ~ 6 alkyl hydrazino group, Show.
However, when Q is the formula (VII) or (VIII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
ただし、Qが式(VII)または(VIII)の場合、R1は水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ~ 6 alkoxy C1 ~ 6 alkoxy group, substituted with C2 ~ 6 alkenyloxy group, C1 ~ 6 alkoxycarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ~ 10 aryl C1 ~ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ~ 6 alkyl substituted with unsubstituted or G 21 Group, C3 ~ 8 cycloalkyloxy C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryloxy C1 ~ 6 alkyl group substituted with G 21, unsubstituted or 3-6 membered heterocyclyl substituted with G 21 oxy C1 ~ 6 alkyl group, C1 ~ 6 alkylidene group, C1 ~ 6 alkoxyimino group, the unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxyimino group substituted by G 21 or C1 ~ 6 alkyl hydrazino group, Show.
However, when Q is the formula (VII) or (VIII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
〔2〕 式(I)中のR4が、水素原子またはアリル基である、前記〔1〕に記載の化合物、またはその互変異性体若しくは塩。
[2] The compound according to [1], or a tautomer or salt thereof, wherein R 4 in formula (I) is a hydrogen atom or an allyl group.
〔3〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する農園芸用殺菌剤。
〔4〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する有害生物防除剤。
〔5〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する殺虫または殺ダニ剤。
〔6〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する外部寄生虫防除剤。 [3] An agricultural and horticultural fungicide containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2] above, and tautomers and salts thereof.
[4] A pest control agent containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
[5] An insecticide or acaricide containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2] above, and tautomers and salts thereof.
[6] An ectoparasite control agent comprising, as an active ingredient, at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
〔4〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する有害生物防除剤。
〔5〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する殺虫または殺ダニ剤。
〔6〕前記〔1〕および〔2〕に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する外部寄生虫防除剤。 [3] An agricultural and horticultural fungicide containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2] above, and tautomers and salts thereof.
[4] A pest control agent containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
[5] An insecticide or acaricide containing as an active ingredient at least one selected from the group consisting of the compounds according to [1] and [2] above, and tautomers and salts thereof.
[6] An ectoparasite control agent comprising, as an active ingredient, at least one selected from the group consisting of the compounds according to [1] and [2], and tautomers and salts thereof.
本発明に係るピリジン化合物は、有害生物防除、殺菌、殺ダニ、殺虫などの効果を有し、植物体に薬害を生じることがなく、人畜魚類に対する毒性や環境への影響が少ない新規化合物である。特に、ムギ病害に対して優れた防除効果を示す。本発明に係るピリジン化合物は、農園芸用殺菌剤、有害生物防除剤、および殺虫または殺ダニ剤の有効成分として有用である。
The pyridine compound according to the present invention is a novel compound that has effects such as pest control, bactericidal, acaricidal, insecticidal and the like, does not cause phytotoxicity on plants, and has little toxicity to human fish and environmental impacts. . In particular, it exhibits an excellent control effect against wheat diseases. The pyridine compound according to the present invention is useful as an active ingredient of agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides.
本発明に係るピリジン化合物は、式(I)で表される化合物(以下、化合物(I)と表記することがある。)、化合物(I)の互変異性体、および化合物(I)の塩である。
The pyridine compound according to the present invention includes a compound represented by formula (I) (hereinafter sometimes referred to as compound (I)), a tautomer of compound (I), and a salt of compound (I). It is.
〔R1〕
R1は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、無置換の若しくはG2で置換されたC6~10アリール基、シアノ基またはハロゲノ基を示す。 [R 1 ]
R 1 is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ~ 6 alkoxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
R1は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、無置換の若しくはG2で置換されたC6~10アリール基、シアノ基またはハロゲノ基を示す。 [R 1 ]
R 1 is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ~ 6 alkoxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
C1~6アルキル基は、直鎖であってもよいし、炭素数が3以上であれば分岐鎖であってもよい。C1~6アルキル基としては、メチル基、エチル基、n-プロピル基、i-プロピル基、n-ブチル基、s-ブチル基、i-ブチル基、t-ブチル基、n-ペンチル基、n-ヘキシル基、i-ペンチル基、ネオペンチル基、2-メチルブチル基、2,2-ジメチルプロピル基、i-ヘキシル基などを挙げることができる。
C2~6アルケニル基としては、ビニル基、1-プロペニル基、2-プロペニル基(アリル基)、1-ブテニル基、2-ブテニル基、3-ブテニル基、1-メチルビニル基(イソプロペニル基)、1-メチル-2-プロペニル基、2-メチル-2-プロペニル基、1-ペンテニル基、2-ペンテニル基、3-ペンテニル基、4-ペンテニル基、1-メチル-2-ブテニル基、2-メチル-2-ブテニル基、1-ヘキセニル基、2-ヘキセニル基、3-ヘキセニル基、4-ヘキセニル基、5-ヘキセニル基などを挙げることができる。
C1~6アルコキシ基としては、メトキシ基、エトキシ基、n-プロポキシ基、n-ブトキシ基、n-ペンチルオキシ基、n-ヘキシルオキシ基、i-プロポキシ基、i-ブトキシ基、s-ブトキシ基、t-ブトキシ基、i-ヘキシルオキシ基などを挙げることができる。
C6~10アリール基としては、フェニル基、ナフチル基、アズレニル基、インデニル基、インダニル基、テトラリニル基などを挙げることができる。
ハロゲノ基としては、フルオロ基、クロロ基、ブロモ基、イオド基を挙げることができる。 The C1-6 alkyl group may be linear or branched if it has 3 or more carbon atoms. Examples of the C1-6 alkyl group include methyl group, ethyl group, n-propyl group, i-propyl group, n-butyl group, s-butyl group, i-butyl group, t-butyl group, n-pentyl group, n -Hexyl group, i-pentyl group, neopentyl group, 2-methylbutyl group, 2,2-dimethylpropyl group, i-hexyl group and the like.
C2-6 alkenyl groups include vinyl, 1-propenyl, 2-propenyl (allyl), 1-butenyl, 2-butenyl, 3-butenyl, 1-methylvinyl (isopropenyl) 1-methyl-2-propenyl group, 2-methyl-2-propenyl group, 1-pentenyl group, 2-pentenyl group, 3-pentenyl group, 4-pentenyl group, 1-methyl-2-butenyl group, 2- Examples thereof include a methyl-2-butenyl group, a 1-hexenyl group, a 2-hexenyl group, a 3-hexenyl group, a 4-hexenyl group, and a 5-hexenyl group.
C1-6 alkoxy groups include methoxy, ethoxy, n-propoxy, n-butoxy, n-pentyloxy, n-hexyloxy, i-propoxy, i-butoxy, s-butoxy , T-butoxy group, i-hexyloxy group and the like.
Examples of the C6-10 aryl group include a phenyl group, a naphthyl group, an azulenyl group, an indenyl group, an indanyl group, and a tetralinyl group.
Examples of the halogeno group include a fluoro group, a chloro group, a bromo group, and an iodo group.
C2~6アルケニル基としては、ビニル基、1-プロペニル基、2-プロペニル基(アリル基)、1-ブテニル基、2-ブテニル基、3-ブテニル基、1-メチルビニル基(イソプロペニル基)、1-メチル-2-プロペニル基、2-メチル-2-プロペニル基、1-ペンテニル基、2-ペンテニル基、3-ペンテニル基、4-ペンテニル基、1-メチル-2-ブテニル基、2-メチル-2-ブテニル基、1-ヘキセニル基、2-ヘキセニル基、3-ヘキセニル基、4-ヘキセニル基、5-ヘキセニル基などを挙げることができる。
C1~6アルコキシ基としては、メトキシ基、エトキシ基、n-プロポキシ基、n-ブトキシ基、n-ペンチルオキシ基、n-ヘキシルオキシ基、i-プロポキシ基、i-ブトキシ基、s-ブトキシ基、t-ブトキシ基、i-ヘキシルオキシ基などを挙げることができる。
C6~10アリール基としては、フェニル基、ナフチル基、アズレニル基、インデニル基、インダニル基、テトラリニル基などを挙げることができる。
ハロゲノ基としては、フルオロ基、クロロ基、ブロモ基、イオド基を挙げることができる。 The C1-6 alkyl group may be linear or branched if it has 3 or more carbon atoms. Examples of the C1-6 alkyl group include methyl group, ethyl group, n-propyl group, i-propyl group, n-butyl group, s-butyl group, i-butyl group, t-butyl group, n-pentyl group, n -Hexyl group, i-pentyl group, neopentyl group, 2-methylbutyl group, 2,2-dimethylpropyl group, i-hexyl group and the like.
C2-6 alkenyl groups include vinyl, 1-propenyl, 2-propenyl (allyl), 1-butenyl, 2-butenyl, 3-butenyl, 1-methylvinyl (isopropenyl) 1-methyl-2-propenyl group, 2-methyl-2-propenyl group, 1-pentenyl group, 2-pentenyl group, 3-pentenyl group, 4-pentenyl group, 1-methyl-2-butenyl group, 2- Examples thereof include a methyl-2-butenyl group, a 1-hexenyl group, a 2-hexenyl group, a 3-hexenyl group, a 4-hexenyl group, and a 5-hexenyl group.
C1-6 alkoxy groups include methoxy, ethoxy, n-propoxy, n-butoxy, n-pentyloxy, n-hexyloxy, i-propoxy, i-butoxy, s-butoxy , T-butoxy group, i-hexyloxy group and the like.
Examples of the C6-10 aryl group include a phenyl group, a naphthyl group, an azulenyl group, an indenyl group, an indanyl group, and a tetralinyl group.
Examples of the halogeno group include a fluoro group, a chloro group, a bromo group, and an iodo group.
置換基G1は、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、シアノ基、またはハロゲノ基を示す。
なお、R1、R2、R3、R4、Ra、Rc、Ga、またはGbのうちの2つ以上がG1で置換された基である場合、係るG1は、相互に同じであっても異なってもよい。 The substituent G 1 is a hydroxyl group, a C1-6 alkoxy group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkoxycarbonyl group, a formyloxy group, a C1-6 alkylcarbonyloxy group, a C1-6 alkoxycarbonyloxy group. , A cyano group, or a halogeno group.
In addition, when two or more of R 1 , R 2 , R 3 , R 4 , R a , R c , G a , or G b are groups substituted with G 1 , G 1 May be the same or different.
なお、R1、R2、R3、R4、Ra、Rc、Ga、またはGbのうちの2つ以上がG1で置換された基である場合、係るG1は、相互に同じであっても異なってもよい。 The substituent G 1 is a hydroxyl group, a C1-6 alkoxy group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkoxycarbonyl group, a formyloxy group, a C1-6 alkylcarbonyloxy group, a C1-6 alkoxycarbonyloxy group. , A cyano group, or a halogeno group.
In addition, when two or more of R 1 , R 2 , R 3 , R 4 , R a , R c , G a , or G b are groups substituted with G 1 , G 1 May be the same or different.
置換基G1における、C1~6アルコキシ基、ハロゲノ基は既に述べたとおりのものである。
C1~6アルコキシC1~6アルコキシ基としては、メトキシメトキシ基、メトキシエトキシ基などを挙げることができる。
C1~6アルコキシカルボニル基としては、メトキシカルボニル基、エトキシカルボニル基、n-プロポキシカルボニル基、i-プロポキシカルボニル基、t-ブトキシカルボニル基などを挙げることができる。
C1~6アルキルカルボニルオキシ基としては、アセチルオキシ基、プロピオニルオキシ基、ブチリルオキシ基などを挙げることができる。
C1~6アルコキシカルボニルオキシ基としては、メトキシカルボニルオキシ基、エトキシカルボニルオキシ基、n-プロポキシカルボニルオキシ基、i-プロポキシカルボニルオキシ基、n-ブトキシカルボニルオキシ基、t-ブトキシカルボニルオキシ基などを挙げることができる。 The C1-6 alkoxy group and the halogeno group in the substituent G 1 are as described above.
Examples of the C1-6 alkoxy group include a methoxymethoxy group and a methoxyethoxy group.
Examples of the C1-6 alkoxycarbonyl group include a methoxycarbonyl group, an ethoxycarbonyl group, an n-propoxycarbonyl group, an i-propoxycarbonyl group, and a t-butoxycarbonyl group.
Examples of the C1-6 alkylcarbonyloxy group include an acetyloxy group, a propionyloxy group, and a butyryloxy group.
Examples of the C1-6 alkoxycarbonyloxy group include a methoxycarbonyloxy group, an ethoxycarbonyloxy group, an n-propoxycarbonyloxy group, an i-propoxycarbonyloxy group, an n-butoxycarbonyloxy group, and a t-butoxycarbonyloxy group. be able to.
C1~6アルコキシC1~6アルコキシ基としては、メトキシメトキシ基、メトキシエトキシ基などを挙げることができる。
C1~6アルコキシカルボニル基としては、メトキシカルボニル基、エトキシカルボニル基、n-プロポキシカルボニル基、i-プロポキシカルボニル基、t-ブトキシカルボニル基などを挙げることができる。
C1~6アルキルカルボニルオキシ基としては、アセチルオキシ基、プロピオニルオキシ基、ブチリルオキシ基などを挙げることができる。
C1~6アルコキシカルボニルオキシ基としては、メトキシカルボニルオキシ基、エトキシカルボニルオキシ基、n-プロポキシカルボニルオキシ基、i-プロポキシカルボニルオキシ基、n-ブトキシカルボニルオキシ基、t-ブトキシカルボニルオキシ基などを挙げることができる。 The C1-6 alkoxy group and the halogeno group in the substituent G 1 are as described above.
Examples of the C1-6 alkoxy group include a methoxymethoxy group and a methoxyethoxy group.
Examples of the C1-6 alkoxycarbonyl group include a methoxycarbonyl group, an ethoxycarbonyl group, an n-propoxycarbonyl group, an i-propoxycarbonyl group, and a t-butoxycarbonyl group.
Examples of the C1-6 alkylcarbonyloxy group include an acetyloxy group, a propionyloxy group, and a butyryloxy group.
Examples of the C1-6 alkoxycarbonyloxy group include a methoxycarbonyloxy group, an ethoxycarbonyloxy group, an n-propoxycarbonyloxy group, an i-propoxycarbonyloxy group, an n-butoxycarbonyloxy group, and a t-butoxycarbonyloxy group. be able to.
置換基G2は、C1~6アルキル基、C2~6アルケニル基、C2~6アルキニル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C1~6アルコキシC1~6ハロアルキル基、C2~6ハロアルケニル基、C2~6ハロアルキニル基、水酸基、C1~6アルコキシ基、C3~8シクロアルキルC1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6ハロアルコキシ基、C1~6ハロアルコキシC1~6ハロアルコキシ基、ホルミル基、C1~6アルキルカルボニル基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、C1~6アルコキシカルボニルアミノ基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシ基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシ基、C1~6アルキルチオ基、C1~6アルキルスルフィニル基、C1~6アルキルスルホニル基、C1~6ハロアルキルチオ基、C1~6ハロアルキルスルフィニル基、C1~6ハロアルキルスルホニル基、ペンタフルオロスルファニル基、無置換の若しくはG21で置換されたC6~10アリールチオ基、無置換の若しくはG21で置換されたC6~10アリールスルフィニル基、無置換の若しくはG21で置換されたC6~10アリールスルホニル基、無置換の若しくはG21で置換されたC6~10アリールアミノ基、ニトロ基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、またはC1~6ハロアルキレンジオキシ基を示す。
なお、R1、R2、R3、R4、Ra、Rc、Ga、Gb、Ar1、Ar2、Ar3、Ar4、Ar5、T1、T2、またはT3のうちの2つ以上がG2で置換された前記の基である場合、係るG2は、相互に同じであっても異なってもよい。 Substituent G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 Alkoxy C1-6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 6 alkoxycarbonyloxy group, C1-6 alkoxycarbonylamino group, unsubstituted or young C6 ~ 10 aryl group substituted by G 21, unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkyl group substituted with G 21, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 21, no substituted with substituted or G 21 a C6 ~ 10 aryl C1 ~ 6 alkoxy group, an unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 21, 3-6 membered substituted with unsubstituted or G 21 Heterocyclyloxy group, C1-6 alkylthio group, C1-6 alkylsulfinyl group, C1-6 alkylsulfonyl group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, C1-6 haloalkylsulfonyl group, pentafluorosulfanyl group, unsubstituted or C6 ~ 10 arylthio group substituted with G 21, which is substituted by unsubstituted or G 21 C6 ~ 10 aryl sulfinyl group, an unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 21, unsubstituted or C6 ~ 10 aryl amino group substituted with G 21, a nitro group, a cyano group, a halogeno group, A C1-6 alkylene group, a C1-6 alkylenedioxy group, or a C1-6 haloalkylenedioxy group;
R 1 , R 2 , R 3 , R 4 , R a , R c , G a , G b , Ar 1 , Ar 2 , Ar 3 , Ar 4 , Ar 5 , T 1 , T 2 , or T 3 If two or more of is the group substituted with G 2, G 2 according may be the same or different from each other.
なお、R1、R2、R3、R4、Ra、Rc、Ga、Gb、Ar1、Ar2、Ar3、Ar4、Ar5、T1、T2、またはT3のうちの2つ以上がG2で置換された前記の基である場合、係るG2は、相互に同じであっても異なってもよい。 Substituent G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 Alkoxy C1-6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 6 alkoxycarbonyloxy group, C1-6 alkoxycarbonylamino group, unsubstituted or young C6 ~ 10 aryl group substituted by G 21, unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkyl group substituted with G 21, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 21, no substituted with substituted or G 21 a C6 ~ 10 aryl C1 ~ 6 alkoxy group, an unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 21, 3-6 membered substituted with unsubstituted or G 21 Heterocyclyloxy group, C1-6 alkylthio group, C1-6 alkylsulfinyl group, C1-6 alkylsulfonyl group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, C1-6 haloalkylsulfonyl group, pentafluorosulfanyl group, unsubstituted or C6 ~ 10 arylthio group substituted with G 21, which is substituted by unsubstituted or G 21 C6 ~ 10 aryl sulfinyl group, an unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 21, unsubstituted or C6 ~ 10 aryl amino group substituted with G 21, a nitro group, a cyano group, a halogeno group, A C1-6 alkylene group, a C1-6 alkylenedioxy group, or a C1-6 haloalkylenedioxy group;
R 1 , R 2 , R 3 , R 4 , R a , R c , G a , G b , Ar 1 , Ar 2 , Ar 3 , Ar 4 , Ar 5 , T 1 , T 2 , or T 3 If two or more of is the group substituted with G 2, G 2 according may be the same or different from each other.
置換基G2における、C1~6アルキル基、C2~6アルケニル基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6アルコキシカルボニル基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、C6~10アリール基、およびハロゲノ基は既に述べたとおりのものである。
In the substituent G 2 , C1-6 alkyl group, C2-6 alkenyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 alkoxycarbonyl group, C1-6 alkylcarbonyloxy group, C1 The -6 alkoxycarbonyloxy group, the C6-10 aryl group, and the halogeno group are as described above.
C2~6アルキニル基としては、エチニル基、1-プロピニル基、2-プロピニル基、1-ブチニル基、2-ブチニル基、3-ブチニル基、1-メチル-2-プロピニル基、2-メチル-3-ブチニル基、1-ペンチニル基、2-ペンチニル基、3-ペンチニル基、4-ペンチニル基、1-メチル-2-ブチニル基、2-メチル-3-ペンチニル基、1-ヘキシニル基、1,1-ジメチル-2-ブチニル基などを挙げることができる。
C3~8シクロアルキル基としては、シクロプロピル基、シクロブチル基、シクロペンチル基、シクロヘキシル基、シクロヘプチル基、2-アダマンチル基などを挙げることができる。 Examples of the C2-6 alkynyl group include ethynyl group, 1-propynyl group, 2-propynyl group, 1-butynyl group, 2-butynyl group, 3-butynyl group, 1-methyl-2-propynyl group, 2-methyl-3 -Butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 2-methyl-3-pentynyl, 1-hexynyl, 1,1 And -dimethyl-2-butynyl group.
Examples of the C3-8 cycloalkyl group include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cycloheptyl group, and a 2-adamantyl group.
C3~8シクロアルキル基としては、シクロプロピル基、シクロブチル基、シクロペンチル基、シクロヘキシル基、シクロヘプチル基、2-アダマンチル基などを挙げることができる。 Examples of the C2-6 alkynyl group include ethynyl group, 1-propynyl group, 2-propynyl group, 1-butynyl group, 2-butynyl group, 3-butynyl group, 1-methyl-2-propynyl group, 2-methyl-3 -Butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 2-methyl-3-pentynyl, 1-hexynyl, 1,1 And -dimethyl-2-butynyl group.
Examples of the C3-8 cycloalkyl group include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cycloheptyl group, and a 2-adamantyl group.
C1~6アルコキシC1~6アルキル基は、既に述べたC1~6アルキル基に、上述のC1~6アルコキシ基が置換したものである。C1~6アルコキシC1~6アルキル基としては、メトキシメチル基、エトキシメチル基、メトキシエチル基、エトキシエチル基、メトキシ-n-プロピル基、エトキシメチル基、エトキシエチル基、n-プロポキシメチル基、i-プロポキシエチル基、s-ブトキシメチル基、t-ブトキシエチル基などを挙げることできる。
C3~8シクロアルキルC1~6アルコキシ基は、既に述べたC1~6アルコキシ基に、上述のC3~8シクロアルキル基が置換したものである。C3~8シクロアルキルC1~6アルコキシ基としては、シクロプロピルメトキシ基、シクロブチルメトキシ基、シクロペンチルメトキシ基、シクロヘキシルメトキシ基、2-(シクロプロピル)-エトキシ基などを挙げることができる。 The C1-6 alkoxy C1-6 alkyl group is the above-described C1-6 alkoxy group substituted on the C1-6 alkyl group already described. C1-6 alkoxy C1-6 alkyl group includes methoxymethyl group, ethoxymethyl group, methoxyethyl group, ethoxyethyl group, methoxy-n-propyl group, ethoxymethyl group, ethoxyethyl group, n-propoxymethyl group, i -Propoxyethyl group, s-butoxymethyl group, t-butoxyethyl group and the like can be mentioned.
The C3-8 cycloalkyl C1-6 alkoxy group is the above-described C3-6 cycloalkyl group substituted on the C1-6 alkoxy group already described. Examples of the C3-8 cycloalkyl C1-6 alkoxy group include a cyclopropylmethoxy group, a cyclobutylmethoxy group, a cyclopentylmethoxy group, a cyclohexylmethoxy group, and a 2- (cyclopropyl) -ethoxy group.
C3~8シクロアルキルC1~6アルコキシ基は、既に述べたC1~6アルコキシ基に、上述のC3~8シクロアルキル基が置換したものである。C3~8シクロアルキルC1~6アルコキシ基としては、シクロプロピルメトキシ基、シクロブチルメトキシ基、シクロペンチルメトキシ基、シクロヘキシルメトキシ基、2-(シクロプロピル)-エトキシ基などを挙げることができる。 The C1-6 alkoxy C1-6 alkyl group is the above-described C1-6 alkoxy group substituted on the C1-6 alkyl group already described. C1-6 alkoxy C1-6 alkyl group includes methoxymethyl group, ethoxymethyl group, methoxyethyl group, ethoxyethyl group, methoxy-n-propyl group, ethoxymethyl group, ethoxyethyl group, n-propoxymethyl group, i -Propoxyethyl group, s-butoxymethyl group, t-butoxyethyl group and the like can be mentioned.
The C3-8 cycloalkyl C1-6 alkoxy group is the above-described C3-6 cycloalkyl group substituted on the C1-6 alkoxy group already described. Examples of the C3-8 cycloalkyl C1-6 alkoxy group include a cyclopropylmethoxy group, a cyclobutylmethoxy group, a cyclopentylmethoxy group, a cyclohexylmethoxy group, and a 2- (cyclopropyl) -ethoxy group.
C1~6アルキルカルボニル基は、カルボニル基に、上述のC1~6アルキル基が結合したものである。C1~6アルキルカルボニル基としては、アセチル基、プロピオニル基、ブチリル基、イソブチリル基、ピバロイル基などを挙げることができる。
The C1-6 alkylcarbonyl group is a group in which the above C1-6 alkyl group is bonded to a carbonyl group. Examples of the C1-6 alkylcarbonyl group include an acetyl group, a propionyl group, a butyryl group, an isobutyryl group, and a pivaloyl group.
C1~6アルコキシカルボニルアミノ基は、アミノ基に、既に述べたC1~6アルコキシカルボニル基が置換したものである。C1~6アルコキシカルボニルアミノ基としては、メトキシカルボニルアミノ基、エトキシカルボニルアミノ基、n-プロポキシカルボニルアミノ基、i-プロポキシカルボニルアミノ基、n-ブトキシカルボニルアミノ基、t-ブトキシカルボニルアミノ基などを挙げることができる。
C6~10アリールC1~6アルキル基は、既に述べたC1~6アルキル基に、上述のC6~10アリール基が置換したものである。C6~10アリールC1~6アルキル基としては、ベンジル基、フェネチル基などを挙げることができる。 The C1-6 alkoxycarbonylamino group is a group in which the above-described C1-6 alkoxycarbonyl group is substituted on the amino group. Examples of the C1-6 alkoxycarbonylamino group include a methoxycarbonylamino group, an ethoxycarbonylamino group, an n-propoxycarbonylamino group, an i-propoxycarbonylamino group, an n-butoxycarbonylamino group, and a t-butoxycarbonylamino group. be able to.
The C6-10 aryl C1-6 alkyl group is obtained by substituting the above-mentioned C6-10 aryl group for the C1-6 alkyl group already described. Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
C6~10アリールC1~6アルキル基は、既に述べたC1~6アルキル基に、上述のC6~10アリール基が置換したものである。C6~10アリールC1~6アルキル基としては、ベンジル基、フェネチル基などを挙げることができる。 The C1-6 alkoxycarbonylamino group is a group in which the above-described C1-6 alkoxycarbonyl group is substituted on the amino group. Examples of the C1-6 alkoxycarbonylamino group include a methoxycarbonylamino group, an ethoxycarbonylamino group, an n-propoxycarbonylamino group, an i-propoxycarbonylamino group, an n-butoxycarbonylamino group, and a t-butoxycarbonylamino group. be able to.
The C6-10 aryl C1-6 alkyl group is obtained by substituting the above-mentioned C6-10 aryl group for the C1-6 alkyl group already described. Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
C6~10アリールオキシ基は、水酸基に、既に述べたC6~10アリール基が置換したものである。C6~10アリールオキシ基としては、フェノキシ基、ナフトキシ基などを挙げることができる。
The C6-10 aryloxy group is a group in which the above-described C6-10 aryl group is substituted on the hydroxyl group. Examples of the C6-10 aryloxy group include a phenoxy group and a naphthoxy group.
C6~10アリールC1~6アルコキシ基は、既に述べたC1~6アルコキシ基に、上述のC6~10アリール基が置換したものである。C6~10アリールC1~6アルコキシ基としては、ベンジルオキシ基などを挙げることができる。
The C6-10 aryl C1-6 alkoxy group is the above-described C1-6 alkoxy group substituted by the above-mentioned C6-10 aryl group. Examples of the C6-10 aryl C1-6 alkoxy group include a benzyloxy group.
3~6員ヘテロシクリル基は、窒素原子、酸素原子および硫黄原子からなる群から選ばれる1~4個のヘテロ原子を環の構成原子として含むものである。ヘテロシクリル基は、単環および多環のいずれであってもよい。多環ヘテロシクリル基は、少なくとも一つの環がヘテロシクリルであれば、残りの環が飽和脂環、不飽和脂環または芳香環のいずれであってもよい。3~6員ヘテロシクリル基としては、3~6員飽和へテロシクリル基、5~6員ヘテロアリール基、5~6員部分不飽和へテロシクリル基などを挙げることができる。
The 3- to 6-membered heterocyclyl group includes 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom, and a sulfur atom as ring constituent atoms. The heterocyclyl group may be monocyclic or polycyclic. In the polycyclic heterocyclyl group, when at least one ring is heterocyclyl, the remaining ring may be a saturated alicyclic ring, an unsaturated alicyclic ring, or an aromatic ring. Examples of the 3- to 6-membered heterocyclyl group include a 3- to 6-membered saturated heterocyclyl group, a 5- to 6-membered heteroaryl group, and a 5- to 6-membered partially unsaturated heterocyclyl group.
3~6員飽和ヘテロシクリル基としては、アジリジニル基、オキシラニル基、アゼチジニル基、オキセタニル基、ピロリジニル基、テトラヒドロフラニル基、チアゾリジニル基、ピペリジル基、ピペラジニル基、モルホリニル基、テトラヒドロピラニル基、ジオキソラニル基、ジオキサニル基などを挙げることができる。
Examples of the 3- to 6-membered saturated heterocyclyl group include aziridinyl group, oxiranyl group, azetidinyl group, oxetanyl group, pyrrolidinyl group, tetrahydrofuranyl group, thiazolidinyl group, piperidyl group, piperazinyl group, morpholinyl group, tetrahydropyranyl group, dioxolanyl group, dioxanyl Examples include groups.
5員ヘテロアリール基としては、ピロリル基、フリル基、チエニル基、イミダゾリル基、ピラゾリル基、オキサゾリル基、イソオキサゾリル基、チアゾリル基、イソチアゾリル基、トリアゾリル基、オキサジアゾリル基、チアジアゾリル基、テトラゾリル基などを挙げることができる。
6員ヘテロアリール基としては、ピリジル基、ピラジニル基、ピリミジニル基、ピリダニジル基、トリアジニル基などを挙げることができる。 Examples of 5-membered heteroaryl groups include pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl, etc. Can do.
Examples of the 6-membered heteroaryl group include a pyridyl group, a pyrazinyl group, a pyrimidinyl group, a pyridanidyl group, and a triazinyl group.
6員ヘテロアリール基としては、ピリジル基、ピラジニル基、ピリミジニル基、ピリダニジル基、トリアジニル基などを挙げることができる。 Examples of 5-membered heteroaryl groups include pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl, etc. Can do.
Examples of the 6-membered heteroaryl group include a pyridyl group, a pyrazinyl group, a pyrimidinyl group, a pyridanidyl group, and a triazinyl group.
3~6員ヘテロシクリルオキシ基は、水酸基に、3~6員ヘテロシクリル基が置換したものである。3~6員ヘテロシクリルオキシ基としては、ピラゾリルオキシ基、ピリジルオキシ基などを挙げることができる。3~6員ヘテロシクリルオキシ基は、5~6員ヘテロシクリルオキシ基が好ましい。
C1~6アルキルチオ基は、SH基に、C1~6アルキル基が置換したものである。C1~6アルキルチオ基としては、メチルチオ基、エチルチオ基、n-プロピルチオ基、n-ブチルチオ基、n-ペンチルチオ基、n-ヘキシルチオ基、i-プロピルチオ基、i-ブチルチオ基などを挙げることができる。
C1~6アルキルスルフィニル基は、スルフィニル基に、C1~6アルキル基が結合したものである。C1~6アルキルスルフィニル基としては、メチルスルフィニル基、エチルスルフィニル基、t-ブチルスルフィニル基などを挙げることができる。
C1~6アルキルスルホニル基は、スルホニル基に、C1~6アルキル基が結合したものである。C1~6アルキルスルホニル基としては、メチルスルホニル基、エチルスルホニル基、t-ブチルスルホニル基などを挙げることができる。 The 3- to 6-membered heterocyclyloxy group is a hydroxyl group substituted with a 3- to 6-membered heterocyclyl group. Examples of the 3- to 6-membered heterocyclyloxy group include a pyrazolyloxy group and a pyridyloxy group. The 3- to 6-membered heterocyclyloxy group is preferably a 5- to 6-membered heterocyclyloxy group.
The C1-6 alkylthio group is obtained by substituting a C1-6 alkyl group for an SH group. Examples of the C1-6 alkylthio group include methylthio group, ethylthio group, n-propylthio group, n-butylthio group, n-pentylthio group, n-hexylthio group, i-propylthio group, i-butylthio group and the like.
The C1-6 alkylsulfinyl group is a C1-6 alkyl group bonded to a sulfinyl group. Examples of the C1-6 alkylsulfinyl group include a methylsulfinyl group, an ethylsulfinyl group, and a t-butylsulfinyl group.
The C1-6 alkylsulfonyl group is a sulfonyl group having a C1-6 alkyl group bonded thereto. Examples of the C1-6 alkylsulfonyl group include a methylsulfonyl group, an ethylsulfonyl group, and a t-butylsulfonyl group.
C1~6アルキルチオ基は、SH基に、C1~6アルキル基が置換したものである。C1~6アルキルチオ基としては、メチルチオ基、エチルチオ基、n-プロピルチオ基、n-ブチルチオ基、n-ペンチルチオ基、n-ヘキシルチオ基、i-プロピルチオ基、i-ブチルチオ基などを挙げることができる。
C1~6アルキルスルフィニル基は、スルフィニル基に、C1~6アルキル基が結合したものである。C1~6アルキルスルフィニル基としては、メチルスルフィニル基、エチルスルフィニル基、t-ブチルスルフィニル基などを挙げることができる。
C1~6アルキルスルホニル基は、スルホニル基に、C1~6アルキル基が結合したものである。C1~6アルキルスルホニル基としては、メチルスルホニル基、エチルスルホニル基、t-ブチルスルホニル基などを挙げることができる。 The 3- to 6-membered heterocyclyloxy group is a hydroxyl group substituted with a 3- to 6-membered heterocyclyl group. Examples of the 3- to 6-membered heterocyclyloxy group include a pyrazolyloxy group and a pyridyloxy group. The 3- to 6-membered heterocyclyloxy group is preferably a 5- to 6-membered heterocyclyloxy group.
The C1-6 alkylthio group is obtained by substituting a C1-6 alkyl group for an SH group. Examples of the C1-6 alkylthio group include methylthio group, ethylthio group, n-propylthio group, n-butylthio group, n-pentylthio group, n-hexylthio group, i-propylthio group, i-butylthio group and the like.
The C1-6 alkylsulfinyl group is a C1-6 alkyl group bonded to a sulfinyl group. Examples of the C1-6 alkylsulfinyl group include a methylsulfinyl group, an ethylsulfinyl group, and a t-butylsulfinyl group.
The C1-6 alkylsulfonyl group is a sulfonyl group having a C1-6 alkyl group bonded thereto. Examples of the C1-6 alkylsulfonyl group include a methylsulfonyl group, an ethylsulfonyl group, and a t-butylsulfonyl group.
C6~10アリールチオ基は、SH基に、C6~10アリール基が置換したものである。C6~10アリールチオ基としては、フェニルチオ基、ナフチルチオ基などを挙げることができる。
C6~10アリールスルフィニル基は、スルフィニル基に、C6~10アリール基が置換したものである。C6~10アリールスルフィニル基としては、フェニルスルフィニル基、ナフチルスルフィニル基などを挙げることができる。
C6~10アリールスルホニル基は、スルホニル基に、C6~10アリール基が置換したものである。C6~10アリールスルホニル基としては、フェニルスルホニル基、ナフチルスルホニル基などを挙げることができる。 The C6-10 arylthio group is a group in which a C6-10 aryl group is substituted on the SH group. Examples of the C6-10 arylthio group include a phenylthio group and a naphthylthio group.
The C6-10 arylsulfinyl group is a sulfinyl group substituted with a C6-10 aryl group. Examples of the C6-10 arylsulfinyl group include a phenylsulfinyl group and a naphthylsulfinyl group.
The C6-10 arylsulfonyl group is a sulfonyl group substituted with a C6-10 aryl group. Examples of the C6-10 arylsulfonyl group include a phenylsulfonyl group and a naphthylsulfonyl group.
C6~10アリールスルフィニル基は、スルフィニル基に、C6~10アリール基が置換したものである。C6~10アリールスルフィニル基としては、フェニルスルフィニル基、ナフチルスルフィニル基などを挙げることができる。
C6~10アリールスルホニル基は、スルホニル基に、C6~10アリール基が置換したものである。C6~10アリールスルホニル基としては、フェニルスルホニル基、ナフチルスルホニル基などを挙げることができる。 The C6-10 arylthio group is a group in which a C6-10 aryl group is substituted on the SH group. Examples of the C6-10 arylthio group include a phenylthio group and a naphthylthio group.
The C6-10 arylsulfinyl group is a sulfinyl group substituted with a C6-10 aryl group. Examples of the C6-10 arylsulfinyl group include a phenylsulfinyl group and a naphthylsulfinyl group.
The C6-10 arylsulfonyl group is a sulfonyl group substituted with a C6-10 aryl group. Examples of the C6-10 arylsulfonyl group include a phenylsulfonyl group and a naphthylsulfonyl group.
C6~10アリールアミノ基は、アミノ基にC6~10アリール基が置換したものである。C6~10アリールアミノ基としては、フェニルアミノ基などが挙げられる。
The C6-10 arylamino group is a group in which an amino group is substituted with a C6-10 aryl group. Examples of the C6-10 arylamino group include a phenylamino group.
C1~6アルキレン基としては、C1~6アルカン中の水素原子2個が外れてなる2価の基である。C1~6アルキレン基としては、メチレン基、エチレン基(ジメチレン基)、トリメチレン基、テトラメチレン基、プロパン-1,2-ジイル基(すなわち、プロピレン基)などを挙げることができる。
C1~6アルキレンジオキシ基は、C1~6アルカン中の水素原子2個がオキシ基で置換されてなる2価の基である。C1~6アルキレンジオキシ基としては、メチレンジオキシ基(-OCH2O-)、エチレンジオキシ基(-OCH2CH2O-)、トリメチレンジオキシ基などを挙げることができる。G2であるC1~6アルキレンジオキシ基で置換されたC6~10アリール基としては、2,3-ジヒドロ-ベンゾ[1,4]ジオキシル基、ベンゾ[1,3]ジオキソリル基などを挙げることができる。 The C1-6 alkylene group is a divalent group formed by removing two hydrogen atoms from the C1-6 alkane. Examples of the C1-6 alkylene group include a methylene group, an ethylene group (dimethylene group), a trimethylene group, a tetramethylene group, and a propane-1,2-diyl group (that is, a propylene group).
The C1-6 alkylenedioxy group is a divalent group formed by replacing two hydrogen atoms in a C1-6 alkane with an oxy group. Examples of the C1-6 alkylenedioxy group include a methylenedioxy group (—OCH 2 O—), an ethylenedioxy group (—OCH 2 CH 2 O—), and a trimethylenedioxy group. Examples of the C6-10 aryl group substituted with a C1-6 alkylenedioxy group as G 2 include a 2,3-dihydro-benzo [1,4] dioxyl group, a benzo [1,3] dioxolyl group, and the like. Can do.
C1~6アルキレンジオキシ基は、C1~6アルカン中の水素原子2個がオキシ基で置換されてなる2価の基である。C1~6アルキレンジオキシ基としては、メチレンジオキシ基(-OCH2O-)、エチレンジオキシ基(-OCH2CH2O-)、トリメチレンジオキシ基などを挙げることができる。G2であるC1~6アルキレンジオキシ基で置換されたC6~10アリール基としては、2,3-ジヒドロ-ベンゾ[1,4]ジオキシル基、ベンゾ[1,3]ジオキソリル基などを挙げることができる。 The C1-6 alkylene group is a divalent group formed by removing two hydrogen atoms from the C1-6 alkane. Examples of the C1-6 alkylene group include a methylene group, an ethylene group (dimethylene group), a trimethylene group, a tetramethylene group, and a propane-1,2-diyl group (that is, a propylene group).
The C1-6 alkylenedioxy group is a divalent group formed by replacing two hydrogen atoms in a C1-6 alkane with an oxy group. Examples of the C1-6 alkylenedioxy group include a methylenedioxy group (—OCH 2 O—), an ethylenedioxy group (—OCH 2 CH 2 O—), and a trimethylenedioxy group. Examples of the C6-10 aryl group substituted with a C1-6 alkylenedioxy group as G 2 include a 2,3-dihydro-benzo [1,4] dioxyl group, a benzo [1,3] dioxolyl group, and the like. Can do.
C1~6ハロアルキル基、C2~6ハロアルケニル基、C2~6ハロアルキニル基、C1~6ハロアルコキシ基、およびC1~6ハロアルキレンジオキシ基は、すでに述べた、C1~6アルキル基、C2~6アルケニル基、C2~6アルキニル基、C1~6アルコキシ基、およびC1~6アルキレンジオキシ基に、ハロゲノ基が置換したものである。
C1~6ハロアルキル基としては、フルオロメチル基、クロロメチル基、ブロモメチル基、ジフルオロメチル基、ジクロロメチル基、ジブロモメチル基、トリフルオロメチル基、トリクロロメチル基、トリブロモメチル基、1-クロロエチル基、2,2,2-トリフルオロエチル基、2,2,2-トリクロロエチル基、ペンタフルオロエチル基、4-フルオロブチル基、4-クロロブチル基、3,3,3-トリフルオロプロピル基、2,2,2-トリフルオロ-1-トリフルオロメチルエチル基、パーフロロヘキシル基、パークロロヘキシル基、2,4,6-トリクロロヘキシル基などを挙げることができる。 The C1-6 haloalkyl group, the C2-6 haloalkenyl group, the C2-6 haloalkynyl group, the C1-6 haloalkoxy group, and the C1-6 haloalkylenedioxy group are the C1-6 alkyl groups, C2-6 A halogeno group is substituted on a 6 alkenyl group, a C2-6 alkynyl group, a C1-6 alkoxy group, and a C1-6 alkylenedioxy group.
C1-6 haloalkyl group includes a fluoromethyl group, a chloromethyl group, a bromomethyl group, a difluoromethyl group, a dichloromethyl group, a dibromomethyl group, a trifluoromethyl group, a trichloromethyl group, a tribromomethyl group, a 1-chloroethyl group, 2,2,2-trifluoroethyl group, 2,2,2-trichloroethyl group, pentafluoroethyl group, 4-fluorobutyl group, 4-chlorobutyl group, 3,3,3-trifluoropropyl group, 2, Examples include 2,2-trifluoro-1-trifluoromethylethyl group, perfluorohexyl group, perchlorohexyl group, 2,4,6-trichlorohexyl group and the like.
C1~6ハロアルキル基としては、フルオロメチル基、クロロメチル基、ブロモメチル基、ジフルオロメチル基、ジクロロメチル基、ジブロモメチル基、トリフルオロメチル基、トリクロロメチル基、トリブロモメチル基、1-クロロエチル基、2,2,2-トリフルオロエチル基、2,2,2-トリクロロエチル基、ペンタフルオロエチル基、4-フルオロブチル基、4-クロロブチル基、3,3,3-トリフルオロプロピル基、2,2,2-トリフルオロ-1-トリフルオロメチルエチル基、パーフロロヘキシル基、パークロロヘキシル基、2,4,6-トリクロロヘキシル基などを挙げることができる。 The C1-6 haloalkyl group, the C2-6 haloalkenyl group, the C2-6 haloalkynyl group, the C1-6 haloalkoxy group, and the C1-6 haloalkylenedioxy group are the C1-6 alkyl groups, C2-6 A halogeno group is substituted on a 6 alkenyl group, a C2-6 alkynyl group, a C1-6 alkoxy group, and a C1-6 alkylenedioxy group.
C1-6 haloalkyl group includes a fluoromethyl group, a chloromethyl group, a bromomethyl group, a difluoromethyl group, a dichloromethyl group, a dibromomethyl group, a trifluoromethyl group, a trichloromethyl group, a tribromomethyl group, a 1-chloroethyl group, 2,2,2-trifluoroethyl group, 2,2,2-trichloroethyl group, pentafluoroethyl group, 4-fluorobutyl group, 4-chlorobutyl group, 3,3,3-trifluoropropyl group, 2, Examples include 2,2-trifluoro-1-trifluoromethylethyl group, perfluorohexyl group, perchlorohexyl group, 2,4,6-trichlorohexyl group and the like.
C2~6ハロアルケニル基としては、2-クロロ-1-プロペニル基、2-フルオロ-1-ブテニル基などを挙げることができる。
C2~6ハロアルキニル基としては、4,4-ジクロロ-1-ブチニル基、4-フルオロ-1-ペンチニル基、5-ブロモ-2-ペンチニル基などを挙げることができる。
C1~6ハロアルコキシ基としては、クロロメトキシ基、ジクロロメトキシ基、ジフルオロメトキシ基、トリクロロメトキシ基、トリフルオロメトキシ基、1-フルオロエトキシ基、1,1-ジフルオロエトキシ基、2,2,2-トリフルオロエトキシ基、1,1,2,2-テトラフルオロエトキシ基、ペンタフルオロエトキシ基、2,2,3,4,4,4-ヘキサフルオロ-ブトキシ基、1-ブロモ-1,1,2,2-テトラフルオロエトキシ基などを挙げることができる。
C1~6ハロアルキレンジオキシ基としては、ジフルオロメチレンジオキシ基(-OCF2O-)、テトラフルオロエチレンジオキシ基(-OCF2CF2O-)などを挙げることができる。G2であるC1~6ハロアルキレンジオキシ基で置換されたC6~10アリール基としては、2,2,3,3-テトラフルオロ-2,3-ジヒドロ-ベンゾ[1,4]ジオキシル基、2,2-ジフルオロ-ベンゾ[1,3]ジオキソリル基などを挙げることができる。 Examples of the C2-6 haloalkenyl group include a 2-chloro-1-propenyl group and a 2-fluoro-1-butenyl group.
Examples of the C2-6 haloalkynyl group include a 4,4-dichloro-1-butynyl group, a 4-fluoro-1-pentynyl group, and a 5-bromo-2-pentynyl group.
C1-6 haloalkoxy groups include chloromethoxy, dichloromethoxy, difluoromethoxy, trichloromethoxy, trifluoromethoxy, 1-fluoroethoxy, 1,1-difluoroethoxy, 2,2,2- Trifluoroethoxy group, 1,1,2,2-tetrafluoroethoxy group, pentafluoroethoxy group, 2,2,3,4,4,4-hexafluoro-butoxy group, 1-bromo-1,1,2 , 2-tetrafluoroethoxy group and the like.
Examples of the C1-6 haloalkylenedioxy group include a difluoromethylenedioxy group (—OCF 2 O—) and a tetrafluoroethylenedioxy group (—OCF 2 CF 2 O—). Examples of the C6-10 aryl group substituted by the C1-6 haloalkylenedioxy group as G 2 include a 2,2,3,3-tetrafluoro-2,3-dihydro-benzo [1,4] dioxyl group, A 2,2-difluoro-benzo [1,3] dioxolyl group and the like can be mentioned.
C2~6ハロアルキニル基としては、4,4-ジクロロ-1-ブチニル基、4-フルオロ-1-ペンチニル基、5-ブロモ-2-ペンチニル基などを挙げることができる。
C1~6ハロアルコキシ基としては、クロロメトキシ基、ジクロロメトキシ基、ジフルオロメトキシ基、トリクロロメトキシ基、トリフルオロメトキシ基、1-フルオロエトキシ基、1,1-ジフルオロエトキシ基、2,2,2-トリフルオロエトキシ基、1,1,2,2-テトラフルオロエトキシ基、ペンタフルオロエトキシ基、2,2,3,4,4,4-ヘキサフルオロ-ブトキシ基、1-ブロモ-1,1,2,2-テトラフルオロエトキシ基などを挙げることができる。
C1~6ハロアルキレンジオキシ基としては、ジフルオロメチレンジオキシ基(-OCF2O-)、テトラフルオロエチレンジオキシ基(-OCF2CF2O-)などを挙げることができる。G2であるC1~6ハロアルキレンジオキシ基で置換されたC6~10アリール基としては、2,2,3,3-テトラフルオロ-2,3-ジヒドロ-ベンゾ[1,4]ジオキシル基、2,2-ジフルオロ-ベンゾ[1,3]ジオキソリル基などを挙げることができる。 Examples of the C2-6 haloalkenyl group include a 2-chloro-1-propenyl group and a 2-fluoro-1-butenyl group.
Examples of the C2-6 haloalkynyl group include a 4,4-dichloro-1-butynyl group, a 4-fluoro-1-pentynyl group, and a 5-bromo-2-pentynyl group.
C1-6 haloalkoxy groups include chloromethoxy, dichloromethoxy, difluoromethoxy, trichloromethoxy, trifluoromethoxy, 1-fluoroethoxy, 1,1-difluoroethoxy, 2,2,2- Trifluoroethoxy group, 1,1,2,2-tetrafluoroethoxy group, pentafluoroethoxy group, 2,2,3,4,4,4-hexafluoro-butoxy group, 1-bromo-1,1,2 , 2-tetrafluoroethoxy group and the like.
Examples of the C1-6 haloalkylenedioxy group include a difluoromethylenedioxy group (—OCF 2 O—) and a tetrafluoroethylenedioxy group (—OCF 2 CF 2 O—). Examples of the C6-10 aryl group substituted by the C1-6 haloalkylenedioxy group as G 2 include a 2,2,3,3-tetrafluoro-2,3-dihydro-benzo [1,4] dioxyl group, A 2,2-difluoro-benzo [1,3] dioxolyl group and the like can be mentioned.
C1~6アルコキシC1~6ハロアルキル基、C1~6ハロアルコキシC1~6ハロアルコキシ基、C1~6ハロアルキルチオ基、C1~6ハロアルキルスルフィニル基、およびC1~6ハロアルキルスルホニル基は、すでに述べた、C1~6アルコキシC1~6アルキル基、C1~6アルコキシC1~6アルコキシ基、C1~6アルキルチオ基、C1~6アルキルスルフィニル基、およびC1~6アルキルスルホニル基に、ハロゲノ基が置換したものである。
C1~6アルコキシC1~6ハロアルキル基としては、ジフルオロ(メトキシ)メチル基、1,1,1,3,3,3-ヘキサフルオロ-2-メトキシプロパン-2-イル基などを挙げることができる。
C1~6ハロアルコキシC1~6ハロアルコキシ基としては、ジフルオロ(メトキシ)メトキシ基、1,2,2-トリフルオロ-2-(トリフルオロメトキシ)エトキシ基などを挙げることができる。
C1~6ハロアルキルチオ基としては、トリフルオロメチルチオ基、2,2,2-トリフルオロエチルチオ基などを挙げることができる。
C1~6ハロアルキルスルフィニル基としては、トリフルオロメチルスルフィニル基、2,2,2-トリフルオロエチルスルフィニル基などを挙げることができる。
C1~6ハロアルキルスルホニル基としては、トリフルオロメチルスルホニル基、2,2,2-トリフルオロエチルスルホニル基などを挙げることができる。 C1-6 alkoxy C1-6 haloalkyl group, C1-6 haloalkoxy C1-6 haloalkoxy group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, and C1-6 haloalkylsulfonyl group are the same as those described above, C1 A halogeno group is substituted on a -6 alkoxy C1-6 alkyl group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkylthio group, a C1-6 alkylsulfinyl group, and a C1-6 alkylsulfonyl group.
Examples of the C1-6 alkoxy C1-6 haloalkyl group include a difluoro (methoxy) methyl group and a 1,1,1,3,3,3-hexafluoro-2-methoxypropan-2-yl group.
Examples of the C1-6 haloalkoxy group include a difluoro (methoxy) methoxy group and a 1,2,2-trifluoro-2- (trifluoromethoxy) ethoxy group.
Examples of the C1-6 haloalkylthio group include a trifluoromethylthio group and a 2,2,2-trifluoroethylthio group.
Examples of the C1-6 haloalkylsulfinyl group include a trifluoromethylsulfinyl group and a 2,2,2-trifluoroethylsulfinyl group.
Examples of the C1-6 haloalkylsulfonyl group include a trifluoromethylsulfonyl group and a 2,2,2-trifluoroethylsulfonyl group.
C1~6アルコキシC1~6ハロアルキル基としては、ジフルオロ(メトキシ)メチル基、1,1,1,3,3,3-ヘキサフルオロ-2-メトキシプロパン-2-イル基などを挙げることができる。
C1~6ハロアルコキシC1~6ハロアルコキシ基としては、ジフルオロ(メトキシ)メトキシ基、1,2,2-トリフルオロ-2-(トリフルオロメトキシ)エトキシ基などを挙げることができる。
C1~6ハロアルキルチオ基としては、トリフルオロメチルチオ基、2,2,2-トリフルオロエチルチオ基などを挙げることができる。
C1~6ハロアルキルスルフィニル基としては、トリフルオロメチルスルフィニル基、2,2,2-トリフルオロエチルスルフィニル基などを挙げることができる。
C1~6ハロアルキルスルホニル基としては、トリフルオロメチルスルホニル基、2,2,2-トリフルオロエチルスルホニル基などを挙げることができる。 C1-6 alkoxy C1-6 haloalkyl group, C1-6 haloalkoxy C1-6 haloalkoxy group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, and C1-6 haloalkylsulfonyl group are the same as those described above, C1 A halogeno group is substituted on a -6 alkoxy C1-6 alkyl group, a C1-6 alkoxy C1-6 alkoxy group, a C1-6 alkylthio group, a C1-6 alkylsulfinyl group, and a C1-6 alkylsulfonyl group.
Examples of the C1-6 alkoxy C1-6 haloalkyl group include a difluoro (methoxy) methyl group and a 1,1,1,3,3,3-hexafluoro-2-methoxypropan-2-yl group.
Examples of the C1-6 haloalkoxy group include a difluoro (methoxy) methoxy group and a 1,2,2-trifluoro-2- (trifluoromethoxy) ethoxy group.
Examples of the C1-6 haloalkylthio group include a trifluoromethylthio group and a 2,2,2-trifluoroethylthio group.
Examples of the C1-6 haloalkylsulfinyl group include a trifluoromethylsulfinyl group and a 2,2,2-trifluoroethylsulfinyl group.
Examples of the C1-6 haloalkylsulfonyl group include a trifluoromethylsulfonyl group and a 2,2,2-trifluoroethylsulfonyl group.
置換基G21は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、C6~10アリール基、またはハロゲノ基を示す。これらは既に述べたとおりのものである。
G2またはG4のうちの2つ以上がG21で置換された前記の基である場合、係るG21は、相互に同じであっても異なってもよい。 The substituent G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group, or a halogeno group. These are as already described.
If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
G2またはG4のうちの2つ以上がG21で置換された前記の基である場合、係るG21は、相互に同じであっても異なってもよい。 The substituent G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group, or a halogeno group. These are as already described.
If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
本発明においては、R1としては、
水素原子、無置換のもしくはG1で置換されたC1~6アルキル基(好ましくは無置換)、無置換のもしくはG1(好ましくはC1~6アルコキシカルボニル基)で置換されたC1~6アルケニル基、無置換のもしくはG2(好ましくはC1~6アルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、シアノ基またはハロゲノ基が好ましく、
無置換のC1~6アルキル基またはハロゲノ基がより好ましい。
〔R2およびR3〕
R2およびR3は、それぞれ独立に、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、ホルミル基、ホルミルオキシ基、無置換の若しくはG1で置換されたC1~6アルキルカルボニルオキシ基、無置換の若しくはG2で置換されたC6~10アリール基、(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基、シアノ基、またはハロゲノ基を示す。 In the present invention, as R 1 ,
Hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1 (preferably unsubstituted), unsubstituted or C1 ~ 6 alkenyl group substituted with G 1 (preferably C1 ~ 6 alkoxycarbonyl group) A C6-10 aryl group (preferably a phenyl group), unsubstituted or substituted with G 2 (preferably a C1-6 alkoxy group), a cyano group or a halogeno group,
An unsubstituted C1-6 alkyl group or a halogeno group is more preferred.
[R 2 and R 3 ]
R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 3-8 cycloalkyl group substituted with G 2 , an unsubstituted group Or a C1-6 alkoxy group substituted with G 1 , a formyl group, a formyloxy group, an unsubstituted or C1-6 alkylcarbonyloxy group substituted with G 1 , an unsubstituted or C6 substituted with G 2 A 10 aryl group, (unsubstituted or G 1 -substituted C1-6 alkoxyimino) -C1-6 alkyl group, cyano group, or halogeno group;
水素原子、無置換のもしくはG1で置換されたC1~6アルキル基(好ましくは無置換)、無置換のもしくはG1(好ましくはC1~6アルコキシカルボニル基)で置換されたC1~6アルケニル基、無置換のもしくはG2(好ましくはC1~6アルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、シアノ基またはハロゲノ基が好ましく、
無置換のC1~6アルキル基またはハロゲノ基がより好ましい。
〔R2およびR3〕
R2およびR3は、それぞれ独立に、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、ホルミル基、ホルミルオキシ基、無置換の若しくはG1で置換されたC1~6アルキルカルボニルオキシ基、無置換の若しくはG2で置換されたC6~10アリール基、(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基、シアノ基、またはハロゲノ基を示す。 In the present invention, as R 1 ,
Hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1 (preferably unsubstituted), unsubstituted or C1 ~ 6 alkenyl group substituted with G 1 (preferably C1 ~ 6 alkoxycarbonyl group) A C6-10 aryl group (preferably a phenyl group), unsubstituted or substituted with G 2 (preferably a C1-6 alkoxy group), a cyano group or a halogeno group,
An unsubstituted C1-6 alkyl group or a halogeno group is more preferred.
[R 2 and R 3 ]
R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 3-8 cycloalkyl group substituted with G 2 , an unsubstituted group Or a C1-6 alkoxy group substituted with G 1 , a formyl group, a formyloxy group, an unsubstituted or C1-6 alkylcarbonyloxy group substituted with G 1 , an unsubstituted or C6 substituted with G 2 A 10 aryl group, (unsubstituted or G 1 -substituted C1-6 alkoxyimino) -C1-6 alkyl group, cyano group, or halogeno group;
R2およびR3における、C1~6アルキル基、C3~8シクロアルキル基、C1~6アルコキシ基、C1~6アルキルカルボニルオキシ基、C6~10アリール基、ハロゲノ基、および置換基G1、G2は、すでに述べたとおりのものである。
(C1~6アルコキシイミノ)-C1~6アルキル基としては、メトキシイミノ-メチル基、1-(エトキシイミノ)-エチル基などを挙げることができる。 In R 2 and R 3 , a C1-6 alkyl group, a C3-8 cycloalkyl group, a C1-6 alkoxy group, a C1-6 alkylcarbonyloxy group, a C6-10 aryl group, a halogeno group, and a substituent G 1 , G 2 is as described above.
Examples of the (C1-6 alkoxyimino) -C1-6 alkyl group include a methoxyimino-methyl group and a 1- (ethoxyimino) -ethyl group.
(C1~6アルコキシイミノ)-C1~6アルキル基としては、メトキシイミノ-メチル基、1-(エトキシイミノ)-エチル基などを挙げることができる。 In R 2 and R 3 , a C1-6 alkyl group, a C3-8 cycloalkyl group, a C1-6 alkoxy group, a C1-6 alkylcarbonyloxy group, a C6-10 aryl group, a halogeno group, and a substituent G 1 , G 2 is as described above.
Examples of the (C1-6 alkoxyimino) -C1-6 alkyl group include a methoxyimino-methyl group and a 1- (ethoxyimino) -ethyl group.
R1とR2は、一緒になってR1およびR2のそれぞれが結合する炭素原子と共に、5~6員環を形成してもよい。係る5~6員環としては、シクロペンテン環、シクロヘキセン環などを挙げることができる。
R 1 and R 2 may together form a 5-6 membered ring with the carbon atom to which each of R 1 and R 2 is attached. Examples of such a 5- to 6-membered ring include a cyclopentene ring and a cyclohexene ring.
本発明のおいては、R2としては、
無置換の若しくはG1で置換されたC1~6アルキル基(好ましくは無置換)が好ましい。 In the present invention, as R 2 ,
An unsubstituted or G 1 -substituted C1-6 alkyl group (preferably unsubstituted) is preferred.
無置換の若しくはG1で置換されたC1~6アルキル基(好ましくは無置換)が好ましい。 In the present invention, as R 2 ,
An unsubstituted or G 1 -substituted C1-6 alkyl group (preferably unsubstituted) is preferred.
本発明のおいては、R3としては、
水素原子、無置換の若しくはG1(好ましくはC1~6アルコキシカルボニルオキシ基)で置換されたC1~6アルキル基、
無置換の若しくはG1で置換されたC1~6アルコキシ基(好ましくは無置換)、
無置換のC3~8シクロアルキル基(好ましくは3~4シクロアルキル基)、
(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基(好ましくは無置換)、
ハロゲノ基、シアノ基またはホルミル基が好ましく、
無置換の若しくはG1(好ましくはC1~6アルコキシカルボニルオキシ基)で置換されたC1~6アルキル基、無置換のC3~8シクロアルキル基(好ましくは3~4シクロアルキル基)またはハロゲノ基がより好ましい。 In the present invention, as R 3 ,
A hydrogen atom, a C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group),
A C1-6 alkoxy group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
Unsubstituted C3-8 cycloalkyl group (preferably 3-4 cycloalkyl group),
(Unsubstituted or G 1 -substituted C 1-6 alkoxyimino) -C 1-6 alkyl group (preferably unsubstituted),
Preferably a halogeno group, a cyano group or a formyl group,
An unsubstituted or substituted C1-6 alkyl group substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group), an unsubstituted C3-8 cycloalkyl group (preferably a 3-4 cycloalkyl group) or a halogeno group; More preferred.
水素原子、無置換の若しくはG1(好ましくはC1~6アルコキシカルボニルオキシ基)で置換されたC1~6アルキル基、
無置換の若しくはG1で置換されたC1~6アルコキシ基(好ましくは無置換)、
無置換のC3~8シクロアルキル基(好ましくは3~4シクロアルキル基)、
(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基(好ましくは無置換)、
ハロゲノ基、シアノ基またはホルミル基が好ましく、
無置換の若しくはG1(好ましくはC1~6アルコキシカルボニルオキシ基)で置換されたC1~6アルキル基、無置換のC3~8シクロアルキル基(好ましくは3~4シクロアルキル基)またはハロゲノ基がより好ましい。 In the present invention, as R 3 ,
A hydrogen atom, a C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group),
A C1-6 alkoxy group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
Unsubstituted C3-8 cycloalkyl group (preferably 3-4 cycloalkyl group),
(Unsubstituted or G 1 -substituted C 1-6 alkoxyimino) -C 1-6 alkyl group (preferably unsubstituted),
Preferably a halogeno group, a cyano group or a formyl group,
An unsubstituted or substituted C1-6 alkyl group substituted with G 1 (preferably a C1-6 alkoxycarbonyloxy group), an unsubstituted C3-8 cycloalkyl group (preferably a 3-4 cycloalkyl group) or a halogeno group; More preferred.
〔R4〕
R4は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC2~6アルキニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG2で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルC1~6アルキル基、ホルミル基、無置換の若しくはG1で置換されたC1~6アルキルカルボニル基、無置換の若しくはG2で置換されたC6~10アリールカルボニル基、無置換の若しくはG1で置換されたC1~6アルコキシカルボニル基、無置換の若しくはG1で置換されたC2~6アルケニルオキシカルボニル基、無置換の若しくはG1で置換されたC1~6アルキルスルホニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノカルボニル基、無置換の若しくはG1で置換された(C1~6アルキルチオ)カルボニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノ(チオカルボニル)基、または式(IX)で表される有機基を示す。 [R 4 ]
R 4 is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2 A 3-6 membered heterocyclyl C1-6 alkyl group, a formyl group, a C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 , a C6-10 arylcarbonyl group which is unsubstituted or substituted with G 2 , unsubstituted or C1 ~ 6 alkoxycarbonyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyloxycarbonyl group substituted with G 1, unsubstituted or G 1 Substituted C1 ~ 6 alkylsulfonyl group, an unsubstituted or C1 ~ 6 alkylaminocarbonyl group substituted with G 1, unsubstituted or substituted with G 1 a (C1 ~ 6 alkylthio) carbonyl group, the unsubstituted Or a C1-6 alkylamino (thiocarbonyl) group substituted with G 1 or an organic group represented by the formula (IX).
R4は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC2~6アルキニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG2で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルC1~6アルキル基、ホルミル基、無置換の若しくはG1で置換されたC1~6アルキルカルボニル基、無置換の若しくはG2で置換されたC6~10アリールカルボニル基、無置換の若しくはG1で置換されたC1~6アルコキシカルボニル基、無置換の若しくはG1で置換されたC2~6アルケニルオキシカルボニル基、無置換の若しくはG1で置換されたC1~6アルキルスルホニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノカルボニル基、無置換の若しくはG1で置換された(C1~6アルキルチオ)カルボニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノ(チオカルボニル)基、または式(IX)で表される有機基を示す。 [R 4 ]
R 4 is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2 A 3-6 membered heterocyclyl C1-6 alkyl group, a formyl group, a C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 , a C6-10 arylcarbonyl group which is unsubstituted or substituted with G 2 , unsubstituted or C1 ~ 6 alkoxycarbonyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyloxycarbonyl group substituted with G 1, unsubstituted or G 1 Substituted C1 ~ 6 alkylsulfonyl group, an unsubstituted or C1 ~ 6 alkylaminocarbonyl group substituted with G 1, unsubstituted or substituted with G 1 a (C1 ~ 6 alkylthio) carbonyl group, the unsubstituted Or a C1-6 alkylamino (thiocarbonyl) group substituted with G 1 or an organic group represented by the formula (IX).
R4における、C1~6アルキル基、C2~6アルケニル基、C2~6アルキニル基、C3~8シクロアルキル基、C6~10アリールC1~6アルキル基、C1~6アルキルカルボニル基、C1~6アルコキシカルボニル基、C1~6アルキルスルホニル基、置換基G1および置換基G2は既に述べたとおりのものである。
In R 4 , C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C6-10 aryl C1-6 alkyl group, C1-6 alkylcarbonyl group, C1-6 alkoxy The carbonyl group, C1-6 alkylsulfonyl group, substituent G 1 and substituent G 2 are as described above.
3~6員ヘテロシクリルC1~6アルキル基は、C1~6アルキル基に、3~6員ヘテロシクリル基が置換したものである。3~6員ヘテロシクリルC1~6アルキル基としては、好ましくは、テトラヒドロフラニルメチル基、テトラヒドロピラニルメチル基、ジオキソラニルメチル基、ジオキサニルメチル基などの5~6員飽和ヘテロシクリルC1~6アルキル基;ピラゾリルメチル基、ピリジルメチル基などの5~6員ヘテロアリールC1~6アルキル基を挙げることができる。
C6~10アリールカルボニル基は、カルボニル基に、C6~10アリール基が結合したものである。C6~10アリールカルボニル基としては、ベンゾイル基などを挙げることができる。 The 3- to 6-membered heterocyclyl C1-6 alkyl group is obtained by substituting a 3- to 6-membered heterocyclyl group for a C1-6 alkyl group. The 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc. Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
The C6-10 arylcarbonyl group is obtained by bonding a C6-10 aryl group to a carbonyl group. Examples of the C6-10 arylcarbonyl group include a benzoyl group.
C6~10アリールカルボニル基は、カルボニル基に、C6~10アリール基が結合したものである。C6~10アリールカルボニル基としては、ベンゾイル基などを挙げることができる。 The 3- to 6-membered heterocyclyl C1-6 alkyl group is obtained by substituting a 3- to 6-membered heterocyclyl group for a C1-6 alkyl group. The 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc. Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
The C6-10 arylcarbonyl group is obtained by bonding a C6-10 aryl group to a carbonyl group. Examples of the C6-10 arylcarbonyl group include a benzoyl group.
C2~6アルケニルオキシカルボニル基としては、ビニルオキシカルボニル基、1-プロペニルオキシカルボニル基、2-プロペニルオキシカルボニル基(アリルオキシカルボニル基)などを挙げることができる。
C1~6アルキルアミノカルボニル基としては、メチルアミノカルボニル基、ジメチルアミノカルボニル基などを挙げることができる。
(C1~6アルキルチオ)カルボニル基としては、(メチルチオ)カルボニル基、(エチルチオ)カルボニル基などを挙げることができる。
C1~6アルキルアミノ(チオカルボニル)基としては、メチルアミノ(チオカルボニル)基、ジメチルアミノ(チオカルボニル)基などを挙げることができる。 Examples of the C2-6 alkenyloxycarbonyl group include a vinyloxycarbonyl group, a 1-propenyloxycarbonyl group, and a 2-propenyloxycarbonyl group (allyloxycarbonyl group).
Examples of the C1-6 alkylaminocarbonyl group include a methylaminocarbonyl group and a dimethylaminocarbonyl group.
Examples of the (C1-6 alkylthio) carbonyl group include (methylthio) carbonyl group and (ethylthio) carbonyl group.
Examples of the C1-6 alkylamino (thiocarbonyl) group include a methylamino (thiocarbonyl) group and a dimethylamino (thiocarbonyl) group.
C1~6アルキルアミノカルボニル基としては、メチルアミノカルボニル基、ジメチルアミノカルボニル基などを挙げることができる。
(C1~6アルキルチオ)カルボニル基としては、(メチルチオ)カルボニル基、(エチルチオ)カルボニル基などを挙げることができる。
C1~6アルキルアミノ(チオカルボニル)基としては、メチルアミノ(チオカルボニル)基、ジメチルアミノ(チオカルボニル)基などを挙げることができる。 Examples of the C2-6 alkenyloxycarbonyl group include a vinyloxycarbonyl group, a 1-propenyloxycarbonyl group, and a 2-propenyloxycarbonyl group (allyloxycarbonyl group).
Examples of the C1-6 alkylaminocarbonyl group include a methylaminocarbonyl group and a dimethylaminocarbonyl group.
Examples of the (C1-6 alkylthio) carbonyl group include (methylthio) carbonyl group and (ethylthio) carbonyl group.
Examples of the C1-6 alkylamino (thiocarbonyl) group include a methylamino (thiocarbonyl) group and a dimethylamino (thiocarbonyl) group.
式(II)中、Gaは、それぞれ独立に、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、または無置換のもしくはG2で置換されたC6~10アリール基を示す。
式(II)中、Gbは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、無置換のもしくはG2で置換されたC6~10アリール基、または無置換のもしくはG2で置換された3~6員ヘテロシクリル基を示す。
式(II)中、Tは、酸素原子、オキシカルボニル基、カルボニルオキシ基、オキシカルボニルオキシ基、硫黄原子、(チオ)カルボニル基、カルボニル(チオ)基、(チオ)カルボニルオキシ基、オキシカルボニル(チオ)基、または-O-C(=O)-N(Gb)-で表される二価の基を示す。
*は式(II)で表される基の結合位置を示す。 Wherein (II), G a are independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, no showing a substituted by a substituted or G 1 a C2 ~ 6 alkynyl, unsubstituted or C3 ~ 8 cycloalkyl group substituted by G 2 or unsubstituted or C6 ~ 10 aryl group substituted by G 2, .
Wherein (II), G b represents a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ~ 6 alkynyl group substituted by one, unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
In formula (II), T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C (═O) —N (G b ) —.
* Indicates the bonding position of the group represented by the formula (II).
式(II)中、Gbは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、無置換のもしくはG2で置換されたC6~10アリール基、または無置換のもしくはG2で置換された3~6員ヘテロシクリル基を示す。
式(II)中、Tは、酸素原子、オキシカルボニル基、カルボニルオキシ基、オキシカルボニルオキシ基、硫黄原子、(チオ)カルボニル基、カルボニル(チオ)基、(チオ)カルボニルオキシ基、オキシカルボニル(チオ)基、または-O-C(=O)-N(Gb)-で表される二価の基を示す。
*は式(II)で表される基の結合位置を示す。 Wherein (II), G a are independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, no showing a substituted by a substituted or G 1 a C2 ~ 6 alkynyl, unsubstituted or C3 ~ 8 cycloalkyl group substituted by G 2 or unsubstituted or C6 ~ 10 aryl group substituted by G 2, .
Wherein (II), G b represents a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ~ 6 alkynyl group substituted by one, unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
In formula (II), T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C (═O) —N (G b ) —.
* Indicates the bonding position of the group represented by the formula (II).
式(II)で表される基の例として、次に示すものが挙げられる。
Examples of the group represented by the formula (II) include the following.
本発明のおいては、R4としては、水素原子、
無置換のもしくはG1(好ましくはC1~6アルキルカルボニルオキシ基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基)で置換されたC1~6アルキル基、
無置換のもしくはG1で置換されたC2~6アルケニル基(好ましくは無置換)、
無置換のもしくはG2(好ましくはC1~6アルキル基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基)で置換されたC6~10アリールC1~6アルキル基(好ましくはベンジル基)、
無置換のもしくはG1で置換されたC1~6アルキルカルボニル基(好ましくは無置換)、
無置換のもしくはG1(好ましくはハロゲノ基)で置換されたC1~6アルコキシカルボニル基、
無置換のもしくはG1で置換された(C1~6アルキルチオ)カルボニル基(好ましくは無置換)、
無置換のもしくはG2で置換された5~6員ヘテロアリールC1~6アルキル基(好ましくはピリジルメチル基)が好ましく、
水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、または無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基がより好ましい。 In the present invention, R 4 is a hydrogen atom,
A C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkylcarbonyloxy group, a C1-6 alkoxy group, a C1-6 alkoxyC1-6 alkoxy group),
A C2-6 alkenyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
C6-10 aryl C1-6 alkyl group (preferably benzyl group) which is unsubstituted or substituted with G 2 (preferably C1-6 alkyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group) ,
A C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
A C1-6 alkoxycarbonyl group which is unsubstituted or substituted with G 1 (preferably a halogeno group),
An unsubstituted or G 1 substituted (C1-6 alkylthio) carbonyl group (preferably unsubstituted),
An unsubstituted or G 2 -substituted 5-6 membered heteroaryl C1-6 alkyl group (preferably a pyridylmethyl group) is preferred,
Hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C1 ~ 6 alkyl group substituted by G 1 C1 ~ 6 substituted or unsubstituted or G 1, An alkoxycarbonyl group is more preferred.
無置換のもしくはG1(好ましくはC1~6アルキルカルボニルオキシ基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基)で置換されたC1~6アルキル基、
無置換のもしくはG1で置換されたC2~6アルケニル基(好ましくは無置換)、
無置換のもしくはG2(好ましくはC1~6アルキル基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基)で置換されたC6~10アリールC1~6アルキル基(好ましくはベンジル基)、
無置換のもしくはG1で置換されたC1~6アルキルカルボニル基(好ましくは無置換)、
無置換のもしくはG1(好ましくはハロゲノ基)で置換されたC1~6アルコキシカルボニル基、
無置換のもしくはG1で置換された(C1~6アルキルチオ)カルボニル基(好ましくは無置換)、
無置換のもしくはG2で置換された5~6員ヘテロアリールC1~6アルキル基(好ましくはピリジルメチル基)が好ましく、
水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、または無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基がより好ましい。 In the present invention, R 4 is a hydrogen atom,
A C1-6 alkyl group which is unsubstituted or substituted with G 1 (preferably a C1-6 alkylcarbonyloxy group, a C1-6 alkoxy group, a C1-6 alkoxyC1-6 alkoxy group),
A C2-6 alkenyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
C6-10 aryl C1-6 alkyl group (preferably benzyl group) which is unsubstituted or substituted with G 2 (preferably C1-6 alkyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group) ,
A C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 (preferably unsubstituted),
A C1-6 alkoxycarbonyl group which is unsubstituted or substituted with G 1 (preferably a halogeno group),
An unsubstituted or G 1 substituted (C1-6 alkylthio) carbonyl group (preferably unsubstituted),
An unsubstituted or G 2 -substituted 5-6 membered heteroaryl C1-6 alkyl group (preferably a pyridylmethyl group) is preferred,
Hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C1 ~ 6 alkyl group substituted by G 1 C1 ~ 6 substituted or unsubstituted or G 1, An alkoxycarbonyl group is more preferred.
〔Q〕
Qは、式(III)~式(XII)で表される有機基のいずれかを示す。なお、*は、式(III)~式(XII)で表される有機基の結合位置を示す。 [Q]
Q represents any one of organic groups represented by the formulas (III) to (XII). Note that * indicates the bonding position of the organic group represented by the formulas (III) to (XII).
Qは、式(III)~式(XII)で表される有機基のいずれかを示す。なお、*は、式(III)~式(XII)で表される有機基の結合位置を示す。 [Q]
Q represents any one of organic groups represented by the formulas (III) to (XII). Note that * indicates the bonding position of the organic group represented by the formulas (III) to (XII).
〈Ra〉
Raは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG2で置換されたC6~10アリール基、またはアミノ基を示す。
RaにおけるC1~6アルキル基、C6~10アリール基、置換基G1および置換基G2はすでに述べたとおりのものである。
Ar1とRaは、相互に繋がって、Ar1とRaが結合する炭素原子とともに5~6員環を形成してもよい。Ar1とRaによって成形される5~6員環としては、シクロペンテン環、シクロヘキセン環などを挙げることができる。
本発明において、Raとしては、無置換のアルキル基または水素原子が好ましい。 <R a >
R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group.
The C1-6 alkyl group, the C6-10 aryl group, the substituent G 1 and the substituent G 2 in R a are as described above.
Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded. The 5- or 6-membered ring which is formed by Ar 1 and R a, may be mentioned cyclopentene ring, a cyclohexene ring.
In the present invention, R a is preferably an unsubstituted alkyl group or a hydrogen atom.
Raは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG2で置換されたC6~10アリール基、またはアミノ基を示す。
RaにおけるC1~6アルキル基、C6~10アリール基、置換基G1および置換基G2はすでに述べたとおりのものである。
Ar1とRaは、相互に繋がって、Ar1とRaが結合する炭素原子とともに5~6員環を形成してもよい。Ar1とRaによって成形される5~6員環としては、シクロペンテン環、シクロヘキセン環などを挙げることができる。
本発明において、Raとしては、無置換のアルキル基または水素原子が好ましい。 <R a >
R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group.
The C1-6 alkyl group, the C6-10 aryl group, the substituent G 1 and the substituent G 2 in R a are as described above.
Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded. The 5- or 6-membered ring which is formed by Ar 1 and R a, may be mentioned cyclopentene ring, a cyclohexene ring.
In the present invention, R a is preferably an unsubstituted alkyl group or a hydrogen atom.
〈X〉
Xは、酸素原子、硫黄原子、スルフィニル基、スルホニル基、または式:NRcで表される二価の基を示す。
ここで、Rcは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、無置換のもしくはG2で置換されたC6~10アリールカルボニル基、無置換のもしくはG2で置換されたC6~10アリールスルホニル基、または無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基または無置換のもしくはG1で置換されたC3~8シクロアルキルオキシカルボニル基を示す。
RcにおけるC1~6アルキル基、C6~10アリール基、C1~6アルキルカルボニル基、C6~10アリールカルボニル基、C6~10アリールスルホニル基、C1~6アルコキシカルボニル基、置換基G1および置換基G2はすでに述べたとおりのものである。
C3~8シクロアルキルオキシカルボニル基としては、シクロプロピルオキシカルボニル基、シクロヘキシルオキシカルボニル基などが挙げられる。
本発明において、Xとしては酸素原子またはNRcで表される二価の基が好ましく、酸素原子がより好ましい。 <X>
X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by the formula: NR c .
Here, R c is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, substituted by unsubstituted or G 1 have been C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryl group substituted with G 2, unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 2 or unsubstituted or G 1, A C1-6 alkoxycarbonyl group substituted with or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 .
C 1-6 alkyl group, C6-10 aryl group, C1-6 alkylcarbonyl group, C6-10 arylcarbonyl group, C6-10 arylsulfonyl group, C1-6 alkoxycarbonyl group, substituent G 1 and substituent in R c G 2 is as already described.
Examples of the C3-8 cycloalkyloxycarbonyl group include a cyclopropyloxycarbonyl group and a cyclohexyloxycarbonyl group.
In the present invention, X is preferably an oxygen atom or a divalent group represented by NR c , and more preferably an oxygen atom.
Xは、酸素原子、硫黄原子、スルフィニル基、スルホニル基、または式:NRcで表される二価の基を示す。
ここで、Rcは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、無置換のもしくはG2で置換されたC6~10アリールカルボニル基、無置換のもしくはG2で置換されたC6~10アリールスルホニル基、または無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基または無置換のもしくはG1で置換されたC3~8シクロアルキルオキシカルボニル基を示す。
RcにおけるC1~6アルキル基、C6~10アリール基、C1~6アルキルカルボニル基、C6~10アリールカルボニル基、C6~10アリールスルホニル基、C1~6アルコキシカルボニル基、置換基G1および置換基G2はすでに述べたとおりのものである。
C3~8シクロアルキルオキシカルボニル基としては、シクロプロピルオキシカルボニル基、シクロヘキシルオキシカルボニル基などが挙げられる。
本発明において、Xとしては酸素原子またはNRcで表される二価の基が好ましく、酸素原子がより好ましい。 <X>
X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by the formula: NR c .
Here, R c is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, substituted by unsubstituted or G 1 have been C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryl group substituted with G 2, unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 2 or unsubstituted or G 1, A C1-6 alkoxycarbonyl group substituted with or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 .
C 1-6 alkyl group, C6-10 aryl group, C1-6 alkylcarbonyl group, C6-10 arylcarbonyl group, C6-10 arylsulfonyl group, C1-6 alkoxycarbonyl group, substituent G 1 and substituent in R c G 2 is as already described.
Examples of the C3-8 cycloalkyloxycarbonyl group include a cyclopropyloxycarbonyl group and a cyclohexyloxycarbonyl group.
In the present invention, X is preferably an oxygen atom or a divalent group represented by NR c , and more preferably an oxygen atom.
〈A〉
Aは、無置換のもしくはG3で置換されたC1~C6アルキレン基、無置換のもしくはG3で置換されたC2~C6アルケニレン基、無置換のもしくはG3で置換されたC2~C6アルキニレン基、無置換のもしくはG3で置換されたC1~C6アルキレンオキシ基、無置換のもしくはG3で置換されたオキシC1~C6アルキレン基、またはカルボニル基を示す。AにおけるC1~C6アルキレン基はすでに述べたとおりのものである。 <A>
A is unsubstituted or C1 ~ C6 alkylene group substituted with G 3, unsubstituted or C2 ~ C6 alkenylene group substituted with G 3, unsubstituted or C2 ~ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group. The C1-C6 alkylene group for A is as described above.
Aは、無置換のもしくはG3で置換されたC1~C6アルキレン基、無置換のもしくはG3で置換されたC2~C6アルケニレン基、無置換のもしくはG3で置換されたC2~C6アルキニレン基、無置換のもしくはG3で置換されたC1~C6アルキレンオキシ基、無置換のもしくはG3で置換されたオキシC1~C6アルキレン基、またはカルボニル基を示す。AにおけるC1~C6アルキレン基はすでに述べたとおりのものである。 <A>
A is unsubstituted or C1 ~ C6 alkylene group substituted with G 3, unsubstituted or C2 ~ C6 alkenylene group substituted with G 3, unsubstituted or C2 ~ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group. The C1-C6 alkylene group for A is as described above.
C2~C6アルケニレン基は、C2~C6アルケン中の水素原子2個が外れてなる2価の基である。C2~C6アルケニレン基としては、エテニレン基、プロペニレン基、ブテニレン基などを挙げることができる。
C2~C6アルキニレン基は、C2~C6アルキン中の水素原子2個が外れてなる2価の基である。C2~C6アルキニレン基としては、エチニレン基、プロピニレン基、ブチニレン基などを挙げることができる。
C1~C6アルキレンオキシ基としては、メチレンオキシ基(-CH2O-)、エチレンオキシ基(-CH2CH2O-)などが挙げられる。
オキシC1~C6アルキレン基としては、オキシメチレン基(-OCH2-)、オキシエチレン基(-OCH2CH2-)などが挙げられる。 The C2-C6 alkenylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkene. Examples of the C2 to C6 alkenylene group include an ethenylene group, a propenylene group, and a butenylene group.
The C2-C6 alkynylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkyne. Examples of the C2 to C6 alkynylene group include an ethynylene group, a propynylene group, a butynylene group, and the like.
Examples of the C1-C6 alkyleneoxy group include a methyleneoxy group (—CH 2 O—) and an ethyleneoxy group (—CH 2 CH 2 O—).
Examples of the oxy C1-C6 alkylene group include an oxymethylene group (—OCH 2 —) and an oxyethylene group (—OCH 2 CH 2 —).
C2~C6アルキニレン基は、C2~C6アルキン中の水素原子2個が外れてなる2価の基である。C2~C6アルキニレン基としては、エチニレン基、プロピニレン基、ブチニレン基などを挙げることができる。
C1~C6アルキレンオキシ基としては、メチレンオキシ基(-CH2O-)、エチレンオキシ基(-CH2CH2O-)などが挙げられる。
オキシC1~C6アルキレン基としては、オキシメチレン基(-OCH2-)、オキシエチレン基(-OCH2CH2-)などが挙げられる。 The C2-C6 alkenylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkene. Examples of the C2 to C6 alkenylene group include an ethenylene group, a propenylene group, and a butenylene group.
The C2-C6 alkynylene group is a divalent group formed by removing two hydrogen atoms from the C2-C6 alkyne. Examples of the C2 to C6 alkynylene group include an ethynylene group, a propynylene group, a butynylene group, and the like.
Examples of the C1-C6 alkyleneoxy group include a methyleneoxy group (—CH 2 O—) and an ethyleneoxy group (—CH 2 CH 2 O—).
Examples of the oxy C1-C6 alkylene group include an oxymethylene group (—OCH 2 —) and an oxyethylene group (—OCH 2 CH 2 —).
G3は、C1~6アルキル基、C1~6アルコキシ基、ホルミル基、C1~6アルキルカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、ハロゲノ基、C1~6アルキレン基、またはオキソ基を示す。G3における、C1~6アルキル基、C1~6アルコキシ基、C1~6アルキルカルボニル基、C1~6アルキルカルボニルオキシ基、ハロゲノ基、およびC1~6アルキレン基はすでに述べたとおりのものである。
G 3 is a C1-6 alkyl group, C1-6 alkoxy group, formyl group, C1-6 alkylcarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, halogeno group, C1-6 alkylene group, or oxo group Indicates. In G 3 , the C1-6 alkyl group, C1-6 alkoxy group, C1-6 alkylcarbonyl group, C1-6 alkylcarbonyloxy group, halogeno group, and C1-6 alkylene group are as described above.
本発明において、Aとしては、無置換のもしくはG3(好ましくはC1~6アルキル基、C1~6アルキレン基)で置換されたC1~C6アルキレン基、無置換のもしくはG3(好ましくはC1~6アルキル基)で置換されたC2~C6アルケニレン基、無置換のC2~C6アルキニレン基が好ましく、
無置換のもしくはC1~6アルキレン基で置換されたC1~C6アルキレン基、無置換のC2~C6アルケニレン基がより好ましい。
C1~6アルキレン基で置換されたC1~C6アルキレン基とは、例えば下記のような2価の基である(*は結合位置を示す。)。 In the present invention, A is a C1-C6 alkylene group which is unsubstituted or substituted with G 3 (preferably a C1-6 alkyl group, C1-6 alkylene group), unsubstituted or G 3 (preferably C1-6 6 alkyl group) is preferably a C2-C6 alkenylene group substituted with an unsubstituted C2-C6 alkynylene group,
An unsubstituted or C1-C6 alkylene group substituted with a C1-6 alkylene group and an unsubstituted C2-C6 alkenylene group are more preferred.
The C1-C6 alkylene group substituted with a C1-6 alkylene group is, for example, a divalent group as shown below (* indicates a bonding position).
無置換のもしくはC1~6アルキレン基で置換されたC1~C6アルキレン基、無置換のC2~C6アルケニレン基がより好ましい。
C1~6アルキレン基で置換されたC1~C6アルキレン基とは、例えば下記のような2価の基である(*は結合位置を示す。)。 In the present invention, A is a C1-C6 alkylene group which is unsubstituted or substituted with G 3 (preferably a C1-6 alkyl group, C1-6 alkylene group), unsubstituted or G 3 (preferably C1-6 6 alkyl group) is preferably a C2-C6 alkenylene group substituted with an unsubstituted C2-C6 alkynylene group,
An unsubstituted or C1-C6 alkylene group substituted with a C1-6 alkylene group and an unsubstituted C2-C6 alkenylene group are more preferred.
The C1-C6 alkylene group substituted with a C1-6 alkylene group is, for example, a divalent group as shown below (* indicates a bonding position).
〈Ar1〉
Ar1は、無置換の若しくはG2で置換されたC6~10アリール基、または無置換の若しくはG2で置換された3~10員ヘテロシクリル基を示す。Ar1におけるC6~10アリール基および置換基G2はすでに述べたとおりのものである。 <Ar 1 >
Ar 1 represents an unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or 3-10 membered heterocyclyl group which is substituted by G 2,. The C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
Ar1は、無置換の若しくはG2で置換されたC6~10アリール基、または無置換の若しくはG2で置換された3~10員ヘテロシクリル基を示す。Ar1におけるC6~10アリール基および置換基G2はすでに述べたとおりのものである。 <Ar 1 >
Ar 1 represents an unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or 3-10 membered heterocyclyl group which is substituted by G 2,. The C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
3~10員ヘテロシクリル基は、窒素原子、酸素原子および硫黄原子からなる群から選ばれる1~4個のヘテロ原子を環の構成原子として含む環状の基である。ヘテロシクリル基は、単環および多環のいずれであってもよい。多環ヘテロシクリル基は、少なくとも一つの環がヘテロシクリルであれば、残りの環が飽和脂環、不飽和脂環または芳香環のいずれであってもよい。3~10員ヘテロシクリル基としては、3~10員飽和へテロシクリル基、5~10員ヘテロアリール基、5~6員部分不飽和へテロシクリル基などを挙げることができる。
既に述べた3~6員ヘテロシクリル基以外のものとしては、インドリル基、イソインドリル基、ベンゾフラニル基、ジヒドロベンゾフラニル基、インダゾリル基、ベンゾオキサゾリル基、ベンゾイソオキサオゾリル基、ベンゾチアゾリル基、ベンゾイソチアゾリル基などの9員ヘテロアリール基;
キノリニル基、イソキノリニル基、シンノリニル基、フタラジニル基、キナゾリニル基、キノキサニル基などの10員ヘテロアリール基;などが挙げられる。 The 3- to 10-membered heterocyclyl group is a cyclic group containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom and a sulfur atom as constituent atoms of the ring. The heterocyclyl group may be monocyclic or polycyclic. In the polycyclic heterocyclyl group, when at least one ring is heterocyclyl, the remaining ring may be a saturated alicyclic ring, an unsaturated alicyclic ring, or an aromatic ring. Examples of the 3- to 10-membered heterocyclyl group include a 3- to 10-membered saturated heterocyclyl group, a 5- to 10-membered heteroaryl group, and a 5- to 6-membered partially unsaturated heterocyclyl group.
Other than the 3 to 6-membered heterocyclyl group already described, there are an indolyl group, an isoindolyl group, a benzofuranyl group, a dihydrobenzofuranyl group, an indazolyl group, a benzoxazolyl group, a benzoisoxazozolyl group, a benzothiazolyl group, a benzoyl group, A 9-membered heteroaryl group such as an isothiazolyl group;
Quinolinyl group, isoquinolinyl group, cinnolinyl group, phthalazinyl group, quinazolinyl group, quinoxanyl group and other 10-membered heteroaryl groups;
既に述べた3~6員ヘテロシクリル基以外のものとしては、インドリル基、イソインドリル基、ベンゾフラニル基、ジヒドロベンゾフラニル基、インダゾリル基、ベンゾオキサゾリル基、ベンゾイソオキサオゾリル基、ベンゾチアゾリル基、ベンゾイソチアゾリル基などの9員ヘテロアリール基;
キノリニル基、イソキノリニル基、シンノリニル基、フタラジニル基、キナゾリニル基、キノキサニル基などの10員ヘテロアリール基;などが挙げられる。 The 3- to 10-membered heterocyclyl group is a cyclic group containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom and a sulfur atom as constituent atoms of the ring. The heterocyclyl group may be monocyclic or polycyclic. In the polycyclic heterocyclyl group, when at least one ring is heterocyclyl, the remaining ring may be a saturated alicyclic ring, an unsaturated alicyclic ring, or an aromatic ring. Examples of the 3- to 10-membered heterocyclyl group include a 3- to 10-membered saturated heterocyclyl group, a 5- to 10-membered heteroaryl group, and a 5- to 6-membered partially unsaturated heterocyclyl group.
Other than the 3 to 6-membered heterocyclyl group already described, there are an indolyl group, an isoindolyl group, a benzofuranyl group, a dihydrobenzofuranyl group, an indazolyl group, a benzoxazolyl group, a benzoisoxazozolyl group, a benzothiazolyl group, a benzoyl group, A 9-membered heteroaryl group such as an isothiazolyl group;
Quinolinyl group, isoquinolinyl group, cinnolinyl group, phthalazinyl group, quinazolinyl group, quinoxanyl group and other 10-membered heteroaryl groups;
本発明においては、Ar1は、無置換のもしくはG2で置換されたC6~10アリール基(好ましくはフェニル基)または無置換のもしくはG2で置換された5~10員ヘテロアリール基(好ましくは5~6員ヘテロアリール基、より好ましくはピリジル基、ピペリジル基、ピラゾリル基)であることが好ましい。
Ar1におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロゲノ基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、C1~6アルコキシカルボニルアミノ基、シアノ基、C1~6ハロアルキレンジオキシ基および/またはハロゲノ基であることが好ましく、
より好ましくは、C1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基) および/またはハロゲノ基である。 In the present invention, Ar 1 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group) or an unsubstituted or G 2 -substituted 5- to 10-membered heteroaryl group (preferably Is preferably a 5- to 6-membered heteroaryl group, more preferably a pyridyl group, a piperidyl group or a pyrazolyl group.
G 2 in Ar 1 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group) An oxy group (preferably a phenoxy group), a 3-6 membered heterocyclyloxy group (preferably a pyridyl group), unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 halogeno group), C1— 6 alkoxycarbonylamino group, cyano group, C1-6 haloalkylenedioxy group and / or halogeno group Preferred,
More preferably, a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, an unsubstituted or C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a haloalkoxy group). An oxy group (preferably a phenoxy group) and / or a halogeno group.
Ar1におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロゲノ基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、C1~6アルコキシカルボニルアミノ基、シアノ基、C1~6ハロアルキレンジオキシ基および/またはハロゲノ基であることが好ましく、
より好ましくは、C1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基) および/またはハロゲノ基である。 In the present invention, Ar 1 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group) or an unsubstituted or G 2 -substituted 5- to 10-membered heteroaryl group (preferably Is preferably a 5- to 6-membered heteroaryl group, more preferably a pyridyl group, a piperidyl group or a pyrazolyl group.
G 2 in Ar 1 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group) An oxy group (preferably a phenoxy group), a 3-6 membered heterocyclyloxy group (preferably a pyridyl group), unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 halogeno group), C1— 6 alkoxycarbonylamino group, cyano group, C1-6 haloalkylenedioxy group and / or halogeno group Preferred,
More preferably, a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, an unsubstituted or C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a haloalkoxy group). An oxy group (preferably a phenoxy group) and / or a halogeno group.
〈Ar2〉
Ar2は、無置換の若しくはG2で置換されたC6~10アリール基、無置換の若しくはG2で置換されたC6~10アリールオキシ基、無置換の若しくはG2で置換された3~6員ヘテロシクリル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルオキシ基、または無置換の若しくはG2で置換された3~6員ヘテロシクリルチオ基を示す。
Ar2における、C6~10アリール基、C6~10アリールオキシ基、3~6員ヘテロシクリル基、3~6員ヘテロシクリルオキシ基、および置換基G2はすでに述べたとおりのものである。 <Ar 2 >
Ar 2 is unsubstituted or C6 ~ 10 aryl group substituted by G 2, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ~ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
In Ar 2 , the C6-10 aryl group, C6-10 aryloxy group, 3-6 membered heterocyclyl group, 3-6 membered heterocyclyloxy group, and substituent G 2 are as described above.
Ar2は、無置換の若しくはG2で置換されたC6~10アリール基、無置換の若しくはG2で置換されたC6~10アリールオキシ基、無置換の若しくはG2で置換された3~6員ヘテロシクリル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルオキシ基、または無置換の若しくはG2で置換された3~6員ヘテロシクリルチオ基を示す。
Ar2における、C6~10アリール基、C6~10アリールオキシ基、3~6員ヘテロシクリル基、3~6員ヘテロシクリルオキシ基、および置換基G2はすでに述べたとおりのものである。 <Ar 2 >
Ar 2 is unsubstituted or C6 ~ 10 aryl group substituted by G 2, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ~ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
In Ar 2 , the C6-10 aryl group, C6-10 aryloxy group, 3-6 membered heterocyclyl group, 3-6 membered heterocyclyloxy group, and substituent G 2 are as described above.
3~6員ヘテロシクリルチオ基は、SH基に3~6員ヘテロシクリル基が置換したものである。3~6員ヘテロシクリルチオ基としては、ピラゾリルチオ基、ピリジルチオ基などの「5~6員ヘテロアリールチオ基」を挙げることができる。
The 3- to 6-membered heterocyclylthio group is a group in which a 3- to 6-membered heterocyclyl group is substituted for the SH group. Examples of the 3- to 6-membered heterocyclylthio group include “5- to 6-membered heteroarylthio groups” such as a pyrazolylthio group and a pyridylthio group.
本発明においては、Ar2は、無置換のもしくはG2で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG2で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換のもしくはG2で置換された5~6員ヘテロアリール基(好ましくはピリジル基、ピペリジル基、ピラゾリル基)、または無置換のもしくはG2で置換された5~6員ヘテロアリールオキシ基(好ましくはピリジルオキシ基)であることが好ましい。
Ar2におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロゲノ基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、シアノ基、C1~6ハロアルキレンジオキシ基および/またはハロゲノ基であることが好ましく、
より好ましくは、C1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基) および/またはハロゲノ基である。 In the present invention, Ar 2 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group), an unsubstituted or G 2 -substituted C 6-10 aryloxy group (preferably phenoxy group), an unsubstituted or 5-6 membered heteroaryl group substituted with G 2 (preferably pyridyl group, piperidyl group, pyrazolyl group), or a 5-6 membered heteroaryl which is substituted by unsubstituted or G 2 An aryloxy group (preferably a pyridyloxy group) is preferable.
G 2 in Ar 2 is, C1 ~ 6 alkyl group, C1 ~ 6 haloalkyl group, C1 ~ 6 alkoxy group, C1 ~ 6 haloalkoxy group, an unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group, C1 ~ 6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group) An oxy group (preferably a phenoxy group), a 3-6 membered heterocyclyloxy group (preferably a pyridyl group) which is unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 halogeno group), a cyano group , C1-6 haloalkylenedioxy group and / or halogeno group,
More preferably, a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, an unsubstituted or C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a haloalkoxy group). An oxy group (preferably a phenoxy group) and / or a halogeno group.
Ar2におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロゲノ基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、シアノ基、C1~6ハロアルキレンジオキシ基および/またはハロゲノ基であることが好ましく、
より好ましくは、C1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、ハロアルコキシ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基) および/またはハロゲノ基である。 In the present invention, Ar 2 is an unsubstituted or G 2 -substituted C 6-10 aryl group (preferably a phenyl group), an unsubstituted or G 2 -substituted C 6-10 aryloxy group (preferably phenoxy group), an unsubstituted or 5-6 membered heteroaryl group substituted with G 2 (preferably pyridyl group, piperidyl group, pyrazolyl group), or a 5-6 membered heteroaryl which is substituted by unsubstituted or G 2 An aryloxy group (preferably a pyridyloxy group) is preferable.
G 2 in Ar 2 is, C1 ~ 6 alkyl group, C1 ~ 6 haloalkyl group, C1 ~ 6 alkoxy group, C1 ~ 6 haloalkoxy group, an unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group, C1 ~ 6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), an unsubstituted or a C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 haloalkoxy group) An oxy group (preferably a phenoxy group), a 3-6 membered heterocyclyloxy group (preferably a pyridyl group) which is unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group, a C1-6 halogeno group), a cyano group , C1-6 haloalkylenedioxy group and / or halogeno group,
More preferably, a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, an unsubstituted or C6-10 aryl substituted with G 21 (preferably a C1-6 haloalkyl group, a haloalkoxy group). An oxy group (preferably a phenoxy group) and / or a halogeno group.
〈Ar3〉
Ar3は、無置換の若しくはG2で置換されたC6~10アリール基を示す。Ar1におけるC6~10アリール基および置換基G2はすでに述べたとおりのものである。
Ar3としては、G2で置換されたフェニル基が好ましい。
Ar3におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、ハロゲノ基、シアノ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールC1~6アルキル基(好ましくはベンジル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールC1~6アルコキシ基(好ましくはベンジルオキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールアミノ基(好ましくはフェニルアミノ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、および/またはハロゲノ基であることが好ましい。
尚、Qが(VII)の場合、R1は、水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 <Ar 3 >
Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 . The C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
Ar 3 is preferably a phenyl group substituted with G 2 .
G 2 in Ar 3 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), unsubstituted or G 21 (preferably a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, a halogeno group A C6-10 aryloxy group (preferably a phenoxy group) substituted with a cyano group, a C6-10 aryl C1-6 alkyl group unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group) ( preferably benzyl group), C6 ~ 10 aryl substituted with unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group) 1-6 alkoxy group (preferably a benzyl group), an unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group) C6 ~ 10 aryl amino group substituted with (preferably phenyl group), a non-substituted or A 3- to 6-membered heterocyclyloxy group (preferably a pyridyl group) substituted with G 21 (preferably a C1-6 haloalkyl group) and / or a halogeno group are preferred.
When Q is (VII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
Ar3は、無置換の若しくはG2で置換されたC6~10アリール基を示す。Ar1におけるC6~10アリール基および置換基G2はすでに述べたとおりのものである。
Ar3としては、G2で置換されたフェニル基が好ましい。
Ar3におけるG2は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、無置換の若しくはG21(好ましくはC1~6ハロアルキル基、C1~6ハロアルコキシ基)で置換されたC6~10アリール基(好ましくはフェニル基)、無置換の若しくはG21(好ましくはC1~6アルキル基、C1~6ハロアルキル基、C1~6ハロアルコキシ基、ハロゲノ基、シアノ基)で置換されたC6~10アリールオキシ基(好ましくはフェノキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールC1~6アルキル基(好ましくはベンジル基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールC1~6アルコキシ基(好ましくはベンジルオキシ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換されたC6~10アリールアミノ基(好ましくはフェニルアミノ基)、無置換の若しくはG21(好ましくはC1~6ハロアルキル基)で置換された3~6員ヘテロシクリルオキシ基(好ましくはピリジル基)、および/またはハロゲノ基であることが好ましい。
尚、Qが(VII)の場合、R1は、水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 <Ar 3 >
Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 . The C6-10 aryl group and the substituent G 2 in Ar 1 are as described above.
Ar 3 is preferably a phenyl group substituted with G 2 .
G 2 in Ar 3 is a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, unsubstituted or G 21 (preferably a C1-6 haloalkyl group, C1-6 A C6-10 aryl group substituted with a haloalkoxy group (preferably a phenyl group), unsubstituted or G 21 (preferably a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 haloalkoxy group, a halogeno group A C6-10 aryloxy group (preferably a phenoxy group) substituted with a cyano group, a C6-10 aryl C1-6 alkyl group unsubstituted or substituted with G 21 (preferably a C1-6 haloalkyl group) ( preferably benzyl group), C6 ~ 10 aryl substituted with unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group) 1-6 alkoxy group (preferably a benzyl group), an unsubstituted or G 21 (preferably C1 ~ 6 haloalkyl group) C6 ~ 10 aryl amino group substituted with (preferably phenyl group), a non-substituted or A 3- to 6-membered heterocyclyloxy group (preferably a pyridyl group) substituted with G 21 (preferably a C1-6 haloalkyl group) and / or a halogeno group are preferred.
When Q is (VII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
〈Ar4〉
Ar4は、無置換の若しくはG2で置換されたC6~10アリーレン基を示す。C6~10アリーレン基は、C6~C10芳香環中の水素原子2個が外れてなる2価の基である。
Ar4におけるC6~C10アリーレン基としては、フェニレン基、ナフチレン基が挙げられる。
Ar4としては、無置換のフェニレン基が好ましい。
尚、Qが(VIII)の場合、R1は、水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 <Ar 4 >
Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G2. The C6-10 arylene group is a divalent group in which two hydrogen atoms in the C6 to C10 aromatic ring are removed.
Examples of the C6 to C10 arylene group in Ar 4 include a phenylene group and a naphthylene group.
Ar 4 is preferably an unsubstituted phenylene group.
When Q is (VIII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
Ar4は、無置換の若しくはG2で置換されたC6~10アリーレン基を示す。C6~10アリーレン基は、C6~C10芳香環中の水素原子2個が外れてなる2価の基である。
Ar4におけるC6~C10アリーレン基としては、フェニレン基、ナフチレン基が挙げられる。
Ar4としては、無置換のフェニレン基が好ましい。
尚、Qが(VIII)の場合、R1は、水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 <Ar 4 >
Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G2. The C6-10 arylene group is a divalent group in which two hydrogen atoms in the C6 to C10 aromatic ring are removed.
Examples of the C6 to C10 arylene group in Ar 4 include a phenylene group and a naphthylene group.
Ar 4 is preferably an unsubstituted phenylene group.
When Q is (VIII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group.
〈Ar5〉
Ar5は、無置換の若しくはG2で置換された3~6員ヘテロシクリル基を示す。Ar5における3~6員ヘテロシクリル基、および置換基G2はすでに述べたとおりのものである。
Ar5としては、無置換の若しくはG2(好ましくはC1~6ハロアルキル基)で置換された5~6員ヘテロアリール基(好ましくはピラゾリル基)が好ましい。 <Ar 5 >
Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 . The 3- to 6-membered heterocyclyl group in Ar 5 and the substituent G 2 are as described above.
Ar 5 is preferably a 5- to 6-membered heteroaryl group (preferably a pyrazolyl group) which is unsubstituted or substituted with G 2 (preferably a C 1-6 haloalkyl group).
Ar5は、無置換の若しくはG2で置換された3~6員ヘテロシクリル基を示す。Ar5における3~6員ヘテロシクリル基、および置換基G2はすでに述べたとおりのものである。
Ar5としては、無置換の若しくはG2(好ましくはC1~6ハロアルキル基)で置換された5~6員ヘテロアリール基(好ましくはピラゾリル基)が好ましい。 <Ar 5 >
Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 . The 3- to 6-membered heterocyclyl group in Ar 5 and the substituent G 2 are as described above.
Ar 5 is preferably a 5- to 6-membered heteroaryl group (preferably a pyrazolyl group) which is unsubstituted or substituted with G 2 (preferably a C 1-6 haloalkyl group).
〈Ba〉
Baは、無置換の若しくはG4で置換されたC1~C6アルキレン基、無置換の若しくはG4で置換されたC2~C6アルケニレン基、無置換の若しくはG4で置換されたC2~C6アルキニレン基、無置換の若しくはG4で置換されたC1~C6アルキレンオキシC1~6アルキレン基、無置換の若しくはG4で置換されたC3~C6シクロアルキレン基、無置換の若しくはG4で置換されたC4~C6シクロアルケニレン基、無置換の若しくはG4で置換された3~6員ヘテロシクリレン基、またはNRdで表される二価の基を示す。Rdは、水素原子、C1~6アルキル基、またはC1~6アルコキシカルボニル基を示す。
Baにおける、C1~C6アルキレン基、C2~C6アルケニレン基、およびC2~C6アルキニレン基は既に述べたとおりのものである。
Rdにおける、C1~C6アルキル基、およびC1~C6アルコキシカルボニル基は既に述べたとおりのものである。
C1~C6アルキレンオキシC1~6アルキレン基は、2つのC1~6アルキレン基が、酸素原子を介して結合してなる基である。C1~C6アルキレンオキシC1~6アルキレン基としては、メチレンオキシメチレン基、メチレンオキシエチレン基、エチレンオキシメチレン基などを挙げることができる。 <B a >
B a is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ~ C6 alkyleneoxy C1 ~ 6 alkylene group substituted with G 4, unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4 A C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown. R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group.
In B a, C1 ~ C6 alkylene group, C2 ~ C6 alkenylene group, and C2 ~ C6 alkynylene radicals are those as already mentioned.
The C1-C6 alkyl group and the C1-C6 alkoxycarbonyl group in R d are as described above.
The C1 to C6 alkyleneoxy C1 to 6 alkylene group is a group formed by bonding two C1 to 6 alkylene groups via an oxygen atom. Examples of the C1-C6 alkyleneoxy C1-6 alkylene group include a methyleneoxymethylene group, a methyleneoxyethylene group, and an ethyleneoxymethylene group.
Baは、無置換の若しくはG4で置換されたC1~C6アルキレン基、無置換の若しくはG4で置換されたC2~C6アルケニレン基、無置換の若しくはG4で置換されたC2~C6アルキニレン基、無置換の若しくはG4で置換されたC1~C6アルキレンオキシC1~6アルキレン基、無置換の若しくはG4で置換されたC3~C6シクロアルキレン基、無置換の若しくはG4で置換されたC4~C6シクロアルケニレン基、無置換の若しくはG4で置換された3~6員ヘテロシクリレン基、またはNRdで表される二価の基を示す。Rdは、水素原子、C1~6アルキル基、またはC1~6アルコキシカルボニル基を示す。
Baにおける、C1~C6アルキレン基、C2~C6アルケニレン基、およびC2~C6アルキニレン基は既に述べたとおりのものである。
Rdにおける、C1~C6アルキル基、およびC1~C6アルコキシカルボニル基は既に述べたとおりのものである。
C1~C6アルキレンオキシC1~6アルキレン基は、2つのC1~6アルキレン基が、酸素原子を介して結合してなる基である。C1~C6アルキレンオキシC1~6アルキレン基としては、メチレンオキシメチレン基、メチレンオキシエチレン基、エチレンオキシメチレン基などを挙げることができる。 <B a >
B a is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ~ C6 alkyleneoxy C1 ~ 6 alkylene group substituted with G 4, unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4 A C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown. R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group.
In B a, C1 ~ C6 alkylene group, C2 ~ C6 alkenylene group, and C2 ~ C6 alkynylene radicals are those as already mentioned.
The C1-C6 alkyl group and the C1-C6 alkoxycarbonyl group in R d are as described above.
The C1 to C6 alkyleneoxy C1 to 6 alkylene group is a group formed by bonding two C1 to 6 alkylene groups via an oxygen atom. Examples of the C1-C6 alkyleneoxy C1-6 alkylene group include a methyleneoxymethylene group, a methyleneoxyethylene group, and an ethyleneoxymethylene group.
C3~C6シクロアルキレン基は、C3~C6シクロアルカン中の水素原子2個が外れてなる2価の基である。C3~C6シクロアルキレン基としては、シクロプロピレン基(1,2-シクロプロピレン基)、シクロブチレン基(1,2-シクロブチレン基、1,3-シクロブチレン基)シクロペンチレン基(1,2-シクロペンチレン基、または1,3-シクロペンチレン基)、シクロヘキシレン基(1,2-シクロヘキシレン基、1,3-シクロヘキシレン基、または1,4-シクロヘキシレン基)などを挙げることができる。
The C3-C6 cycloalkylene group is a divalent group formed by removing two hydrogen atoms from a C3-C6 cycloalkane. Examples of the C3-C6 cycloalkylene group include a cyclopropylene group (1,2-cyclopropylene group), a cyclobutylene group (1,2-cyclobutylene group, 1,3-cyclobutylene group), a cyclopentylene group (1,2 -Cyclopentylene group or 1,3-cyclopentylene group), cyclohexylene group (1,2-cyclohexylene group, 1,3-cyclohexylene group, or 1,4-cyclohexylene group). Can do.
C4~C6シクロアルケニレン基は、C4~C6シクロアルケン中の水素原子2個が外れてなる2価の基である。C4~C6シクロアルケニレン基としては、シクロブテニレン基(1,2-シクロブテニレン基、1,3-シクロブテニレン基、または3,4-シクロブテニレン基)、シクロペンテニレン基(1,2-シクロペンテニレン基、1,3-シクロペンテニレン基、1,4-シクロペンテニレン基、または1,5-シクロペンテニレン基)、シクロヘキセニレン基(1,2-シクロヘキセニレン基、1,3-シクロヘキセニレン基、1,4-シクロヘキセニレン基、)などを挙げることができる。
3~6員ヘテロシクリレン基は、ヘテロ脂環式化合物中の水素原子2個が外れてなる2価の基である。ヘテロ脂環式化合物は、窒素原子、酸素原子および硫黄原子からなる群から選ばれる1~4個のヘテロ原子を環の構成原子として含む非芳香族化合物である。 The C4-C6 cycloalkenylene group is a divalent group formed by removing two hydrogen atoms from a C4-C6 cycloalkene. Examples of the C4 to C6 cycloalkenylene group include a cyclobutenylene group (1,2-cyclobutenylene group, 1,3-cyclobutenylene group, or 3,4-cyclobutenylene group), a cyclopentenylene group (1,2-cyclopentenylene group). 1,3-cyclopentenylene group, 1,4-cyclopentenylene group or 1,5-cyclopentenylene group), cyclohexenylene group (1,2-cyclohexenylene group, 1,3 -Cyclohexenylene group, 1,4-cyclohexenylene group)) and the like.
A 3- to 6-membered heterocyclylene group is a divalent group formed by removing two hydrogen atoms from a heteroalicyclic compound. The heteroalicyclic compound is a non-aromatic compound containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom, and a sulfur atom as constituent atoms of the ring.
3~6員ヘテロシクリレン基は、ヘテロ脂環式化合物中の水素原子2個が外れてなる2価の基である。ヘテロ脂環式化合物は、窒素原子、酸素原子および硫黄原子からなる群から選ばれる1~4個のヘテロ原子を環の構成原子として含む非芳香族化合物である。 The C4-C6 cycloalkenylene group is a divalent group formed by removing two hydrogen atoms from a C4-C6 cycloalkene. Examples of the C4 to C6 cycloalkenylene group include a cyclobutenylene group (1,2-cyclobutenylene group, 1,3-cyclobutenylene group, or 3,4-cyclobutenylene group), a cyclopentenylene group (1,2-cyclopentenylene group). 1,3-cyclopentenylene group, 1,4-cyclopentenylene group or 1,5-cyclopentenylene group), cyclohexenylene group (1,2-cyclohexenylene group, 1,3 -Cyclohexenylene group, 1,4-cyclohexenylene group)) and the like.
A 3- to 6-membered heterocyclylene group is a divalent group formed by removing two hydrogen atoms from a heteroalicyclic compound. The heteroalicyclic compound is a non-aromatic compound containing 1 to 4 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom, and a sulfur atom as constituent atoms of the ring.
3~6員ヘテロシクリレン基としては、3~6員飽和へテロシクリレン基または5~6員部分不飽和へテロシクリレン基などを挙げることができる。
3~6員ヘテロシクリレン基としては、ジヒドロフリレン基、テトラヒドロフリレン基、ピロリニレン基、ピロリジニレン基、ピラゾリニレン基、ピラゾリジニレン基、イミダゾリニレン基、イミダゾリジニレン基、オキサゾリニレン基、オキサゾリジニレン基、チアゾリニレン基、チアゾリジニレン基、イソオキサゾリジニレン基、イソチアゾリジニレン基、ジヒドロピラニレン基、テトラヒドロピラニレン基、ピペリジニレン基、ピペラジニレン基、モルホリニレン基などを挙げることができる。
なお、特定の実施態様においては、3~6員ヘテロシクリレン基はC1~6アルキレン基で、架橋されていてよい。 Examples of the 3- to 6-membered heterocyclylene group include a 3- to 6-membered saturated heterocyclylene group or a 5- to 6-membered partially unsaturated heterocyclylene group.
Examples of the 3- to 6-membered heterocyclylene group include dihydrofurylene group, tetrahydrofurylene group, pyrrolinylene group, pyrrolidinylene group, pyrazolinylene group, pyrazolidinylene group, imidazolinylene group, imidazolidinylene group, oxazolinylene group, oxazolidinylene group, Examples include thiazolinylene group, thiazolidinylene group, isoxazolidinylene group, isothiazolidinylene group, dihydropyranylene group, tetrahydropyranylene group, piperidinylene group, piperazinylene group, morpholinylene group and the like.
In certain embodiments, the 3-6 membered heterocyclylene group may be a C1-6 alkylene group, which may be cross-linked.
3~6員ヘテロシクリレン基としては、ジヒドロフリレン基、テトラヒドロフリレン基、ピロリニレン基、ピロリジニレン基、ピラゾリニレン基、ピラゾリジニレン基、イミダゾリニレン基、イミダゾリジニレン基、オキサゾリニレン基、オキサゾリジニレン基、チアゾリニレン基、チアゾリジニレン基、イソオキサゾリジニレン基、イソチアゾリジニレン基、ジヒドロピラニレン基、テトラヒドロピラニレン基、ピペリジニレン基、ピペラジニレン基、モルホリニレン基などを挙げることができる。
なお、特定の実施態様においては、3~6員ヘテロシクリレン基はC1~6アルキレン基で、架橋されていてよい。 Examples of the 3- to 6-membered heterocyclylene group include a 3- to 6-membered saturated heterocyclylene group or a 5- to 6-membered partially unsaturated heterocyclylene group.
Examples of the 3- to 6-membered heterocyclylene group include dihydrofurylene group, tetrahydrofurylene group, pyrrolinylene group, pyrrolidinylene group, pyrazolinylene group, pyrazolidinylene group, imidazolinylene group, imidazolidinylene group, oxazolinylene group, oxazolidinylene group, Examples include thiazolinylene group, thiazolidinylene group, isoxazolidinylene group, isothiazolidinylene group, dihydropyranylene group, tetrahydropyranylene group, piperidinylene group, piperazinylene group, morpholinylene group and the like.
In certain embodiments, the 3-6 membered heterocyclylene group may be a C1-6 alkylene group, which may be cross-linked.
これらの中でも、Baとしては、無置換の若しくはG4で置換されたC1~C6アルキレン基またはNRdで表される二価の基が好ましい。
Among them, B a, divalent groups represented by substituted C1 ~ C6 alkylene or NR d in unsubstituted or G 4 is preferred.
G4は、C1~6アルキル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C2~6アルケニルオキシC1~6アルキル基、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C2~6アルケニルオキシ基、C1~6アルコキシカルボニル基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、オキソ基、C3~8シクロアルキルC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルC1~6アルキル基、C3~8シクロアルキルオキシC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシC1~6アルキル基、C1~6アルキリデン基、C1~6アルコキシイミノ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシイミノ基、またはC1~6アルキルヒドラジノ基を示す。
G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ~ 6 alkoxy C1 ~ 6 alkoxy group, substituted with C2 ~ 6 alkenyloxy group, C1 ~ 6 alkoxycarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ~ 10 aryl C1 ~ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ~ 6 alkyl substituted with unsubstituted or G 21 Group, C3 ~ 8 cycloalkyloxy C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryloxy C1 ~ 6 alkyl group substituted with G 21, unsubstituted or 3-6 membered heterocyclyl substituted with G 21 oxy C1 ~ 6 alkyl group, C1 ~ 6 alkylidene group, C1 ~ 6 alkoxyimino group, the unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxyimino group substituted by G 21 or C1 ~ 6 alkyl hydrazino group, Show.
G4における、C1~6アルキル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6アルコキシカルボニル基、C1~6アルキレンジオキシ基、C6~10アリール基、3~6員ヘテロシクリル基、ハロゲノ基、C1~6アルキレン基、および置換基G21は既に述べたとおりのものである。
In G 4 , a C1-6 alkyl group, a C3-8 cycloalkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy C1-6 alkyl group, a C1-6 alkoxy group, a C1-6 alkoxy C1-6 alkoxy group, C1 1-6 alkoxycarbonyl group, C1 to 6 alkylenedioxy group, C6 ~ 10 aryl group, 3- to 6-membered heterocyclyl group, a halogeno group, C1 ~ 6 alkylene group and substituents G 21, is of as already mentioned .
C2~6アルケニルオキシ基は、水酸基に、C2~6アルケニル基が置換したものである。C2~6アルケニルオキシ基としては、ビニルオキシ基、1-プロペニルオキシ基、2-プロペニルオキシ基(アリルオキシ基)などを挙げることができる。
C2~6アルケニルオキシC1~6アルキル基は、C1~6アルキル基に、C2~6アルケニルオキシ基が置換したものである。C2~6アルケニルオキシC1~6アルキル基としては、ビニルオキシメチル基、アリルオキシメチル基などを挙げることができる。
C3~8シクロアルキルC1~6アルキル基としては、シクロプロピルメチル基、シクロペンチルメチル基などを挙げることができる。
C6~10アリールC1~6アルキル基としては、ベンジル基、フェネチル基などを挙げることができる。 The C2-6 alkenyloxy group is a hydroxyl group substituted with a C2-6 alkenyl group. Examples of the C2-6 alkenyloxy group include a vinyloxy group, a 1-propenyloxy group, and a 2-propenyloxy group (allyloxy group).
The C2-6 alkenyloxy C1-6 alkyl group is a C1-6 alkyl group substituted with a C2-6 alkenyloxy group. Examples of the C2-6 alkenyloxy C1-6 alkyl group include a vinyloxymethyl group and an allyloxymethyl group.
Examples of the C3-8 cycloalkyl C1-6 alkyl group include a cyclopropylmethyl group and a cyclopentylmethyl group.
Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
C2~6アルケニルオキシC1~6アルキル基は、C1~6アルキル基に、C2~6アルケニルオキシ基が置換したものである。C2~6アルケニルオキシC1~6アルキル基としては、ビニルオキシメチル基、アリルオキシメチル基などを挙げることができる。
C3~8シクロアルキルC1~6アルキル基としては、シクロプロピルメチル基、シクロペンチルメチル基などを挙げることができる。
C6~10アリールC1~6アルキル基としては、ベンジル基、フェネチル基などを挙げることができる。 The C2-6 alkenyloxy group is a hydroxyl group substituted with a C2-6 alkenyl group. Examples of the C2-6 alkenyloxy group include a vinyloxy group, a 1-propenyloxy group, and a 2-propenyloxy group (allyloxy group).
The C2-6 alkenyloxy C1-6 alkyl group is a C1-6 alkyl group substituted with a C2-6 alkenyloxy group. Examples of the C2-6 alkenyloxy C1-6 alkyl group include a vinyloxymethyl group and an allyloxymethyl group.
Examples of the C3-8 cycloalkyl C1-6 alkyl group include a cyclopropylmethyl group and a cyclopentylmethyl group.
Examples of the C6-10 aryl C1-6 alkyl group include a benzyl group and a phenethyl group.
3~6員ヘテロシクリルC1~6アルキル基としては、好ましくは、テトラヒドロフラニルメチル基、テトラヒドロピラニルメチル基、ジオキソラニルメチル基、ジオキサニルメチル基などの5~6員飽和ヘテロシクリルC1~6アルキル基;ピラゾリルメチル基、ピリジルメチル基などの5~6員ヘテロアリールC1~6アルキル基を挙げることができる。
C3~8シクロアルキルオキシC1~6アルキル基としては、シクロプロピルオキシメチル基、シクロヘキシルオキシメチル基などを挙げることができる。 The 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc. Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
Examples of the C3-8 cycloalkyloxy C1-6 alkyl group include a cyclopropyloxymethyl group and a cyclohexyloxymethyl group.
C3~8シクロアルキルオキシC1~6アルキル基としては、シクロプロピルオキシメチル基、シクロヘキシルオキシメチル基などを挙げることができる。 The 3- to 6-membered heterocyclyl C1-6 alkyl group is preferably a 5- to 6-membered saturated heterocyclyl C1-6 alkyl such as tetrahydrofuranylmethyl group, tetrahydropyranylmethyl group, dioxolanylmethyl group, dioxanylmethyl group, etc. Groups; 5- to 6-membered heteroaryl C1-6 alkyl groups such as a pyrazolylmethyl group and a pyridylmethyl group.
Examples of the C3-8 cycloalkyloxy C1-6 alkyl group include a cyclopropyloxymethyl group and a cyclohexyloxymethyl group.
C6~10アリールオキシC1~6アルキル基としては、フェノキシメチル基、ナフチルオキシメチル基などを挙げることができる。
Examples of the C6-10 aryloxy C1-6 alkyl group include a phenoxymethyl group and a naphthyloxymethyl group.
3~6員ヘテロシクリルオキシC1~6アルキル基としては、好ましくは、ピラゾリルオキシメチル基、ピリジルオキシメチル基などの5~6員ヘテロアリールメチル基を挙げることができる。
C1~6アルキリデン基としては、メチリデン基、プロパン-2-イリデン基などを挙げることができる。
C1~6アルコキシイミノ基としては、メトキシイミノ基(MeO-N=)、エトキシイミノ基(EtO-N=)などを挙げることができる。
C6~10アリールC1~6アルコキシイミノ基としては、ベンジルオキシイミノ基(BnO-N=)などを挙げることができる。
C1~6アルキルヒドラジノ基としては、メチルヒドラジノ基(MeNH-N=)、エチルヒドラジノ基(EtNH-N=)などを挙げることができる。 The 3- to 6-membered heterocyclyloxy C1-6 alkyl group is preferably a 5- to 6-membered heteroarylmethyl group such as a pyrazolyloxymethyl group and a pyridyloxymethyl group.
Examples of the C1-6 alkylidene group include a methylidene group and a propane-2-ylidene group.
Examples of the C1-6 alkoxyimino group include a methoxyimino group (MeO-N =) and an ethoxyimino group (EtO-N =).
Examples of the C6-10 aryl C1-6 alkoxyimino group include a benzyloxyimino group (BnO-N =).
Examples of the C1-6 alkyl hydrazino group include a methyl hydrazino group (MeNH-N =) and an ethyl hydrazino group (EtNH-N =).
C1~6アルキリデン基としては、メチリデン基、プロパン-2-イリデン基などを挙げることができる。
C1~6アルコキシイミノ基としては、メトキシイミノ基(MeO-N=)、エトキシイミノ基(EtO-N=)などを挙げることができる。
C6~10アリールC1~6アルコキシイミノ基としては、ベンジルオキシイミノ基(BnO-N=)などを挙げることができる。
C1~6アルキルヒドラジノ基としては、メチルヒドラジノ基(MeNH-N=)、エチルヒドラジノ基(EtNH-N=)などを挙げることができる。 The 3- to 6-membered heterocyclyloxy C1-6 alkyl group is preferably a 5- to 6-membered heteroarylmethyl group such as a pyrazolyloxymethyl group and a pyridyloxymethyl group.
Examples of the C1-6 alkylidene group include a methylidene group and a propane-2-ylidene group.
Examples of the C1-6 alkoxyimino group include a methoxyimino group (MeO-N =) and an ethoxyimino group (EtO-N =).
Examples of the C6-10 aryl C1-6 alkoxyimino group include a benzyloxyimino group (BnO-N =).
Examples of the C1-6 alkyl hydrazino group include a methyl hydrazino group (MeNH-N =) and an ethyl hydrazino group (EtNH-N =).
これらの中でもG4としては、C1~6アルキル基、オキソ基、C1~6アルコキシイミノ基が好ましい。
Among these, G 4 is preferably a C 1-6 alkyl group, an oxo group, or a C 1-6 alkoxyimino group.
また、式(V)中、BaとRaは、相互に繋がって、BaとRaが結合する炭素原子とともに5~6員環を形成してもよい。BaとRaによって形成される5~6員環としては、シクロペンテン環、シクロヘキセン環などを挙げることができる。
In the formula (V), B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached. The 5- or 6-membered ring formed by B a and R a, may be mentioned cyclopentene ring, a cyclohexene ring.
〈T1~T3〉
T1は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-メチル基、1-(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-エチル基、または無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基を示す。
T2は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-ホルミル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ)-C1~6アルキル基、または(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基を示す。
T3は、無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基、または無置換のもしくはG2で置換されたC6~10アリールアミノカルボニル-C1~6アルキル基を示す。 <T 1 to T 3 >
T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ~ 10 aryl C1 ~ 6 alkyl)-C1 ~ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ~ 10 aryl C1 which is substituted by G 2 ~ shows the 6 alkoxy)-C1 ~ 6 alkyl group or a (unsubstituted or C6 ~ 10 aryl C1-6 alkyl substituted with G 2) -3 ~ 6-membered heterocyclyl group.
T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
T1は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-メチル基、1-(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-エチル基、または無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基を示す。
T2は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-ホルミル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ)-C1~6アルキル基、または(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基を示す。
T3は、無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基、または無置換のもしくはG2で置換されたC6~10アリールアミノカルボニル-C1~6アルキル基を示す。 <T 1 to T 3 >
T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ~ 10 aryl C1 ~ 6 alkyl)-C1 ~ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ~ 10 aryl C1 which is substituted by G 2 ~ shows the 6 alkoxy)-C1 ~ 6 alkyl group or a (unsubstituted or C6 ~ 10 aryl C1-6 alkyl substituted with G 2) -3 ~ 6-membered heterocyclyl group.
T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
T1における「(C6~10アリールC1~6アルコキシイミノ)-メチル基」としては、ベンジルオキシイミノ-メチル基などを挙げることができる。
T1における「1-(C6~10アリールC1~6アルコキシイミノ)-エチル基」としては、1-(ベンジルオキシイミノ)-エチル基などを挙げることができる。
T1における「C6~10アリールC1~6アルコキシ基」としては、ベンジルオキシ基、フェネチルオキシ基、2-フェニル-1-メチルプロポキシ基などを挙げることができる。 Examples of the “(C6-10 aryl C1-6 alkoxyimino) -methyl group” in T 1 include a benzyloxyimino-methyl group.
Examples of the “1- (C6-10 aryl C1-6 alkoxyimino) -ethyl group” for T 1 include a 1- (benzyloxyimino) -ethyl group.
Examples of the “C6-10 aryl C1-6 alkoxy group” for T 1 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
T1における「1-(C6~10アリールC1~6アルコキシイミノ)-エチル基」としては、1-(ベンジルオキシイミノ)-エチル基などを挙げることができる。
T1における「C6~10アリールC1~6アルコキシ基」としては、ベンジルオキシ基、フェネチルオキシ基、2-フェニル-1-メチルプロポキシ基などを挙げることができる。 Examples of the “(C6-10 aryl C1-6 alkoxyimino) -methyl group” in T 1 include a benzyloxyimino-methyl group.
Examples of the “1- (C6-10 aryl C1-6 alkoxyimino) -ethyl group” for T 1 include a 1- (benzyloxyimino) -ethyl group.
Examples of the “C6-10 aryl C1-6 alkoxy group” for T 1 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
T2における「(C6~10アリールC1~6アルキル)-アミノ基」としては、ベンジルアミノ基などを挙げることができる。
T2における「(C6~10アリールC1~6アルキル)-ホルミル-アミノ基」としては、ベンジル-ホルミルアミノ基などを挙げることができる。
T2における「(C6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基」としては、ベンジル-アセチルアミノ基などを挙げることができる。
T2における「(C6~10アリールC1~6アルコキシ)-C1~6アルキル基」としては、ベンジルオキシメチル基、2-ベンジルオキシ-1-メチルプロピル基などを挙げることができる。
T2における「(C6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基」としては、1-ベンジル-ピロリジン-3-イル基などを挙げることができる。 Examples of the “(C6-10 aryl C1-6 alkyl) -amino group” for T 2 include a benzylamino group.
Examples of the “(C6-10 aryl C1-6 alkyl) -formyl-amino group” at T 2 include a benzyl-formylamino group.
Examples of the “(C6-10 aryl C1-6 alkyl) -C1-6 alkylcarbonyl-amino group” for T 2 include a benzyl-acetylamino group.
Examples of the “(C6-10 aryl C1-6 alkoxy) -C1-6 alkyl group” for T 2 include a benzyloxymethyl group, a 2-benzyloxy-1-methylpropyl group, and the like.
Examples of the “(C6-10 aryl C1-6 alkyl) -3-6 membered heterocyclyl group” for T 2 include a 1-benzyl-pyrrolidin-3-yl group.
T2における「(C6~10アリールC1~6アルキル)-ホルミル-アミノ基」としては、ベンジル-ホルミルアミノ基などを挙げることができる。
T2における「(C6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基」としては、ベンジル-アセチルアミノ基などを挙げることができる。
T2における「(C6~10アリールC1~6アルコキシ)-C1~6アルキル基」としては、ベンジルオキシメチル基、2-ベンジルオキシ-1-メチルプロピル基などを挙げることができる。
T2における「(C6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基」としては、1-ベンジル-ピロリジン-3-イル基などを挙げることができる。 Examples of the “(C6-10 aryl C1-6 alkyl) -amino group” for T 2 include a benzylamino group.
Examples of the “(C6-10 aryl C1-6 alkyl) -formyl-amino group” at T 2 include a benzyl-formylamino group.
Examples of the “(C6-10 aryl C1-6 alkyl) -C1-6 alkylcarbonyl-amino group” for T 2 include a benzyl-acetylamino group.
Examples of the “(C6-10 aryl C1-6 alkoxy) -C1-6 alkyl group” for T 2 include a benzyloxymethyl group, a 2-benzyloxy-1-methylpropyl group, and the like.
Examples of the “(C6-10 aryl C1-6 alkyl) -3-6 membered heterocyclyl group” for T 2 include a 1-benzyl-pyrrolidin-3-yl group.
T3における「C6~10アリールC1~6アルコキシ基」としては、ベンジルオキシ基、フェネチルオキシ基、2-フェニル-1-メチルプロポキシ基などを挙げることができる。
T3における「C6~10アリールアミノカルボニル-C1~6アルキル基」としては、1-フェニルカルバモイル-2-メチルプロピル基などを挙げることができる。 Examples of the “C6-10 aryl C1-6 alkoxy group” for T 3 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
Examples of the “C6-10 arylaminocarbonyl-C1-6 alkyl group” for T 3 include a 1-phenylcarbamoyl-2-methylpropyl group.
T3における「C6~10アリールアミノカルボニル-C1~6アルキル基」としては、1-フェニルカルバモイル-2-メチルプロピル基などを挙げることができる。 Examples of the “C6-10 aryl C1-6 alkoxy group” for T 3 include a benzyloxy group, a phenethyloxy group, a 2-phenyl-1-methylpropoxy group, and the like.
Examples of the “C6-10 arylaminocarbonyl-C1-6 alkyl group” for T 3 include a 1-phenylcarbamoyl-2-methylpropyl group.
本発明に係るピリジン化合物には、水和物、各種溶媒和物や結晶多形なども含まれる。さらに、本発明に係るピリジン化合物は、不斉炭素原子、二重結合などに基づく立体異性体およびそれらの混合物、互変異性体を包含する。
The pyridine compound according to the present invention includes hydrates, various solvates and crystal polymorphs. Furthermore, the pyridine compound according to the present invention includes stereoisomers based on asymmetric carbon atoms, double bonds, etc., and mixtures and tautomers thereof.
〔互変異性体〕
本発明に係るピリジン化合物は、R4が水素原子である場合、以下に示す互変異性体 ピリジン-4-オン化合物を生じる。なお、式中のR1、R2、R3、およびQは、式(I)中の規定と同様の意味を示す。本発明は、当該互変異性体 ピリジン-4-オン化合物を包含する。 [Tautomers]
When R 4 is a hydrogen atom, the pyridine compound according to the present invention yields the tautomeric pyridine-4-one compound shown below. In the formula, R 1 , R 2 , R 3 , and Q have the same meaning as defined in the formula (I). The present invention includes such tautomeric pyridine-4-one compounds.
本発明に係るピリジン化合物は、R4が水素原子である場合、以下に示す互変異性体 ピリジン-4-オン化合物を生じる。なお、式中のR1、R2、R3、およびQは、式(I)中の規定と同様の意味を示す。本発明は、当該互変異性体 ピリジン-4-オン化合物を包含する。 [Tautomers]
When R 4 is a hydrogen atom, the pyridine compound according to the present invention yields the tautomeric pyridine-4-one compound shown below. In the formula, R 1 , R 2 , R 3 , and Q have the same meaning as defined in the formula (I). The present invention includes such tautomeric pyridine-4-one compounds.
〔立体異性体〕
本発明は、同一の構造式で表される化合物であるが、構造中の原子または置換基の空間的配置が異なる化合物、例えば、光学異性体、ジアステレオ異性体、幾何異性体などの全ての立体異性体を包含する。立体異性体は、単一物であっても混合物であってもよい。 [Stereoisomer]
The present invention is a compound represented by the same structural formula, but all compounds such as optical isomers, diastereoisomers, geometrical isomers, etc. having different spatial arrangements of atoms or substituents in the structure. Includes stereoisomers. The stereoisomer may be a single substance or a mixture.
本発明は、同一の構造式で表される化合物であるが、構造中の原子または置換基の空間的配置が異なる化合物、例えば、光学異性体、ジアステレオ異性体、幾何異性体などの全ての立体異性体を包含する。立体異性体は、単一物であっても混合物であってもよい。 [Stereoisomer]
The present invention is a compound represented by the same structural formula, but all compounds such as optical isomers, diastereoisomers, geometrical isomers, etc. having different spatial arrangements of atoms or substituents in the structure. Includes stereoisomers. The stereoisomer may be a single substance or a mixture.
〔塩〕
本発明に係る化合物(I)の塩としては、農園芸学的に許容される塩であれば、特に制限されない。例えば、塩酸、硫酸などの無機酸の塩;酢酸、乳酸などの有機酸の塩;リチウム、ナトリウム、カリウムなどのアルカリ金属の塩;カルシウム、マグネシウムなどのアルカリ土類金属の塩;鉄、銅などの遷移金属の塩;アンモニア、トリエチルアミン、トリブチルアミン、ピリジン、ヒドラジンなどの有機塩基の塩などを挙げることができる。化合物(I)の塩は、化合物(I)から公知の手法によって得ることができる。 〔salt〕
The salt of the compound (I) according to the present invention is not particularly limited as long as it is an agro-horticulturally acceptable salt. For example, salts of inorganic acids such as hydrochloric acid and sulfuric acid; salts of organic acids such as acetic acid and lactic acid; salts of alkali metals such as lithium, sodium and potassium; salts of alkaline earth metals such as calcium and magnesium; iron and copper And salts of organic metals such as ammonia, triethylamine, tributylamine, pyridine, hydrazine, and the like. The salt of compound (I) can be obtained from compound (I) by a known method.
本発明に係る化合物(I)の塩としては、農園芸学的に許容される塩であれば、特に制限されない。例えば、塩酸、硫酸などの無機酸の塩;酢酸、乳酸などの有機酸の塩;リチウム、ナトリウム、カリウムなどのアルカリ金属の塩;カルシウム、マグネシウムなどのアルカリ土類金属の塩;鉄、銅などの遷移金属の塩;アンモニア、トリエチルアミン、トリブチルアミン、ピリジン、ヒドラジンなどの有機塩基の塩などを挙げることができる。化合物(I)の塩は、化合物(I)から公知の手法によって得ることができる。 〔salt〕
The salt of the compound (I) according to the present invention is not particularly limited as long as it is an agro-horticulturally acceptable salt. For example, salts of inorganic acids such as hydrochloric acid and sulfuric acid; salts of organic acids such as acetic acid and lactic acid; salts of alkali metals such as lithium, sodium and potassium; salts of alkaline earth metals such as calcium and magnesium; iron and copper And salts of organic metals such as ammonia, triethylamine, tributylamine, pyridine, hydrazine, and the like. The salt of compound (I) can be obtained from compound (I) by a known method.
〔製造中間体〕
式(I)中のR4が、水素原子またはアリル基である化合物は、本発明においては、製造中間体として有用である。 [Production intermediate]
The compound in which R 4 in formula (I) is a hydrogen atom or an allyl group is useful as a production intermediate in the present invention.
式(I)中のR4が、水素原子またはアリル基である化合物は、本発明においては、製造中間体として有用である。 [Production intermediate]
The compound in which R 4 in formula (I) is a hydrogen atom or an allyl group is useful as a production intermediate in the present invention.
逆合成の概念を示す上記の式中、R4aは、上記のR4のうちで、水素原子およびアリル基以外の有機基を示す。R1~R3、およびQは、上記の規定と同様の意味を示す。
式(A)で表される化合物は、化合物(I)のうちで、R4が、水素原子およびアリル基以外の有機基である化合物を示す(以下、化合物(A)と言うことがある。)。
式(B)で表される化合物は、化合物(I)のうちで、R4が、水素原子である化合物を示し(以下、化合物(A)と言うことがある。)、化合物(B)は、化合物(A)を製造する際の製造中間体となりうる。
化合物(A)は、式:R4a-Lで表される試薬、式:(R4a)2Oで表される試薬などを用いることで、化合物(B)から製造できる。ここで、Lは、ハロゲノ基などの脱離基を示す。
式(C)で表される化合物は、化合物(I)のうちで、R4が、アリル基である化合物を示し(以下、化合物(C)と言うことがある。)、化合物(C)は、化合物(B)を製造する際の製造中間体となりうる。
化合物(B)は、通常のアリル基の脱保護法、例えば、接触還元法、パラジウム触媒(テトラキストリフェニルホスフィンパラジウムやジベンジリデンアセトンパラジウムを用いる方法により、化合物(C)から製造できる。 In said formula which shows the concept of reverse synthesis, R <4a> shows organic groups other than a hydrogen atom and an allyl group among said R < 4 >. R 1 to R 3 and Q have the same meaning as defined above.
The compound represented by the formula (A) is a compound in which R 4 is an organic group other than a hydrogen atom and an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (A)). ).
The compound represented by the formula (B) is a compound in which R 4 is a hydrogen atom in the compound (I) (hereinafter sometimes referred to as the compound (A)), and the compound (B) is And can be a production intermediate in the production of compound (A).
Compound (A) can be produced from compound (B) by using a reagent represented by the formula: R 4a -L, a reagent represented by the formula: (R 4a ) 2 O, and the like. Here, L represents a leaving group such as a halogeno group.
The compound represented by the formula (C) is a compound in which R 4 is an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (C)), and the compound (C) is And can be a production intermediate in the production of compound (B).
The compound (B) can be produced from the compound (C) by an ordinary allyl group deprotection method, for example, a catalytic reduction method or a palladium catalyst (a method using tetrakistriphenylphosphine palladium or dibenzylideneacetone palladium).
式(A)で表される化合物は、化合物(I)のうちで、R4が、水素原子およびアリル基以外の有機基である化合物を示す(以下、化合物(A)と言うことがある。)。
式(B)で表される化合物は、化合物(I)のうちで、R4が、水素原子である化合物を示し(以下、化合物(A)と言うことがある。)、化合物(B)は、化合物(A)を製造する際の製造中間体となりうる。
化合物(A)は、式:R4a-Lで表される試薬、式:(R4a)2Oで表される試薬などを用いることで、化合物(B)から製造できる。ここで、Lは、ハロゲノ基などの脱離基を示す。
式(C)で表される化合物は、化合物(I)のうちで、R4が、アリル基である化合物を示し(以下、化合物(C)と言うことがある。)、化合物(C)は、化合物(B)を製造する際の製造中間体となりうる。
化合物(B)は、通常のアリル基の脱保護法、例えば、接触還元法、パラジウム触媒(テトラキストリフェニルホスフィンパラジウムやジベンジリデンアセトンパラジウムを用いる方法により、化合物(C)から製造できる。 In said formula which shows the concept of reverse synthesis, R <4a> shows organic groups other than a hydrogen atom and an allyl group among said R < 4 >. R 1 to R 3 and Q have the same meaning as defined above.
The compound represented by the formula (A) is a compound in which R 4 is an organic group other than a hydrogen atom and an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (A)). ).
The compound represented by the formula (B) is a compound in which R 4 is a hydrogen atom in the compound (I) (hereinafter sometimes referred to as the compound (A)), and the compound (B) is And can be a production intermediate in the production of compound (A).
Compound (A) can be produced from compound (B) by using a reagent represented by the formula: R 4a -L, a reagent represented by the formula: (R 4a ) 2 O, and the like. Here, L represents a leaving group such as a halogeno group.
The compound represented by the formula (C) is a compound in which R 4 is an allyl group in the compound (I) (hereinafter sometimes referred to as the compound (C)), and the compound (C) is And can be a production intermediate in the production of compound (B).
The compound (B) can be produced from the compound (C) by an ordinary allyl group deprotection method, for example, a catalytic reduction method or a palladium catalyst (a method using tetrakistriphenylphosphine palladium or dibenzylideneacetone palladium).
〔農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤〕
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、化合物(I)、化合物(I)の互変異性体若しくは化合物(I)の塩(以下、これらを「本発明化合物」ということがある。)から選ばれる少なくとも1つを有効成分として含有するものである。 [Agricultural and horticultural fungicides, pesticides, and insecticides or acaricides]
The agricultural and horticultural fungicide, pesticide, and insecticide or acaricide of the present invention are compound (I), a tautomer of compound (I), or a salt of compound (I) (hereinafter referred to as "this" It may contain at least one selected from “inventive compounds”) as an active ingredient.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、化合物(I)、化合物(I)の互変異性体若しくは化合物(I)の塩(以下、これらを「本発明化合物」ということがある。)から選ばれる少なくとも1つを有効成分として含有するものである。 [Agricultural and horticultural fungicides, pesticides, and insecticides or acaricides]
The agricultural and horticultural fungicide, pesticide, and insecticide or acaricide of the present invention are compound (I), a tautomer of compound (I), or a salt of compound (I) (hereinafter referred to as "this" It may contain at least one selected from “inventive compounds”) as an active ingredient.
本発明の農園芸用殺菌剤は、広範囲の種類の糸状菌、例えば、藻菌類(Oomycetes)、子のう(嚢)菌類(Ascomycetes)、不完全菌類(Deuteromycetes)、担子菌類(Basidiomycetes)、接合菌類(Zygomycetes)に属する菌に由来する植物病害の防除に使用できる。
Agricultural and horticultural fungicides of the present invention include a wide variety of filamentous fungi, such as algae (Oomycetes), Ascomycetes, incomplete fungi (Deuteromycetes), basidiomycetes, mating It can be used for controlling plant diseases derived from fungi belonging to fungi (Zygomycetes).
防除の対象となる植物病害と病原菌の例を以下に示す。
テンサイ:褐斑病(Cercospora beticola)、黒根病(Aphanomyces cochlloides)、根腐病(Thanatephorus cucumeris)、葉腐病(Thanatephorus cucumeris)など
ラッカセイ:褐斑病(Mycosphaerella arachidis)、汚斑病(Ascochyta sp.)、さび病(Puccinia arachidis)、立枯病(Pythium debaryanum)、さび斑病(Alternaria alternata)、白絹病(Sclerotium rolfsii)黒渋病(Mycosphaerella berkeleyi)など
キュウリ:うどんこ病(Sphaerotheca fuliginea)、べと病(Pseudoperonospora cubensis)、つる枯病(Mycosphaerella melonis)、つる割病(Fusarium oxysporum)、菌核病(Sclerotinia sclerotiorum)、灰色かび病(Botrytis cinerea)、炭そ病(Colletotrichum orbiculare)、黒星病(Cladosporium cucumerinum)、褐斑病(Corynespora cassicola)、苗立枯病(Pythium debaryanam、Rhizoctonia solani Kuhn)、ホモプシス根腐病(Phomopsis sp.)斑点細菌病(Pseudomonas syringae pv. Lecrymans)など
トマト:灰色かび病(Botrytis cinerea)、葉かび病(Cladosporium fulvum)、疫病(Phytophthora infestans)、半身萎凋病(Verticillium albo-atrum)、うどんこ病(Oidium neolycopersici)、輪紋病(Alternaria solani)、すすかび病(Pseudocercospora fuligena)など
ナス:灰色かび病(Botrytis cinerea)、黒枯病(Corynespora melongenae)、うどんこ病(Erysiphe cichoracearum)、すすかび病(Mycovellosiella nattrassii)、菌核病(Sclerotinia sclerotiorum)など
イチゴ:灰色かび病(Botrytis cinerea)、うどんこ病(Sohaerotheca humuli)、炭そ病(Colletotrichum acutatum、Colletotrichum fragariae)、疫病(Phytophthora cactorum)、軟腐病(Rhizopus stolonifer)、萎黄病(Fusarium oxysporum)など
タマネギ:灰色腐敗病(Botrytis allii)、灰色かび病(Botrytis cinerea)、白斑葉枯病(Botrytis squamosa)、べと病(Peronospora destructor)、白色疫病(Phytophthora porri)など
キャベツ:根こぶ病(Plasmodiophora brassicae)、軟腐病(Erwinia carotovora)、黒腐病(Xanthomonas campesrtis pv. campestris)、黒斑細菌病(Pseudomonas syringae pv. maculicala、Pseudomonas syringae pv. alisalensis)、べと病(Peronospora parasitica)、菌核病(Sclerotinia sclerotiorum)、黒すす病(Alternaria brassicicola)、灰色かび病(Botrytis cinerea)など
インゲン:菌核病(Sclerotinia sclerotiorum)、灰色かび病(Botrytis cinerea)、炭疽病(Colletotrichum lindemuthianum)、角斑病(Phaeoisariopsis griseola)など Examples of plant diseases and pathogens to be controlled are shown below.
Sugar beet: brown spot disease (Cercospora beticola), black root disease (Aphanomyces cochlloides), root rot (Thanatephorus cucumeris), leaf rot (Thanatephorus cucumeris), etc. ), Rust disease (Puccinia arachidis), withering disease (Pythium debaryanum), rust spot disease (Alternaria alternata), white silk disease (Sclerotium rolfsii) black astringency (Mycosphaerella berkeleyi), cucumber: powdery mildew (Sphaerotheca fuliginea), Downy mildew (Pseudoperonospora cubensis), vine blight (Mycosphaerella melonis), vine split disease (Fusarium oxysporum), mycorrhizal disease (Sclerotinia sclerotiorum), gray mold disease (Botrytis cinerea), anthracnose disease (Colletotrichum orbiculare), black star disease (Cladosporium cucumerinum), brown spot disease (Corynespora cassicola), seedling blight (Pythium debaryanam, Rhizoctonia solani Kuhn), homopsis root rot (Phomopsis sp.) Spot bacterial disease (Pseudomonas syringa) e pv. Lecrymans) Tomato: Gray mold disease (Botrytis cinerea), leaf mold disease (Cladosporium fulvum), plague (Phytophthora infestans), half body wilt disease (Verticillium albo-atrum), powdery mildew (Oidium neolycopersici), ring crest Diseases (Alternaria solani), Subtilis (Pseudocercospora fuligena), etc. Eggplant: Gray mold (Botrytis cinerea), Black blight (Corynespora melongenae), Powdery mildew (Erysiphe cichoracearum), Subtilis (Mycovellosiella nattrassii), Mycovellosiella nattrassii Strawberries: Botrytis cinerea, powdery mildew (Sohaerotheca humuli), anthracnose (Colletotrichum acutatum, Colletotrichum fragariae), plague (Phytophthora cactorum), soft rot (Rhizopus stoifer) (Fusarium oxysporum) Onions: gray rot (Botrytis allii), gray mold (Botrytis cinerea), white leaf blight (Botrytis squamosa), downy mildew (Peronospora d estructor), white plague (Phytophthora porri), etc. cabbage: root-knot disease (Plasmodiophora brassicae), soft rot disease (Erwinia carotovora), black rot disease (Xanthomonas campesrtis pv. campestris), black spot bacterial disease (Pseudomonas syringae pv. maculicalas, Pseudomonas syringae pv. alisalensis), downy mildew (Peronospora parasitica), mycorrhizal disease (Sclerotinia sclerotiorum), black soot disease (Alternaria brassicicola), gray mold disease (Botrytis cinerea), etc. Kidney: Sclerotinia sclerotiorum, gray mold Diseases (Botrytis cinerea), anthrax (Colletotrichum lindemuthianum), keratosis (Phaeoisariopsis griseola), etc.
テンサイ:褐斑病(Cercospora beticola)、黒根病(Aphanomyces cochlloides)、根腐病(Thanatephorus cucumeris)、葉腐病(Thanatephorus cucumeris)など
ラッカセイ:褐斑病(Mycosphaerella arachidis)、汚斑病(Ascochyta sp.)、さび病(Puccinia arachidis)、立枯病(Pythium debaryanum)、さび斑病(Alternaria alternata)、白絹病(Sclerotium rolfsii)黒渋病(Mycosphaerella berkeleyi)など
キュウリ:うどんこ病(Sphaerotheca fuliginea)、べと病(Pseudoperonospora cubensis)、つる枯病(Mycosphaerella melonis)、つる割病(Fusarium oxysporum)、菌核病(Sclerotinia sclerotiorum)、灰色かび病(Botrytis cinerea)、炭そ病(Colletotrichum orbiculare)、黒星病(Cladosporium cucumerinum)、褐斑病(Corynespora cassicola)、苗立枯病(Pythium debaryanam、Rhizoctonia solani Kuhn)、ホモプシス根腐病(Phomopsis sp.)斑点細菌病(Pseudomonas syringae pv. Lecrymans)など
トマト:灰色かび病(Botrytis cinerea)、葉かび病(Cladosporium fulvum)、疫病(Phytophthora infestans)、半身萎凋病(Verticillium albo-atrum)、うどんこ病(Oidium neolycopersici)、輪紋病(Alternaria solani)、すすかび病(Pseudocercospora fuligena)など
ナス:灰色かび病(Botrytis cinerea)、黒枯病(Corynespora melongenae)、うどんこ病(Erysiphe cichoracearum)、すすかび病(Mycovellosiella nattrassii)、菌核病(Sclerotinia sclerotiorum)など
イチゴ:灰色かび病(Botrytis cinerea)、うどんこ病(Sohaerotheca humuli)、炭そ病(Colletotrichum acutatum、Colletotrichum fragariae)、疫病(Phytophthora cactorum)、軟腐病(Rhizopus stolonifer)、萎黄病(Fusarium oxysporum)など
タマネギ:灰色腐敗病(Botrytis allii)、灰色かび病(Botrytis cinerea)、白斑葉枯病(Botrytis squamosa)、べと病(Peronospora destructor)、白色疫病(Phytophthora porri)など
キャベツ:根こぶ病(Plasmodiophora brassicae)、軟腐病(Erwinia carotovora)、黒腐病(Xanthomonas campesrtis pv. campestris)、黒斑細菌病(Pseudomonas syringae pv. maculicala、Pseudomonas syringae pv. alisalensis)、べと病(Peronospora parasitica)、菌核病(Sclerotinia sclerotiorum)、黒すす病(Alternaria brassicicola)、灰色かび病(Botrytis cinerea)など
インゲン:菌核病(Sclerotinia sclerotiorum)、灰色かび病(Botrytis cinerea)、炭疽病(Colletotrichum lindemuthianum)、角斑病(Phaeoisariopsis griseola)など Examples of plant diseases and pathogens to be controlled are shown below.
Sugar beet: brown spot disease (Cercospora beticola), black root disease (Aphanomyces cochlloides), root rot (Thanatephorus cucumeris), leaf rot (Thanatephorus cucumeris), etc. ), Rust disease (Puccinia arachidis), withering disease (Pythium debaryanum), rust spot disease (Alternaria alternata), white silk disease (Sclerotium rolfsii) black astringency (Mycosphaerella berkeleyi), cucumber: powdery mildew (Sphaerotheca fuliginea), Downy mildew (Pseudoperonospora cubensis), vine blight (Mycosphaerella melonis), vine split disease (Fusarium oxysporum), mycorrhizal disease (Sclerotinia sclerotiorum), gray mold disease (Botrytis cinerea), anthracnose disease (Colletotrichum orbiculare), black star disease (Cladosporium cucumerinum), brown spot disease (Corynespora cassicola), seedling blight (Pythium debaryanam, Rhizoctonia solani Kuhn), homopsis root rot (Phomopsis sp.) Spot bacterial disease (Pseudomonas syringa) e pv. Lecrymans) Tomato: Gray mold disease (Botrytis cinerea), leaf mold disease (Cladosporium fulvum), plague (Phytophthora infestans), half body wilt disease (Verticillium albo-atrum), powdery mildew (Oidium neolycopersici), ring crest Diseases (Alternaria solani), Subtilis (Pseudocercospora fuligena), etc. Eggplant: Gray mold (Botrytis cinerea), Black blight (Corynespora melongenae), Powdery mildew (Erysiphe cichoracearum), Subtilis (Mycovellosiella nattrassii), Mycovellosiella nattrassii Strawberries: Botrytis cinerea, powdery mildew (Sohaerotheca humuli), anthracnose (Colletotrichum acutatum, Colletotrichum fragariae), plague (Phytophthora cactorum), soft rot (Rhizopus stoifer) (Fusarium oxysporum) Onions: gray rot (Botrytis allii), gray mold (Botrytis cinerea), white leaf blight (Botrytis squamosa), downy mildew (Peronospora d estructor), white plague (Phytophthora porri), etc. cabbage: root-knot disease (Plasmodiophora brassicae), soft rot disease (Erwinia carotovora), black rot disease (Xanthomonas campesrtis pv. campestris), black spot bacterial disease (Pseudomonas syringae pv. maculicalas, Pseudomonas syringae pv. alisalensis), downy mildew (Peronospora parasitica), mycorrhizal disease (Sclerotinia sclerotiorum), black soot disease (Alternaria brassicicola), gray mold disease (Botrytis cinerea), etc. Kidney: Sclerotinia sclerotiorum, gray mold Diseases (Botrytis cinerea), anthrax (Colletotrichum lindemuthianum), keratosis (Phaeoisariopsis griseola), etc.
りんご:うどんこ病(Podosphaera leucotricha)、黒星病(Venturia inaequalis)、モニリア病(Monilinia mali)、黒点病(Mycosphaerella pomi)、腐らん病(Valsa mali)、斑点落葉病(Alternaria mali)、赤星病(Gymnosporangium yamadae)、輪紋病(Botryosphaeria berengeriana)、炭そ病(Glomerella cingulata、Colletotrichum acutatum)、褐斑病(Diplocarpon mali)、すす点病(Zygophiala jamaicensis)、すす斑病(Gloeodes pomigena)、紫紋羽病(Helicobasidium mompa)、灰色かび病(Botrytis cinerea)など
ウメ:黒星病(Cladosporium carpophilum)、灰色かび病(Botrytis cinerea)、灰星病(Monilinia mumecola)など
カキ:うどんこ病(Phyllactinia kakicola)、炭そ病(Gloeosporium kaki)、角斑落葉病(Cercospora kaki)など
モモ:灰星病(Monilinia fructicola)、黒星病(Cladosporium carpophilum)、ホモプシス腐敗病(Phomopsis sp.)、穿孔細菌病(Xanthomonas campestris pv. pruni)など
アーモンド:灰星病(Monilinia laxa)、斑点病(Stigmina carpophila)、黒星病(Cladosporium carpophilum)、葉ぶくれ病(Polystigma rubrum)、斑点落葉病(Alternaria alternata)、炭疽病(Colletotrichum gloeospoides)など
オウトウ:灰星病(Monilinia fructicola)、炭そ病(Colletotrichum acutatum)、黒斑病(Alternaria sp.)、幼果菌核病(Monilinia kusanoi)など
ブドウ:灰色かび病(Botrytis cinerea)、うどんこ病(Uncinula necator)、晩腐病(Glomerella cingulata、Colletotrichum acutatum)、べと病(Plasmopara viticola)、黒とう病(Elsinoe ampelina)、褐斑病(Pseudocercospora vitis)、黒腐病(Guignardia bidwellii)、白腐病(Coniella castaneicola)など
ナシ:黒星病(Venturia nashicola)、赤星病(Gymnosporangium asiaticum)、黒斑病(Alternaria kikuchiana)、輪紋病(Botryosphaeria berengeriana)、うどんこ病(Phyllactinia mali)、胴枯病(Phomopsis fukushii)、褐色斑点病(Stemphylium vesicarium)、炭そ病(Glomerella cingulata)など
チャ:輪斑病(Pestalotia theae)、炭そ病(Colletotrichum theae-sinensis)など
カンキツ:そうか病(Elsinoe fawcetti)、青かび病(Penicillium italicum)、緑かび病(Penicillium digitatum)、灰色かび病(Botrytis cinerea)、黒点病(Diaporthe citri)、かいよう病(Xanthomonas campestris pv.Citri)、うどんこ病(Oidium sp.)など Apples: powdery mildew (Podosphaera leucotricha), black spot disease (Venturia inaequalis), monilinia disease (Monilinia mali), black spot disease (Mycosphaerella pomi), rot disease (Valsa mali), spotted leaf disease (Alternaria mali), red star disease (Gymnosporang) yamadae), ring rot (Botryosphaeria berengeriana), anthracnose (Glomerella cingulata, Colletotrichum acutatum), brown spot (Diplocarpon mali), soot spot (Zygophiala jamaicensis), soot spot (Gloeodes pomigena), purple coat rot (Helicobasidium mompa), gray mold disease (Botrytis cinerea), etc. Ume: black rot (Cladosporium carpophilum), gray mold disease (Botrytis cinerea), gray star disease (Monilinia mumecola), etc. Oysters: powdery mildew (Phyllactinia kakicola), anthrax Diseases such as Gloeosporium kaki, Cercospora kaki, peaches: Monilinia fructicola, black scab (Cladosporium carpophilum), homopsis spoilage (Phomopsis sp.), Perforated bacteria (Xanthomonas campestris pv. Pruni) and other almonds: Monilinia laxa, spot disease (Stigmina carpophila), black spot disease (Cladosporium carpophilum), leaf blight disease (Polystigma rubrum), spotted leaf disease (Alternaria alternata), anthrax Sugar beetle (Colletotrichum gloeospoides), etc. Sweet potato: Monilinia fructicola, anthracnose (Colletotrichum acutatum), black spot (Alternaria sp.), Larvae nuclear disease (Monilinia kusanoi), etc. Grapes: Gray mold disease (Botrytis) cinerea), powdery mildew (Uncinula necator), late rot (Glomerella cingulata, Colletotrichum acutatum), downy mildew (Plasmopara viticola), black mildew (Elsinoe ampelina), brown spot (Pseudocercospora vitis), black rot ( Guignardia bidwellii), white rot (Coniella castaneicola), etc. Pear: Venturia nashicola, Red Star Disease (Gymnosporangium asiaticum), Black Spot Disease (Alternaria kikuchiana), Ring Ring Disease (Botryosphaeria berengeriana) Powdery mildew (Phyllactinia mali), head blight (Phomopsis fukushii), brown spot (Stemphylium vesicarium), anthracnose (Glomerella cingulata), etc. Cha: Ring-to-leaf (Pestalotia theae), anthracnose (Colletotrichum theae-sinensis) ) Citrus, etc .: Scab (Elsinoe fawcetti), blue mold (Penicillium italicum), green mold (Penicillium digitatum), gray mold (Botrytis cinerea), sunspot (Diaporthe citri), scab (Xanthomonas campestris pv.Citri) ), Powdery mildew (Oidium sp.), Etc.
ウメ:黒星病(Cladosporium carpophilum)、灰色かび病(Botrytis cinerea)、灰星病(Monilinia mumecola)など
カキ:うどんこ病(Phyllactinia kakicola)、炭そ病(Gloeosporium kaki)、角斑落葉病(Cercospora kaki)など
モモ:灰星病(Monilinia fructicola)、黒星病(Cladosporium carpophilum)、ホモプシス腐敗病(Phomopsis sp.)、穿孔細菌病(Xanthomonas campestris pv. pruni)など
アーモンド:灰星病(Monilinia laxa)、斑点病(Stigmina carpophila)、黒星病(Cladosporium carpophilum)、葉ぶくれ病(Polystigma rubrum)、斑点落葉病(Alternaria alternata)、炭疽病(Colletotrichum gloeospoides)など
オウトウ:灰星病(Monilinia fructicola)、炭そ病(Colletotrichum acutatum)、黒斑病(Alternaria sp.)、幼果菌核病(Monilinia kusanoi)など
ブドウ:灰色かび病(Botrytis cinerea)、うどんこ病(Uncinula necator)、晩腐病(Glomerella cingulata、Colletotrichum acutatum)、べと病(Plasmopara viticola)、黒とう病(Elsinoe ampelina)、褐斑病(Pseudocercospora vitis)、黒腐病(Guignardia bidwellii)、白腐病(Coniella castaneicola)など
ナシ:黒星病(Venturia nashicola)、赤星病(Gymnosporangium asiaticum)、黒斑病(Alternaria kikuchiana)、輪紋病(Botryosphaeria berengeriana)、うどんこ病(Phyllactinia mali)、胴枯病(Phomopsis fukushii)、褐色斑点病(Stemphylium vesicarium)、炭そ病(Glomerella cingulata)など
チャ:輪斑病(Pestalotia theae)、炭そ病(Colletotrichum theae-sinensis)など
カンキツ:そうか病(Elsinoe fawcetti)、青かび病(Penicillium italicum)、緑かび病(Penicillium digitatum)、灰色かび病(Botrytis cinerea)、黒点病(Diaporthe citri)、かいよう病(Xanthomonas campestris pv.Citri)、うどんこ病(Oidium sp.)など Apples: powdery mildew (Podosphaera leucotricha), black spot disease (Venturia inaequalis), monilinia disease (Monilinia mali), black spot disease (Mycosphaerella pomi), rot disease (Valsa mali), spotted leaf disease (Alternaria mali), red star disease (Gymnosporang) yamadae), ring rot (Botryosphaeria berengeriana), anthracnose (Glomerella cingulata, Colletotrichum acutatum), brown spot (Diplocarpon mali), soot spot (Zygophiala jamaicensis), soot spot (Gloeodes pomigena), purple coat rot (Helicobasidium mompa), gray mold disease (Botrytis cinerea), etc. Ume: black rot (Cladosporium carpophilum), gray mold disease (Botrytis cinerea), gray star disease (Monilinia mumecola), etc. Oysters: powdery mildew (Phyllactinia kakicola), anthrax Diseases such as Gloeosporium kaki, Cercospora kaki, peaches: Monilinia fructicola, black scab (Cladosporium carpophilum), homopsis spoilage (Phomopsis sp.), Perforated bacteria (Xanthomonas campestris pv. Pruni) and other almonds: Monilinia laxa, spot disease (Stigmina carpophila), black spot disease (Cladosporium carpophilum), leaf blight disease (Polystigma rubrum), spotted leaf disease (Alternaria alternata), anthrax Sugar beetle (Colletotrichum gloeospoides), etc. Sweet potato: Monilinia fructicola, anthracnose (Colletotrichum acutatum), black spot (Alternaria sp.), Larvae nuclear disease (Monilinia kusanoi), etc. Grapes: Gray mold disease (Botrytis) cinerea), powdery mildew (Uncinula necator), late rot (Glomerella cingulata, Colletotrichum acutatum), downy mildew (Plasmopara viticola), black mildew (Elsinoe ampelina), brown spot (Pseudocercospora vitis), black rot ( Guignardia bidwellii), white rot (Coniella castaneicola), etc. Pear: Venturia nashicola, Red Star Disease (Gymnosporangium asiaticum), Black Spot Disease (Alternaria kikuchiana), Ring Ring Disease (Botryosphaeria berengeriana) Powdery mildew (Phyllactinia mali), head blight (Phomopsis fukushii), brown spot (Stemphylium vesicarium), anthracnose (Glomerella cingulata), etc. Cha: Ring-to-leaf (Pestalotia theae), anthracnose (Colletotrichum theae-sinensis) ) Citrus, etc .: Scab (Elsinoe fawcetti), blue mold (Penicillium italicum), green mold (Penicillium digitatum), gray mold (Botrytis cinerea), sunspot (Diaporthe citri), scab (Xanthomonas campestris pv.Citri) ), Powdery mildew (Oidium sp.), Etc.
コムギ:うどんこ病(Erysiphe graminis f.sp.Tritici)、赤かび病(Gibberella zeae)、赤さび病(Puccinia recondita)、褐色雪腐病(Pythium iwayamai)、紅色雪腐病(Monographella nivalis)、眼紋病(Pseudocercosporella herpotrichoides)、葉枯病(Septoria tritici)、ふ枯病(Leptosphaeria nodorum)、雪腐小粒菌核病(Typhula incarnata)、雪腐大粒菌核病(Myriosclerotinia borealis)、立枯病(Gaeumanomyces graminis)、麦角病(Claviceps purpurea)、なまぐさ黒穂病(Tilletia caries)、裸黒穂病(Ustilago nuda)など
オオムギ:斑葉病(Pyrenophora graminea)、網斑病(Pyrenophora teres)、雲形病(Rhynchosporium secalis)、裸黒穂病(Ustilago tritici、U.nuda)など
イネ:いもち病(Pyricularia oryzae)、紋枯病(Rhizoctonia solani)、馬鹿苗病(Gibberella fujikuroi)、ごま葉枯病(Cochliobolus miyabeanus)、苗立枯病(Pythium graminicolum)、白葉枯病(Xanthomonas oryzae)、苗立枯細菌病(Burkholderia plantarii)、褐条病(Acidovorax avenae)、もみ枯細菌病(Burkholderia glumae)、すじ葉枯病(Cercospora oryzae)、稲こうじ病(Ustilaginoidea virens)、褐色米(Alternaria alternata、Curvularia intermedia)、腹黒米(Alternaria padwickii)、紅変米(Epicoccam purpurascenns)など
タバコ:菌核病(Sclerotinia sclerotiorum)、うどんこ病(Erysiphe cichoracearum)、疫病(Phytophthora nicotianae)、など
チューリップ:灰色かび病(Botrytis cinerea)など
ヒマワリ:べと病(Plasmopara halstedii)、菌核病(Sclerotinia sclerotiorum)など
ベントグラス:雪腐大粒菌核病(Sclerotinia borealis)、ラージパッチ(Rhizoctonia solani)、ダラースポット(Sclerotinia homoeocarpa)、いもち病(Pyricularia sp.)、赤焼病(Pythium aphanidermatum)、炭そ病(Colletotrichum graminicola)など
オーチャードグラス:うどんこ病(Erysiphe graminis)など
ダイズ:紫斑病(Cercospora kikuchii)、べと病(Peronospora manshurica)、茎疫病(Phytophthora sojae)、さび病(Phakopsora pachyrhizi)、菌核病(Sclerotinia sclerotiorum)、炭そ病(Colletotrichum truncatum)、灰色かび病(Botrytis cinerea)など
ジャガイモ:疫病(Phytophthora infestans)、夏疫病(Aleternaria solani)、黒あざ病(Thanatephorus cucumeris)など
バナナ:パナマ病(Fusarium oxysporum)、シガトカ病(Mycosphaerella fijiensis、Mycosphaerella musicola)など
ナタネ:菌核病(Sclerotinia sclerotiorum)、根朽病(Phoma lingam)、黒斑病(Alternaria brassicae)など
コーヒー:さび病(Hemileia vastatrix)、炭疽病(Colletotrichum coffeanum)、褐眼病(Cercospora coffeicola)など
サトウキビ:褐さび病(Puccinia melanocephala)など
トウモロコシ:ひょう紋病(Gloecercospora sorghi)、さび病(Puccinia sorghi)、南方さび病(Puccinia polysora)、黒穂病(Ustilago maydis)、ごま葉枯病(Cochliobolus heterostrophus)、すす紋病(Setophaeria turcica)など
ワタ:苗立枯病(Pythium sp)、さび病(Phakopsora gossypii)、白かび病(Mycosphaerella areola)、炭疽病(Glomerella gossypii)など Wheat: powdery mildew (Erysiphe graminis f.sp.Tritici), red mold (Gibberella zeae), red rust (Puccinia recondita), brown snow rot (Pythium iwayamai), red snow rot (Monographella nivalis), eyeprint Disease (Pseudocercosporella herpotrichoides), leaf blight (Septoria tritici), blight (Leptosphaeria nodorum), snow rot microbe nuclei (Typhula incarnata), snow rot large bacilli (Myriosclerotinia borealis), blight (Gaeumanomyces graminis) ), Ergot disease (Claviceps purpurea), lintel scab (Tilletia caries), bare scab (Ustilago nuda), etc. barley: leafy disease (Pyrenophora graminea), reticulosis (Pyrenophora teres), cloud disease (Rhynchosporium secalis), Bare smut (Ustilago tritici, U.nuda), etc. Rice: Rice blast (Pyricularia oryzae), blight (Rhizoctonia solani), idiot seedling (Gibberella fujikuroi), sesame leaf blight (Cochliobolus miyabeanus), seedling blight (Pythium graminicolum , White leaf blight (Xanthomonas oryzae), seedling blight (Burkholderia plantarii), brown streak (Acidovorax avenae), blight blight (Burkholderia glumae), streak blight (Cercospora oryzae), rice leaf blight (Ustilaginoidea) virens), brown rice (Alternaria alternata, Curvularia intermedia), belly black rice (Alternaria padwickii), red rice (Epicoccam purpurascenns), etc. Tobacco: Sclerotinia sclerotiorum, powdery mildew (Erysiphe cichoracearum), plague ), Etc. Tulip: Gray mold disease (Botrytis cinerea) Sunflower: Downy mildew (Plasmopara halstedii), Mycorrhizal disease (Sclerotinia sclerotiorum), etc. Bentgrass: Snow rot (Sclerotinia borealis), Large patch (Rhizoctonia solani) , Dollar spot (Sclerotinia homoeocarpa), blast (Pyricularia sp.), Red blight (Pythium aphanidermatum), anthracnose (Colletotrichum graminicola) Orchardgrass: powdery mildew (Erysiphe graminis), etc. Soybean: Purpura (Cercospora kikuchii), downy mildew (Peronospora manshurica), stem blight (Phytophthora sojae), rust (Phakopsora pachyrhizi), sclerotia (Sclerotinia sclerotiorum) Anthracnose (Colletotrichum truncatum), gray mold (Botrytis cinerea), etc. Potato: Phytophthora infestans, summer plague (Aleternaria solani), black rot (Thanatephorus cucumeris), etc. Banana: Panama disease (Fusarium oxysporum), Shiga (Mycosphaerella fijiensis, Mycosphaerella musicola), etc. Rapeseed: Sclerotinia sclerotiorum, root rot (Phoma lingam), black spot (Alternaria brassicae), etc. Coffee: Rust (Hemileia vastatrix), Anthrax (Colletotrichum coffeanum) Brown eye disease (Cercospora coffeicola), etc. Sugar cane: Brown rust (Puccinia melanocephala), etc. Corn: Leopard disease Gloecercospora sorghi), rust disease (Puccinia sorghi), southern rust disease (Puccinia polysora), smut (Ustilago maydis), sesame leaf blight (Cochliobolus heterostrophus), soot rot (Setophaeria turcica), etc. Pythium sp), rust (Phakopsora gossypii), mildew (Mycosphaerella areola), anthrax (Glomerella gossypii), etc.
オオムギ:斑葉病(Pyrenophora graminea)、網斑病(Pyrenophora teres)、雲形病(Rhynchosporium secalis)、裸黒穂病(Ustilago tritici、U.nuda)など
イネ:いもち病(Pyricularia oryzae)、紋枯病(Rhizoctonia solani)、馬鹿苗病(Gibberella fujikuroi)、ごま葉枯病(Cochliobolus miyabeanus)、苗立枯病(Pythium graminicolum)、白葉枯病(Xanthomonas oryzae)、苗立枯細菌病(Burkholderia plantarii)、褐条病(Acidovorax avenae)、もみ枯細菌病(Burkholderia glumae)、すじ葉枯病(Cercospora oryzae)、稲こうじ病(Ustilaginoidea virens)、褐色米(Alternaria alternata、Curvularia intermedia)、腹黒米(Alternaria padwickii)、紅変米(Epicoccam purpurascenns)など
タバコ:菌核病(Sclerotinia sclerotiorum)、うどんこ病(Erysiphe cichoracearum)、疫病(Phytophthora nicotianae)、など
チューリップ:灰色かび病(Botrytis cinerea)など
ヒマワリ:べと病(Plasmopara halstedii)、菌核病(Sclerotinia sclerotiorum)など
ベントグラス:雪腐大粒菌核病(Sclerotinia borealis)、ラージパッチ(Rhizoctonia solani)、ダラースポット(Sclerotinia homoeocarpa)、いもち病(Pyricularia sp.)、赤焼病(Pythium aphanidermatum)、炭そ病(Colletotrichum graminicola)など
オーチャードグラス:うどんこ病(Erysiphe graminis)など
ダイズ:紫斑病(Cercospora kikuchii)、べと病(Peronospora manshurica)、茎疫病(Phytophthora sojae)、さび病(Phakopsora pachyrhizi)、菌核病(Sclerotinia sclerotiorum)、炭そ病(Colletotrichum truncatum)、灰色かび病(Botrytis cinerea)など
ジャガイモ:疫病(Phytophthora infestans)、夏疫病(Aleternaria solani)、黒あざ病(Thanatephorus cucumeris)など
バナナ:パナマ病(Fusarium oxysporum)、シガトカ病(Mycosphaerella fijiensis、Mycosphaerella musicola)など
ナタネ:菌核病(Sclerotinia sclerotiorum)、根朽病(Phoma lingam)、黒斑病(Alternaria brassicae)など
コーヒー:さび病(Hemileia vastatrix)、炭疽病(Colletotrichum coffeanum)、褐眼病(Cercospora coffeicola)など
サトウキビ:褐さび病(Puccinia melanocephala)など
トウモロコシ:ひょう紋病(Gloecercospora sorghi)、さび病(Puccinia sorghi)、南方さび病(Puccinia polysora)、黒穂病(Ustilago maydis)、ごま葉枯病(Cochliobolus heterostrophus)、すす紋病(Setophaeria turcica)など
ワタ:苗立枯病(Pythium sp)、さび病(Phakopsora gossypii)、白かび病(Mycosphaerella areola)、炭疽病(Glomerella gossypii)など Wheat: powdery mildew (Erysiphe graminis f.sp.Tritici), red mold (Gibberella zeae), red rust (Puccinia recondita), brown snow rot (Pythium iwayamai), red snow rot (Monographella nivalis), eyeprint Disease (Pseudocercosporella herpotrichoides), leaf blight (Septoria tritici), blight (Leptosphaeria nodorum), snow rot microbe nuclei (Typhula incarnata), snow rot large bacilli (Myriosclerotinia borealis), blight (Gaeumanomyces graminis) ), Ergot disease (Claviceps purpurea), lintel scab (Tilletia caries), bare scab (Ustilago nuda), etc. barley: leafy disease (Pyrenophora graminea), reticulosis (Pyrenophora teres), cloud disease (Rhynchosporium secalis), Bare smut (Ustilago tritici, U.nuda), etc. Rice: Rice blast (Pyricularia oryzae), blight (Rhizoctonia solani), idiot seedling (Gibberella fujikuroi), sesame leaf blight (Cochliobolus miyabeanus), seedling blight (Pythium graminicolum , White leaf blight (Xanthomonas oryzae), seedling blight (Burkholderia plantarii), brown streak (Acidovorax avenae), blight blight (Burkholderia glumae), streak blight (Cercospora oryzae), rice leaf blight (Ustilaginoidea) virens), brown rice (Alternaria alternata, Curvularia intermedia), belly black rice (Alternaria padwickii), red rice (Epicoccam purpurascenns), etc. Tobacco: Sclerotinia sclerotiorum, powdery mildew (Erysiphe cichoracearum), plague ), Etc. Tulip: Gray mold disease (Botrytis cinerea) Sunflower: Downy mildew (Plasmopara halstedii), Mycorrhizal disease (Sclerotinia sclerotiorum), etc. Bentgrass: Snow rot (Sclerotinia borealis), Large patch (Rhizoctonia solani) , Dollar spot (Sclerotinia homoeocarpa), blast (Pyricularia sp.), Red blight (Pythium aphanidermatum), anthracnose (Colletotrichum graminicola) Orchardgrass: powdery mildew (Erysiphe graminis), etc. Soybean: Purpura (Cercospora kikuchii), downy mildew (Peronospora manshurica), stem blight (Phytophthora sojae), rust (Phakopsora pachyrhizi), sclerotia (Sclerotinia sclerotiorum) Anthracnose (Colletotrichum truncatum), gray mold (Botrytis cinerea), etc. Potato: Phytophthora infestans, summer plague (Aleternaria solani), black rot (Thanatephorus cucumeris), etc. Banana: Panama disease (Fusarium oxysporum), Shiga (Mycosphaerella fijiensis, Mycosphaerella musicola), etc. Rapeseed: Sclerotinia sclerotiorum, root rot (Phoma lingam), black spot (Alternaria brassicae), etc. Coffee: Rust (Hemileia vastatrix), Anthrax (Colletotrichum coffeanum) Brown eye disease (Cercospora coffeicola), etc. Sugar cane: Brown rust (Puccinia melanocephala), etc. Corn: Leopard disease Gloecercospora sorghi), rust disease (Puccinia sorghi), southern rust disease (Puccinia polysora), smut (Ustilago maydis), sesame leaf blight (Cochliobolus heterostrophus), soot rot (Setophaeria turcica), etc. Pythium sp), rust (Phakopsora gossypii), mildew (Mycosphaerella areola), anthrax (Glomerella gossypii), etc.
本発明の殺虫若しくは殺ダニ剤は、植物の生育に影響する各種の農業害虫およびダニ類などの有害生物の防除効果に優れている。本発明の殺虫若しくは殺ダニ剤は、感受性系統のみならず、従来の薬剤、たとえば、有機リン剤やカーバメート剤に対する抵抗性が発達した系統の害虫にも有効である。抵抗性系統の害虫の代表例としては、コナガ、ウンカ、ヨコバイ、アブラムシなどを挙げることができる。
また、本発明に係るピリジン化合物は、防除の対象となる生物のすべての発育ステージにおいて効力を示し、例えば、ダニ、昆虫などの卵、若虫、幼虫、成虫に対して優れた防除効果を示す。 The insecticide or acaricide of the present invention is excellent in controlling pests such as various agricultural pests and mites that affect the growth of plants. The insecticide or acaricide of the present invention is effective not only for susceptible strains but also for pests of strains that have developed resistance to conventional agents such as organophosphorus agents and carbamate agents. Representative examples of pests of resistant strains include diamondback moth, planthopper, leafhopper and aphids.
Moreover, the pyridine compound according to the present invention is effective at all stages of development of organisms to be controlled, and exhibits an excellent control effect on eggs, nymphs, larvae and adults such as mites and insects.
また、本発明に係るピリジン化合物は、防除の対象となる生物のすべての発育ステージにおいて効力を示し、例えば、ダニ、昆虫などの卵、若虫、幼虫、成虫に対して優れた防除効果を示す。 The insecticide or acaricide of the present invention is excellent in controlling pests such as various agricultural pests and mites that affect the growth of plants. The insecticide or acaricide of the present invention is effective not only for susceptible strains but also for pests of strains that have developed resistance to conventional agents such as organophosphorus agents and carbamate agents. Representative examples of pests of resistant strains include diamondback moth, planthopper, leafhopper and aphids.
Moreover, the pyridine compound according to the present invention is effective at all stages of development of organisms to be controlled, and exhibits an excellent control effect on eggs, nymphs, larvae and adults such as mites and insects.
本発明の有害生物防除剤は、農業害虫、ダニ類以外の有害生物の防除に効果を示す。有害生物として、たとえば、外部寄生虫、衛生害虫などを挙げることができる。
The pest control agent of the present invention is effective for controlling pests other than agricultural pests and mites. Examples of pests include ectoparasites and sanitary pests.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、穀物類;野菜類;根菜類;イモ類;果樹類、茶、コーヒー、カカオなどの樹木類;牧草類;芝類;ワタなどの植物に対して用いることが好ましい。
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、植物類の各部位、たとえば、葉、茎、柄、花、蕾、果実、種子、スプラウト、根、塊茎、塊根、苗条、挿し木などに施用することができる。また、これら植物類の改良品種・変種、栽培品種、さらには突然変異体、ハイブリッド体、遺伝子組み換え体(GMO)を対象とすることもできる。 The agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention include grains; vegetables; root vegetables; potatoes; trees such as fruit trees, tea, coffee, cacao; Turf; preferably used for plants such as cotton.
The agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention are plant parts such as leaves, stems, stalks, flowers, buds, fruits, seeds, sprout, roots, tubers, It can be applied to tuberous roots, shoots and cuttings. In addition, improved varieties and varieties of these plants, cultivated varieties, and mutants, hybrids, and genetically modified organisms (GMO) can also be targeted.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、植物類の各部位、たとえば、葉、茎、柄、花、蕾、果実、種子、スプラウト、根、塊茎、塊根、苗条、挿し木などに施用することができる。また、これら植物類の改良品種・変種、栽培品種、さらには突然変異体、ハイブリッド体、遺伝子組み換え体(GMO)を対象とすることもできる。 The agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention include grains; vegetables; root vegetables; potatoes; trees such as fruit trees, tea, coffee, cacao; Turf; preferably used for plants such as cotton.
The agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention are plant parts such as leaves, stems, stalks, flowers, buds, fruits, seeds, sprout, roots, tubers, It can be applied to tuberous roots, shoots and cuttings. In addition, improved varieties and varieties of these plants, cultivated varieties, and mutants, hybrids, and genetically modified organisms (GMO) can also be targeted.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、花卉、芝、牧草を含む農園芸作物に発生する種々の病害の防除をするために行われる種子処理、茎葉散布、土壌施用、水面施用などに使用することができる。
The agricultural and horticultural fungicides, pesticides, and insecticides or acaricides of the present invention are seed treatments and foliage that are used to control various diseases occurring in agricultural and horticultural crops including flowers, turf, and grass. It can be used for spraying, soil application, water surface application, etc.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、殺菌、殺虫・殺ダニ、殺線虫、殺土壌害虫などの効果を有する他の農園芸用薬剤; 植物生長調節剤、共力剤、肥料、土壌改良剤、動物用飼料などと混用または併用してもよい。
以下にその一例を示す。 The agricultural and horticultural fungicide, pest control agent, and insecticide or acaricide of the present invention are other agricultural and horticultural agents having effects such as bactericidal, insecticidal / miticidal, nematicidal, soil-killing insect pests; You may mix or use together with a regulator, a synergist, a fertilizer, a soil conditioner, animal feed, etc.
An example is shown below.
以下にその一例を示す。 The agricultural and horticultural fungicide, pest control agent, and insecticide or acaricide of the present invention are other agricultural and horticultural agents having effects such as bactericidal, insecticidal / miticidal, nematicidal, soil-killing insect pests; You may mix or use together with a regulator, a synergist, a fertilizer, a soil conditioner, animal feed, etc.
An example is shown below.
殺菌剤:
(1)核酸生合成阻害剤:
(a)RNAポリメラーゼI阻害剤: ベナラキシル、ベナラキシル-M、フララキシル、メタラキシル、メタラキシル-M;オキサジキシル;クロジラコン、オフレース;
(b)アデノシンデアミナーゼ阻害剤: ブピリメート、ジメチリモール、エチリモール;
(c)DNA/RNA合成阻害剤: ハイメキサゾール、オクチリノン;
(d)DNAトポイソメラーゼII阻害剤: オキソリン酸; Fungicide:
(1) Nucleic acid biosynthesis inhibitors:
(A) RNA polymerase I inhibitor: benalaxyl, benalaxyl-M, furaxyl, metalaxyl, metalaxyl-M; oxadixil; cloziracone, off-race;
(B) adenosine deaminase inhibitor: bupilimate, dimethylylmol, ethylimol;
(C) DNA / RNA synthesis inhibitors: Himexazole, octirinone;
(D) DNA topoisomerase II inhibitor: oxophosphate;
(1)核酸生合成阻害剤:
(a)RNAポリメラーゼI阻害剤: ベナラキシル、ベナラキシル-M、フララキシル、メタラキシル、メタラキシル-M;オキサジキシル;クロジラコン、オフレース;
(b)アデノシンデアミナーゼ阻害剤: ブピリメート、ジメチリモール、エチリモール;
(c)DNA/RNA合成阻害剤: ハイメキサゾール、オクチリノン;
(d)DNAトポイソメラーゼII阻害剤: オキソリン酸; Fungicide:
(1) Nucleic acid biosynthesis inhibitors:
(A) RNA polymerase I inhibitor: benalaxyl, benalaxyl-M, furaxyl, metalaxyl, metalaxyl-M; oxadixil; cloziracone, off-race;
(B) adenosine deaminase inhibitor: bupilimate, dimethylylmol, ethylimol;
(C) DNA / RNA synthesis inhibitors: Himexazole, octirinone;
(D) DNA topoisomerase II inhibitor: oxophosphate;
(2)有糸核分裂阻害剤および細胞分裂阻害剤:
(a)β-チューブリン重合阻害剤: ベノミル、カルベンダジム、クロルフェナゾール、フベリダゾール、チアベンダゾール;チオファネート、チオファネートメチル(thiophanate-methyl);ジエトフェンカルブ;ゾキサミド;エタボキサム;
(b)細胞分裂阻害剤: ペンシクロン;
(c)スペクトリン様タンパク質の非局在化阻害剤: フルオピコリド; (2) Mitotic fission inhibitor and cell division inhibitor:
(A) β-tubulin polymerization inhibitor: benomyl, carbendazim, chlorphenazole, fuberidazole, thiabendazole; thiophanate, thiophanate-methyl; dietofencarb; zoxamide; ethaboxam;
(B) Cell division inhibitor: Penciclone;
(C) Delocalization inhibitor of spectrin-like protein: fluopicolide;
(a)β-チューブリン重合阻害剤: ベノミル、カルベンダジム、クロルフェナゾール、フベリダゾール、チアベンダゾール;チオファネート、チオファネートメチル(thiophanate-methyl);ジエトフェンカルブ;ゾキサミド;エタボキサム;
(b)細胞分裂阻害剤: ペンシクロン;
(c)スペクトリン様タンパク質の非局在化阻害剤: フルオピコリド; (2) Mitotic fission inhibitor and cell division inhibitor:
(A) β-tubulin polymerization inhibitor: benomyl, carbendazim, chlorphenazole, fuberidazole, thiabendazole; thiophanate, thiophanate-methyl; dietofencarb; zoxamide; ethaboxam;
(B) Cell division inhibitor: Penciclone;
(C) Delocalization inhibitor of spectrin-like protein: fluopicolide;
(3)呼吸阻害剤:
(a)複合体I NADH酸化還元酵素阻害剤: ジフルメトリム;トルフェンピラド;
(b)複合体IIコハク酸脱水素酵素阻害剤: ベノダニル、フルトラニル、メプロニル;イソフェタミド;フルオピラム;フェンフラム、フルメシクロックス;カルボキシン、オキシカルボキシン;チフルザミド;ベンゾビンジフルピル、ビキサフェン、フルキサピロキサド、フラメトピル、イソピラザム、ペンフルフェン、ペンチオピラド、セダキサン;ボスカリド;
(c)複合体IIIユビキノールオキシダーゼQo阻害剤: アゾキシストロビン、クモキシストロビン、クメトキシストロビン、エノキサストロビン、フルフェノキシストロビン、ピクオキシストロビン、ピラオキシストロビン;ピラクロストロビン、ピラメトストロビン、トリクロピリカルブ;クレソキシム-メチル、トリフロキシストロビン;ジモキシストロビン、フェナミンストロビン、メトミノストロビン、オリサストロビン;ファモキサドン;フルオキサストロビン;フェンアミドン;ピリベンカルブ;
(d)複合体IIIユビキノール還元酵素Qi阻害剤: シアゾファミド;アミスルブロム;
(e)酸化的リン酸化の脱共役剤: ビナパクリル、メプチルジノカップ、ジノカップ;フルアジナム;フェリムゾン;
(f)酸化的リン酸化阻害剤(ATP 合成酵素の阻害剤): フェンチンアセテート、塩化フェンチン、水酸化フェンチン;
(g)ATP生産阻害剤: シルチオファム;
(h)複合体III:チロクロームbc1(ユビキノン還元酵素)のQx(未知)阻害剤: アメトクトラジン; (3) Respiratory inhibitor:
(A) Complex I NADH oxidoreductase inhibitor: diflumetrim; tolfenpyrad;
(B) Complex II succinate dehydrogenase inhibitors: benodanyl, flutolanil, mepronil; isofetamide; fluopyram; fenfram, flumecyclox; carboxin, oxycarboxyl; tifluzamide; , Furametopyr, isopyrazam, penflufen, penthiopyrad, sedaxane; boscalid;
(C) Complex III ubiquinol oxidase Qo inhibitor: azoxystrobin, cumoxystrobin, cumethoxystrobin, enoxastrobin, fluphenoxystrobin, picoxystrobin, pyroxystrobin; Piramethostrobin, triclopyricarb; Cresoxime-methyl, trifloxystrobin; Dimoxystrobin, Phenaminestrobin, Metominostrobin, Orisastrobin; Famoxadone; Fluoxastrobin; Fenamidon;
(D) Complex III ubiquinol reductase Qi inhibitor: cyazofamide; amisulbrom;
(E) Uncoupler of oxidative phosphorylation: Binapacryl, meptyldinocup, dinocup; fluazinam; ferrimzone;
(F) Oxidative phosphorylation inhibitor (inhibitor of ATP synthase): fentin acetate, fentin chloride, fentin hydroxide;
(G) ATP production inhibitor: silthiofam;
(H) Complex III: Qx (unknown) inhibitor of cytochrome bc1 (ubiquinone reductase): Amethoctrazine;
(a)複合体I NADH酸化還元酵素阻害剤: ジフルメトリム;トルフェンピラド;
(b)複合体IIコハク酸脱水素酵素阻害剤: ベノダニル、フルトラニル、メプロニル;イソフェタミド;フルオピラム;フェンフラム、フルメシクロックス;カルボキシン、オキシカルボキシン;チフルザミド;ベンゾビンジフルピル、ビキサフェン、フルキサピロキサド、フラメトピル、イソピラザム、ペンフルフェン、ペンチオピラド、セダキサン;ボスカリド;
(c)複合体IIIユビキノールオキシダーゼQo阻害剤: アゾキシストロビン、クモキシストロビン、クメトキシストロビン、エノキサストロビン、フルフェノキシストロビン、ピクオキシストロビン、ピラオキシストロビン;ピラクロストロビン、ピラメトストロビン、トリクロピリカルブ;クレソキシム-メチル、トリフロキシストロビン;ジモキシストロビン、フェナミンストロビン、メトミノストロビン、オリサストロビン;ファモキサドン;フルオキサストロビン;フェンアミドン;ピリベンカルブ;
(d)複合体IIIユビキノール還元酵素Qi阻害剤: シアゾファミド;アミスルブロム;
(e)酸化的リン酸化の脱共役剤: ビナパクリル、メプチルジノカップ、ジノカップ;フルアジナム;フェリムゾン;
(f)酸化的リン酸化阻害剤(ATP 合成酵素の阻害剤): フェンチンアセテート、塩化フェンチン、水酸化フェンチン;
(g)ATP生産阻害剤: シルチオファム;
(h)複合体III:チロクロームbc1(ユビキノン還元酵素)のQx(未知)阻害剤: アメトクトラジン; (3) Respiratory inhibitor:
(A) Complex I NADH oxidoreductase inhibitor: diflumetrim; tolfenpyrad;
(B) Complex II succinate dehydrogenase inhibitors: benodanyl, flutolanil, mepronil; isofetamide; fluopyram; fenfram, flumecyclox; carboxin, oxycarboxyl; tifluzamide; , Furametopyr, isopyrazam, penflufen, penthiopyrad, sedaxane; boscalid;
(C) Complex III ubiquinol oxidase Qo inhibitor: azoxystrobin, cumoxystrobin, cumethoxystrobin, enoxastrobin, fluphenoxystrobin, picoxystrobin, pyroxystrobin; Piramethostrobin, triclopyricarb; Cresoxime-methyl, trifloxystrobin; Dimoxystrobin, Phenaminestrobin, Metominostrobin, Orisastrobin; Famoxadone; Fluoxastrobin; Fenamidon;
(D) Complex III ubiquinol reductase Qi inhibitor: cyazofamide; amisulbrom;
(E) Uncoupler of oxidative phosphorylation: Binapacryl, meptyldinocup, dinocup; fluazinam; ferrimzone;
(F) Oxidative phosphorylation inhibitor (inhibitor of ATP synthase): fentin acetate, fentin chloride, fentin hydroxide;
(G) ATP production inhibitor: silthiofam;
(H) Complex III: Qx (unknown) inhibitor of cytochrome bc1 (ubiquinone reductase): Amethoctrazine;
(4)アミノ酸およびタンパク質合成阻害剤
(a)メチオニン生合成阻害剤: アンドプリム、シプロジニル、メパニピリム、ピリメタニル;
(b)タンパク質合成阻害剤: ブラストサイジン-S;カスガマイシン、カスガマイシン塩酸塩;ストレプトマイシン;オキシテトラサイクリン; (4) Amino acid and protein synthesis inhibitors (a) Methionine biosynthesis inhibitors: Andoprim, cyprodinil, mepanipyrim, pyrimethanil;
(B) Protein synthesis inhibitor: blasticidin-S; kasugamycin, kasugamycin hydrochloride; streptomycin; oxytetracycline;
(a)メチオニン生合成阻害剤: アンドプリム、シプロジニル、メパニピリム、ピリメタニル;
(b)タンパク質合成阻害剤: ブラストサイジン-S;カスガマイシン、カスガマイシン塩酸塩;ストレプトマイシン;オキシテトラサイクリン; (4) Amino acid and protein synthesis inhibitors (a) Methionine biosynthesis inhibitors: Andoprim, cyprodinil, mepanipyrim, pyrimethanil;
(B) Protein synthesis inhibitor: blasticidin-S; kasugamycin, kasugamycin hydrochloride; streptomycin; oxytetracycline;
(5)シグナル伝達阻害剤:
(a)シグナル伝達阻害剤: キノキシフェン、プロキナジド;
(b)浸透圧シグナル伝達におけるMAP・ヒスチジンキナーゼ阻害剤: フェンピクロニル、フルジオキソニル;クロゾリメート、イプロジオン、プロシミドン、ビンクロゾリン; (5) Signaling inhibitor:
(A) Signaling inhibitor: quinoxyphene, proquinazide;
(B) MAP • histidine kinase inhibitor in osmotic signal transduction: fenpiclonil, fludioxonil; clozolimate, iprodione, procymidone, vinclozolin;
(a)シグナル伝達阻害剤: キノキシフェン、プロキナジド;
(b)浸透圧シグナル伝達におけるMAP・ヒスチジンキナーゼ阻害剤: フェンピクロニル、フルジオキソニル;クロゾリメート、イプロジオン、プロシミドン、ビンクロゾリン; (5) Signaling inhibitor:
(A) Signaling inhibitor: quinoxyphene, proquinazide;
(B) MAP • histidine kinase inhibitor in osmotic signal transduction: fenpiclonil, fludioxonil; clozolimate, iprodione, procymidone, vinclozolin;
(6)脂質および細胞膜合成阻害剤:
(a)りん脂質生合成、メチルトランス-フェラーゼ阻害剤: エジフェンホス、イプロベンホス、ピラゾホス;イソプロチオラン;
(b)脂質の過酸化剤: ビフェニル、クロロネブ、ジクロラン、キンドゼン、テクナゼン、トルクロホスメチル;エトリジアゾール;
(c)細胞膜に作用する剤: ヨードカルブ、プロパモカルブ、プロパモカルブ塩酸塩、プロパモカルブホセチレート、プロチオカルブ;
(d)病原菌細胞膜を撹乱する微生物: バチルスズブチリス菌、バチルス ズブチリスQST713 株、バチルス ズブチリスFZB24 株、バチルス ズブチリスMBI600 株、バチルス ズブチリスD747株;
(e)細胞膜を撹乱する剤: ゴセイカユプテ(ティーツリー)の抽出物; (6) Lipid and cell membrane synthesis inhibitors:
(A) Phospholipid biosynthesis, methyltransferase-inhibitors: edifenphos, iprobenphos, pyrazophos; isoprothiolane;
(B) lipid peroxidants: biphenyl, chloroneb, dichlorane, kinden, technazene, tolcrofosmethyl; etridiazole;
(C) Agents that act on cell membranes: iodocarb, propamocarb, propamocarb hydrochloride, propamocarbfocetylate, prothiocarb;
(D) Microorganisms that disturb the cell membrane of pathogenic bacteria: Bacillus subtilis, Bacillus subtilis QST713 strain, Bacillus subtilis FZB24 strain, Bacillus subtilis MBI600 strain, Bacillus subtilis D747 strain;
(E) Agents that disrupt the cell membrane: an extract of Goseika Yupte (Tea Tree);
(a)りん脂質生合成、メチルトランス-フェラーゼ阻害剤: エジフェンホス、イプロベンホス、ピラゾホス;イソプロチオラン;
(b)脂質の過酸化剤: ビフェニル、クロロネブ、ジクロラン、キンドゼン、テクナゼン、トルクロホスメチル;エトリジアゾール;
(c)細胞膜に作用する剤: ヨードカルブ、プロパモカルブ、プロパモカルブ塩酸塩、プロパモカルブホセチレート、プロチオカルブ;
(d)病原菌細胞膜を撹乱する微生物: バチルスズブチリス菌、バチルス ズブチリスQST713 株、バチルス ズブチリスFZB24 株、バチルス ズブチリスMBI600 株、バチルス ズブチリスD747株;
(e)細胞膜を撹乱する剤: ゴセイカユプテ(ティーツリー)の抽出物; (6) Lipid and cell membrane synthesis inhibitors:
(A) Phospholipid biosynthesis, methyltransferase-inhibitors: edifenphos, iprobenphos, pyrazophos; isoprothiolane;
(B) lipid peroxidants: biphenyl, chloroneb, dichlorane, kinden, technazene, tolcrofosmethyl; etridiazole;
(C) Agents that act on cell membranes: iodocarb, propamocarb, propamocarb hydrochloride, propamocarbfocetylate, prothiocarb;
(D) Microorganisms that disturb the cell membrane of pathogenic bacteria: Bacillus subtilis, Bacillus subtilis QST713 strain, Bacillus subtilis FZB24 strain, Bacillus subtilis MBI600 strain, Bacillus subtilis D747 strain;
(E) Agents that disrupt the cell membrane: an extract of Goseika Yupte (Tea Tree);
(7)細胞膜のステロール生合成阻害剤:
(a)ステロール生合成におけるC14位の脱メチル化阻害剤: トリホリン;ピリフェノックス、ピリイソキサゾール;フェナリモル、フルルプリミドール、ヌアリモル;イマザリル、イマザリル硫酸塩、オキスポコナゾール、ペフラゾエート、プロクロラズ、トリフルミゾール、ビニコナゾール;
アザコナゾール、ビテルタノール、ブロムコナゾール、シプロコナゾール、ジクロブトラゾール、ジフェノコナゾール、ジニコナゾール、ジニコナゾール-M、エポキシコナゾール、エタコナゾール、フェンブコナゾール、フルキンコナゾール、フルシラゾール、フルトリアホール)、フルコナゾール、フルコナゾール-シス、ヘキサコナゾール、イミベンコナゾール、イプコナゾール、メトコナゾール、ミクロブタニル、ペンコナゾール、プロピコナゾール、キンコナゾール、シメコナゾール、テブコナゾール、テトラコナゾール、トリアジメホン、トリアジメノール、トリチコナゾール;プロチオコナゾール、ボリコナゾール;
(b)ステロール生合成におけるΔ14還元酵素およびΔ8→Δ7-イソメラーゼの阻害剤: アルジモルフ、ドデモルフ、ドデモルフ酢酸塩、フェンプロピモルフ、トリデモルフ;フェンプロピジン、ピペラリン;スピロキサミン;
(c)ステロール生合成系のC4位脱メチル化における3-ケト還元酵素阻害剤: フェンヘキサミド;フェンピラザミン;
(d)ステロール生合成系のスクワレンエポキシダーゼ阻害剤: ピリブチカルブ;ナフチフェン、テルビナフィン; (7) Cell membrane sterol biosynthesis inhibitors:
(A) Demethylation inhibitor at the C14 position in sterol biosynthesis: Trifolin; Triflumizole, biniconazole;
Azaconazole, viteltanol, bromconazole, cyproconazole, diclobutrazole, difenoconazole, diniconazole, diniconazole-M, epoxiconazole, etaconazole, fenbuconazole, fluquinconazole, flusilazole, flutriazole), fluconazole, fluconazole- Cis, hexaconazole, imibenconazole, ipconazole, metconazole, microbutanyl, penconazole, propiconazole, quinconazole, cimeconazole, tebuconazole, tetraconazole, triadimethone, triadimenol, triticonazole; prothioconazole, voriconazole;
(B) Inhibitors of Δ14 reductase and Δ8 → Δ7-isomerase in sterol biosynthesis: aldimorph, dodemorph, dodemorph acetate, fenpropimorph, tridemorph; fenpropidin, piperalin; spiroxamine;
(C) 3-keto reductase inhibitor in C4-demethylation of sterol biosynthesis system: phenhexamide; fenpyrazamine;
(D) Sterol biosynthetic squalene epoxidase inhibitors: Pyributicarb; Naftifen, Terbinafine;
(a)ステロール生合成におけるC14位の脱メチル化阻害剤: トリホリン;ピリフェノックス、ピリイソキサゾール;フェナリモル、フルルプリミドール、ヌアリモル;イマザリル、イマザリル硫酸塩、オキスポコナゾール、ペフラゾエート、プロクロラズ、トリフルミゾール、ビニコナゾール;
アザコナゾール、ビテルタノール、ブロムコナゾール、シプロコナゾール、ジクロブトラゾール、ジフェノコナゾール、ジニコナゾール、ジニコナゾール-M、エポキシコナゾール、エタコナゾール、フェンブコナゾール、フルキンコナゾール、フルシラゾール、フルトリアホール)、フルコナゾール、フルコナゾール-シス、ヘキサコナゾール、イミベンコナゾール、イプコナゾール、メトコナゾール、ミクロブタニル、ペンコナゾール、プロピコナゾール、キンコナゾール、シメコナゾール、テブコナゾール、テトラコナゾール、トリアジメホン、トリアジメノール、トリチコナゾール;プロチオコナゾール、ボリコナゾール;
(b)ステロール生合成におけるΔ14還元酵素およびΔ8→Δ7-イソメラーゼの阻害剤: アルジモルフ、ドデモルフ、ドデモルフ酢酸塩、フェンプロピモルフ、トリデモルフ;フェンプロピジン、ピペラリン;スピロキサミン;
(c)ステロール生合成系のC4位脱メチル化における3-ケト還元酵素阻害剤: フェンヘキサミド;フェンピラザミン;
(d)ステロール生合成系のスクワレンエポキシダーゼ阻害剤: ピリブチカルブ;ナフチフェン、テルビナフィン; (7) Cell membrane sterol biosynthesis inhibitors:
(A) Demethylation inhibitor at the C14 position in sterol biosynthesis: Trifolin; Triflumizole, biniconazole;
Azaconazole, viteltanol, bromconazole, cyproconazole, diclobutrazole, difenoconazole, diniconazole, diniconazole-M, epoxiconazole, etaconazole, fenbuconazole, fluquinconazole, flusilazole, flutriazole), fluconazole, fluconazole- Cis, hexaconazole, imibenconazole, ipconazole, metconazole, microbutanyl, penconazole, propiconazole, quinconazole, cimeconazole, tebuconazole, tetraconazole, triadimethone, triadimenol, triticonazole; prothioconazole, voriconazole;
(B) Inhibitors of Δ14 reductase and Δ8 → Δ7-isomerase in sterol biosynthesis: aldimorph, dodemorph, dodemorph acetate, fenpropimorph, tridemorph; fenpropidin, piperalin; spiroxamine;
(C) 3-keto reductase inhibitor in C4-demethylation of sterol biosynthesis system: phenhexamide; fenpyrazamine;
(D) Sterol biosynthetic squalene epoxidase inhibitors: Pyributicarb; Naftifen, Terbinafine;
(8)細胞壁合成阻害
(a)トレハラーゼ阻害剤: バリダマイシン;
(b)キチン合成酵素阻害剤: ポリオキシン、ポリオクソリム;
(c)セルロース合成酵素阻害剤: ジメトモルフ、フルモルフ、ピリモルフ;ベンチアバリカルブ、イプロバリカルブ、トルプロカルブ、バリフェナレート;マンジプロパミド; (8) Cell wall synthesis inhibition (a) Trehalase inhibitor: Validamycin;
(B) chitin synthase inhibitor: polyoxin, polyoxolim;
(C) Cellulose synthase inhibitor: dimethomorph, fulmorph, pyrimorph; Bench Avaricarb, Iprovaricarb, Toluprocarb, Variphenate; Mandipropamide;
(a)トレハラーゼ阻害剤: バリダマイシン;
(b)キチン合成酵素阻害剤: ポリオキシン、ポリオクソリム;
(c)セルロース合成酵素阻害剤: ジメトモルフ、フルモルフ、ピリモルフ;ベンチアバリカルブ、イプロバリカルブ、トルプロカルブ、バリフェナレート;マンジプロパミド; (8) Cell wall synthesis inhibition (a) Trehalase inhibitor: Validamycin;
(B) chitin synthase inhibitor: polyoxin, polyoxolim;
(C) Cellulose synthase inhibitor: dimethomorph, fulmorph, pyrimorph; Bench Avaricarb, Iprovaricarb, Toluprocarb, Variphenate; Mandipropamide;
(9)メラニン生合成阻害剤
(a)メラニン生合成の還元酵素阻害剤: フサライド;ピロキロン;トリシクラゾール;
(b)メラニン生合成の脱水酵素阻害剤: カルプロパミド;ジクロシメット;フェノキサニル; (9) Melanin biosynthesis inhibitor (a) Reductase inhibitor of melanin biosynthesis: Fusaride; Pyroxylone; Tricyclazole;
(B) Dehydrase inhibitor of melanin biosynthesis: carpropamide; diclocimet; phenoxanyl;
(a)メラニン生合成の還元酵素阻害剤: フサライド;ピロキロン;トリシクラゾール;
(b)メラニン生合成の脱水酵素阻害剤: カルプロパミド;ジクロシメット;フェノキサニル; (9) Melanin biosynthesis inhibitor (a) Reductase inhibitor of melanin biosynthesis: Fusaride; Pyroxylone; Tricyclazole;
(B) Dehydrase inhibitor of melanin biosynthesis: carpropamide; diclocimet; phenoxanyl;
(10)宿主植物の抵抗性誘導剤:
(a)サリチル酸合成経路に作用する剤: アシベンゾラル-S-メチル;
(b)その他: プロベナゾール;チアジニル;イソチアニル;ラミナリン;オオイタドリ抽出液; (10) Host plant resistance inducer:
(A) Agents acting on the salicylic acid synthesis pathway: Acibenzoral-S-methyl;
(B) Others: Probenazole; thiazinyl; isotianil; laminarin;
(a)サリチル酸合成経路に作用する剤: アシベンゾラル-S-メチル;
(b)その他: プロベナゾール;チアジニル;イソチアニル;ラミナリン;オオイタドリ抽出液; (10) Host plant resistance inducer:
(A) Agents acting on the salicylic acid synthesis pathway: Acibenzoral-S-methyl;
(B) Others: Probenazole; thiazinyl; isotianil; laminarin;
(11)作用性が不明な剤: シモキサニル、ホセチルアルミニウム、リン酸(リン酸塩)、テクロフタラム、トリアゾキシド、フルスルファミド、ジクロメジン、メタスルホカルブ、シフルフェナミド、メトラフェノン、ピリオフェノン、ドジン、ドジン遊離塩基、フルチアニル;
(11) Agents with unknown activity: Simoxanyl, fosetylaluminum, phosphoric acid (phosphate), teclophthalam, triazoxide, fursulfamide, dichromedin, metasulfocarb, cyflufenamide, metolaphenone, pyriophenone, dodin, dodin free base, fluthianyl;
(12)多作用点を有する剤: 銅(銅塩)、ボルドー液、水酸化銅、銅ナフタレート、酸化銅、オキシ塩化銅、硫酸銅、硫黄、硫黄製品、多硫化カルシウム;ファーバム、マンコゼブ、マネブ、マンカッパー、メチラム、ポリカーバメート、プロピネブ、チラム、ジネブ、ジラム;キャプタン、カプタホール、フォルペット;クロロタロニル;ジクロフルアニド、トリルフルアニド;グアザチン、イミノクタジン酢酸塩、イミノクタジンアルベシル酸塩;アニラジン;ジチアノン;キノメチオネート;フルオルイミド;
(12) Agents having multiple action points: copper (copper salt), Bordeaux liquid, copper hydroxide, copper naphthalate, copper oxide, copper oxychloride, copper sulfate, sulfur, sulfur products, calcium polysulfide; farbum, mancozeb, maneb, Mankappa, methylam, polycarbamate, propineb, thiram, dineb, ziram; captan, captahol, phorpet; chlorothalonil; diclofluuride, tolylfluanid; guazatine, iminotadine acetate, iminoctadine albecate; anilazine; dithianone; Fluorimide;
(13)その他の剤: DBEDC、フルオロフォルペット、グアザチンアセテート、ビス(8-キノリノラト)銅(II)、プロパミジン、クロロピクリン、シプロフラム、アグロバクテリウム、ベトキサジン、ジフェニルアミン、メチルイソチアネート(MITC)、ミルデオマイシン、カプサイシン、クフラネブ、シプロスルファミド、ダゾメット、デバカルブ、ジクロロフェン、ジフェンゾクワット、ジフェンゾクワットメチルスルホネート、フルメトベル、ホセチルカルシウム、ホセチルナトリウム、イルママイシン、ナタマイシン、ニトロタールイソプロピル、オキサモカルブ、プロパモシンナトリウム、ピロールニトリン、テブフロキン、トルニファニド、ザリラミド、アルゴフェーズ(Algophase)、アミカルチアゾール(Amicarthiazol)、オキサチアピプロリン(Oxathiapiprolin)、メチラム亜鉛、ベンチアゾール、トリクラミド、ユニコナゾール、ミルデオマイシン、オキシフェンチイン(Oxyfenthiin)、ピカルブトラゾクス(picarbutrazox);
(13) Other agents: DBEDC, fluorophorpet, guazatine acetate, bis (8-quinolinolato) copper (II), propamidine, chloropicrin, ciprofuram, Agrobacterium, betoxazine, diphenylamine, methyl isothiocyanate (MITC) ), Mildeomycin, Capsaicin, Cufraneb, Cyprosulfamide, Dazomet, Debacarb, Dichlorophen, Diphenzoquat, Diphenzoquat methylsulfonate, Flumetober, Focetyl calcium, Focetyl sodium, Irumamycin, Natamycin, Nitrotal isopropyl , Oxamocarb, sodium propamosine, pyrrolnitrin, tebufloquine, torniphanide, zaliramide, Algophase, amicalhiazole (Amicarthiazol), oxachi Pipurorin (Oxathiapiprolin), metiram zinc, benches azole, trichlamide, uniconazole, mill Deo mycin, oxyphencyclimine Ji-in (Oxyfenthiin), Pical -but Ratho box (picarbutrazox);
殺虫・殺ダニ剤、殺線虫剤、殺土壌害虫剤:
(1)アセチルコリンエステラーゼ阻害剤:
(a)カーバメート系: アラニカルブ、アルジカルブ、ベンジオカルブ、ベンフラカルブ、ブトカルボキシム、ブトキシカルボキシム、カルバリル、カルボフラン、カルボスルファン、エチオフェンカルブ、フェノブカルブ、ホルメタネート、フラチオカルブ、イソプロカルブ、メチオカルブ、メソミル、オキサミル、ピリミカルブ、プロポキサル、チオジカルブ、チオファノックス、トリアザメート、トリメタカルブ、XMC、キシリルカルブ;フェノチオカルブ、MIPC、MPMC、MTMC、アルドキシカルブ、アリキシカルブ、アミノカルブ、ブフェンカルブ、クロエトカルブ、メタム・ナトリウム、プロメカルブ; Insecticides, acaricides, nematicides, soil insecticides:
(1) Acetylcholinesterase inhibitor:
(A) Carbamate series: Alanicarb, Aldicarb, Bengiocarb, Benfuracarb, Butcarboxyme, Butoxycarboxyme, Carbaryl, Carbofuran, Carbosulfan, Ethiophenecarb, Fenobucarb, Formethanate, Furatiocarb, Isoprocarb, Methiocarb, Mesomil, Oxamyl, Pirimicarb, Propoxycarb Thiodicarb, thiophanox, triazamate, trimetacarb, XMC, xylylcarb; phenothiocarb, MIPC, MPMC, MTMC, aldoxicarb, alixicarb, aminocarb, bufencarb, cloetocarb, metam sodium, promecarb;
(1)アセチルコリンエステラーゼ阻害剤:
(a)カーバメート系: アラニカルブ、アルジカルブ、ベンジオカルブ、ベンフラカルブ、ブトカルボキシム、ブトキシカルボキシム、カルバリル、カルボフラン、カルボスルファン、エチオフェンカルブ、フェノブカルブ、ホルメタネート、フラチオカルブ、イソプロカルブ、メチオカルブ、メソミル、オキサミル、ピリミカルブ、プロポキサル、チオジカルブ、チオファノックス、トリアザメート、トリメタカルブ、XMC、キシリルカルブ;フェノチオカルブ、MIPC、MPMC、MTMC、アルドキシカルブ、アリキシカルブ、アミノカルブ、ブフェンカルブ、クロエトカルブ、メタム・ナトリウム、プロメカルブ; Insecticides, acaricides, nematicides, soil insecticides:
(1) Acetylcholinesterase inhibitor:
(A) Carbamate series: Alanicarb, Aldicarb, Bengiocarb, Benfuracarb, Butcarboxyme, Butoxycarboxyme, Carbaryl, Carbofuran, Carbosulfan, Ethiophenecarb, Fenobucarb, Formethanate, Furatiocarb, Isoprocarb, Methiocarb, Mesomil, Oxamyl, Pirimicarb, Propoxycarb Thiodicarb, thiophanox, triazamate, trimetacarb, XMC, xylylcarb; phenothiocarb, MIPC, MPMC, MTMC, aldoxicarb, alixicarb, aminocarb, bufencarb, cloetocarb, metam sodium, promecarb;
(b)有機リン系: アセフェート、アザメチホス、アジンホス-エチル、アジンホス-メチル、カズサホス、クロルエトキシホス、クロルフェンビンホス、クロルメホス、クロルピリホス、クロルピリホス-メチル、クマホス、シアノホス、ジメトン-S-メチル、ダイアジノン、ジクロルボス/DDVP、ジクロトホス、ジメトエート、ジメチルビンホス、ジスルホトン、EPN、エチオン、エトプロホス、ファムフール、フェナミホス、フェニトロチオン、フェンチオン、ホスチアゼート、ヘプテノホス、イミシアホス、イソフェンホス、イソカルボホス、イソキサチオン、マラチオン、メカルバム、メタミドホス、メチダチオン、メビンホス、モノクロトホス、ナレド、オメトエート、オキシジメトン-メチル、パラチオン、パラチオン-メチル、フェントエート、ホレート、ホサロン、ホスメット、ホスファミドン、ホキシム、ピリミホス-メチル、プロフェノホス、プロペタムホス、プロチオホス、ピラクロホス、ピリダフェンチオン、キナルホス、スルホテップ、テブピリンホス、テメホス、テルブホス、テトラクロルビンホス、チオメトン、トリアゾホス、トリクロルホン、バミドチオン;ブロモホス・エチル、BRP、カルボフェノチオン、シアノフェンホス、CYAP、ジメトン-S-メチルスルホン、ジアリホス、ジクロフェンチオン、ジオキサベンゾホス、エトリムホス、フェンスルホチオン、フルピラゾホス、ホノホス、ホルモチオン、ホスメチラン、イサゾホス、ヨードフェンホス、メタクリホス、ピリミホス-エチル、ホスホカルブ、プロパホス、プロトエート、スルプロホス;
(B) Organophosphorus: Acephate, azamethiphos, azinephos-ethyl, azinephos-methyl, kazusafos, chlorethoxyphos, chlorfenvinphos, chlormefos, chlorpyrifos, chlorpyrifos-methyl, coumaphos, cyanophos, dimethone-S-methyl, diazinon, Dichlorvos / DDVP, dicrotophos, dimethoate, dimethylvinphos, disulfoton, EPN, ethione, etoprophos, famfur, phenamiphos, fenitrothion, fenthion, phostiazet, heptenophos, imisiaphos, isofenphos, isocarbophos, isoxathione, malathione, methalmethione, mecarbamethione Monocrotophos, nared, ometoate, oxydimethone-methyl, parathion, parathion-methyl, fento Ate, folate, hosalon, phosmet, phosphamidone, phoxime, pyrimiphos-methyl, propenophos, propetamphos, prothiophos, pyracrofos, pyridafenthion, quinalphos, sulfotep, tebupyrine phos, temefos, terbufos, tetrachlorbinphos, thiomethone, triazophos, trichlorfos, bamidthione;・ Ethyl, BRP, carbophenothion, cyanophenphos, CYAP, dimeton-S-methylsulfone, diariphos, diclofenthion, dioxabenzophos, etrimphos, fensulfothion, flupyrazophos, phonofos, formotethione, phosmethylan, isazophos, iodofenphos, methacliphos , Pyrimifos-ethyl, phosphocarb, propaphos, protoate, sulfophos;
(2)GABA-作動性塩素イオンチャネルアンタゴニスト: クロルデン、エンドスルファン、エチプロール、フィプロニル、ピラフルプロール、ピリプロール;カンフェクロル、ヘプタクロル、ジエノクロル;
(3)ナトリウムチャンネルモジュレーター: アクリナトリン、d-シス-トランス アレスリン、d-トランスアレスリン、ビフェントリン、ビオアレスリン、ビオアレスリンS-シクロペンチル異性体、ビオレスメトリン、シクロプロトリン、シフルトリン、ベータ-シフルトリン、シハロトリン、ラムダ-シハロトリン、ガンマ-シハロトリン、シペルメトリン、アルファ-シペルメトリン、ベータ-シペルメトリン、シータ-シペルメトリン、ゼータ-シペルメトリン、シフェノトリン[(1R)-トランス異性体]、デルタメトリン、エンペントリン[(EZ)-(1R)-異性体]、エスフェンバレレート、エトフェンプロックス、フェンプロパトリン、フェンバレレート、フルシトリネート、フルメトリン、タウ-フルバリネート、ハルフェンプロックス、イミプリトリン、カデスリン、ペルメトリン、フェノトリン[(1R)-トランス異性体]、プラレトリン、ピレスラム、レスメトリン、シラフルオフェン、テフルスリン、テトラメスリン、テトラメトリン[(1R)-異性体]、トラロメトリン、トランスフルトリン;アレスリン、ピレトリン、ピレトリンI、ピレトリンII、プロフルトリン、ジメフルトリン、ビオエタノメトリン、ビオペルメトリン、トランスペルメトリン、フェンフルトリン、フェンピリトリン、フルブロシトリネート、フルフェンプロックス、メトフルトリン、プロトリフェンブト、ピレスメトリン、テラレトリン; (2) GABA-agonist chloride channel antagonists: chlordane, endosulfan, etiprole, fipronil, pyrafluprole, pyriprole; camfechlor, heptachlor, dienochlor;
(3) Sodium channel modulators: Acrinatrin, d-cis-trans allethrin, d-transarethrin, bifenthrin, bioareslin, bioareslin isomers, violesmethrin, cycloprotorin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda- Cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, ciphenothrin [(1R) -trans isomer], deltamethrin, enpentrin [(EZ)-( 1R) -isomers], esfenvalerate, etofenprox, fenpropatoline, fenvalerate, flucitrinate, flumethrin, tau-fulvalinate, halfenprox, imiprito , Cadreslin, permethrin, phenothrin [(1R) -trans isomer], praretrin, pyrethram, resmethrin, silafluophene, tefluthrin, tetramethrin, tetramethrin [(1R) -isomer], tralomethrin, transfluthrin; allethrin, pyrethrin, pyrethrin I, pyrethrin II, profluthrin, dimefluthrin, bioethanomethrin, biopermethrin, transpermethrin, fenfluthrin, fenpyritrin, fulbrocitrinate, flufenprox, methfluthrin, profolifenbut, pyrethmethrin, terraretrin;
(3)ナトリウムチャンネルモジュレーター: アクリナトリン、d-シス-トランス アレスリン、d-トランスアレスリン、ビフェントリン、ビオアレスリン、ビオアレスリンS-シクロペンチル異性体、ビオレスメトリン、シクロプロトリン、シフルトリン、ベータ-シフルトリン、シハロトリン、ラムダ-シハロトリン、ガンマ-シハロトリン、シペルメトリン、アルファ-シペルメトリン、ベータ-シペルメトリン、シータ-シペルメトリン、ゼータ-シペルメトリン、シフェノトリン[(1R)-トランス異性体]、デルタメトリン、エンペントリン[(EZ)-(1R)-異性体]、エスフェンバレレート、エトフェンプロックス、フェンプロパトリン、フェンバレレート、フルシトリネート、フルメトリン、タウ-フルバリネート、ハルフェンプロックス、イミプリトリン、カデスリン、ペルメトリン、フェノトリン[(1R)-トランス異性体]、プラレトリン、ピレスラム、レスメトリン、シラフルオフェン、テフルスリン、テトラメスリン、テトラメトリン[(1R)-異性体]、トラロメトリン、トランスフルトリン;アレスリン、ピレトリン、ピレトリンI、ピレトリンII、プロフルトリン、ジメフルトリン、ビオエタノメトリン、ビオペルメトリン、トランスペルメトリン、フェンフルトリン、フェンピリトリン、フルブロシトリネート、フルフェンプロックス、メトフルトリン、プロトリフェンブト、ピレスメトリン、テラレトリン; (2) GABA-agonist chloride channel antagonists: chlordane, endosulfan, etiprole, fipronil, pyrafluprole, pyriprole; camfechlor, heptachlor, dienochlor;
(3) Sodium channel modulators: Acrinatrin, d-cis-trans allethrin, d-transarethrin, bifenthrin, bioareslin, bioareslin isomers, violesmethrin, cycloprotorin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda- Cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, ciphenothrin [(1R) -trans isomer], deltamethrin, enpentrin [(EZ)-( 1R) -isomers], esfenvalerate, etofenprox, fenpropatoline, fenvalerate, flucitrinate, flumethrin, tau-fulvalinate, halfenprox, imiprito , Cadreslin, permethrin, phenothrin [(1R) -trans isomer], praretrin, pyrethram, resmethrin, silafluophene, tefluthrin, tetramethrin, tetramethrin [(1R) -isomer], tralomethrin, transfluthrin; allethrin, pyrethrin, pyrethrin I, pyrethrin II, profluthrin, dimefluthrin, bioethanomethrin, biopermethrin, transpermethrin, fenfluthrin, fenpyritrin, fulbrocitrinate, flufenprox, methfluthrin, profolifenbut, pyrethmethrin, terraretrin;
(4)ニコチン性アセチルコリン受容体アゴニスト: アセタミプリド、クロチアニジン、ジノテフラン、イミダクロプリド、ニテンピラム、ニチアジン、チアクロプリド、チアメトキサム、スルフォキサフロール、ニコチン;フルピラジフロン;
(5)ニコチン性アセチルコリン受容体アロステリックモジュレーター: スピネトラム、スピノサド;
(6)クロライドチャンネル活性化剤: アバメクチン、エマメクチン安息香酸塩、レピメクチン、ミルベメクチン;イベルメクチン、セラメクチン、ドラメクチン、エプリノメクチン、モキシデクチン、ミルベマイシン、ミルベマイシンオキシム;
(7)幼若ホルモン様物質: ヒドロプレン、キノプレン、メソプレン、フェノキシカルブ、ピリプロキシフェン;ジオフェノラン、エポフェノナン、トリプレン;
(8)その他非特異的阻害剤: 臭化メチル、クロルピクリン、フッ化スルフリル、ホウ砂、吐酒石;
(9)同翅目選択的摂食阻害剤: フロニカミド、ピメトロジン、ピリフルキナゾン; (4) Nicotinic acetylcholine receptor agonists: acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, nithiazine, thiacloprid, thiamethoxam, sulfoxafurol, nicotine;
(5) Nicotinic acetylcholine receptor allosteric modulators: spinetoram, spinosad;
(6) Chloride channel activator: abamectin, emamectin benzoate, repimectin, milbemectin; ivermectin, selamectin, doramectin, eprinomectin, moxidectin, milbemycin, milbemycin oxime;
(7) Juvenile hormone-like substances: hydroprene, quinoprene, mesoprene, phenoxycarb, pyriproxyfen; geofenolan, epofenonane, triprene;
(8) Other non-specific inhibitors: methyl bromide, chloropicrin, sulfuryl fluoride, borax, tartar;
(9) Homogeneous selective feeding inhibitors: flonicamid, pymetrozine, pyrifluquinazone;
(5)ニコチン性アセチルコリン受容体アロステリックモジュレーター: スピネトラム、スピノサド;
(6)クロライドチャンネル活性化剤: アバメクチン、エマメクチン安息香酸塩、レピメクチン、ミルベメクチン;イベルメクチン、セラメクチン、ドラメクチン、エプリノメクチン、モキシデクチン、ミルベマイシン、ミルベマイシンオキシム;
(7)幼若ホルモン様物質: ヒドロプレン、キノプレン、メソプレン、フェノキシカルブ、ピリプロキシフェン;ジオフェノラン、エポフェノナン、トリプレン;
(8)その他非特異的阻害剤: 臭化メチル、クロルピクリン、フッ化スルフリル、ホウ砂、吐酒石;
(9)同翅目選択的摂食阻害剤: フロニカミド、ピメトロジン、ピリフルキナゾン; (4) Nicotinic acetylcholine receptor agonists: acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, nithiazine, thiacloprid, thiamethoxam, sulfoxafurol, nicotine;
(5) Nicotinic acetylcholine receptor allosteric modulators: spinetoram, spinosad;
(6) Chloride channel activator: abamectin, emamectin benzoate, repimectin, milbemectin; ivermectin, selamectin, doramectin, eprinomectin, moxidectin, milbemycin, milbemycin oxime;
(7) Juvenile hormone-like substances: hydroprene, quinoprene, mesoprene, phenoxycarb, pyriproxyfen; geofenolan, epofenonane, triprene;
(8) Other non-specific inhibitors: methyl bromide, chloropicrin, sulfuryl fluoride, borax, tartar;
(9) Homogeneous selective feeding inhibitors: flonicamid, pymetrozine, pyrifluquinazone;
(10)ダニ類生育阻害剤: クロフェンテジン、ジフロビダジン、ヘキシチアゾクス、エトキサゾール;
(11)微生物由来昆虫中腸内膜破壊剤: バチルス・チューリンゲンシス亜種イスラエレンシ、バチルス・スファエリクス、バチルス・チューリンゲンシス亜種アイザワイ、バチルス・チューリンゲンシス亜種クルスタキ、バチルス・チューリンゲンシス亜種テネブリオニス、Bt作物タンパク質:Cry1Ab、Cry1Ac、Cry1Fa、Cry1A.105、Cry2Ab、Vip3A、mCry3A、Cry3Ab、Cry3Bb、Cry34Ab1/Cry35Ab1;
(12)ミトコンドリアATP生合成酵素阻害剤: ジアフェンチウロン、アゾシクロチン、シヘキサチン、酸化フェンブタスズ、プロパルギット、テトラジホン;
(13)酸化的リン酸化脱共役剤: クロルフェナピル、スルフラミド、DNOC;ビナパクリル、ジノブトン、ジノカップ;
(14)ニコチン性アセチルコリン受容体チャンネルブロッカー: ベンスルタップ、カルタップ塩酸塩;ネライストキシン;チオスルタップ一ナトリウム塩、チオシクラム;
(15)キチン合成阻害剤: ビストリフルロン、クロルフルアズロン、ジフルベンズロン、フルシクロクスロン、フルフェノクスロン、ヘキサフルムロン、ルフェヌロン、ノバルロン、ノビフルムロン、テフルベンズロン、トリフルムロン、ブプロフェジン、フルアズロン;
(16)双翅目脱皮かく乱剤: シロマジン;
(17)脱皮ホルモン受容体アゴニスト: クロマフェノジド、ハロフェノジド、メトキシフェノジド、テブフェノジド;
(18)オクトパミン受容体アゴニスト: アミトラズ、デミジトラズ、クロルジメホルム;
(19)ミトコンドリア電子伝達系複合体III阻害剤: アセキノシル、フルアクリピリム、ヒドラメチルノン;
(20)ミトコンドリア電子伝達系複合体I阻害剤: フェナザキン、フェンプロキシメート、ピリミジフェン、ピリダベン、テブフェンピラド、トルフェンピラド、ロテノン; (10) Mite growth inhibitor: clofentezin, diflovidazine, hexythiazox, etoxazole;
(11) Microbial-derived insect midgut mesentery: Bacillus thuringiensis subsp. Isla elensis, Bacillus sphaericus, Bacillus thuringiensis subsp. Aisawai, Bacillus thuringiensis subsp. Kurstaki, Bacillus thuringiensis subsp. Crop proteins: Cry1Ab, Cry1Ac, Cry1Fa, Cry1A.105, Cry2Ab, Vip3A, mCry3A, Cry3Ab, Cry3Bb, Cry34Ab1 / Cry35Ab1;
(12) Mitochondrial ATP biosynthetic enzyme inhibitors: diafenthiuron, azocyclotin, cyhexatin, phenbutasine oxide, propargite, tetradiphone;
(13) Oxidative phosphorylation uncoupler: chlorfenapyr, sulframide, DNOC; binapacryl, dinobutone, dinocup;
(14) Nicotinic acetylcholine receptor channel blockers: bensultap, cartap hydrochloride; nereistoxin; thiosultap monosodium salt, thiocyclam;
(15) Chitin synthesis inhibitor: bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novallon, nobiflumuron, teflubenzuron, triflumuron, buprofezin, fluazuron;
(16) Diptera molting disruptors: cyromazine;
(17) Molting hormone receptor agonists: Chromafenozide, halofenozide, methoxyphenozide, tebufenozide;
(18) Octopamine receptor agonist: Amitraz, demiditraz, chlordimeform;
(19) Mitochondrial electron transport system complex III inhibitor: acequinosyl, fluacrylpyrim, hydramethylnon;
(20) Mitochondrial electron transport system complex I inhibitor: phenazaquin, fenproxymate, pyrimidifen, pyridaben, tebufenpyrad, tolfenpyrad, rotenone;
(11)微生物由来昆虫中腸内膜破壊剤: バチルス・チューリンゲンシス亜種イスラエレンシ、バチルス・スファエリクス、バチルス・チューリンゲンシス亜種アイザワイ、バチルス・チューリンゲンシス亜種クルスタキ、バチルス・チューリンゲンシス亜種テネブリオニス、Bt作物タンパク質:Cry1Ab、Cry1Ac、Cry1Fa、Cry1A.105、Cry2Ab、Vip3A、mCry3A、Cry3Ab、Cry3Bb、Cry34Ab1/Cry35Ab1;
(12)ミトコンドリアATP生合成酵素阻害剤: ジアフェンチウロン、アゾシクロチン、シヘキサチン、酸化フェンブタスズ、プロパルギット、テトラジホン;
(13)酸化的リン酸化脱共役剤: クロルフェナピル、スルフラミド、DNOC;ビナパクリル、ジノブトン、ジノカップ;
(14)ニコチン性アセチルコリン受容体チャンネルブロッカー: ベンスルタップ、カルタップ塩酸塩;ネライストキシン;チオスルタップ一ナトリウム塩、チオシクラム;
(15)キチン合成阻害剤: ビストリフルロン、クロルフルアズロン、ジフルベンズロン、フルシクロクスロン、フルフェノクスロン、ヘキサフルムロン、ルフェヌロン、ノバルロン、ノビフルムロン、テフルベンズロン、トリフルムロン、ブプロフェジン、フルアズロン;
(16)双翅目脱皮かく乱剤: シロマジン;
(17)脱皮ホルモン受容体アゴニスト: クロマフェノジド、ハロフェノジド、メトキシフェノジド、テブフェノジド;
(18)オクトパミン受容体アゴニスト: アミトラズ、デミジトラズ、クロルジメホルム;
(19)ミトコンドリア電子伝達系複合体III阻害剤: アセキノシル、フルアクリピリム、ヒドラメチルノン;
(20)ミトコンドリア電子伝達系複合体I阻害剤: フェナザキン、フェンプロキシメート、ピリミジフェン、ピリダベン、テブフェンピラド、トルフェンピラド、ロテノン; (10) Mite growth inhibitor: clofentezin, diflovidazine, hexythiazox, etoxazole;
(11) Microbial-derived insect midgut mesentery: Bacillus thuringiensis subsp. Isla elensis, Bacillus sphaericus, Bacillus thuringiensis subsp. Aisawai, Bacillus thuringiensis subsp. Kurstaki, Bacillus thuringiensis subsp. Crop proteins: Cry1Ab, Cry1Ac, Cry1Fa, Cry1A.105, Cry2Ab, Vip3A, mCry3A, Cry3Ab, Cry3Bb, Cry34Ab1 / Cry35Ab1;
(12) Mitochondrial ATP biosynthetic enzyme inhibitors: diafenthiuron, azocyclotin, cyhexatin, phenbutasine oxide, propargite, tetradiphone;
(13) Oxidative phosphorylation uncoupler: chlorfenapyr, sulframide, DNOC; binapacryl, dinobutone, dinocup;
(14) Nicotinic acetylcholine receptor channel blockers: bensultap, cartap hydrochloride; nereistoxin; thiosultap monosodium salt, thiocyclam;
(15) Chitin synthesis inhibitor: bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novallon, nobiflumuron, teflubenzuron, triflumuron, buprofezin, fluazuron;
(16) Diptera molting disruptors: cyromazine;
(17) Molting hormone receptor agonists: Chromafenozide, halofenozide, methoxyphenozide, tebufenozide;
(18) Octopamine receptor agonist: Amitraz, demiditraz, chlordimeform;
(19) Mitochondrial electron transport system complex III inhibitor: acequinosyl, fluacrylpyrim, hydramethylnon;
(20) Mitochondrial electron transport system complex I inhibitor: phenazaquin, fenproxymate, pyrimidifen, pyridaben, tebufenpyrad, tolfenpyrad, rotenone;
(21)電位依存性ナトリウムチャネルブロッカー: インドキサカルブ、メタフルミゾン;
(22)アセチルCoAカルボキシラーゼ阻害剤: スピロジクロフェン、スピロメシフェン、スピロテトラマト;
(23)ミトコンドリア電子伝達系複合体IV阻害剤: リン化アルミニウム、リン化カルシウム、ホスフィン、リン化亜鉛、シアニド;
(24)ミトコンドリア電子伝達系複合体II阻害剤: シエノピラフェン、シフルメトフェン、ピフルブミド;
(25)リアノジン受容体モジュレーター: クロラントラニリプロール、シアントラニプロール、フルベンジアミド、シクラニリプロール、テトラニリプロール;
(26)混合機能オキシダーゼ阻害剤化合物: ピペロニルブトキシド;
(27)ラトロフィリン受容体作用薬: デプシペプチド、環状デプシペプチド、24員環状デプシペプチド、エモデプシド;
(28)その他の剤(作用機構が未知): アザジラクチン、ベンゾキシメート、ビフェナゼート、ブロモプロピレート、キノメチオネート、クリオライト、ジコホル、ピリダリル、;ベンクロチアズ、硫黄、アミドフルメット、1,3-ジクロロプロペン、DCIP、フェニソブロモレート、ベンゾメート、メタアルデヒド、クロルベンジレート、クロチアゾベン、ジシクラニル、フェノキサクリム、フェントリファニル、フルベンジミン、フルフェナジン、ゴシップルア、ジャポニルア、メトキサジアゾン、石油、オレイン酸カリウム、テトラスル、トリアラセン;アフィドピロペン(afidopyropen)、フロメトキン、フルフィプロル(flufiprole)、フルエンスルフォン、メペルフルスリン、テトラメチルフルスリン、トラロピリル、ジメフルスリン、メチルネオデカンアミド;フルララネル、アフォキソラネル、フルキサメタミド、5-[5-(3,5-ジクロロフェニル)-5-トリフルオロメチル-4,5-ジヒドロイソオキサゾール-3-イル]-2-(1H-1,2,4-トリアゾール-1-イル)ベンゾニトリル(CAS:943137-49-3)、ブロフラニリド、その他のメタジアミド類、; (21) Voltage-gated sodium channel blockers: indoxacarb, metaflumizone;
(22) Acetyl CoA carboxylase inhibitor: spirodiclofen, spiromesifen, spirotetramat;
(23) Mitochondrial electron transport system complex IV inhibitor: aluminum phosphide, calcium phosphide, phosphine, zinc phosphide, cyanide;
(24) Mitochondrial electron transport system complex II inhibitor: sienopyrafen, cyflumetofene, pivlumide;
(25) Ryanodine receptor modulator: chlorantraniliprole, cyantraniprolol, fulvendiamide, cyclaniliprol, tetraniliprol;
(26) Mixed function oxidase inhibitor compound: piperonyl butoxide;
(27) Latrophilin receptor agonist: depsipeptide, cyclic depsipeptide, 24-membered cyclic depsipeptide, emodepside;
(28) Other agents (the mechanism of action is unknown): azadirachtin, benzoxymate, biphenazate, bromopropyrate, quinomethionate, cryolite, dicofol, pyridalyl, benclothiaz, sulfur, amidoflumet, 1,3-dichloropropene, DCIP , Phenisobromolate, benzomate, metaldehyde, chlorbenzilate, clothiazoben, dicyclanyl, phenoxacrime, fentriphanyl, flubenzimine, fluphenazine, gossip lure, japonyla, methoxadiazone, petroleum, potassium oleate, tetrasul, trialacene; afidopyropen), flometokin, flufiprole, fluenesulfone, meperfluthrin, tetramethylfluthrin, tralopyril, dimefluthrin, me Luneodecanamide; fluralanel, afoxolanel, floxamethamide, 5- [5- (3,5-dichlorophenyl) -5-trifluoromethyl-4,5-dihydroisoxazol-3-yl] -2- (1H-1,2, , 4-Triazol-1-yl) benzonitrile (CAS: 943137-49-3), brofuranilide, other metadiamides;
(22)アセチルCoAカルボキシラーゼ阻害剤: スピロジクロフェン、スピロメシフェン、スピロテトラマト;
(23)ミトコンドリア電子伝達系複合体IV阻害剤: リン化アルミニウム、リン化カルシウム、ホスフィン、リン化亜鉛、シアニド;
(24)ミトコンドリア電子伝達系複合体II阻害剤: シエノピラフェン、シフルメトフェン、ピフルブミド;
(25)リアノジン受容体モジュレーター: クロラントラニリプロール、シアントラニプロール、フルベンジアミド、シクラニリプロール、テトラニリプロール;
(26)混合機能オキシダーゼ阻害剤化合物: ピペロニルブトキシド;
(27)ラトロフィリン受容体作用薬: デプシペプチド、環状デプシペプチド、24員環状デプシペプチド、エモデプシド;
(28)その他の剤(作用機構が未知): アザジラクチン、ベンゾキシメート、ビフェナゼート、ブロモプロピレート、キノメチオネート、クリオライト、ジコホル、ピリダリル、;ベンクロチアズ、硫黄、アミドフルメット、1,3-ジクロロプロペン、DCIP、フェニソブロモレート、ベンゾメート、メタアルデヒド、クロルベンジレート、クロチアゾベン、ジシクラニル、フェノキサクリム、フェントリファニル、フルベンジミン、フルフェナジン、ゴシップルア、ジャポニルア、メトキサジアゾン、石油、オレイン酸カリウム、テトラスル、トリアラセン;アフィドピロペン(afidopyropen)、フロメトキン、フルフィプロル(flufiprole)、フルエンスルフォン、メペルフルスリン、テトラメチルフルスリン、トラロピリル、ジメフルスリン、メチルネオデカンアミド;フルララネル、アフォキソラネル、フルキサメタミド、5-[5-(3,5-ジクロロフェニル)-5-トリフルオロメチル-4,5-ジヒドロイソオキサゾール-3-イル]-2-(1H-1,2,4-トリアゾール-1-イル)ベンゾニトリル(CAS:943137-49-3)、ブロフラニリド、その他のメタジアミド類、; (21) Voltage-gated sodium channel blockers: indoxacarb, metaflumizone;
(22) Acetyl CoA carboxylase inhibitor: spirodiclofen, spiromesifen, spirotetramat;
(23) Mitochondrial electron transport system complex IV inhibitor: aluminum phosphide, calcium phosphide, phosphine, zinc phosphide, cyanide;
(24) Mitochondrial electron transport system complex II inhibitor: sienopyrafen, cyflumetofene, pivlumide;
(25) Ryanodine receptor modulator: chlorantraniliprole, cyantraniprolol, fulvendiamide, cyclaniliprol, tetraniliprol;
(26) Mixed function oxidase inhibitor compound: piperonyl butoxide;
(27) Latrophilin receptor agonist: depsipeptide, cyclic depsipeptide, 24-membered cyclic depsipeptide, emodepside;
(28) Other agents (the mechanism of action is unknown): azadirachtin, benzoxymate, biphenazate, bromopropyrate, quinomethionate, cryolite, dicofol, pyridalyl, benclothiaz, sulfur, amidoflumet, 1,3-dichloropropene, DCIP , Phenisobromolate, benzomate, metaldehyde, chlorbenzilate, clothiazoben, dicyclanyl, phenoxacrime, fentriphanyl, flubenzimine, fluphenazine, gossip lure, japonyla, methoxadiazone, petroleum, potassium oleate, tetrasul, trialacene; afidopyropen), flometokin, flufiprole, fluenesulfone, meperfluthrin, tetramethylfluthrin, tralopyril, dimefluthrin, me Luneodecanamide; fluralanel, afoxolanel, floxamethamide, 5- [5- (3,5-dichlorophenyl) -5-trifluoromethyl-4,5-dihydroisoxazol-3-yl] -2- (1H-1,2, , 4-Triazol-1-yl) benzonitrile (CAS: 943137-49-3), brofuranilide, other metadiamides;
〔外部寄生虫防除剤〕
本発明化合物は、人獣に害を及ぼす外部寄生虫の防除効果に優れている。また、薬害が少なく、魚類や温血動物への毒性が低いため、安全性の高い化合物である。そのため、外部寄生虫防除剤の有効成分として有用である。
外部寄生虫としては、ダニ類、シラミ類、ノミ類などが挙げられる。
本発明の外部寄生虫防除剤の処理の対象となる宿主動物としては、イヌ、ネコなどの愛玩動物;愛玩鳥;ウシ、ウマ、ブタ、ヒツジなどの家畜;家禽; などの温血動物が挙げられる。その他にも、ミツバチが挙げられる。
外部寄生虫は、宿主動物、特には温血動物の中および上に寄生する。詳しくは、宿主動物の背、脇下、下腹部、内股部などに寄生して動物から血液やフケなどの栄養源を得て生息する。
本発明の外部寄生虫防除剤は、公知の獣医学的な手法(局所、経口、非経口または皮下投与)で施用することができる。その方法として、錠剤、カプセル、飼料混入などにより動物に経口的に投与する方法; 浸漬液、坐薬、注射(筋肉内、皮下、静脈内、腹腔内など)などにより動物に投与する方法; 油性または水性液剤を噴霧、ポアオン、スポットオンなどにより局所的に投与する方法; 樹脂に外部寄生虫防除剤を練り込み、前記混練物を首輪、耳札などの適当な形状に成形し、それを動物に装着し局所的に投与する方法; などが挙げられる。 [External parasite control agent]
The compound of the present invention is excellent in the control effect of ectoparasites that are harmful to humans. In addition, it is a highly safe compound due to its low phytotoxicity and low toxicity to fish and warm-blooded animals. Therefore, it is useful as an active ingredient of an ectoparasite control agent.
Examples of ectoparasites include ticks, lice and fleas.
Examples of host animals to be treated with the ectoparasite control agent of the present invention include pets such as dogs and cats; pets; domestic animals such as cattle, horses, pigs, and sheep; It is done. In addition, a bee is mentioned.
Ectoparasites parasitize in and on host animals, particularly warm-blooded animals. Specifically, it infests the back, underarms, lower abdomen, and inner crotch of the host animal and obtains nutrients such as blood and dandruff from the animal.
The ectoparasite control agent of the present invention can be applied by a known veterinary technique (topical, oral, parenteral or subcutaneous administration). As the method, it is administered orally to animals by tablet, capsule, feed mixing, etc .; it is administered to animals by immersion liquid, suppository, injection (intramuscular, subcutaneous, intravenous, intraperitoneal, etc.); A method of locally administering an aqueous solution by spraying, pour-on, spot-on, etc .; kneading an ectoparasite-controlling agent into a resin, shaping the kneaded product into an appropriate shape such as a collar or ear tag, and applying it to an animal A method of wearing and administering locally;
本発明化合物は、人獣に害を及ぼす外部寄生虫の防除効果に優れている。また、薬害が少なく、魚類や温血動物への毒性が低いため、安全性の高い化合物である。そのため、外部寄生虫防除剤の有効成分として有用である。
外部寄生虫としては、ダニ類、シラミ類、ノミ類などが挙げられる。
本発明の外部寄生虫防除剤の処理の対象となる宿主動物としては、イヌ、ネコなどの愛玩動物;愛玩鳥;ウシ、ウマ、ブタ、ヒツジなどの家畜;家禽; などの温血動物が挙げられる。その他にも、ミツバチが挙げられる。
外部寄生虫は、宿主動物、特には温血動物の中および上に寄生する。詳しくは、宿主動物の背、脇下、下腹部、内股部などに寄生して動物から血液やフケなどの栄養源を得て生息する。
本発明の外部寄生虫防除剤は、公知の獣医学的な手法(局所、経口、非経口または皮下投与)で施用することができる。その方法として、錠剤、カプセル、飼料混入などにより動物に経口的に投与する方法; 浸漬液、坐薬、注射(筋肉内、皮下、静脈内、腹腔内など)などにより動物に投与する方法; 油性または水性液剤を噴霧、ポアオン、スポットオンなどにより局所的に投与する方法; 樹脂に外部寄生虫防除剤を練り込み、前記混練物を首輪、耳札などの適当な形状に成形し、それを動物に装着し局所的に投与する方法; などが挙げられる。 [External parasite control agent]
The compound of the present invention is excellent in the control effect of ectoparasites that are harmful to humans. In addition, it is a highly safe compound due to its low phytotoxicity and low toxicity to fish and warm-blooded animals. Therefore, it is useful as an active ingredient of an ectoparasite control agent.
Examples of ectoparasites include ticks, lice and fleas.
Examples of host animals to be treated with the ectoparasite control agent of the present invention include pets such as dogs and cats; pets; domestic animals such as cattle, horses, pigs, and sheep; It is done. In addition, a bee is mentioned.
Ectoparasites parasitize in and on host animals, particularly warm-blooded animals. Specifically, it infests the back, underarms, lower abdomen, and inner crotch of the host animal and obtains nutrients such as blood and dandruff from the animal.
The ectoparasite control agent of the present invention can be applied by a known veterinary technique (topical, oral, parenteral or subcutaneous administration). As the method, it is administered orally to animals by tablet, capsule, feed mixing, etc .; it is administered to animals by immersion liquid, suppository, injection (intramuscular, subcutaneous, intravenous, intraperitoneal, etc.); A method of locally administering an aqueous solution by spraying, pour-on, spot-on, etc .; kneading an ectoparasite-controlling agent into a resin, shaping the kneaded product into an appropriate shape such as a collar or ear tag, and applying it to an animal A method of wearing and administering locally;
〔製剤処方〕
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、剤型によって特に限定されない。たとえば、水和剤、乳剤、粉剤、粒剤、水溶剤、懸濁剤、顆粒水和剤、錠剤などの剤型を挙げることができる。製剤への調製方法は、特に制限されず、剤形に応じて公知の調製方法を採用することができる。
以下に、製剤実施例を若干示す。なお、以下に示す製剤処方は単なる例示であり、本発明の主旨に反しない範囲で修正することができ、本発明は以下の製剤実施例によって何ら制限されるものではない。「部」は特段の断りが無い限り「重量部」を意味する。 [Prescription formulation]
The agricultural and horticultural fungicide, pesticide, and insecticide or acaricide of the present invention are not particularly limited depending on the dosage form. For example, dosage forms such as wettable powder, emulsion, powder, granule, aqueous solvent, suspension, granular wettable powder, and tablet can be exemplified. The preparation method to a formulation is not specifically limited, A well-known preparation method can be employ | adopted according to a dosage form.
The following are some formulation examples. In addition, the pharmaceutical formulation shown below is only an example, and can be modified without departing from the gist of the present invention, and the present invention is not limited by the following pharmaceutical examples. “Parts” means “parts by weight” unless otherwise specified.
本発明の農園芸用殺菌剤、有害生物防除剤、および殺虫若しくは殺ダニ剤は、剤型によって特に限定されない。たとえば、水和剤、乳剤、粉剤、粒剤、水溶剤、懸濁剤、顆粒水和剤、錠剤などの剤型を挙げることができる。製剤への調製方法は、特に制限されず、剤形に応じて公知の調製方法を採用することができる。
以下に、製剤実施例を若干示す。なお、以下に示す製剤処方は単なる例示であり、本発明の主旨に反しない範囲で修正することができ、本発明は以下の製剤実施例によって何ら制限されるものではない。「部」は特段の断りが無い限り「重量部」を意味する。 [Prescription formulation]
The agricultural and horticultural fungicide, pesticide, and insecticide or acaricide of the present invention are not particularly limited depending on the dosage form. For example, dosage forms such as wettable powder, emulsion, powder, granule, aqueous solvent, suspension, granular wettable powder, and tablet can be exemplified. The preparation method to a formulation is not specifically limited, A well-known preparation method can be employ | adopted according to a dosage form.
The following are some formulation examples. In addition, the pharmaceutical formulation shown below is only an example, and can be modified without departing from the gist of the present invention, and the present invention is not limited by the following pharmaceutical examples. “Parts” means “parts by weight” unless otherwise specified.
(製剤1:水和剤)
本発明化合物 40部
珪藻土 53部
高級アルコール硫酸エステル 4部
アルキルナフタレンスルホン酸塩 3部
以上を均一に混合して微細に粉砕して、有効成分40%の水和剤を得る。 (Formulation 1: wettable powder)
Compound of the present invention 40 parts Diatomaceous earth 53 parts Higher alcohol sulfate 4 parts Alkyl naphthalene sulfonate 3 parts The above is uniformly mixed and finely pulverized to obtain a wettable powder of 40% active ingredient.
本発明化合物 40部
珪藻土 53部
高級アルコール硫酸エステル 4部
アルキルナフタレンスルホン酸塩 3部
以上を均一に混合して微細に粉砕して、有効成分40%の水和剤を得る。 (Formulation 1: wettable powder)
Compound of the present invention 40 parts Diatomaceous earth 53 parts Higher alcohol sulfate 4 parts Alkyl naphthalene sulfonate 3 parts The above is uniformly mixed and finely pulverized to obtain a wettable powder of 40% active ingredient.
(製剤2:乳剤)
本発明化合物 30部
キシレン 33部
ジメチルホルムアミド 30部
ポリオキシエチレンアルキルアリルエーテル 7部
以上を混合溶解して、有効成分30%の乳剤を得る。 (Formulation 2: Emulsion)
Compound of the present invention 30 parts Xylene 33 parts Dimethylformamide 30 parts Polyoxyethylene alkylallyl ether 7 parts The above components are mixed and dissolved to obtain an emulsion containing 30% active ingredient.
本発明化合物 30部
キシレン 33部
ジメチルホルムアミド 30部
ポリオキシエチレンアルキルアリルエーテル 7部
以上を混合溶解して、有効成分30%の乳剤を得る。 (Formulation 2: Emulsion)
Compound of the present invention 30 parts Xylene 33 parts Dimethylformamide 30 parts Polyoxyethylene alkylallyl ether 7 parts The above components are mixed and dissolved to obtain an emulsion containing 30% active ingredient.
(製剤3:粉剤)
本発明化合物 10部
クレー 90部
以上を均一に混合して微細に粉砕し、有効成分10%の粉剤を得る。 (Formulation 3: Powder)
Compound of the present invention 10 parts Clay 90 parts The above is uniformly mixed and finely pulverized to obtain a powder of 10% active ingredient.
本発明化合物 10部
クレー 90部
以上を均一に混合して微細に粉砕し、有効成分10%の粉剤を得る。 (Formulation 3: Powder)
Compound of the present invention 10 parts Clay 90 parts The above is uniformly mixed and finely pulverized to obtain a powder of 10% active ingredient.
(製剤4:粒剤)
本発明化合物 5部
クレー 73部
ベントナイト 20部
ジオクチルスルホサクシネートナトリウム塩 1部
リン酸カリウム 1部
以上をよく粉砕混合し、水を加えてよく練り合せた後、造粒乾燥して有効成分5%の粒剤を得る。 (Formulation 4: Granules)
Compound of the present invention 5 parts Clay 73 parts Bentonite 20 parts Dioctyl sulfosuccinate sodium salt 1 part Potassium phosphate 1 part The above components are pulverized and mixed well, mixed well with water, granulated and dried, and 5% active ingredient. Get the granules.
本発明化合物 5部
クレー 73部
ベントナイト 20部
ジオクチルスルホサクシネートナトリウム塩 1部
リン酸カリウム 1部
以上をよく粉砕混合し、水を加えてよく練り合せた後、造粒乾燥して有効成分5%の粒剤を得る。 (Formulation 4: Granules)
Compound of the present invention 5 parts Clay 73 parts Bentonite 20 parts Dioctyl sulfosuccinate sodium salt 1 part Potassium phosphate 1 part The above components are pulverized and mixed well, mixed well with water, granulated and dried, and 5% active ingredient. Get the granules.
(製剤5:懸濁剤)
本発明化合物 10部
ポリオキシエチレンアルキルアリルエーテル 4部
ポリカルボン酸ナトリウム塩 2部
グリセリン 10部
キサンタンガム 0.2部
水 73.8部
以上を混合し、粒度が3ミクロン以下になるまで湿式粉砕し、有効成分10%の懸濁剤を得る。 (Formulation 5: Suspension)
Compound of the present invention 10 parts Polyoxyethylene alkyl allyl ether 4 parts Polycarboxylic acid sodium salt 2 parts Glycerin 10 parts Xanthan gum 0.2 parts Water 73.8 parts A suspension with 10% active ingredient is obtained.
本発明化合物 10部
ポリオキシエチレンアルキルアリルエーテル 4部
ポリカルボン酸ナトリウム塩 2部
グリセリン 10部
キサンタンガム 0.2部
水 73.8部
以上を混合し、粒度が3ミクロン以下になるまで湿式粉砕し、有効成分10%の懸濁剤を得る。 (Formulation 5: Suspension)
Compound of the present invention 10 parts Polyoxyethylene alkyl allyl ether 4 parts Polycarboxylic acid sodium salt 2 parts Glycerin 10 parts Xanthan gum 0.2 parts Water 73.8 parts A suspension with 10% active ingredient is obtained.
(製剤6:顆粒水和剤)
本発明化合物 40部
クレー 36部
塩化カリウム 10部
アルキルベンゼンスルホン酸ナトリウム塩 1部
リグニンスルホン酸ナトリウム塩 8部
アルキルベンゼンスルホン酸ナトリウム塩のホルムアルデヒド縮合物
5部
以上を均一に混合して微細に粉砕後,適量の水を加えてから練り込んで粘土状にする。粘土状物を造粒した後乾燥し、有効成分40%の顆粒水和剤を得る。
実施例
次に、化合物実施例を示し、本発明をより具体的に説明する。ただし、本発明は以下の化合物実施例によって何ら制限されるものではない。 (Formulation 6: Granule wettable powder)
Compound of the present invention 40 parts Clay 36 parts Potassium chloride 10 parts Alkylbenzenesulfonic acid sodium salt 1 part Lignin sulfonic acid sodium salt 8 parts Formaldehyde condensate of alkylbenzenesulfonic acid sodium salt 5 parts
After mixing the above uniformly and finely grinding, add an appropriate amount of water and knead to make clay. The clay-like product is granulated and then dried to obtain a granule wettable powder containing 40% of the active ingredient.
Examples Next, compound examples will be shown to explain the present invention more specifically. However, this invention is not restrict | limited at all by the following compound examples.
本発明化合物 40部
クレー 36部
塩化カリウム 10部
アルキルベンゼンスルホン酸ナトリウム塩 1部
リグニンスルホン酸ナトリウム塩 8部
アルキルベンゼンスルホン酸ナトリウム塩のホルムアルデヒド縮合物
5部
以上を均一に混合して微細に粉砕後,適量の水を加えてから練り込んで粘土状にする。粘土状物を造粒した後乾燥し、有効成分40%の顆粒水和剤を得る。
実施例
次に、化合物実施例を示し、本発明をより具体的に説明する。ただし、本発明は以下の化合物実施例によって何ら制限されるものではない。 (Formulation 6: Granule wettable powder)
Compound of the present invention 40 parts Clay 36 parts Potassium chloride 10 parts Alkylbenzenesulfonic acid sodium salt 1 part Lignin sulfonic acid sodium salt 8 parts Formaldehyde condensate of alkylbenzenesulfonic acid sodium salt 5 parts
After mixing the above uniformly and finely grinding, add an appropriate amount of water and knead to make clay. The clay-like product is granulated and then dried to obtain a granule wettable powder containing 40% of the active ingredient.
Examples Next, compound examples will be shown to explain the present invention more specifically. However, this invention is not restrict | limited at all by the following compound examples.
〔実施例1〕 メチル[2,3,6-トリメチル-5-[[4-(4-トリフルオロメトキシ)フェニル]シクロヘキソキシ]-4-ピリジル]カーボネートの製造
(工程1) 4-ヒドロキシ-2,5,6-トリメチルニコチン酸エチルの合成 Example 1 Production of methyl [2,3,6-trimethyl-5-[[4- (4-trifluoromethoxy) phenyl] cyclohexoxy] -4-pyridyl] carbonate (Step 1) 4-hydroxy-2 Of ethyl 5,5,6-trimethylnicotinate
(工程1) 4-ヒドロキシ-2,5,6-トリメチルニコチン酸エチルの合成 Example 1 Production of methyl [2,3,6-trimethyl-5-[[4- (4-trifluoromethoxy) phenyl] cyclohexoxy] -4-pyridyl] carbonate (Step 1) 4-hydroxy-2 Of ethyl 5,5,6-trimethylnicotinate
3-アミノクロトン酸エチル45gおよび2-メチルアセト酢酸エチル50gをキシレン100mlに溶解させた。この溶液を一晩加熱還流した。次いで、反応液を室温まで冷却して、析出した結晶をろ過、ジエチルエーテルで洗浄し、乾燥させて、目的化合物24.2g(収率33%)を得た。
1H-NMR(CDCl3,δppm) 1.21(3H,t),2.14(3H,s),2.46(3H,s),2.71(3H,s),4.45(2H,q),12.14(3H,s) 45 g of ethyl 3-aminocrotonate and 50 g of ethyl 2-methylacetoacetate were dissolved in 100 ml of xylene. This solution was heated to reflux overnight. Next, the reaction solution was cooled to room temperature, and the precipitated crystals were filtered, washed with diethyl ether, and dried to obtain 24.2 g (yield 33%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.21 (3H, t), 2.14 (3H, s), 2.46 (3H, s), 2.71 (3H, s), 4.45 (2H, q), 12.14 (3H, s )
1H-NMR(CDCl3,δppm) 1.21(3H,t),2.14(3H,s),2.46(3H,s),2.71(3H,s),4.45(2H,q),12.14(3H,s) 45 g of ethyl 3-aminocrotonate and 50 g of ethyl 2-methylacetoacetate were dissolved in 100 ml of xylene. This solution was heated to reflux overnight. Next, the reaction solution was cooled to room temperature, and the precipitated crystals were filtered, washed with diethyl ether, and dried to obtain 24.2 g (yield 33%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.21 (3H, t), 2.14 (3H, s), 2.46 (3H, s), 2.71 (3H, s), 4.45 (2H, q), 12.14 (3H, s )
(工程2) 4-アリルオキシ-2,5,6-トリメチルニコチン酸エチルの合成
4-ヒドロキシ-2,5,6-トリメチルニコチン酸エチル12.2gをアセトニトリル120mlに溶解させた。この溶液に、炭酸カリウム16.1gを加え、次いで臭化アリル14.1gを加え、8時間加熱還流した。次いで、反応液を室温まで冷却し、水に注加し、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物10.0g(収率77%)を得た。
1H-NMR(CDCl3,δppm) 1.36(3H,t),2.15(3H,s),2.46(6H,s),4.35-4.40(5H,m),5.25-5.39(2H,m),5.92-6.11(1H,m) (Step 2) Synthesis of ethyl 4-allyloxy-2,5,6-trimethylnicotinate 12.2 g of ethyl 4-hydroxy-2,5,6-trimethylnicotinate was dissolved in 120 ml of acetonitrile. To this solution, 16.1 g of potassium carbonate was added, and then 14.1 g of allyl bromide was added, and the mixture was heated to reflux for 8 hours. The reaction was then cooled to room temperature, poured into water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 10.0 g (yield 77%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.36 (3H, t), 2.15 (3H, s), 2.46 (6H, s), 4.35-4.40 (5H, m), 5.25-5.39 (2H, m), 5.92 -6.11 (1H, m)
4-ヒドロキシ-2,5,6-トリメチルニコチン酸エチル12.2gをアセトニトリル120mlに溶解させた。この溶液に、炭酸カリウム16.1gを加え、次いで臭化アリル14.1gを加え、8時間加熱還流した。次いで、反応液を室温まで冷却し、水に注加し、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄し、無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物10.0g(収率77%)を得た。
1H-NMR(CDCl3,δppm) 1.36(3H,t),2.15(3H,s),2.46(6H,s),4.35-4.40(5H,m),5.25-5.39(2H,m),5.92-6.11(1H,m) (Step 2) Synthesis of ethyl 4-allyloxy-2,5,6-trimethylnicotinate 12.2 g of ethyl 4-hydroxy-2,5,6-trimethylnicotinate was dissolved in 120 ml of acetonitrile. To this solution, 16.1 g of potassium carbonate was added, and then 14.1 g of allyl bromide was added, and the mixture was heated to reflux for 8 hours. The reaction was then cooled to room temperature, poured into water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 10.0 g (yield 77%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.36 (3H, t), 2.15 (3H, s), 2.46 (6H, s), 4.35-4.40 (5H, m), 5.25-5.39 (2H, m), 5.92 -6.11 (1H, m)
(工程3) (4-アリルオキシ-2,5,6-トリメチルピリジン-3-イル)-メタノールの合成
水素化リチウムアルミニウム2.8gをテトラヒドロフラン300mlに加えた。この液に、氷冷下、4-メトキシ-2,5,6-トリメチルニコチン酸エチル10.0gを滴下した。滴下終了後、室温で2時間攪拌した。その後、反応液を氷冷し、未反応の水素化リチウムナトリウムが分解されるまで水を加えた。次いで、無水硫酸マグネシウムで乾燥させ、溶媒を減圧留去して、目的化合物7.9g(収率97%)を得た。
1H-NMR(CDCl3,δppm) 2.18(3H,s),2.45(3H,s),2.56(3H,s),4.38(2H,d),4.70(2H,s),5.25-5.39(2H,m),5.92-6.11(1H,m) (Step 3) Synthesis of (4-allyloxy-2,5,6-trimethylpyridin-3-yl) -methanol 2.8 g of lithium aluminum hydride was added to 300 ml of tetrahydrofuran. To this solution, 10.0 g of ethyl 4-methoxy-2,5,6-trimethylnicotinate was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was ice-cooled, and water was added until unreacted lithium sodium hydride was decomposed. Subsequently, it was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 7.9 g (yield 97%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.18 (3H, s), 2.45 (3H, s), 2.56 (3H, s), 4.38 (2H, d), 4.70 (2H, s), 5.25-5.39 (2H , m), 5.92-6.11 (1H, m)
水素化リチウムアルミニウム2.8gをテトラヒドロフラン300mlに加えた。この液に、氷冷下、4-メトキシ-2,5,6-トリメチルニコチン酸エチル10.0gを滴下した。滴下終了後、室温で2時間攪拌した。その後、反応液を氷冷し、未反応の水素化リチウムナトリウムが分解されるまで水を加えた。次いで、無水硫酸マグネシウムで乾燥させ、溶媒を減圧留去して、目的化合物7.9g(収率97%)を得た。
1H-NMR(CDCl3,δppm) 2.18(3H,s),2.45(3H,s),2.56(3H,s),4.38(2H,d),4.70(2H,s),5.25-5.39(2H,m),5.92-6.11(1H,m) (Step 3) Synthesis of (4-allyloxy-2,5,6-trimethylpyridin-3-yl) -methanol 2.8 g of lithium aluminum hydride was added to 300 ml of tetrahydrofuran. To this solution, 10.0 g of ethyl 4-methoxy-2,5,6-trimethylnicotinate was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was ice-cooled, and water was added until unreacted lithium sodium hydride was decomposed. Subsequently, it was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 7.9 g (yield 97%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.18 (3H, s), 2.45 (3H, s), 2.56 (3H, s), 4.38 (2H, d), 4.70 (2H, s), 5.25-5.39 (2H , m), 5.92-6.11 (1H, m)
(工程4) 4-アリルオキシ-3-(クロロメチル)-2,5,6-トリメチルピリジンの合成
(4-メトキシ-2,5,6-トリメチルピリジン-3-イル)-メタノール7.9gをジクロロメタン100mlに溶解させた。この溶液に、氷冷下、塩化チオニル9.0gを滴下した。滴下終了後、室温で2時間攪拌した。その後、反応液を飽和重曹水に注加し、次いでジクロロメタンで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物7.7g(収率90%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.46(3H,s),2.57(3H,s),4.42(2H,d),4.67(2H,s),5.32-5.49(2H,m),6.06-6.14(1H,m) (Step 4) Synthesis of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine (7.9 g of (4-methoxy-2,5,6-trimethylpyridin-3-yl) -methanol in dichloromethane Dissolved in 100 ml. To this solution, 9.0 g of thionyl chloride was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was poured into saturated aqueous sodium hydrogen carbonate, and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 7.7 g (yield 90%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.16 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.42 (2H, d), 4.67 (2H, s), 5.32-5.49 (2H , m), 6.06-6.14 (1H, m)
(4-メトキシ-2,5,6-トリメチルピリジン-3-イル)-メタノール7.9gをジクロロメタン100mlに溶解させた。この溶液に、氷冷下、塩化チオニル9.0gを滴下した。滴下終了後、室温で2時間攪拌した。その後、反応液を飽和重曹水に注加し、次いでジクロロメタンで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物7.7g(収率90%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.46(3H,s),2.57(3H,s),4.42(2H,d),4.67(2H,s),5.32-5.49(2H,m),6.06-6.14(1H,m) (Step 4) Synthesis of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine (7.9 g of (4-methoxy-2,5,6-trimethylpyridin-3-yl) -methanol in dichloromethane Dissolved in 100 ml. To this solution, 9.0 g of thionyl chloride was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours. Thereafter, the reaction solution was poured into saturated aqueous sodium hydrogen carbonate, and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 7.7 g (yield 90%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.16 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.42 (2H, d), 4.67 (2H, s), 5.32-5.49 (2H , m), 6.06-6.14 (1H, m)
(工程5) 4-アリルオキシ-2,3,6-トリメチル-5-[[4-[4-(トリフルオロメトキシ)フェニル]シクロヘキソキシ]ピリジンの合成
(Step 5) Synthesis of 4-allyloxy-2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] pyridine
4-[4-(トリフルオロメトキシ)フェニル]シクロヘキサノール5.24gをN,N-ジメチルホルムアミド50mlに溶解させた。この溶液に氷冷下で55%水素化ナトリウム2.2gを加えた。次いで、室温で1時間攪拌した。得られた反応液に、氷冷下で、4-アリルオキシ-3-(クロロメチル)-2,5,6-トリメチルピリジン6.82gのN,N-ジメチルホルムアミド溶液(10ml)を滴下した。滴下終了後、室温で2時間攪拌、次いで50℃で一晩攪拌した。得られた反応液を氷水に注加し、次いでジクロロメタンで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物7.39g(収率82%)を得た。
1H-NMR(CDCl3,δppm) 1.38-1.51(4H,m),1.88-2.26(7H,m),2.44-2.55(7H,m),3.37-3.48(1H,m),4.39(2H,d),4.55(2H,s),5.29-5.46(2H,m),6.04-6.14(1H,m),7.10-7.21(4H,m) 4- [4- (trifluoromethoxy) phenyl] cyclohexanol (5.24 g) was dissolved in 50 ml of N, N-dimethylformamide. To this solution, 2.2 g of 55% sodium hydride was added under ice cooling. Subsequently, it stirred at room temperature for 1 hour. To the resulting reaction solution, a solution of 6.82 g of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine in N, N-dimethylformamide (10 ml) was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours, and then stirred at 50 ° C. overnight. The resulting reaction solution was poured into ice water and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 7.39 g (yield 82%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.38-1.51 (4H, m), 1.88-2.26 (7H, m), 2.44-2.55 (7H, m), 3.37-3.48 (1H, m), 4.39 (2H, d), 4.55 (2H, s), 5.29-5.46 (2H, m), 6.04-6.14 (1H, m), 7.10-7.21 (4H, m)
1H-NMR(CDCl3,δppm) 1.38-1.51(4H,m),1.88-2.26(7H,m),2.44-2.55(7H,m),3.37-3.48(1H,m),4.39(2H,d),4.55(2H,s),5.29-5.46(2H,m),6.04-6.14(1H,m),7.10-7.21(4H,m) 4- [4- (trifluoromethoxy) phenyl] cyclohexanol (5.24 g) was dissolved in 50 ml of N, N-dimethylformamide. To this solution, 2.2 g of 55% sodium hydride was added under ice cooling. Subsequently, it stirred at room temperature for 1 hour. To the resulting reaction solution, a solution of 6.82 g of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine in N, N-dimethylformamide (10 ml) was added dropwise under ice cooling. After completion of dropping, the mixture was stirred at room temperature for 2 hours, and then stirred at 50 ° C. overnight. The resulting reaction solution was poured into ice water and then extracted with dichloromethane. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 7.39 g (yield 82%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.38-1.51 (4H, m), 1.88-2.26 (7H, m), 2.44-2.55 (7H, m), 3.37-3.48 (1H, m), 4.39 (2H, d), 4.55 (2H, s), 5.29-5.46 (2H, m), 6.04-6.14 (1H, m), 7.10-7.21 (4H, m)
(工程6) 2,3,6-トリメチル-5-[[4-[4-(トリフルオロメトキシ)フェニル]シクロヘキソキシ]メチル]ピリジン-4-オールの合成
4-アリルオキシ-2,3,6-トリメチル-5-[[4-[4-(トリフルオロメトキシ)フェニル]シクロヘキソキシ]ピリジン0.5gをメタノール10mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.03g、および炭酸カリウム0.31gを加え、室温で2時間攪拌した。反応液を氷水に注ぎ、次いでクロロホルムで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.4g(収率88%)を得た。
1H-NMR(CDCl3,δppm) 1.31-1.49(2H,m),1.84-2.55(14H,m),3.33-3.42(1H,m),4.59(2H,s),7.08-7.17(4H,m) (Step 6) Synthesis of 2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] methyl] pyridin-4-ol 4-allyloxy-2,3,6- 0.5 g of trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] pyridine was dissolved in 10 ml of methanol. To this solution, 0.03 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate were added, and the mixture was stirred at room temperature for 2 hours. The reaction solution was poured into ice water and then extracted with chloroform. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.4 g (yield 88%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.31-1.49 (2H, m), 1.84-2.55 (14H, m), 3.33-3.42 (1H, m), 4.59 (2H, s), 7.08-7.17 (4H, m)
4-アリルオキシ-2,3,6-トリメチル-5-[[4-[4-(トリフルオロメトキシ)フェニル]シクロヘキソキシ]ピリジン0.5gをメタノール10mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.03g、および炭酸カリウム0.31gを加え、室温で2時間攪拌した。反応液を氷水に注ぎ、次いでクロロホルムで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.4g(収率88%)を得た。
1H-NMR(CDCl3,δppm) 1.31-1.49(2H,m),1.84-2.55(14H,m),3.33-3.42(1H,m),4.59(2H,s),7.08-7.17(4H,m) (Step 6) Synthesis of 2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] methyl] pyridin-4-ol 4-allyloxy-2,3,6- 0.5 g of trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] pyridine was dissolved in 10 ml of methanol. To this solution, 0.03 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate were added, and the mixture was stirred at room temperature for 2 hours. The reaction solution was poured into ice water and then extracted with chloroform. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.4 g (yield 88%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.31-1.49 (2H, m), 1.84-2.55 (14H, m), 3.33-3.42 (1H, m), 4.59 (2H, s), 7.08-7.17 (4H, m)
(工程7) メチル[2,3,6-トリメチル-5-[[4-(4-トリフルオロメトキシ)フェニル]シクロヘキソキシ]-4-ピリジル]カーボネートの合成
(Step 7) Synthesis of methyl [2,3,6-trimethyl-5-[[4- (4-trifluoromethoxy) phenyl] cyclohexoxy] -4-pyridyl] carbonate
2,3,6-トリメチル-5-[[4-[4-(トリフルオロメトキシ)フェニル]シクロヘキソキシ]メチル]ピリジン-4-オール0.4gをクロロホルム10mlに溶解させた。この溶液にトリエチルアミン0.12gを加えた。その後、氷冷下、クロロギ酸メチル0.11gを加えた。室温で一晩撹拌した。得られた反応液を飽和重曹水に注ぎ、クロロホルムで抽出した。有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物0.41g(収率90%)を得た。
1H-NMR(CDCl3,δppm) 1.31-1.51(2H,m),1.89-2.58(14H,m),3.29-3.37(1H,m),3.91(3H,s),4.52(2H,s),7.10-7.20(4H,m) 0.4 g of 2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] methyl] pyridin-4-ol was dissolved in 10 ml of chloroform. To this solution, 0.12 g of triethylamine was added. Thereafter, 0.11 g of methyl chloroformate was added under ice cooling. Stir overnight at room temperature. The resulting reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.41 g (yield 90%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.31-1.51 (2H, m), 1.89-2.58 (14H, m), 3.29-3.37 (1H, m), 3.91 (3H, s), 4.52 (2H, s) , 7.10-7.20 (4H, m)
1H-NMR(CDCl3,δppm) 1.31-1.51(2H,m),1.89-2.58(14H,m),3.29-3.37(1H,m),3.91(3H,s),4.52(2H,s),7.10-7.20(4H,m) 0.4 g of 2,3,6-trimethyl-5-[[4- [4- (trifluoromethoxy) phenyl] cyclohexoxy] methyl] pyridin-4-ol was dissolved in 10 ml of chloroform. To this solution, 0.12 g of triethylamine was added. Thereafter, 0.11 g of methyl chloroformate was added under ice cooling. Stir overnight at room temperature. The resulting reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.41 g (yield 90%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.31-1.51 (2H, m), 1.89-2.58 (14H, m), 3.29-3.37 (1H, m), 3.91 (3H, s), 4.52 (2H, s) , 7.10-7.20 (4H, m)
〔実施例2〕 3-[[2-[3,5-ビス(トリフルオロメチル)フェノキシ]-1-メチルプロピリデン]アミノ]オキシメチル]-2,5,6-トリメチル-4-ピリジル]アセテートの製造
(工程1) 2-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]イソインドリン-1,3-ジオンの合成 Example 2 3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-4-pyridyl] acetate (Step 1) Synthesis of 2-[(4-Allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] isoindoline-1,3-dione
(工程1) 2-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]イソインドリン-1,3-ジオンの合成 Example 2 3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-4-pyridyl] acetate (Step 1) Synthesis of 2-[(4-Allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] isoindoline-1,3-dione
N-ヒドロキシフタルイミド3.73gと4-アリルオキシ-3-(クロロメチル)-2,5,6-トリメチルピリジン4.29gをN,N-ジメチルホルムアミド100mlに溶解させた。この溶液に、氷冷下で、トリエチルアミン4.22gを加えた。室温で一晩攪拌した。得られた反応液を氷水に注加した。析出した結晶をろ過、乾燥させて、目的化合物4.54g(収率68%)を得た。
1H-NMR(CDCl3,δppm) 2.17(3H,s),2.48(3H,s),2.75(3H,s),4.63(2H,d),5.29(2H,s),5.31-5.54(2H,m),6.13-6.25(1H,m),7.74-7.83(4H,m) 3.73 g of N-hydroxyphthalimide and 4.29 g of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine were dissolved in 100 ml of N, N-dimethylformamide. To this solution, 4.22 g of triethylamine was added under ice cooling. Stir overnight at room temperature. The obtained reaction liquid was poured into ice water. The precipitated crystals were filtered and dried to obtain 4.54 g (yield 68%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.17 (3H, s), 2.48 (3H, s), 2.75 (3H, s), 4.63 (2H, d), 5.29 (2H, s), 5.31-5.54 (2H , m), 6.13-6.25 (1H, m), 7.74-7.83 (4H, m)
1H-NMR(CDCl3,δppm) 2.17(3H,s),2.48(3H,s),2.75(3H,s),4.63(2H,d),5.29(2H,s),5.31-5.54(2H,m),6.13-6.25(1H,m),7.74-7.83(4H,m) 3.73 g of N-hydroxyphthalimide and 4.29 g of 4-allyloxy-3- (chloromethyl) -2,5,6-trimethylpyridine were dissolved in 100 ml of N, N-dimethylformamide. To this solution, 4.22 g of triethylamine was added under ice cooling. Stir overnight at room temperature. The obtained reaction liquid was poured into ice water. The precipitated crystals were filtered and dried to obtain 4.54 g (yield 68%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.17 (3H, s), 2.48 (3H, s), 2.75 (3H, s), 4.63 (2H, d), 5.29 (2H, s), 5.31-5.54 (2H , m), 6.13-6.25 (1H, m), 7.74-7.83 (4H, m)
(工程2) O-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メチル]ヒドロキシルアミンの合成
(Step 2) Synthesis of O-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methyl] hydroxylamine
2-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]イソインドリン-1,3-ジオン2.00gをジクロロメタン40mlに溶解させた。この溶液に、氷冷下で、メチルヒドラジン0.53gを加え、0℃で2時間攪拌した。析出した結晶を濾別し、濾液を減圧濃縮して目的化合物1.43g(収率100%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.46(3H,s),2.57(3H,s),4.38(2H,d),4.75(2H,s),5.29-5.45(2H,m),6.04-6.14(1H,m) 2.00 g of 2-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] isoindoline-1,3-dione was dissolved in 40 ml of dichloromethane. To this solution, 0.53 g of methyl hydrazine was added under ice cooling, and the mixture was stirred at 0 ° C. for 2 hours. The precipitated crystals were separated by filtration, and the filtrate was concentrated under reduced pressure to obtain 1.43 g (yield 100%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.16 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.38 (2H, d), 4.75 (2H, s), 5.29-5.45 (2H , m), 6.04-6.14 (1H, m)
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.46(3H,s),2.57(3H,s),4.38(2H,d),4.75(2H,s),5.29-5.45(2H,m),6.04-6.14(1H,m) 2.00 g of 2-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] isoindoline-1,3-dione was dissolved in 40 ml of dichloromethane. To this solution, 0.53 g of methyl hydrazine was added under ice cooling, and the mixture was stirred at 0 ° C. for 2 hours. The precipitated crystals were separated by filtration, and the filtrate was concentrated under reduced pressure to obtain 1.43 g (yield 100%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.16 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.38 (2H, d), 4.75 (2H, s), 5.29-5.45 (2H , m), 6.04-6.14 (1H, m)
(工程3) N-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]-3-[3,5-ビス(トリフルオロメチル)フェノキシ]ブタン-2-イミンの合成
(Step 3) Synthesis of N-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] -3- [3,5-bis (trifluoromethyl) phenoxy] butan-2-imine
3-[3,5-ビス(トリフルオロメチル)フェノキシ]ブタン-2-オン1.11gをジクロロメタン10mlに溶解させた。この溶液に、氷冷下で、O-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メチル]ヒドロキシルアミン0.54gおよびトリフルオロ酢酸数滴を加え、室温で一晩攪拌した。得られた反応液から溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物0.97g(収率76%)を得た。
1H-NMR(CDCl3,δppm) 1.39-1.57(3H,m),1.73(3H,m),2.10-2.47(9H,m),4.32-4.41(2H,m),4.96-5.58(5H,m),5.97-6.13(1H,m),7.19-7.46(3H,m) 1.11 g of 3- [3,5-bis (trifluoromethyl) phenoxy] butan-2-one was dissolved in 10 ml of dichloromethane. To this solution was added 0.54 g of O-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methyl] hydroxylamine and a few drops of trifluoroacetic acid under ice cooling, and the mixture was stirred overnight at room temperature. did. The solvent was distilled off from the obtained reaction solution under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.97 g (yield 76%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.39-1.57 (3H, m), 1.73 (3H, m), 2.10-2.47 (9H, m), 4.32-4.41 (2H, m), 4.96-5.58 (5H, m), 5.97-6.13 (1H, m), 7.19-7.46 (3H, m)
1H-NMR(CDCl3,δppm) 1.39-1.57(3H,m),1.73(3H,m),2.10-2.47(9H,m),4.32-4.41(2H,m),4.96-5.58(5H,m),5.97-6.13(1H,m),7.19-7.46(3H,m) 1.11 g of 3- [3,5-bis (trifluoromethyl) phenoxy] butan-2-one was dissolved in 10 ml of dichloromethane. To this solution was added 0.54 g of O-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methyl] hydroxylamine and a few drops of trifluoroacetic acid under ice cooling, and the mixture was stirred overnight at room temperature. did. The solvent was distilled off from the obtained reaction solution under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.97 g (yield 76%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 1.39-1.57 (3H, m), 1.73 (3H, m), 2.10-2.47 (9H, m), 4.32-4.41 (2H, m), 4.96-5.58 (5H, m), 5.97-6.13 (1H, m), 7.19-7.46 (3H, m)
(工程4) 3-[[2-[3,5-ビス(トリフルオロメチル)フェノキシ]-1-メチルプロピリデン]アミノ]オキシメチル]-2,5,6-トリメチル-ピリジン-4-オールの合成
N-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]-3-[3,5-ビス(トリフルオロメチル)フェノキシ]ブタン-2-イミン0.97gをメタノール20mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.022gおよび炭酸カリウム0.78gを加え、室温で2時間攪拌した。得られた反応液を氷水に注ぎ、クロロホルムで抽出し、有機層を飽和食塩水で洗浄した。分液し、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.82g(収率92%)を得た。
1H-NMR(CDCl3,δppm) 1.39-1.53(3H,m),1.67-1.73(3H,m),2.00-2.28(9H,m),4.91-5.58(3H,m),7.18-7.40(3H,m) (Step 4) 3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-pyridin-4-ol Synthesis N-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] -3- [3,5-bis (trifluoromethyl) phenoxy] butan-2-imine (0.97 g) in methanol (20 ml) Dissolved in. To this solution were added 0.022 g of tetrakistriphenylphosphine palladium and 0.78 g of potassium carbonate, and the mixture was stirred at room temperature for 2 hours. The obtained reaction solution was poured into ice water, extracted with chloroform, and the organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.82 g (yield 92%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.39-1.53 (3H, m), 1.67-1.73 (3H, m), 2.00-2.28 (9H, m), 4.91-5.58 (3H, m), 7.18-7.40 ( (3H, m)
N-[(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)メトキシ]-3-[3,5-ビス(トリフルオロメチル)フェノキシ]ブタン-2-イミン0.97gをメタノール20mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.022gおよび炭酸カリウム0.78gを加え、室温で2時間攪拌した。得られた反応液を氷水に注ぎ、クロロホルムで抽出し、有機層を飽和食塩水で洗浄した。分液し、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.82g(収率92%)を得た。
1H-NMR(CDCl3,δppm) 1.39-1.53(3H,m),1.67-1.73(3H,m),2.00-2.28(9H,m),4.91-5.58(3H,m),7.18-7.40(3H,m) (Step 4) 3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-pyridin-4-ol Synthesis N-[(4-allyloxy-2,5,6-trimethyl-3-pyridyl) methoxy] -3- [3,5-bis (trifluoromethyl) phenoxy] butan-2-imine (0.97 g) in methanol (20 ml) Dissolved in. To this solution were added 0.022 g of tetrakistriphenylphosphine palladium and 0.78 g of potassium carbonate, and the mixture was stirred at room temperature for 2 hours. The obtained reaction solution was poured into ice water, extracted with chloroform, and the organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.82 g (yield 92%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.39-1.53 (3H, m), 1.67-1.73 (3H, m), 2.00-2.28 (9H, m), 4.91-5.58 (3H, m), 7.18-7.40 ( (3H, m)
(工程5) [3-[[2-[3,5-ビス(トリフルオロメチル)フェノキシ]-1-メチルプロピリデン]アミノ]オキシメチル]-2,5,6-トリメチル-4-ピリジル]アセテートの合成
(Step 5) [3-[[2- [3,5-Bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-4-pyridyl] acetate Synthesis of
3-[[2-[3,5-ビス(トリフルオロメチル)フェノキシ]-1-メチルプロピリデン]アミノ]オキシメチル]-2,5,6-トリメチル-ピリジン-4-オール0.82gをクロロホルム10mlに溶解させた。この溶液にトリエチルアミン0.21gを加えた。次いで、氷冷下、塩化アセチル0.17gを加えた。室温で一晩撹拌した。反応液を飽和重曹水に注ぎ、クロロホルムで抽出した。有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製し、目的化合物0.65g(収率72%)を得た。
1H-NMR(CDCl3,δppm) 1.39-1.53(3H,m),1.67-1.73(3H,m),2.03-2.50(12H,m),4.91-5.52(3H,m),7.21-7.44(3H,m) 3-[[2- [3,5-bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-pyridin-4-ol 0.82 g in chloroform Dissolved in 10 ml. To this solution, 0.21 g of triethylamine was added. Next, 0.17 g of acetyl chloride was added under ice cooling. Stir overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.65 g (yield 72%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.39-1.53 (3H, m), 1.67-1.73 (3H, m), 2.03-2.50 (12H, m), 4.91-5.52 (3H, m), 7.21-7.44 ( (3H, m)
1H-NMR(CDCl3,δppm) 1.39-1.53(3H,m),1.67-1.73(3H,m),2.03-2.50(12H,m),4.91-5.52(3H,m),7.21-7.44(3H,m) 3-[[2- [3,5-bis (trifluoromethyl) phenoxy] -1-methylpropylidene] amino] oxymethyl] -2,5,6-trimethyl-pyridin-4-ol 0.82 g in chloroform Dissolved in 10 ml. To this solution, 0.21 g of triethylamine was added. Next, 0.17 g of acetyl chloride was added under ice cooling. Stir overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.65 g (yield 72%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.39-1.53 (3H, m), 1.67-1.73 (3H, m), 2.03-2.50 (12H, m), 4.91-5.52 (3H, m), 7.21-7.44 ( (3H, m)
(実施例3) [3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチル-4-ピリジル]メチルカーボネートの製造
(工程1) 4-アリルオキシ-2,5,6-トリメチルピリジン-3-カルバルデヒドの合成 Example 3 [3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethyl-4-pyridyl] Production of methyl carbonate (Step 1) Synthesis of 4-allyloxy-2,5,6-trimethylpyridine-3-carbaldehyde
(工程1) 4-アリルオキシ-2,5,6-トリメチルピリジン-3-カルバルデヒドの合成 Example 3 [3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethyl-4-pyridyl] Production of methyl carbonate (Step 1) Synthesis of 4-allyloxy-2,5,6-trimethylpyridine-3-carbaldehyde
(4-アリルオキシ-2,5,6-トリメチルピリジン-3-イル)-メタノール7.9gをベンゼン90mlに溶解させた。この溶液に、二酸化マンガン15.2gを加え、一晩加熱還流した。反応液を室温まで冷却し、セライトろ過した。ろ液を減圧濃縮して、目的化合物5.98g(収率77%)を得た。
1H-NMR(CDCl3,δppm) 2.20(3H,s),2.51(3H,s),2.73(3H,s),4.42(2H,d),5.25-5.39(2H,m),5.92-6.11(1H,m),10.48(1H,s) 7.9 g of (4-allyloxy-2,5,6-trimethylpyridin-3-yl) -methanol was dissolved in 90 ml of benzene. To this solution, 15.2 g of manganese dioxide was added and heated to reflux overnight. The reaction was cooled to room temperature and filtered through celite. The filtrate was concentrated under reduced pressure to obtain 5.98 g (yield 77%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.20 (3H, s), 2.51 (3H, s), 2.73 (3H, s), 4.42 (2H, d), 5.25-5.39 (2H, m), 5.92-6.11. (1H, m), 10.48 (1H, s)
1H-NMR(CDCl3,δppm) 2.20(3H,s),2.51(3H,s),2.73(3H,s),4.42(2H,d),5.25-5.39(2H,m),5.92-6.11(1H,m),10.48(1H,s) 7.9 g of (4-allyloxy-2,5,6-trimethylpyridin-3-yl) -methanol was dissolved in 90 ml of benzene. To this solution, 15.2 g of manganese dioxide was added and heated to reflux overnight. The reaction was cooled to room temperature and filtered through celite. The filtrate was concentrated under reduced pressure to obtain 5.98 g (yield 77%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.20 (3H, s), 2.51 (3H, s), 2.73 (3H, s), 4.42 (2H, d), 5.25-5.39 (2H, m), 5.92-6.11. (1H, m), 10.48 (1H, s)
(工程2) エチル(E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エノエートの合成
(Step 2) Synthesis of ethyl (E) -3- (4-allyloxy-2,5,6-trimethyl-3-pyridyl) prop-2-enoate
ジエチルホスホノ酢酸エチル8.2gをテトラヒドロフラン80mlに溶解させた。この溶液に、氷冷下、60%水素化ナトリウム1.4gを加えた。0℃で30分間攪拌した。その後、4-アリルオキシ-2,5,6-トリメチルピリジン-3-カルバルデヒド5.0gを滴下した。滴下終了後、室温で2時間攪拌した。得られた反応液を氷水に注ぎ、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄した。分液し、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物4.21g(収率64%)を得た。
1H-NMR(CDCl3,δppm) 1.33(3H,t),2.17(3H,s),2.46(3H,s),2.57(3H,s),4.23-4.28(4H,m),5.28-5.40(2H,m),5.96-6.05(1H,m),6.54(1H,d),7.80(1H,d) 8.2 g of ethyl diethylphosphonoacetate was dissolved in 80 ml of tetrahydrofuran. To this solution, 1.4 g of 60% sodium hydride was added under ice cooling. Stir at 0 ° C. for 30 minutes. Thereafter, 5.0 g of 4-allyloxy-2,5,6-trimethylpyridine-3-carbaldehyde was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 2 hours. The resulting reaction solution was poured into ice water and extracted with ethyl acetate. The organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 4.21 g (yield: 64%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.33 (3H, t), 2.17 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.23-4.28 (4H, m), 5.28-5.40 (2H, m), 5.96-6.05 (1H, m), 6.54 (1H, d), 7.80 (1H, d)
1H-NMR(CDCl3,δppm) 1.33(3H,t),2.17(3H,s),2.46(3H,s),2.57(3H,s),4.23-4.28(4H,m),5.28-5.40(2H,m),5.96-6.05(1H,m),6.54(1H,d),7.80(1H,d) 8.2 g of ethyl diethylphosphonoacetate was dissolved in 80 ml of tetrahydrofuran. To this solution, 1.4 g of 60% sodium hydride was added under ice cooling. Stir at 0 ° C. for 30 minutes. Thereafter, 5.0 g of 4-allyloxy-2,5,6-trimethylpyridine-3-carbaldehyde was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 2 hours. The resulting reaction solution was poured into ice water and extracted with ethyl acetate. The organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 4.21 g (yield: 64%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.33 (3H, t), 2.17 (3H, s), 2.46 (3H, s), 2.57 (3H, s), 4.23-4.28 (4H, m), 5.28-5.40 (2H, m), 5.96-6.05 (1H, m), 6.54 (1H, d), 7.80 (1H, d)
(工程3) (E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エン-1-オールの合成
エチル(E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エノエート4.21gをジクロロメタン100mlに溶解させた。この溶液に、寒剤冷却下、1N水素化ジイソブチルアルミニウムトルエン溶液30mlを滴下した。滴下終了後、0℃で2時間攪拌した。その後、反応液を水酸化ナトリウム水溶液に注加した。ジクロロメタンで抽出し、有機層を飽和食塩水で洗浄した。分液し、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物3.3g(収率96%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.44(3H,s),2.48(3H,s),4.24-4.33(4H,m),5.22-5.40(2H,m),5.97-6.06(1H,m),6.28-6.37(1H,m),6.58(1H,d) (Step 3) Synthesis of (E) -3- (4-allyloxy-2,5,6-trimethyl-3-pyridyl) prop-2-en-1-ol Ethyl (E) -3- (4-allyloxy- 4.21 g of 2,5,6-trimethyl-3-pyridyl) prop-2-enoate was dissolved in 100 ml of dichloromethane. To this solution, 30 ml of 1N diisobutylaluminum hydride toluene solution was added dropwise under cooling with a cryogen. After completion of dropping, the mixture was stirred at 0 ° C. for 2 hours. Thereafter, the reaction solution was poured into an aqueous sodium hydroxide solution. The mixture was extracted with dichloromethane, and the organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 3.3 g (yield 96%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 2.16 (3H, s), 2.44 (3H, s), 2.48 (3H, s), 4.24-4.33 (4H, m), 5.22-5.40 (2H, m), 5.97 -6.06 (1H, m), 6.28-6.37 (1H, m), 6.58 (1H, d)
エチル(E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エノエート4.21gをジクロロメタン100mlに溶解させた。この溶液に、寒剤冷却下、1N水素化ジイソブチルアルミニウムトルエン溶液30mlを滴下した。滴下終了後、0℃で2時間攪拌した。その後、反応液を水酸化ナトリウム水溶液に注加した。ジクロロメタンで抽出し、有機層を飽和食塩水で洗浄した。分液し、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去して、目的化合物3.3g(収率96%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.44(3H,s),2.48(3H,s),4.24-4.33(4H,m),5.22-5.40(2H,m),5.97-6.06(1H,m),6.28-6.37(1H,m),6.58(1H,d) (Step 3) Synthesis of (E) -3- (4-allyloxy-2,5,6-trimethyl-3-pyridyl) prop-2-en-1-ol Ethyl (E) -3- (4-allyloxy- 4.21 g of 2,5,6-trimethyl-3-pyridyl) prop-2-enoate was dissolved in 100 ml of dichloromethane. To this solution, 30 ml of 1N diisobutylaluminum hydride toluene solution was added dropwise under cooling with a cryogen. After completion of dropping, the mixture was stirred at 0 ° C. for 2 hours. Thereafter, the reaction solution was poured into an aqueous sodium hydroxide solution. The mixture was extracted with dichloromethane, and the organic layer was washed with saturated brine. The layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 3.3 g (yield 96%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 2.16 (3H, s), 2.44 (3H, s), 2.48 (3H, s), 4.24-4.33 (4H, m), 5.22-5.40 (2H, m), 5.97 -6.06 (1H, m), 6.28-6.37 (1H, m), 6.58 (1H, d)
(工程4) 4-アリルオキシ-3-[(E)-3-ブロモプロプ-1-エニル]-2,5,6-トリメチルピリジンの合成
(E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エン-1-オール3.58gをジクロロメタン70mlに溶解させた。この溶液に、氷冷下、四臭化炭素5.34gおよびトリフェニルホスフィン4.22gを加え、室温で一晩攪拌した。得られた反応液から溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物3.5g(収率79%)を得た。
1H-NMR(CDCl3,δppm) 2.15(3H,s),2.44(3H,s),2.50(3H,s),4.15(3H,d),4.26(2H,d),5.24-5.41(2H,m),5.99-6.08(1H,m),6.39-6.47(1H,m),6.24(1H,d) (Step 4) Synthesis of 4-allyloxy-3-[(E) -3-bromoprop-1-enyl] -2,5,6-trimethylpyridine (E) -3- (4-allyloxy-2,5,6 -Trimethyl-3-pyridyl) prop-2-en-1-ol 3.58 g was dissolved in 70 ml of dichloromethane. To this solution, 5.34 g of carbon tetrabromide and 4.22 g of triphenylphosphine were added under ice cooling, and the mixture was stirred overnight at room temperature. The solvent was distilled off from the obtained reaction solution under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 3.5 g of the target compound (yield 79%).
1 H-NMR (CDCl 3 , δppm) 2.15 (3H, s), 2.44 (3H, s), 2.50 (3H, s), 4.15 (3H, d), 4.26 (2H, d), 5.24–5.41 (2H , m), 5.99-6.08 (1H, m), 6.39-6.47 (1H, m), 6.24 (1H, d)
(E)-3-(4-アリルオキシ-2,5,6-トリメチル-3-ピリジル)プロプ-2-エン-1-オール3.58gをジクロロメタン70mlに溶解させた。この溶液に、氷冷下、四臭化炭素5.34gおよびトリフェニルホスフィン4.22gを加え、室温で一晩攪拌した。得られた反応液から溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物3.5g(収率79%)を得た。
1H-NMR(CDCl3,δppm) 2.15(3H,s),2.44(3H,s),2.50(3H,s),4.15(3H,d),4.26(2H,d),5.24-5.41(2H,m),5.99-6.08(1H,m),6.39-6.47(1H,m),6.24(1H,d) (Step 4) Synthesis of 4-allyloxy-3-[(E) -3-bromoprop-1-enyl] -2,5,6-trimethylpyridine (E) -3- (4-allyloxy-2,5,6 -Trimethyl-3-pyridyl) prop-2-en-1-ol 3.58 g was dissolved in 70 ml of dichloromethane. To this solution, 5.34 g of carbon tetrabromide and 4.22 g of triphenylphosphine were added under ice cooling, and the mixture was stirred overnight at room temperature. The solvent was distilled off from the obtained reaction solution under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 3.5 g of the target compound (yield 79%).
1 H-NMR (CDCl 3 , δppm) 2.15 (3H, s), 2.44 (3H, s), 2.50 (3H, s), 4.15 (3H, d), 4.26 (2H, d), 5.24–5.41 (2H , m), 5.99-6.08 (1H, m), 6.39-6.47 (1H, m), 6.24 (1H, d)
(工程5) 4-アリルオキシ-3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジンの合成
(Step 5) Synthesis of 4-allyloxy-3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridine
2,4-ビストリフルオロメチルベンジルアルコール0.73gをN,N-ジメチルホルムアミド5mlおよびテトラヒドロフラン5mlからなる混合溶媒に溶解させた。この溶液に、氷冷下、60%水素化ナトリウム0.13gを加えた。室温で30分間攪拌した。得られた反応液に、氷冷下で、4-アリルオキシ-3-[(E)-3-ブロモプロプ-1-エニル]-2,5,6-トリメチルピリジン0.6gを滴下した。滴下終了後、室温で一晩攪拌した。反応液を氷水に注ぎ、酢酸エチルで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製し、目的化合物0.6g(収率65%)を得た。
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.44(3H,s),2.50(3H,s),4.26-4.29(4H,m),4.81(2H,s),5.18-5.38(2H,m),5.95-6.05(1H,m),6.24-6.68(2H,m),7.81-7.95(3H,m) 0.73 g of 2,4-bistrifluoromethylbenzyl alcohol was dissolved in a mixed solvent consisting of 5 ml of N, N-dimethylformamide and 5 ml of tetrahydrofuran. To this solution, 0.13 g of 60% sodium hydride was added under ice cooling. Stir at room temperature for 30 minutes. To the resulting reaction solution, 0.6 g of 4-allyloxy-3-[(E) -3-bromoprop-1-enyl] -2,5,6-trimethylpyridine was added dropwise under ice cooling. After completion of dropping, the mixture was stirred overnight at room temperature. The reaction mixture was poured into ice water and extracted with ethyl acetate. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The resulting residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.6 g (yield 65%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 2.16 (3H, s), 2.44 (3H, s), 2.50 (3H, s), 4.26-4.29 (4H, m), 4.81 (2H, s), 5.18-5.38 (2H, m), 5.95-6.05 (1H, m), 6.24-6.68 (2H, m), 7.81-7.95 (3H, m)
1H-NMR(CDCl3,δppm) 2.16(3H,s),2.44(3H,s),2.50(3H,s),4.26-4.29(4H,m),4.81(2H,s),5.18-5.38(2H,m),5.95-6.05(1H,m),6.24-6.68(2H,m),7.81-7.95(3H,m) 0.73 g of 2,4-bistrifluoromethylbenzyl alcohol was dissolved in a mixed solvent consisting of 5 ml of N, N-dimethylformamide and 5 ml of tetrahydrofuran. To this solution, 0.13 g of 60% sodium hydride was added under ice cooling. Stir at room temperature for 30 minutes. To the resulting reaction solution, 0.6 g of 4-allyloxy-3-[(E) -3-bromoprop-1-enyl] -2,5,6-trimethylpyridine was added dropwise under ice cooling. After completion of dropping, the mixture was stirred overnight at room temperature. The reaction mixture was poured into ice water and extracted with ethyl acetate. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The resulting residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.6 g (yield 65%) of the target compound.
1 H-NMR (CDCl 3 , δ ppm) 2.16 (3H, s), 2.44 (3H, s), 2.50 (3H, s), 4.26-4.29 (4H, m), 4.81 (2H, s), 5.18-5.38 (2H, m), 5.95-6.05 (1H, m), 6.24-6.68 (2H, m), 7.81-7.95 (3H, m)
(工程6) 3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジン-4-オールの合成
4-アリルオキシ-3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジン0.52gをメタノール10mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.01gおよび炭酸カリウム0.31gを加え、室温で2時間攪拌した。反応液を氷水に注ぎ、クロロホルムで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.35g(収率82%)を得た。
1H-NMR(CDCl3,δppm) 2.00(3H,s),2.27(3H,s),2.36(3H,s),4.24(2H,d),4.77(2H,s),6.57-7.03(2H,m),7.79-7.95(3H,m) (Step 6) Synthesis of 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol 4-Allyloxy-3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridine (0.52 g) in 10 ml of methanol Dissolved in. To this solution were added 0.01 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate, and the mixture was stirred at room temperature for 2 hours. The reaction solution was poured into ice water and extracted with chloroform. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.35 g (yield 82%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.00 (3H, s), 2.27 (3H, s), 2.36 (3H, s), 4.24 (2H, d), 4.77 (2H, s), 6.57-7.03 (2H , m), 7.79-7.95 (3H, m)
4-アリルオキシ-3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジン0.52gをメタノール10mlに溶解させた。この溶液にテトラキストリフェニルホスフィンパラジウム0.01gおよび炭酸カリウム0.31gを加え、室温で2時間攪拌した。反応液を氷水に注ぎ、クロロホルムで抽出した。有機層を飽和食塩水で洗浄した。分液して、有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をジエチルエーテルで洗浄して、目的化合物0.35g(収率82%)を得た。
1H-NMR(CDCl3,δppm) 2.00(3H,s),2.27(3H,s),2.36(3H,s),4.24(2H,d),4.77(2H,s),6.57-7.03(2H,m),7.79-7.95(3H,m) (Step 6) Synthesis of 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol 4-Allyloxy-3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridine (0.52 g) in 10 ml of methanol Dissolved in. To this solution were added 0.01 g of tetrakistriphenylphosphine palladium and 0.31 g of potassium carbonate, and the mixture was stirred at room temperature for 2 hours. The reaction solution was poured into ice water and extracted with chloroform. The organic layer was washed with saturated brine. Liquid separation was performed, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was washed with diethyl ether to obtain 0.35 g (yield 82%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.00 (3H, s), 2.27 (3H, s), 2.36 (3H, s), 4.24 (2H, d), 4.77 (2H, s), 6.57-7.03 (2H , m), 7.79-7.95 (3H, m)
(工程7) [3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチル-4-ピリジル]メチルカーボネートの合成
(Step 7) [3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethyl-4-pyridyl] methyl Carbonate synthesis
3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジン-4-オール0.15gをクロロホルム10mlに溶解させた。この溶液にトリエチルアミン0.07gを加えた。次いで、氷冷下で、クロロギ酸メチル0.07gを加えた。得られた液を室温で一晩撹拌した。反応液を飽和重曹水に注ぎ、クロロホルムで抽出した。有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物0.14g(収率81%)を得た。
1H-NMR(CDCl3,δppm) 2.11(3H,s),2.49(3H,s),2.50(3H,s),3.83(3H,s),4.25(2H,d),4.80(2H,s),6.08-6.54(2H,m),7.82-7.95(3H,m) 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol (0.15 g) in chloroform (10 ml) Dissolved in. To this solution, 0.07 g of triethylamine was added. Next, 0.07 g of methyl chloroformate was added under ice cooling. The resulting solution was stirred overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.14 g (yield 81%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.11 (3H, s), 2.49 (3H, s), 2.50 (3H, s), 3.83 (3H, s), 4.25 (2H, d), 4.80 (2H, s ), 6.08-6.54 (2H, m), 7.82-7.95 (3H, m)
1H-NMR(CDCl3,δppm) 2.11(3H,s),2.49(3H,s),2.50(3H,s),3.83(3H,s),4.25(2H,d),4.80(2H,s),6.08-6.54(2H,m),7.82-7.95(3H,m) 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol (0.15 g) in chloroform (10 ml) Dissolved in. To this solution, 0.07 g of triethylamine was added. Next, 0.07 g of methyl chloroformate was added under ice cooling. The resulting solution was stirred overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.14 g (yield 81%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 2.11 (3H, s), 2.49 (3H, s), 2.50 (3H, s), 3.83 (3H, s), 4.25 (2H, d), 4.80 (2H, s ), 6.08-6.54 (2H, m), 7.82-7.95 (3H, m)
(実施例4) [3-[3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロピル]-2,5,6-トリメチル-4-ピリジル]メチルカーボネートの製造
(工程1) 3-[3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロピル]-2,5,6-トリメチルピリジン-4-オール Example 4 Production of [3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethyl-4-pyridyl] methyl carbonate (Step 1) 3- [3-[[2,4-Bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethylpyridin-4-ol
(工程1) 3-[3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロピル]-2,5,6-トリメチルピリジン-4-オール Example 4 Production of [3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethyl-4-pyridyl] methyl carbonate (Step 1) 3- [3-[[2,4-Bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethylpyridin-4-ol
3-[(E)-3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロプ-1-エニル]-2,5,6-トリメチルピリジン-4-オール0.2gをメタノール20mlに溶解させた。この溶液に10%パラジウム炭素0.02gを加え、水素雰囲気下、室温で一晩攪拌した。反応液をセライト濾過した。濾液を減圧濃縮して、目的化合物0.2g(収率100%)を得た。
1H-NMR(CDCl3,δppm) 1.82-1.92(2H,m),2.00(3H,s),2.24(3H,s),2.27(3H,s),2.59-2.64(2H,m),3.57-3.60(2H,m),4.71(2H,s),7.78-7.95(3H,m) 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol 0.2 g was added to 20 ml of methanol. Dissolved in. To this solution was added 0.02 g of 10% palladium carbon, and the mixture was stirred overnight at room temperature under a hydrogen atmosphere. The reaction solution was filtered through celite. The filtrate was concentrated under reduced pressure to obtain 0.2 g (yield 100%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.82-1.92 (2H, m), 2.00 (3H, s), 2.24 (3H, s), 2.27 (3H, s), 2.59-2.64 (2H, m), 3.57 -3.60 (2H, m), 4.71 (2H, s), 7.78-7.95 (3H, m)
1H-NMR(CDCl3,δppm) 1.82-1.92(2H,m),2.00(3H,s),2.24(3H,s),2.27(3H,s),2.59-2.64(2H,m),3.57-3.60(2H,m),4.71(2H,s),7.78-7.95(3H,m) 3-[(E) -3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] prop-1-enyl] -2,5,6-trimethylpyridin-4-ol 0.2 g was added to 20 ml of methanol. Dissolved in. To this solution was added 0.02 g of 10% palladium carbon, and the mixture was stirred overnight at room temperature under a hydrogen atmosphere. The reaction solution was filtered through celite. The filtrate was concentrated under reduced pressure to obtain 0.2 g (yield 100%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.82-1.92 (2H, m), 2.00 (3H, s), 2.24 (3H, s), 2.27 (3H, s), 2.59-2.64 (2H, m), 3.57 -3.60 (2H, m), 4.71 (2H, s), 7.78-7.95 (3H, m)
(工程2) [3-[3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロピル]-2,5,6-トリメチル-4-ピリジル]メチルカーボネートの合成
(Step 2) Synthesis of [3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethyl-4-pyridyl] methyl carbonate
3-[3-[[2,4-ビス(トリフルオロメチル)フェニル]メトキシ]プロピル]-2,5,6-トリメチルピリジン-4-オール0.23gをクロロホルム10mlに溶解させた。この溶液にトリエチルアミン0.11gを加えた。次いで、氷冷下で、クロロギ酸メチル0.1gを加えた。得られた液を室温で一晩撹拌した。反応液を飽和重曹水に注ぎ、クロロホルムで抽出した。有機層を無水硫酸マグネシウムで乾燥させた。溶媒を減圧留去した。得られた残渣をシリカゲルカラムクロマトグラフィー(展開溶媒:n-ヘキサン/酢酸エチル)にて精製して、目的化合物0.16g(収率60%)を得た。
1H-NMR(CDCl3,δppm) 1.81-1.89(2H,m),2.06(3H,s),2.47(3H,s),2.51(3H,s),2.64-2.69(2H,m),3.56-3.60(2H,m),3.84(3H,s),4.73(2H,s),7.80-7.92(3H,m) 0.23 g of 3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethylpyridin-4-ol was dissolved in 10 ml of chloroform. To this solution, 0.11 g of triethylamine was added. Next, 0.1 g of methyl chloroformate was added under ice cooling. The resulting solution was stirred overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.16 g (yield 60%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.81-1.89 (2H, m), 2.06 (3H, s), 2.47 (3H, s), 2.51 (3H, s), 2.64-2.69 (2H, m), 3.56 -3.60 (2H, m), 3.84 (3H, s), 4.73 (2H, s), 7.80-7.92 (3H, m)
1H-NMR(CDCl3,δppm) 1.81-1.89(2H,m),2.06(3H,s),2.47(3H,s),2.51(3H,s),2.64-2.69(2H,m),3.56-3.60(2H,m),3.84(3H,s),4.73(2H,s),7.80-7.92(3H,m) 0.23 g of 3- [3-[[2,4-bis (trifluoromethyl) phenyl] methoxy] propyl] -2,5,6-trimethylpyridin-4-ol was dissolved in 10 ml of chloroform. To this solution, 0.11 g of triethylamine was added. Next, 0.1 g of methyl chloroformate was added under ice cooling. The resulting solution was stirred overnight at room temperature. The reaction solution was poured into saturated aqueous sodium hydrogen carbonate and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane / ethyl acetate) to obtain 0.16 g (yield 60%) of the target compound.
1 H-NMR (CDCl 3 , δppm) 1.81-1.89 (2H, m), 2.06 (3H, s), 2.47 (3H, s), 2.51 (3H, s), 2.64-2.69 (2H, m), 3.56 -3.60 (2H, m), 3.84 (3H, s), 4.73 (2H, s), 7.80-7.92 (3H, m)
上記の実施例と同様の方法で製造した化合物を第1表~第13表に示す。併せて、化合物の物性データを「物性」の欄に記載した。物性データとしては、融点〔mp(℃)〕またはその性状を記載した。
表中、Meはメチル基、Etはエチル基、nPrはノルマルプロピル基、iPrはイソプロピル基、cPrはシクロプロピル基、nBuはノルマルブチル基、tBuはターシャリーブチル基、nPenはノルマルペンチル基、nHexはノルマルヘキシル基、cHexはシクロヘキシル基、nNonはノルマルノニル基Phはフェニル基、Pyはピリジル基、Acはアシル基を示す。 Compounds prepared by the same method as in the above examples are shown in Tables 1 to 13. In addition, the physical property data of the compounds are described in the “Physical Properties” column. As the physical property data, the melting point [mp (° C.)] or its property was described.
In the table, Me is a methyl group, Et is an ethyl group, n Pr is a normal propyl group, i Pr is an isopropyl group, c Pr is a cyclopropyl group, n Bu is a normal butyl group, t Bu is a tertiary butyl group, n Pen Is a normal pentyl group, n Hex is a normal hexyl group, c Hex is a cyclohexyl group, n Non is a normal nonyl group Ph is a phenyl group, Py is a pyridyl group, and Ac is an acyl group.
表中、Meはメチル基、Etはエチル基、nPrはノルマルプロピル基、iPrはイソプロピル基、cPrはシクロプロピル基、nBuはノルマルブチル基、tBuはターシャリーブチル基、nPenはノルマルペンチル基、nHexはノルマルヘキシル基、cHexはシクロヘキシル基、nNonはノルマルノニル基Phはフェニル基、Pyはピリジル基、Acはアシル基を示す。 Compounds prepared by the same method as in the above examples are shown in Tables 1 to 13. In addition, the physical property data of the compounds are described in the “Physical Properties” column. As the physical property data, the melting point [mp (° C.)] or its property was described.
In the table, Me is a methyl group, Et is an ethyl group, n Pr is a normal propyl group, i Pr is an isopropyl group, c Pr is a cyclopropyl group, n Bu is a normal butyl group, t Bu is a tertiary butyl group, n Pen Is a normal pentyl group, n Hex is a normal hexyl group, c Hex is a cyclohexyl group, n Non is a normal nonyl group Ph is a phenyl group, Py is a pyridyl group, and Ac is an acyl group.
第1表は、式(I-1)で表される化合物における置換基を示す。
なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と示す。 Table 1 shows substituents in the compound represented by the formula (I-1).
In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond of the imine in the compound is in the E configuration is indicated as “E”, the case where the imine is in the Z configuration is indicated as “Z”, and the case where both the configurations are mixed is indicated as “EZ”.
なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と示す。 Table 1 shows substituents in the compound represented by the formula (I-1).
In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond of the imine in the compound is in the E configuration is indicated as “E”, the case where the imine is in the Z configuration is indicated as “Z”, and the case where both the configurations are mixed is indicated as “EZ”.
第1表中のA-12とA-13は立体異性体である。第1表中の、A-17とA-18、A-21とA-22、A-23とA-24は、立体異性体である。
A-12 and A-13 in Table 1 are stereoisomers. In Table 1, A-17 and A-18, A-21 and A-22, and A-23 and A-24 are stereoisomers.
第2表は、式(I-2)で表される化合物における置換基を示す。
なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。A中の二重結合が、E配置である場合を「E」、Z配置である場合を「Z」と表記する。 Table 2 shows substituents in the compound represented by the formula (I-2).
In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。A中の二重結合が、E配置である場合を「E」、Z配置である場合を「Z」と表記する。 Table 2 shows substituents in the compound represented by the formula (I-2).
In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
第3表は、式(I-3)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。A中の二重結合が、E配置である場合を「E」、Z配置である場合を「Z」と表記する。
Table 3 shows substituents in the compound represented by the formula (I-3). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
第4表は、式(I-4)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と表記する。
Table 4 shows substituents in the compound represented by the formula (I-4). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. The case where the double bond of the imine in the compound is in the E configuration is referred to as “E”, the case where it is in the Z configuration as “Z”, and the case where both configurations are mixed as “EZ”.
第5表は、式(I-5)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。Bbが非対称な部分構造の場合、構造式中の*の付された炭素はXと結合する炭素を示し、**の付された炭素はAr2と結合する炭素を示す。A中の二重結合が、E配置である場合を「E」、Z配置である場合を「Z」と表記する。
化合物E-5~E-51、E-58、E-59、E-110~E-114、E-116、E-159、またはE-160において、Bb中の1,4-シクロへキシレンの立体配置が、トランス配置である場合を「trans」、シス配置である場合を「cis」と表記する。
化合物E-63、E-66、E-119、E-122、またはE-162におぴて、Bb中の架橋部分と、*または**を付した炭素上の置換基の立体配置が、シス配置である場合を「cis」と表記する。 Table 5 shows substituents in the compound represented by the formula (I-5). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. In the case where B b is an asymmetric partial structure, a carbon marked with * in the structural formula represents a carbon bonded to X, and a carbon marked with ** represents a carbon bonded to Ar 2 . The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
In compounds E-5 to E-51, E-58, E-59, E-110 to E-114, E-116, E-159, or E-160, 1,4-cyclohexylene in B b When the steric configuration is a trans configuration, it is expressed as “trans”, and when it is a cis configuration, it is expressed as “cis”.
In the compound E-63, E-66, E-119, E-122, or E-162, the configuration of the bridging moiety in B b and the substituent on the carbon marked with * or ** is In the case of a cis configuration, it is expressed as “cis”.
化合物E-5~E-51、E-58、E-59、E-110~E-114、E-116、E-159、またはE-160において、Bb中の1,4-シクロへキシレンの立体配置が、トランス配置である場合を「trans」、シス配置である場合を「cis」と表記する。
化合物E-63、E-66、E-119、E-122、またはE-162におぴて、Bb中の架橋部分と、*または**を付した炭素上の置換基の立体配置が、シス配置である場合を「cis」と表記する。 Table 5 shows substituents in the compound represented by the formula (I-5). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. In the case where B b is an asymmetric partial structure, a carbon marked with * in the structural formula represents a carbon bonded to X, and a carbon marked with ** represents a carbon bonded to Ar 2 . The case where the double bond in A is E configuration is expressed as “E”, and the case where it is Z configuration is expressed as “Z”.
In compounds E-5 to E-51, E-58, E-59, E-110 to E-114, E-116, E-159, or E-160, 1,4-cyclohexylene in B b When the steric configuration is a trans configuration, it is expressed as “trans”, and when it is a cis configuration, it is expressed as “cis”.
In the compound E-63, E-66, E-119, E-122, or E-162, the configuration of the bridging moiety in B b and the substituent on the carbon marked with * or ** is In the case of a cis configuration, it is expressed as “cis”.
第6表は、式(I-6)で表される化合物における置換基を示す。
化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と示す。 Table 6 shows substituents in the compound represented by the formula (I-6).
The case where the double bond of the imine in the compound is in the E configuration is indicated as “E”, the case where the imine is in the Z configuration is indicated as “Z”, and the case where both the configurations are mixed is indicated as “EZ”.
化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と示す。 Table 6 shows substituents in the compound represented by the formula (I-6).
The case where the double bond of the imine in the compound is in the E configuration is indicated as “E”, the case where the imine is in the Z configuration is indicated as “Z”, and the case where both the configurations are mixed is indicated as “EZ”.
第7表は、式(I-7)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。
Table 7 shows the substituents in the compound represented by the formula (I-7). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
第8表は、式(I-8)で表される化合物における置換基を示す。化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と表記する。
Table 8 shows the substituents in the compound represented by the formula (I-8). The case where the double bond of the imine in the compound is in the E configuration is referred to as “E”, the case where it is in the Z configuration as “Z”, and the case where both configurations are mixed as “EZ”.
第9表は、式(I-9)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。Bbが非対称な部分構造の場合、構造式中の*の付された炭素はXと結合する炭素を示す。
化合物I-2~I-8、I-36~I-38、またはI-40においてBb中の1,4-シクロへキシレンの立体配置が、トランス配置である場合を「trans」、シス配置である場合を「cis」と表記する。 Table 9 shows substituents in the compound represented by the formula (I-9). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. In the case where B b is an asymmetric partial structure, the carbon marked with * in the structural formula represents a carbon bonded to X.
When the configuration of 1,4-cyclohexylene in B b in compound I-2 to I-8, I-36 to I-38, or I-40 is trans configuration, “trans”, cis configuration The case of “is” is expressed as “cis”.
化合物I-2~I-8、I-36~I-38、またはI-40においてBb中の1,4-シクロへキシレンの立体配置が、トランス配置である場合を「trans」、シス配置である場合を「cis」と表記する。 Table 9 shows substituents in the compound represented by the formula (I-9). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine. In the case where B b is an asymmetric partial structure, the carbon marked with * in the structural formula represents a carbon bonded to X.
When the configuration of 1,4-cyclohexylene in B b in compound I-2 to I-8, I-36 to I-38, or I-40 is trans configuration, “trans”, cis configuration The case of “is” is expressed as “cis”.
第10表は、式(I-10)で表される化合物における置換基を示す。化合物中のイミンの二重結合が、E配置である場合を「E」、Z配置である場合を「Z」、および両配置が混合している場合を「EZ」と表記する。
Table 10 shows the substituents in the compound represented by the formula (I-10). The case where the double bond of the imine in the compound is in the E configuration is referred to as “E”, the case where it is in the Z configuration as “Z”, and the case where both configurations are mixed as “EZ”.
第12表は、式(I-12)で表される化合物における置換基を示す。
Table 12 shows substituents in the compound represented by the formula (I-12).
第14表は、式(I-13)で表される化合物における置換基を示す。
Table 14 shows substituents in the compound represented by the formula (I-13).
第15表は、式(I-14)で表される化合物における置換基を示す。なお、Ar3の構造式中*の付された炭素は隣接する基と結合する炭素を示す。
Table 15 shows substituents in the compound represented by the formula (I-14). In addition, the carbon marked with * in the structural formula of Ar 3 represents carbon bonded to an adjacent group.
第16表は、式(I-15)で表される化合物における置換基を示す。なお、Aが非対称な部分構造の場合、構造式中の*の付された炭素はピリジンと結合する炭素を示す。
Table 16 shows substituents in the compound represented by Formula (I-15). In the case where A is an asymmetric partial structure, the carbon marked with * in the structural formula represents the carbon bonded to pyridine.
上記の第1表~第16表に記載した化合物のいくつかについて、1H-NMRデータを、第17表に示す。
Table 1 shows the 1 H-NMR data for some of the compounds described in Tables 1 to 16 above.
〔生物試験〕
本発明化合物が、農園芸用殺菌剤、殺虫剤または殺ダニ剤の有効成分として有用であることを以下の試験例で示す。 [Biological test]
The following test examples show that the compounds of the present invention are useful as active ingredients of agricultural and horticultural fungicides, insecticides or acaricides.
本発明化合物が、農園芸用殺菌剤、殺虫剤または殺ダニ剤の有効成分として有用であることを以下の試験例で示す。 [Biological test]
The following test examples show that the compounds of the present invention are useful as active ingredients of agricultural and horticultural fungicides, insecticides or acaricides.
(試験例1)コムギうどんこ病防除試験-1
本発明化合物5部、ポリオキシエチレンソルビタンモノラウレート1.5部、ジメチルホルムアミド93.5部を混合溶解して、有効成分5%の乳剤を調製した。該乳剤を本発明化合物が100ppmになるように水で希釈した。
続いて素焼きポットで栽培したコムギ幼苗(品種「チホク」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギうどんこ病菌(Erysiphe graminis f.sp.tritici)の分生胞子を振り払い接種し、20℃の温室に静置した。4日後に葉上の病斑出現状態を調査した(処理区)。一方、該希釈溶液を散布せずコムギを栽培し、同様に病斑出現状態を調査した(無処理区)。処理区と無処理区を比較調査し、下記式0000より防除価を算出した。
防除価(%)=100-{病斑が出現した面積(処理区)/病斑が出現した面積(無処理区)}×100
第18表に示す化合物についてコムギうどんこ病防除試験を行った。いずれの化合物も防除価が75%以上であった。 (Test Example 1) Wheat powdery mildew control test-1
An emulsion of 5% active ingredient was prepared by mixing and dissolving 5 parts of the compound of the present invention, 1.5 parts of polyoxyethylene sorbitan monolaurate and 93.5 parts of dimethylformamide. The emulsion was diluted with water so that the compound of the present invention was 100 ppm.
Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C. Four days later, the appearance of lesions on the leaves was examined (treatment area). On the other hand, wheat was cultivated without spraying the diluted solution, and the lesion appearance state was similarly investigated (untreated section). The treated area and the untreated area were compared and the control value was calculated from the following formula 0000.
Control value (%) = 100− {area where lesions appear (treated area) / area where lesions appear (untreated area)} × 100
The compounds shown in Table 18 were subjected to wheat powdery mildew control test. All the compounds had a control value of 75% or more.
本発明化合物5部、ポリオキシエチレンソルビタンモノラウレート1.5部、ジメチルホルムアミド93.5部を混合溶解して、有効成分5%の乳剤を調製した。該乳剤を本発明化合物が100ppmになるように水で希釈した。
続いて素焼きポットで栽培したコムギ幼苗(品種「チホク」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギうどんこ病菌(Erysiphe graminis f.sp.tritici)の分生胞子を振り払い接種し、20℃の温室に静置した。4日後に葉上の病斑出現状態を調査した(処理区)。一方、該希釈溶液を散布せずコムギを栽培し、同様に病斑出現状態を調査した(無処理区)。処理区と無処理区を比較調査し、下記式0000より防除価を算出した。
防除価(%)=100-{病斑が出現した面積(処理区)/病斑が出現した面積(無処理区)}×100
第18表に示す化合物についてコムギうどんこ病防除試験を行った。いずれの化合物も防除価が75%以上であった。 (Test Example 1) Wheat powdery mildew control test-1
An emulsion of 5% active ingredient was prepared by mixing and dissolving 5 parts of the compound of the present invention, 1.5 parts of polyoxyethylene sorbitan monolaurate and 93.5 parts of dimethylformamide. The emulsion was diluted with water so that the compound of the present invention was 100 ppm.
Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C. Four days later, the appearance of lesions on the leaves was examined (treatment area). On the other hand, wheat was cultivated without spraying the diluted solution, and the lesion appearance state was similarly investigated (untreated section). The treated area and the untreated area were compared and the control value was calculated from the following formula 0000.
Control value (%) = 100− {area where lesions appear (treated area) / area where lesions appear (untreated area)} × 100
The compounds shown in Table 18 were subjected to wheat powdery mildew control test. All the compounds had a control value of 75% or more.
(試験例2)コムギ赤さび病防除試験-1
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が100ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「農林61号」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギ赤さび病菌(Puccinia recondita)の夏胞子を振り払い接種し、20℃の温室に静置した。10日後に葉上の病斑出現状態を調査した(処理区)。試験例1と同様に葉上の病斑出現状態を無処理区と比較調査し、防除価を算出した。
第19表に示す化合物についてコムギ赤さび病防除試験を行った。いずれの化合物も防除価が75%以上であった。 (Test Example 2) Wheat red rust control test-1
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 100 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section). In the same manner as in Test Example 1, the lesion appearance state on the leaves was compared with the untreated group, and the control value was calculated.
The compound shown in Table 19 was subjected to a wheat red rust control test. All the compounds had a control value of 75% or more.
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が100ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「農林61号」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギ赤さび病菌(Puccinia recondita)の夏胞子を振り払い接種し、20℃の温室に静置した。10日後に葉上の病斑出現状態を調査した(処理区)。試験例1と同様に葉上の病斑出現状態を無処理区と比較調査し、防除価を算出した。
第19表に示す化合物についてコムギ赤さび病防除試験を行った。いずれの化合物も防除価が75%以上であった。 (Test Example 2) Wheat red rust control test-1
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 100 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section). In the same manner as in Test Example 1, the lesion appearance state on the leaves was compared with the untreated group, and the control value was calculated.
The compound shown in Table 19 was subjected to a wheat red rust control test. All the compounds had a control value of 75% or more.
(試験例3)コムギうどんこ病防除試験-2
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が125ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「チホク」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギうどんこ病菌(Erysiphe graminis f.sp.tritici)の分生胞子を振り払い接種し、20℃の温室に静置した。4日後に葉上の病斑出現状態を調査した(処理区)。
試験例1と同様に葉上の病斑出現状態を無処理と比較調査し、防除価を算出した。 (Test Example 3) Wheat powdery mildew control test-2
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C. Four days later, the appearance of lesions on the leaves was examined (treatment area).
In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が125ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「チホク」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギうどんこ病菌(Erysiphe graminis f.sp.tritici)の分生胞子を振り払い接種し、20℃の温室に静置した。4日後に葉上の病斑出現状態を調査した(処理区)。
試験例1と同様に葉上の病斑出現状態を無処理と比較調査し、防除価を算出した。 (Test Example 3) Wheat powdery mildew control test-2
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Chihoku”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, conidia spores of wheat powdery mildew (Erysiphe graminis f. Sp. Tritici) were shaken off and inoculated, and left in a greenhouse at 20 ° C. Four days later, the appearance of lesions on the leaves was examined (treatment area).
In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
第20表に示す化合物についてコムギうどんこ病防除試験を行った。いずれの化合物も75%以上の防除価を示した。
A wheat powdery mildew control test was conducted on the compounds shown in Table 20. All the compounds showed a control value of 75% or more.
(試験例4)コムギ赤さび病防除試験-2
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が125ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「農林61号」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギ赤さび病菌(Puccinia recondita)の夏胞子を振り払い接種し、20℃の温室に静置した。10日後に葉上の病斑出現状態を調査した(処理区)。試験例1と同様に葉上の病斑出現状態を無処理と比較調査し、防除価を算出した。 (Test Example 4) Wheat red rust control test-2
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section). In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
試験例1と同じ方法で乳剤を調製した。該乳剤を本発明化合物が125ppmになるように水で希釈した。続いて素焼きポットで栽培したコムギ幼苗(品種「農林61号」、1~2葉期)に該希釈溶液を散布した。風乾後、コムギ赤さび病菌(Puccinia recondita)の夏胞子を振り払い接種し、20℃の温室に静置した。10日後に葉上の病斑出現状態を調査した(処理区)。試験例1と同様に葉上の病斑出現状態を無処理と比較調査し、防除価を算出した。 (Test Example 4) Wheat red rust control test-2
An emulsion was prepared in the same manner as in Test Example 1. The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Subsequently, the diluted solution was sprayed on wheat seedlings (variety “Noribayashi No. 61”, 1-2 leaf stage) cultivated in an unglazed pot. After air drying, summer spores of wheat rust (Puccinia recondita) were shaken off and inoculated, and left in a 20 ° C. greenhouse. Ten days later, the appearance of lesions on the leaves was examined (treatment section). In the same manner as in Test Example 1, the lesion appearance state on the leaf was compared with no treatment, and the control value was calculated.
第21表に示す化合物についてコムギ赤さび病防除試験を行った。いずれの化合物も75%以上の防除価を示した。
The compound shown in Table 21 was subjected to a wheat red rust control test. All the compounds showed a control value of 75% or more.
(試験例5)ハスモンヨトウに対する効力確認試験
試験例1と同じ方法で、有効成分5%の乳剤を調製した。
該乳剤を本発明化合物が125ppmになるように水で希釈した。キャベツ葉を前記希釈液に30秒間浸漬した。このキャベツ葉を、シャーレに入れ、ハスモントウ2齢幼虫5頭を放した。シャーレを温度25℃、湿度60%の恒温室内に置いた。放虫から6日間経過したときに生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 5) Efficacy confirmation test against Japanese scallop In the same manner as in Test Example 1, an emulsion of 5% active ingredient was prepared.
The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Cabbage leaves were immersed in the diluted solution for 30 seconds. The cabbage leaves were placed in a petri dish and 5 second instar larvae were released. The petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 6 days had passed since the release, life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
試験例1と同じ方法で、有効成分5%の乳剤を調製した。
該乳剤を本発明化合物が125ppmになるように水で希釈した。キャベツ葉を前記希釈液に30秒間浸漬した。このキャベツ葉を、シャーレに入れ、ハスモントウ2齢幼虫5頭を放した。シャーレを温度25℃、湿度60%の恒温室内に置いた。放虫から6日間経過したときに生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 5) Efficacy confirmation test against Japanese scallop In the same manner as in Test Example 1, an emulsion of 5% active ingredient was prepared.
The emulsion was diluted with water so that the compound of the present invention was 125 ppm. Cabbage leaves were immersed in the diluted solution for 30 seconds. The cabbage leaves were placed in a petri dish and 5 second instar larvae were released. The petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 6 days had passed since the release, life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
第22表に示す化合物についてハスモンヨトウに対する効力確認試験を行った。いずれの化合物も殺虫率が100%であった。
The compound shown in Table 22 was tested for efficacy against Spodoptera litura. All compounds had an insecticidal rate of 100%.
(試験例6)ワタアブラムシに対する効力確認試験
3寸鉢でキュウリを育苗し、第一本葉上にワタアブラムシ若虫を接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をキュウリ苗に散布した。該キュウリを温度25℃、湿度60%の恒温室内に置いた。散布から4日間経過したときにワタアブラムシの生死判定を行い、殺虫率を算出した。試験は2反復で行った。
第23表に示す化合物についてワタアブラムシに対する効力確認試験を行った。いずれの化合物も殺虫率が100%であった。 (Test Example 6) Efficacy confirmation test for cotton aphids Cucumbers were raised in 3-inch pots, and cotton aphid nymphs were inoculated on the first true leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cucumber seedlings. The cucumber was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, cotton aphids were evaluated for life and death, and the insecticidal rate was calculated. The test was performed in duplicate.
The compounds shown in Table 23 were tested for efficacy against cotton aphids. All compounds had an insecticidal rate of 100%.
3寸鉢でキュウリを育苗し、第一本葉上にワタアブラムシ若虫を接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をキュウリ苗に散布した。該キュウリを温度25℃、湿度60%の恒温室内に置いた。散布から4日間経過したときにワタアブラムシの生死判定を行い、殺虫率を算出した。試験は2反復で行った。
第23表に示す化合物についてワタアブラムシに対する効力確認試験を行った。いずれの化合物も殺虫率が100%であった。 (Test Example 6) Efficacy confirmation test for cotton aphids Cucumbers were raised in 3-inch pots, and cotton aphid nymphs were inoculated on the first true leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cucumber seedlings. The cucumber was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, cotton aphids were evaluated for life and death, and the insecticidal rate was calculated. The test was performed in duplicate.
The compounds shown in Table 23 were tested for efficacy against cotton aphids. All compounds had an insecticidal rate of 100%.
(試験例7)ナミハダニに対する効力確認試験
3寸鉢でインゲンを育苗し、初生葉上に、カンザワハダニ雌成虫を10頭接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をインゲン幼苗に散布した。該インゲンを温度25℃、湿度65%の恒温室内に置いた。散布から3日間経過したときに成虫の生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 7) Efficacy confirmation test against nymph spider mung bean seedlings were grown in 3 inch pots, and 10 Kanzawa spider mite female adults were inoculated on primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings. The green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 3 days had passed since the spraying, the life and death of adults were determined, and the insecticidal rate was calculated. The test was performed in duplicate.
3寸鉢でインゲンを育苗し、初生葉上に、カンザワハダニ雌成虫を10頭接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をインゲン幼苗に散布した。該インゲンを温度25℃、湿度65%の恒温室内に置いた。散布から3日間経過したときに成虫の生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 7) Efficacy confirmation test against nymph spider mung bean seedlings were grown in 3 inch pots, and 10 Kanzawa spider mite female adults were inoculated on primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings. The green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 3 days had passed since the spraying, the life and death of adults were determined, and the insecticidal rate was calculated. The test was performed in duplicate.
第24表に示す化合物についてナミハダニに対する効力確認試験を行った。いずれの化合物も殺虫率が100%であった。
The compound shown in Table 24 was tested for efficacy against urticae. All compounds had an insecticidal rate of 100%.
(試験例8)アワヨトウに対する効力確認試験
試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈した。トウモロコシ葉を前記希釈液に30秒間浸漬した。このトウモロコシ葉を、シャーレに入れ、アワヨトウ2齢幼虫5頭を放した。シャーレを温度25℃、湿度60%の恒温室内に置いた。放虫から6日間経過したときに生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 8) Efficacy Confirmation Test for Ayayoto An emulsion prepared by the same method as Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm. Corn leaves were immersed in the diluent for 30 seconds. This corn leaf was put into a petri dish and 5 larvae of the second instar larvae were released. The petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 6 days had passed since the release, life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈した。トウモロコシ葉を前記希釈液に30秒間浸漬した。このトウモロコシ葉を、シャーレに入れ、アワヨトウ2齢幼虫5頭を放した。シャーレを温度25℃、湿度60%の恒温室内に置いた。放虫から6日間経過したときに生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 8) Efficacy Confirmation Test for Ayayoto An emulsion prepared by the same method as Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm. Corn leaves were immersed in the diluent for 30 seconds. This corn leaf was put into a petri dish and 5 larvae of the second instar larvae were released. The petri dish was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 6 days had passed since the release, life / death determination was performed and the insecticidal rate was calculated. The test was performed in duplicate.
第25表に示す化合物についてアワヨトウに対する効力確認試験を行った。いずれの化合物も殺虫率が100%であった。
The compound shown in Table 25 was subjected to a potency confirmation test against Amanita. All compounds had an insecticidal rate of 100%.
(試験例9)マメアブラムシに対する効力確認試験
3寸鉢でササゲを育苗し、初生葉上にマメアブラムシ若虫を接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をササゲ苗に散布した。該ササゲを温度25℃、湿度60%の恒温室内に置いた。散布から4日間経過したときにマメアブラムシの生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 9) Efficacy confirmation test for bean aphids Cowpeas were raised in 3-inch pots, and bean aphid nymphs were inoculated on the primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cowpea seedlings. The cowpea was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, the aphid of the bean aphid was determined and the insecticidal rate was calculated. The test was performed in duplicate.
3寸鉢でササゲを育苗し、初生葉上にマメアブラムシ若虫を接種した。試験例1と同じ方法で調製した乳剤を、本発明化合物が125ppmになるように水で希釈し、該希釈液をササゲ苗に散布した。該ササゲを温度25℃、湿度60%の恒温室内に置いた。散布から4日間経過したときにマメアブラムシの生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 9) Efficacy confirmation test for bean aphids Cowpeas were raised in 3-inch pots, and bean aphid nymphs were inoculated on the primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on cowpea seedlings. The cowpea was placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 60%. When 4 days have passed since spraying, the aphid of the bean aphid was determined and the insecticidal rate was calculated. The test was performed in duplicate.
第26表に示す化合物についてマメアブラムシに対する効力確認試験を行った。いずれの化合物も殺虫率が80%以上であった。
The compounds shown in Table 26 were tested for efficacy against bean aphids. All compounds had an insecticidal rate of 80% or more.
(試験例10)カンザワハダニに対する効力確認試験
3寸鉢でインゲンを育苗し、初生葉上に、カンザワハダニ雌成虫を10頭接種した。試験例1と同じ方法で調製した乳剤を本発明化合物が125ppmになるように水で希釈し、該希釈液をインゲン幼苗に散布した。該インゲンを温度25℃、湿度65%の恒温室内に置いた。散布から10日間経過したときに成虫の生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 10) Efficacy Confirmation Test for Kanzawa Spider Mite Green beans were bred in 3 sized pots, and 10 adult female Kanzawa spider mites were inoculated on the primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings. The green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 10 days have passed since spraying, adults were determined to be alive or dead, and the insecticidal rate was calculated. The test was performed in duplicate.
3寸鉢でインゲンを育苗し、初生葉上に、カンザワハダニ雌成虫を10頭接種した。試験例1と同じ方法で調製した乳剤を本発明化合物が125ppmになるように水で希釈し、該希釈液をインゲン幼苗に散布した。該インゲンを温度25℃、湿度65%の恒温室内に置いた。散布から10日間経過したときに成虫の生死判定を行い、殺虫率を算出した。試験は2反復で行った。 (Test Example 10) Efficacy Confirmation Test for Kanzawa Spider Mite Green beans were bred in 3 sized pots, and 10 adult female Kanzawa spider mites were inoculated on the primary leaves. An emulsion prepared by the same method as in Test Example 1 was diluted with water so that the compound of the present invention was 125 ppm, and the diluted solution was sprayed on kidney seedlings. The green beans were placed in a constant temperature room at a temperature of 25 ° C. and a humidity of 65%. When 10 days have passed since spraying, adults were determined to be alive or dead, and the insecticidal rate was calculated. The test was performed in duplicate.
第27表に示す化合物についてカンザワハダニに対する効力確認試験を行った。いずれの化合物も殺虫率が80%以上であった。
The compounds shown in Table 27 were tested for efficacy against the Kanzawa spider mite. All compounds had an insecticidal rate of 80% or more.
本発明に係るピリジン化合物は、有害生物防除、殺菌、殺ダニ、殺虫などの効果を有し、植物体に薬害を生じることがなく、人畜魚類に対する毒性や環境への影響が少ない新規化合物である。特に、ムギ病害に対して優れた防除効果を示す。本発明に係るピリジン化合物は、農園芸用殺菌剤、有害生物防除剤、および殺虫または殺ダニ剤の有効成分として有用であり、産業上有用である。
The pyridine compound according to the present invention is a novel compound that has effects such as pest control, bactericidal, acaricidal, insecticidal and the like, does not cause phytotoxicity on plants, and has little toxicity to human fish and environmental impacts. . In particular, it exhibits an excellent control effect against wheat diseases. The pyridine compound according to the present invention is useful as an active ingredient of agricultural and horticultural fungicides, pest control agents, and insecticides or acaricides, and is industrially useful.
Claims (6)
- 式(I)で表される化合物、またはその互変異性体若しくは塩。
R1は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、無置換の若しくはG2で置換されたC6~10アリール基、シアノ基またはハロゲノ基を示す。
R2およびR3は、それぞれ独立に、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG1で置換されたC1~6アルコキシ基、ホルミル基、ホルミルオキシ基、無置換の若しくはG1で置換されたC1~6アルキルカルボニルオキシ基、無置換の若しくはG2で置換されたC6~10アリール基、(無置換の若しくはG1で置換されたC1~6アルコキシイミノ)-C1~6アルキル基、シアノ基、またはハロゲノ基を示す。
ここで、R1とR2は、相互に繋がって、R1およびR2のそれぞれが結合する炭素原子とともに5~6員環を形成してもよい。
R4は、水素原子、無置換の若しくはG1で置換されたC1~6アルキル基、無置換の若しくはG1で置換されたC2~6アルケニル基、無置換の若しくはG1で置換されたC2~6アルキニル基、無置換の若しくはG2で置換されたC3~8シクロアルキル基、無置換の若しくはG2で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルC1~6アルキル基、ホルミル基、無置換の若しくはG1で置換されたC1~6アルキルカルボニル基、無置換の若しくはG2で置換されたC6~10アリールカルボニル基、無置換の若しくはG1で置換されたC1~6アルコキシカルボニル基、無置換の若しくはG1で置換されたC2~6アルケニルオキシカルボニル基、無置換の若しくはG1で置換されたC1~6アルキルスルホニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノカルボニル基、無置換の若しくはG1で置換された(C1~6アルキルチオ)カルボニル基、無置換の若しくはG1で置換されたC1~6アルキルアミノ(チオカルボニル)基、または式(II)で表される有機基を示す。
式(II)中、Gbは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC2~6アルキニル基、無置換のもしくはG2で置換されたC3~8シクロアルキル基、無置換のもしくはG2で置換されたC6~10アリール基、または無置換のもしくはG2で置換された3~6員ヘテロシクリル基を示す。
式(II)中、Tは、酸素原子、オキシカルボニル基、カルボニルオキシ基、オキシカルボニルオキシ基、硫黄原子、(チオ)カルボニル基、カルボニル(チオ)基、(チオ)カルボニルオキシ基、オキシカルボニル(チオ)基、または-O-C(=O)-N(Gb)-で表される二価の基を示す。
*は式(II)で表される基の結合位置を示す。
Qは、式(III)~式(XII)で表される有機基のいずれかを示す。
Ar1は、無置換の若しくはG2で置換されたC6~10アリール基、または無置換の若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Ar2は、無置換の若しくはG2で置換されたC6~10アリール基、無置換の若しくはG2で置換されたC6~10アリールオキシ基、無置換の若しくはG2で置換された3~6員ヘテロシクリル基、無置換の若しくはG2で置換された3~6員ヘテロシクリルオキシ基、または無置換の若しくはG2で置換された3~6員ヘテロシクリルチオ基を示す。
Ar3は、無置換若しくはG2で置換されたC6~10アリール基を示す。
Ar4は、無置換若しくはG2で置換されたC6~10アリーレン基を示す。
Ar5は、無置換若しくはG2で置換された3~6員ヘテロシクリル基を示す。
Raは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG2で置換されたC6~10アリール基、またはアミノ基を示す。ここで、Ar1とRaは、相互に繋がって、Ar1とRaが結合する炭素原子とともに5~6員環を形成してもよい。
Xは、酸素原子、硫黄原子、スルフィニル基、スルホニル基、またはNRcで表される二価の基を示す。Rcは、水素原子、無置換のもしくはG1で置換されたC1~6アルキル基、無置換のもしくはG1で置換されたC2~6アルケニル基、無置換のもしくはG1で置換されたC1~6アルキルカルボニル基、無置換のもしくはG2で置換されたC6~10アリールカルボニル基、無置換のもしくはG2で置換されたC6~10アリールスルホニル基、無置換のもしくはG1で置換されたC1~6アルコキシカルボニル基、または無置換のもしくはG1で置換されたC3~8シクロアルキルオキシカルボニル基を示す。
Aは、無置換のもしくはG3で置換されたC1~C6アルキレン基、無置換のもしくはG3で置換されたC2~C6アルケニレン基、無置換のもしくはG3で置換されたC2~C6アルキニレン基、無置換のもしくはG3で置換されたC1~C6アルキレンオキシ基、無置換のもしくはG3で置換されたオキシC1~C6アルキレン基、またはカルボニル基を示す。
Baは、無置換の若しくはG4で置換されたC1~C6アルキレン基、無置換の若しくはG4で置換されたC2~C6アルケニレン基、無置換の若しくはG4で置換されたC2~C6アルキニレン基、無置換の若しくはG4で置換されたC1~C6アルキレンオキシC1~6アルキレン基、無置換の若しくはG4で置換されたC3~C6シクロアルキレン基、無置換の若しくはG4で置換されたC4~C6シクロアルケニレン基、無置換の若しくはG4で置換された3~6員ヘテロシクリレン基、またはNRdで表される二価の基を示す。Rdは、水素原子、C1~6アルキル基、またはC1~6アルコキシカルボニル基を示す。また、式(V)中、BaとRaは、相互に繋がって、BaとRaが結合する炭素原子とともに5~6員環を形成してもよい。
Bbは、無置換のもしくはG4で置換されたC1~C6アルキレン基、無置換のもしくはG4で置換されたC2~C6アルケニレン基、無置換のもしくはG4で置換されたC2~C6アルキニレン基、無置換のもしくはG4で置換されたC3~C6シクロアルキレン基、無置換のもしくはG4で置換されたC4~C6シクロアルケニレン基、無置換のもしくはG4で置換された3~6員ヘテロシクリレン基、またはカルボニル基を示す。
T1は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-メチル基、1-(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシイミノ)-エチル基、または無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基を示す。
T2は、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-ホルミル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-C1~6アルキルカルボニル-アミノ基、(無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ)-C1~6アルキル基、または(無置換のもしくはG2で置換されたC6~10アリールC1~6アルキル)-3~6員ヘテロシクリル基を示す。
T3は、無置換のもしくはG2で置換されたC6~10アリールC1~6アルコキシ基、または無置換のもしくはG2で置換されたC6~10アリールアミノカルボニル-C1~6アルキル基を示す。
*は、式(III)~式(XII)で表される有機基の結合位置を示す。
G1は、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、シアノ基、またはハロゲノ基を示す。R1、R2、R3、R4、Ra、Rc、Ga、またはGbのうちの2つ以上がG1で置換された前記の基である場合、係るG1は、相互に同じであっても異なってもよい。
G2は、C1~6アルキル基、C2~6アルケニル基、C2~6アルキニル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C1~6アルコキシC1~6ハロアルキル基、C2~6ハロアルケニル基、C2~6ハロアルキニル基、水酸基、C1~6アルコキシ基、C3~8シクロアルキルC1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C1~6ハロアルコキシ基、C1~6ハロアルコキシC1~6ハロアルコキシ基、ホルミル基、C1~6アルキルカルボニル基、C1~6アルコキシカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、C1~6アルコキシカルボニルオキシ基、C1~6アルコキシカルボニルアミノ基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシ基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシ基、C1~6アルキルチオ基、C1~6アルキルスルフィニル基、C1~6アルキルスルホニル基、C1~6ハロアルキルチオ基、C1~6ハロアルキルスルフィニル基、C1~6ハロアルキルスルホニル基、ペンタフルオロスルファニル基、無置換の若しくはG21で置換されたC6~10アリールチオ基、無置換の若しくはG21で置換されたC6~10アリールスルフィニル基、無置換の若しくはG21で置換されたC6~10アリールスルホニル基、無置換の若しくはG21で置換されたC6~10アリールアミノ基、ニトロ基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、またはC1~6ハロアルキレンジオキシ基を示す。R1、R2、R3、R4、Ra、Rc、Ga、Gb、Ar1、Ar2、Ar3、Ar4、Ar5、T1、T2、またはT3のうちの2つ以上がG2で置換された前記の基である場合、係るG2は、相互に同じであっても異なってもよい。
G21は、C1~6アルキル基、C1~6ハロアルキル基、C1~6アルコキシ基、C1~6ハロアルコキシ基、C6~10アリール基またはハロゲノ基を示す。G2またはG4のうちの2つ以上がG21で置換された前記の基である場合、係るG21は、相互に同じであっても異なってもよい。
G3は、C1~6アルキル基、C1~6アルコキシ基、ホルミル基、C1~6アルキルカルボニル基、ホルミルオキシ基、C1~6アルキルカルボニルオキシ基、ハロゲノ基、C1~6アルキレン基、またはオキソ基を示す。
G4は、C1~6アルキル基、C3~8シクロアルキル基、C1~6ハロアルキル基、C1~6アルコキシC1~6アルキル基、C2~6アルケニルオキシC1~6アルキル基、水酸基、C1~6アルコキシ基、C1~6アルコキシC1~6アルコキシ基、C2~6アルケニルオキシ基、C1~6アルコキシカルボニル基、無置換の若しくはG21で置換されたC6~10アリール基、無置換の若しくはG21で置換された3~6員ヘテロシクリル基、シアノ基、ハロゲノ基、C1~6アルキレン基、C1~6アルキレンジオキシ基、オキソ基、C3~8シクロアルキルC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルC1~6アルキル基、C3~8シクロアルキルオキシC1~6アルキル基、無置換の若しくはG21で置換されたC6~10アリールオキシC1~6アルキル基、無置換の若しくはG21で置換された3~6員ヘテロシクリルオキシC1~6アルキル基、C1~6アルキリデン基、C1~6アルコキシイミノ基、無置換の若しくはG21で置換されたC6~10アリールC1~6アルコキシイミノ基、またはC1~6アルキルヒドラジノ基を示す。
ただし、Qが式(VII)または(VIII)の場合、R1は水素原子、ハロゲノ基、シアノ基、メトキシ基、トリフルオロメチル基ではない。 A compound represented by formula (I), or a tautomer or salt thereof.
R 1 is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 ~ 6 alkoxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, a cyano group or a halogeno group.
R 2 and R 3 are each independently a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or C3-8 cycloalkyl group substituted with G 2 , C1-6 alkoxy group unsubstituted or substituted with G 1 , formyl group, formyloxy group, C1-6 alkyl unsubstituted or substituted with G 1 carbonyloxy group, an unsubstituted or C6 ~ 10 aryl group substituted by G 2, (unsubstituted or C1 ~ 6 alkoxyimino substituted with G 1) -C1 ~ 6 alkyl group, a cyano group, or a halogeno group, Indicates.
Here, R 1 and R 2 may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which each of R 1 and R 2 is bonded.
R 4 is a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C2 substituted 1-6 alkynyl group, an unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl C1 to 6 alkyl group substituted with G 2, unsubstituted or with G 2 A 3-6 membered heterocyclyl C1-6 alkyl group, a formyl group, a C1-6 alkylcarbonyl group which is unsubstituted or substituted with G 1 , a C6-10 arylcarbonyl group which is unsubstituted or substituted with G 2 , unsubstituted or C1 ~ 6 alkoxycarbonyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyloxycarbonyl group substituted with G 1, unsubstituted or with G 1 Conversion has been C1 ~ 6 alkylsulfonyl group, an unsubstituted or C1 ~ 6 alkylaminocarbonyl group substituted with G 1, unsubstituted or substituted with G 1 (C1 ~ 6 alkylthio) carbonyl group, the unsubstituted Or a C1-6 alkylamino (thiocarbonyl) group substituted by G 1 or an organic group represented by the formula (II).
Wherein (II), G b represents a hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, unsubstituted or G C2 ~ 6 alkynyl group substituted by one, unsubstituted or C3 ~ 8 cycloalkyl group substituted with G 2, unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or G, 3 to 6-membered heterocyclyl group substituted by 2 .
In formula (II), T represents an oxygen atom, an oxycarbonyl group, a carbonyloxy group, an oxycarbonyloxy group, a sulfur atom, a (thio) carbonyl group, a carbonyl (thio) group, a (thio) carbonyloxy group, an oxycarbonyl ( (Thio) group or a divalent group represented by —O—C (═O) —N (G b ) —.
* Indicates the bonding position of the group represented by the formula (II).
Q represents any one of organic groups represented by the formulas (III) to (XII).
Ar 1 represents an unsubstituted or C6 ~ 10 aryl group substituted by G 2 or unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 2,.
Ar 2 is unsubstituted or C6 ~ 10 aryl group substituted by G 2, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 2, 3 substituted with unsubstituted or G 2 ~ 6 It shows a membered heterocyclyl group, an unsubstituted or 3-6 membered heterocyclyloxy group substituted with G 2 or unsubstituted or 3-6 membered heterocyclylthio group substituted with G 2,.
Ar 3 represents a C6-10 aryl group which is unsubstituted or substituted with G 2 .
Ar 4 represents a C6-10 arylene group which is unsubstituted or substituted with G 2 .
Ar 5 represents a 3-6 membered heterocyclyl group which is unsubstituted or substituted with G 2 .
R a represents a hydrogen atom, an unsubstituted or substituted C 1-6 alkyl group substituted with G 1 , an unsubstituted or substituted C 2-10 aryl group substituted with G 2 , or an amino group. Here, Ar 1 and R a may be connected to each other to form a 5- to 6-membered ring together with the carbon atom to which Ar 1 and R a are bonded.
X represents an oxygen atom, a sulfur atom, a sulfinyl group, a sulfonyl group, or a divalent group represented by NR c . R c is hydrogen atom, an unsubstituted or C1 ~ 6 alkyl group substituted with G 1, unsubstituted or C2 ~ 6 alkenyl group substituted with G 1, which is substituted by unsubstituted or G 1 C1 1-6 alkylcarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted with G 2, unsubstituted or C6 ~ 10 arylsulfonyl group substituted with G 2, substituted with unsubstituted or G 1 A C1-6 alkoxycarbonyl group or a C3-8 cycloalkyloxycarbonyl group which is unsubstituted or substituted with G 1 is shown.
A is unsubstituted or C1 ~ C6 alkylene group substituted with G 3, unsubstituted or C2 ~ C6 alkenylene group substituted with G 3, unsubstituted or C2 ~ C6 alkynylene group substituted with G 3 , An unsubstituted or G 3 substituted C1-C6 alkyleneoxy group, an unsubstituted or G 3 substituted oxy C1-C6 alkylene group, or a carbonyl group.
B a is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C1 ~ C6 alkyleneoxy C1 ~ 6 alkylene group substituted with G 4, unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, which is substituted unsubstituted or at G 4 A C4-C6 cycloalkenylene group, an unsubstituted or 3- to 6-membered heterocyclylene group substituted with G 4 , or a divalent group represented by NR d is shown. R d represents a hydrogen atom, a C1-6 alkyl group, or a C1-6 alkoxycarbonyl group. In the formula (V), B a and R a are connected to each other, they may form a 5- to 6-membered ring together with the carbon atom to which B a and R a are attached.
B b is unsubstituted or C1 ~ C6 alkylene group substituted with G 4, unsubstituted or C2 ~ C6 alkenylene group substituted with G 4, unsubstituted or C2 ~ C6 alkynylene substituted with G 4 group, an unsubstituted or C3 ~ C6 cycloalkylene group substituted by G 4, unsubstituted or C4 ~ C6 cycloalkenylene group substituted with G 4, unsubstituted or 3-6 membered substituted by G 4 A heterocyclylene group or a carbonyl group is shown.
T 1 is (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxyimino) -methyl group, 1- (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy Imino) -ethyl, or an unsubstituted or G 2 substituted C6-10 aryl C1-6 alkoxy group.
T 2 represents (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -amino group, (unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkyl) -formyl - amino group, (unsubstituted or which is substituted G 2 a C6 ~ 10 aryl C1 ~ 6 alkyl)-C1 ~ 6 alkyl-carbonyl - amino group, (unsubstituted or C6 ~ 10 aryl C1 which is substituted by G 2 ~ shows the 6 alkoxy)-C1 ~ 6 alkyl group or a (unsubstituted or C6 ~ 10 aryl C1-6 alkyl substituted with G 2) -3 ~ 6-membered heterocyclyl group.
T 3 represents an unsubstituted or G 2 -substituted C 6-10 aryl C 1-6 alkoxy group, or an unsubstituted or G 2 -substituted C 6-10 aryl aminocarbonyl-C 1-6 alkyl group.
* Indicates the bonding position of the organic group represented by formula (III) to formula (XII).
G 1 is a hydroxyl group, C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxycarbonyloxy group, cyano A group or a halogeno group. R 1, R 2, R 3 , R 4, R a, R c, when it is the group more than two substituted by G 1 of G a or G b,, G 1 according the mutual May be the same or different.
G 2 is a C1-6 alkyl group, C2-6 alkenyl group, C2-6 alkynyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C1-6 alkoxyC1 ~ 6 haloalkyl group, C2-6 haloalkenyl group, C2-6 haloalkynyl group, hydroxyl group, C1-6 alkoxy group, C3-8 cycloalkyl C1-6 alkoxy group, C1-6 alkoxy C1-6 alkoxy group, C1 ~ 6 haloalkoxy group, C1-6 haloalkoxy C1-6 haloalkoxy group, formyl group, C1-6 alkylcarbonyl group, C1-6 alkoxycarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, C1-6 alkoxy carbonyloxy group, C1 ~ 6 alkoxycarbonylamino group, an unsubstituted or G 21 Substituted C6 ~ 10 aryl group, an unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkyl group substituted with G 21, unsubstituted or C6 ~ 10 aryloxy group which is substituted by G 21, unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxy group substituted by G 21, unsubstituted or 3-6 membered heterocyclyl group which is substituted by G 21, unsubstituted or 3-6 membered heterocyclyloxy group which is substituted by G 21 C1-6 alkylthio group, C1-6 alkylsulfinyl group, C1-6 alkylsulfonyl group, C1-6 haloalkylthio group, C1-6 haloalkylsulfinyl group, C1-6 haloalkylsulfonyl group, pentafluorosulfanyl group, unsubstituted or C6 ~ 10 arylthio group substituted with G 21, C6 substituted with unsubstituted or G 21 ~ 10 arylsulfinyl group, C6-10 arylsulfonyl group unsubstituted or substituted with G 21 , C6-10 arylamino group unsubstituted or substituted with G 21 , nitro group, cyano group, halogeno group, C1— A 6 alkylene group, a C1-6 alkylenedioxy group, or a C1-6 haloalkylenedioxy group; Of R 1 , R 2 , R 3 , R 4 , R a , R c , G a , G b , Ar 1 , Ar 2 , Ar 3 , Ar 4 , Ar 5 , T 1 , T 2 , or T 3 If more than one is a said group substituted with G 2, G 2 according may be the same or different from each other.
G 21 represents a C1-6 alkyl group, a C1-6 haloalkyl group, a C1-6 alkoxy group, a C1-6 haloalkoxy group, a C6-10 aryl group or a halogeno group. If more than one of G 2 or G 4 is a said group substituted with G 21, G 21 according may be the same or different from each other.
G 3 is a C1-6 alkyl group, C1-6 alkoxy group, formyl group, C1-6 alkylcarbonyl group, formyloxy group, C1-6 alkylcarbonyloxy group, halogeno group, C1-6 alkylene group, or oxo group Indicates.
G 4 is a C1-6 alkyl group, C3-8 cycloalkyl group, C1-6 haloalkyl group, C1-6 alkoxy C1-6 alkyl group, C2-6 alkenyloxy C1-6 alkyl group, hydroxyl group, C1-6 alkoxy group, C1 ~ 6 alkoxy C1 ~ 6 alkoxy group, substituted with C2 ~ 6 alkenyloxy group, C1 ~ 6 alkoxycarbonyl group, an unsubstituted or C6 ~ 10 aryl group substituted by G 21, unsubstituted or G 21 3-6 membered heterocyclyl group, cyano group, halogeno group, C1-6 alkylene group, C1-6 alkylenedioxy group, oxo group, C3-8 cycloalkyl C1-6 alkyl group, unsubstituted or at G 21 substituted C6 ~ 10 aryl C1 ~ 6 alkyl group, 3- to 6-membered heterocyclyl C1 ~ 6 alkyl substituted with unsubstituted or G 21 Group, C3 ~ 8 cycloalkyloxy C1 ~ 6 alkyl group, unsubstituted or C6 ~ 10 aryloxy C1 ~ 6 alkyl group substituted with G 21, unsubstituted or 3-6 membered heterocyclyl substituted with G 21 oxy C1 ~ 6 alkyl group, C1 ~ 6 alkylidene group, C1 ~ 6 alkoxyimino group, the unsubstituted or C6 ~ 10 aryl C1 ~ 6 alkoxyimino group substituted by G 21 or C1 ~ 6 alkyl hydrazino group, Show.
However, when Q is the formula (VII) or (VIII), R 1 is not a hydrogen atom, a halogeno group, a cyano group, a methoxy group, or a trifluoromethyl group. - 式(I)中のR4が、水素原子またはアリル基である、請求項1に記載の化合物、またはその互変異性体若しくは塩。 The compound according to claim 1, or a tautomer or salt thereof, wherein R 4 in formula (I) is a hydrogen atom or an allyl group.
- 請求項1および2に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する農園芸用殺菌剤。 An agricultural and horticultural fungicide containing as an active ingredient at least one selected from the group consisting of the compound according to claim 1 and 2, and tautomers and salts thereof.
- 請求項1および2に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する有害生物防除剤。 A pest control agent comprising at least one selected from the group consisting of the compound according to claim 1 and 2 and tautomers and salts thereof as an active ingredient.
- 請求項1および2に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する殺虫または殺ダニ剤。 An insecticide or acaricide containing as an active ingredient at least one selected from the group consisting of the compound according to claim 1 and 2 and tautomers and salts thereof.
- 請求項1および2に記載の化合物、並びにその互変異性体および塩からなる群から選ばれる少なくとも1つを有効成分として含有する外部寄生虫防除剤。 An ectoparasite control agent comprising as an active ingredient at least one selected from the group consisting of the compound according to claim 1 and 2 and tautomers and salts thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014184671A JP2017206440A (en) | 2014-09-10 | 2014-09-10 | Pyridine compound and use therefor |
| JP2014-184671 | 2014-09-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016039048A1 true WO2016039048A1 (en) | 2016-03-17 |
Family
ID=55458807
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/072141 WO2016039048A1 (en) | 2014-09-10 | 2015-08-04 | Pyridine compound and use thereof |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP2017206440A (en) |
| TW (1) | TW201609655A (en) |
| WO (1) | WO2016039048A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017155052A1 (en) * | 2016-03-09 | 2017-09-14 | 日本曹達株式会社 | Pyridine compound and use thereof |
| WO2019083008A1 (en) * | 2017-10-27 | 2019-05-02 | 住友化学株式会社 | Pyridine compound and pest control composition containing same |
| WO2019083007A1 (en) * | 2017-10-27 | 2019-05-02 | 住友化学株式会社 | Pyridine compound and harmful-arthropod control agent containing same |
| JPWO2018169045A1 (en) * | 2017-03-17 | 2020-01-16 | Meiji Seikaファルマ株式会社 | Pest control agent for the midges |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5148671A (en) * | 1974-08-28 | 1976-04-26 | Lilly Co Eli | 33 fueniruu4*1h* piridon oyobi pirijinchionrui narabini soreranoenruinoseiho |
| JPS5179725A (en) * | 1974-12-28 | 1976-07-12 | Yoshio Katsuta | SATSUCHUZAIO YOBISONOSEIHO |
| JPS552689A (en) * | 1978-06-19 | 1980-01-10 | Lilly Co Eli | Pyridinium salt derivative and herbicide containing it |
| JPS63243074A (en) * | 1987-03-13 | 1988-10-07 | ルセル−ユクラフ | Novel derivatives of pyridine, manufacture and intermediate, use as drug and composition |
| JPH05140107A (en) * | 1991-02-11 | 1993-06-08 | Imperial Chem Ind Plc <Ici> | Pyridine compound |
| WO2001053288A1 (en) * | 2000-01-20 | 2001-07-26 | Eisai Co., Ltd. | Novel piperidine compounds and drugs containing the same |
| WO2002066434A1 (en) * | 2001-02-20 | 2002-08-29 | Nippon Soda Co.,Ltd. | Oxime ether compound and agricultural or horticultural bactericide |
| WO2003087056A1 (en) * | 2002-04-15 | 2003-10-23 | Nippon Soda Co.,Ltd. | Novel oxime o-ether compound, production process, and agricultural or horticultural bactericide |
| JP2004501903A (en) * | 2000-06-29 | 2004-01-22 | 藤沢薬品工業株式会社 | Benzhydryl derivatives |
| JP2005520838A (en) * | 2002-03-19 | 2005-07-14 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Contains at least one N ′-(2-pyridinyl) methyl-3-pyridinecarboxamide derivative and one or more additional fungicides that are useful to protect against fungal and fungal plant diseases Synergistic fungicidal and fungicidal composition |
| WO2006137389A1 (en) * | 2005-06-21 | 2006-12-28 | Meiji Seika Kaisha Ltd. | Novel insecticidal/miticidal agent |
| JP2007186434A (en) * | 2006-01-12 | 2007-07-26 | Astellas Pharma Inc | Medicinal composition |
| JP2009215221A (en) * | 2008-03-11 | 2009-09-24 | Kumiai Chem Ind Co Ltd | Disease controlling agent for agriculture and horticulture |
| JP2011529464A (en) * | 2008-07-29 | 2011-12-08 | ビーエーエスエフ ソシエタス・ヨーロピア | Piperazine compounds with herbicidal activity |
| WO2013064521A1 (en) * | 2011-11-04 | 2013-05-10 | Syngenta Participations Ag | Pesticidal compounds |
| WO2014007228A1 (en) * | 2012-07-03 | 2014-01-09 | 小野薬品工業株式会社 | Compound having agonistic activity on somatostatin receptor, and use thereof for medical purposes |
| WO2014084330A1 (en) * | 2012-11-30 | 2014-06-05 | 協和発酵キリン株式会社 | Nitrogen-containing heterocyclic compound |
-
2014
- 2014-09-10 JP JP2014184671A patent/JP2017206440A/en active Pending
-
2015
- 2015-08-04 WO PCT/JP2015/072141 patent/WO2016039048A1/en active Application Filing
- 2015-08-04 TW TW104125166A patent/TW201609655A/en unknown
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5148671A (en) * | 1974-08-28 | 1976-04-26 | Lilly Co Eli | 33 fueniruu4*1h* piridon oyobi pirijinchionrui narabini soreranoenruinoseiho |
| JPS5179725A (en) * | 1974-12-28 | 1976-07-12 | Yoshio Katsuta | SATSUCHUZAIO YOBISONOSEIHO |
| JPS552689A (en) * | 1978-06-19 | 1980-01-10 | Lilly Co Eli | Pyridinium salt derivative and herbicide containing it |
| JPS63243074A (en) * | 1987-03-13 | 1988-10-07 | ルセル−ユクラフ | Novel derivatives of pyridine, manufacture and intermediate, use as drug and composition |
| JPH05140107A (en) * | 1991-02-11 | 1993-06-08 | Imperial Chem Ind Plc <Ici> | Pyridine compound |
| WO2001053288A1 (en) * | 2000-01-20 | 2001-07-26 | Eisai Co., Ltd. | Novel piperidine compounds and drugs containing the same |
| JP2004501903A (en) * | 2000-06-29 | 2004-01-22 | 藤沢薬品工業株式会社 | Benzhydryl derivatives |
| WO2002066434A1 (en) * | 2001-02-20 | 2002-08-29 | Nippon Soda Co.,Ltd. | Oxime ether compound and agricultural or horticultural bactericide |
| JP2005520838A (en) * | 2002-03-19 | 2005-07-14 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Contains at least one N ′-(2-pyridinyl) methyl-3-pyridinecarboxamide derivative and one or more additional fungicides that are useful to protect against fungal and fungal plant diseases Synergistic fungicidal and fungicidal composition |
| WO2003087056A1 (en) * | 2002-04-15 | 2003-10-23 | Nippon Soda Co.,Ltd. | Novel oxime o-ether compound, production process, and agricultural or horticultural bactericide |
| WO2006137389A1 (en) * | 2005-06-21 | 2006-12-28 | Meiji Seika Kaisha Ltd. | Novel insecticidal/miticidal agent |
| JP2007186434A (en) * | 2006-01-12 | 2007-07-26 | Astellas Pharma Inc | Medicinal composition |
| JP2009215221A (en) * | 2008-03-11 | 2009-09-24 | Kumiai Chem Ind Co Ltd | Disease controlling agent for agriculture and horticulture |
| JP2011529464A (en) * | 2008-07-29 | 2011-12-08 | ビーエーエスエフ ソシエタス・ヨーロピア | Piperazine compounds with herbicidal activity |
| WO2013064521A1 (en) * | 2011-11-04 | 2013-05-10 | Syngenta Participations Ag | Pesticidal compounds |
| WO2014007228A1 (en) * | 2012-07-03 | 2014-01-09 | 小野薬品工業株式会社 | Compound having agonistic activity on somatostatin receptor, and use thereof for medical purposes |
| WO2014084330A1 (en) * | 2012-11-30 | 2014-06-05 | 協和発酵キリン株式会社 | Nitrogen-containing heterocyclic compound |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017155052A1 (en) * | 2016-03-09 | 2017-09-14 | 日本曹達株式会社 | Pyridine compound and use thereof |
| JPWO2017155052A1 (en) * | 2016-03-09 | 2019-01-17 | 日本曹達株式会社 | Pyridine compounds and uses thereof |
| US10781177B2 (en) | 2016-03-09 | 2020-09-22 | Nippon Soda Co., Ltd. | Pyridine compound and use thereof |
| JPWO2018169045A1 (en) * | 2017-03-17 | 2020-01-16 | Meiji Seikaファルマ株式会社 | Pest control agent for the midges |
| JP7090071B2 (en) | 2017-03-17 | 2022-06-23 | Meiji Seikaファルマ株式会社 | Controlling agents for suborder mites |
| US12108761B2 (en) | 2017-03-17 | 2024-10-08 | Meiji Seika Pharma Co., Ltd. | Mesostigmata mite control agent |
| WO2019083008A1 (en) * | 2017-10-27 | 2019-05-02 | 住友化学株式会社 | Pyridine compound and pest control composition containing same |
| WO2019083007A1 (en) * | 2017-10-27 | 2019-05-02 | 住友化学株式会社 | Pyridine compound and harmful-arthropod control agent containing same |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201609655A (en) | 2016-03-16 |
| JP2017206440A (en) | 2017-11-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6159906B2 (en) | Mesoionic compounds | |
| CN102573478B (en) | As 1-(pyridin-3-yl)-pyrazoles and 1-(pyrimidine-5-the base)-pyrazoles of insecticide | |
| CN106573894B (en) | Diaryl imidazole compound and pest control agent | |
| JP6508540B2 (en) | Pyridine compound and use thereof | |
| JP2013028588A (en) | Pest-controlling agent | |
| WO2019022061A1 (en) | Oxadiazole compound and fungicide for agricultural and horticultural use | |
| JP6837052B2 (en) | Pyridine compounds and their uses | |
| WO2019031384A1 (en) | 1,3,5,6-tetrasubstituted thieno[2,3-d]pyrimidine-2,4-(1h,3h)dione compound and agricultural or horticultural bactericide | |
| JP2021008403A (en) | 1,3,5,6-TETRA-SUBSTITUTED THIENO[2,3-d]PYRIMIDINE-2,4(1H,3H)DIONE COMPOUND AND AGRICULTURAL/HORTICULTURAL FUNGICIDE | |
| JP2021028298A (en) | 1,3,5,6-tetrasubstituted thieno[2,3-d]pyrimidine-2,4(1h,3h)dione compound, and agricultural and horticultural fungicide | |
| TW201817729A (en) | Novel mesoionic insecticidal compound | |
| WO2016039048A1 (en) | Pyridine compound and use thereof | |
| JPWO2019065516A1 (en) | Quinoline compounds and fungicides for agriculture and horticulture | |
| JP6221189B2 (en) | Pyridine compounds and uses thereof | |
| JP5322296B2 (en) | Method for using plant disease control agent containing tetrazoyloxime derivative | |
| WO2016047550A1 (en) | Agricultural and horticultural fungicide containing aryl amidine compound | |
| JP2016079175A (en) | Germicide for agriculture and horticulture containing amidinopyridine compound | |
| EA019857B1 (en) | Insecticidal compounds | |
| JP2020097575A (en) | 1,3,5,6-TETRA SUBSTITUTED THIENO[2,3-d]PYRIMIDINE-2,4(1H,3H)DIONE COMPOUND AND HORTICULTURAL BACTERICIDE | |
| JP2019163223A (en) | 1,3,5,6-TETRASUBSTITUTED THIENO[2,3-d]PYRIMIDINE-2,4(1H,3H)DIONE COMPOUND, AND AGRICULTURAL AND HORTICULTURAL BACTERICIDE | |
| WO2018097126A1 (en) | Guanidine compound and fungicide | |
| JP2019026575A (en) | Novel compound and bactericide for agricultural and horticultural use containing the same as active ingredient | |
| JP2020040947A (en) | 1,3,5,6-tetra-substituted thieno [2,3-d]pyrimidine-2,4(1h,3h) dione compound and bactericide for agricultural and horticultural use | |
| EP3939975A1 (en) | Heterocyclic compound and agricultural or horticultural bactericide | |
| JP2022035834A (en) | 2,4-dioxo-1,4-dihydrothienopyrimidine compound and bactericide for agricultural and horticultural use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15840432 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15840432 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |